- Department of Clinical Pharmacy, Xiangtan Central Hospital, Xiangtan, China
Background: Stevens-Johnson syndrome (SJS) is considered a hypersensitivity syndrome affecting the skin and mucous membranes. It has been reported that an anticonvulsant drug, oxcarbazepine, may cause Stevens-Johnson syndrome and toxic epidermal necrolysis (TEN). However, the clinical features of oxcarbazepine-induced Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) remain ambiguous. This article aims to explore the clinical features of SJS/TEN.
Methods: Systematic searches of several Chinese and English databases were conducted for case reports published on PubMed, EMBASE, Web of Science, MEDLINE, CNKI from January 1, 2007 to March 1, 2023.
Results: A total of seventeen patients (10 males and 7 females) were included in this study, including nine adult patients and eight pediatric patients. The results showed that males seem to have a higher prevalence of SJS/TEN than females, and SJS/TEN usually occurs within 2 weeks after administration of oxcarbazepine (OXC). The main clinical manifestations among the included patients were rashes or maculopapules (17 cases, 100%), fever (11 cases, 64.7%), mucosal lesions (15 cases, 88.2%), conjunctivitis with/without ocular discharge (12 cases, 70.6%), and blisters (12 cases, 70.6%). After stopping OXC or switching to other drugs that treat primary disease as well as treatment with IVIG, glucocorticoid, anti-allergy, and fluid replacement, eight of the included patients recovered completely, and another eight of the included patients reported symptomatic improvement, while the prognosis of one of the included patients was not reported.
Conclusion: Diverse clinical signs and symptoms of SJS/TEN might result in misinterpretation and delayed diagnosis. It should be identified and treated immediately to avoid significant consequences and potentially jeopardize patients’ lives.
Introduction
As an anticonvulsant medication, oxcarbazepine (OXC) is structurally similar to carbamazepine (CBZ), which was initially utilized in patients with neuralgia and epilepsy. OXC is an inactive prodrug converted into a pharmacologically active intermediate — monohydroxycarbazepine (MHD). OXC and its metabolites can suppress repetitive neuronal discharge, stabilize the membrane of highly stimulated nerve cells, block voltage-sensitive sodium channels, and lessen the synaptic transmission of nerve impulses (1). Previous studies indicated that the most common adverse events of OXC were drowsiness, headache, dizziness, diplopia, nausea, vomiting, and fatigue, with an incidence of 10% (2). Over the past few years, it has been reported that OXC may cause Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) (3).
SJS and TEN were described as serious mucocutaneous allergic reactions, mainly caused by certain medications such as antibiotics, NSAIDs (non-steroidal anti-inflammatory drugs), antiepileptics, or other medicines. SJS and TEN were categorized as belonging to the same spectrum of diseases and were classified according to the body surface area embroiled; skin injury surface area less than 10% is defined as SJS, more than 30% is considered as TEN and 10–30% is thought to be SJS-TEN overlap (4, 5). The primary clinical signs of SJS and TEN were maculopapules, which spread rapidly and merged to form blisters and epidermal necrosis. Despite the rare incidence of drug-induced SJS and TEN, the mortality rate is high, up to 7.5% in children and 20–25% in adults (6). In addition, the recovery period of SJS/TEN may also leave sequelae, including cutaneous problems (84.3%), ocular problems (59.5%), and oral mucosal problems (50.8%) (7). Because of the severity of SJS/TEN, early identification, diagnosis, and therapy of SJS/TEN are incredibly significant in reducing mortality. However, the timely identification and effective management of this condition are challenges for healthcare professionals. The clinical features reported in different case reports are inconsistent and variable and the diagnosis and treatment of SJS/TEN vary greatly among different specialists, and the data related to the treatment outcome are limited. There is still a lack of adequate evidence-based evaluation for OXC-induced SJS/TEN. This review aimed to investigate the clinical features of OXC-induced SJS/TEN by collecting relevant cases to provide a reference for accurate diagnosis, appropriate treatment, and prognosis of SJS/TEN.
Methods
Search strategy
The oxcarbazepine induced SJS/TEN case reports were collected by searching Chinese and English databases, including CNKI, Pubmed, Embase, and Web of Science, from 2007 to March 1, 2023. The following search terms were used to establish the search strategy, including ‘Oxcarbazepine’, ‘10,11-Dihydro-10-oxo-5H-dibenz(b,f)azepine-5-carboxamide’, ‘Trileptal’, ‘Timox’, ‘GP 47680’, ‘OXC’, ‘MHD’, ‘Monohydroxycarbazepine’ and ‘Stevens-Johnson Syndrome’, ‘Stevens Johnson Syndrome’, ‘Toxic Epidermal Necrolysis’, ‘Drug-Induced Stevens Johnson Syndrome’, ‘SJS’, ‘Epidermal Necrolysis, Toxic’, ‘Toxic Epidermal Necrolyses’, ‘TEN’.
Inclusion and exclusion criteria
Inclusion criteria: (1) studies published by case reports of OXC-induced SJS and TEN. (2) the full article was written in Chinese or English.
Exclusion criteria: studies published by review, mechanistic studies, only abstract, no full-text researches, animal studies, and duplicate cases.
Data extraction
The data extraction was conducted independently by two reviewers using self-designed tables. Data were extracted on the first author, publication year, age, disease history, primary disease treatment drugs, the onset time after administration, clinical manifestations, laboratory examinations, body examination, treatment, and prognosis.
