- 1Institute of Edible Fungi, Shanghai Academy of Agricultural Sciences, National Engineering Research Center of Edible Fungi, Shanghai, China
- 2Engineering Research Centre of Chinese Ministry of Education for Edible and Medicinal Fungi, Jilin Agricultural University, Changchun, China
- 3Biotechnology Research Institute, Shanghai Academy of Agricultural Sciences, Shanghai, China
Lentinula edodes, also known as Xiang’gu, is commonly eaten in cultures around the world. However, L. edodes is particularly susceptible to enrichment with heavy metals, particularly cadmium (Cd), which is toxic to human health. Understanding the molecular mechanism and mining key genes involved in Cd enrichment will facilitate genetic modification of L. edodes strains. Two L. edodes genotypes, Le4625 (with higher Cd enrichment capability) and Le4606 (with lower Cd enrichment capability) were used in this study. The Cd concentrations in the mycelia of the tested genotypes differed significantly after Cd (0.1 mg/L) exposure; and the Cd content in Le4625 (1.390 ± 0.098 mg/kg) was approximately three-fold that in Le4606 (0.440 ± 0.038 mg/kg) after 7 h of Cd exposure. A total of 24,592 transcripts were assessed by RNA-Seq to explore variance in Cd accumulation. Firstly, differentially expressed genes (DEGs) were analyzed separately following Cd exposure. In comparison with Ld4625, Ld4606 showed a greater number of Cd-induced changes in transcription. In Ld4606, DEGs following Cd exposure were associated with transmembrane transport, glutathione transfer and cytochrome P450, indicating that these genes could be involved in Cd resistance in L. edodes. Next, Le4606 and Le4625 were exposed to Cd, after which DEGs were identified to explore genetic factors affecting Cd accumulation. After Cd exposure, DEGs between Le4606 and Le4625 encoded proteins involved in multiple biological pathways, including transporters on the membrane, cell wall modification, oxidative stress response, translation, degradation, and signaling pathways. Cadmium enrichment in cells may activate MAPK signaling and the anti-oxidative stress response, which can subsequently alter signal transduction and the intracellular oxidation/reduction balance. Furthermore, several possible candidate genes involved in the Cd accumulation were identified, including the major facilitator superfamily genes, heat shock proteins, and laccase 11, a multicopper oxidase. This comparison of the transcriptomes of two L. edodes strains with different capacities for Cd accumulation provides valuable insight into the cultivation of mushrooms with less Cd enrichment and also serves as a reference for the construction of engineered strains for environmental pollution control.
Introduction
Lentinula edodes, known as Xiang’gu in China and Shiitake in Japan, is gaining popularity because of its high nutritional and medicinal value. L. edodes is rich in essential amino acids, vitamins, and minerals (Chang, 1980), and it contains many pharmaceutical compounds with antibacterial, antioxidant, and immune-enhancing properties (Cao et al., 2013; Dai et al., 2015). L. edodes is one of the most economically important cultivated mushrooms, and China is the largest producer and exporter in the world, with an output of 10.43 million tons in 2018 (China Edible Fungi Association [CEFA], 2019).
Heavy metals efficiently accumulate in various edible mushrooms (Liu et al., 2015). Cadmium (Cd) is one of the most toxic heavy metals and may decrease intelligence quotients in children (Khani et al., 2017). Cadmium combines with sulfhydryl groups in proteins and inhibits enzyme activity (Thomet et al., 1999), affecting the cardiovascular system and kidneys (Khani et al., 2017). As Cd can accumulate over the lifetime of an organism (ATSDR, 2007), even a small overdose of Cd can be hazardous to human health. L. edodes is readily enriched in Cd (Hatvani and Mécs, 2003), and its high Cd content may adversely affect human health (Mleczek et al., 2017).
Extrinsic and intrinsic factors can affect the accumulation of heavy metals in edible mushrooms. The cultivation medium is the main external factor affecting heavy metal accumulation (Huang et al., 2015; Wang et al., 2016). Mushrooms can produce various organic acids and active enzymes to degrade organic matter, leading to the release of heavy metals, and increasing their absorption. Additionally, trace metal concentrations vary among mushroom species, indicating that genetics are a major intrinsic factor influencing heavy metal accumulation (Huang et al., 2015). Mleczek et al. (2018) analyzed trace elements in 14 mushrooms and found that the contents of toxic deleterious elements varied widely among different mushroom genotypes.
The accumulation of heavy metals (particularly Cd) in fungi has been extensively studied. Some studies report that essential metal ions are replaced by heavy metals through ion exchange (Zadrazil and Brunnert, 1985). In addition, heavy metals are enriched through biosorption processes, such as the complexation of heavy metals with biological macromolecular active groups in cells (Plaza et al., 1996; Marin et al., 1997). For example, metallothionein, which is common in edible mushrooms, may complex with heavy metals, leading to heavy metal accumulation (Qin, 2016). Additionally, active groups on the cell wall, such as sulfhydryl, carboxyl, and hydroxyl groups may react with heavy metal ions to form insoluble substances and deposit heavy metals via physical adsorption or the formation of inorganic precipitates (Xu et al., 2001).
Proteomic and genomic methods have been used to identify key proteins involved in Cd accumulation by L. edodes (Zhao et al., 2015; Chen et al., 2016; Dong et al., 2019; Yoo et al., 2019). Although certain oxidases and metallothionein are known to play roles in Cd metabolism, the precise molecular mechanisms underlying Cd accumulation in mushrooms remains unclear. Knowledge of the expression patterns of Cd-related genes in different strains of L. edodes following Cd exposure could facilitate the development of improved edible strains with less heavy metal content, as well as strains that readily absorb Cd and could be used for environmental remediation of heavy metal pollution. To investigate Cd response mechanisms in L. edodes, two L. edodes genotypes with different Cd enrichment capabilities, Le4606 (low enrichment capacity for Cd) and Le4625 (high enrichment capacity for Cd), were cultivated and exposed to Cd, after which RNA-Seq was performed to identify genes involved in Cd transport and metabolism.
Materials and Methods
L. edodes Mycelium Cultivation and Treatment
Lentinula edodes genotypesLe4606 and Le4625 were obtained from the Improved and Standardized Spawn Breeding Center at the Shanghai Academy of Agricultural Sciences (Shanghai, China). Equal doses of both genotypes were inoculated into 100 mL of potato-dextrose broth and cultivated for 7 days with shaking at 120 rpm (25°C). On day 7, CdCl2⋅2H2O was added to the medium to a final concentration of 0.1 mg/L, as a higher concentration is known to inhibit the growth of mycelium. After Cd exposure for 0, 0.5, and 7 h, mycelial samples were collected and frozen in liquid nitrogen for transcriptome sequencing (three repeats). The mycelium was washed with ddH2O and dried at 37°C, after which the dry weight of each sample was determined. The mycelium biomass (four repeats) of Le4606 and Le4625 was 0.23 ± 0.04, 0.37 ± 0.02, 0.39 ± 0.04 g and 0.45 ± 0.02, 0.47 ± 0.19, 0.52 ± 0.04 g after 0, 0.5, and 7 h exposure time. Cadmium content was analyzed by the Shanghai Jiao Tong University Analysis and Testing Center according to the atomic absorption spectrometry method specified in GB5009.15-2014 (National Food Safety Standard-Determination of Cadmium in Food, National Health Commission of the People’s Republic of China).
Total RNA Isolation, cDNA Library Preparation, and Illumina Sequencing
Total RNA was isolated from each mycelium sample using TRIzol Reagent (Invitrogen, Carlsbad, CA, United States) according to the manufacturer’s instructions. RNA integrity was analyzed by 1.5% denaturing agarose gel electrophoresis, and the RNA concentration and purity were determined with a NanoDrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA, United States). For each sample, 0.75 μg (40 ng/μL) RNA was used for library construction. Libraries were generated using the TruSeq RNA Sample Preparation Kit (Illumina, San Diego, CA, United States). Each mRNA sample was purified and enriched by polyA tail selection and then fragmented. Random hexamer primers were used for first-strand cDNA synthesis, followed by second-strand cDNA synthesis. The cDNA fragments were blunted and ligated to sequencing adaptors. The libraries were then selected according to size (200 bp), and the selected cDNA was amplified via polymerase chain reaction (PCR; 15 cycles) using the Illumina PCR Primer Cocktail. The PCR products were purified (AMPure XP system) and quantified on a Bioanalyzer 2100 system (Agilent Technologies, Santa Clara, CA, United States). The sequencing library was sequenced using a Hiseq Xten system (Illumina). Raw sequencing data were submitted to the National Center for Biotechnology Information (NCBI) Sequencing Read Archive (accession number: SRP256428).
Data Filtering and Read Mapping
The quality of the raw sequences was evaluated by the FastQC quality control tool1. The raw paired-end reads were filtered according to the Phred quality score (Q ≥ 30) and read length (≥50 bp) using SolexaQA software2. A reference genome index was established using Bowtie2 software (Langmead et al., 2009), and the clean reads were used for reference (BGI L. edodes genomic sequences)-guided transcriptome assembly with Tophat2 software.
DEG Enrichment Analysis and GO Annotation
The mapped reads were assembled according to the reference genome. Cufflinks software was used to calculate the expression value of each gene, and the gene expression level was standardized by fragments per kb per million mapped reads. DEGSeq was performed to identify the DEGs in Le4606 and Le4625 after Cd stress. The significance and reliability of the multiple-comparison tests were evaluated by calculating the false discovery rate (FDR). The thresholds for significant gene expression differences were FDR ≤ 0.01 and | log2 fold-change| ≥ 1. GO and KEGG function analyses were implemented based on sequence similarities (e-value ≤ 1e–10) with proteins in the GO (Gene Ontology)3 and KEGG databases (Kyoto Encyclopedia of Genes and Genomes)4.
Validation of Gene Expression by qRT-PCR
To validate the RNA-Seq results, four genes were selected randomly from the significant gene expression patterns and analyzed by quantitative real-time PCR (qRT-PCR). The four selected genes were hypothetical protein (Le_10013468), aspartate aminotransferase (Le_10009629), amino hydrolase (Le_10009215), and hydrophobin 2 (Le_10001088). The primer pairs were designed using Primer 3 (V.0.4.0)5 as shown in Supplementary Table S1. The actin gene was used as an internal standard.
RNA was isolated as described above. Total RNA (2 μg) was reverse-transcribed using cDNA Synthesis SuperMix (TransGen Biotech, Beijing, China) after genomic DNA was removed (TransScript One-Step gDNA Removal). SYBR green fluorescent dye (TaKaRa, Shiga, Japan) was used to perform the qPCR reactions on an ABI Prism 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, United States). The PCR conditions were as follows: 95°C for 2 min, 95°C for 15 s (40 cycles), 60°C for 15 s, and 72°C for 30 s. The relative expression value of each gene was calculated using the 2–ΔΔCt method. The experiment was performed using three biological replicates.
Statistical Analysis
Independent sample t-test was performed to analyze the difference between Cd concentrations in two L. edodes genotypes. Differences were considered as statistically significant difference when P < 0.01. Regression analysis was performed between qPCR and RNA sequencing including all genes for both genotypes at the three time points of Cd treatment. Statistical analysis was performed using SPSS 23.0 (SPSS, Inc., Chicago, IL, United States).
