- 1Department of Pulmonary and Critical Care Medicine, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 2Department of Pulmonary and Critical Care Medicine, 363 Hospital, Chengdu, Sichuan, China
Nocardia species are gram-positive, acid-fast, saprophytic, aerobic bacilli, predominantly resulting in opportunistic infections in immunocompromised individuals. Here, we reported a case of Nocardia infection in a 27-year-old woman with normal immunocompetence, who presented as a solitary neoplasm in the left principal bronchus with a chief complaint of postural dyspnea. By electrotomy via bronchoscopy, the neoplasm was successfully removed, and it was further identified as Nocardia farcinica by metagenomic next-generation sequencing.
Introduction
Nocardia species are gram-positive, acid-fast, saprophytic, aerobic bacilli, widely found in soil, decomposing vegetation, and other organic matter (1). Among all of the Nocardia species, Nocardia asteroides, Nocardia brasiliensis, and Nocardia otitidiscaviarum were documented to be the most common pathogenic strains (2), predominantly causing opportunistic infections in immunocompromised individuals (3). However, in the present report, we described a case of Nocardia farcinica infection in an immunocompetent adult, presenting as a solitary neoplasm in the left principal bronchus with a chief complaint of postural dyspnea.
Case presentation
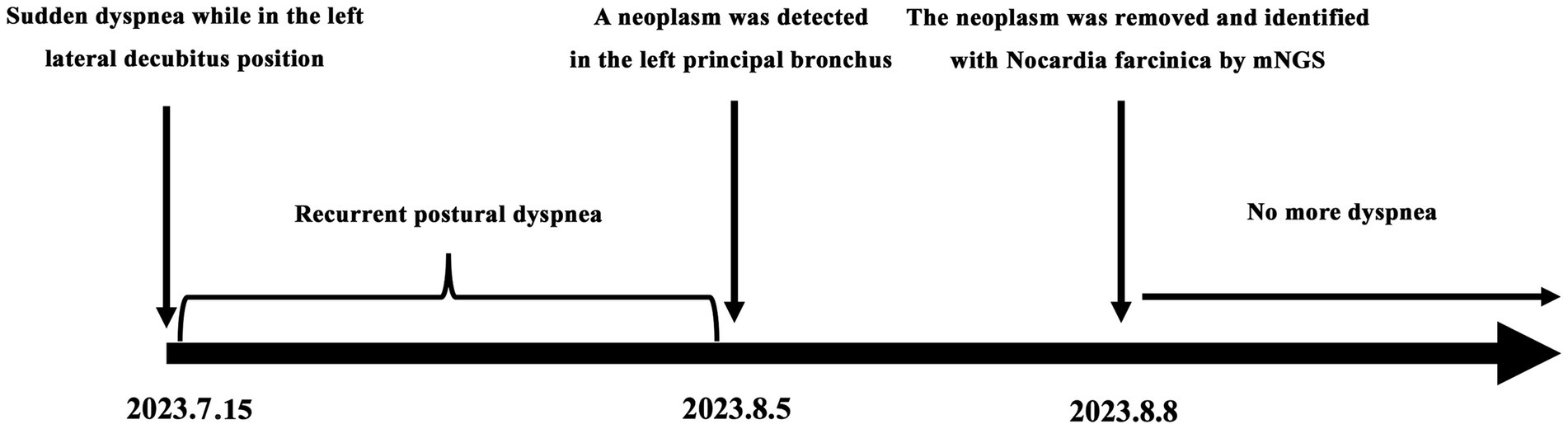
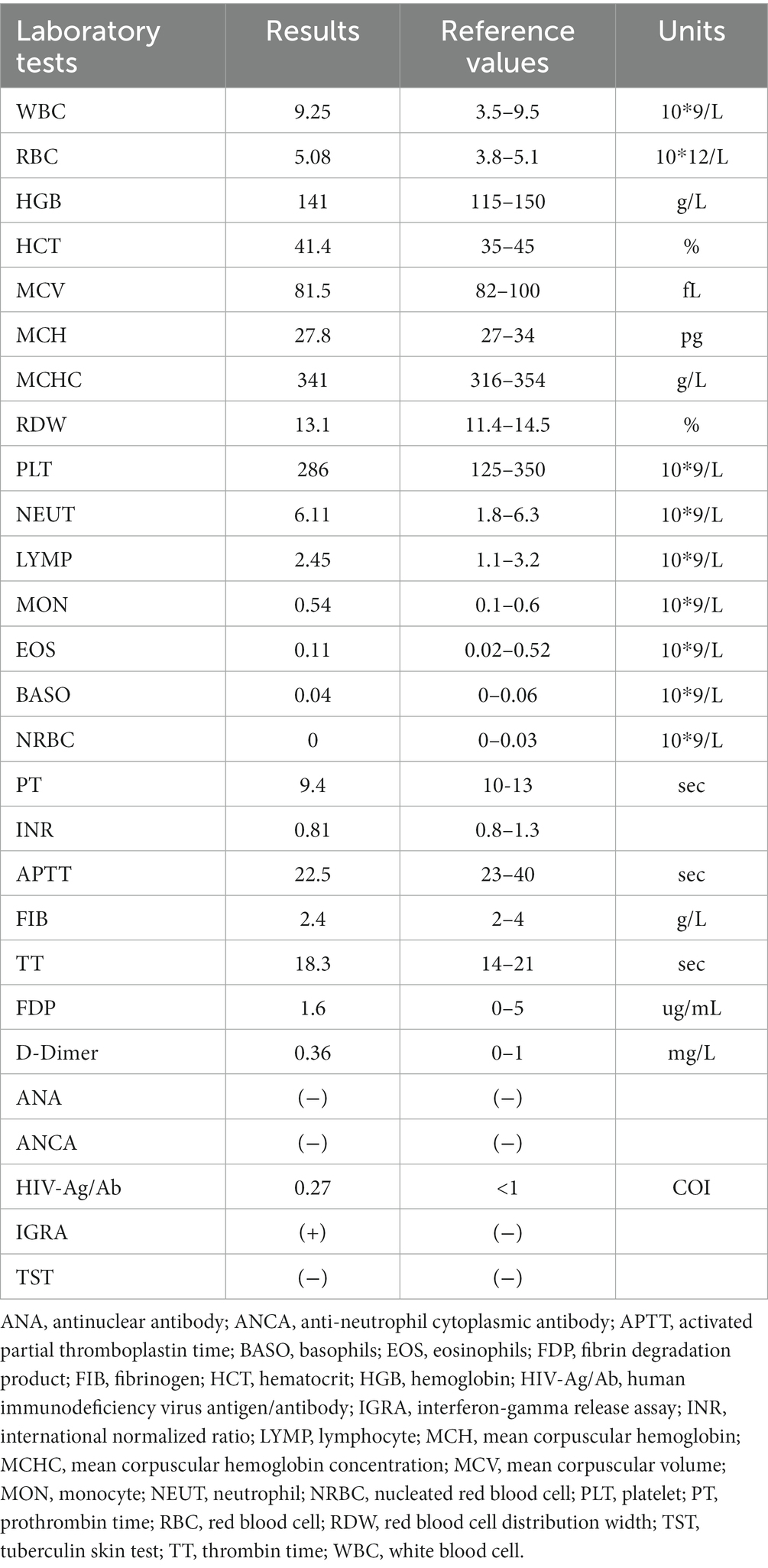
A 27-year-old woman was admitted to the local hospital because of recurrent dyspnea while in the left lateral decubitus position for 3 weeks. No other symptoms such as fever, cough, wheezing, expectoration, hemoptysis, and chest pain were reported. Moreover, she had a history of surgery in the right talus due to cartilage damage 5 months ago and denied any history of asthma, bronchiectasis, pulmonary tuberculosis, allergic diseases, anemia, autoimmune diseases, acquired immune deficiency syndrome, and tumors. On physical examination, no positive signs were revealed. The timeline of history for the present illness was showcased in Figure 1. In laboratory tests, the interferon-gamma release assay was positive, but the tuberculin skin test was negative. Furthermore, there were no positive results in the blood routine examination, antinuclear and anti-neutrophil cytoplasmic antibody test, and human immunodeficiency virus antibody test (Table 1). Importantly, chest tomography (CT) displayed a small hyperdense nodule of approximately 5 mm × 6 mm in the left principal bronchus with mild intensification in the enhanced images (Figures 2A,B). No significant lesions were detected in the mediastina, lung lobes, and pleural cavities.
