- 1Department of Pathobiology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
- 2Department of Bacteriology and Virology, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
- 3Department of Bacteriology, Pasteur Institute of Iran, Tehran, Iran
- 4Department of Microbiology, Islamic Azad University, Arak, Iran
- 5Nervous System Stem Cells Research Center, Semnan University of Medical Sciences, Semnan, Iran
- 6Department of Medical Microbiology, School of Medicine, Shahed University, Tehran, Iran
- 7Pediatric Pulmonary Disease and Sleep Medicine Research Center, Pediatric Center of Excellence, Children's Medical Center, Tehran, Iran
- 8Cystic Fibrosis Research Center, Iran CF Foundation (ICFF), Tehran, Iran
Cystic fibrosis (CF) is a genetic ailment caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. This autosomal recessive disorder is characterized by diverse pathobiological abnormalities, such as the disorder of CFTR channels in mucosal surfaces, caused by inadequate clearance of mucus and sputum, in addition to the malfunctioning of mucous organs. However, the primary motive of mortality in CF patients is pulmonary failure, which is attributed to the colonization of opportunistic microorganisms, formation of resistant biofilms, and a subsequent decline in lung characteristics. In December 2019, the World Health Organization (WHO) declared the outbreak of the radical coronavirus disease 2019 (COVID-19) as a worldwide public health crisis, which unexpectedly spread not only within China but also globally. Given that the respiration system is the primary target of the COVID-19 virus, it is crucial to investigate the impact of COVID-19 on the pathogenesis and mortality of CF patients, mainly in the context of acute respiratory distress syndrome (ARDS). Therefore, the goal of this review is to comprehensively review the present literature on the relationship between cystic fibrosis, COVID-19 contamination, and development of ARDS. Several investigations performed during the early stages of the virus outbreak have discovered unexpected findings regarding the occurrence and effectiveness of COVID-19 in individuals with CF. Contrary to initial expectancies, the rate of infection and the effectiveness of the virus in CF patients are lower than those in the overall population. This finding may be attributed to different factors, including the presence of thick mucus, social avoidance, using remedies that include azithromycin, the fairly younger age of CF patients, decreased presence of ACE-2 receptors, and the effect of CFTR channel disorder on the replication cycle and infectivity of the virus. However, it is important to notice that certain situations, which include undergoing a transplant, can also doubtlessly boost the susceptibility of CF patients to COVID-19. Furthermore, with an increase in age in CF patients, it is vital to take into account the prevalence of the SARS-CoV-2 virus in this population. Therefore, ordinary surveillance of CF patients is vital to evaluate and save the population from the capability of transmission of the virus given the various factors that contribute to the spread of the SARS-CoV-2 outbreak in this precise organization.
Introduction
Cystic fibrosis (CF) is a genetic ailment resulting from an autosomal recessive trait. In the United States of America, the estimated prevalence of CF disease is 0.797 per 10,000 individuals; at the same time, among Caucasians who are born alive, the superiority is 1 in 2,500 (Stern, 1997). An association related to CF has expressed concerns about the potential impact of COVID-19 on CF patients, as previous viral infections have been associated with severe outcomes and consequences. Furthermore, a significant number of CF patients already have impaired lung function (Lambrecht et al., 2001). In the United States of America, the estimated prevalence of CF disease is 0.797 per 10,000 individuals, while in Caucasians who are born alive, the prevalence is 1 in 2,500 (Stern, 1997). The CF association is expressing significant concerns about the potential impact of the coronavirus disease on individuals with CF, as previous viral infections have been linked to more severe outcomes. Additionally, a significant proportion of CF patients already have impaired lung function (Lambrecht et al., 2001).
Etiopathology of cystic fibrosis (CF)
The prevalence of CF has increased due to a genetic mutation within a specific gene located on chromosome 7. This gene encodes a protein referred to as the cystic fibrosis transmembrane conductance regulator (CFTR), which consists of 1,480 amino acids. The CFTR protein has a vital function in regulating the movement of electrolytes at some point of epithelial-cell membranes, and it is also believed to have an effect on intracellular membranes (Stern, 1997; Terlizzi et al., 2022a,b).
One of the foremost complications related to CF is continuous pulmonary infections, which might be the leading cause of respiratory failure in individuals with CF (Meng et al., 2020). A study carried out with the aid of the Cystic Fibrosis Foundation Patient Registry (CFFPR) from 2017 to 2021 centered on newborn infants and found that CF patients have a median lifespan of ~53 years (Taylor-Cousar et al., 2023). In individuals with CF, the presence of two faulty CF genes and malfunctioning elements of the genetic machinery can result in multiplied stages of mucus in diverse organs, which can cause similar complications and impair the regular functioning of these organs.
This situation could have detrimental effects on the lungs, pancreas, liver, intestines, and salivary glands. On the other hand, although the CFTR channel and chloride ions are not functioning properly due to insufficient shipping, it causes the production of thick mucus. Mucous production obstructs the airways, making it hard to breathe and increasing the probability of bacterial lung infections in affected individuals (Blevings et al., 2022).
Immune responses in cystic fibrosis
CF patients affected with COVID-19 show an exaggerated inflammatory reaction characterized by the release of positive chemical compounds and cells such as IL-8, TNF, mucin, polymorphonuclear leukocytes (PMNs), and serine proteases. These robust inflammatory reactions contribute to the further development of CF symptoms. The abnormal innate immune responses found in CF patients may be attributed to the CFTR mutation, which negatively affects the function of the epithelial innate immune system.
Under these circumstances, the NF-κB pathway, which induces activation of the positive genes, becomes active, resulting in a multiplied secretion of IL-8. The law of TLR4 and IFN-γ expression and accumulation is compromised in a weakened immune system. Additionally, the activation of pulmonary dendritic cells (DCs), which play a crucial role in T-cell-established immunity and immune surveillance, is decreased (Lambrecht et al., 2001).
