- 1Suzhou TCM Hospital Affiliated to Nanjing University of Chinese Medicine, Suzhou, Jiangsu, China
- 2Nanjing University of Chinese Medicine, Nanjing, Jiangsu, China
- 3Ruikang Hospital Affiliated with Guangxi University of Chinese Medicine, Nanning, Guangxi, China
- 4Guangxi University of Chinese Medicine, Nanning, Guangxi, China
- 5Suzhou Academy of Wumen Chinese Medicine, Suzhou, Jiangsu, China
Lumbar disc herniation (LDH) is a common and frequently occurring condition primarily caused by lumbar intervertebral disc degeneration (LIVDD) and protrusion of the nucleus pulposus (NP), with low back pain and lower limb pain as the main clinical manifestations. It is characterized by a prolonged disease course and a high recurrence rate, with patients often experiencing long-term suffering, significantly impairing their quality of life and mental health. Studies have found that some LDH patients, without undergoing surgery or chemonucleolysis, experience spontaneous shrinkage and resorption of the intervertebral disc (IVD) tissue, along with relief of back and leg pain-a phenomenon referred to as LDH reabsorption. Modern medical research suggests that this reabsorption process is closely related to factors such as inflammatory responses, macrophage activation, extracellular matrix enzyme balance, neovascularization, ferroptosis, mitochondrial function, oxidative stress, and cellular autophagy. However, the precise mechanisms of LDH reabsorption and commonly used clinical therapies remain unclear, leading to suboptimal treatment outcomes. This study systematically reviews the relevant literature on LDH reabsorption, focusing on clinical diagnosis, underlying mechanisms, and common therapeutic strategies, aiming to summarize recent research progress and provide theoretical references for future clinical prevention and treatment of LDH.
1 Introduction
Lumbar disc herniation (LDH) is a syndrome primarily characterized by low back pain (LBP) and lower extremity pain, resulting from nerve root and cauda equina compression and irritation due to lumbar intervertebral disc degeneration (LIVDD), rupture of the annulus fibrosus (AF), and protrusion of the nucleus pulposus (NP) (1). Due to its chronic course, high recurrence rate, and poor prognosis-with severe cases even resulting in lower limb paralysis and urinary incontinence (2)-LDH imposes a heavy economic burden on families and society. Currently, authoritative epidemiological studies on LDH are lacking; however, numerous studies have demonstrated that the prevalence of LDH increases with age and is closely related to the widespread use of computers and changes in work patterns (3). Recent studies have revealed a phenomenon termed “LDH reabsorption,” in which patients experience spontaneous shrinkage and absorption of intervertebral disc (IVD) tissue, along with alleviation of low back and leg pain, despite receiving no surgical intervention or chemonucleolysis (4). Several clinical studies have gradually confirmed that the phenomenon of LDH reabsorption is widely present in patients with LDH (5, 6), and is associated with factors such as the patient’s age, sex, and body weight. Younger patients are more likely to experience reabsorption compared to older patients. Due to differences in hormone levels, females, influenced by estrogen, have a lower probability of reabsorption than males. Patients with lower body weight are more likely to undergo reabsorption than those with higher body weight (7, 8). Additionally, the larger the protrusion of the IVD tissue, the higher the likelihood of reabsorption occurring. In summary, LDH reabsorption occurs across all age groups in both male and female patients and is present throughout the entire process of the onset and progression of LDH disease. Therefore, studying the mechanisms of LDH reabsorption and its clinical diagnosis and treatment holds significant value.
In systematically reviewing recent studies related to LDH reabsorption, the author found that its occurrence is closely associated with the size of the disc protrusion in LDH, which can be confirmed by imaging techniques such as computed tomography (CT) and magnetic resonance imaging (MRI). Its underlying mechanisms are closely related to inflammatory reactions, tissue vascularization, tissue dehydration, hematoma absorption, autoimmune responses, and cellular autophagy. Therapies such as bed rest, pharmacological treatment, exercise therapy, and epidural injections can facilitate this reabsorption process (9, 10). This article systematically analyzes LDH reabsorption from perspectives of clinical diagnosis, underlying mechanisms, and therapeutic approaches, aiming to provide insights and references for exploring the significance of LDH reabsorption in the clinical management of LDH.
2 Search method
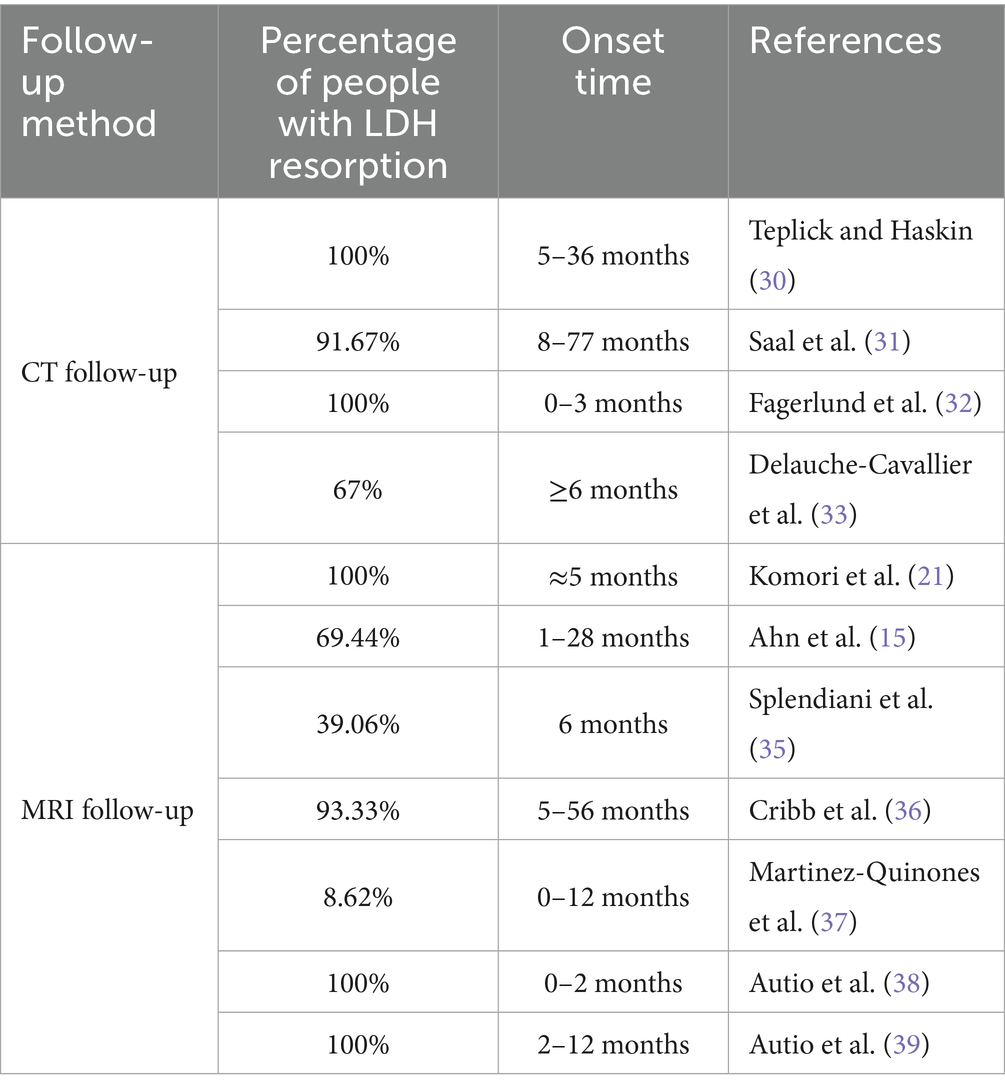
We searched for all reports published in English in the PubMed database from January 1980 to April 2025. The literature search was conducted from May 1 to May 10, 2025. The following medical subject headings were used: (“lumbar disc herniation” OR “lumbar disc protrusion”) AND (“disease classification” OR “imaging findings” OR “common onset time” OR “mechanisms” OR “conservative treatment” OR “spontaneous regression” OR “resorption” OR “natural resolution” OR “herniation regression”). Additionally, we reviewed the references from the extracted papers, and after de-duplication, two independent reviewers screened the titles and abstracts. Studies whose titles and abstracts did not meet the inclusion criteria were excluded from this review. After careful screening, a total of 163 articles were finally included in the review, as shown in Figure 1.
2.1 Clinical diagnosis of LDH reabsorption
2.1.1 Common classification of LDH reabsorption
The occurrence of LDH reabsorption is closely related to the structure of IVD tissue. The protruded IVD tissue includes the AF, cartilage endplate (CEP), and NP. The reabsorption phenomenon is closely related to the water content of the NP, whereby gradual dehydration leads to a reduction in the volume of the protruded IVD tissue (11). The likelihood of LDH reabsorption is closely associated with clinical classification. Relevant studies have combined clinical experience with experimental analyses to evaluate the relationship between LDH reabsorption and LDH classification. MRI is utilized to detect bone marrow lesions in vertebrae adjacent to the CEP. Factors indicating reabsorption are identified through darker signals on T1-weighted (T1W) imaging and brighter contrasts on T2-weighted (T2W) imaging. LDH is classified into non-ruptured types, including degenerative, bulging, protruded, and subligamentous types, and ruptured types. Normal IVD tissue is avascular, which separates the NP from the host immune system and suppresses the infiltration of immune cells and cytokines. In non-herniated IVD tissue, the morphology remains intact. However, when herniation occurs, the blood and NP barrier is damaged, exposing the IVD to the immune microenvironment, triggering an autoimmune response, and leading to various pathological processes, such as angiogenesis and immune cell infiltration (12). These mechanisms all play a role in the reabsorption of non-herniated LDH. The posterior longitudinal ligament type and free-type 2 are classified as ruptured types (13, 14). Compared to non-ruptured types, ruptured types are more likely to undergo reabsorption, with a higher incidence in free-type patients than protruding-type patients, and a higher incidence in lateral-type compared to central-type (11). Under the same conservative treatment, the reduction rate in free-type LDH patients is higher than that in the ligamentous and subligamentous types (15). The likelihood of absorption is higher in large protrusions compared to medium and small protrusions, and patients with normal intervertebral spaces are more likely to experience reabsorption than those with narrowed intervertebral spaces (16). Extruded LDH has a larger contact surface area for macrophage adhesion, resulting in a higher number of macrophages compared to protruding LDH (17). Moreover, in ruptured-type LDH, the NP begins to contact external tissues, making it more likely to trigger the body’s immune-inflammatory response and angiogenesis. This, in turn, induces inflammatory responses and vascularization mechanisms. Additionally, cell membrane rupture and necrosis lead to the occurrence of ferroptosis, which, together with various mechanisms, promotes LDH reabsorption (18–20).
In summary, LDH resorption occurs at various stages of LDH, but is most common in ruptured-type LDH. The larger the area of Intervertebral Disc Herniation (IDH), the faster the resorption rate. In the early stages of LDH, ligamentous-type and free-type protrusions cause the annulus fibrosus to rupture and form hematomas in the epidural venous plexus. MRI shows that the reduction in protruding IVD is associated with hematoma resorption and dehydration of the NP after it protrudes into the epidural space. LDH reabsorption or reduction is more common in free-type, posterior longitudinal ligament-type, and ruptured-type LDH. This is related to whether the posterior longitudinal ligament is intact, the amount of NP in the epidural space, and the distance of the free NP. The larger the protruding NP, the more NP components protrude into the epidural space, and the further the free NP, the more likely resorption occurs (11, 21). Therefore, imaging examination is of significant importance in assessing LDH resorption.
2.1.2 Imaging manifestations of LDH reabsorption
The proposal of the LDH reabsorption theory is closely associated with advancements in imaging technologies. LDH reabsorption was first observed through CT in 1984 and subsequently received more extensive investigation with the advancement of MRI techniques (22–24), gradually becoming an essential standard for diagnosing LDH and related lumbar intervertebral disc (LIVD) (25). MRI images demonstrate that as the peripheral signal ring around the protrusion intensifies, greater edge thickness corresponds to higher signal intensity. Significant enhancement of reabsorption at the margins of protruded tissue is an important factor for evaluating spontaneous regression of IDH (26–28), as shown in Figure 2. LDH tissues have a high cartilage content, and subtle changes associated with reabsorption can be detected by MRI. During the natural progression of LDH, imaging features of protruded tissues play a critical role in determining reabsorption status. When LDH tissue has a relatively high cartilage component or when MRI displays only subtle changes, spontaneous LDH absorption is usually impeded. Conversely, if contrast-enhanced MRI reveals free IVD fragments breaking through into the epidural space, an autoimmune response can be stimulated, inducing inflammation and subsequently leading to peripheral granulation tissue formation with annular enhancement. Notably, the non-enhancing free IVD fragment, known as the “bull’s eye sign,” serves as an important imaging marker predictive of favorable LDH reabsorption. Additionally, the greater the proportion of protruded IVD tissue within the spinal canal, the higher the likelihood of reabsorption (29). In summary, MRI is crucial for the diagnosis and evaluation of LDH reabsorption.
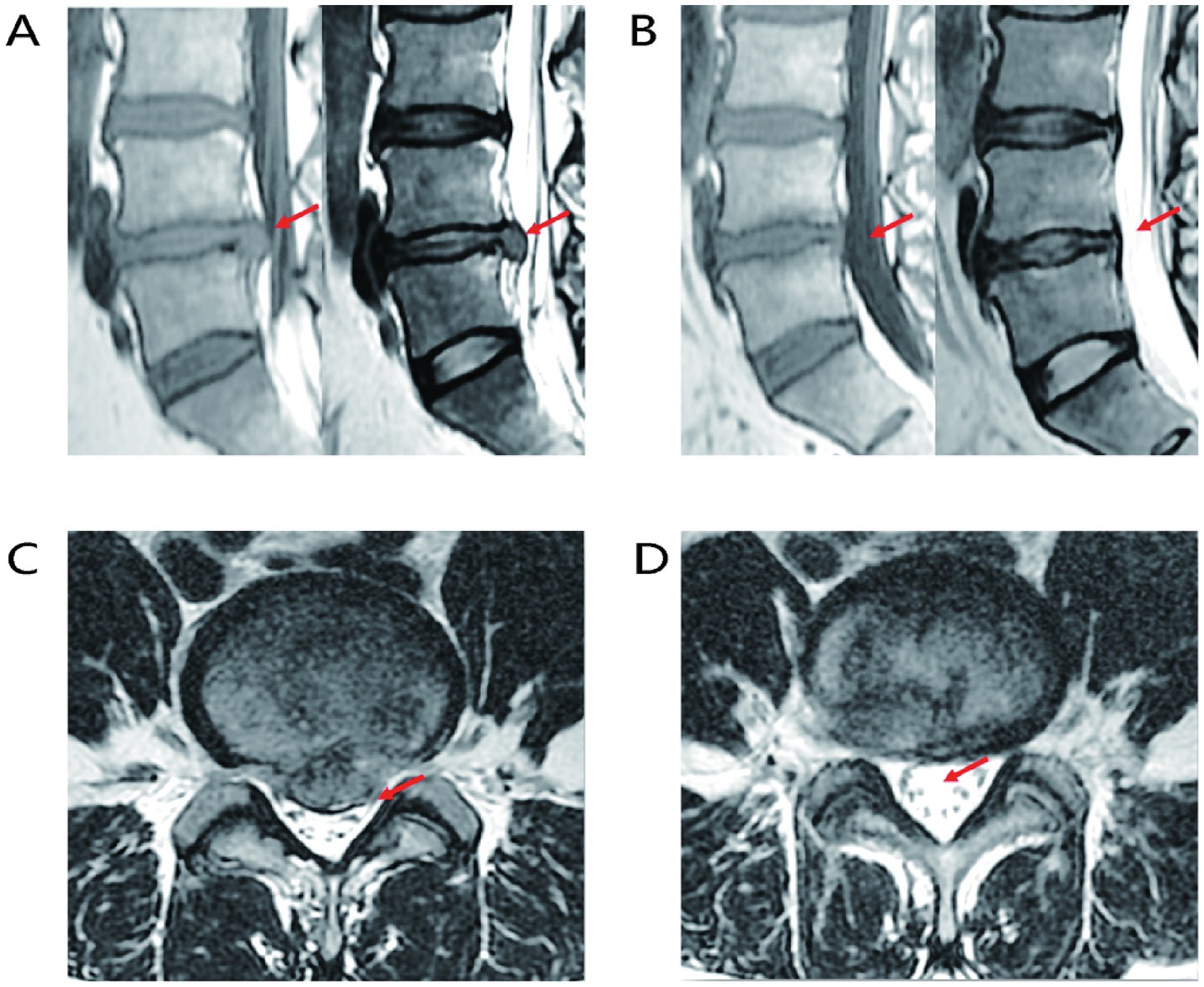
Figure 2. Representative MRI images of LIVD reabsorption. (A) Sagittal MRI image of LIVD before treatment. (B) Sagittal MRI image after LIVD reabsorption. (C) Axial MRI image of LIVD before treatment. (D) Axial MRI image after LIVD reabsorption.
