- Department of Hepatobiliary, Pancreas & Spleen Surgery, Beijing Chao-Yang Hospital, Capital Medical University, Beijing, China
Background: The assessment of resectability in borderline resectable pancreatic cancer (BRPC) primarily relies on preoperative imaging, which examines the spatial association between the tumor and adjacent vital veins, particularly the portal venous system. However, venous invasion detected via imaging is rarely confirmed by postoperative pathology. This research seeks to assess the long-term results in patients with pathologically verified venous invasion and to explore the related risk factors.
Methods: This study involved 248 individuals who underwent radical pancreaticoduodenectomy (PD) from January 2011 to July 2023. Patients were classified into three categories based on preoperative imaging and postoperative pathology: no venous invasion (n = 99), imaging-suspected (n = 30), and pathology-confirmed (n = 119). Clinical features and prognoses were compared among groups. In the cohort with pathology-confirmed venous invasion, patients were further classified by invasion depth into intimal (n = 51) and non-intimal (n = 68) subgroups for risk factor analysis.
Results: Patients with pathologically confirmed venous invasion exhibited significantly reduced disease-free survival (DFS) and overall survival (OS) when compared to those with either imaging-suspected or absent venous invasion (p < 0.001). Further analysis of pathology-confirmed venous invasion patients showed significantly lower DFS (p = 0.008) and OS (p < 0.001) compared to non-intimal invasion patients. Independent risk variables for poor DFS included age ≥60 years, tumor diameter >3 cm, and intimal invasion. Independent risk factors for poor OS in patients with venous invasion included age ≥60 years, poorly differentiated tumors, intimal invasion, and TNM stage III.
Conclusion: Intimal venous invasion emerged as a distinct risk factor influencing postoperative survival. Radical surgical resection remains essential for attaining favorable long-term outcomes in pancreatic cancers.
Introduction
Pancreatic carcinoma, being a malignancy of high aggressiveness, has seen an increasing incidence and mortality rate globally and has a mere 8% five-year survival rate (1, 2). Due to its unique anatomic location, pancreatic cancer easily invades the adjacent portal venous system. Additionally, patients often exhibit subtle symptoms, and 80% are already in the locally advanced stage at the time of diagnosis, further increasing the difficulty of surgical resection (3). Although chemotherapy and immunotherapy have rapidly advanced in cancer treatment over the past decades, their efficacy in pancreatic cancer remains limited (4–6). Radical surgical resection remains essential for attaining favorable long-term outcomes in pancreatic cancers.
Pancreatic neoplasms are stratified into three distinct surgical feasibility categories—“Resectable,” “Borderline Resectable,” and “Locally Advanced”—through systematic evaluation of anatomical interactions between malignant masses and critical vascular structures in the abdominal region (7). Extensive evidence has demonstrated the safety and effectiveness of radical PD combined with venous resection and reconstruction (8, 9). While neoadjuvant therapy can facilitate downstaging in certain BRPC patients, the conversion rate for neoadjuvant sequential therapy ranges from 12 to 27% (10, 11). Certain patients with BRPC may still achieve a surgical cure at specialized pancreatic centers by upfront surgery accompanied by venous resection and reconstruction.
Currently, the definition of BRPC is predominantly reliant on preoperative imaging to assess the interaction between the pancreatic tumor and surrounding venous structures, hence defining the degree of venous invasion to establish the cancer’s resectability. Nonetheless, a certain level of false positives persists in the diagnosis of BRPC, even among proficient radiologists or pancreatic surgeons. Pathology, regarded as the gold standard, is hardly utilized in assessing the degree of vascular invasion in pancreatic ductal adenocarcinoma (PDAC) patients. The present research conducted a retrospective analysis of the clinical data from individuals diagnosed with pancreatic carcinoma, integrating preoperative imaging assessments and postoperative pathology findings, to further explore the predictive outcomes of individuals receiving radical resection.
Materials and methods
Patient selection
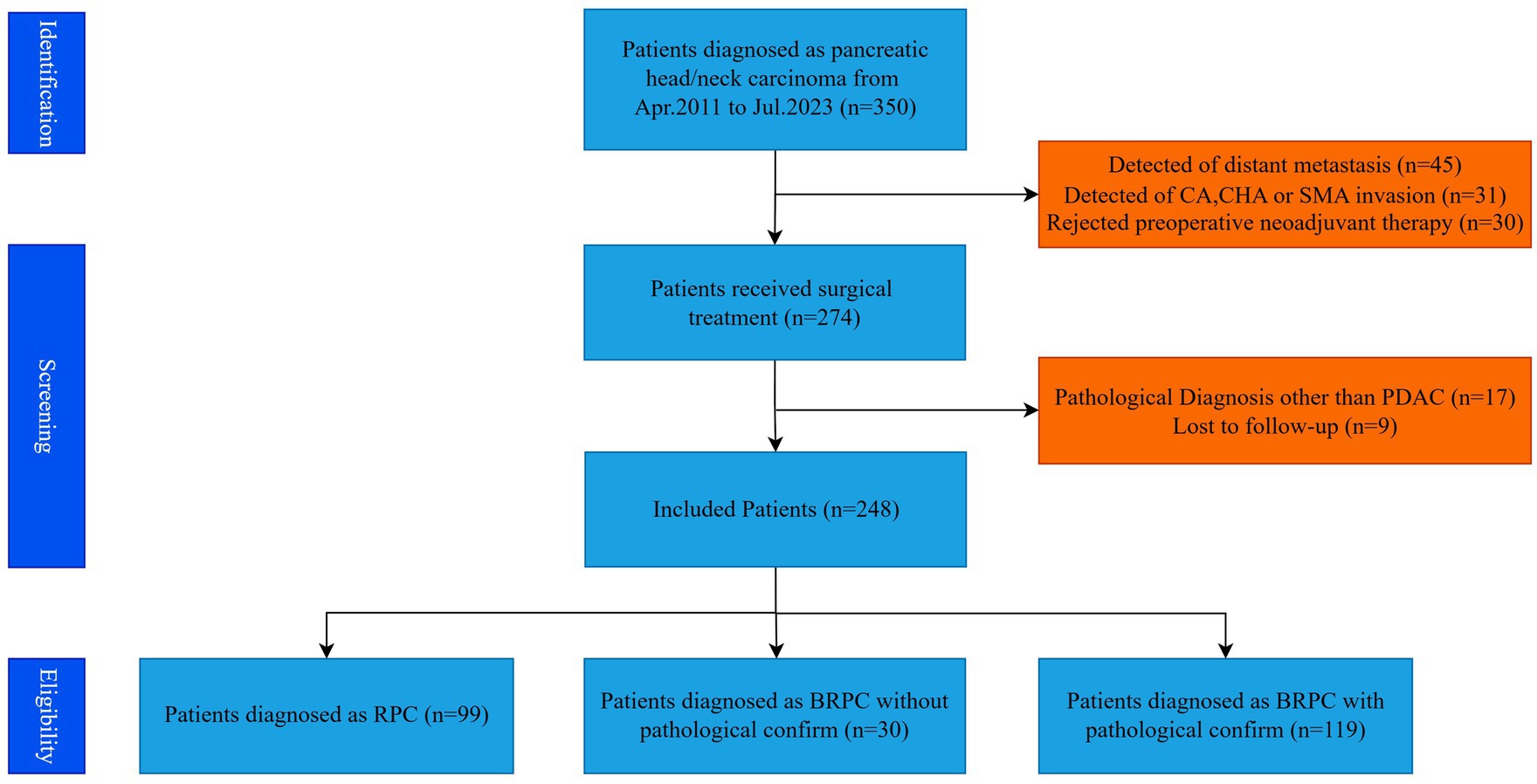
A retrospective analysis was performed on the clinical data of 248 pancreatic cancer patients who underwent radical PD at the Department of Hepatobiliary Surgery, Beijing Chaoyang Hospital, Capital Medical University, from Jan 2011 to July 2023. The standards for patient inclusion and exclusion in this particular study are depicted in Figure 1. Criteria for inclusion: (1) Preoperative imaging identifies resectable or borderline resectable pancreatic cancer, as defined by the 2014 National Comprehensive Cancer Network (NCCN) guidelines (7); (2) Tumor situated in the pancreatic head or neck; (3) Postoperative pathology confirms pancreatic adenocarcinoma; (4) Informed consent for the surgical procedure was secured from the patient and their family. Exclusion criteria: (1) Administration of neoadjuvant therapy before surgery; (2) Intraoperative identification of vascular invasion; (3) Intraoperative identification of distant metastases; (4) Loss to follow-up. The research adhered to the Declaration of Helsinki (2013 revision) and received approval from the Ethics Committee at Beijing Chao-Yang Hospital (No. 2021-D-16) for the use of allogeneic venous vascular replacement procedures; as a retrospective study, it was exempt from informed consent requirements.
