- 1Department of Ophthalmology, Peking University Third Hospital, Beijing, China
- 2Beijing Key Laboratory of Restoration of Damaged Ocular Nerve, Peking University Third Hospital, Beijing, China
- 3Peking University Institute of Laser Medicine, Beijing, China
Introduction: Keratoconus (KC) is a vision-threatening corneal disorder predominantly affecting young males, significantly impairing their quality of life. We aim to evaluate tear cortisol levels in KC and compare with controls, and to determine the correlation of tear cortisol levels with morphological and biomechanical parameters of KC.
Methods: Age- and sex-matched 42 eyes of 42 patients were enrolled. The levels of tear cortisol were obtained by liquid chromatography–tandem mass spectrometry. Pentacam HR and Corvis ST II were used to detect the corneal morphological and biomechanical parameters. Spearman correlations between tear cortisol levels and corneal parameters were calculated for all patients.
Results: Comparing with the control group, the level of tear cortisol significantly increased in the KC group (1660.95 [1175.01–2408.81] vs. 945.60 [550.36–1699.32], p = 0.023). It was positively correlated with Belin-Ambrosio Display D value (BAD-D), inferior–superior value (IS-value), keratoconus index (KI), Pentacam random forest index (PRFI), Corvis biomechanical index (CBI), and negatively correlated with central corneal thickness (CCT) and thinnest corneal thickness (TCT). No significant association was found between tear cortisol levels and maximum K value (Kmax), tomographic and biomechanical index (TBI), and stress–strain index (SSI).
Conclusion: Our findings demonstrate that tear cortisol levels are significantly associated with some corneal morphological and biomechanical parameters in KC, suggesting its potential role as a pathogenic factor, thereby providing new insights into the exploration of disease pathogenesis.
1 Introduction
Keratoconus (KC) is a progressive bilateral corneal ectatic disorder characterized by corneal thinning, irregular astigmatism, and visual impairment (1). Corneal morphological and biomechanical assessments are critical for the early diagnosis and monitoring of disease progression in KC. Commonly evaluated morphological parameters include central corneal thickness (CCT), thinnest corneal thickness (TCT), maximum K value (Kmax), Belin-Ambrosio Display D value (BAD-D), inferior–superior value (IS-value), keratoconus index (KI), and Pentacam random forest index (PRFI) (2–5). In more advanced stages of KC, reduced corneal thickness, increased corneal curvature, and greater morphological deviation are typically observed. Among the biomechanical parameters, the most widely studied include Corneal Biomechanical Index (CBI), Tomographic Biomechanical Index (TBI), and Stress–Strain Index (SSI). In severe KC, CBI and TBI values are typically elevated, whereas SSI, which reflects corneal stiffness, tends to be decreased (6).
Numerous hypotheses have been proposed to explain the pathogenesis of KC, including genetic predisposition, oxidative stress, immunological disorders, corneal stroma proteolytic degradation, mechanical injury, and environmental pollution (7, 8). However, the precise pathophysiological mechanisms underlying KC remain incompletely characterized. Emerging evidence indicates that steroid hormones exert pleiotropic effects on corneal homeostasis. Melatonin demonstrates antioxidant efficacy and alleviates ocular surface inflammatory response in dry eye disease (9). Women undergoing in vitro fertilization treatment, with serum estradiol levels increased dramatically (10 to 50x), were found to have significant improvement in ocular symptoms and tear film associated with dry eye disease (10). Regarding cortisol, previous studies have investigated the association between hair cortisol concentration and KC, suggesting a positive correlation (11, 12). Dutta et al. (13) documented bilateral KC with right corneal scarring in a 52-year-old male diagnosed with adrenal myelolipoma. However, the relationship between tear cortisol levels and the morphological and biomechanical parameters of KC has not been systematically investigated. This study bridges this gap by establishing associations between tear cortisol levels and morphological and biomechanical characteristics in KC.
Cortisol, the primary glucocorticoid secreted by the adrenal cortex, regulates blood glucose levels, modulates the endocrine and immune systems, combats stress, and promotes the metabolism of fats, proteins, and carbohydrates (14, 15). Cortisol quantification is feasible in multiple biofluids, including serum, saliva, urine, and tears (14, 16). The substrate for cortisol synthesis is cholesterol, which is converted to pregnenolone (P5) and then undergoes a series of enzymatic reactions to generate progesterone (P4), 17α-hydroxypregnenolone (17-OHP5), 17α-hydroxyprogesterone (17-OHP), 11-deoxycortisol (11-DF), 21-deoxycortisol (21-DF), and ultimately cortisol (17–20). Liquid chromatography–tandem mass spectrometry (LC–MS/MS) methods, a specific and selective method, were used for the simultaneous quantification of the steroids.
We aimed to evaluate the tear cortisol levels in KC using LC-MS/MS, to investigate their associations with morphological and biomechanical parameters of KC.
2 Materials and methods
2.1 Ethical approval
This cross-sectional study was approved by the Ethics Committee of Peking University Third Hospital and followed the tenets of the Declaration of Helsinki. Informed written consents were obtained from all subjects.
2.2 Participants
The sample size calculation for this study was performed using PASS 15.0, with the primary outcome measure being the difference in tear cortisol levels between the KC group and the control group. Based on the mean concentration and standard deviation of tear cortisol levels, and assuming an alpha error of 0.05 and a power of 0.90, the calculated sample size required for statistical significance was 20 participants per group.
