- 1Department of Clinical Medicine, Qinghai University, Xining, China
- 2Respiratory and Critical Care Medicine Department, Luoyang First People's Hospital, Luoyang, China
- 3Respiratory and Critical Care Medicine Department, Qinghai Provincial People's Hospital, Xining, China
Objective: To investigate the clinical characteristics and risk factors of overlap syndrome (OVS) patients with metabolic syndrome (Mets) in middle-high altitude areas.
Methods: A retrospective analysis was performed on adult (≥40 years) OVS patients and healthy controls from Qinghai Provincial People’s Hospital (January 2017–January 2024), including general and laboratory data.
Results: 1. OVS patients had a higher rate of Diabetes, Hypertension, and Pulmonary Hypertension than healthy individuals; 2. OVS patients had significantly higher levels of inflammatory markers, hematologic, and lipid than Healthy individuals; 3. The proportion of OVS patients who also had Mets was 55.24%; 4. Compared to OVS patients without Mets, OVS patients with Mets had significantly higher levels of neutrophils, hemoglobin, red blood cell distribution width, C-reactive protein, NHR, and NLR, as well as a higher percentage of time with pulse oxygen saturation (SpO2) less than 80%, while the average and lowest SpO2 were significantly lower; 5. Hypoxic index, average SpO2, baseline SpO2, SpO2 less than 90%, and SpO2 less than 80% may be risk factors for the co-occurrence of OVS and Mets; 6. The rate of Mets among OVS patients who lived at an altitude of ≥2,500 meters was 63.79%, higher than OVS patients who lived at an altitude of <2,500 meters (44.68%).
Conclusion: Over half of middle-high altitude OVS patients have Mets, with higher rates at higher altitudes. Hypoxia may drive OVS-Mets comorbidity, while inflammation appears less significant.
1 Introduction
Chronic Obstructive Pulmonary Disease (COPD) is a chronic respiratory disorder defined by irreversible airflow limitation. Globally, it ranks as the third leading cause of death, underscoring its substantial public health burden. Obstructive Sleep Apnea/Hypoventilation Syndrome (OSA), by contrast, is primarily characterized by recurrent breathing pauses during sleep—episodes that trigger intermittent hypoxia (IH), alongside nocturnal hypoxemia and fluctuations in intrathoracic pressure. Clinically, OSA is closely linked to an increased risk of comorbidities such as diabetes mellitus and hypertension; epidemiologically, it is highly prevalent, with an estimated 936 million adults aged 30–69 worldwide affected by mild-to-severe obstructive sleep apnea. The concept of Overlap Syndrome (OVS) was first proposed in 1985, referring to the coexistence of COPD and OSA. Critically, these two conditions do not merely present as a “simple sum” of their individual manifestations-instead, they exert a mutually exacerbating effect that accelerates disease progression and deterioration. Consequently, OVS is associated with a far worse prognosis than either COPD or OSA alone.
Research has shown that Mets may be one of the most clinically relevant factors for COPD (1). In patients with chronic obstructive pulmonary disease (COPD), the prevalence of Mets ranges from 21 to 58%, depending on disease severity, geographical location, and the assessment indicators and methods used (2). Intermittent hypoxia is a fundamental feature of OSA. In patients with OSA, repeated episodes of hypoxemia and hypercapnia during sleep can damage multiple systems, including the endocrine-metabolic, nervous, digestive, and circulatory systems, leading to abnormalities in metabolic, cognitive, immune, and other functions. Additionally, the hypoxic environment specific to high altitudes significantly reduces the blood SpO2 of healthy individuals compared to those at low altitudes. This altitude-related hypoxia may further increase the incidence of OVS by impairing systems such as the endocrine-metabolic system. Therefore, this study aims to analyze the clinical characteristics of patients with OVS in middle-to-high altitude areas and identify the risk factors for the coexistence of Mets in these patients.
2 Objectives and methods
2.1 Study population
This study retrospectively included adult patients (≥40 years old) with COPD who were treated at the Qinghai provincial people’s hospital from January 1, 2017-a Grade A Tertiary Hospital located in Xining, Qinghai, China, with a comprehensive sleep medicine department and a large-scale general outpatient clinic.
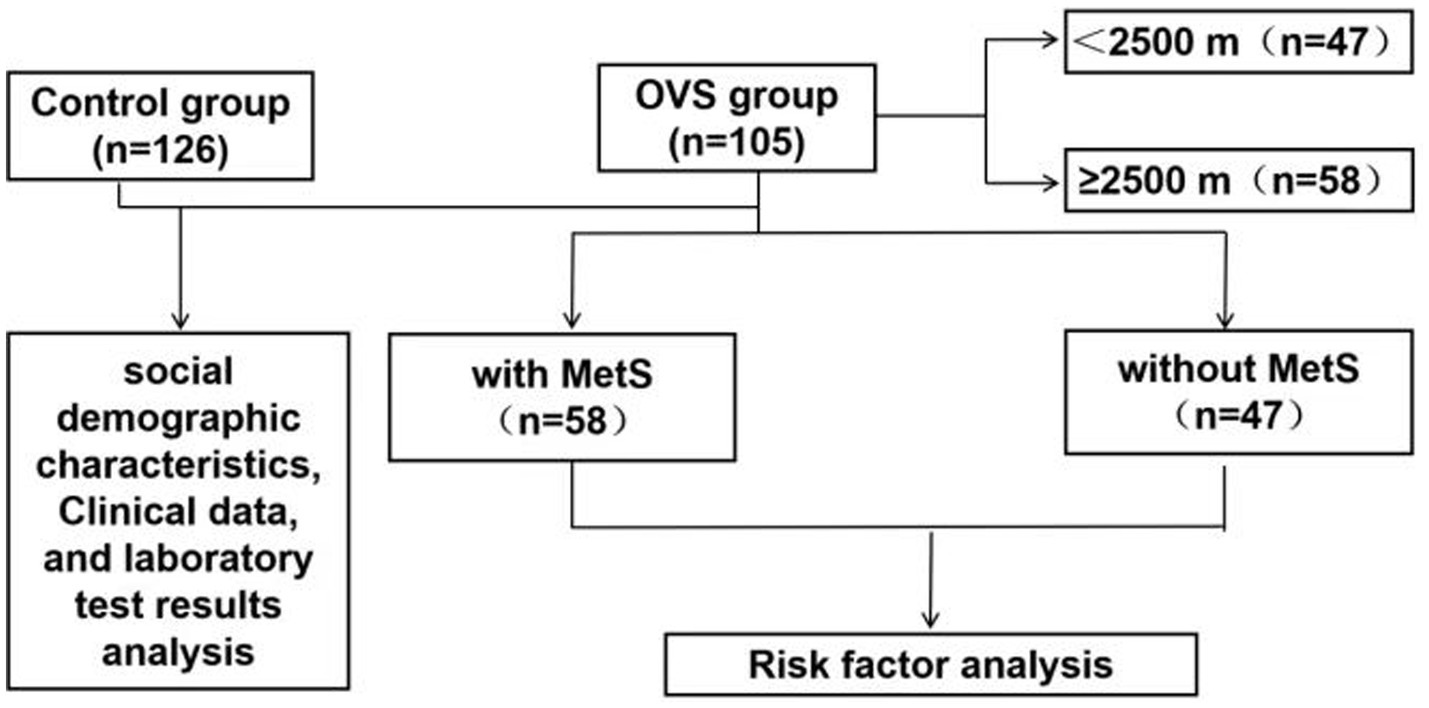
Patients were consecutively enrolled in the study. A total of 467 COPD patients and 126 healthy individuals were initially screened from the hospital’s electronic medical record system (inpatient and outpatient departments). After applying the exclusion criteria-including patients who refused to undergo Polysomnography (PSG), echocardiography, pulmonary function tests, or had significant missing covariates-the final cohort consisted of 105 eligible OVS participants and 126 healthy individuals. We have also supplemented a post-hoc power analysis based on the existing sample size, and the results show that the statistical power of this study in the analysis of the risk factor of OVS with Mets (SpO2 at baseline Proportion of time when SpO2 ≤ 90% during night sleep) reaches 0.96, meeting the basic requirements of clinical research. The PSG scores were analyzed according to the standardized criteria recommended by the American Academy of Sleep Medicine (3).