Patient grouping and definition
Patients were categorized according to preoperative imaging and postoperative pathology findings. Ninety-nine patients had radical PD, with preoperative imaging and postoperative pathology indicating the absence of venous invasion, and were categorized into the no venous invasion group (Group 1, n = 99). Thirty patients had radical PD together with resection and repair of the involved vein. Preoperative imaging indicated potential invasion of the portal venous system; however, postoperative pathology did not substantiate venous invasion, resulting in classification into the imaging-suspected venous invasion group (Group 2, n = 30). One hundred and nineteen patients had radical PD together with resection and repair of the involved vein. Preoperative imaging revealed invasion of the portal venous system, and postoperative pathology corroborated venous invasion, so categorizing it into the pathology-confirmed venous invasion group (Group 3, n = 119). Patients with pathology-confirmed venous invasion were classified into two subgroups depending on the level of invasion: the intimal invasion group (n = 51) and the non-intimal invasion group (n = 68).
Surgical strategy
All patients underwent complete tumor excision during surgery, succeeded by radical PD with lymphadenectomy. In individuals suspected of having venous invasion associated with pancreatic cancer (Figure 2A), following the resection of the invaded vein. Vascular reconstruction may be executed by directly suturing the vein with 6-0 or 7-0 Prolene vascular sutures, therefore maintaining vascular tension and hemodynamic stability. Alternatively, preserved allogenic vessels may be employed, adjusted to the requisite length and configuration, and sutured at both extremities using 6-0 or 7-0 Prolene vascular sutures. The posterior and anterior walls are constantly sutured to finalize the anastomosis between the recipient and allogenic vessels (Figure 2B). Postoperatively, low-molecular-weight heparin is supplied to avert venous thrombosis in the repaired vein, contingent upon the exclusion of postoperative bleeding risk.
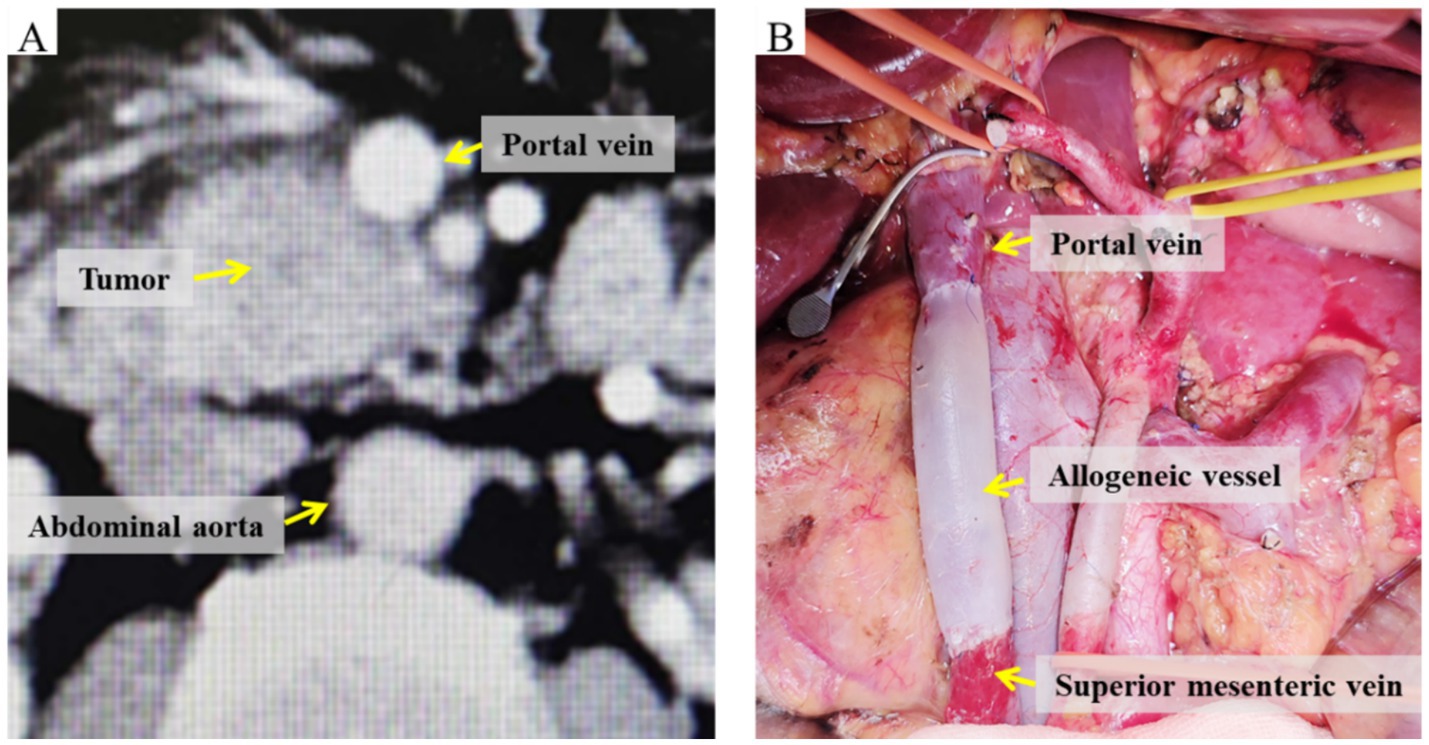
Figure 2. Images of the involved vein before and after radical PD with venous resection and reconstruction. (A) Preoperative CT demonstrating tumor invasion of the portal vein. (B) Intraoperative view of allogeneic vein graft reconstruction.
Follow-up strategy
Patients underwent re-evaluation at 1 month and 3 months post-surgery, followed by assessments every 3 months during the initial 2 years after the operation. If the results remained stable, the follow-up frequency could be adjusted to once a year. Follow-up evaluations comprised blood tests and imaging procedures, incorporating contrast-enhanced computed tomography (CT) imaging of the abdomen and thorax. OS was defined as the duration from the date of surgery to either the date of death or the last follow-up, while DFS was defined as the time from the surgery date to the identification of local recurrence, metastasis, or the last follow-up assessment. The follow-up period ended in July 2024, with the primary endpoint being patient death.
