- 1The Affiliated Hospital of Qingdao University, Qingdao University, Qingdao, China
- 2Department of Hepatopancreatobiliary Surgery, Qingdao Municipal Hospital, Qingdao University, Qingdao, China
- 3Qingdao Hospital, University of Health and Rehabilitation Sciences (Qingdao Municipal Hospital), Qingdao, China
Background: Owing to advances in diagnostic technology, the diagnosis of T1 colorectal cancers (CRCs) continues to increase. However, the optimal management of T1 CRCs in the Western Hemisphere remains unclear due to limited population-based data directly comparing the efficacy of endoscopic therapy (ET) and surgical resection (SR). The purpose of this study was to report outcome data from a large Western cohort of patients who underwent ET or SR for early CRCs.
Methods: The SEER-18 database was used to identify patients with T1 CRCs diagnosed from 2004 to 2018 treated with ET or SR. Multivariable logistic regression models were employed to identify variables related to lymph node metastasis (LNM). Rates of ET and 1-year relative survival were calculated for each year. Effect of ET or SR on overall survival and cancer-specific survival was compared using Kaplan–Meier method stratified by tumor size and site.
Results: A total of 28,430 T1 CRCs patients were identified from 2004 to 2018 in US, with 22.7% undergoing ET and 77.3% undergoing SR. The incidence of T1 CRCs was 6.15 per 100,000 person-years, with male patients having a higher incidence. Left-sided colon was the most frequent location of tumors. The utilization of ET increased significantly from 2004 to 2018, with no significant change in 1-year relative survival rate. Predictors of LNM were age at diagnosis, sex, race, tumor size, histology, grade, and location. The 5-year relative survival rates were 91.4 and 95.4% for ET and SR, respectively. Subgroup analysis showed that OS and CSS were similar between ET and SR in T1N0M0 left-sided colon cancers with tumors 2 cm or less and in rectal cancers with tumors 1 cm or less.
Conclusion: Our study showed that ET was feasible and safe for patients with left-sided T1N0M0 colon cancers and tumors of 2 cm or less, as well as T1N0M0 rectal cancers and tumors of 1 cm or less. Therefore, the over- and under-use of ET should be avoided by carefully selecting patients based on tumor size and site.
1. Introduction
Colorectal cancers (CRCs) rank as the fourth most frequently diagnosed cancer and second leading cause of cancer-related death overall. An estimated 153,020 people in the United States will be diagnosed with CRCs and it will result in the death of 52,550 individuals as of 2023 (1). Although the incidence of CRCs has remained stable or decreased in highly developed countries over the last several decades, recent advances in screening and diagnostic technologies have led to an increase in the detection of early-stage CRCs, including tumors classified as T1 (2). The current treatment options for T1 CRCs include surgical resection (SR) and endoscopic therapy (ET). SR has traditionally been the mainstay of curative intent treatment, but ET is being increasingly adopted for patients with superficial CRCs due to its advantages in reducing treatment-related adverse events, compared to colorectal surgery in clinical practice, especially in the elderly pupulation (3, 4). Several studies have demonstrated that endoscopic removal of tumors is both feasible and suitable for many T1 cancers (5–8). However, ET is technically and clinically challenging due to the varied risks of lymph node metastasis (LNM) among T1 CRCs. Approximately 10% of T1 diseases are found to have LNM at the time of diagnosis, and these patients are candidates for radical surgery. For the remaining patients, endoscopic removal is considered sufficient owing to the low risk of LNM. Moreover, the characteristics of colorectal lesions, such as tumor size, location, and histology, may affect the optimal removal method. Therefore, comparing the long-term survival outcomes of ET and SR is crucial for determining the optimal treatment for T1 CRCs. However, there is a lack of population-based studies that have examined the outcomes of ET versus SR among patients with T1 CRCs in the United States.
Early-stage CRCs present several unresolved clinical questions, including the optimal treatment for T1N0M0 CRCs, which is currently unclear due to the limited population-based data comparing the efficacy of ET and SR in Western hemisphere. Therefore, our study aims to evaluate the relative prevalence, demographics, tumor characteristics, treatment, and survival of patients with T1 CRCs in the United States. In addition, we will analyze the effectiveness of ET versus SR in treating T1 CRCs stratified by tumor size and location.
Methods
Data source and patient population
The Surveillance, Epidemiology, and End Results (SEER)-18 registries were used to identify patients with T1 CRCs diagnosed between 2004 and 2018, based on the International Classification of Diseases for Oncology, Third Edition (ICD-O-3). The institutional review board of Qingdao municipal hospital deemed this cohort study exempt from review and informed consent requirement as the data was deidentified and publicly available. And the work has been reported in line with the STROCSS criteria (9).
Patients were then divided into two groups according to their surgical approach used: ET group and SR group. Other studied variables included patient demographic and clinical characteristics. Patient race was coded as white, black, and other. Age at diagnosis was categorized into two groups of <50 years and ≥ 50 years. This age threshold allows us to better capture the disparities in disease characteristics and outcomes between early-onset and late-onset CRCs in our analysis. Tumor characteristics included tumor grade (well-differentiated and poorly-differentiated), size (≤1 cm, ≤2 cm, ≤3 cm, and > 3 cm), histology (adenocarcinoma, mucous, and other), lymph node status (positive and negative), and tumor location (right-sided colon, left-sided colon, and rectum). The primary endpoints were OS and CSS. Patients were excluded if showed a distant metastasis at baseline. And those lacking data on tumor size, surgery, or survival were also not include in our study.
Statistical analysis
Using the SEER*Stat statistical software, we calculated age-adjusted incidence rates for patients with T1 CRCs overall and specific to sex (male and female) and age groups (0–24, 25–29, 30–34, 35–39, 40–44, 45–49, 50–54, 55–59, 60–64, 65–69, 70–74, 75–79, 80–84, and ≥ 85) from 2004 to 2018. All incidence rates were standardized to the 2000 US standard population and reported per 100,000 person-years. Categorical variables were reported as frequency and percentage, and the distribution of variables between groups were analyzed using χ2 or Fisher exact tests. Univariate and multivariate logistic regression models were utilized to identify factors associated with lymph node metastasis in T1 CRCs patients. The rates of ET utilization in patients were calculated for each year during the study period of 2004 to 2018. Differences in survival outcomes between ET and SR groups were examined using Kaplan–Meier method with log-rank test. To investigate the effect of surgical approaches on patient survival based on tumor characteristics, we conducted a subgroup analysis stratified by tumor size and site (right-sided colon, left-sided colon, and rectum). Two-sided p values and 95% CIs were reported, and p < 0.05 was considered as having statistical significance. All statistical analyses were performed by SPSS, version 22.0 and R, version 4.1.1.
