- 1AIDS Research Center, National Institute of Infectious Diseases, Tokyo, Japan
- 2Department of AIDS Vaccine, The Institute of Medical Science, The University of Tokyo, Tokyo, Japan
Neutralizing antibody (NAb) responses are promising immune effectors for control of human immunodeficiency virus (HIV) infection. Protective activity and mechanisms of immunodeficiency virus-specific NAbs have been increasingly scrutinized in animals infected with simian immunodeficiency virus (SIV), chimeric simian/human immunodeficiency virus (SHIV) and related viruses. Studies on such models have unraveled a previously underscored protective potential against in vivo immunodeficiency virus replication. Pre-challenge NAb titers feasibly provide sterile protection from SIV/SHIV infection by purging the earliest onset of viral replication and likely modulate innate immune cell responses. Sufficient sub-sterile NAb titers after established infection also confer dose-dependent reduction of viremia, and in certain earlier time frames augment adaptive immune cell responses and even provide rebound-free viral control. Here, we provide an overview of the obtained patterns of SIV/SHIV protection and viral control by various types of NAb passive immunizations and discuss how these notions may be extrapolated to NAb-based clinical control of HIV infection.
Introduction
Viral infections overcoming the host sentinel hurdles of cell-intrinsic and innate immune responses are met with well-concerted adaptive immune responses. Adaptive immune responses comprise cellular and humoral effectors, and the central players for each are CD8+ cytotoxic T lymphocytes (CTLs) targeting infected cells and neutralizing antibodies (NAbs) targeting viral envelopes (Envs). Normally, a combinational response of these two effectors, initiated and assisted by professional antigen-presenting cells (APCs) such as dendritic cells (DCs) and CD4+ helper T lymphocytes (Th), effectively kill and neutralize infected cells and cell-free virus, respectively, resulting in elimination of virus from the infected host.
Unfitting such an optimal course, CCR5+ (R5) memory CD4+ T cell-tropic (R5-tropic) human immunodeficiency virus (HIV) and pathogenic simian immunodeficiency virus (SIV) infections are met with inefficient adaptive immune responses, resulting in persistent viral replication (Figure 1A). CTL responses play a still incomplete yet central role in primary resolution of viremia (Goulder and Watkins, 2008), whereas there is a more major impairment in early NAb responses in typical HIV/SIV infections (Tomaras et al., 2008). Delayed HIV/SIV-specific NAb induction also accompanies very distinct traits, contrasting other viral infections; firstly, germinal center formation itself is delayed for more than 1 month (Levesque et al., 2009; Peruchon et al., 2009). Approximately past 3 months post-infection NAbs appear, which is severely delayed, and they repetitively succumb to viral escape (Richman et al., 2003). Along this course of NAb development, cross-reactivity against other HIV strains is gradually acquired by NAbs at about year 1 post-infection (Mikell et al., 2011). Later on, certain rare patients further proceed to eliciting NAbs showing very extensive cross-reactivity, which became defined as broadly neutralizing antibodies (bNAbs) (Burton et al., 1994). These bNAbs can now be identified and characterized by single-cell B-cell receptor cloning (Scheid et al., 2009).
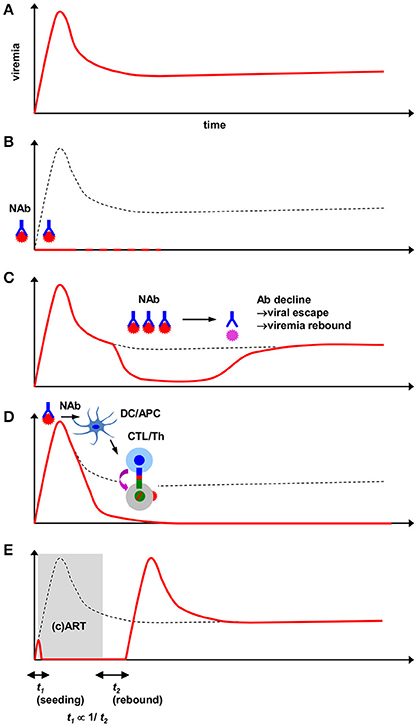
Figure 1. Viremia patterns in HIV/SIV/SHIV-infected animals receiving interventions including passive NAbs. (A) Course of persistent viremia in naïve infection. (B) Sterile protection against HIV/SIV/SHIV challenge by pre-challenge NAbs. Depending on the type of antibody, anti-HIV bNAbs can provide such protection at modest serum titers. Superacute-phase (1–10d) NAbs similarly mediate elimination of viral reservoirs. Complete elimination of virus (dotted lines) may be evaluated by experimental interventions such as CD8+-cell depletion. (C) Viremia reduction by subacute-phase post-challenge NAbs. An antibody dose-dependent attainment of viral load reduction (generally 10–1000-fold) is observed. Pharmacological decline of NAb titers and subsequent epitope-specific viral NAb escape results in rebound of viremia. (D) Early passive NAb-mediated sustained SIV viremia control by augmented virus-specific T-cell responses. NAb administration near peak viremia provides viral accumulation in DCs and functional augmentation in CTLs(/Th). Depending on combination of host/virus, B-cell responses are alternatively augmented. Neutralizing activity is critical for this modulation. (E) Antiretroviral therapy-based transient suppression of SIV viremia for comparison with NAb-based viremia suppression. Early in vivo viral dissemination (proportionate to t1) inversely correlates with the time to post-therapy cessation viremia rebound (t2). Judged from the uniform outcome of viral rebound, antiretroviral therapy likely does not modulate endogenous host immune responses.
NAb absence in early infection conversely suggests the importance of identifying anti-HIV antibody defense mechanisms as well as induction strategies. A practical approach for mechanistically analyzing NAb-based HIV control is antibody passive immunization. Here, recent progress on antibody passive immunization experiments in several types of animal AIDS models will be discussed (Table 1).
