- 1Department of Pancreatic Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Sino-German Laboratory of Personalized Medicine for Pancreatic Cancer, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Introduction: Pancreatic abscesses resulting from gastrointestinal fishbone migration represent rare yet clinically challenging surgical emergencies, with standardized management protocols remaining undefined.
Methods: We analyzed three consecutive cases (2024–2025) treated via anatomical landmark-guided laparoscopy alongside 11 PubMed-indexed cases (2004–2025). This study evaluates a novel surgical paradigm for complete foreign body retrieval and abscess resolution.
Results: The laparoscopic strategy achieved technical precision with minimal operative duration (73 ± 6 min) and blood loss (6.67 ± 4.71 mL), eliminating pancreatic fistula or hemorrhagic complications. Postoperative hospitalization was reduced by 43% compared to conventional interventions (5.3 ± 1.5 vs. 9.3 ± 3.1 days; *p* < 0.01). Crucially, this strategy attained hemostatic efficacy equivalent to augmented reality navigation (ARN)-assisted techniques without preoperative conditioning.
Conclusion: These findings establish a reproducible framework integrating anatomical landmark navigation for emergency pancreatic abscess management. The approach offers clinically validated advantages in procedural safety, visceral preservation, and accelerated recovery trajectories compared to existing strategies.
1 Introduction
Gastrointestinal foreign body impaction constitutes a critical surgical emergency, carrying well-established life-threatening risks (1, 2). Fishbone impactions display unique clinical behavior among gastrointestinal foreign bodies: the migration patterns exhibit extraordinary anatomical adaptability, capable of transmural penetration with subsequent infiltration of adjacent structures including the hepatic caudate lobe and pancreatic compartment (3, 4). The diagnostic and therapeutic complexity escalates dramatically when fishbones traverse the gastrointestinal wall into pancreatic tissue. This transmural migration creates a clinical triad of challenges: subtle symptom onset, intricate peripancreatic anatomy, and elevated risk of lethal sequelae like pancreatic abscess formation (4, 5). Global literature documents fewer than 30 reported cases of pancreatic fishbone migration, typically presenting with non-specific symptoms such as epigastric pain and fever that overlap with acute pancreatitis (6). Diagnostic uncertainty is compounded by the frequent radiological mimicry of pancreatic abscess on conventional imaging modalities, coupled with technical limitations in detecting subcentimeter foreign bodies within edematous parenchyma via standard CT or MRI protocols.
The clinical consequences of delayed intervention are profound, with retained foreign bodies precipitating pancreatic necrosis, systemic sepsis, and multiorgan dysfunction in advanced cases (7, 8). Although thin-section CT with multiplanar reconstruction has enhanced preoperative detection rates, intraoperative localization remains technically demanding due to inflammatory adhesions and necrotic tissue obscuring anatomical planes (9). Current management strategies lack standardization, with existing evidence limited to isolated case reports. Three critical knowledge gaps persist: (1) pathophysiological mechanisms enabling bony fragment migration through the gastroduodenal-pancreatic interface; (2) optimized multimodal imaging protocols; (3) evidence-based criteria for selecting minimally invasive versus open surgical approaches.
In this study, we conducted a retrospective analysis of clinical data pertaining to a series of cases involving peripancreatic abscesses induced by fishbone, managed at our institution. The objective was to synthesize the diagnostic and therapeutic strategies employed in addressing peripancreatic abscesses resulting from fishbones embedded in the pancreas and to underscore the clinical relevance of novel laparoscopic strategies for this patient cohort. Our findings further contribute to the understanding of foreign body migration patterns, including rare extrapancreatic destinations, through systematic anatomical landmark analysis and comparative outcome benchmarking.
2 Case series description
This case series details management protocols for three consecutive patients presenting with pancreatic abscesses secondary to migratory fishbone perforation at our institution (January 2024–January 2025). Diagnostic evaluation prioritized abdominal pain localization, febrile episodes, and recent dietary fish consumption. Advanced imaging protocols included triphasic pancreatic CT for foreign body characterization (18–42 mm), abscess mapping, and perforation site identification, supplemented by MRCP/ERCP for ductal integrity assessment and EUS-Doppler for vascular proximity stratification (≤ 2 mm from major vessels). Laboratory confirmation required elevated acute-phase reactants (CRP ≥ 50 mg/L, PCT ≥ 0.5 ng/mL) and pancreatic enzyme derangements (amylase > 3 × ULN, lipase > 5 × ULN).
