- 1School of Medicine, Royal College of Surgeons in Ireland - Medical University of Bahrain, Busaiteen, Bahrain
- 2Research Department, Royal College of Surgeons in Ireland - Medical University of Bahrain, Busaiteen, Bahrain
Septic arthritis (SA) is an orthopedic emergency characterized by joint inflammation secondary to infectious etiologies, most commonly Staphylococcus aureus. The prompt recognition of SA is crucial due to its significant morbidity and mortality. Fever, along with a swollen, painful joint and limited range of motion, are typical manifestations; however, presentations can vary. The incidence of SA in adult populations is rising, accompanied by unfavorable mortality rates. This trend is further exacerbated by comorbid conditions that substantially influence outcomes. Among the literature, diabetes mellitus (DM), rheumatoid arthritis (RA), and chronic kidney disease (CKD) have emerged as key prognostic factors in SA. DM exacerbates the severity of SA through impairment of polymorphonuclear function, ultimately leading to increased susceptibility to infection and a higher risk of acquiring infection from atypical pathogens. CKD causes uremia-induced immune dysfunction leading to an immunocompromised state as well as repeated vascular access increasing infection susceptibility, leading to increased mortality. Patients with RA harbor an elevated risk of SA, attributed to immune dysregulation, immunosuppressive therapy, and diagnostic challenges. Additionally, these comorbidities can complicate the surgical management of SA and increase the likelihood of treatment failure. Therefore, given the rising burden of comorbid conditions worldwide and their impact on SA prognosis, healthcare professionals should remain vigilant when managing these factors. A holistic, multidisciplinary approach to management is vital to ensure that SA patients with these certain comorbid conditions experience fewer complications and improved survival. This mini-review aims to highlight the key comorbid conditions that impact the prognosis of SA patients.
Introduction
Septic arthritis (SA), also known as infectious arthritis, is an inflammation of joint(s) secondary to an infectious etiology and is regarded as an orthopedic emergency (1, 2). In the United States, between 4 and 10 individuals per 100,000 are affected annually (2). Infectious agents are often bacterial, but can also involve viral, fungal, and mycobacterial pathogens (3). The most common organism to cause SA in roughly 37%–56% of cases is Staphylococcus aureus (4–6). SA typically presents as a single-joint infection; however, multiple joints can be affected in roughly 20% of cases, termed polyarticular septic arthritis (PASA), especially among patients who are immunocompromised or have severe sepsis, rheumatoid arthritis (RA), or multiple comorbid conditions (4, 7). PASA is further complicated by a higher mortality rate, higher treatment failure and diagnostic challenges due to the overlapping clinical features seen with other arthritic presentation such as RA (8–11).
In adults with SA, the knee joint is most often affected, comprising over 50% of cases whereas, in children, the hip joint is most affected. Other joints, such as the shoulder and elbow, are less frequently affected and can be more severe due to a delay in diagnosis (4, 7, 12, 13). This is more likely to occur in immunocompromised individuals, such as elderly patients, those with diabetes, intravenous drug users and those who are immunosuppressed.
Clinically, SA presents with an acute onset of fever and a warm, painful, swollen joint exhibiting a limited range of motion; however, presentation can vary between patients (7, 14). A systematic review involving 14 studies, revealed that in more than 50% of patients, the symptoms of joint pain, joint swelling, and fever occur with sensitivities of 85, 78, and 57% respectively (7, 15).
The gold standard used for diagnosis of SA involves bacterial isolation from the synovial fluid, with synovial culture being the most important test in all those with synovial fluid aspiration (7, 16). Additionally, real-time polymerase chain reaction (PCR) targeting the 16s rRNA gene has emerged as a potential modality in the diagnosis of bacterial SA. In comparison to results of synovial fluid culture, PCR harbors a sensitivity of 95% and a specificity of 97% (17). However, its definitive role in clinical performance is limited by its diagnostic performance, time to results, and inability to provide information on antibiotic sensitivity (18, 19). The management of SA is often aimed at reducing morbidity and mortality through prompt diagnosis and treatment, which often entails the use of broad-spectrum antibiotics until culture results are available, with organism identification to later narrow the choice of antibiotic (7, 14). Furthermore, joint drainage should be employed in the form of closed or open arthrotomy (3).
The rate of mortality associated with SA is significant; one national cohort study from Taiwan involving 31,491 patients found mortality rates of 4.3% at 30 days, 8.6% at 90 days, and 16.4% at 1 year (20). By comparison, a study from Denmark reported a 30-day mortality rate of 9.3% (21). Furthermore, in adult populations, the incidence of SA appears to be increasing and can be attributed to older age, increased use of immunosuppressants, underlying joint diseases and a rise in medical comorbidities (6, 22–25).
Certain prognostic factors, such as advanced age or the presence of certain comorbidities such as RA and diabetes mellitus (DM), significantly worsen outcomes. Therefore, the exploration of these factors is vital for the treatment and identification of SA (26–28).
A dramatic increase in 90-day mortality was found in one cohort study in patients who were 80 years and older (22%–69% compared to 7% in patients <80 years) (26, 28). Another cohort study identified a significant association between mortality and the presence of certain comorbidities such as DM, RA, bacteremia and low creatinine clearance, among others (27, 28). Furthermore, a systematic review of over 126 peer-reviewed studies in the adult population in community settings revealed a rise in multimorbidity, which is defined as having 2 or more chronic conditions simultaneously (29). The worldwide prevalence of multimorbidity is 37.2%, rising to a prevalence of 51% in those aged >60 (29, 30). Other systematic reviews based on adults in the community and in a healthcare-based setting report a prevalence of 42.4% (29, 31).
Therefore, these findings underscore the fact that comorbidities play a pivotal role in determining the prognosis of patients with SA. As the global burden of comorbid conditions continues to rise, the need to understand their underlying pathogenesis and impact on SA prognosis will only intensify. This mini-review therefore aims to highlight key comorbidities that play a significant role in the clinical trajectory of patients with SA, emphasizing the underlying pathogenesis and its clinical implications for patient care.
Methods
Search strategy
A literature search was conducted between December 2024 and January 2025 utilizing PubMed and Google Scholar. PubMed was used to retrieve peer-reviewed articles from indexed sources including MEDLINE. Google Scholar was also used to identify other relevant peer-reviewed journal articles from indexed databases such as ScienceDirect. The search included keywords such as “rheumatoid arthritis,” “chronic kidney disease,” “diabetes mellitus,” “mortality,” and “prognosis,” in relation to “septic arthritis.” Only articles written in the English language were considered. Articles were selected based on relevance, with no limitation on publication type or date.
Diabetes mellitus
Diabetes mellitus is a chronic metabolic disorder characterized by hyperglycemia (32, 33). Type 1 DM results from the autoimmune destruction of pancreatic β cells, ultimately leading to insulin deficiency. By contrast, type 2 DM arises from the impaired ability of insulin-sensitive tissues to respond effectively to insulin, combined with impaired secretion of insulin by β cells in the pancreas (34–36). Given its global prevalence of approximately 537 million people, projected to rise to 783 million cases by 2045, it is an important disease to consider in terms of SA prognosis (37).
One study performed in Taiwan found that, among patients with Staphylococcus aureus SA, 41 (44.1%) had DM as the most common underlying disease (38). Of the five deaths reported, all occurred in immunocompromised patients with DM. Notably, DM was the only risk factor identified for mortality and was mentioned as the only significant poor prognostic factor (38). Similarly, a retrospective study in Qatar of patients aged 15 years or older with SA reported that DM was the most prevalent concomitant condition, present in 24 of the 56 patients (42.8%) (39). Another study in Paraguay investigating adult patients with SA reported that DM was again the most predominant comorbidity, affecting 63.6% of the 66 included cases (40).
