- 1Hyperbaric Medical Solutions, New York, NY, United States
- 2Orlando College of Osteopathic Medicine, Winter Garden, FL, United States
- 3Greenwich Hospital, Yale New Haven Health System, New Haven, CT, United States
- 4University of Alabama at Birmingham, Heersink School of Medicine, Birmingham, AL, United States
Background: The World Health Organization defines long COVID as “the continuation or development of new symptoms 3 months after the initial SARS-CoV-2 infection, with these symptoms lasting for at least 2 months with no other explanation.” Estimations of approximately 50 million individuals suffer from long COVID, reporting low health-related quality of life. Patients develop ongoing persistent symptoms that continue for more than 12 weeks that are not explained by another alternative diagnosis. To date, no current therapeutics are effective in treating the underlying pathophysiology of long COVID.
Discussion: A comprehensive literature search using PubMed and Google Scholar was conducted and all available articles from November 2021 to January 2024 containing keywords long covid and hyperbaric oxygen were reviewed. These published studies, including case series and randomized trials, demonstrate that utilizing Hyperbaric Oxygen Therapy (HBO) provided significant improvement in patients with long COVID.
Conclusion: A large cohort of patients suffer from long COVID or post-COVID-19 syndrome after recovery from their acute infection with no effective treatment options. HBO is a safe treatment and may provide benefit for this population and should continue to be researched for adjunctive treatment of long COVID.
Introduction
As the worldwide COVID epidemic continues, a large cohort of patients suffer from long COVID or post-COVID-19 syndrome after recovery from their acute infection (1). Estimations of approximately 50 million individuals (2), or 10–20% of patients initially diagnosed (3), suffer from long COVID reporting low health related quality of life. The World Health Organization defines long COVID as “the continuation or development of new symptoms 3 months after the initial SARS-CoV-2 infection, with these symptoms lasting for at least 2 months with no other explanation” (4). Patients develop ongoing persistent symptoms including dyspnea, cough, fatigue, “brain-fog,” cognitive dysfunction, anxiety, depression, sleep disturbances, palpitations, postural tachycardia syndrome (POTS), and rashes that continue for more than 12 weeks not explained by another alternative diagnosis. Decreased exercise capacity, hypoxia, reduced diffusion capacity, restrictive pulmonary physiology, ground-glass opacities, and fibrotic changes on imaging have been noted after initial COVID infection has resolved (1). Thromboembolic events, hair loss and renal impairment have all been noted in follow up. Symptoms can be severe and hinder productivity, most often in economically active adults (1, 5).
Post-COVID syndrome is well described worldwide with symptoms affecting quality of life and productivity. To date, no current therapeutics are effective in treating the underlying pathophysiology of long COVID. Recent studies, including case series and randomized trials, demonstrate that Hyperbaric Oxygen Therapy (HBO) treated patients had significant improvement in global cognitive function, fatigue, attention, executive function, energy, sleep, psychiatric symptoms, cardiopulmonary function, endurance and pain. HBO is beneficial and safe to treat patients with long COVID.
Discussion
Presentation and pathophysiology of long COVID
An observational cohort study from 38 hospitals in Michigan evaluated the outcomes of 1,250 patients through record review and telephone surveys. 488 patients completed the telephone survey with 32.6% of patients reporting persistent symptoms, including dyspnea while walking up the stairs (22.9%), cough (15.4%) and persistent loss of taste and/or smell (13.1%) (6). The CDC, in a multivariate regression model, studied adults and found that the risk of developing long COVID was higher in those in the age range of 40–54, female, with co-morbidities, and black people. The results of a sample size of 366 people are consistent with clinical observations. The economic impact of removing people who otherwise would be at the peak of their productive years is profound (7).
