- 1Lab of Medication Adherence and Interprofessionality, School of Pharmaceutical Sciences, University of Geneva, Geneva, Switzerland
- 2Institute of Pharmaceutical Sciences of Western Switzerland, University of Geneva, Geneva, Switzerland
- 3Center for Primary Care and Public Health (Unisanté), University of Lausanne, Lausanne, Switzerland
- 4Pharma24, An Academic Community Pharmacy and Living Lab Located at the Exit of the Geneva University Hospitals, Geneva, Switzerland
Adherence to prescribed medication is suboptimal in 50% of the chronic population, resulting in negative medical and economic outcomes. With the widespread use of mobile phones worldwide, medication adherence apps for mobile phones become promising medication adherence aids thanks to simplicity, user-friendliness, and accessibility for the public. Yet, until today, there is insufficient evidence in favor of using mobile health (mHealth) apps to increase medication adherence. This study aims to develop a methodology for scientific and end-user (patient) mHealth evaluation (a) to identify medication adherence apps search terms, (b) to evaluate identified apps based on scientific criteria, and (c) to report best smartphone apps evaluated by patients. Search terms were identified via literature review and expertise. Firstly, an online questionnaire was developed to identify frequently used search terms by recruited patients. Related medication adherence apps were identified and selected using predefined inclusion criteria. Secondly, identified apps were evaluated thanks to a scientific evaluation method and a created online questionnaire for patient feedback. Recruited patients were invited to test and evaluate the selected apps. Out of 1,833 free-of-charge and 307 paid apps identified, only four free-of-charge and three paid apps remained included in the study after eligibility criteria. None of the selected app reached a high score. Looking at the overall scores, Medisafe (59%), MyTherapy (56%), and Meds on time (44%) received the highest scores in the scientific app evaluation. In the patient evaluation, Dosecast (3.83 out of five points), Medisafe (3.62), and SwissMeds (3.50) received the highest scores. None of the apps in this research has undergone a process for certification, for example, CE marking, through a notified body. Security and data protection aspects of existing apps highly contribute to these low evaluation scores through little information on patient's data processing and storage. This might be corrected through the introduction of General Data Protection Regulation (GDPR) in the European Economic Area (EEA) and more scrutiny through regulatory bodies in the EU/EEA and the USA. None of the applications should be recommended by healthcare providers. In addition, clinical studies with chronic patients are necessary to measure long-term app impacts.
Introduction
Medication adherence is highly associated with healthcare clinical outcomes such as treatment effectiveness, medical visits, hospitalization, and morbidity and mortality rates. This is especially true when it comes to chronic disease treatments (1–4). Optimal medication adherence rates bring benefit to both patients and the healthcare system (5). The World Health Organization (WHO) reports 50% of the population being non-adherent, resulting in negative medical and economic outcomes. This percentage is reportedly increasing in parallel with the rise in the prevalence of chronic diseases worldwide (6–9). Thanks to scientific innovation, numerous medication plans have changed from acute to chronic, increasing life expectancies, quality of life, patient autonomy, and self-determination. To improve patients' long-term adequate drug management, healthcare providers need to partner with patients to define clear treatment objectives and to reinforce patients' autonomous and active role in adhering to their treatment plans.
Numerous initiatives aim to support patients' medication adherence by targeting the four identified major influencing factors: (i) education, (ii) behavior, (iii) cognitive behavior, and (iv) multi-approach (10–14). Recent studies underline high adherence rates in conjunction with improved interprofessional communication and collaboration of the medical sector (15, 16). Defined as the process of a patient's intake of prescribed treatments, medication adherence includes the three phases of treatment initiation, implementation, and persistence (17). “Implementation” describes the daily drug intake during the time span of “persistence,” which is defined as the duration between treatment “initiation” and “discontinuation.” To correlate medication adherence with clinical outcomes, measurement methods are necessary (9). In the literature, indirect measures such as electronic monitoring are reported with high validity (18). Simultaneously, aiming to increase medication adherence, different tools are being developed in the digital health (or eHealth) sector with constantly growing profile and use.
Mobile health (mHealth) is an integral part of the digital health sector and is defined by the WHO as a healthcare and public health service delivery supported by digital health tools on mobile devices (19). Health applications (apps), also described as mHealth software, deliver health information and communication technologies in support of health fields, collect healthcare data, and may be connected to wearables, also described as mHealth hardware (20). While digital technologies are becoming important resources for the entire healthcare sector, mobile wireless technologies are particularly relevant, thanks to their user-friendliness, broad reach, cost-effectiveness, and popular public acceptance. In 2018, according to the International Telecommunication Union (ITU), 5.3 billion active mobile-broadband phone subscriptions were in use globally, demonstrating sustained growth (21). On the market for 10 years, mHealth apps have steadily increased with 78,000 new health apps added to major app stores in 1 year (2017) resulting in a total of 325,000 mHealth apps available in 2017. Of those, 10,000 apps focused on medication reminders (22, 23).
If an mHealth app is able to increase medication adherence, it has promising potential to improve a patient's quality of life and life expectancy while also monitoring healthcare cost (24). However, patients' data collection and related GDPR issues may limit such an app's usability. Sensitive personalized data may be transferred to third parties, be modified, or even get lost. Further, the quality of the medical information may be poor or misleading (25). To tackle these issues, medical regulations and guidelines (26, 27), data protection regulations (28), and national (29) and European (20) recommendations have been developed.
Until today, there is insufficient evidence in favor of using mHealth apps (30) although, in 2015, a review identified a positive impact on medication adherence through mobile phone applications (31). For mHealth to be adopted as part of routine clinical practice, collaboration with healthcare practitioners is essential. To encourage patient use of mHealth technologies, healthcare providers need to understand and recommend adapted technologies. However, a recent study reported that healthcare providers have high concerns: first, about digital health technologies' lack of official approval from government or similar regulatory bodies and, second, about the sparse studies demonstrating safety or effectiveness of such technology (32).
This study aims to propose a novel methodology enabling (a) to identify search terms and smartphone applications for medication adherence of chronic diseases, (b) to evaluate identified apps and establish a ranking based on scientific criteria, and (c) to report the best smartphone applications evaluated by patients. The results of the study should support healthcare providers with criteria for advising and recommending medication adherence applications.