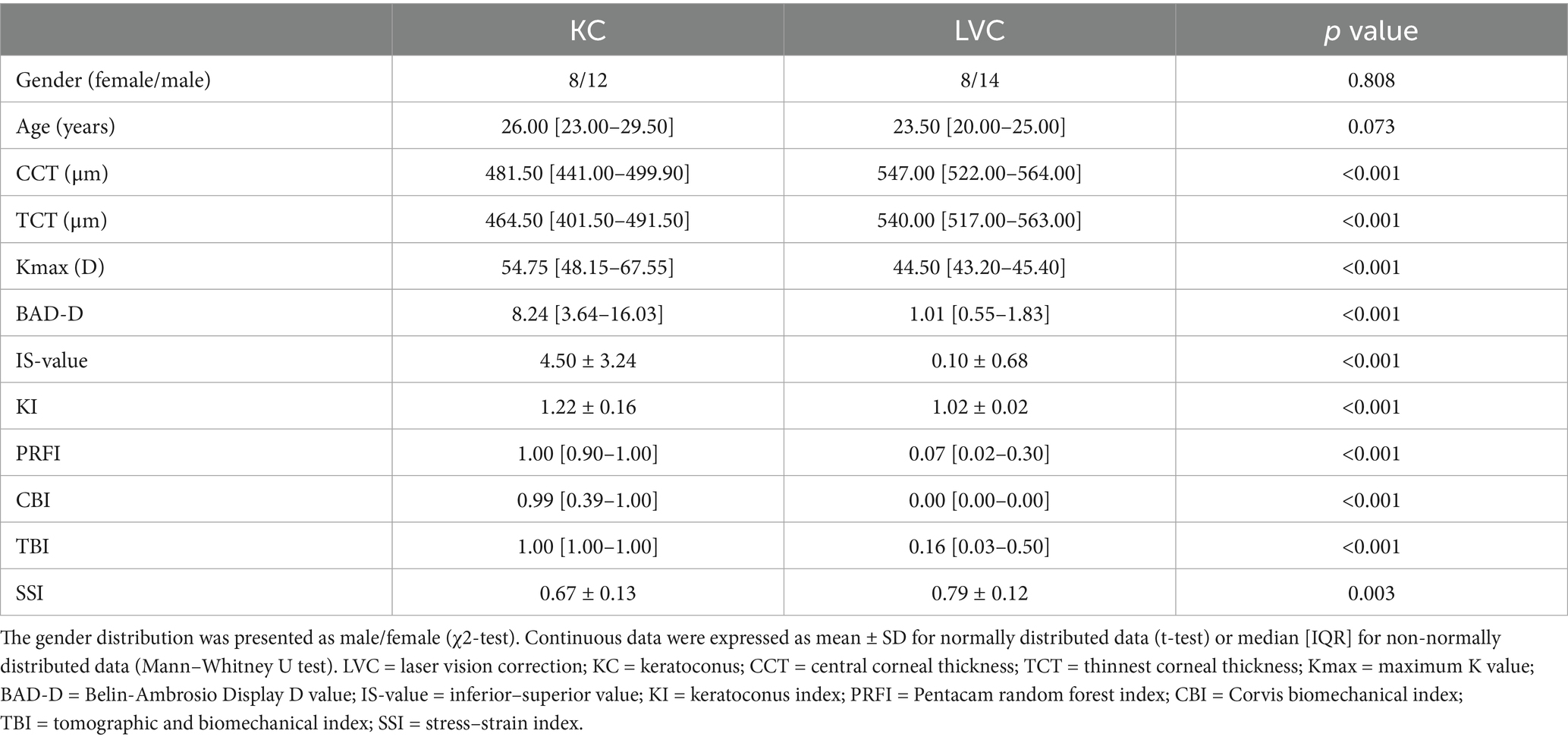
This study consecutively enrolled 42 participants from the Peking University Third Hospital Eye Center. Patients with bilateral KC were included in the KC group (n = 20), and those with bilateral mild-to-moderate myopia undergoing laser vision correction were included in the laser vision correction group (LVC) (n = 22). All patients were newly diagnosed and had not used any ocular or systemic medications within the 3 months prior to the visit. Both eyes underwent ocular examinations, with one eye randomly selected for enrollment. The diagnosis of KC was based on the stage I-IV on Amsler–Krumeich classification, with corneal tomography confirmation, and was confirmed by two experienced directors specializing in corneal surgery. Exclusion criteria included: ocular inflammation or infection, previous ocular surgery, ocular trauma, systemic hormone supplementation therapy, or connective systemic diseases. Contact lens discontinuation periods were required as follows: ≥ 14 days for soft lenses and ≥ 28 days for rigid gas permeable lenses.
2.3 Clinical examinations
All patients underwent a standardized bilateral ophthalmic evaluation, including medical history, corrected distance visual acuity, slit-lamp biomicroscopy, scheimpflug tomography (Pentacam HR; Oculus Instruments, Wetzlar, Germany), and dynamic biomechanical analysis (Corvis ST II; Oculus Instruments, Wetzlar, Germany). All examinations were conducted by the same certified technician under standardized conditions and using quality-controlled protocols to minimized bias. Corneal morphological parameters, including CCT, TCT, Kmax, BAD-D, IS-value, KI, PRFI, as well as biomechanical characteristics, including CBI, TBI, and SSI, were collected and analyzed.
