- 1Department of Histology, West Kazakhstan Marat Ospanov Medical University, Aktobe, Kazakhstan
- 2Department of Anatomy, School of Biomedical Sciences, Medicine and Health, University of New South Wales, Sydney, NSW, Australia
- 3Department of Oncology, West Kazakhstan Marat Ospanov Medical University, Aktobe, Kazakhstan
- 4Department of Pathological Anatomy and Forensic Medicine, West Kazakhstan Marat Ospanov Medical University, Aktobe, Kazakhstan
Gastric cancer remains a significant global health challenge, ranking fifth worldwide in both incidence and mortality worldwide. Early detection and accurate prognosis are crucial for effective management, yet current diagnostic methods, including tumor markers, often exhibit less sensitivity. This bibliometric analysis investigates trends and key contributions in research on tumor markers for gastric cancer diagnosis from 2019 to 2024. Using Scopus and Web of Science databases, 2,940 articles were analyzed to assess publication trends, prominent authors, institutions, and emerging research themes. Results highlight East Asia, particularly institutions like Fudan University and Nanjing Medical University, as a hub for groundbreaking research. The study identifies key tumor markers and advances in molecular diagnostics, with emphasis on personalized medicine and early detection strategies. Visualization of global collaborations reveals extensive networks driving innovation in this field. While this analysis underscores progress in gastric cancer biomarker research, it also identifies limitations, including language bias and a narrow temporal scope. Future research should prioritize novel biomarkers, integrate advanced technologies like AI, and enhance international cooperation to further improve outcomes for gastric cancer patients.
1 Introduction
Stomach cancer is a heterogeneous malignancy associated with environmental and genetic predisposing factors and is the fifth most common cancer worldwide. There were over 968,000 new cases of stomach cancer in 2022 and nearly 660,000 deaths, ranking the disease as fifth in terms of both incidence and mortality worldwide. Thus, according to the Global Cancer Observatory (2022), stomach cancer (4.9%) ranks fifth after lung cancer (12.4%), breast cancer (11.6%), colorectal cancer (9.6%), and prostate cancer (7.3%). Recent indicators have seen a decline in the incidence of stomach cancer, but mortality from this manifestation remains high. For 2022, according to the Global Cancer Observatory, mortality from stomach cancer in the world ranks fifth after lung cancer (18.7%), colorectal cancer (9.3%), and liver cancer (7.8%), female breast (6.9%) (1–7).
Currently, the main challenge in diagnosing stomach cancer is the less sensitivity of the existing methods available for detecting small lesions in the early stages or after radiotherapy and chemotherapy. Moreover, the markers for diagnosing stomach cancer achieved unsatisfactory efficacy. Thus, there is an urgent need to identify new biomarkers to enhance treatment effectiveness in patients with GC (8–10). Today, to predict the clinical course of GC, morphological criteria for the malignancy of the tumor process, such as size, depth of invasion, macroscopic and histological type, are widely used (11, 12). It should be noted that the course of the disease varies significantly within one histological type. In cancer, immunohistochemical (IHC) techniques can predict the clinical course of the disease in different individuals. In this regard, it is necessary to select the most informative markers while also considering complications arising from cancers in other organs (13, 14).
Bibliometric analysis is a quantitative method which applies mathematical and statistical tools to evaluate the inter-relationships and impacts of publications, authors, institutions and countries in a specific research area. Through extracting and analyzing the metrics of each publication including author, institution, country, and keywords, bibliometric analysis is able to determine the development trends or future research directions. Compared with conventional narrative reviews by experts, which often subjectively focus on the progress in a specific research field, bibliometric analysis is advantageous in objectively, comprehensively, and quantitatively summarizing the whole topic based on the best available data (15).
In this study, we conducted a bibliometric analysis and generated visual knowledge maps of relevant publications to analyze the research landscape and trends regarding applying tumor markers for stomach cancer diagnosis from 2019 to 2024, using the Scopus and Web of Science databases. We aim to identify additional promising tumor markers with high sensitivity for this purpose.
2 Materials and methods
2.1 Eligibility criteria and data source
This bibliometric analysis aimed to analyze the application of tumor markers in stomach cancer diagnosis from 2019 to 2024. The eligibility criteria included original research articles and reviews published in peer-reviewed journals, with only English-language articles considered. Data were collected from major citation databases, Scopus, which were chosen due to comprehensive coverage of biomedical research. The search was conducted in November 2024. Metadata for each article were downloaded in BibTeX format from Scopus, and subsequently converted for analysis using the RStudio as an excel file (Table 1).
2.2 Search strategy
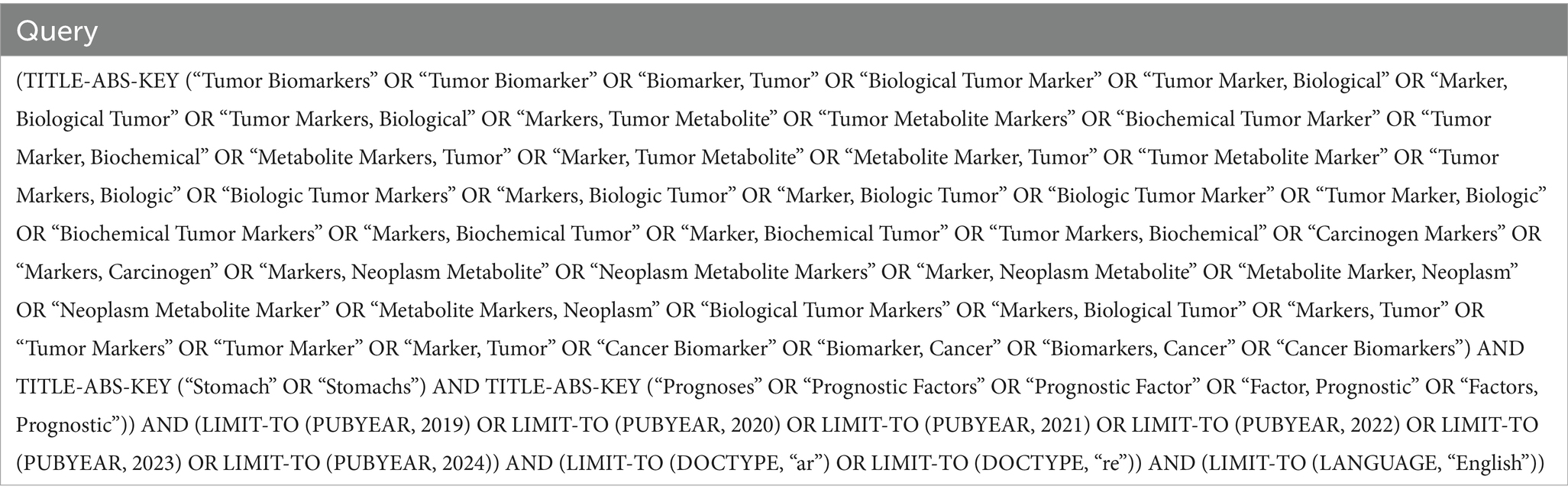
The search strategy involved the use of advanced search features in Scopus, with the complete search strategy detailed in Figure 1. Boolean and Wildcard search operators were employed to capture variations in terminology related to tumor markers and gastric cancer. The complete search strategy for databases is outlined in Table 2. Articles deemed irrelevant based on title, abstract, or full-text review were excluded. After obtaining the results, we imported 2,940 articles for bibliometric analyses.