Materials and Methods
The study focused in a first step on free-of-charge medication adherence apps. In a second step, the methodology was replicated and adapted to paid apps. Most importantly, a method for scientific (objective) and patient evaluation (subjective) to assess medication adherence apps was developed.
Identification of Search Terms for Medication Adherence Apps
A list of primary search terms (French or French and English) was created by establishing a preliminary list of search terms based on previously published articles (24, 33–35) and mHealth expertise (CM, MS, CB, CR). Subsequently, the list and a questionnaire were sent to patients online to identify the main search terms used by patients when searching for medication adherence apps (Appendix 2). Patients also rated how likely they would use already identified search terms.
Smartphone Application Identification
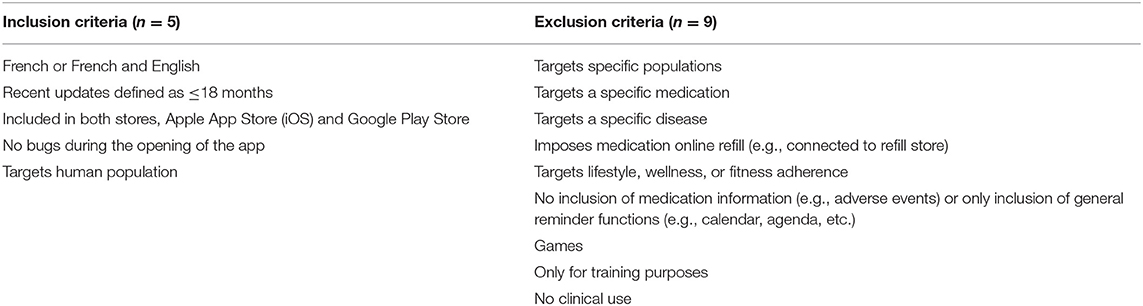
The retained search terms allowed for the identification of medication adherence apps for smartphones in Swiss stores, specifically the Apple App Store (iOS, Apple Inc.) and Google Play Store (Google LLC.). Medication adherence app inclusion and exclusion criteria were adapted based on previously published criteria (24, 33) (Table 1). The first 50 apps occurring from each search term were included.
Scientific App Evaluation Criteria
A list of criteria to scientifically evaluate app quality was created based on various criteria collected from literature research (24, 36–38), national recommendations (39), and European recommendations (20). A panel of three experts and one e-patient assessed these criteria, categorizing each as “very important,” “important,” and “not important.” Only “important” and “very important” criteria were kept.
These criteria are divided into six categories: (i) security and privacy (five items), (ii) quality of the health-related content (4), (iii) quality of the app information management (6), (iv) functionality (11), (v) user interface, and (vi) acceptability of the app (Table 2). For each criterion, a definition was developed on what circumstances the scientific evaluation requirement was not met (zero point) and was met (one point). Each included application was tested for 10 days by an investigator (CM, CR) with the help of these scientific evaluation criteria.
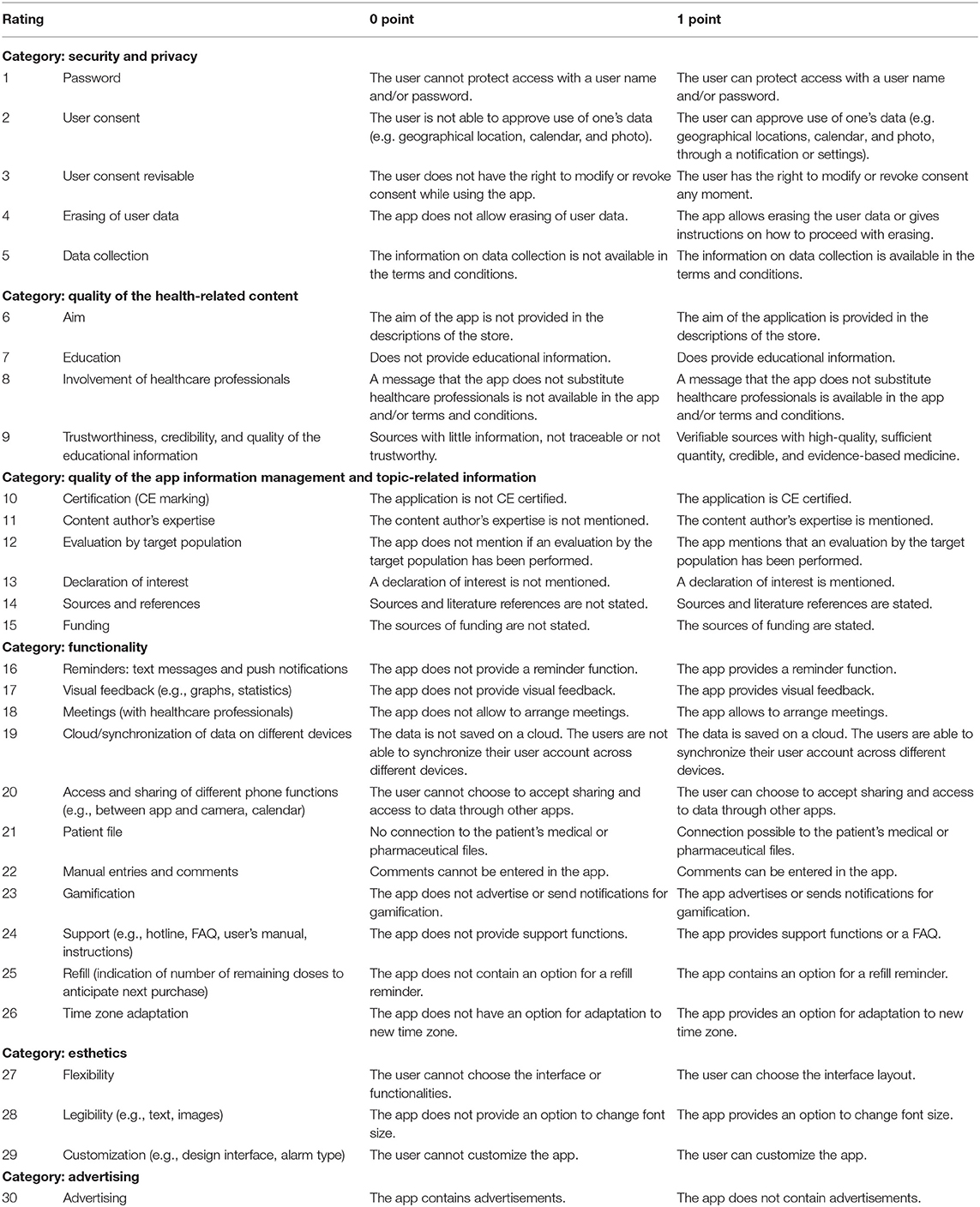
Table 2. Definition of categories and point criteria for scientific evaluation of the quality of the smartphone apps.