Therapeutic interventions were stratified by clinical severity: conservative management (fasting, antibiotics, PPI) for stable cases versus anatomical landmark-guided laparoscopy for definitive management. The standardized laparoscopic surgical protocol entailed meticulous exploration of the lesser sac through gastrocolic ligament dissection, leveraging this anatomical landmark to expedite localization of fishbone foreign bodies migrating from the gastric wall toward pancreatic regions. Critical technical elements included preservation of gastroduodenal and pancreaticoduodenal vascular integrity, ultrasonic dissection-guided abscess cavity access with concurrent necrotic tissue debridement and foreign body extraction, and stepwise drain placement augmented by perioperative somatostatin analog therapy. Elevated amylase levels in drain fluid exceeding 3 × ULN serum amylase levels warrant close clinical attention.
Comparative analysis integrated 11 literature-derived cases (PubMed/Web of Science, 2004–2025) using search terms: (“pancreatic abscess” OR “foreign body pancreatitis”) AND (“fishbone”). Parameters included operative duration, blood loss, complications, and hospitalization length. Ethical approval was obtained from the Institutional Review Board of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, with written consent from all participants.
3 Results
3.1 Case series characteristics
This study comprised 14 clinical cases, including three prospectively enrolled from our tertiary referral center and 11 retrospective cases extracted from global medical records (2004–2025). The institutional cohort (n = 3) consisted of one male and two female patients aged 49–57 years (median 53). All three cases in our institution demonstrated confirmed 30 days piscine dietary exposure antecedents. Radiological confirmation revealed transmural gastric migration pathways terminating in pancreatic parenchymal embedding. The definitive management involved standardized laparoscopic retrieval procedures performed under the direction of a surgeon with 10 years of specialized experience in pancreatic surgery. Comprehensive demographic and clinical characteristics of the institutional cohort are summarized in Table 1.
3.2 Diagnostic algorithm
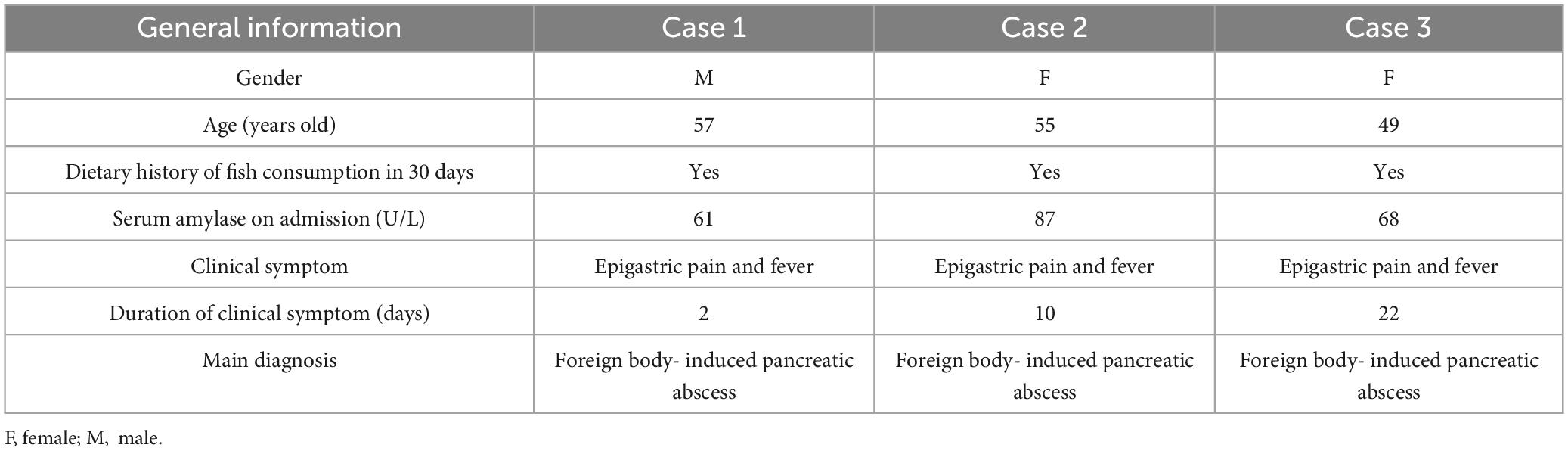
The preoperative diagnostic protocol encompassed a tripartite approach: hematological profiling, advanced cross-sectional imaging, and endoscopic ultrasonography. Each patient underwent systematic evaluation through these procedures, including standardized laboratory panels [complete blood count, C-reactive protein (CRP), amylase/lipase quantification], multiphase contrast-enhanced abdominal CT imaging, and linear-array endoscopic ultrasound (EUS) with Doppler interrogation (Figure 1).