2.1.3 Favorable time for LDH reabsorption
Clinical observations have shown that over time, all patients with LDH exhibited resorption of the protruded IVD tissue on CT imaging, accompanied by clinical symptom improvement, and in some cases, complete resolution (30). Follow-up CT scans revealed that in conservatively treated LDH patients, the protruded IVD retracted to some extent, with a reduction exceeding 50% in most cases (31). This phenomenon occurred more frequently within the first 3 months (32). After 6 months of conservative treatment, the NP protrusion in most patients either completely resolved or decreased in size, with corresponding improvement in clinical symptoms (33). With the widespread clinical application of MRI, research on LDH resorption from both clinical and imaging perspectives has become increasingly precise and comprehensive. MRI observations have confirmed that the early stage of LDH represents a period of active resorption, with the degree of resorption correlating with improvements in clinical symptoms and signs (21). As clinical diagnosis and treatment have improved, the understanding of LDH resorption has become more refined beyond initial CT-based studies.
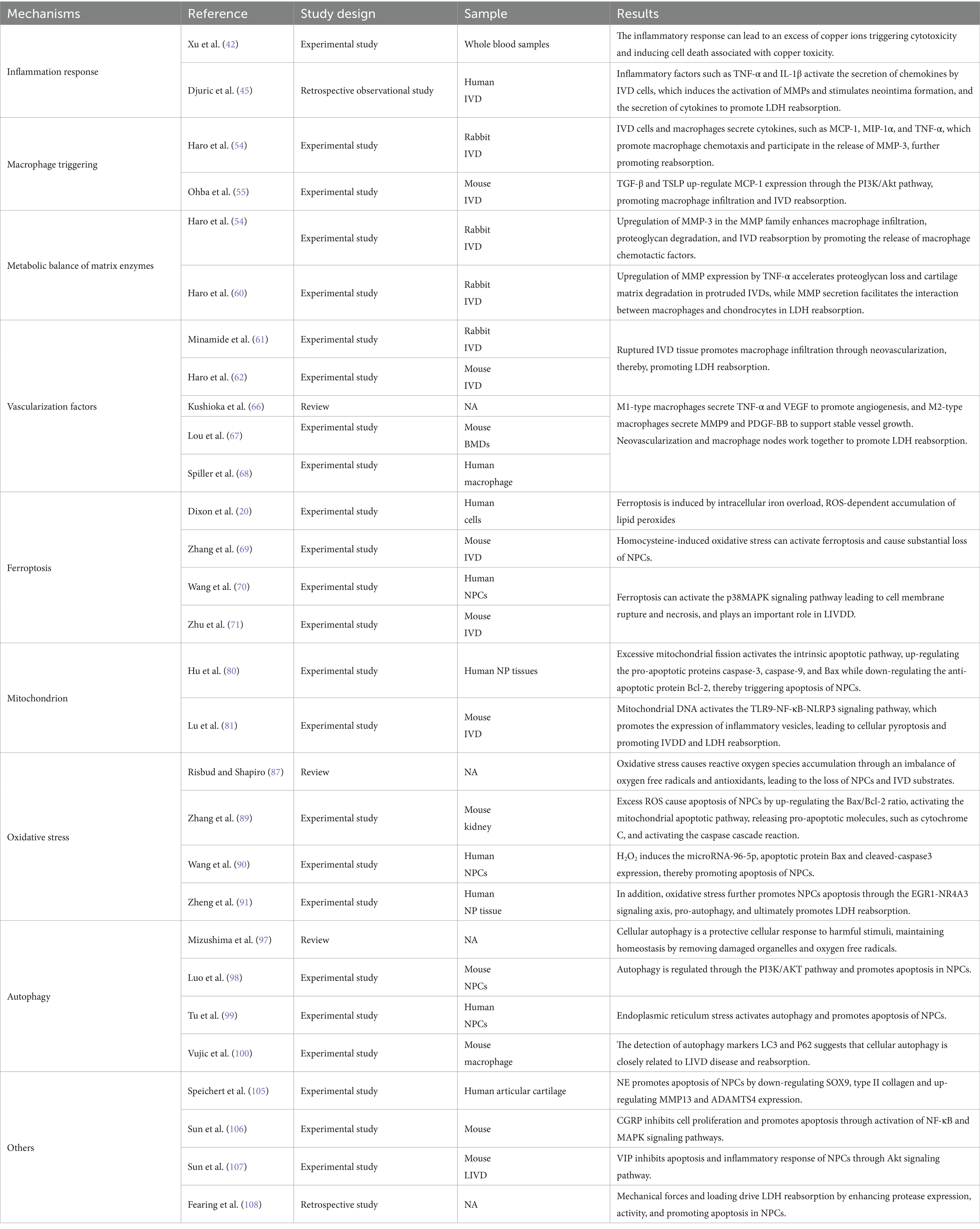
In related clinical studies (15, 21, 34–39) MRI was used for regular follow-up of LDH patients, measuring parameters such as the volume of the herniated disc, the extent of marginal thickening, and the percentage of thickening relative to the original disc height for a comprehensive assessment of treatment efficacy. The follow-up periods in these studies have been progressively shortened from 8 to 77 months (as in earlier CT studies) to 1–28 months. Ultimately, most clinical studies indicate that LDH resorption commonly begins after 2 months, typically occurring within the first 2–12 months, with the 3–6 month period being the most frequent, as shown in Table 1.
2.2 Mechanism of action of LDH reabsorption
2.2.1 Mechanisms of inflammatory response in LDH reabsorption
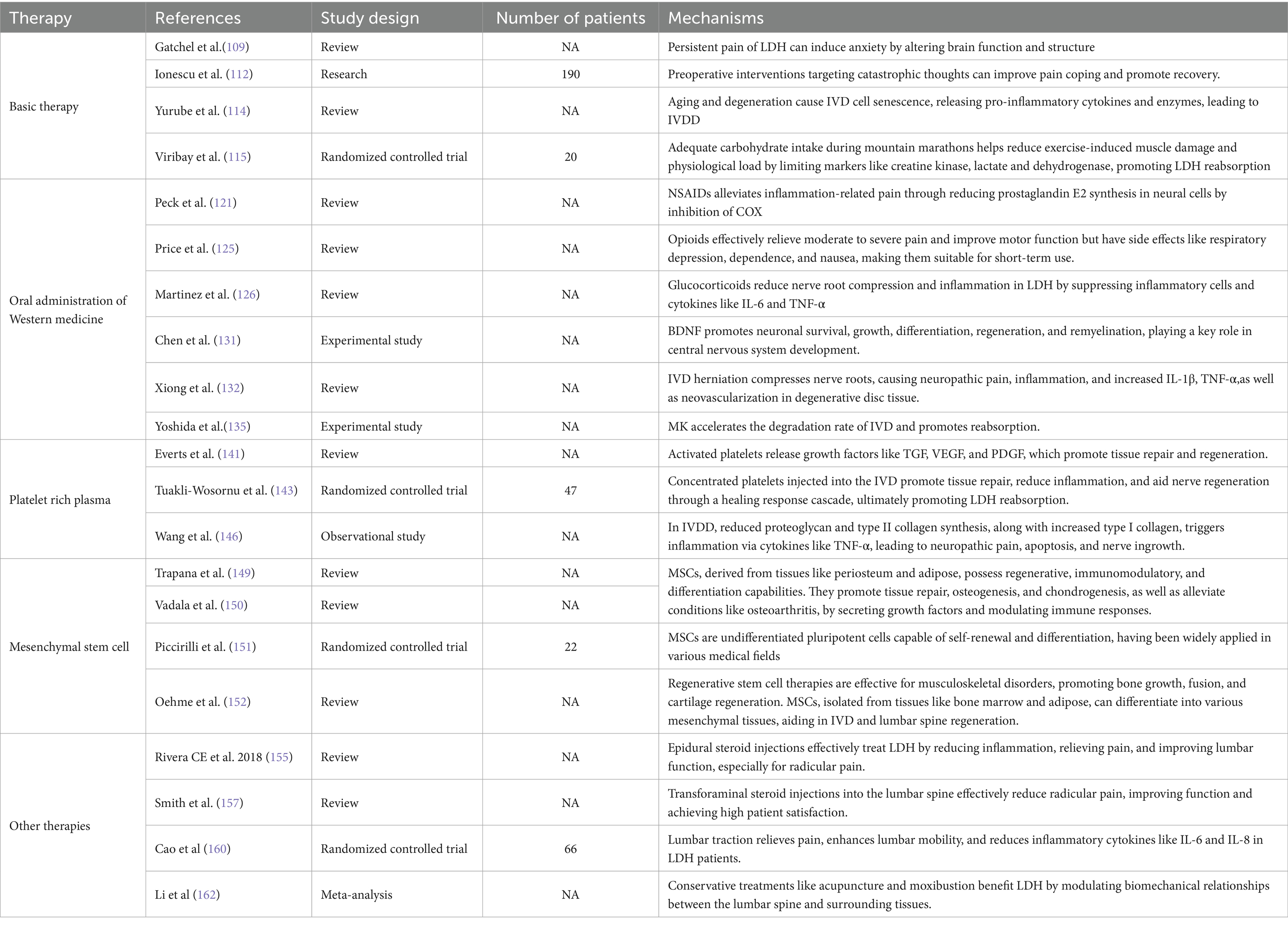
The IVD is an avascular, enclosed structure composed of three parts: the AF, the cartilage endplate (CEP), and the NP. The AF is a ligament-like lamellar structure composed of type I collagen fibers that encircle the NP. The CEP consists of a small amount of hyaline cartilage located between the vertebral endplate and the NP. A unique blood–nucleus pulposus barrier, along with the expression of Fas ligand (FasL), isolates the NP from the immune system. When IVD degeneration occurs or is influenced by an inflammatory microenvironment, it often leads to discogenic pain (40). IVD disorders are not caused by a single factor, and some patients with LBP caused by intervertebral disc degeneration (IVDD) continue to experience chronic pain even after lumbar IVD discectomy. This persistent pain is closely related to inflammation-induced remodeling of the endplate, which is rich in sensory nerve endings (41).
Machine learning analysis indicates that LDH is influenced by inflammatory cell infiltration and the release of atypical cytokines. When copper ion levels gradually exceed a certain threshold, the resulting cellular hyper-respiration increases cytotoxicity, ultimately leading to copper-induced cell death. The elevated immune infiltration observed in LDH patients is closely associated with copper-related gene (CRG) clusters that regulate this process (42). Both LDH and LBP-related disorders are strongly linked to inflammation and may result in adverse symptoms such as stimulation of nociceptive nerve fibers (19). In clinical practice, patients with protruding or free-type LIVD exhibit significantly elevated levels of inflammatory mediators such as Tumor Necrosis Factor-α (TNF-α), Interleukin-1 beta (IL-1β), and Interleukin-6 (IL-6) (43, 44). Pro-inflammatory factors such as TNF-α stimulate the IVD to produce chemokines, inducing the activation of Matrix Metalloproteinases (MMPs), which indirectly promotes angiogenesis, leading to a functional inflammatory response that facilitates LDH reabsorption. The latter secretes anti-inflammatory factors such as Interleukin-4 and Interleukin-10, which promote phagocytosis and reduce the inflammatory response, thereby promoting IVD reabsorption (45). Therefore, the inflammatory response can further promote LDH reabsorption by activating MMPs and promoting angiogenesis. Furthermore, postoperative levels of IL-1β and IL-6 are significantly elevated in the herniated regions and spinal nerves in both the puncture and lateral puncture groups (46). When herniated IVD tissue breaches the AF, it is recognized as an antigen by the immune system, triggering an autoimmune response. Research on demethoxycurcumin has revealed its therapeutic potential for inflammation-related diseases through immunomodulation. It achieves this by suppressing pro-inflammatory cytokines via inhibition of the mitogen-activated protein kinase (MAPK)/nuclear factor-κB (NF-κB) signaling pathway and related transcriptional activators (47). Additionally, TNF-α and IL-1β secreted by macrophages activate the p38MAPK signaling pathway (48, 49), which further promotes inflammatory mediator expression and contributes to the development of radicular pain (50). When NP tissue protrudes into the epidural space, it triggers an autoimmune response, leading to a significant increase in pro-inflammatory factors such as TNF-α, IL-1β, and IL-6. This, in turn, results in immune cell infiltration. The recruited immune cells interact with the intervertebral disc cells to secrete various factors that promote LDH reabsorption.
