- 1Department of Pathology, Second Affiliated Hospital of Jiujiang University, Jiujiang, China
- 2Jiangxi Provincial Key Laboratory of Cell Precision Therapy, School of Basic Medical Sciences, Jiujiang University, Jiujiang, China
- 3Department of General Surgery, Second Affiliated Hospital of Jiujiang University, Jiujiang, China
- 4Department of Pathology, Jiujiang First People’s Hospital, Jiujiang, China
- 5Department of Pathology, Yangpu Hospital, School of Medicine, Tongji University, Shanghai, China
Introduction: Small intestinal NK/T-cell lymphoma (NK/T-L) is a rare condition, and cases presenting with malignant peritoneal effusion as the initial symptoms are not well-documented. We report a unique case that contributes to the understanding of this rare disease.
Case report: A 47-year-old man was admitted to the hospital with a chief complaint of abdominal distension and weight loss persisting for over 2 months. Imaging studies revealed ascites and localized thickening of the small intestine. Paracentesis cytology revealed a significant presence of small-to-medium-sized lymphoid cells. Immunohistochemistry confirmed a diagnosis of T-cell lymphoma. Histological examination confirmed primary NK/T-L of the small intestine after PET-CT excluded metastases from other sites. Despite aggressive chemotherapy, the patient’s condition deteriorated, resulting in his death 4 months later.
Discussion: This case highlights the importance of considering small intestinal NK/T-L in patients with abdominal symptoms and malignant peritoneal effusion. The aggressive nature and poor prognosis of this disease pose challenges in diagnosis and treatment. Increased awareness among clinicians and pathologists is crucial for early detection and improved patient outcomes.
Introduction
NK/T-cell lymphoma (NK/T-L) is more common, affecting the nasal cavity, with infrequent reports of cases originating in the intestine, whereas NK/T-L presenting with malignant peritoneal effusion is even rarer; to our knowledge, there is only one case that has been reported worldwide (1). Therefore, we report the clinical course, diagnostic challenges, and treatment outcomes of a case of small intestine primary NK/T-L with malignant peritoneal effusion as the first symptom. Through a comprehensive review of the relevant literature, we aim to emphasize the significance of recognizing this atypical presentation and contribute to the body of knowledge for small intestine NK/T-L and raise awareness among clinicians and pathologists, ultimately leading to improved patient outcomes.
Case report
Methods
A 47-year-old man presented with a 2-month history of abdominal discomfort and weight loss. The abdominal distension worsened after meals and was occasionally accompanied by non-specific abdominal pain without fever, vomiting, diarrhea, or constipation. The patient underwent abdominal color Doppler ultrasonography, computed tomography (CT), and laboratory examination.
Results
Abdominal color Doppler ultrasonography showed moderate ascites that did not improve with anti-inflammatory and symptomatic treatments. The patient had no relevant personal or family medical histories and appeared healthy on physical examination. The abdomen was slightly distended with mild tenderness, rebound tenderness, shifting dullness to percussion, and no enlargement of superficial lymph nodes.
Laboratory examination showed elevated levels of C-reactive protein (CRP) at 68.21 mg/L, erythrocyte sedimentation rate (ESR) at 87 mm/h, decreased levels of albumin at 31.7 g/L, and prealbumin at 103 g/L. Quantitative EBV DNA was measured at 2.74 × 103 U/mL. The tumor marker cancer antigen 125 (CA125) was elevated to 104.2 U/mL, and CEA and AFP were normal. The fecal occult blood test yielded positive results. Various tests, including tuberculosis antibody, PPD, ANCA, Vita reaction, hepatitis viruses, blood culture, ENA, and immunoglobulin, were within normal limits. CT of the upper abdomen revealed peritoneal effusion with small intestinal adhesions, jejunal wall thickening, and multiple abdominal and retroperitoneal lymphadenopathies (Figure 1a). Ascites examination showed light yellow, turbid fluid with a positive Rivalta test. Total protein was 36.0 g/L, white blood cell count was 4.021 × 109/L, multinucleated cell ratio was 0.17, lymphocyte ratio was 0.83, ascites Adenosine deaminase (ADA) was 55 U/L, lactate dehydrogenase (LDH) was 730 U/L, and CA125 was 874.6 U/mL. No bacteria were detected by blue staining, and acid-fast bacilli were absent from the smear.