Results
Difference in Cd Concentration Between Both Genotypes
Mycelia were collected for measurement of Cd content after Cd exposure for 0, 0.5, and 7 h. Initially, the Cd concentrations in Le4606 and Le4625 were 0.035 ± 0.017 and 0.068 ± 0.010 mg/kg, respectively (P = 0.024). As shown in Figure 1, a significant difference in the Cd concentration of the selected strains began to appear at 0.5 h, when the Cd concentrations in Le4606 and Le4625 were 0.343 ± 0.097 and 0.593 ± 0.041 mg/kg, respectively (n = 4, P = 0.009). The difference between the Cd concentrations of the selected strains increased after exposure to Cd for 7 h. The Cd concentration in Le4625 (1.390 ± 0.098 mg/kg) was ∼3-fold that in Le4606 (0.440 ± 0.038 mg/kg; n = 4, P < 0.001), indicating that Le4625 had a greater capacity for Cd enrichment.
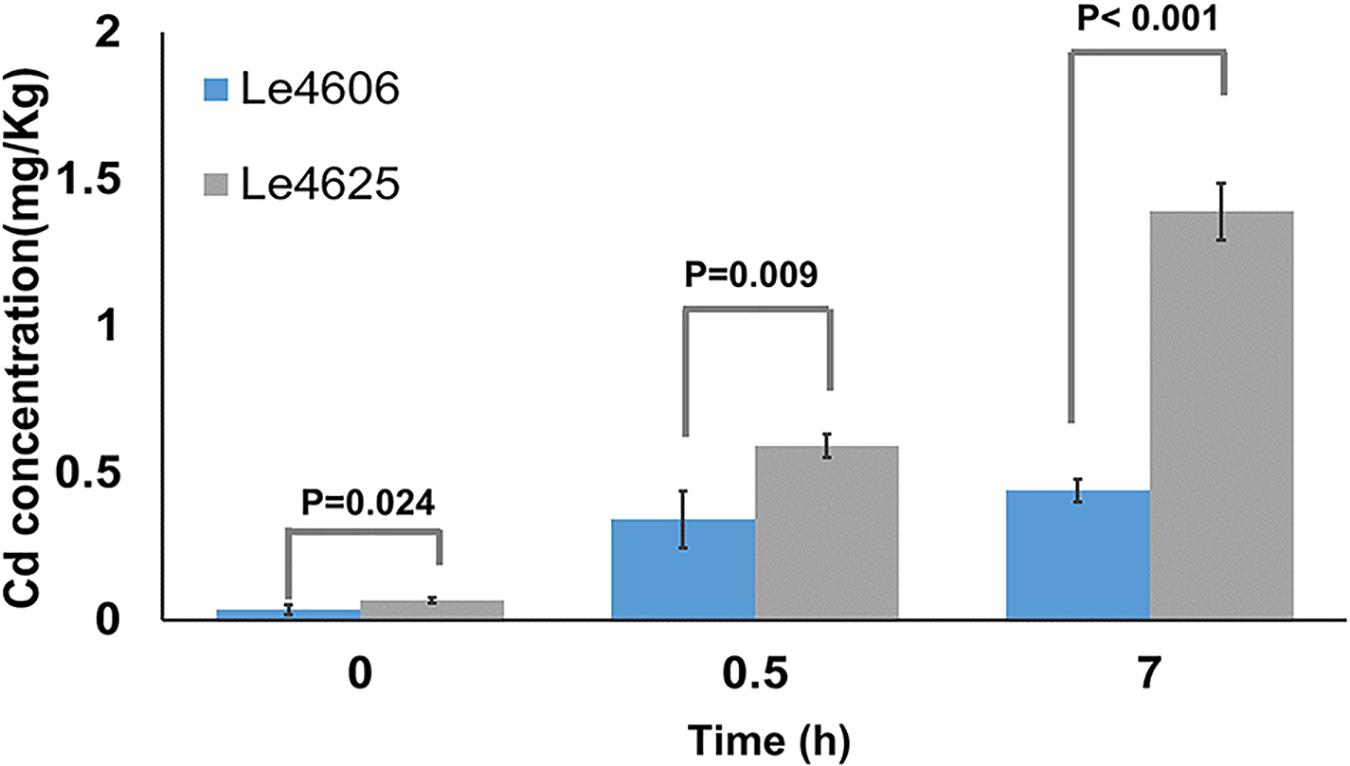
Figure 1. Cd concentrations in L.edodes genotypes Le4606 and Le4625 after exposure to Cd (0.1 mg/L) for 0, 0.5, and 7 h.
Transcriptome Sequencing of L. edodes and Functional Annotation
RNA-Seq was performed using 18 L. edodes cDNA libraries, yielding 797.61 million clean reads. A total of 120.03 Gb of clean data was obtained, and the Q30 value of the base ratio was higher than 90.82% (Supplementary Table S2). The proportion of reads mapping to the L. edodes genome was 86.18–90.35% (Supplementary Table S3). The saturation curve of the sequencing data indicated that the sequencing depth was sufficient for transcriptome assembly and expression detection (Supplementary Figure S1). A total of 24,592 transcripts were detected and the genes were annotated via the GO and KEGG pathway databases. The annotated genes were enriched into 14 GO classifications. In the molecular function category, >400 genes were annotated as “binding,” >200 genes were annotated as “catalytic activity,” and >50 genes were annotated as “localization.” Kyoto Encyclopedia of Genes and Genomes pathway analysis showed that the annotated genes were classified into 30 KEGG pathways. Most genes were involved in the “carbohydrate and amino acid metabolism,” “genetic information processing,” and “cellular processes” pathways (Supplementary Figure S2).
Gene Expression Pattern and RNA-seq Validation by qPCR
Based on gene expression patterns, DEGs of Le4606 and Le4625 at different time points were clustered in eight profiles. The profiles displayed considerable difference in the gene expression patterns of the selected genotypes after Cd exposure (Figure 2). In Le4606, 349 DEGs were significantly up-regulated at 0.5 h (Profiles 6 and 5, P < 0.001) (Figure 2A). In Le4625, 134 genes were significantly down-regulated and 90 genes were significantly up-regulated at 7 h (Profiles 3 and 4, P < 0.001) (Figure 2B). These results suggest a delay in the transcriptional responses of Le4625 to Cd stress, as compared to Le4606.
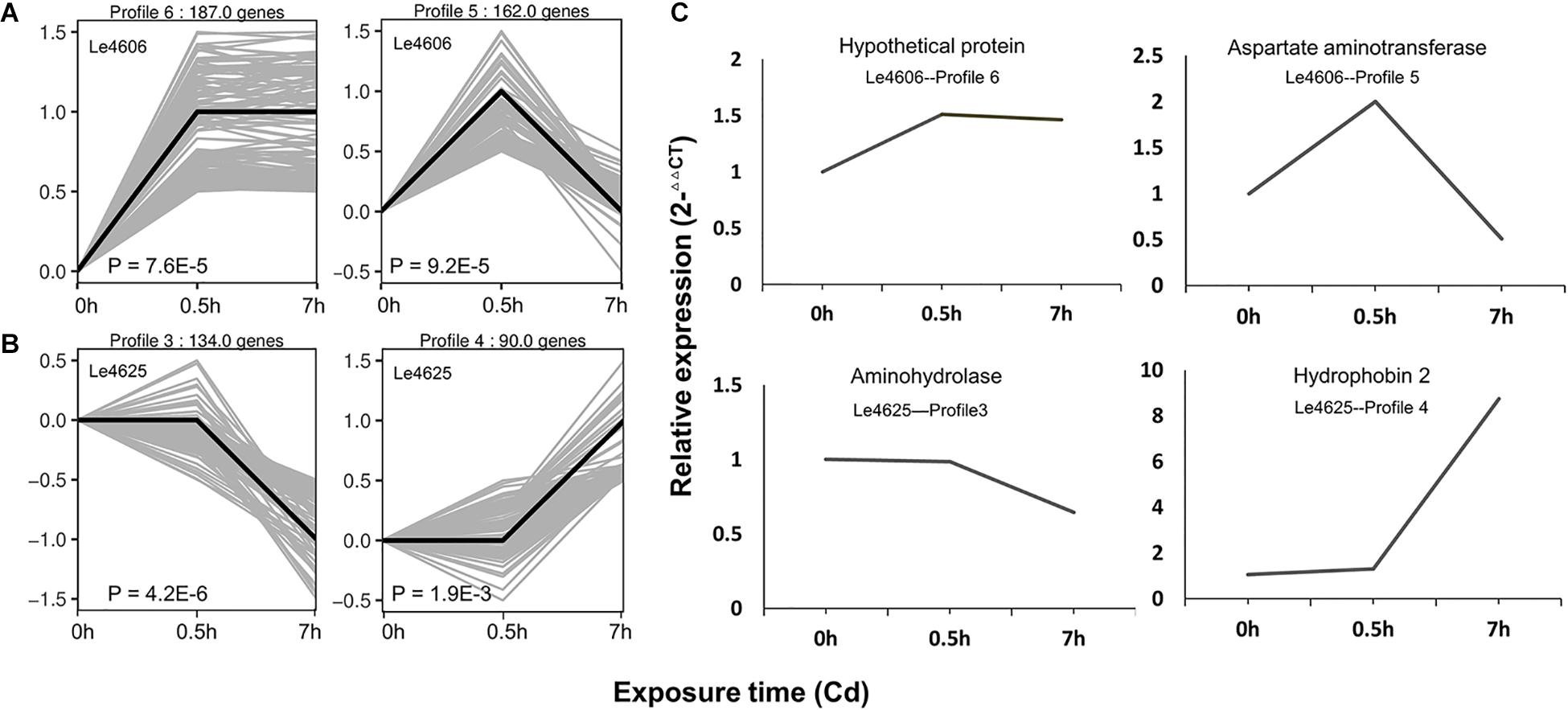
Figure 2. Expression patterns of DEGs and the qRT-PCR validation. (A) Significant expression patterns of DEGs in Le4606. (B) Significant expression patterns of DEGs in Le4625. (C) qRT-PCR validation of DEGs in Le4606 (patterns 5 and 6) and 4605 (patterns 3 and 4).
To validate the expression data obtained by RNA sequencing, one gene from each gene expression pattern was randomly selected for qPCR validation. For each gene, the expression count values from the transcriptome data exhibited an expression profile similar to that shown by the qPCR results (Figure 2C), suggesting that reliable expression results were generated via RNA-seq. The results of this analysis revealed a strong correlation between the RNA-seq and qPCR data (Supplementary Figure S3).