Epidemiology and clinical outcomes of COVID-19
Among the extremely good human coronaviruses (HCoVs), the novel coronavirus (SARS-CoV-2) is significant. HCoVs, which are RNA-enveloped viruses, can be transmitted from animals, such as rodent and bat families, to human beings. In December 2019, the World Health Organization (WHO) identified SARS-CoV-2 in Wuhan, China, ultimately leading to the prevalence of acute respiration distress syndrome (ARDS). On 11 March 2020, the novel coronavirus, known as COVID-19, was declared an international pandemic. It is crucial to highlight that SARS-CoV-2 is a newly diagnosed strain that has not been previously detected in humans. The outbreak ended with over 381,000 individuals being infected across 195 countries, with the number of deaths exceeding 16,000. In patients having inflammation with severe acute respiration syndrome coronavirus 2 (SARS-CoV-2), common signs and symptoms include low-grade fever, fatigue, dry cough, sore throat, diarrhea, anosmia, and loss of flavor or scent. While many people with this contamination might also be asymptomatic or experience slightly higher breathing tract symptoms, there are times when they will develop intense and acute respiratory distress syndrome (ARDS; Chams et al., 2020; Chen et al., 2020). Contracting COVID-19 can bring about numerous organ-related troubles, such as acute kidney injury, vascular blood clots, endothelial cell damage, and shock. These issues and headaches can substantially increase pressure on society and healthcare systems (Bradley et al., 2020; Chams et al., 2020; Yohannes, 2021).
Microbiome changes linked to COVID-19
The microbiome consists of a wide variety of microorganisms, including bacteria, fungi, viruses, and protozoans, that live in distinct organs at some point in the human body. These microorganisms have a substantial function in regulating cell metabolisms and biological signaling pathways (Gilbert et al., 2018). Evidence supports the critical involvement of the microbiome community, especially intestinal microbiota, in maintaining a properly functioning bodily system, immune responses, and metabolic functions. In cystic fibrosis patients, there is regular dysbiosis of the intestinal microbiota over time, characterized by a reduction in the abundance of useful bacteria and an increase in pathogens. This imbalance can bring about inflammation and compromise immune responses. However, the impact of this dysbiosis on the susceptibility to or transmission of COVID-19 in these patients remains inadequately explored. Subsequent medical evaluations have found the prevalence of intestinal dysbiosis and changes in the respiration microbiome in people affected by COVID-19 (Segal et al., 2020). An observation in 2022 potentiated that the depletion of Faecalibacterium prausnitzii ought to contribute to respiratory and lung disorders along with allergies and cystic fibrosis (Vernocchi et al., 2018; Demirci et al., 2019). F. prausnitzii is believed to possess anti-inflammatory properties, protecting against various gastrointestinal illnesses, including Crohn's disease (Parada Venegas et al., 2019; Leylabadlo et al., 2020). Furthermore, more recent research on COVID-19-recovered patients with persistent symptoms discovered that post-exercising chest tightness becomes inversely associated with the relative abundance of F. prausnitzii (Zhou et al., 2021). Throughout the duration of hospitalization in Hong Kong, the stool samples of the 15 COVID-19 patients displayed the growth of opportunistic bacteria such as Rothia, Streptococcus, Actinomyces, and Veillonella, as indicated by similar examinations. Additionally, a lower bacterial range was found in these samples (Gu et al., 2020). Moreover, alterations in the gut and lung microbiomes may facilitate the infiltration of the SARS-CoV-2 virus into lung tissue. The lung microbiomes play critical roles in the onset, development, and efficacy of therapeutic interventions (Ye et al., 2020).
Entry of SARS-CoV-2 of SARSCoV-2; outcome in CF patients
The SARS-CoV-2 virus enters cells by using the spike (S) protein, which is located on its surface. This protein is made up of two parts, S1 and S2. The S1 subunit attaches to the angiotensin-converting enzyme 2 (ACE2), which is the main receptor on the surface of certain cells in the airway epithelium. These cells include airway epithelial cells, goblet secretory cells, and type II cells during pneumocystis infection. When the virus enters a host cell, the S1 domain of the S protein binds to ACE2, causing the S1 subunit to be cut by cellular proteases. Then, the S2 subunit helps the viral membrane fuse with the host cell membrane, allowing viral components to be released into the host cell's cytoplasm (Shirato et al., 2017; Chams et al., 2020; Touret et al., 2020; Zhao et al., 2022).
