- 1Department of Cardiology, Ganzhou Hospital of Traditional Chinese Medicine, Ganzhou, Jiangxi, China
- 2Department of Cardiology, Ganzhou People’s Hospital, Ganzhou, Jiangxi, China
Background: Increased arterial stiffness is an important marker of target organ damage in hypertension. Estimated pulse wave velocity (ePWV) is a noninvasive assessment of arterial stiffness based on blood pressure and age calculations, but its association with albuminuria, an early indicator of renal function impairment, still needs to be validated. The aim of this study was to investigate the association of ePWV with albuminuria and its dose–response properties in hypertensive patients.
Methods: Hypertensive patients who attended the cardiology department of Ganzhou Hospital of Traditional Chinese Medicine in China from July 2024 to April 2025 were retrospectively enrolled. ePWV was calculated from systolic and diastolic blood pressure and age. Albuminuria was detected using a dipstick test. Logistic regression was used to analyze the association between ePWV and albuminuria, and dose–response relationship was assessed using restricted cubic spline (RCS).
Results: A total of 761 hypertensive patients were included in the final analysis. The rate of positive albuminuria was 19.6%. High ePWV group (≥10.74 m/s) was associated with albuminuria compared to low ePWV group (OR = 2.79, 95% CI: 1.18–6.71). And for per 1-m/s increase in ePWV, the risk of ePWV association with albuminuria increased by 42% (OR = 1.42, 95% CI: 1.14–1.78). RCS analysis showed a linear dose–response relationship between ePWV and albuminuria (p non-linear >0.05).
Conclusion: ePWV was independently associated with albuminuria in hypertensive patients with a linear dose–response relationship, suggesting that arterial stiffness may be a measurable risk factor for early renal injury, and that ePWV may serve as a simple tool for primary renal function assessment.
Introduction
Hypertension is one of the leading chronic diseases worldwide, affecting approximately 31.1% of the adult population and significantly increasing the risk of cardiovascular and cerebrovascular disease and end-stage renal disease (1). Although antihypertensive therapy has improved the prognosis of patients to some extent, renal damage is still prevalent in hypertensive patients, and albuminuria is a sensitive indicator of early renal impairment (2–5).
The dipstick method is a semi-quantitative dry chemistry assay based on the tetra bromophenol blue (TBPB) colorimetric reaction, which enables rapid detection of albuminuria by leveraging the specific binding of albumin to TBPB under alkaline conditions, resulting in a color transition from green to yellow that correlates with albumin concentration (6, 7). This method has been widely adopted in clinical practice and epidemiologic studies due to its advantages of rapidity, simplicity, and cost-effectiveness, making it particularly valuable in resource-limited settings or large-scale screening programs where sophisticated laboratory equipment is unavailable (8, 9). Beyond its practical utility, accumulating evidence supports the clinical significance of dipstick-detected albuminuria. For instance, albuminuria detected by the dipstick method on admission has been recognized as a poor prognostic factor in patients with acute stroke (10). According to Kim et al., the dipstick method can be used as a simple and useful method for all-cause mortality risk assessment in patients with hypertensive crisis (11). Specifically in hypertensive patients, dipstick-detected albuminuria is widely accepted as a sensitive indicator of early renal impairment, with consistent findings across multiple studies linking its presence to progressive renal damage and increased cardiovascular risk (12–14). Its ability to provide immediate results allows for timely identification of at-risk individuals, facilitating early intervention and management in clinical practice.
Previous studies have demonstrated that arterial stiffness assessed by brachial-ankle PWV (baPWV) or carotid-femoral PWV (cfPWV) is strongly associated with proteinuria and deterioration of renal function (15–17). However, the measurement of baPWV and cfPWV requires specialized equipment and operating techniques, which are difficult to generalize in resource-limited areas or large-scale epidemiological surveys. Meanwhile, estimated pulse wave velocity (ePWV), a proxy for noninvasive arterial stiffness calculated based on age, systolic and diastolic blood pressures, has been shown to correlate with atherosclerosis, cardiovascular events, and decreased renal function (18–20). However, studies on whether ePWV independently associates with albuminuria levels in hypertensive patients and whether there is a dose–response relationship between the two are still limited.
Therefore, to address these gaps, we aimed to assess the association between ePWV and albuminuria development by utilizing consultation data from Ganzhou Hospital of Traditional Chinese Medicine in China. This finding provides a potential biological basis for renal function monitoring in hypertensive patients.