2.2.2 Mechanisms of macrophage triggering in LDH reabsorption
Macrophages, as key immune regulatory cells, play an important role in the infiltration and activation of LDH reabsorption. Both protruded and free LDH exhibit abundant macrophage infiltration, which is closely related to the differentiation of macrophage precursors into classically activated M1-type and alternatively activated M2-type macrophages. M1-and M2-type cytokines undergo phenotypic and functional differentiation under the influence of various cytokines. M1-type macrophages primarily produce pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β, which regulate the inflammatory response that mediates pain, and are closely associated with IVDD and sciatica. M2-type macrophages possess anti-inflammatory and healing-promoting functions, playing roles in tissue repair, fibrosis, and tissue regeneration by modulating functional inflammatory effects. Studies have shown that anti-inflammatory cytokines secreted by M2-type macrophages, such as IL-4 and IL-10, stimulate protruded IVD absorption by promoting phagocytosis and reducing inflammation (51, 52). Cytokines and chemokines secreted by IVD cells and macrophages in the IDH, such as Monocyte Chemoattractant Protein (MCP)-1, MCP-3, MCP-4, Chemokine (C-C motif) ligand 5, Macrophage Inflammatory Protein-1α (MIP-1α), and Interferon-γ-inducible protein 10, promote the chemotaxis of macrophages. When LDH occurs, IVD cells rapidly produce TNF-α, IL-1β, and MCP-1 (53), thereby recruiting macrophages. The produced TNF-α works in conjunction with MMP-3 produced by chondrocytes to promote the release of chemotactic factors and macrophage migration, further enhancing IVD cell reabsorption (54). Additionally, the protruded IVD is unable to interact with complexes formed by Transforming Growth Factor-beta (TGF-β) and Extracellular Matrix (ECM) foundation molecules, preventing the inhibition of the NF-κB signaling pathway. This, in turn, upregulates the level of Thymic Stromal Lymphopoietin (TSLP) in IVD tissue, which promotes MCP-1 expression through the regulation of the PI3K/Akt signaling pathway. When stimuli promote the recruitment of macrophages to the IVD, the protruded IVD interacts with macrophages to induce the production of inflammatory cytokines (55). This initiates immune-inflammatory responses and phagocytosis, along with the alternating facilitation of reabsorption by M1 and M2 macrophages, ultimately inducing LDH reabsorption.
2.2.3 Mechanism of matrix enzyme metabolic balance in LDH reabsorption
The development of IVDD is closely associated with mitochondrial dysfunction and ECM metabolic imbalance in nucleus pulposus cells (NPCs), caused by the excessive accumulation of reactive oxygen species (ROS). MMPs, a group of zinc-dependent intracellular proteases, are involved in the degradation of ECM and other proteins and have potential roles in tissue remodeling. The expression of MMP-1 in the IVD is significantly associated with histological degeneration in patients with cervical spondylosis and IVDD. MMP-1 is the most abundant and predominant catabolic enzyme involved in IVD degeneration (56), playing a key role in the pathogenesis of IVDD. As a group of dependent enzymes, MMPs facilitate tissue resorption and ECM remodeling. In protruded IVD tissue, infiltrating macrophages secrete pro-inflammatory cytokines such as IL-1 and TNF-α, which in turn upregulate MMP expression and accelerate proteoglycan loss in the protruded disc. Among the MMP family, upregulation of MMP-3 promotes the release of macrophage chemotactic factors, thereby enhancing macrophage infiltration, proteoglycan degradation, and IVD resorption (54). Studies have shown that in patients with intervertebral disc herniation (IDH), the activity of matrix-degrading enzymes in the IVD increases while the activity of inhibitory enzymes decreases, thereby accelerating the resorption of protruded IVD tissue (57). MMPs contribute to resorption by regulating macrophage infiltration, modulating inflammatory mediators, and maintaining tissue and cellular homeostasis. Recombinant human MMP-7 (rhMMP-7) has already been applied in the treatment of IVD degeneration, offering a minimally invasive alternative that partially avoids the complications associated with surgical intervention (58, 59). On one hand, the upregulation of MMP expression induced by TNF-α accelerates the loss of proteoglycans in the protruded IVD and promotes the degradation of the cartilage matrix. On the other hand, the secretion of MMPs is involved in the complex interaction between macrophages and chondrocytes in LDH reabsorption (60). The contact between macrophages and the IVD promotes a cascade reaction between macrophages and chondrocytes. MMP-3 and MMP-7 play a role in the macrophage-chondrocyte interaction, indirectly affecting cartilage matrix degradation and disc absorption.
2.2.4 Mechanism of vascularization factors in LDH reabsorption
IVD tissue lacks a direct blood supply, and its nutrients are primarily derived from diffusion through the CEP and AF. The degree of vascularization in herniated NP tissue is closely correlated with the extent of herniation resorption. When ruptured IVD material enters the epidural space, neovascularization facilitates macrophage infiltration and phagocytosis, resulting in the reduction or even disappearance of the herniated tissue (61, 62), thereby promoting LDH reabsorption (63). Clinical studies have shown that during the progression of IVDD, the polarization state of macrophages surrounding neovessels may change, with an increased presence of CD16 + perivascular macrophages being associated with chronic inflammation (64). Pro-angiogenic mediators secreted by different macrophage subtypes are key regulators of neovascularization during inflammation. TNF-α secreted by macrophages activates the NF-κB signaling pathway, upregulating the expression of vascular endothelial growth factor (VEGF) and thereby promoting neovessel formation (65). This angiogenic process involves early pro-inflammatory cytokines released by M1 macrophages, while anti-inflammatory M2 macrophages, which are associated with tissue regeneration, promote endothelial cell proliferation and stabilize vascular growth through the secretion of MMP-9 and platelet-derived growth factor-BB (PDGF-BB). Failure of M1-to-M2 repolarization results in persistent chronic inflammation, increased osteoclast activation, reduced osteoblast formation, and impaired stem cell function, ultimately diminishing bone regeneration capacity by increasing bone resorption and decreasing bone formation during healing (66–68). It can be concluded that macrophage infiltration in the IVD is involved in the regulation of angiogenesis. The formation of new blood vessels promotes macrophage infiltration and the regulation of M1 macrophages with pro-inflammatory effects and M2 macrophages with anti-inflammatory effects, thereby promoting endothelial cell growth and stabilizing blood vessel formation. This leads to the reduction or disappearance of the protruded IVD tissue, facilitating LDH reabsorption.
2.2.5 The role of ferroptosis in the mechanism of LDH reabsorption
Ferroptosis is a newly identified form of iron-dependent cell death, distinguished from classical cell death modalities by its unique mitochondrial abnormalities-such as mitochondrial shrinkage, reduced cristae, and increased membrane density. It is driven by intracellular iron overload and accumulation of ROS-dependent lipid peroxides, leading to plasma membrane rupture, cytoplasmic content leakage, and eventual necrosis (20). Ferroptosis is closely associated with the NP tissue in LIVDD. In vitro studies have shown that homocysteine-induced oxidative stress can activate ferroptosis and cause substantial loss of NPCs (69). Moreover, NPCs apoptosis is strongly linked to the activation of the p38 MAPK signaling pathway, which promotes the reabsorption of ruptured LDH (70, 71). IL-6 in LIVDD can also induce oxidative stress in chondrocytes, disrupt iron homeostasis, and trigger ferroptosis, further contributing to NPCs-associated degeneration (72).
Experimental findings further support that tert-butyl hydroperoxide can induce ferroptosis in AF cells and NPCs, characterized by downregulation of glutathione peroxidase 4 (GPX4) and ferritin heavy chain, and upregulation of cyclooxygenase-2 (COX-2) and acyl-CoA synthetase long-chain family member 4 (ACSL4) (73). Wang et al. (74) observed that in mice fed with high-iron diets, CEP cells exhibited decreased GPX4 and SLC7A11 expression, increased ROS production, elevated 4-hydroxynonenal levels, and enhanced lipid peroxidation, suggesting iron overload-mediated oxidative stress as a key driver of ferroptosis in CEP cells. Additionally, in LDH patients, ruptured peripheral vasculature surrounding NP tissues results in erythrocyte extravasation and elevated heme levels. Heme breakdown further increases local ROS and reduces GPX4, promoting ferroptosis in NPCs and thereby accelerating LIVDD progression (75). The newly formed NP blood vessels may expose tissues to a large amount of hemoglobin, thereby triggering cytotoxicity and ferroptosis, accelerating progressive degeneration. When iron ions excessively accumulate in cells and tissues, they disrupt the redox balance, catalyzing ROS production and promoting ferroptosis. Related studies have shown that when ferritin autophagy or BMSCs ferroptosis is inhibited, cell viability increases, BMSCs dysfunction is reduced compared to before, and bone damage is reversed (76).
2.2.6 Mechanism of mitochondrial action in LDH reabsorption
Mitochondria are highly dynamic membrane-bound organelles that provide the majority of the chemical energy required for biochemical reactions in eukaryotic cells. Cells maintain mitochondrial health through a mitochondrial quality control mechanism, which includes selectively removing damaged mitochondria and balancing mitochondrial biosynthesis. These organelles communicate with the cell nucleus and other cellular structures to help maintain cellular homeostasis, enabling the cell to adapt to stress, promote development, and support metabolism, energy, and genetic regulation within the cell (77). Mitochondrial fission and fusion not only safeguard mitochondrial genetics and function but are also intimately linked to energy metabolism, aging, and cell death (78, 79). Accordingly, mitochondria act as central regulators of apoptosis, autophagy, and necroptosis. Excessive mitochondrial fission activates the intrinsic apoptotic pathway, up-regulating the pro-apoptotic proteins caspase-3, caspase-9, and Bax while down-regulating the anti-apoptotic protein Bcl-2, thereby triggering apoptosis of NPCs (80). Moreover, mitochondrial DNA (mtDNA), acting as a damage-associated molecular pattern, activates the TLR9–NF-κB–NLRP3 signaling cascade, promotes NLRP3 inflammasome expression, and induces pyroptosis of NPCs, thus facilitating the reabsorption of LDH (81). Given that NPCs apoptosis is pivotal in IVDD-with compelling evidence highlighting the mitochondrial pathway as a key mediator (82, 83). In summary, NPC apoptosis is closely associated with LDH reabsorption, and mitochondrial activation and apoptosis play critical roles in the initiation and progression of IVDD. When mitochondrial dysfunction leads to cellular impairment and reduced activity, mitochondrial regulatory function becomes unbalanced in degenerated NPCs. Excessive activation of degenerative cells leads to the mitochondrial unfolded protein response, initiating non-selective mitochondrial autophagy to salvage damaged mitochondria. Over-activation exacerbates mitochondrial dysfunction. Mitochondria in NPCs are more vulnerable to cellular interference, resulting in mitochondrial dysfunction (84). When the mitochondrial respiratory chain is disrupted, excessive ROS triggers oxidative stress, disrupting the proton gradient and the stability of the adenosine nucleotide phosphorylation process. Antioxidants may enhance mitochondrial antioxidant capacity by neutralizing free radicals and alleviating oxidative stress, thus reducing oxidative stress on mitochondria, improving the survival and regeneration of IVD cells, and promoting LDH reabsorption.
2.2.7 Mechanism of oxidative stress in LDH reabsorption
Oxidative stress refers to the accumulation of ROS resulting from an imbalance between oxidants and antioxidants within cells and tissues. This redox disequilibrium-triggered by excessive production of oxygen free radicals, reactive metabolites, or oxidants relative to antioxidant defense mechanisms-damages essential biomolecules and cellular structures, posing potential harm to the organism. It has been closely implicated in the pathogenesis of numerous diseases, including IVDD and atherosclerosis (85, 86). In the context of disrupted IVD homeostasis, oxidative stress primarily exerts its deleterious effects by inhibiting the synthesis of ECM by NPCs, promoting the secretion of matrix-degrading enzymes and proinflammatory cytokines. These processes accelerate matrix degradation, intensify inflammatory responses in the IVD microenvironment, and induce non-physiological death of NPCs, leading to cell dysfunction and tissue injury (87). Ultimately, the imbalance in oxygen radical metabolism within NPCs disrupts the structural and functional homeostasis of the IVD, causing ECM degradation and water loss, thereby driving the onset and progression of IVDD (88).
Moreover, mitochondria are the primary source of ROS during electron transport chain activity, and excessive ROS levels increase the Bax/Bcl-2 ratio by upregulating the pro-apoptotic protein Bax relative to the anti-apoptotic protein Bcl-2. This altered ratio enhances mitochondrial membrane permeability and compromises its structural integrity, leading to the release of apoptosis-related molecules such as cytochrome c. These molecules subsequently activate the caspase cascade, initiating programmed cell death via the mitochondrial apoptotic pathway (89). Studies have shown that hydrogen peroxide (H2O2) can induce the expression of microRNA-96-5p, Bax, and cleaved caspase-3, thereby promoting NPCs apoptosis (90). Additionally, oxidative stress stimulates the expression of Early Growth Response 1 (EGR1) in NPCs, which activates the NR4A3 signaling axis, increasing the levels of Bax and caspase-3 while suppressing the expression of Bcl-2, aggrecan, collagen II, and ECM-related proteins, ultimately promoting NPCs apoptosis (91). Oxidative stress also upregulates Beclin-1 expression and facilitates the conversion of LC3B-I to LC3B-II, promoting autophagosome formation and contributing to the degeneration of rat NPCs (92, 93). Animal experiments further reveal that oxidative stress significantly enhances METTL16 expression while downregulating MAT2A in NPCs, disrupting the balance of MAT2A pre-mRNA splicing, maturation, and degradation, thereby accelerating NPCs apoptosis (94). Overall, oxidative stress-induced NPCs apoptosis represents a core mechanism in the development of intervertebral disc degeneration. It is intricately linked to inflammatory pathways and mitochondrial apoptosis, forming a self-amplifying loop that intensifies NPCs death and contributes to the process of LDH reabsorption. The IVD lacks direct blood supply and is in a hypoxic environment, but IVD cells still undergo aerobic metabolism and produce ROS. During IVD degeneration, intracellular metabolites cannot be efficiently transported, leading to the accumulation of metabolic waste, which in turn impairs cell function. Oxidative stress disrupts cellular homeostasis by increasing ROS, triggering the release of pro-inflammatory cytokines and degrading extracellular matrix components. This, in turn, induces apoptosis, autophagy, and senescence in disc cells. Trace elements, as important cofactors in enzyme-catalyzed reactions and redox processes, may contribute to the metabolic imbalance caused by spinal degeneration. Antioxidant trace elements help regulate inflammation and prevent oxidative damage (95), thereby promoting LDH reabsorption.
2.2.8 Mechanisms of cellular autophagy and LDH reabsorption
Autophagy is a coordinated self-degradation mechanism that serves as a protective response against harmful stimuli such as nutrient deprivation and hypoxia (96). It plays a critical role in maintaining cellular homeostasis by removing damaged or excessive ROS, peroxisomes, endoplasmic reticulum, mitochondria, and other cellular components, thereby reducing the accumulation of abnormal proteins and organelles (97). The CEP contributes to delaying degeneration by regulating the PI3K/Akt/autophagy signaling pathway through exosomal communication, which in turn affects the progression of IVDD (98). Moreover, studies by Tu et al. (99) revealed that endoplasmic reticulum stress can promote autophagy and ECM degradation in NPCs, ultimately enhancing NPCs apoptosis. Among autophagy markers, LC3 is widely recognized as a key indicator of autophagic activity and serves as a structural component of the autophagosome membrane (100). The p62 protein binds to LC3 and is selectively incorporated into autophagosomes, where it undergoes effective degradation during the autophagic process (101). Autophagy and apoptosis act on the same set of cellular regulatory proteins, exhibiting a bidirectional effect of both inhibiting and promoting apoptosis. Autophagy in NPCs is closely related to LIVD and reabsorption. When the normal metabolic processes of NPCs are disrupted, it leads to the accumulation of harmful substances and an upregulation of apoptosis levels, thereby accelerating IVDD. Autophagy prevents the occurrence of LIVD by regulating apoptosis and alleviating inflammation, thus mitigating the progression of IVDD (102, 103).
