- 1Academy of Integrative Medicine, Fujian University of Traditional Chinese Medicine, Fuzhou, China
- 2Fujian Key Laboratory of Integrative Medicine on Geriatrics, Fujian University of Traditional Chinese Medicine, Fuzhou, China
- 3Department of Sports Medicine (Orthopedics), Fujian University of Traditional Chinese Medicine Subsidiary Rehabilitation Hospital, Fuzhou, China
- 4College of Integrative Medicine, Fujian University of Traditional Chinese Medicine, Fuzhou, China
Osteoporosis (OP), osteoarthritis (OA), and rheumatoid arthritis (RA) are common bone and joint diseases with a high incidence and long duration. Thus, these conditions can affect the lives of middle-aged and elderly people. Tea drinking is a traditional lifestyle in China, and the long-term intake of tea and its active ingredients is beneficial to human health. However, the mechanisms of action of tea and its active ingredients against OP, OA, and RA are not completely elucidated. This study aimed to assess the therapeutic role and related mechanisms of tea and its active ingredients in OP, OA, and RA. Moreover, it expanded the potential mechanisms of tea efficacy based on network pharmacology and molecular docking. Results showed that tea has potential anti-COX properties and hormone-like effects. Compared with a single component, different tea components synergize or antagonize each other, thereby resulting in a more evident dual effect. In conclusion, tea has great potential in the medical and healthcare fields. Nevertheless, further research on the composition, proportion, and synergistic mechanism of several tea components should be performed.
1 Introduction
Musculoskeletal disorders, such as osteoporosis (OP), osteoarthritis (OA), and rheumatoid arthritis (RA), are prevalent health issues with substantial global impact. Epidemiological data have revealed the widespread development of these bone-related conditions across various age groups (1–3). OP is defined as bone brittleness, which is associated with fracture susceptibility (4). OA is characterized by cartilage degradation, leading to pain and impaired mobility (5). RA is a condition causing joint deformities and other organ pathologies (6). Current pharmacological therapies (such as bisphosphonates for OP, non-steroidal anti-inflammatory drugs for OA, and disease-modifying antirheumatic drugs for RA) do not provide a fundamental solution to issues with significant safety risks (7–9). Therefore, safer and more promising alternatives should be investigated.
Tea (Camellia sinensis), a traditional beverage, has several benefits for the human body (10, 11). In a randomized placebo-controlled trial, postmenopausal women with osteopenia who received green tea polyphenols exhibited better bone health (12). In another randomized controlled trial involving 50 participants, individuals who supplemented their diclofenac tablets with green tea had significantly lower pain scores, as measured using the visual analog scale, and better OA physical function scores compared with controls (13). In addition, a case–control study has revealed that high tea consumption can have a protective effect on smokers and individuals with anti-citrullinated protein autoantibody-positive RA (14). Nonetheless, the comprehensive roles of tea and its extracts in OP, OA, and RA must be systematically elucidated.
This review aimed to examine the current therapeutic mechanisms of tea and its extracts against OP, OA, and RA. In addition, the key active components and target proteins of tea were identified via computer simulations, thereby providing a theoretical foundation for its potential medical and healthcare applications.
2 Effects of tea against OP, OA, and RA
Tea has remarkable performance due to its antioxidant and anti-inflammatory properties. Therefore, it can be a promising candidate when used as a novel anti-inflammatory or antioxidant agent (15, 16). Furthermore, contemporary research can provide substantial evidence supporting the role of tea in preventing various diseases, particularly joint diseases, inhibiting disease progression, and promoting pain relief (17, 18). Catechins, which encompass (+)-catechin (C), (−)-epigallocatechin (EC), (−)-gallocatechin (GC), (−)-epigallocatechin gallate (ECG), (−)-epigallocatechin (EGC), and (−)-epigallocatechin-3-gallate (EGCG), are the primary components of tea (19, 20). These compounds, which are naturally consumed via tea consumption, play an essential role in maintaining bodily health. Current research focuses on the anti-inflammatory and antioxidant activities of tea and its components. That is, they promote osteoblast growth and inhibit osteoclast formation, thereby counteracting OP. In addition, these activities inhibit chondrocyte damage and synovial inflammation, which then promote resistance to OA and RA.
In this context, the current study primarily aimed to provide an overview of the potential mechanisms underlying the effects of tea against OP, OA, and RA.
2.1 Studies of tea treating osteoporosis
OP, characterized by low bone mass (osteopenia) and deterioration of bone microarchitecture, leads to compromised bone strength and an increased risk of fractures (21). There are several effective strategies against OP. These include the maintenance of bone homeostasis, which enhances bone density, microarchitecture, and strength and reduces the risk of OP and associated fractures (22–25). We gathered clinical studies of tea-treating OP, which are summarized in Table 1. A study showed that in postmenopausal women, additional intake of green tea polyphenol supplementation can improve the serum and urinary levels of oxidative damage biomarkers (31). Furthermore, it can increase the production of bone formation markers and improve bone turnover rates (12). In particular, the intake of active ingredients such as tea water extracts and tea polyphenols leads to significant improvements in bone mineral density, microstructure deterioration, and biological properties in ovariectomized or orchiectomized rats (32–35).
Maintaining bone homeostasis is important for addressing OP by regulating osteoblasts and mesenchymal stem/stromal cells to achieve a balance between bone formation and resorption (36–38). Tea extracts have antioxidant effects that enhance osteoclastogenesis, improve cell survival, and mitigate inflammation (39). Moreover, tea extracts exhibit potent phytoestrogenic effects by upregulating ESR1 expression (40, 41). EGC significantly upregulated the expression of key markers of bone formation, including Runt-related transcription factor 2 (RUNX2), alkaline phosphatase, osteonectin, and osteopontin (42). (−)-Epiafzelechin and (−)-epicatechin promote osteoblast proliferation and differentiation via their antioxidant properties (43). In addition, (−)-epicatechin gallate stimulates osteoblast differentiation by activating the PDZ-binding motif (TAZ) and RUNX2 (44). EGCG has antioxidant effects via the Nrf2 pathway, thereby protecting osteoblasts from apoptosis and attenuating bone microstructure deterioration (45). Theaflavin-3,3′-digallate activates several signaling pathways, including the tumor necrosis factor-α (TNF-α)-inhibited mitogen-activated protein kinase (MAPK), Wnt/β-catenin, and BMP/Smad pathways. This mechanism ultimately promotes the transcription of osteogenesis-associated factors such as RUNX2 and Osterix, leading to osteoblast differentiation and maturation (46). Furthermore, various tea extracts and tea polyphenols inhibit osteoclast formation, with EGCG being the most effective (47–51). Notably, EGCG downregulates the expression of NFATc1, directly binds to RANK, blocks the interaction between RANK and RANKL, and inhibits multiple pathways, including the HO-1-HMGB1-AGE pathway, nuclear factor kappa B (NF-κB) pathway, MAPK signaling pathway, and RANK/RANKL/OPG pathway, ultimately reducing osteoclast formation (52–54). In addition, tea extracts and EGCG enhance the osteogenic differentiation capacity of stem cells (55–57), thereby underscoring the anti-osteoporotic potential of tea and its compounds.
2.2 Studies of tea treating osteoarthritis
OA is a prevalent chronic joint disorder primarily characterized by joint cartilage degeneration, synovial inflammation, and pain. Moreover, it is often associated with the aging process (63, 64). We gathered clinical studies of tea treating OA and summarized in Table 2. Several studies have reported that high green tea intake is associated with a low incidence of OA (65, 66). In a short-term randomized, double-blind pilot study, a mixture of baicalin and catechin was as effective as naproxen in controlling the signs and symptoms of knee OA (67). Green tea has anti-inflammatory properties (58). Hence, tea and its extracts can mitigate the pathological progression of OA by decreasing the expression of inflammatory factors, including interleukins and matrix metalloproteinases, in the articular cartilage and synovium, thereby suppressing the inflammatory response.
Research on the effects of tea beverages on OA dates has been conducted since 1991 (68). Haqqi et al. have made significant contributions by focusing on the pharmacodynamic mechanisms of tea and its components for treating OA. They discovered that tea polyphenols when added to water, can be effective in preventing the development and progression of arthritis (69). Furthermore, they found that EGCG can reduce the expression and activity of various factors, including cyclooxygenase-2 (COX-2), nitric oxide synthase-2 (NOS-2) (70), matrix metalloproteinase (MMP)-1, MMP-13 (71), and TNF-α (72). In addition, EGCG can globally suppress the inflammatory response in human chondrocytes, possibly via the inhibition of NF-κB and c-Jun N-terminal kinase (JNK)-MAPK activation (73–75). A previous study has consistently revealed the protective effect of EGCG against OA (76). Furthermore, its mechanisms have been found to be involved in various processes such as microRNA regulation (e.g., microRNA-140-3p, microRNA-199a-3p, and microRNA-29b) (77–79) and oxidative stress (77, 78). In addition to its chondroprotective effects, EGCG alleviates synovial inflammation (80). Another study has explored the role of other tea components, such as theaflavin-3,3′-digallate (81) and theaflavin (82), both of which have the ability to inhibit cartilage damage. In recent years, previous studies have focused on enhancing the anti-inflammatory effects of tea and its components. Several studies have improved the efficacy of EGCG in the cartilage and synovium by introducing novel materials or altering the mode of application. These mechanisms involve the modulation of autophagy, the production of reactive oxygen species, mitochondrial repair, and synovial macrophage polarization (83–86).
2.3 Studies of tea treating rheumatoid arthritis
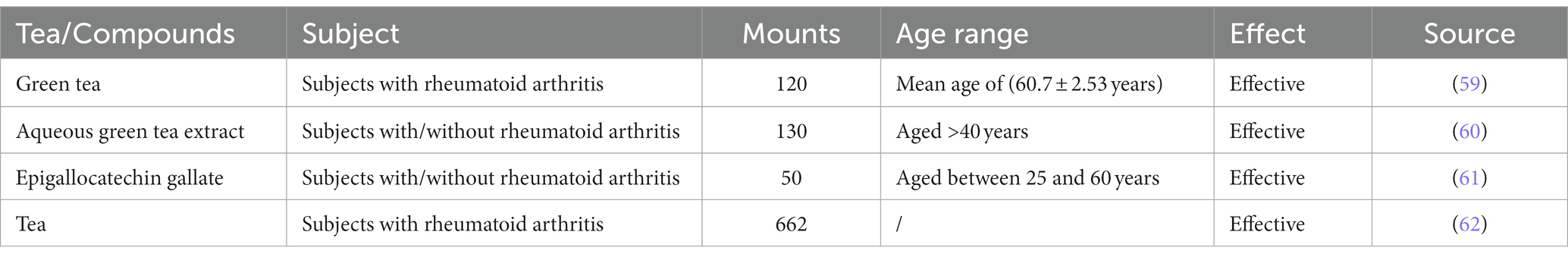
In a review published in 2001, the authors proposed that green tea can be a prophylactic agent against chronic inflammatory diseases, including RA (87). We gathered Clinical trial of tea or components treating rheumatoid arthritis in Table 3. A case–control study showed that consuming more than one cup of green tea per month can have a preventive effect against RA (88). Maintaining a daily intake of 4–6 cups of green tea over a period of up to 6 months has a positive effect on RA disease activity in patients with RA (59). In addition, a Swedish case–control study showed that heavy tea consumption has a protective effect against RA in smokers and anti-citrullinated protein autoantibody-positive individuals (14). Numerous experiments have revealed that tea water extracts or the polyphenolic components of tea can reduce RA in experimental animals. The investigation of its mechanism has predominantly revolved around its antioxidant and anti-inflammatory properties.