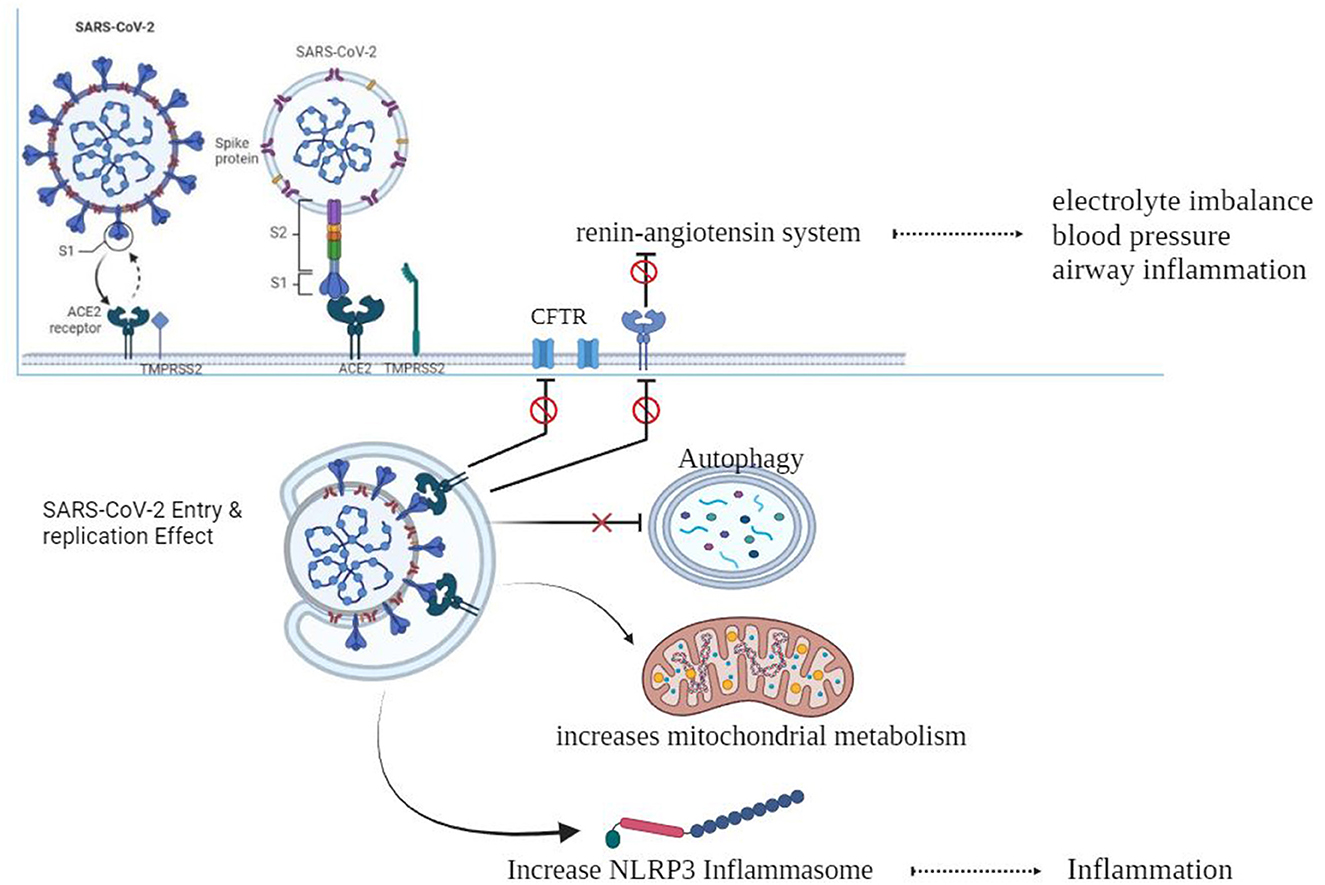
As shown in Figure 1, When the spike protein attaches to the ACE2 cell membrane protein, an association is formed between the virus and the cell. The enzymes TMPRSS2 and furin help in the entry of the virus into the cell, and individuals with CF may have altered versions of these enzymes (Chams et al., 2020; Peckham et al., 2020). There is a z report of a decreased level of TMPRSS2 among CF patients compared to the control group, although it was not significant, and there is also an increase in the TMPRSS2 enzyme in CF patients by flagellin of Pseudomonas aeruginosa (Bitossi et al., 2021; Ruffin et al., 2021).
After entering the cell, the virus can be affected by excessive inflammation and CF-related cellular processes like autophagy, mitophagy, endosomal function, and cellular metabolism. SARS-CoV-2 may take advantage of these cellular processes to replicate itself (Peckham et al., 2020).
CF can result in electrolyte abnormalities, while the SARS-CoV-2 infection hampers the functioning of pulmonary ACE2, resulting in the disruption of the renin–angiotensin system (RAS). This disruption has detrimental outcomes on fluid and electrolyte balance, blood pressure, and airway irritation. Furthermore, it additionally complements vascular permeability in the airways, as indicated in previous studies (Scurati-Manzoni et al., 2014; Bekassy et al., 2021).
Replication of SARSCoV-2; outcome in CF patients
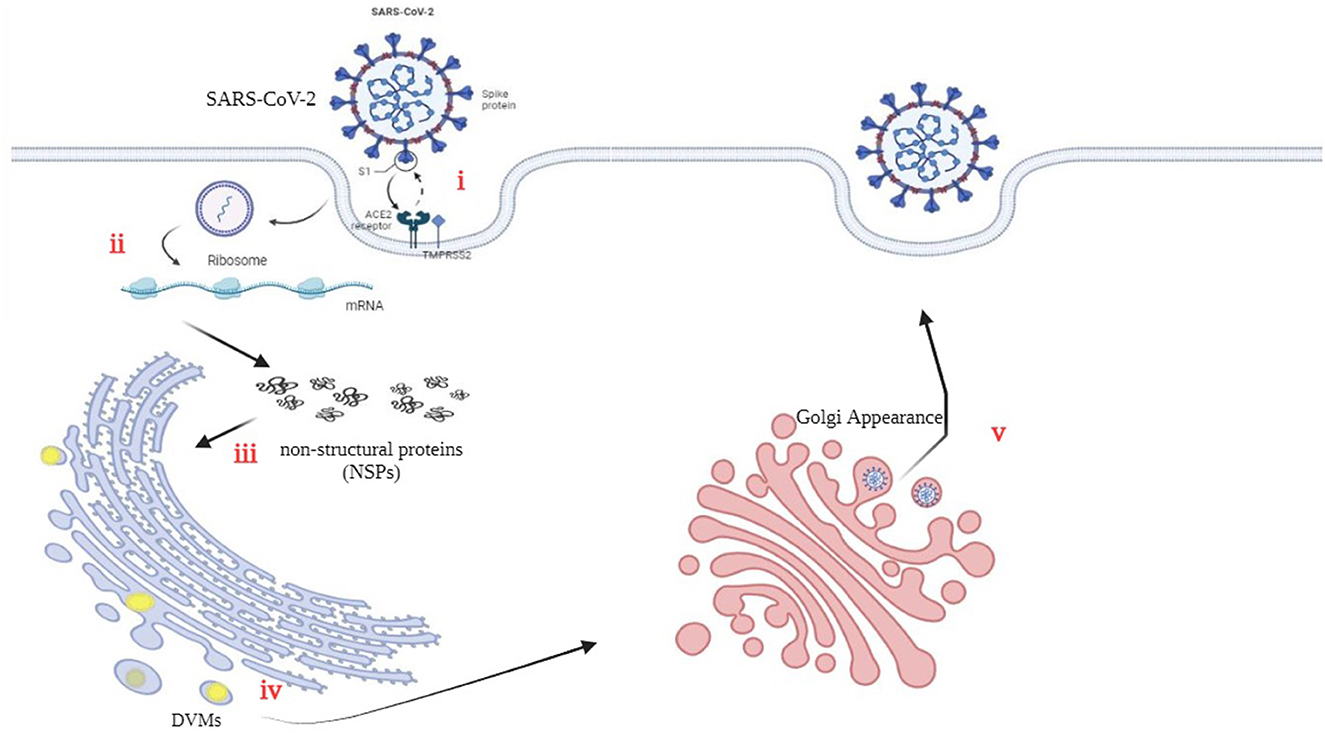
The three-chymotrypsin-like cysteine protease (3CLpro) of SARSCoV-2 is a vital component of the virus that plays a key function in virus replication and is conserved. This protease cleaves the polyproteins to generate 16 useful non-structural proteins (NSPs). The cleaved NSPs have essential functions in assembly and replication (Mody et al., 2021). Figure 2 illustrates the precise interaction between viral S-glycoproteins and the cell ACE2 receptor, which allows the entry of SARS-CoV-2 into cells. The spike glycoprotein is cleaved through TMPRSS2, which helps fusion among the host cellular membrane and the virus envelope (Hoffmann et al., 2020). Viral RNA is translated into non-structural proteins (NSPs). (iii) Furthermore, the virus utilizes the host cellular machinery to translate viral proteins. The RNA genome is replicated and translated into double-membrane vesicles (DMVs), which resemble bubble-like endoplasmic structures. (v) Finally, the mature virion is transported to the Golgi bodies, where it is launched through exocytosis (Yuan et al., 2020; Roingeard et al., 2022).