One large prospective study determined that DM was an independent risk factor for the development of SA, among others (41). A retrospective study of 186 patients reported that DM carried 15.33 times higher odds of mortality compared to those without DM (42). However, another study involving 215 patients with SA concluded that DM was not a statistically significant risk factor for predicting 30-day mortality (21).
One mechanism through which DM can worsen the prognosis of SA is through its effect on the immune system by leading to an immunocompromised state. It is known that DM has a negative physiological impact on polymorphonuclear function, leading to increased susceptibility to infection (41). Notably, the rate of infection in those with type 2 DM was 47%–50% higher compared to the general population, with a positive correlation between infection rate and HbA1c (43–48). Previous studies have revealed that, in hyperglycemic conditions, neutrophil antibacterial activity, chemotaxis and adherence are impaired, while phagocytosis and bacterial killing mechanisms are also decreased (49–53). Attenuated neutrophil antibacterial activity can be explained by decreased ROS production, impaired superoxide generation, impaired neutrophil extracellular trap formation and degranulation, among others (54–58). Moreover, reduction in cytokine generation is also highlighted as a mechanism toward infection susceptibility, where decreased levels of type 1 interferon, interleukin (IL)-6, IL-2, and IL-10, among others, are impacted (58–60). Furthermore, monocyte respiratory burst activity was significantly reduced in individuals with blood glucose levels >11 mmol/L compared with those who had well-controlled DM or were healthy individuals (50, 61). Collectively, these processes lead DM patients to an immunocompromised state predisposing them to an increased infection risk. With regards to pathogen prevalence in DM patients, Staphylococcus aureus infection is typically more pronounced (21, 41). Furthermore, patients with DM are at increased risk for rarer, atypical organism infections in SA, including Burkholderia pseudomallei, Candida albicans and Aspergillus species, all of which can delay diagnosis and complicate treatment (62–68). DM predisposes patients to fungal infections through skin barrier integrity disruption and impaired antibody function (66, 69). Furthermore, microvascular and macrovascular complications in DM lead to a reduction of oxygen and blood supply, which negatively impacts the delivery of antibiotics and immune cells, amongst other factors (66, 69, 70). In addition, DM patients may be predisposed to rare joint involvement, such as the sternoclavicular joint, which is associated with complications and treatment challenges (71).
A study investigating 128 patients with acute SA undergoing surgical debridement identified that DM, among other risk factors, was an independent clinical predictor for single surgical debridement failure, with an odds ratio of 2.6 (72). However, conflicting evidence exists, where a recent systematic review of 30 studies comprising 8,586 native joint SA published in 2023 found that DM was not associated with surgical treatment failure (72–78). Importantly, patients with DM often require thorough preoperative evaluation and optimization to reduce perioperative morbidity and mortality. However, this crucial preparation may delay joint drainage, which may ultimately impact the prognosis (79).
Although effective management of these patients is paramount, the evidence regarding DM’s role in treatment failure is conflicting. Nevertheless, DM has a substantial impact on prognosis and disease management (Figure 1). Therefore, healthcare professionals should utilize a multidisciplinary approach aiming for meticulous glycemic control and consistent monitoring to ensure the best possible outcomes for these patients.
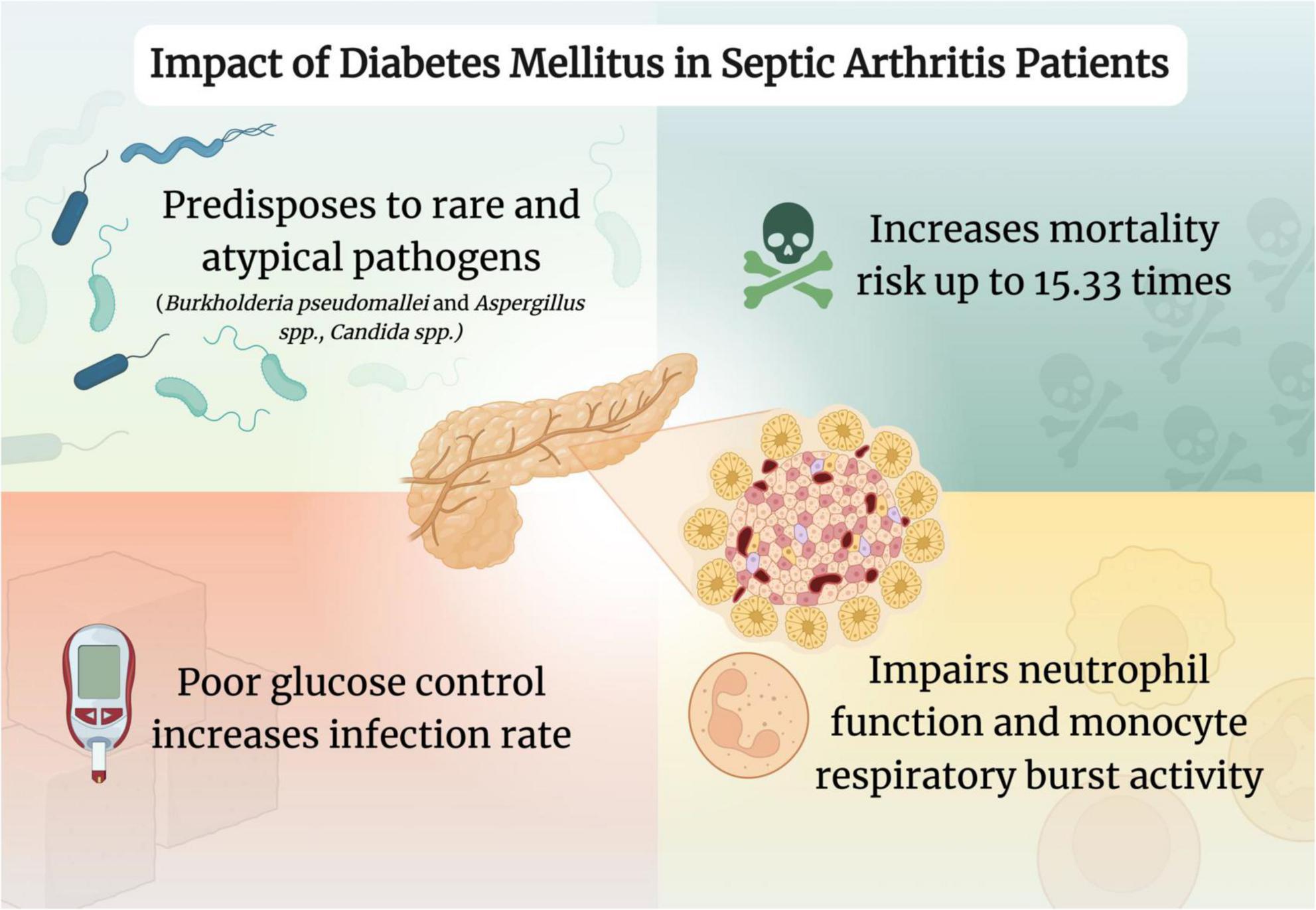
Figure 1. Impact of diabetes mellitus in septic arthritis patients. Created in www.biorender.com.