2.2.9 Other factors
LDH reabsorption is a complex physicochemical process that not only involves the individual actions of the mechanisms mentioned above but also their interactions. For example, inflammation can stimulate the production of MMPs, which indirectly promotes angiogenesis. The production of MMPs and angiogenesis can further regulate the infiltration of macrophages and other immune cells, thereby modulating the inflammatory response, resulting in a synergistic effect that promotes LDH reabsorption. In addition to the mechanisms mentioned above, resorption may also be influenced by neurotransmitters and mechanical forces. Tu et al. (104) demonstrated that human NPCs, particularly fibrotic NPCs, are innervated by nerve fibers. Speichert et al. (105) found that norepinephrine (NE), under IL-1β stimulation, significantly downregulates the gene expression of Sex Determining Region Y-box 9 (SOX9), type II collagen, cartilage oligomeric matrix protein (COMP), and aggrecan, while upregulating the expression of matrix metalloproteinase 13 (MMP13) and a disintegrin and metalloproteinase with thrombospondin motifs 4 (ADAMTS4) in chondrocytes, thereby promoting apoptosis of NPCs. Studies have shown that calcitonin gene-related peptide (CGRP) can inhibit NPCs proliferation and promote apoptosis, inflammation, and ECM degeneration by activating the NF-κB and MAPK signaling pathways (106). Moreover, vasoactive intestinal peptide (VIP), a sympathetic neurotransmitter, can target fibroblast growth factor 18 (FGF18) to activate the Akt signaling pathway, thereby suppressing apoptosis and inflammatory responses in NPCs (107). Therefore, multiple neurotransmitters play critical roles in the process of LDH reabsorption. Mechanical forces and loading also affect LDH reabsorption. Under low levels of mechanical stress, NPCs exhibit anabolic responses, whereas high levels of stress induce catabolic responses characterized by increased protease expression and activity (108). Thus, high-intensity mechanical stress can promote NPCs apoptosis, thereby accelerating LDH reabsorption. The summary table of LDH reabsorption mechanisms can be found in Table 2. The summary diagram of LDH reabsorption mechanisms can be found in Figure 3.
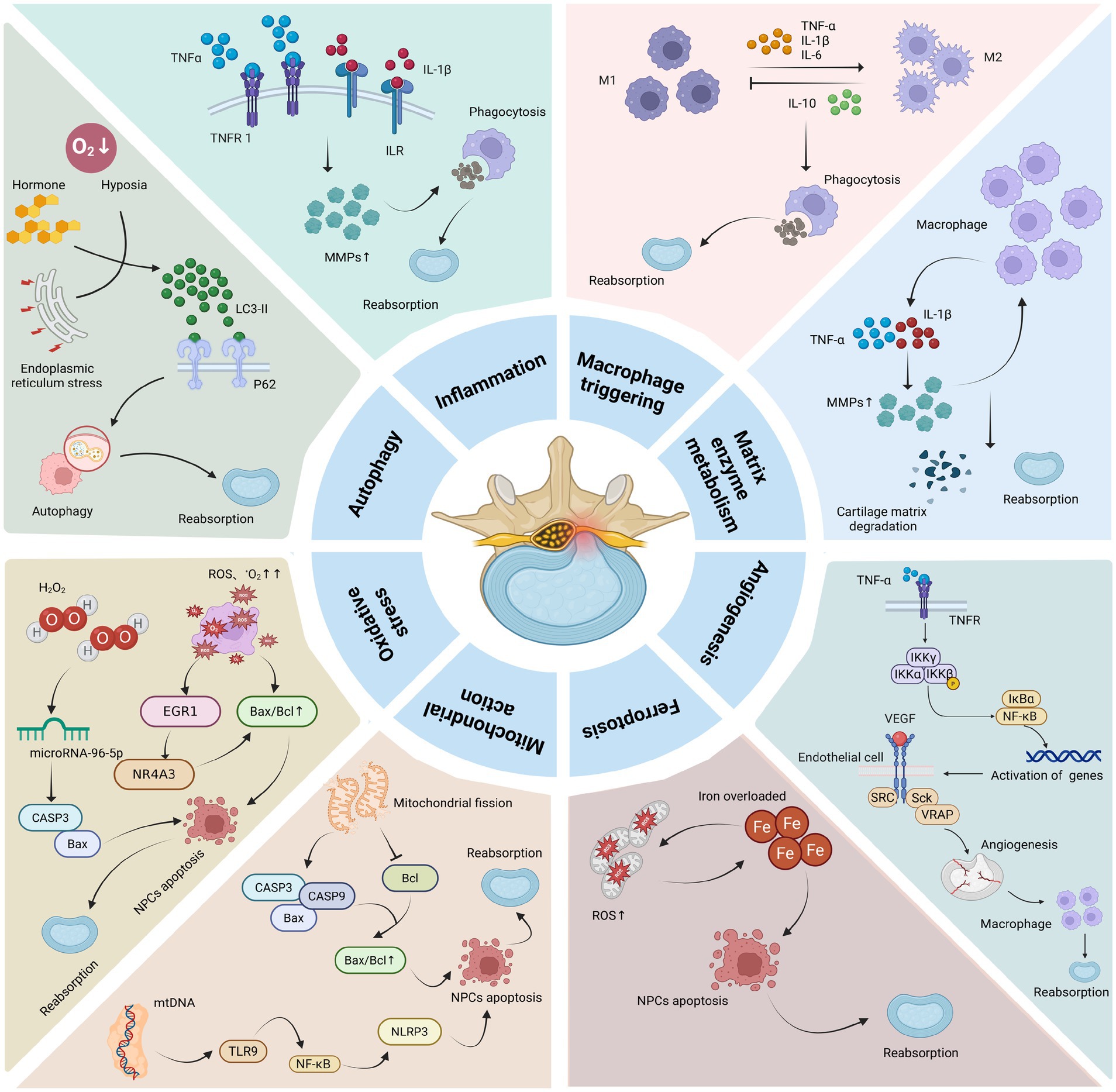
Figure 3. Mechanism of action of LDH reabsorption. Macrophages can secrete pro-inflammatory factors such as TNF-α, IL-1β, and IL-6, which induce inflammatory responses while also upregulating the expression of MMPs. The latter can, in turn, promote macrophage infiltration. M1 and M2 macrophages can regulate each other through the secretion of different cytokines, ultimately promoting phagocytosis and ECM degradation, thereby facilitating LDH reabsorption. In addition, pro-inflammatory factors can stimulate angiogenesis, which further enhances macrophage infiltration and promotes LDH reabsorption. Oxidative stress and mitochondrial fission can upregulate the expression of Bax/Bcl, promoting NPC apoptosis through NF-κB and other related signaling pathways, and also induce iron overload leading to ferroptosis, which further accelerates NPCs apoptosis and promotes LDH reabsorption. Protective autophagy can regulate NPCs apoptosis and directly promote LDH reabsorption (The graphic was created with BioRender.com).
2.3 Research progress on LDH treatment
2.3.1 Mechanisms of action of basic therapy in LDH reabsorption
LDH resorption refers to the spontaneous shrinkage or disappearance of protruded NP tissue in patients with LDH without undergoing surgery, chemonucleolysis, or endoscopic NP removal. Conventional conservative therapies in clinical practice include health education, dietary regulation, exercise therapy, and rehabilitation treatment. LDH is a chronic condition, and the resulting persistent pain can induce anxiety by altering brain function and structure (109). Pain and anxiety share common neural substrates, contributing to their close association (110). Pain is a multidimensional experience that involves sensory-discriminative, affective-motivational, and cognitive-evaluative components (111). Pain is commonly present throughout the course of LDH, often impairing mental health and diminishing quality of life. It can lead to dysfunctional symptoms such as depression, anxiety, pain catastrophizing, and rumination. Preoperative interventions targeting patients’ catastrophic thoughts can help them cope more effectively with pain and accelerate recovery (112). Furthermore, postoperative education via mobile applications significantly enhances patients’ understanding of LDH, leading to improved attitudes and practical self-management skills (113). Therefore, providing psychological counseling and education to LDH patients is beneficial at all stages of clinical diagnosis and treatment.
IVD is the largest avascular tissue in the human body, and its nutrient supply primarily relies on diffusion from peripheral capillaries and the endplates. The notochordal phenotype of NP cells disappears during adolescence, accompanied by cell death and chondrocyte proliferation. As the largest avascular organ in the body, the IVD is particularly susceptible to nutrient deficiency, which may contribute to disc degeneration. During aging and degenerative processes, IVD cells undergo senescence and growth arrest, leading to the release of pro-inflammatory cytokines and matrix-degrading enzymes (114), ultimately resulting in IVDD. Relevant studies have shown that adequate carbohydrate intake during mountain marathons can limit markers such as creatine kinase, lactate dehydrogenase, and aspartate aminotransferase, thereby reducing exercise-induced muscle damage and physiological load (115). Properly managed exercise training has been shown to relieve pain in non-surgically treated patients and partially restore motor function (116). For LDH patients suffering from neuropathic pain, yoga-based interventions aimed at enhancing mobility, core strength, and spinal and hamstring flexibility can significantly reduce pain and disability (117). Furthermore, rehabilitation studies following unilateral microdiscectomy have revealed that long-term regular physical activity helps alleviate pain, accelerates postoperative recovery, and plays a vital role in improving quality of life and preventing occupational impairment (118). In summary, conservative clinical treatments for LDH-emphasizing mental well-being, exercise, and rehabilitation-may promote LDH resorption by limiting muscle injury biomarkers such as creatine kinase and lactate dehydrogenase, thus achieving improved therapeutic outcomes.
2.3.2 Mechanism of action of orally administered drugs in LDH reabsorption
In clinical practice, conservative treatment of LDH primarily relies on oral pharmacotherapy. Commonly prescribed medications include non-steroidal anti-inflammatory drugs (NSAIDs), opioids, muscle relaxants, analgesics, glucocorticoids, and neurotrophic agents, which mainly function to relieve pain and suppress inflammation. Resorption is frequently observed in cases of large IDH, where enzymatic degradation and phagocytosis of cartilaginous tissues are driven by IVDD, inflammatory responses, and neovascularization within the herniated tissue. The proliferation of cells and blood vessels within the herniated LIVD is a key factor promoting the resorption process (119). NSAIDs are commonly recommended for short-term use in patients with LBP without nerve root compression (120), demonstrating good efficacy in relieving pain and suppressing postoperative inflammatory pain. Compared to opioids, NSAIDs are more economical and have fewer side effects. Their therapeutic effect in LDH is mediated by inhibition of COX, which reduces prostaglandin E2 synthesis in neural cells and thereby alleviates inflammation-related pain. However, NSAIDs may pose risks to gastrointestinal and renal health, particularly in elderly patients (121). As such, in patients with a history or risk of peptic ulcers, co-administration of COX-2 inhibitors or enteric-coated aspirin is recommended to minimize gastrointestinal side effects (122, 123). Diclofenac sodium, a widely used NSAID, also exerts its effects by inhibiting COX-2 activity, thereby reducing local pain and restoring lumbar spine mobility (124).
For lumbosacral pain that is poorly controlled by NSAIDs, opioid analgesics and muscle relaxants may be considered. Opioids are effective in relieving moderate to severe pain and can moderately improve motor function. However, they are associated with numerous side effects, including respiratory depression, potential drug dependence, nausea, dizziness, headache, and drowsiness. As such, they are typically reserved for short-term treatment and should be used with caution (125). When patients exhibit significant pathological changes or experience severe pain that is unresponsive to other medications, muscle relaxants are often used as alternatives. These agents relieve muscle spasms, help restore range of motion, and improve pain tolerance, while having fewer side effects and being suitable for short-term use. Glucocorticoids alleviate nerve root compression and inflammation caused by herniated IVDs by suppressing the activation of inflammatory cells and the release of inflammatory mediators. Inflammatory cytokines such as IL-6 and TNF-α play pivotal roles in the pathogenesis of LDH, and glucocorticoids help reduce their levels (126). Additionally, glucocorticoids can regulate immune responses, and their long-term use may promote neovascularization by persistently inhibiting inflammatory and immune processes, thereby indirectly facilitating the resorption of the herniated NP (127). Most patients with massive LDH prefer non-surgical treatment. The resorption of IDH is closely related to capillary infiltration of the large, free herniated tissue. VEGF, a major pro-angiogenic factor, promotes neovascularization at the edges of the IVD and facilitates disc resorption. Clinically, a combination of oral celecoxib, spinal cord demyelination decoction, and acupuncture is used. This integrated approach-combining oral and external therapies-enhances NP resorption and reduces nerve root edema. More than half of patients treated with this regimen exhibit evidence of LIVD resorption (128).
Recent studies have revealed that the deficiency of brain-derived neurotrophic factor (BDNF) can cause neurodegeneration in central motor structures, subsequently triggering motor neuron diseases (129). Nerve growth factor, initially investigated for its capacity to promote neuronal growth and treat neurological disorders (130), has been shown to repair damaged neural tissues. Among these neurotrophic factors, BDNF plays a crucial role in the development of the central nervous system by promoting neuronal survival, growth, differentiation, and regeneration, as well as facilitating remyelination of injured neurons (131). Damage to LIVD and endplates can result in pathological nerve ingrowth. Herniation of IVDs compressing nerve roots may lead to traumatic neuropathic pain. Such injuries activate lumbar sensory receptors, initiating inflammation, upregulating IL-1β and TNF-α in degenerative disc tissue, and enhancing neovascularization (132, 133), thereby alleviating pain and accelerating disc resorption through improved blood supply. Additionally, Zhou et al. (134) found in a rabbit IVD model that midkine (MK) treatment led to a significantly greater reduction in disc weight, increased neovascularization, and elevated inflammatory cell infiltration compared to controls. MK also accelerated IVD degradation and promoted resorption. As a heparin-binding growth and differentiation factor, MK plays a key role in angiogenesis and wound healing. Epidural MK injection has shown therapeutic promise for LDH, outperforming conventional treatments such as NSAIDs and intrathecal corticosteroids (135, 136). With advancements in technology and pharmacological delivery, novel minimally invasive treatments-such as radiofrequency ozone therapy and targeted injections of anti-inflammatory agents into the nerve root foramina-are emerging as safe and effective options (137). Although pharmacotherapy effectively reduces inflammation and pain, anti-inflammatory drugs like NSAIDs and glucocorticoids, whether administered systemically or locally, may impair the body’s natural healing process, prolonging the duration of LDH and causing adverse effects on renal, gastrointestinal, and cardiovascular systems (138), which require further research to address.