Criteria for App Evaluation by Patients Based Upon uMARS
A new online uMARS (user version of the Mobile Application Rating Scale) questionnaire was created in French (36) (Appendix 3) and sent to recruited patients via Google Sheets. This questionnaire was based on various uMARS versions: an Italian version, the non-validated French version, and the original English uMARS version. The evaluation based on uMARS aims to assess the usability and suitability of the previously identified apps in chronic patients.
Inclusion of Patients
Patients voluntarily participated in the study and were selected by defined inclusion criteria (Appendix 4). Patients having accepted replying to the questionnaire about frequently used search terms (section identification of search terms for medication adherence apps, Appendix 2) were asked to volunteer to test an app at random.
For the first step evaluation of free-of-charge apps, we included e-patients. These apps were evaluated by e-patients for 10 days. The e-patient is defined as a patient who is an expert in his disease field and highly at ease in using digital health tools and information. An e-patient is part of the investigators of the study; she is a professional patient working at a university medical institution and leading a voluntary e-patient platform, participating in various research studies.
In the second step evaluation, to increase external validity in the general population, paid apps were not tested by e-patients but by regular chronic patients recruited via flyers, web ads, and the Facebook page of Pharma24, the academic community pharmacy located at the exit of the University Hospitals of Geneva, Switzerland. Ethics committee approval from the canton of Geneva, Switzerland, was obtained through application 2018-01398. The University paid for the app fees. These patients then tested the applications for 14 days, with randomization of which patient was testing which app (Appendix 5).
Results
Search Terms Identified
A total of 147 search terms were identified. Through elimination of duplicates and terms with little relation to medication adherence, a total of 17 English and 65 French search terms have been identified for the search of medication adherence apps (Appendix 6).
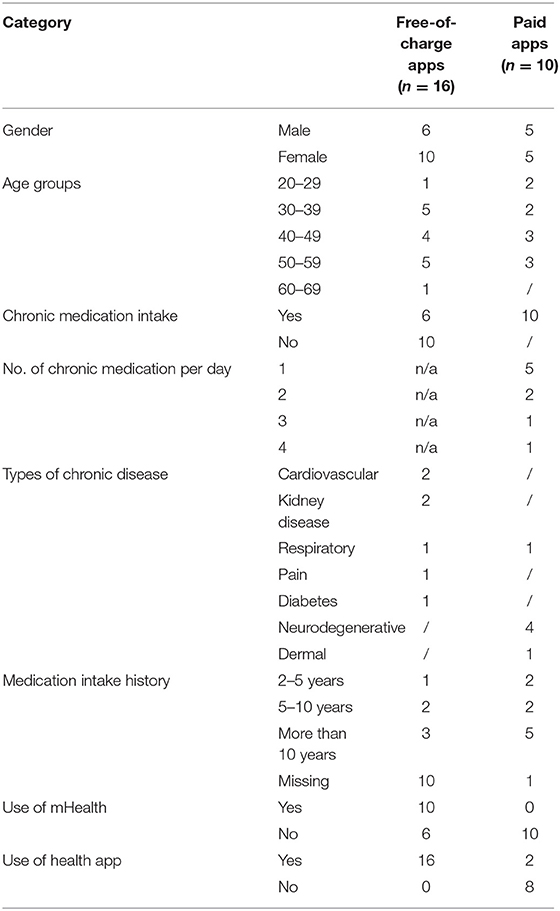
The patients included in this study (N = 16 for free-of-charge app testing and N = 10 for paid app testing) were mainly between 30 and 60 years of age (n = 14), taking medication since more than 10 years (n = 8) (Table 3).
The search terms most likely used by patients are described in Figure 1. “Medication reminder” (French: “rappel médicament”), “medication alarm” (“alarme médicament”), and “treatment reminder” (“rappel traitement”) were mainly used. Only one English search term was selected, “medication reminder.” The search terms with at least five nominations from patients and mHealth experts have been retained for the next steps.
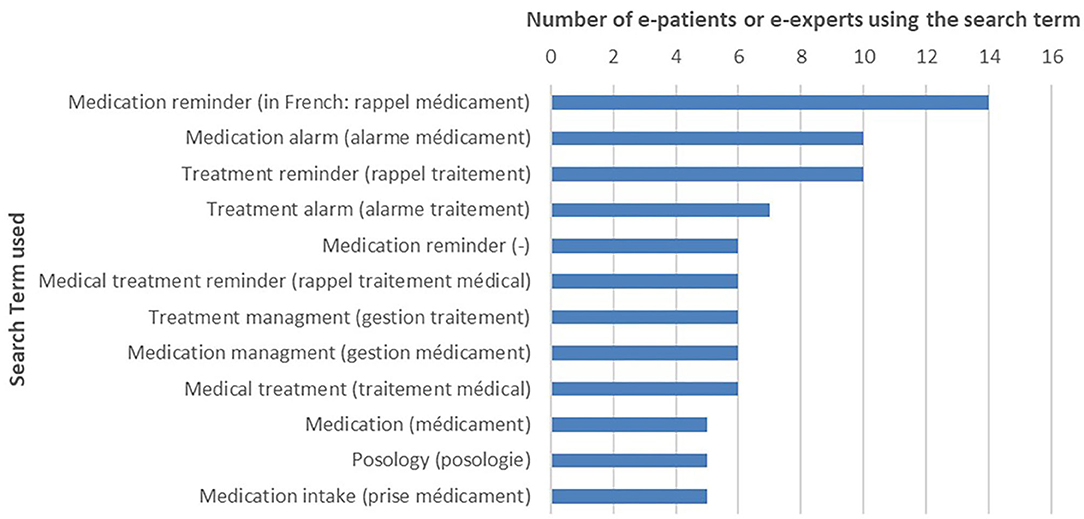
Figure 1. Medication adherence apps: search terms selected by e-patients (N = 16) and eHealth experts (search term nominated at least five times).