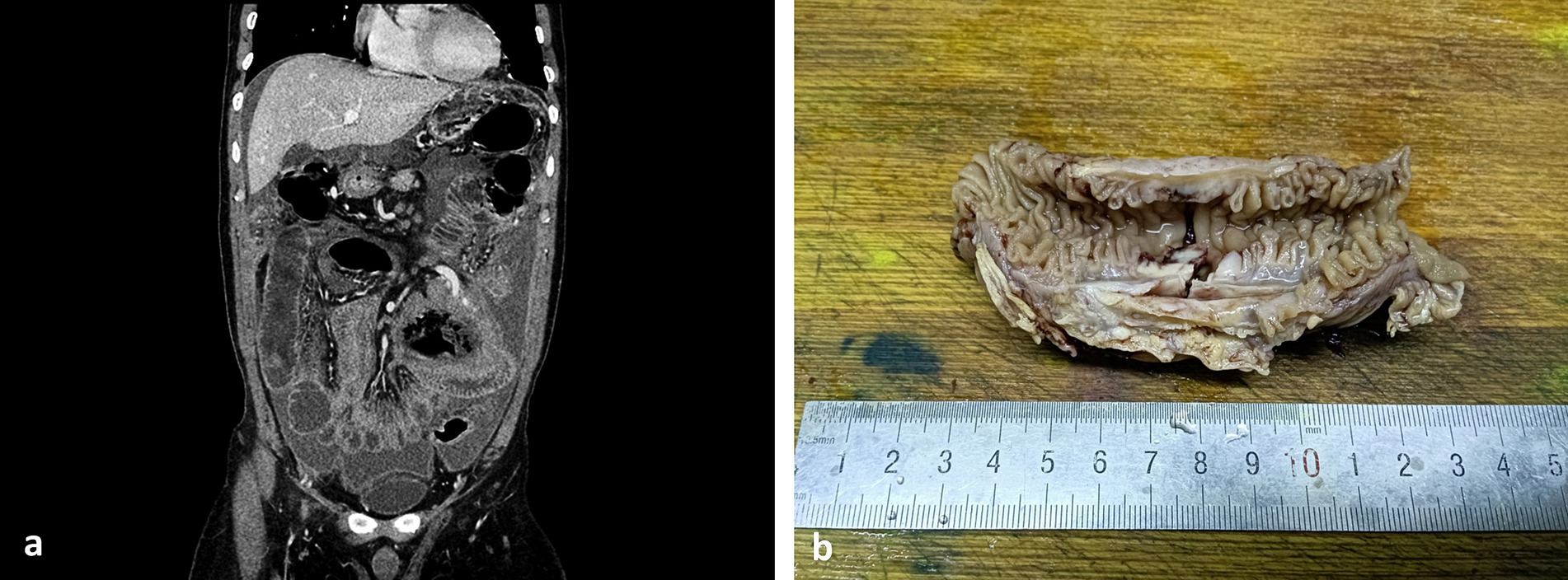
Figure 1. CT image and large body picture of the case. (a) CT of the upper abdomen showed peritoneal effusion with small intestinal adhesions, jejunal wall thickening, and multiple abdominal and retroperitoneal lymphadenopathies; and (b) ulcer could be seen in the membrane of the small intestine.
As shown in Figure 1b, ulcer could be seen in the membrane of the small intestine. Ascites cytopathological smear revealed diffuse distribution of mildly atypical medium-to-small lymphocytes with scant cytoplasm, deep chromatin, and cytoplasmic granules that were present in some tumor cells. A large number of necrotic fragments are visible in the background (Figure 2). Microscopic examination of the cell sediment section showed a large number of small lymphocytes with uniform shape, deep chromatin, and irregular and vesicular karyotypes (Figures 2a,b). Immunohistochemical markers were positive for CD3ε(+) (Figure 2c), CD43(+), CD19(−), CD20(−) (Figure 2d), CD5(−), MOC31(−), CK20(−), CR(−), WT-1(−), and Ki67:50%(+), supporting a diagnosis of small bowel T-cell lymphoma. The patient and his family members refused enteroscopy and opted for surgical exploration. Intraoperatively, ulcerative lesions and serosal layer involvement were observed in the jejunum, and the hard part had extended through the serosal layer. A section of the bowel was excised for pathological examination (Figures 2e–h), which revealed full-thickness infiltration of atypical lymphocytes with extensive necrosis in the small intestine (Figures 2e–h). The patient was followed up with CT postoperatively, and PET-CT showed no other lesions. Immunohistochemical staining was positive for CD3ε(+), CD43(+), CD56(+) (Figure 2i), GranB(+), TIA-1(+), and Ki67: 70% (+), whereas it was negative for CD4(−), CD5(−), CD8(−), CD20(−), and EMA(−). In situ hybridization detected Epstein–Barr virus (EBV) with positive EBV-encoded small RNA (EBER) staining (Figure 2j), and the molecular testing was positive for TCR-γ and negative for TCR-δ, leading to the final diagnosis of small intestinal NK/T-L. The patient received two courses of the R-CHOP regimen (cyclophosphamide + doxorubicin + vincristine + prednisone + rituximab) postoperatively but succumbed to disease progression 4 months later.