DEGs in the Same Genotype After Cd Exposure
To detect genes that may respond to Cd exposure, DEGs before and after Cd exposure were analyzed, with the filtration criteria of ≥2 fold-change and P ≤ 0.05. The number of DEGs identified for Le4606 (Figures 3A–C) was greater than that of Le4625 (Figures 3D–F). As shown in Figures 3J,K, comparisons of gene expression at 0, 0.5, and 7 h revealed sets of 51 DEGs (0 h vs 0.5 h) and 17 DEGs (0 h vs 7 h) in Le4606, and four DEGs were detected both at 0.5 and 7 h. These DEGs mainly involved in six GO classifications (Figure 4A), and the transcript abundance of the genes is shown in the heatmap in Figure 4B. Le_10001689, and Le_10002245 were annotated as MFS general substrate transporters with the identity of 72.94 and 92.9%, respectively. Le_10008273 was annotated as an MFS transporter (identity = 100%) (Table 1). The transcripts of Le_10008273 and Le_10002245 were significantly enriched in Le4606 after Cd exposure, but their abundance did not change in response to Cd exposure in Le4625. Le_10001689 showed similar expression patterns in Le4606 and Le4625; it was abundant before Cd exposure, but its abundance was greatly reduced at 0.5 h and recovered at 7 h. Le_10004805 was annotated as a transmembrane protein (identity = 99.57%); it was not detected in Le4606 at 0 h, but its expression was significantly increased at 0.5 h and 7 h.
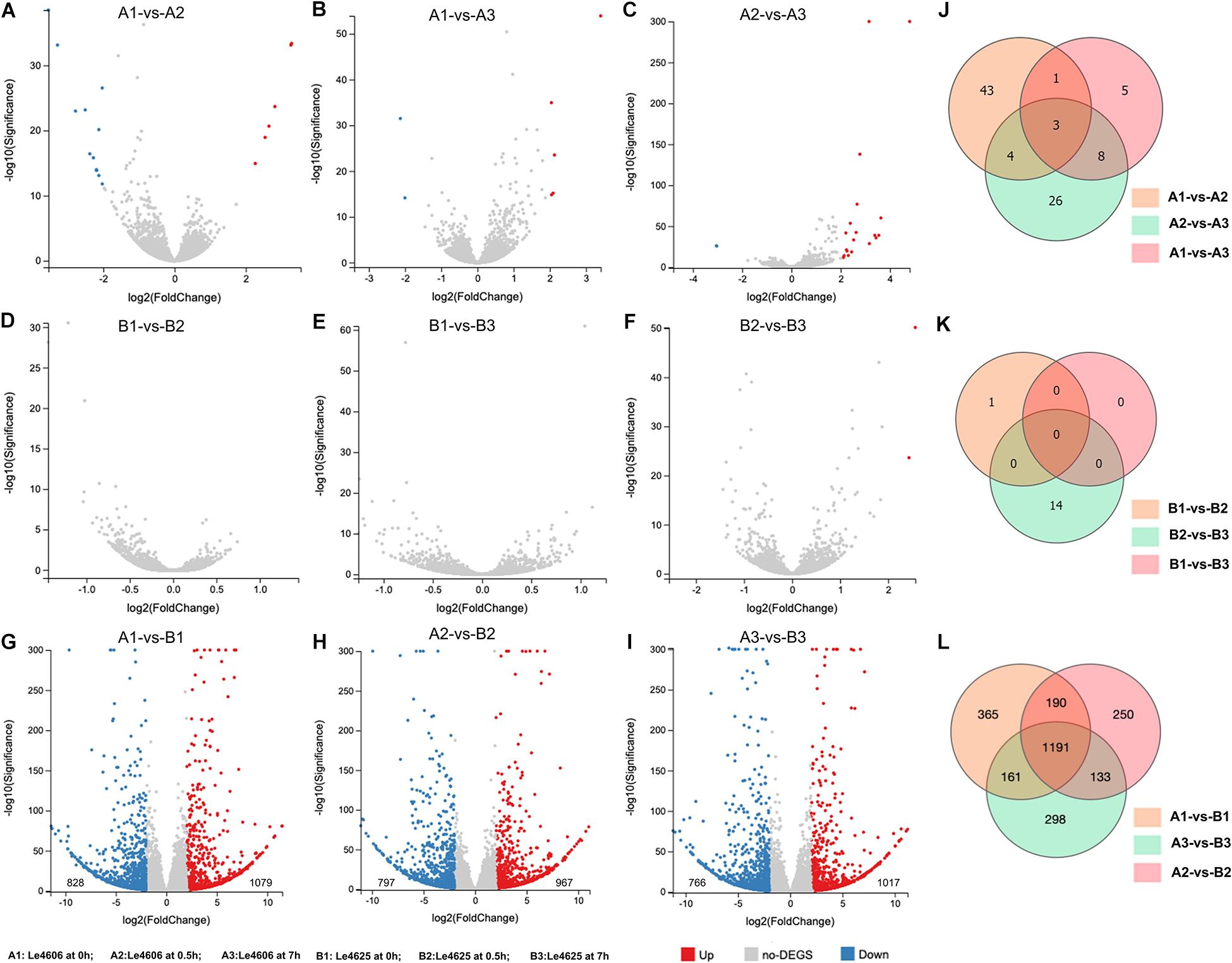
Figure 3. Statistics for differentially expressed genes between Le4606 and Le4625. (A–C) Volcano plots of the pairwise comparison of DEGs between three treatments (Cd exposure for 0, 0.5, and 7 h) in the Le4606 genotype. (D–F) Volcano plots of the pairwise comparisons of DEGs between three treatments (Cd exposure for 0, 0.5, and 7 h) in the Le4625 genotype. (G–I) Volcano plots of DEGs between Le4606 and Le4625 after Cd exposure for 0, 0.5, and 7 h. (J–L) Venn analysis of intra-genotype and inter-genotype DEGs for Le4606 and Le4625.
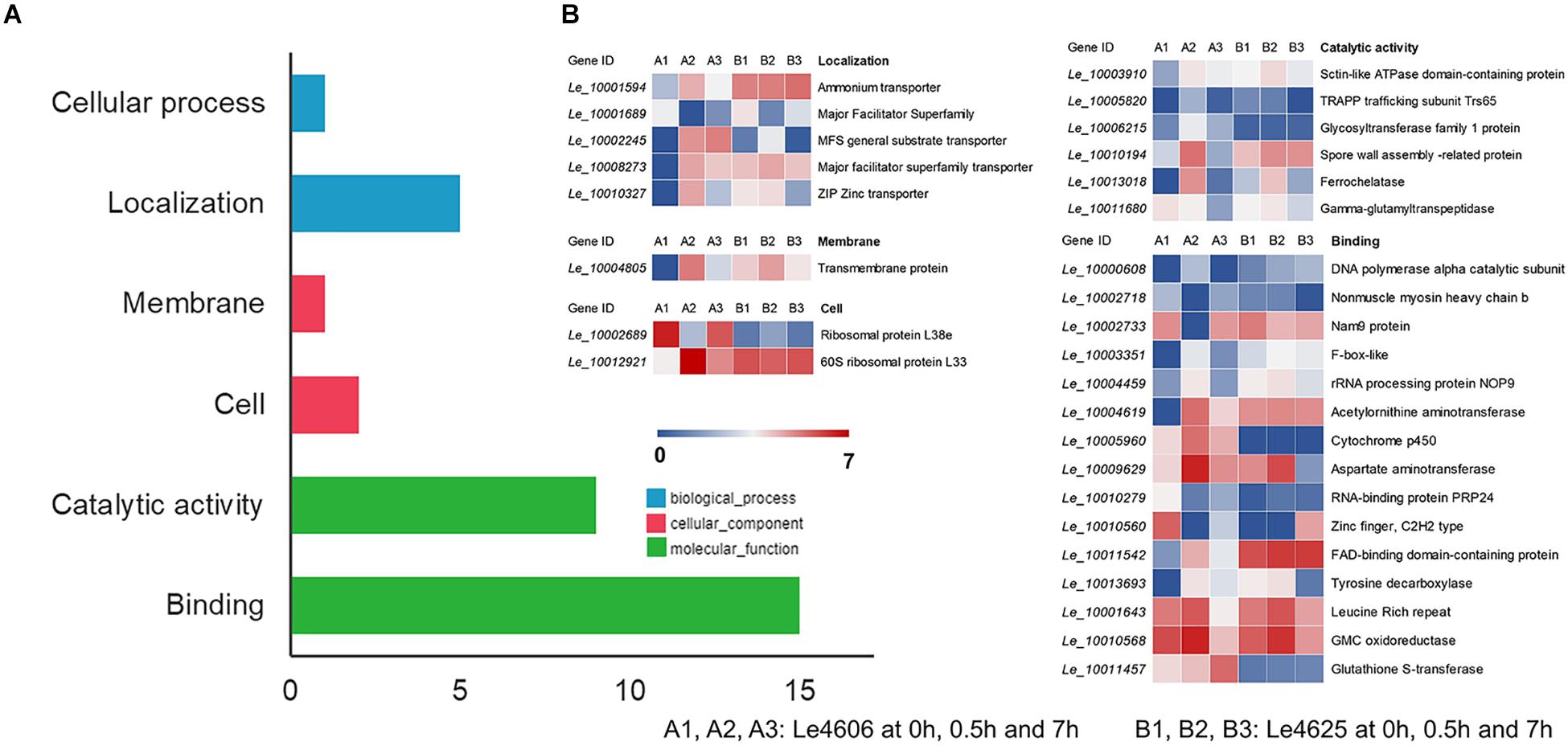
Figure 4. GO classification and transcript abundance of DEGs in Le4606 after Cd exposure. (A) GO classifications of DEGs. (B) Heatmap of genes in different GO classifications.