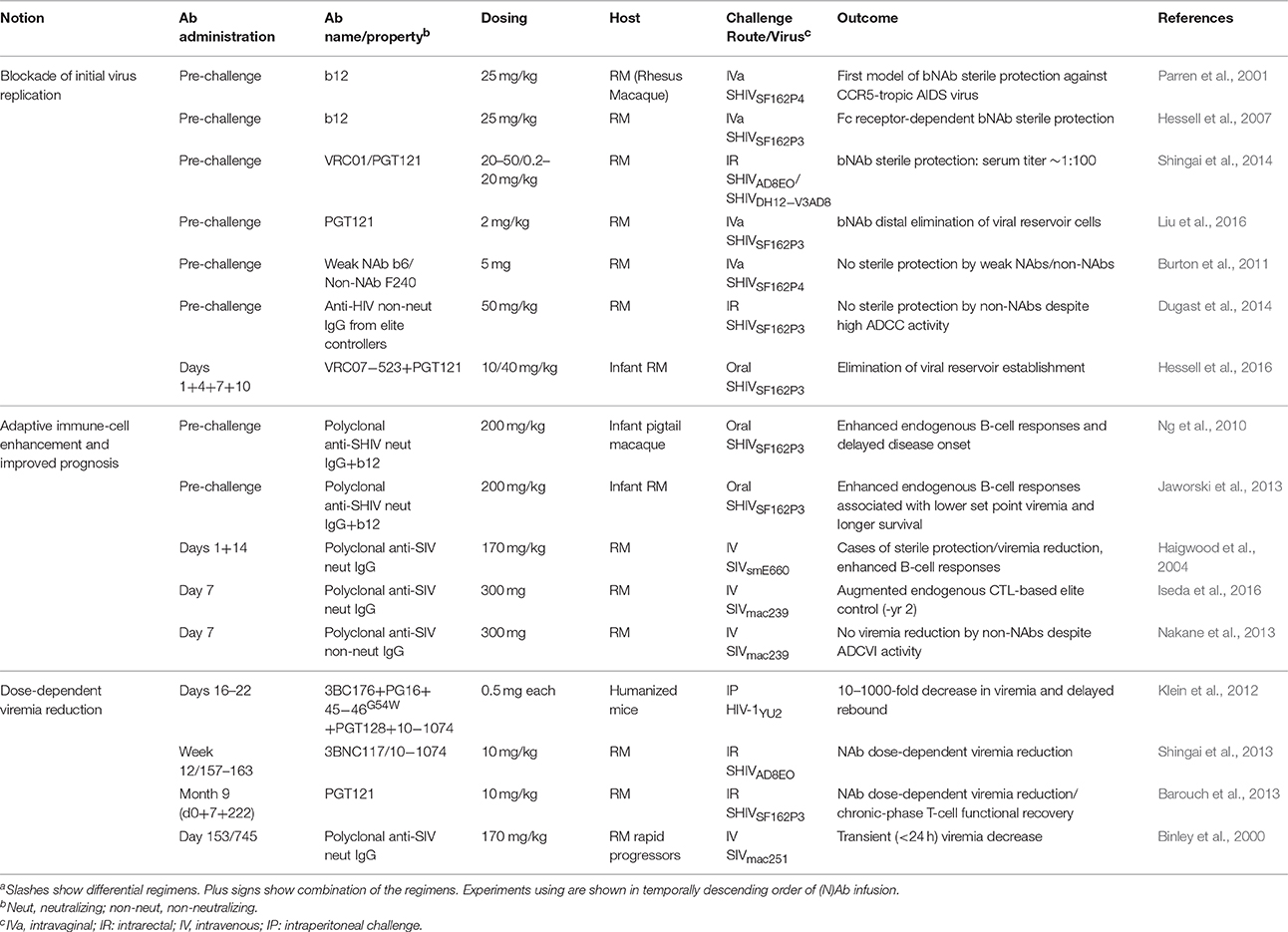
Table 1. Anti-HIV/SIV antibody passive immunization in CCR5-tropic immunodeficiency virus-infected animalsa.
Sterile Protection and Viremia Suppression by Passive NAbs
The initial interest in the field of HIV antibodies was whether pre-challenge NAbs may actually provide sterile protection against the incoming virus. Early implications had been obtained in a report on SIV-challenged cynomolgus macaques (Putkonen et al., 1991), while viral quantitation (and thus evaluation of protectivity) was relatively suboptimal at that time. Later work demonstrated NAb sterile protection against CXCR4-tropic (X4-tropic) HIV in human lymphocyte-reconstituted immunodeficient mice (Gauduin et al., 1997) and X4-tropic SHIV (X4-SHIV) challenge in pigtail macaques (Shibata et al., 1999) and rhesus macaques (Mascola et al., 1999, 2000; Baba et al., 2000). Further analysis on X4-SHIV models proposed theoretical requisite NAb titers (Nishimura et al., 2002) and also showed that the temporal window period permitting NAb-mediated sterile protection was very stringent (<24 h) (Nishimura et al., 2003).
Next addressed was the fundamental question on how NAbs may protect against the more difficult-to-protect CCR5-tropic (R5-tropic) immunodeficiency viruses which propagate more dominantly in vivo in the acute phase as transmitted/founder strains (Keele et al., 2008). An early challenging work (Parren et al., 2001) showed that, as like against X4-SHIVs, bNAb b12 with sufficient titers can exert complete protection against R5-tropic SHIV (R5-SHIV) challenge (Table 1 and Figure 1B). This concept of R5-tropic virus sterile protection was further confirmed by several combinations of bNAbs and viral challenge routes (Veazey et al., 2003; Hessell et al., 2009a,b, 2010). While it was first speculated that attainment of such sterile protection-conferring titers was rather difficult, recent studies have suggested that depending on the choice and potency of the passive bNAb, sterile protection may well be a feasible goal (Rudicell et al., 2014; Shingai et al., 2014). Another related new study has discovered that the biological half-life of the bNAbs determine the longevity of sterile protection against repeated SHIV challenges afforded by a single administration (Gautam et al., 2016). Based on the robust HIV/SIV-blocking efficacy in vitro as well as theoretically maximal “breadth,” interest had also been taken in blocking the CD4 entry receptor itself by antibodies, while recently it was shown that straightforwardly blocking the viral Env by bNAbs was more effective (Pegu et al., 2014).