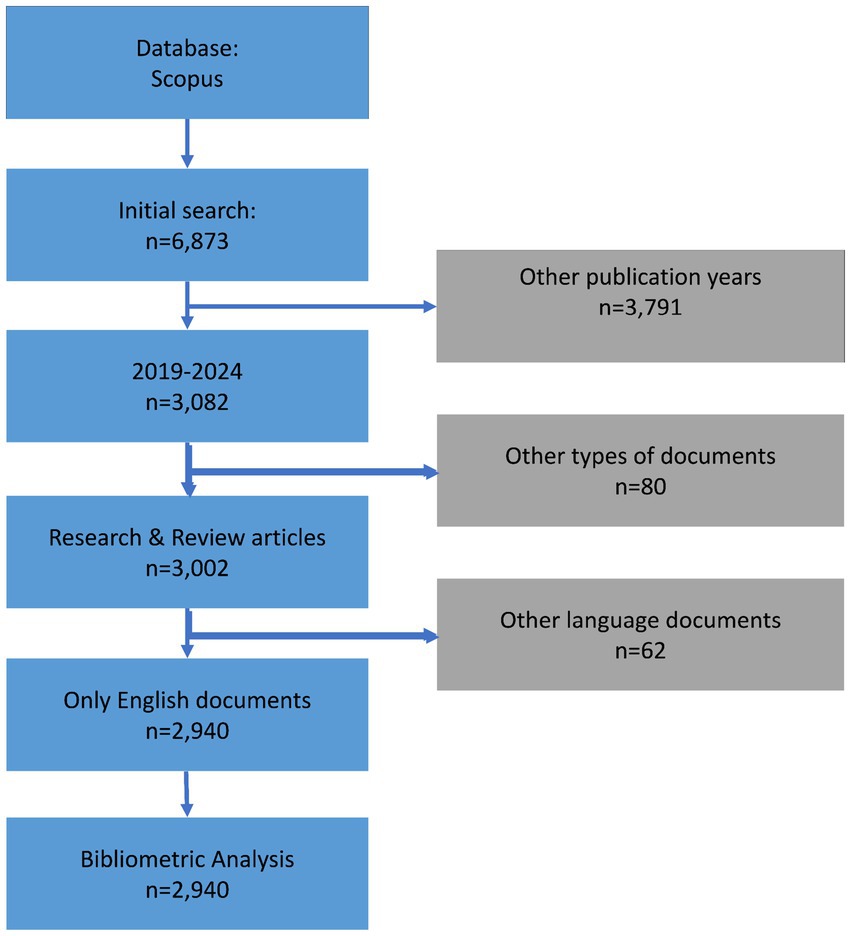
Figure 1. Flowchart depicting the article selection process, from initial retrieval to final inclusion.
2.3 Bibliometric analyses
Data management and bibliometric analysis were conducted using the bibliometric package (version 4.3.2) and Biblioshiny, a web-based interface for bibliometric visualization (RStudio 2024.09.0–375, PBC, Boston, MA). The analysis covered a 5-year period for research articles, focusing on publication and citation metrics and citation trends (JOSHI S, 2021, CA CANCER J CLIN). Prolific institutions were identified based on the number of articles related to the applying tumor markers in stomach cancer diagnosis within this timeframe.
This investigation explored interactions among 10 prominent journals, fields of study, and countries contributing to research on the application of tumor markers in stomach cancer diagnosis over the past 5 years. To illustrate global research collaboration, we visualized connections on a world map. A TreeMap visualization depicted the 20 most frequently used keywords in articles published on this topic. Lastly, a “Thematic Map” categorized topics into four domains: primary subjects, specialized subjects, emerging or declining subjects, and overarching foundational subjects.
3 Results
Additionally, we created collaborative networks among 606 research journals for research articles using clustering algorithms and data normalization from 2,392 research articles. This process was guided by an association parameter to identify meaningful connections. We identified authors with significant contributions based on the frequency of their published articles. The assessment also included identifying the top 10 most frequently cited documents and leading journals.
3.1 Search results
We initially retrieved 6,873 papers from the Scopus database. After applying eligibility criteria and excluding 3,933 ineligible studies, we considered a total of 2,940 papers for this bibliometric analysis (Figure 1).
3.2 Key attributes of the included studies
Among the 2,940 articles, a majority of the studies were original research articles (81.4%), with the remainder comprising reviews (18.6%). The most common research areas included molecular biology, clinical oncology, and pathomorphology, reflecting the interdisciplinary nature of tumor marker research in gastric cancer diagnosis.
3.3 Trend of publication and citation
Figure 2 presents the trends in average citations and publication counts for research and review articles related to gastric cancer and biomarkers from 2019 to 2024. Graph A illustrates the average annual citations of research articles over this period, showing a consistent decline, with citations steadily decreasing each year. Graph B displays the global number of research article publications, which, despite some fluctuations, remains relatively stable, with a slight decrease in recent years. Graph C highlights the average annual citations of review articles, revealing an upward trend from 2019 to 2022, followed by a sharp decline thereafter. Graph D shows the number of published review articles, peaking around 2022 before steadily dropping.
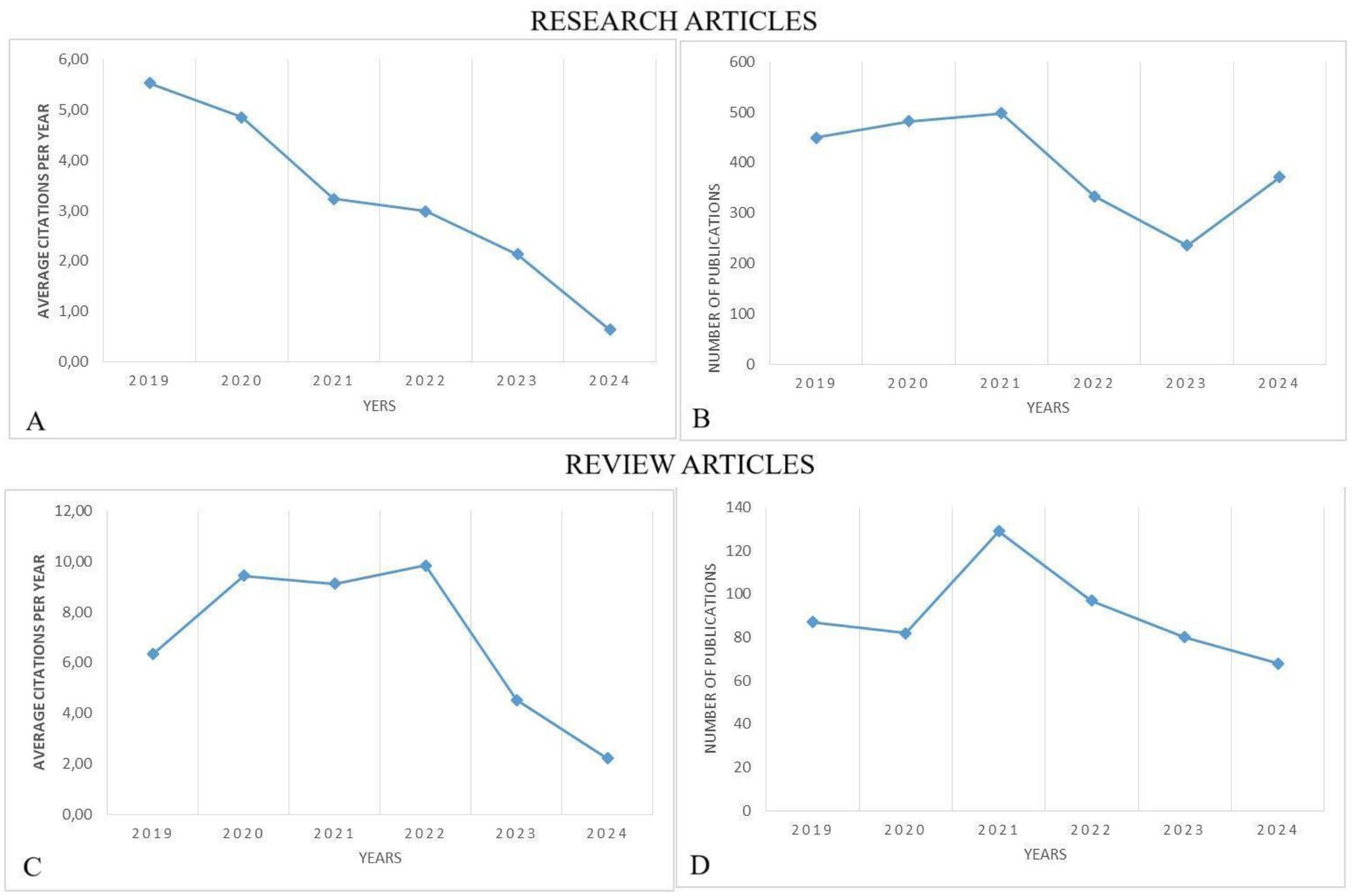
Figure 2. Average citations in (A) research study publications and (B) global annual trends, as well as (C) review article citations and (D) publications, biomarkers and GC research from 2019 to 2024.