2. Results
2.1. Patient characteristics and incidence
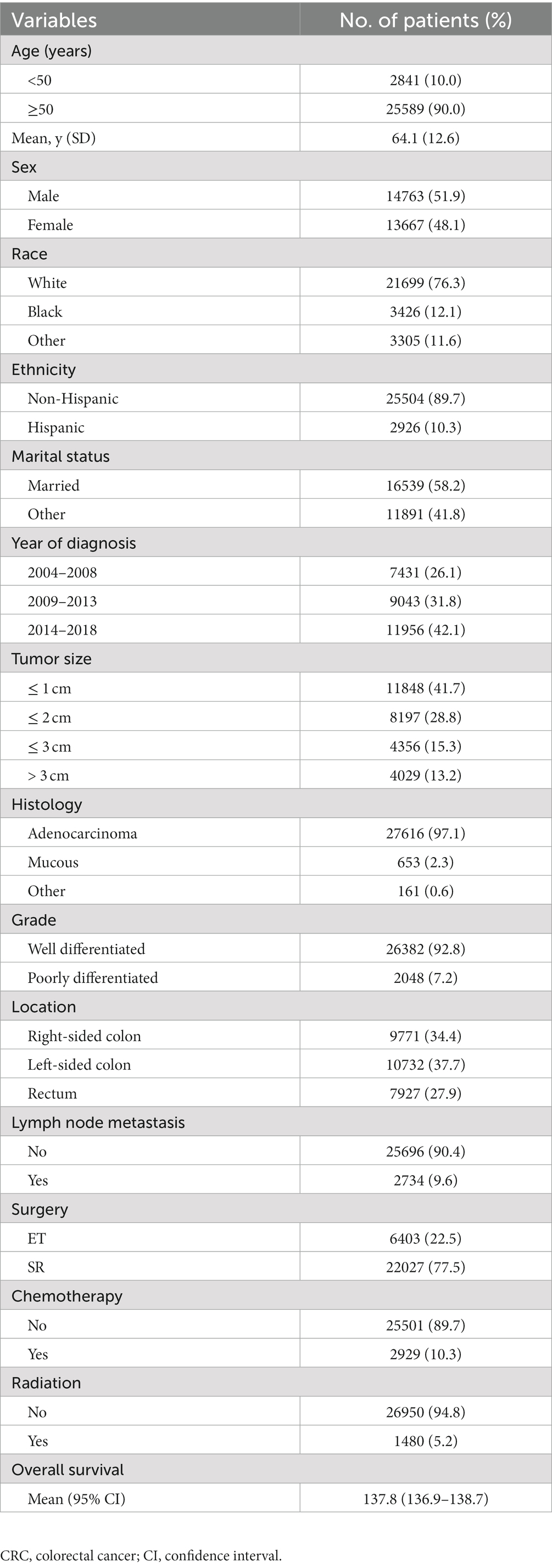
After rigorous screening of patients based on inclusion and exclusion criteria, we identified 28,430 eligible patients with T1 CRCs from 2004 to 2018 in the US. Among them, 51.9% were male and the mean age was 64.1 years (SD: 12.6). The majority of patients were White individuals (76.3%) and non-Hispanic (89.7%). LNM was present in only 9.6% of cases. Among these patients, 22.5% underwent ET and 77.5% underwent SR for the tumor. Table 1 shows the detailed demographic and clinical characteristics of all enrolled patients. The overall age-adjusted incidence of T1 CRCs from 2004 to 2018 was 6.15 per 100,000 person-years (Figure 1). Male patients had a higher incidence of T1 CRCs than female patients (7.22 vs. 5.26 per 100,000 person-years). The incidence increased with age, with the highest incidence occurring between 80–84 years old for both male and female patients.
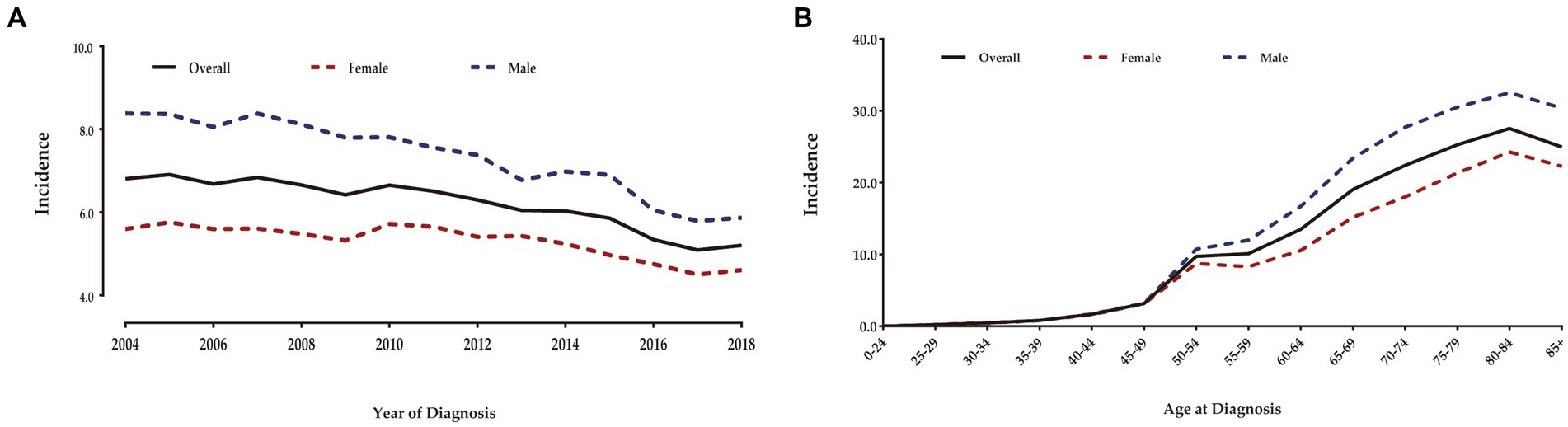
Figure 1. Annual age-adjusted incidence of patients with T1 CRCs in US (Per 100,000 person-years) (A). Age-wise incidence of patients with T1 CRCs in US (B).
Tumor characteristics and treatment
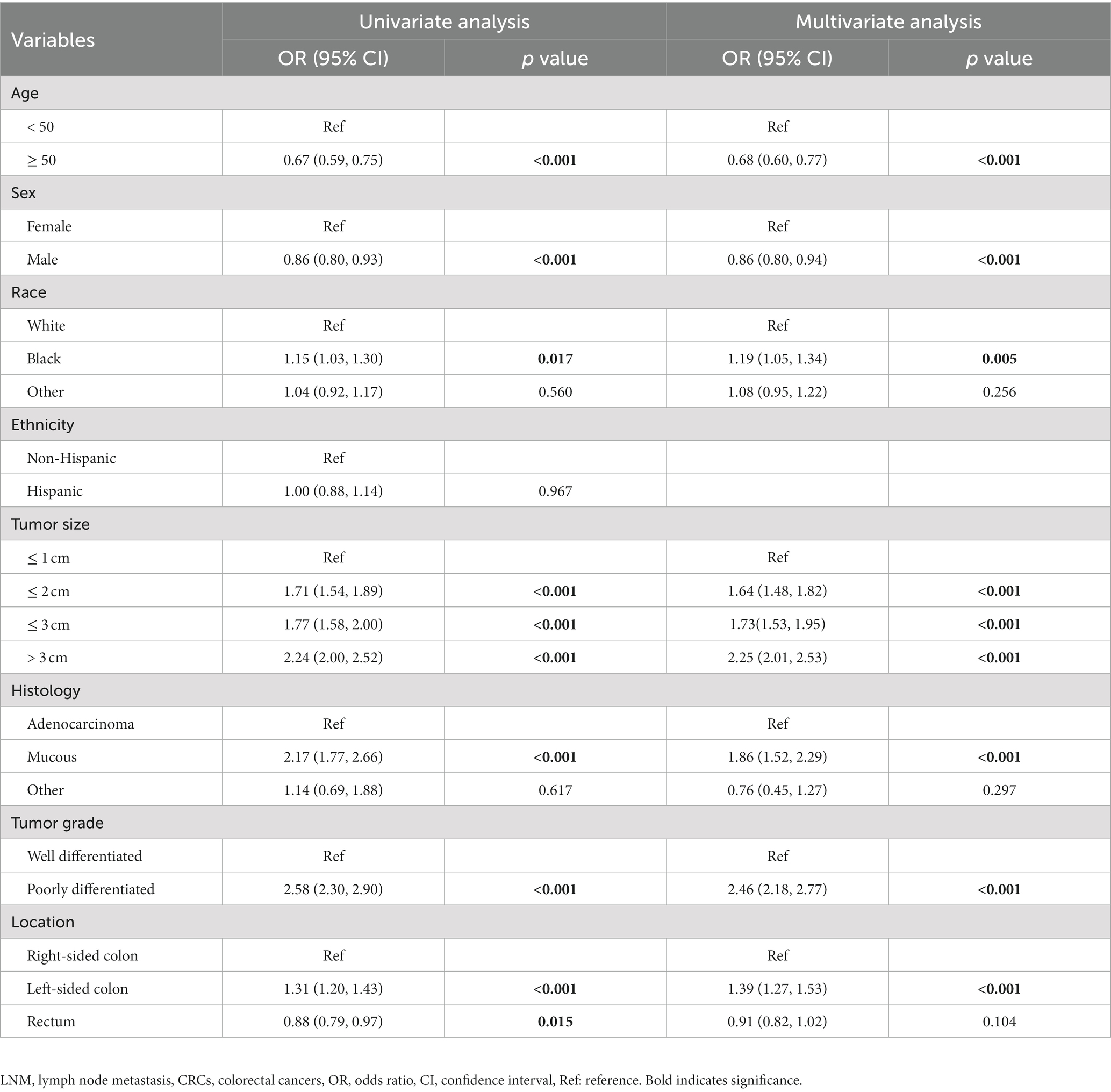
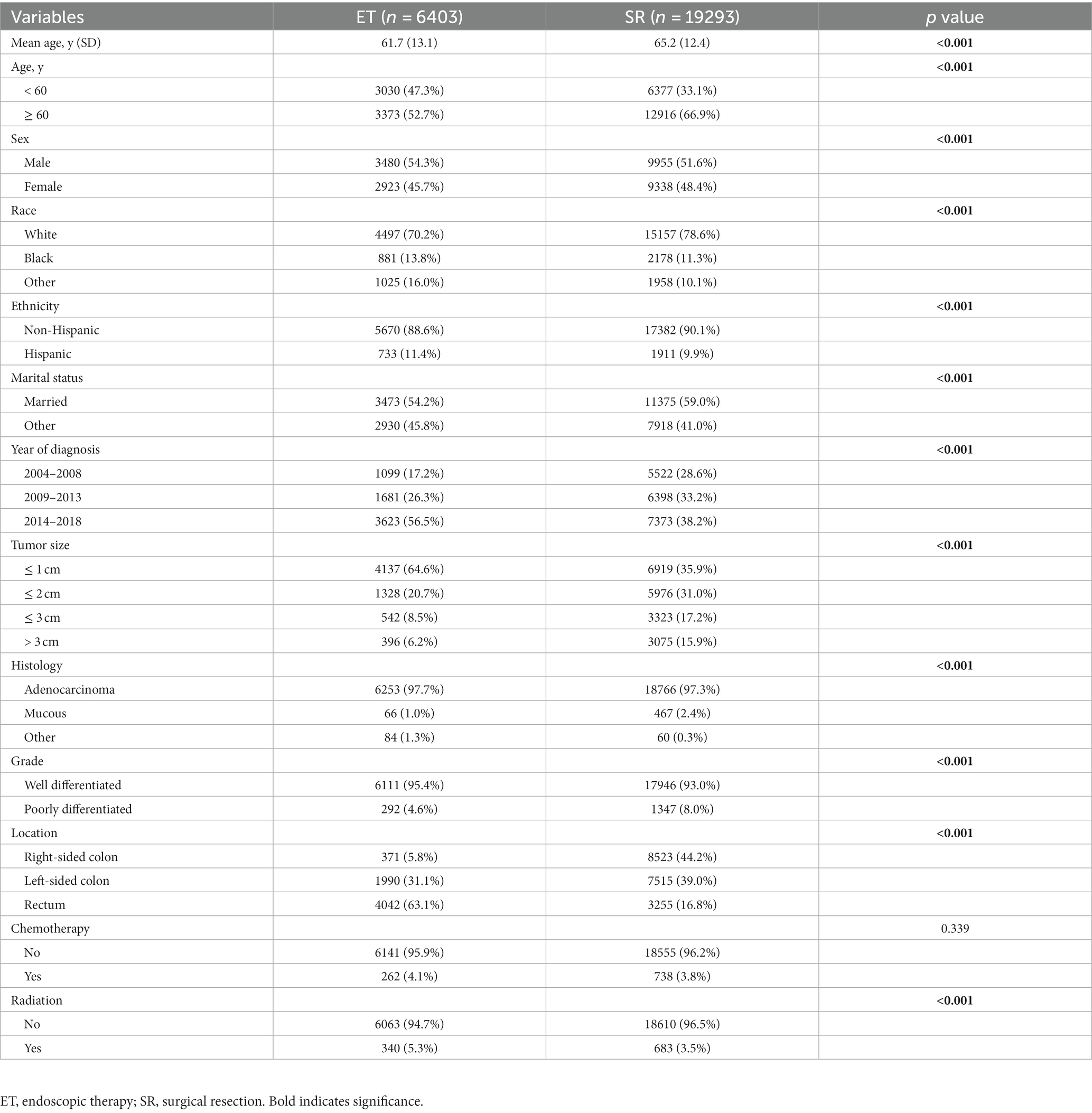
