- 1Department of Clinical Laboratory, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China
- 2Graduate School, Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing, China
Objectives: The performance of mainstream commercial antimicrobial susceptibility testing systems on polymyxins has not been well evaluated in China. In this study, three antimicrobial susceptibility testing systems were evaluated for polymyxin B and colistin.
Methods: The MICs of 257 Gram-negative strains collected from clinical cases and livestock were determined and analyzed. Using Broth Microdilution as the gold standard, the performance of VITEK 2® COMPACT, PhoenixTM M50, and Bio-kont AST System were evaluated. Essential agreement (EA), category agreement (CA), very major error (VME), and major error (ME) were calculated for comparison. The results of mcr-1 positive strains were separately discussed.
Results: The EA, CA, VME, and ME to polymyxin B for Bio-kont were 83.5, 95.6, 13.1, and 0.6%, respectively. The EAs, CAs, VMEs, and MEs to colistin were as follows: Bio-kont, 86.7%/96.5%/7.2%/1.7%; Vitek 2, 64.2%/86.8%/41.0%/0%, and Phoenix M50, 92.9%/92.9%/21.7%/0%. The performance of Bio-kont to polymyxin B and colistin for Pseudomonas spp. (EA, CA < 90%, VME > 1.5%, ME = 5.6%/10%) and Enterobacter spp. (EA, CA < 90%, VME > 1.5% and ME = 0%), Vitek to colistin for most genera, and Phoenix to colistin for Enterobacter spp. (EA, CA < 90%, VME > 1.5%, ME = 0%) were unsatisfactory compared with other genera. The performance of Bio-kont to polymyxins for Escherichia spp. and Phoenix to colistin for Citrobacter spp., Escherichia spp., and Klebsiella spp., which all met the CLSI standard, were satisfactory. When the susceptibility of mcr-1 positive E. coli was tested, Bio-kont and Phoenix M50 presented excellent performance with no category errors, while Vitek 2 performed a high VME (25.5%).
Conclusion: With relatively more accurate results for polymyxin B and colistin and lower VME, Bio-kont has an advantage in polymyxin antimicrobial susceptibility testing, especially for Escherichia spp., Klebsiella spp., Citrobacter spp. and Acinetobacter spp.
Introduction
Polymyxin B and polymyxin E (colistin) are components of antibiotics produced by fermentation of Paenibacillus polymyxa. Despite the high incidence of nephrotoxicity and neurotoxicity associated with these agents, the family of polymyxins includes A, B, C, D, and E (Nation et al., 2014). Polymyxins have gained more attention in clinical practice due to the high susceptibility of multidrug-resistant (MDR) Gram-negative bacteria. Colistin and polymyxin B differ by just one amino acid in the peptide ring (Nation et al., 2014). Furthermore, polymyxin B is administered as an active form, while colistin in the form of an inactive prodrug, colistin methanesulfonate (CMS), which is also known as colistimethate (Tran et al., 2016).
Colistin or polymyxin B was first used in the 1950s for treating infections caused by Gram-negative MDR pathogens. These antibiotics fell out of favor and were replaced by agents with wider therapeutic indexes and less toxicity (Loho and Dharmayanti, 2015). The polymyxin class has recently re-emerged as a last-line agent in the treatment of multi-drug resistant pathogens non-susceptible to fluoroquinolones, aminoglycosides, and beta lactams (Bialvaei and Samadi Kafil, 2015). However, limited experience and lack of reliable consensus guidelines could potentially result in inappropriate use of these last-line antibiotics by clinicians.
As resistance to polymyxins has posed a great challenge in the treatment of infectious diseases, rapid and accurate detection of polymyxin-resistant strains is needed to prevent and control the outbreak of resistant strains. At present, the broth microdilution (BMD) method is the standard reference method for antimicrobial susceptibility testing (AST). However, the whole procedure is cumbersome and the testing requirements are strict, so it is rarely performed manually in clinical practice. At present, VITEK 2® COMPACT (BioMérieux) and PhoenixTM M50 (Becton Dickson Diagnostics) are the most commonly used AST systems in China. However, these systems have unsatisfactory performance when utilized to determine polymyxin susceptibilities. The purpose of this study is to evaluate the performance of three mainstream antimicrobial susceptibility systems in China on colistin and polymyxin B and to select the most accurate one.
Materials and Methods
Strains
A total of 257 non-repetitive Gram-negative strains were collected from clinical cases (202 mcr-1 negative strains) and livestock (55 mcr-1 positive strains) in China with different polymyxin MICs, including Escherichia spp. (n = 136), Klebsiella spp. (n = 27), Citrobacter spp. (n = 23), Enterobacter spp. (n = 25), Acinetobacter spp. (n = 23), and Pseudomonas spp. (n = 23). All strains were identified by the Vitek MS MALDI-TOF (BioMérieux) system.