2.3.3 Mechanism of action of biological agents in LDH reabsorption
2.3.3.1 Platelet rich plasma
Treatment with biological agents can significantly improve pain symptoms, physical function, and overall quality of life in patients with IVD disorders. Commonly used biological agents in clinical practice include mesenchymal stem cells (MSCs), platelet-rich plasma (PRP), and α2-macroglobulin, all of which exhibit significant potential in promoting chondrogenesis within the IVD. These agents are highly effective in treating IVD-related LBP and lower limb pain, with minimal associated risk, resulting in notable improvements in patients’ pain relief, physical capabilities, and quality of life (139, 140). PRP therapy utilizes platelet concentrates obtained from autologous whole blood via centrifugation. Upon activation, the platelets release large quantities of growth factors, such as transforming growth factor, VEGF and platelet-derived growth factor (141, 142). These concentrated platelets are directly injected into the IVD, initiating a cascade of healing responses that promote tissue repair and regeneration, reduce inflammation, and aid in the repair of damaged nerves (143). Clinical studies have shown that IVD herniation is a leading cause of LBP. PRP has been demonstrated to significantly alleviate LBP in patients with IVD herniation, with long-lasting therapeutic effects, representing a safe and promising alternative to epidural local anesthetics and corticosteroids (144, 145).
In IVDD, there is a reduction in the synthesis of proteoglycans and type II collagen, alongside an increase in type I collagen synthesis. Herniation of the NP, which compresses the nerve roots, is frequently accompanied by inflammatory responses. The major inflammatory mediators originate from degenerative disc cells and include cytokines such as p38 MAPK, COX-2, and TNF-α. TNF-α is also secreted by endoneurial macrophages and neuroglial cells. Once activated, TNF-α stimulates the dorsal root ganglion to produce a large number of inflammatory mediators, resulting in neuropathic pain. Additionally, when TNF-α and IL-1β act on degenerative disc cells, they increase apoptosis rates and promote neovascularization and nerve ingrowth into the degenerated disc, exacerbating pain (146–148). PRP, through the anti-inflammatory and immunomodulatory actions of its platelets, regulates growth and immune factors in damaged nerves, thereby reducing inflammation and pain and facilitating the resorption of LDH.
2.3.3.2 Mesenchymal stem cell
MSCs, first discovered in the 20th century and derived from periosteum, adipose tissue, and muscle, possess multidirectional differentiation potential, immunomodulatory capacity, and regenerative properties. These characteristics enable them to promote osteogenesis, chondrogenesis, and tissue repair, making them widely used in muscle recovery, meniscus tears, tendon and ligament injuries, and IVDD, where they have shown potential for cartilage regeneration and alleviating osteoarthritis. MSCs regulate immune responses and promote tissue repair by secreting growth factors and nutrients such as VEGF. In addition, they exert immunomodulatory and anti-inflammatory effects by inhibiting dendritic cell maturation, T and B lymphocyte activation, and reducing the cytotoxicity of natural killer cells (149, 150). MSCs are undifferentiated pluripotent cells capable of self-renewal and differentiation, and have been widely applied in various medical fields (151). Studies have shown that regenerative stem cell therapies are particularly suitable for treating musculoskeletal disorders, as they promote bone growth and fusion as well as cartilage regeneration. These therapies also facilitate IVD cartilage regeneration and have demonstrated significant therapeutic effects in lumbar spine disorders. MSCs can be isolated from bone marrow, synovium, adipose tissue, umbilical cord, and other tissues. With appropriate intervention, they can differentiate into bone, cartilage, fat, and other mesenchymal tissues (152), thereby contributing to the treatment of LDH by promoting reabsorption. In a clinical study on MSC therapy for IVDD, direct injection of bone marrow-derived MSCs (BM-MSCs) into the NP led to significant relief of LBP, improved quality of life, and increased water content in the lumbar intervertebral disc as shown by MRI (153). In studies using autologous biological agents to repair damaged tissues, BM-MSCs have been applied for their anti-inflammatory, immunomodulatory, and regenerative properties. Their therapeutic efficacy and safety have been investigated in patients with severe chronic LBP. Interventions targeting multiple structures, including IVD, facet joints, nerve roots, and sacroiliac joints, based on patients’ clinical symptoms, showed favorable outcomes after treatment (154). In summary, MSCs are undifferentiated pluripotent cells that can promote symptom relief and disc reabsorption in patients with LDH through their self-renewal and differentiation capabilities.
2.3.4 Other therapies
LDH has a complex etiology and involves multiple pathogenic mechanisms, with a variety of clinical treatment options available. Among these, epidural steroid injections are widely used to treat LDH due to their significant anti-inflammatory effects, pain relief, and improvement in lumbar function. They are particularly effective in alleviating radicular pain and axial LBP associated with LDH (155, 156). In clinical studies, transforaminal steroid injections into the lumbar spine effectively relieved radicular pain, with more than half of the patients experiencing pain reduction, functional improvement, and high satisfaction rates (157). Hong et al. (158) conducted MRI evaluations before and after treatment in LDH patients who received transforaminal epidural steroid injections. The results showed that most patients experienced a reduction in herniated IVD volume, and even in cases without significant MRI changes, clinical symptoms improved, thereby avoiding the trauma and cost associated with surgery. Furthermore, changes in the human lumbar spine are neither singular nor isolated. The onset of LDH is closely associated with structural abnormalities such as facet joint hypertrophy, loss of IVD height, and the formation of adjacent osteophytes. There are complex biomechanical interactions among the lumbar spine, adjacent joints, and surrounding muscles. Massage therapy guided by musculoskeletal assessment models has been shown to effectively relieve pain and reduce functional impairment in LDH patients (159). Lumbar traction, which improves spinal biomechanics, can relieve pain and enhance lumbar mobility in LDH patients. It also significantly reduces serum concentrations of inflammatory cytokines such as IL-6 and IL-8 following treatment (160). Mechanical traction has demonstrated significant efficacy in alleviating lumbar and leg pain, improving clinical symptoms, and reducing the need for surgical intervention in LDH patients (161). In addition, conservative treatments such as acupuncture and moxibustion also show therapeutic benefits in LDH, primarily by modulating the biomechanical relationships between the lumbar spine and adjacent tissues (162, 163). The summary table of research progress on conservative treatment for LDH reabsorption can be found in Table 3. The summary diagram of research progress on conservative treatment for LDH reabsorption can be found in Figure 4.
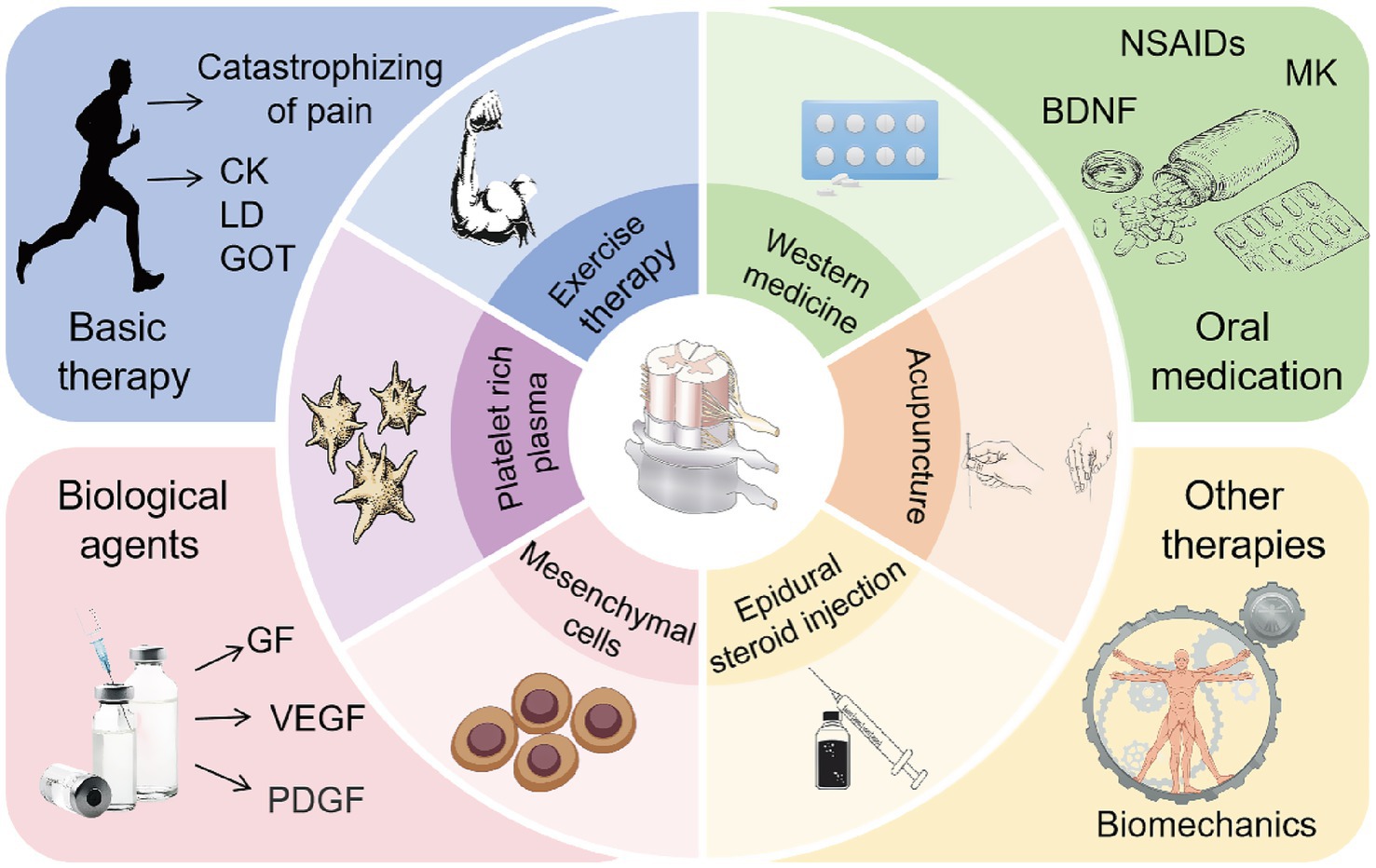
Figure 4. Common conservative clinical treatments to promote LDH reabsorption. Rational exercise control training can alleviate pain in patients, reduce muscle damage and exercise load, thereby promoting LDH reabsorption. Oral medications such as NSAIDs, corticosteroids, or opioids, as well as Western medical treatments like MK epidural injections, exert anti-inflammatory effects, relieve pain, and promote LDH reabsorption. The application of biological agents such as (PRP) and MSCs can exhibit anti-inflammatory properties, regulate immune responses, promote tissue repair, and enhance LDH reabsorption. Epidural steroid injections, lumbar traction, massage therapy, and acupuncture can relieve lower back pain, improve lumbar function, and further promote LDH reabsorption.
3 Conclusion
LDH reabsorption exists at various stages of the onset and progression of LDH, being more pronounced in patients with large and free types of LDH. Relevant studies have shown that conservative treatments, including health education, dietary regulation, exercise therapy, oral and topical medications, and biologics such as epidural steroid injections, can modulate mechanisms such as ferroptosis, mitochondrial fission, oxidative stress, tissue vascularization, inflammatory response, macrophage activation, apoptosis, and autophagy of NPCs, thereby alleviating LDH pain, improving lumbar function, and promoting LDH reabsorption to treat LDH effectively. Early implementation of conservative treatment can promote LDH reabsorption, thus avoiding the heavy burden that surgical treatments may place on families and society. Therefore, systematic research into the mechanisms of LDH reabsorption and the implementation of relevant conservative treatments are of significant importance and value for the treatment of LDH.
Author contributions
YC: Writing – original draft, Conceptualization. XS: Data curation, Writing – original draft. ZiW: Investigation, Writing – review & editing. XZh: Writing – review & editing, Supervision. ZhW: Visualization, Writing – review & editing. XZu: Writing – review & editing, Formal analysis. JL: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Third Batch of Leading Talents in Traditional Chinese Medicine in Jiangsu Province (Su TCM Science and Education [2023] No. 17), Jiangsu Province Science and Education Capability Enhancement Project: Jiangsu Province Medical Key Discipline and Medical Key Laboratory Construction Project (Suwei Science and Education [2022] No. 17), Jiangsu Province Traditional Chinese Medicine Technology Development Project (MS2022080), National Natural Science Foundation of China Project (82474529), Liu 2024, National Natural Science Foundation of China Project (82374220), Jiang 2023, Jiangsu Provincial Natural Science Foundation Project (No.: BK20241802), The 29th Batch of Suzhou Science and Technology Development Plan for 2022 (New Key Laboratory Construction) Project (SZS2022019), and Jiangsu Province Leading Talents Cultivation Project for Traditional Chinese Medicine (SLJ0310).