Statistical analysis
Continuous data were expressed as either the mean ± standard deviation (SD) or the median with interquartile range (IQR). For categorical variables, chi-square or Fisher’s exact tests were employed. Survival outcomes were evaluated using Kaplan–Meier curves, supplemented by log-rank tests. Univariate and multivariate analyses were conducted using the Cox proportional hazards model to determine independent prognostic indicators. A p-value of less than 0.05 was considered statistically significant.
Results
Baseline and clinical features of patients
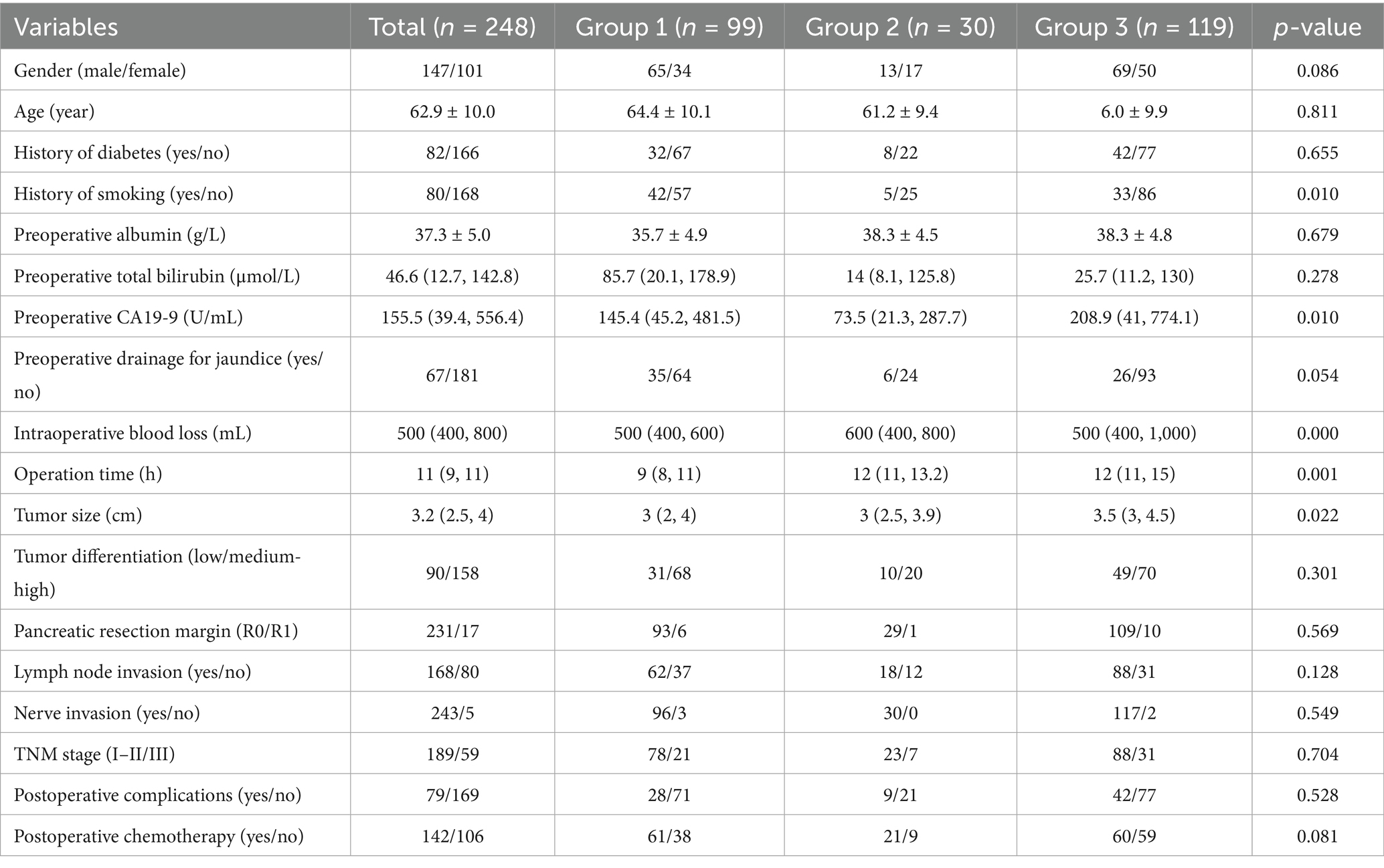
This study included a cohort of 248 consecutive patients, comprising 147 males and 101 females, with a mean age of 62.9 ± 10.0 years. The primary symptoms at the onset for these patients were as follows: jaundice was reported in 119 cases, abdominal pain in 87 cases, and non-typical gastrointestinal symptoms in 12 cases. Additionally, a diabetic history was identified in 82 participants (33.1%). Among those presenting with jaundice, 67 received preoperative treatments aimed at reducing jaundice, which included endoscopic retrograde cholangiopancreatography (n = 10) and percutaneous transhepatic biliary drainage (n = 57).
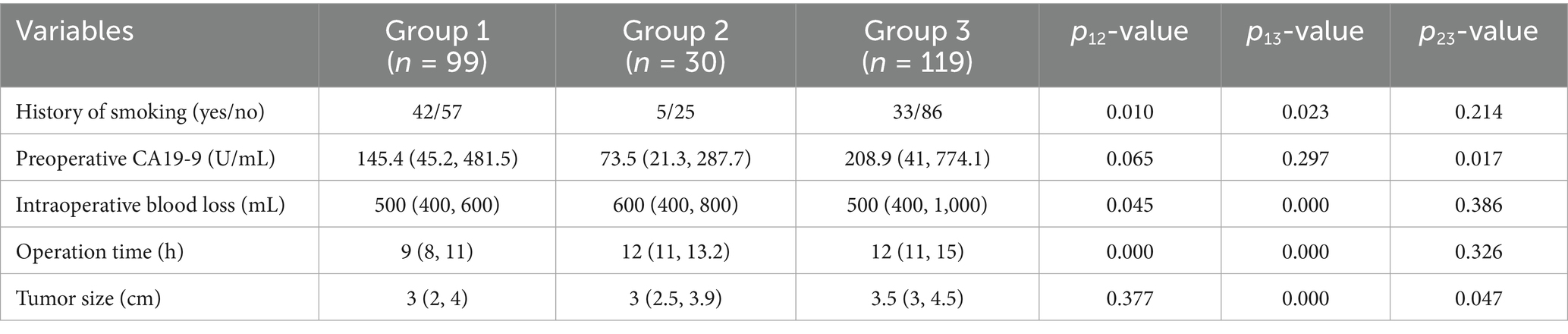
As is shown in Tables 1, 2, compared to patients in Group 1, a higher proportion of patients in Groups 2 and 3 had a history of smoking, with p-values of 0.01 and 0.023, respectively. Concurrently, as observed in Table 2, due to the combined resection and reconstruction of involved veins, patients in Groups 2 and 3 required longer operative times and experienced greater intraoperative blood loss compared to Group 1, with p-values less than 0.05. Although the overall comparison in Table 1 revealed significant differences in CA19-9 levels and tumor diameter among the three groups, pairwise comparisons indicated that preoperative CA19-9 levels showed notable variations between individuals in Groups 2 and 3, and significant differences in tumor diameter between patients in Groups 1 and 3, as well as between Groups 2 and 3.
Survival outcomes of patients in three groups
In this study, the median duration of DFS was 15 months, with DFS rates of 55.4, 34.4, and 22.4% at 1, 2, and 3 years, respectively. The median OS duration was 18 months, with OS rates following radical surgery of 67.4, 37.6, and 23.4% at 1, 2, and 3 years, respectively.