The healthy control group was recruited following strict inclusion and exclusion criteria: (1) no self-reported history of sleep disturbances (including snoring, daytime somnolence, or previously diagnosed sleep disorders such as OSA); (2) no history of chronic diseases closely associated with OSA pathogenesis (e.g., obesity with body mass index ≥30 kg/m2, or upper airway structural abnormalities such as tonsillar hypertrophy); (3) normal findings in routine physical examinations (including vital signs, cardiopulmonary auscultation, and head–neck region inspection for obvious upper airway narrowing). Notably, objective diagnostic tests for OSA—including overnight polysomnography (PSG, the gold standard for OSA diagnosis) were not performed for participants in the healthy control group. This decision was made due to two key constraints: first, the large sample size of the healthy cohort (n = 126), which would have resulted in excessive resource consumption (e.g., equipment, personnel, and financial costs) for universal objective testing; second, ethical and practical considerations regarding the burden of overnight monitoring on asymptomatic healthy participants, which could have reduced recruitment efficiency and follow-up compliance.
COPD was diagnosed in accordance with the GOLD 2023 guidelines, which include spirometric criteria (post-bronchodilator FEV1/FVC < 0.7) as well as relevant clinical manifestations. OSA was defined by an apnea-hypopnea index (AHI) of 5 events or more per hour based on AASM 2012 criteria. Therefore, OVS was defined as the coexistence of COPD and OSA, with specific reference to the diagnostic thresholds for both conditions (AHI ≥ 5 events/h for OSA and meeting COPD spirometric criteria as above). The diagnosis of Mets in our study was based on the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) guidelines (2005). This study was approved by the Research Ethics Committee of the Qinghai provincial people’s hospital (No. 2024–024-02). The flowchart was shown in Figure 1.
2.2 Data collection
Collecting all social demographic characteristics of the patients, including age, gender, and smoking status, etc. Clinical data, including patient history, such as diagnosis of hypertension, metabolic diseases, pulmonary arterial hypertension; laboratory test results, including complete blood count, lipid profile, etc.; and collect anthropometric indices. After patients use bronchodilators, measure lung function using a lung function instrument (Jaeger; Germany). Parameters include FEV1, FVC, FEV1/FVC, and the predicted percentage of FEV1 (FEV1%pre). Record polysomnography parameters, including apnea-hypopnea index (AHI), sleep stages, oxygen desaturation index, SpO2, mean SpO2, lowest SpO2, etc. According to the Qinghai International Consensus Scoring System (4), divide patients with OVS into those at altitudes of 1500-2499 meters and those at altitudes≥2,500 meters.
2.3 Statistical analysis
The analysis of the study results were conducted by SPSS 19.0 software. Count data were compared between groups using the chi-square test. Continuous data that passed the normality test were expressed as mean ± standard deviation ( ± s), and independent sample t-tests were used to compare two groups. Non-normally distributed continuous data were expressed as M (Q1, Q3). For non-normally distributed or heteroscedastic data, the Mann–Whitney U test was used. The comparison of rates between two groups was conducted using the chi-square test for a 2×2 table. Body mass index, oxygen deficit index, lowest SpO2, and mean SpO2 were included in a binary logistic regression model to assess the risk factors for patients with OVS and Mets. Multicollinearity among the independent variables also was evaluated in the logistic regression model. p < 0.05 is considered statistically significant.