Acknowledgments
We are grateful to Jintao Liu from the Nanjing University of Chinese Medicine and Xiaoyun Zhang from the Affiliated Hospital of Guangxi University of Chinese Medicine for their help in all stages of this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wang, Z, Liu, X, Gao, K, Tuo, H, Zhang, X, and Liu, W. Clinical effects and biological mechanisms of exercise on lumbar disc herniation. Front Physiol. (2024) 15:1309663. doi: 10.3389/fphys.2024.1309663
2. Demirel, A, Yorubulut, M, and Ergun, N. Regression of lumbar disc herniation by physiotherapy. Does non-surgical spinal decompression therapy make a difference? Double-blind randomized controlled trial. J Back Musculoskelet Rehabil. (2017) 30:1015–22. doi: 10.3233/BMR-169581
3. Collaborators GDAI. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet. (2020) 396:1204–22. doi: 10.1016/S0140-6736(20)30925-9
4. Kesikburun, B, Eksioglu, E, Turan, A, Adiguzel, E, Kesikburun, S, and Cakci, A. Spontaneous regression of extruded lumbar disc herniation: correlation with clinical outcome. Pak J Med Sci. (2019) 35:974–80. doi: 10.12669/pjms.35.4.346
5. Ozturk, B, Gunduz, OH, Ozoran, K, and Bostanoglu, S. Effect of continuous lumbar traction on the size of herniated disc material in lumbar disc herniation. Rheumatol Int. (2006) 26:622–6. doi: 10.1007/s00296-005-0035-x
6. El Barzouhi, A, Vleggeert-Lankamp, CL, Lycklama, ANG, Van der Kallen, BF, van den Hout, WB, Jacobs, WC, et al. Magnetic resonance imaging in follow-up assessment of sciatica. N Engl J Med. (2013) 368:999–1007. doi: 10.1056/NEJMoa1209250
7. Ghaffari-Rafi, A, Nosova, K, Kim, K, and Goodarzi, A. Intradural disc herniation in the setting of congenital lumbar spinal stenosis. Neurochirurgie. (2022) 68:335–41. doi: 10.1016/j.neuchi.2021.04.006
8. Elkholy, AR, Farid, AM, and Shamhoot, EA. Spontaneous resorption of herniated lumbar disk: observational retrospective study in 9 patients. World Neurosurg. (2019) 124:e453–9. doi: 10.1016/j.wneu.2018.12.115
9. Zhong, M, Liu, JT, Jiang, H, Mo, W, Yu, PF, Li, XC, et al. Incidence of spontaneous resorption of lumbar disc herniation: a meta-analysis. Pain Physician. (2017) 20:E45–52. doi: 10.36076/ppj.2017.1.E45
10. Sun, K, Huang, F, Qi, B, Yin, H, Tang, B, Yang, B, et al. A systematic review and meta-analysis for Chinese herbal medicine duhuo jisheng decoction in treatment of lumbar disc herniation: a protocol for a systematic review. Medicine (Baltimore). (2020) 99:e19310. doi: 10.1097/MD.0000000000019310
11. Mochida, K, Komori, H, Okawa, A, Muneta, T, Haro, H, and Shinomiya, K. Regression of cervical disc herniation observed on magnetic resonance images. Spine. (1998) 23:990–5, 996–997. doi: 10.1097/00007632-199805010-00005
12. Yu, P, Mao, F, Chen, J, Ma, X, Dai, Y, Liu, G, et al. Characteristics and mechanisms of resorption in lumbar disc herniation. Arthritis Res Ther. (2022) 24:205. doi: 10.1186/s13075-022-02894-8
13. Compte, R, Granville, SI, Isaac, A, Danckert, N, Mcsweeney, T, Liantis, P, et al. Are current machine learning applications comparable to radiologist classification of degenerate and herniated discs and modic change? A systematic review and meta-analysis. Eur Spine J. (2023) 32:3764–87. doi: 10.1007/s00586-023-07718-0
14. Lee, J, Kim, J, Shin, JS, Lee, YJ, Kim, MR, Jeong, SY, et al. Long-term course to lumbar disc resorption patients and predictive factors associated with disc resorption. Evid Based Complement Alternat Med. (2017) 2017:2147408. doi: 10.1155/2017/2147408
15. Ahn, SH, Ahn, MW, and Byun, WM. Effect of the transligamentous extension of lumbar disc herniations on their regression and the clinical outcome of sciatica. Spine (Phila Pa 1976). (2000) 25:475–80. doi: 10.1097/00007632-200002150-00014
16. Dullerud, R, and Nakstad, PH. Ct changes after conservative treatment for lumbar disk herniation. Acta Radiol. (1994) 35:415–9. doi: 10.1177/028418519403500503
17. Arango, DG, and Descoteaux, A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. (2014) 5:491. doi: 10.3389/fimmu.2014.00491
18. Kobayashi, S, Chowdhury, JU, Tokuno, H, Nahar, NS, and Iino, S. A smooth muscle nodule producing 10-12 cycle/min regular contractions at the mesenteric border of the pacemaker area in the guinea-pig colon. Arch Histol Cytol. (1996) 59:159–68. doi: 10.1679/aohc.59.159
19. Cunha, C, Silva, AJ, Pereira, P, Vaz, R, Goncalves, RM, and Barbosa, MA. The inflammatory response in the regression of lumbar disc herniation. Arthritis Res Ther. (2018) 20:251. doi: 10.1186/s13075-018-1743-4
20. Dixon, SJ, Lemberg, KM, Lamprecht, MR, Skouta, R, Zaitsev, EM, Gleason, CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. (2012) 149:1060–72. doi: 10.1016/j.cell.2012.03.042
21. Komori, H, Shinomiya, K, Nakai, O, Yamaura, I, Takeda, S, and Furuya, K. The natural history of herniated nucleus pulposus with radiculopathy. Spine (Phila Pa 1976). (1996) 21:225–9. doi: 10.1097/00007632-199601150-00013
22. Guinto, FJ, Hashim, H, and Stumer, M. Ct demonstration of disk regression after conservative therapy. AJNR Am J Neuroradiol. (1984) 5:632–3.
23. Hong, J, and Ball, PA. Images in clinical medicine. Resolution of lumbar disk herniation without surgery. N Engl J Med. (2016) 374:1564. doi: 10.1056/NEJMicm1511194
24. Sucuoglu, H, and Barut, AY. Clinical and radiological follow-up results of patients with sequestered lumbar disc herniation: a prospective cohort study. Med Princ Pract. (2021) 30:244–52. doi: 10.1159/000515308
25. Dutta, S, Bhave, A, and Patil, S. Correlation of 1.5 tesla magnetic resonance imaging with clinical and intraoperative findings for lumbar disc herniation. Asian Spine J. (2016) 10:1115–21. doi: 10.4184/asj.2016.10.6.1115
26. Yamashita, K, Hiroshima, K, and Kurata, A. Gadolinium-DTPA--enhanced magnetic resonance imaging of a sequestered lumbar intervertebral disc and its correlation with pathologic findings. Spine (Phila Pa 1976). (1994) 19:479–82. doi: 10.1097/00007632-199402001-00021
27. Sakai, T, Tsuji, T, Asazuma, T, Yato, Y, Matsubara, O, and Nemoto, K. Spontaneous resorption in recurrent intradural lumbar disc herniation. Case report. J Neurosurg Spine. (2007) 6:574–8. doi: 10.3171/spi.2007.6.6.11
28. Komori, H, Okawa, A, Haro, H, Muneta, T, Yamamoto, H, and Shinomiya, K. Contrast-enhanced magnetic resonance imaging in conservative management of lumbar disc herniation. Spine (Phila Pa 1976). (1998) 23:67–73. doi: 10.1097/00007632-199801010-00015
29. Zeng, Z, Qin, J, Guo, L, Hirai, T, Gui, Z, Liu, T, et al. Prediction and mechanisms of spontaneous resorption in lumbar disc herniation: narrative review. Spine Surg Relat Res. (2024) 8:235–42. doi: 10.22603/ssrr.2023-0152
30. Teplick, JG, and Haskin, ME. Spontaneous regression of herniated nucleus pulposus. AJR Am J Roentgenol. (1985) 145:371–5. doi: 10.2214/ajr.145.2.371
31. Saal, JA, Saal, JS, and Herzog, RJ. The natural history of lumbar intervertebral disc extrusions treated nonoperatively. Spine (Phila Pa 1976). (1990) 15:683–6. doi: 10.1097/00007632-199007000-00013
32. Fagerlund, MK, Thelander, U, and Friberg, S. Size of lumbar disc hernias measured using computed tomography and related to sciatic symptoms. Acta Radiol. (1990) 31:555–8. doi: 10.1080/02841859009173096
33. Delauche-Cavallier, MC, Budet, C, Laredo, JD, Debie, B, Wybier, M, Dorfmann, H, et al. Lumbar disc herniation. Computed tomography scan changes after conservative treatment of nerve root compression. Spine (Phila Pa 1976). (1992) 17:927–33. doi: 10.1097/00007632-199208000-00010
34. Bozzao, A, Gallucci, M, Masciocchi, C, Aprile, I, Barile, A, and Passariello, R. Lumbar disk herniation: mr imaging assessment of natural history in patients treated without surgery. Radiology. (1992) 185:135–41. doi: 10.1148/radiology.185.1.1523297
35. Splendiani, A, Puglielli, E, De Amicis, R, Barile, A, Masciocchi, C, and Gallucci, M. Spontaneous resolution of lumbar disk herniation: predictive signs for prognostic evaluation. Neuroradiology. (2004) 46:916–22. doi: 10.1007/s00234-004-1232-0
36. Cribb, GL, Jaffray, DC, and Cassar-Pullicino, VN. Observations on the natural history of massive lumbar disc herniation. J Bone Joint Surg Br. (2007) 89:782–4. doi: 10.1302/0301-620X.89B6.18712
37. Martinez-Quinones, JV, Aso-Escario, J, Consolini, F, and Arregui-Calvo, R. Spontaneous regression from intervertebral disc herniation. Propos of a series of 37 cases. Neurocirugia (Astur). (2010) 21:108–17. doi: 10.1016/S1130-1473(10)70065-8
38. Autio, RA, Karppinen, J, Niinimaki, J, Ojala, R, Kurunlahti, M, Haapea, M, et al. Determinants of spontaneous resorption of intervertebral disc herniations. Spine (Phila Pa 1976). (2006) 31:1247–52. doi: 10.1097/01.brs.0000217681.83524.4a
39. Autio, RA, Karppinen, J, Kurunlahti, M, Haapea, M, Vanharanta, H, and Tervonen, O. Effect of periradicular methylprednisolone on spontaneous resorption of intervertebral disc herniations. Spine (Phila Pa 1976). (2004) 29:1601–7. doi: 10.1097/01.BRS.0000132511.46818.67
40. Wang, L, He, T, Liu, J, Tai, J, Wang, B, Zhang, L, et al. Revealing the immune infiltration landscape and identifying diagnostic biomarkers for lumbar disc herniation. Front Immunol. (2021) 12:666355. doi: 10.3389/fimmu.2021.666355
41. Chen, H, Zhou, Q, Pu, X, Wang, N, Wang, S, Feng, Z, et al. Association between vertebral endplate defects and patient-reported symptoms: an immunohistochemical study investigating the cox-2/pge-2/ep-4 axis. Spine J. (2024) 24:1407–15. doi: 10.1016/j.spinee.2024.04.003
42. Xu, H, Jiang, Y, Wen, Y, Liu, Q, Du, HG, and Jin, X. Identification of copper death-associated molecular clusters and immunological profiles for lumbar disc herniation based on the machine learning. Sci Rep. (2024) 14:19294. doi: 10.1038/s41598-024-69700-0
43. Shamji, MF, Setton, LA, Jarvis, W, So, S, Chen, J, Jing, L, et al. Proinflammatory cytokine expression profile in degenerated and herniated human intervertebral disc tissues. Arthritis Rheum. (2010) 62:1974–82. doi: 10.1002/art.27444
44. Le Maitre, CL, Hoyland, JA, and Freemont, AJ. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: il-1beta and tnfalpha expression profile. Arthritis Res Ther. (2007) 9:R77. doi: 10.1186/ar2275
45. Djuric, N, Yang, X, El Barzouhi, A, Ostelo, R, van Duinen, SG, Lycklama, ANG, et al. Lumbar disc extrusions reduce faster than bulging discs due to an active role of macrophages in sciatica. Acta Neurochir. (2020) 162:79–85. doi: 10.1007/s00701-019-04117-7
46. Jin, L, Xiao, L, Ding, M, Pan, A, Balian, G, Sung, SJ, et al. Heterogeneous macrophages contribute to the pathology of disc herniation induced radiculopathy. Spine J. (2022) 22:677–89. doi: 10.1016/j.spinee.2021.10.014
47. Lu, B, Chen, X, Chen, H, Li, Q, Li, H, Xu, Y, et al. Demethoxycurcumin mitigates inflammatory responses in lumbar disc herniation via MAPK and NF-κB pathways in vivo and in vitro. Int Immunopharmacol. (2022) 108:108914. doi: 10.1016/j.intimp.2022.108914
48. Suzuki, K, Hino, M, Kutsuna, H, Hato, F, Sakamoto, C, Takahashi, T, et al. Selective activation of p38 mitogen-activated protein kinase cascade in human neutrophils stimulated by il-1beta. J Immunol. (2001) 167:5940–7. doi: 10.4049/jimmunol.167.10.5940
49. Ichijo, H, Nishida, E, Irie, K, Ten, DP, Saitoh, M, Moriguchi, T, et al. Induction of apoptosis by ask1, a mammalian mapkkk that activates sapk/jnk and p38 signaling pathways. Science. (1997) 275:90–4.