The availability of the CFTR protein for the SARS-CoV 3CL protease may result in its cleavage (Peckham et al., 2020). Additionally, evidence suggests that the airway tract microbiota, thick secretions, and autophagy induction in CF patients may play a protective role against viral infections. Autophagy, as an immune mechanism in CF disease, could be heightened and actively contributes to the antiviral response (van Ewijk et al., 2005; Junkins et al., 2014; Mingione et al., 2020; Pehote and Vij, 2020).
Immune system status in patients affected with COVID-19 simultaneously with CF
At the onset of contamination, SARS-CoV-2 penetrates host cells, permitting its genome to enter the cytoplasm and initiate a pro-inflammatory reaction via diverse signaling pathways. During the preliminary levels of viral infection in airway epithelial cells, regulated and programmed cell death responses known as pyroptosis take place, releasing seasoned-inflammatory cytokines and chemokines such as IL-6, IFNγ, MCP1, and IP-10. In the presence of inflammatory cytokines, immune cells are recruited to eliminate host epithelial cells, thereby diminishing inflammatory immune responses in the lungs. Nevertheless, in SARS-COV-2 positive patients, induction of a cytokine storm, a deadly immune system reaction that may damage multiple organs, including the coronary heart, liver, and kidneys, in the long run may result in organ failure (Tay et al., 2020; Yapasert et al., 2021).
The innate immune system plays an essential role in the preliminary defense in opposition to COVID-19 (Diamond and Kanneganti, 2022). Among the various cytokines being considered for the improvement of COVID-19, interleukin-6 (IL-6) often emerges as the most significant. Elevated levels of IL-6 are directly associated with the development and mortality rates of this viral infection, which may be triggered by the initiation of SARS-CoV-2. Importantly, this research shows that lowering IL-6 levels ought to serve as a protective measure against SARS-CoV-2 infection, specifically in the context of cytokine storms (Chams et al., 2020; Coomes and Haghbayan, 2020; McGonagle et al., 2020). In a comprehensive study involving 39 CF patients with persistent pulmonary infections, an evaluation of accumulated sputum found a decrease in IL-6 and interleukin-10 (IL-10) levels, followed by an increase in interleukin-8 (IL-8) levels. The production of IL-6 within localized sputum was found to decrease, even as systemic IL-6 levels remained unaffected (Majka et al., 2021).
The airway tract of people with cystic fibrosis suffering lung damage indicates an increased concentration of neutrophil elastase, a dangerous pathogen, leading to a decrease in pulmonary function and activities. The incidence of pulmonary infections, together with acute respiratory distress syndrome (ARDS), related to COVID-19, has been linked to the increased secretion of excess neutrophil elastase. According to available evidence, the capacity treatment (therapy, cure, medication, repair, solution, medicine, fix, correct, solutions, solve, prevent, remedies, recourse) for lung damage and ARDS includes the use of neutrophil elastase inhibitors (Mohamed et al., 2020; Sahebnasagh et al., 2020; Yang and Montgomery, 2021; Terlizzi et al., 2022a,b). Nebulized dornase alfa, which breaks down DNA and promotes the discharge of extracellular traps of neutrophils, has been identified as having a useful function in the development of lung disease. Its use has shown promise in improving lung characteristics in cystic fibrosis patients and can be taken into consideration for trials for COVID-19 treatment. Although the current research proposes against the persistent use of nebulized dornase alfa, the administration of azithromycin has been indicated as an effective remedy for COVID-19 in some research (Kournoutou and Dinos, 2022).
Epidemiological and clinical outcomes of SARS-CoV-2 in CF patients
Recent findings propose that the majority of children infected with the quite contagious SARS-CoV-2 virus exhibit mild signs of inflammation. However, it remains uncertain whether children with continual respiratory symptoms experience exacerbated symptoms due to SARS-CoV-2. Nevertheless, initial studies suggest that most children infected with SARS-CoV-2 exhibit mild signs and symptoms (Borch et al., 2022). The recent findings revealed that children recognized with bronchial asthma and cystic fibrosis, who have reduced weight and contracted SARS-CoV-2, did not display a considerable decrease in lung characteristics. This finding is especially important as many children with bronchopulmonary dysplasia (BPD) and other breathing disorders require ventilator assistance, making them more prone to infections inside this group (Ward, 2016; Kaore and Kaore, 2021). A study carried out across more than one country, together with the United States, verified that the duration of infection in people with CF corresponds to that of the general population. However, due to the small sample size of the studies, definitive and particular conclusions were hard to draw. Therefore, it is widely suggested that people with CF adhere strictly to public health policies to safeguard themselves from infections (Jin et al., 2020). In cases in which people are recognized as having cystic fibrosis (CF) and sooner or later are identified as having SARS-CoV-2, the severity of infection can be further intensified, potentially leading to an escalation in signs and symptoms (Hong et al., 2020). Several investigations were conducted in this specific context. In February 2020, a total of 13 individuals recognized with cystic fibrosis (CF) were found to be suffering from COVID-19 (Fainardi et al., 2020).