The precise pathophysiology of long COVID is unknown and may vary between individuals. Symptoms are thought to be related to possible auto-immune disease due to dysregulated T-cell activation, chronic inflammation, chronic oxidative stress, mitochondrial dysfunction, endothelial dysfunction, thrombotic disease, tissue hypoxia, and direct brain invasion by the virus (8, 9). In a recent prospective study, a cohort of 31 patients who reported the presence of one of the following symptoms: dyspnea, fatigue, chest pain, were matched with 31 individuals who had prior COVID infection but no evidence of long COVID10. Those with long COVID symptoms showed increased frequency of activated CD14 + CD16+ monocytes and plasmacytoid dendritic cells, compared with control individuals (10). The individuals studied demonstrated persistent elevation in the levels of type I (IFNβ) and type III (IFNλ1) interferon 8 months post-infection. The combination of IFNβ, pentraxin 3, IFNγ, IFNλ2/3 and IL-6 was associated with long COVID symptoms, with an accuracy ranging from 78.5 to 81.6% (10). The levels observed have been associated with acute, severe disease, suggesting that the long COVID symptoms are a result of delayed or defective resolution of inflammation (10).
T-cell dysfunction may promote long COVID pathophysiology. Consistently, autopsy examinations of deceased COVID-19 patients demonstrated that infiltrates in the lungs and other organs were enriched with CD8+ T cells (11). Thyroid dysfunction has been detected in 15–20% of patients with COVID-19, suggesting that thyroid effect on T cell-mediated autoimmunity may play a role in the autoimmunity pathophysiology of long COVID (12).
B-cells may also be involved in long COVID autoimmunity. In severe cases of COVID-19, it has been shown that COVID-19 infection causes lymphopenia (i.e., B-cell and T-cell lymphocytes deficiency) that causes hyperinflammation (12). Subsequently, as B-cell and T-cell lymphocytes are renewed, elevated inflammation may develop, leading to symptoms of long COVID (12).
Elevated IL-6 levels have been observed in severe and moderate cases of COVID-19 infection causing inflammation and oxidative stress resulting from excessive reactive oxygen species (ROS) production and depleted antioxidant systems (13). Because inflammation and oxidative stress mutually reinforce one another, the elevation of IL-6 and ROS leads to a state of hyperinflammation post COVID infection (14).
Potential treatment options
Studied treatments for long COVID include anti-inflammatory agents, specific diets, cognitive behavioral therapy, rehabilitation, and hyperbaric oxygen therapy (15, 16). No universally effective treatments for long COVID have been identified. However, treatments aimed at symptom categories have shown efficacy in certain groups, such as pharmacological options targeting symptoms such as β-blockers for POTS, low-dose naltrexone for neuroinflammation and intravenous immunoglobulin for immune dysfunction. H1 and H2 antihistamines may relieve symptoms involving mast cell activation and anticoagulant regimens can counteract abnormal clotting (17). Many non-pharmacological options have been utilized including cognitive pacing for ME/CFS symptoms, increasing salt intake and compression stockings for POTS, and probiotics and elimination diets for gastrointestinal symptoms (17). Some supplements have shown promise in treating long COVID including coenzyme Q10 and D-ribose (17).
Mechanism of action and rationale for the use of HBO in long COVID
The mechanism of action of HBO involves both increased pressure and elevated partial pressure of O2. The former causes a reduction of bubble size related to Boyle’s Law however much of the clinical efficacy of HBO is derived from the high O2 partial pressures and hyperoxia that increase the production of reactive O2 species (ROS) and of reactive nitrogen species (RNS). HBO promotes the synthesis of growth factors and mitigates post-ischemic and post-inflammatory responses (18).