The average citations for both research and review articles on gastric cancer and biomarkers have declined in recent years, with review articles initially experiencing growth before decreasing. The number of publications shows slight variations, potentially reflecting shifts in research focus or citation patterns.
3.4 Most relevant affiliations
In the context of research articles focused on the investigation of the applying tumor markers in stomach cancer, an assessment of institutional productivity reveals Fudan University as the leading institution in terms of output. This institution has made significant contributions to the field by presenting a total of 106 research papers on the subject (Table 3). Other top institutions included Nanjing Medical University and Sun Yat-Sen University, indicating a strong concentration of research output in East Asia.
Table 3. The top 10 affiliations that published research and review articles on biomarkers and GC research from 2019 to 2024.
Table 3 offers an evaluation of institutional productivity within research on the application of tumor markers in stomach cancer. It identifies Mashhad University of Medical Sciences as a primary contributor, with a total output of 36 review articles. Additional prominent institutions include Shahid Beheshti University of Medical Sciences and Tabriz University of Medical Sciences, suggesting a significant concentration of research activity in this field in Iran. This distribution underscores the role of these institutions in advancing knowledge on tumor markers in stomach cancer research.
3.5 Most contributing authors and their collaboration network
Leading authors such as Wang Y and Zhang Y had the highest number of published research articles on tumor markers in gastric cancer, with 168 and 115 articles and review articles for both 18, respectively (Figures 3A,B). Their collaboration networks were analyzed, revealing key partnerships with researchers across Europe and North America.
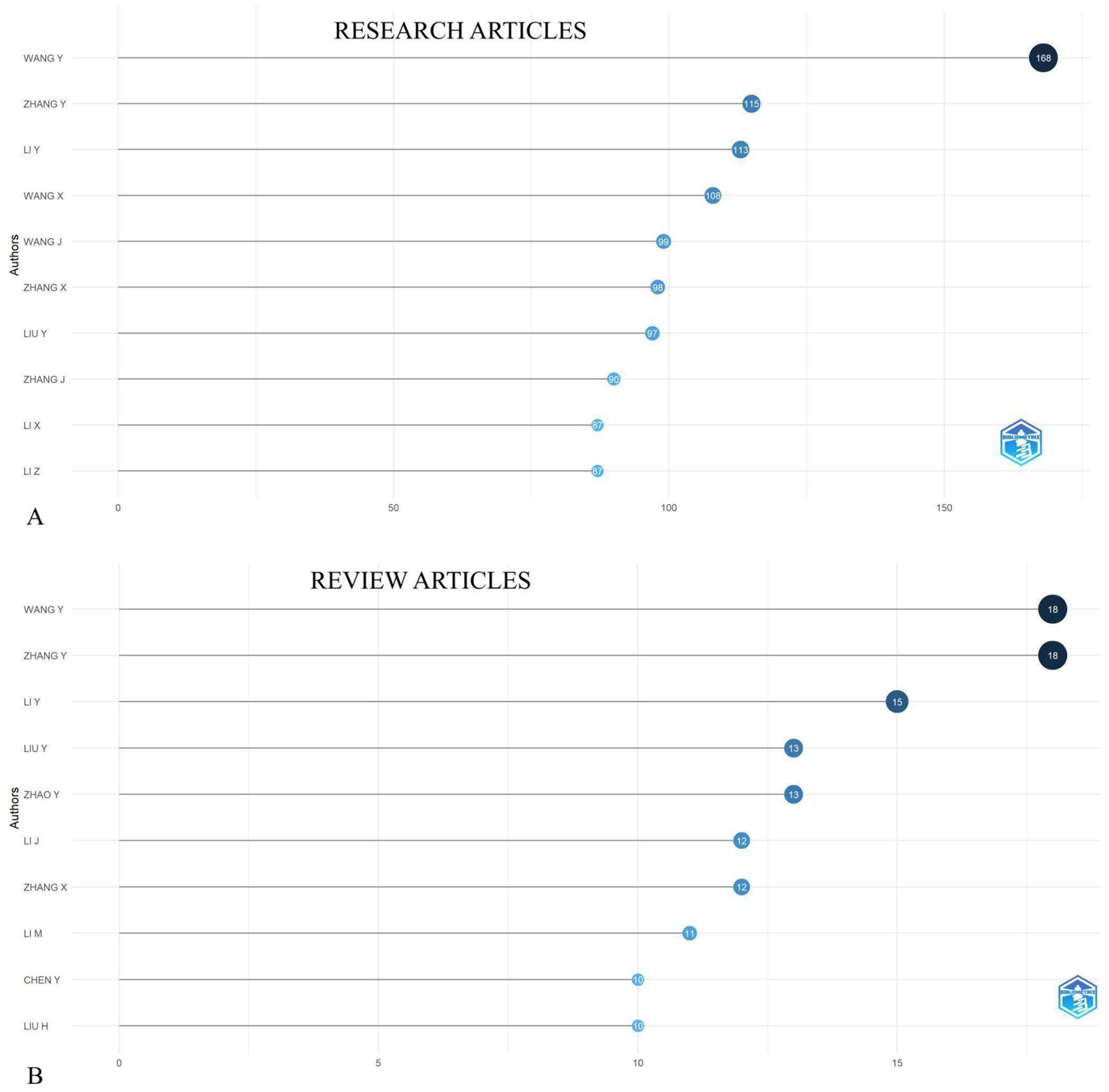
Figure 3. Top 10 most prolific authors of research articles (A) and review articles (B) in the field of biomarkers and GC research from 2019 to 2024.
Figure 4 presents a graphical representation of the top 10 authors over time, spotlighting the most prolific contributors in the field. These prolific authors are those who have made substantial contributions to the field, as determined by their h-index, indicating that each author has amassed at least “h” citations for their published papers. In Figure 4, the size of the circles corresponds to the number of articles authored, with larger circles denoting a higher volume of publications. Furthermore, the color of the circles represents the total citations per year, where a darker shade indicates a greater number of citations.