Alternatively, it was also found that polyclonal neutralizing IgG existing at viral challenge can confer slower disease onset as well as enhanced survival in orally R5-SHIV-infected newborn macaques, suggestive of a lasting protective effect by preexisting NAb titers (Ng et al., 2010; Jaworski et al., 2013).
Extended from these, recently it was found that even in established chronic-phase R5-SHIV infection (post-set point), potent anti-HIV bNAbs at high titers can also provide viremia suppression (Barouch et al., 2013; Shingai et al., 2013) (Table 1 and Figure 1C). In such models, plasma bNAb titers, viral suppression and rebound basically are linked in a pharmacological manner; i.e., decline in NAb titers results in viral escape and viremia recrudescence. Furthermore, a recent report of HIV viremia suppression in stably infected (~20 days) humanized mice (Klein et al., 2012) (Table 1) showed that, compared with bNAb monotherapy, tri-mix or penta-mix administration of bNAbs resulted in a much sharper decline in viremia as well as delay in viremia rebound; i.e., an increase in the number of simultaneous Env targeting resulted in additive and/or synergistic containment of viral replication. This result also provided a rationale of inducing and/or administering multiple epitope-specific (b) NAbs for maximal efficacy. Thus, pre-challenge NAb titers do provide sterile protection against AIDS virus challenge and viremia suppression can also be transiently attained by sufficient post-challenge NAb titers.
Innate Immune-Cell Boosting by Passive NAbs
How passive Ab administration may affect endogenous immune responses has been a long-standing important question. In the report on R5-SHIV sterile protection by b12 (Parren et al., 2001), the authors noted that NAbs, at sub-sterilizing titers, also derived cases of decreased viremia levels later on. Later, the group showed that the b12 sterile protection occurred in vivo in an Fc receptor- but not complement-dependent manner (Hessell et al., 2007), and associated with antibody-dependent cellular viral inhibition (ADCVI) (Forthal et al., 2001) activity in vitro. These innate (mainly natural killer) cell-dependent mechanisms were also explanatory of the above non-sterile protective effects. Another line of work showed engineered Fc receptor-binding properties of bNAb VRC01 being related with extended bioavailability, altered localization (antibody transcytosis) and improved protection against SHIV challenge (Ko et al., 2014). Recently, treatment-naïve HIV-infected patients manifested evidence for selective pressure by antibody-dependent cellular cytotoxicity (ADCC) even without exogenous Ab infusion (Chung et al., 2011), suggesting that augmenting this innate cell-dependent molecular/cellular axis may indeed be a promising strategy. Indeed, in HIV-1-infected humanized mice, enhanced binding against activating Fc receptors increased while its complete abrogation notably decreased bNAb-mediated viremia suppression (Bournazos et al., 2014), suggesting that innate cell modulation may also occur in established infection.
Adaptive Immune-Cell Boosting by Passive NAbs
Intimately linked with the above is how NAbs may influence adaptive cellular responses. In our SIV challenge-NAb passive immunization model, we found that early (day 7) passive polyclonal NAb infusion in SIVmac239-challenged rhesus macaques resulted in elevated myeloid DC-associated viral loads (Yamamoto et al., 2007), temporally followed by elevation of Gag-specific polyfunctional CD4+ T-cell responses and increased in vitro viral suppressive activity in CD8+ cells (Yamamoto et al., 2009) (Table 1 and Figure 1D). Extended from such findings, in this model we recently identified that these NAb-mediated CD8+ cells also acquired enhanced suppressive activity against a panel of immunodominant CTL escape mutants, providing stringent T cell-based SIV control for up to 2 years without accumulation of viral CTL escape mutations (Iseda et al., 2016). This poses a possibility that the total virus-specific CTL population in NAb-infused animals became resistant against arousal of SIVs with CTL escape mutations in vivo, contributing to the prevention of CTL escape mutation accumulation. This early CTL “functional broadening” in NAb-infused macaques also may be related with the identified direct DC-mediated CTL cross-priming activity of the infused NAbs, suggestive of Ab/APC-dependent epitope spreading. Upon attainment of this stable SIV control, the originally immunodominant epitope-specific CTLs became preserved (presumably due to early CTL broadening) and showed stimuli-specific metabolic quiescence, as defined by enrichment of a phosphorylated AMP kinase-low CTL subpopulation which is indicative of exhaustion-free T-cell qualitative preservation (Blagih et al., 2015). Thus, NAb-boosted T cell-based primary SIV control also secondarily results in functional preservation of the most potent CTLs, which in turn may have further stabilized the viral control.
The above results collectively well explained SIV control in the animals, i.e., by passive NAb-mediated improved acute-phase CD4+/CD8+ T-cell priming. Alternatively, in another two-dose (days 1 plus 14) polyclonal NAb infusion model, cases of sterile protection and set-point viremia reduction were obtained in SIVsmE660-challenged macaques, and this associated with enhanced endogenous de novo NAb responses (Haigwood et al., 1996, 2004). These similar phenotypes triggering different endogenous immune effector responses may stem from the different properties of the challenge virus strain (i.e., SIVmac239 is highly NAb-resistant and induces NAb responses only rarely and in the chronic phase). Importantly, this pattern of synergism between endogenous adaptive immune cells and early short-term NAb administration has also become well-conceptualized in murine retrovirus-infected mice (Gros et al., 2008; Michaud et al., 2010; Nasser et al., 2010), further emphasizing the importance of actively modifying T-cell and/or B-cell responses through coexisting NAbs.