Chronic kidney disease
Chronic kidney disease (CKD) affects over 10% of the global population and is defined as decreased renal function as evidenced by a glomerular filtration rate of less than 60 mL/min per 1.73 m2 occurring for 3 months or more (80–82). In high-, middle-, and certain low-income countries, DM and hypertension are the primary causes of CKD (81, 83). Due to the progressive nature of CKD, patients ultimately require either dialysis or transplantation, both serving as forms of renal replacement therapy (82). In one study investigating end-stage renal disease (ESRD) patients, the incidence of SA was more than 50 times that of the general population (514.8 per 100,000 persons per year) (84). This finding was corroborated by other studies (85, 86). One proposed mechanism is uremia-induced immune dysfunction, which leads to a chronic immunocompromised state (84). In addition, the need for chronic vascular access increases susceptibility to infection (84, 87, 88).
Hematogenous spread, the commonest route of infection in SA occurs in over 70% of cases, thereby placing patients on hemodialysis at an increased risk (86, 89, 90). Furthermore, peritoneal dialysis may also be implicated (91). Due to frequent healthcare exposure, methicillin-resistant Staphylococcus aureus (MRSA) colonization is common among ESRD patients (92). One study determined that MRSA was implicated in 57.4% of SA cases in dialysis patients, with another reporting a rate of 58.3% in these patients (84, 93). The significance of this finding is immense as, in one study, those patients with native joint SA due to MRSA incurred a rate of treatment failure of 33.3% compared to those with methicillin-sensitive Staphylococcus aureus (MSSA) at 11.3% (94). Additionally, cases of MRSA SA were also associated with higher mortality, increased length of hospital stay and poorer clinical outcomes compared to MSSA SA (85, 86, 95–97). Moreover, common comorbid conditions in those with CKD, such as DM, further predispose to increased infection risk (98, 99).
In one study, hemodialysis-associated SA carried a 22% mortality rate, whereas another study reported a rate of only 7% (86, 100). The authors of the latter study owed these favorable outcomes to a multidisciplinary approach involving renal, orthopedic and infectious disease teams (86, 100). Therefore, this highlights the importance of coordinated care to improve prognosis in patients with ESRD with SA. Furthermore, dialysis access plays a pivotal role in prognosis. In a study by Yeh et al., the use of tunneled cuffed catheters was an independent predictor of positive blood cultures and in-hospital mortality (odds ratio 14.33) (93). Furthermore, elevated blood urea nitrogen (BUN) levels were associated with a higher likelihood of requiring a repeat washout procedure in one study (75). Additionally, chronic renal failure was strongly associated with mortality, with an odds ratio of 81.27 (42). Moreover, a Korean study that analyzed 89,120 hospitalizations for knee SA identified CKD as a risk factor for mortality (101).
Given the significant morbidity and mortality associated with SA in CKD patients, early recognition and a multidisciplinary approach are crucial for improving outcomes. Prioritizing infection prevention strategies, optimizing dialysis access and implementing prompt, focused management is essential toward improving the prognosis of SA in CKD patients.
Inflammatory arthropathy
Inflammatory arthropathies are a group of joint diseases marked by joint inflammation, with RA being the most common (102–104). Spondyloarthropathies, including psoriatic arthritis, reactive arthritis and ankylosing spondylitis, are less common (103). RA is a chronic inflammatory disorder primarily involving the synovial joints that usually presents with swelling, pain, stiffness of joints, fever and malaise (105). Furthermore, it can also present with extra-articular manifestations involving the kidney, lung, heart, eye, skin, gastrointestinal and nervous system (106–109). RA predominantly affects females in a 3:1 ratio to males, with a prevalence of around 0.5%–1% in the adult population (110).
Of note, studies demonstrate that up to 40% of patients with SA have RA (12, 111). Additionally, in those with RA, the risk of SA, irrespective of therapy, increases by four- to fifteen-fold (21, 41, 112–114). Patients with seropositive RA treated with tumor necrosis factor (TNF) inhibitors, regardless of type, had a notable increased incidence of SA after the first year post-treatment commencement (115). This finding was also reported by another study which showed that the risk of SA doubled in RA patients treated with anti-TNF therapy (112). Another study confirmed that those taking disease-modifying antirheumatic drugs (DMARDs) compared to those without had a significantly increased risk of SA (114). A recent systematic literature review published in 2022 highlighted the increased risk for serious infection that the usage of biologic DMARDs carry in comparison to conventional synthetic DMARDs (116). Additionally, a recent narrative review also shared the same findings in regards to biologic DMARDs and encourages shared decision-making between healthcare providers and patients in regards to their own comorbidites and medication selection (117). According to the British Society for Rheumatology (BSR), they recommend that in those with an active infection, biological agents should not be started, while those carrying a heightened infection risk should utilize these agents with caution (118). Subsequently, the BSR recommends utilizing etanercept or adalimumab as first choice agents among patients with high infection risk (118).
This increased risk can be explained by decreased bactericidal activity within the synovial fluid and impaired phagocytosis due to the underlying disease and/or medications (12, 42, 119, 120). A proposed explanation for the increased SA risk in RA patients is the use of immunosuppressive medications, such as DMARDs and TNF inhibitors, which may predispose these patients to infection (42, 111, 112, 114, 121). In fact, individuals with RA are twice as likely to develop confirmed infection compared to those without the condition while matching for age and sex (111, 113). However, studies also show that patients who have not yet received steroids remain at a higher risk of infection, further alluding to the fact that underlying immune dysfunction along with the concomitant joint damage may be greater risk factors than the immunomodulatory drugs employed (121–123).
The treatment of SA in RA patients may also be impacted, as a study performed by Hunter et al., revealed that single surgical debridement failure had the highest odds ratio of 7.3 in those with a history of inflammatory arthropathy (72). However, a systematic review by Walinga et al. determined that the evidence regarding inflammatory arthritis being a positive risk factor for surgical treatment failure rate was conflicting (72, 73, 75, 124).
Besides the increased susceptibility to infection, diagnosing SA in RA patients can prove to ber challenging. A hot, inflamed joint could be mistaken for an RA flare-up rather than the development of SA, hence delaying the emergent diagnosis of SA (111, 114). A study by Favero et al., found that the time of onset on average was 25.07 ± 2.40 days in those with RA, compared to 14.30 ± 32.47 days in those without pre-existing joint disease (111). Another hindrance to early diagnosis is that RA patients often present late with normal white cell count and temperature (114). These factors all lead to a suboptimal prognosis. In terms of mortality prediction, one study concluded that RA was not a statistically significant predictor of 30-day mortality (21).
RA remains a critical factor in determining the prognosis in patients with SA (Figure 2). The prompt detection of SA in those with RA, alongside proper management, can ensure favorable outcomes in those with increased risk. Further evidence can help determine the best course of action to optimize the prognosis of SA in these patients.