Materials and methods
Study population
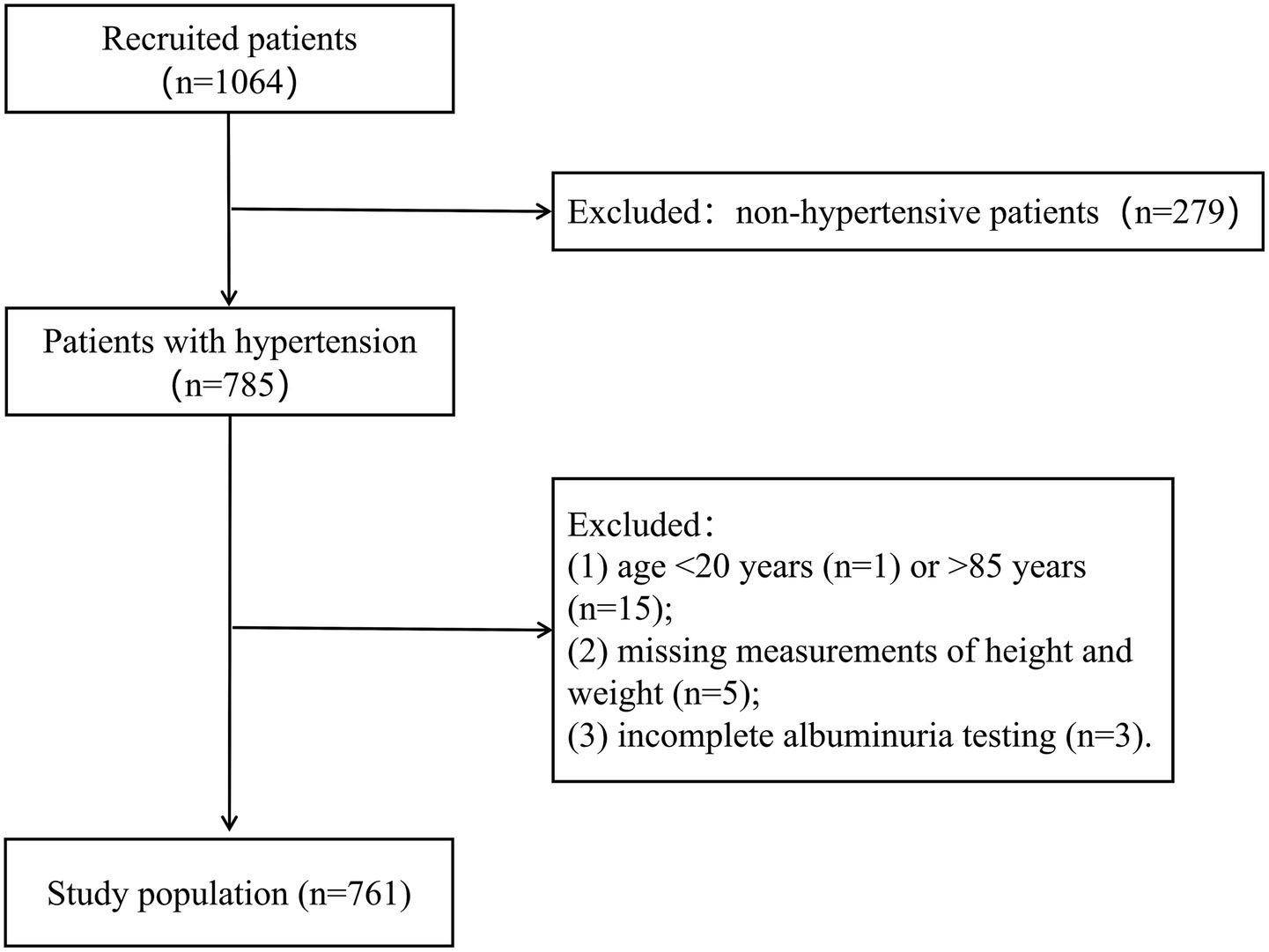
This study used a retrospective cohort design, and 1,067 patients attended in the Department of Cardiology between July 2024 and April 2025 were consecutively included by accessing the electronic medical record system of Ganzhou Hospital of Traditional Chinese Medicine, Jiangxi Province, China. All enrolled patients completed standardized blood pressure measurement and detailed history taking within 24 h of consultation. The study strictly adhered to the following inclusion and exclusion criteria: inclusion criteria were (1) patients with confirmed hypertension and (2) age 20–85 years. Exclusion criteria included (1) patients without hypertension (n = 279); (2) age <20 years (n = 1) or >85 years (n = 15); (3) missing measurements of height and weight (n = 5); and (4) incomplete albuminuria testing (n = 3). Through rigorous screening, 761 hypertensive patients finally met the study criteria and were included in the analysis (Figure 1). The study was approved by the Ethics Committee of Ganzhou Hospital of Traditional Chinese Medicine (no. GZSZYYKYLL20250024), strictly following the ethical guidelines of the Declaration of Helsinki. Given the nature of the study as a retrospective data analysis, the Institutional Review Board exempted patients from the requirement of written informed consent.
Diagnostic criteria of hypertension
Previous history of hypertension, or currently taking antihypertensive medication, or blood pressure measurement at the first visit, systolic blood pressure (SBP) ≥140 mmHg and/or diastolic blood pressure (DBP) ≥90 mmHg (21). Blood pressure was measured in a seated position after a 5-min quiet rest, with all participants choosing the right arm.
Calculation of ePWV
ePWV is a noninvasive hemodynamic parameter used to assess arterial stiffness, derived from age and mean blood pressure (MBP). The mathematical formula for ePWV is expressed as follows: ePWV (m/s) = 9.587–0.402 × age + 4.560 × 10−3 × age2 − 2.621 × 10−5 × age2 × MBP (Mean Arterial Pressure) + 3.176 × 10−3 × age × MBP − 1.832 × 10−2 × MBP, where MBP (mean arterial pressure, mmHg) is calculated using DBP and SBP according to: MBP=DBP + 0.4 (SBP-DBP) (22).
Measurement of albuminuria
In this study, semi-quantitative analysis of albuminuria was performed using the Mejer 700 I urine chemistry analyzer (Shenzhen Meiqiao Medical Technology Co., Ltd., China) by the dry chemistry dipstick method. This method is based on the tetra bromophenol blue (TBPB) colorimetric assay. Under alkaline conditions, albumin binds to TBPB, producing a chromogenic reaction that transitions from green to yellow, with the intensity of coloration directly proportional to the albumin concentration. According to the manufacturer’s colorimetric grading criteria, a qualitative proteinuria reading of ≥1 + was defined as albuminuria (23).
Covariates
The following covariates were included in this study: age, sex, smoking status, history of previous illnesses (including diabetes, coronary heart disease, and stroke), as well as medication use (antihypertensive medications, hypoglycemic medications, and hyperlipidemia medications), body mass index (BMI, calculated using the formula weight in kg/height in m2), SBP, DBP, total cholesterol (TC), low density lipoprotein cholesterol (LDL-C), and renal function indexes (eGFR, calculated according to the Chronic Kidney Disease Epidemiology Collaboration 2021 (CKD-EPI) formula in mL/min/1.73 m2) (24).