2.4 Laboratory investigations
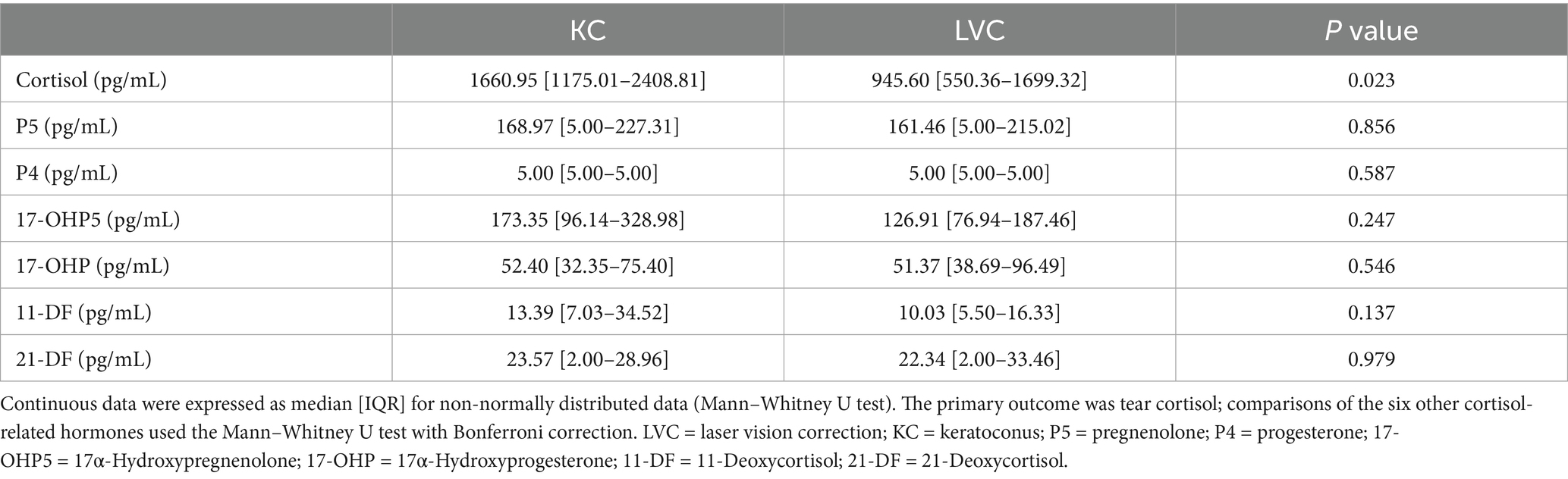
In this study, tear cortisol, P5, P4, 17-OHP5, 17-OHP, 11-DF, and 21-DF were quantified, with cortisol designated as the primary outcome measure. Considering the secretion rhythm of endogenous cortisol, tear sampling was consistently performed at the same time (9–10 a.m.). For the selected eye per participant, approximately 20 μL of tears fluid were collected from the inferior lateral meniscus using a sterile glass micropipette under slit-lamp guidance. The procedure was completed within a continuous 5-min period to minimize reflex secretion and ensure sample integrity for all participants. The samples were then stored at −80 °C until analysis. For LC–MS/MS analysis, 20 μL of each sample was aliquoted into a 1.5 mL Eppendorf tube, followed by the sequential addition of 20 μL of internal standard, 80 μL of ultrapure water, and 400 μL of tert-butyl methyl ether. The mixture was vortex-mixed for 5 min, then centrifuged at 12000 rpm for 8 min, and 350 μL of the supernatant was transferred to a 96-well plate. The extraction was repeated with tert-butyl methyl ether, the supernatants were combined, dried under nitrogen at room temperature, and dissolve in 40 μL of 10% acetonitrile solution for analysis. Tear steroid quantification was performed using the Waters ACQUITY UPLC I-Class/Xevo TQ-XS Absolute system (Waters Corporation, Milford, Massachusetts, United States). Tear extracts were analyzed on a Waters TQ-XS micro tandem mass spectrometer equipped with a Waters ACQUITY I-Class UPLC system. Analytical separation was performed using a Waters BEH C18 column (1.7 μm, 2.1 × 100 mm). The chromatographic flow rate was 0.3 mL/min. Nitrogen was used as the desolvation gas at 550 °C with a flow of 1,100 L/h. The lower limits of quantification for the analytes in tears were as follows: cortisol, 30 pg./mL; P5, 5 pg./mL; P4, 5 pg./mL; 17-OHP5, 10 pg./mL; 17-OHP, 2 pg./mL; 11-DF, 2 pg./mL; and 21-DF, 2 pg./mL. The recovery rates for all analytes ranged from 85 to 115%. The intra- and inter-assay coefficients of variation for all analytes were less than 15%.
2.5 Statistical analysis
All statistical analyses were conducted in SPSS (Version 26.0, IBM Corporation, Armonk, New York, United States) and GraphPad Prism (Version 10.0, GraphPad, San Diego, California, United States) with two-tailed tests and α = 0.05. The Shapiro–Wilk test was used to test for normality. Normally distributed data were expressed as mean ± SD and compared using the independent samples t-test between the KC group and the LVC group; non-normally distributed data were reported as median [IQR] and analyzed with the Mann–Whitney U test. Notably, as cortisol was the primary outcome, Bonferroni correction was applied when comparing the six other cortisol-related measures. The χ2-test was used in the analysis of the gender ratio. Spearman rank correlation analysis was used to explore the correlations between tear cortisol levels and different corneal parameters. p < 0.05 was considered statistically significant.
3 Results
Table 1 presented comparative analyses of demographic characteristics, tomographic indices, and biomechanical parameters between KC (n = 20) and LVC (n = 22) groups. The cohorts were age- and sex-matched (KC vs. LVC: 26.00 [23.00–29.50] vs. 23.50 [20.00–25.00] years; 63.64% vs. 60.00% male; p > 0.05) with comparable baseline characteristics. KC patients exhibited characteristic ectatic changes: reduced corneal thickness (CCT, TCT), elevated biomechanical indices (CBI, TBI, SSI), and abnormal tomographic markers (Kmax, BAD-D, IS-value, KI, PRFI).