In the Kaplan–Meier analysis of DFS and OS based on the three groups, patients in Group 3 displayed significantly worse DFS (Figure 3A) and OS (Figure 3B) compared to those in Group 1 and Group 2 (p < 0.001). The median DFS for patients in Group 1 and Group 2 was 21 and 16 months, with corresponding 1-, 2-, and 3-year DFS rates of 77.4, 47.5, 34.4, and 59.5%, 45.2, 31.7%, respectively. The median OS for the two groups was 24 months and 17 months, respectively. The OS rates at 1, 2, and 3 years were 80.7, 50.0, and 34.4% for the Group 1, and 70.0, 46.9, and 41.7% for the Group 2.
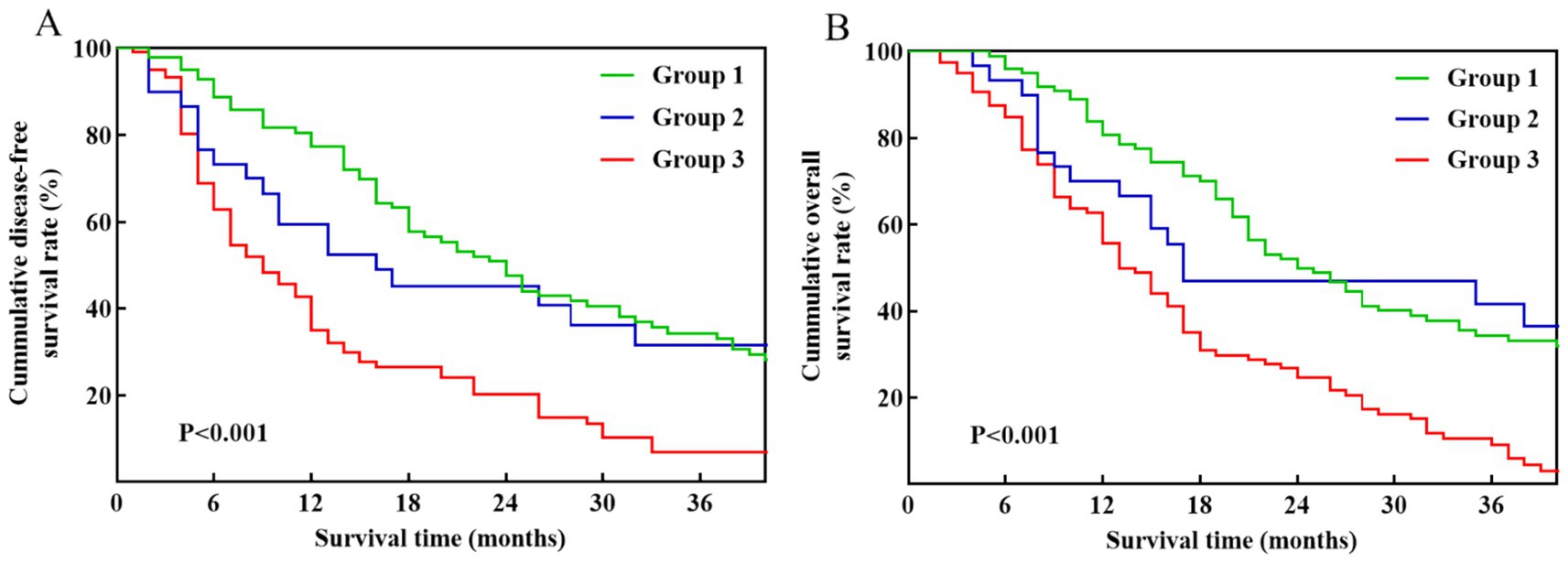
Figure 3. Kaplan–Meier survival curves among pancreatic cancer patients stratified by venous invasion status: Group 1 (no venous invasion), Group 2 (imaging-suspected venous invasion), and Group 3 (pathology-confirmed venous invasion). (A) DFS. (B) OS.
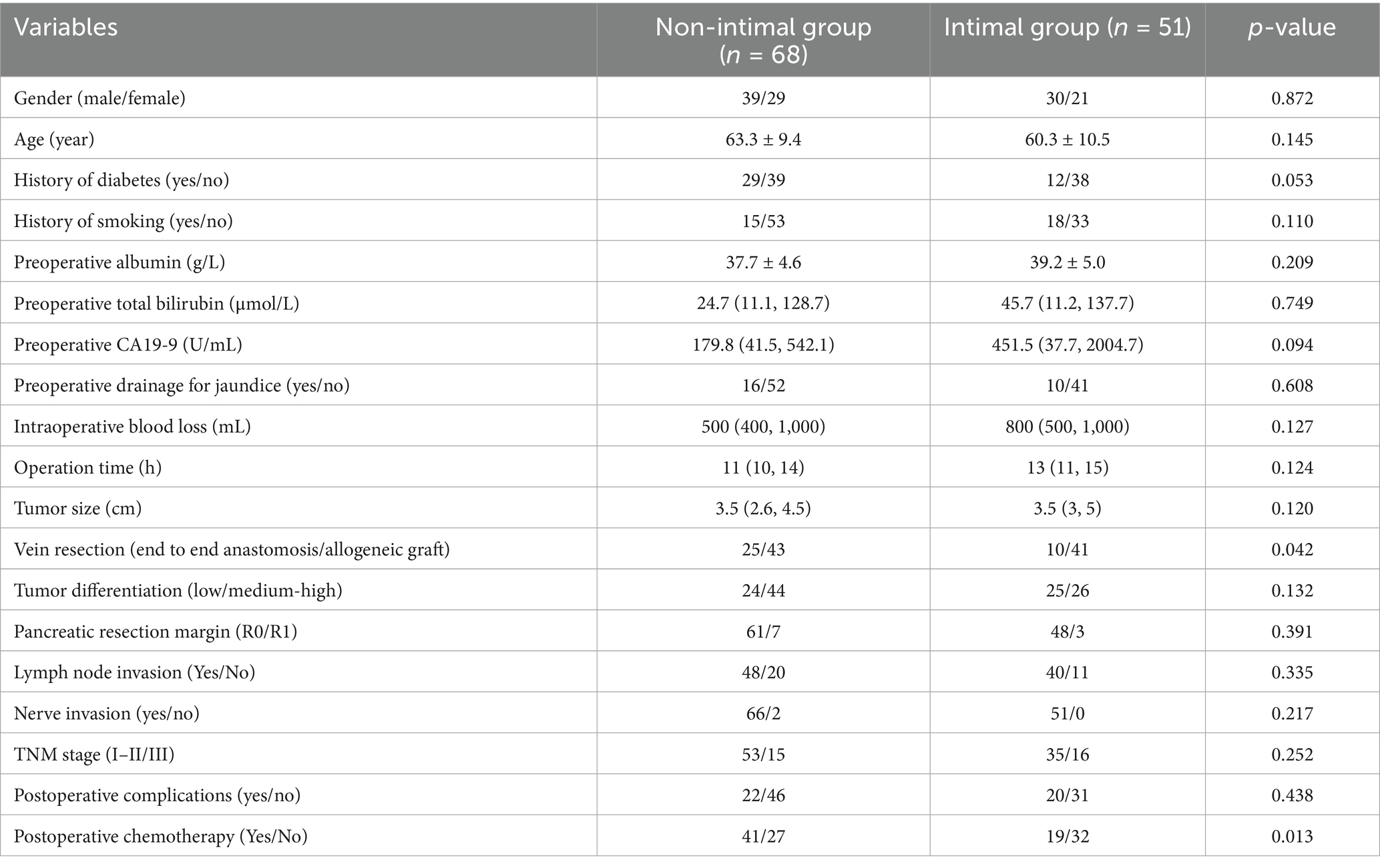
Table 3 illustrates that there are no notable variations were observed in clinical and pathological characteristics between the intimal and non-intimal groups. Among patients with pathology-confirmed venous invasion, those in the non-intimal group demonstrated improved DFS (p = 0.008) and OS (p < 0.001) compared to their counterparts in the intimal group (Figure 4). The median DFS and OS for the intimal group were 9 months and 13 months, respectively.