Statistical analysis
All statistical analyses were performed using R studio software (4.2.1) and a two-sided p < 0.05 was considered statistically significant. Participants were categorized into three groups based on the tertiles of ePWV. Continuous data are shown as means (25–75th percentile) and differences between groups were compared using ANOVA. Categorical data are shown as number (n) and percentage (%) and differences between groups were examined using Chi-square tests. Logistic regression analyses and ordinal multinomial logistic regression were used to explore the association between ePWV and urinary albuminuria in the hypertensive population, and results are expressed as odds ratios (ORs) and 95% confidence intervals (95% CIs). We constructed three model, Model 1 was adjusted for age and sex. Model 2 was adjusted for age, sex, smoking status, coronary heart disease, stroke, and diabetes. Model 3 was adjusted for the variables in model 2 plus antihypertensive medication, hypoglycemic medications, hyperlipidemia medications, BMI, TC and LDL-C. Linear trends were tested by treating the median value of each tertile as a continuous variable. Restricted cubic spline (RCS) analysis was used to evaluate the potential nonlinear relationship between ePWV and albuminuria. Knots were placed at the 10th, 50th, and 90th percentiles of ePWV distribution, based on Harrell’s recommendations for balanced flexibility and stability of spline curves. The nonlinearity was tested by comparing the model fit with and without the nonlinear terms using likelihood ratio tests. All covariates in model 3 were adjusted in the spline regression. Subgroup analyses of the association between ePWV and albuminuria were performed, with stratification factors including age, sex, smoking status, diabetes, coronary heart disease, and stroke.
Results
Study participants and baseline characteristics
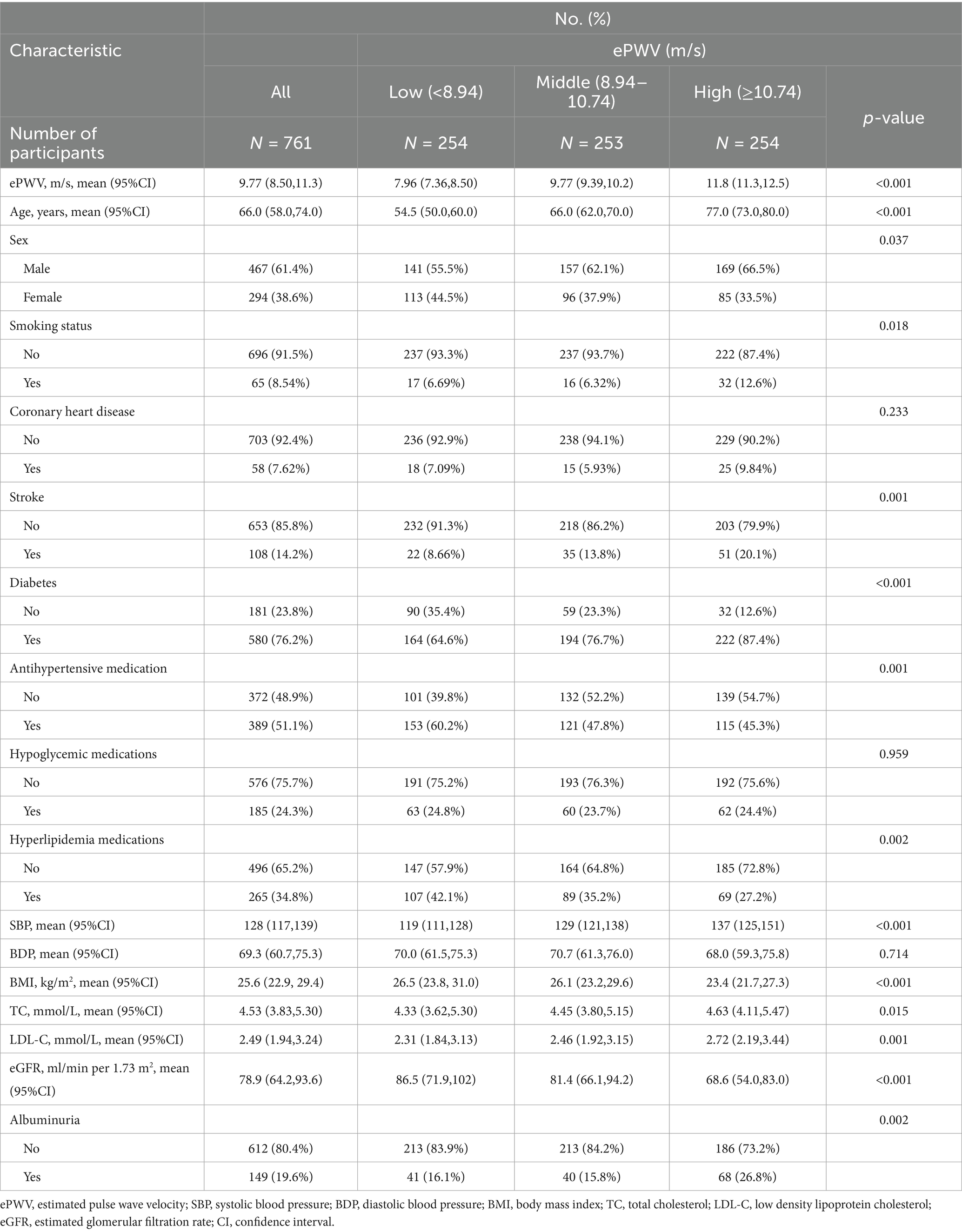
A total of 761 hypertensive patients were included in this study in which the positivity rate of albuminuria was at 19.6% and a total of 294 (38.6%) were females. ePWV had a mean velocity of 9.77 m/s. ePWV was categorized into three groups by tertiles as low (<8.94 m/s), middle (8.94–10.74 m/s) and high (≥10.74 m/s) (Table 1). Age, sex, BMI, SBP, DBP, stroke, smoking status, antihypertensive medications, hyperlipidemia medications, diabetes, TC and LDL-C differed between groups (Table 1).