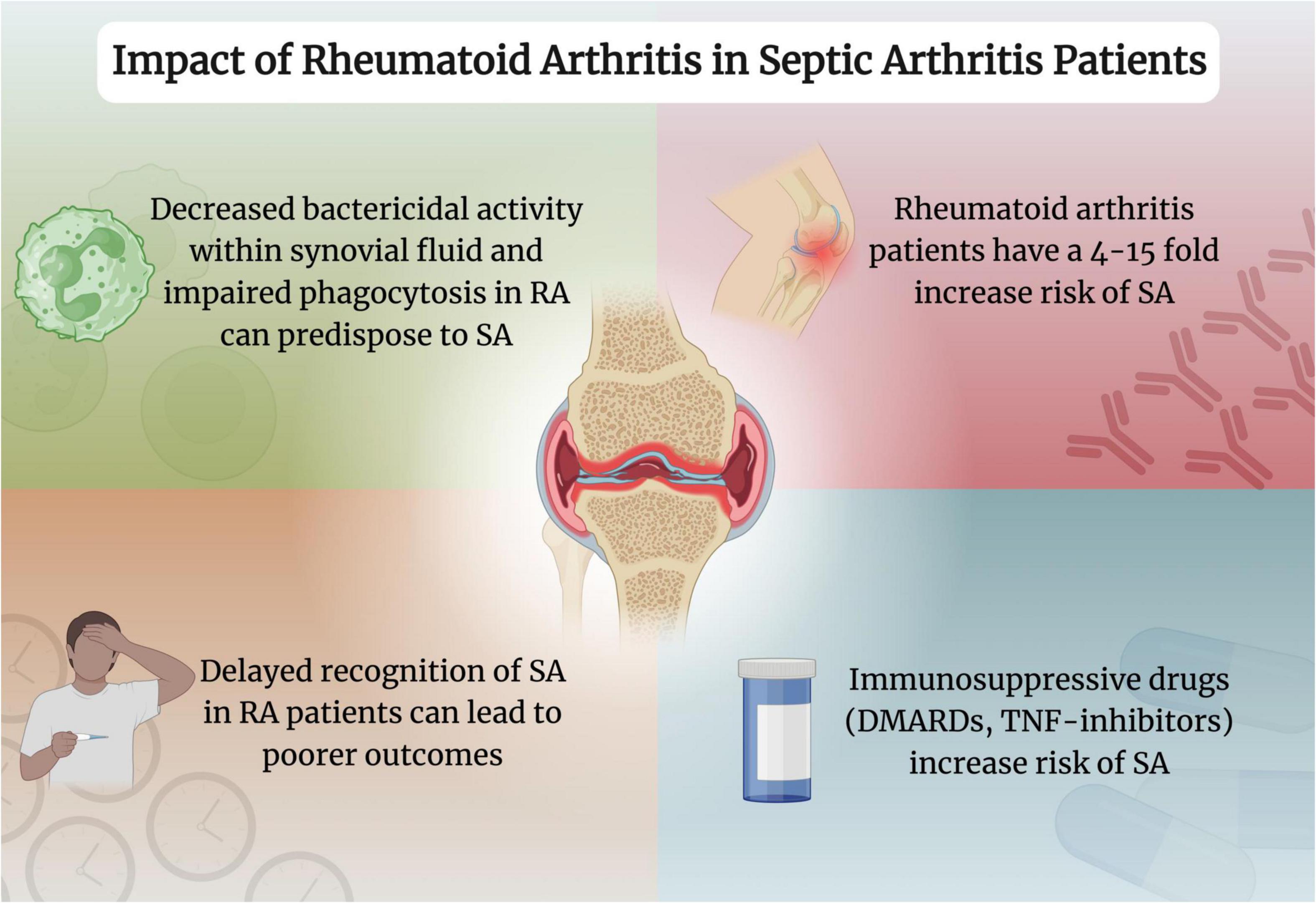
Figure 2. Impact of rheumatoid arthritis in septic arthritis patients. Created with www.biorender.com. RA, rheumatoid arthritis; SA, septic arthritis; DMARDs, disease-modifying antirheumatic drugs; TNF, tumor necrosis factor.
Cirrhosis
Cirrhosis, a chronic condition, is characterized by fibrosis and nodule formation in the liver as a result of chronic injury (125, 126). Some etiologies include non-alcoholic fatty liver disease, excessive alcohol consumption, hepatitis B or C infection, autoimmune and cholestatic diseases (127). Clinically, patients with cirrhosis may present with jaundice, ascites, hepatomegaly, spider nevi or encephalopathy (126). A study conducted in Taiwan aimed to analyze the occurrence of native SA in non-cirrhotic and cirrhotic patients, revealing that cirrhotic patients possessed a significantly higher risk of developing native SA compared to non-cirrhotic patients (128). Furthermore, patients with complicated cirrhosis (defined as those with refractory ascites, episodes of esophageal/gastric variceal bleeding, or hepatic encephalopathy episodes) were more susceptible to developing native SA compared to the patients with non-complicated cirrhosis (128). This may be attributed to the impaired phagocytic function of neutrophils and the innate immune system (128, 129). As such, cirrhosis represents an important comorbidity that can significantly affect the prognosis of SA and should be diligently considered in its management.
Limitations
One limitation of our review is the relatively short search window conducted between December 2024 and January 2025. This narrow time frame could have potentially excluded newly published studies.
Conclusion
Septic arthritis, an inflammation of one or more joints secondary to an infectious cause, remains a critical orthopedic emergency, particularly plaguing those with certain comorbidities. Studies indicate that CKD, DM and RA not only increase the risk of acquiring SA but also lead to poorer clinical prognosis with increased mortality or failure of treatment. The exploration of the complex interplay between these comorbidities and SA allows a deeper understanding for healthcare professionals to refine management strategies. Proactive assessment and management of these comorbidities is critical to minimize their negative implications on SA prognosis. A multidisciplinary approach involving a wide range of specialties including, but not limited to rheumatologists, nephrologists, orthopedics and infectious disease specialists, alongside prompt detection and timely treatment is imperative for an optimal patient prognosis. Further research is needed to determine the effective therapeutic regimens that are optimal in managing the comorbid conditions and preventing both the onset and progression of SA.
Author contributions
AiK: Conceptualization, Visualization, Writing – original draft, Writing – review and editing. AK: Visualization, Writing – original draft, Writing – review and editing. AB: Supervision, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Colavite P, Sartori A. Septic arthritis: immunopathogenesis, experimental models and therapy. J Venom Anim Toxins Incl Trop Dis. (2014) 20:19. doi: 10.1186/1678-9199-20-19
2. He M, Arthur Vithran D, Pan L, Zeng H, Yang G, Lu B, et al. An update on recent progress of the epidemiology, etiology, diagnosis, and treatment of acute septic arthritis: a review. Front Cell Infect Microbiol. (2023) 13:1193645. doi: 10.3389/fcimb.2023.1193645
3. Momodu I, Savaliya V. Septic Arthritis. StatPearls. Treasure Island (FL): StatPearls Publishing (2024).
4. Hassan A, Rao A, Manadan A, Block J. Peripheral bacterial septic arthritis: review of diagnosis and management. J Clin Rheumatol. (2017) 23:435–42. doi: 10.1097/RHU.0000000000000588
6. Mathews C, Coakley G. Septic arthritis: current diagnostic and therapeutic algorithm. Curr Opin Rheumatol. (2008) 20:457–62. doi: 10.1097/BOR.0b013e3283036975
7. Long B, Koyfman A, Gottlieb M. Evaluation and management of septic arthritis and its mimics in the emergency department. West J Emerg Med. (2019) 20:331–41. doi: 10.5811/westjem.2018.10.40974
8. Miller A, Abduljabbar F, Jarzem P. Polyarticular septic arthritis in an immunocompetent adult: a case report and review of the literature. Case Rep Orthop. (2015) 2015:602137. doi: 10.1155/2015/602137
9. Katz A, Marte D, Charles K. Polyarticular septic arthritis secondary to septic thrombophlebitis of the brachiocephalic vein: a rare case report. J Emerg Med. (2022) 63:414–6. doi: 10.1016/j.jemermed.2022.06.010
10. Lieber S, Fowler M, Zhu C, Moore A, Shmerling R, Paz Z. Clinical characteristics and outcomes in polyarticular septic arthritis. Joint Bone Spine. (2018) 85:469–73. doi: 10.1016/j.jbspin.2017.09.001
11. Christodoulou C, Gordon P, Coakley G. Polyarticular septic arthritis. BMJ. (2006) 333:1107–8. doi: 10.1136/bmj.39020.401331.68
12. Goldenberg D. Septic arthritis and other infections of rheumatologic significance. Rheum Dis Clin North Am. (1991) 17:149–56.