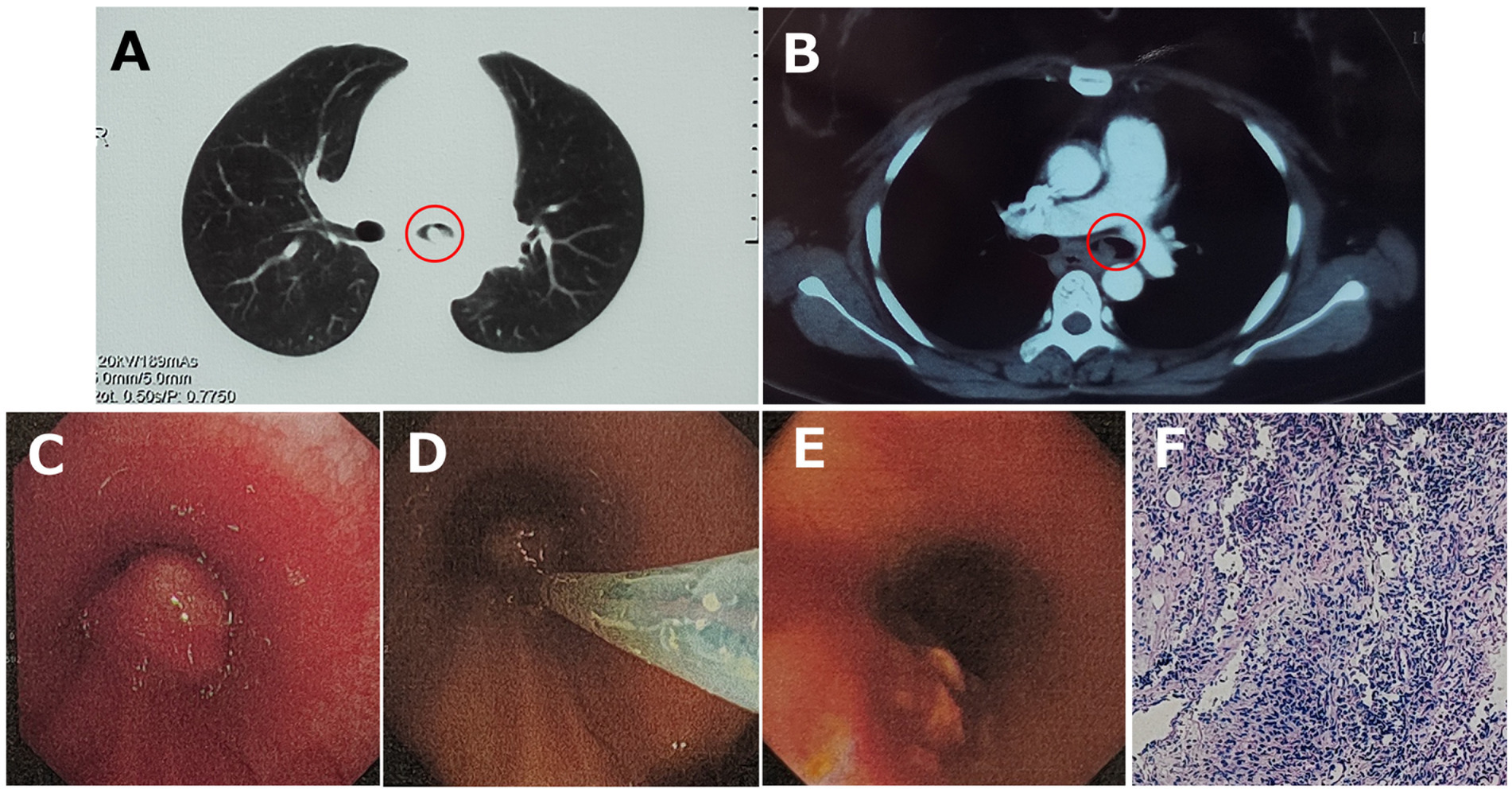
Figure 2. (A) Chest tomography (CT) displayed a small hyperdense nodule (red circles) in the left principal bronchus, and (B) mild intensification was detected in the enhanced CT image. (C) Bronchoscopy visualized a smooth-surfaced neoplasm with airway obstruction in the left principal bronchus, and (D) it was excised with electrotomy and (E) removed successfully. (F) HE stain showed chronic active inflammation with histiocyte aggregation.