Various tea-related ingredients, including tea aqueous extract (89), catechin (90), EGCG, and gallic acid (91), are significantly effective in alleviating RA symptoms. The imbalance between oxidation and reduction is an important mechanism in the development of RA (92). Reactive species oxidize cellular biomolecules, leading to DNA damage (93). Therefore, reducing oxidative stress in RA is an effective therapeutic strategy (94). Physiological antioxidant enzymes such as superoxide dismutase (SOD), glutathione (GSH), and peroxiredoxins counteract the possible damaging effects of these reactive species by scavenging or neutralizing free radicals and oxidizing substances. Research has shown that green tea extract can increase the SOD and GSH levels while decreasing the levels of lipid peroxides (LPO), nitric oxide (NO), and PGE2 in a rat RA model. Hence, it can be beneficial in both the liver and brain (95, 96). EGCG-fed mice exhibited higher levels of heme oxygenase-1 (HO-1) and nuclear factor erythroid2-related factor 2 (Nrf2) (97, 98), and the significant activation of HO-1 and Nrf2 has anti-arthritic effects (99). In addition to their antioxidant effects, tea and its compounds have significant anti-inflammatory effects. Sabrina Fechtner has revealed that EGCG, epigallocatechin (EGC), and EC occupy the active site of the TAK1 kinase domain, with EGCG being the most dominant, interfering with the IL-1β signaling pathway that regulates the expression of IL-6, IL-8, and Cox-2 in primary human RA synovial fibroblasts (100). Another study revealed that EGCG targets TAK1 for treating RA by inhibiting TAK1 phosphorylation at Thr (184/187), suppressing K(63)-linked autoubiquitination of TRAF6, and enhancing proteasome-associated deubiquitinase expression to rescue proteins from proteasomal degradation (101). In addition, green tea extract and EGCG modulate the production of chemokine (102) and immune cells (97), leading to RA improvements.
3 Assessment of the potential active components of tea in OP, OA, and RA
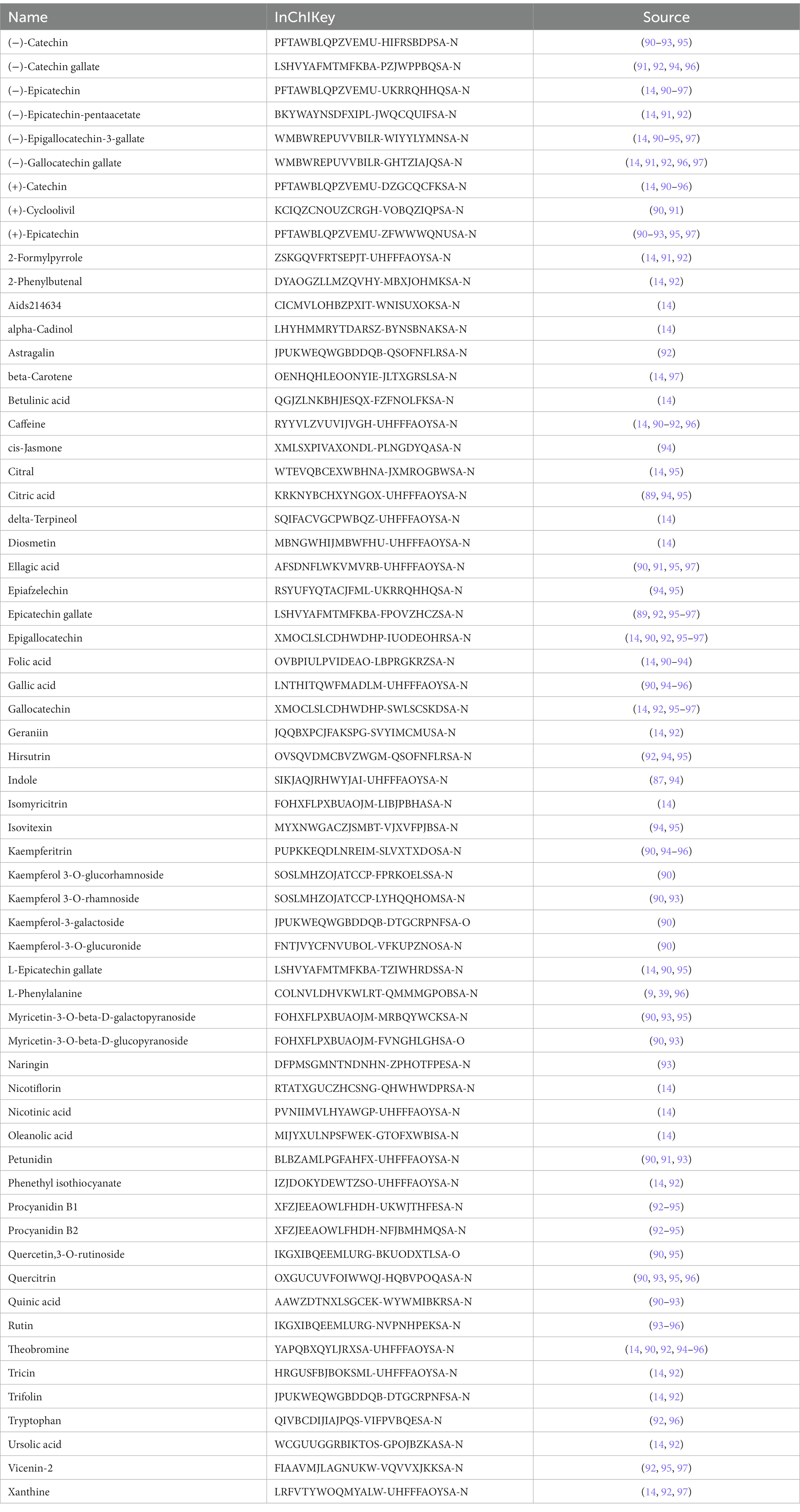
Since tea performed well not only in OP but also in OA and RA, searching for targets in OP, OA, and RA may have a practical meaning in providing guidance for the prevention and control of OP, OA, and RA. With the help of bioinformatics analysis methods, we summarized the ingredients of tea (Table 4) and discovered the relationship between tea ingredients and diseases. We screened the genes of OP, OA, and RA related to tea ingredients and then performed Gene Ontology (GO) analysis and enrichment analysis of the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway.
3.1 Oxidative stress mitigation: unraveling pathways
Due to aging, traumatic injury, or immune dysfunction, various tissue cells are exposed to a range of pathophysiological mediators, including reactive oxygen species (ROS) and reactive nitrogen species (NOS). ROS-mediated stress, by inducing functional impairments in osteoblasts, osteoclasts, chondrocytes, and synovial cells, contributes to the pathological progression of OP, OA, and RA.
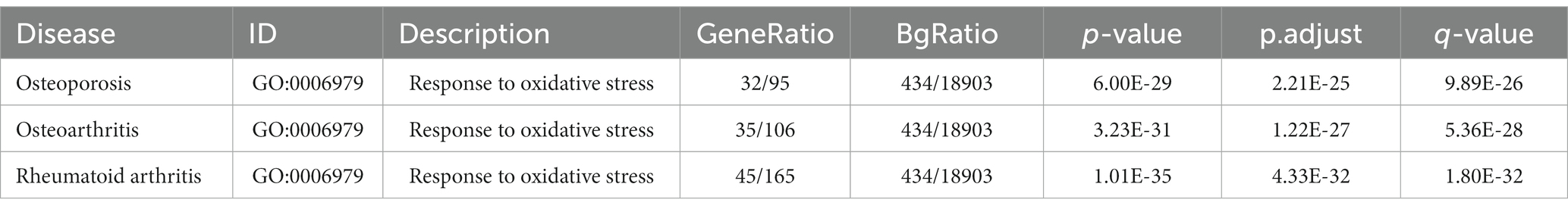
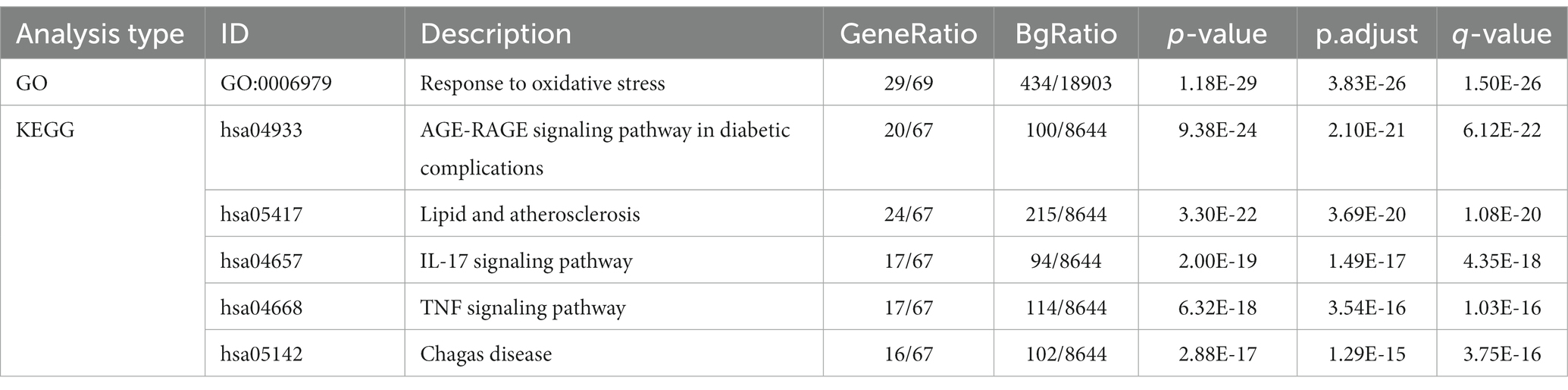
Tea exhibits robust antioxidant effects, a perspective supported by network pharmacology results. The active ingredient–gene target networks of the active ingredients for each disease were mapped (Figure 1). Gene Ontology (GO) enrichment analysis reveals that genes associated with OP, targeted by active components of tea, are primarily enriched in response to oxidative stress (Figure 2A). In the GO analysis results of networks between tea and OA and between tea and RA, genes linked to response to oxidative stress occupy the top positions (Figures 2B,C). The frequency ranking of responses to oxidative stress across the three diseases is detailed in Table 5. Among the 37 tea components targeting genes related to this biological process, EGCG, caffeine, ursolic acid, beta-carotene, and (−)-epicatechin emerge as the top five components. KEGG pathway analysis indicates enrichment in pathways such as lipid and atherosclerosis and the AGE-RAGE signaling pathway in diabetic complications (Figure 3 and Table 6). Although direct evidence of oxidative stress is not explicitly shown in the KEGG results, the enrichment of pathways closely related to oxidative stress responses underscores the pivotal role of tea’s antioxidant action in the treatment of OP, OA, and RA.