HBO also effects the expression of immune-modulatory cytokines by decreasing proinflammatory cytokines such as IL-1, IL-6, and TNF-α and elevating the anti-inflammatory cytokine IL-10 (19). Many HBO protocols call for the intermittent fluctuation of O2 levels (from 100 to 20.9% for brief periods). These fluctuations serve to induce the Hyperoxic-Hypoxic Paradox which increases oxidative stress scavenger transcription factors and subsequently increases the production of antioxidant enzymes (20). HBO elevates ROS productions, especially via mitochondrial function but also elevates antioxidant levels and activity, thereby reducing overall ROS level. Conversion of oxygen to ROS is a function of metabolic rate and the mitochondria serve as the main source of oxidative stress. HBO causes an increase in ATP production levels, decreased mitochondria-mediated apoptosis signaling, and reduced mitochondrial membrane potential. It has become clearer that mitochondrial dysfunction drives many disease processes, so the effects of HBO on oxidative phosphorylation and ROS likely contribute to its therapeutic benefits (20).
Long COVID pathophysiology is characterized by dysregulated T-cell activation, chronic inflammation, chronic oxidative stress, mitochondrial dysfunction, endothelial dysfunction, thrombotic disease, and tissue hypoxia (8, 9). The beneficial effects of HBO on mitochondrial function likely contribute to the mechanism of action when treating many of the symptoms of long COVID. Another possible mechanism of action of HBO in the treatment of long COVID is reduced production of proinflammatory cytokines (21). HBO increases the mobilization of stem cells (21). It is through this mechanism that HBO can inhibit the abnormal activation of T lymphocytes and macrophages and decreases the secretion of proinflammatory cytokines. HBO provides benefits to sufferers of long COVID through the enhanced mitochondrial function, reduction in inflammation, mobilization of stem cells, improvement in thrombotic disease and the relief of hypoxia (21) (see Table 1).
Review of literature regarding use of HBO in long COVID
HBO has been studied for patients with long COVID syndrome. A comprehensive literature search using PubMed and Google Scholar was conducted and all available articles from November 2021 to January 2024 containing keywords “long covid” and “hyperbaric oxygen” were reviewed.