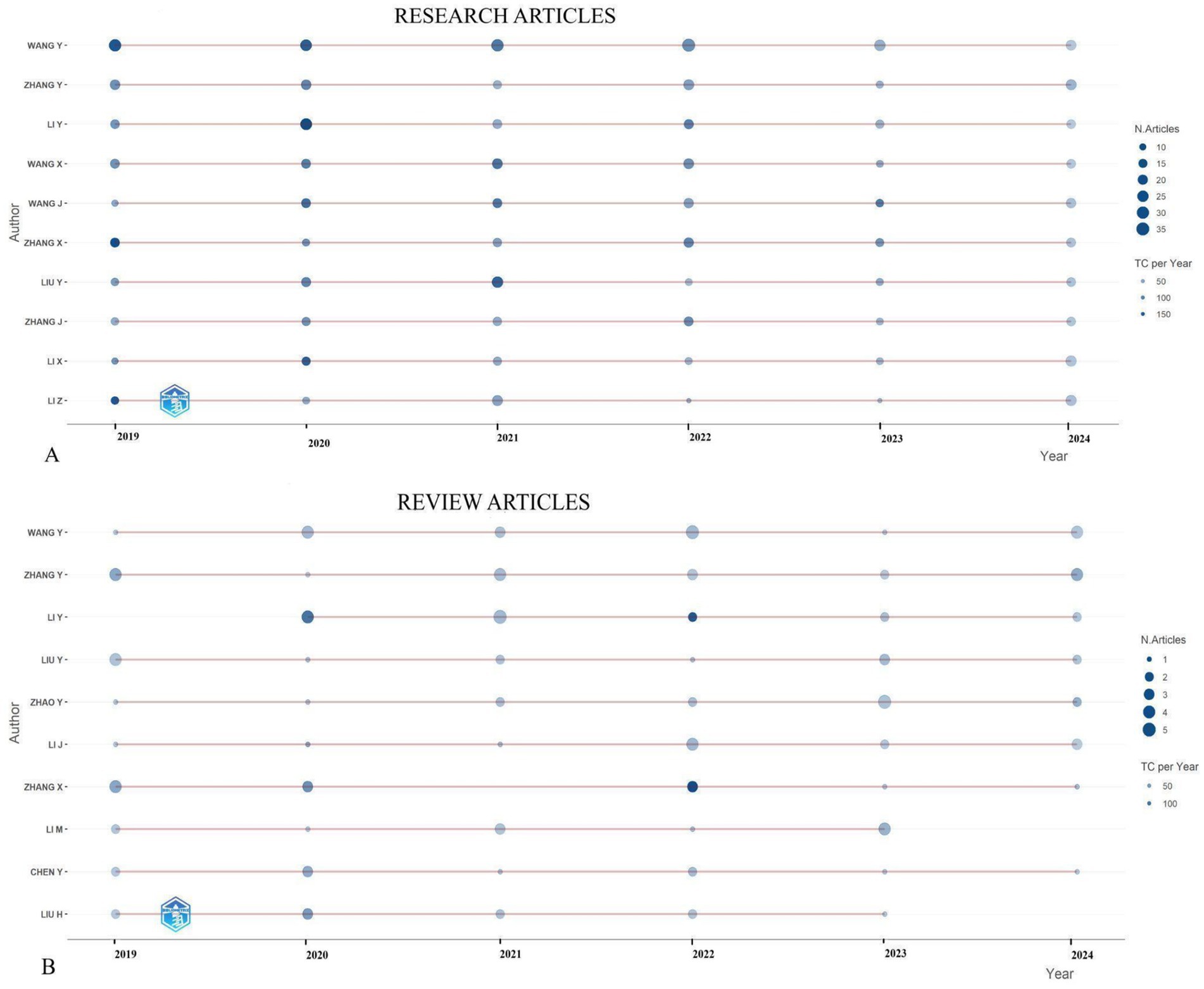
Figure 4. Top 10 most prolific authors’ research article (A) and review article (B) production over time (2019–2024) regarding biomarkers and GC research.
3.6 Most productive journals
Among the published research articles in our field of interest, 75 research articles were featured in the journal “BMC Cancer” followed by 68, 55, 54, 50 research articles in the journals “Gastric Cancer,” “Medicine (United States),” “Scientific Reports,” “Pathology Research and Practice” respectively as shown in Figure 5A.
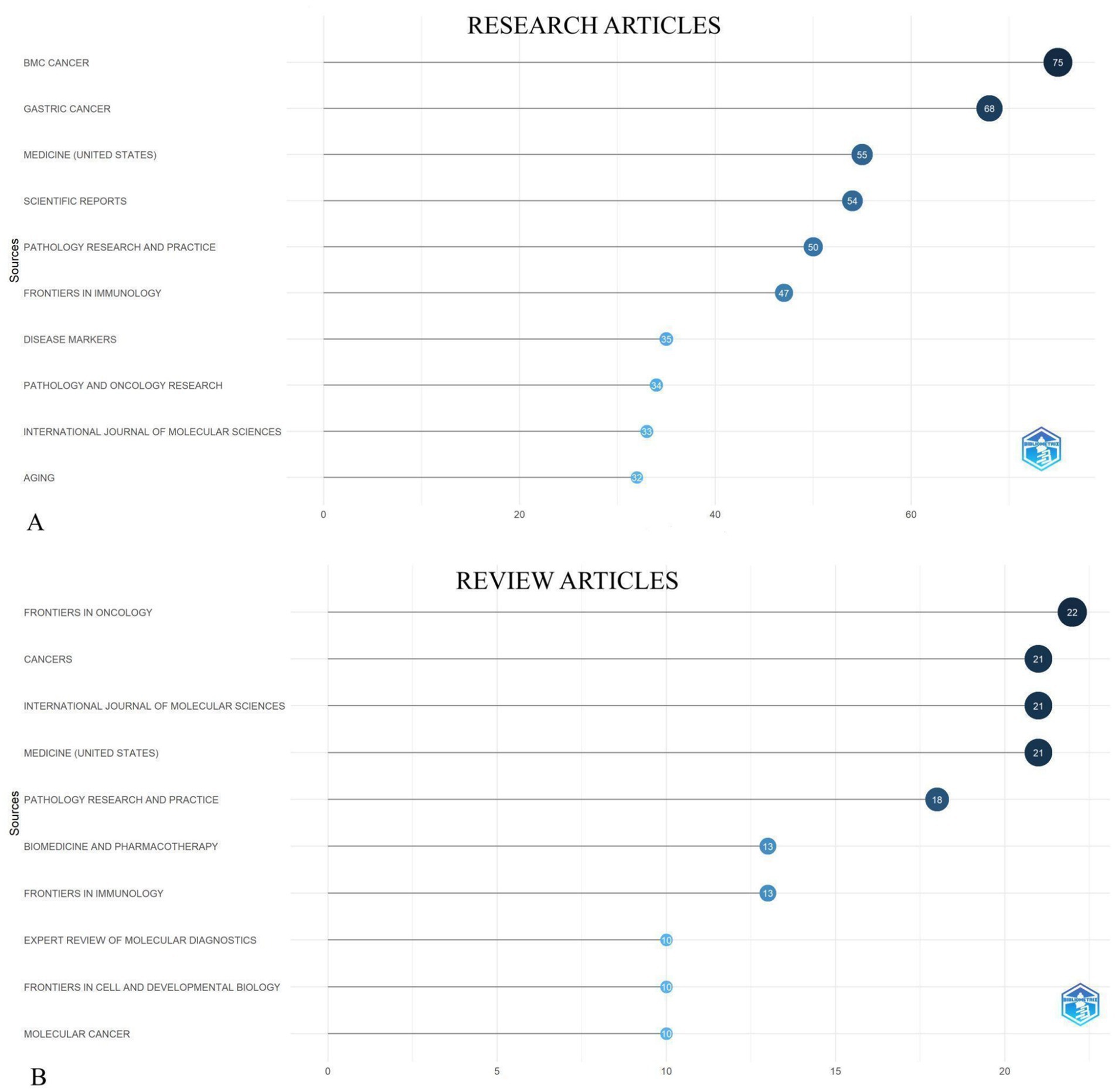
Figure 5. The top 10 most productive journals on biomarkers and GC in research articles (A) and review articles (B), respectively from 2019 to 2024.
Most productive journals between 2019 and 2024, as shown in Figure 5B were “Frontiers in oncology” with 22 articles, “Cancers,” “International Journal of Molecular Sciences,” “Medicine (United States)” each with 21 articles and “Pathology Research and Practice”with 18 review articles published on GC and biomarkers field. The network diagram of international collaboration in publications on the studied topic shows that China holds a leading position in terms of both the number of publications and the degree of collaboration. Other active contributors include the United States, the United Kingdom, Germany, and Australia (Figure 6).

Figure 6. A visual representation of countries’ collaborative networks in the publication of research articles (A) and review (B) focusing on the application of the tumor markers in stomach cancer diagnosis from 2019 to 2024. The map highlights collaborative efforts between various countries, illustrating the global nature of research in this field.
3.7 World research production and collaborations
To depict the contributions from the top 10 journals, authors, and keywords in exploring the applying tumor markers in stomach cancer diagnosis, we have created a graphical representation that illustrates the interactions within these domains. This depiction illustrates the inbound and outbound interactions among these respective domains in Figure 7.