The number of T1 CRCs patients increased with year synchronously in males and females, with the most pronounced increase appearing in tumors that were 1 cm or smaller (Figure 2A). Most tumors diagnosed were well-differentiated (92.8%), while the remaining 7.2% were poorly differentiated (Table 1). The left-sided colon was the most common location of tumors (37.7%), followed by the right-sided colon (34.4%) and rectum (27.9%). Figure 2 illustrates the trends in tumor size, differentiation, location, and LNM over the study period from 2004 to 2018. The proportion of patients with LNM remained relatively constant throughout the study period. However, there was a significant increase in the percentage of patients receiving ET during the follow-up period, from 14.7% in 2004 to 35.3% in 2018. Despite this, there was no significant change in the 1-year relative survival rate of T1 CRCs patients between 2004 and 2018 (Figure 3). These findings suggest that ET is increasingly being used as a treatment option for T1 CRCs patients.
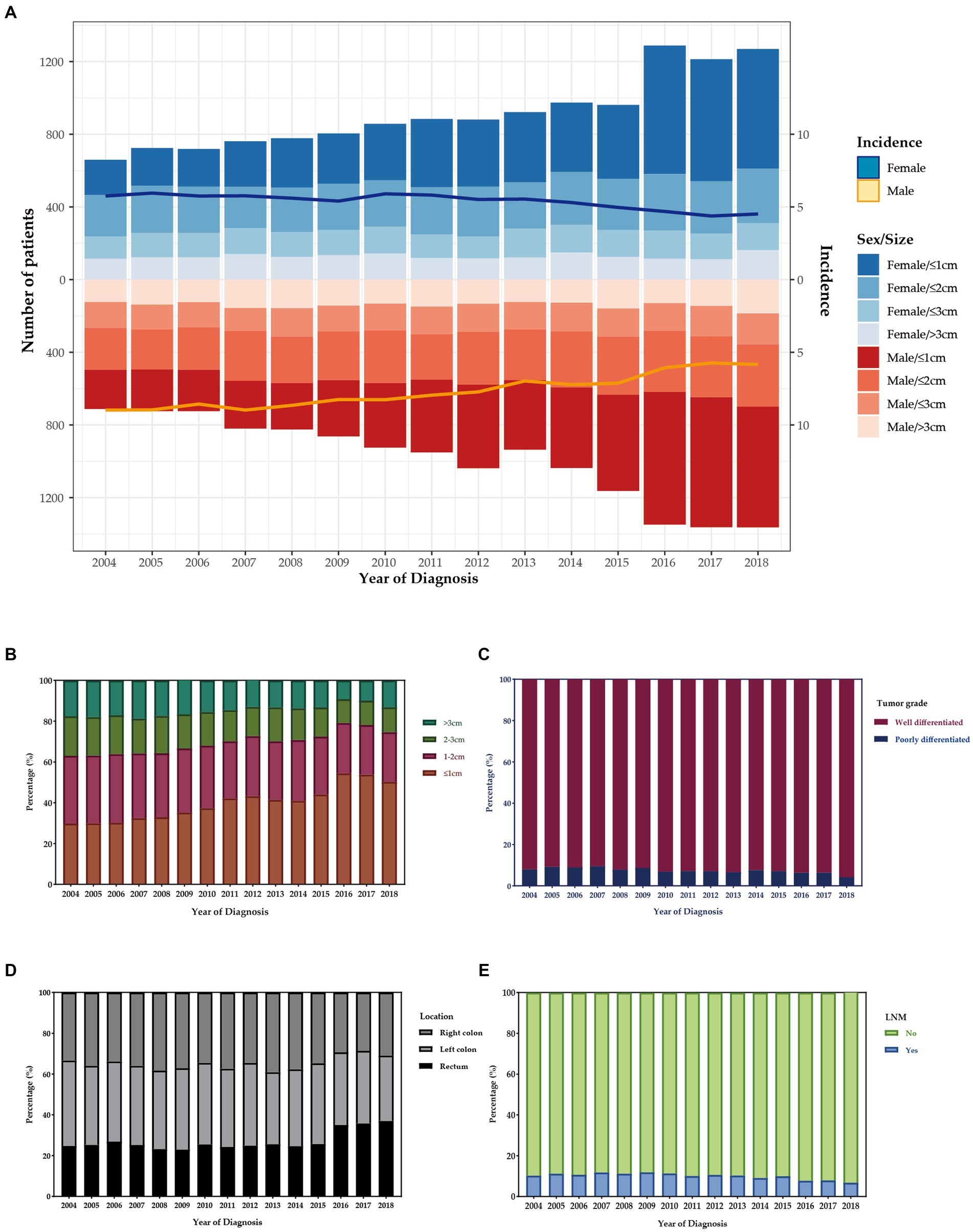
Figure 2. (A) The age distribution of cases with T1 CRCs from 2004 to 2018 in US. The bar was the number of cases in females and males, and the line with 95% CI represents age-adjusted incidence. Trends of distribution in tumor size (B), differentiation (C), location (D), and LNM (E) over the study period.