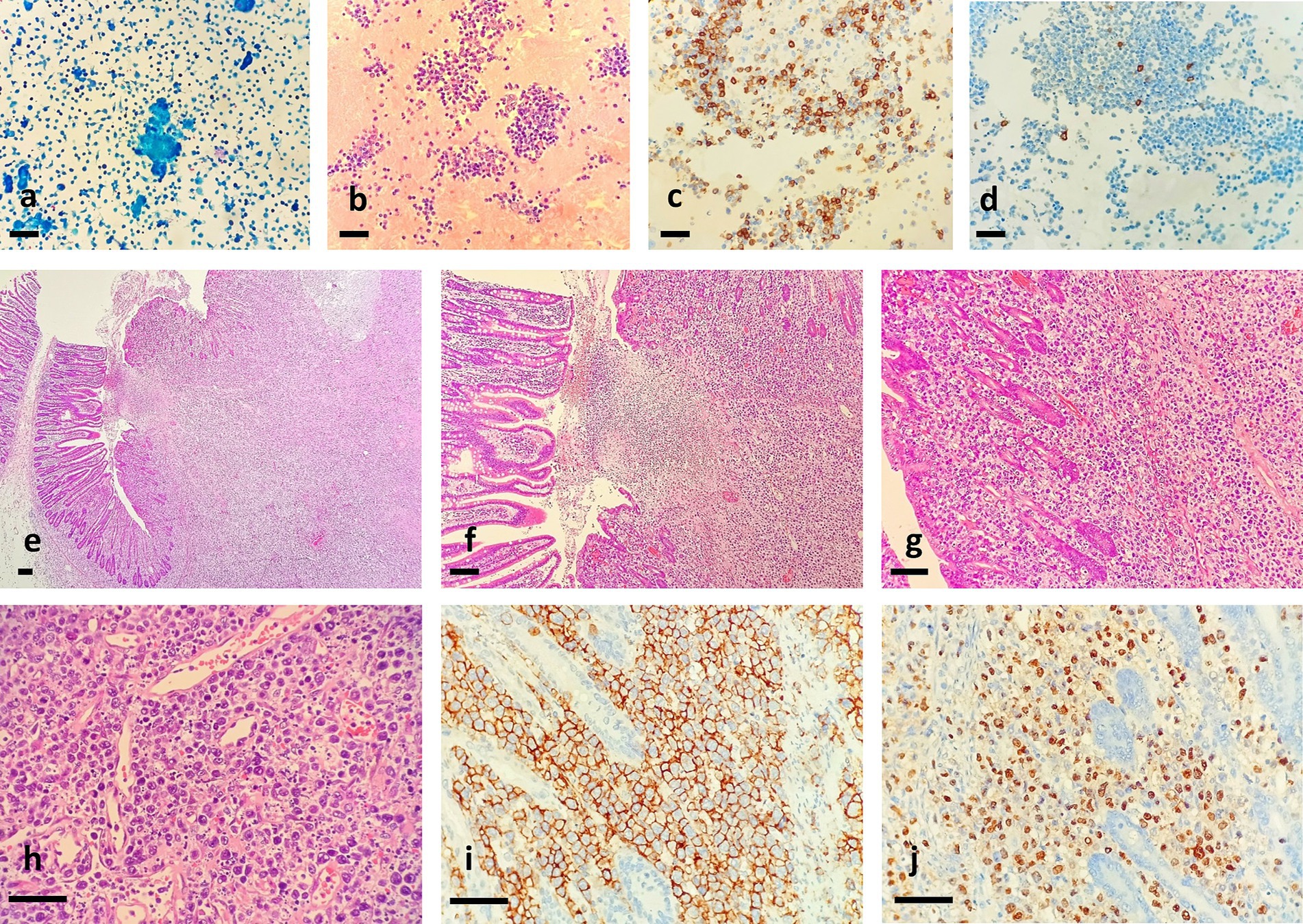
Figure 2. Ascites cytopathological smear revealed diffuse distribution of mildly atypical medium-to-small lymphocytes with scant cytoplasm and deep chromatin, and A section of the bowel revealed full-thickness infiltration of atypical lymphocytes with extensive necrosis in the small intestine. (a) Pap staining, (b) HE staining for cell mass, (c) tumor cells were positive for CD3ε, and D. tumor cells were negative for CD20, bar = 200 μm. (e,×40; f,×100; g,×200; h,×400). Immunohistochemical analysis showed that tumor cells were positive for CD56 (i,×400). In situ hybridization for EBER showed strong positivity in most of the tumor cells (j,×400). Bar = 200 μm.