50. Zhong, Y, Huang, Y, Hu, Y, Xu, M, Zhu, L, and Deng, Z. Sfks/p38 pathway is involved in radicular pain by promoting spinal expression of pro-inflammatory cytokines in a rat model of lumbar disc herniation. Spine (Phila Pa 1976). (2019) 44:E1112–21. doi: 10.1097/BRS.0000000000003076
51. Wang, K, Bao, JP, Yang, S, Hong, X, Liu, L, Xie, XH, et al. A cohort study comparing the serum levels of pro- or anti-inflammatory cytokines in patients with lumbar radicular pain and healthy subjects. Eur Spine J. (2016) 25:1428–34. doi: 10.1007/s00586-015-4349-4
52. Peluffo, H, Solari-Saquieres, P, Negro-Demontel, ML, Francos-Quijorna, I, Navarro, X, Lopez-Vales, R, et al. Cd300f immunoreceptor contributes to peripheral nerve regeneration by the modulation of macrophage inflammatory phenotype. J Neuroinflammation. (2015) 12:145. doi: 10.1186/s12974-015-0364-y
53. Yoshida, M, Nakamura, T, Sei, A, Kikuchi, T, Takagi, K, and Matsukawa, A. Intervertebral disc cells produce tumor necrosis factor alpha, interleukin-1beta, and monocyte chemoattractant protein-1 immediately after herniation: an experimental study using a new hernia model. Spine (Phila Pa 1976). (2005) 30:55–61. doi: 10.1097/01.brs.0000149194.17891.bf
54. Haro H CHFB. Matrix metalloproteinase-3 – dependent generation of a macrophage chemoattractant in a model of herniated disc resorption. J Clin Invest. (2000) 105:133–41. doi: 10.1172/JCI7090
55. Ohba, T, Haro, H, Ando, T, Koyama, K, Hatsushika, K, Suenaga, F, et al. A potential role of thymic stromal lymphopoietin in the recruitment of macrophages to mouse intervertebral disc cells via monocyte chemotactic protein 1 induction: implications for herniated discs. Arthritis Rheum. (2008) 58:3510–9. doi: 10.1002/art.23965
56. Song, Y, Ren, S, Chen, X, Li, X, Chen, L, Zhao, S, et al. Inhibition of mfn 1 restores tamoxifen-induced apoptosis in resistant cells by disrupting aberrant mitochondrial fusion dynamics. Cancer Lett. (2024) 590:216847. doi: 10.1016/j.canlet.2024.216847
57. Roberts, S, Caterson, B, Menage, J, Evans, EH, Jaffray, DC, and Eisenstein, SM. Matrix metalloproteinases and aggrecanase: their role in disorders of the human intervertebral disc. Spine (Phila Pa 1976). (2000) 25:3005–13. doi: 10.1097/00007632-200012010-00007
58. Haro, H. Translational research of herniated discs: current status of diagnosis and treatment. J Orthop Sci. (2014) 19:515–20. doi: 10.1007/s00776-014-0571-x
59. Haro, H, Nishiga, M, Ishii, D, Shimomoto, T, Kato, T, Takenouchi, O, et al. Experimental chemonucleolysis with recombinant human matrix metalloproteinase 7 in human herniated discs and dogs. Spine J. (2014) 14:1280–90. doi: 10.1016/j.spinee.2013.11.039
60. Haro, H, Komori, H, Kato, T, Hara, Y, Tagawa, M, Shinomiya, K, et al. Experimental studies on the effects of recombinant human matrix metalloproteinases on herniated disc tissues--how to facilitate the natural resorption process of herniated discs. J Orthop Res. (2005) 23:412–9. doi: 10.1016/j.orthres.2004.08.020
61. Minamide, A, Hashizume, H, Yoshida, M, Kawakami, M, Hayashi, N, and Tamaki, T. Effects of basic fibroblast growth factor on spontaneous resorption of herniated intervertebral discs. An experimental study in the rabbit. Spine (Phila Pa 1976). (1999) 24:940–5. doi: 10.1097/00007632-199905150-00003
62. Haro, H, Kato, T, Komori, H, Osada, M, and Shinomiya, K. Vascular endothelial growth factor (vegf)-induced angiogenesis in herniated disc resorption. J Orthop Res. (2002) 20:409–15. doi: 10.1016/S0736-0266(01)00150-4
63. Ratsep, T, Minajeva, A, and Asser, T. Relationship between neovascularization and degenerative changes in herniated lumbar intervertebral discs. Eur Spine J. (2013) 22:2474–80. doi: 10.1007/s00586-013-2842-1
64. Xiao, L, Matharoo, J, Chi, J, Ma, J, Chen, M, Manley, B, et al. Transient depletion of macrophages alters local inflammatory response at the site of disc herniation in a transgenic mouse model. Osteoarthr Cartil. (2023) 31:894–907. doi: 10.1016/j.joca.2023.01.574
65. Ohba, T, Haro, H, Ando, T, Wako, M, Suenaga, F, Aso, Y, et al. Tnf-alpha-induced nf-kappab signaling reverses age-related declines in vegf induction and angiogenic activity in intervertebral disc tissues. J Orthop Res. (2009) 27:229–35. doi: 10.1002/jor.20727
66. Kushioka, J, Chow, SK, Toya, M, Tsubosaka, M, Shen, H, Gao, Q, et al. Bone regeneration in inflammation with aging and cell-based immunomodulatory therapy. Inflamm Regen. (2023) 43:29. doi: 10.1186/s41232-023-00279-1
67. Lou, R, Chen, J, Zhou, F, Zhang, T, Chen, X, Wang, C, et al. Exosomal mirna-155-5p from m1-polarized macrophages suppresses angiogenesis by targeting gdf 6 to interrupt diabetic wound healing. Mol Ther Nucleic Acids. (2023) 34:102074. doi: 10.1016/j.omtn.2023.102074
68. Spiller, KL, Anfang, RR, Spiller, KJ, Ng, J, Nakazawa, KR, Daulton, JW, et al. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials. (2014) 35:4477–88. doi: 10.1016/j.biomaterials.2014.02.012
69. Zhang, Y, Han, S, Kong, M, Tu, Q, Zhang, L, and Ma, X. Single-cell rna-seq analysis identifies unique chondrocyte subsets and reveals involvement of ferroptosis in human intervertebral disc degeneration. Osteoarthr Cartil. (2021) 29:1324–34. doi: 10.1016/j.joca.2021.06.010
70. Wang, Z, Wang, G, Zhu, X, Geng, D, and Yang, H. Interleukin-2 is upregulated in patients with a prolapsed lumbar intervertebral disc and modulates cell proliferation, apoptosis and extracellular matrix metabolism of human nucleus pulposus cells. Exp Ther Med. (2015) 10:2437–43. doi: 10.3892/etm.2015.2809
71. Zhu, Y, Liu, JT, Yang, LY, Du, WP, Li, XC, Qian, X, et al. P 38 mitogen-activated protein kinase inhibition modulates nucleus pulposus cell apoptosis in spontaneous resorption of herniated intervertebral discs: an experimental study in rats. Mol Med Rep. (2016) 13:4001–6. doi: 10.3892/mmr.2016.5039
72. Bin, S, Xin, L, Lin, Z, Jinhua, Z, Rui, G, and Xiang, Z. Targeting mir-10a-5p/il-6r axis for reducing il-6-induced cartilage cell ferroptosis. Exp Mol Pathol. (2021) 118:104570. doi: 10.1016/j.yexmp.2020.104570
73. Yang, RZ, Xu, WN, Zheng, HL, Zheng, XF, Li, B, Jiang, LS, et al. Involvement of oxidative stress-induced annulus fibrosus cell and nucleus pulposus cell ferroptosis in intervertebral disc degeneration pathogenesis. J Cell Physiol. (2021) 236:2725–39. doi: 10.1002/jcp.30039
74. Wang, W, Jing, X, Du, T, Ren, J, Liu, X, Chen, F, et al. Iron overload promotes intervertebral disc degeneration via inducing oxidative stress and ferroptosis in endplate chondrocytes. Free Radic Biol Med. (2022) 190:234–46. doi: 10.1016/j.freeradbiomed.2022.08.018
75. Shan, L, Xu, X, Zhang, J, Cai, P, Gao, H, Lu, Y, et al. Increased hemoglobin and heme in maldi-tof ms analysis induce ferroptosis and promote degeneration of herniated human nucleus pulposus. Mol Med. (2021) 27:103. doi: 10.1186/s10020-021-00368-2
76. Xu, Y, Xu, X, Chai, R, and Wu, X. Targeting ferroptosis to enhance the efficacy of mesenchymal stem cell-based treatments for intervertebral disc degeneration. Int J Biol Sci. (2025) 21:1222–41. doi: 10.7150/ijbs.107021
77. He, L, and Maheshwari, A. Mitochondria in early life. Curr Pediatr Rev. (2023) 19:395–416. doi: 10.2174/1573396319666221221110728
78. Ren, L, Chen, X, Chen, X, Li, J, Cheng, B, and Xia, J. Mitochondrial dynamics: fission and fusion in fate determination of mesenchymal stem cells. Front Cell Dev Biol. (2020) 8:580070. doi: 10.3389/fcell.2020.580070
79. Seo, BJ, Yoon, SH, and Do, JT. Mitochondrial dynamics in stem cells and differentiation. Int J Mol Sci. (2018) 19:3893. doi: 10.3390/ijms19123893
80. Hu, B, Wang, P, Zhang, S, Liu, W, Lv, X, Shi, D, et al. Hsp70 attenuates compression-induced apoptosis of nucleus pulposus cells by suppressing mitochondrial fission via upregulating the expression of sirt3. Exp Mol Med. (2022) 54:309–23. doi: 10.1038/s12276-022-00745-9
81. Lu, P, Zheng, H, Meng, H, Liu, C, Duan, L, Zhang, J, et al. Mitochondrial DNA induces nucleus pulposus cell pyroptosis via the TLR 9-NF-kappaB-NLRP3 axis. J Transl Med. (2023) 21:389. doi: 10.1186/s12967-023-04266-5
82. Ding, F, Shao, ZW, and Xiong, LM. Cell death in intervertebral disc degeneration. Apoptosis. (2013) 18:777–85. doi: 10.1007/s10495-013-0839-1
83. Xiang, Q, Cheng, Z, Wang, J, Feng, X, Hua, W, Luo, R, et al. Allicin attenuated advanced oxidation protein product-induced oxidative stress and mitochondrial apoptosis in human nucleus pulposus cells. Oxidative Med Cell Longev. (2020) 2020:6685043. doi: 10.1155/2020/6685043
84. Ji, Y, Hu, Y, Feng, Y, Liu, L, Chen, Z, Shen, H, et al. Mitochondrial 'birth-death' coordinator: an intelligent hydrogen nanogenerator to enhance intervertebral disc regeneration. Biomaterials. (2025) 313:122764. doi: 10.1016/j.biomaterials.2024.122764
85. Zhao, Y, Xiang, Q, Lin, J, Jiang, S, and Li, W. Oxidative stress in intervertebral disc degeneration: new insights from bioinformatic strategies. Oxidative Med Cell Longev. (2022) 2022:2239770. doi: 10.1155/2022/2239770
86. Durackova, Z. Some current insights into oxidative stress. Physiol Res. (2010) 59:459–69. doi: 10.33549/physiolres.931844
87. Risbud, MV, and Shapiro, IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. (2014) 10:44–56. doi: 10.1038/nrrheum.2013.160
88. Cannizzo, ES, Clement, CC, Sahu, R, Follo, C, and Santambrogio, L. Oxidative stress, inflamm-aging and immunosenescence. J Proteome. (2011) 74:2313–23. doi: 10.1016/j.jprot.2011.06.005
89. Zhang, B, Zhang, X, Zhang, C, Shen, Q, Sun, G, and Sun, X. Notoginsenoside R1 protects db/db mice against diabetic nephropathy via upregulation of Nrf2-mediated HO-1 expression. Molecules. (2019) 24:247. doi: 10.3390/molecules24020247
90. Wang, J, Xia, Z, Su, Z, Xue, H, Huang, C, and Su, M. Knockdown of microrna-96-5p resists oxidative stress-induced apoptosis in nucleus pulposus cells. Am J Transl Res. (2023) 15:4912–21.
91. Zheng, SK, Zhao, XK, Wu, H, He, DW, Xiong, L, and Cheng, XG. Oxidative stress-induced egr1 upregulation promotes nr4a3-mediated nucleus pulposus cells apoptosis in intervertebral disc degeneration. Aging (Albany NY). (2024) 16:10216–38. doi: 10.18632/aging.205920
92. Ma, KG, Shao, ZW, Yang, SH, Wang, J, Wang, BC, Xiong, LM, et al. Autophagy is activated in compression-induced cell degeneration and is mediated by reactive oxygen species in nucleus pulposus cells exposed to compression. Osteoarthr Cartil. (2013) 21:2030–8. doi: 10.1016/j.joca.2013.10.002
93. Xu, HG, Yu, YF, Zheng, Q, Zhang, W, Wang, CD, Zhao, XY, et al. Autophagy protects end plate chondrocytes from intermittent cyclic mechanical tension induced calcification. Bone. (2014) 66:232–9. doi: 10.1016/j.bone.2014.06.018
94. Chen, PB, Shi, GX, Liu, T, Li, B, Jiang, SD, Zheng, XF, et al. Oxidative stress aggravates apoptosis of nucleus pulposus cells through m (6) a modification of mat2a pre-mrna by mettl16. Oxidative Med Cell Longev. (2022) 2022:4036274. doi: 10.1155/2022/4036274
95. Dabrowski, M, Labedz, W, Kubaszewski, L, Walczak, MK, Ziola-Frankowska, A, and Frankowski, M. Trace element concentrations in degenerative lumbar spine tissues: insights into oxidative stress. Antioxidants (Basel). (2025) 14:485. doi: 10.3390/antiox14040485
96. Klionsky, DJ, Petroni, G, Amaravadi, RK, Baehrecke, EH, Ballabio, A, Boya, P, et al. Autophagy in major human diseases. EMBO J. (2021) 40:e108863. doi: 10.15252/embj.2021108863
97. Mizushima, N, and Levine, B. Autophagy in human diseases. N Engl J Med. (2020) 383:1564–76. doi: 10.1056/NEJMra2022774
98. Luo, L, Jian, X, Sun, H, Qin, J, Wang, Y, Zhang, J, et al. Cartilage endplate stem cells inhibit intervertebral disc degeneration by releasing exosomes to nucleus pulposus cells to activate akt/autophagy. Stem Cells. (2021) 39:467–81. doi: 10.1002/stem.3322
99. Tu, J, Li, W, Li, S, Liu, W, Zhang, Y, Wu, X, et al. Sestrin-mediated inhibition of stress-induced intervertebral disc degradation through the enhancement of autophagy. Cell Physiol Biochem. (2018) 45:1940–54. doi: 10.1159/000487970
100. Vujic, N, Bradic, I, Goeritzer, M, Kuentzel, KB, Rainer, S, Kratky, D, et al. Atg7 is dispensable for lc3-pe conjugation in thioglycolate-elicited mouse peritoneal macrophages. Autophagy. (2021) 17:3402–7. doi: 10.1080/15548627.2021.1874132
101. Lamark, T, Svenning, S, and Johansen, T. Regulation of selective autophagy: the p 62/sqstm1 paradigm. Essays Biochem. (2017) 61:609–24. doi: 10.1042/EBC20170035
102. Huang, SJ, Yan, JQ, Luo, H, Zhou, LY, and Luo, JG. Il-33/st2 signaling contributes to radicular pain by modulating mapk and nf-kappab activation and inflammatory mediator expression in the spinal cord in rat models of noncompressive lumber disk herniation. J Neuroinflammation. (2018) 15:12. doi: 10.1186/s12974-017-1021-4
103. Chen, JW, Ni, BB, Li, B, Yang, YH, Jiang, SD, and Jiang, LS. The responses of autophagy and apoptosis to oxidative stress in nucleus pulposus cells: implications for disc degeneration. Cell Physiol Biochem. (2014) 34:1175–89. doi: 10.1159/000366330
104. Tu, J, Li, W, Yang, S, Yang, P, Yan, Q, Wang, S, et al. Single-cell transcriptome profiling reveals multicellular ecosystem of nucleus pulposus during degeneration progression. Adv Sci (Weinh). (2022) 9:e2103631. doi: 10.1002/advs.202103631
105. Speichert, S, Molotkov, N, El, BK, Meurer, A, Zaucke, F, and Jenei-Lanzl, Z. Role of norepinephrine in IL-1beta-induced chondrocyte dedifferentiation under physioxia. Int J Mol Sci. (2019) 20:1212. doi: 10.3390/ijms20051212
106. Sun, JS, Yang, DJ, Kinyua, AW, Yoon, SG, Seong, JK, Kim, J, et al. Ventromedial hypothalamic primary cilia control energy and skeletal homeostasis. J Clin Invest. (2021) 131:e138107. doi: 10.1172/JCI138107
107. Sun, K, Sun, J, Yan, C, Sun, J, Xu, X, and Shi, J. Sympathetic neurotransmitter, vip, delays intervertebral disc degeneration via fgf18/fgfr2-mediated activation of akt signaling pathway. Adv Biol (Weinh). (2024) 8:e2300250. doi: 10.1002/adbi.202300250
108. Fearing, BV, Hernandez, PA, Setton, LA, and Chahine, NO. Mechanotransduction and cell biomechanics of the intervertebral disc. JOR Spine. (2018) 1:e1026. doi: 10.1002/jsp2.1026
109. Gatchel, RJ, Peng, YB, Peters, ML, Fuchs, PN, and Turk, DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull. (2007) 133:581–624. doi: 10.1037/0033-2909.133.4.581
110. Meerwijk, EL, Ford, JM, and Weiss, SJ. Brain regions associated with psychological pain: implications for a neural network and its relationship to physical pain. Brain Imaging Behav. (2013) 7:1–14. doi: 10.1007/s11682-012-9179-y
111. Mercer, LN, Chen, C, Gilam, G, Mackey, S, and Scherrer, G. Brain circuits for pain and its treatment. Sci Transl Med. (2021) 13:eab–j7360. doi: 10.1126/scitranslmed.abj7360
112. Ionescu, D, Iacob, CI, Brehar, FM, and Avram, E. The role of catastrophizing and basic psychological needs satisfaction on health-related quality of life and pain in patients with lumbar disc herniation. Front Psychol. (2023) 14:1147254. doi: 10.3389/fpsyg.2023.1147254
113. Sadeghpour, ES, Zarei, F, and Haghani, S. Evaluation of a mobile-application educational intervention on the knowledge, attitude, and practice of patients in postoperative care for lumbar disk herniation surgery: a randomized control trial. SAGE Open Med. (2023) 11:372912796. doi: 10.1177/20503121231203684
114. Yurube, T, Takeoka, Y, Kanda, Y, Kuroda, R, and Kakutani, K. Intervertebral disc cell fate during aging and degeneration: apoptosis, senescence, and autophagy. N Am Spine Soc J. (2023) 14:100210. doi: 10.1016/j.xnsj.2023.100210
115. Viribay, A, Arribalzaga, S, Mielgo-Ayuso, J, Castaneda-Babarro, A, Seco-Calvo, J, and Urdampilleta, A. Effects of 120 g/h of carbohydrates intake during a mountain marathon on exercise-induced muscle damage in elite runners. Nutrients. (2020) 12:1367. doi: 10.3390/nu12051367
116. Pourahmadi, M, Delavari, S, Hayden, JA, Keshtkar, A, Ahmadi, M, Aletaha, A, et al. Does motor control training improve pain and function in adults with symptomatic lumbar disc herniation? A systematic review and meta-analysis of 861 subjects in 16 trials. Br J Sports Med. (2022) 56:1230–40. doi: 10.1136/bjsports-2021-104926
117. Yildirim, P, and Gultekin, A. The effect of a stretch and strength-based yoga exercise program on patients with neuropathic pain due to lumbar disc herniation. Spine (Phila Pa 1976). (2022) 47:711–9. doi: 10.1097/BRS.0000000000004316
118. Uysal, E, Cine, HS, and Cetin, E. The necessity and timing of exercise after lumbar disc herniation surgery. Eur Rev Med Pharmacol Sci. (2023) 27:9521–9. doi: 10.26355/eurrev_202310_34125
119. Yu, PF, Jiang, H, Liu, JT, Li, XC, Qian, X, Han, S, et al. Traditional chinese medicine treatment for ruptured lumbar disc herniation: clinical observations in 102 cases. Orthop Surg. (2014) 6:229–35. doi: 10.1111/os.12120
120. Katz, JN, Zimmerman, ZE, Mass, H, and Makhni, MC. Diagnosis and management of lumbar spinal stenosis: a review. JAMA. (2022) 327:1688–99. doi: 10.1001/jama.2022.5921
121. Peck, J, Urits, I, Peoples, S, Foster, L, Malla, A, Berger, AA, et al. A comprehensive review of over the counter treatment for chronic low back pain. Pain Ther. (2021) 10:69–80. doi: 10.1007/s40122-020-00209-w
122. van der Gaag, WH, Roelofs, PD, Enthoven, WT, van Tulder, MW, and Koes, BW. Non-steroidal anti-inflammatory drugs for acute low back pain. Emergencias. (2022) 34:66–7.