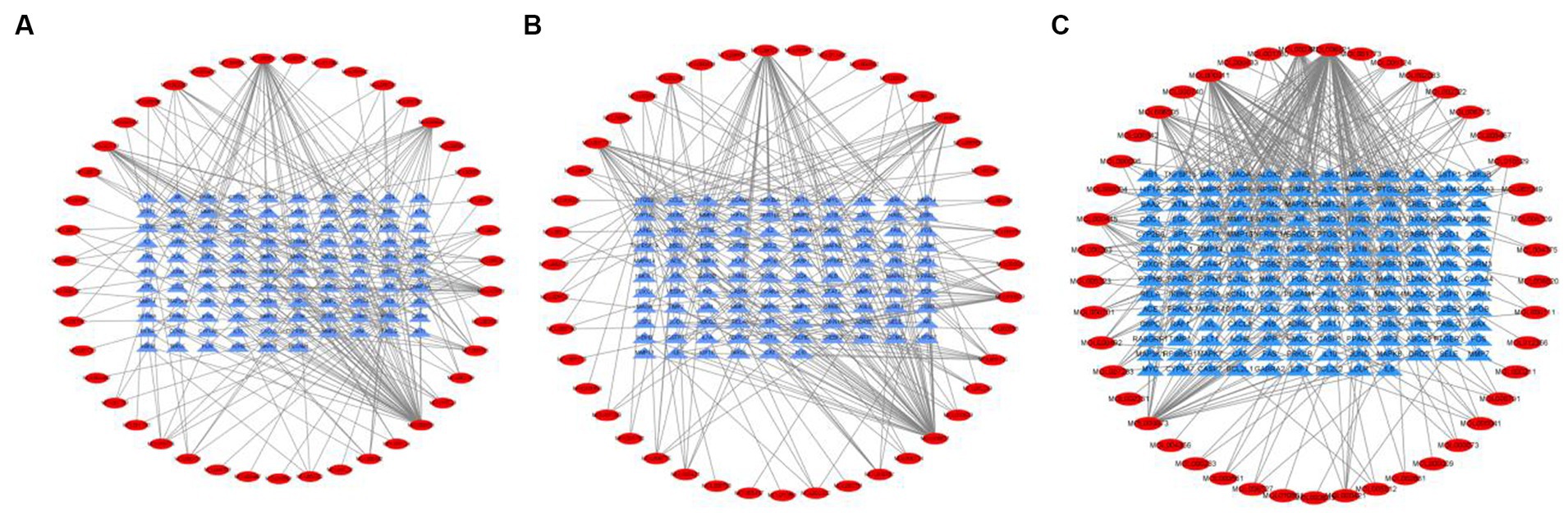
Figure 1. Networks of tea components targeting OP, OA and RA. (A) Network of tea treating OP; (B) Network of tea treating OA; (C) Network of tea treating RA.
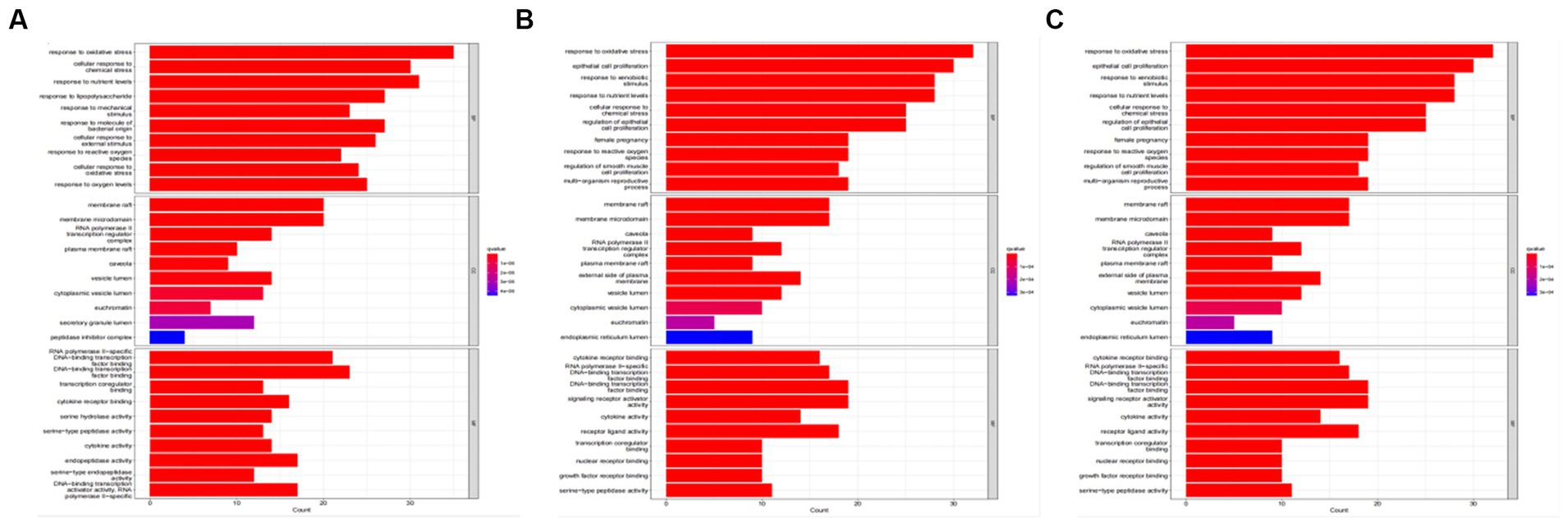
Figure 2. GO analysis of tea targeting OP, OA, and RA. (A) GO analysis of tea targeting OP; (B) GO analysis of tea targeting OA; (C) GO analysis of tea targeting RA.
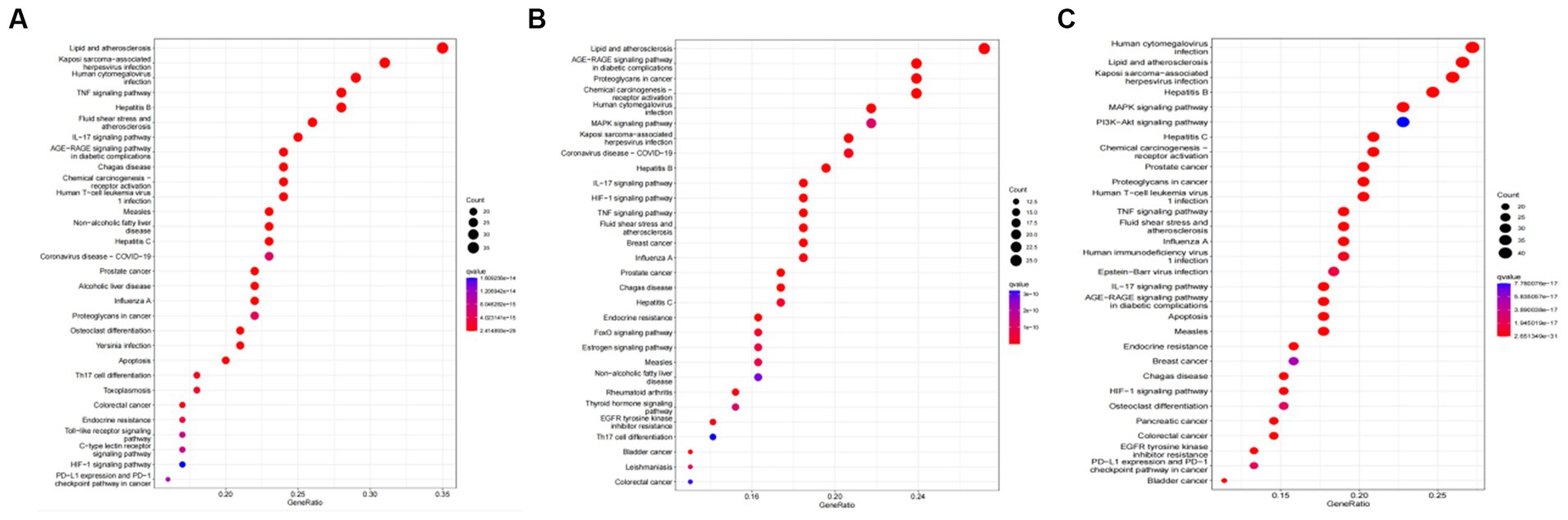
Figure 3. KEGG analysis of tea targeting OP, OA, and RA. (A) KEGG analysis of tea targeting OP; (B) KEGG analysis of tea targeting OA; (C) KEGG analysis of tea targeting RA.
3.2 Inflammatory modulation: an alternate pathway for tea impact
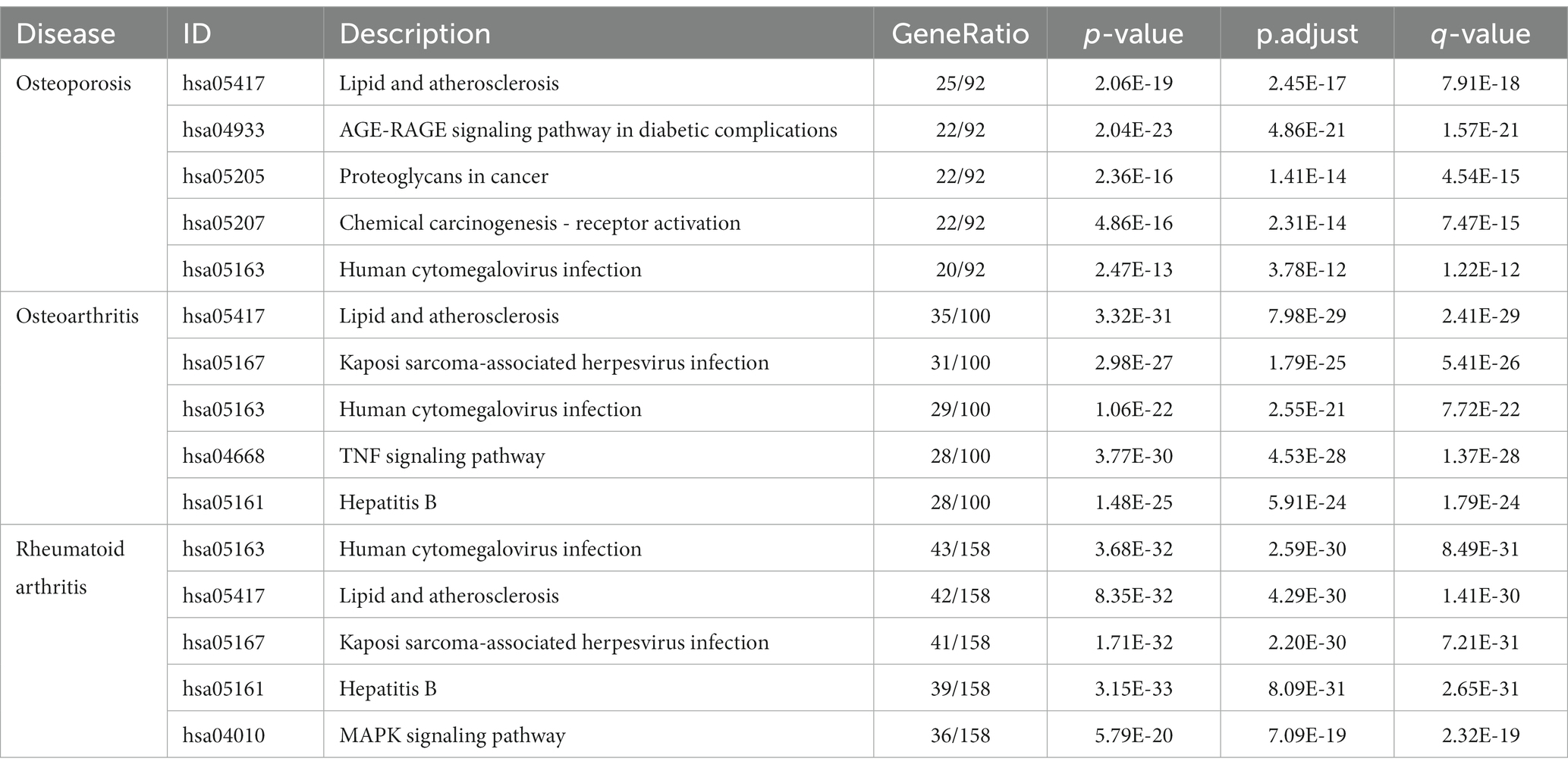
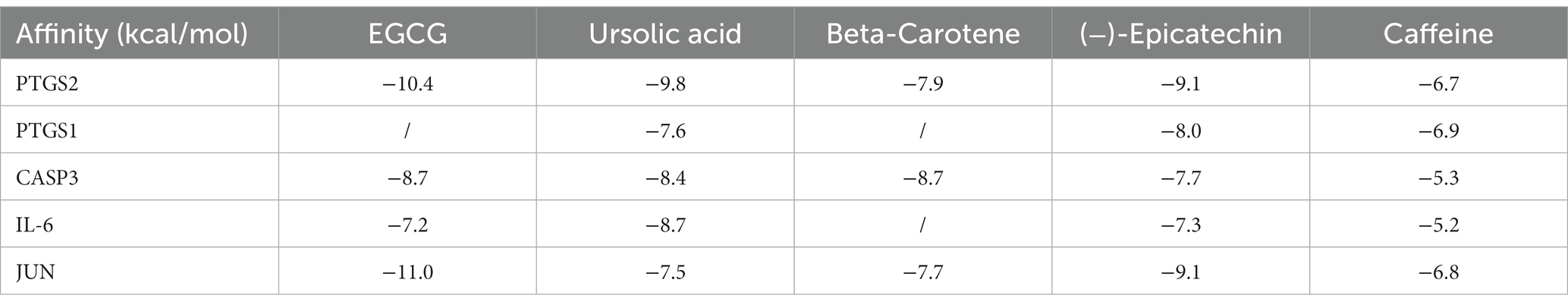
To further investigate the therapeutic effects of tea in treating OP, OA, and RA, network pharmacology was applied to analyze the targeted relationship between tea and commonly associated genes. The analysis results not only confirm the involvement of oxidative stress in line with previous findings but also reveal enrichment in pathways such as lipid and atherosclerosis, the AGE-RAGE signaling pathway in diabetic complications, the IL-17 signaling pathway, and the TNF signaling pathway through KEGG analysis (Figure 4 and Table 7). This demonstrates the anti-inflammatory effects of tea, with core genes such as PTGS2, PTGS1, CASP3, JUN, and IL-6 remaining central in these pathways. A total of 36 tea components target genes related to these pathways, with (−)-epigallocatechin-3-gallate, caffeine, ursolic acid, beta-carotene, and (−)-epicatechin ranking as the top five components targeting the highest number of genes. This suggests that these components play a core role in anti-inflammatory action. Importantly, these components also play a significant role in the previously mentioned antioxidative effects. Therefore, we utilized computer-simulated molecular docking to further validate the relationships between these components and core proteins.