Association of ePWV and albuminuria
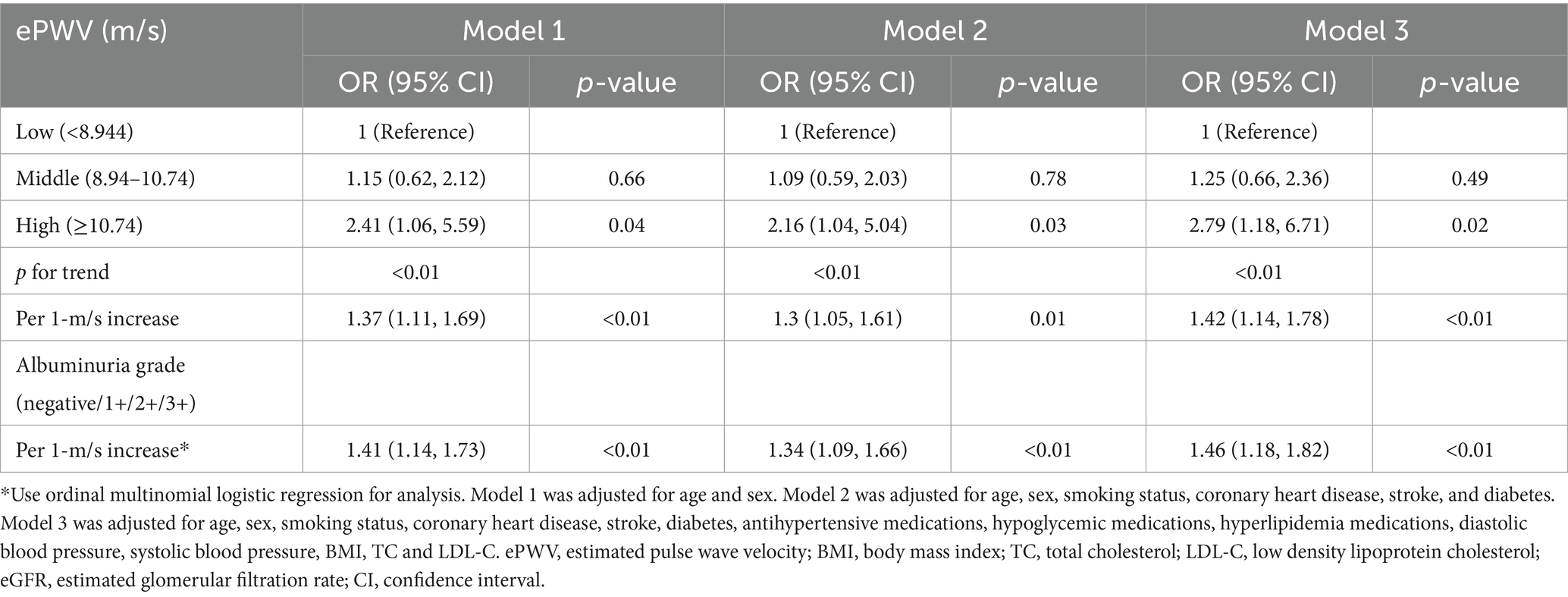
There was a linear dose–response relationship between ePWV and albuminuria after multivariate adjustment (nonlinear p > 0.05) (Figure 2). We analyzed the association between ePWV and albuminuria using logistic regression, and the results are shown in Table 2. In model 1, the OR for albuminuria was 2.41 (95% CI, 1.06–5.59) in hypertensive patients with high ePWV compared with the low ePWV group. This association persisted even after all covariates (OR 2.79, 0.95% CI, 1.18–6.71), and in addition, the risk of association of ePWV with albuminuria was increased by 42% (OR = 1.42, 95% CI: 1.14–1.78) for per 1-m/s increase in ePWV (Model 3). The ordinal multinomial logistic regression analysis showed that ePWV was significantly associated with the albuminuria grade (negative/1+/2+/3+). For per 1-m/s increase in ePWV, the risk of a higher albuminuria grade (progression to a more severe level) increased by 46% (OR = 1.46, 95% CI: 1.18–1.82) (Model 3).
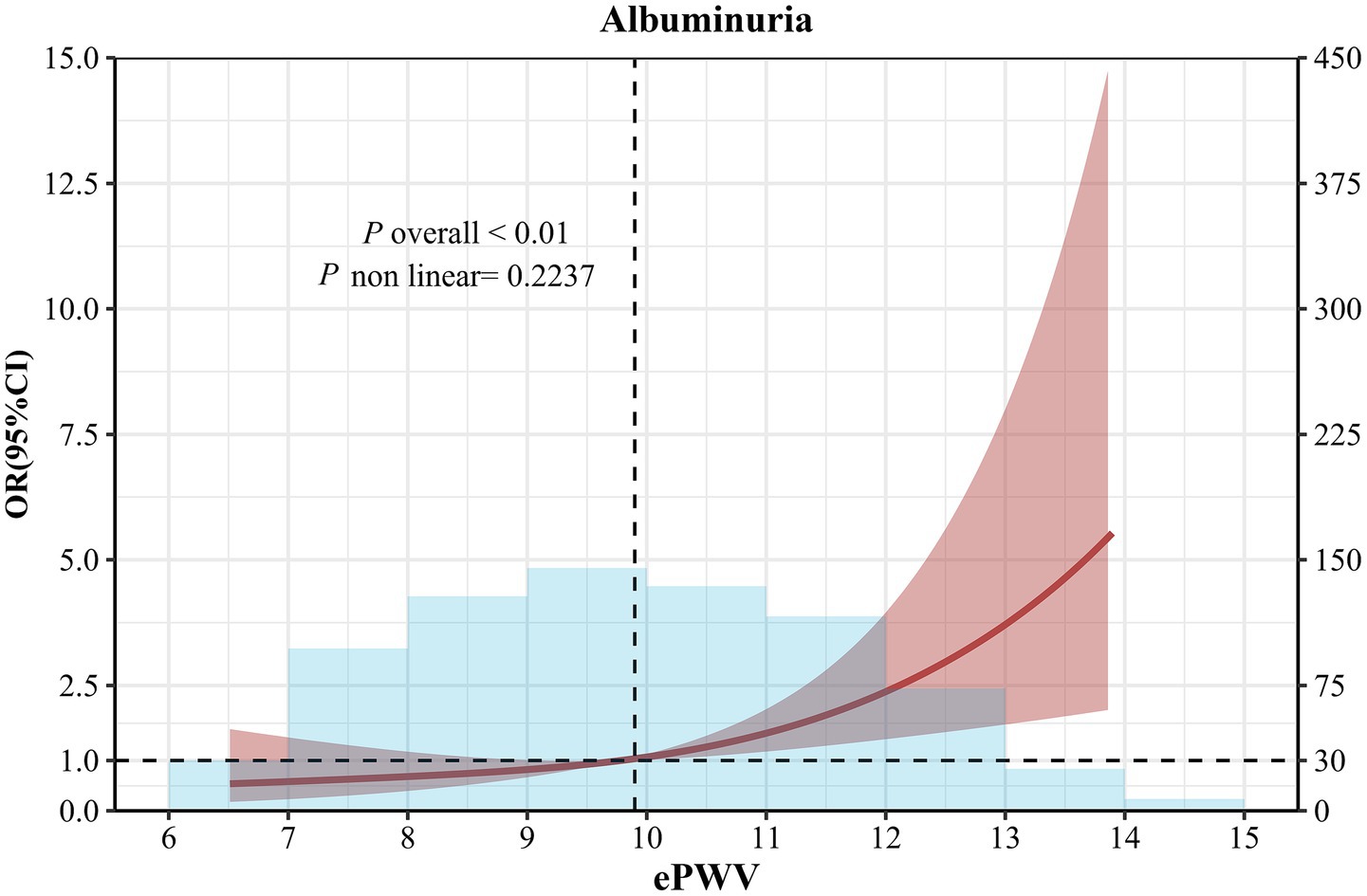
Figure 2. Restricted cubic spline curve analysis for the association of ePWV with albuminuria in hypertensive patients. Solid lines were odds ratios with 95% CI in shaded areas. Knot locations were the 10th, 50th, and 90th percentiles of ePWV. All models were adjusted for age, sex, smoking status, coronary heart disease, stroke, diabetes, antihypertensive medications, hypoglycemic medications, hyperlipidemia medications, diastolic blood pressure, systolic blood pressure, BMI, TC and LDL-C.