3 Result
3.1 Sociodemographic characteristics and disease history comparison
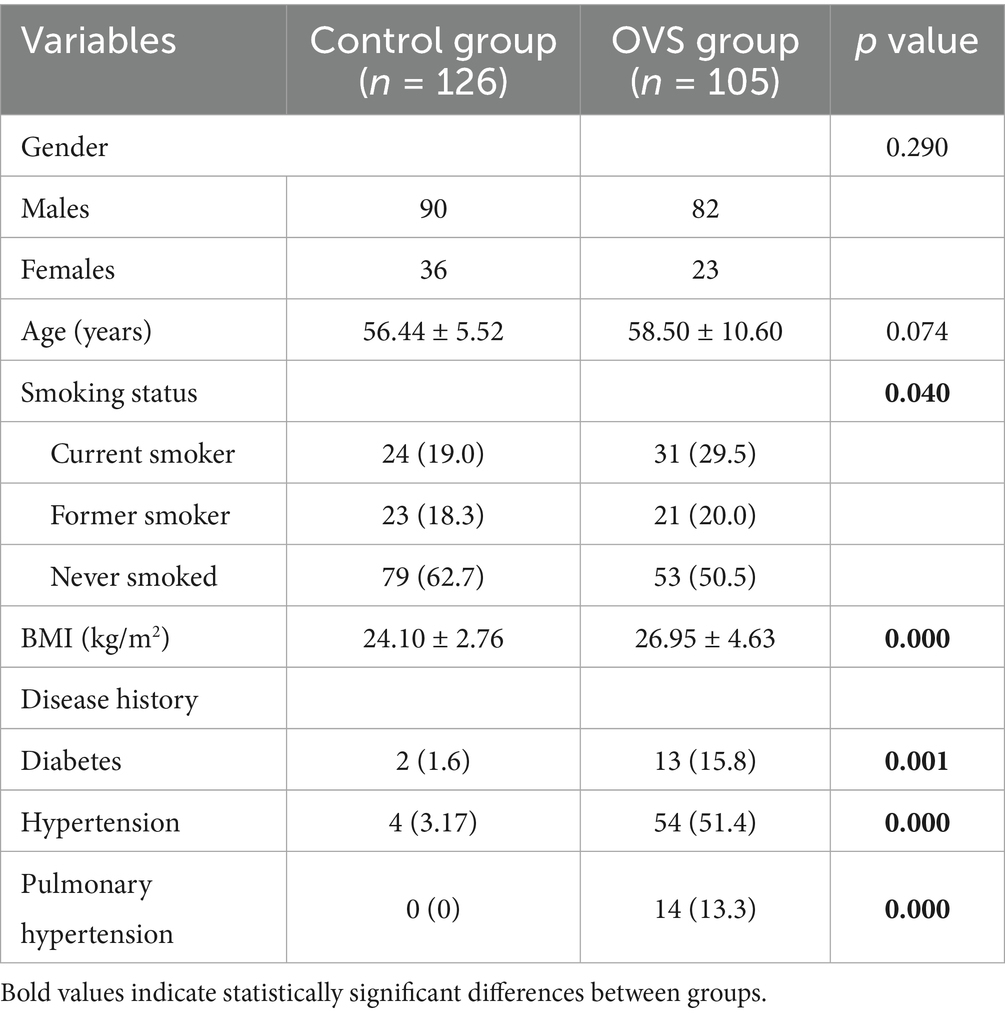
As shown in Table 1, there were no statistically significant differences in gender or age between the control group and patients with OVS. However, a difference was observed in smoking history. Patients with OVS had a higher body mass index (BMI) and a greater likelihood of comorbidities—including diabetes, hypertension, and pulmonary arterial hypertension-and these differences were statistically significant (p < 0.05).
3.2 Distribution of laboratory examination indexes of OVS patients
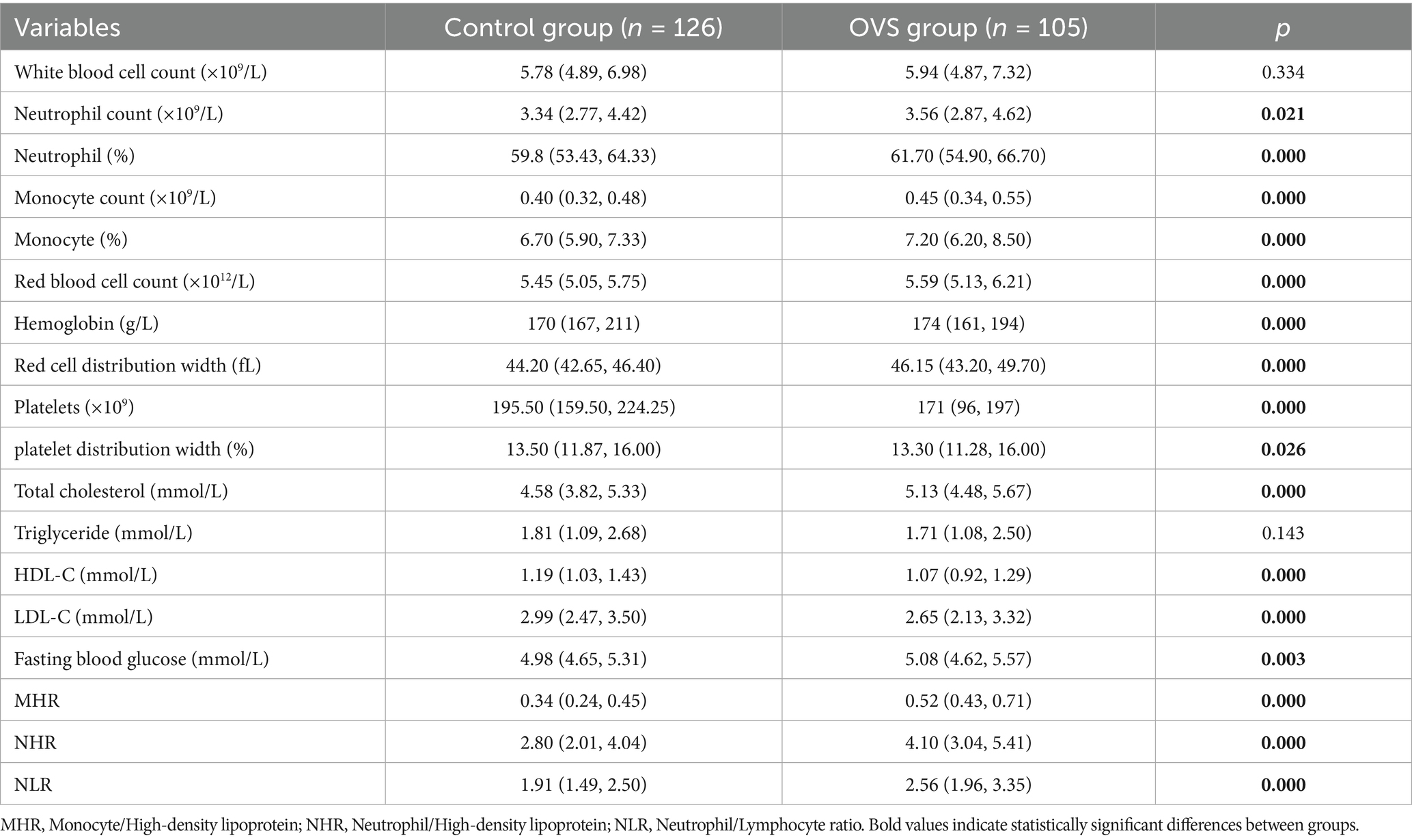
As shown in Table 2, compared with the control group, the levels of neutrophils, neutrophil percentage, monocytes, monocyte percentage, red blood cells, hemoglobin, red cell distribution width, platelets, platelet distribution width, total cholesterol, fasting blood glucose, creatinine, uric acid, monocyte/high-density lipoprotein (MHR), neutrophil/high-density lipoprotein (NHR), and neutrophil/lymphocyte ratio (NLR) were significantly increased in patients with OVS, while the number of lymphocytes decreased, with statistical significance, suggesting that OVS may be closely related to hyperglycemia, hyperuricemia, polycythemia et al. In addition, elevated levels of inflammatory markers such as neutrophils, neutrophil percentage, MHR, NHR, and NLR in OVS patients indicated an increased inflammatory level in OVS patients, which may participate in the progression of the disease in patients with OVS.