13. Tarkowski A. Infection and musculoskeletal conditions: infectious arthritis. Best Pract Res Clin Rheumatol. (2006) 20:1029–44. doi: 10.1016/j.berh.2006.08.001
14. Ross J. Septic arthritis of native joints. Infect Dis Clin North Am. (2017) 31:203–18. doi: 10.1016/j.idc.2017.01.001
15. Margaretten M, Kohlwes J, Moore D, Bent S. Does this adult patient have septic arthritis? JAMA. (2007) 297:1478–88. doi: 10.1001/jama.297.13.1478
16. Fowler M, Zhu C, Byrne K, Lieber S, Moore A, Shmerling R, et al. Pathogen or contaminant? Distinguishing true infection from synovial fluid culture contamination in patients with suspected septic arthritis. Infection. (2017) 45:825–30. doi: 10.1007/s15010-017-1051-y
17. Yang S, Ramachandran P, Hardick A, Hsieh Y, Quianzon C, Kuroki M, et al. Rapid PCR-based diagnosis of septic arthritis by early Gram-type classification and pathogen identification. J Clin Microbiol. (2008) 46:1386–90. doi: 10.1128/JCM.02305-07
18. Coiffier G, David C, Gauthier P, Le Bars H, Guggenbuhl P, Jolivet-Gougeon A, et al. Broad-range 16 s rDNA PCR in synovial fluid does not improve the diagnostic performance of septic arthritis in native joints in adults: cross-sectional single-center study in 95 patients. Clin Rheumatol. (2019) 38:1985–92. doi: 10.1007/s10067-019-04492-7
19. Carter K, Doern C, Jo C, Copley L. The clinical usefulness of polymerase chain reaction as a supplemental diagnostic tool in the evaluation and the treatment of children with septic arthritis. J Pediatr Orthop. (2016) 36:167–72. doi: 10.1097/BPO.0000000000000411
20. Huang Y, Ho C, Lin Y, Chen H, Liu S, Wang C, et al. Site-specific mortality in native joint septic arthritis: a national population study. Rheumatology (Oxford). (2020) 59:3826–33. doi: 10.1093/rheumatology/keaa162
21. Andreasen R, Andersen N, Just S, Christensen R, Hansen I. Prognostic factors associated with mortality in patients with septic arthritis: a descriptive cohort study. Scand J Rheumatol. (2017) 46:27–32. doi: 10.3109/03009742.2016.1164241
22. Alexandersson H, Dehlin M, Jin T. Increased incidence and clinical features of septic arthritis in patients aged 80 years and above: a comparative analysis with younger cohorts. Pathogens. (2024) 13:891. doi: 10.3390/pathogens13100891
23. Rutherford A, Subesinghe S, Bharucha T, Ibrahim F, Kleymann A, Galloway JB. A population study of the reported incidence of native joint septic arthritis in the United Kingdom between 1998 and 2013. Rheumatology (Oxford). (2016) 55:2176–80. doi: 10.1093/rheumatology/kew323
24. García-Arias M, Balsa A, Mola E. Septic arthritis. Best Pract Res Clin Rheumatol. (2011) 25:407–21. doi: 10.1016/j.berh.2011.02.001
25. Kennedy N, Chambers S, Nolan I, Gallagher K, Werno A, Browne M, et al. Native joint septic arthritis: epidemiology, clinical features, and microbiological causes in a New Zealand population. J Rheumatol. (2015) 42:2392–7. doi: 10.3899/jrheum.150434
26. Abram S, Alvand A, Judge A, Beard D, Price A. Mortality and adverse joint outcomes following septic arthritis of the native knee: a longitudinal cohort study of patients receiving arthroscopic washout. Lancet Infect Dis. (2020) 20:341–9. doi: 10.1016/S1473-3099(19)30419-0
27. Ferrand J, El Samad Y, Brunschweiler B, Grados F, Dehamchia-Rehailia N, Séjourne A, et al. Morbimortality in adult patients with septic arthritis: a three-year hospital-based study. BMC Infect Dis. (2016) 16:239. doi: 10.1186/s12879-016-1540-0
28. Earwood, J, Walker T, Sue G. Septic arthritis: diagnosis and treatment. Am Fam Physician. (2021) 104:589–97.
29. Chowdhury S, Chandra Das D, Sunna T, Beyene J, Hossain A. Global and regional prevalence of multimorbidity in the adult population in community settings: a systematic review and meta-analysis. EClinicalMedicine. (2023) 57:101860. doi: 10.1016/j.eclinm.2023.101860
30. Moffat K, Mercer S. Challenges of managing people with multimorbidity in today’s healthcare systems. BMC Fam Pract. (2015) 16:129. doi: 10.1186/s12875-015-0344-4
31. Ho I, Azcoaga-Lorenzo A, Akbari A, Davies J, Hodgins P, Khunti K, et al. Variation in the estimated prevalence of multimorbidity: systematic review and meta-analysis of 193 international studies. BMJ Open. (2022) 12:e057017. doi: 10.1136/bmjopen-2021-057017
32. Banday M, Sameer A, Nissar S. Pathophysiology of diabetes: an overview. Avicenna J Med. (2020) 10:174–88. doi: 10.4103/ajm.ajm_53_20