By endotracheal endoscopy, a smooth-surfaced neoplasm with airway obstruction was visualized in the left principal bronchus (Figure 2C). Subsequently, the neoplasm was successfully removed with electrotomy (Figures 2D,E), and pathological biopsy with metagenomic next-generation sequencing (mNGS) was then carried out for precise diagnosis.
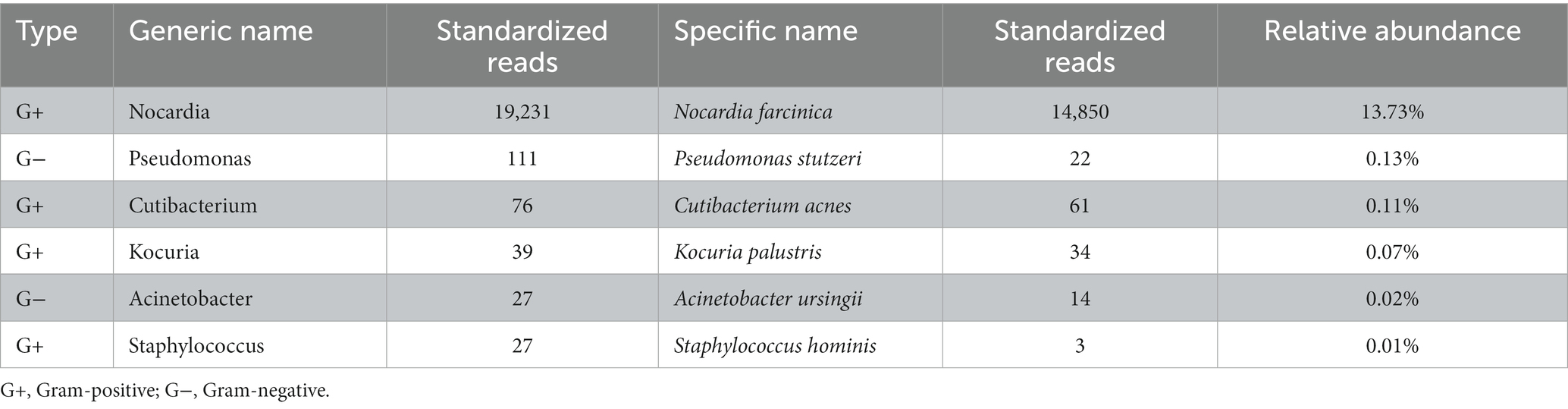
The hematoxylin-eosin (HE) staining showed chronic active inflammation, evidenced by granulation tissue hyperplasia, inflammatory necrosis, and exudation, as well as histiocyte aggregation with a tendency of granuloma formation (Figure 2F). mNGS was conducted by Beijing Genomics Institute (BGI), Shenzhen. First, total DNA from the tissue sample was isolated, and after DNA library construction, sequencing was performed by the MGISEQ-2000 platform (BGI) (4, 5). Then, short reads, duplicates, and human host sequences were removed using the Burrows-Wheeler Alignment Tool. Finally, the remaining sequences were compared with the PMseq metagenomic database (PMDB, BGI), consisting of bacteria, fungi, viruses, and parasites, which indicated the significant presence of Nocardia farcinica (14,850 reads), with possible contamination or colonization by Pseudomonas stutzeri (22 reads), Cutibacterium acnes (61 reads), Kocuria palustris (34 reads), Acinetobacter ursingii (14 reads), and Staphylococcus hominis (3 reads; Table 2).