Table 2 delineates tear steroids profiles quantified via LC–MS/MS, encompassing P5, P4, 17-OHP5, 17-OHP, 11-DF, 21-DF and cortisol. Tear cortisol level in KC group was higher than that in LVC group (1660.95 [1175.01–2408.81] vs. 945.60 [550.36–1699.32], p = 0.023). The tear levels of P5, P4, 17-OHP5, 17-OHP, 11-DF, 21-DF showed no significant between these two groups.
Spearman correlation analysis was used to find the relationships between the tear cortisol, corneal morphological parameters, and corneal biomechanical parameters for all patients (Figure 1). Among them, tear cortisol levels demonstrated statistically significant positive correlations with BAD-D (r = 0.34, p = 0.030), IS-value (r = 0.41, p = 0.007), KI (r = 0.35, p = 0.022), PRFI (r = 0.31, p = 0.047), and CBI (r = 0.35, p = 0.023). Conversely, significant inverse correlations were observed between tear cortisol levels and CCT (r = −0.34, p = 0.030) and TCT (r = −0.38, p = 0.014). No significant association was found between tear cortisol levels and Kmax (r = 0.30, p = 0.057), TBI (r = 0.28, p = 0.070), and SSI (r = −0.14, p = 0.391).
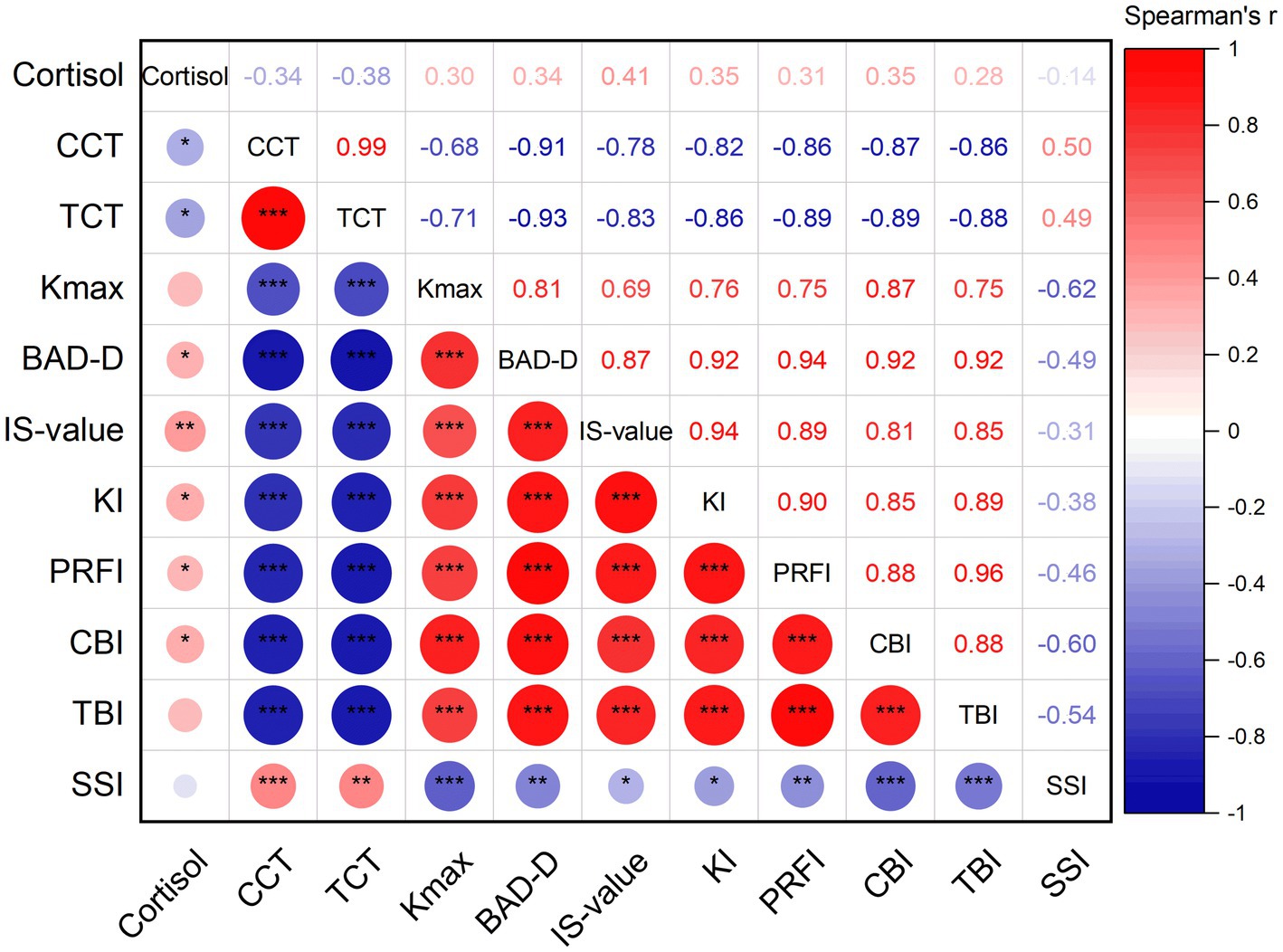
Figure 1. Spearman correlation analysis between cortisol, the corneal morphological parameters, and corneal biomechanical parameters for all patients (CCT: central corneal thickness; TCT: thinnest corneal thickness; Kmax: maximum K value; BAD-D: Belin-Ambrosio Display D value; IS-value: inferior–superior value; KI: keratoconus index; PRFI: Pentacam random forest index; CBI: Corvis biomechanical index; TBI: tomographic and biomechanical index; SSI: stress–strain index; *, p < 0.05; **, p < 0.01; ***, p < 0.001).
4 Discussion
This study revealed significantly elevated tear cortisol levels in patients with KC compared to those with mild-to-moderate myopia (control group). Cortisol levels were significantly positively correlated with parameters indicative of KC severity (Kmax, BAD-D, IS-value, KI, PRFI, CBI and TBI) and negatively correlated with parameters reflecting corneal structural integrity (CCT, TCT and SSI).