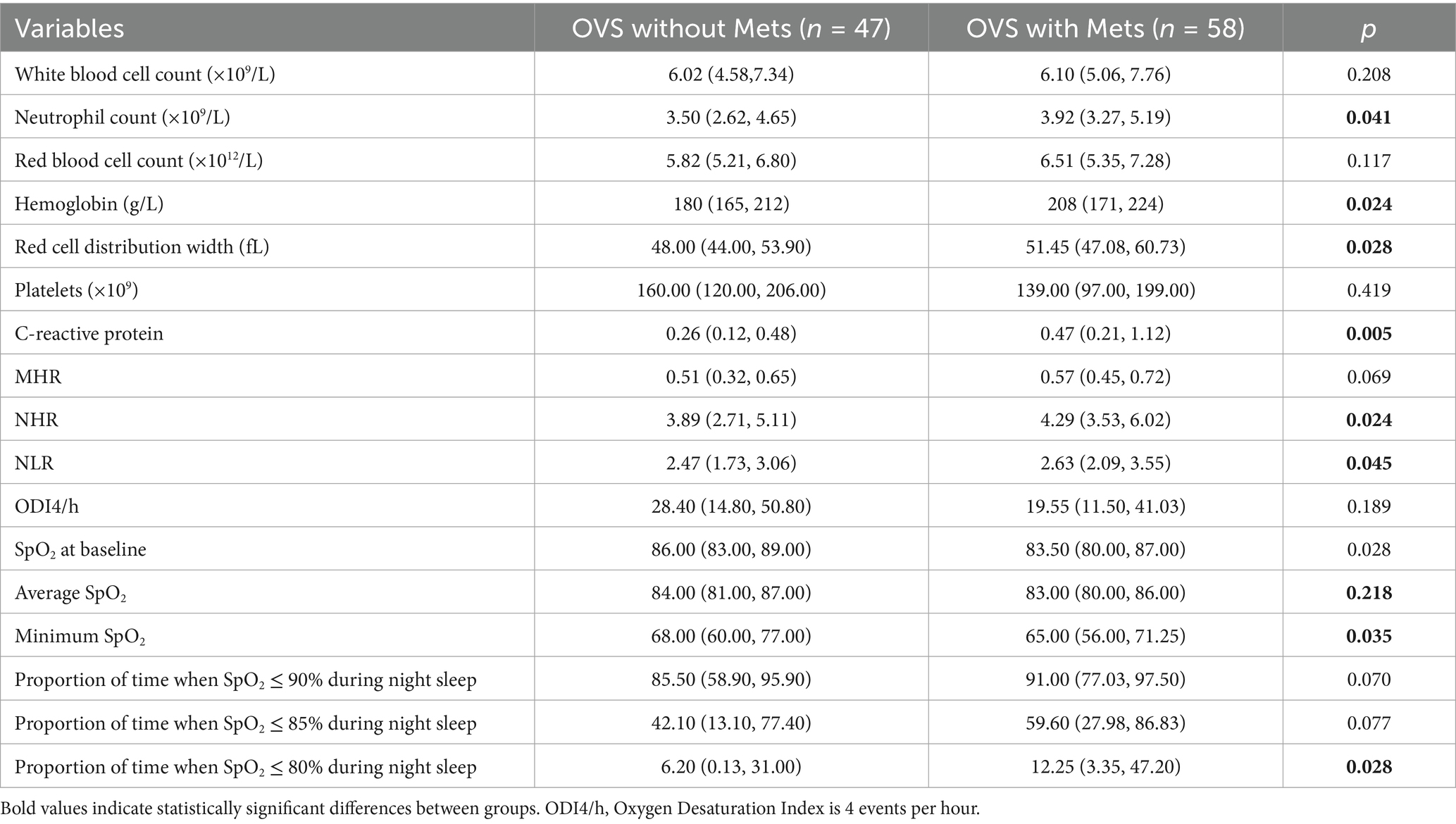
3.3 Sociodemographic characteristics and distribution of laboratory examination indexes of OVS patients with or without Mets
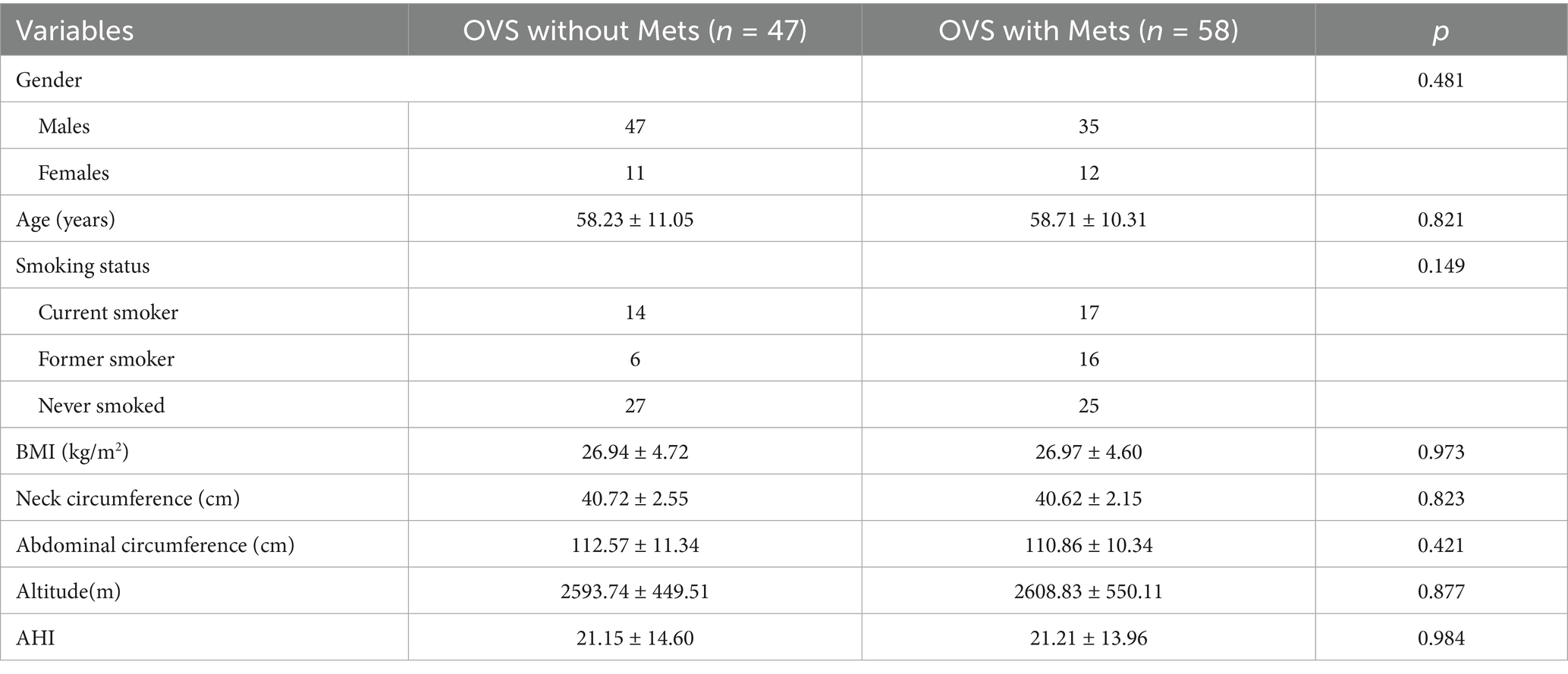
The OVS patients were divided into two groups based on whether they were accompanied by Mets: OVS patients with Mets group and OVS patients without Mets group. Results showed that the proportion of OVS patients with Mets was 55.24%. There were no statistically significant differences in gender, age, ethnicity, smoking history, body mass index, neck circumference, and waist circumference between the two groups (p > 0.05). In addition, the levels of neutrophils, hemoglobin, red cell distribution width (RDW), C-reactive protein (CRP), NHR, and NLR, as well as proportion of time when SpO2 ≤ 80% during night sleep in the OVS patients with Mets were significantly higher than in the OVS patients without Mets, while the mean SpO2 and the lowest SpO2 were significantly lower in OVS patients with Mets than in the OVS patients without Mets (p < 0.05). Although proportion of time when SpO2 ≤ 90% and SpO2 ≤ 85% during night sleep was higher in the OVS patients with Mets than in the OVS patients without Mets, the differences were not statistically significant (Tables 3, 4).
3.4 Analysis of risk factors in OVS patients with Mets
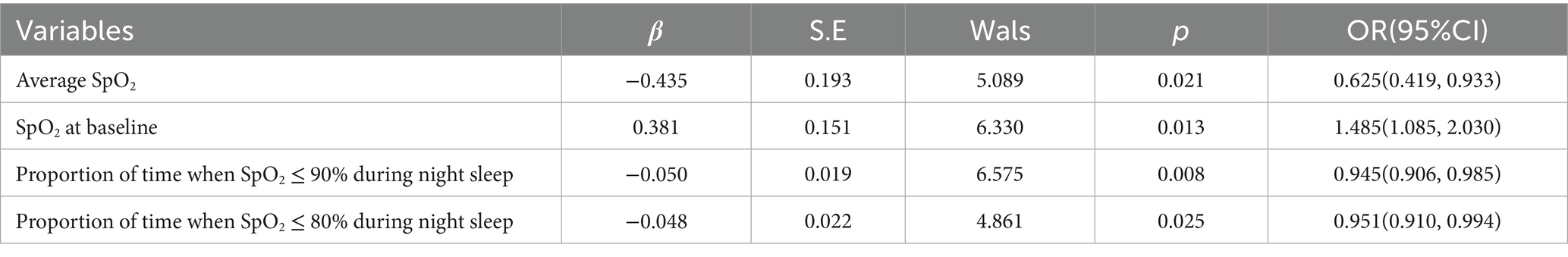