Results
Basic information
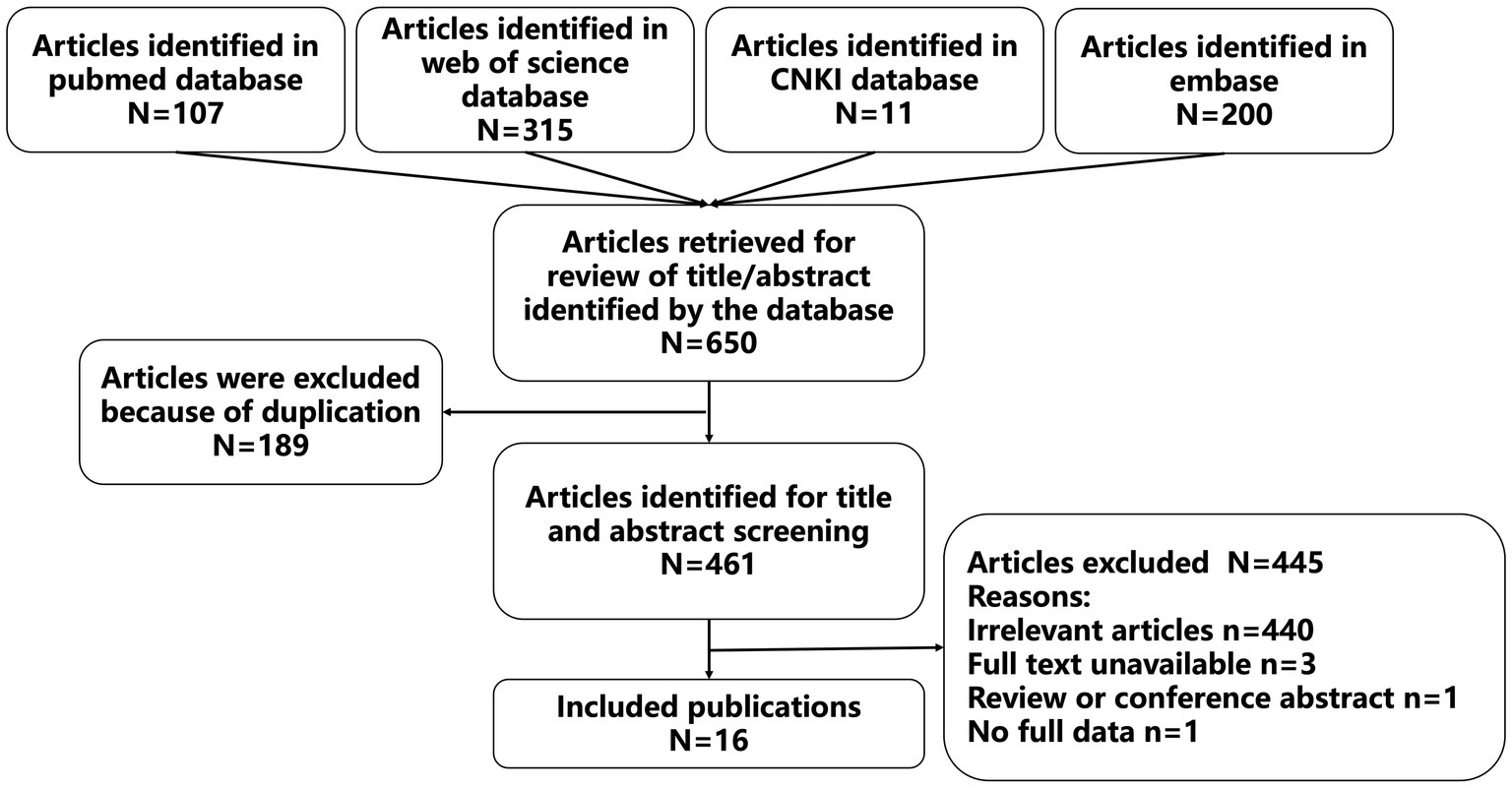
Sixteen studies were included according to the inclusion and exclusion criteria. The details of the search results were shown in Figure 1, and information for these studies were presented in Table 1. Of the 16 included studies, eleven of them were reported in English, and five of them were reported in Chinese. A total of 17 patients were reported in the 16 included studies (including 10 males and 7 females), who were from China (8 cases, 47.1%), India (5 cases, 29.4%), USA (1 case, 5.9%), Mexico (1 case, 5.9%), Peru (1 case, 5.9%), and Turkey (1 case, 5.9%). In terms of age, nine of the included patients were adults (52.9%) and eight of them were pediatric patients (47.1%). In addition, 15 of the cases were treated with OXC because of epilepsy (88.2%), one cases was treated with OXC because of trigeminal neuralgia (5.9%), and one case was treated with OXC because of oppositional defiant disorder and a bipolar disorder variant (5.9%). The primary diseases of 11 patients were treated with other drugs and received or added OXC treatment due to poor therapeutic effect and other reasons.
Administration of oxcarbazepine
Two patients reported that the drug dose was calculated in units of tablets, and no accurate dose was reported (2 cases, 11.8%). The dose of 4 pediatric patients was calculated by body weight; the initial dose of OXC ranged from 10 mg/kg/day- 20 mg/kg/day, then the dose was increased to 18 mg/kg/day-25 mg/kg/day within 1 week. For the other patients, the daily dose of OXC was used at a standard dose(100-900 mg/day), and no overdose were used. The precision dose of OXC for different patients were shown in Table 1. Most of the patients (15 cases, 88.2%) developed SJS/TEN within 2 weeks after OXC exposure, of which three patients (3 cases, 17.6%) developed SJS/TEN within 1 week. SJS/TEN occurred in the other three patients (2cases, 11.8%) who treated with OXC for over 2 weeks.