Antimicrobial Susceptibility Testing
BMD, the gold-standard reference method for AST, was performed strictly in accordance with the CLSI M7-A10 document (Clinical and Laboratory Standards Institute, 2015). The minimum inhibitory concentrations (MICs) of colistin or polymyxin B were measured only when the growth control was acceptable. Escherichia coli ATCC25922 and Pseudomonas aeruginosa ATCC27853 were used as quality controls. The performance of three commercial methods, including VITEK 2® COMPACT (BioMérieux) with AST-N335 card (colistin), PhoenixTM M50 (Becton Dickson Diagnostics) with NMIC-413 card (colistin), and Bio-kont AST System (Wenzhou Bio-kont) with polymyxin AST card (colistin and polymyxin B) were evaluated. The AST results of each test strain were considered accurate only when the MIC results of quality control strains were in the QC range.
Data Analysis
MICs were analyzed according to the CLSI M100-E30 document (CLSI, 2020) and EUCAST clinical breakpoints-bacteria (v 10.0) (EUCAST, 2020). To date, the MIC of 2 mg/L has been the CLSI intermediate breakpoints of polymyxin B and colistin for Enterobacteriaceae, Pseudomonas, and Acinetobacter. Given that there is no breakpoint for the susceptible, results that were interpreted as “intermediate” were treated as “susceptible” in order to better calculate ME and VME, which is in accordance with the EUCAST standard. We interpreted the results in this manner: susceptible ≤2mg/L, resistance ≥4mg/L (Humphries et al., 2019).
Using BMD as the reference method, the following parameters are included in the assessment: Essential agreement (EA) was defined as a percentage of MICs measured by the system within ± 1 dilution of reference MICs. Category agreement (CA) represents the percentage of results with the same susceptibility categorization as BMD. Very major error (VME) stands for the percentage of false susceptible results compared to BMD. Major error (ME) means the percentage of false resistant results compared to BMD. According to CLSI recommendations, a new system can be acceptable when it meets the standards as follows: CA ≥ 90%, EA ≥ 90%, VME ≤ 1.5%, and ME ≤ 3% (CLSI, 2015).
Results