OVS patients were divided into those with and without Mets based on the presence of Mets. Binary logistic regression models were employed to assess risk factors for OVS with Mets, incorporating neutrophil count, red blood cell distribution width, NLR, NHR, oxygen desaturation index, minimum SpO2, mean SpO2, baseline SpO2, proportion of time when SpO2 ≤ 90 and 80% during night sleep. Multicollinearity analysis indicated that the variance inflation factor (VIF) and tolerance values for all variables fall within acceptable ranges, with all VIFs<10 and tolerances>0.1. In addition, results showed that the oxygen desaturation index, mean SpO2, baseline SpO2, proportion of time when SpO2 ≤ 90%, and SpO2 ≤ 80% may serve as risk factors for overlapping syndrome with Mets (Table 5).
3.5 Incidence of Mets in patients with overlapping syndrome at different altitudes
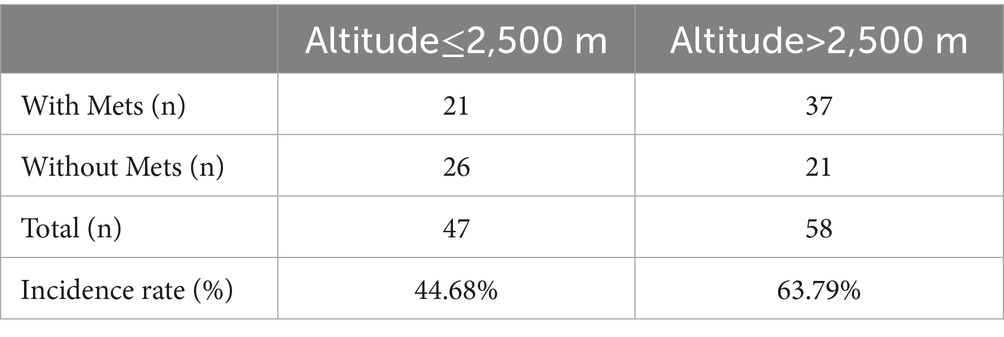
To clarify whether there are differences in the incidence of Mets in patients with overlapping syndrome at different altitudes, this study took 2,500 meters as the boundary and explored the incidence of Mets in patients with overlapping syndrome at different altitudes. The results showed (Table 6) that the incidence of Mets in patients with overlapping syndrome above 2,500 meters was 63.79%, higher than that in the lower altitude group below 2,500 meters (44.68%, p = 0.05).