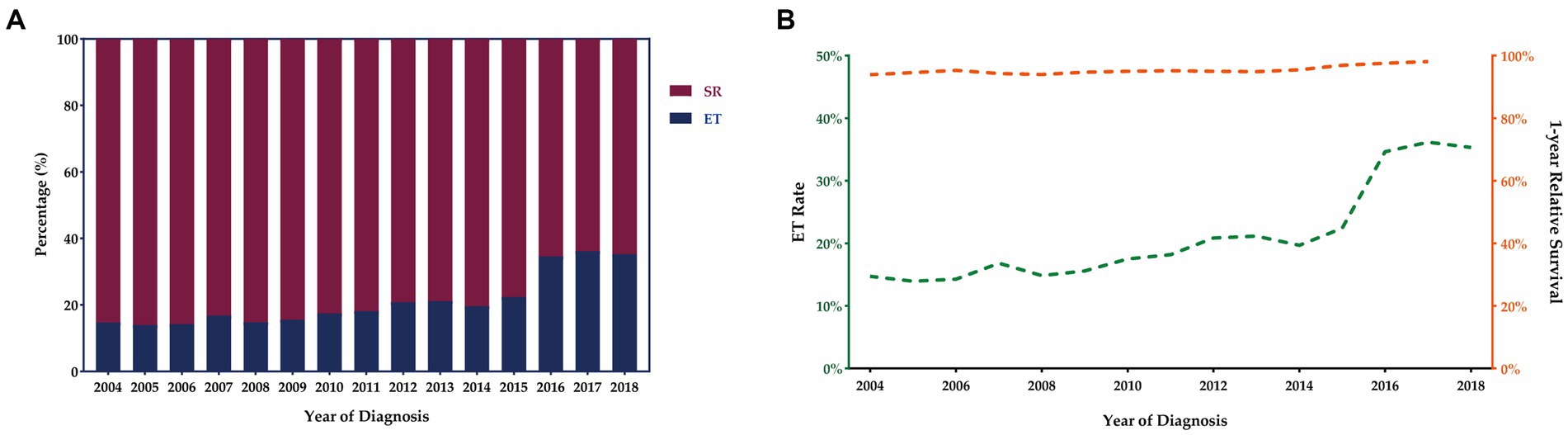
Figure 3. Trends in treatment modalities for T1 CRCs in US from 2004 to 2018 (A). ET rates and 1-year relative survival rate for patients with T1 CRCs from 2004 to 2018 (B).
Predictors of LNM
There were 2734 out of 28487 patients (9.6%) with T1 CRCs who were found to have LNM. The odds of LNM in patients with T1 CRCs were analyzed by a logistic regression model. Factors associated with LNM in multivariate analysis were age at diagnosis [≥50 (OR: 0.68, 95%CI: 0.60–0.77, p < 0.001)], sex [male (OR: 0.86, 95%CI: 0.80–0.94, p < 0.001)], race [Black (OR: 1.19, 95%CI: 1.05–1.34, p = 0.005)], tumor size [size≤2 cm (OR: 1.64, 95%CI: 1.48–1.82, p < 0.001), size≤3 cm (OR: 1.73, 95%CI: 1.53–1.95, p < 0.001), size>3 cm (OR: 2.25, 95%CI: 2.01–2.53, p < 0.001)], histology [mucous (OR: 1.86, 95%CI: 1.52–2.29, p < 0.001)], tumor grade (OR: 2.46, 95%CI: 2.18–2.77, p < 0.001), and location [left-sided colon (OR: 1.39, 95%CI: 1.27–1.53, p < 0.001)] (Table 2).
2.2. Survival
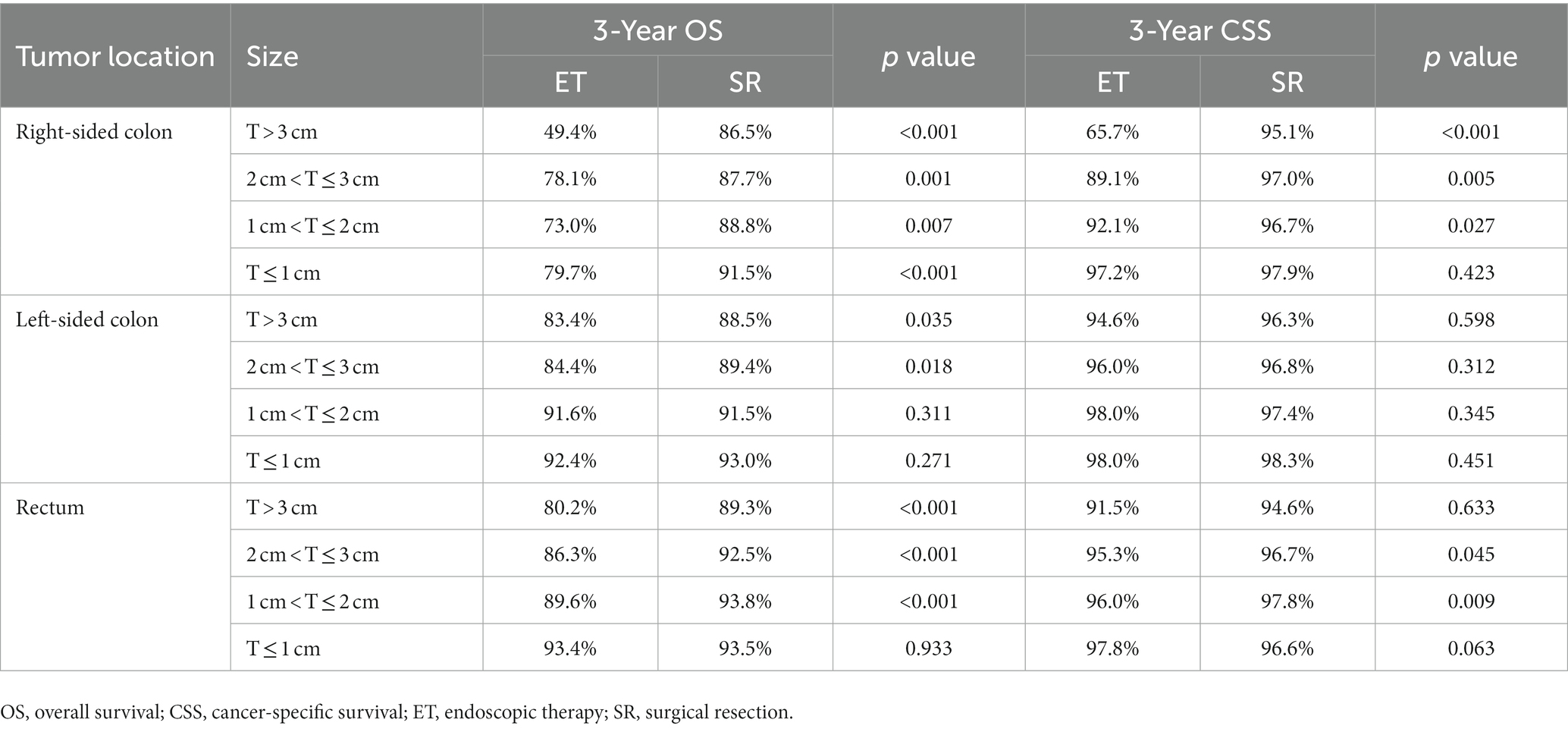
The 5-year relative survival (RS) rates for T1 CRCs were 91.4 and 95.4% for patients who underwent ET and SR, respectively, while the 5-year cause-specific survival (CSS) rates were 94.6% for both groups (Figure 4).