Antimicrobial Susceptibility Test
The MICs of quality control strains were all within the expected reference ranges specified by CLSI M100-S30 (CLSI, 2020). Susceptibilities to polymyxin B and colistin were tested in all 257 strains by BMD. Due to differences in AST cards, both drugs were not always tested by each system. Susceptibilities to polymyxin B were not reported by Vitek 2 and Phoenix M50. Due to loss in the tests, not all strains were reported by each system.
The general agreement between BMD and three AST systems for polymyxin B and colistin is shown in Figure 1. The performance of Bio-kont for polymyxin B and colistin both showed acceptable CAs (both 99.4%) and unacceptable EAs (77.8 and 82.2%, respectively) for susceptible strains identified by BMD. For resistant strains, the performances resulted in lower CAs (88.1 and 92.6%, respectively) and improved EAs (89.3 and 93.8%, respectively). The Vitek 2 system performed poorly with only 86.8% of results classified as CA and 62.6% classified as EA compared to the reference method. The performance of Phoenix M50 was better especially for susceptible strains with 100% results in both CA and EA. And for resistant strains, CA was 77.8% and EA was 70.4%.
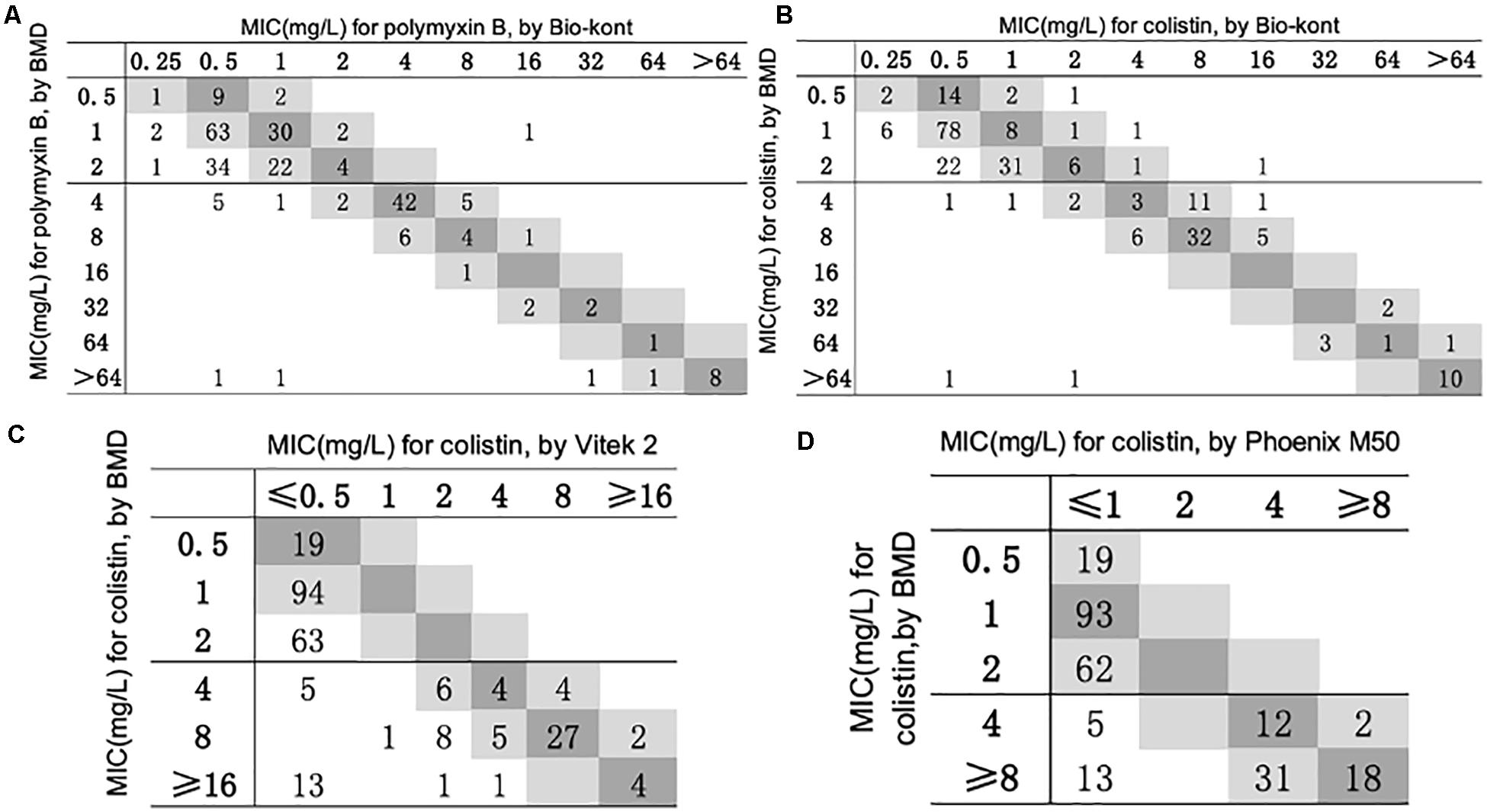
Figure 1. Scatterplot of three AST methods versus reference MIC obtained from BMD. The BMD MICs have been normalized in the scatterplots of Phoenix M50 and Vitek 2. When compared with Vitek 2, the maximum test limit is ≥ 16 mg/L, while compared with Phoenix M50, it is ≥ 8mg/L. (A) Scatterplot of Bio-kont versus reference MIC obtained from BMD for polymyxin B. (B) Scatterplot of Bio-kont versus reference MIC obtained from BMD for colistin. (C) Scatterplot of Vitek 2 versus reference MIC obtained from BMD for colistin. (D) Scatterplot of Phoenix M50 versus reference MIC obtained from BMD for colistin.
The detailed statistics of three AST systems for 257 strains to polymyxin B and colistin are presented in Tables 1, 2.
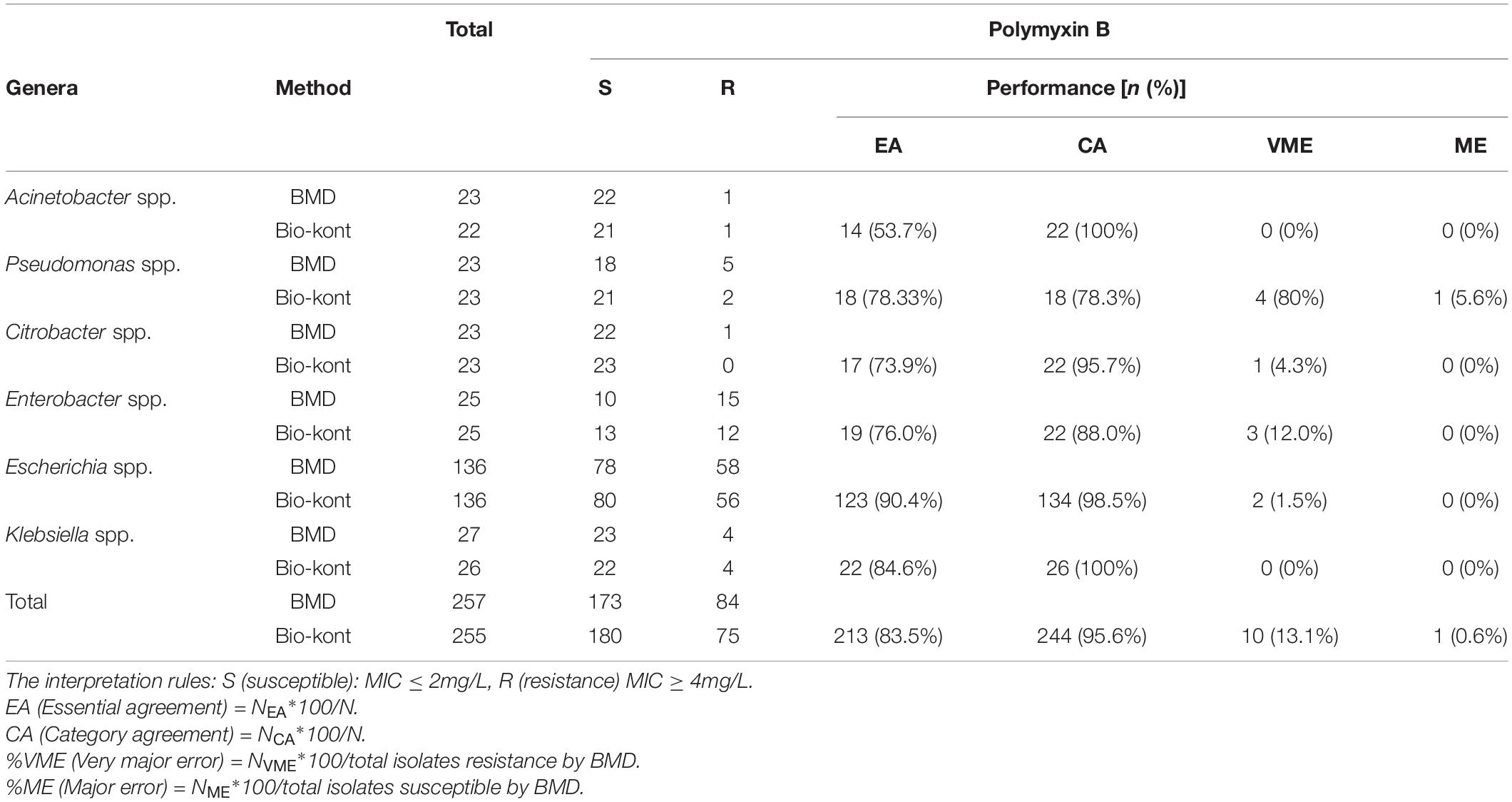
Table 1. Comparison of performance characteristics to polymyxin B between Bio-kont AST System and BMD method for the seven genera in the 257 strains.