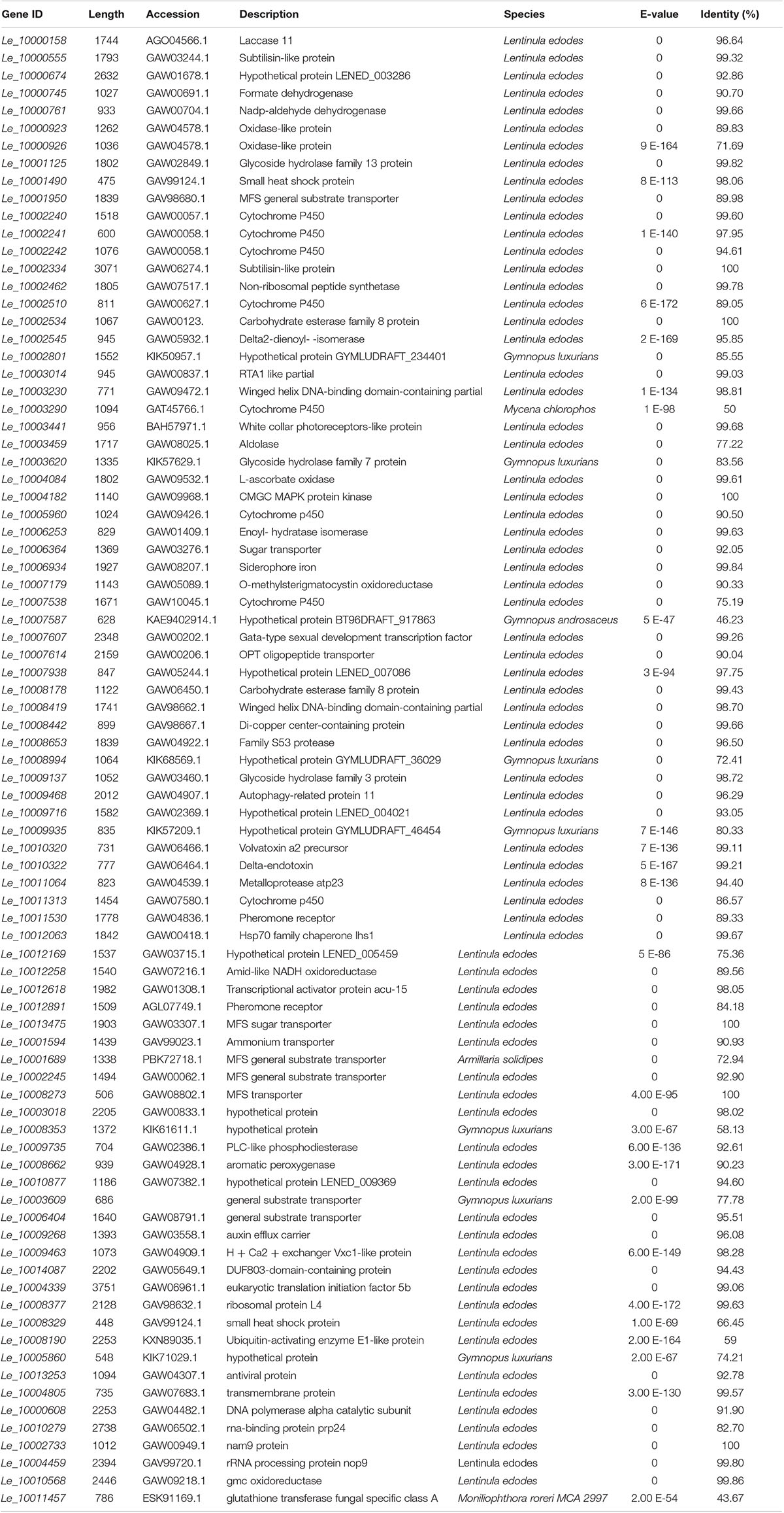
Table 1. BLASTX results for function description of DEGs involved in different GO classification and KEGG pathways.
The BLASTX results showed that Le_10011457 has a thioredoxin-like superfamily domain, and the protein has 43.67% identity with a glutathione transferase (GST) fungal specific class A protein (Table 1). Glutathione transferases are a family of multifunctional enzymes that play important roles in combating abiotic stresses, including heavy metal stress, through amelioration of oxidative damage (Bartling et al., 1993). Before Cd exposure, Le_10011457 transcript abundance in Le4606 was greater than that in Le4625. After Cd exposure for 7 h, the Le_10011457 expression level in Le4606 was twice that observed at 0 h. In addition, the expression pattern of cytochrome P450 gene Le_10005960 was similar to that of Le_10011457. The Le_10005960 transcript abundance in Le4606 was higher than that in Le4625, and Le_10005960 expression was up-regulated nearly two-fold after Cd exposure for 0.5 h in Le4606. Cytochrome P450 enzymes can detoxify natural and environmental pollutants and allow fungi to grow under a relatively wide range of conditions (Crešnar and Petrič, 2011).
The abundance of Le_10010568 was down-regulated in Le4606 and Le4625 after 7 h of Cd exposure. Le_10010568 is a glucose–methanol–choline (GMC) oxidoreductase that is expressed by many organisms and plays an auxiliary roles in lignocellulose degradation in fungi (Sützl et al., 2019). The genes annotated as processing binding functions also participate in DNA synthesis (Le_10000608, DNA polymerase alpha catalytic subunit), RNA splicing (Le_10010279, RNA-binding protein prp24), and protein translation (Le_10002733, Nam9 protein; Le_10004459, rRNA processing protein Nop9). In Le4606, Le_10010279 and Le_10002733 were significantly down-regulated at 0.5 h, whereas Le_10000608 and Le_10004459 expression levels were increased by 100% in comparison with those measured at 0 h. However, Cd exposure did not induce changes in the transcript abundance of Le_10010279, Le_10002733, Le_10000608 or Le_10004459 in Le4625.
GO and KEGG Analysis of DEGs Between Le4606 and Le4625
Genetics are an important factor affecting Cd accumulation, and differences in the transcription levels of particular genes may result in differences in Cd accumulation in fungi. Therefore, DEGs between Le4606 and Le4625 were analyzed. After Cd exposure for 0, 0.5, and 7 h, Le4625 showed 1079, 967, and 1017 up-regulated genes, respectively, in comparison with Le4606, whereas 828, 797, and 766 down-regulated genes were identified, respectively (Figures 3G–I). A Venn diagram was drawn to illustrate the DEGs between the two genotypes after Cd exposure for different time periods (Figure 3L).
After Cd exposure for 0, 0.5, and 7 h, 365, 250, and 298 DEGs, respectively, were identified between Le4606 and Le4625 (Figure 3L). These sets of DEGs were subjected to GO and KEGG analysis (Supplementary Figure S4). At 0.5 and 7 h, the DEGs were mainly annotated as “binding,” “catalytic activity,” and “localization.” At 0.5 h, the KEGG pathway analysis showed that the genes were involved in “translation,” “cell growth and death,” “folding sorting and degradation,” and “carbohydrate metabolism.” The DEGs were mainly involved in “carbohydrate metabolism,” “cell growth and death,” “folding sorting and degradation,” and “amino metabolism” at 7 h.
After Cd exposure for 0, 0.5, and 7 h, 1191 DEGs (fold-change ≥ 2, P ≤ 0.05) were identified between Le4625 and Le4606. Among these 1191 DEGs, 680 genes were up-regulated, and 511 genes were down-regulated. The GO classifications of these DEGs revealed their involvement in “metabolic process,” “molecular functions,” “catalytic activity,” “membrane,” and “binding” (Figure 5A). KEGG analysis showed that the DEGs were primarily involved in “carbohydrate metabolism,” “amino acid metabolism,” “lipid metabolism transport and catabolism,” “signal transduction,” and “fold sorting and degradation” (Figure 5B). GO classification and KEGG pathway analysis were performed for the 680 up-regulated genes (Supplementary Figures S5A,B) and 511 down-regulated genes (Supplementary Figures S5C,D) in Le4625. The GO membrane and antioxidant activity functions were specifically enriched in the set of up-regulated genes in Le4625. The KEGG analysis showed that the up- and down-regulated genes were enriched in different metabolic pathways. The up-regulated genes were involved in biosynthesis of other secondary metabolites, as well as metabolism of terpenoids and polyketides.
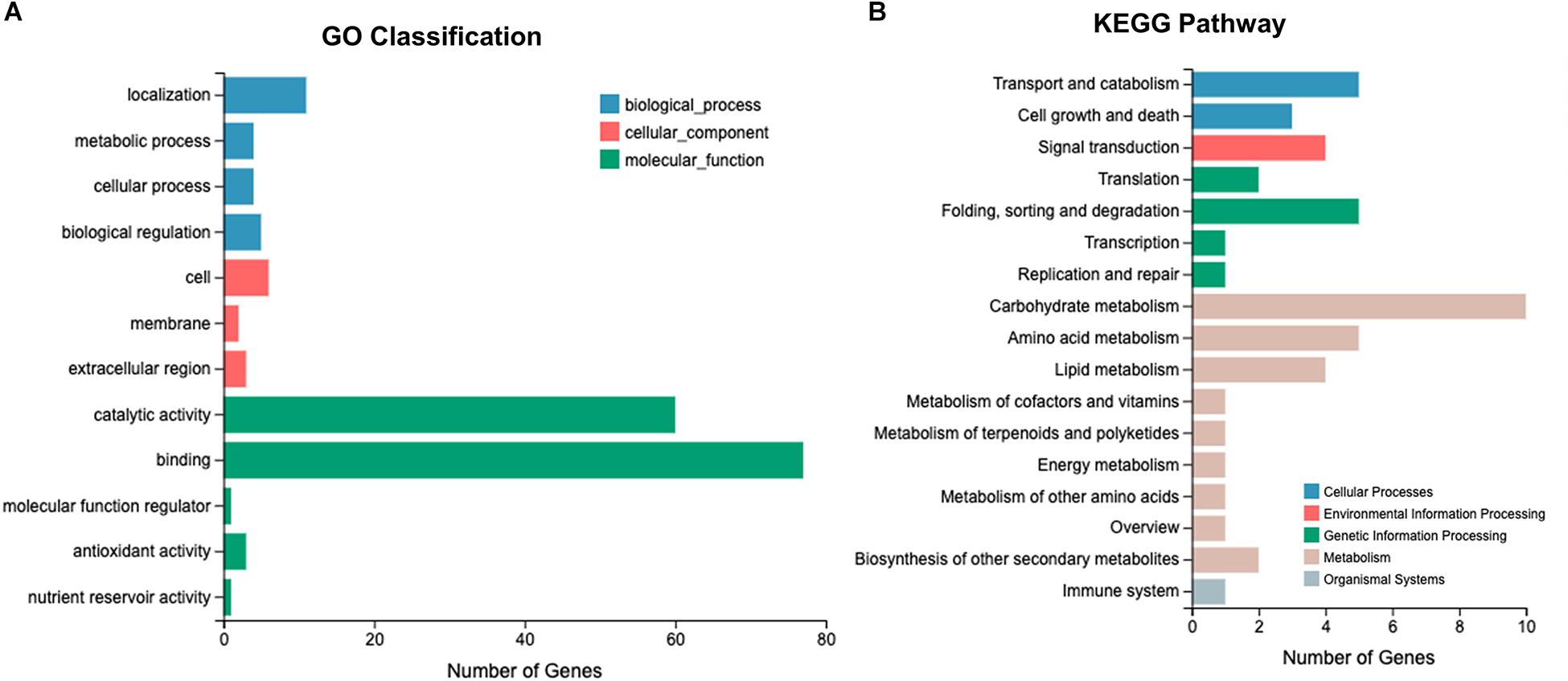
Figure 5. O and KEGG analysis of 1191 DEGs. (A) Genes involved in different GO classifications. (B) Genes involved in different KEGG pathways.
Expression of DEGs in Different GO Functions and KEGG Pathways
To further explore the functions of the DEGs mentioned above, BLASTX analysis was performed based on their sequences (Table 1). Heatmaps of genes involved in GO functions (Supplementary Table S4) and KEGG pathways (Supplementary Table S5) were generated (Figure 6).