In the aforementioned chronic-phase SHIV viremia suppression study (Barouch et al., 2013), bNAb infusion in macaques similarly provided an increase in CTL viral suppressive activity. Given that virus-specific CTLs show considerable functional exhaustion due to antigen load in chronic infection (Streeck et al., 2008), this post-NAb infusion increase in viral suppression may reflect a functional recovery, and hence may also involve viremia decrease itself in the recovery process.
Another well-designed AIDS model utilizes infant macaques to evaluate the direct impact of NAbs on impeding pathogenic progression with a clear-cut disease phenotype. Here, pre-challenge polyclonal and monoclonal NAbs conferred enhanced endogenous B-cell responses against oral R5-SHIV challenge and protection against disease onset (Ng et al., 2010; Jaworski et al., 2013). This may be a consequence of CD4+ T-cell protection, or due to some pattern of indirect B-cell modulation, such as NAb-mediated Env antigenic modulation (Schoofs et al., 2016). Conversely, late-phase polyclonal NAb infusion in immunocompromised SIV-infected rapid progressors showed no protective effect (Binley et al., 2000), suggesting the importance of intact endogenous immunity for NAb-mediated viral suppression. Taken together, results collectively show that NAbs provide a wide spectrum of protective mechanisms in vivo, particularly those involving Fc receptors (Lambour et al., 2016), against AIDS virus replication.
Comparison between Passive NAbs and Drug Therapy
How passive NAbs, which indeed are soluble effectors, may be pharmacologically compared for its impact with (combined) antiretroviral therapy [(c)ART] is another important point. One work on the effect of acute-phase ART on SIV-infected rhesus macaques (Kubo et al., 2009) showed that initiation of ART as early as day 2 (and up to day 28) still does not eliminate in vivo virus, as demonstrated by rapid rebound of viremia. Another further systematic analysis on temporal establishment of in vivo viral reservoir seeding showed that past day 3 post-SIV challenge, the time until initiation of cART is proportionate to the speed of viremia rebound after therapy cessation (Whitney et al., 2014) (Figure 1E). This implicates that the acknowledged notion of “hit early and hard” for drug therapy (Ho, 1995) also partially applies to the superacute phase, while attainment of complete viral eradication by ART is still an independent and extremely high final hurdle. This potentially may require host dispositions different from ones associated with elite HIV control such as possession of protective major histocompatibility complex class I (MHC-I) alleles, as implicated in the VISCONTI study in which early cART-treated patients with no viral rebound did not possess them (Sáez-Cirión et al., 2013).
In stark contrast, a recent important report showed that superacute-phase (days 1–10) administration of bNAbs (Hessell et al., 2016) results in an elimination in virus-detectable tissue compartments throughout the body for 6 months. Another cutting-edge report (Liu et al., 2016) tracked previously underscored post-infection viral replication in situ deriving transcriptomic signatures of antiviral gene up-regulation in infected foci (Barouch et al., 2016), and its abortion by in vivo titers of bNAb PGT121 at viral challenge. These two essentially may highlight a common notion of literally purging the eclipse phase of initial viral infection/dissemination by NAbs. It remains to be clarified whether the slightly earlier moment of NAb infusion derives the protection not obtained by early cART, or if other effector functions of NAbs unavailable by drugs mediate the protective effect. Our model of an acute-phase single NAb infusion resulting in sustained SIV control (Iseda et al., 2016) is most suggestive for the latter. Implications are also provided by a report showing that a combined bNAb single shot at day 10 post-SHIV infection derives protective effects substituting and comparable to that of a daily ART regimen for the next 11 days (Bolton et al., 2016). Taken together, NAbs do appear to exert protective effects unavailable by antiretroviral therapy.
Requisite of Neutralizing Activity in Passive Antibody-Based Viral Prevention and Control
Whether direct virus-neutralizing activity is required for antibody-mediated AIDS virus control is critical. A very important report showed that for attaining stringent sterile protection against R5-SHIV challenge, neutralizing activity is indispensable (Burton et al., 2011). This notion was confirmed by another group, which showed that regardless of possessing potent Fc effector functions [ADCC and antibody-dependent cellular phagocytosis (ADCP) (Pelegrin et al., 2015)], antibody sterile protection was not obtained by pre-infection non-NAbs (Dugast et al., 2014) (Table 1). Similarly, in acute-phase infection, polyclonal non-NAb infusion at day 7 achieved no SIV viremia control in our model (Nakane et al., 2013). We very recently compared viral suppressive activity in our aforementioned polyclonal NAb- and non-NAb-infused rhesus macaques, and found that CD8+-cell viral suppressive activity is selectively enhanced in NAb-infused but not in non-NAb-infused animals (Yamamoto et al., 2016). This means that in addition to the availability of direct virus neutralization, such property of antiviral antibodies may also directly affect modulation patterns of cellular immune responses and further impact disease prognosis.
Passive NAb Immunotherapy in HIV-Infected Humans
Based on recent characterization of various bNAbs, their application as immunotherapeutic agents has started to extensively proceed in human trials. Preceding this current trend, insights had been obtained by one early clinical trial (Trkola et al., 2005), which showed that earlier administration of bNAbs against HIV-infected humans resulted in more delayed viremia rebound in several patients. In addition to considerations on such endogenous baseline status of the patients to be treated, another current interest is how the previously monitored association of virological control and (b) NAb pharmacological properties can be extrapolated from mice and non-human primates to humans.
In one representative study, single administration of a well-characterized potent CD4 binding site-specific bNAb VRC01 to HIV-infected patients was reported to exert 1.1–1.8 log10 reduction in viremia (Lynch et al., 2015). This single injection also derived emergence of VRC01-resistant strains, suggesting the necessity of either modifying or combining the NAbs to be infused.