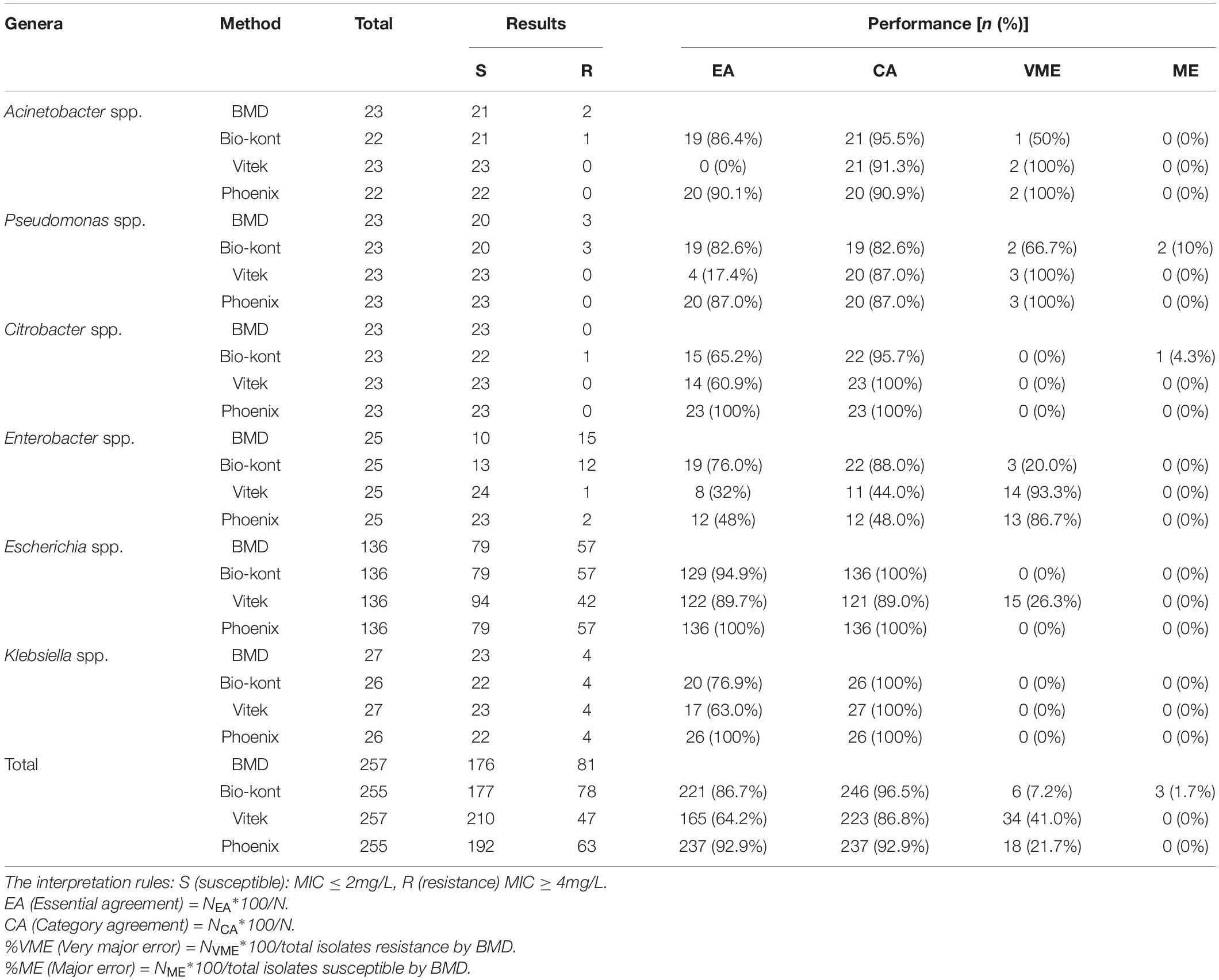
Table 2. Comparison of performance characteristics to colistin between each of the three AST systems and BMD method for the seven genera in the 257 strains.