Discussion
Extranodal NK/T-L of the nasal type is a rare subtype of non-Hodgkin lymphoma derived from extranodal NK cells and T cells. It accounts for only 0.2–0.4% of all cases and is closely related to EBV infection. Extranodal lymphoma with malignant effusion as the primary feature is rare; the pathological type is mainly B-cell lymphoma (2). Due to the literature on cytological findings related to NK/T-L in effusions is limited, in order to have a comprehensive understanding of the cytomorphology, treatment and outcome characteristics this disease, including our case, we summarized a total of 13 reported cases of NK/T-L with pleural, peritoneal, pericardial, or cerebrospinal effusions that were documented and compared with those reported in the literature (Table 1) (1, 3–11). The male-to-female ratio was 11:3, with a median of 48.5 years. The primary sites were diverse, and the most common nasal cavity with malignant effusion was in only two cases. This may also be related to the fact that NK/T-L in the primary sites, such as the lung, intestine, and pericardium, were closer to the serosal cavity. In 14 cases, 10 cases could not be diagnosed before malignant effusion. This evidence supports that it is difficult to directly diagnose NK/T-L at other primary sites. Our case is similar to the above report, which may be due to the fact that NK/T-L has an insidious onset, a low incidence, and the internal information of deep tumors cannot be effectively obtained.
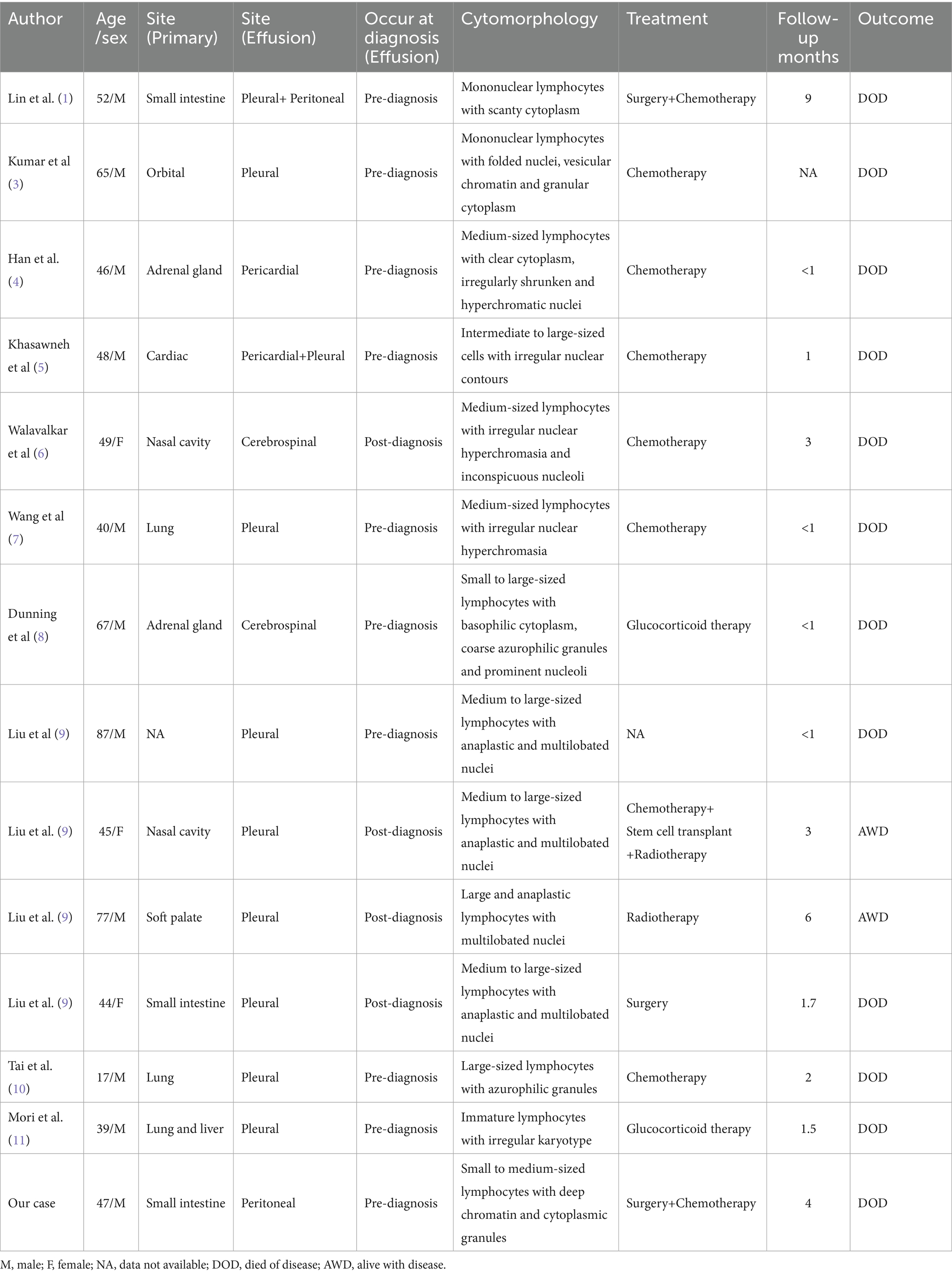
Table 1. Summarization of the clinicopathological features of body cavity effusion involved by NK/T-cell lymphoma.