Administration of another extremely potent CD4 binding site-specific bNAb 3BNC117, at a modest dose (30 mg/kg), also resulted in significant 0.8–2.5 log10 reduction in viremia (Caskey et al., 2015). 3BNC117 administration in HIV-infected humans also resulted in suppression of viral rebound during cART interruption (Scheid et al., 2016), highlighting the feasibility of and strong selective pressure exerted by this bNAb infusion. Furthermore, 3BNC117 administration in HIV-infected viremic individuals resulted in altered endogenous B-cell responses, as analyzed by changes in viral Env and B-cell receptor phylogenetic polymorphisms (Schoofs et al., 2016).
In another cohort, administration of an Env variable region 3 (V3)-specific bNAb KD-247 resulted in decreased viremia in HIV-infected patients, as well as an obtained case of ongoing viremia control after NAb decline (Matsushita et al., 2015), showing that a V3-specific bNAb can also mediate viral suppression. This bNAb further holds promise for host protection in that it may give synergistic protective effects with CD4-mimetic chemical compounds (Yoshimura et al., 2010). This and other types of NAb/chemical compound synergisms (Yoshimura et al., 2014; Madani et al., 2016) may become important strategies, particularly in NAb-based immunotherapies.
Collectively, these reports are now starting to provide a proof-of-concept for the notions initially obtained in animal AIDS models of NAb passive immunization.
Concluding Remarks
NAbs, when present by passive immunization, are being recognized as capable of playing a central role in sterile protection against and post-infection control of SIV and SHIV infection. Future studies aiming for NAb immunotherapy-based HIV clinical control shall proceed with a pharmacological perspective on the in vivo spectrum/dosage as well as Fc-mediated effector functions of the (b) NAbs to be infused. For prophylactic vaccine induction of HIV-specific NAbs, in addition to the rational design of vaccine Env antigen, designing attainment of synergism with concomitantly induced T-cell responses may delineate protective responses much more potent than what is currently expected.
Author Contributions
HY conceived and drafted manuscript; TM co-drafted manuscript.
Funding
This work was supported by Japan Agency for Medical Research and Development, the Ministry of Health, Labor, and Welfare and the Ministry of Education, Culture, Sports, Science, and Technology in Japan ([JSPS] KAKENHI).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Baba, T. W., Liska, V., Hofmann-Lehmann, R., Vlasak, J., Xu, W., Ayehunie, S., et al. (2000). Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6, 200–206. doi: 10.1038/72309
Barouch, D. H., Ghneim, K., Bosche, W. J., Li, Y., Berkemeier, B., Hull, M., et al. (2016). Rapid inflammasome activation following mucosal SIV infection of Rhesus monkeys. Cell 165, 656–667. doi: 10.1016/j.cell.2016.03.021
Barouch, D. H., Whitney, J. B., Moldt, B., Klein, F., Oliveira, T. Y., Liu, J., et al. (2013). Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature 503, 224–228. doi: 10.1038/nature12744
Binley, J. M., Clas, B., Gettie, A., Vesanen, M., Montefiori, D. C., Sawyer, L., et al. (2000). Passive infusion of immune serum into simian immunodeficiency virus-infected rhesus macaques undergoing a rapid disease course has minimal effect on plasma viremia. Virology 270, 237–249. doi: 10.1006/viro.2000.0254
Blagih, J., Coulombe, F., Vincent, E. E., Dupuy, F., Galicia-Vázquez, G., Yurchenko, E., et al. (2015). The energy sensor AMPK regulates T cell metabolic adaptation and effector responses in vivo. Immunity 42, 41–54. doi: 10.1016/j.immuni.2014.12.030
Bolton, D. L., Pegu, A., Wang, K., McGinnis, K., Nason, M., Foulds, K., et al. (2016). Human immunodeficiency virus type 1 monoclonal antibodies suppress acute simian-human immunodeficiency virus viremia and limit seeding of cell-associated viral reservoirs. J. Virol. 90, 1321–1332. doi: 10.1128/JVI.02454-15
Bournazos, S., Klein, F., Pietzsch, J., Seaman, M. S., Nussenzweig, M. C., and Ravetch, J. V. (2014). Broadly neutralizing anti-HIV-1 antibodies require Fc effector functions for in vivo activity. Cell 158, 1243–1253. doi: 10.1016/j.cell.2014.08.023
Burton, D. R., Hessell, A. J., Keele, B. F., Klasse, P. J., Ketas, T. A., Moldt, B., et al. (2011). Limited or no protection by weakly or nonneutralizing antibodies against vaginal SHIV challenge of macaques compared with a strongly neutralizing antibody. Proc. Natl. Acad. Sci. U.S.A. 108, 11181–11186. doi: 10.1073/pnas.1103012108
Burton, D. R., Pyati, J., Koduri, R., Sharp, S. J., Thornton, G. B., Parren, P. W., et al. (1994). Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science 266, 1024–1027. doi: 10.1126/science.7973652
Caskey, M., Klein, F., Lorenzi, J. C., Seaman, M. S., West, A. P. Jr., Buckley, N., et al. (2015). Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature 522, 487–491. doi: 10.1038/nature14411
Chung, A. W., Isitman, G., Navis, M., Kramski, M., Center, R. J., Kent, S. J., et al. (2011). Immune escape from HIV-specific antibody-dependent cellular cytotoxicity (ADCC) pressure. Proc. Natl. Acad. Sci. U.S.A. 108, 7505–7510. doi: 10.1073/pnas.1016048108
Dugast, A. S., Chan, Y., Hoffner, M., Licht, A., Nkolola, J., Li, H., et al. (2014). Lack of protection following passive transfer of polyclonal highly functional low-dose non-neutralizing antibodies. PLoS ONE 9:e97229. doi: 10.1371/journal.pone.0097229
Forthal, D. N., Landucci, G., and Daar, E. S. (2001). Antibody from patients with acute human immunodeficiency virus (HIV) infection inhibits primary strains of HIV type 1 in the presence of natural-killer effector cells. J. Virol. 75, 6953–6961. doi: 10.1128/JVI.75.15.6953-6961.2001
Gauduin, M. C., Parren, P. W., Weir, R., Barbas, C. F., Burton, D. R., and Koup, R. A. (1997). Passive immunization with a human monoclonal antibody protects hu-PBL-SCID mice against challenge by primary isolates of HIV-1. Nat. Med. 3, 1389–1393. doi: 10.1038/nm1297-1389