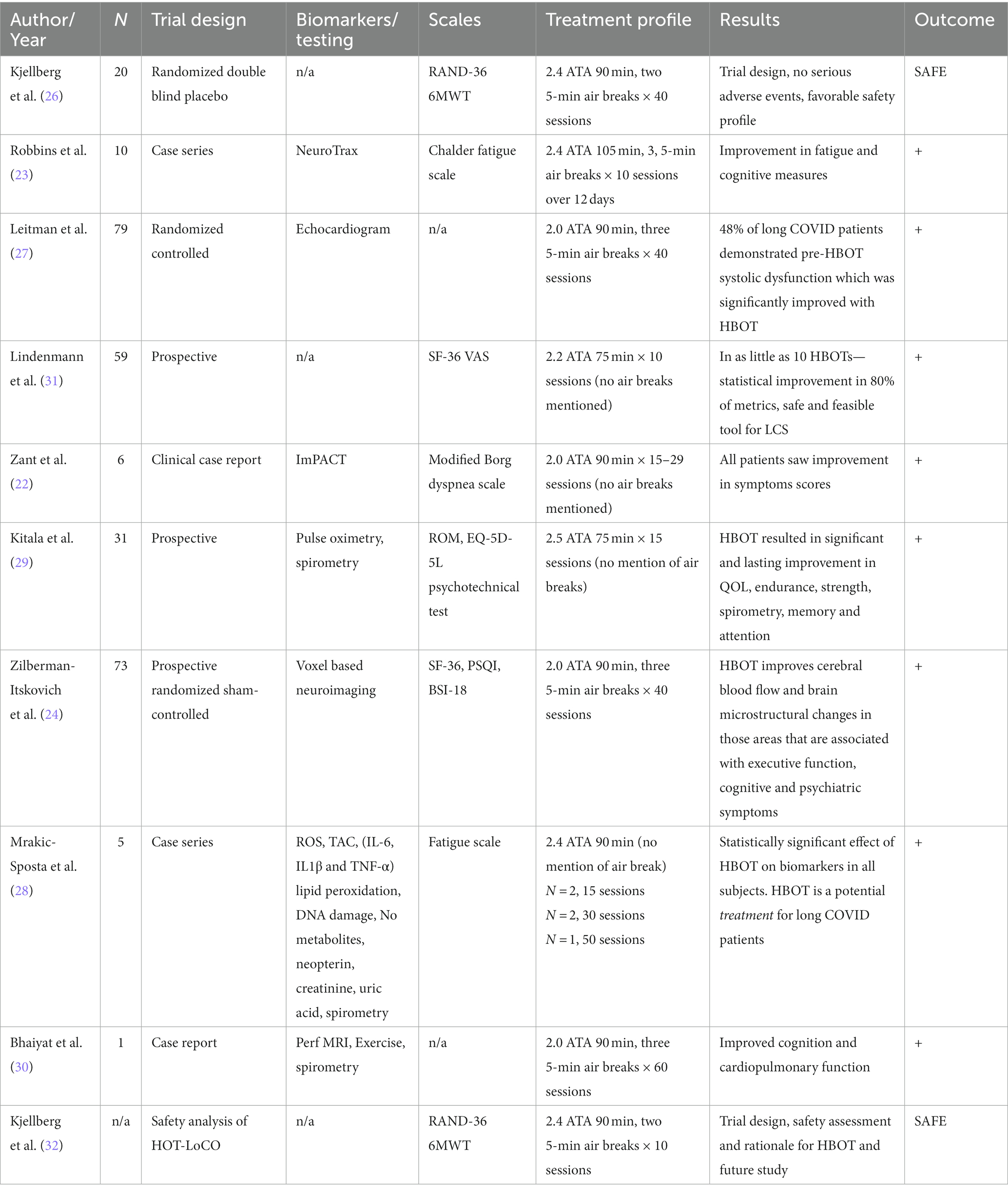
In a trial of six patients with long COVID symptoms treated with HBO, 6/6 patients saw improvement in symptoms, 5/6 of whom returned to pre-infection levels of illness (22). All the patients studied had developed dyspnea symptoms in the slight to moderate range of the modified Borg scale (average dyspnea score: 3.81). After completing 15 to 29 HBO treatments, dyspnea scores were significantly reduced (average dyspnea score post-HBO2: 0.17) in all patients (22). A case series of 10 patients treated with HBO yielded statistically significant improvements in fatigue, global cognition, executive function, attention, information processing speed, and verbal function (23). No adverse effects of HBO on these patients were noted.
Zilberman-Itskovich et al. in a randomized, sham-controlled, double-blind trial recently reported similar results (24). Seventy-three patients were randomized to receive HBO treatments vs. sham treatment. These patients were treated with HBO for 40 sessions. HBO treated patients had significant improvement in global cognitive function, attention, executive function, energy, sleep, psychiatric symptoms, and pain. Improvements in brain MRI perfusion and microstructural changes were noted, highlighting HBO’s beneficial effect on inducing neuroplasticity (25).
An on-going Swedish study looking at HBO for long COVID (HOT-LoCO) recently published an interim safety report from their ongoing trial, reporting mostly mild adverse events, indicating that HBO can be safely utilized in long COVID patients; outcome measures have not yet been reported (26).
A recent randomized, sham-controlled, double-blind trial addressed the effects of long COVID on cardiac dysfunction. Sixty patients who demonstrated ongoing left ventricular dysfunction symptoms for at least three months after COVID infection were randomized to receive 40 HBO or sham sessions. Echocardiography was performed at baseline and 1–3 weeks after the last HBO session. Twenty-nine (48.3%) patients had reduced global longitudinal strain (GLS) at baseline. Compared to the sham group, GLS significantly increased following treatment with HBO, illustrating that HBO enhances left ventricular systolic function recovery in patients suffering from long COVID-19 induced subclinical left ventricular dysfunction (27).