The COVID-19 outbreak in Northern Italy had a substantial effect on CF patients, especially those living in areas with an excessive occurrence of the ailment. It is known that every CF patient who contracted the virus had been infected through their circle of relatives, such as close people or related people. Among those patients, 61.5% experienced slight breathing difficulties; at the same time, the remaining 38.5% required more intensive clinical interventions. Furthermore, out of the 13 patients, COVID-19 was detected in others as well. However, in May 2020, a significant number of patients, especially 85%, had recovered (Fainardi et al., 2020).
In contrast to European nations with similar populations, the areas in northern Italy, namely, Lombardia, Emilia-Romagna, Veneto, and Piemont, witnessed a substantially higher mortality rate due to the referred infection.
COVID-19 has been observed to have an excessive effect on the elderly population, with men being more vulnerable to infection than women. Consequently, a considerable number of elderly individuals in Italy have a higher mortality rate (Salzberger et al., 2021). Moreover, people with underlying health conditions, which include diabetes, cardiovascular diseases, or most cancers, are at a higher risk of death than those without these health conditions. Recent studies have additionally suggested cases of COVID-19 infections in kids (Mehrabani, 2020). Notably, the general population tested for an additional occurrence of SARS-CoV-2 (0.15%) in comparison to CF patients (0.07%; Cosgriff et al., 2020). This unexpected discovery could potentially be ascribed to the relatively younger age of patients affected with CF. The utilization of antibiotic treatment among CF patients confers protection against SARS-CoV-2.
CF patients also take precautions to prevent the spread of infection, including self-isolation, which probably contributes to the low occurrence of SARS-CoV-2 infection in this population (Bezzerri et al., 2020; Biondo et al., 2022). The Cystic Fibrosis Center in Parma endorsed self-isolation measures, including hand hygiene, sporting face masks, and remote verbal exchange, to reveal and avoid group activities throughout the pandemic period in Italy. Based on the results, it could be concluded that individuals suffering from extreme respiration infection as a result of the coronavirus may be correctly managed and guarded (Fainardi et al., 2020).
In an examination conducted in France, the findings imply that patients with excessive lung ailments resulting from COVID-19 may be affected. A study conducted in France determined that, out of 31 CF patients who have mild COVID-19, only 0.41% of them had inflammation, which resulted in a significant decrease (93% less) compared to the total number of members (Corvol et al., 2020). The study also found that CF patients with COVID-19 had a higher average age than the general population (Mathew et al., 2021), suggesting a correlation between age and coinfection with the virus. Symptoms such as fever, fatigue, and aggravated cough were mentioned in 28 patients, while the other three patients did not display any signs (Corvol et al., 2020).
Another study in Spain, at some stage in the peak of the preliminary wave of the pandemic, used RT-qPCR to confirm the presence of COVID-19 in CF patients (Mondejar-Lopez et al., 2020). CF patients with COVID-19 had a higher likelihood of being hospitalized as compared to the general population. Moreover, people with CF are more likely to experience worsening of their persistent lung ailment, which may lead to respiratory tract viral infections. Research has shown that CF patients have a lower prevalence of COVID-19 infection than the overall population (Mondejar-Lopez et al., 2020).
Cystic fibrosis treatment: outcome in SARS-CoV-2 infection
Prolonged usage of antibiotics, such as azithromycin, acknowledged for its anti-inflammatory properties, can suppress viral infections in CF patients. Moreover, this antibiotic reveals antiviral consequences in a model of continuous obstructive pulmonary disorder (COPD; Khezri et al., 2021; Suarez-Reyes and Villegas-Valverde, 2021). Azithromycin, an often prescribed antibiotic for cystic fibrosis (CF), holds promise for decreasing the severity of COVID-19 because of its potential to modulate the immune response and show slight antiviral properties (Echeverria-Esnal et al., 2020; Ghazy et al., 2020; Touret et al., 2020). Furthermore, mutations in the CFTR gene may impact the performance of ACE2 and TMPRSS2 proteins, resulting in reduced susceptibility to SARS-CoV-2 infection (Stanton et al., 2020).
The significance of microorganisms, mainly Pseudomonas aeruginosa and Staphylococcus aureus, in the treatment of continual and acute infections is noteworthy. When infection arises inside the lungs, it leads to pulmonary exacerbation (PEx), inflicting a decline in lung characteristics and negatively affecting normal, excellent lifestyles (Lam et al., 2015).
Previous research has indicated that bacterial infections can affect immune responses to viral infections (Nilashi et al., 2020). A previous study found that viral infections currently do not have a sizeable impact on lung features in patients with CF (Smith et al., 1980). In fact, it can be asserted that the suppression of bacterial colonization, in particular Pseudomonas aeruginosa as formerly stated, is essential in dealing with CF infection. The presence of viral microorganisms played a considerable position in the exacerbation of CF (Wark et al., 2012).
Medications and specialized treatments administered to people with CF can relieve the severity of COVID-19. It is plausible that pills used in the treatment of CF sufferers may be connected to a reduction in COVID-19 symptoms, playing an important function in mitigating the severity of the disorder (Gaudio, 2020; Porter et al., 2023).
