- 1Department of Dermatology, University Hospital Tuebingen, Tuebingen, Germany
- 2Institute of Human Genetics, Medical Center, University of Freiburg, Freiburg, Germany
Pityriasis rubra pilaris is a rare inflammatory papulosquamous skin disease without any approved treatment options. Variants in the CARD14 (caspase recruitment domain family member 14) gene have been identified to play a role in the pathophysiology of atypical juvenile PRP by activating the IL-23/IL-17A cytokine axis, highlighting this pathway as a potential target of therapy. Here, we present a case of successful treatment with ixekizumab, a humanized monoclonal anti-IL-17A antibody, in an atypical juvenile PRP (type V) patient with a novel variant of CARD14 mutation.
1 Introduction
Pityriasis rubra pilaris is a rare inflammatory disease characterized by red-orange scaling plaques with classic “islands of sparing” of unaffected skin, keratotic follicular papules, and palmoplantar keratoderma. PRP can be divided into six clinical subtypes affecting pediatric and adult patients (1). CARD14 (caspase recruitment domain family member 14) gene variants have been found to be involved in the pathophysiology of atypical juvenile (type V) PRP leading to activation of the IL-23/IL-17A cytokine axis (2–4). Ixekizumab is a humanized monoclonal antibody targeting IL-17A. Here, we present a case of an atypical juvenile PRP (type V) patient with a novel variant of CARD14 mutation successfully treated with ixekizumab.
2 Case description
A 3-years-old caucasian girl presented to our dermatology department at the University Hospital Tuebingen with generalized well-demarcated confluent scaly orange-red plaques with distinct “islands of sparing” of unaffected skin. The further physical examination revealed prominent facial involvement of cheeks and ears with erythematosquamous plaques, palmar and plantar waxy orange-red keratoderma, bilateral ectropion and extensive thick scaly plaques on the scalp (Figure 1A).