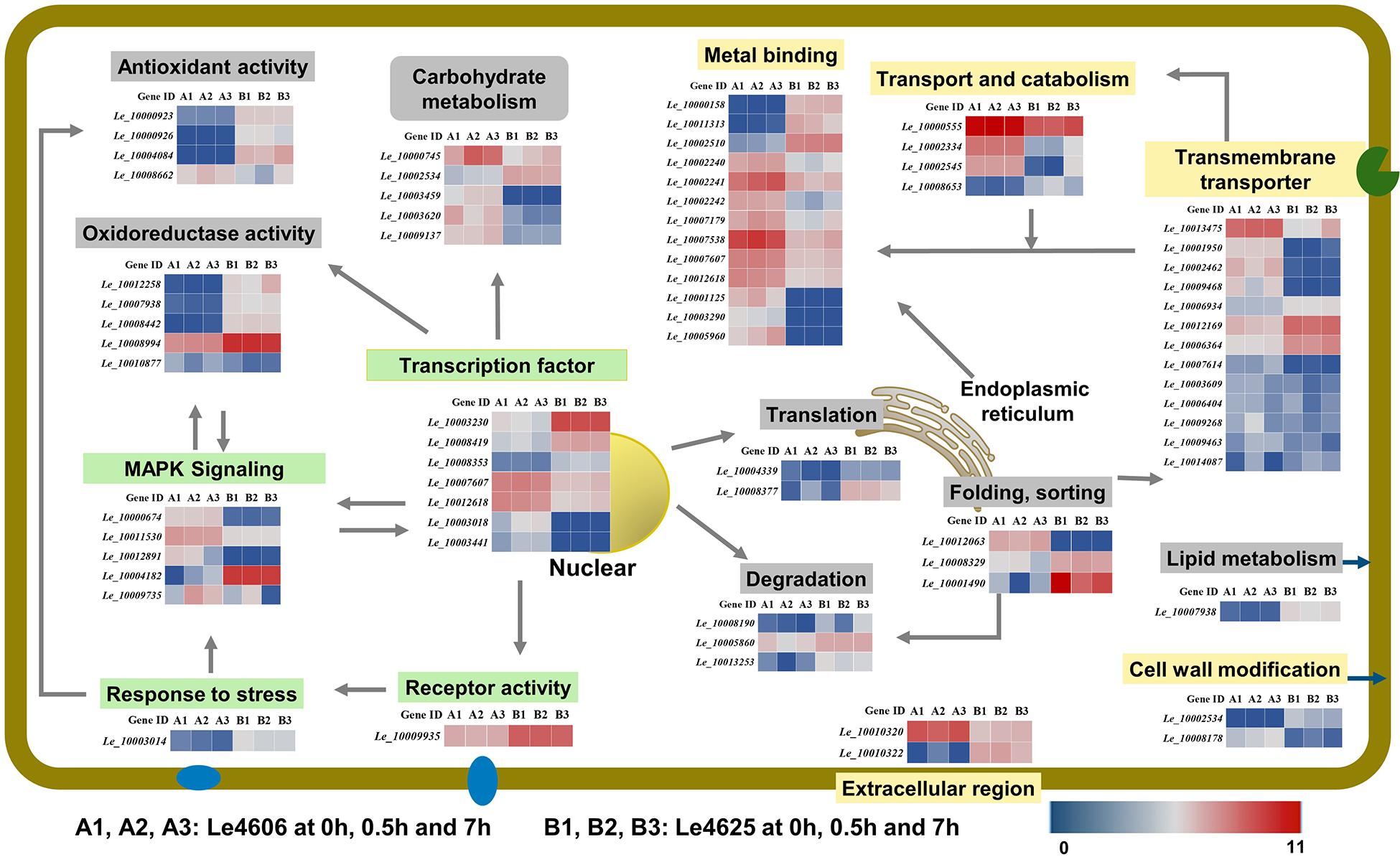
Figure 6. Transcriptional changes of genes involved in Cd enrichment in the two L. edodes genotypes. DEGs involved in Cd enrichment are represented in different colors. Genes that may directly respond to heavy metals are shown in a yellow box; Genes involves in signal transduction are shown in a green box. Other genes are shown in gray. For signal transduction, the arrows represent the direction of signaling. For transporters, the arrows represented the directions of Cd transport. For transcriptional factors, the arrows pointed to the products of transcriptions.
Expression of Genes Involved in the GO Binding Function
Metal ion binding proteins and transcription factors involved in the GO binding function were selected for analysis. Laccase 11 (Le_10000158) gene was involved in copper ion binding, and its transcript was enriched in Le4625. GATA-type sexual development transcription factor (Le_10007607), transcriptional activator protein ACU15 (Le_10012618), and white-collar-photoreceptor-like protein (Le_10003441) were annotated as zinc ion binding genes with transcription factor activity. The expression patterns of Le_10007607, Le_10012618, and Le_10003441 were similar, and the transcript abundance of these genes in Le4606 was higher than that in Le4625. Interestingly, Le_10003441 was expressed in response to Cd stress at 0.5 h in Le4606 but this transcript was not detected in Le4625. In addition, winged-helix DNA-binding domain-containing genes Le_10003230, Le_10008419, and Le_10008353, which encode three transcription factors, were relatively enriched in Le4625 at 0 h, 0.5 h and 7 h. A transcript encoding a hypothetical protein annotated as a DNA-binding protein (Le_10003018) was enriched in Le4606, but this transcript was not detected in Le4625. Le_10001125, which encodes glycoside hydrolase (GH) family 13, an enzyme that bind with cations, was not detected in Le4625 (Figure 6).
Expression of Genes Involved in the GO Cellular Component and Localization Function
According to the GO annotation, the volvatoxin a2 precursor (Le_10010320) and delta-endotoxin (Le_10010322) were identified as being localized in the extracellular region. Both genes showed different expression patterns in Le4606 and Le4625. Le_10010320 was nearly two-fold more abundant in Le4606 than in Le4625. However, two genes annotated as membrane components, Le_10003014 (an RTA1-like-gene expressed in response to stress), and Le_10009935 (a hypothetical protein with receptor activity), were enriched in Le4625 in comparison with Le4606 (Figure 6).
Interestingly, three heat shock protein (HSP)-encoding genes showed different expression levels in Le4606 and Le4625. The transcript abundance of Hsp70 family chaperone LHS1 (Le_10012063) was relatively low in Le4625, but higher in Le4606. Two small HSP genes, Le_10008329 and Le_10001490, were identified. The transcript abundance of Le_10001490 and Le_10008329 showed no change in Le4625 after Cd exposure, but they are down-regulated in Le4606 after Cd exposure for 0.5 and 7 h, respectively. Their transcript abundance was 2-fold higher in Le4625 in comparison with Le4606 at 0 h (Figure 6).
Expression of Genes Involved in GO Transmembrane Transporter Activity and Cell Wall Modification Functions
Eight genes were annotated as transmembrane transporters. Le_10012169 (hypothetical protein) and Le_10006934 (siderophore iron) were enriched in Le4625, whereas the other six genes showed higher transcript abundance in Le4606. In addition, one MFS sugar transporter gene (Le_10013475) was up-regulated at 7 h in Le4625. The transcript abundance of another MFS general substrate transporter gene, Le_10001950 was >6-fold higher in Le4606 in comparison with Le4625. Two carbohydrate esterase family 8 (CE8) genes, Le_10002534 and Le_10008178, may be involved in cell wall modification, but their expression patterns were different. Le_10008178 was nearly five-fold more enriched in Le4606 in comparison with Le4625, whereas Le_10002534 showed very low abundance in Le4606, suggesting that these genes have different functions (Figure 6).
Expression of Genes Involved in GO Antioxidant and Catalytic Activity Functions
Genes encoding two oxidase-like proteins (Le_10000923 and Le_10000926) and a L-ascorbate oxidase (Le_10004084) were annotated as antioxidant genes. All three genes have peroxidase activity and had higher transcript abundance in Le4625; the expression level of Le_10000923 in Le4625 was three-fold higher than that in Le4606. In contrast, the di-copper-center containing gene Le_10008442, amide-like NADH oxidoreductase gene Le_10012258, and genes encoding two hypothetical proteins (Le_10008994 and Le_10007938) were annotated as oxidoreductase genes. Interestingly, the peroxidase and oxidoreductase genes were more enriched in Le4625 than in Le4606 at the transcript level. The transcript abundance of Le_10008662 (aromatic peroxygenase) in Le4606 was two times higher than that in Le4625 at 0.5 h. The transcript abundance of Le_10010877 (hypothetical protein) in Le4606 was three times higher than that in Le4625 at 7 h (Figure 6).
Expression of Genes Involved in the KEGG Metabolism, Translation and Degradation Pathway
Carbohydrate and lipid metabolism pathways were analyzed. Carbohydrate metabolism supplies energy allowing cells to grow or resist stress, and lipid metabolism may affect cell membrane activity. As shown in Figure 6 and Supplementary Table S5, formate dehydrogenase (Le_10000745), CE8 (Le_10002534), aldolase (Le_10003459), GH7 (Le_10003620), and GH3 (Le_10009137) were identified as differentially expressed carbohydrate metabolism genes. With the exception of CE8, the transcripts levels of these genes in Le4606 were greater than those in Le4625. Additionally, in Le4625, the transcript abundance of a hypothetical protein-encoding gene (Le_10007938) in a fatty acid elongation pathway involved in cell membrane synthesis was >5-fold higher than that in Le4606 (Figure 6).
Ubiquitin-activating enzyme E1-like protein (Le_10008190) and a hypothetical protein gene (Le_10005860) were revealed to be involved in ubiquitin mediated proteolysis. Le_10008190 was down-regulated at 0.5 h (2-fold) in Le4625. Le_10005860 was down-regulated at 0.5 h in Le4606, and its abundance in Le4625 was not affected by Cd exposure. An antiviral protein (Le_10013253) was found to be involved in RNA degradation, and its transcript abundance was lower at 0.5 h in Le4606, but unchanged in Le4625 (Figure 6).
Translation initiation factor 5b (Le_10004339) and ribosomal protein L4 (Le_10008377), which are both involved in translation, showed higher expression levels in Le4625. Le_10004339 was down-regulated at 0.5 h both in Le4606 and Le4625, but recovered in Le4625 at 7 h.
Expression of Genes Involved in the KEGG Transport and Signaling Pathways
Genes involved in the KEGG signal transduction pathway were annotated as members of the MAPK signaling pathway. Two pheromone receptor genes (Le_10011530 and Le_10012891) and a hypothetical protein-encoding gene (Le_10000674) were more highly expressed in Le4606 than in Le4625. The expression levels of both pheromone receptor genes at 0 h were higher than those measured at 7 h in Le4606, and these differences could partially explain the early response of Le4606 to Cd stress. PLC-like phosphodiesterase gene (Le_10009735) was up-regulated in Le4606; at 0.5 and 7 h, its transcript abundance was 2 times and 30 times higher, respectively, than that of Le4625 (Figure 6).
Genes involved in transport and catabolism were annotated as members of lysosome- or peroxisome-related biological pathways. The lysosome is an important mediator of metal homeostasis (Blabyhaas and Merchant, 2014). Three lysosome-related genes identified were two subtilisin-like proteins (Ld_10000555 and Ld_10002334) and a family S53 protease (Ld_10008653), were identified. The transcript abundance of Le_10002334 in Le4606 was 3∼5-fold higher than that in Le4625. In contrast, Le_10002334 expression was enriched in Le4625 after Cd exposure for 7 h. The transcript abundance of Le_10008653 in Le4625 was 4∼10-fold higher than that in Le4606. The other gene involved in transport and catabolism, Le_10002545, was involved in peroxisome. Le_10002545 expression was enriched in Le4606, and it was up-regulated in Le4625 after Cd exposure for 7 h (Figure 6).