Overall, the patient was definitely diagnosed with Nocardia farcinica infection localized in the left principal bronchus. Moreover, since no clinical evidence supported the possibilities, tuberculosis and tumors were excluded. After removal of the neoplasm and during the follow-up, the patient had no more dyspnea. Therefore, no antibiotic therapy was administrated all the way.
This case report was approved by the Institutional Review Board of West China Hospital of Sichuan University, and the informed consent was obtained.
Discussion
Nocardia species are highly opportunistic pathogens and typically cause infections in immunocompromised individuals (6–9). Unlike the other strains, Nocardia farcinica often exhibits pulmonary infection via inhalation with a greater propensity for dissemination and a unique pattern of antibiotic susceptibility (10). Consequently, it is prone to develop into severe pneumonia or sepsis, leading to a deteriorating prognosis (11, 12).
However, it has been reported that pulmonary infection by Nocardia farcinica may occur in immunocompetent subjects (Table 3) (13–16). Although these reported cases typically presented with fever, cough, and expectoration, as well as leukocytosis and elevated C-reactive protein (CRP), radiological findings usually demonstrated the localized presence of nodular shadows and pleural effusion, which was more easily recovered after antibiotic treatment. Differently, this patient, an immunocompetent host, complained of postural dyspnea but no fever and other respiratory symptoms, and the Nocardia farcinica infection was strictly limited within the left principal bronchus, presenting as a solitary neoplasm.
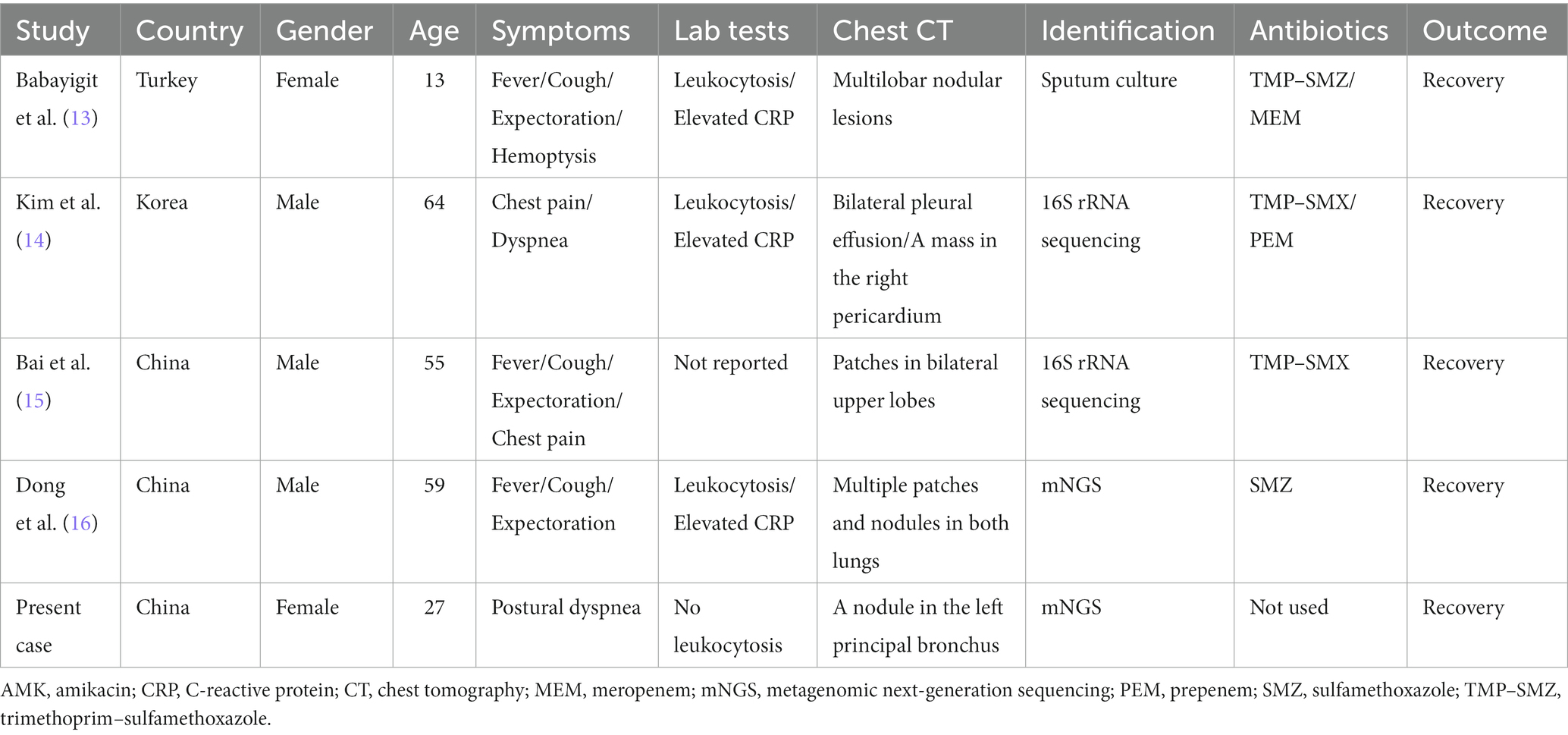
Table 3. Clinical characteristics of pulmonary infection by Nocardia farcinica in immunocompetent cases.