Effect of host factors of CF patients on SARS-CoV-2 infection
The variation in COVID-19 and mortality rates among distinct countries, in conjunction with the various clinical manifestations of the viral infection in patients, has been drastically documented (Sorci et al., 2020). Host genetic elements were recognized as vital elements influencing the pathogenicity of COVID-19 (Tharappel et al., 2020; Jafarpour et al., 2021). Specifically, individuals carrying single pathogenic versions of the CFTR gene (CF vendors) are more susceptible to respiratory tract infections and severe COVID-19 (Baldassarri et al., 2021). ACE polymorphisms have additionally been related to COVID-19, as studies have indicated that patients with the ACE D/D polymorphism showcase advanced medical signs and symptoms and a higher danger of lung damage in comparison to people with I/I or D/I polymorphisms (Karakaş Çelik et al., 2021). Furthermore, studies have validated that ACE polymorphisms can affect ACE2 expression, leading to the CF phenotype and pulmonary inflammation associated with the development of COVID-19 (Vitiello et al., 2023). It is critical to highlight that the angiotensin-converting enzyme 2 (ACE2) serves as the crucial host receptor for SARS-CoV-2 entry via the spike (S) protein on the virus surface (Zhang et al., 2020).
Impact of COVID-19 on individuals with cystic fibrosis
To gain a complete knowledge of the effect of COVID-19 on individuals with cystic fibrosis, it is essential to accumulate additional proof and records. The European Cystic Fibrosis Society (ECFS) performs an important role in this regard by gathering proof statistics and disseminating well-timed and vital documents from various areas across Europe (Colombo et al., 2020). Respiratory infections affecting the respiratory system are more intense in cystic fibrosis patients than the overall population (Yu and Kotsimbos, 2023). Furthermore, complications and destructive effects on lung characteristics were determined to be increasing among individuals with cystic fibrosis (Flume et al., 2019). While a few individuals with cystic fibrosis may additionally experience respiratory fitness troubles, others may also be afflicted by a chronic airway ailment characterized by intense signs and symptoms (Hisert et al., 2017). It is crucial to note that the signs of cystic fibrosis differ significantly from the clinical manifestations of COVID-19 (Al Lawati et al., 2023).
Investigations have confirmed that organ transplantation is a determinant of COVID-19 prevalence. Consequently, it has been found that lung transplant recipients with COVID-19 infection have a higher mortality rate than those note affected with COVID-19 (Pereira et al., 2020; Hall et al., 2022). In patients with cystic fibrosis (CF), mainly those experiencing extreme respiratory infections, it is important that we no longer miss the possibility of COVID-19 contamination because of reduced immunity. Despite the incredibly low prevalence of SARS-CoV-2 infections in CF people, it is more important to impress upon this population the importance of using numerous measures together with effective somatic and public distancing, as well as using structures and practices aimed toward infection management. These measures should be incorporated into ordinary CF care. Additionally, different elements can also make a contribution to the management of COVID-19 in CF patients (Mathew et al., 2021).
Hence, it is more feasible for people with mild COVID-19 to be mistakenly diagnosed as having CF, while those with slight signs and symptoms can be perceived as healthy individuals. To tackle this trouble, a low-threshold trial is proposed to be implemented, which will facilitate early detection. Given the cutting-edge instances, a wide variety of households have expressed issues concerning the right of entry to medicinal drugs and food supplies (Burgel and Goss, 2021; Lim et al., 2022). To maintain excellent health, patients with cystic fibrosis and their families must adhere to certain principles. It has been observed that CF patients have been successful in averting infection with SARS-CoV-19 (Colombo et al., 2020). Researchers are presently accumulating various statistics to determine the elements that affect the severity of COVID-19 within the cystic fibrosis patients. With the spread of the SARS-CoV-2 pandemic, it is essential for us to accumulate diverse records to understand how this viral infection influences specific patient groups with unique illnesses, which includes cystic fibrosis (Carr et al., 2022). One study found that 181 cystic fibrosis patients (32 post-transplant) from 19 countries were infected with SARS-CoV, and infection with SARS-CoV-2 had similar effects as determined within the general population (McClenaghan et al., 2020). One study demonstrated that only a small number of individuals from the Cystic Fibrosis Registry had been diagnosed with or examined for COVID-19 on a month-to-month basis as of January 2020 (Berardis et al., 2020; Colombo et al., 2020; Corvol et al., 2020; Cosgriff et al., 2020; McClenaghan et al., 2020; Scagnolari et al., 2020; Bain et al., 2021; Naehrlich et al., 2021).
The association between scientific cycles and certain elements, which include older age, CF-associated diabetes, decreased lung features within the 12 months before contamination, and having gone through an organ transplant, has been observed (Jardel et al., 2018; Mainbourg et al., 2019). Despite the higher-than-anticipated effects in a large cohort, probably because of the noticeably younger CF population as compared to other continual situations, it is critical to say that SARS-CoV-2 is not a benign ailment for all individuals in this group of affected persons (Hadi et al., 2021). The COVID-19 global pandemic as a result of SARS-CoV-2 has had a huge impact and continues to unfold globally, with CF being identified as a potential factor for negative effects (Taylor-Cousar et al., 2023). Multiple national CF registries from diverse countries have documented a lower prevalence of SARS-CoV-2 contamination in people with cystic fibrosis (PwCF) in comparison to the general population (Cosgriff et al., 2020; Flume et al., 2022). Furthermore, it has been discovered that more young PwCF tend to have milder signs and symptoms and inflammation with SARS-CoV-2. However, individuals with compromised immune systems and people with impaired lung function are more likely to experience intense results (Burgel and Goss, 2021). Another study applied the multicenter research community TriNETX method to research the medical outcomes of COVID-19 contamination in a large cohort of PwCF in an evaluation of the general populace. The findings found that, out of the 507,810 individuals aged 6 years or older, women constituted the majority (n = 225, 53.32%), and the common age at COVID-19 analysis among CF patients was ~46.6 years. The majority of the participants were of Caucasian ethnicity (n = 309, 73.22%). Higher rates of hospitalization, acute renal damage, and critical care needs were found in PwCF following robust propensity matching.