Stratified analyses
Stratified analyses according to age (<60 years, ≥60 years), sex, smoking status, diabetes, coronary heart disease, and stroke were shown in Figure 3. There was no evidence of an association between ePWV and albuminuria that varied by age (<60 years, ≥60 years), sex, smoking status, diabetes, coronary heart disease, and stroke.
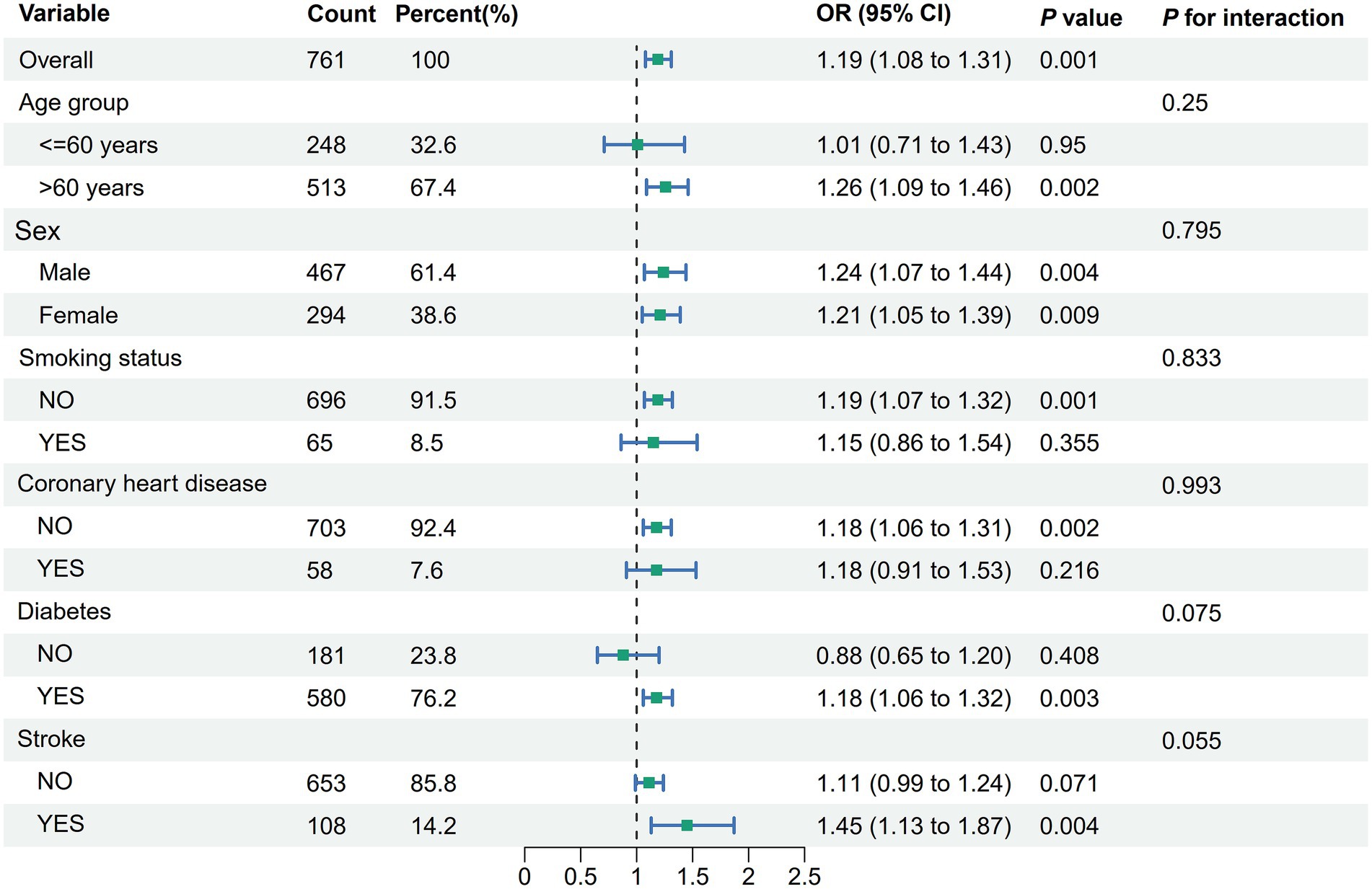
Figure 3. Stratified analyses of the association between ePWV and albuminuria in hypertensive patients. Model was adjusted for age, sex, smoking status, coronary heart disease, stroke, diabetes, antihypertensive medications, hypoglycemic medications, hyperlipidemia medications, diastolic blood pressure, systolic blood pressure, BMI, TC and LDL-C. ePWV, estimated pulse wave velocity; BMI, body mass index; TC, total cholesterol; LDL-C, low density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; CI, confidence interval.