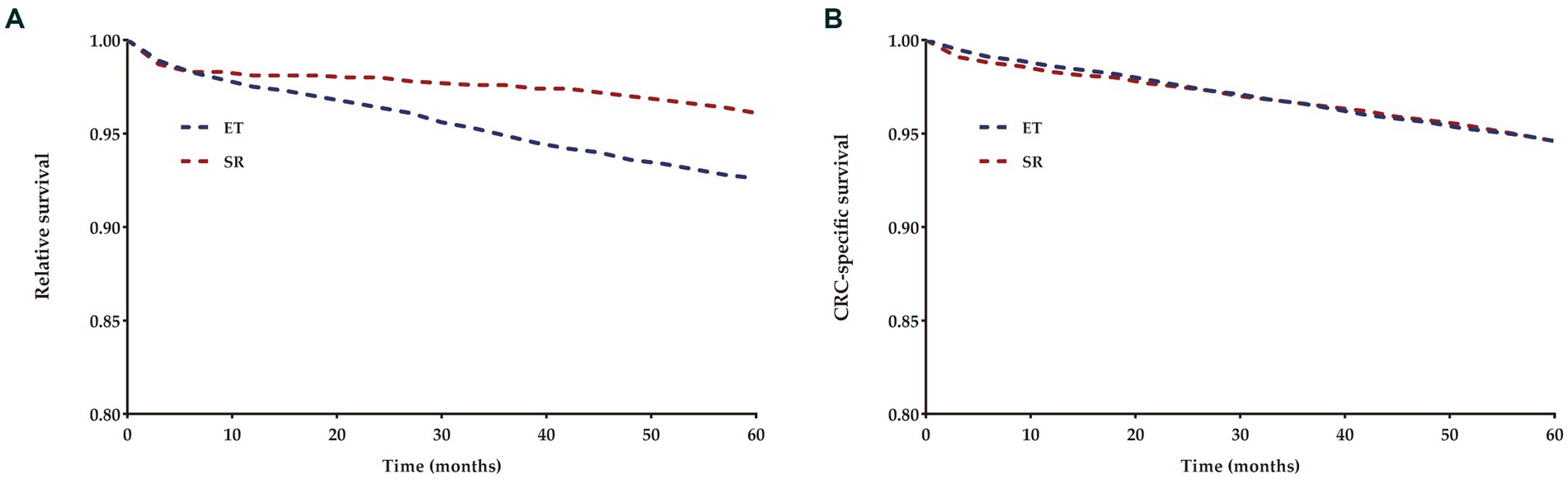
Figure 4. Relative (A) and cancer-specific (B) survival for each treatment group compared to US population.
2.3. Subgroup analysis according to tumor size
The study included 25696 patients with T1N0M0 CRCs, classified into four groups based on tumor size: T ≤ 1 cm (n = 11056), 1 cm < T ≤ 2 cm (n = 7304), 2 cm < T ≤ 3 cm (n = 3865), and > 3 cm (n = 3471). The baseline characteristics of all patients, stratified by treatment group, are presented in Table 3. Significant differences were observed in all clinical features between these two cohorts. As expected, patients with small (≤1 cm) and rectal cancer were more likely to receive ET. During the follow-up period, there were 1005 deaths among the 6403 patients in the ET group and 3996 deaths among the 19293 patients in the SR group. To determine the effect of ET on OS and CSS, we analyzed the survival outcomes using the Kaplan–Meier method (Figure 5). Our analysis revealed that OS was comparable between patients who received ET and SR for tumors 2 cm or less (1 cm < T ≤ 2 cm: HR, 0.90, 95%CI, 0.79–1.03, p = 0.120; ≤1 cm: HR, 0.97, 95%CI, 0.87–1.08, p = 0.540), but SR was associated with significantly better OS in tumors larger than 2 cm (>3 cm: HR, 0.69, 95%CI, 0.57–0.83, p < 0.001; 2 cm < T ≤ 3 cm: HR, 0.75, 95%CI, 0.63–0.89, p = 0.001). Meanwhile, for CSS, there was no significant difference between ET and SR in tumors 1 cm or less (HR, 0.94, 95%CI, 0.75–1.18, p = 0.610), but SR was associated with better CSS in patients with tumors larger than 1 cm (>3 cm: HR, 0.65, 95%CI, 0.47–0.90, p = 0.008; 2 cm < T ≤ 3 cm: HR, 0.52, 95%CI, 0.38–0.71, p < 0.001; 1 cm < T ≤ 2 cm: HR, 0.70, 95%CI, 0.55–0.91, p = 0.006).
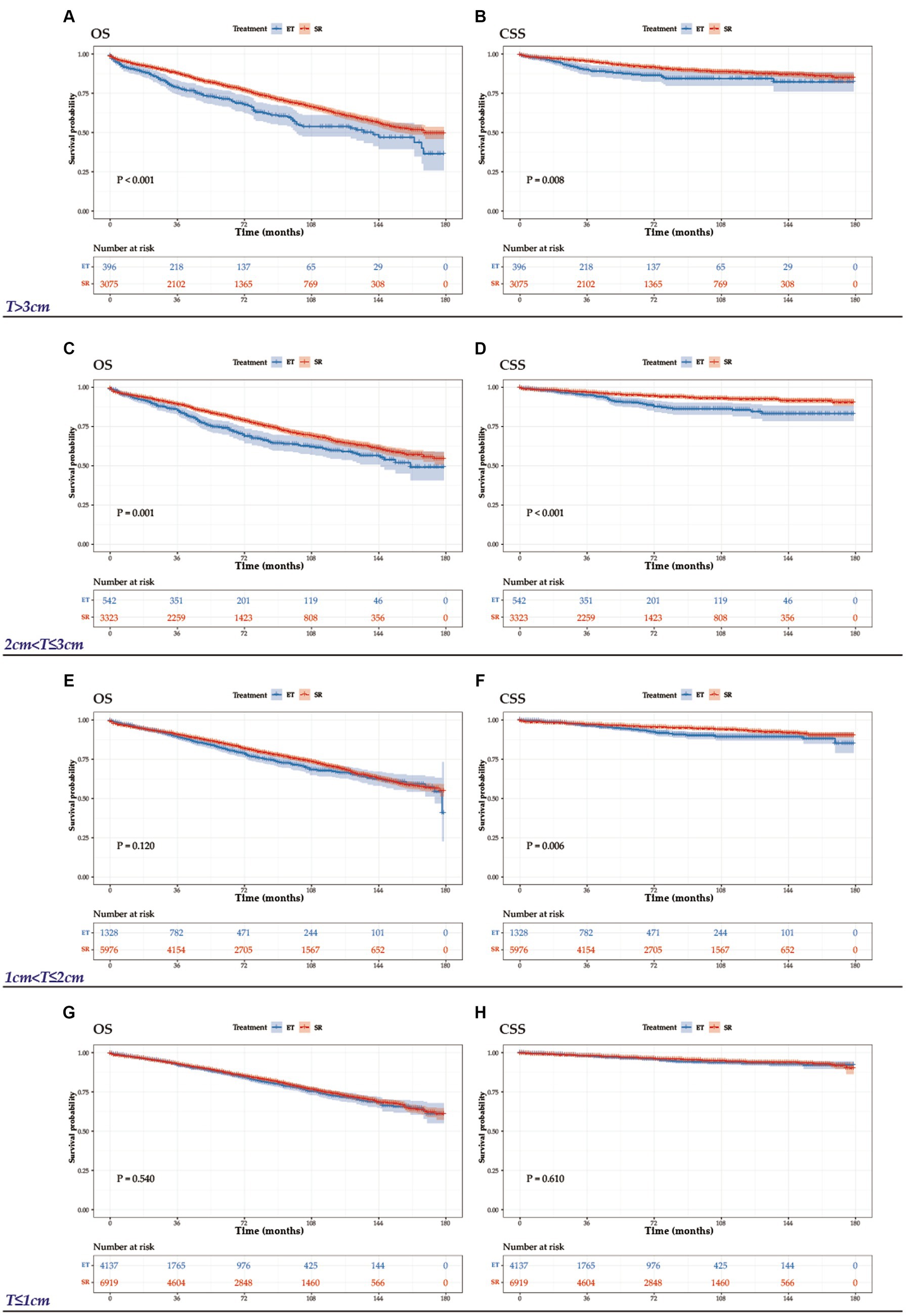
Figure 5. OS and CSS graphs by treatment for tumors >3 cm (A, B), 2 cm < T ≤ 3 cm (C, D), 1 < T ≤ 2 cm (E, F), and T ≤ 1 cm (G, H).