Cortisol’s role in ocular pathophysiology has been well-documented across multiple diseases, spanning anterior to posterior segment of ocular diseases. In Cushing’s syndrome, patients had significantly lower central macular thickness compared to those with normal cortisol levels, and increased cortisol levels were correlated with lesser central macular thickness and thicker central choroidal thickness (21). Endogenous hypercortisolism in Cushing’s syndrome confers a higher risk of ocular hypertension, which suggests cortisol screening should be considered in the assessment of glaucoma (22). Chronically elevated hair cortisol correlates with KC progression and epithelial thickness vatiation (23). Topical corticosteroids inhibit corneal wound strength in rabbits and humans (24). Larissa et al. (12) established progression KC as a hypercortisolemic state mediated through tear interleukin (IL)-6 level.
Our preliminary findings suggest an association between cortisol and KC progression, although the causal relationship remains unclear. We propose two hypothetical pathways.
First, cortisol may play a pathogenic role in KC development through the following two potential mechanisms: (a) Cortisol may exacerbate ocular surface oxidative stress and inflammation, ultimately worsening KC. Patients with Cushing’s syndrome exhibit profound oxidative imbalance, as demonstrated by elevated levels of plasma 15-F2t-Isoprostane compared to healthy controls (25). Concurrently, vitamin E levels were found to be reduced and were inversely correlated with urinary free cortisol levels (25). Exogenous cortisol provokes profound oxidative stress in human platelets, characterized by increased reactive oxygen species (ROS), elevated superoxide anions, and lipid peroxidation (26). This oxidative burst coincided with depletion of reduced glutathione, thereby compromising cellular redox homeostasis (26). Oxidative stress plays a critical role in the pathogenesis and progression of KC. KC corneas demonstrate sustained oxidative overload, with ROS and reactive nitrogen species levels significantly higher than those of healthy controls (27). Oxidative insult induces mitochondrial DNA (mtDNA) deletions and a reduction in mtDNA copy number in KC keratocytes (28). Cumulative oxidative damage triggers keratocyte apoptosis and disorganization of the extracellular matrix (ECM) (29). Mechanistically, oxidative stress may remodel the ECM by upregulating matrix metalloproteinase-2 and downregulating tissue inhibitor of metalloproteinases-1, thereby disrupting the architecture of corneal stromal collagen, particularly type IV and V collagen, and ultimately promoting the development of KC (30). Cortisol may modulate corneal stromal collagen homeostasis in KC through proinflammatory cytokines and matrix metalloproteinases (MMPs) expression. In coronary artery disease, evening cortisol levels have been shown to have strong positive correlations with the levels of total and active MMP-9, which are significantly elevated in the corneal epithelium and tears of KC. Serum inflammatory markers IL-6 was strongly positively correlated with the levels of evening cortisol, which also has been found elevated in KC (31). A previous study confirmed that in patients with progressive KC, levels of IL-6 in tear and cortisol in hair were significantly higher (12). However, this regulatory effect appears to be dose-dependent. A study found that low-concentration hydrocortisone increased the release of IL-6 and IL-8 in human corneal epithelial cell lines, while high-concentration hydrocortisone can inhibit their expression (32). (b) Cortisol might cause systemic metabolic disturbances leading to obesity, which alters corneal surface pressure gradients, thereby worsening KC. Elevated endogenous cortisol serves as a significant etiological factor not only for neuropsychiatric disorders but also for metabolic derangements, including metabolic syndrome (characterized by insulin resistance and dyslipidemia) and central obesity. Crucially, these pathological alterations may reciprocally exacerbate cortisol dysregulation, thereby establishing a self-reinforcing vicious cycle. Obesity has been implicated in modifying periocular anatomical architecture, including palpebral fat pad hypertrophy, orbital septal adipose expansion, and reduced orbital volume. These structural alterations amplify damage on the cornea through anterior corneal surface pressure gradient distortion induced by orbital space compression, intraocular pressure fluctuations secondary to retrobulbar adipose accumulation, and also chronic low-grade ocular inflammation (33, 34).
Second, patients with KC may experience significant psychological stress, which in turn alters the release of cortisol. Vision loss in patients with KC is a significant contributing factor to increased psychological burden. A psychiatric comorbidity burden was observed in KC cohorts: among 57 KC patients, 63.2% had anxiety disorders, 56.1% had depression, 10.5% had schizophrenia, and 1.8% had bipolar disorder, suggesting that there is an association between psychiatric disorders and KC (35). Cortisol, as one of the body’s primary stress hormones, is released in response to psychological stress. When an individual experiences psychological stress, the hypothalamus triggers the release of corticotropin-releasing hormone, which stimulates the anterior pituitary gland to secrete adrenocorticotropic hormone, ultimately leading to cortisol production by the adrenal cortex (36, 37). In patients experiencing their first episode of psychiatric disorders, heightened cortisol levels correlate with the severity of clinical symptoms, which suggests that psychiatric disorders and psychological stress are associated with cortisol levels (36, 38–40).
It is worth noting that patients with depression may exhibit behaviors or habits such as eye-rubbing or neglecting ocular care, which could exacerbate KC or accelerate its progression (33, 41–44). The mechanical effects of eye rubbing and their potential causative mechanisms may involve: elevated corneal temperature, thinning of the epithelial layer, heightened inflammatory mediator levels in pre-corneal tear fluid, dysregulated enzymatic activity, transient intraocular pressure surges, elevated tissue hydrostatic pressure, thixotropic reduction in ECM viscosity, transient displacement of matrix components from the corneal apex, fibril deformation and buckling induced by corneal indentation waves, biomechanical redistribution of curvature toward the cone apex, inter-fibrillar slippage at the conical apex, keratocyte alterations caused by mechanical stress and/or elevated hydrostatic pressure, alongside scar tissue development (45–51).