Gautam, R., Nishimura, Y., Pegu, A., Nason, M. C., Klein, F., Gazumyan, A., et al. (2016). A single injection of anti-HIV-1 antibodies protects against repeated SHIV challenges. Nature 533, 105–109. doi: 10.1038/nature17677
Goulder, P. J. R., and Watkins, D. I. (2008). Impact of MHC class I diversity on immune control of immunodeficiency virus replication. Nat. Rev. Immunol. 8, 619–630. doi: 10.1038/nri2357
Gros, L., Pelegrin, M., Michaud, H. A., Bianco, S., Hernandez, J., Jacquet, C., et al. (2008). Endogenous cytotoxic T-cell response contributes to the long-term antiretroviral protection induced by a short period of antibody-based immunotherapy of neonatally infected mice. J. Virol. 82, 1339–1349. doi: 10.1128/JVI.01970-07
Haigwood, N. L., Montefiori, D. C., Sutton, W. F., McClure, J., Watson, A., Voss, G., et al. (2004). Passive immunotherapy in simian immunodeficiency virus-infected macaques accelerates the development of neutralizing antibodies. J. Virol. 78, 5983–5995. doi: 10.1128/JVI.78.11.5983-5995.2004
Haigwood, N. L., Watson, A., Sutton, W. F., McClure, J., Lewis, A., Ranchalis, J., et al. (1996). Passive immune globulin therapy in the SIV/macaque model: early intervention can alter disease profile. Immunol. Lett. 51, 107–114. doi: 10.1016/0165-2478(96)02563-1
Hessell, A. J., Hangartner, L., Hunter, M., Havenith, C. E., Beurskens, F. J., Bakker, J. M., et al. (2007). Fc receptor but not complement binding is important in antibody protection against HIV. Nature 449, 101–104. doi: 10.1038/nature06106
Hessell, A. J., Jaworski, J. P., Epson, E., Matsuda, K., Pandey, S., Kahl, C., et al. (2016). Early short-term treatment with neutralizing human monoclonal antibodies halts SHIV infection in infant macaques. Nat. Med. 22, 362–368. doi: 10.1038/nm.4063
Hessell, A. J., Poignard, P., Hunter, M., Hangartner, L., Tehrani, D. M., Bleeker, W. K., et al. (2009b). Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat. Med. 15, 951–954. doi: 10.1038/nm.1974
Hessell, A. J., Rakasz, E. G., Poignard, P., Hangartner, L., Landucci, G., Forthal, D. N., et al. (2009a). Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog. 5:e1000433. doi: 10.1371/journal.ppat.1000433
Hessell, A. J., Rakasz, E. G., Tehrani, D. M., Huber, M., Weisgrau, K. L., Landucci, G., et al. (2010). Broadly neutralizing monoclonal antibodies 2F5 and 4E10 directed against the human immunodeficiency virus type 1 gp41 membrane-proximal external region protect against mucosal challenge by simian-human immunodeficiency virus SHIVBa-L. J. Virol. 84, 1302–1313. doi: 10.1128/JVI.01272-09
Ho, D. D. (1995). Time to hit HIV, early and hard. N. Engl. J. Med. 333, 450–451. doi: 10.1056/NEJM199508173330710
Iseda, S., Takahashi, N., Poplimont, H., Nomura, T., Seki, S., Nakane, T., et al. (2016). Biphasic CD8+ T-cell defense in simian immunodeficiency virus control by acute-phase passive neutralizing antibody immunization. J. Virol. 90, 6276–6290. doi: 10.1128/JVI.00557-16
Jaworski, J. P., Kobie, J., Brower, Z., Malherbe, D. C., Landucci, G., Sutton, W. F., et al. (2013). Neutralizing polyclonal IgG present during acute infection prevents rapid disease onset in simian-human immunodeficiency virus SHIVSF162P3-infected infant rhesus macaques. J. Virol. 87, 10447–10459. doi: 10.1128/JVI.00049-13
Keele, B. F., Giorgi, E. E., Salazar-Gonzalez, J. F., Decker, J. M., Pham, K. T., Salazar, M. G., et al. (2008). Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl. Acad. Sci. U.S.A. 105, 7552–7557. doi: 10.1073/pnas.0802203105
Klein, F., Halper-Stromberg, A., Horwitz, J. A., Gruell, H., Scheid, J. F., Bournazos, S., et al. (2012). HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature 492, 118–122. doi: 10.1038/nature11604
Ko, S. Y., Pegu, A., Rudicell, R. S., Yang, Z. Y., Joyce, M. G., Chen, X., et al. (2014). Enhanced neonatal Fc receptor function improves protection against primate SHIV infection. Nature 514, 642–645. doi: 10.1038/nature13612
Kubo, M., Nishimura, Y., Shingai, M., Lee, W., Brenchley, J., Lafont, B., et al. (2009). Initiation of antiretroviral therapy 48 h after infection with simian immunodeficiency virus potently suppresses acute-phase viremia and blocks the massive loss of memory CD4+ T cells but fails to prevent disease. J. Virol. 83, 7099–7108. doi: 10.1128/JVI.02522-08
Lambour, J., Naranjo-Gomez, M., Piechaczyk, M., and Pelegrin, M. (2016). Converting monoclonal antibody-based immunotherapies from passive to active: bringing immune complexes into play. Emerg. Microbes Infect. 5:e92. doi: 10.1038/emi.2016.97
Levesque, M. C., Moody, M. A., Hwang, K. K., Marshall, D. J., Whitesides, J. F., Amos, J. D., et al. (2009). Polyclonal B cell differentiation and loss of gastrointestinal tract germinal centers in the earliest stages of HIV-1 infection. PLoS Med. 6:e1000107. doi: 10.1371/journal.pmed.1000107
Liu, J., Ghneim, K., Sok, D., Bosche, W. J., Li, Y., Chipriano, E., et al. (2016). Antibody-mediated protection against SHIV challenge includes systemic clearance of distal virus. Science 353, 1045–1049. doi: 10.1126/science.aag0491
Lynch, R. M., Boritz, E., Coates, E. E., DeZure, A., Madden, P., Costner, P., et al. (2015). Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Sci. Transl. Med. 7:319ra206. doi: 10.1126/scitranslmed.aad5752
Madani, N., Princiotto, A. M., Easterhoff, D., Bradley, T., Luo, K., Williams, W. B., et al. (2016). Antibodies elicited by multiple envelope glycoprotein immunogens in primates neutralize primary human immunodeficiency viruses (HIV-1) sensitized by CD4-mimetic compounds. J. Virol. 90, 5031–5046. doi: 10.1128/JVI.03211-15
Mascola, J. R., Lewis, M. G., Stiegler, G., Harris, D., VanCott, T. C., Hayes, D., et al. (1999). Protection of Macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol. 73, 4009–4018.