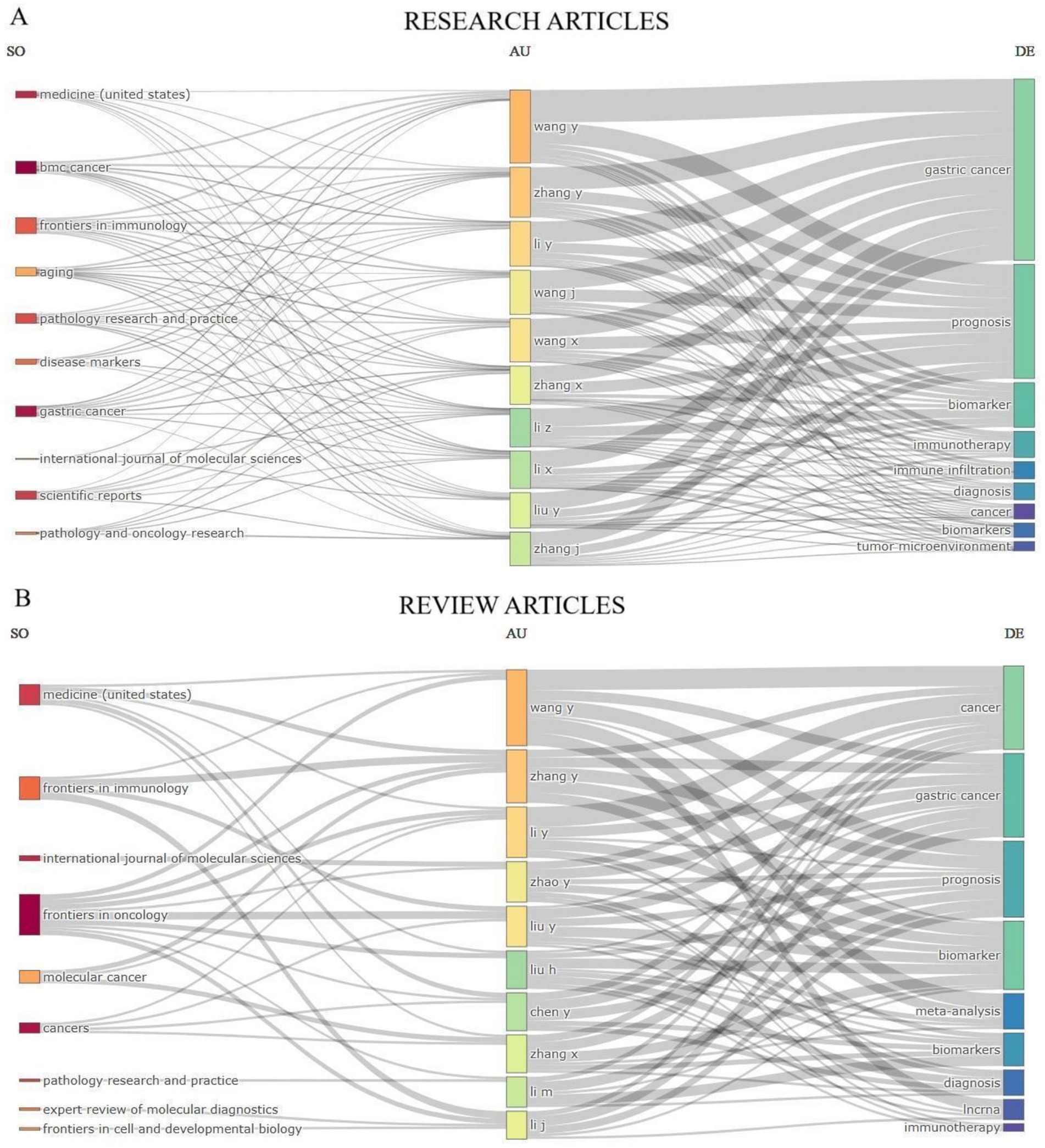
Figure 7. Three-Fields Plot that illustrates the interconnections between the top 10 journals, authors, and keywords that have made contributions to research articles (A) and review articles (B) on the application of the tumor markers in stomach cancer diagnosis. This plot visualizes the incoming and outgoing flows of influence among these key elements in the research field.
This visualization effectively maps the collaboration between scientific output, key contributors, and focal research topics, providing insights into influential authors and trending areas within cancer research.
3.8 TreeMap and thematic map
Figure 8A displays a TreeMap visualization that highlights the top 20 keywords frequently used by authors in research articles related to applying tumor markers in stomach cancer diagnosis. It is noteworthy that three keywords, namely, “gastric cancer” (constituting 36% of usage), “prognosis” (comprising 20% of usage), and “biomarker” (comprising 8% of usage), prominently recur within this research domain. While Figure 8B presents a showcasing the top 20 keywords in review articles notably, three keywords including “cancer” (17%), “gastric cancer” (making up 13% of usage), and “biomarker” (representing 14% of usage) stand out as recurring themes in this field.
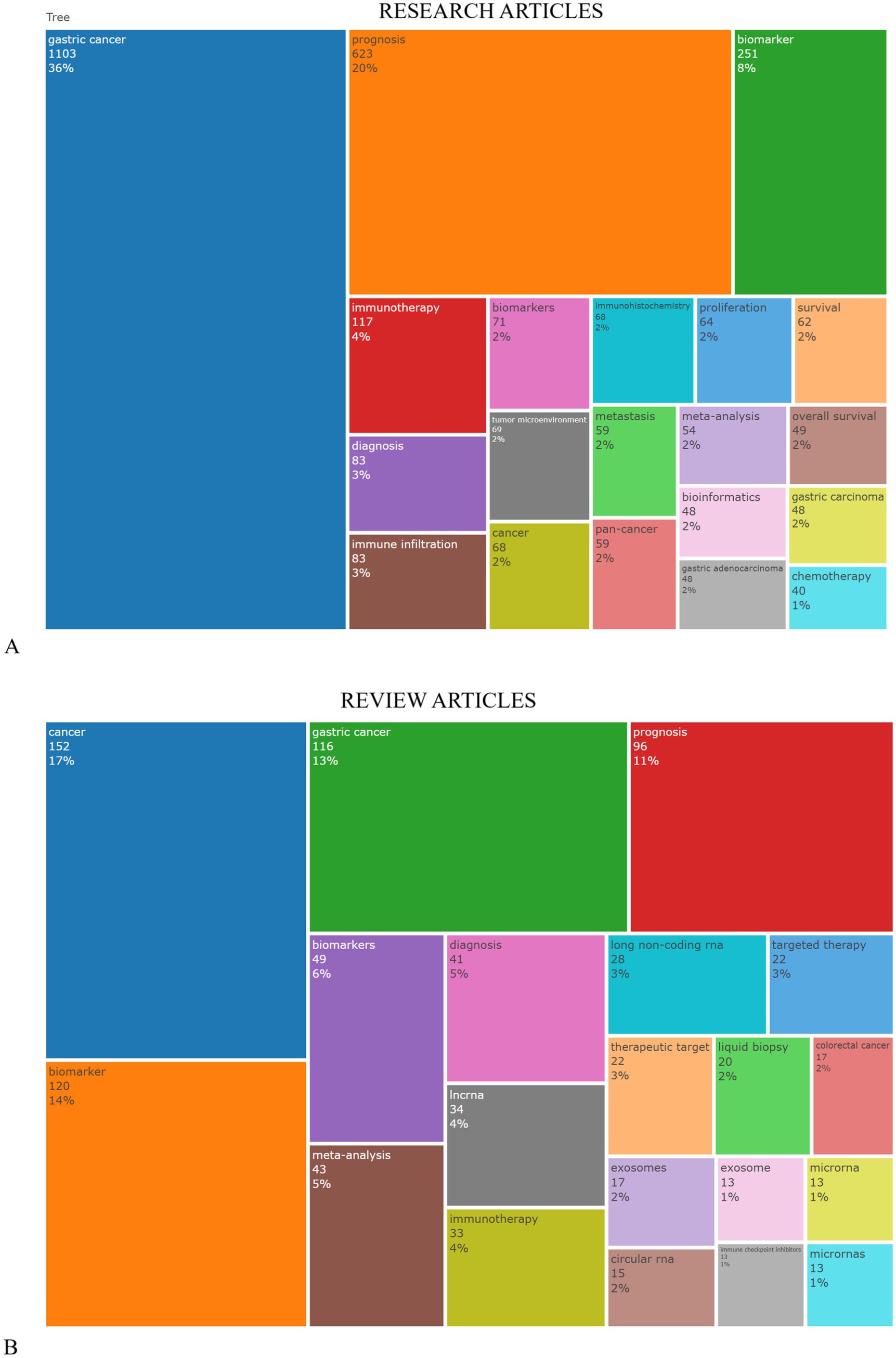
Figure 8. TreeMap visualization that highlights the top 20 author’s keywords commonly found in research articles related to tumor markers in stomach cancer. (A) refers to data from original research articles, while part (B) presents data from review articles, with an emphasis on the use of keywords related to the diagnosis of gastric cancer.