Diagnosis and adverse reaction causality evaluation
All the included patients were diagnosed as SJS/TEN according to clinical symptoms and laboratory tests after consultation in the department of dermatology. Of the included patients, fifteen of them (15 cases, 88.2%) were diagnosed as SJS, one patient (1 cases, 5.9%) were diagnosed with SJS/TEN overlap, and one patient (1 case, 5.9%) was diagnosed as TEN. Moreover, SJS occurred in seven patients (7 cases, 41.2%) after OXC monotherapy because of epilepsy and one patient (1 case, 5.9%) developed SJS after taking OXC due to mental illness. Six patients (6 cases, 35.3%) experienced SJS after converting to OXC from other antiepileptic medications due to poor seizure control of the initial drug and two patients (2 cases, 11.8%) developed SJS after adding OXC. One patient developed SJS after turning from CBZ to OXC due to trigeminal neuralgia.
Clinical symptoms
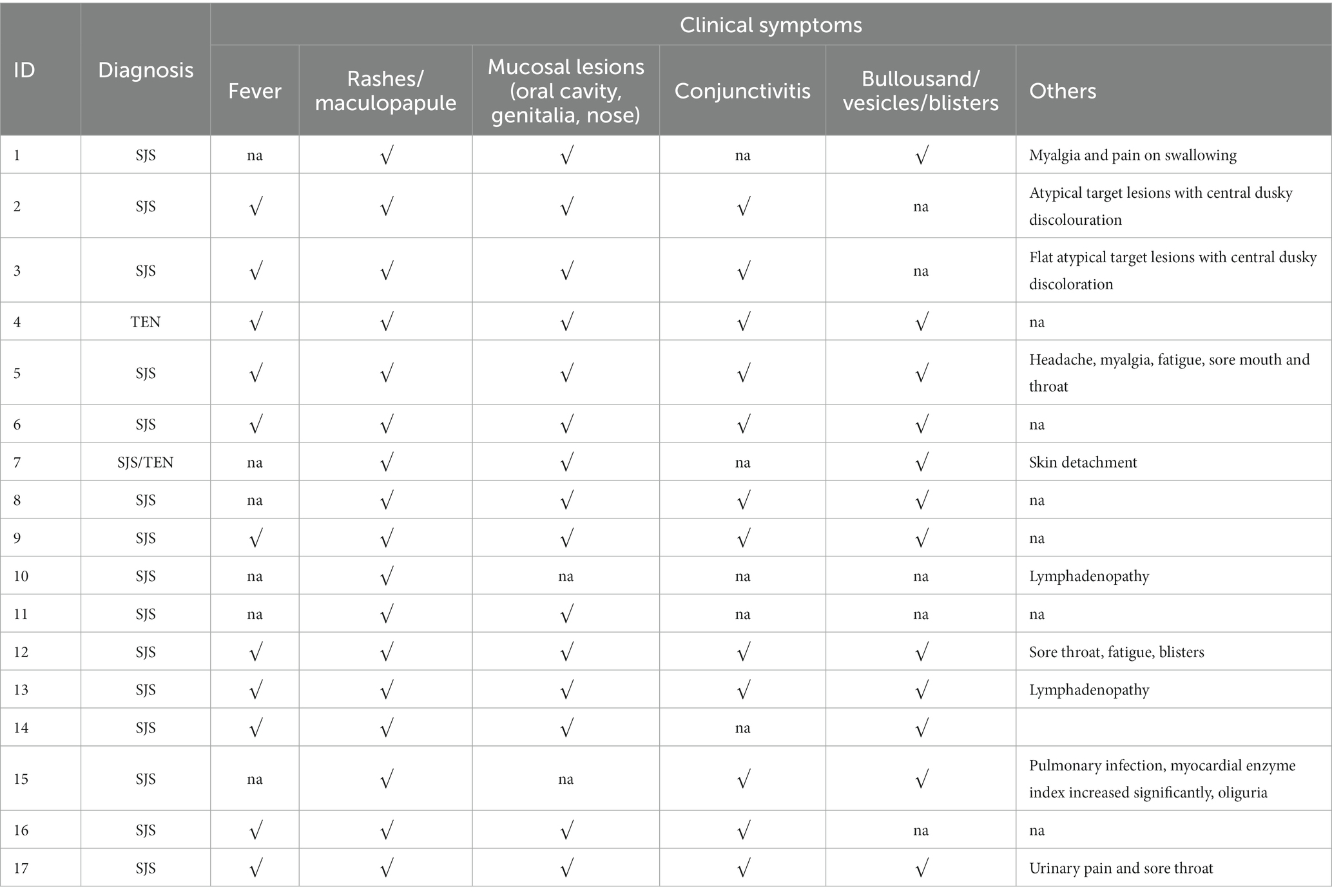
The majority of patients were prescribed OXC for seizure control and SJS/TEN was observed within 4–22 days (median time was 11 days) after exposure to OXC. When SJS/TEN occurs, the main clinical symptoms were as follows: rashes or maculopapule (17 cases, 100%), fever (11 cases, 64.7%), mucosal lesions including oral cavity, genitalia, and nose (15 cases, 88.2%), conjunctivitis with/without ocular discharge (12 cases, 70.6%), blisters (12 cases, 70.6%). Moreover, some patients also have other symptoms such as myalgia and pain on swallowing,flat atypical target lesions with central dusky discoloration, Headache, myalgia, fatigue and Lymphadenopathy, etc. The clinical symptoms of the 17 patients were summarized in Table 2.