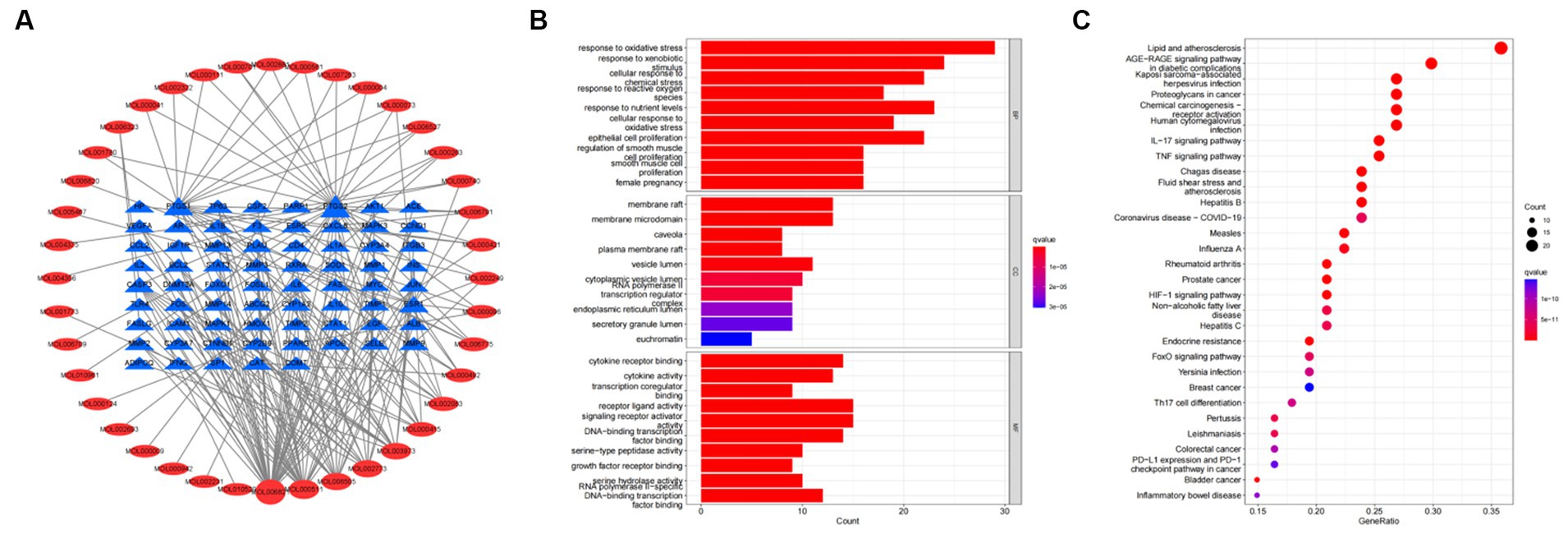
Figure 4. Bioinformatics analysis for tea targeting common genes of OP, OA, and RA. (A) Network of tea treating common genes, (B) Gene ontology (GO) enrichment analysis; (C) Kyoto encyclopedia of genes and genomes (KEGG) pathway enrichment analysis.
We conducted molecular docking for the aforementioned components (EGCG, ursolic acid, beta-carotene, (−)-epicatechin, and caffeine) and proteins (PTGS2, PTGS1, CASP3, IL-6, and JUN) with established targeting relationships. The affinity for each combination was below −5 kCal ∙ mol-1. Thus, it has favorable binding activity. Furthermore, most combinations exhibited an affinity below −7 kCal ∙ mol-1, which indicated a robust binding activity (Table 8). Figure 5 shows the combinations featuring hydrogen bonds whose affinity is below −9 kCal∙mol-1.
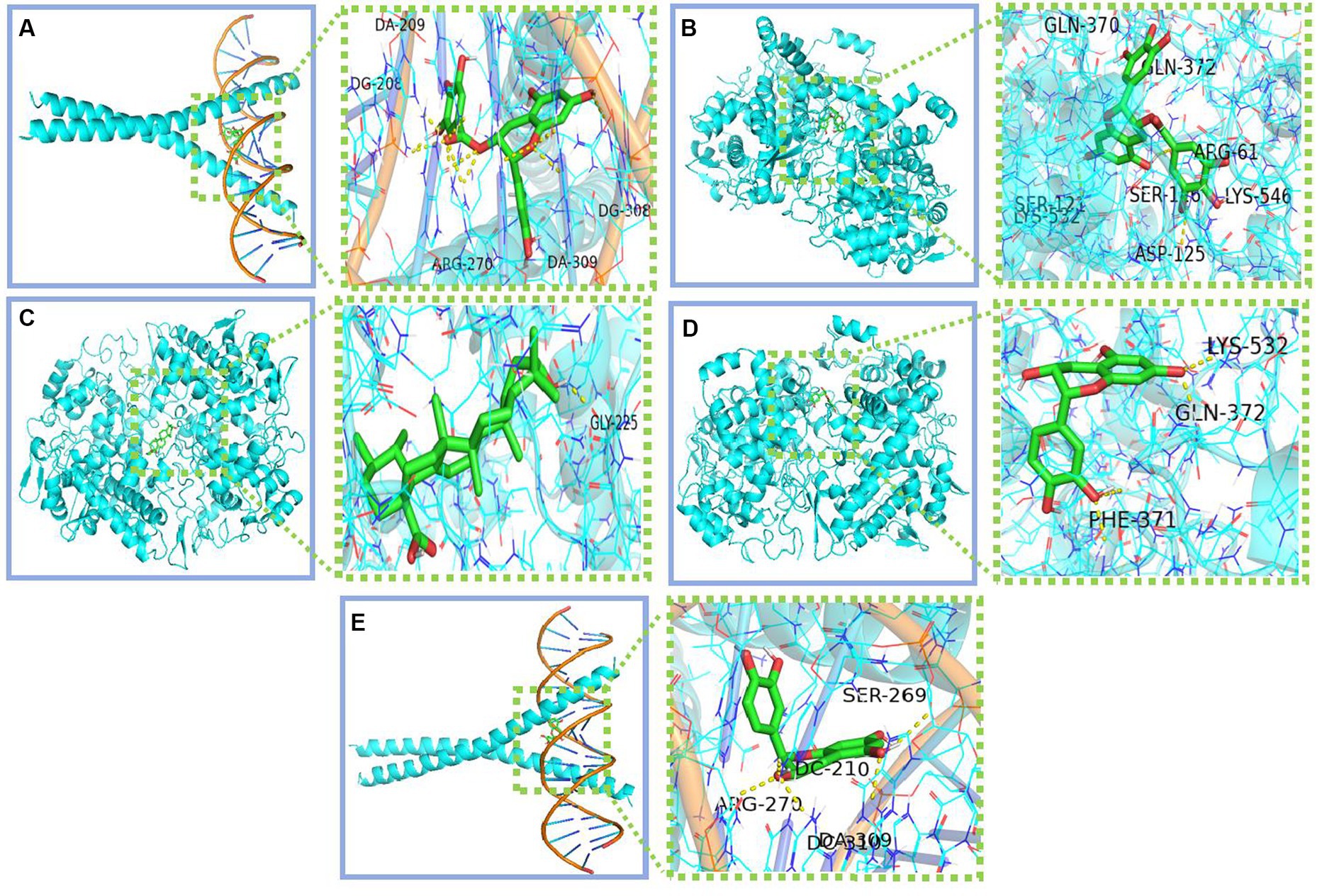
Figure 5. Combinations with affinity below −9 kCal∙mol-1 (A) Jun-EGCG; (B) PTGS2-EGCG; (C) PTGS2-Ursolic acid; (D) PTGS2-Epicatechin; (E) Jun-Epicatechin.
4 Discussion
The Chinese have been drinking tea for hundreds of years. Therefore, most people believe that tea can reduce the risk of various diseases. With the development of modern medicine, the efficacy and mechanism of action of tea have been comprehensively explored. However, the results of clinical and animal studies are still inconclusive. This review aimed to explore the possibility and mechanism of action of tea for treating OP, OA, and RA by evaluating previous studies and constructing a network for the association between tea and different diseases. Current studies have focused on the anti-inflammatory and antioxidant effects of tea. Tea and its components affect the activation of various enzymes, transcription of inflammation-related genes, and release of inflammatory factors in bone and joint tissues via the Nrf2-related pathway, MAPK pathway, and NF-κB pathway. Moreover, they regulate oxidative stress and inflammation in tissues and cells in OP, OA, and RA.