Apps Meeting the Inclusion Criteria
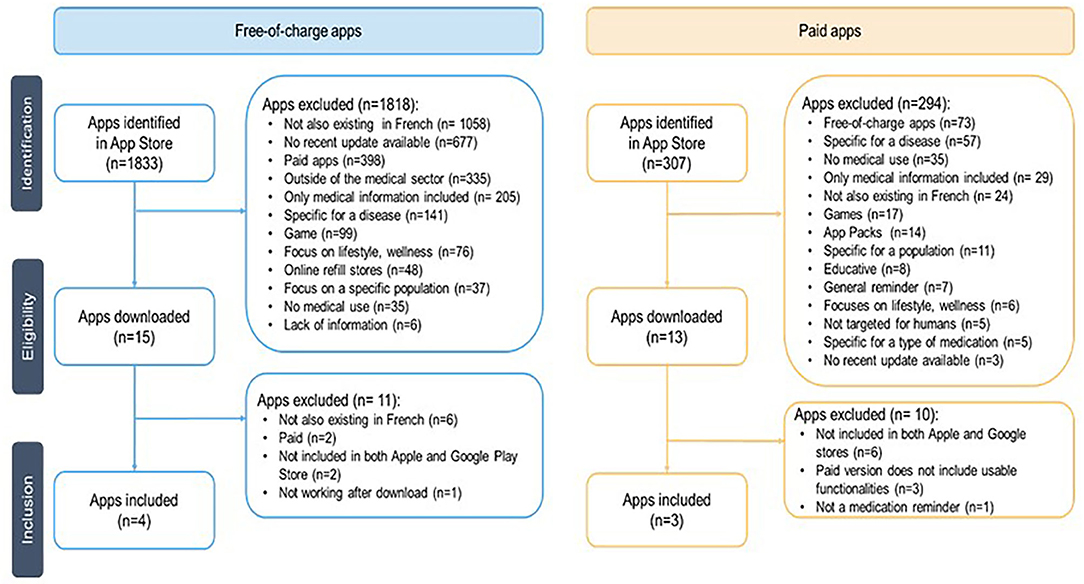
The flowchart (Figure 2) presents the systematic selection of apps included and evaluated in this study. A total of 1,833 free-of-charge apps have been identified in the Swiss Apple App Store, 567 paid apps in Google Play Store, and 307 paid apps in the Apple App Store.
Having identified these apps, the search terms for this study (Appendix 6) were revisited looking at what search terms matched the later included free-of-charge apps Medisafe, MyTherapy, Meds on time, and Médi'rappel (Appendix 1). Out of the 82 initial search terms, 26 led to the free-of-charge apps included in the study, 33 did not meet the eligibility criteria, and 23 terms did not provide any result. Additionally, SwissMeds (no pricing yet defined as it is currently at the end of its development stage by the Geneva University Hospitals) was added to the list.
Evaluation of Quality of the Smartphone Apps Through Scientific Criteria and Patient Evaluation
Free-of-charge apps included are Medisafe (free version tested; premium version available), MyTherapy, Meds on time, and Médi'rappel. Paid apps included are Dosecast (pricing: service fee of 2.99 CHF/month), Rappel de medicament (2 CHF/download), and Suivre ma Rx (7 CHF/download). At the time of evaluation, SwissMeds was not yet available in the app stores as it was only made available for testing as a prototype, and yet, it fulfilled all the inclusion criteria described in Table 1.
Scientific Evaluation Based on Quality Criteria
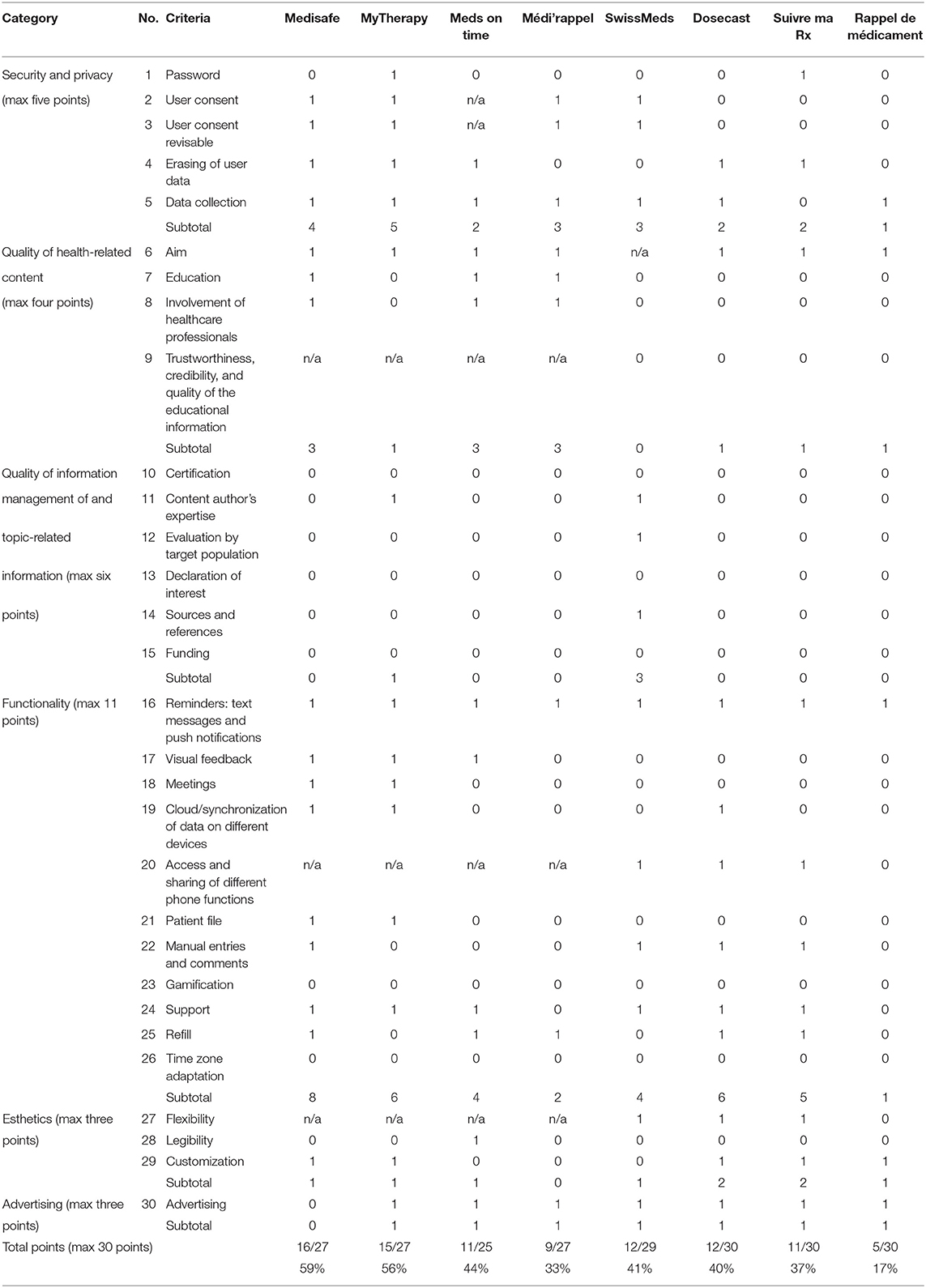
Using the scientific evaluation criteria (Table 2), the apps were rated (Table 4). The highest score was achieved by Medisafe (59%), followed by MyTherapy (56%), Meds on time (44%), and Médi'rappel (33%). For the paid apps, the highest score was achieved by Dosecast (40%), Suivre ma Rx (37%), and Rappel de medicament (17%). SwissMeds received a score of 41%.