Laboratory tests
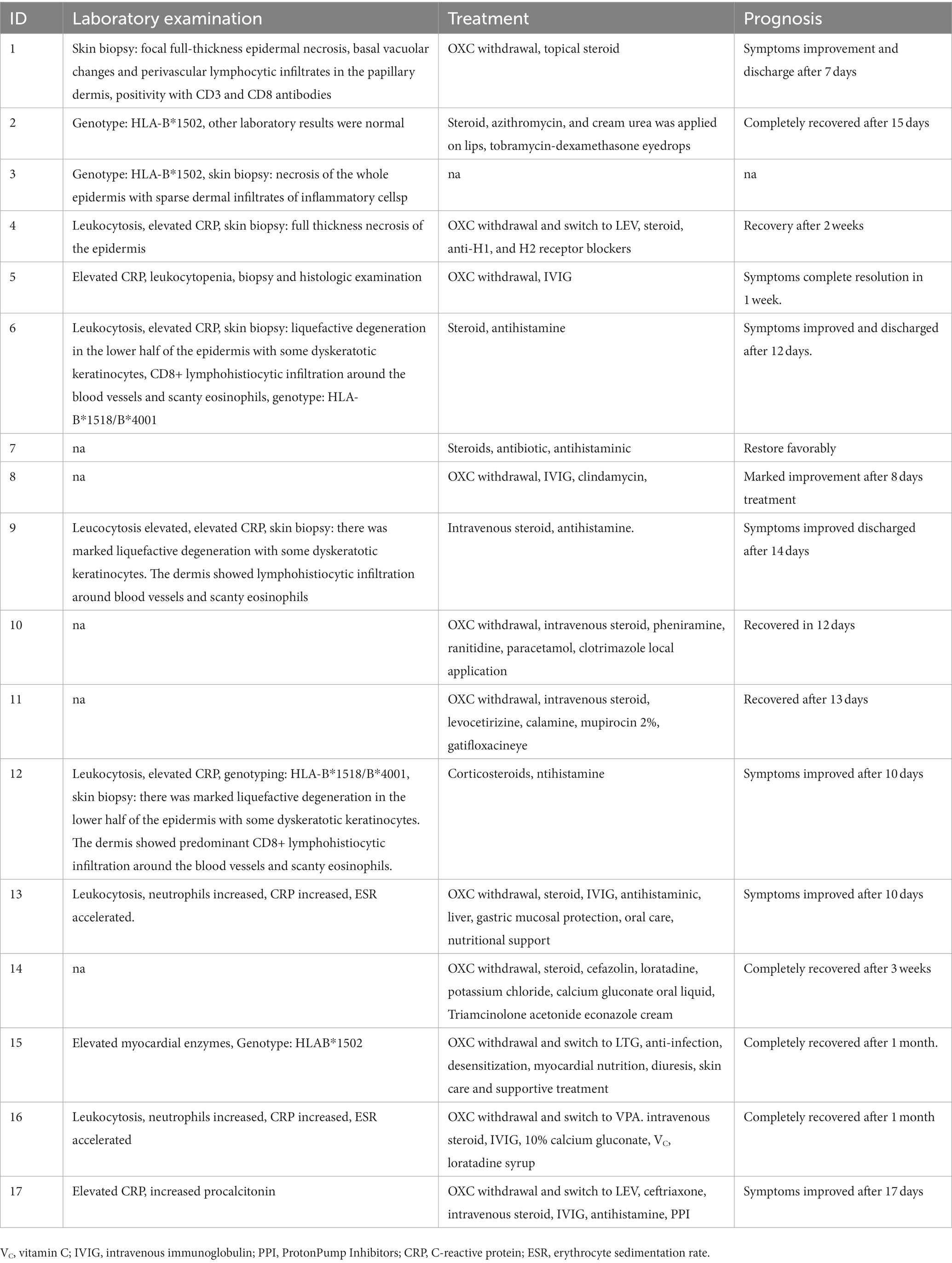
Two patients (2 cases, 11.8%) were reported with no significant changes in whole blood cell count and biochemical markers. Three patients (3 cases, 17.6%) were genotyped for HLA-B*1502, and two patients (2 case, 11.8%) were genotyped for HLA-B*1518/B*4001. Infection indicators such as white blood cells, CRP, and granulocytes were increased in 8 patients (8 cases, 47.1%). Seven patients (7 cases, 41.2%) were performed skin biopsy to further confirm the SJS/TEN, the primary histological characteristics were focal full thickness epidermal necrosis, basal vacuolar changes, and perivascular lymphocytic infiltrates in the papillary dermis, positivity with CD3 and CD8 antibodies. Partial of patients who did not perform skin biopsy were diagnosed by dermatologists after consultation with clinical symptoms and other laboratory tests.
Treatment and prognosis
The treatment and prognosis of the 17 patients were summarized in Table 3. After developing SJS/TEN, OXC was discontinued or switched to other antiepileptic drugs, including LEV, LTG, and VPA in eleven patients (11 cases, 64.7%). It was not reported whether the other 6 patients discontinued OXC. The other most common therapies for SJS/TEN were steroids (13 cases, 76.5%), antihistamine (10 cases, 55.8%), and IVIG (6 cases, 35.3%). Moreover, total parenteral nutrition, electrolyte, antibiotics, oral care, and other supportive therapy were used to treat the SJS/TEN. Finally, eight patients (8 cases, 47.1%) recovered completely, the symptoms of eight patients (8 cases, 47.1%) were improved, and the prognosis of one patient was not reported.