4 Discussion
Sleep exerts a significant influence on respiration and gas exchange-effects that can have adverse impacts on patients with COPD (5). Notably, certain respiratory disorders (including OSA) exhibit specific associations with sleep. Given the high prevalence of both COPD and OSA, OVS-defined as the coexistence of these two conditions—is relatively common in clinical practice.
Extensive evidence confirms that COPD and OSA interact significantly, with these interactions shaping key clinical outcomes such as disease epidemiology, comorbidity profiles, and patient survival rates. Epidemiologically, OVS affects 1–3.6% of the general population; however, this proportion rises markedly when assessed within clinical cohorts of patients with OSA or COPD alone (6). A critical mechanistic link between the two diseases lies in their shared ability to induce local and systemic inflammatory responses. These inflammatory processes may act synergistically to drive the development of comorbidities such as Mets (7).
COPD and OSA are associated with heightened cardiovascular morbidity and mortality (8, 9), primarily due to a combination of factors: intermittent hypoxia, systemic inflammation, elevated oxidative stress, metabolic abnormalities, and activation of the sympathetic nervous system (10–12).
Numerous studies have reported that high levels of red blood cell distribution width (RDW) are similar to inflammatory factors such as C-reactive protein (CRP), interleukin, and tumor necrosis factor-α, and can reflect the body’s inflammatory levels, making it a novel inflammatory marker (13). In addition, the MHR, NHR, and NLR are also considered inflammatory markers (14, 15). Additionally, studies have demonstrated the predictive value of RDW in relation to pulmonary embolism, as well as its association with the severity and prognosis of this condition (16). The present study revealed significantly higher levels of neutrophils, neutrophil percentage, monocytes, monocyte percentage, RDW, MHR, NHR, and NLR in OVS patients compared to healthy individuals. These findings indicate an enhanced inflammatory response in OVS patients, which may contribute to the development of cardiovascular complications (17). Consistent with this inflammatory profile, the study also found that the incidence of hypertension and pulmonary arterial hypertension was significantly higher in OVS patients than in healthy controls.
A growing body of research highlights a bidirectional correlation between COPD and Mets, with the latter exacerbating the progression and prognosis of the former. Additionally, COPD may be a significant risk factor for the development and persistence of Mets. This correlation is primarily reflected in the presence of hypoxia and chronic inflammation in patients with COPD-two key pathological features that may contribute to the development of Mets. Furthermore, diabetes and hypertension are two major complications of OSA. Studies have shown that the prevalence of Mets in patients with moderate to severe OSA is 17.2%, a 2.5-fold increase in risk compared to control groups (18). Intermittent hypoxia (IH) is the primary cause of metabolic dysfunction in these patients. IH may induce metabolic disorders by impairing pancreatic β-cell function, causing inflammatory responses in adipose tissue, and increasing glucose production in the liver, which interferes with insulin signaling pathways and disrupts glucose metabolism (19, 20). As a result, patients with overlap syndrome may be more susceptible to metabolic disorders. The findings of this study revealed significantly higher levels of erythrocytes, hemoglobin, platelets, platelet distribution width, total cholesterol, and fasting blood glucose in overlap syndrome patients. Additionally, the prevalence of Mets in overlap syndrome patients was 55.24%, suggesting that overlap syndrome may be more prone to metabolic disorders and is closely associated with the development of conditions such as hyperglycemia. However, larger-scale studies are needed.
To clarify the risk factors associated with the development of metabolic syndrome (Mets) in patients with OVS, this study categorized participants into two groups: those with Mets and those without. The results showed that OVS patients with Mets exhibited significantly higher levels of inflammation markers, along with more severe hypoxic state than OVS without Mets patients. This is consistent with that OSA patients with more severe intermittent hypoxia are more prone to developing metabolic syndrome, while patients with Mets exhibit higher levels of inflammation markers (21, 22). As a key pathological feature, the core components of Mets—including obesity, insulin resistance, and dyslipidemia—collectively trigger a state of chronic low-grade inflammation in the body. For example, adipocytes, particularly visceral adipocytes, secrete large amounts of proinflammatory cytokines such as tumor necrosis factor-α and interleukin-6. These cytokines stimulate hepatic synthesis of CRP, thereby contributing to elevated circulating levels of this inflammatory marker (23). Studies showed that an elevated CRP level serves as a key marker indicating an increased risk of cardiovascular disease in patients with metabolic syndrome (24).
Our results also revealed that the mean SpO2, baseline SpO2, SpO2 below 90%, and SpO2 below 80% may be risk factors for Mets in OVS patients, indicating that hypoxia may play a role in the development of Mets in these patients. However, inflammatory indicators such as Neutrophil cell, RDW, MHR, NHR, NLR, and CRP were not identified as risk factors for OVS with Mets. Our findings suggest that inflammation may not play a significant role in the development of Mets associated with OVS, or it may only be a minor factor. Notably, the observed lack of statistical significance for inflammatory markers in our analysis could partially reflect limitations in our study’s sample size. A modest sample size may reduce statistical power, potentially masking subtle but biologically relevant associations between specific inflammatory pathways and Mets risk in OVS patients. This is particularly relevant given that some inflammatory indices did show numerical elevations, even if they did not reach statistical significance—hinting at a potential signal that requires further validation. Recognizing this uncertainty, larger cohorts needed for more robust subgroup analyses (e.g., stratifying by OVS severity or altitude) and may help clarify whether inflammation exerts context-dependent effects that were not detectable in our current dataset. Additionally, future studies could incorporate more comprehensive assessments of inflammatory mediators (e.g., cytokines, acute-phase proteins) and longitudinal designs to better characterize the temporal relationship between inflammation and Mets onset in OVS.