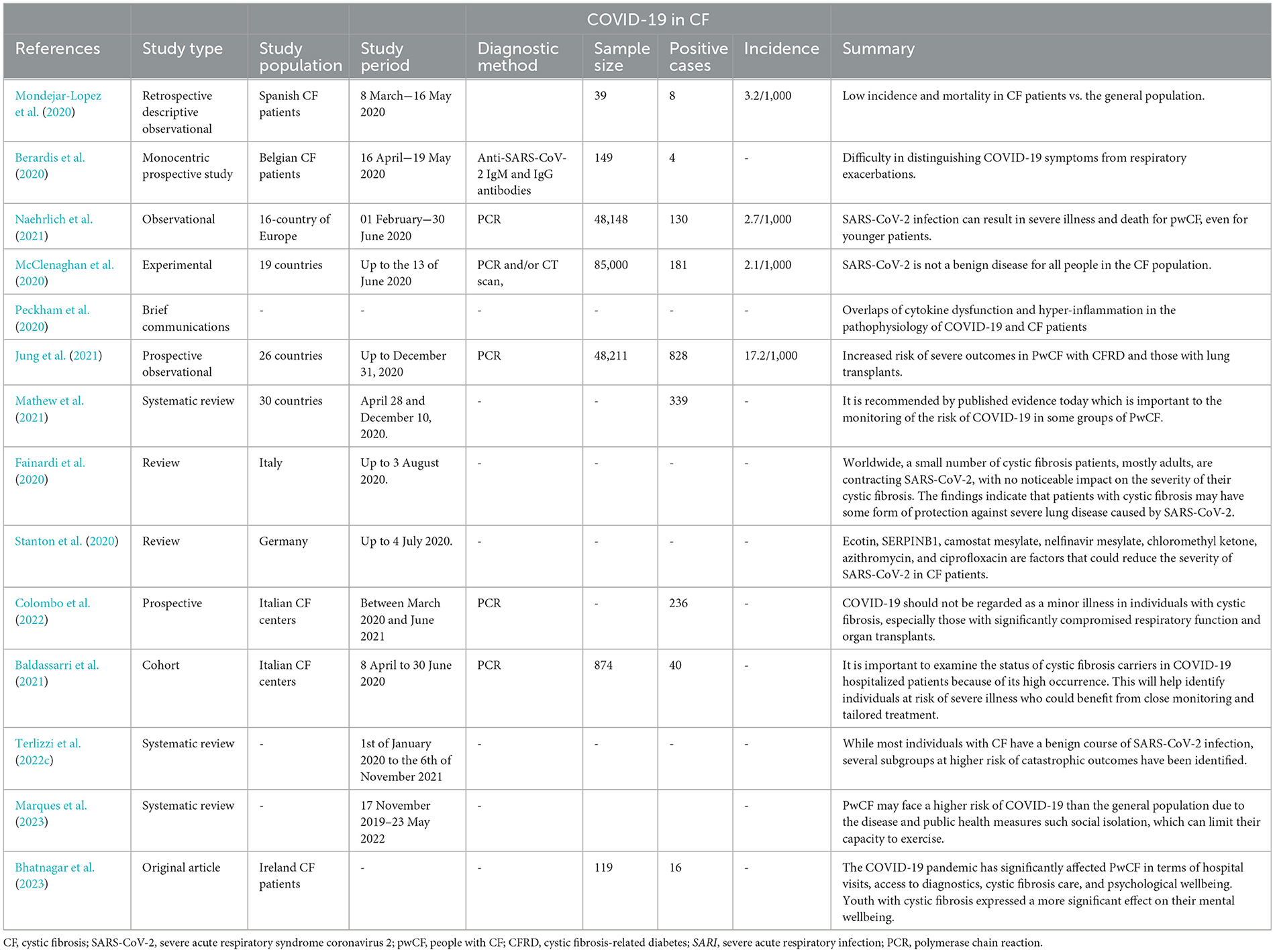
Hospitalization became vital for 10% of patients with COVID-19 (Hadi et al., 2021). Despite the reality that CF patients have a decreased prevalence of obesity and a lesser median age, these elements no longer offer protection against intense disease. A prospective multicenter cohort study, which involved 32 CF centers and 6,597 patients, was conducted to study the symptoms and scientific path of SARS-CoV-2 infection in PwCF. It was found that dysfunctional kidneys, respiration systems, dying facts, and unrivaled reviews were also improved in PwCF at 30 days (Colombo et al., 2021). To ensure proper follow-up, facilities reached out to individuals showing symptoms indicative of COVID-19. According to a recent publication (Tedbury et al., 2023), CFTR may additionally affect the severity of SARS-CoV-2 infection and COVID-19 ailments in CF patients (Vitiello et al., 2023). Meanwhile, a literature evaluation (Marques et al., 2023) emphasizes the restricted understanding regarding the effects of COVID-19 on CF patients. The evaluation indicates that CF patients may be at a greater risk of intense infection from COVID-19 due to their underlying lung disease and other comorbidities (Marques et al., 2023). Additionally, an examination carried out in Brazil indicates using observational studies to assess the results of COVID-19 on individuals diagnosed with cystic fibrosis (Table 1; Sorci et al., 2020).