Mascola, J. R., Stiegler, G., VanCott, T. C., Katinger, H., Carpenter, C. B., Hanson, C. E., et al. (2000). Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6, 207–210. doi: 10.1038/72318
Matsushita, S., Yoshimura, K., Ramirez, K. P., Pisupati, J., Murakami, T., and KD-1002 Study Group (2015). Passive transfer of neutralizing mAb KD-247 reduces plasma viral load in patients chronically infected with HIV-1. AIDS 29, 453–462. doi: 10.1097/QAD.0000000000000570
Michaud, H. A., Gomard, T., Gros, L., Thiolon, K., Nasser, R., Jacquet, C., et al. (2010). A crucial role for infected-cell/antibody immune complexes in the enhancement of endogenous antiviral immunity by short passive immunotherapy. PLoS Pathog. 6:e1000948. doi: 10.1371/journal.ppat.1000948
Mikell, I., Sather, D. N., Kalams, S. A., Altfeld, M., Alter, G., and Stamatatos, L. (2011). Characteristics of the earliest cross-neutralizing antibody response to HIV-1. PLoS Pathog. 7:e1001251. doi: 10.1371/journal.ppat.1001251
Nakane, T., Nomura, T., Shi, S., Nakamura, M., Naruse, T. K., Kimura, A., et al. (2013). Limited impact of passive non-neutralizing antibody immunization in acute SIV infection on viremia control in rhesus macaques. PLoS ONE 8:e73453. doi: 10.1371/journal.pone.0073453
Nasser, R., Pelegrin, M., Michaud, H. A., Plays, M., Piechaczyk, M., and Gros, L. (2010). Long-lasting protective antiviral immunity induced by passive immunotherapies requires both neutralizing and effector functions of the administered monoclonal antibody. J. Virol. 84, 10169–10181. doi: 10.1128/JVI.00568-10
Ng, C. T., Jaworski, J. P., Jayaraman, P., Sutton, W. F., Delio, P., Kuller, L., et al. (2010). Passive neutralizing antibody controls SHIV viremia and enhances B cell responses in infant macaques. Nat. Med. 16, 1117–1119. doi: 10.1038/nm.2233
Nishimura, Y., Igarashi, T., Haigwood, N. L., Sadjadpour, R., Donau, O. K., Buckler, C., et al. (2003). Transfer of neutralizing IgG to macaques 6 h but not 24 h after SHIV infection confers sterilizing protection: implications for HIV-1 vaccine development. Proc. Natl. Acad. Sci. U.S.A. 100, 15131–15136. doi: 10.1073/pnas.2436476100
Nishimura, Y., Igarashi, T., Haigwood, N., Sadjadpour, R., Plishka, R. J., Buckler-White, A., et al. (2002). Determination of a statistically valid neutralization titer in plasma that confers protection against simian-human immunodeficiency virus challenge following passive transfer of high-titered neutralizing antibodies. J. Virol. 76, 2123–2130. doi: 10.1128/jvi.76.5.2123-2130.2002
Parren, P. W., Marx, P. A., Hessell, A. J., Luckay, A., Harouse, J., Cheng-Mayer, C., et al. (2001). Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J. Virol. 75, 8340–8347. doi: 10.1128/JVI.75.17.8340-8347.2001
Pegu, A., Yang, Z. Y., Boyington, J. C., Wu, L., Ko, S. Y., Schmidt, S. D., et al. (2014). Neutralizing antibodies to HIV-1 envelope protect more effectively in vivo than those to the CD4 receptor. Sci. Transl. Med. 6:243ra88. doi: 10.1126/scitranslmed.3008992
Pelegrin, M., Naranjo-Gomez, M., and Piechaczyk, M. (2015). Antiviral monoclonal antibodies: can they be more than simple neutralizing agents? Trends Microbiol. 23, 653–665. doi: 10.1016/j.tim.2015.07.005
Peruchon, S., Chaoul, N., Burelout, C., Delache, B., Brochard, P., Laurent, P., et al. (2009). Tissue-specific B-cell dysfunction and generalized memory B-cell loss during acute SIV infection. PLoS ONE 4:e5966. doi: 10.1371/journal.pone.0005966
Putkonen, P., Thorstensson, R., Ghavamzadeh, L., Albert, J., Hild, K., Biberfeld, G., et al. (1991). Prevention of HIV-2 and SIVsm infection by passive immunization in cynomolgus monkeys. Nature 352, 436–438. doi: 10.1038/352436a0
Richman, D. D., Wrin, T., Little, S. J., and Petropoulos, C. J. (2003). Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl. Acad. Sci. U.S.A. 100, 4144–4149. doi: 10.1073/pnas.0630530100
Rudicell, R. S., Kwon, Y. D., Ko, S. Y., Pegu, A., Louder, M. K., Georgiev, I. S., et al. (2014). Enhanced potency of a broadly neutralizing HIV-1 antibody in vitro improves protection against lentiviral infection in vivo. J. Virol. 88, 12669–12682. doi: 10.1128/JVI.02213-14
Sáez-Cirión, A., Bacchus, C., Hocqueloux, L., Avettand-Fenoel, V., Girault, I., Lecuroux, C., et al. (2013). Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 9:e1003211. doi: 10.1371/journal.ppat.1003211