Network pharmacology results show that PTGS2, PTGS1, CASP3, IL-6, and JUN are the potential targets of tea when regulating OP, OA, and RA. PTGS2 (also referred to as COX-2) and PTGS1 (also known as COX-1) have been extensively and intensively evaluated. COX inhibitors, or non-steroidal anti-inflammatory drugs, inhibit the production of COX-2 and COX-1 to achieve anti-inflammatory, analgesic, and antipyretic effects. In addition, they are commonly used in the treatment of OA and RA (103). The anti-inflammatory and analgesic efficacies of COX inhibitors are significant. However, they also increase the risk of gastrointestinal ulcers, hemorrhage, and renal and cardiovascular adverse events (104). In the molecular target regulatory network of tea and disease (OP, OA, and RA), 31 molecules can bind to PTGS2 targets, and 18 molecules can bind to PTGS1 targets. Therefore, tea can possibly play a role in COX inhibition. However, epidemiological studies have shown that tea consumption reduces the risk of cardiovascular mortality through mechanisms associated with the lowering of lipid levels, mitigation of ischemia/reperfusion injury (105, 106), inhibition of oxidative stress, enhancement of endothelial function, attenuation of inflammation, and protection of cardiomyocyte function (107). The tea polyphenol EGCG exerted a protective effect on patients with 5-aminosalicylic acid and/or azathioprine-refractory ulcerative colitis (108). According to the report, TIMP1, PTGS2, ICAM1, MMP9, IL1B, CXCL8, IL-6, and RELA were identified as hub genes in ulcerative colitis (109), which had been found in the target collection of tea components. A new study, processed by integrating network pharmacology and metabolomics, demonstrated that Jasminum elongatum reverses ulcerative colitis in mice via the IκB/p65/COX-2/arachidonic acid pathway (110). The tea aqueous extract inhibited experimentally induced colitis and liver injury in mice (111). Tea and its extracts confer protective effects against alcoholic liver disease, non-alcoholic fatty liver disease, CCL4-induced liver injury, and inflammatory liver damage. The mechanisms underlying these protective effects involve modulation of signaling pathways such as the NF-κB signal pathway, TGFβ/p-ERK/p-Smad1/2 signal pathway, Nrf2 signaling activation, and autophagy restoration (112–117). Tea consumption has been associated with a reduced risk of renal cell carcinoma (118) and improved kidney function in diabetic patients (119). Studies indicate that effective components such as L-theanine, tea polyphenols, and EGCG can ameliorate renal cell damage through the modulation of related pathways, including the AGEs/RAGE signaling pathway (120), CYP450s/ROS/MAPK/NF-κB pathway (121), TGFβ/Smad3 signaling pathway (122), and ferroptosis (123). The abovementioned studies have revealed the potential COX inhibitory effects of tea and its ability to fight diseases such as OP, OA, and RA with minimal cardiovascular, gastrointestinal, hepatic, and renal damage.
Via a network pharmacological analysis, a number of tea ingredients target disease genes in OP, OA, and RA, proving that tea has therapeutic or adjunctive therapeutic effects against OP, OA, and RA. Current clinical studies do not provide clear conclusions. Some reports have shown that tea can be effective in treating OP, OA, or RA. However, there are limitations in terms of the study population, the size of the population, or the quality of the data. Meanwhile, some clinical studies or meta-analyses have revealed that tea consumption does not improve the clinical performance of patients or reduce the risk of OP, OA, or RA (124). Considering the diversity of active ingredients in tea, in addition to ingredients such as EGCG and EC, which play a positive role, other ingredients, such as caffeine, increase the risk of fracture, OP, or OA and can be an influencing factor in the therapeutic effects of tea. Therefore, further studies on the role of tea must be performed.
Studies on effective treatment strategies against OP, OA, and RA are still conducted by the medical community. Tea is rich in various natural compounds that can be used for disease treatment. This study aimed to evaluate the potential mechanisms of action of tea and its related components for treating OP, OA, and RA. These mechanisms of action mainly focus on the antioxidant and anti-inflammatory responses of tea components. In previous experimental studies, tea and tea extracts and their active ingredients mainly acted on OA inflammatory factors to alleviate OA cartilage degeneration. Furthermore, they are mainly used to promote osteoblast growth, inhibit osteoclast formation in OP, and inhibit inflammation mainly via their antioxidant effects in RA. The network pharmacological results revealed targets and pathways not covered by existing experimental studies. Moreover, they were validated by molecular docking. The network pharmacology results showed that tea has an anti-COX capacity, hormone-like properties, and cardiovascular, gastrointestinal, hepatic, and renal protective effects. This is because tea has various components that synergize or antagonize each other, which has a more pronounced dual effect than a single component. In the network pharmacology analysis, we comprehensively collected data on the compounds of tea and did not screen the compounds for bioavailability and drug-like properties so that we could analyze the mechanism of action of tea against OP, OA, and RA analyzed without omission. However, different kinds of tea have different compound compositions; for example, black tea contains theaflavins, thearubigins, and other components, and lower levels of polyphenols compared with green tea, which cannot be represented in network pharmacological analysis results. The network pharmacological analysis results may conceal the specific effects of tea on certain disease genes.
Nevertheless, there is still a need for future research on the composition of tea and the development of standardized tea beverages, which will lead to efficacy studies. Research on the relationship between the use of standardized tea beverages and disease would be helpful to clarify the efficacy of tea. In conclusion, the use of tea has great potential in the medical and healthcare fields.
Author contributions
XX: Data curation, Formal analysis, Writing – original draft. JF: Investigation, Methodology, Writing – original draft. WG: Conceptualization, Investigation, Writing – original draft. YQ: Investigation, Writing – original draft. DW: Methodology, Writing – original draft. ZH: Formal analysis, Validation, Writing – original draft. LW: Supervision, Writing – review & editing. XL: Funding acquisition, Project administration, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (No. 82374495), the Science and Technology Programs Pilot Project of Fujian Province (No. 2021Y0032). The Major Project for Young and Middle-aged Fund of Fujian Provincial Health Commission (No. 2023ZQNZD016).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lobo, RA, and Gompel, A. Management of menopause: a view towards prevention. Lancet Diab. Endocrinol. (2022) 10:457–70. doi: 10.1016/S2213-8587(21)00269-2
2. Sarzi-Puttini, P, Zen, M, Arru, F, Giorgi, V, and Choy, EA. Residual pain in rheumatoid arthritis: is it a real problem? Autoimmun Rev. (2023) 22:103423. doi: 10.1016/j.autrev.2023.103423
3. Muñoz Laguna, J, Puhan, MA, Rodríguez Artalejo, F, De Pauw, R, Wyper, GMA, Devleesschauwer, B, et al. Certainty of the global burden of disease 2019 modelled prevalence estimates for musculoskeletal conditions: a meta-epidemiological study. Int J Public Health. (2023) 68:1605763. doi: 10.3389/ijph.2023.1605763
4. Li, J, Chen, X, Lu, L, and Yu, X. The relationship between bone marrow adipose tissue and bone metabolism in postmenopausal osteoporosis. Cytokine Growth Factor Rev. (2020) 52:88–98. doi: 10.1016/j.cytogfr.2020.02.003
5. Giorgino, R, Albano, D, Fusco, S, Peretti, GM, Mangiavini, L, and Messina, C. Knee osteoarthritis: epidemiology, pathogenesis, and mesenchymal stem cells: what else is new? An update. Int J Mol Sci. (2023) 24:6405. doi: 10.3390/ijms24076405
6. Smolen, JS . Insights into the treatment of rheumatoid arthritis: a paradigm in medicine. J Autoimmun. (2020) 110:102425. doi: 10.1016/j.jaut.2020.102425
7. Ensrud, KE . Bisphosphonates for postmenopausal osteoporosis. Jama-J. Am. Med. Assoc. (2021) 325:96. doi: 10.1001/jama.2020.2923
8. Da, CB, Pereira, TV, Saadat, P, Rudnicki, M, Iskander, SM, Bodmer, NS, et al. Effectiveness and safety of non-steroidal anti-inflammatory drugs and opioid treatment for knee and hip osteoarthritis: network meta-analysis. Bmj-Brit. Med. J. (2021) 375:n2321. doi: 10.1136/bmj.n2321
9. Kerschbaumer, A, Sepriano, A, Bergstra, SA, Smolen, JS, van der Heijde, D, Caporali, R, et al. Efficacy of synthetic and biological dmards: a systematic literature review informing the 2022 update of the eular recommendations for the management of rheumatoid arthritis. Ann Rheum Dis. (2023) 82:95–06. doi: 10.1136/ard-2022-223365
10. Zhai, X, Zhang, L, Granvogl, M, Ho, CT, and Wan, X. Flavor of tea (camellia sinensis): a review on odorants and analytical techniques. Compr Rev Food Sci Food Saf. (2022) 21:3867–09. doi: 10.1111/1541-4337.12999
11. Ahammed, GJ, and Li, X. Hormonal regulation of health-promoting compounds in tea (camellia sinensis l.). Plant Physiol Biochem. (2022) 185:390–400. doi: 10.1016/j.plaphy.2022.06.021
12. Shen, CL, Chyu, MC, Yeh, JK, Zhang, Y, Pence, BC, Felton, CK, et al. Effect of green tea and tai chi on bone health in postmenopausal osteopenic women: a 6-month randomized placebo-controlled trial. Osteoporos Int. (2012) 23:1541–52. doi: 10.1007/s00198-011-1731-x
13. Hashempur, MH, Sadrneshin, S, Mosavat, SH, and Ashraf, A. Green tea (camellia sinensis) for patients with knee osteoarthritis: a randomized open-label active-controlled clinical trial. Clin Nutr. (2018) 37:85–90. doi: 10.1016/j.clnu.2016.12.004
14. Westerlind, H, Palmqvist, I, Saevarsdottir, S, Alfredsson, L, Klareskog, L, and Di Giuseppe, D. Is tea consumption associated with reduction of risk of rheumatoid arthritis? A swedish case-control study. Arthritis Res Ther. (2021) 23:209. doi: 10.1186/s13075-021-02583-y
15. Xing, L, Zhang, H, Qi, R, Tsao, R, and Mine, Y. Recent advances in the understanding of the health benefits and molecular mechanisms associated with green tea polyphenols. J Agric Food Chem. (2019) 67:1029–43. doi: 10.1021/acs.jafc.8b06146
16. Zhao, T, Li, C, Wang, S, and Song, X. Green tea (camellia sinensis): a review of its phytochemistry, pharmacology, and toxicology. Molecules. (2022) 27:909. doi: 10.3390/molecules27123909
17. Luk, HY, Appell, C, Chyu, MC, Chen, CH, Wang, CY, Yang, RS, et al. Impacts of green tea on joint and skeletal muscle health: prospects of translational nutrition. Antioxidants. (2020) 9:1050. doi: 10.3390/antiox9111050
18. Negah, SS, Ghazavi, H, Vafaee, F, Rashidi, R, Aminian, AR, and Forouzanfar, F. The potential role of green tea and its main constituent (epigallocatechin −3-gallate) in pain relief: a mechanistic review. Curr Drug Discov Technol. (2021) 18:e130921189586. doi: 10.2174/1570163817666201229121033
19. Engelhardt, UH . Tea chemistry - what do and what don't we know? - a micro review. Food Res Int. (2020) 132:109120. doi: 10.1016/j.foodres.2020.109120