Figure 1. Representative X-ray (A) and computed tomography (CT) (B) images of pancreatic fishbone impaction.
3.3 Therapeutic management
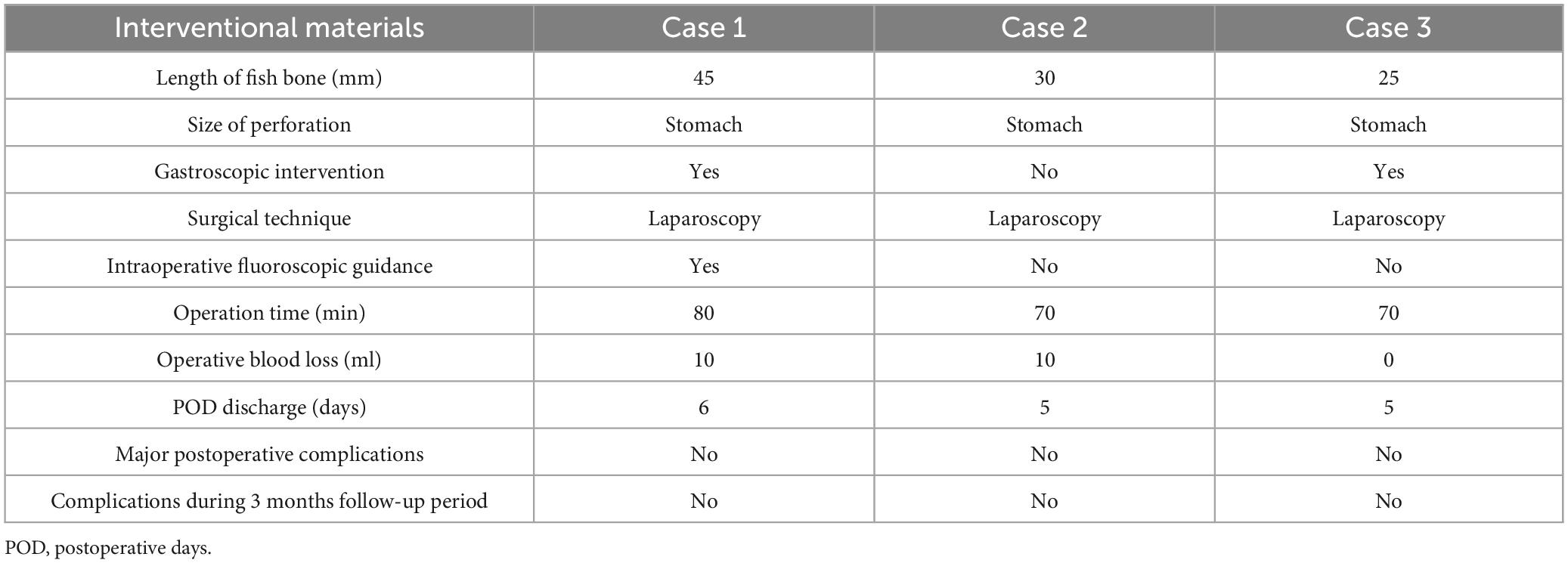
All enrolled patients received institutionally standardized supportive care protocols upon admission. Clinical management pathways were determined by endoscopic feasibility assessments, resulting in two distinct therapeutic strategies. Two patients initially underwent endoscopic ultrasonography-guided extraction attempts, which were subsequently converted to laparoscopic pancreatic retrieval procedures due to technical limitations; these cases were categorized as endoscopic-laparoscopic conversions. In contrast, one patient with definitive radiological confirmation of surgical indication underwent primary laparoscopic extraction without endoscopic intervention. Figure 2 demonstrates representative images of the laparoscopic localization and retrieval of the fishbone foreign body.