Discussion
Lentinula edodes has high nutritional value and is commonly eaten in many cultures around the world (Cao et al., 2013; Dai et al., 2015). However, L. edodes. easily absorbs heavy metals, especially Cd (Hatvani and Mécs, 2003). Cadmium in edible mushrooms can have deleterious effects on human health (Mleczek et al., 2017) and seriously affects the quality of mushrooms (Chiu et al., 1998). Consequently, strict control of Cd content in L.edodes is an important consideration in mushroom production. Genetic factors play an important role in Cd enrichment, so determining the molecular mechanism involved in Cd enrichment may facilitate genetic modification of L. edodes strains. Therefore, two L. edodes genotypes with different capabilities for Cd enrichment, Le4606 and Le4625, were subjected to RNA-Seq analysis. By analyzing the transcriptomes of Le4606 and Le4625, we found candidate genes and pathways that may participate in Cd enrichment.
The Cd enrichment capabilities of Le4606 and Le4625 were analyzed. Firstly, we analyzed the Cd content in Le4606 and Le4625 after Cd exposure for 0, 0.5, 1, 2, 3, 5, 7, and 10 h. Cadmium concentration in mycelium increased rapidly at 0.5 h and it was relative stable in Le4606 after 0.5 h. In addition, the Cd content in Le4625 was highest at 7 h. Therefore, 0.5 and 7 h was apparently important for evaluating the effect of Cd on L. edodes. After Cd exposure for 0, 0.5, and 7 h, the Cd concentrations of both strains increased gradually. The Cd content in Le4606 and Le4625 at 0 h was much lower than that at 0.5 and 7 h. A difference in the Cd content of the two genotypes was observed at 0 h. We speculate that the PDB medium may contain a low concentration of Cd, which was absorbed by the mycelium of Le4625. However, the Cd concentration in Le4625 reached 0.593 ± 0.041 mg/kg at 0.5 h, which was ∼9-fold higher than that measured at 0 h, indicating the rapid Cd enrichment capability of the mycelium. The Cd concentration in Le4625 was 1.7-fold or 3.2-fold higher than that in Le4606 after Cd exposure for 0.5 or 7 h, respectively. These results indicate that Le4606 has a low Cd enrichment capacity, whereas Le4625 has a strong ability to absorb and accumulate Cd.
The transcriptomes of both genotypes differed with regard to DEG expression patterns and the number of DEGs. In Le4606, the DEGs were up-regulated at 0.5 h of Cd exposure, whereas changes in DEG expression tended to occur as late as 7 h of Cd exposure in Le4625 (Figure 2). In addition, the number of DEGs in Le4606 was higher than that in Le4625 after Cd exposure for 0.5 and 7 h (Figures 3A–F). These findings suggest that the response of Le4606 to Cd exposure is faster and stronger than that of Le4625. The DEGs induced by Cd exposure in Le4606 were analyzed, and 51 and 17 genes were found to respond to Cd at 0.5 and 7 h, respectively. GO analysis showed that these genes were mainly involved in transporter, binding, and catalytic activity. Three MFS transporters genes (Le_10001689, Le_10008273, and Le_10002245), a transmembrane protein gene (Le_10004805), cytochrome and a GST gene (Le_10011457) respond to Cd at the early time of 0.5 h. These findings are accordance with a previous study, in which the genes involved in early response to Cd exposure were identified, including MFS transporter, mitogenactivated protein (MAP) kinase, cytochromec peroxidase genes (Zhao et al., 2015). In addition, because genetic factors play an important role in Cd enrichment by L. edodes, differences between Le4606 and Le4625 were analyzed on the transcript level. According to the GO and KEGG results, the DEGs participate in many biological pathways, including metal binding, transmembrane transportation, and antioxidant activity. These findings indicate that several biological pathways may play roles in Cd accumulation in L. edodes.
The accumulation of heavy metals in edible mushrooms can be summarized as follows: heavy metals are intercepted by the cell wall (Vimala and Das, 2011) and transported via carriers located in the plasma membrane (Severoglu et al., 2013), or they may be chelated by bioactive compounds in the plasma membrane (Bellion et al., 2007). It has been reported that metalloproteins are involved in the uptake, binding, release, and transport of heavy metals in vivo (Degtyarenko, 2000). Hence, we selected gene clusters involved in the cell wall, cell membrane, signal transfer, transport, metal ion binding, and other functions based on the GO and KEGG annotations. Next, we analyzed the expression patterns of these gene clusters in the two genotypes using a heatmap. We found that cell wall modification, transmembrane transporter, and metal-binding genes showed different expression patterns in the two genotypes. To understand the biological functions of these DEGs, we conducted a BLASTX search in the NCBI database using their coding DNA sequences (Table 1). The results of this analysis are discussed below.
Cell Wall Modification Genes May be Involved in Cd Accumulation
Based on reports that heavy metals can be adsorbed onto the cell wall and enriched in fungi (Mo et al., 2010), genes involved in cell wall modification were analyzed. Most carbohydrate esterase family (CE) proteins have been predicted to be involved in the synthesis and remodeling of cell wall components (Lao et al., 2003). A CE8 protein (Le_10002534) enriched in Le4625 was homologous to pectin esterase (identity = 68.21%). Pectin methyl esterases (PMEs) belong to the CE8 family and catalyze de-esterification of the homogalacturonan chain of pectin, resulting in de-esterified homogalacturonan. PMEs defend plants from fungal infections, and the tobacco mosaic virus employs PMEs for its systemic spread in plants (Rajulapati et al., 2018). CE8 proteins may respond to enrichment of Cd by remodeling the cell walls of L. edodes.
Genes With Transmembrane Transport Activity
Metal transporters play an important role in metal homeostasis (Bolchi et al., 2011) and can remove toxic metal ions from the cytosol by extrusion or intracellular sequestration (Hall, 2002). Thus, we analyzed genes involved in transmembrane transport, as these protein carriers may participate in transporting Cd (Severoglu et al., 2013). In Le4606, the abundance of four genes annotated as transmembrane transporters was changed after Cd exposure, and three of these genes (Le_10001689, Le_10002245, and Le_10008273) were found to be members of the major facilitator superfamily (MFS). Le_10002245 and Le_10008273 were up-regulated in Le4606 after Cd exposure, but not affected in Le4625. In addition, thirteen genes with transmembrane transport activity were differentially expressed between Le4606 and Le4625, and one MFS sugar transporter gene (Le_10013475) was gradually enriched in Le4625 by Cd exposure. Le_10006934 was homologous with a siderophore in L. edodes (identity = 99.84%) and the MFS general substrate transporter in Dendrothele bispora (identity = 70.02%). The MFS family of transport proteins is essential for the movement of a wide range of substrates, including inorganic anions and cations, across biological membranes (Quistgaard et al., 2016). However, there are distinct families within the MFS, each of which generally transports a single class of compounds. The subfamilies of the MFS genes identified in this study must be determined, and their roles in Cd accumulation will be explored in future studies.
Genes With Binding Functions
Analysis of the DEGs annotated as binding proteins revealed that most of the identified ion binding genes were zinc binding transcription factors. For example, transcriptional activator protein acu-15 (Le_10012618) is located in the nucleus and might function in activating gene expression. The manner in which these transcription factors influence Cd enrichment merits further study. Metallothionein and phytochelatin (cysteine-rich polypeptides with affinity for heavy metals) are involved in regulating metal homeostasis and facilitate cellular detoxification in some edible mushrooms (Haq et al., 2003; Collin et al., 2007). Le_10000158, a transcript specifically enriched in Le4625, was found to be homologous to laccase 11 (identity = 96.64%), multicopper oxidase (identity = 96.65%), and ferroxidase (identity = 94.01%) in L. edodes. Laccases are Cu-containing glycoproteins that are capable of degrading xenobiotic compounds (Pandey et al., 2018) and are employed by microbes to resist metal ion toxicity by controlling the oxidation states of ions under aerobic conditions (Kaur et al., 2019), but there is no direct evidence support the role it played in Cd response. The role of laccase in Cd metabolism merits further study. In addition, the transcript abundance of several cytochrome P450 enzymes (Le_10002240, Le_10002241, Le_10002242, and Le_10005960) was elevated in Le4606 compared with Le4625. Cytochromes P450 monooxygenases are implicated as key enzymes in many fungal processes and mediate detoxification/degradation of xenobiotics (Crešnar and Petrič, 2011). In some studies, a high Cd concentration induced CYP450 in white rot fungus Phanerochaete chrysosporium (Zhang et al., 2015) and Mactra chinensis (Zhang et al., 2016a). However, the CYP450 superfamily is relatively large, and the subgroups of the CYP450 genes identified in this study and their functions in Cd metabolism merit further study.
Genes Involved in Protein Translation, Folding, Sorting, and Degradation
In addition to ion binding proteins, genes involved in DNA binding and RNA binding were also identified. After Cd exposure, genes involved in DNA synthesis, RNA splicing, and protein translation were identified in Le4606. Le_10000608 encodes the DNA polymerase alpha catalytic subunit, which playes an essential role in the initiation of DNA synthesis (Stadlbauer et al., 1994). Le_10010279 encodes RNA-binding protein PRP24, an essential yeast U6 snRNP protein involved in spliceosome assembly that removes introns from precursor messenger RNA (Kwan and Brow, 2005). Le_10002733 encodes a Nam9 protein, which is homologous to S4 ribosomal proteins from chloroplasts (Boguta et al., 1992). Le_10004459 encodes RNA binding protein Nop9, which is required for the production of mature small ribosomal subunit 18S rRNA in yeast (Zhang et al., 2016b). These findings suggest that L. edodes responds to Cd exposure at the DNA, RNA and protein levels.
Translation initiation factor 5B (Le_10004339) is a universally conserved GTPase that promotes joining of the large and small ribosomal subunits during translation initiation (Murakami et al., 2018). Ribosomal protein L4 (Le_10008377) is a typical ribosomal protein that is assembled into the pre-ribosome (Stelter et al., 2015). The abundance of Le_10004339 and Le_10008377 in Le4625 was higher than that in Le4606. In addition, in comparison with Le4606, two ubiquitin-mediated proteolysis genes, Le_10008190 and Le_10005860, showed higher transcript abundance in Le4625. Based on these results, the DEGs encoding translation initiation factors and ribosomal proteins play important roles in protein synthesis, and the proteolysis genes are involved in protein degradation. We infer that these DEGs may help maintain the protein balance, or change the protein composition, in mushrooms.