33. Goyal R, Singhal M, Jialal I. Type 2 diabetes. StatPearls. Treasure Island, FL: StatPearls Publishing (2025).
34. Syed F. Type 1 diabetes mellitus. Ann Intern Med. (2022) 175:ITC33–48. doi: 10.7326/AITC202203150
35. Lucier J, Mathias P. Type 1 diabetes. StatPearls. Treasure Island, FL: StatPearls Publishing (2025).
36. Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe K, et al. Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci. (2020) 21:6275. doi: 10.3390/ijms21176275
37. Hossain M, Al-Mamun M, Islam M. Diabetes mellitus, the fastest growing global public health concern: early detection should be focused. Health Sci Rep. (2024) 7:e2004. doi: 10.1002/hsr2.2004
38. Lin W, Wu C, Cheng S, Chiu C, Tseng C, Chan H, et al. High prevalence of methicillin-resistant staphylococcus aureus among patients with septic arthritis caused by Staphylococcus aureus. PLoS One. (2015) 10:e0127150. doi: 10.1371/journal.pone.0127150
39. Khan F, Abu-Khattab M, Baagar K, Mohamed S, Elgendy I, Anand D, et al. Characteristics of patients with definite septic arthritis at Hamad General Hospital, Qatar: a hospital-based study from 2006 to 2011. Clin Rheumatol. (2013) 32:969–73. doi: 10.1007/s10067-013-2211-9
40. Real Delor R, Martínez Giménez E. Clinical characteristics of septic arthritis in adult patients hospitalized at the Hospital Nacional between 2015 and 2019. Rev Nac Itauguá. (2020) 12:14–27. doi: 10.18004/rdn2020.0012.01.014-027
41. Kaandorp C, Van Schaardenburg D, Krijnen P, Habbema J, van de Laar M. Risk factors for septic arthritis in patients with joint disease. A prospective study. Arthritis Rheum. (1995) 38:1819–25. doi: 10.1002/art.1780381215
42. Maneiro J, Souto A, Cervantes E, Mera A, Carmona L, Gomez-Reino J. Predictors of treatment failure and mortality in native septic arthritis. Clin Rheumatol. (2015) 34:1961–7. doi: 10.1007/s10067-014-2844-3
43. Carey I, Critchley J, DeWilde S, Harris T, Hosking F, Cook D. Risk of infection in type 1 and type 2 diabetes compared with the general population: a matched cohort study. Diabetes Care. (2018) 41:513–21. doi: 10.2337/dc17-2131
44. Benfield T, Jensen J, Nordestgaard B. Influence of diabetes and hyperglycaemia on infectious disease hospitalisation and outcome. Diabetologia. (2007) 50:549–54. doi: 10.1007/s00125-006-0570-3
45. Hine J, de Lusignan S, Burleigh D, Pathirannehelage S, McGovern A, Gatenby P, et al. Association between glycaemic control and common infections in people with Type 2 diabetes: a cohort study. Diabet Med. (2017) 34:551–7. doi: 10.1111/dme.13205
46. Balintescu A, Lind M, Franko M, Oldner A, Cronhjort M, Svensson A, et al. Glycemic control and risk of sepsis and subsequent mortality in type 2 diabetes. Diabetes Care. (2022) 45:127–33. doi: 10.2337/dc21-1050
47. Critchley J, Carey I, Harris T, DeWilde S, Hosking F, Cook D. Glycemic control and risk of infections among people with type 1 or type 2 diabetes in a large primary care cohort study. Diabetes Care. (2018) 41:2127–35. doi: 10.2337/dc18-0287
48. Zhou Z, Wang H, Tan S, Zhang H, Zhu Y. The alterations of innate immunity and enhanced severity of infections in diabetes mellitus. Immunology. (2024) 171:313–23. doi: 10.1111/imm.13706
49. Delamaire M, Maugendre D, Moreno M, Le Goff M, Allannic H, Genetet B. Impaired leucocyte functions in diabetic patients. Diabet Med. (1997) 14:29–34. doi: 10.1002/(SICI)1096-9136(199701)14:1<29::AID-DIA300>3.0.CO;2-V
50. Knapp S. Diabetes and infection: is there a link? – A mini-review. Gerontology. (2013) 59:99–104. doi: 10.1159/000345107
51. Joshi N, Caputo G, Weitekamp M, Karchmer A. Infections in patients with diabetes mellitus. N Engl J Med. (1999) 341:1906–12. doi: 10.1056/NEJM199912163412507
52. Valerius N, Eff C, Hansen N, Karle H, Nerup J, Søeberg B, et al. Neutrophil and lymphocyte function in patients with diabetes mellitus. Acta Med Scand. (1982) 211:463–7. doi: 10.1111/j.0954-6820.1982.tb01983.x
53. Gallacher S, Thomson G, Fraser W, Fisher B, Gemmell C, MacCuish A. Neutrophil bactericidal function in diabetes mellitus: evidence for association with blood glucose control. Diabet Med. (1995) 12:916–20. doi: 10.1111/j.1464-5491.1995.tb00396.x
54. Chao W, Yen C, Wu Y, Chen S, Hsieh C, Chang T, et al. Increased resistin may suppress reactive oxygen species production and inflammasome activation in type 2 diabetic patients with pulmonary tuberculosis infection. Microbes Infect. (2015) 17:195–204. doi: 10.1016/j.micinf.2014.11.009
55. Perner A, Nielsen S, Rask-Madsen J. High glucose impairs superoxide production from isolated blood neutrophils. Intensive Care Med. (2003) 29:642–5. doi: 10.1007/s00134-002-1628-4
56. Stegenga M, van der Crabben S, Blümer R, Levi M, Meijers J, Serlie M, et al. Hyperglycemia enhances coagulation and reduces neutrophil degranulation, whereas hyperinsulinemia inhibits fibrinolysis during human endotoxemia. Blood. (2008) 112:82–9. doi: 10.1182/blood-2007-11-121723
57. Joshi M, Lad A, Bharath Prasad A, Balakrishnan A, Ramachandra L, Satyamoorthy K. High glucose modulates IL-6 mediated immune homeostasis through impeding neutrophil extracellular trap formation. FEBS Lett. (2013) 587:2241–6. doi: 10.1016/j.febslet.2013.05.053
58. Berbudi A, Rahmadika N, Tjahjadi A, Ruslami R. Type 2 diabetes and its impact on the immune system. Curr Diabetes Rev. (2020) 16:442–9. doi: 10.2174/1573399815666191024085838
59. Reinhold D, Ansorge S, Schleicher ED. Elevated glucose levels stimulate transforming growth factor-beta 1 (TGF-beta 1), suppress interleukin IL-2, IL-6 and IL-10 production and DNA synthesis in peripheral blood mononuclear cells. Horm Metab Res. (1996) 28:267–70. doi: 10.1055/s-2007-979789
60. Hu R, Xia C, Butfiloski E, Clare-Salzler M. Effect of high glucose on cytokine production by human peripheral blood immune cells and type I interferon signaling in monocytes: implications for the role of hyperglycemia in the diabetes inflammatory process and host defense against infection. Clin Immunol. (2018) 195:139–48. doi: 10.1016/j.clim.2018.06.003
61. Chang F, Shaio M. Respiratory burst activity of monocytes from patients with non-insulin-dependent diabetes mellitus. Diabetes Res Clin Pract. (1995) 29:121–7. doi: 10.1016/0168-8227(95)01123-4
62. Priyadharshini J, Banu M, Ramya P, Senita S. A case report on septic arthritis caused by Burkholderia pseudomallei. J Clin Diagn Res. (2025) 17:DD04–06. doi: 10.7860/JCDR/2023/64073.18738
63. Kung C, Li K, Hung S, Lee C. Melioidotic septic arthritis in the ED–an easily delayed diagnosis. Am J Emerg Med. (2010) 28:843.e1-2. doi: 10.1016/j.ajem.2009.11.018.
64. Kosuwon W, Taimglang T, Sirichativapee W, Jeeravipoolvarn P. Melioidotic septic arthritis and its risk factors. J Bone Joint Surg Am. (2003) 85:1058–61. doi: 10.2106/00004623-200306000-00011
65. Wooten M, Panwalker A. Septic arthritis caused by Burkholderia pseudomallei: case report and review of the literature. J Clin Rheumatol. (2001) 7:242–7. doi: 10.1097/00124743-200108000-00010
66. Tucker A, Matthews S, Wilson A. Mycotic Septic Arthritis of the Ankle Joint. Am J Orthop (Belle Mead NJ). (2016) 45:E478–80.