As for polymyxin B, the Bio-kont AST System showed a CA ≥ 90% and a ME = 0% for most genera except Pseudomonas spp. and Enterobacter spp. However, immense diversity in EA was observed from 53.7 to 90.4% and high VMEs were showed in Pseudomonas spp. (80%), Citrobacter spp. (4.3%), and Enterobacter spp. (12%). The only species meeting the requirement of an acceptable system (CA = 90.4%, EA = 98.5%, VME = 1.5%, and ME = 0%) was Escherichia spp. against polymyxin B. EA for strains of Acinetobacter spp. for polymyxin B was merely 53.7%, though with high CA of 100%. The MICs of these strains tested by Bio-kont AST System are generally lower than those of BMD. The total EA rate of Bio-kont for polymyxin B was 83.5%, while the CA rate was 95.6%. Additionally, several VMEs (13.1%) and few ME (0.6%) were observed. CA and ME of Bio-kont met the required standards, and EA and VME did not meet the standards.
As for colistin, compared with BMD, the total EA was 86.7% for Bio-kont, 64.2% for Vitek 2, and 92.9% for Phoenix M50. Among the three AST systems, Vitek 2 showed the worst performance with the lowest EAs for each genus tested in the study, especially in Acinetobacter spp., Pseudomonas spp., and Enterobacter spp. In general, Phoenix M50 presented better EAs, followed by Bio-kont. The total CA was 96.5% for Bio-kont, 86.8% for Vitek 2 and 92.9% for Phoenix M50. Of particular attention, the systems yielded numerous VMEs: 7.2% for Bio-kont, 41.0% for Phoenix M50, and 21.7% for Vitek 2, mainly in Acinetobacter spp., Pseudomonas spp. and Enterobacter spp. along with a high VME (26.3%) from Vitek 2 in Escherichia spp. Very limited ME rates of 1.7% from Bio-kont and 0% from both Vitek 2 and Phoenix M50 were observed. The respective MEs were all 0% except Pseudomonas spp. from Bio-kont (10%) and Citrobacter spp. from Bio-kont (4.3%). On the whole, only the EA of Phoenix M50, CAs of Bio-kont and Phoenix M50, and MEs of three systems met the required standards.
To be more specific, although CAs were all >90%, the particularly high VMEs of Acinetobacter spp. from three systems were worth exploring. Additionally, the performance characteristics of Pseudomonas spp. to colistin were barely satisfactory with low EAs (Bio-kont 82.6%, Vitek 2 17.4%, Phoenix M50 87.0%), low CAs (Bio-kont both 82.6%, Vitek 2 and Phoenix M50 both 87.0%), and high VMEs (Bio-kont 66.7%, Vitek 2 and Phoenix M50 both 100%) and MEs (Bio-kont 10%, Vitek 2 0%, Phoenix M50 0%). The similar unsatisfactory performance of Enterobacter spp. to colistin was presented (low EAs: Bio-kont 76.0%, Vitek 2 32%, Phoenix M50 48%, low CAs: Bio-kont 88.0%, Vitek 2 44.0%, Phoenix M50 48.0%; high VMEs: Bio-kont 20.0%, Vitek 2 93.3%, Phoenix M50 86.7%).
The EAs for Escherichia spp. to colistin were highest (Bio-kont 94.9%, Vitek 2 89.7%, Phoenix M50 100%). No VMEs or MEs for Escherichia spp. to colistin from Bio-kont and Phoenix M50 were observed to have fully consistent agreement with BMD, which indicated the agreement of Bio-kont and Phoenix M50 with the rules (CA ≥ 90%, EA ≥ 90%, VME ≤ 1.5%, ME ≤ 3%).
Except for EAs, the performance characteristics of Vitek 2 and Phoenix M50 were similar in terms of colistin in three genera (for Acinetobacter spp.: CA ≥ 90%, VME = 100%, and ME = 0%; for Citrobacter spp. and Klebsiella spp.: CA = 100%, VME = 0%, ME = 0%).
In all, Bio-kont presented the best performance in the tested genus. However, the performance of Pseudomonas spp. and Enterobacter spp. requires further research and a larger sample size.
Detection of mcr-1 Gene and Performance Evaluation on mcr-1-Positive/Negative Strains
Among 257 Gram-negative bacteria strains, 55 strains were positive for mcr-1 gene and all of them were E. coli. As mcr-1 gene strongly indicates the resistance of colistin and polymyxin B, the susceptibilities of 55 mcr-1-positive strains are verified according to BMD. The MIC distribution of 55 E. coli strains is as follows. For polymyxin B, the MICs of 45 mcr-1-positive strains were 4 mg/L and those of 10 strains were 8 mg/L. For colistin, the MICs of 12 strains were 4 mg/L and those of 43 strains were 8 mg/L. All mcr-1-positive strains were low-level resistant to colistin (MIC: 4–8 mg/L) and polymyxin B (MIC: 4–8 mg/L). These strains can be classified as colistin-resistant and polymyxin B-resistant strains according to either CLSI standard document (CLSI, 2020) or EUCAST standard (EUCAST, 2020), which corresponds to the genomic explanation.
Utilizing BMD as standard, the performance of three AST systems for 55 mcr-1 positive E. coli strains and other mcr-1-negative strains to polymyxin B and colistin are presented in Table 3.