Subgroup analysis according to tumor site
Table 4 presents the 3-year OS and CSS rates of patients with T1N0M0 CRCs categorized by tumor location. The results of the site-specific analysis indicate that for patients with left-sided colon cancer and tumors 2 cm or less, as well as for patients with rectal cancer and tumors 1 cm or less, the survival outcomes including OS and CSS were similar between ET and SR treatment options.
Discussion
Our study provides a comprehensive analysis on the topic of T1 CRCs, including examining the epidemiology, clinicopathologic characteristics, treatment, and survival from 2004 to 2018 in the United States. To the best of our knowledge, this study is the first to explore two key aspects of T1 CRCs using the SEER-18 database: (1) the epidemiology of T1 CRCs and (2) the subclassifications of treatment modalities for T1N0M0 CRCs based on tumor size and site. We found that the incidence of T1 CRCs was 6.15 per 100,000 person-years, with higher rates observed in males than females (7.22 vs. 5.26 per 100,000 person-years). Incidence increased with age and peaked between ages 80–84 for both genders. Additionally, 9.6% of the patients were found to have LNM. Factors associated with LNM in multivariate analysis were age at diagnosis, sex, race, tumor size, histology, tumor grade, and location. Our findings also suggest an increasing trend in the use of ET as a treatment option for T1 CRCs patients. However, subgroup analysis according to tumor size and site demonstrates that ET was associated with similar survival outcomes to SR only in T1N0M0 patients with left-sided colon cancer and tumors 2 cm or less or in patients with rectal cancer and tumors 1 cm or less.
With the advancement of colonoscopic techniques, endoscopic removal of neoplasms has become one of the preferred treatments for T1 disease. However, the presence of LNM is a crucial factor in determining the feasibility and suitability of ET, as it is only safe for patients in the absence of nodal metastasis. Our study found that LNM occurred in 9.6% of T1 CRCs patients, consistent with previous studies, indicating that nearly 90% of this population can benefit from ET (10–12). Identifying those at low risk of LNM could help balance the potential risks and benefits of ET for treating early-stage CRCs. Multivariable analyses in our study revealed that age, sex, race, tumor size, histology, tumor differentiation, and location were significantly associated with LNM in patients with submucosal invasive CRCs. This information can be used to make evidence-based decisions regarding active surveillance or additional radical resection following endoscopic resection. While previous studies have identified several pathological high-risk features, such as deep submucosal invasion, lymphovascular invasion, positive resection margin, and tumor budding, as predictors of presence of LNM in early CRCs, our study could not investigate these factors due to limitations of the SEER database (13–15). Accurately evaluating the risk of LNM is critical in determining whether further radical resection is necessary for patients who have undergone ET. Kudo et al. developed an algorithm using artificial intelligence that includes patients’ age, tumor size, grade, location, lymphatic invasion, and vascular invasion to identify those with T1 CRCs who are at higher risk for LNM (16). This model could help in providing appropriate care without excess or deficient treatment for patients. Future studies should investigate the associations between patient characteristics and LNM to provide more comprehensive guidance for clinical decision-making.
Our study also found a significant increase in the use of ET for T1 CRCs in US from 2004 to 2018, with utilization rates rising from 14.7 to 35.3%. This trend may reflect the growing acceptance of ET as a feasible alternative to SR for treating early-stage invasive CRCs, likely driven by improving endoscopic techniques as well as the higher incidence of T1 disease as a result of population-based screening programs. Similar trends have been observed in the treatment of early-stage esophageal and gastric cancers (17, 18). Considering the increasing detection of CRCs at early stages, we are not surprised to find that the utilization of endoscopic removal for tumors will continue to rise in the future.
Most published studies on the outcomes of ET versus SR for early gastrointestinal cancers have been conducted in Asian countries (19–22). Therefore, it is essential to validate these findings in the Western population. Our study, based on a population-based registry from US, suggests that ET may be a promising treatment option for T1N0M0 left-sided colon cancers of 2 cm or less and T1N0M0 rectal cancers of 1 cm or less. A systematic review and meta-analysis by Yeh et al. involving 17 studies and 19,979 patients with colorectal cancers demonstrated comparable long-term survival outcomes between ET and SR for T1 CRCs (23). Additionally, Jang et al. utilized a Markov model to assess the cost-effectiveness of various treatment strategies in T1 CRCs based on biomarker profiles and concluded that ET was more suitable for patients with less aggressive biomarkers (24). Refinements in the endoscopic technology have significantly improved the management of colorectal lesions. However, one of the main challenges in treating early-stage CRCs is choosing the most suitable method of ET that ensures complete resection, reduces recurrence risk, and mitigates complications. A large meta-analysis, comprising 50 studies and involving 6,442 patients, was carried out to assess the effectiveness and safety of ET, and the results revealed that ET emerged as an exceedingly effective and safe intervention (25). It achieved an initial success rate of 92%, with only 8% of patients ultimately undergoing surgery due to the inadequacy of endoscopic resection for cure. It’s worth noting that the incidence of perforation and delayed bleeding stood at 1.5 and 6.5%, respectively. These results collectively underscore the remarkable effectiveness and safety profile of ET in clinical practice. Endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) are two main minimally invasive techniques used to remove superficially invasive CRCs, with ESD facilitating a higher en-bloc resection rate regardless of tumor size (26). However, ESD is associated with certain drawbacks. It is a technically demanding and time-consuming procedure, necessitating specialized training and dedicated equipment. Moreover, ESD entails elevated costs and poses higher risks of bleeding and perforation, especially when applied to colonic cases. Consequently, its execution should be entrusted to experienced gastroenterologists and surgeons operating in well-equipped centers with rigorous quality control protocols. Regrettably, ESD adoption remains relatively scarce in Western countries. A recent meta-analysis, encompassing 238 publications published between 1990 and 2016, investigated the efficacy of ESD on a global scale. Of note, 90% of the included studies originated from Eastern countries, while only 10% were conducted in Western countries (27). The findings revealed that ESD outcomes were significantly superior in Eastern countries compared to Western countries, prompting the authors to emphasize the importance of considering local ESD expertise and regional outcomes when treating gastrointestinal lesions with ESD. Similarly, another meta-analysis involving 97 studies, with 71 conducted in Asia, indicated that the standard ESD technique in non-Asian countries is still falling short in achieving satisfactory performance levels (28). In a retrospective analysis of ESD procedures performed in a high-volume US referral center, Zhang et al. observed that Western practitioners commonly faced a longer learning curve in comparison to their Asian counterparts. This disparity may be attributed in part to the limited availability of experienced trainers and training programs, as well as scarcity of easier lesions (29, 30). Prior studies showed that ESD for CRCs in the western countries achieves en-bloc lesion resection removal in 50–84% and curative resection rate in 74–92%, with a perforation and bleeding rate of 1.3–20% and 7.9–12%, respectively, which is not as favorable as in Eastern hemisphere studies (31–33). Given these limitations, there is a pressing need for developing an endoscopic technique superior to conventional EMR in terms of en bloc resection and local recurrence prevention, while minimizing the occurrence of adverse events. In this context, Underwater endoscopic mucosal resection (UEMR) has emerged as a compelling and oncologically safe alternative to EMR for the treatment of colorectal cancers (34–40). The findings reported by Nagl et al. are valuable additions to the literature, as they suggest that UEMR could serve as an intermediate approach, bridging the gap between smaller lesions suitable for conventional EMR and larger lesions where ESD might be the preferred procedure (36). Further research is warranted to compare the long-term outcomes and cost-effectiveness of various ET methods and to identify the optimal management approach for T1 CRCs.