123. Zhang, Z, Xu, H, Zhang, Y, Li, W, Yang, Y, Han, T, et al. Nonsteroidal anti-inflammatory drugs for postoperative pain control after lumbar spine surgery: a meta-analysis of randomized controlled trials. J Clin Anesth. (2017) 43:84–9. doi: 10.1016/j.jclinane.2017.08.030
124. Cashin, AG, Wand, BM, O'Connell, NE, Lee, H, Rizzo, RR, Bagg, MK, et al. Pharmacological treatments for low back pain in adults: an overview of cochrane reviews. Cochrane Database Syst Rev. (2023) 4:CD13815. doi: 10.1002/14651858.CD013815.pub2
125. Price, MR, Cupler, ZA, Hawk, C, Bednarz, EM, Walters, SA, and Daniels, CJ. Systematic review of guideline-recommended medications prescribed for treatment of low back pain. Chiropr Man Therap. (2022) 30:26. doi: 10.1186/s12998-022-00435-3
126. Martinez, FO, Helming, L, and Gordon, S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. (2009) 27:451–83. doi: 10.1146/annurev.immunol.021908.132532
127. Wang, Y, Dai, G, Jiang, L, and Liao, S. The incidence of regression after the non-surgical treatment of symptomatic lumbar disc herniation: a systematic review and meta-analysis. BMC Musculoskelet Disord. (2020) 21:530. doi: 10.1186/s12891-020-03548-z
128. Ma, Z, Yu, P, Jiang, H, Li, X, Qian, X, Yu, Z, et al. Conservative treatment for giant lumbar disc herniation: clinical study in 409 cases. Pain Physician. (2021) 24:E639–48. doi: 10.36076/ppj.2021.24.E639
129. Li, Q, Liu, Y, Chu, Z, Chen, J, Dai, F, Zhu, X, et al. Brain-derived neurotrophic factor expression in dorsal root ganglia of a lumbar spinal stenosis model in rats. Mol Med Rep. (2013) 8:1836–44. doi: 10.3892/mmr.2013.1723
130. Eftimiadi, G, Soligo, M, Manni, L, Di Giuda, D, Calcagni, ML, and Chiaretti, A. Topical delivery of nerve growth factor for treatment of ocular and brain disorders. Neural Regen Res. (2021) 16:1740–50. doi: 10.4103/1673-5374.306062
131. Chen, X, Xiao, JW, Cao, P, Zhang, Y, Cai, WJ, Song, JY, et al. Brain-derived neurotrophic factor protects against acrylamide-induced neuronal and synaptic injury via the trkb-mapk-erk1/2 pathway. Neural Regen Res. (2021) 16:150–7. doi: 10.4103/1673-5374.286976
132. Xiong, HY, Hendrix, J, Schabrun, S, Wyns, A, Campenhout, JV, Nijs, J, et al. The role of the brain-derived neurotrophic factor in chronic pain: links to central sensitization and neuroinflammation. Biomol Ther. (2024) 14:71. doi: 10.3390/biom14010071
133. Fathy, W, Hussein, M, Magdy, R, Elmoutaz, H, Youssef, NA, Abd, AM, et al. Predictive value of s100b and brain derived neurotrophic factor for radiofrequency treatment of lumbar disc prolapse. BMC Anesthesiol. (2024) 24:161. doi: 10.1186/s12871-024-02527-4
134. Zhou, G, Dai, L, Jiang, X, Ma, Z, Ping, J, Li, J, et al. Effects of human midkine on spontaneous resorption of herniated intervertebral discs. Int Orthop. (2010) 34:103–8. doi: 10.1007/s00264-009-0740-2
135. Yoshida, Y, Goto, M, Tsutsui, J, Ozawa, M, Sato, E, Osame, M, et al. Midkine is present in the early stage of cerebral infarct. Brain Res Dev Brain Res. (1995) 85:25–30. doi: 10.1016/0165-3806(94)00183-Z
136. Yu, PF, Jiang, FD, Liu, JT, and Jiang, H. Outcomes of conservative treatment for ruptured lumbar disc herniation. Acta Orthop Belg. (2013) 79:726–30.
137. Zhang, W, Wang, Z, Yin, J, Bai, Y, Qiu, F, Zhang, H, et al. Clinical treatment of lumbar disc herniation. Ann Ital Chir. (2022) 93:457–62.
138. Albert, HB, Sayari, AJ, Barajas, JN, Hornung, AL, Harada, G, Nolte, MT, et al. The impact of novel inflammation-preserving treatment towards lumbar disc herniation resorption in symptomatic patients: a prospective, multi-imaging and clinical outcomes study. Eur Spine J. (2024) 33:964–73. doi: 10.1007/s00586-023-08064-x
139. Manchikanti, L, Knezevic, E, Knezevic, NN, Kaye, AD, Atluri, S, Sanapati, MR, et al. Effectiveness of intradiscal regenerative medicine therapies for long-term relief of chronic low back pain: a systematic review and meta-analysis. Pain Physician. (2024) 27:E995–E1032.
140. R, NB, Ethiraj, P, Ramachandraiah, MK, Kumaar, A, and S, NJ. Association between spinopelvic parameters and clinical outcomes of patients treated with transforaminal platelet-rich plasma injections for lumbar disc prolapse. Cureus. (2024) 16:e66995. doi: 10.7759/cureus.66995
141. Everts, P, Onishi, K, Jayaram, P, Lana, JF, and Mautner, K. Platelet-rich plasma: new performance understandings and therapeutic considerations in 2020. Int J Mol Sci. (2020) 21:7794. doi: 10.3390/ijms21207794
142. Navani, A, Manchikanti, L, Albers, SL, Latchaw, RE, Sanapati, J, Kaye, AD, et al. Responsible, safe, and effective use of biologics in the management of low back pain: american society of interventional pain physicians (asipp) guidelines. Pain Physician. (2019) 22:S1–S74. doi: 10.36076/ppj/2019.22.s1
143. Tuakli-Wosornu, YA, Terry, A, Boachie-Adjei, K, Harrison, JR, Gribbin, CK, Lasalle, EE, et al. Lumbar intradiskal platelet-rich plasma (prp) injections: a prospective, double-blind, randomized controlled study. PM R. (2016) 8:1–10. doi: 10.1016/j.pmrj.2015.08.010
144. Gupta, A, Chhabra, HS, Singh, V, and Nagarjuna, D. Lumbar transforaminal injection of steroids versus platelet-rich plasma for prolapse lumbar intervertebral disc with radiculopathy: a randomized double-blind controlled pilot study. Asian Spine J. (2024) 18:58–65. doi: 10.31616/asj.2023.0115
145. Singh, GK, Talawar, P, Kumar, A, Sharma, RS, Purohit, G, and Bhandari, B. Effect of autologous platelet-rich plasma (prp) on low back pain in patients with prolapsed intervertebral disc: a randomised controlled trial. Indian J Anaesth. (2023) 67:277–82. doi: 10.4103/ija.ija_821_22
146. Wang, AM, Cao, P, Yee, A, Chan, D, and Wu, EX. Detection of extracellular matrix degradation in intervertebral disc degeneration by diffusion magnetic resonance spectroscopy. Magn Reson Med. (2015) 73:1703–12. doi: 10.1002/mrm.25289
147. Otoshi, K, Kikuchi, S, Konno, S, and Sekiguchi, M. The reactions of glial cells and endoneurial macrophages in the dorsal root ganglion and their contribution to pain-related behavior after application of nucleus pulposus onto the nerve root in rats. Spine (Phila Pa 1976). (2010) 35:10–7. doi: 10.1097/BRS.0b013e3181c67f1e
148. Shamji, MF, Jing, L, Chen, J, Hwang, P, Ghodsizadeh, O, Friedman, AH, et al. Treatment of neuroinflammation by soluble tumor necrosis factor receptor type ii fused to a thermally responsive carrier. J Neurosurg Spine. (2008) 9:221–8. doi: 10.3171/SPI/2008/9/8/221
149. Trapana, J, Weinerman, J, Lee, D, Sedani, A, Constantinescu, D, Best, TM, et al. Cell-based therapy in the treatment of musculoskeletal diseases. Stem Cells Transl Med. (2024) 13:959–78. doi: 10.1093/stcltm/szae049
150. Vadala, G, Ambrosio, L, Russo, F, Papalia, R, and Denaro, V. Stem cells and intervertebral disc regeneration overview-what they can and can't do. Int J Spine Surg. (2021) 15:40–53. doi: 10.14444/8054
151. Piccirilli, M, Delfinis, CP, Santoro, A, and Salvati, M. Mesenchymal stem cells in lumbar spine surgery: a single institution experience about red bone marrow and fat tissue derived mscs. J Neurosurg Sci. (2017) 61:124–33. doi: 10.23736/S0390-5616.16.03266-X
152. Oehme, D, Goldschlager, T, Rosenfeld, JV, Ghosh, P, and Jenkin, G. The role of stem cell therapies in degenerative lumbar spine disease: a review. Neurosurg Rev. (2015) 38:429–45. doi: 10.1007/s10143-015-0621-7
153. Orozco, L, Soler, R, Morera, C, Alberca, M, Sanchez, A, and Garcia-Sancho, J. Intervertebral disc repair by autologous mesenchymal bone marrow cells: a pilot study. Transplantation. (2011) 92:822–8. doi: 10.1097/TP.0b013e3182298a15
154. Atluri, S, Murphy, MB, Dragella, R, Herrera, J, Boachie-Adjei, K, Bhati, S, et al. Evaluation of the effectiveness of autologous bone marrow mesenchymal stem cells in the treatment of chronic low back pain due to severe lumbar spinal degeneration: a 12-month, open-label, prospective controlled trial. Pain Physician. (2022) 25:193–207.
155. Rivera, CE. Lumbar epidural steroid injections. Phys Med Rehabil Clin N Am. (2018) 29:73–92. doi: 10.1016/j.pmr.2017.08.007
156. Sariyildiz, MA, Batmaz, I, and Hattapoglu, S. Predictors of successful treatment after transforaminal epidural steroid injections in patients with lumbar disc herniation. J Back Musculoskelet Rehabil. (2024) 37:327–35. doi: 10.3233/BMR-230051
157. Smith, CC, Mccormick, ZL, Mattie, R, Macvicar, J, Duszynski, B, and Stojanovic, MP. The effectiveness of lumbar transforaminal injection of steroid for the treatment of radicular pain: a comprehensive review of the published data. Pain Med. (2020) 21:472–87. doi: 10.1093/pm/pnz160
158. Hong, SJ, Kim, DY, Kim, H, Kim, S, Shin, KM, and Kang, SS. Resorption of massive lumbar disc herniation on mri treated with epidural steroid injection: a retrospective study of 28 cases. Pain Physician. (2016) 19:381–8. doi: 10.36076/ppj/2016.19.381
159. Liang, Z, Dai, X, Li, W, Chen, W, Shi, Q, Wei, Y, et al. Development of a spinopelvic complex finite element model for quantitative analysis of the biomechanical response of patients with degenerative spondylolisthesis. Med Biol Eng Comput. (2025) 63:575–94. doi: 10.1007/s11517-024-03218-5
160. Cao, B, Zhou, X, Zhang, SP, Zhu, QG, Kong, LJ, and Fang, M. Effect of traditional Chinese manual therapy on alleviating pain and dysfunction of lumbar disc herniation: a randomized controlled pilot study. Am J Transl Res. (2022) 14:6941–52.
161. Wang, W, Long, F, Wu, X, Li, S, and Lin, J. Clinical efficacy of mechanical traction as physical therapy for lumbar disc herniation: a meta-analysis. Comput Math Methods Med. (2022) 2022:5670303. doi: 10.1155/2022/5670303
162. Li, X, Han, Y, Cui, J, Yuan, P, Di, Z, and Li, L. Efficacy of warm needle moxibustion on lumbar disc herniation: a meta-analysis. J Evid Based Complementary Altern Med. (2016) 21:311–9. doi: 10.1177/2156587215605419
Keywords: lumbar disc herniation, reabsorption, mechanisms of action, treatment, research progress
Citation: Chai Y, Shen X, Wang Z, Zhang X, Wang Z, Zuo X and Liu J (2025) Lumbar disc herniation reabsorption: a review of clinical manifestations, mechanisms, and conservative treatments. Front. Med. 12:1633762. doi: 10.3389/fmed.2025.1633762
Edited by:
Taotao Xu, Zhejiang Chinese Medical University, ChinaReviewed by:
Qiu-Shi Wei, Guangzhou University of Chinese Medicine, ChinaGuanyi Liu, Department of Spine Surgery, China
Lei Liang, Second Military Medical University, China
Copyright © 2025 Chai, Shen, Wang, Zhang, Wang, Zuo and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jintao Liu, b2tkb2N0b3JAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Yuan Chai
Yuan Chai Xuyu Shen4†
Xuyu Shen4† Xiaoyun Zhang
Xiaoyun Zhang Jintao Liu
Jintao Liu