Interestingly, the abundance of two HSP-encoding genes identified in this study differed in Le4606 and Le4625. These HSPs are involved in protein folding, sorting, and degradation to maintain intracellular redox balance under stress. HSPs play important roles in stress responses (Mandelli et al., 2017) and have been found to respond to Cd stress in Pleurotus eryngii (Li et al., 2018). Hsp70 family chaperone LHS1 (Le_10012063) was enriched in Le4606 and almost undetectable in Le4625. LHS1 is a chaperon of endoplasmic reticulum in yeast and is involved in protein folding, its expression was higher in the Cd hypersensitive strain (Gardarin et al., 2010). We infer that the Le4606 was sensitive to the increasement of Cd concentration, and LHS1 was involved in Cd stress and might protect the cells by protein folding. In addition, two small HSPs Le_10008329 and Le_10001490, were identified. The transcript abundance of Le_10008329 and Le_10001490 was relatively stable in Le4625. In Le4606, Le_10001490 was down-regulated at 0.5 h and Le_10008329 was down-regulated at 7 h. The changes in gene expression suggested a functional diversity between the sHSPs in Cd enrichment. SHSPs are widely present in all kingdoms of life, and they promote protein homeostasis and proteome stability under stressful conditions (Carra et al., 2017). Martín-Folgar and Martínez-Guitarte (2017) analyzed the expression profile of sHSPs under Cd exposure in C. riparius larvae. Cadmium exposure will change the expression of sHSPs, some sHSPs genes are rapidly activated by Cd, and some sHSPs are induced under long-term cadmium stress. In this study, HSP27 was downregulated at early time of Cd exposure, but significant upregulated after 24 h of Cd treatment. The authors proved the function for HSP27 during the long-term response after Cd exposure. The similar expression of Le_10001490 may indicate its function in the long-term of Cd exposure. In addition, the expression pattern of Le_10008329 may suggest a relevant function in the early response to Cd enrichment.
MAPK Signaling Pathway
Plants respond to heavy metal stress by inducing several distinct MAPK pathways (Jonak et al., 2004) that enhance tolerance to Cd stress. Cadmium may activate MPK3 and MPK6 via the accumulation of reactive oxygen species in Arabidopsis (Liu et al., 2010). In a previous study, a MAP kinase was found to be involved in responses to 24 h of Cd exposure in L. edodes (Zhao et al., 2015). In this study, we identified changes in the expression levels of genes participating in the MAPK signaling pathway following Cd exposure. Le_10004182 was annotated as a CMGC MAPK protein kinase, a superfamily that is mainly composed of the catalytic domains of serine/threonine-specific and tyrosine-specific protein kinases. The potential role of Le_10004182 in Cd enrichment will be verified in future studies.
Anti-Oxidative Stress Response
Peroxidation can facilitate Cd removal via coagulation under alkaline conditions (Wang et al., 2016). Several studies have reported that the antioxidant system contributes to microbial resistance to heavy metals (Guo et al., 2016), thereby allowing fungi to counteract reactive oxygen species accumulation initiated by metals (Zhao et al., 2015). Genes annotated as antioxidants had different expression patterns in the two genotypes investigated in this study. Genes involved in oxidoreductase activity showed greater abundance in Le4625 in comparison with Le4606, with the exception of Le_10008994. Additionally, the redox reaction can change intracellular pH and facilitate a reduction in the intracellular Cd concentration. This finding indicates that a high Cd concentration is a stress factor for mushrooms that activates antioxidant mechanisms.
Glutathione transferases have been reported in all organisms, including fungi, and are notable for their roles in protecting cells from oxidative damage. Moreover, GSTs play important roles in abiotic stress responses and heavy metal detoxification reactions (Kumar and Trivedi, 2018). In a previously study, overexpression of a GST gene in tobacco resulted in plants that were tolerant to Cd and showed reduces Cd accumulation in the plant biomass (Dixit et al., 2011). In this study, a GST gene (Le_10011457) was up-regulated after Cd exposure. The role of Le_10011457 in Cd enrichment in L. edodes will be verified in future studies.
In conclusion, L. edodes genotypes (Le4606 and Le4625) showed different Cd accumulation capacities, which were attributed to genetic differences. The DEGs between these two genotypes were found to be involved in a variety of biological processes. MFS transporters (Le_10006934, Le_10013475, Le_10001689, Le_10002245, and Le_10008273), CYP450 enzymes (Le_10002240, Le_10002241, Le_10002242, and Le_10005960), and a GST (Le_10011457) may be involved in Cd accumulation in mushrooms. Additionally, Cd enrichment in cells may activate MAPK signaling and the anti-oxidative stress response, which play roles in signal transduction and maintaining intracellular redox balance. Further work should be conducted to delineate the functions of the identified genes and elucidate their regulation mechanisms in Cd enrichment. The candidate genes may be applied in gene engineer to alter the Cd enrichment ability of L. edodes, to develop edible strains with less Cd.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, SRP256428.
Author Contributions
BL and XDS conceived the study design. QL was responsible for the sample preparation and RT-PCR. HY, LZ, and JL performed the bioinformatic analysis. HY completed the initial manuscript. QT, YL, and XFS revised the manuscript. All the authors read and approved the final version of the manuscript.
Funding
This study was supported by Shanghai Agriculture Commission, Shanghai Agriculture Applied Technology Development Program China (2019-02-08-00-08-F01114); China Agriculture Research System (CARS20); Science and Technology Commission of Shanghai Municipality (18391900200); and Shanghai Academy of Agricultural Sciences (SAAS) Program for Excellent Research Team.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2020.558104/full#supplementary-material
Footnotes
- ^ http://www.bioinformatics.babraham.ac.uk/projects/fastqc/
- ^ http://solexaqa.sourceforge.net/
- ^ http://geneontology.org/
- ^ http://www.genome.jp/kegg
- ^ http://bioinfo.ut.ee/primer3-0.4.0/
References
ATSDR (2007). Toxilogical Profile for Cadmium; U. S. Department of Health and Human Services. Atlanta, GA: Public Health Services.
Bartling, D., Radzio, R., Steiner, U., and Weiler, E. W. (1993). A glutathione S-transferase with glutathione-peroxidase activity from Arabidopsis thaliana. Molecular cloning and functional characterization. Eur. J. Biochem. 216, 579–586. doi: 10.1111/j.1432-1033.1993.tb18177.x
Bellion, M., Courbot, M., Jacob, C., Guinet, F., Blaudez, D., and Chalot, M. (2007). Metal induction of a Paxillus involutus metallothionein and its heterologous expression in Hebeloma cylindrosporum. New Phytol. 174, 151–158. doi: 10.1111/j.1469-8137.2007.01973.x
Blabyhaas, C. E., and Merchant, S. S. (2014). Lysosome-related organelles as mediators of metal homeostasis. J. Biol. Chem. 289, 28129–28136. doi: 10.1074/jbc.r114.592618
Boguta, M., Dmochowska, A., Borsuk, P., Wrobel, K., Gargouri, A., Lazowska, J., et al. (1992). NAM9 nuclear suppressor of mitochondrial ochre mutations in Saccharomyces cerevisiae codes for a protein homologous to S4 ribosomal proteins from chloroplasts, bacteria, and eucaryotes. Mol. Cell. Biol. 12, 402–412. doi: 10.1128/mcb.12.1.402
Bolchi, A., Ruotolo, R., Marchini, G., Vurro, E., di Toppi, L. S., Kohler, A., et al. (2011). Genome-wide inventory of metal homeostasis-related gene products including a functional phytochelatin synthase in the hypogeous mycorrhizal fungus Tuber melanosporum. Fungal Genet. Biol. 48, 573–584. doi: 10.1016/j.fgb.2010.11.003
Cao, X., Liu, R., Liu, J., Huo, Y., Yang, W., Zeng, M., et al. (2013). A novel polysaccharide from Lentinus edodes Mycelia exhibits potential antitumor activity on laryngeal squamous cancer cell line Hep-2. Appl. Biochem. Biotechnol. 171, 1444–1453. doi: 10.1007/s12010-013-0441-6
Carra, S., Alberti, S., Arrigo, P. A., Benesch, J. L., Benjamin, I. J., Boelens, W., et al. (2017). The growing world of small heat shock proteins: from structure to functions. Cell Stress Chaperones 22, 601–611.
Chen, L., Gong, Y., Cai, Y., Liu, W., Zhou, Y., Xiao, Y., et al. (2016). Genome sequence of the edible cultivated mushroom Lentinula edodes (Shiitake) reveals insights into Lignocellulose Degradation. PLoS One 11:e0160336. doi: 10.1371/journal.pone.0160336
China Edible Fungi Association [CEFA] (2019). The National Edible Fungi Statistical Survey in 2018. Available online at: http://hz.cefa.com.cn/UploadFiles/news/2020/3/202003191756353016.pdf
Chiu, S. W., Chan, Y. H., Law, S. C., Cheung, K. T., and Moore, D. (1998). Cadmium and manganese in contrast to calcium reduce yield and nutritional values of the edible mushroom Pleurotus pulmonarius. Fungal Biol. 102, 449–457. doi: 10.1017/s0953756297005728
Collin, H. C., Pedersen, S. A., Andersen, R. A., and Steinnes, E. (2007). First report of phytochelatins in a mushroom: induction of phytochelatins by metal exposure in Boletus edulis. Mycologia 99, 161–174. doi: 10.3852/mycologia.99.2.161
Crešnar, B., and Petrič, S. (2011). Cytochrome P450 enzymes in the fungal kingdom. Biochim. Biophys. Acta 1814, 29–35. doi: 10.1016/j.bbapap.2010.06.020
Dai, X., Stanilka, J. M., Rowe, C. A., Esteves, E. A., Nieves, C. Jr., Spaiser, S. J., et al. (2015). Consuming Lentinula edodes (Shiitake) mushrooms daily improves human immunity: a randomized dietary intervention in healthy young adults. J. Am. Coll. Nutr. 34, 478–487. doi: 10.1080/07315724.2014.950391
Degtyarenko, K. (2000). Bioinorganic motifs: towards functional classification of metalloproteins. Bioinformatics 16, 851–864. doi: 10.1093/bioinformatics/16.10.851
Dixit, P., Mukherjee, P. K., Ramachandran, V., and Eapen, S. (2011). Glutathione transferase from Trichoderma virens enhances cadmium tolerance without enhancing its accumulation in transgenic Nicotiana tabacum. PLoS One 6:e16360. doi: 10.1371/journal.pone.0016360
Dong, X. B., Huang, W., Bian, Y. B., Feng, X., Ibrahim, S. A., Shi, D. F., et al. (2019). Remediation and mechanisms of cadmium biosorption by a cadmium-binding protein from Lentinula edodes. J. Agric. Food Chem. 67, 11373–11379. doi: 10.1021/acs.jafc.9b04741
Gardarin, A., Chédin, S., Lagniel, G., Aude, J. C., Godat, E., Catty, P., et al. (2010). Endoplasmic reticulum is a major target of cadmium toxicity in yeast. Mol. Microbiol. 76, 1034–1048. doi: 10.1111/j.1365-2958.2010.07166.x
Guo, S., Yao, Y., Zuo, L., Shi, W., Gao, N., and Xu, H. (2016). Enhancement of tolerance of Ganoderma lucidum to cadmium by nitric oxide. J. Basic Microbiol. 56, 36–43. doi: 10.1002/jobm.201500451
Hall, J. L. (2002). Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 53, 1–11. doi: 10.1093/jexbot/53.366.1
Haq, F., Mahoney, M., and Koropatnick, J. (2003). Signaling events for metallothionein induction. Mutat. Res. 533, 211–226. doi: 10.1016/j.mrfmmm.2003.07.014
Hatvani, N., and Mécs, I. (2003). Effects of certain heavy metals on the growth, dye decolorization, and enzyme activity of Lentinula edodes. Ecotoxicol. Environ. Safe. 55, 199–203. doi: 10.1016/s0147-6513(02)00133-1
Huang, Q., Jia, Y., Wan, Y., Li, H., and Jiang, R. (2015). Market survey and risk assessment for trace metals in Edible Fungi and the substrate role in accumulation of heavy metals. J. Food Sci. 80, H1612–H1618.