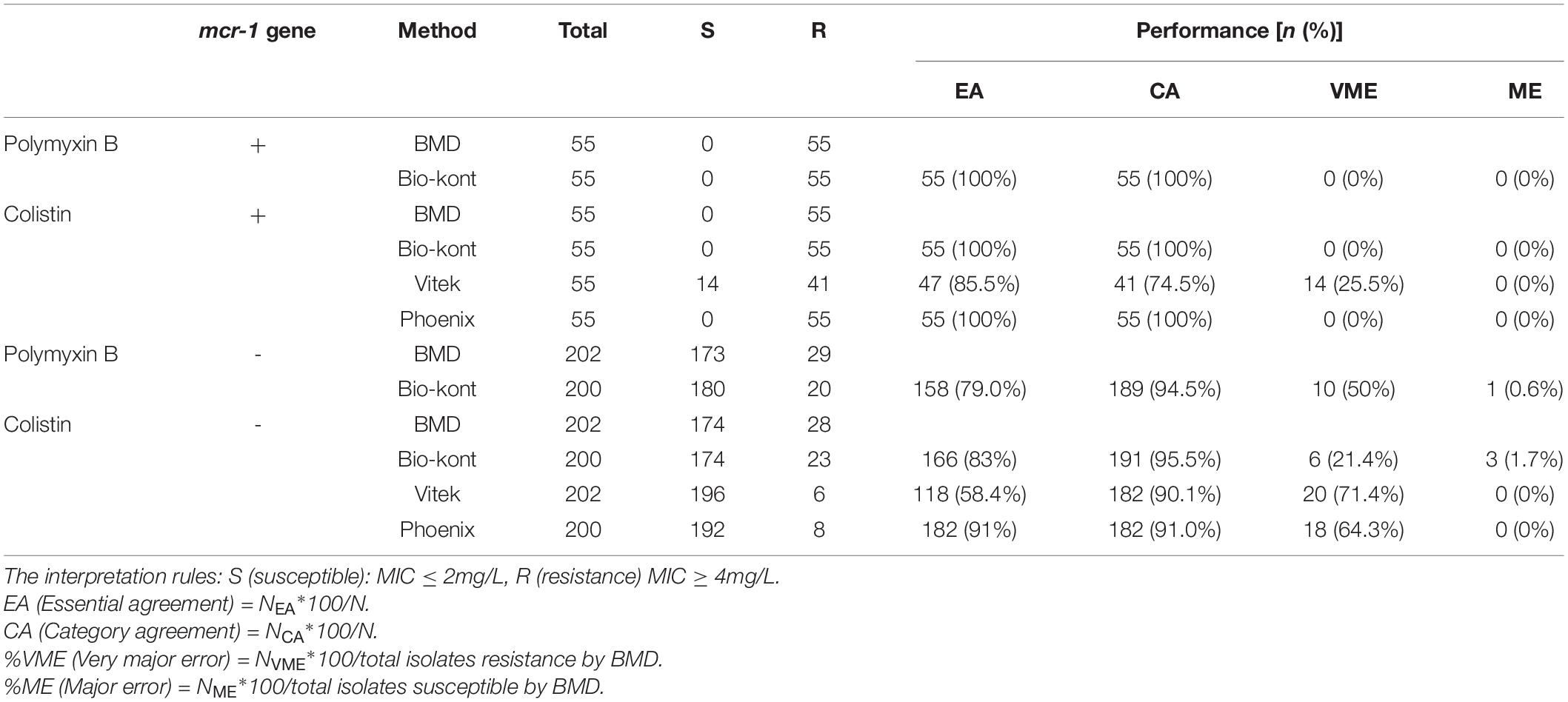
Table 3. Comparison of performance characteristics to colistin and polymyxin B between each of the three AST systems and BMD method for strains of different mcr-1 genetic conditions.
Compared with BMD, Bio-kont system and Phoenix M50 system presented excellent performance with no category errors when the susceptibility of mcr-1-positive E. coli was tested. It was worth noting that high VME rates (14/55, 25.5%) were observed from Vitek 2 system in mcr-1-positive E. coli strains. As for mcr-1-negative strains, each system showed acceptable rates of CA with several errors. The performance of Bio-kont was better than the other two systems with fewer errors. Noticeably high error rates were presented in both Vitek 2 system and Phoenix M50 system in mcr-1-negative strains. In conclusion, influence from mcr-1 gene to Bio-kont and Phoenix M50 is minor, while that to Vitek 2 maybe need more data for analysis.
Discussion
In this study, we evaluated the performance of three systems: Vitek 2, Phoenix M50, and Bio-kont. Vitek 2 and Phoenix M50 are two of the most commonly used AST systems in China while Bio-kont is a newly developed Chinese system that has obtained a marketing license. A joint EUCAST and CLSI polymyxin breakpoint working group recommended standard BMD as the reference method for the MIC testing of colistin (Carretto et al., 2018; Tsuji et al., 2019). However, diversity in instrument manufacturers, software stability, and even AST panels with different concentration gradients could make a difference in MIC results. With the preclinical PK/PD, clinical PK/TD, and MIC distribution data reviewed, the category of susceptibility was deemed to be inappropriate by CLSI, and an intermediate-only category was established as this category identifies isolates “that approach usually attainable blood and tissue levels and/or for which response rates may be lower than for susceptible isolates” (CLSI, 2020; Satlin et al., 2020). The absence of a susceptible category of the CLSI standard (CLSI, 2020) promoted us to cast light on the analysis referred to EUCAST standard (EUCAST, 2020).