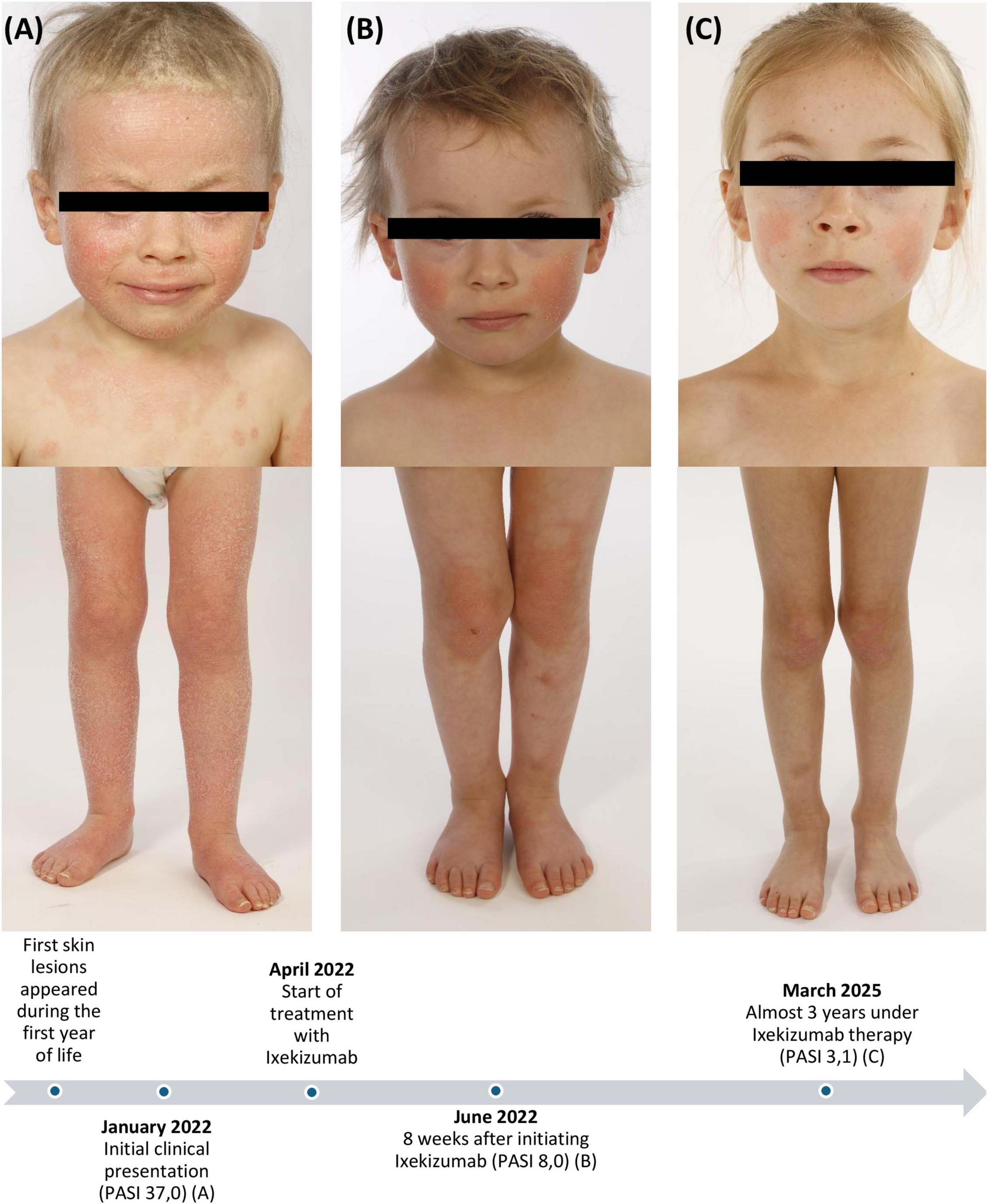
Figure 1. Clinical images and case timeline: Initial clinical presentation with generalized well-demarcated confluent scaly orange-red plaques, thick scaly plaques on the scalp and bilateral ectropion before treatment (A), 8 weeks after initiating ixekizumab (B), and almost 3 years under ixekizumab therapy (C). PASI, Psoriasis Area and Severity Index.
According to the family, initial skin lesions appeared during the first year of life. Prior to the patient’s presentation at our department, the patient had been diagnosed with a
mixed form of psoriasiform dermatitis and atopic eczema. She had been treated with topical steroids, but without sufficient response. Her growth and development were regular, with no signs suggesting a syndromic disorder. Family history was negative for psoriasis or other erythrosquamous skin diseases.
We took a skin biopsy from an affected area of the back. Histology showed regular but plump acanthosis of the epidermis with checkerboard-like alternating orthokeratosis and parakeratosis and sparse dermal perivascular lymphohistiocytic infiltrates (Figure 2).
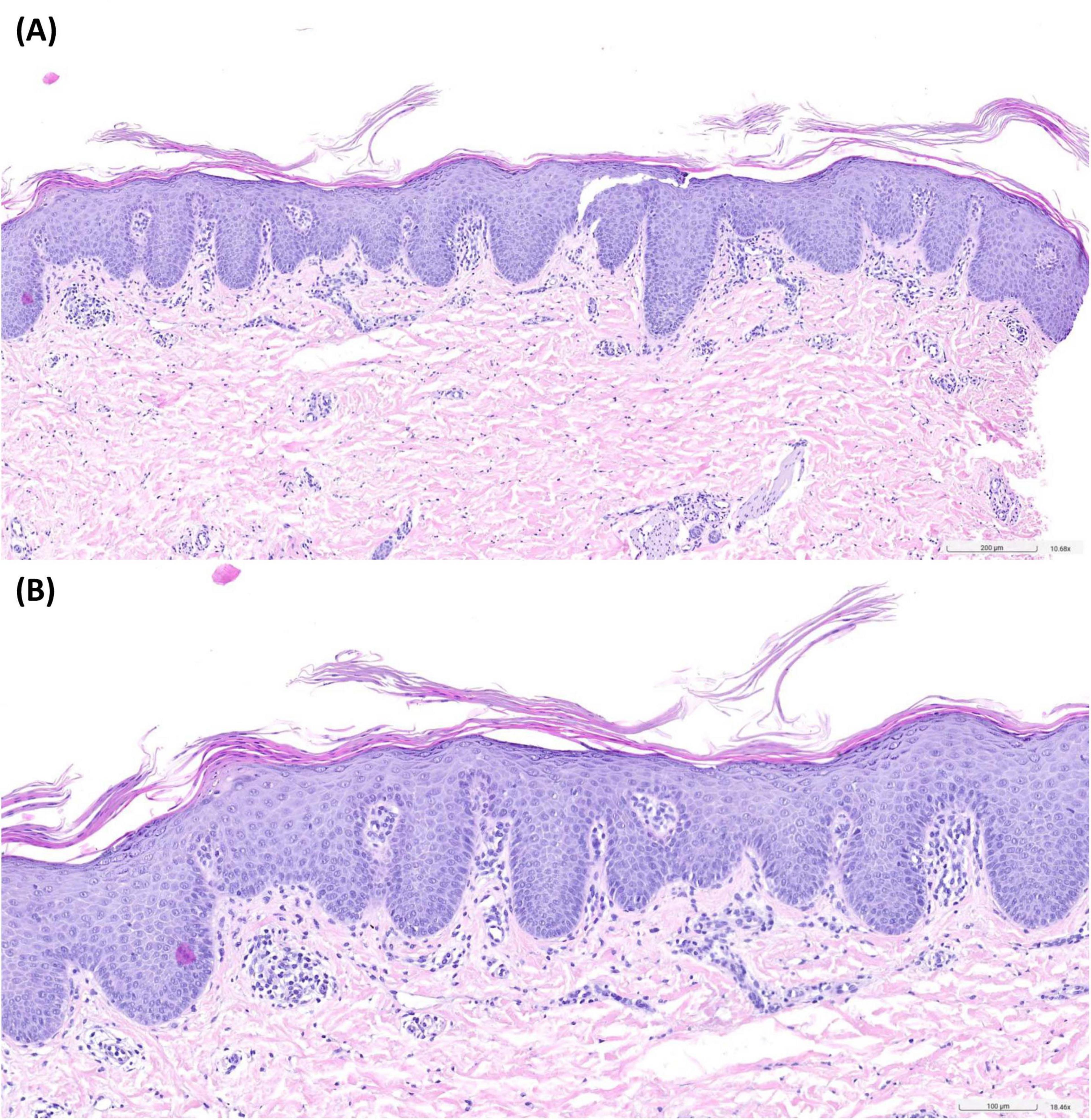
Figure 2. Histopathology of an affected area of the back revealed regular but plump acanthosis of the epidermis with checkerboard-like alternating orthokeratosis and parakeratosis. Sparse dermal perivascular lymphohistiocytic infiltrates. Hematoxylin-eosin-staining, 10-fold (A) and 18-fold (B) magnification.