Discussion
Key findings
It is generally believed that OXC induced cutaneous adverse reactions less than CBZ (24). According to the previous report, the estimated relative risk of CBZ-SJS was 30 to 40 fold than that of OXC-SJS among Han Chinese in Taiwan, the incidence of CBZ-SJS was 2.6–3.4 cases per thousand person-years and OXC-SJS is only 0.08 cases per thousand person-years (10). VPA, LTG, CBZ, and other antiepileptic drugs can also cause SJS/TEN, with the highest absolute risk among new users of LTG and PHT (both approximately 45 cases/100,000 new users), followed by CBZ (20 cases/100,000 new users) (25). In addition, SJS/TEN may induced by some macrolide antibiotics such as azithromycin, clarithromycin, erythromycin, roxithromycin or telithromycin (26). However, there is a lack of research on whether there are differences in the clinical characteristics of SJS/TEN caused by different types of materials. More research is needed to further explore the clinical characteristics of SJS/TEN caused by different types of substance, so that to stop suspicious materials in time when patients take multiple drugs.
As SJS/TEN occurs, the risk of secondary infection, multiple organ failure, liver and kidney damage, aplastic anemia, and about half of the patients may have long-term skin and eye sequelae, and even mortality is exceptionally high; with early treatment, the survival rate for SJS/TEN patients may exceed 90% (27). But, the diagnosis of SJS/TEN is based on the clinical manifestations of acute episodes, including rapidly expanding target-like erythema, epidermal necrosis and exfoliation, and erythema, erosion, and crusting on two or more mucosal surfaces,remains difficult after excluding other interference factors such as SSSS (staphylococcal scalded skin syndrome), TSS (toxic shock syndrome), MCLS (mucocutaneous lymph node syndrome),autoimmune disease. In addition, attention should be paid to the differential diagnosis between SJS/TEN, DRESS (drug reaction with eosinophilia and systemic symptom) and AGEP (acute generalized exanthematous pustulosis). DRESS and AGEP can be caused by antimicrobials, antipyretic and analgesic agents, antihypertensive medications, chemotherapeutic drugs, biological preparation antiepileptic drug and other drugs (28–30). Clinically, AGEP is characterized by pinpoint-sized non-follicular aseptic pustular plaques based on erythema, as well as systemic symptoms such as leukocytosis and elevated neutrophils, with no or minimal damage to the mucous membranes (31). DRESS is characterized as an adverse drug reaction based on erythema, fever, lymph node enlargement, and eosinophilia with variable system and organ involvement (32). In OXC induced-SJS/TEN patients, who treated with recommended dose of OXC, indicating that SJS/TEN may not be related to the dose of OXC. The primary clinical symptoms are dermatological manifestations such as reddish pruritic rashes, erythematous maculopapular eruptions, mucosal lesions on the lip, nose, and exulcerations in the genital region and then fusion to form bullae or blisters, as well as the non-dermatological manifestation such as high fever, myalgia, conjunctivitis, CD8+ antibody positive, white blood cells, CRP, neutrophils and other inflammatory indicators increased leading to pain, unable to eat, loss of a large number of body fluids and electrolytes (26, 33). When mucosal lesions occur in severe cases of AGEP or SJS/TEN-like exfoliative dermatitis occurs in patients with severe DRESS, it may be difficult to distinguish SJS/TEN among them. Generally, AGEP typically undergoes eruption within 24–48 h, compared with a longer incubation period within 2–6 weeks for DRESS (28), whereas the results in this study showed that SJS/TEN generally occurs within 4–22 days after OXC exposure, the median time was 11 days, which was shorter than the instructions’ recorded time (a median of 19 days). The median time reported in our study was also shorter than the median time in Mockenhaupt’s study on CBZ (15 days), PHT (24 days), VPA (more than 30 weeks), phenobarbital (17 days) (34). In terms of cutaneous features, SJS/TEN presented as bullae and mucosal (including oral cavity, eye, and genital) lesions, AGEP is characterized by sterile pustules on erythematous base with minimal mucous membrane involvement, the skin features of DRESS are morbilliform diffuse, pruritic, macular exanthema. In histopathology, the OXC-SJS/TEN patients in this study were mainly manifested as focal full thickness epidermal necrosis, basal vacuolar changes, and perivascular lymphocytic infiltrates in the papillary dermis, while DRESS exhibits perivascular lymphocytic infiltration, and AGEP shows intracorneal, subcorneal, or intraepidermal pustules according to previous study (28).
Pathogenesis of OXC-SJS/TEN
Medications induce more than 80% of SJS/TEN; at least 200 drugs including OXC may cause SJS/TEN (35), AEDs are among the most frequently reported triggers of SJS/TEN. However, the SJS/TEN pathogenesis is still not completely clarified. It is acknowledged that the apoptosis of keratinocytes is one of the mechanisms, which involved in the Fas/FasL interaction, cytotoxic T cells, TNF-α, and nitric oxide synthase (36). Studies have shown that pathogenesis of drug-induced SJS/TEN is abnormal drug metabolism in some patients, who have different detoxification abilities to active metabolites, or this active metabolite may directly produce toxicity or may form antigens with host tissues, thereby inducing T cell-mediated cytotoxicity of intracellular drug antigens (37). OXC is a structural derivative of carbamazepine, it is rapidly and almost completely converted into pharmacologically active metabolites(10-monohydroxy derivatives, MHD) under the catalysis of liver cell enzymes after taking OXC, and further metabolized by the glucuronate pathway, about 4% of the OXC was oxidized to non-pharmacological activity metabolites─10,11-dihydroxy derivative (DHD) (38). Moreover, CBZ, LTG, PB, and PHT are all metabolized to arene oxide metabolites, these highly reactive intermediate metabolites binding with cellular macromolecules to produce toxic antigens, it is considered to be a type 4 hypersensitivity reaction, which have been hypothesized to cause skin adverse reactions (39). Additionally, there may be cross hypersensitivity between these drugs that the metabolites are arene oxide metabolites. For previous SJS/TEN patients, some AEDs (LEV, topiramate, clonazepam, etc.) may be safer options for antiepileptic therapy (25). Consistent with the results of our study, SJS/TEN did not trigger again when OXC was switched to another antiepileptic drug in some patients. Hence, it may be considered to switch to medications whose metabolites are not arene oxide metabolites, such as LEV, topiramate, and clonazepam, but more scientific evidence is needed to support this. It has also been reported that SJS/TEN is associated with the cell-mediated inflammatory response and immune system. Macrophages, CD8+ T cells, CD25+ T cells, and CD4+ T cells in the epidermis and dermis release inflammatory factors such as interferon-γ and TNF-α to promote an immune response to epidermal and keratin damage (40). Part of the patients included in this study did not perform histopathological examination, and the patients who examined histopathology were mainly manifested as focal full thickness epidermal necrosis, basal vacuolar changes, and perivascular lymphocytic infiltrates in the papillary dermis.