The precise identification of Nocardia species has been challenging. In the past decades, traditional methods including microscopic examination, microbial culture, and biochemical analyses were widely used, but with more false-negative results than expected (17, 18). Subsequently, 16S rRNA sequencing seems to improve the testing efficiency; however, this technique might miss the potential non-bacterial infections that are usually considered depending on the clinical assumption (19, 20). More importantly, the clinical presentation of this patient was so atypic that we could not exclude the possibility of non-bacterial infections. Thus, for this case, 16S rRNA sequencing may not be the best choice for microorganism identification, and relatively, mNGS is a more ideal method that, regardless of clinical assumption, identifies pathogenic microorganisms/Nocardia strains comprehensively, quickly, and accurately, providing an opportunity for precise interventions at the early stage (21–23). As demonstrated in this case, the significant presence of Nocardia farcinica was detected by mNGS from the resected tissue, which confirmed its pathogenic role according to the Johns Hopkins ABX Guide (24). However, it should be noted that mNGS reports must be cautiously interpreted before accurate differentiation of pathogenic, contaminated, and colonized subgroups (19).
Moreover, antimicrobial therapy plays a key role in the treatment of Nocardia infections. Trimethoprim–sulfamethoxazole (TMP–SMX) is the most recommended antibiotic, usually combined with imipenem and amikacin (25). However, as suggested in this case, antibiotics are not always necessary to treat the localized infections, but surgical resection can bring about a significant improvement and recovery.