3.9 Overview of gastric cancer literature trends (2019–2024)
For a focused examination of GC literature in recent years, we conducted an in-depth analysis of publications spanning the period 2019 to 2024. Our investigation revealed a notable surge in research interest and publications related to GC during the specified timeframe (Figure 9).
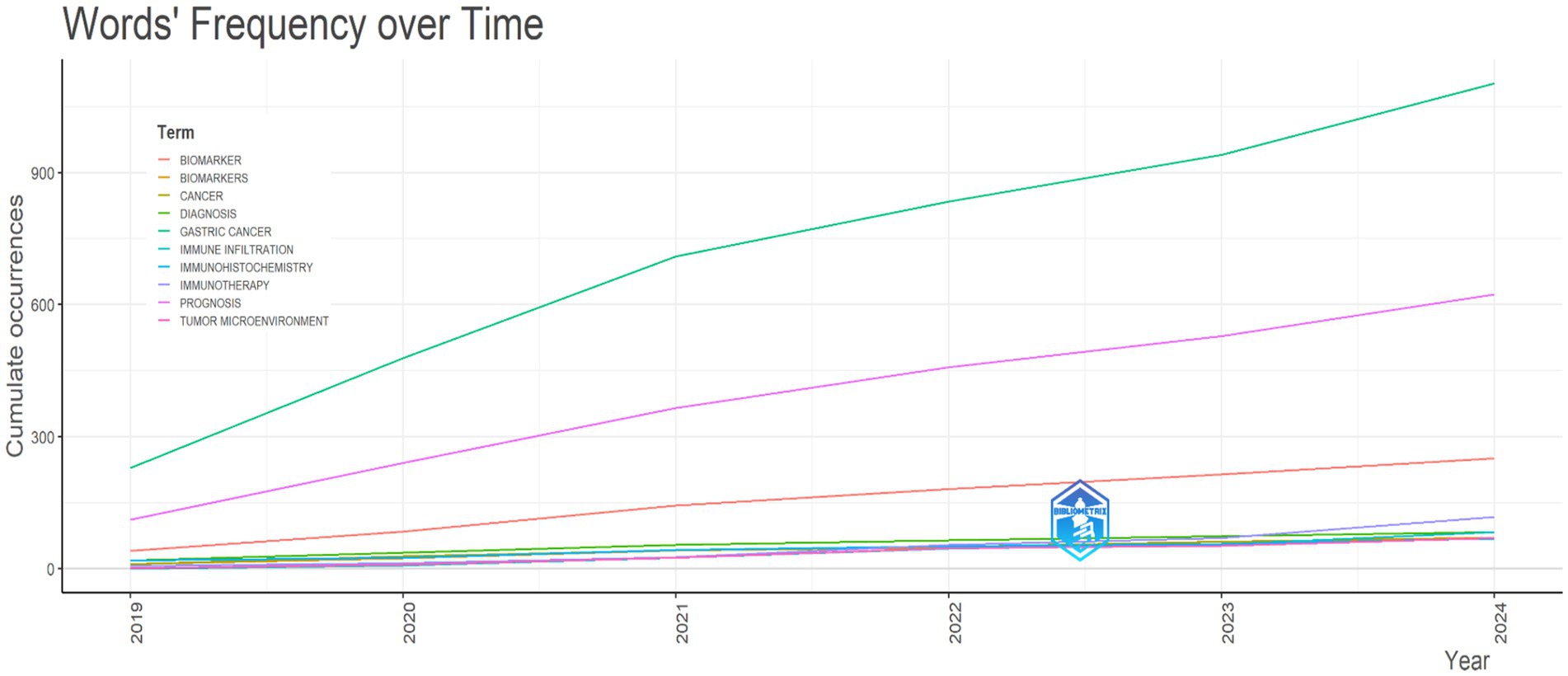
Figure 9. Trends and emerging themes in gastric cancer research studies (2019–2024). This figure highlights the evolving attention of researchers and clinicians toward prognosis within the context of GC studies during the period 2019–2024.
4 Discussion
The current bibliometric analysis provides a comprehensive overview of research trends in tumor marker applications for gastric cancer (GC) diagnosis from 2019 to 2024, highlighting significant advancements in molecular diagnostics, institutional contributions, and emerging research themes that shape the field. The increasing number of publications in the recent years underscores the growing interest of tumor biomarkers and their potential to transform early detection and personalized treatment strategies.
4.1 Global research trends
The analysis reveals an increased trend in publications on GC biomarkers, with 81.4% original research articles and 18.6% reviews, confirming heightened research activity driven by the high global burden of GC and advancements in molecular diagnostics (16–21). Moreover, a prominent portion of the research is contributed from East Asia including China, Japan and South Korea due to their high GC incidence rate (1, 2). Institutions such as Fudan University, Nanjing Medical University, and Sun Yat-Sen University lead in publication volume, contributing pioneering work in molecular biology and clinical oncology (22–26). North America and Europe also play significant roles, fostering robust international contribution that enhance knowledge exchange and innovation (27–31). These global networks, visualized in collaborative maps, underscore the interdisciplinary and transnational nature of GC biomarker research and advancements in the identification and validation of novel tumor markers (16–21).
4.2 Institutional and author contributions
Fudan University, Nanjing Medical University, and Sun Yat-Sen University remain at the forefront, driving innovation in the field of GC biomarkers. Their vast contributions include pioneering works in the fields of molecular diagnostics and clinical oncology, which have supported the advancement of methods for early detection and treatment approaches (22–26).
Among these are highly cited authors such as Wang Y and Zhang Y. Their extensive collaboration networks, spanning Asia, Europe, and North America, highlight the importance of interdisciplinary partnerships in advancing GC research. The increasing prevalence of multi-center studies and cross-institutional collaborations further illustrates the value of shared expertise and resources in driving scientific progress (27–31).
4.3 Key research themes and emerging trends
The bibliometric mapping of keywords, authors, and journals reveals main and emerging themes in GC biomarker research, with gastric cancer (36%), prognosis (20%), and biomarker (8%) as the most frequent keywords. These themes emphasize the critical role of tumor biomarkers in predicting disease progression and guiding personalized treatment strategies. Genetic and epigenetic markers, such as HER2, microsatellite instability (MSI), and circulating tumor DNA (ctDNA) play crucial roles in diagnosis and prognosis (22). For instance, HER2-targeted therapies have shown promise in improving outcomes for HER2-positive GC patients, though challenges like heterogeneity in expression persist (19, 32).
Emerging trends include the integration of novel technologies to enhance biomarker sensitivity and specificity. Nanotechnology, for example, is being explored for early detection of small lesions, addressing the limitations of traditional diagnostic methods (8). Additionally, the application of artificial intelligence (AI) and machine learning is gaining traction, with models being developed to analyze large-scale biomarker data and refine diagnostic algorithms (24). These innovations enable automated identification of tumor markers, improving diagnostic efficiency and reducing observer bias (33).
Immunohistochemistry (IHC) techniques continue to be widely used for improving diagnostic specificity, with recent advances focusing on novel markers like Claudin-18 and Ki-67 to enhance prognostic accuracy across histological subtypes (9, 13, 14). The integration of IHC with digital pathology and imaging-based analysis is enhancing precision in GC subtype differentiation (31).