Based on our study, none of the systems in this study met the standards for colistin and polymyxin B AST compared to BMD. Generally, Bio-kont was the most satisfactory system with the highest CA and least errors; it was followed by Phoenix M50, which had higher EA (92.9%), acceptable CA (92.9%), but more errors (21.7%). Vitek 2 showed the worst performance with low EA (64.2%), unacceptable CA (86.8%), and unexpectedly high VME (41.0%). The performances of the Vitek 2 and Phoenix M50 systems for colistin susceptibility test have been estimated before by Vourli et al. with a similar CA as this study (89.7% vs. 86.8% for Vitek 2 and 88.9% vs. 92.9% for Phoenix M50) (Vourli et al., 2017). In a study conducted by Ka Lip Chew et al., a VME rate of 36% for colistin testing by Vitek 2 was demonstrated (Chew et al., 2017). However, research into the Bio-kont system is limited as it is mainly applied in China. The evolution results of this system in this study has proved that this system—with satisfactory CA and an acceptable error rate—has better promotion value.
The problem of considerable errors in the detection of Pseudomonas spp. and Enterobacter spp. existed in all systems. In terms of Pseudomonas spp., the MICs of these error-prone strains were 4 mg/L which were interpreted as ATU (EUCAST) or R—the dividing value (CLSI). It is worth mentioning that the error-prone Enterobacter spp. strains tested by Vitek 2 and Phoenix M50 were all highly resistant to colistin (MIC>64 mg/L). False susceptibility in polymyxin B for Enterobacteriaceae from Vitek 2 and Phoenix M50 has been reported before, which was suspected to be the result of a smaller inoculum size effect (Doern et al., 2011; Lat et al., 2011; Bobenchik et al., 2015; Zhou et al., 2018). Currently, the CLSI still felt it important to acknowledge that available data suggested limited clinical effectiveness of the polymyxins for the Enterobacterales, P. aeruginosa, and Acinetobacter spp. (Satlin et al., 2020). The results of this study require validation through further research.
The notable errors in Escherichia spp. from Vitek 2 were mainly from mcr-1-positive strains. In 2017, colistin was formally forbidden from animal feeds in China (China, 2017). Whether poor reliability of colistin susceptibility testing by Vitek 2 for mcr-1-positive E. coli is an objective existence or an occasional occurrence remains unknown.
Meanwhile, it could be observed that the MICs of colistin in several susceptible isolates tested by the Vitek 2 system were one- to two-fold dilutions lower than those of BMD, indicating that strains classified as S and I by Vitek 2 system should be verified by BMD (Vourli et al., 2017). More studies are needed to further interpret the poor performance of systems and to support the interpretation of AST results.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The Human Research Ethics Committee of Peking Union Medical College Hospital approved this study and waived the need for consent (Ethics Approval Number: S-K771).
Author Contributions
YZ wrote the manuscript. PJ, MZ, QY, and YX revised the manuscript. JZ, GZ, WK, SD, and TW performed the experiments. All authors approved the final version of the manuscript.
Funding
This study was supported by National Natural Science Foundation of China (82072318), National Key Research and Development Program of China (2018YFE0101800 and 2018YFC1200105), CAMS Initiative for Innovative Medicine (Grant No. 2016-I2M-3-014), and Beijing Key Clinical Specialty for Laboratory Medicine – Excellent Project (No. ZK201000).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Bialvaei, A. Z., and Samadi Kafil, H. (2015). Colistin, mechanisms and prevalence of resistance. Curr. Med. Res. Opin. 31, 707–721. doi: 10.1185/03007995.2015.1018989
Bobenchik, A. M., Deak, E., Hindler, J. A., Charlton, C. L., and Humphries, R. M. (2015). Performance of Vitek 2 for antimicrobial susceptibility testing of Enterobacteriaceae with Vitek 2 (2009 FDA) and 2014 CLSI breakpoints. J. Clin. Microbiol. 53, 816–823. doi: 10.1128/JCM.02697-14
Carretto, E., Brovarone, F., Russello, G., Nardini, P., El-Bouseary, M. M., Aboklaish, A. F., et al. (2018). Clinical validation of SensiTest colistin, a broth microdilution-based method to evaluate colistin MICs. J. Clin. Microbiol. 56, e1523–17. doi: 10.1128/JCM.01523-17
Chew, K. L., La, M. V., Lin, R. T. P., and Teo, J. W. P. (2017). Colistin and polymyxin B susceptibility testing for carbapenem-resistant and mcr-positive Enterobacteriaceae: comparison of sensititre, microscan, Vitek 2, and Etest with broth microdilution. J. Clin. Microbiol. 55, 2609–2616. doi: 10.1128/JCM.00268-17
China (2017). Announcement of the Ministry of Agriculture of the People’s Republic of China No. 2428. Available online at: http://www.moa.gov.cn/nybgb/2016/dibaqi/201712/t20171219_6102822.htm (accessed December 01, 2017)
Clinical and Laboratory Standards Institute (2015). Methods for Dilution Antimicrobial Susceptibility Test for Bacteria That Grow Aerobically Document in M07-A9, 10th Edn. Wayne, PA: Clinical and Laboratory Standards Institute.