67. Dal T. Septic arthritis caused by Aspergillus fumigatus in an immunosuppressive patient: A case report and review of the literature. J Microbiol Infect Dis. (2012) 2:296–232. doi: 10.5799/jmid2012.01.0038
68. Frincy K, Biswajyoti B, Saikia S, Baruah M, Devi U. Burkholderia pseudomallei septic arthritis in Type-2 diabetes mellitus patients: report of two cases. Indian J Med Microbiol. (2020) 38:222–5. doi: 10.4103/ijmm.IJMM_20_74
70. Spampinato S, Caruso G, De Pasquale R, Sortino M, Merlo S. The treatment of impaired wound healing in diabetes: looking among old drugs. Pharmaceuticals (Basel). (2020) 13:60. doi: 10.3390/ph13040060
71. Shioya N, Ishibe Y, Kan S, Masuda T, Matsumoto N, Takahashi G, et al. Sternoclavicular joint septic arthritis following paraspinal muscle abscess and septic lumbar spondylodiscitis with epidural abscess in a patient with diabetes: a case report. BMC Emerg Med. (2012) 12:7. doi: 10.1186/1471-227X-12-7
72. Hunter J, Gross J, Dahl J, Amsdell S, Gorczyca J. Risk factors for failure of a single surgical debridement in adults with acute septic arthritis. J Bone Joint Surg Am. (2015) 97:558–64. doi: 10.2106/JBJS.N.00593
73. Walinga A, Stornebrink T, Emanuel K, Kievit A, Janssen S, Kerkhoffs G. Failure rates in surgical treatment in adults with bacterial arthritis of a native joint: a systematic review of 8,586 native joints. Arch Orthop Trauma Surg. (2023) 143:6547–59. doi: 10.1007/s00402-023-04958-z
74. Joo Y, Lee W, Shin H, Kim K, Kim Y. Risk factors for failure of eradicating infection in a single arthroscopic surgical procedure for septic arthritis of the adult native shoulder with a focus on the volume of irrigation. J Shoulder Elbow Surg. (2020) 29:497–501. doi: 10.1016/j.jse.2019.07.014
75. Stake S, Scully R, Swenson S, Lee D, Lee R, Sparks A, et al. Repeat irrigation & debridement for patients with acute septic knee arthritis: incidence and risk factors. J Clin Orthop Trauma. (2020) 11:S177–83. doi: 10.1016/j.jcot.2019.12.006
76. Böhler C, Dragana M, Puchner S, Windhager R, Holinka J. Treatment of septic arthritis of the knee: a comparison between arthroscopy and arthrotomy. Knee Surg Sports Traumatol Arthrosc. (2016) 24:3147–54. doi: 10.1007/s00167-015-3659-8
77. Böhler C, Pock A, Waldstein W, Staats K, Puchner S, Holinka J, et al. Surgical treatment of shoulder infections: a comparison between arthroscopy and arthrotomy. J Shoulder Elbow Surg. (2017) 26:1915–21. doi: 10.1016/j.jse.2017.04.001
78. Kao F, Hsu Y, Liu P, Tu Y, Jou I. High 2-year mortality and recurrent infection rates after surgical treatment for primary septic arthritis of the hip in adult patients: an observational study. Medicine (Baltimore). (2019) 98:e16765. doi: 10.1097/MD.0000000000016765
79. Galway U, Chahar P, Schmidt M, Araujo-Duran J, Shivakumar J, Turan A, et al. Perioperative challenges in management of diabetic patients undergoing non-cardiac surgery. World J Diabetes. (2021) 12:1255–66. doi: 10.4239/wjd.v12.i8.1255
80. Kovesdy C. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl. (2022) 2022:7–11. doi: 10.1016/j.kisu.2021.11.003
81. Webster A, Nagler E, Morton R, Masson P. Chronic Kidney Disease. Lancet. (2017) 389:1238–52. doi: 10.1016/S0140-6736(16)32064-5
82. Vaidya S, Aeddula N. Chronic kidney disease. StatPearls. Treasure Island, FL: StatPearls Publishing (2025).
83. Chen T, Knicely D, Grams M. Chronic kidney disease diagnosis and management: a review. JAMA. (2019) 322:1294–304. doi: 10.1001/jama.2019.14745
84. Aitkens L, Winn M, Waller J, Huber L, Baer S, Mohammed A, et al. Septic arthritis in the end-stage renal disease population. J Investig Med. (2022) 70:383–90. doi: 10.1136/jim-2021-001869
85. Ross J. Septic arthritis. Infect Dis Clin North Am. (2005) 19:799–817. doi: 10.1016/j.idc.2005.07.004
86. Al-Nammari S, Gulati V, Patel R, Bejjanki N, Wright M. Septic arthritis in haemodialysis patients: a seven-year multi-centre review. J Orthop Surg (Hong Kong). (2008) 16:54–7. doi: 10.1177/230949900801600114
87. Kumbar L, Yee J. Current concepts in hemodialysis vascular access infections. Adv Chronic Kidney Dis. (2019) 26:16–22. doi: 10.1053/j.ackd.2018.10.005
88. Sullivan R, Samuel V, Le C, Khan M, Alexandraki I, Cuhaci B, et al. Hemodialysis vascular catheter-related bacteremia. Am J Med Sci. (2007) 334:458–65. doi: 10.1097/MAJ.0b013e318068b24c
89. Morgan D, Fisher D, Merianos A, Currie B. An 18 year clinical review of septic arthritis from tropical Australia. Epidemiol Infect. (1996) 117:423–8. doi: 10.1017/s0950268800059070
90. Powe N, Jaar B, Furth S, Hermann J, Briggs W. Septicemia in dialysis patients: incidence, risk factors, and prognosis. Kidney Int. (1999) 55:1081–90. doi: 10.1046/j.1523-1755.1999.0550031081.x
91. Civelek G, Atalay A, Turhan N. Septic arthritis of the shoulder in a peritoneal dialysis patient. Turk J Phys Med Rehab. (2011) 57:57–9. doi: 10.4274/tftr.57.11
92. Centers for Disease Control and Prevention (CDC). Invasive methicillin-resistant Staphylococcus aureus infections among dialysis patients–United States, 2005. MMWR Morb Mortal Wkly Rep. (2007) 56:197–9.
93. Yeh H, Liau S, Niu K, Hsiao C, Yeh C, Lu J, et al. Clinical characteristics and in-hospital outcomes in dialysis patients with septic arthritis. Medicina (Kaunas). (2022) 58:401. doi: 10.3390/medicina58030401
94. Kim J, Park S, Sohn K, Kim B, Joo E. Methicillin resistance increased the risk of treatment failure in native joint septic arthritis caused by Staphylococcus aureus. Antibiotics (Basel). (2023) 12:1628. doi: 10.3390/antibiotics12111628
95. Wang J, Wang L. Novel therapeutic interventions towards improved management of septic arthritis. BMC Musculoskelet Disord. (2021) 22:530. doi: 10.1186/s12891-021-04383-6
96. Mínguez S, Molinos S, Mateo L, Gimenez M, Mateu L, Cabello J, et al. Septic arthritis due to methylcyllin-resistant Staphylococcus aureus in adults. Reumatol Clin. (2015) 11:381–6. doi: 10.1016/j.reuma.2014.12.009
97. Combs K, Cox K. Clinical outcomes involving patients that develop septic arthritis with methicillin sensitive staphylococcus aureus versus methicillin resistant staphylococcus aureus. J Orthop. (2018) 15:9–12. doi: 10.1016/j.jor.2017.11.007
98. MacRae C, Mercer S, Guthrie B, Henderson D. Comorbidity in chronic kidney disease: a large cross-sectional study of prevalence in Scottish primary care. Br J Gen Pract. (2021) 71:e243–9. doi: 10.3399/bjgp20X714125
99. Darwitz B, Genito C, Thurlow L. Triple threat: how diabetes results in worsened bacterial infections. Infect Immun. (2024) 92:e0050923. doi: 10.1128/iai.00509-23
100. Slaughter S, Dworkin R, Gilbert D, Leggett J, Jones S, Bryant R, et al. Staphylococcus aureus septic arthritis in patients on hemodialysis treatment. West J Med. (1995) 163:128–32.