Based on clinical and histopathological findings, the diagnosis of PRP was made. To validate this, we performed a genetic analysis of CARD14, identifying the heterozygous variant c.373A>C, p.(Thr125Pro) in exon 4. This variant has not yet been described in databases such as gnomAD or HGMD, and is currently classified as a variant of unknown significance (VUS).
Due to the severity and lack of response in spite of intensive topical therapy, we opted for targeted systemic therapy. Ixekizumab is a humanized monoclonal anti-IL-17A antibody with EMA approval for treatment of moderate-to-severe plaque psoriasis in children from the age of 6 years. Ixekizumab was chosen due to the approval for psoriasis in children with beneficial safety and positive data for treatment of PRP in adults (5). Due to the absence of approved therapeutic options for PRP, the use of ixekizumab was off-label. The treatment was weight-adjusted, started with a 40 mg loading dose, followed by 20 mg every 4 weeks. Monitoring was carried out in accordance with the in-label treatment protocol for psoriasis. Given the absence of a validated severity score of PRP, the Psoriasis Area and Severity Index (PASI) was used due to the clinical overlap with psoriasis, particulary the shared presentation of erythematosquamous plaques on the extensor surfaces. A noticeable clinical response was observed in the first follow-up after 8 weeks with reduction in PASI from 37,0 to 8,0 and declining degree of ectropion (Figure 1B). According to the parents, the quality of life had been clearly improved. Almost 3 years later, we still observe a good response with residual lesions on the cheeks and elbows (PASI 3,1) (Figure 1C). So far, no side effects have been observed. Blood testing was conducted every 6 months with no relevant abnormalities. Although PRP can be self-limiting in contrast to psoriasis, we plan to continue the therapy with ixekizumab, as the patient still presents residual lesions.