This study represents the first to quantify tear cortisol in KC using validated UPLC-MS/MS, revealing its great significance for exploring the role of cortisol in the pathogenesis of KC. This study has several limitations. First, the sample size of this study is relatively small. Second, the use of a mild-to-moderate myopic population as controls limits generalizability; future studies should consider enrolling patients without ocular or systemic comorbidities. Third, the study design is cross-sectional and correlational, which cannot establish causality or directionality. Further studies are warranted to elucidate the interplay between tear and blood cortisol levels at different time points, inflammatory mediators (e.g., cytokines), and oxidative stress markers in KC pathogenesis.
In conclusion, UPLC-MS/MS-based tear cortisol profiling identifies of KC progression, offering new insights into the exploration of the pathogenesis of KC.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Peking University Third Hospital Medical Science Research Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XZ: Writing – original draft, Conceptualization, Writing – review & editing. TS: Writing – review & editing, Conceptualization, Writing – original draft. YY: Writing – review & editing, Data curation. YZ: Data curation, Writing – review & editing. YC: Writing – review & editing, Supervision, Conceptualization. JH: Writing – review & editing, Data curation, Supervision, Project administration, Validation.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhao, X, Yuan, Y, Sun, T, Zhang, Y, and Chen, Y. Associations between keratoconus and the level of sex hormones: a cross-sectional study. Front Med (Lausanne). (2022) 9:828233. doi: 10.3389/fmed.2022.828233
2. Randleman, JB, Susanna, BN, Hammoud, B, Dutra, BAL, Scarcelli, G, Santhiago, MR, et al. Evaluating the global consensus on Keratoconus and Ectatic diseases agreements reached on subclinical Keratoconus. Am J Ophthalmol. (2025) 275:27–35. doi: 10.1016/j.ajo.2025.03.013
3. Liu, Y, Zhang, Y, Wang, Y, Dong, R, and Chen, Y. The role of Pentacam random Forest index in detecting subclinical keratoconus in a Chinese cohort. Diagnostics (Basel). (2024) 14:1–12. doi: 10.3390/diagnostics14202304
4. Lu, NJ, Koppen, C, Ni Dhubhghaill, S, Wang, QM, Chen, SH, Cui, LL, et al. A novel optical coherence tomography-based Keratoconus diagnostic index incorporating stromal and epithelial features. J Refract Surg. (2025) 41:e748–59. doi: 10.3928/1081597X-20250602-02
5. Gomes, JAP, Tan, D, Rapuano, CJ, Belin, MW, Ambrósio, R, Guell, JL, et al. Global consensus on keratoconus and ectatic diseases. Cornea. (2015) 34:359–69. doi: 10.1097/ICO.0000000000000408
6. Huo, Y, Chen, X, Cao, H, Li, J, Hou, J, and Wang, Y. Biomechanical properties analysis of forme fruste keratoconus and subclinical keratoconus. Graefes Arch Clin Exp Ophthalmol. (2023) 261:1311–20. doi: 10.1007/s00417-022-05916-y
7. Said, OM, Iqbal, M, El-Massry, A, Elgharieb, ME, Mady, M, Sharawy, AM, et al. Thyroid gland dysfunction and keratoconus. Med Hypothesis Discov Innov Ophthalmol. (2024) 13:104–11. doi: 10.51329/mehdiophthal1501
8. El-Massry, A, Doheim, MF, Iqbal, M, Fawzy, O, Said, OM, Yousif, MO, et al. Association between keratoconus and thyroid gland dysfunction: a cross-sectional case-control study. J Refract Surg. (2020) 36:253–7. doi: 10.3928/1081597X-20200226-03
9. Yang, X, Wang, B, Zeng, H, Liang, L, Zhang, R, Deng, W, et al. A modified polydopamine nanoparticle loaded with melatonin for synergistic ROS scavenging and anti-inflammatory effects in the treatment of dry eye disease. Adv Healthc Mater. (2025) 14:e2404372. doi: 10.1002/adhm.202404372
10. Boga, A, Stapleton, F, Chapman, M, and Golebiowski, B. Effects of elevated serum estrogen on dry eye in women undergoing in vitro fertilisation. Ocul Surf. (2023) 29:511–20. doi: 10.1016/j.jtos.2023.06.015
11. Lenk, J, Spoerl, E, Stalder, T, Schmiedgen, S, Herber, R, Pillunat, LE, et al. Increased hair cortisol concentrations in patients with progressive keratoconus. J Refract Surg. (2017) 33:383–8. doi: 10.3928/1081597X-20170413-01
12. Stival, LR, Avila, LP, Araujo, DC, Chaves, LF, Toledo, MC, Silva, AC, et al. Correlation of hair cortisol and interleukin 6 with structural change in the active progression of keratoconus. J Cataract Refract Surg. (2022) 48:591–8. doi: 10.1097/j.jcrs.0000000000000809
13. Dutta, D, Shivaprasad, K, Ghosh, S, Mukhopadhyay, S, and Chowdhury, S. Adrenal myelolipoma with keratoconus: a novel clinical association. Indian J Endocrinol Metab. (2012) 16:S364–6. doi: 10.4103/2230-8210.104094