It has been reported that individuals with pre-existing asymptomatic obstructive sleep apnea (OSA) are more likely to experience reduced SpO2 and clinical deterioration (25). Additionally, hypobaric hypoxia at high altitudes impairs systemic tissue oxygenation, which in turn induces insulin resistance and a metabolic shift-marked by decreased oxidative phosphorylation and glucose storage, together with enhanced glycolysis. Notably, the induction of insulin resistance is not exclusive to hypobaric hypoxia; it also occurs in healthy individuals exposed to normobaric hypoxia and in patients with obstructive sleep apnea (26). Intermittent hypoxia, one of the primary pathologies of OSA, subjects cells to repeated cycles of hypoxia and normoxia, leading to oxidative stress and systemic inflammation. This, in turn, may contribute to the development of type 2 diabetes/insulin resistance, obesity, hypertension, and dyslipidemia in Mets through the regulation of gene expression and oxidative stress (20, 27). Furthermore, hypoxia can also lead to metabolic dysfunction (28–30). Studies have shown that hypoxia-induced transcription factors can contribute to the development of non-alcoholic fatty liver disease by regulating lipid metabolism in hepatocytes (30), and impaired carbohydrate and lipid metabolism (31). Therefore, the low-pressure and hypoxic environment at middle and high altitudes may further promote the development of Mets in OVS patients by reducing their blood SpO2 levels. The results of this study showed a higher prevalence of Mets in patients living above 2,500 meters compared to those living below 2,500 meters.
In conclusion, the prevalence of Mets in OVS patients in middle and high altitude areas was 55.24%, with a higher incidence of Mets in OVS patients living above 2,500 meters compared to those living below that altitude. Additionally, mean SpO2, baseline SpO2, and SpO2 levels below 90 and 80% may be risk factors for Mets in overlap syndrome patients.
5 Limitations
A major and unavoidable limitation of this study lies in its retrospective design, which inherently introduces several critical biases that significantly constrain the interpretation of our results. First, information bias is intrinsic to this approach: the data were extracted from clinical records compiled for patient care rather than research purposes, leading to inconsistencies in the documentation of variables such as symptom onset, treatment adherence, sociodemographic characteristics, and the distribution of laboratory test results. Second, selection bias represents a key concern: our sample was derived from patients who sought care at our institution, potentially overrepresenting those with more severe disease while excluding individuals with milder symptoms or undiagnosed cases. This limits the generalizability of our findings to broader populations. Most importantly, retrospective designs cannot establish causality. While we observed an association between high altitude and metabolic syndrome, unmeasured confounding factors—such as dietary habits, physical activity levels, and other unhealthy lifestyle behaviors not documented in the records—may account for this relationship. This represents a key limitation that prospective, controlled studies are uniquely positioned to resolve. These constraints underscore the necessity of future prospective investigations to validate our findings.
Author contributions
YQ: Formal analysis, Visualization, Writing – original draft. AA: Conceptualization, Data curation, Formal analysis, Visualization, Writing – original draft. YW: Conceptualization, Data curation, Formal analysis, Visualization, Writing – original draft. MS: Methodology, Software, Visualization, Writing – original draft. XH: Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. JL: Methodology, Software, Visualization, Writing – review & editing. XS: Funding acquisition, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by Qinghai Science and Technology Department (No. 2024-ZJ-934), 2022 Kunlun Elite of Qinghai Province High-End Innovation and Entrepreneurship leading Talents (No. 2022), Qinghai Clinical Research Center for Respiratory Diseases (No. 2019-SF-L4) and National Key Clinical Specialty Project: Respiratory and Critical Care Department (No. 2023).
Acknowledgments
The authors thank the Department of Respiratory and Critical Care Medicine of Qinghai Provincial People’s Hospital for providing data and technical assistance for this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Clini, E, Crisafulli, E, and Radaeli, A. COPD and the metabolic syndrome: an intriguing association. Intern Emerg Med. (2013) 8:283–9. doi: 10.1007/s11739-011-0700-x
2. James, BD, Jones, AV, and Trethewey, RE. Obesity and metabolic syndrome in COPD: Is exercise the answer? Chronic Respiratory Dis. (2018) 15:173–81. doi: 10.1177/1479972317736294
3. Kapur, VK, Auckley, DH, Chowdhuri, S, Kuhlmann, DC, Mehra, R, Ramar, K, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. (2017) 13:479–504. doi: 10.5664/jcsm.6506
4. Villafuerte, FC, and Corante, N. Chronic Mountain Sickness: Clinical Aspects, Etiology, Management, and Treatment. High Alt Med Biol. (2016) 17:61–9. doi: 10.1089/ham.2016.0031
5. Crinion, SJ, and McNicholas, WT. Sleep-related disorders in chronic obstructive pulmonary disease. Expert Rev Respir Med. (2014) 8:79–88. doi: 10.1586/17476348.2014.860357
6. Shawon, MS, Perret, JL, Senaratna, CV, Shawon, MSR, Lodge, C, Hamilton, GS, et al. Current evidence on prevalence and clinical outcomes of co-morbid obstructive sleep apnea and chronic obstructive pulmonary disease: a systematic review. Sleep Med Rev. (2017) 32:58–68. doi: 10.1016/j.smrv.2016.02.007
7. Alam, I, Lewis, K, Stephens, JW, and Baxter, JN. Obesity, metabolic syndrome and sleep apnoea: all pro-inflammatory states. Obes Rev. (2007) 8:119–27. doi: 10.1111/j.1467-789X.2006.00269.x