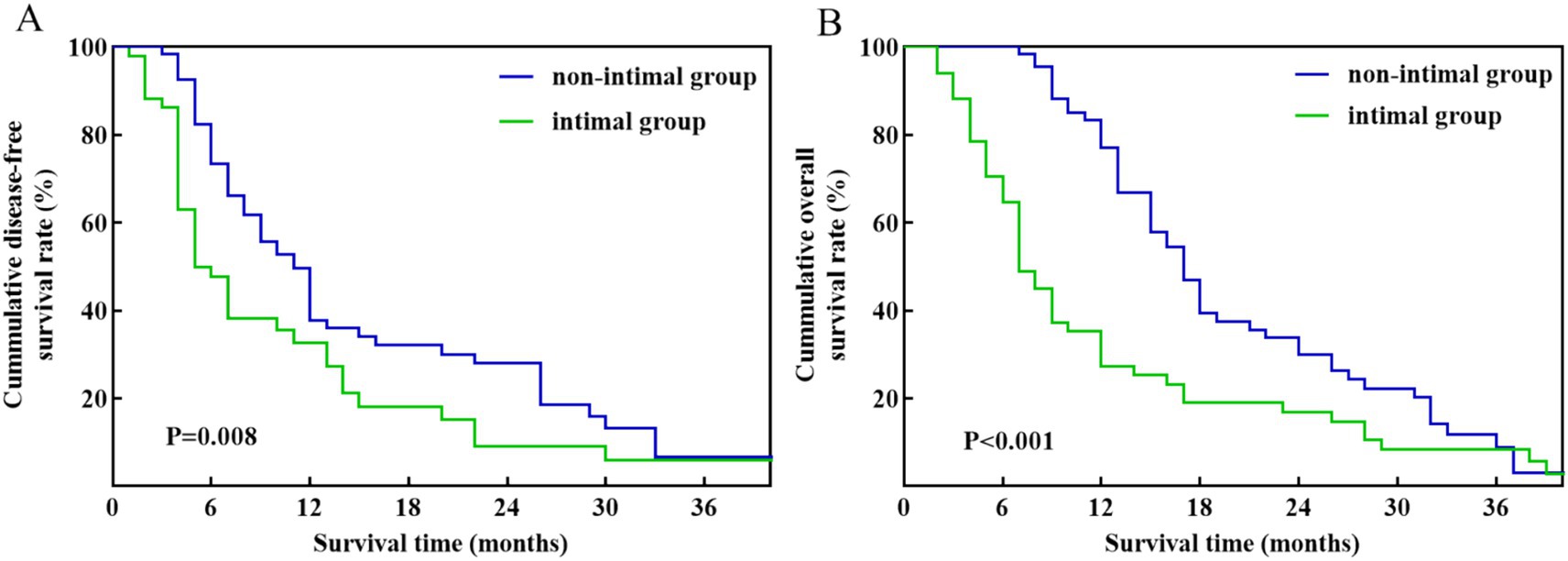
Figure 4. Kaplan–Meier survival curves between the intimal group and non-intimal group patients with pathology-confirmed venous invasion. (A) DFS. (B) OS.
Risk factors associated with prognosis in patients with pathology-confirmed venous invasion
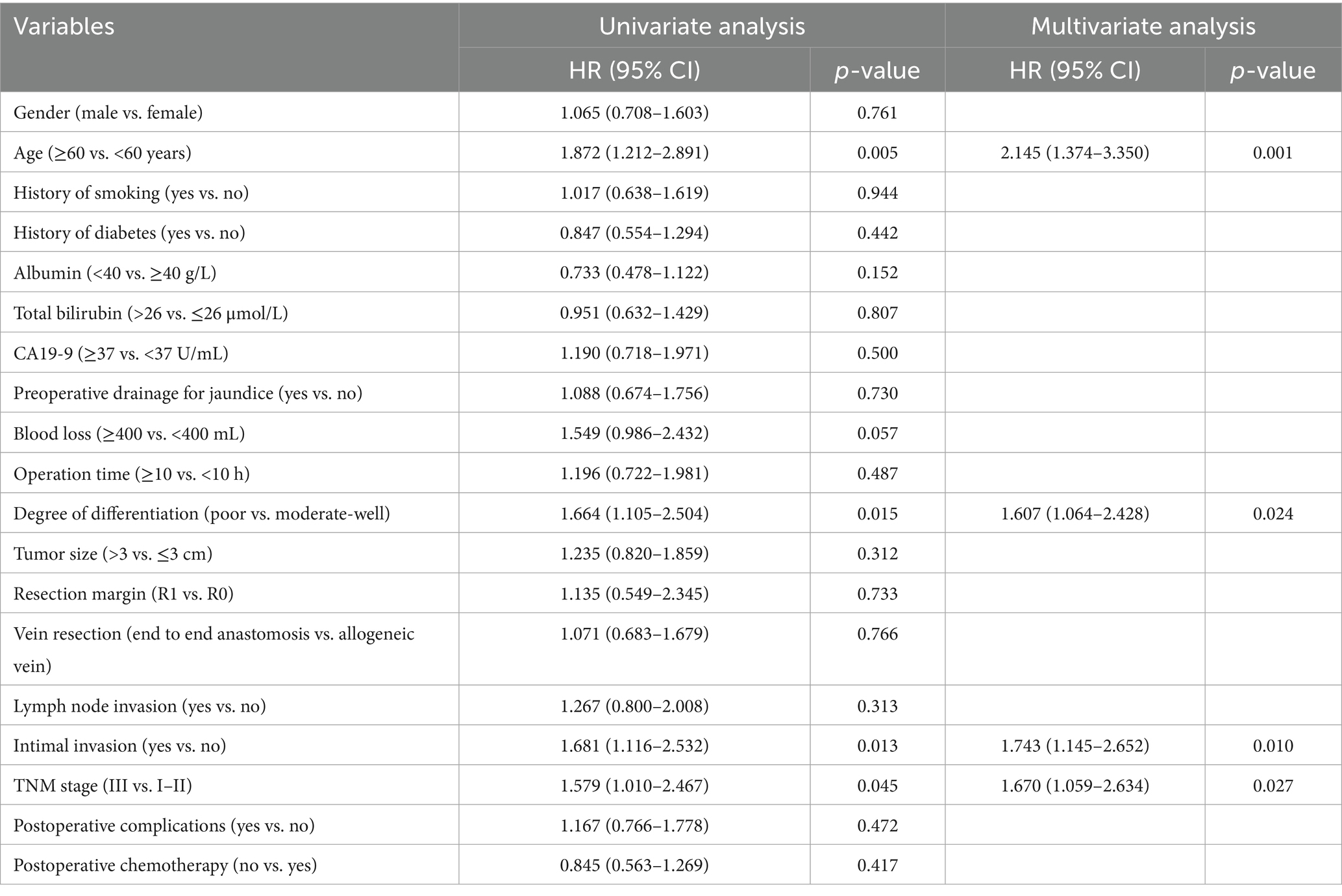
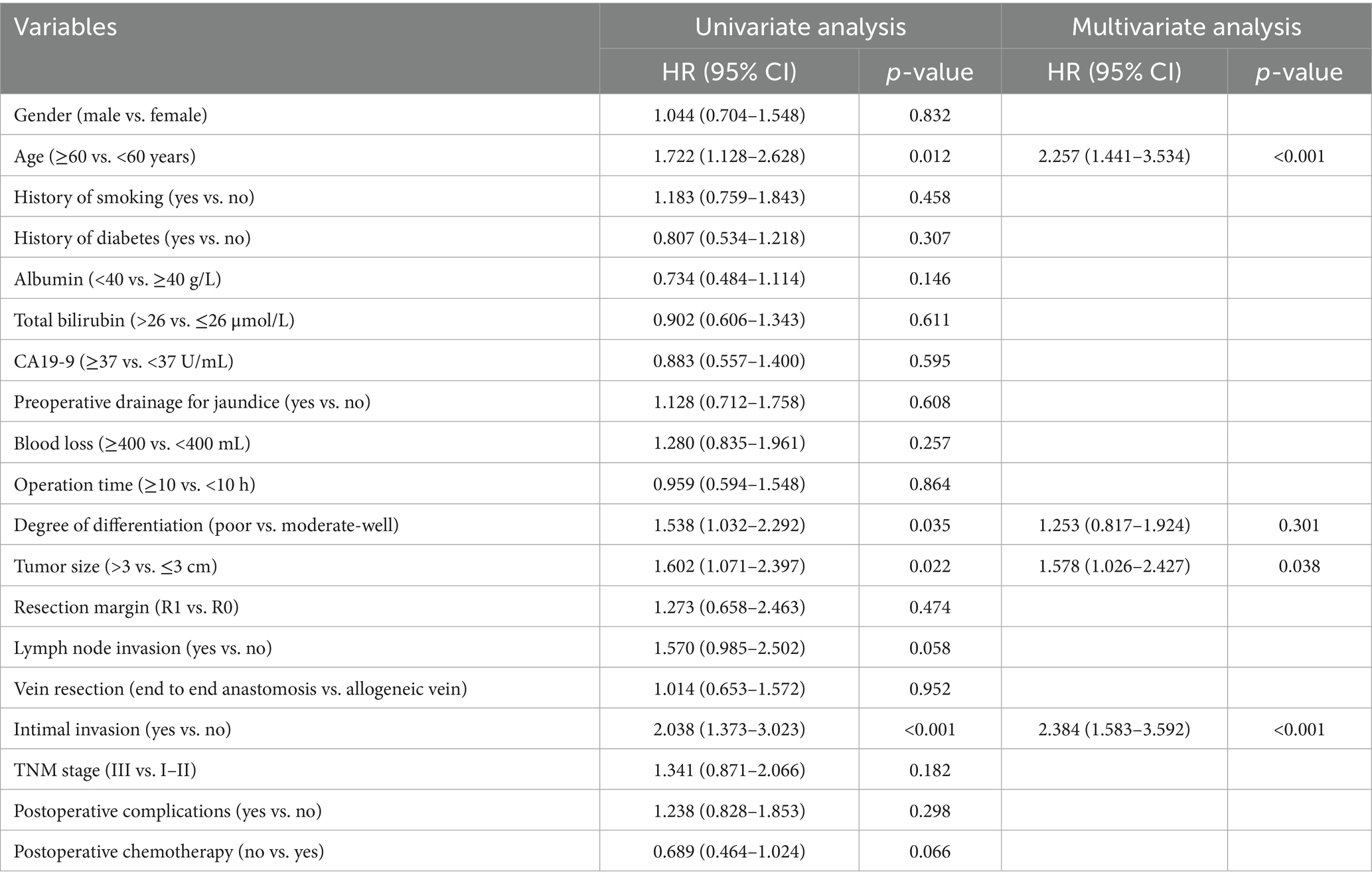
In univariate Cox regression, intimal invasion, age, tumor differentiation, and TNM stage were significant for DFS (p < 0.05). In the multivariate Cox regression of DFS, age (HR: 2.145; 95% CI: 1.374–3.350; p = 0.001), degree of differentiation (HR: 1.607; 95% CI: 1.064–2.428; p = 0.024), intimal invasion (HR: 1.734; 95% CI: 1.145–2.652; p = 0.010), and TNM stage (HR: 1.670; 95% CI: 1.059–2.634; p = 0.027) were independent risk factors (Table 4).
In univariate Cox regression, intimal invasion, age, degree of differentiation, and tumor size were significant for OS (p < 0.05). In the multivariate Cox regression of OS, age (HR: 2.257; 95% CI: 1.441–3.534; p < 0.001), tumor size (HR: 1.578; 95% CI: 1.026–2.427; p = 0.038), intimal invasion (HR: 2.384; 95% CI: 1.583–3.592; p = 0.010) were independent risk factors (Table 5).
Discussion
Pancreatic cancer is susceptible to early local vascular invasion and distant metastases due to its high malignancy and proximity to the portal venous system. Statistics indicate that 17 to 32% of patients demonstrate portal venous system invasion at diagnosis, whereas 50% present with distant metastasis at that time, rendering them ineligible for surgical intervention (12, 13). Siriwardana et al. examined 52 clinical studies encompassing 6,333 patients, of whom 26% exhibited venous invasion during surgery and subsequently underwent simultaneous resection and reconstruction of the involved portal vein or mesenteric vein (14). A meta-analysis of 5,242 samples revealed that 1,218 samples