14. Li, Z, Luo, D, Zhang, Y, Niu, X, and Liu, H. Smart health monitoring: review of electrochemical biosensors for cortisol monitoring. Adv Healthc Mater. (2025) 14:e2404454. doi: 10.1002/adhm.202404454
15. Nampeng, J, Vongmanee, N, Pintavirooj, C, Chiu, WT, and Visitsattapongse, S. Electrochemical biosensors by means of molecularly imprinted polymers (MIPs) cortisol recognition. Polymers (Basel). (2025) 17:545. doi: 10.3390/polym17040545
16. Alkozi, HA, Alhudhayf, HA, and Alawad, NMA. Association between dry eye disease with anxiety and depression among medical sciences students in qassim region: cortisol levels in tears as a stress biomarker. J Multidiscip Healthc. (2024) 17:4549–57. doi: 10.2147/JMDH.S488956
17. Comito, R, Ciavarella, C, Astolfi, G, Conti, M, Porru, E, Violante, FS, et al. Tear sampling and biomarker discovery: a robust workflow for routine clinical applications using UHPLC-MS/MS and schirmer strips. Int J Mol Sci. (2025) 26:2041. doi: 10.3390/ijms26052041
18. Coeli-Lacchini, FB, Mermejo, LM, Bodoni, AF, Elias, LLK, Silva, WA Jr, Antonini, SR, et al. Clinical, molecular, functional, and structural characterization of CYP17A1 mutations in Brazilian patients with 17-hydroxylase deficiency. Horm Metab Res. (2020) 52:186–93. doi: 10.1055/a-1100-7066
19. Olthof, A, Hillebrand, JJ, Wickenhagen, WV, Boelen, A, and Heijboer, AC. Stability of steroid hormones in dried blood spots (DBS). Clin Chem Lab Med. (2024) 62:2469–76. doi: 10.1515/cclm-2024-0142
20. Revanasiddappa, S, Dhananjaya, MS, Kansal, N, Lila, A, and Sarathi, V. Baseline and corticotropin-stimulated blood steroid profiles in women of reproductive age in India: a cross-sectional study and global literature review. Cureus. (2025) 17:e77748. doi: 10.7759/cureus.77748
21. Duan, J, Shen, S, Lei, C, Gao, S, Chang, T, Zhang, Y, et al. Choroidal and retinal abnormalities in Cushing syndrome: correlation with the cortisol level. Retina. (2024) 44:861–7. doi: 10.1097/IAE.0000000000004023
22. Ma, Y, Chen, Z, Ma, Z, Ye, H, Zhang, Z, Wang, Y, et al. Increased risk of ocular hypertension in patients with Cushing's disease. J Glaucoma. (2022) 31:941–6. doi: 10.1097/IJG.0000000000002113
23. Santhiago, MR, Stival, LR, Araujo, DC, Antunes-Foschini, R, Toledo, MC, Nunes, ILS, et al. Relationship of inflammatory mediators (interleukin and cortisol concentrations) with corneal epithelial quantifiable metrics. Ophthalmol Sci. (2025) 5:100624. doi: 10.1016/j.xops.2024.100624
24. Yin, H, Lu, Q, Wang, X, Majumdar, S, Jun, AS, Stark, WJ, et al. Tissue-derived microparticles reduce inflammation and fibrosis in cornea wounds. Acta Biomater. (2019) 85:192–202. doi: 10.1016/j.actbio.2018.12.027
25. Karamouzis, I, Berardelli, R, D'Angelo, V, Fussotto, B, Zichi, C, Giordano, R, et al. Enhanced oxidative stress and platelet activation in patients with Cushing's syndrome. Clin Endocrinol. (2015) 82:517–24. doi: 10.1111/cen.12524
26. Signorello, MG, Ravera, S, and Leoncini, G. Oxidative stress induced by cortisol in human platelets. Int J Mol Sci. (2024) 25:3776. doi: 10.3390/ijms25073776
27. López-López, M, Regueiro, U, Bravo, SB, Chantada-Vázquez, MP, Pena, C, Díez-Feijoo, E, et al. Shotgun proteomics for the identification and profiling of the tear proteome of keratoconus patients. Invest Ophthalmol Vis Sci. (2022) 63:12. doi: 10.1167/iovs.63.5.12
28. López-López, M, Regueiro, U, Bravo, SB, Chantada-Vázquez, MP, Varela-Fernández, R, Ávila-Gómez, P, et al. Tear proteomics in keratoconus: a quantitative SWATH-MS analysis. Invest Ophthalmol Vis Sci. (2021) 62:30. doi: 10.1167/iovs.62.10.30
29. Loukovitis, E, Kozeis, N, Gatzioufas, Z, Kozei, A, Tsotridou, E, Stoila, M, et al. The proteins of Keratoconus: a literature review exploring their contribution to the pathophysiology of the disease. Adv Ther. (2019) 36:2205–22. doi: 10.1007/s12325-019-01026-0
30. Dammak, A, Pastrana, C, Martin-Gil, A, Carpena-Torres, C, Peral Cerda, A, Simovart, M, et al. Oxidative stress in the anterior ocular diseases: diagnostic and treatment. Biomedicine. (2023) 11:292. doi: 10.3390/biomedicines11020292
31. Degroote, C, von Kanel, R, Thomas, L, Zuccarella-Hackl, C, Messerli-Burgy, N, Saner, H, et al. Lower diurnal HPA-axis activity in male hypertensive and coronary heart disease patients predicts future CHD risk. Front Endocrinol (Lausanne). (2023) 14:1080938. doi: 10.3389/fendo.2023.1080938
32. Kadmiel, M, Janoshazi, A, Xu, X, and Cidlowski, JA. Glucocorticoid action in human corneal epithelial cells establishes roles for corticosteroids in wound healing and barrier function of the eye. Exp Eye Res. (2016) 152:10–33. doi: 10.1016/j.exer.2016.08.020