8. Tietjens, JR, Claman, D, and Kezirian, EJ. Obstructive Sleep Apnea in Cardiovascular Disease: A Review of the Literature and Proposed Multidisciplinary Clinical Management Strategy. J Am Heart Assoc. (2019) 8:e010440. doi: 10.1161/JAHA.118.010440
9. André, S, Conde, B, Fragoso, E, Boléo-Tomé, JP, Areias, V, and Cardoso, J. COPD and Cardiovascular disease. Pulmonology. (2019) 25:168–76. doi: 10.1016/j.pulmoe.2018.09.006
10. Balbirsingh, V, Mohammed, AS, Turner, AM, and Newnham, M. Cardiovascular disease in chronic obstructive pulmonary disease: a narrative review. Thorax. (2022) 77:e2021018333. doi: 10.1136/thoraxjnl-2021-218333
11. Tobaldini, E, Costantino, G, and Solbiati, M. Sleep, sleep deprivation, autonomic nervous system and cardiovascular diseases. Neurosci Biobehav Rev. (2017) 74:321–9. doi: 10.1016/j.neubiorev.2016.07.004
12. Orrù, G, Storari, M, Scano, A, Piras, V, Taibi, R, and Viscuso, D. Obstructive sleep apnea, oxidative stress, inflammation and endothelial dysfunction-an overview of predictive laboratory biomarkers. Eur Rev Med Pharmacol Sci. (2020) 24:6939–48. doi: 10.26355/eurrev_202006_21685
13. Zhang, Y, Xing, Z, and Zhou, K. The Predictive Role of Systemic Inflammation Response Index (SIRI) in the Prognosis of Stroke Patients. Clin Interv Aging. (2021) 16:1997–2007. doi: 10.2147/CIA.S339221
14. Liu, Z, Fan, Q, and Wu, S. Compared with the monocyte to high-density lipoprotein ratio (MHR) and the neutrophil to lymphocyte ratio (NLR), the neutrophil to high-density lipoprotein ratio (NHR) is more valuable for assessing the inflammatory process in Parkinson’s disease. Lipids Health Dis. (2021) 20:35. doi: 10.1186/s12944-021-01462-4
15. Kwak, IH, Kim, YE, and Kim, YJ. Monocyte to high-density lipoprotein cholesterol ratio reflects the peripheral inflammatory state in parkinsonian disorders. Parkinsonism Relat Disord. (2024) 129:107155. doi: 10.1016/j.parkreldis.2024.107155
16. Hammons, L, Filopei, J, Steiger, D, and Bondarsky, E. A narrative review of red blood cell distribution width as a marker for pulmonary embolism. J Thromb Thrombolysis. (2019) 48:638–47. doi: 10.1007/s11239-019-01906-w
17. Xie, J, Li, F, and Wu, X. Prevalence of pulmonary embolism in patients with obstructive sleep apnea and chronic obstructive pulmonary disease: The overlap syndrome. Heart Lung. (2019) 48:261–5. doi: 10.1016/j.hrtlng.2018.11.001
18. Haile, SR, Guerra, B, Soriano, JB, and Puhan, MA. Multiple score comparison: a network meta-analysis approach to comparison and external validation of prognostic scores. BMC Med Res Methodol. (2017) 17:172. doi: 10.1186/s12874-017-0433-2
19. Murphy, AM, Thomas, A, and Crinion, SJ. Intermittent hypoxia in obstructive sleep apnoea mediates insulin resistance through adipose tissue inflammation. Eur Respir J. (2017) 49:1601731. doi: 10.1183/13993003.01731-2016
20. Shobatake, R, Ota, H, and Takahashi, N. The Impact of Intermittent Hypoxia on Metabolism and Cognition. Int J Mol Sci. (2022) 23:12957. doi: 10.3390/ijms232112957
21. Reddy, P, Lent-Schochet, D, and Ramakrishnan, N. Metabolic syndrome is an inflammatory disorder: A conspiracy between adipose tissue and phagocytes. Clin Chim Acta. (2019) 496:35–44. doi: 10.1016/j.cca.2019.06.019
22. Lee, JY, Kim, CW, Lee, KC, Lee, J-H, Kang, S-H, Li, S-W, et al. Effect of intermittent hypoxia on metabolic syndrome and insulin resistance in the general male population. Medicina (Kaunas). (2021) 57:668. doi: 10.3390/medicina57070668
23. Kim, MJ, and Kim, IW. Self-rated health may be a predictor for metabolic syndrome and high hs-CRP prevalences in healthy adults in South Korea: Based on the 2015 Korea National Health and Nutrition Examination Survey. Nutr Res. (2022) 102:71–83. doi: 10.1016/j.nutres.2022.03.003
24. Chen, Y, Ju, H, and Xie, K. Association of inflammatory score with all-cause and cardiovascular mortality in patients with metabolic syndrome: NHANES longitudinal cohort study. Front Immunol. (2024) 15:1410871. doi: 10.3389/fimmu.2024.1410871
25. Ortiz-Naretto, AE, Pereiro, MP, and Ernst, G. Effect of mild obstructive sleep apnea in mountaineers during the climb to Mount Aconcagua. Sleep Sci. (2020) 13:138–44. doi: 10.5935/1984-0063.20190146
26. Adeva-Andany, MM, Adeva-Contreras, L, Carneiro-Freire, N, Ameneiros-Rodríguez, E, Vila-Altesor, M, and Calvo-Castro, I. The impact of high altitude (hypobaric hypoxia) on insulin resistance in humans. J Physiol Biochem. (2025) 81:35–55. doi: 10.1007/s13105-025-01069-8
27. Baffi, CW, Wood, L, and Winnica, D. Metabolic Syndrome and the Lung. Chest. (2016) 149:1525–34. doi: 10.1016/j.chest.2015.12.034
28. Wang, Q, Wang, P, and Qin, Z. Altered glucose metabolism and cell function in keloid fibroblasts under hypoxia. Redox Biol. (2021) 38:101815. doi: 10.1016/j.redox.2020.101815
29. Hermes-Lima, M, Moreira, DC, and Rivera-Ingraham, GA. Preparation for oxidative stress under hypoxia and metabolic depression: Revisiting the proposal two decades later. Free Radic Biol Med. (2015) 89:1122–43. doi: 10.1016/j.freeradbiomed.2015.07.156
30. Suzuki, T, Shinjo, S, and Arai, T. Hypoxia and fatty liver. World J Gastroenterol: WJG. (2014) 20:15087–97. doi: 10.3748/wjg.v20.i41.15087
Keywords: middle-high altitude, overlapping syndromes, metabolic syndrome, risk factors, clinical characteristics
Citation: Qin Y, Aju A, Wan Y, Song M, Hao X, Li J and Shi X (2025) Clinical characteristics and risk factor analysis of overlapping syndromes of COPD-OSA with metabolic syndrome in middle-to-high altitude areas. Front. Med. 12:1680584. doi: 10.3389/fmed.2025.1680584
Edited by:
Ling Zhou, Huazhong University of Science and Technology, ChinaReviewed by:
Minh Sang Nguyen, Vietnam National Lung Hospital, VietnamDuy-Thai Nguyen, NICVB, Ministry of Health, Vietnam
Oral Menteş, Gülhane Askerî Tıp Akademisi, Türkiye
Copyright © 2025 Qin, Aju, Wan, Song, Hao, Li and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuefeng Shi, c2hpeHVlZmVuZzEyOEAxNjMuY29t
†These authors have contributed equally to this work
 Yisha Qin
Yisha Qin Aniu Aju1†
Aniu Aju1† Yilin Wan
Yilin Wan Xingxing Hao
Xingxing Hao Xuefeng Shi
Xuefeng Shi