Moreover, genotype is closely related to the occurrence of SJS/TEN, especially when the patient’s genotype is HLA-B*1502, who had the higher incidence of SJS/TEN. In Asian populations, the risk of SJS/TEN seems to be higher in patients with HLA-B*1502 (41), (OR = 27.90; 95% CI: 7.84–99.23) in Chinese. The positive and negative predictive values of HLA-B*1502 for OXC-SJS/TEN were 0.73 and 99.97%, respectively. The incidence and mortality of OXC-SJS/TEN was lower than CBZ-STS/TEN in new users (p = 0.003; relative risk 0.212; 95%CI 0.077–0.584) (42). Moreover, it is worth noting that HLA-B*1502 is associated with OXC-SJS/TEN but not OXC-DRESS, and HLA-A*3101 has been previously reported to be associated with CBZ-DRESS; however, there was no significant association with OXC-DRESS in Asians (42, 43). Therefore, it is necessary to detect the genotype of patients when taking drugs that may cause SJS/TEN such as OXC, LTG, VPA, etc. If the genetic information were known a priori, physicians can formulate a personalized treatment plan based on this to prevent SJS/TEN. The primary treatment for SJS/TEN is discontinuation of OXC, and other treatments include pulsed corticosteroids and intravenous IVIG in some severe patients. Secondly, supportive treatment is the focus of treatment for SJS/TEN patients, including fluid and electrolyte supplementation, infection control and trauma care. Most patients can completely recover after reasonable and timely treatment. For patients who still need antiepileptic, the alternative is to switch to other relative drugs such as pregabalin, LEV, and VPA, etc.
Limitations
This study has some limitations such as cannot be used to determine the incidence of events due to the fact that it was based on available cases in the literature. Secondly, we excluded studies that were not written by English and Chinese. The quality of most of these included studies was low or moderate, mainly due to the lack of a large amount of important information in these case reports such as SJS/TEN was diagnosed with the assistance of dermatologists, some patients did not perform skin biopsy. Thirdly, these cases from diverse clinical setting, and missing some important information. There are differences in diagnostic tools, medical standards, and reporting quality result in the data in this article may still be biased. We should call on researchers to provide as much detailed information as possible improve the quality of case reports when reporting cases. Nevertheless, this study provides some preliminary insights into the clinical features of SJS/TEN patients caused by OXC, which helps in the improved recognition and management of OXC-induced adverse reactions.
Conclusion
To sum up, SJS/TEN is a rare complication of OXC. Patients treated with OXC should always pay attention to their symptoms, such as unexplained rash or maculopapule, fever, oral ulcer, and other symptoms. If any of the symptoms mentioned above appear when OXC is being administered, the medicine should be stopped immediately and followed up regularly throughout the medication period. Clinicians should promptly identify SJS/TEN when skin or fever-related adverse reactions occur so that timely intervention and early treatment minimize the deleterious influences of OXC induced-SJS/TEN.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
QY designed the study, wrote the manuscript, and prepared the original draft. XL provided the clinical support and reviewed the final version of the manuscript. RL and HL collected the data. YH analyzed the data. All authors have agreed to the submission of the final manuscript.