33. Ren, S, Tu, R, Xu, L, Gu, Y, Fan, Q, Wang, Q, et al. A high body mass index strengthens the association between the time of eye rubbing and keratoconus in a Chinese population: a case control study. BMC Public Health. (2023) 23:2032. doi: 10.1186/s12889-023-16937-5
34. Wang, J, Liu, F, Gong, D, Su, J, Zheng, F, Ding, S, et al. Mendelian randomization reveals that abnormal lipid metabolism mediates the causal relationship between body mass index and keratoconus. Sci Rep. (2024) 14:23698. doi: 10.1038/s41598-024-74455-9
35. Alfardan, F, Alsanad, MH, and Altoub, HA. Prevalence of psychiatric illness among keratoconus patients. Cureus. (2023) 15:e42141. doi: 10.7759/cureus.42141
36. Dziurkowska, E, and Wesolowski, M. Cortisol as a biomarker of mental disorder severity. J Clin Med. (2021) 10:5204. doi: 10.3390/jcm10215204
37. van der Valk, ES, Savas, M, and van Rossum, EFC. Stress and obesity: are there more susceptible individuals? Curr Obes Rep. (2018) 7:193–203. doi: 10.1007/s13679-018-0306-y
38. Touskova, TP, Bob, P, Pec, O, Mishara, A, Vanickova, Z, Raboch, J, et al. Insight and cortisol responses in women with first episode psychosis. Schizophr Res. (2018) 201:428–9. doi: 10.1016/j.schres.2018.06.002
39. Shah, JL, and Malla, AK. Much ado about much: stress, dynamic biomarkers and HPA axis dysregulation along the trajectory to psychosis. Schizophr Res. (2015) 162:253–60. doi: 10.1016/j.schres.2015.01.010
40. Kogler, L, Wang, R, Luther, T, Hofer, A, Frajo-Apor, B, and Derntl, B. Cortisol in schizophrenia spectrum disorders: a comprehensive meta-analysis. Front Neuroendocrinol. (2025) 77:101186. doi: 10.1016/j.yfrne.2025.101186
41. Moshfeghinia, R, Arman, A, Sobhi, N, Mahmoudinezhad, G, and Molavi Vardanjani, H. Depression among keratoconus patients: a systematic review and meta-analysis. Front Public Health. (2024) 12:1477411. doi: 10.3389/fpubh.2024.1477411
42. Krachmer, JH. Eye rubbing can cause keratoconus. Cornea. (2004) 23:539–40. doi: 10.1097/01.ico.0000137168.24883.3e
43. Yin, S, Xu, L, Yang, K, Fan, Q, Gu, Y, Yin, C, et al. Gene–environment interaction between CAST gene and eye-rubbing in the Chinese keratoconus cohort study: a case-only study. Invest Ophthalmol Vis Sci. (2024) 65:36–6. doi: 10.1167/iovs.65.10.36
44. Hage, A, Knoeri, J, Leveziel, L, Majoulet, A, Blanc, J-V, Buffault, J, et al. Eyerubbics: the eye rubbing cycle study. J Clin Med. (2023) 12:1529. doi: 10.3390/jcm12041529
45. McMonnies, CW. Mechanisms of rubbing-related corneal trauma in keratoconus. Cornea. (2009) 28:607–15. doi: 10.1097/ICO.0b013e318198384f
46. Fukuoka, S, Adachi, N, Ouchi, E, Ikemoto, H, Okumo, T, Ishikawa, F, et al. Mechanoreceptor Piezo1 channel-mediated interleukin expression in conjunctival epithelial cells: linking mechanical stress to ocular inflammation. Ocul Surf. (2025) 36:56–68. doi: 10.1016/j.jtos.2025.01.001
47. Bosic, V, Mudassar, H, Seitz, B, and Flockerzi, E. Is there a predisposition for developing corneal hydrops in keratoconus?—tomographic and biomechanical analysis of the fellow eyes. Acta Ophthalmol. (2025) 103:e281–9. doi: 10.1111/aos.17474
48. Alqasimi, NA, Aljohani, LH, Ambrosio, R Jr, AlQahtani, BS, Al Haydar, NS, Alanazi, BR, et al. Assessment of awareness of keratoconus and its relation to eye rubbing among Saudi Arabia population. Front Ophthalmol (Lausanne). (2025) 5:1545030. doi: 10.3389/fopht.2025.1545030
49. Cheung, IMY, Lize, A, Akilesh, G, and Ziaei, M. Non-genetic risk factors for keratoconus and its progression. Clin Exp Optom. (2025) 108:648–56. doi: 10.1080/08164622.2024.2443454
50. Wan Abdul Halim, WH, Jamaludin, MI, and Cheng, TC. Corneal biomechanics in patients with allergic conjunctivitis. Indian J Ophthalmol. (2024) 72:S728–33. doi: 10.4103/IJO.IJO_654_24
Keywords: keratoconus, cortisol, pathogenesis, morphological parameters, biomechanical parameters
Citation: Zhao X, Sun T, Yuan Y, Zhang Y, Chen Y and Hong J (2025) Correlation of tear cortisol levels with morphological and biomechanical parameters of keratoconus. Front. Med. 12:1648334. doi: 10.3389/fmed.2025.1648334
Edited by:
Vivek Singh, LV Prasad Eye Institute, IndiaReviewed by:
Phillip Thomas Yuhas, The Ohio State University, United StatesYineng Chen, University at Albany—Downtown Campus, United States
Copyright © 2025 Zhao, Sun, Yuan, Zhang, Chen and Hong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yueguo Chen, Y2hlbnl1ZWd1b0AyNjMubmV0; Jing Hong, aG9uZ2ppbmcxOTY0MDFAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Xiaorui Zhao1,2,3†
Xiaorui Zhao1,2,3† Tong Sun
Tong Sun Yu Zhang
Yu Zhang Yueguo Chen
Yueguo Chen