3 Discussion
Pityriasis rubra pilaris has been classified into 6 subtypes, which are differentiated by clinical features, age at onset, and disease duration. These subtypes are classical adult-onset (Type I); atypical adult-onset (Type II); classical juvenile-onset (Type III); circumscribed juvenile-onset (Type IV); atypical juvenile-onset (Type V); and HIV-associated (Type VI) (1, 6, 7). Atypical juvenile (Type V) PRP is most commonly associated with familial forms of PRP and variants of the CARD14 gene (8). While the novel variant p.(Thr125Pro) can currently only be classified as VUS, pathogenic missense variants in the neighboring region between amino acids 124 and 127 have already been identified in patients with PRP.
Additionally, CARD14 variants also have been found to be associated with forms of psoriasis, including (generalized) pustular psoriasis, suggesting that these conditions share pathophysiological mechanisms with PRP (9, 10). CARD14 variants have been identified to be a predisposing factor for autoinflammatory keratinization. These gain-of-function variants enhance nuclear factor κB (NF-κB) activation in keratinocytes, resulting in recruitment and differentiation of inflammatory cells with increased production of IL-17 and IL-22 by T cells and IL-23 by dendritic cells (11). However, in PRP patients without CARD14 mutations, NF-κB is also activated through IL-1ß signaling, resulting in upregulation of CCL20 expression and subsequent activation of TH17 cells (12). It remains unknown whether specific CARD14 variants are associated with particular phenotypes, because wide ranging Genome-Wide Association Study (GWAS) are lacking.
Due to the shared pathophysiological mechanisms, PRP and psoriasis present similar morphological features, characterized by well-demarcated erythematosquamous plaques predominantly affecting the extensor surfaces. In 2018, the term CARD14-associated papulosquamous eruption (CAPE) was proposed as a separate entity to describe a spectrum of patients with clinical characteristics of psoriasis and PRP (13). CAPE is characterized by an early age of onset, prominent facial involvement, and insufficient response to conventional therapies. In our patient, some clinical characteristics of CAPE are also met, such as manifestation within the first year of life and prominent facial involvement.
As there are significant histopathological similarities between PRP and CAPE, clinical classification of juvenile patients with PRP might be challenging (14). Many patients are diagnosed as type V, which is sometimes used as a general category for all juvenile and familial cases of PRP. Therefore, we would like to emphasize the importance of a genetic testing for CARD14 mutations in patients with familiar and early-onset PRP. It is necessary to establish clear and standardized diagnostic criteria for CAPE and type V PRP to ensure unambiguous and accurate classification.
Since PRP, CAPE and psoriasis share common pathophysiological mechanisms, targeting the IL-23/IL-17 signaling pathway offers a promising strategy for therapeutic intervention (3). Currently, ustekinumab, a combined IL-12 and IL-23 inhibitor, holds the most evidence for the therapy of PRP with CARD14 gene variations or CAPE (15, 16). However, targeted inhibition of IL-17A with secukinumab also showed promising results (17, 18). A single-arm, investigator-initiated trial treating 12 adult patients with moderate to severe PRP, including one with juvenile-onset, with ixekizumab showed improvement in their skin condition and improvement of quality of life (5). Furthermore, several case reports of successful treatment with ixekizumab in adult patients with CAPE have been published (19, 20).
In children with plaque psoriasis, ixekizumab has demonstrated long-term effectiveness and a favorable safety profile, which led to approval from the age of 6 (21). As the diagnosis PRP is rarely made early in children, it is difficult to carry out a prospective clinical study. Similar to the reported improvement in adults, a case report of a 6-years-old boy with juvenile PRP showed rapid response after starting treatment with ixekizumab (22). However, it must be emphasized that the CARD14 gene status was unknown.
It is uncertain if therapeutic responses to different biological molecules can be predicted based on CARD14 variations. A study involving 19 patients with PRP, of whom 10 carried CARD14 variants, found no correlation between genetic background and treatment success (23). Due to the small numbers of participants, further investigations are necessary.
To our knowledge, this is the first case of a pediatric patient with a CARD14-mutated and histological confirmed PRP successfully treated for 3 years with ixekizumab. Additionally, we found a novel CARD14 gene variant in this patient. Despite promising case reports, therapy guidelines and long-term data of children with PRP treated with modern targeted therapies are needed.