Intestinal involvement in NK/T-L is even less common, with fewer than 7% of all cases, and clinical manifestations are often non-specific, including abdominal pain, blood in the stool, fever, and weight loss (12). Early endoscopic examination may not yield high positive rates due to submucosal origin, and misdiagnosis as enteritis, intestinal tuberculosis, or inflammatory bowel disease is common in the later stages when polyps or ulcerative lesions develop (13). Ascites with primary features is predominantly caused by abdominal tuberculosis and liver cirrhosis. Different from infection and hypoproteinemia in benign ascites, lymphoma with malignant effusion is mainly caused by advanced lymphoma cells’ direct invasion or metastasis of the serosa and lymph nodes, causing tubal circulation disorders (14). In this case, the patient had no history of tuberculosis or hepatitis, and CT findings showed small bowel wall thickening, adhesions, and multiple abdominal lymph nodes, indicating the possibility of tumor ascites.
Blood routine examination of lymphoma patients often shows mild to moderate anemia. CRP and ESR, as indicators of non-specific inflammatory activity, are both elevated during lymphoma disease activity but lack specificity for diagnosis (15). Ascitic fluid ADA levels ≥40 IU/L showed excellent sensitivity for the diagnosis of tuberculous peritonitis, but a large retrospective study found that lymphoma-related ascites is an important mimic of tuberculous peritonitis that can result in high ascitic fluid ADA levels with similar clinical manifestations (16).
Therefore, when ADA and LDH were increased simultaneously, it was necessary to broaden our thinking and be alert to the possibility of lymphoma. It has been reported in the literature that elevated levels of LDH and CA125 in the serum and ascites are commonly observed in advanced lymphoma cases with intestinal serosal layer involvement, which can reflect the invasion ability of lymphoma (17). The higher the levels, the worse the prognosis, which has important reference value in judging the clinical stage, disease progression, and therapeutic outcome of patients (15). In our case, LDH and CA125 levels were consistent with the reported literature, and quantification of EBV DNA was significantly increased, indicating a high suspicion of NK/T-L with ascites.
Cytological examination of ascitic fluid is a mature diagnostic technology, with malignant cells observed in 60% of malignant ascites. A large sample study reported that in 197 samples of patients with lymphomatous effusion, the positive rate of malignant cells found in cytology was 56.6%. It is confirmed that there are some missed diagnoses and misdiagnoses, which are related to the collection of tumor cells and the diagnosis level of pathologists (18). The cytologic smears of the Extranodal NK/T-cell lymphoma (ENKTL) effusion specimens were highly cellular, and the tumor cells were small- to large-sized with pleomorphic nuclei and coarse chromatin. A moderate amount of eccentric basophilic cytoplasm and azurophilic granules was also seen (Table 1) (1, 3–11). Microscopic cytological examination of the ascitic fluid in this case showed many small and medium lymphocytes with consistent morphology and some atypia. Proliferative lymphocytic lesions were also considered. The patient had no history of lymphoma, and it was difficult to diagnose lymphoma by cytology alone. After the cell sediment was collected by centrifugation, T-cell lymphoma was diagnosed based on cell morphology and immunohistochemical markers. Therefore, ascites of unknown cause should be checked with a cell wax block as much as possible, which can not only determine benignity and malignancy but also be combined with immunohistochemical markers to identify common adenocarcinoma cells, mesothelial cells, and lymphocytes in ascites. The final diagnosis of ENKTCL requires the help of histology, which shows diffuse and consistent lymphocyte infiltrating growth in the small intestinal wall, and vascular destruction and necrosis as its unique histological features; immunohistochemical markers CD2, CD3ε, CD56, TiA-1, granzyme B, and perforin positive, and EBER in situ hybridization positive can be definitively confirmed (19). The differential diagnosis of small intestinal NK/T-L is particularly important because of the differences in treatment and prognosis. It is necessary to differentiate from intestinal inert T-lymphoproliferative disorders, Enteropathy-associated T-cell lymphoma (EATL), Monomorphic–epitheliotropic intestinal T-cell lymphoma (MEITL), peripheral T-cell lymphoma of the gastrointestinal tract, angioimmunoblastic T-cell lymphoma, and B-cell lymphoma. These are lymphoid disorders occurring in the small intestine and are associated with clinical histories, defined microscopic morphology, specific immunohistochemical markers, EBERs, and TCR gene detection, which can help us make a final diagnosis.