4.4 Productive journals and collaborative networks
The most impactful articles have been published within prestigious journals like the BMC Cancer, Gastric Cancer, and Scientific Reports, serving as an important source for the diffusion of research output. The growing number of international research collaborations further strengthens knowledge exchange, fostering innovation and accelerating the translation of scientific discoveries into clinical practice. Finally, the collaborative networks, as depicted by the bibliometric maps, are very significant in terms of institutions and countries’ partnerships for knowledge flow and creation (32–37).
4.5 Strong and weak points of the analysis
This bibliometric analysis provides an extensive quantitative overview of the field, taking into consideration influential researchers, institutions, and tendencies.
However, certain limitations should be acknowledged. The limitations the exclusive focus on English-language publications and the Scopus database, which may exclude significant non-English studies or those indexed elsewhere. The five-year temporal scope, while focused, may miss longer-term trends or nascent fields. Despite these constraints, this study provides critical insights into the current landscape of GC biomarker research, highlighting key advancements and areas for further exploration.
4.6 Clinical and research implications
The findings emphasize the potential of tumor markers to revolutionize gastric cancer management. Clinically, novel biomarkers promise improved early detection, addressing the challenge of identifying small lesions at treatable stages (8, 12). Personalized Treatment Strategies, driven by molecular insights, are poised to enhance therapeutic efficacy, particularly through targeted therapies and immunotherapies (22–26). From a research perspective, strengthening global collaborations can accelerate innovation and address disparities in research funding and access to advanced diagnostics.
5 Conclusion
This bibliometric analysis demonstrates the significant progress made in the application of tumor markers for gastric cancer diagnosis between 2019 and 2024. Key institutions and researchers are driving innovation, and the global collaborative networks revealed through this analysis highlight the importance of international cooperation. Future research should continue to explore novel biomarkers and diagnostic techniques, with the aim of improving early detection and personalized treatment strategies for gastric cancer.
Data availability statement
The datasets presented in this article are not readily available because this bibliometric analysis aimed to analyze the application of tumor markers in stomach cancer diagnosis from 2019 to 2024. The eligibility criteria included original research articles and reviews published in peer-reviewed journals, with only English-language articles considered. Data were collected from major citation databases, Scopus, which were chosen due to comprehensive coverage of biomedical research. The search was conducted in November 2024. Requests to access the datasets should be directed to Zhanat Komekbay, WmhhbmF0LnJ1QGluYm94LnJ1.
Author contributions
ZK: Conceptualization, Data curation, Methodology, Supervision, Writing – original draft, Writing – review & editing. RS: Conceptualization, Formal analysis, Writing – review & editing. GY: Data curation, Methodology, Visualization, Writing – original draft. AG: Conceptualization, Data curation, Methodology, Visualization, Writing – review & editing. AT: Conceptualization, Project administration, Supervision, Writing – review & editing. NK: Conceptualization, Investigation, Project administration, Supervision, Writing – review & editing. SA: Data curation, Methodology, Resources, Writing – review & editing. AK: Data curation, Formal analysis, Resources, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The research materials were conducted as part of the scientific project IRN AR23490776, “Prognostic value of gastric cancer biomarkers in relation to Lauren’s classification,” which was funded by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Jokhadze, N, Das, A, and Dizon, DS. Global cancer statistics: a healthy population relies on population health. CA Cancer J Clin. (2024) 74:224–6. doi: 10.3322/caac.21838
2. Ferlay, J, Ervik, M, Lam, F, Laversanne, M, Colombet, M, Mery, L, et al. Global Cancer observatory: Cancer today (version 1.0). International Agency for Research on Cancer. (2024). Available at: https://gco.iarc.who.int/today (Accessed February 1, 2024).
3. Chen, S, Cao, Z, Prettner, K, Kuhn, M, Yang, J, Jiao, L, et al. Estimates and projections of the global economic cost of 29 cancers in 204 countries and territories from 2020 to 2050. JAMA Oncol. (2023) 9:465–72. doi: 10.1001/jamaoncol.2022.7826
4. Freitas, MB, Gullo, I, Leitão, D, Águas, L, Oliveira, C, Polónia, A, et al. HER2 and PD-L1 expression in gastric and gastroesophageal junction Cancer: insights for combinatorial targeting approaches. Cancers. (2024) 16:1227. doi: 10.3390/cancers16061227
5. Qiuying, Q, Lingchuan, G, Lili, H, Zhiju, L, Tianwei, G, Yu, S, et al. Expression and clinical significance of PD-L1 and infiltrated immune cells in the gastric adenocarcinoma microenvironment. Medicine (Baltimore). (2023) 102:e36323. doi: 10.1097/MD.0000000000036323
6. Niloofar, N, Mehdi, M, Simin, A, Bahare, S, and Abbas, G. Expression of PD-1 in tumor cells is associated with shorter survival in non-metastatic intestinal-type gastric adenocarcinoma. Iran J Allergy Asthma Immunol. (2022) 21:600–15. doi: 10.18502/ijaai.v21i6.11519
7. Hui, L, Longsong, L, Nan, Z, Zixin, W, Ning, X, Enqiang, L, et al. Relationship between HER2 overexpression and long-term outcomes of early gastric cancer: a prospective observational study with a 6-year follow-up. BMC Gastroenterol. 22:238. doi: 10.1186/s12876-022-02309-7
8. Deng, S, Gu, J, Jiang, Z, Cao, Y, Mao, F, Xue, Y, et al. Application of nanotechnology in the early diagnosis and comprehensive treatment of gastrointestinal cancer. J Nanobiotechnol. (2022) 20:415. doi: 10.1186/s12951-022-01613-4
9. Xingbin, H, Wenhao, O, Haizhu, C, Zhihong, L, Zijia, L, and Herui, Y. Claudin-9 (CLDN9) promotes gastric cancer progression by enhancing the glycolysis pathway and facilitating PD-L1 lactylation to suppress CD8þ T cell anti-tumor immunity. Cancer Pathog Therapy. (2024). doi: 10.1016/j.cpt.2024.09.006
10. Min, H, Xianglan, Z, Minkyu, J, Jung, I, Park, HS, Beom, SH, et al. Immunohistochemistry biomarkers predict survival in stage II/III gastric Cancer patients: from a prospective clinical trial. Cancer Res Treat. (2019) 51:819–31. doi: 10.4143/crt.2018.331
11. Liu, F, Wu, X, Wang, W, and Chang, J. A novel immunohistochemical score predicts the postoperative prognosis of gastric cancer patients. World J Surg Oncol. (2023) 21:220. doi: 10.1186/s12957-023-03113-7