Data availability statement
The original contributions presented in this study are included in this article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
LM: Visualization, Writing – original draft, Formal Analysis, Conceptualization, Data curation, Writing – review and editing. MH: Resources, Conceptualization, Writing – review and editing, Supervision, Methodology. JF: Writing – review and editing, Data curation. SV: Supervision, Funding acquisition, Writing – review and editing, Methodology, Project administration, Conceptualization.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. We acknowledge support from the Open Access Publication Fund of the University of Tübingen.
Acknowledgments
We would like to thank our patient and his family for their consent to the publication of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Griffiths W. Pityriasis Rubra Pilaris. Clin Exp Dermatol. (1980) 5:105–12. doi: 10.1111/j.1365-2230.1980.tb01676.x
2. Fuchs-Telem D, Sarig O, van Steensel M, Isakov O, Israeli S, Nousbeck J, et al. Familial Pityriasis Rubra Pilaris is caused by mutations in CARD14. Am J Hum Genet. (2012) 91:163–70. doi: 10.1016/j.ajhg.2012.05.010
3. Feldmeyer L, Mylonas A, Demaria O, Mennella A, Yawalkar N, Laffitte E, et al. Interleukin 23-helper T cell 17 axis as a treatment target for Pityriasis Rubra Pilaris. JAMA Dermatol. (2017) 153:304–8. doi: 10.1001/jamadermatol.2016.5384
4. Yoshikawa T, Takeichi T, Hirabayashi T, Muro Y, Miyasaka Y, Ohno T, et al. Hyperactivation of the IL-17 axis and IL-36 signaling in card14-mutant Pityriasis Rubra Pilaris mouse model. J Invest Dermatol. (2025) 145:427–32. doi: 10.1016/j.jid.2024.04.036
5. Haynes D, Strunck J, Topham C, Ortega-Loayza A, Kent G, Cassidy P, et al. Evaluation of ixekizumab treatment for patients with Pityriasis Rubra Pilaris: a single-arm trial. JAMA Dermatol. (2020) 156:668–75. doi: 10.1001/jamadermatol.2020.0932
6. Misery I, Faure M, Claidy A. Pityriasis Rubra Pilaris and human immunodeficiency virus infection–type 6 Pityriasis Rubra Pilaris? Br J Dermatol. (1996) 135:1008–9. doi: 10.1046/j.1365-2133.1996.d01-1114.x
7. Wang D, Chong V, Chong W, Oon HH. A review on Pityriasis Rubra Pilaris. Am J Clin Dermatol. (2018) 19:377–90. doi: 10.1007/s40257-017-0338-1
8. Takeichi T, Sugiura K, Nomura T, Sakamoto T, Ogawa Y, Oiso N, et al. Pityriasis Rubra Pilaris type V as an autoinflammatory disease by CARD14 mutations. JAMA Dermatol. (2017) 153:66–70. doi: 10.1001/jamadermatol.2016.3601
9. Msafiri Makene A, Liu J. Association between CARD14 gene polymorphisms and psoriasis vulgaris in Hainan Han population based on exon sequencing: a case-control study. Medicine. (2022) 101:E30890. doi: 10.1097/MD.0000000000030890
10. Berki D, Liu L, Choon S, David Burden A, Griffiths C, Navarini A, et al. Activating CARD14 mutations are associated with generalized pustular psoriasis but rarely account for familial recurrence in psoriasis vulgaris. J Invest Dermatol. (2015) 135:2964–70. doi: 10.1038/jid.2015.288
11. Mellett M, Meier B, Mohanan D, Schairer R, Cheng P, Satoh T, et al. CARD14 gain-of-function mutation alone is sufficient to drive IL-23/IL-17-mediated psoriasiform skin inflammation in vivo. J Invest Dermatol. (2018) 138:2010–23. doi: 10.1016/j.jid.2018.03.1525
12. Schmauch E, Severin Y, Xing X, Mangold A, Conrad C, Johansen P, et al. Targeting IL-1 controls refractory Pityriasis Rubra Pilaris. Sci Adv. (2024) 10:eado2365. doi: 10.1126/sciadv.ado2365