In the context of our primary focus on comparing the efficacy of ET and SR, it’s worth noting that studies conducted by Marcellinaro and colleagues shed light on potential avenues for improving postoperative care and reducing complications in surgical interventions for CRCs. Marcellinaro et al. reported encouraging results with the Microbiota Implementation to Reduce Anastomotic Colorectal Leaks (MIRACLe) protocol in patients undergoing colorectal surgery for cancer (41). The MIRACLe protocol significantly reduced anastomotic leaks (AL), with an incidence as low as 1.7% in the post-matched MIRACLe group compared to 6.5% in the post-matched Control group. Similarly, a pilot study by the same group observed positive trends with the MIRACLe protocol, with a 1.7% AL incidence in the MIRACLe group compared to 6.4% in the control group (42). These findings suggest the potential benefits of microbiota manipulation as a complementary strategy to enhance surgical outcomes in CRCs patients.
It is important to acknowledge the limitations of our study, which include the reliance on data from the SEER database, limited availability of detailed clinical information, and the absence of prospective clinical trials for validation. The SEER database did not provide information regarding medical comorbidities, complications, lymphovascular invasion, margin status, or disease recurrence. Additionally, the lack of data on ulceration prevented us from determining the effect of this factor on long-term survival outcomes in patients after ET or SR. Patients who were ill or older were more likely to undergo ET due to its less invasive nature. As this was a retrospective study, inherent selection biases could not be fully avoided.
Our study revealed an increasing utilization of ET and promising survival outcomes for patients with T1 CRCs in US, which corresponds with the evolution of endoscopic techniques. Further analysis showed that ET was feasible and safe for patients with left-sided T1 colon cancers and tumors of 2 cm or less, as well as T1 rectal cancers and tumors of 1 cm or less. Therefore, the over- and under-use of ET should be avoided by carefully selecting patients based on tumor size and site. Future studies are required to examine the effectiveness of EMR and ESD at a population level in the Western hemisphere.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Author contributions
KL: conception. KS: data analysis. ZY: writing. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Shandong Provincial Postdoctoral Innovation Project (SDCX-ZG-202203025), Shandong Provincial Medical and Health Science and Technology Development Plan Project (202104080658), Shandong Provincial Geriatrics Medical Association Science and Technology Tackling Plan Project (LKJGG2021Y035), Qingdao City Pharmaceutical Research Guidance Program (2020-WJZD025).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel, RL, Miller, KD, Wagle, NS, and Jemal, A. Cancer statistics, 2023. CA Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763
2. Keum, N, and Giovannucci, E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. (2019) 16:713–32. doi: 10.1038/s41575-019-0189-8
3. Iversen, LH, Nielsen, H, Pedersen, L, Harling, H, and Laurberg, S. Seasonal variation in short-term mortality after surgery for colorectal cancer? Colorectal Dis. (2010) 12:e31–6. doi: 10.1111/j.1463-1318.2009.01888.x
4. Paulson, EC, Mitra, N, Sonnad, S, Armstrong, K, Wirtalla, C, Kelz, RR, et al. National Cancer Institute designation predicts improved outcomes in colorectal cancer surgery. Ann Surg. (2008) 248:675–86. doi: 10.1097/SLA.0b013e318187a757
5. Jayanna, M, Burgess, NG, Singh, R, Hourigan, LF, Brown, GJ, Zanati, SA, et al. Cost analysis of endoscopic mucosal resection vs surgery for large laterally spreading colorectal lesions. Clin Gastroenterol Hepatol. (2016) 14:271–278.e2. doi: 10.1016/j.cgh.2015.08.037
6. Ahlenstiel, G, Hourigan, LF, Brown, G, Zanati, S, Williams, SJ, Singh, R, et al. Actual endoscopic versus predicted surgical mortality for treatment of advanced mucosal neoplasia of the colon. Gastrointest Endosc. (2014) 80:668–76. doi: 10.1016/j.gie.2014.04.015
7. Law, R, Das, A, Gregory, D, Komanduri, S, Muthusamy, R, Rastogi, A, et al. Endoscopic resection is cost-effective compared with laparoscopic resection in the management of complex colon polyps: an economic analysis. Gastrointest Endosc. (2016) 83:1248–57. doi: 10.1016/j.gie.2015.11.014
8. Raju, GS, Lum, PJ, Ross, WA, Thirumurthi, S, Miller, E, Lynch, PM, et al. Outcome of EMR as an alternative to surgery in patients with complex colon polyps. Gastrointest Endosc. (2016) 84:315–25. doi: 10.1016/j.gie.2016.01.067
9. Agha, R, Abdall-Razak, A, Crossley, E, Dowlut, N, Iosifidis, C, and Mathew, G. STROCSS 2019 guideline: strengthening the reporting of cohort studies in surgery. Int J Surgery. (2019) 72:156–65. doi: 10.1016/j.ijsu.2019.11.002
10. Choi, JY, Jung, SA, Shim, KN, Cho, WY, Keum, B, Byeon, JS, et al. Meta-analysis of predictive clinicopathologic factors for lymph node metastasis in patients with early colorectal carcinoma. J Korean Med Sci. (2015) 30:398–406. doi: 10.3346/jkms.2015.30.4.398
11. Nascimbeni, R, Burgart, LJ, Nivatvongs, S, and Larson, DR. Risk of lymph node metastasis in T1 carcinoma of the colon and rectum. Dis Colon Rectum. (2002) 45:200–6. doi: 10.1007/s10350-004-6147-7
12. Ueno, H, Mochizuki, H, Hashiguchi, Y, Shimazaki, H, Aida, S, Hase, K, et al. Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology. (2004) 127:385–94. doi: 10.1053/j.gastro.2004.04.022
13. Lee, SJ, Kim, A, Kim, YK, Park, WY, Kim, HS, Jo, HJ, et al. The significance of tumor budding in T1 colorectal carcinoma: the most reliable predictor of lymph node metastasis especially in endoscopically resected T1 colorectal carcinoma. Hum Pathol. (2018) 78:8–17. doi: 10.1016/j.humpath.2018.02.001
14. Cracco, N, Todaro, V, Pedrazzi, G, Del Rio, P, Haboubi, N, and Zinicola, R. The risk of lymph node metastasis in T1 colorectal cancer: new parameters to assess the degree of submucosal invasion. Int J Color Dis. (2021) 36:41–5. doi: 10.1007/s00384-020-03738-0
15. Nishimura, T, Oka, S, Tanaka, S, Asayama, N, Nagata, S, Tamaru, Y, et al. Clinical significance of immunohistochemical lymphovascular evaluation to determine additional surgery after endoscopic submucosal dissection for colorectal T1 carcinoma. Int J Color Dis. (2021) 36:949–58. doi: 10.1007/s00384-020-03795-5
16. Kudo, SE, Ichimasa, K, Villard, B, Mori, Y, Misawa, M, Saito, S, et al. Artificial intelligence system to determine risk of T1 colorectal cancer metastasis to lymph node. Gastroenterology. (2021) 160:1075–84.e2. doi: 10.1053/j.gastro.2020.09.027
17. Ngamruengphong, S, Wolfsen, HC, and Wallace, MB. Survival of patients with superficial esophageal adenocarcinoma after endoscopic treatment vs surgery. Clin Gastroenterol Hepatol. (2013) 11:1424–1429.e2. doi: 10.1016/j.cgh.2013.05.025