Figure 2. (A) Representative images demonstrating fishbone sizes. (B) Representative intraoperative images demonstrating fishbone retrieval.
Perioperative outcome analyses were stratified according to therapeutic strategy, with comprehensive evaluation of procedure-specific complications and quantitative postoperative recovery parameters. Comparative data encompassing operative duration, intraoperative blood loss, postoperative pain management requirements, and length of hospitalization are systematically presented in Table 2.
4 Comparative analysis
4.1 Demographic and clinical benchmarking
A systematic literature review of PubMed-indexed cases (2004–2025) identified six comparable pancreatic fishbone-induced abscess cases. Comparative analysis of our institutional case series (n = 3) versus published cases (n = 6) revealed no significant correlation between foreign body dimensions and surgical approach selection (9–14). Notably, our novel surgical protocol demonstrated statistically superior outcomes in hospitalization duration (5.3 ± 0.6 vs. 9.3 ± 1.9 days; P < 0.01), as delineated in Table 3.

Table 3. Clinical data comparison of fishbone-induced peri-pancreatic abscess: our center vs. global case series.
4.2 Minimally invasive approach benchmarking
A systematic PubMed review identified six international cases of pancreatic fishbone migration managed laparoscopically, with two providing analyzable data for comparative analysis. Comparative analysis of our institutional case series (n = 3) versus these two published cases revealed significant advantages of this novel laparoscopic protocol in hemorrhagic control and operative efficiency, as summarized in Table 4 (4, 5, 14–17).

Table 4. Comparison of laparoscopic surgery outcomes in pancreatic fishbone impaction: our center vs. published external center data.
Notably, while the ARN-assisted laparoscopic technique described by Li et al. demonstrated reduced operative time, it showed comparable blood loss metrics to conventional approaches. Furthermore, this method necessitates complex preoperative conditioning including: prolonged bed rest with confined bowel evacuation protocols, and mandatory 2 h positional rotation. Our optimized laparoscopic strategy eliminates such preparatory burdens while maintaining therapeutic effectiveness, particularly for abscessed cases where no successful ARN-assisted interventions have been documented.
5 Discussion
The epidemiological profile of pancreatic foreign bodies remains clinically uncommon, with PubMed-indexed literature documents only 32 confirmed cases of pancreatic foreign bodies through 2024, representing 0.2%–0.5% of all gastrointestinal perforation events. The foreign body predominantly comprises as sharp objects such as fine needles and fishbones, alongside organic materials including duck bones and toothpicks, with metallic and osseous fragments demonstrating particular clinical prevalence (18–20).
Peripancreatic abscesses secondary to fishbone migration were confirmed in three consecutive cases at our institution. Endoscopic retrieval attempts failed in two cases due to complete transmural penetration of the fishbone into the pancreatic parenchyma. All three patients ultimately underwent laparoscopic procedures for foreign body extraction, abscess drainage, and pancreatic repair. Although preoperative CT and ultrasonography provided two-dimensional localization, intraoperative determination of the fishbone’s spatial relationship with adjacent tissues remained challenging. In the first case, intraoperative X-ray imaging was utilized twice to confirm the foreign body’s position. Systematic analysis of anatomical landmarks, including the potential dissection plane between the posterior gastric antrum and the anterior pancreatic head, enabled rapid localization in subsequent cases without additional imaging, significantly improving surgical efficiency. Technical refinements, such as ultrasonic scalpel-mediated precise dissection to minimize thermal injury and standardized suture reinforcement of gastric perforations with peripancreatic drainage, ensured postoperative safety. Intraoperative blood loss was limited to 0–10 mL, with a mean postoperative hospitalization duration of 5.3 ± 0.6 days and no incidence of pancreatic fistula or hemorrhage.