Discussion
In this cross-sectional study, we investigated the association between ePWV and albuminuria. Our study found that high ePWV was associated with an increased incidence of albuminuria in hypertensive patients, after adjusting for important potential confounders. This result suggests that increased arterial stiffness may be strongly associated with early renal damage and provides a new theoretical basis for the prevention and control of hypertensive target organ damage.
Previous studies have shown that ePWV can be used as a surrogate for cfPWV and baPWV. Kevin S Heffernan et al. found that ePWV was as useful a tool for assessing vascular aging as cfPWV (25). A study of 881 patients from Kaohsiung Hospital in Taiwan, China, found that ePWV had a better additive predictive value for cardiovascular mortality than baPWV and a similar predictive value for all-cause mortality as baPWV (26). According to Sara V Greve et al., ePWV and cfPWV have similar predictive value for cardiovascular events (22). The results of this study are in general agreement with previous knowledge on the relationship between arterial stiffness and renal function. A Japanese Trial of Prognostic Effects of Pulse Wave Velocity (J-TOPP) found a 19.2% increase in the risk of microproteinuria for every 2 m/s increase in baPWV (22). Hypertensive patients with high baPWV levels had a 1.8-fold increased risk of albuminuria compared with hypertensive patients with low baPWV (27), as reported by Yoshitsugu Sunagawa et al. It is noteworthy that although our study used ePWV rather than device-measured PWV, the trends of the results obtained were in close agreement, which somewhat validates the reliability of ePWV as an assessment of arterial stiffness.
Our findings support the current pathophysiologic understanding. Increased arterial stiffness may affect renal function through multiple pathways: (1) directly increasing intraglomerular pressure, leading to filtration barrier damage (28, 29); (2) causing systemic blood pressure fluctuations, resulting in renal microcirculatory damage (30); and (3) promoting a chronic state of renal inflammation and oxidative stress (31–33). Of particular note, a linear dose–response relationship between ePWV and albuminuria was found in this study. This result implies that lowering arterial stiffness in hypertensive patients may have a corresponding renoprotective effect. This is in good agreement with the trend observed in antihypertensive treatment studies of greater cardiovascular protection with lower arterial stiffness (34).
It is worth noting that, unlike cfPWV, ePWV does not yet have standardized percentile reference values for the general population. Given that ePWV is derived directly from age and blood pressure, it inherently increases with aging and may reflect both vascular aging and the degree of blood pressure control (22). The absence of population-based calibration may limit its precision in individual-level risk stratification. Future studies are warranted to establish ePWV reference percentiles based on large-scale epidemiological cohorts, which would enhance its clinical applicability and comparability across populations. The results of this study have important clinical practice implications. ePWV, as a simple and readily available estimator of arterial stiffness, may help clinicians to identify subgroups of hypertensive patients at higher risk of renal damage. Especially in primary care or resource-limited areas, ePWV may be a practical risk assessment tool when sophisticated vascular function tests are not available.
Despite the importance of the findings of this study, several important limitations must be recognized. First, the cross-sectional study design prevented us from determining the direction of causality between ePWV and albuminuria. Although increased arterial stiffness may theoretically lead to renal injury, improved renal function may in turn attenuate atherosclerosis (35), a bidirectional relationship that needs to be further validated by prospective cohort studies. Second, we used a dipstick method for the detection of albuminuria, and although this method is widely used and convenient in clinical practice, there is a gap in its sensitivity compared with quantitative laboratory methods (e.g., urine albumin/creatinine ratio) (36). This may lead to an underestimation of the true strength of the association, especially in the detection of proteinuria at low concentrations (37). However, from another perspective, it is due to the relatively high specificity of the dipstick method that the significant associations observed in this study may reflect more definitive renal damage. Third, the study samples were all from a single medical center and were convenience samples, which may affect the external validity of the findings. Fourthly, This study did not consider other factors that may affect the relationship between ePWV and albuminuria, such as ARB, ACEI and SGLT2i. Fifthly, due to the retrospective nature of our study and incomplete documentation of hypertension onset dates in medical records, we were unable to reliably extract this information for analysis. This limitation restricts our ability to disentangle the effects of disease duration from those of arterial stiffness on albuminuria. Sixthly, it should be noted that dipstick-based methods (paper-analytical devices, PADs) have only moderate sensitivity for detecting low-grade microalbuminuria (38). Therefore, our study may have underestimated the true prevalence of albuminuria due to the limited sensitivity of the detection method. While the dipstick method remains a widely used and cost-effective screening tool in routine clinical practice, this limitation should be considered when interpreting the findings. Seventhly, we acknowledge that direct PWV measurements (cfPWV or baPWV) remain the gold standard for assessing arterial stiffness, and head-to-head comparisons between ePWV and these direct methods in the same patient population would provide valuable insights into their relative performance. Future studies with access to both ePWV and direct PWV data are warranted to further validate the utility of ePWV as a surrogate marker, particularly in relation to albuminuria and renal function outcomes.