18. Pourmousavi, MK, Wang, R, Kerdsirichairat, T, Kamal, A, Akshintala, VS, Hajiyeva, G, et al. Comparable cancer-specific mortality of patients with early gastric cancer treated with endoscopic therapy vs surgical resection. Clin Gastroenterol Hepatol. (2020) 18:2824–32.e1. doi: 10.1016/j.cgh.2020.04.085
19. Choi, IJ, Lee, JH, Kim, YI, Kim, CG, Cho, SJ, Lee, JY, et al. Long-term outcome comparison of endoscopic resection and surgery in early gastric cancer meeting the absolute indication for endoscopic resection. Gastrointest Endosc. (2015) 81:333–41.e1. doi: 10.1016/j.gie.2014.07.047
20. Chung, IK, Lee, JH, Lee, SH, Kim, SJ, Cho, JY, Cho, WY, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD study group multicenter study. Gastrointest Endosc. (2009) 69:1228–35. doi: 10.1016/j.gie.2008.09.027
21. Isomoto, H, Shikuwa, S, Yamaguchi, N, Fukuda, E, Ikeda, K, Nishiyama, H, et al. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut. (2009) 58:331–6. doi: 10.1136/gut.2008.165381
22. Sugimoto, T, Okamoto, M, Mitsuno, Y, Kondo, S, Ogura, K, Ohmae, T, et al. Endoscopic submucosal dissection is an effective and safe therapy for early gastric neoplasms: a multicenter feasible study. J Clin Gastroenterol. (2012) 46:124–9. doi: 10.1097/MCG.0b013e31822f3988
23. Yeh, JH, Tseng, CH, Huang, RY, Lin, CW, Lee, CT, Hsiao, PJ, et al. Long-term outcomes of primary endoscopic resection vs surgery for T1 colorectal cancer: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. (2020) 18:2813–23.e5. doi: 10.1016/j.cgh.2020.05.060
24. Jang, SR, Truong, H, Oh, A, Choi, J, Tramontano, AC, Laszkowska, M, et al. Cost-effectiveness evaluation of targeted surgical and endoscopic therapies for early colorectal adenocarcinoma based on biomarker profiles. JAMA Netw Open. (2020) 3:e1919963. doi: 10.1001/jamanetworkopen.2019.19963
25. Hassan, C, Repici, A, Sharma, P, Correale, L, Zullo, A, Bretthauer, M, et al. Efficacy and safety of endoscopic resection of large colorectal polyps: a systematic review and meta-analysis. Gut. (2016) 65:806–20. doi: 10.1136/gutjnl-2014-308481
26. Pimentel-Nunes, P, Libânio, D, Bastiaansen, BAJ, Bhandari, P, Bisschops, R, Bourke, MJ, et al. Endoscopic submucosal dissection for superficial gastrointestinal lesions: European Society of Gastrointestinal Endoscopy (ESGE) guideline - update 2022. Endoscopy. (2022) 54:591–622. doi: 10.1055/a-1811-7025
27. Daoud, DC, Suter, N, Durand, M, Bouin, M, Faulques, B, and von Renteln, D. Comparing outcomes for endoscopic submucosal dissection between eastern and Western countries: a systematic review and meta-analysis. World J Gastroenterol. (2018) 24:2518–36. doi: 10.3748/wjg.v24.i23.2518
28. Fuccio, L, Hassan, C, Ponchon, T, Mandolesi, D, Farioli, A, Cucchetti, A, et al. Clinical outcomes after endoscopic submucosal dissection for colorectal neoplasia: a systematic review and meta-analysis. Gastrointest Endosc. (2017) 86:74–86.e17. doi: 10.1016/j.gie.2017.02.024
29. Schlachterman, A, Yang, D, Goddard, A, Gotoda, T, and Draganov, PV. Perspectives on endoscopic submucosal dissection training in the United States: a survey analysis. Endosc Int Open. (2018) 6:E399–e409. doi: 10.1055/s-0044-101452
30. Zhang, X, Ly, EK, Nithyanand, S, Modayil, RJ, Khodorskiy, DO, Neppala, S, et al. Learning curve for endoscopic submucosal dissection with an untutored, prevalence-based approach in the United States. Clin Gastroenterol Hepatol. (2020) 18:580–8.e1. doi: 10.1016/j.cgh.2019.06.008
31. Probst, A, Golger, D, Anthuber, M, Märkl, B, and Messmann, H. Endoscopic submucosal dissection in large sessile lesions of the rectosigmoid: learning curve in a European center. Endoscopy. (2012) 44:660–7. doi: 10.1055/s-0032-1309403
32. Ohata, K, Kobayashi, N, Sakai, E, Takeuchi, Y, Chino, A, Takamaru, H, et al. Long-term outcomes after endoscopic submucosal dissection for large colorectal epithelial neoplasms: a prospective, Multicenter, Cohort Trial from Japan. Gastroenterology. (2022) 163:1423–34.e2. doi: 10.1053/j.gastro.2022.07.002
33. Lee, EJ, Lee, JB, Lee, SH, Kim, DS, Lee, DH, Lee, DS, et al. Endoscopic submucosal dissection for colorectal tumors—1,000 colorectal ESD cases: one specialized institute’s experiences. Surg Endosc. (2013) 27:31–9. doi: 10.1007/s00464-012-2403-4
34. Coutinho, LMA, Lenz, L, Kawaguti, FS, Martins, BC, Baba, E, Gusmon, C, et al. Underwater endoscopic mucosal resection for small rectal neuroendocrine TUMORS. Arq Gastroenterol. (2021) 58:210–3. doi: 10.1590/s0004-2803.202100000-37
35. Rodríguez Sánchez, J, Uchima Koecklin, H, González López, L, Cuatrecasas, M, de la Santa, BE, Olivencia Palomar, P, et al. Short and long-term outcomes of underwater EMR compared to the traditional procedure in the real clinical practice. Rev Esp Enferm Dig. (2019) 111:543–9. doi: 10.17235/reed.2019.6009/2018
36. Nagl, S, Ebigbo, A, Goelder, SK, Roemmele, C, Neuhaus, L, Weber, T, et al. Underwater vs conventional endoscopic mucosal resection of large sessile or flat colorectal polyps: a prospective randomized controlled trial. Gastroenterology. (2021) 161:1460–74.e1. doi: 10.1053/j.gastro.2021.07.044
37. Saito, Y, and Ono, A. Underwater endoscopic mucosal resection for colorectal lesions: a bridge between conventional endoscopic mucosal resection and endoscopic submucosal dissection. Gastroenterology. (2021) 161:1369–71. doi: 10.1053/j.gastro.2021.08.039
38. Sundaram, S. Conventional endoscopic mucosal resection versus underwater endoscopic mucosal resection: is the choice straightforward? Gastroenterology. (2022) 162:1775. doi: 10.1053/j.gastro.2021.08.034
39. Leung, FW. Incomplete resection after macroscopic radical endoscopic resection of T1 colorectal cancer-should a paradigm-changing approach to address the risk be considered? Transl Gastroenterol Hepatol. (2017) 2:69. doi: 10.21037/tgh.2017.08.04
40. Backes, Y, de Vos tot Nederveen Cappel, WH, van Bergeijk, J, ter Borg, F, Schwartz, MP, Spanier, BWM, et al. Risk for incomplete resection after macroscopic radical endoscopic resection of T1 colorectal cancer: a Multicenter cohort study. Am J Gastroenterol. (2017) 112:785–96. doi: 10.1038/ajg.2017.58
41. Marcellinaro, R, Grieco, M, Spoletini, D, Troiano, R, Avella, P, Brachini, G, et al. How to reduce the colorectal anastomotic leakage? The MIRACLe protocol experience in a cohort in a single high-volume Centre. Updat Surg. (2023) 75:1559–67. doi: 10.1007/s13304-023-01588-3
Keywords: T1 colorectal cancers, characteristics, lymph node metastasis, survival, endoscopic therapy, surgical resection
Citation: Shi K, Yang Z and Leng K (2023) Treatment for T1 colorectal cancers substratified by site and size: “horses for courses”. Front. Med. 10:1230844. doi: 10.3389/fmed.2023.1230844
Edited by:
Aldo Rocca, University of Molise, ItalyReviewed by:
Alessandro Gambella, University of Pittsburgh, United StatesPasquale Avella, University of Naples Federico II, Italy
Yu Cheng, Yantai Affiliated Hospital of Binzhou Medical University, China
Jie Li, Shandong Provincial Qianfoshan Hospital, China
Copyright © 2023 Shi, Yang and Leng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kaiming Leng, bGVuZ2thaW1pbmcxMTI4QGhvdG1haWwuY29t
†These authors have contributed equally to this work
 Kexin Shi1†
Kexin Shi1† Zhen Yang
Zhen Yang