20. Zeeb, DJ, Nelson, BC, Albert, K, and Dalluge, JJ. Separation and identification of twelve catechins in tea using liquid chromatography/atmospheric pressure chemical ionization-mass spectrometry. Anal Chem. (2000) 72:5020–6. doi: 10.1021/ac000418f
21. de Villiers, TJ . Bone health and menopause: osteoporosis prevention and treatment. Best Pract Res Clin Endocrinol Metab. (2023) 10:101782. doi: 10.1016/j.beem.2023.101782
22. Lisco, G, Triggiani, D, Giagulli, VA, De Pergola, G, Guastamacchia, E, Piazzolla, G, et al. Endocrine, metabolic, and immune pathogenesis of postmenopausal osteoporosis. Is there a therapeutic role in natural products? Endocrine Metabolic & Immune Disorders-Drug. Targets. (2023) 23:1278–90. doi: 10.2174/1871530323666230330121301
23. Li, X, Qiao, Y, Yu, C, Guo, Y, Bian, Z, Yang, L, et al. Tea consumption and bone health in chinese adults: a population-based study. Osteoporos Int. (2019) 30:333–41. doi: 10.1007/s00198-018-4767-3
24. Lee, DB, Song, HJ, Paek, YJ, Park, KH, Seo, YG, and Noh, HM. Relationship between regular green tea intake and osteoporosis in korean postmenopausal women: a nationwide study. Nutrients. (2021) 14:87. doi: 10.3390/nu14010087
25. Huang, YP, Chen, LS, Feng, SH, Liang, YS, and Pan, SL. Tea consumption and the risks of osteoporosis and hip fracture: a population-based longitudinal follow-up study. Osteoporos Int. (2023) 34:101–9. doi: 10.1007/s00198-022-06569-7
26. Ni, S, Wang, L, Wang, G, Lin, J, Ma, Y, Zhao, X, et al. Drinking tea before menopause is associated with higher bone mineral density in postmenopausal women. Eur J Clin Nutr. (2021) 75:1454–64. doi: 10.1038/s41430-021-00856-y
27. Chen, Z, Pettinger, MB, Ritenbaugh, C, LaCroix, AZ, Robbins, J, Caan, BJ, et al. Habitual tea consumption and risk of osteoporosis: a prospective study in the women's health initiative observational cohort. Am J Epidemiol. (2003) 158:772–81. doi: 10.1093/aje/kwg214
28. Hamdi, KI, Aydin, S, Gemalmaz, A, Akturk, Z, Yaman, H, Bozdemir, N, et al. Habitual tea drinking and bone mineral density in postmenopausal turkish women: investigation of prevalence of postmenopausal osteoporosis in Turkey (ippot study). Int J Vitam Nutr Res. (2007) 77:389–97. doi: 10.1024/0300-9831.77.6.389
29. Duan, P, Zhang, J, Chen, J, Liu, Z, Guo, P, Li, X, et al. Oolong tea drinking boosts calcaneus bone mineral density in postmenopausal women: a population-based study in southern China. Arch Osteoporos. (2020) 15:49. doi: 10.1007/s11657-020-00723-6
30. Myers, G, Prince, RL, Kerr, DA, Devine, A, Woodman, RJ, Lewis, JR, et al. Tea and flavonoid intake predict osteoporotic fracture risk in elderly australian women: a prospective study. Am J Clin Nutr. (2015) 102:958–65. doi: 10.3945/ajcn.115.109892
31. Qian, G, Xue, K, Tang, L, Wang, F, Song, X, Chyu, MC, et al. Mitigation of oxidative damage by green tea polyphenols and tai chi exercise in postmenopausal women with osteopenia. PLoS One. (2012) 7:e48090. doi: 10.1371/journal.pone.0048090
32. Wang, MY, Shen, C, An, MF, Xie, CQ, Wu, X, Zhu, QQ, et al. Combined treatment with dendrobium candidum and black tea extract promotes osteoprotective activity in ovariectomized estrogen deficient rats and osteoclast formation. Life Sci. (2018) 200:31–41. doi: 10.1016/j.lfs.2018.03.025
33. Liu, T, Xiang, Z, Chen, F, Yin, D, Huang, Y, Xu, J, et al. Theabrownin suppresses in vitro osteoclastogenesis and prevents bone loss in ovariectomized rats. Biomed Pharmacother. (2018) 106:1339–47. doi: 10.1016/j.biopha.2018.07.103
34. Shen, CL, Smith, BJ, Li, J, Cao, JJ, Song, X, Newhardt, MF, et al. Effect of long-term green tea polyphenol supplementation on bone architecture, turnover, and mechanical properties in middle-aged ovariectomized rats. Calcif Tissue Int. (2019) 104:285–300. doi: 10.1007/s00223-018-0489-y
35. Yildirim, M, Saral, S, Mercantepe, T, Iskender, H, Tumkaya, L, Atak, M, et al. White tea reduced bone loss by suppressing the trap/ctx pathway in ovariectomy-induced osteoporosis model rats. Cells Tissues Organs. (2020) 209:64–74. doi: 10.1159/000507791
36. Siddiqui, JA, and Partridge, NC. Physiological bone remodeling: systemic regulation and growth factor involvement. Physiology. (2016) 31:233–45. doi: 10.1152/physiol.00061.2014
37. Kim, JM, Lin, C, Stavre, Z, Greenblatt, MB, and Shim, JH. Osteoblast-osteoclast communication and bone homeostasis. Cell. (2020) 9:9. doi: 10.3390/cells9092073
38. Wang, XD, Li, SY, Zhang, SJ, Gupta, A, Zhang, CP, and Wang, L. The neural system regulates bone homeostasis via mesenchymal stem cells: a translational approach. Theranostics. (2020) 10:4839–50. doi: 10.7150/thno.43771
39. Holzer, N, Braun, KF, Ehnert, S, Egana, JT, Schenck, TL, Buchholz, A, et al. Green tea protects human osteoblasts from cigarette smoke-induced injury: possible clinical implication. Langenbecks Arch. Surg. (2012) 397:467–74. doi: 10.1007/s00423-011-0882-8
40. Shalan, NA, Mustapha, NM, and Mohamed, S. Noni leaf and black tea enhance bone regeneration in estrogen-deficient rats. Nutrition. (2017) 33:42–51. doi: 10.1016/j.nut.2016.08.006
41. Das, AS, Das, D, Mukherjee, M, Mukherjee, S, and Mitra, C. Phytoestrogenic effects of black tea extract (camellia sinensis) in an oophorectomized rat (rattus norvegicus) model of osteoporosis. Life Sci. (2005) 77:3049–57. doi: 10.1016/j.lfs.2005.02.035
42. Ko, CH, Siu, WS, Wong, HL, Shum, WT, Fung, KP, San, LC, et al. Pro-bone and antifat effects of green tea and its polyphenol, epigallocatechin, in rat mesenchymal stem cells in vitro. J Agric Food Chem. (2011) 59:9870–6. doi: 10.1021/jf202015t
43. Zeng, X, Tian, J, Cai, K, Wu, X, Wang, Y, Zheng, Y, et al. Promoting osteoblast differentiation by the flavanes from Huangshan maofeng tea is linked to a reduction of oxidative stress. Phytomedicine. (2014) 21:217–24. doi: 10.1016/j.phymed.2013.08.026
44. Byun, MR, Sung, MK, Kim, AR, Lee, CH, Jang, EJ, Jeong, MG, et al. (−)-epicatechin gallate (ecg) stimulates osteoblast differentiation via runt-related transcription factor 2 (runx2) and transcriptional coactivator with pdz-binding motif (taz)-mediated transcriptional activation. J Biol Chem. (2014) 289:9926–35. doi: 10.1074/jbc.M113.522870
45. Liu, S, Yang, L, Mu, S, and Fu, Q. Epigallocatechin-3-gallate ameliorates glucocorticoid-induced osteoporosis of rats in vivo and in vitro. Front Pharmacol. (2018) 9:447. doi: 10.3389/fphar.2018.00447
46. Ge, G, Yang, S, Hou, Z, Gan, M, Tao, H, Zhang, W, et al. Theaflavin-3,3′-digallate promotes the formation of osteoblasts under inflammatory environment and increases the bone mass of ovariectomized mice. Front Pharmacol. (2021) 12:648969. doi: 10.3389/fphar.2021.648969
47. Oka, Y, Iwai, S, Amano, H, Irie, Y, Yatomi, K, Ryu, K, et al. Tea polyphenols inhibit rat osteoclast formation and differentiation. J Pharmacol Sci. (2012) 118:55–64. doi: 10.1254/jphs.11082FP
48. Liu, T, Ding, S, Yin, D, Cuan, X, Xie, C, Xu, H, et al. Pu-erh tea extract ameliorates ovariectomy-induced osteoporosis in rats and suppresses osteoclastogenesis in vitro. Front Pharmacol. (2017) 8:324. doi: 10.3389/fphar.2017.00324
49. Xu, H, Yin, D, Liu, T, Chen, F, Chen, Y, Wang, X, et al. Tea polysaccharide inhibits rankl-induced osteoclastogenesis in raw264.7 cells and ameliorates ovariectomy-induced osteoporosis in rats. Biomed Pharmacother. (2018) 102:539–48. doi: 10.1016/j.biopha.2018.03.125
50. Liang, Q, Lv, M, Zhang, X, Hu, J, Wu, Y, Huang, Y, et al. Effect of black tea extract and thearubigins on osteoporosis in rats and osteoclast formation in vitro. Front Physiol. (2018) 9:1225. doi: 10.3389/fphys.2018.01225
51. Wu, X, Xie, CQ, Zhu, QQ, Wang, MY, Sun, B, Huang, YP, et al. Green tea (camellia sinensis) aqueous extract alleviates postmenopausal osteoporosis in ovariectomized rats and prevents rankl-induced osteoclastogenesis in vitro. Food & Nutrition. Research. (2018) 62:1478. doi: 10.29219/fnr.v62.1478
52. Chen, ST, Kang, L, Wang, CZ, Huang, PJ, Huang, HT, Lin, SY, et al. (−)-epigallocatechin-3-gallate decreases osteoclastogenesis via modulation of rankl and osteoprotegrin. Molecules. (2019) 24:156. doi: 10.3390/molecules24010156
53. Nishioku, T, Kubo, T, Kamada, T, Okamoto, K, Tsukuba, T, Uto, T, et al. (−)-epigallocatechin-3-gallate inhibits rankl-induced osteoclastogenesis via downregulation of nfatc1 and suppression of ho-1-hmgb1-rage pathway. Biomed Res Tokyo. (2020) 41:269–77. doi: 10.2220/biomedres.41.269
54. Xu, H, Liu, T, Jia, Y, Li, J, Jiang, L, Hu, C, et al. (−)-epigallocatechin-3-gallate inhibits osteoclastogenesis by blocking rankl-rank interaction and suppressing nf-kappab and mapk signaling pathways. Int Immunopharmacol. (2021) 95:107464. doi: 10.1016/j.intimp.2021.107464
55. Jin, P, Li, M, Xu, G, Zhang, K, Zheng, LI, and Zhao, J. Role of (−)-epigallocatechin-3-gallate in the osteogenic differentiation of human bone marrow mesenchymal stem cells: an enhancer or an inducer? Exp Ther Med. (2015) 10:828–34. doi: 10.3892/etm.2015.2579
56. Lin, SY, Kang, L, Wang, CZ, Huang, HH, Cheng, TL, Huang, HT, et al. (−)-epigallocatechin-3-gallate (egcg) enhances osteogenic differentiation of human bone marrow mesenchymal stem cells. Molecules. (2018) 23:3221. doi: 10.3390/molecules23123221
57. Lao, W, Zhao, Y, Tan, Y, Johnson, M, Li, Y, Xiao, L, et al. Regulatory effects and mechanism of action of green tea polyphenols on osteogenesis and adipogenesis in human adipose tissue-derived stem cells. Curr Issues Mol Biol. (2022) 44:6046–58. doi: 10.3390/cimb44120412
58. Sala-Climent, M, Coras, R, Cedeno, M, Murilla-Saich, J, Quan, A, Kalli Hose, M, et al. Clinical changes in knee osteoarthritis (koa) patients exposed to an anti-inflammatory (itis)-diet. Osteoarthr Cartil. (2023) 31:S192–3. doi: 10.1016/j.joca.2023.01.167
59. Alghadir, AH, Gabr, SA, and Al-Eisa, ES. Green tea and exercise interventions as nondrug remedies in geriatric patients with rheumatoid arthritis. J Phys Ther Sci. (2016) 28:2820–9. doi: 10.1589/jpts.28.2820
60. Sami, AG, Ahmad, HA, Gehan, AG, Xiao-Wan, Z, Yeong-Ho, C, Youn-Jin, P, et al. Regulation of cartilage and inflammatory biomarkers in rheumatoid arthritis patients treated with green tea therapy. Afr J Pharm Pharmacol. (2014) 8:263–73. doi: 10.5897/AJPP2013.3710
61. Liu, Y, Xie, Y, Liu, M, and Yang, J. Epigallocatechin gallate (egcg) restores 25-hydroxy vitamin d levels in rheumatoid arthritis patients by attenuating ros-mediated activation of nf-κb. Trop J Pharm Res. (2022) 20:1395–02. doi: 10.4314/tjpr.v20i7.11
62. Jin, J, Li, J, Gan, Y, Liu, J, Zhao, X, Chen, J, et al. Tea consumption is associated with decreased disease activity of rheumatoid arthritis in a real-world, large-scale study. Ann Nutr Metab. (2020) 76:54–61. doi: 10.1159/000505952
63. Ghouri, A, Muzumdar, S, Barr, AJ, Robinson, E, Murdoch, C, Kingsbury, SR, et al. The relationship between meniscal pathologies, cartilage loss, joint replacement and pain in knee osteoarthritis: a systematic review. Osteoarthr Cartil. (2022) 30:1287–27. doi: 10.1016/j.joca.2022.08.002
64. Sharma, L . Osteoarthritis of the knee. N Engl J Med. (2021) 384:51–9. doi: 10.1056/NEJMcp1903768
65. Takiguchi, R, Komatsu, R, Kitamura, K, Watanabe, Y, Takahashi, A, Kobayashi, R, et al. Modifiable factors associated with symptomatic knee osteoarthritis: the Murakami cohort study. Maturitas. (2019) 128:53–9. doi: 10.1016/j.maturitas.2019.06.013
66. Kacar, C, Gilgil, E, Tuncer, T, Butun, B, Urhan, S, Sunbuloglu, G, et al. The association of milk consumption with the occurrence of symptomatic knee osteoarthritis. Clin Exp Rheumatol. (2004) 22:473–6.