Conclusion
In this study, we found an independent and robust association between ePWV and albuminuria results detected by the dipstick method with a linear dose–response relationship. This finding not only supports the important role of arterial stiffness in hypertension-associated renal damage, but also suggests that ePWV may be a simple tool for assessing renal risk in clinical practice.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ganzhou Traditional Chinese Medicine Hospital Medical Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XZ: Data curation, Project administration, Writing – original draft, Methodology, Investigation. CX: Project administration, Supervision, Methodology, Writing – original draft. WX: Data curation, Writing – original draft, Conceptualization, Investigation. QZ: Methodology, Writing – original draft, Visualization, Writing – review & editing, Validation. JW: Validation, Writing – original draft, Supervision, Software. JL: Investigation, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Science and Technology Bureau of Ganzhou City, China (no. GZ2024YLJ154).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mills, KT, Stefanescu, A, and He, J. The global epidemiology of hypertension. Nat Rev Nephrol. (2020) 16:223–37. doi: 10.1038/s41581-019-0244-2
2. Musini, VM, Gueyffier, F, Puil, L, Salzwedel, DM, and Wright, JM. Pharmacotherapy for hypertension in adults aged 18 to 59 years. Cochrane Database Syst Rev. (2017) 8:CD008276. doi: 10.1002/14651858.CD008276.pub2
3. Gnjidic, D, Langford, AV, Jordan, V, Sawan, M, Sheppard, JP, Thompson, W, et al. Withdrawal of antihypertensive drugs in older people. Cochrane Database Syst Rev. (2025) 3:CD012572. doi: 10.1002/14651858.CD012572.pub3
4. Russo, E, Bussalino, E, Macciò, L, Verzola, D, Saio, M, Esposito, P, et al. Non-haemodynamic mechanisms underlying hypertension-associated damage in target kidney components. Int J Mol Sci. (2023) 24:9422. doi: 10.3390/ijms24119422
5. Zeng, D, Wang, B, Xiao, Z, Wang, X, Tang, X, Yao, X, et al. Early diagnosis and treatment of kidney injury: a focus on urine protein. Int J Mol Sci. (2024) 25:11171. doi: 10.3390/ijms252011171
6. Kavuru, V, Vu, T, Karageorge, L, Choudhury, D, Senger, R, and Robertson, J. Dipstick analysis of urine chemistry: benefits and limitations of dry chemistry-based assays. Postgrad Med. (2020) 132:225–33. doi: 10.1080/00325481.2019.1679540
7. Alao, MA, Asinobi, OA, Ibrahim, OR, and Lagunju, IA. Spot urine protein-to-creatinine ratio compared with dipstick proteinuria as a primary screening tool for renal disease in a community setting. Saudi J Kidney Dis Transpl. (2021) 32:1655–65. doi: 10.4103/1319-2442.352426
8. Sumida, K, Nadkarni, GN, Grams, ME, Sang, Y, Ballew, SH, Coresh, J, et al. Conversion of urine protein-creatinine ratio or urine dipstick protein to urine albumin-creatinine ratio for use in chronic kidney disease screening and prognosis: an individual participant-based meta-analysis. Ann Intern Med. (2020) 173:426–35. doi: 10.7326/M20-0529
9. Salinas, M, López-Garrigós, M, Flores, E, Lugo, J, and Leiva-Salinas, CPRIMary Care-LABoratory (PRIMLAB) working group. Urinary albumin strip assay as a screening test to replace quantitative technology in certain conditions. Clin Chem Lab Med. (2018) 57:204–9. doi: 10.1515/cclm-2018-0546
10. You, S, Xu, J, Ou, Z, Zhong, C, Han, Q, Chen, J, et al. Prognostic significance of urinary protein and urinary ketone bodies in acute ischemic stroke. Nutr Metab Cardiovasc Dis. (2021) 31:3152–60. doi: 10.1016/j.numecd.2021.07.010
11. Kim, BS, Yu, MY, Park, JK, Shin, J, and Shin, JH. Association of Dipstick Proteinuria with Long-term mortality among patients with hypertensive crisis in the emergency department. J Pers Med. (2022) 12:971. doi: 10.3390/jpm12060971
12. Mantula, PS, Outinen, TK, Clement, JPG, Huhtala, HSA, Pörsti, IH, Vaheri, A, et al. Glomerular proteinuria predicts the severity of acute kidney injury in Puumala hantavirus-induced Tubulointerstitial nephritis. Nephron. (2017) 136:193–201. doi: 10.1159/000459634
13. Song, JJ, Lee, KB, Hyun, YY, and Kim, H. Trace albumin in the urine dipstick test is associated with coronary artery calcification in Korean adults. Nephron. (2018) 140:169–74. doi: 10.1159/000490954
14. Wei, XB, Liu, YH, He, PC, Yu, DQ, Zhou, YL, Tan, N, et al. The relationship between albuminuria and poor clinical outcomes in patients with infective endocarditis. Clin Chim Acta. (2016) 462:28–32. doi: 10.1016/j.cca.2016.08.017
15. Munakata, M, Miura, Y, and Yoshinaga, KJ-TOPP study group. Higher brachial-ankle pulse wave velocity as an independent risk factor for future microalbuminuria in patients with essential hypertension: the J-TOPP study. J Hypertens. (2009) 27:1466–71. doi: 10.1097/HJH.0b013e32832b4740
16. Wang, T, Fan, F, Gong, Y, Gao, L, Liu, Z, Jia, J, et al. Comparison of brachial-ankle pulse wave velocity and carotid-femoral pulse wave velocity in association with albuminuria in a community of Beijing: a cross-sectional study. J Hum Hypertens. (2023) 37:412–8. doi: 10.1038/s41371-022-00697-7
17. Sanchez, RA, Sanchez, MJ, and Ramirez, AJ. Renal function, albumin-creatinine ratio and pulse wave velocity predict silent coronary artery disease and renal outcome in type 2 diabetic and Prediabetic subjects. Curr Hypertens Rev. (2021) 17:131–6. doi: 10.2174/1573402116999201210194817