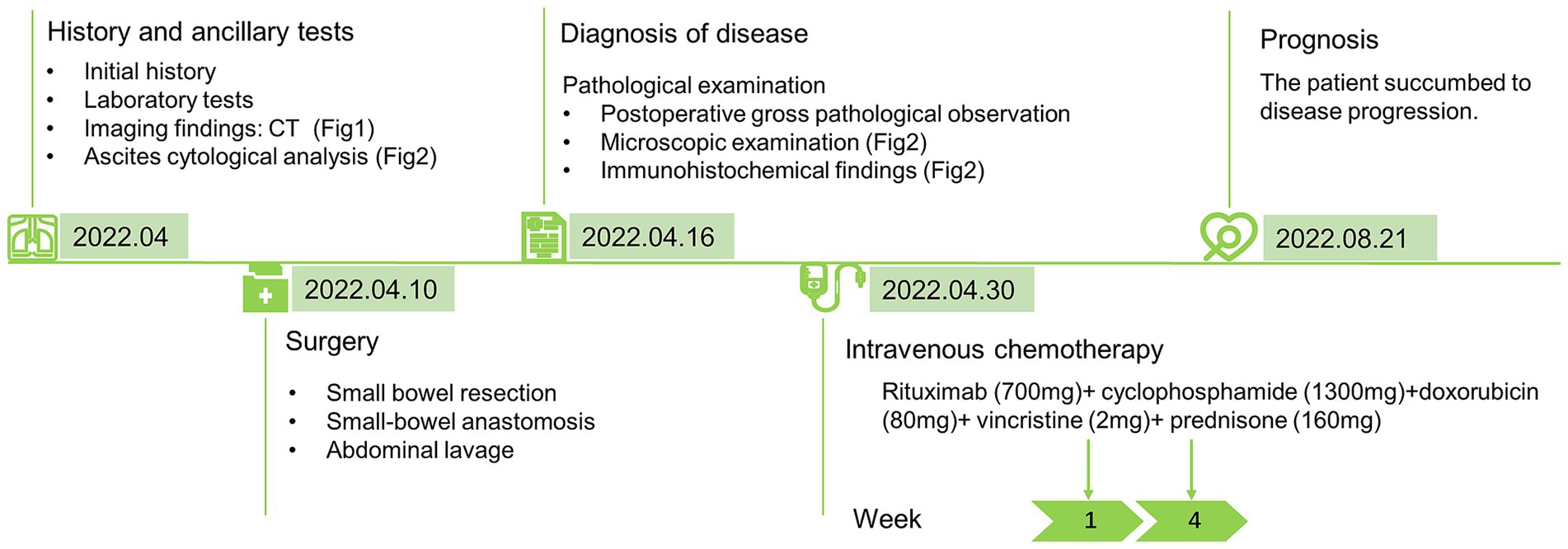
Currently, there are no standard treatments for NK/T-L. The total effective rate of the conventional Cyclophosphamide, Hydroxydoxorubicin, Oncovin and Prednisone (CHOP) regimen is approximately 36%, and the total effective rate for relapsed and refractory patients is less than 10% (20). In this case, the patient received two courses of the R-CHOP regimen but succumbed to disease progression rapidly 4 months later, which might be related to the failure to select the standardized treatment. CHOP chemotherapy is not an optimal treatment for NK/T-L, and L-asparaginase-containing chemotherapy is a key component of first-line treatments for systemic NK/T-L. Standard treatments for refractory or relapsed NK/T-L, dexamethasone, methotrexate, ifosfamide, l-asparaginase, and etoposide (SMILE) regimen and sequential chemoradiotherapy is recommended (21), or hematopoietic stem cell transplantation may be considered (22). The NK/T-L with malignant effusion was in the advanced stage of the disease. In the 14 patients we collected, it was found that the treatment effect was not significant, whether it was surgery, chemoradiotherapy, or stem cell transplantation. Recently, next-generation sequencing (NGS) was performed in ENKTL, and the missense mutation of STAT3, STAT5B, and RNA helicase gene DDX3X was found, which may be potential therapeutic targets for treatment (7, 23). Given that NK/T-L can destroy intestinal adhesions, when patients present with unexplained abdominal pain and hematochezia, conventional diagnostic methods such as traditional colonoscopy fail to identify the cause. Consideration should be given to performing small bowel endoscopy, and biopsy can be considered to effectively improve the early detection rate of lesions. Nevertheless, the prognosis of small-bowel NK/T-L remains poor, with a median survival time of only 3–6 months (24, 25). The patient received R-CHOP chemotherapy after surgery and died of secondary symptoms 4 months later. A timeline figure summarizing the case diagnosis and treatment pathway is shown in Figure 3.