Patient App Evaluation Using an Online Questionnaire
Two e-patients agreed to review the top three free-of-charge apps identified by a scientific scoring of at least 35%. Their evaluation resulted in Medisafe (3.62) receiving the highest scores, followed by MyTherapy (3.37) and Meds on time (3.27) (Table 5).
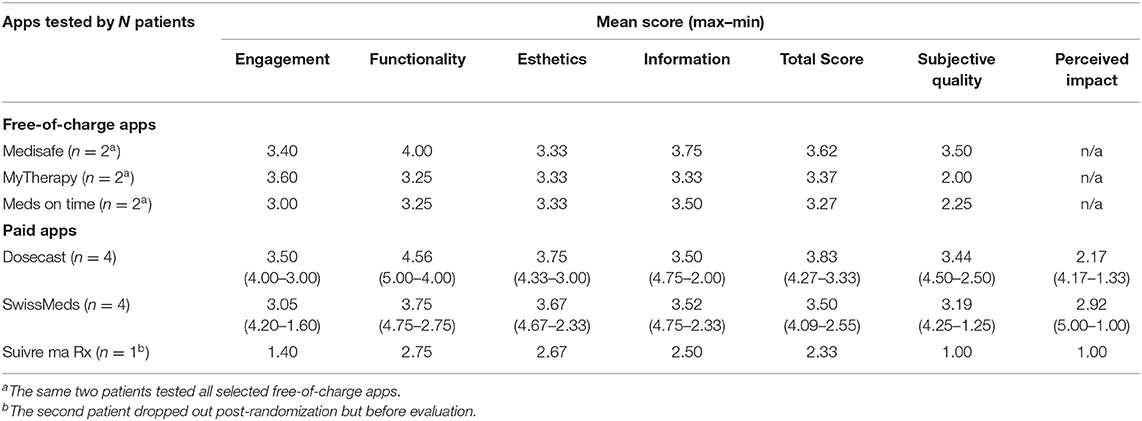
Table 5. Scoring of the apps by patients (N = 2 of free-of-charge app testing; N = 10 for paid app testing).
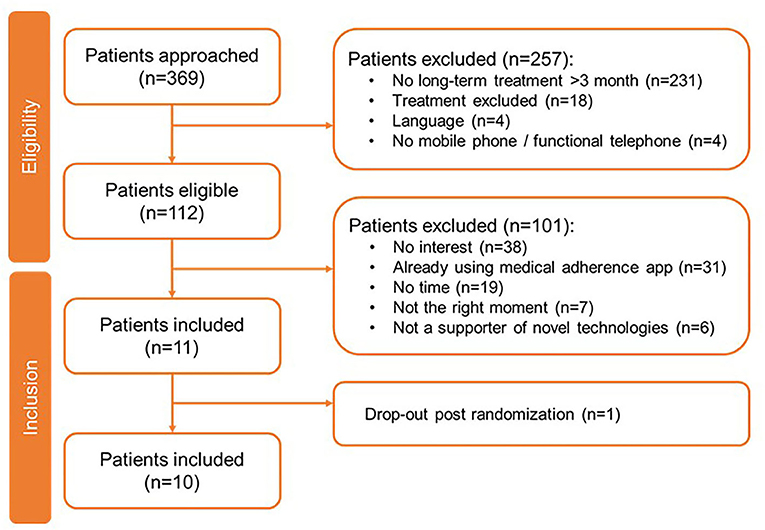
For the paid apps, 369 patients have been approached of whom 257 were excluded, primarily because they were not undergoing chronic or long-term (>3 months) treatment (n = 231) (Figure 3). Of the 112 eligible patients, 10 were included in this study. Their demographics are shown in Table 3. Of the 10 patients, the majority used Apple's iOS as operating system (n = 9) and did not have experience with health-related apps (n = 8). One patient on Suivre ma Rx dropped out post-randomization. During their evaluation, patients (N = 10) allocated the highest scoring on Dosecast (3.83), followed by SwissMeds (3.50) and Suivre ma Rx (2.33) (Table 5).