Funding
This study was funded by the project of the Hunan Medical Association (No. HMA202001021).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gambeta, E , Chichorro, JG , and Zamponi, GW . Trigeminal neuralgia: an overview from pathophysiology to pharmacological treatments. Mol Pain. (2020) 16:1744806920901890. doi: 10.1177/1744806920901890
2. Abou-Khalil, BW . Update on antiepileptic drugs 2019. Continuum. (2019) 25:508–36. doi: 10.1212/CON.0000000000000715
3. Mullan, KA , Anderson, A , Illing, PT , Kwan, P , Purcell, AW , and Mifsud, NA . HLA-associated antiepileptic drug-induced cutaneous adverse reactions. HLA. (2019) 93:417–35. doi: 10.1111/tan.13530
4. Mockenhaupt, M . Stevens-Johnson syndrome and toxic epidermal necrolysis: clinical patterns, diagnostic considerations, etiology, and therapeutic management. Semin Cutan Med Surg. (2014) 33:10–6. doi: 10.12788/j.sder.0058
5. Dodiuk-Gad, RP , Chung, WH , Valeyrie-Allanore, L , and Shear, NH . Stevens-Johnson syndrome and toxic epidermal necrolysis: an update. Am J Clin Dermatol. (2015) 16:475–93. doi: 10.1007/s40257-015-0158-0
6. Hsu, DY , Brieva, J , Silverberg, NB , and Silverberg, JI . Morbidity and mortality of Stevens-Johnson syndrome and toxic epidermal necrolysis in United States adults. J Invest Dermatol. (2016) 136:1387–97. doi: 10.1016/j.jid.2016.03.023
7. Hoffman, M , Chansky, PB , Bashyam, AR , Boettler, MA , Challa, N , Dominguez, A, et al. Long-term physical and psychological outcomes of Stevens-Johnson syndrome/toxic epidermal necrolysis. JAMA Dermatol. (2021) 157:712–5. doi: 10.1001/jamadermatol.2021.1136
8. Beken, B , Can, C , Örencik, A , Can, N , and Yazıcıoğlu, M . Oxcarbazepine-induced Stevens-Johnson syndrome: a pediatric case report. Oxf Med Case Reports. (2017) 2017:omx028. doi: 10.1093/omcr/omx028
9. Chen, M , Yang, B , Wang, C , Liu, H , and Zhang, F . Oxcarbazepine-induced Stevens-Johnson syndrome in a patient with HLA-B*1502 genotype. Australas J Dermatol. (2016) 57:e137–8. doi: 10.1111/ajd.12368
10. Chen, YC , Chu, CY , and Hsiao, CH . Oxcarbazepine-induced Stevens-Johnson syndrome in a patient with HLA-B*1502 genotype. J European Acad Dermatol Venereol. (2009) 23:702–3. doi: 10.1111/j.1468-3083.2008.02988.x
11. Guleria, VS , Sharda, C , Rana, T , and Sood, AK . Oxcarbazepine induced toxic epidermal necrolysis – a rare case report. Indian J Pharmacol. (2015) 47:459–61. doi: 10.4103/0253-7613.161279
12. Khalid, K , Kwak, BS , and Leo, RJ . Oxcarbazepine-induced Stevens-Johnson syndrome. Prim Care Companion CNS Disord. (2018) 20:18l02304. doi: 10.4088/PCC.18l02304
13. Lin, L-C , Lai, P-C , Yang, S-F , and Yang, R-C . Oxcarbazepine-induced Stevens-Johnson syndrome: a case report. Kaohsiung J Med Sci. (2009) 25:82–6. doi: 10.1016/S1607-551X(09)70045-2
14. Poletti-Jabbour, J , Wiegering-Rospigliosi, A , Pereyra-Elías, R , and Elías-Barrera, CC . Carbamazepine and oxcarbazepine: reflections after an oxcarbazepine-induced Stevens-Johnson syndrome/toxic epidermal necrolysis overlap. Eur J Clin Pharmacol. (2016) 72:1031–2. doi: 10.1007/s00228-016-2066-5
15. Romero-Tapia, SJ , Cámara-Combaluzier, HH , Baeza-Bacab, MA , Cerino-Javier, R , Bulnes-Mendizabal, DP , and Virgen-Ortega, C . Use of intravenous immunoglobulin for Stevens-Johnson syndrome and toxic epidermal necrolysis in children: report of two cases secondary to anticonvulsants. Allergol Immunopathol. (2015) 43:227–9. doi: 10.1016/j.aller.2013.12.008
16. Sharma, SR , Sharma, N , and Yeolekar, ME . Oxcarbazepine-induced Stevens Johnson syndrome: a rare case report. Indian Dermatol Online J. (2011) 2:13–5. doi: 10.4103/2229-5178.79861
17. Trivedi, BS , Darji, NH , Malhotra, SD , and Patel, PR . Antiepileptic drugs-induced Stevens-Johnson syndrome: a case series. J Basic Clin Pharm. (2016) 8:42–4. doi: 10.4103/0976-0105.195130
18. Wal, P , Wal, A , Pandey, U , Rai, AK , and Bhandari, A . Genetic predisposition to oxcarbazepine induced Stevens-Johnson syndrome. Indian J Crit Care Med. (2011) 15:173–5. doi: 10.4103/0972-5229.84904
19. Liang, Y , Hao, X , Wang, J , and Liang, D . Oxcarbazepine induced Stevens-Johnson syndrome in children: a case report. J Apoplexy Nervous Dis. (2014) 31:365. doi: 10.19845/j.cnki.zfysjjbzz.2014.04.026
20. Jia, X . Oxcarbazepine induced Stevens-Johnson syndrome: 1 case report. Proceedings of the 2011 National Symposium on Allergic Reactions of the Chinese Medical Association. (2011). p. 120.