67. Levy, RM, Saikovsky, R, Shmidt, E, Khokhlov, A, and Burnett, BP. Flavocoxid is as effective as naproxen for managing the signs and symptoms of osteoarthritis of the knee in humans: a short-term randomized, double-blind pilot study. Nutr Res. (2009) 29:298–04. doi: 10.1016/j.nutres.2009.04.003
68. Ferraz, MB, Pereira, RB, Coelho, AL, and Atra, E. The effectiveness of tipi in the treatment of hip and knee osteoarthritis: a preliminary report. Memorias Do Instituto Oswaldo Cruz. (1991) 86:241–3. doi: 10.1590/S0074-02761991000600054
69. Haqqi, TM, Anthony, DD, Gupta, S, Ahmad, N, Lee, MS, Kumar, GK, et al. Prevention of collagen-induced arthritis in mice by a polyphenolic fraction from green tea. Proc Natl Acad Sci U S A. (1999) 96:4524–9. doi: 10.1073/pnas.96.8.4524
70. Ahmed, S, Rahman, A, Hasnain, A, Lalonde, M, Goldberg, VM, and Haqqi, TM. Green tea polyphenol epigallocatechin-3-gallate inhibits the il-1 beta-induced activity and expression of cyclooxygenase-2 and nitric oxide synthase-2 in human chondrocytes. Free Radic Biol Med. (2002) 33:1097–05. doi: 10.1016/S0891-5849(02)01004-3
71. Ahmed, S, Wang, N, Lalonde, M, Goldberg, VM, and Haqqi, TM. Green tea polyphenol epigallocatechin-3-gallate (egcg) differentially inhibits interleukin-1 beta-induced expression of matrix metalloproteinase-1 and -13 in human chondrocytes. J Pharmacol Exp Ther. (2004) 308:767–73. doi: 10.1124/jpet.103.059220
72. Rasheed, Z, Anbazhagan, AN, Akhtar, N, Ramamurthy, S, Voss, FR, and Haqqi, TM. Green tea polyphenol epigallocatechin-3-gallate inhibits advanced glycation end product-induced expression of tumor necrosis factor-alpha and matrix metalloproteinase-13 in human chondrocytes. Arthritis Res Ther. (2009) 11:R71. doi: 10.1186/ar2700
73. Akhtar, N, and Haqqi, TM. Epigallocatechin-3-gallate suppresses the global interleukin-1beta-induced inflammatory response in human chondrocytes. Arthritis Res Ther. (2011) 13:R93. doi: 10.1186/ar3368
74. Singh, R, Ahmed, S, Islam, N, Goldberg, VM, and Haqqi, TM. Epigallocatechin-3-gallate inhibits interleukin-1beta-induced expression of nitric oxide synthase and production of nitric oxide in human chondrocytes: suppression of nuclear factor kappab activation by degradation of the inhibitor of nuclear factor kappab. Arthritis Rheum. (2002) 46:2079–86. doi: 10.1002/art.10443
75. Singh, R, Ahmed, S, Malemud, CJ, Goldberg, VM, and Haqqi, TM. Epigallocatechin-3-gallate selectively inhibits interleukin-1beta-induced activation of mitogen activated protein kinase subgroup c-Jun n-terminal kinase in human osteoarthritis chondrocytes. J Orthop Res. (2003) 21:102–9. doi: 10.1016/S0736-0266(02)00089-X
76. Adcocks, C, Collin, P, and Buttle, DJ. Catechins from green tea (camellia sinensis) inhibit bovine and human cartilage proteoglycan and type ii collagen degradation in vitro. J Nutr. (2002) 132:341–6. doi: 10.1093/jn/132.3.341
77. Rasheed, Z, Rasheed, N, and Al-Shaya, O. Epigallocatechin-3-o-gallate modulates global microrna expression in interleukin-1beta-stimulated human osteoarthritis chondrocytes: potential role of egcg on negative co-regulation of microrna-140-3p and adamts5. Eur J Nutr. (2018) 57:917–28. doi: 10.1007/s00394-016-1375-x
78. Rasheed, Z, Rasheed, N, and Al-Shobaili, HA. Epigallocatechin-3-o-gallate up-regulates microrna-199a-3p expression by down-regulating the expression of cyclooxygenase-2 in stimulated human osteoarthritis chondrocytes. J Cell Mol Med. (2016) 20:2241–8. doi: 10.1111/jcmm.12897
79. Yang, D, Cao, G, Ba, X, and Jiang, H. Epigallocatechin-3-o-gallate promotes extracellular matrix and inhibits inflammation in il-1beta stimulated chondrocytes by the pten/mirna-29b pathway. Pharm Biol. (2022) 60:589–99. doi: 10.1080/13880209.2022.2039722
80. Huang, GS, Tseng, CY, Lee, CH, Su, SL, and Lee, HS. Effects of (−)-epigallocatechin-3-gallate on cyclooxygenase 2, pge(2), and il-8 expression induced by il-1beta in human synovial fibroblasts. Rheumatol Int. (2010) 30:1197–03. doi: 10.1007/s00296-009-1128-8
81. Xu, C, Ni, S, Xu, N, Yin, G, Yu, Y, Zhou, B, et al. Theaflavin-3,3′-digallate inhibits erastin-induced chondrocytes ferroptosis via the nrf2/gpx4 signaling pathway in osteoarthritis. Oxidative Med Cell Longev. (2022) 2022:1–17. doi: 10.1155/2022/3531995
82. Xu, XX, Zheng, G, Tang, SK, Liu, HX, Hu, YZ, and Shang, P. Theaflavin protects chondrocytes against apoptosis and senescence via regulating nrf2 and ameliorates murine osteoarthritis. Food Funct. (2021) 12:1590–02. doi: 10.1039/D0FO02038A
83. Huang, HT, Cheng, TL, Ho, CJ, Huang, HH, Lu, CC, Chuang, SC, et al. Intra-articular injection of (−)-epigallocatechin 3-gallate to attenuate articular cartilage degeneration by enhancing autophagy in a post-traumatic osteoarthritis rat model. Antioxidants. (2020) 10:8. doi: 10.3390/antiox10010008
84. Li, H, Xiang, D, Gong, C, Wang, X, and Liu, L. Naturally derived injectable hydrogels with ros-scavenging property to protect transplanted stem cell bioactivity for osteoarthritic cartilage repair. Front Bioeng Biotechnol. (2022) 10:1109074. doi: 10.3389/fbioe.2022.1109074
85. Xu, S, Chang, L, Zhao, X, Hu, Y, Lin, Y, Chen, Z, et al. Preparation of epigallocatechin gallate decorated au-ag nano-heterostructures as nir-sensitive nano-enzymes for the treatment of osteoarthritis through mitochondrial repair and cartilage protection. Acta Biomater. (2022) 144:168–82. doi: 10.1016/j.actbio.2022.03.038
86. Wei, H, Qin, J, Huang, Q, Jin, Z, Zheng, L, Zhao, J, et al. Epigallocatechin-3-gallate (egcg) based metal-polyphenol nanoformulations alleviates chondrocytes inflammation by modulating synovial macrophages polarization. Biomed Pharmacother. (2023) 161:114366. doi: 10.1016/j.biopha.2023.114366
87. Sueoka, N, Suganuma, M, Sueoka, E, Okabe, S, Matsuyama, S, Imai, K, et al. A new function of green tea: prevention of lifestyle-related diseases. Ann N Y Acad Sci. (2001) 928:274–80. doi: 10.1111/j.1749-6632.2001.tb05656.x
88. Rambod, M, Nazarinia, M, and Raieskarimian, F. The impact of dietary habits on the pathogenesis of rheumatoid arthritis: a case-control study. Clin Rheumatol. (2018) 37:2643–8. doi: 10.1007/s10067-018-4151-x
89. Ramadan, G, El-Beih, NM, Talaat, RM, and Abd, EE. Anti-inflammatory activity of green versus black tea aqueous extract in a rat model of human rheumatoid arthritis. Int J Rheum Dis. (2017) 20:203–13. doi: 10.1111/1756-185X.12666
90. Tang, LQ, Wei, W, and Wang, XY. Effects and mechanisms of catechin for adjuvant arthritis in rats. Adv Ther. (2007) 24:679–90. doi: 10.1007/BF02848793
91. Yoon, CH, Chung, SJ, Lee, SW, Park, YB, Lee, SK, and Park, MC. Gallic acid, a natural polyphenolic acid, induces apoptosis and inhibits proinflammatory gene expressions in rheumatoid arthritis fibroblast-like synoviocytes. Joint Bone Spine. (2013) 80:274–9. doi: 10.1016/j.jbspin.2012.08.010
92. Lopez-Armada, MJ, Fernandez-Rodriguez, JA, and Blanco, FJ. Mitochondrial dysfunction and oxidative stress in rheumatoid arthritis. Antioxidants. (2022) 11:1151. doi: 10.3390/antiox11061151
93. Minguzzi, M, Cetrullo, S, D'Adamo, S, Silvestri, Y, Flamigni, F, and Borzi, RM. Emerging players at the intersection of chondrocyte loss of maturational arrest, oxidative stress, senescence and low-grade inflammation in osteoarthritis. Oxidative Med Cell Longev. (2018) 2018:1–17. doi: 10.1155/2018/3075293
94. Islam, MT, Sarkar, C, Hossain, R, Bhuia, MS, Mardare, I, Kulbayeva, M, et al. Therapeutic strategies for rheumatic diseases and disorders: targeting redox imbalance and oxidative stress. Biomed Pharmacother. (2023) 164:114900. doi: 10.1016/j.biopha.2023.114900
95. de Almeida, GG, de Sa-Nakanishi, AB, Wendt, MM, Comar, JF, Bersani, AC, Bracht, A, et al. Green tea extract improves the oxidative state of the liver and brain in rats with adjuvant-induced arthritis. Food Funct. (2015) 6:2701–11. doi: 10.1039/C5FO00548E
96. Meki, AR, Hamed, EA, and Ezam, KA. Effect of green tea extract and vitamin c on oxidant or antioxidant status of rheumatoid arthritis rat model. Indian J Clin Biochem. (2009) 24:280–7. doi: 10.1007/s12291-009-0053-7