A review of 11 published cases involving pancreatic fishbone migration revealed six cases of pancreatic abscess formation. Five were managed via open laparotomy, while one employed laparoscopic intervention, with a mean postoperative hospitalization duration of 9.3 ± 1.9 days—significantly longer than our cohort (5.3 ± 0.6 days, *p < 0.01). Among six laparoscopic cases addressing pancreatic fishbone migration, hospitalization ranged from 4 to 7 days, though only two reported operative metrics. Augmented reality navigation (ARN)-assisted laparoscopic techniques achieved favorable outcomes (60 min operative time, 10 mL blood loss), yet their reliance on complex preoperative protocols may increase patient burden. Notably, no data exist on ARN’s efficacy in fishbone-induced pancreatic abscesses.
Compared to existing approaches, our anatomical landmark-guided laparoscopic strategy demonstrates superior intraoperative hemostasis, operative efficiency, and reduced hospitalization. By standardizing anatomical plane identification and procedural workflows, this protocol eliminates the need for resource-intensive preoperative preparations required by ARN-assisted techniques. These findings establish a reproducible minimally invasive framework for managing pancreatic abscesses secondary to migrant fishbones, balancing therapeutic efficacy with operational pragmatism. This case series has several limitations that warrant acknowledgment. First, the small case series size (n = 3) may restrict the generalizability of our findings, necessitating validation through larger multicenter studies. Second, while comparisons with published literature provided contextual insights, the absence of a formal systematic review methodology (e.g., adherence to PRISMA guidelines) precludes comprehensive evidence synthesis. Future investigations should incorporate rigorous systematic approaches to strengthen cross-study comparisons. Despite these limitations, our work advances clinical management paradigms for migrant fishbone-induced pancreatic abscesses.
In summary, this case series proposes a novel laparoscopic anatomical landmark-guided strategy that enables precise foreign body retrieval and abscess drainage. Furthermore, the optimized diagnostic algorithm provides a standardized framework for early detection and intervention. These innovations establish a minimally invasive therapeutic roadmap for this rare yet critical surgical emergency, demonstrating both technical feasibility and clinical efficacy.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Union Hospital, Tongji Medical College, Huazhong University of Science and Technology. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
CZ: Writing – review and editing, Writing – original draft. LH: Writing – review and editing. HW: Writing – original draft. CD: Data curation, Writing – original draft. YL: Writing – original draft, Data curation. JW: Writing – original draft, Methodology. TY: Writing – original draft, Conceptualization, Investigation, Validation, Writing – review and editing, Formal Analysis, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ARN, augmented reality navigation; CT, computed tomography; MRCP, magnetic resonance cholangiopancreatography; EUS, endoscopic ultrasonography; ERCP, endoscopic retrograde cholangiopancreatography; PCT, procalcitonin.
References
1. Yadav T, Singh H, Sah R. Unusual foreign body of pancreas: Surgical management. JOP. (2013) 14:669–70. doi: 10.6092/1590-8577/1902
2. Dal F, Hatipo glu E, Teksoz S. Foreign body: A sewing needle migrating from the gastrointestinal tract to pancreas. Turk J Surg. (2018) 34:256–8. doi: 10.5152/turkjsurg.2017.3391
3. Kehagias D, Mulita F, Maroulis I, Benetatos N. Caudate lobe: The last barrier - an unusual place for a foreign body. ANZ J Surg. (2022) 92:1218–20. doi: 10.1111/ans.17226
4. Mima K, Sugihara H, Kato R, Matsumoto C, Nomoto D, et al. Laparoscopic removal of an ingested fishbone that penetrated the stomach and was embedded in the pancreas: A case report. Surg Case Rep. (2018) 4:149. doi: 10.1186/s40792-018-0559-4
5. Mulita F, Papadopoulos G, Tsochatzis S, Kehagias I. Laparoscopic removal of an ingested fishbone from the head of the pancreas: Case report and review of literature. Pan Afr Med J. (2020) 36:123. doi: 10.11604/pamj.2020.36.123.23948
6. Díaz Granados A, Dabán López P, Jurado Prieto F, Mirón Pozo B. Acute pancreatitis due to fishbone. Rev Esp Enferm Dig. (2024): doi: 10.17235/reed.2024.10944/2024 Epub ahead of print.