were pathologically proven to demonstrate tumor infiltration in the superior mesenteric vein or portal vein based on histopathological analysis following surgery, resulting in an invasion rate of 23.2% (15). For these patients, the key to surgical treatment lies in the rational and effective resection and reconstruction of the invaded veins.
Advancements in surgical procedures and perioperative management have demonstrated that pancreatic cancer resection, along with the removal and reconstruction of invaded veins, is a safe approach. In 2006, Varadhachary et al. (16) from the University of Texas MD Anderson Cancer Center first proposed the criteria for BRPC. Three years later, the American Hepato-Pancreato-Biliary Association published expert consensus guidelines on BRPC (17). Since that time, the resection of pancreatic cancer, in conjunction with vascular resection and repair, has emerged as the primary strategy for managing BRPC. A meta-analysis of 19 trials and 2,247 patients evaluated the outcomes of standard PD against PD with venous invasion resection. The findings indicated no substantial difference in perioperative complication rates or mortality rates between the two cohorts. Additionally, the overall survival rates over 5 years were comparable between the two groups of patients (OR: 0.57; 95% CI: 0.32–1.02; p = 0.06) (18). This further substantiates the safety of PD in conjunction with venous invasion resection and reconstruction, while also offering the potential for long-term survival for pancreatic cancer patients with portal venous system involvement. Our study additionally evaluated the long-term prognostic results of PD with concomitant venous resection in comparison to standard PD. In pancreatic cancer patients lacking pathology-confirmed venous invasion, the execution of PD with venous resection and repair did not influence their long-term survival outcomes.