97. Lee, SY, Jung, YO, Ryu, JG, Oh, HJ, Son, HJ, Lee, SH, et al. Epigallocatechin-3-gallate ameliorates autoimmune arthritis by reciprocal regulation of t helper-17 regulatory t cells and inhibition of osteoclastogenesis by inhibiting stat3 signaling. J Leukoc Biol. (2016) 100:559–68. doi: 10.1189/jlb.3A0514-261RR
98. Karatas, A, Dagli, AF, Orhan, C, Gencoglu, H, Ozgen, M, Sahin, N, et al. Epigallocatechin 3-gallate attenuates arthritis by regulating nrf2, ho-1, and cytokine levels in an experimental arthritis model. Biotechnol Appl Biochem. (2020) 67:317–22. doi: 10.1002/bab.1860
99. Alcaraz, MJ, and Ferrandiz, ML. Relevance of nrf2 and heme oxygenase-1 in articular diseases. Free Radic Biol Med. (2020) 157:83–93. doi: 10.1016/j.freeradbiomed.2019.12.007
100. Fechtner, S, Singh, A, Chourasia, M, and Ahmed, S. Molecular insights into the differences in anti-inflammatory activities of green tea catechins on il-1beta signaling in rheumatoid arthritis synovial fibroblasts. Toxicol Appl Pharmacol. (2017) 329:112–20. doi: 10.1016/j.taap.2017.05.016
101. Singh, AK, Umar, S, Riegsecker, S, Chourasia, M, and Ahmed, S. Regulation of transforming growth factor beta-activated kinase activation by epigallocatechin-3-gallate in rheumatoid arthritis synovial fibroblasts: suppression of k(63) -linked autoubiquitination of tumor necrosis factor receptor-associated factor 6. Arthrit Rheumatol. (2016) 68:347–58. doi: 10.1002/art.39447
102. Marotte, H, Ruth, JH, Campbell, PL, Koch, AE, and Ahmed, S. Green tea extract inhibits chemokine production, but up-regulates chemokine receptor expression, in rheumatoid arthritis synovial fibroblasts and rat adjuvant-induced arthritis. Rheumatology. (2010) 49:467–79. doi: 10.1093/rheumatology/kep397
103. Ribeiro, H, Rodrigues, I, Napoleao, L, Lira, L, Marques, D, Verissimo, M, et al. Non-steroidal anti-inflammatory drugs (nsaids), pain and aging: adjusting prescription to patient features. Biomed Pharmacother. (2022) 150:112958. doi: 10.1016/j.biopha.2022.112958
104. Stiller, CO, and Hjemdahl, P. Lessons from 20 years with cox-2 inhibitors: importance of dose-response considerations and fair play in comparative trials. J Intern Med. (2022) 292:557–74. doi: 10.1111/joim.13505
105. Zhang, C, Qin, YY, Wei, X, Yu, FF, Zhou, YH, and He, J. Tea consumption and risk of cardiovascular outcomes and total mortality: a systematic review and meta-analysis of prospective observational studies. Eur J Epidemiol. (2015) 30:103–13. doi: 10.1007/s10654-014-9960-x
106. Shin, S, Lee, JE, Loftfield, E, Shu, XO, Abe, SK, Rahman, MS, et al. Coffee and tea consumption and mortality from all causes, cardiovascular disease and cancer: a pooled analysis of prospective studies from the asia cohort consortium. Int J Epidemiol. (2022) 51:626–40. doi: 10.1093/ije/dyab161
107. Cao, SY, Zhao, CN, Gan, RY, Xu, XY, Wei, XL, Corke, H, et al. Effects and mechanisms of tea and its bioactive compounds for the prevention and treatment of cardiovascular diseases: an updated review. Antioxidants. (2019) 8:166. doi: 10.3390/antiox8060166
108. Dryden, GW, Lam, A, Beatty, K, Qazzaz, HH, and McClain, CJ. A pilot study to evaluate the safety and efficacy of an oral dose of (−)-epigallocatechin-3-gallate-rich polyphenon e in patients with mild to moderate ulcerative colitis. Inflamm Bowel Dis. (2013) 19:1904–12. doi: 10.1097/MIB.0b013e31828f5198
109. Ding, H, Liu, XC, Jian-Ming, X, and Qiao, M. Identification of crucial genes and related transcription factors in ulcerative colitis. Ann Clin Lab Sci. (2021) 51:245–54.
110. Qiu, J, Xiao, G, Yang, M, Huang, X, Cai, D, Xie, C, et al. Integrated network pharmacology and metabolomics reveal the mechanisms of jasminum elongatum in anti-ulcerative colitis. Sci Rep. (2023) 13:22449. doi: 10.1038/s41598-023-49792-w
111. Liu, H, Chen, R, Wen, S, Li, Q, Lai, X, Zhang, Z, et al. Tea (camellia sinensis) ameliorates dss-induced colitis and liver injury by inhibiting tlr4/nf-kappab/nlrp3 inflammasome in mice. Biomed Pharmacother. (2023) 158:114136. doi: 10.1016/j.biopha.2022.114136
112. Zhang, YP, Yang, XQ, Yu, DK, Xiao, HY, and Du, JR. Nrf2 signalling pathway and autophagy impact on the preventive effect of green tea extract against alcohol-induced liver injury. J Pharm Pharmacol. (2021) 73:986–95. doi: 10.1093/jpp/rgab027
113. Li, N, Zhou, X, Wang, J, Chen, J, Lu, Y, Sun, Y, et al. White tea alleviates non-alcoholic fatty liver disease by regulating energy expenditure and lipid metabolism. Gene. (2022) 833:146553. doi: 10.1016/j.gene.2022.146553
114. Zhan, J, Cao, H, Hu, T, Shen, J, Wang, W, Wu, P, et al. Efficient preparation of black tea extract (bte) with the high content of theaflavin mono- and digallates and the protective effects of bte on ccl(4)-induced rat liver and renal injury. J Agric Food Chem. (2021) 69:5938–47. doi: 10.1021/acs.jafc.1c01851
115. Wu, Z, Sun, L, Chen, R, Wen, S, Li, Q, Lai, X, et al. Chinese tea alleviates ccl(4)-induced liver injury through the nf-kappabornrf2signaling pathway in c57bl-6j mice. Nutrients. (2022) 14:972. doi: 10.3390/nu14050972
116. Wang, D, Zhang, M, Wang, T, Cai, M, Qian, F, Sun, Y, et al. Green tea polyphenols prevent lipopolysaccharide-induced inflammatory liver injury in mice by inhibiting nlrp3 inflammasome activation. Food Funct. (2019) 10:3898–08. doi: 10.1039/C9FO00572B
117. Mostafa-Hedeab, G, Ewaiss, HM, and Ahmed WF,. Epigallocatechin gallate ameliorates tetrahydrochloride-induced liver toxicity in rats via inhibition of tgfbeta / p-erk/p-smad1/2 signaling, antioxidant, anti-inflammatory activity. Saudi Pharmac J. (2022) 30:1293–00. doi: 10.1016/j.jsps.2022.06.021
118. Chen, Y, Abe, SK, Inoue, M, Yamaji, T, Iwasaki, M, Nomura, S, et al. Green tea and coffee consumption and risk of kidney cancer in japanese adults. Sci Rep. (2022) 12:20274. doi: 10.1038/s41598-022-24090-z
119. Barocio-Pantoja, M, Quezada-Fernandez, P, Cardona-Muller, D, Jimenez-Cazarez, MB, Larios-Cardenas, M, Gonzalez-Radillo, OI, et al. Green tea extract increases soluble rage and improves renal function in patients with diabetic nephropathy. J Med Food. (2021) 24:1264–70. doi: 10.1089/jmf.2020.0212
120. Zeng, L, Lin, L, Xiao, W, and Li, Y. L-theanine protects rat kidney from d-galactose-induced injury via inhibition of the ages/rage signaling pathway. Eur J Pharmacol. (2022) 927:175072. doi: 10.1016/j.ejphar.2022.175072
121. Zhao, X, Shi, X, Liu, Q, and Li, X. Tea polyphenols alleviates acetochlor-induced apoptosis and necroptosis via ros/mapk/nf-kappab signaling in ctenopharyngodon idellus kidney cells. Aquat Toxicol. (2022) 246:106153. doi: 10.1016/j.aquatox.2022.106153
122. Zhu, QQ, Yang, XY, Zhang, XJ, Yu, CJ, Pang, QQ, Huang, YW, et al. Egcg targeting notch to attenuate renal fibrosis via inhibition of tgfbeta/smad3 signaling pathway activation in streptozotocin-induced diabetic mice. Food Funct. (2020) 11:9686–95. doi: 10.1039/D0FO01542C
123. Yue, L, Yang, YR, Ma, WX, Wang, HY, Fan, QW, Wang, YY, et al. Epigallocatechin gallate attenuates gentamicin-induced nephrotoxicity by suppressing apoptosis and ferroptosis. Molecules. (2022) 27:564. doi: 10.3390/molecules27238564
Keywords: Camellia sinensis , osteoporosis, osteoarthritis, rheumatoid arthritis, cyberpharmacology, molecular docking
Citation: Xie X, Fu J, Gou W, Qin Y, Wang D, Huang Z, Wang L and Li X (2024) Potential mechanism of tea for treating osteoporosis, osteoarthritis, and rheumatoid arthritis. Front. Med. 11:1289777. doi: 10.3389/fmed.2024.1289777
Edited by:
Claudio Lucchiari, University of Milan, ItalyReviewed by:
Lifeng Jiang, University of California, Davis, United StatesYanqiu Liu, Shandong University of Traditional Chinese Medicine, China
Hongting Jin, Zhejiang Chinese Medical University, China
Copyright © 2024 Xie, Fu, Gou, Qin, Wang, Huang, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xihai Li, MjAxMDAzMUBmanRjbS5lZHUuY24=
†These authors have contributed equally to this work
 Xinyu Xie
Xinyu Xie Jiehui Fu3†
Jiehui Fu3† Lili Wang
Lili Wang Xihai Li
Xihai Li