Discussion
This study established a novel methodology to evaluate mHealth applications for medication adherence, comprising first a scientific appraisal and second an end-user appraisal. Firstly, we evaluated free-of-charge apps to get an overview of apps accessible for all. Since the results from this first evaluation were not satisfactory, we decided to pursue our study with paid apps. This method enabled the withholding of four out of 1,853 free-of-charge medication adherence apps (0.2%) and three out of 307 paid apps (0.9%). These results underline the high availably of medication apps on the market, as well as the exceptionally low number of apps which met the study inclusion criteria and that could be recommended by healthcare providers. The evaluation resulted in a wide range of average quality scores (17–57%) showing a high variability of quality and usability and a need to evaluate apps individually. Moreover, scoring levels were quite low (max 57%), suggesting significant room for improvements. The apps were evaluated through six identified evaluation domains: (i) security and privacy, (ii) quality of the health-related content, (iii) quality of the app information management, (iv) functionality, (v) user interface, and (vi) acceptability of the app. To our knowledge, this is the first study in this context conducted in this area in Switzerland. Due to the repeatability of the proposed method, this study can also be used in other environments and populations. The study is based upon a qualitative and quantitative evaluation including patient feedback allowing for the retrieval of additional information (e.g. data protection). The study was carried out with chronic disease patients who require long-term treatment and can thus benefit from medication adherence.
Security and data protection aspects of existing apps highly contribute to the low evaluation scores. Introduced on May, 25, 2018, the GDPR of the EU currently includes specific regulations for mHealth apps (28). For the US apps, e.g., Dosecast, US data protection laws, widely considered less strict than the European laws, apply. The free-of-charge apps score higher in the data protection category than the paid apps, similar to what Loy et al. described. This can be attributed to the fact that paid apps are more often developed by individuals than companies (40). For Dosecast, the terms and conditions include a statement on data collection that asserts that data is not transferred to third parties. However, a closer look reveals that data on the drugs used may be accessed by partnering companies. For end users, this ambiguity may be difficult to discern.
On the quality of the health-related content, the free-of-charge apps score higher than the paid ones as they have a clear aim and educational information and further involve healthcare professionals.
Quality of information management is handled poorly with the exception for SwissMeds, which was specifically programmed for this aspect. None of the apps in this research has undergone a certification process, such as CE marking, through an accredited body. In Europe, medical software, of which mHealth is a part of, is regulated through the medical device directive 2017/745/EC resulting in CE marking and will be required from 26 May 2021 onwards (26). In the USA, Food and Drug Administration (FDA) guidelines exist (41), providing information about regulatory oversight of mobile health apps that satisfy the definition of a medical device. An independent European industry label in digital health exists, called “mHealth Quality,” which evaluates apps on their medical value, ethics, privacy, security, and regulatory conformity (42). Looking at these regulations, the apps may need to be improved, which would raise the scores in this category.
None of the apps listed focuses on quality of health-related and medication adherence contents. The only app declaring a collaboration with a credible content author is MyTherapy, which has a collaboration with Charité—Universitätsmedizin Berlin (43). Conflicts of interest, financing, and sources for the content are not declared in the apps except for SwissMeds. This is concerning as the app Meds on time is part of the Japanese pharmaceutical company Daiichi Sankyo (44) and Médi'rappel is offered by the French generics pharmaceutical company Biogaran (45). The user may question these companies' interests and/or motives, as these apps are free and offer no declaration on data privacy and security. Further research and regulation are urgently needed. By supporting medication adherence, apps can be considered as intervention devices. Their constitutive components should be investigated, especially the way medication adherence is scored for patient's feedback. Moreover, the intervention content provided to the users and healthcare providers must be developed from research findings.
The functionality of medication adherence apps is determined on the basis of medication reminders. Only Suivre ma Rx does not provide adaptability for weekly adherence monitoring. Tailoring the app to the patient's treatment regime is essential and ideal for supporting patient's adherence (25). Some apps also provide cloud storage and sharing across several devices, allowing for information (including dedicated patient files) to be shared with other people. None of the tested apps provided a gamification mode. However, the literature has described gamification modes, for instance through trophies or leveling, as a means of improving patient engagement with apps (46).
Looking at the overall scores, Medisafe (59%), MyTherapy (56%), and Meds on time (44%) received the highest scores in the scientific app evaluation. In the patient evaluation, Dosecast (3.83 out of five points), Medisafe (3.62), and SwissMeds (3.50) received the highest scores. These relatively average quality scores, even among the highest scoring apps, show the difficulty that patients face in finding a high-quality app. Comparing these results to the literature, similar results have been found in a study for the Australian market, rating Medisafe as the best and Dosecast as the second best app (33). In another study in the USA, Medisafe was rated highest and Dosecast in the midfield (47). This confirms the proposed method being in accordance with the findings of other similar studies.
The uMARS does not evaluate questions regarding data privacy and security, and patients may not provide feedback on these aspects. In addition, it does not evaluate the existence and credibility of sources, which is an integral part of a scientific evaluation. Thus, uMARS should be used with end users only after a scientific, professional, and patients' app evaluation, including security and privacy aspects, as presented in this study.
The most important limitation of this study is the constant evolution of the technology and delivery of updates. Every day, some apps are removed, while others are added. Even the apps tested in this study may disappear in the future. Furthermore, apps require frequent updates, which may add new content and functionalities, improving the score in this study. Upcoming CE marking or other labeling might be possible for the apps as well. Nevertheless, this study aimed at developing a methodology that can be replicated with new apps, thus allowing comparison of the apps' performance over time.
Another limitation is the constraint to only the top 50 search results for each search term. So, apps listed outside of this constraint might be overlooked. To reduce this risk, the search was executed with several search terms reducing this risk. We mimicked patient search, hypothesizing that they would not search beyond a certain number of apps and we limited the threshold at 50. In addition, the ranking of search terms in the app stores is changed non-transparently, diminishing repeatability. Also, apps could have been excluded and not tested because of their vague description without having been tested. Apps only available on one of the two stores might have been excluded even though they may have had a high quality. However, most of the high-quality apps are offered in both stores. The evaluation of medication adherence apps is linked to the local context including the language and the app store availability (here: the French and the Swiss stores) and, thus, represents an inherent constraint of our study. However, our methodology is reproducible whatever the languages.
Scientific evaluation was based upon assigning zero or one point per category. These categories could be adapted to higher granularity of scoring to allow for weighting for some aspects (e.g. prioritizing data protection over esthetics). This needs to be investigated in future studies.