From a radiological standpoint, pancreatic cancer characterized by low vascular involvement yet still suitable for surgical resection is classified as BRPC. The NCCN recommendations characterize BRPC as tumor involvement in the superior mesenteric vein-portal venous system exhibiting segmental constriction, tortuosity, or blockage (7), while yet allowing for safe reconstruction following resection. In these individuals, preoperative imaging is predominantly utilized to evaluate vascular invasion in pancreatic cancer. Previous studies suggest that the accuracy in detecting and the ability to rule out vascular invasion in PDAC using CT range from 0.58 to 0.63 and 0.92 to 0.95, respectively, whereas for EUS, the sensitivity and specificity range from 0.72 to 0.87 and 0.89 to 0.93, respectively (19–21). Despite the significantly superior sensitivity of preoperative contrast-enhanced ultrasonography (CEUS) in evaluating vascular invasion in pancreatic cancer, a notable incidence of misinterpretation persists. The proximity of inflammatory adhesions between specific arteries and tumor tissue complicates the differentiation of vascular invasion from a singular imaging viewpoint. In recent years, image-based machine learning algorithms have demonstrated significant potential in the prompt identification of pancreatic cancer (22–24), and we can similarly anticipate that machine learning will contribute to the identification of vascular invasion in pancreatic cancer.
The early clinical manifestations of pancreatic cancer lack specificity and often present as latent, nonspecific symptoms (25). These nonspecific symptoms lead to most patients being diagnosed at a borderline resectable or local advanced stage, with the tumor potentially involving major vascular structures, gradually infiltrating from the adventitia to the intima. Intimal invasion is often accompanied by the disappearance of the fat space between the pancreas and the vessel. Invasion of the muscular layer may lead to significant morphological changes in the vessels. When the tumor invades the vascular intima, it is typically associated with significant narrowing or morphological abnormalities of the portal vein or superior mesenteric vein, and may even lead to complete occlusion. Meanwhile, CD133, which is associated with cell polarity, is significantly elevated during the invasion of veins in pancreatic cancer (26–28). Patients with these characteristics generally have a poorer prognosis. In our study, we also observed that patients with pathology-confirmed venous invasion had worse overall survival and progression-free survival compared to RBC patients or BRPC with imaging-suspected venous invasion. Intimal invasion is associated with a worse prognosis compared to non-intimal invasion patients. In a retrospective study by Boggi et al. (29), involving 110 patients who underwent PD with venous resection and reconstruction for pancreatic cancer, it was observed that those with the intimal invasion of the portal venous system typically experienced a markedly poorer prognosis, with a median survival of 11 months. These findings align with the results of our research.
The findings of this study indicate that patients with confirmed pathological invasion have a somewhat unfavorable prognosis, even undergoing intensive surgical interventions that involve resection and repair of affected veins. This may arise from the significant proliferation of circulating tumor cells due to vascular invasion, complicating surgery and increasing the likelihood of postoperative recurrence and metastasis. Fundamental surgical intervention may not yield optimal therapeutic outcomes. Therefore, improving the sensitivity of preoperative imaging in assessing vascular invasion is crucial for informing the comprehensive treatment approach for these patients. The 2017 NCCN guidelines emphasize the need for neoadjuvant therapy in the treatment of non-metastatic pancreatic cancer, including borderline resectable and locally advanced cases (30). Neoadjuvant therapy provides the advantage of eliminating potential circulating tumor cells and micrometastases, hence reducing the likelihood of rapid tumor progression post-surgery and enhancing the surgical benefit. A multicenter phase III clinical trial showed that neoadjuvant sequential chemotherapy employing gemcitabine as the principal agent for resectable or borderline resectable pancreatic cancer significantly improves overall survival in patients (31). Therefore, those demonstrating confirmed vascular invasion via imaging and pathology may derive a more significant surgical benefit from neoadjuvant sequential therapy.
Constraints of this research
The patient enrollment duration in this study was extensive, and the application of early single-slice spiral CT may have influenced the imaging assessment outcomes for certain individuals. Secondly, despite preoperative imaging evaluations of vascular invasion conducted by several imaging and pancreatic surgery specialists, a certain false positive rate persists. Third, as a single-center retrospective investigation, the sample size was comparatively limited, potentially introducing certain biases in the results. Consequently, multicenter large-sample trials are essential to further corroborate the correctness of the study findings.
Conclusion
Pancreatic cancer patients with pathology-confirmed venous invasion have worse overall survival and disease-free survival compared to those with no invasion of the portal vein or those with imaging-suspected vascular invasion. Intimal invasion serves as an independent prognostic factor affecting postoperative survival in pancreatic cancer patients who exhibit vascular invasion.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Beijing Chao-Yang Hospital (No. 2021-D-16). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
J-CH: Software, Formal analysis, Writing – original draft. C-RZ: Writing – original draft, Software, Formal analysis. H-XW: Data curation, Writing – original draft. S-CL: Writing – review & editing, Methodology. RL: Conceptualization, Supervision, Project administration, Writing – review & editing. TJ: Writing – review & editing, Project administration, Supervision, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported through the Research Program of the Multidisciplinary Clinical Research Innovation Team Project of Beijing Chao-Yang Hospital (CYDXK202212).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AUC, Area under the curve; BRPC, Borderline resectable pancreatic cancer; CI, Confidence interval; CT, Computed tomography; HR, Hazard ratio; NCCN, National Comprehensive Cancer Network; OS, Overall survival; PD, Pancreaticoduodenectomy; PDAC, Pancreatic ductal adenocarcinoma; RFS, Recurrence-free survival.
References
1. Ilic, M, and Ilic, I. Epidemiology of pancreatic cancer. World J Gastroenterol. (2016) 22:9694–705. doi: 10.3748/wjg.v22.i44.9694
2. Siegel, RL, Miller, KD, and Jemal, A. Cancer statistics, 2018. CA Cancer J Clin. (2018) 68:7–30. doi: 10.3322/caac.21442
3. Li, D, Xie, K, Wolff, R, and Abbruzzese, JL. Pancreatic cancer. Lancet. (2004) 363:1049–57. doi: 10.1016/S0140-6736(04)15841-8
4. Bear, AS, Vonderheide, RH, and O’Hara, MH. Challenges and opportunities for pancreatic cancer immunotherapy. Cancer Cell. (2020) 38:788–802. doi: 10.1016/j.ccell.2020.08.004