Conclusion
Overall, this case report indicates (i) immunocompetent individuals are the target for pulmonary infection by Nocardia, which may present as atypical manifestations and localized lesions; (ii) adoption of mNGS can benefit for accurate identification and early diagnosis of Nocardia infections; and (iii) surgical management but not antibiotic therapy could be the first choice for these patients with localized lesions.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YT: Writing – original draft, Data curation, Software, Visualization. MY: Writing – original draft, Data curation, Visualization. CW: Writing – original draft, Data curation, Formal Analysis. ST: Formal Analysis, Writing – original draft. LL: Writing – review & editing, Conceptualization. LC: Writing – review & editing, Conceptualization, Supervision, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wilson, JW. Nocardiosis: updates and clinical overview. Mayo Clin Proc. (2012) 87:403–7. doi: 10.1016/j.mayocp.2011.11.016
2. Brown-Elliott, BA, Brown, JM, Conville, PS, and Wallace, RJ Jr. Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin Microbiol Rev. (2006) 19:259–82. doi: 10.1128/CMR.19.2.259-282.2006
3. Rahim, Y, Khan, J, Shahid, S, Awan, S, and Irfan, M. Clinical characteristics, outcomes, and factors associated with mortality in Nocardia pneumonia: 18 years' real-world data from a tertiary care hospital in Karachi. Pak Respir Invest. (2023) 61:254–60. doi: 10.1016/j.resinv.2022.11.004
4. Foox, J, Tighe, SW, Nicolet, CM, Zook, JM, Byrska-Bishop, M, Clarke, WE, et al. Performance assessment of DNA sequencing platforms in the ABRF next-generation sequencing study. Nat Biotechnol. (2021) 39:1129–40. doi: 10.1038/s41587-021-01049-5
5. Jeon, SA, Park, JL, Park, SJ, Kim, JH, Goh, SH, Han, JY, et al. Comparison between MGI and Illumina sequencing platforms for whole genome sequencing. Genes Genom. (2021) 43:713–24. doi: 10.1007/s13258-021-01096-x
6. Lerner, PJ. “Nocardia species,”in Principles and Practice of Infectious Diseases, Vol 2, 5th Ed. Eds. Mandell GL, Douglas RG, Bennett JE (New York, NY: Churchill Livingstone ). (1999) 2637–2643.
7. Ando, T, Usa, T, Ide, A, Abe, Y, Sera, N, Tominaga, T, et al. Pulmonary nocardiosis associated with idiopathic thrombocytopenic purpura. Intern Med. (2001) 40:246–9. doi: 10.2169/internalmedicine.40.246
8. Parande, MV, Shinde, RS, Mantur, BG, Parande, AM, Chandrashekhar, MR, Aralikatti, PS, et al. A fatal case of empyema thoracis by Nocardia farcinica in an immunocompromised patient. Indian J Med Microbiol. (2010) 28:390–2. doi: 10.4103/0255-0857.71831
9. King, AS, Castro, JG, and Dow, GC. Nocardia farcinica lung abscess presenting in the context of advanced HIV infection: spontaneous resolution in response to highlyactive antiretroviral therapy alone. Can J Infect Dis Med Microbiol. (2009) 20:e103–6. doi: 10.1155/2009/181750
10. Hershko, Y, Levytskyi, K, Rannon, E, Assous, MV, Ken-Dror, S, Amit, S, et al. Phenotypic and genotypic analysis of antimicrobial resistance in Nocardia species. J Antimicrob Chemother. (2023) 78:2306–14. doi: 10.1093/jac/dkad236
11. Wang, H, Zhu, Y, Cui, Q, Wu, W, Li, G, Chen, D, et al. Epidemiology and antimicrobial resistance profiles of the Nocardia species in China, 2009 to 2021. Microbiol Spectr. (2022) 10:e0156021. doi: 10.1128/spectrum.01560-21
12. Martínez, R, Reyes, S, and Menéndez, R. Pulmonary nocardiosis: risk factors, clinical features, diagnosis and prognosis. Curr Opin Pulm Med. (2008) 14:219–27. doi: 10.1097/MCP.0b013e3282f85dd3