A recent trial evaluated oxy-inflammation biomarkers in long COVID-19 subjects treated with HBO. The study examined five subjects who received 100% O2 at 2.4 ATA for 90 min. Three of the patients received 15 sessions, one received 30 sessions and one received 50 sessions, with daily sessions, 5 times per week. Reactive oxygen species (ROS), antioxidant capacity, cytokines, lipids peroxidation, DNA damage, and renal status were assessed pre-treatment and after completion of HBO. The data showed reduction of ROS production, lipid peroxidation and DNA damage. There was a reduction of nitric oxide metabolites and inflammatory biomarkers (28). The results demonstrate that HBO may effectively mitigate the COVID-19-induced inflammation.
A prospective trial published in 2022 treated 31 patients with 15 sessions at HBO, reporting significant and sustained improvement in quality of life, endurance and strength, spirometry parameters, and working memory and attention (29).
A published case report of a 55-year-old male who received HBOT with pre and post perfusion MRI also demonstrated significant improvements in brain perfusion, white matter brain microstructure, and cognitive and cardiopulmonary function (30) (see Table 2).
Conclusion
Long COVID-related effects can be debilitating and often affect people who are economically productive. Eight published studies show that HBO has significant effects in improving the lives of patients diagnosed with long COVID. There are no other treatment options currently available that improve symptoms. HBO directly addresses the pathophysiology of long COVID including chronic inflammation, small vessel injury, disrupted neural pathways and mitochondrial dysfunction. There is increasing evidence which supports the use of HBO in treating patients suffering from the effects of long COVID. HBO has been documented as safe to use in patients suffering from long COVID. HBO may provide benefit to those suffering from long COVID symptoms and should continue to be researched for adjunctive treatment of long COVID.
Author contributions
AK: Conceptualization, Writing – original draft, Writing – review & editing. SW: Writing – original draft, Writing – review & editing. MK: Writing – original draft. PA: Writing – original draft. RB: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to acknowledge all the article authors and treating HBO units along with their medical providers and staff for treating patients with long COVID. They clearly helped to show the efficacy and safety of using HBO to treat long COVID. Additionally, a special thank you to Emma Belluscio for table design and formatting.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Marshall, M. The four most urgent questions about long COVID. Nature. (2021) 594:168–70. doi: 10.1038/d41586-021-01511-z,
2. Nalbandian, A, Sehgal, K, Gupta, A, Madhavan, MV, McGroder, C, Stevens, JS, et al. Post-acute COVID-19 syndrome. Nat Med. (2021) 27:601–15. doi: 10.1038/s41591-021-01283-z
3. Venkatesan, P. NICE guideline on long COVID. Lancet Respir Med. (2021) 9:129. doi: 10.1016/S2213-2600(21)00031-X
4. World Health Organization (2022). Post COVID-19 condition (long COVID). Available at: https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition
5. Mandal, S, Barnett, J, Brill, SE, Brown, JS, Denneny, EK, Hare, SS, et al. 'Long-COVID': a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax. (2021) 76:396–8. doi: 10.1136/thoraxjnl-2020-215818