12. Necula, L, Matei, L, Dragu, D, Neagu, AI, Mambet, C, Nedeianu, S, et al. Recent advances in gastric cancer early diagnosis. World J Gastroenterol. (2019) 25:2029–44. doi: 10.3748/wjg.v25.i17.2029
13. Almabrouk, NM, MNE, E-M, Mohy Eldin Badr, A, Meckawy, GR, and Shakweer, MM. Prognostic utility of Ki-67 in gastric carcinoma. Immunopathol. Persa. (2022) 8:e14. doi: 10.34172/ipp.2022.14
14. Xuesong, Y, Yan, W, Anqiang, W, Ma, X, Zhou, K, Ji, K, et al. Immunohistochemical characteristics and potential therapeutic regimens of hepatoid adenocarcinoma of the stomach: a study of 139 cases. J Pathol Clin Res. (2024) 10:e343. doi: 10.1002/cjp2.343
15. Donthu, N, Kumar, S, Mukherjee, D, Pandey, N, and Lim, WM. How to conduct abibliometric analysis: an overview and guidelines. J Bus Res. (2021) 133:285–96. doi: 10.1016/j.jbusres.2021.04.070
16. Meng, X, Zheng, Y, Wang, PP, Zhao, XK, Song, X, Xu, RH, et al. Novel metabolic biomarker for early detection and diagnosis to the patients with gastric cardia adenocarcinoma. Cancer Med. (2024) 13:e7015. doi: 10.1002/cam4.7015
17. Kim, I-H. Emerging targets for systemic treatment of gastric cancer: HER2 and beyond. J Gastric Cancer. (2024) 24:29–56. doi: 10.5230/jgc.2024.24.e6
18. Xuesong, Y, Yan, W, Anqiang, W, Xiaoyi, C, Yanyan, L, Jialin, L, et al. Tracking circulating PD-L1-positive cells to monitor the outcome of patients with gastric cancer receiving anti-HER2 plus anti-PD-1 therapy. Hum Cell. (2024) 37:258–70. doi: 10.1007/s13577-023-00990-8
19. Kyunghye, B, Jaekyung, C, Young, S, Kim, HD, Ryu, MH, Park, Y, et al. Association between HER2 heterogeneity and clinical outcomes of HER2-positive gastric cancer patients treated with trastuzumab. Gastric Cancer. (2022) 25:794–803. doi: 10.1007/s10120-022-01298-6
20. Euno, C, Mee Soo, C, Sun-ju, B, Jin, H, Jung, KC, Kim, H, et al. Prognostic perspectives of PD-L1 combined with tumor-infiltrating lymphocytes, Epstein-Barr virus, and microsatellite instability in gastric carcinomas. Diagn Pathol. (2020) 15:69. doi: 10.1186/s13000-020-00979-z
21. Dong, H, Go, E, Kwang, S, Ryuman, D, Song, KS, Kim, JS, et al. Clinical significance of tumor and immune cell PD-L1 expression in gastric adenocarcinoma. In Vivo. (2020) 34:3171–80. doi: 10.21873/invivo.12152
22. Yu-Ting, S, Shi-Xun, L, Ming-Yu, L, Yang, X, Guan, WL, Yang, LQ, et al. Clinical outcomes and biomarker exploration of first-line PD-1 inhibitors plus chemotherapy in patients with low PD-L1-expressing of gastric or gastroesophageal junction adenocarcinoma. Cancer Immunol Immunother. (2024) 73:144. doi: 10.1007/s00262-024-03721-6
23. Chenchen, W, Ying, C, Ru, Z, Yanan, Y, and Yantian, F. Systematic analysis of tumor stem cell-related gene characteristics to predict the PD-L1 immunotherapy and prognosis of gastric Cancer. Curr Med Chem. (2024) 31:2467–82. doi: 10.2174/0109298673278775231101064235
24. Qiuyu, J, Zhixue, C, Fansheng, M, Zhang, H, Chen, H, Xue, J, et al. CD36-BATF2\MYB Axis predicts anti-PD-1 immunotherapy response in gastric Cancer. Int J Biol Sci. (2023) 19:4476–92. doi: 10.7150/ijbs.87635
25. Hong Jian, ZH, Huang, C, Mei, L, Mingqing, ZH, and Chunjin, H. Dynamic detection of HER2 of circulating tumor cells in patients with gastric carcinoma and its clinical application. Mol Med Rep. (2022) 25:187. doi: 10.3892/mmr.2022.12703
26. Ji-Bin, L, Ming-Yu, L, Zhuo-Chen, L, Guan, WL, Sun, YT, Yang, J, et al. The optimal threshold of PD-L1 combined positive score to predict the benefit of PD-1 antibody plus chemotherapy for patients with HER2-negative gastric adenocarcinoma: a meta-analysis. Cancer Immunol Immunother. (2024) 73:132. doi: 10.1007/s00262-024-03726-1
27. Huan, X, Zou, K, Zhang, P, Ding, H, Luo, C, Xiang, C, et al. Neoadjuvant chemotherapy is linked to an amended anti-tumorigenic microenvironment in gastric cancer. Int Immunopharmacol. (2024) 127:111352. doi: 10.1016/j.intimp.2023.111352
28. Xu, S, Chen, W, Wang, Y, Zhang, Y, Xia, R, Shen, J, et al. N6-methyladenosine-related lnc RNAs identified as potential biomarkers for predicting the overall survival of Asian gastric cancer patients. BMC Cancer. (2022) 22:721. doi: 10.1186/s12885-022-09801-z
29. Zhao, W, Zhang, Y, Zhang, W, Sun, Y, Zheng, B, Wang, J, et al. Exosomal LINC00355 promotes the malig nant progression of gastric cancer through histone deacetylase HDAC3-mediated TP53INP1 transcriptional inhibition. Life Sci. (2023) 315:121387. doi: 10.1016/j.lfs.2023.121387
30. Chen, X, Luo, Q, Xiao, Y, Zhu, J, Zhang, Y, Ding, J, et al. LINC00467: an oncogenic long noncoding RNA. Cancer Cell Int. (2022) 22:303. doi: 10.1186/s12935-022-02733-5
31. Huang, Z, Li, Y, Qian, Y, Zhai, E, Zhao, Z, Zhang, T, et al. Tumor-secreted LCN2 impairs gastric cancer progression via autocrine inhibition of the 24p3R/JNK/c-Jun/SPARC axis. Cell Death Dis. (2024) 15:756. doi: 10.1038/s41419-024-07153-z
32. Yusuke, G, Yuka, N, Yusuke, O, Takahiro, O, Keiichiro, H, Kenji, S, et al. Prognostic significance of low HER2 expression in gastric cancer: a retrospective, single-center analysis. BMC Cancer. (2024) 24:1081. doi: 10.1186/s12885-024-12749-x
33. Toshiaki, M, Shinji, K, and Nobuhiko, K. PD-L1 expression combined with microsatellite instability/CD8+ tumor infiltrating lymphocytes as a useful prognostic biomarker in gastric cancer. Sci Rep. (2019) 9:4633. doi: 10.1038/s41598-019-41177-2
34. Gang, N, Qihui, Z, Wonyoung, K, Hamin, L, Leigh, M, Yun-S, MM, et al. A novel treatment strategy for lapatinib resistance in a subset of HER2-amplified gastric cancer. Ning et al. BMC Cancer. (2021) 21:923. doi: 10.1186/s12885-021-08283-9
35. Shuangfen, T, Jianchun, G, Qing, W, and Leizhen, Z. Translational control of Bcl-2 promotes apoptosis of gastric carcinoma cells. BMC Cancer. (2021) 21:12. doi: 10.1186/s12885-020-07711-6
36. Haruki, O, Hiroyuki, A, Koichi, Y, Yasuyuki, S, and Ushiku, T. Claudin-18 status and its correlation with HER2 and PD-L1 expression in gastric cancer with peritoneal dissemination. Gastric Cancer. (2024) 27:802–10. doi: 10.1007/s10120-024-01505-6
Keywords: tumor marker, gastric cancer, prognosis, immunohistochemistry, diagnosis
Citation: Komekbay Z, Shirazi R, Yessultanova G, Garifollin A, Tulyayeva A, Kereyeva N, Akhmetova S and Kaliev A (2025) Bibliometric analysis of tumor marker application in gastric cancer diagnosis from 2019 to 2024. Front. Med. 12:1547850. doi: 10.3389/fmed.2025.1547850
Edited by:
Alessandro Mangogna, University of Udine, ItalyReviewed by:
Chao Yang, Wuhan University, ChinaJIanchun Yu, Peking Union Medical College Hospital (CAMS), China
Zehua Zhang, Tongji University, China
Copyright © 2025 Komekbay, Shirazi, Yessultanova, Garifollin, Tulyayeva, Kereyeva, Akhmetova and Kaliev. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhanat Komekbay, emhhbmF0LnJ1QGluYm94LnJ1
 Zhanat Komekbay
Zhanat Komekbay Reza Shirazi
Reza Shirazi Gulnaz Yessultanova
Gulnaz Yessultanova Alisher Garifollin
Alisher Garifollin Anar Tulyayeva
Anar Tulyayeva Nurgul Kereyeva
Nurgul Kereyeva Saule Akhmetova
Saule Akhmetova Abdiraman Kaliev
Abdiraman Kaliev