21. Wang, J , Tao, Y , Hongtao Cao, H , and Zhang, JZ . Oxcarbazepine-induced Stevens-Johnson syndrome: a case report. J Shanxi Med Univ. (2013) 44:501–2. doi: 10.3969/J.ISSN.1007-6611.2013.06.023
22. Xin, F , and Guanghua, W . Pharmacists participated in the treatment of oxcarbazepine induced Steven-Johnson syndrome in children: a case report. J Pharmacoepidemiol. (2018) 27:131–3. doi: 10.19960/j.cnki.issn1005-0698.2018.02.013
23. Tian, L . Oxcarbazepine-induced Stevens-Johnson syndrome: a case report. Chin J Rural Med Pharm. (2023) 30:54–5. doi: 10.19542/j.cnki.1006-5180.007047
24. Xu, C , Pan, L , Zhang, Y , Zhang, J , Sun, Q , and Fang, W . Assessing carbamazepine and oxcarbazepine-associated Stevens-Johnson syndrome/toxic epidermal necrolysis: data mining the public version of the FDA adverse event reporting system. Int J Clin Pract. (2021) 75:e14273. doi: 10.1111/ijcp.14273
25. Frey, N , Bodmer, M , Bircher, A , Rüegg, S , Jick, SS , Meier, CR, et al. The risk of Stevens-Johnson syndrome and toxic epidermal necrolysis in new users of antiepileptic drugs. Epilepsia. (2017) 58:2178–85. doi: 10.1111/epi.13925
26. Pejcic, AV . Stevens-Johnson syndrome and toxic epidermal necrolysis associated with the use of macrolide antibiotics: a review of published cases. Int J Dermatol. (2021) 60:12–24. doi: 10.1111/ijd.15144
27. Kirchhof, MG , Miliszewski, MA , Sikora, S , Papp, A , and Dutz, JP . Retrospective review of Stevens-Johnson syndrome/toxic epidermal necrolysis treatment comparing intravenous immunoglobulin with cyclosporine. J Am Acad Dermatol. (2014) 71:941–7. doi: 10.1016/j.jaad.2014.07.016
28. Szatkowski, J , and Schwartz, RA . Acute generalized exanthematous pustulosis (AGEP): a review and update. J Am Acad Dermatol. (2015) 73:843–8. doi: 10.1016/j.jaad.2015.07.017
29. Husain, Z , Reddy, BY , and Schwartz, RA . DRESS syndrome: part I. Clinical perspectives. J Am Acad Dermatol. (2013) 68:693.e1–14; quiz 706–708. doi: 10.1016/j.jaad.2013.01.033
30. Schwartz, RA , McDonough, PH , and Lee, BW . Toxic epidermal necrolysis: part II. Prognosis, sequelae, diagnosis, differential diagnosis, prevention, and treatment. J Am Acad Dermatol. (2013) 69:187.e1–16; quiz 203–204. doi: 10.1016/j.jaad.2013.05.002
31. Błaszczyk, B , Lasoń, W , and Czuczwar, SJ . Antiepileptic drugs and adverse skin reactions: an update. Pharmacol Rep. (2015) 67:426–34. doi: 10.1016/j.pharep.2014.11.009
32. Isaacs, M , Cardones, AR , and Rahnama-Moghadam, S . DRESS syndrome: clinical myths and pearls. Cutis. (2018) 102:322–6.
33. Rashid, M , Kashyap, A , and Undela, K . Valproic acid and Stevens-Johnson syndrome: a systematic review of descriptive studies. Int J Dermatol. (2019) 58:1014–22. doi: 10.1111/ijd.14411
34. Mockenhaupt, M , Viboud, C , Dunant, A , Naldi, L , Halevy, S , Bouwes Bavinck, JN, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. J Invest Dermatol. (2008) 128:35–44. doi: 10.1038/sj.jid.5701033
35. Lerch, M , Mainetti, C , Terziroli Beretta-Piccoli, B , and Harr, T . Current perspectives on Stevens-Johnson syndrome and toxic epidermal necrolysis. Clin Rev Allergy Immunol. (2018) 54:147–76. doi: 10.1007/s12016-017-8654-z
36. Nassif, A , Bensussan, A , Boumsell, L , Deniaud, A , Moslehi, H , Wolkenstein, P, et al. Toxic epidermal necrolysis: effector cells are drug-specific cytotoxic T cells. J Allergy Clin Immunol. (2004) 114:1209–15. doi: 10.1016/j.jaci.2004.07.047
37. Friedmann, PS , Strickland, I , Pirmohamed, M , and Park, BK . Investigation of mechanisms in toxic epidermal necrolysis induced by carbamazepine. Arch Dermatol. (1994) 130:598–604. doi: 10.1001/archderm.1994.01690050066011
39. Chang, WC , Abe, R , Anderson, P , Anderson, W , Ardern-Jones, MR , Beachkofsky, TM, et al. SJS/TEN 2019: from science to translation. J Dermatol Sci. (2020) 98:2–12. doi: 10.1016/j.jdermsci.2020.02.003
40. Ko, TM , Chung, WH , Wei, CY , Shih, HY , Chen, JK , Lin, CH, et al. Shared and restricted T-cell receptor use is crucial for carbamazepine-induced Stevens-Johnson syndrome. J Allergy Clin Immunol. (2011) 128:e11:1266–1276.e11. doi: 10.1016/j.jaci.2011.08.013
41. Tangamornsuksan, W , Chaiyakunapruk, N , Somkrua, R , Lohitnavy, M , and Tassaneeyakul, W . Relationship between the HLA-B*1502 allele and carbamazepine-induced Stevens-Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. JAMA Dermatol. (2013) 149:1025–32. doi: 10.1001/jamadermatol.2013.4114
42. Chen, C-B , Hsiao, Y-H , Wu, T , Hsih, M-S , Tassaneeyakul, W , Jorns, TP, et al. Risk and association of HLA with oxcarbazepine-induced cutaneous adverse reactions in Asians. Neurology. (2017) 88:78–86. doi: 10.1212/WNL.0000000000003453
Keywords: oxcarbazepine, drug adverse reaction, Stevens-Johnson syndrome, toxic epidermal necrolysis, severe cutaneous adverse reactions
Citation: Yan Q, Liu X, Lei H, Liu R and Hu Y (2023) Analysis of clinical features of oxcarbazepine-induced Stevens-Johnson syndrome and toxic epidermal necrolysis. Front. Med. 10:1232969. doi: 10.3389/fmed.2023.1232969
Edited by:
Giusto Trevisan, University of Trieste, ItalyReviewed by:
Malathi Munisamy, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), IndiaNicola Di Meo, University of Trieste, Italy
Copyright © 2023 Yan, Liu, Lei, Liu and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingzi Yan, MjAxNTMzNjBAc3R1LmhudWNtLmVkdS5jbg==
 Qingzi Yan
Qingzi Yan Xiang Liu
Xiang Liu Renzhu Liu
Renzhu Liu