13. Babayigit, A, Olmez, D, Sozmen, SC, Makay, B, Uzuner, N, Karaman, O, et al. Infection caused by Nocardia farcinica mimicking pulmonary metastasis in an adolescent girl. Pediatr Emerg Care. (2010) 26:203–5. doi: 10.1097/PEC.0b013e3181d1e3f7
14. Kim, J, Kang, M, Kim, J, Jung, S, Park, J, Lee, D, et al. A case of Nocardia farcinica pneumonia and Mediastinitis in an immunocompetent patient. Tuberc Respir Dis. (2016) 79:101–3. doi: 10.4046/trd.2016.79.2.101
15. Bai, Y, Liu, K, Chen, Y, Zhao, H, Wang, Y, Liu, X, et al. Disseminated infection of Nocardia farcinica in an immunocompetent adult: mistaken for tuberculosis Bacilli in acid-fast staining of Bronchoalveolar lavage fluid. J Cytol. (2021) 38:106–8. doi: 10.4103/JOC.JOC_208_20
16. Dong, S, Lin, Q, Dai, X, and Zhang, B. Nocardia farcinica pneumonia with Sepsis and a bronchial neoplasm in a healthy patient: a case report. Clin Med Insights Circ Respir Pulmon Med. (2023) 17:117954842211463. doi: 10.1177/11795484221146370
17. Kandi, V. Human Nocardia infections: a review of pulmonary nocardiosis. Cureus. (2015) 7:e304. doi: 10.7759/cureus
18. Duggal, SD, and Chugh, TD. Nocardiosis: a neglected disease. Med Princ Pract. (2020) 29:514–23. doi: 10.1159/000508717
19. Gu, W, Miller, S, and Chiu, CY. Clinical metagenomic next-generation sequencing for pathogen detection. Annu Rev Pathol. (2019) 14:319–38. doi: 10.1146/annurev-pathmechdis-012418-012751
20. Ding, J, Ma, B, Wei, X, and Li, Y. Detection of Nocardia by 16S ribosomal RNA gene PCR and metagenomic next-generation sequencing (mNGS). Front Cell Infect Microbiol. (2022) 11:768613. doi: 10.3389/fcimb.2021.768613
21. Xu, Y, Lian, QY, Chen, A, Zhang, JH, Xu, X, Huang, DX, et al. Clinical characteristics and treatment strategy of nocardiosis in lung transplant recipients: a single-center experience. IDCases. (2023) 32:e01758. doi: 10.1016/j.idcr.2023.e01758
22. Liang, Y, Lin, M, Qiu, L, Chen, M, Tan, C, Tu, C, et al. Clinical characteristics of hospitalized patients with Nocardia genus detection by metagenomic next generation sequencing in a tertiary hospital from southern China. BMC Infect Dis. (2023) 23:772. doi: 10.1186/s12879-023-08615-z
23. Miao, Q, Ma, Y, Wang, Q, Pan, J, Zhang, Y, Jin, W, et al. Microbiological diagnostic performance of metagenomic next-generation sequencing when applied to clinical practice. Clin Infect Dis. (2018) 67:S231–40. doi: 10.1093/cid/ciy693
24. Melia, M., and Bartlett, J. G. Nocardia. In Johns Hopkins ABX Guide. The Johns Hopkins University. (2017). Available at: https://www.hopkinsguides.com/hopkins/view/Johns_Hopkins_ABX_Guide/540389/all/Nocardia
Keywords: bronchial neoplasm, metagenomic next-generation sequencing, Nocardia farcinica, postural dyspnea, immunocompetent adult
Citation: Tan Y, Yang M, Wan C, Tang S, Liu L and Chen L (2024) Nocardia farcinica infection presenting as a solitary bronchial neoplasm in an immunocompetent adult: a case report. Front. Med. 10:1337303. doi: 10.3389/fmed.2023.1337303
Edited by:
John-David Aubert, Centre Hospitalier Universitaire Vaudois (CHUV), SwitzerlandReviewed by:
Leslie Noirez, Centre Hospitalier Universitaire Vaudois (CHUV), SwitzerlandTohru Gonoi, Chiba University, Japan
Copyright © 2024 Tan, Yang, Wan, Tang, Liu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Chen, bGNoZW5zQDEyNi5jb20=; Lin Liu, bGxpbml1QDEyNi5jb20=
†These authors have contributed equally to this work
 Yuying Tan1†
Yuying Tan1† Lei Chen
Lei Chen