101. Choi H, Yoon H, Oh H, Hong J, Choi T, Park S. Mortality of septic knee arthritis in Korea: risk factors analysis of a large national database. Sci Rep. (2022) 12:14008. doi: 10.1038/s41598-022-18420-4
102. Pisetsky D, Ward M. Advances in the treatment of inflammatory arthritis. Best Pract Res Clin Rheumatol. (2012) 26:251–61. doi: 10.1016/j.berh.2012.03.001
103. Ledingham J, Snowden N, Ide Z. Diagnosis and early management of inflammatory arthritis. BMJ. (2017) 358:j3248. doi: 10.1136/bmj.j3248
104. Firestein G, McInnes I. Immunopathogenesis of rheumatoid arthritis. Immunity. (2017) 46:183–96. doi: 10.1016/j.immuni.2017.02.006
106. Chauhan K, Jandu JS, Brent LH, Al-Dhahir MA. Rheumatoid arthritis. StatPearls. Treasure Island, FL: StatPearls Publishing (2025).
107. Radu A, Bungau S. Management of rheumatoid arthritis: an overview. Cells. (2021) 10:2857. doi: 10.3390/cells10112857
108. Conforti A, Di Cola I, Pavlych V, Ruscitti P, Berardicurti O, Ursini F, et al. Beyond the joints, the extra-articular manifestations in rheumatoid arthritis. Autoimmun Rev. (2021) 20:102735. doi: 10.1016/j.autrev.2020.102735
109. Cojocaru M, Cojocaru I, Silosi I, Vrabie C, Tanasescu R. Extra-articular manifestations in rheumatoid arthritis. Maedica (Bucur). (2010) 5:286–91.
110. Venetsanopoulou A, Alamanos Y, Voulgari P, Drosos A. Epidemiology and risk factors for rheumatoid arthritis development. Mediterr J Rheumatol. (2023) 34:404–13. doi: 10.31138/mjr.301223.eaf
111. Favero M, Schiavon F, Riato L, Carraro V, Punzi L. Rheumatoid arthritis is the major risk factor for septic arthritis in rheumatological settings. Autoimmun Rev. (2008) 8:59–61. doi: 10.1016/j.autrev.2008.07.018
112. Galloway J, Hyrich K, Mercer L, Dixon W, Ustianowski A, Helbert M, et al. Risk of septic arthritis in patients with rheumatoid arthritis and the effect of anti-TNF therapy: results from the British society for rheumatology biologics register. Ann Rheum Dis. (2011) 70:1810–4. doi: 10.1136/ard.2011.152769
113. Doran M, Crowson C, Pond G, O’Fallon W, Gabriel S. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. (2002) 46:2287–93. doi: 10.1002/art.10524
114. Edwards C, Cooper C, Fisher D, Field M, van Staa T, Arden N. The importance of the disease process and disease-modifying antirheumatic drug treatment in the development of septic arthritis in patients with rheumatoid arthritis. Arthritis Rheum. (2007) 57:1151–7. doi: 10.1002/art.23003
115. Kim H, Han M, Jung I, Ahn S. Incidence of septic arthritis in patients with ankylosing spondylitis and seropositive rheumatoid arthritis following TNF inhibitor therapy. Rheumatology (Oxford). (2023) 62:2740–7. doi: 10.1093/rheumatology/keac721
116. Sepriano A, Kerschbaumer A, Bergstra S, Smolen J, van der Heijde D, Caporali R, et al. Safety of synthetic and biological DMARDs: a systematic literature review informing the 2022 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis. (2023) 82:107–18. doi: 10.1136/ard-2022-223357
117. Sen R, Riofrio M, Singh JA. A narrative review of the comparative safety of disease-modifying anti-rheumatic drugs used for the treatment of rheumatoid arthritis. Expert Opin Drug Saf. (2024) 23:687–714. doi: 10.1080/14740338.2024.2348575
118. Chaplin S. Summary of the BSR biological DMARD safety guidelines. Prescriber. (2019) 30:34–7. doi: 10.1002/psb.1749
119. Dolganiuc A, Stavaru C, Anghel M, Baltaru D, Georgescu E, Olinescu A. The migratory and phagocytic activity of polymorphonuclear leukocytes in rheumatoid arthritis and osteoarthritis patients. Roum Arch Microbiol Immunol. (2000) 59:43–53.
120. Turner R, Schumacher R, Myers A. Phagocytic function of polymorphonuclear leukocytes in rheumatic diseases. J Clin Invest. (1973) 52:1632–5. doi: 10.1172/JCI107342
121. Strangfeld A, Listing J. Infection and musculoskeletal conditions: bacterial and opportunistic infections during anti-TNF therapy. Best Pract Res Clin Rheumatol. (2006) 20:1181–95. doi: 10.1016/j.berh.2006.08.010
122. Baum J. Infection in rheumatoid arthritis. Arthritis Rheum. (1971) 14:135–7. doi: 10.1002/art.1780140119
123. Stuck A, Minder C, Frey F. Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis. (1989) 11:954–63. doi: 10.1093/clinids/11.6.954
124. Besnard M, Babusiaux D, Garaud P, Rosset P, Bernard L, Le Nail L, et al. Impact of setting up a bone and joint infection referral center on arthroscopic treatment of septic arthritis of the knee and shoulder: retrospective study. Orthop Traumatol Surg Res. (2018) 104:1265–9. doi: 10.1016/j.otsr.2018.08.006
125. Sharma B, John S. Hepatic cirrhosis. StatPearls. Treasure Island, FL: StatPearls Publishing (2025).
126. Wilson R, Williams D. Cirrhosis. Med Clin North Am. (2022) 106:437–46. doi: 10.1016/j.mcna.2021.12.001
127. Ginès P, Krag A, Abraldes J, Solà E, Fabrellas N, Kamath P. Liver cirrhosis. Lancet. (2021) 398:1359–76. doi: 10.1016/S0140-6736(21)01374-X
128. Hung T, Hsieh M, Lay C, Tsai C, Tsai C. Increased occurrence of native septic arthritis in adult cirrhotic patients: a population-based three-year follow-up study in Taiwan. Prz Gastroenterol. (2014) 9:342–7. doi: 10.5114/pg.2014.47896
Keywords: septic arthritis, comorbidities, diabetes mellitus, rheumatoid arthritis, chronic kidney disease, prognosis, mortality
Citation: Khudair A, Khudair A and Butler AE (2025) The prognostic implications of comorbidities in septic arthritis. Front. Med. 12:1566384. doi: 10.3389/fmed.2025.1566384
Received: 24 January 2025; Accepted: 14 July 2025;
Published: 29 July 2025.
Edited by:
João Eurico Fonseca, University of Lisbon, PortugalReviewed by:
Saad Ahmed, University of Cambridge, United KingdomThanit Foocharoen, Khon Kaen University, Thailand
Copyright © 2025 Khudair, Khudair and Butler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexandra E. Butler, YWViOTEwMTFAZ21haWwuY29t; YWJ1dGxlckByY3NpLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Aiman Khudair
Aiman Khudair Ahmed Khudair
Ahmed Khudair Alexandra E. Butler
Alexandra E. Butler