6. Chopra, V, Flanders, SA, and O’Malley, M. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med. (2020) 174:576–8. doi: 10.7326/M20-5661
7. Yomogida, K, Zhu, S, Rubino, F, Figueroa, W, Balanji, N, and Holman, E. Post-Acute Sequelae of SARS-CoV-2 Infection Among Adults Aged ≥18 Years - Long Beach, California, April 1-December 10, 2020. MMWR Morb Mortal Wkly Rep. (2021) 70:1274–1277. doi: 10.15585/mmwr.mm7037a2
8. Mehandru, S, and Merad, M. Pathological sequelae of long-haul COVID. Nat Immunol. (2022) 23:194–202. doi: 10.1038/s41590-021-01104-y
9. Yong, SJ. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infect Dis (Lond). (2021) 53:737–54. doi: 10.1080/23744235.2021.1924397
10. Phetsouphanh, C, Darley, DR, Wilson, DB, Howe, A, Munier, CML, Patel, SK, et al. Immunological dysfunction persists for 8 months following initial mild–moderate SARS-CoV-2 infection. Nat Immunol. (2022) 23:210–6. doi: 10.1038/s41590-021-01113-x
11. Karlsson, AC, Humbert, M, and Buggert, M. The known unknowns of T cell immunity to COVID. Sci Immunol. (2020) 5:19. doi: 10.1126/sciimmunol.abe8063
12. Tavakolpour, S, Rakhshandehroo, T, Wei, EX, and Rashidian, M. Lymphopenia during the COVID-19 infection: what it shows and what can be learned. Immunol Lett. (2020) 225:31–2. doi: 10.1016/j.imlet.2020.06.013
13. Grifoni, E, Valoriani, A, Cei, F, Lamanna, R, Gelli, AMG, Ciambotti, B, et al. Interleukin-6 as prognosticator in patients with COVID-19. J Infect. (2020) 81:452–82. doi: 10.1016/j.jinf.2020.06.008
14. Passos, FRS, Heimfarth, L, Monteiro, BS, Corrêa, CB, Moura, TR, AAS, A, et al. Oxidative stress and inflammatory markers in patients with COVID-19: potential role of RAGE, HMGB1, GFAP and COX-2 in disease severity. Int Immunopharmacol. (2022) 104:108502. doi: 10.1016/j.intimp.2021.108502
15. Rossato, MS, Brilli, E, Ferri, N, Giordano, G, and Tarantino, G. Observational study on the benefit of a nutritional supplement, supporting immune function and energy metabolism, on chronic fatigue associated with the SARS-CoV-2 post-infection progress. Clin Nutr ESPEN. (2021) 46:510–8. doi: 10.1016/j.clnesp.2021.08.031
16. Oronsky, B, Larson, C, Hammond, TC, Oronsky, A, Kesari, S, Lybeck, M, et al. A review of persistent post-COVID syndrome (PPCS). Clin Rev Allergy Immunol. (2023) 64:66–74. doi: 10.1007/s12016-021-08848-3
17. Davis, HE, McCorkell, L, Vogel, JM, and Topol, EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. (2023) 21:133–46. doi: 10.1038/s41579-022-00846-2
18. Camporesi, EM, and Bosco, G. Mechanisms of action of hyperbaric oxygen therapy. Undersea Hyperb Med. (2014) 41:247–52.
19. Hedetoft, M, Garred, P, Madsen, MB, and Hyldegaard, O. Hyperbaric oxygen treatment is associated with a decrease in cytokine levels in patients with necrotizing soft-tissue infection. Physiol Rep. (2021) 9:e14757. doi: 10.14814/phy2.14757
20. Schottlender, N, Gottfried, I, and Ashery, U. Hyperbaric oxygen treatment: effects on mitochondrial function and oxidative stress. Biomol Ther. (2021) 11:1827. doi: 10.3390/biom11121827
21. Feldmeier, JJ, Kirby, JP, Buckey, JC, Denham, DW, Evangelista, JS, Gelly, H, et al. Physiologic and biochemical rationale for treating COVID-19 patients with hyperbaric oxygen. Undersea Hyperb Med. (2021) 48:1–12. doi: 10.22462/01.03.2021.1
22. Zant, AE, Figueroa, XA, Paulson, CP, and Wright, JK. Hyperbaric oxygen therapy to treat lingering COVID-19 symptoms. Undersea Hyperb Med. (2022) 49:333–9. doi: 10.22462/05.06.2022.7