18. Cheng, W, Kong, F, Pan, H, Luan, S, Yang, S, and Chen, S. Superior predictive value of estimated pulse wave velocity for all-cause and cardiovascular disease mortality risk in U.S. general adults. BMC Public Health. (2024) 24:600. doi: 10.1186/s12889-024-18071-2
19. Liu, G, Sha, W, Wu, Y, Luo, J, Cai, Y, Zhang, T, et al. The association between estimated pulse wave velocity and cardio-cerebrovascular disease risk: a cohort study. Eur J Med Res. (2025) 30:16. doi: 10.1186/s40001-024-02217-4
20. Wei, S, Pan, X, Xiao, Y, and Wei, J. The unique association between estimated pulse wave velocity and the prevalence of diabetic kidney disease: a cross-sectional study. BMC Cardiovasc Disord. (2025) 25:46. doi: 10.1186/s12872-024-04453-2
21. Wang, JG. Chinese guidelines for the prevention and treatment of hypertension (2024 revision). J Geriatr Cardiol. (2025) 22:1–149. doi: 10.26599/1671-5411.2025.01.008
22. Greve, SV, Blicher, MK, Kruger, R, Sehestedt, T, Gram-Kampmann, E, Rasmussen, S, et al. Estimated carotid-femoral pulse wave velocity has similar predictive value as measured carotid-femoral pulse wave velocity. J Hypertens. (2016) 34:1279–89. doi: 10.1097/HJH.0000000000000935
23. Robinson, JL, Venner, AA, and Seiden-Long, I. Urine protein detection by dipstick: no interference from alkalinity or specific gravity. Clin Biochem. (2019) 71:77–80. doi: 10.1016/j.clinbiochem.2019.07.005
24. Inker, LA, Eneanya, ND, Coresh, J, Tighiouart, H, Wang, D, Sang, Y, et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. (2021) 385:1737–49. doi: 10.1056/NEJMoa2102953
25. Heffernan, KS, Stoner, L, London, AS, Augustine, JA, and Lefferts, WK. Estimated pulse wave velocity as a measure of vascular aging. PLoS One. (2023) 18:e0280896. doi: 10.1371/journal.pone.0280896
26. Hsu, PC, Lee, WH, Tsai, WC, Chen, YC, Chu, CY, Yen, HW, et al. Comparison between estimated and brachial-ankle pulse wave velocity for cardiovascular and overall mortality prediction. J Clin Hypertens (Greenwich). (2021) 23:106–13. doi: 10.1111/jch.14124
27. Sunagawa, Y, Ishida, A, Yamazato, M, Ohya, Y, and Kusunose, K. Combined effects of hypertension and arterial stiffness on target organ damage among community-based screening participants. Hypertens Res. (2025) 48:1342–50. doi: 10.1038/s41440-025-02163-6
28. van Varik, BJ, Vossen, LM, Rennenberg, RJ, Stoffers, HE, Kessels, AG, de Leeuw, PW, et al. Arterial stiffness and decline of renal function in a primary care population. Hypertens Res. (2017) 40:73–8. doi: 10.1038/hr.2016.113
29. Butt, L, Unnersjö-Jess, D, Höhne, M, Edwards, A, Binz-Lotter, J, Reilly, D, et al. A molecular mechanism explaining albuminuria in kidney disease. Nat Metab. (2020) 2:461–74. doi: 10.1038/s42255-020-0204-y
30. Ruiz-Hurtado, G, and Ruilope, LM. Microvascular injury and the kidney in hypertension. Hipertens Riesgo Vasc. (2018) 35:24–9. doi: 10.1016/j.hipert.2017.03.002
31. Li, Z, Wu, H, Yao, F, Li, Y, Li, Y, Xie, SA, et al. Runx2-NLRP3 axis orchestrates matrix stiffness-evoked vascular smooth muscle cell inflammation. Am J Physiol Cell Physiol. (2025) 328:C467–82. doi: 10.1152/ajpcell.00448.2024
32. Inserra, F, Forcada, P, Castellaro, A, and Castellaro, C. Chronic kidney disease and arterial stiffness: a two-way path. Front Med (Lausanne). (2021) 8:765924. doi: 10.3389/fmed.2021.765924
33. Gil-Ortega, M, Vega-Martín, E, Martín-Ramos, M, González-Blázquez, R, Pulido-Olmo, H, Ruiz-Hurtado, G, et al. Finerenone reduces intrinsic arterial stiffness in Munich Wistar Frömter rats, a genetic model of chronic kidney disease. Am J Nephrol. (2020) 51:294–303. doi: 10.1159/000506275
34. Cardoso, CRL, and Salles, GF. Prognostic value of changes in aortic stiffness for cardiovascular outcomes and mortality in resistant hypertension: a cohort study. Hypertension. (2022) 79:447–56. doi: 10.1161/HYPERTENSIONAHA.121.18498
35. Korogiannou, M, Xagas, E, Marinaki, S, Sarafidis, P, and Boletis, JN. Arterial stiffness in patients with renal transplantation; associations with co-morbid conditions, evolution, and prognostic importance for cardiovascular and renal outcomes. Front Cardiovasc Med. (2019) 6:67. doi: 10.3389/fcvm.2019.00067
36. Résimont, G, Piéroni, L, Bigot-Corbel, E, Cavalier, E, and Delanaye, P. Urinary strips for protein assays: easy to do but difficult to interpret! J Nephrol. (2021) 34:411–32. doi: 10.1007/s40620-020-00735-y
37. White, SL, Yu, R, Craig, JC, Polkinghorne, KR, Atkins, RC, and Chadban, SJ. Diagnostic accuracy of urine dipsticks for detection of albuminuria in the general community. Am J Kidney Dis. (2011) 58:19–28. doi: 10.1053/j.ajkd.2010.12.026
Keywords: pulse wave analysis, albuminuria, hypertension, vascular stiffness, cross-sectional study
Citation: Zeng X, Xiao C, Xu W, Zeng Q, Wu J and Luo J (2025) Estimated pulse wave velocity as a potential predictor of albuminuria in hypertension. Front. Med. 12:1636846. doi: 10.3389/fmed.2025.1636846
Edited by:
Humberto Muzi-Filho, Federal University of Rio de Janeiro, BrazilReviewed by:
Diego Chemello, Federal University of Santa Maria, BrazilKen-ichi Aihara, Tokushima University, Japan
Copyright © 2025 Zeng, Xiao, Xu, Zeng, Wu and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianping Luo, bHVvamlhbnBpbmdAbWFpbC5nenNybXl5LmNvbQ==
†These authors have contributed equally to this work
 Xianghui Zeng
Xianghui Zeng Chunqing Xiao2†
Chunqing Xiao2† Qingfeng Zeng
Qingfeng Zeng Jincheng Wu
Jincheng Wu Jianping Luo
Jianping Luo