5. Qin, C, Yang, G, Yang, J, Ren, B, Wang, H, Chen, G, et al. Metabolism of pancreatic cancer: paving the way to better anticancer strategies. Mol Cancer. (2020) 19:50. doi: 10.1186/s12943-020-01169-7
6. Strobel, O, Neoptolemos, J, Jager, D, and Buchler, MW. Optimizing the outcomes of pancreatic cancer surgery. Nat Rev Clin Oncol. (2019) 16:11–26. doi: 10.1038/s41571-018-0112-1
7. Tempero, MA, Malafa, MP, Behrman, SW, Benson, AB 3rd, Casper, ES, Chiorean, EG, et al. Pancreatic adenocarcinoma, version 2.2014: featured updates to the NCCN guidelines. J Natl Compr Cancer Netw. (2014) 12:1083–93. doi: 10.6004/jnccn.2014.0106
8. Ravikumar, R, Sabin, C, Abu Hilal, M, Al-Hilli, A, Aroori, S, Bond-Smith, G, et al. Impact of portal vein infiltration and type of venous reconstruction in surgery for borderline resectable pancreatic cancer. Br J Surg. (2017) 104:1539–48. doi: 10.1002/bjs.10580
9. Vuorela, T, Vikatmaa, P, Kokkola, A, Mustonen, H, Salmiheimo, A, Eurola, A, et al. Long term results of pancreatectomy with and without venous resection: a comparison of safety and complications of spiral graft, end-to-end and tangential/patch reconstruction techniques. Eur J Vasc Endovasc Surg. (2022) 64:244–53. doi: 10.1016/j.ejvs.2022.04.006
10. Katz, MH, Fleming, JB, Bhosale, P, Varadhachary, G, Lee, JE, Wolff, R, et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer. (2012) 118:5749–56. doi: 10.1002/cncr.27636
11. Katz, MH, Shi, Q, Ahmad, SA, Herman, JM, Marsh, RW, Collisson, E, et al. Preoperative modified FOLFIRINOX treatment followed by capecitabine-based chemoradiation for borderline resectable pancreatic cancer: Alliance for Clinical Trials in Oncology trial A021101. JAMA Surg. (2016) 151:e161137. doi: 10.1001/jamasurg.2016.1137
12. Bockhorn, M, Uzunoglu, FG, Adham, M, Imrie, C, Milicevic, M, Sandberg, AA, et al. Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. (2014) 155:977–88. doi: 10.1016/j.surg.2014.02.001
14. Siriwardana, HP, and Siriwardena, AK. Systematic review of outcome of synchronous portal-superior mesenteric vein resection during pancreatectomy for cancer. Br J Surg. (2006) 93:662–73. doi: 10.1002/bjs.5368
15. Song, A, Liu, F, Wu, L, Si, X, and Zhou, Y. Histopathologic tumor invasion of superior mesenteric vein/portal vein is a poor prognostic indicator in patients with pancreatic ductal adenocarcinoma: results from a systematic review and meta-analysis. Oncotarget. (2017) 8:32600–7. doi: 10.18632/oncotarget.15938
16. Varadhachary, GR, Tamm, EP, Abbruzzese, JL, Xiong, HQ, Crane, CH, Wang, H, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. (2006) 13:1035–46. doi: 10.1245/ASO.2006.08.011
17. Vauthey, JN, and Dixon, E. AHPBA/SSO/SSAT consensus conference on resectable and borderline resectable pancreatic cancer: rationale and overview of the conference. Ann Surg Oncol. (2009) 16:1725–6. doi: 10.1245/s10434-009-0409-5
18. Zhou, Y, Zhang, Z, Liu, Y, Li, B, and Xu, D. Pancreatectomy combined with superior mesenteric vein-portal vein resection for pancreatic cancer: a meta-analysis. World J Surg. (2012) 36:884–91. doi: 10.1007/s00268-012-1461-z
19. Li, JH, He, R, Li, YM, Cao, G, Ma, QY, and Yang, WB. Endoscopic ultrasonography for tumor node staging and vascular invasion in pancreatic cancer: a meta-analysis. Dig Surg. (2014) 31:297–305. doi: 10.1159/000368089
20. Si, K, Wu, H, Yang, M, Guo, Y, Zhang, X, Ding, C, et al. Utility of dark-blood dual-energy CT images for predicting vascular involvement and R0 resection in patients with pancreatic cancer. AJR Am J Roentgenol. (2023) 220:838–48. doi: 10.2214/AJR.22.28640
21. Yang, R, Lu, M, Qian, X, Chen, J, Li, L, Wang, J, et al. Diagnostic accuracy of EUS and CT of vascular invasion in pancreatic cancer: a systematic review. J Cancer Res Clin Oncol. (2014) 140:2077–86. doi: 10.1007/s00432-014-1728-x
22. Cao, K, Xia, Y, Yao, J, Han, X, Lambert, L, Zhang, T, et al. Large-scale pancreatic cancer detection via non-contrast CT and deep learning. Nat Med. (2023) 29:3033–43. doi: 10.1038/s41591-023-02640-w
23. Mukherjee, S, Patra, A, Khasawneh, H, Korfiatis, P, Rajamohan, N, Suman, G, et al. Radiomics-based machine-learning models can detect pancreatic cancer on prediagnostic computed tomography scans at a substantial lead time before clinical diagnosis. Gastroenterology. (2022) 163:1435–1446.e3. doi: 10.1053/j.gastro.2022.06.066
24. Yuan, L, Yang, L, Zhang, S, Xu, Z, Qin, J, Shi, Y, et al. Development of a tongue image-based machine learning tool for the diagnosis of gastric cancer: a prospective multicentre clinical cohort study. EClinicalMedicine. (2023) 57:101834. doi: 10.1016/j.eclinm.2023.101834
25. Kawai, M, Fukuda, A, Otomo, R, Obata, S, Minaga, K, Asada, M, et al. Early detection of pancreatic cancer by comprehensive serum miRNA sequencing with automated machine learning. Br J Cancer. (2024) 131:1158–68. doi: 10.1038/s41416-024-02794-5
26. Ding, Q, Miyazaki, Y, Tsukasa, K, Matsubara, S, Yoshimitsu, M, and Takao, S. CD133 facilitates epithelial-mesenchymal transition through interaction with the ERK pathway in pancreatic cancer metastasis. Mol Cancer. (2014) 13:15. doi: 10.1186/1476-4598-13-15
27. Hermann, PC, Huber, SL, Herrler, T, Aicher, A, Ellwart, JW, Guba, M, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. (2007) 1:313–23. doi: 10.1016/j.stem.2007.06.002
28. Immervoll, H, Hoem, D, Sakariassen, PO, Steffensen, OJ, and Molven, A. Expression of the “stem cell marker” CD133 in pancreas and pancreatic ductal adenocarcinomas. BMC Cancer. (2008) 8:48. doi: 10.1186/1471-2407-8-48
29. Boggi, U, Del Chiaro, M, Croce, C, Vistoli, F, Signori, S, Moretto, C, et al. Prognostic implications of tumor invasion or adhesion to peripancreatic vessels in resected pancreatic cancer. Surgery. (2009) 146:869–81. doi: 10.1016/j.surg.2009.04.029
30. Tempero, MA, Malafa, MP, Al-Hawary, M, Asbun, H, Bain, A, Behrman, SW, et al. Pancreatic adenocarcinoma, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. (2017) 15:1028–61. doi: 10.6004/jnccn.2017.0131
31. Versteijne, E, van Dam, JL, Suker, M, Janssen, QP, Groothuis, K, Akkermans-Vogelaar, JM, et al. Neoadjuvant chemoradiotherapy versus upfront surgery for resectable and borderline resectable pancreatic cancer: long-term results of the Dutch randomized PREOPANC trial. J Clin Oncol. (2022) 40:1220–30. doi: 10.1200/JCO.21.02233
Keywords: pancreatic cancer, venous invasion, pathology, prognosis, pancreaticoduodenectomy
Citation: Huang J-C, Zhang C-R, Wang H-X, Lyu S-C, Lang R and Jiang T (2025) Prognostic implications of venous invasion in pancreatic cancer following radical surgical resection: a pathological perspective. Front. Med. 12:1613153. doi: 10.3389/fmed.2025.1613153
Edited by:
Ihsan Ullah, Khyber Medical University, PakistanReviewed by:
Federica Pedica, San Raffaele Scientific Institute (IRCCS), ItalyXiaolong Tian, Yale University, United States
Copyright © 2025 Huang, Zhang, Wang, Lyu, Lang and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ren Lang, ZHJfbGFuZ3JlbjEyM0AxMjYuY29t; Tao Jiang, ZHJfamlhbmcyMDIzQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Jin-Can Huang
Jin-Can Huang Cheng-Run Zhang
Cheng-Run Zhang Han-Xuan Wang
Han-Xuan Wang Shao-Cheng Lyu
Shao-Cheng Lyu Ren Lang
Ren Lang Tao Jiang*
Tao Jiang*