Jonak, C., Nakagami, H., and Hirt, H. (2004). Heavy metal stress. Activation of distinct mitogen-activated protein kinase pathways by copper and cadmium. Plant Physiol. 136, 3276–3283. doi: 10.1104/pp.104.045724
Kaur, K., Sharma, A., Capalash, N., and Sharma, P. (2019). Multicopper oxidases: biocatalysts in microbial pathogenesis and stress management. Microbiol. Res. 222, 1–13. doi: 10.1016/j.micres.2019.02.007
Khani, R., Moudi, M., and Khojeh, V. (2017). Contamination level, distribution and health risk assessment of heavy and toxic metallic and metalloid elements in a cultivated mushroom Pleurotus florida (Mont.) singer. Environ. Sci. Pollut. Res. Int. 24, 4699–4708. doi: 10.1007/s11356-016-8222-8
Kumar, S., and Trivedi, P. K. (2018). Glutathione S-transferases: role in combating abiotic stresses including arsenic detoxification in plants. Front Plant Sci. 9:751. doi: 10.3389/fpls.2018.00751
Kwan, S. S., and Brow, D. A. (2005). The N- and C-terminal RNA recognition motifs of splicing factor Prp24 have distinct functions in U6 RNA binding. RNA 11, 808–820. doi: 10.1261/rna.2010905
Langmead, B., Trapnell, C., Pop, M., and Salzberg, S. L. (2009). Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25.
Lao, N. T., Long, D., Kiang, S., Coupland, G., Shoue, D. A., Carpita, N. C., et al. (2003). Mutation of a family 8 glycosyltransferase gene alters cell wall carbohydrate composition and causes a humidity-sensitive semi-sterile dwarf phenotype in Arabidopsis. Plant Mol. Biol. 53, 647–661.
Li, Q., Huang, W., Xiong, C., and Zhao, J. (2018). Transcriptome analysis reveals the role of nitric oxide in Pleurotus eryngii responses to Cd2+ stress. Chemosphere 201, 294–302. doi: 10.1016/j.chemosphere.2018.03.011
Liu, B., Huang, Q., Cai, H., Guo, X., Wang, T., and Gui, M. (2015). Study of heavy metal concentrations in wild edible mushrooms in Yunnan Province, China. Food Chem. 188, 294–300. doi: 10.1016/j.foodchem.2015.05.010
Liu, X. M., Kim, K. E., Kim, K. C., Nguyen, X. C., Han, H. J., Jung, M. S., et al. (2010). Cadmium activates Arabidopsis MPK3 and MPK6 via accumulation of reactive oxygen species. Phytochemistry 71, 614–618. doi: 10.1016/j.phytochem.2010.01.005
Mandelli, F., Couger, M. B., Paixão, D., Machado, C. B., Carnielli, C. M., Aricetti, J. A., et al. (2017). Thermal adaptation strategies of the extremophile bacterium Thermus filiformis based on multi-omics analysis. Extremophiles 21, 775–788. doi: 10.1007/s00792-017-0942-2
Marin, A., Conti, C., and Gobbi, G. (1997). Sorption of lead and caesium by mushrooms grown in natural conditions. Resour. Environ. Biotechnol. 2, 35–49.
Martín-Folgar, R., and Martínez-Guitarte, J.-L. (2017). Cadmium alters the expression of small heat shock protein genes in the aquatic midge Chironomus riparius. Chemosphere 169, 485–492. doi: 10.1016/j.chemosphere.2016.11.067
Mleczek, M., Rzymski, P., Budka, A., Siwulski, M., Jasinska, A., Kalac, P., et al. (2018). Elemental characteristics of mushroom species cultivated in China and Poland. J. Food Composit. Anal. 66, 168–178. doi: 10.1016/j.jfca.2017.12.018
Mleczek, M., Siwulski, M., Rzymski, P., Niedzielski, P., Gąsecka, M., Jasińska, A., et al. (2017). Multi-elemental analysis of Lentinula edodes mushrooms available in trade. J. Environ. Sci. Health B 52, 196–205. doi: 10.1080/03601234.2017.1261551
Mo, Y., Pan, R., Huang, H. W., Cao, L. X., and Zhang, R. D. (2010). Biosorption of Cd (II), Cu (II), Pb (II) and Zn (II) in aqueous solutions by fruiting bodies of macrofungi (Auricularia polytricha and Tremella fuciform-is). Environ. Sci. 31, 1566.
Murakami, R., Singh, C. R., Morris, J., Tang, L., Harmon, I., Takasu, A., et al. (2018). The interaction between the ribosomal stalk proteins and translation initiation factor 5B promotes translation initiation. Mol. Cell. Biol. 38, e00067–18.
Pandey, R. K., Tewari, S., and Tewari, L. (2018). Lignolytic mushroom Lenzites elegans WDP2: laccase production, characterization, and bioremediation of synthetic dyes. Ecotoxicol. Environ. Safe. 158, 50–58. doi: 10.1016/j.ecoenv.2018.04.003
Plaza, G., Lukasik, W., and Ulfig, K. (1996). Sorption of cadmium by filamentous soil fungi. Acta Microbiol. Pol 45, 193–201.
Qin, P. (2016). Biosorption of Heavy Metal Ions by Metallothionein Flocculant Yeast. Ph.D. thesis, Dalian University of Technology, Dalian.
Quistgaard, E. M., Löw, C., Guettou, F., and Nordlund, P. (2016). Understanding transport by the major facilitator superfamily (MFS): structures pave the way. Nat. Rev. Mol. Cell Biol. 17, 123–132. doi: 10.1038/nrm.2015.25
Rajulapati, V., Sharma, K., Dhillon, A., and Goyal, A. (2018). SAXS and homology modelling based structure characterization of pectin methylesterase a family 8 carbohydrate esterase from Clostridium thermocellum ATCC 27405. Arch. Biochem. Biophys. 641, 39–49. doi: 10.1016/j.abb.2018.01.015
Severoglu, Z., Sumer, S., Yalcin, B., Leblebici, Z., and Aksoy, A. (2013). Trace metal levels in edible wild fungi. Int. J. Environ. Sci. Technol. 10, 295–304. doi: 10.1007/s13762-012-0139-2
Stadlbauer, F., Brueckner, A., Rehfuess, C., Eckerskorn, C., Lottspeich, F., Förster, V., et al. (1994). DNA replication in vitro by recombinant DNA-polymerase-alpha-primase. Eur. J. Biochem. 222, 781–793. doi: 10.1111/j.1432-1033.1994.tb18925.x
Stelter, P., Huber, F. M., Kunze, R., Flemming, D., Hoelz, A., and Hurt, E. (2015). Coordinated ribosomal L4 protein assembly into the pre-ribosome is regulated by its eukaryote-specific extension. Mol. Cell 58, 854–862. doi: 10.1016/j.molcel.2015.03.029
Sützl, L., Foley, G., Gillam, E., Bodén, M., and Haltrich, D. (2019). The GMC superfamily of oxidoreductases revisited: analysis and evolution of fungal GMC oxidoreductases. Biotechnol. Biofuels 12, 118.
Thomet, U., Vogel, E., and Krhenbühl, U. (1999). The uptake of cadmium and zinc by mycelia and their ac-cumulation in mycelia and fruiting bodies of edible mushrooms. Eur. Food Res. Technol. 209, 317–324. doi: 10.1007/s002170050502
Vimala, R., and Das, N. (2011). Mechanism of Cd (II) adsorption by macrofungus Pleurotus platypus. J. Environ. Sci. China 23, 288–293. doi: 10.1016/s1001-0742(10)60405-6
Wang, Y., Wang, C., Cheng, W., Bian, Y., and Guo, P. (2016). Removal of cadmium from contaminated Lentinula edodes by optimized complexation and coagulation. Food Sci. Nutr. 5, 215–222. doi: 10.1002/fsn3.384
Xu, Z., Wu, Q., and Yi, Y. (2001). Research progress of heavy metal phytochelatin (PC). J. Agro Environ. Sci. 20, 468–470.
Yoo, S. I., Lee, H. Y., Markkandan, K., Moon, S., Ahn, Y. J., Ji, S., et al. (2019). Comparative transcriptome analysis identified candidate genes involved in mycelium browning in Lentinula edodes. BMC Genomics 20:121. doi: 10.1186/s12864-019-5509-4
Zadrazil, F., and Brunnert, H. (1985). The influence of zinc on the translocation of cadmium and mercury in the fungus Agrocybe aegerita (a model system). J. Appl. Bot. Angew. Botanik 59, 469–477.
Zhang, J., Li, H., Qin, Y., Ye, S., and Liu, M. (2016a). Identification of functional genes involved in Cd (2+) response of Chinese surf clam (Mactra chinensis) through transcriptome sequencing. Environ. Toxicol. Pharmacol. 41, 113–120. doi: 10.1016/j.etap.2015.11.006
Zhang, J., McCann, K. L., Qiu, C., Gonzalez, L. E., Baserga, S. J., and Hall, T. M. (2016b). Nop9 is a PUF-like protein that prevents premature cleavage to correctly process pre-18S rRNA. Nat. Commun. 7, 13085.
Zhang, Q., Zeng, G., Chen, G., Yan, M., Chen, A., Du, J., et al. (2015). The effect of heavy metal-induced oxidative stress on the enzymes in white rot fungus Phanerochaete chrysosporium. Appl. Biochem. Biotechnol. 175, 1281–1293. doi: 10.1007/s12010-014-1298-z
Keywords: Lentinula edodes, Cd accumulation, RNA-Seq, transporter, MAPK
Citation: Yu H, Li Q, Shen X, Zhang L, Liu J, Tan Q, Li Y, Lv B and Shang X (2020) Transcriptomic Analysis of Two Lentinula edodes Genotypes With Different Cadmium Accumulation Ability. Front. Microbiol. 11:558104. doi: 10.3389/fmicb.2020.558104
Received: 01 May 2020; Accepted: 24 August 2020;
Published: 16 September 2020.
Edited by:
Siu Mui Tsai, University of São Paulo, BrazilReviewed by:
Ying Liu, Huazhong Agricultural University, ChinaQuaiser Saquib, College of Science, King Saud University, Saudi Arabia
Copyright © 2020 Yu, Li, Shen, Zhang, Liu, Tan, Li, Lv and Shang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Beibei Lv, beibeiyu0264@126.com; Xiaodong Shang, xdshang@163.com
†These autors have contributed equally to this work
 Hailong Yu1,2†
Hailong Yu1,2† Qiaozhen Li
Qiaozhen Li Qi Tan
Qi Tan Yu Li
Yu Li Beibei Lv
Beibei Lv