CLSI (2015). Verification of Commercial Microbial Identification and Antimicrobial Susceptibility Testing Systems–First Edition: M52-Ed1. Wayne, PA: Clinical and Laboratory Standards Institute.
CLSI (2020). Performance Standards for Antimicrobial Susceptibility Testing, Twenty-Fifth Informational Supplement in M100-S30. Wayne, PA: Clinical and Laboratory Standards Institute.
Doern, C. D., Dunne, W. M. Jr., and Burnham, C. A. (2011). Detection of Klebsiella pneumoniae carbapenemase (KPC) production in non-Klebsiella pneumoniae Enterobacteriaceae isolates by use of the Phoenix, Vitek 2, and disk diffusion methods. J. Clin. Microbiol. 49, 1143–1147. doi: 10.1128/JCM.02163-10
EUCAST (2020). EUCAST Clinical Breakpoints – Bacteria (v 10.0). Växjö: European Committee on Antimicrobial Susceptibility Testing.
Humphries, R. M., Green, D. A., Schuetz, A. N., Bergman, Y., Lewis, S., Yee, R., et al. (2019). Multicenter evaluation of colistin broth disk elution and colistin agar test: a report from the clinical and laboratory standards institute. J. Clin. Microbiol. 57, e1269–19. doi: 10.1128/JCM.01269-19
Lat, A., Clock, S. A., Wu, F., Whittier, S., Della-Latta, P., Fauntleroy, K., et al. (2011). Comparison of polymyxin B, tigecycline, cefepime, and meropenem MICs for KPC-producing Klebsiella pneumoniae by broth microdilution, Vitek 2, and Etest. J. Clin. Microbiol. 49, 1795–1798. doi: 10.1128/JCM.02534-10
Loho, T., and Dharmayanti, A. (2015). Colistin: an antibiotic and its role in multiresistant Gram-negative infections. Acta Med. Indones. 47, 157–168.
Nation, R. L., Velkov, T., and Li, J. (2014). Colistin and polymyxin B: peas in a pod, or chalk and cheese? Clin. Infect. Dis. 59, 88–94. doi: 10.1093/cid/ciu213
Satlin, M. J., Lewis, J. S., Weinstein, M. P., Patel, J., Humphries, R. M., Kahlmeter, G., et al. (2020). Clinical and Laboratory Standards Institute (CLSI) and European Committee on Antimicrobial Susceptibility Testing (EUCAST) position statements on polymyxin B and colistin clinical breakpoints. Clin. Infect. Dis. 71, e523–e529. doi: 10.1093/cid/ciaa121
Tran, T. B., Velkov, T., Nation, R. L., Forrest, A., Tsuji, B. T., Bergen, P. J., et al. (2016). Pharmacokinetics/pharmacodynamics of colistin and polymyxin B: are we there yet? Int. J. Antimicrob. Agents 48, 592–597. doi: 10.1016/j.ijantimicag.2016.09.010
Tsuji, B. T., Pogue, J. M., Zavascki, A. P., Paul, M., Daikos, G. L., Forrest, A., et al. (2019). International consensus guidelines for the optimal use of the polymyxins: endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti−infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacother. J. Hum. Pharmacol. Drug Ther. 39, 10–39. doi: 10.1002/phar.2209
Vourli, S., Dafopoulou, K., Vrioni, G., Tsakris, A., and Pournaras, S. (2017). Evaluation of two automated systems for colistin susceptibility testing of carbapenem-resistant Acinetobacter baumannii clinical isolates. J. Antimicrob. Chemother. 72, 2528–2530. doi: 10.1093/jac/dkx186
Keywords: antimicrobial susceptibility testing, polymyxin, essential agreement, category agreement, very major error, major error
Citation: Zhu Y, Jia P, Zhou M, Zhang J, Zhang G, Kang W, Duan S, Wang T, Xu Y and Yang Q (2021) Evaluation of the Clinical Systems for Polymyxin Susceptibility Testing of Clinical Gram-Negative Bacteria in China. Front. Microbiol. 11:610604. doi: 10.3389/fmicb.2020.610604
Received: 07 October 2020; Accepted: 15 December 2020;
Published: 22 February 2021.
Edited by:
Alberto Quesada, University of Extremadura, SpainReviewed by:
David Burgess, University of Kentucky, United StatesRaquel Girardello, Sao Francisco University, Brazil
Copyright © 2021 Zhu, Jia, Zhou, Zhang, Zhang, Kang, Duan, Wang, Xu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiwen Yang, yangqiwen81@vip.163.com
 Ying Zhu
Ying Zhu Peiyao Jia
Peiyao Jia Menglan Zhou
Menglan Zhou Jingjia Zhang
Jingjia Zhang Ge Zhang
Ge Zhang Wei Kang1
Wei Kang1 Simeng Duan
Simeng Duan Yingchun Xu
Yingchun Xu Qiwen Yang
Qiwen Yang