Finally, even though higher-quality applications have been identified, until today, there is no evidence that they increase medication adherence (30). There is currently no scoring system in place to analyze the app quality in relation to medication adherence measures and feedback to the patients. This is crucial and needs to be developed. Finally, clinical studies with chronic patients are needed to measure the impact of the app's long-term utilization on outcomes such as medication adherence (both implementation and persistence), patient satisfaction, and app-mediated exchange of information on medication adherence between patients and healthcare providers.
Currently, we would discourage healthcare providers from recommending these free-of-charge and paid apps because of security issues. In the long term, we underline the need of further medication app evaluations including other languages and countries, as well as larger patient groups, and hope that high-quality apps will be available in the healthcare system. SwissMeds seems promising in this regard. This will allow patients to take personal control of their own treatment through mHealth tools while supporting and strengthening patient collaboration with healthcare providers.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics committee approval from the canton of Geneva, Switzerland, was obtained through application 2018-01398. The patients/participants provided their written informed consent to participate in this study.
Author's Note
This article is a synthesis of the master theses in pharmaceutical sciences of Carla Moyano and Camille Rimaud, which have been supervised by Claudine Backes and Marie-Paule Schneider.
Author Contributions
CBa is the main author of this manuscript and coordinated the research. This article is a synthesis of the master theses by CM and CR. CBa and MS supervised this work. CBi, an e-patient, provided crucial insight on the patient's perspective for mHealth apps. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Katherine Blondon, directorate of the Geneva University Hospitals, and the SwissMed team for having allowed our research team to test the SwissMeds app although this application was not yet available on the app stores. We also thank all the e-patients and patients who participated in this study and tested the apps.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmedt.2020.616242/full#supplementary-material
References
1. Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA. (2007) 297:177–86. doi: 10.1001/jama.297.2.177
2. Schneider M-P, Gertsch A, Bugnon O. Cyberhealth serving to support individual intake of medication. Swiss Med Weekly. (2013) 143:w13827. doi: 10.4414/smw.2013.13827
3. Lelubre M, Kamal S, Genre N, Celio J, Gorgerat S, Hugentobler Hampai D, et al. Interdisciplinary medication adherence program: the example of a University Community Pharmacy in Switzerland. BioMed Res Int. (2015) 2015:103546. doi: 10.1155/2015/103546
4. Glass TR, Sterne JA, Schneider M-P, De Geest S, Nicca D, Furrer H, et al. Self-reported non-adherence to antiretroviral therapy as a predictor of viral failure and mortality. Aids. (2015) 29:2195–200. doi: 10.1097/QAD.0000000000000782
5. Morrissey EC, Casey M, Glynn LG, Walsh JC, Molloy GJ. Smartphone apps for improving medication adherence in hypertension: patients' perspectives. Patient Preference Adherence. (2018) 12:813. doi: 10.2147/PPA.S145647
6. Ho PM, Magid DJ, Shetterly SM, Olson KL, Maddox TM, Peterson PN, et al. Medication non-adherence is associated with a broad range of adverse outcomes in patients with coronary artery disease. Am Heart J. (2008) 155:772–9. doi: 10.1016/j.ahj.2007.12.011
7. Scheen A, Giet D. Non-observance thérapeutique: causes, conséquences, solutions. Revue Médicale de Liège. (2010) 65:239–45.
8. Aitken M, Gorokhovich L. Advancing the responsible use of medicines: applying levers for change. SSRN. (2012) 2222541. doi: 10.2139/ssrn.2222541
9. Sabaté E.Adherence to Long-Term Therapies: Evidence for Action. Geneva: World Health Organization (2003).
10. Costa E, Giardini A, Savin M, Menditto E, Lehane E, Laosa O, et al. Interventional tools to improve medication adherence: review of literature. Patient Preference Adherence. (2015) 9:1303. doi: 10.2147/PPA.S87551
11. Axelsson M, Lötvall J. Recent educational interventions for improvement of asthma medication adherence. Asia Pacific Allergy. (2012) 2:67–75. doi: 10.5415/apallergy.2012.2.1.67
12. Lavielle M, Puyraimond-Zemmour D, Romand X, Gossec L, Senbel E, Pouplin S, et al. Methods to improve medication adherence in patients with chronic inflammatory rheumatic diseases: a systematic literature review. RMD Open. (2018) 4:684. doi: 10.1136/rmdopen-2018-000684
13. Chan W, Chen A, Tiao D, Selinger C, Leong R. Medication adherence in inflammatory bowel disease. Intestinal Res. (2017) 15:434. doi: 10.5217/ir.2017.15.4.434
14. Krousel-Wood M, Hyre A, Muntner P, Morisky D. Methods to improve medication adherence in patients with hypertension: current status and future directions. Curr Opin Cardiol. (2005) 20:296–300. doi: 10.1097/01.hco.0000166597.52335.23
15. Zullig LL, Blalock DV, Dougherty S, Henderson R, Ha CC, Oakes MM, et al. The new landscape of medication adherence improvement: where population health science meets precision medicine. Patient Preference Adherence. (2018) 12:1225. doi: 10.2147/PPA.S165404
16. Thakkar J, Kurup R, Laba T-L, Santo K, Thiagalingam A, Rodgers A, et al. Mobile telephone text messaging for medication adherence in chronic disease: a meta-analysis. JAMA Internal Med. (2016) 176:340–9. doi: 10.1001/jamainternmed.2015.7667
17. Vrijens B, De Geest S, Hughes DA, Przemyslaw K, Demonceau J, Ruppar T, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. (2012) 73:691–705. doi: 10.1111/j.1365-2125.2012.04167.x
18. Lehmann A, Aslani P, Ahmed R, Celio J, Gauchet A, Bedouch P, et al. Assessing medication adherence: options to consider. Int J Clin Pharm. (2014) 36:55–69. doi: 10.1007/s11096-013-9865-x
19. World Health Organization (WHO) Global Observatory for eHealth, mHealth. New Horizons for Health through mobile Technologies. Geneva: World Health Organization (2011). p. viii.
20. European Commission. Green Paper on Mobile Health (“m-health”) (Paper No. 219), Brussels: European Economic and Social Committee (2014).
21. International Telecommunication Union (ITU). ITU releases 2018 global and regional ICT estimates. In: ICT for Sustainable Development, editor. For the First Time, More Than Half of the World's Population Is Using the Internet. Geneva: ITU. (2018).