Scheid, J. F., Horwitz, J. A., Bar-On, Y., Kreider, E. F., Lu, C. L., Lorenzi, J. C., et al. (2016). HIV-1 antibody 3BNC117 suppresses viral rebound in humans during treatment interruption. Nature 535, 556–560. doi: 10.1038/nature18929
Scheid, J. F., Mouquet, H., Feldhahn, N., Seaman, M. S., Velinzon, K., Pietzsch, J., et al. (2009). Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature 458, 636–640. doi: 10.1038/nature07930
Schoofs, T., Klein, F., Braunschweig, M., Kreider, E. F., Feldmann, A., Nogueira, L., et al. (2016). HIV-1 therapy with monoclonal antibody 3BNC117 elicits host immune responses against HIV-1. Science 352, 997–1001. doi: 10.1126/science.aaf0972
Shibata, R., Igarashi, T., Haigwood, N., Buckler-White, A., Ogert, R., Ross, W., et al. (1999). Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat. Med. 5, 204–210. doi: 10.1038/5568
Shingai, M., Donau, O. K., Plishka, R. J., Buckler-White, A., Mascola, J. R., Nabel, G. J., et al. (2014). Passive transfer of modest titers of potent and broadly neutralizing anti-HIV monoclonal antibodies block SHIV infection in macaques. J. Exp. Med. 211, 2061–2074. doi: 10.1084/jem.20132494
Shingai, M., Nishimura, Y., Klein, F., Mouquet, H., Donau, O. K., Plishka, R., et al. (2013). Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature 503, 277–280. doi: 10.1038/nature12746
Streeck, H., Brumme, Z. L., Anastario, M., Cohen, K. W., Jolin, J. S., Meier, A., et al. (2008). Antigen load and viral sequence diversification determine the functional profile of HIV-1-specific CD8+ T cells. PLoS Med. 5:e100. doi: 10.1371/journal.pmed.0050100
Tomaras, G. D., Yates, N. L., Liu, P., Qin, L., Fouda, G. G., Chavez, L. L., et al. (2008). Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J. Virol. 82, 12449–12463. doi: 10.1128/JVI.01708-08
Trkola, A., Kuster, H., Rusert, P., Joos, B., Fischer, M., Leemann, C., et al. (2005). Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat. Med. 11, 615–622. doi: 10.1038/nm1244
Veazey, R. S., Shattock, R. J., Pope, M., Kirijan, J. C., Jones, J., Hu, Q., et al. (2003). Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat. Med. 9, 343–346. doi: 10.1038/nm833
Whitney, J. B., Hill, A. L., Sanisetty, S., Penaloza-MacMaster, P., Liu, J., Shetty, M., et al. (2014). Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature 512, 74–77. doi: 10.1038/nature13594
Yamamoto, H., Iseda, S., Nakane, T., Nomura, T., Takahashi, N., Seki, S., et al. (2016). Augmentation of anti-simian immunodeficiency virus activity in CD8+ cells by neutralizing but not nonneutralizing antibodies in the acute phase. AIDS 30, 2391–2394. doi: 10.1097/QAD.0000000000001221
Yamamoto, H., Kawada, M., Takeda, A., Igarashi, H., and Matano, T. (2007). Post-infection immunodeficiency virus control by neutralizing antibodies. PLoS ONE 2:e540. doi: 10.1371/journal.pone.0000540
Yamamoto, T., Iwamoto, N., Yamamoto, H., Tsukamoto, T., Kuwano, T., Takeda, A., et al. (2009). Polyfunctional CD4+ T-cell induction in neutralizing antibody-triggered control of simian immunodeficiency virus infection. J. Virol. 83, 5514–5524. doi: 10.1128/JVI.00145-09
Yoshimura, K., Harada, S., Boonchawalit, S., Kawanami, Y., and Matsushita, S. (2014). Impact of maraviroc-resistant and low-CCR5-adapted mutations induced by in vitro passage on sensitivity to anti-envelope neutralizing antibodies. J. Gen. Virol. 95, 1816–1826. doi: 10.1099/vir.0.062885-0
Keywords: HIV, SIV, neutralizing antibodies, passive immunization, CD8+ T cells
Citation: Yamamoto H and Matano T (2016) Patterns of HIV/SIV Prevention and Control by Passive Antibody Immunization. Front. Microbiol. 7:1739. doi: 10.3389/fmicb.2016.01739
Received: 15 September 2016; Accepted: 18 October 2016;
Published: 02 November 2016.
Edited by:
Akio Adachi, Tokushima University, JapanReviewed by:
Takamasa Ueno, Kumamoto University, JapanBernard A. P. Lafont, National Institute of Allergy and Infectious Diseases, USA
Takeo Kuwata, Kumamoto University Hospital, Japan
Copyright © 2016 Yamamoto and Matano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hiroyuki Yamamoto, aC15YW1hdG9AbmloLmdvLmpw
 Hiroyuki Yamamoto
Hiroyuki Yamamoto Tetsuro Matano
Tetsuro Matano