13. Craiglow B, Boyden L, Hu R, Virtanen M, Su J, Rodriguez G, et al. CARD14 – associated papulosquamous eruption (CAPE): a spectrum including features of psoriasis and Pityriasis Rubra pilaris. J Am Acad Dermatol. (2018) 79:487–94. doi: 10.1016/J.JAAD.2018.02.034
14. Ring N, Craiglow B, Panse G, Antaya R, Ashack K, Ashack R, et al. Histopathologic findings characteristic of CARD14-associated papulosquamous eruption. J Cutan Pathol. (2020) 47:425–30. doi: 10.1111/CUP.13633
15. Guenther J, Novack D, Kamath S, Worswick S. Treatment options for juvenile Pityriasis Rubra Pilaris. Paediatr Drugs. (2023) 25:151–64. doi: 10.1007/s40272-022-00549-4
16. Niedźwiedź M, Narbutt J, Siekierko A, Skibińska M, Kwiek B, Sobolewska-Sztychny D, et al. Case report: successful treatment with biologics in a pediatric patient with a severe inflammatory skin disease and novel CARD14 mutation. Front. Med. (2024) 11:1360248. doi: 10.3389/fmed.2024.1360248
17. Liang J, Ye R, Tian X, Zhang S, Zhang X. Secukinumab monotherapy successfully treated severe refractory type V (atypical juvenile) Pityriasis Rubra Pilaris: a case report and literature review. Dermatol Ther. (2020) 33:e14097. doi: 10.1111/dth.14097
18. Boudreaux B, Pincelli T, Bhullar P, Patel M, Brumfiel C, Li X, et al. Secukinumab for the treatment of adult-onset Pityriasis Rubra Pilaris: a single-arm clinical trial with transcriptomic analysis. Br J Dermatol. (2022) 187:650–8. doi: 10.1111/bjd.21708
19. Klein B, Treudler R, Dumann K, Boldt A, Schumann I, Simon J, et al. Clinical response of CARD14-associated papulosquamous eruption to an anti-interleukin-17A antibody. Br J Dermatol. (2022) 187:419–22. doi: 10.1111/bjd.21229
20. Ouyang X, Zhang D, Wang X, Wu L, Xiao Z, Zhu Y, et al. A novel variant with a severe phenotype in a patient with CARD14-associated papulosquamous eruption successfully treated with ixekizumab. Clin Exp Dermatol. (2024) 49:661–4. doi: 10.1093/ced/llae019
21. Paller A, Seyger M, Magariños G, Pinter A, Cather J, Rodriguez-Capriles C, et al. Long-term efficacy and safety of up to 108 weeks of Ixekizumab in pediatric patients with moderate to severe plaque psoriasis: the IXORA-PEDS randomized clinical trial. JAMA Dermatol. (2022) 158:533–41. doi: 10.1001/jamadermatol.2022.0655
22. Kim E, Corey K, Damji Y. Rapid clearance of extensive juvenile Pityriasis Rubra Pilaris with ixekizumab. Pediatr Dermatol. (2024) 41:1235–7. doi: 10.1111/pde.15700
Keywords: Pityriasis rubra pilaris, case report, ixekizumab, CARD14, pediatric dermatology
Citation: Millak L, Hahn M, Fischer J and Volc S (2025) Case Report: Successful treatment of a novel variant of CARD14-mutated juvenile Pityriasis rubra pilaris with ixekizumab. Front. Med. 12:1637045. doi: 10.3389/fmed.2025.1637045
Received: 28 May 2025; Accepted: 03 July 2025;
Published: 22 July 2025.
Edited by:
Dennis Niebel, University Medical Center Regensburg, GermanyReviewed by:
Maurizio Romagnuolo, IRCCS Ca ‘Granda Foundation Maggiore Policlinico Hospital, ItalyAntonios Kolios, University Hospital Zürich, Switzerland
Michał Niedźwiedź, Bieganski Hospital, Poland
Copyright © 2025 Millak, Hahn, Fischer and Volc. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matthias Hahn, TWF0dGhpYXMuSGFobkBtZWQudW5pLXR1ZWJpbmdlbi5kZQ==
 Laura Millak
Laura Millak Matthias Hahn
Matthias Hahn Judith Fischer
Judith Fischer Sebastian Volc
Sebastian Volc