22. Aitken M, Clancy B, Nass D. The Growing Value of Digital Health Parsippany, NJ: IQVIA Institute for Human Data Science (2017). p.1–76.
23. Aitken M, Lyle J. Patient Adoption of mHealth: Use, Evidence and Remaining Barriers to Mainstream Acceptance. Parsippany, NJ: IMS Institute for Healthcare Informatics (2015).
24. Dayer L, Heldenbrand S, Anderson P, Gubbins PO, Martin BC. Smartphone medication adherence apps: potential benefits to patients and providers. JAPhA. (2013) 53:172–81. doi: 10.1331/JAPhA.2013.12202
25. Dehling T, Gao F, Schneider S, Sunyaev A. Exploring the far side of mobile health: information security and privacy of mobile health apps on iOS and Android. JMIR mHealth uHealth. (2015) 3:e8. doi: 10.2196/mhealth.3672
26. European Parliament and of The Council. European Parliament. Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices, amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing Council Directives 90/385/EEC and 93/42/EEC. Off J Eur Union. (2017) 1:L177.
27. Food Drug Administration. Mobile Medical Applications: Guidance for Industry and Food and Drug Administration Staff. Rockville, MD (2015). p. 1:2016.
28. EU General Data Protection Regulation (GDPR). Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data, and repealing Directive 95/46. Off J Eur Union. (2016) 59:294.
29. Haute Autorité de Santé (HAS). Référentiel de bonnes pratiques sur les applications et les objets connectés en santé, Saint-Denis La Plaine (2016).
30. Byambasuren O, Sanders S, Beller E, Glasziou P. Prescribable mHealth apps identified from an overview of systematic reviews. NPJ Digital Med. (2018) 1:1–12. doi: 10.1038/s41746-018-0021-9
31. Choi A, Lovett AW, Kang J, Lee K, Choi L. Mobile applications to improve medication adherence: existing apps, quality of life and future directions. Adv Pharmacol Pharmacy. (2015) 3:64–74. doi: 10.13189/app.2015.030302
32. Leigh S, Ashall-Payne L. The role of health-care providers in mHealth adoption. Lancet Digital Health. (2019) 1:e58–9. doi: 10.1016/S2589-7500(19)30025-1
33. Santo K, Richtering SS, Chalmers J, Thiagalingam A, Chow CK, Redfern J. Mobile phone apps to improve medication adherence: a systematic stepwise process to identify high-quality apps. JMIR mHealth uHealth. (2016) 4:e132. doi: 10.2196/mhealth.6742
34. Santo K, Chow CK, Thiagalingam A, Rogers K, Chalmers J, Redfern J. MEDication reminder APPs to improve medication adherence in Coronary Heart Disease (MedApp-CHD) Study: a randomised controlled trial protocol. BMJ Open. (2017) 7:e017540. doi: 10.1136/bmjopen-2017-017540
35. Ahmed A, Ahmad NS, Ali S, Ali S, George A, Saleem Danish H, et al. Medication adherence apps: review and content analysis. JMIR mHealth uHealth. (2018) 6:e62. doi: 10.2196/mhealth.6432
36. Stoyanov SR, Hides L, Kavanagh DJ, Wilson H. Development and validation of the user version of the mobile application rating scale (uMARS). JMIR mHealth uHealth. (2016) 4:e72. doi: 10.2196/mhealth.5849
37. Stoyanov SR, Hides L, Kavanagh DJ, Zelenko O, Tjondronegoro D, Mani M. Mobile app rating scale: a new tool for assessing the quality of health mobile apps. JMIR mHealth uHealth. (2015) 3:e27. doi: 10.2196/mhealth.3422
38. Domnich A, Arata L, Amicizia D, Signori A, Patrick B, Stoyanov S, et al. Development and validation of the Italian version of the Mobile Application Rating Scale and its generalisability to apps targeting primary prevention. BMC Med Informat Decision Making. (2016) 16:83. doi: 10.1186/s12911-016-0323-2
39. Haute Autorité de Santé (HAS). Assessment and Improvement of Practice. Good Practice Guidelines on Health Apps and Smart Devices (Mobile Health or MHealth), Saint Denis: Haute Autorité de Santé (2016).
40. Loy JS, Ali EE, Yap KY. Quality assessment of medical apps that target medication-related problems. J Manag Care Specialty Pharm. (2016) 22:1124–40. doi: 10.18553/jmcp.2016.22.10.1124
41. US Food and Drug Administration (FDA). Policy for Device Software Functions and Mobile Medical Applications. Rockville, MD: US Department of Health and Human Services (2015).
42. Yasini M, Beranger J, Desmarais P, Perez L, Marchand G. mHealth quality: a process to seal the qualified mobile health apps. Stud Health Technol Informat. (2016) 228:205–9. doi: 10.3233/978-1-61499-678-1-205
45. Biogaran. Lancement Medi'Rappel. Available online at: https://play.google.com/store/apps/details?id=com.biogaran.medirappel&hl=fr&gl=US
46. Anderson K, Burford O, Emmerton L. Mobile health apps to facilitate self-care: a qualitative study of user experiences. PLoS ONE. (2016) 11:e0156164. doi: 10.1371/journal.pone.0156164
Keywords: medication adherence, mHealth, eHealth, chronic diseases, pharmacists, app evaluation, healthcare provider
Citation: Backes C, Moyano C, Rimaud C, Bienvenu C and Schneider MP (2021) Digital Medication Adherence Support: Could Healthcare Providers Recommend Mobile Health Apps? Front. Med. Technol. 2:616242. doi: 10.3389/fmedt.2020.616242
Received: 11 October 2020; Accepted: 29 December 2020;
Published: 17 February 2021.
Edited by:
Joao Almeida Fonseca, Universidade Do Porto, PortugalReviewed by:
Guenka Ivanova Petrova, Medical University Sofia, BulgariaGarrett Greene, Royal College of Surgeons in Ireland, Ireland
Copyright © 2021 Backes, Moyano, Rimaud, Bienvenu and Schneider. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marie P. Schneider, TWFyaWUuU2NobmVpZGVyQHVuaWdlLmNo
 Claudine Backes
Claudine Backes Carla Moyano2
Carla Moyano2 Camille Rimaud
Camille Rimaud