7. Lin J, Tao H, Wang Z, Chen R, Chen Y, Lin W, et al. Augmented reality navigation facilitates laparoscopic removal of foreign body in the pancreas that cause chronic complications. Surg Endosc. (2022) 36:6326–30. doi: 10.1007/s00464-022-09195-w
8. Lim D, Ho C. Appendicitis-mimicking presentation in fishbone induced microperforation of the distal duodenum: A case report. World J Gastrointest Surg. (2020) 12:77–84. doi: 10.4240/wjgs.v12.i2.77
9. Huang YH, Siao FY, Yen HH. Pre-operative diagnosis of pancreatic abscess from a penetrating fishbone. QJM. (2013) 106:955–6. doi: 10.1093/qjmed/hcs166
10. Goh BK, Jeyaraj P-R, Chan H-S, Waikeong W, Agasthian T, Chang KT, et al. A case of fishbone perforation of the stomach mimicking a locally advanced pancreatic carcinoma. Dig. Dis. Sci. (2004) 49:1935–7. doi: 10.1007/s10620-004-9595-y
11. Goh BK, Yong WS, Yeo AW. Pancreatic and hepatic abscess secondary to fishbone perforation of the duodenum. Dig. Dis. Sci. (2005) 50:1103–6. doi: 10.1007/s10620-005-2712-8
12. Wang WL, Liu KL, Wang HP. Clinical challenges and images in GI. Pancreatic abscess resulting from a fishbone penetration of the stomach. Gastroenterology. (2008) 135:1865–2160. doi: 10.1053/j.gastro.2008.10.067
13. Wang WJ, Zhang Y, Wang L, Chen Y, Tian BL. Chronic pancreatic inflammatory granuloma caused by foreign body presenting as a pancreatic pseudotumor: A case report and literature review. Pancreatology. (2015) 15:573–5. doi: 10.1016/j.pan.2015.05.474
14. Wu YJ, Chen YY, Hsieh YC. Unusual pancreatic abscess secondary to embedded fishbone: A challenging clinical scenario. Diagnostics (Basel). (2022) 12:2999. doi: 10.3390/diagnostics12122999
15. Xie R, Tuo B, Wu H. Unexplained abdominal pain due to a fishbone penetrating the gastric antrum and migrating into the neck of the pancreas: A case report. World J Clin Cases. (2019) 7:805–8. doi: 10.12998/wjcc.v7.i6.805
16. Wang Y, Luo X, Zhang J. Successful laparoscopic treatment for sustained abdominal pain due to fishbone migrating into the neck of the pancreas: A case report and thinking about surgical approach through the literature review. Surg Case Rep. (2021) 7:91. doi: 10.1186/s40792-021-01174-y
17. Li S, Liu C, Wen S, Geng Y. Minimally invasive removal of a foreign body in the pancreas using digital intelligent technology: A case report. J Int Med Res. (2024) 52:3000605241266548. doi: 10.1177/03000605241266548
18. Nasri B, Yuu K, Tada M. A case report of successful removal of multiples sewing needles in the gastrointestinal tract and pancreas using intraoperative C-arm fluoroscopy. Int J Surg Case Rep. (2016) 24:166–71. doi: 10.1016/j.ijscr.2016.05.043
19. Kim K, Jo S, Song S. Laparoscopic removal of a broken acupuncture needle in pancreatic head: A case report. J Surg Case Rep. (2024) 2024:rjae714. doi: 10.1093/jscr/rjae714
Keywords: pancreatic foreign body, fishbone, pancreatic abscess, novel laparoscopic strategies, case series
Citation: Zhao C, Wei H, He L, Deng C, Lu Y, Wang J and Yin T (2025) Anatomical landmark-guided laparoscopy for migrant fishbone - induced pancreatic abscesses: a case series study and review of the literature. Front. Med. 12:1598619. doi: 10.3389/fmed.2025.1598619
Received: 23 March 2025; Accepted: 26 May 2025;
Published: 02 July 2025.
Edited by:
Hiroo Uchida, Nagoya University Graduate School of Medicine, JapanReviewed by:
Dimitrios Kehagias, University of Patras, GreeceAndreas Antzoulas, General University Hospital of Patras, Greece
Copyright © 2025 Zhao, Wei, He, Deng, Lu, Wang and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Yin, eXR3aHVuQGh1c3QuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Chuanbing Zhao1,2†
Chuanbing Zhao1,2† Jingjie Wang
Jingjie Wang Tao Yin
Tao Yin