In summary, the clinical manifestations of small-intestinal NK/T-L are atypical, with ascites being the first symptom. When refractory ascites is accompanied by elevated serum LDH, CA125, and EBV, lymphomatous disease should be considered after excluding other common diseases. The possibility of ascites, especially NK/T-L, combined with pleural and ascites cytology to provide diagnostic clues, active deep colonoscopy biopsy or laparoscopy, and finally combined with immunohistochemical staining and EBER in situ hybridization should be considered to establish a pathological diagnosis and provide clinical treatment.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by the ethics committee of Second Affiliated Hospital of Jiujiang University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
BZ: Conceptualization, Investigation, Writing – original draft. QZ: Formal Analysis, Resources, Writing – original draft. XL: Data curation, Resources, Writing – original draft. HL: Data curation, Resources, Writing – original draft. HH: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Project of College-level Key Discipline of Yangpu Hospital Affiliated to Tongji University (No. 2023YJXK01), the Fund of Yangpu Hospital Affiliated to Tongji University (No. Se1202318), the Project of Yangpu District Health and Wellness Committee (No. YPM202414), and the Science and Technology Project of Health Commission of Jiangxi Province (No. 202510881).
Acknowledgments
We thank Editage (www.editage.cn) for its linguistic assistance during the preparation of this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lin, YN, Chou, JW, Chuang, PH, Cheng, KS, Peng, CY, and Chiang, IP. Primary small intestinal natural killer/T cell lymphoma mimicking tuberculous peritonitis: report of a case and review of the literature. Intern Med. (2011) 50:515–8. doi: 10.2169/internalmedicine.50.4435
2. Zhang, S, Chen, X, Bo, J, Zhu, X, Zhang, T, Gao, Z, et al. Clinical and cytological characteristics of serous effusions in 69 cases of lymphoma patients. Diagn Cytopathol. (2024) 52:649–61. doi: 10.1002/dc.25379
3. Kumar, C, Jain, G, Gupta, A, Pramanik, R, and Chopra, A. Extra-nasal Nk/T-cell lymphoma: a rare case with a rarer presentation. Cytopathology. (2022) 33:518–21. doi: 10.1111/cyt.13095
4. Han, Y, Kim, KH, and Choi, IH. Cytological findings of Nk/T-cell lymphoma in pericardial effusion: a case report with a review of the literature. Indian J Pathol Microbiol. (2019) 62:473–6. doi: 10.4103/ijpm.ijpm_540_18
5. Khasawneh, A, McGuinness, G, and Ward, N. Initial diagnosis of Extranodal Nk/T-cell lymphoma in pericardial fluid with concomitant Hemophagocytic Lymphohistiocytosis (Hlh). J Hematop. (2024) 17:27–36. doi: 10.1007/s12308-023-00572-5
6. Walavalkar, V, Oak, J, and Gu, M. Cytological diagnosis of Extranodal Nk/T-cell lymphoma, nasal type, in cerebrospinal fluid. Cytopathology. (2013) 24:342–4. doi: 10.1111/j.1365-2303.2012.00999.x
7. Wang, Y, Wang, Z, Wu, C, Zhao, X, Ji, N, and Huang, M. Primary pulmonary Extranodal Nk/T-cell lymphoma: a case report and literature review. Transl Cancer Res. (2020) 9:7359–65. doi: 10.21037/tcr-20-2151
8. Dunning, KK, Wudhikarn, K, Safo, AO, Holman, CJ, McKenna, RW, and Pambuccian, SE. Adrenal extranodal NK/T-cell lymphoma diagnosed by fine-needle aspiration and cerebrospinal fluid cytology and immunophenotyping: a case report. Diagn Cytopathol. (2009) 37:686–95. doi: 10.1002/dc.21077
9. Liu, CY, Chen, BJ, and Chuang, SS. Malignant effusions from extranodal NK/T-cell lymphomas are frequently of anaplastic morphology with azurophilic granules and of T-cell lineage. Diagn Cytopathol. (2020) 48:453–63. doi: 10.1002/dc.24388
10. Tai, CF, Chang, LY, Lin, DT, Lin, KH, Jou, ST, and Yang, YL. A case of natural killer cell lymphoma presenting with bilateral pleural effusions and Hemophagocytic Lymphohistocytosis. Pediatr Blood Cancer. (2009) 52:666–9. doi: 10.1002/pbc.21943
11. Mori, H, Ebisawa, K, Nishimura, M, and Kanazawa, K. Late diagnosis: a case of rapidly progressive Extranodal Nk/T cell lymphoma, nasal type. BMJ Case Rep. (2018) 19. doi: 10.1136/bcr-2017-221019
12. Guo, N, Zhou, C, Wang, Y, Fu, J, Chen, Y, Wang, F, et al. Primary intestinal T-cell and natural killer-cell lymphomas: Clinicopathologic and prognostic features of 79 cases in South China. Am J Clin Pathol. (2025) 163:121–33. doi: 10.1093/ajcp/aqae102
13. Wan Ahmad Kammal, WS, Mohd Rose, I, Md Zin, RR, Raja Ali, RA, and Masir, N. Extranodal NK/T-cell lymphoma mimicking Crohn's colitis. Malays J Pathol. (2019) 41:195–9.