23. Robbins, T, Gonevski, M, Clark, C, Baitule, S, Sharma, K, Magar, A, et al. Hyperbaric oxygen therapy for the treatment of long COVID: early evaluation of a highly promising intervention. Clin Med (Lond). (2021) 21:e629–32. doi: 10.7861/clinmed.2021-0462
24. Zilberman-Itskovich, S, Catalogna, M, Sasson, E, Elman-Shina, K, Hadanny, A, Lang, E, et al. Hyperbaric oxygen therapy improves neurocognitive functions and symptoms of post-COVID condition: randomized controlled trial. Sci Rep. (2022) 12:11252. doi: 10.1038/s41598-022-15565-0
25. Catalogna, M, Sasson, E, Hadanny, A, Parag, Y, Zilberman-Itskovich, S, and Efrati, S. Effects of hyperbaric oxygen therapy on functional and structural connectivity in post-COVID-19 condition patients: a randomized, sham-controlled trial. Neuroimage Clin. (2022) 36:103218. doi: 10.1016/j.nicl.2022.103218
26. Kjellberg, A, Hassler, A, Boström, E, El Gharbi, S, Al-Ezerjawi, S, Kowalski, J, et al. Hyperbaric oxygen therapy for long COVID (HOT-LoCO), an interim safety report from a randomised controlled trial. BMC Infect Dis. (2023) 23:33. doi: 10.1186/s12879-023-08002-8
27. Leitman, M, Fuchs, S, Tyomkin, V, Hadanny, A, Zilberman-Itskovich, S, and Efrati, S. The effect of hyperbaric oxygen therapy on myocardial function in post-COVID-19 syndrome patients: a randomized controlled trial. Sci Rep. (2023) 13:9473. doi: 10.1038/s41598-023-36570-x
28. Mrakic-Sposta, S, Vezzoli, A, Garetto, G, Paganini, M, Camporesi, E, Giacon, TA, et al. Hyperbaric oxygen therapy counters oxidative stress/inflammation-driven symptoms in long COVID-19 patients: preliminary outcomes. Meta. (2023) 13:1032. doi: 10.3390/metabo13101032
29. Kitala, D, Łabuś, W, Kozielski, J, Strzelec, P, Nowak, M, Knefel, G, et al. Preliminary research on the effect of hyperbaric oxygen therapy in patients with post-COVID-19 syndrome. J Clin Med. (2023) 12:308. doi: 10.3390/jcm12010308
30. Bhaiyat, AM, Sasson, E, Wang, Z, Khairy, S, Ginzarly, M, Qureshi, U, et al. Hyperbaric oxygen treatment for long coronavirus disease-19: a case report. J Med Case Rep. (2022) 16:80. doi: 10.1186/s13256-022-03287-w
31. Lindenmann, J, Porubsky, C, Okresa, L, Klemen, H, Mykoliuk, I, Roj, A, et al. Immediate and Long-Term Effects of Hyperbaric Oxygenation in Patients with Long COVID-19 Syndrome Using SF-36 Survey and VAS Score: A Clinical Pilot Study. J Clin Med. (2023) 12:6253. doi: 10.3390/jcm12196253
32. Kjellberg, A, Abdel-Halim, L, Hassler, A, El Gharbi, S, Al-Ezerjawi, S, Boström, E, et al. Hyperbaric oxygen for treatment of long COVID-19 syndrome (HOT-LoCO): protocol for a randomised, placebo-controlled, double-blind, phase II clinical trial. BMJ Open. (2022) 12:e061870. doi: 10.1136/bmjopen-2022-061870
Keywords: long COVID, hyperbaric, hyperbaric oxygen therapy, post-COVID-19 syndrome, HBO
Citation: Katz AA, Wainwright S, Kelly MP, Albert P and Byrne R (2024) Hyperbaric oxygen effectively addresses the pathophysiology of long COVID: clinical review. Front. Med. 11:1354088. doi: 10.3389/fmed.2024.1354088
Edited by:
César Fernández-de-las-Peñas, Rey Juan Carlos University, SpainReviewed by:
Harpal Singh Randeva, University Hospitals Coventry and Warwickshire NHS Trust, United KingdomSimona Mrakic-Sposta, Institute of Clinical Physiology, Italy
Jacek Kot, Medical University of Gdansk, Poland
Copyright © 2024 Katz, Wainwright, Kelly, Albert and Byrne. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rosemary Byrne, cm9zZW1hcnlAaG1zaGJvdC5jb20=
 Alan A. Katz
Alan A. Katz Sandra Wainwright3
Sandra Wainwright3 Rosemary Byrne
Rosemary Byrne