14. Chaves, FP, Quillen, K, and Xu, D. Pericardial effusion: a rare presentation of adult T-cell leukemia/lymphoma. Am J Hematol. (2004) 77:381–3. doi: 10.1002/ajh.20229
15. Herishanu, Y, Perry, C, Braunstein, R, Metser, U, Goor, O, Rogowski, O, et al. Early-mid treatment C-reactive protein level is a prognostic factor in aggressive non-Hodgkin's lymphoma. Eur J Haematol. (2007) 79:150–4. doi: 10.1111/j.1600-0609.2007.00894.x
16. Kumabe, A, Hatakeyama, S, Kanda, N, Yamamoto, Y, and Matsumura, M. Utility of ascitic fluid adenosine deaminase levels in the diagnosis of tuberculous peritonitis in general medical practice. Can J Infect Dis Med Microbiol. (2020) 2020:5792937. doi: 10.1155/2020/5792937
17. Bairey, O, Blickstein, D, Stark, P, Prokocimer, M, Nativ, HM, Kirgner, I, et al. Serum ca 125 as a prognostic factor in non-Hodgkin's lymphoma. Leuk Lymphoma. (2003) 44:1733–8. doi: 10.1080/1042819031000104079
18. Santos, GC, Longatto-Filho, A, de Carvalho, LV, Neves, JI, and Alves, AC. Immunocytochemical study of malignant lymphoma in serous effusions. Acta Cytol. (2000) 44:539–42. doi: 10.1159/000328526
19. Fang, JC, Xia, ZX, Wang, CN, and Li, Z. Clinicopathologic and Immunophenotypic features of primary intestinal Extranodal Nk/T-cell lymphoma, nasal type. Int J Surg Pathol. (2015) 23:609–16. doi: 10.1177/1066896915595863
20. Liang, R. Advances in the management and monitoring of Extranodal Nk/T-cell lymphoma, nasal type. Br J Haematol. (2009) 147:13–21. Epub 2009/07/17. doi: 10.1111/j.1365-2141.2009.07802.x
21. Yamaguchi, M, Suzuki, R, and Oguchi, M. Advances in the treatment of extranodal NK/T-cell lymphoma, nasal type. Blood. (2018) 131:2528–40. doi: 10.1182/blood-2017-12-791418
22. Philippe Walter, L, Couronné, L, Jais, JP, Nguyen, PD, Blaise, D, Pigneux, A, et al. Outcome after hematopoietic stem cell transplantation in patients with Extranodal natural killer/T-cell lymphoma, nasal type: a French study from the Société francophone De Greffe De Moelle et De Thérapie Cellulaire (Sfgm-Tc). Am J Hematol. (2021) 96:834–45. doi: 10.1002/ajh.26200
23. Lin, GW, Xu, C, Chen, K, Huang, HQ, Chen, J, Song, B, et al. Genetic risk of Extranodal natural killer T-cell lymphoma: a genome-wide association study in multiple populations. Lancet Oncol. (2020) 21:306–16. doi: 10.1016/s1470-2045(19)30799-5
24. Sun, ZH, Zhou, HM, Song, GX, Zhou, ZX, and Bai, L. Intestinal T-cell lymphomas: a retrospective analysis of 68 cases in China. World J Gastroenterol. (2014) 20:296–302. doi: 10.3748/wjg.v20.i1.296
Keywords: small intestinal NK/T-cell lymphoma, malignant peritoneal effusion, T-cell lymphoma diagnosis, treatment, case report
Citation: Zhou B, Zhao Q, Li X, Liu H and Hao H (2025) Small intestinal NK/T-cell lymphoma with malignant peritoneal effusion as the first symptom: a case report and literature review. Front. Med. 12:1610820. doi: 10.3389/fmed.2025.1610820
Edited by:
Alessandro Poggi, San Martino Hospital (IRCCS), ItalyReviewed by:
Wenjun Meng, Sichuan University, ChinaVishal Busa, Upstate Medical University, United States
Copyright © 2025 Zhou, Zhao, Li, Liu and Hao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hua Hao, aGFvaHVhNDEwQHRvbmdqaS5lZHUuY24=
 Bing Zhou
Bing Zhou Qihan Zhao2
Qihan Zhao2 Hua Hao
Hua Hao