Conclusion
Various breathing infections have posed a hazard to individuals with CF, regularly leading to excessive consequences. However, the contribution of viral infections to CF pulmonary decline remains a subject of research. Research suggests that CF patients are prone to acute breathing viral infections, yet it appears that most people have not been critically laid low with SARS-CoV-2. Conversely, reports advise that CF patients who have undergone transplantation and finally got mild infections have been laid low with the severe SARS-CoV-2 strain. Another cause of the particularly low occurrence of this virus among CF patients is the commonly lower age of this populace. Given that many CF patients are young people and have constrained receptors for the SARS-CoV-2 virus, the occurrence and severity of the ailment are generally decreasing in this group. Recent research has proposed numerous protective mechanisms for SARS-CoV-2 infection in CF patients. These investigations have highlighted the complete function of CFTR in SARS-CoV-2 replication, as CFTR deficiency results in reduced viral replication. Panou (2018) and Lotti et al. (2022) have said that inhibiting CFTR in primary kidney cells inflamed with BK polyomavirus (BKPyV) drastically reduces the transportation of virions to the ER. Recent studies have indicated that impaired CFTR features may also have a huge effect on viral replication. Specifically, the activation of the SARS-CoV-2 S protein depends on endolysosomal proteases in acidification, and deacidification of this organelle has been shown to prevent viral infection. Mutations in the CFTR gene can result in an increase in organelle pH, resulting in altered glycosylation patterns of ACE-2 and/or TMPRSS-2, which could affect the results of SARS-CoV-2 infection. Furthermore, modifications in ionic balance can modify intracellular pH, leading to large modifications in viral protein assembly and structure (Stanton et al., 2020).
From a pathogenic perspective, increased stages of neutrophil elastase are associated with extended lung damage and reduced pulmonary characteristics in CF (Dittrich et al., 2018; Barth et al., 2020). Consequently, neutrophil elastase inhibitors are being actively examined in trials for CF treatments (Barth et al., 2020). It is worth noting that an imbalance of seasoned-inflammatory neutrophil elastase is also implicated in the improvement of acute respiratory distress syndrome (ARDS) associated with COVID-19 (Polverino et al., 2017). Therefore, neutrophil elastase inhibitors were proposed as capacity-repurposed treatments for ARDS and the associated lung damage (Sahebnasagh et al., 2020).
Additionally, nebulized dornase alfa, a typically used CF treatment, is presently undergoing trials for COVID-19 treatment (Southern et al., 2019; Earhart et al., 2020). Its proposed protective impact is attributed to its clearance of neutrophil extracellular traps, which play a pathogenic function in SARS-CoV-2 infection (Okur et al., 2020). Interestingly, preliminary studies show that dornase alfa is effective in proscribing the in vitro contamination of green monkey and bovine kidney cell strains with the aid of SARS-CoV-2 (Okur et al., 2020).
In conclusion, azithromycin, another usually prescribed antimicrobial agent, has been proven to have antiviral effects against SARS-CoV-2. In the preliminary research, the use and effectiveness of nebulized dornase alfa have not yet been truly defined, but the use of azithromycin was referred to in four research studies (Corvol et al., 2020; Moeller et al., 2020; Mondejar-Lopez et al., 2020; Bain et al., 2021), both as a pre-present treatment or as a capability treatment for COVID-19. It is possible that CF patients exposed to SARS-CoV-2 can be on medications that could alleviate the severity of COVID-19.
Overall, the studies referred to in this evaluation advise that different factors might also help lessen the severity of SARS-CoV-2 in CF sufferers and identify potential goals for further studies to mitigate the severity of SARS-CoV-2 in both CF patients and the general population. These factors encompass ecotin, SERPINB1, camostat mesylate, nelfinavir mesylate, chloromethyl ketone, azithromycin, and ciprofloxacin, some of which are presently being tested in scientific trials (Stanton et al., 2020). Additionally, the composition of the lung microbiome in CF patients, which includes opportunistic pathogens, non-tuberculosis mycobacteria (NTM), and fungi, may play a role in the functioning of COVID-19. Further research in those areas ought to offer valuable insights and future prospects for investigation.
While the findings advocate that CF patients can be truly covered in opposition to extreme lung disorders resulting from SARS-CoV-2, the medium and long-term effects of SARS-CoV-2 on infected CF patients remain unknown. Further research on a larger population of CF patients is needed to determine the proper impact of SARS-CoV-2 on CF lung ailment. Additionally, given the different factors contributing to the spread of SARS-CoV-2, it is encouraged that CF patients be closely monitored to shield them from the risk of COVID-19 contamination.
Author contributions
FA: Data curation, Software, Writing – original draft. NR: Data curation, Writing – original draft. HA: Conceptualization, Supervision, Writing – original draft. PS: Writing – review & editing. MS: Project administration, Supervision, Validation, Writing – review & editing. MMod: Writing – review & editing, Visualization. MMoe: Data curation, Conceptualization, investigation, Software, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Al Lawati, A., Al Balushi, A., and Al Salimi, S. (2023). COVID-19 in a cystic fibrosis patient. Oman Med. J. 38:e490. doi: 10.5001/omj.2023.10
Bain, R., Cosgriff, R., Zampoli, M., Elbert, A., Burgel, P. R., Carr, S. B., et al. (2021). Clinical characteristics of SARS-CoV-2 infection in children with cystic fibrosis: an international observational study. J. Cyst. Fibr. 20, 25–30. doi: 10.1016/j.jcf.2020.11.021
Baldassarri, M., Fava, F., Fallerini, C., Daga, S., Benetti, E., Zguro, K., et al. (2021). Severe COVID-19 in hospitalized carriers of single CFTR pathogenic variants. J. Personal. Med. 11:558. doi: 10.3390/jpm11060558
Barth, P., Bruijnzeel, P., Wach, A., Kessler, O. S., Hooftman, L., Zimmermann, J., et al. (2020). Single dose escalation studies with inhaled POL6014, a potent novel selective reversible inhibitor of human neutrophil elastase, in healthy volunteers and subjects with cystic fibrosis. J. Cyst. Fibr. 19, 299–304. doi: 10.1016/j.jcf.2019.08.020
Bekassy, Z., Lopatko Fagerström, I., Bader, M., and Karpman, D. (2021). Crosstalk between the renin-angiotensin, complement and kallikrein-kinin systems in inflammation. Nat. Rev. Immunol. 2021, 1–18. doi: 10.1038/s41577-021-00634-8
Berardis, S., Verroken, A., Vetillart, A., Struyf, C., Gilbert, M., Gruson, D., et al. (2020). SARS-CoV-2 seroprevalence in a Belgian cohort of patients with cystic fibrosis. J. Cyst. Fibr. 19, 872–874. doi: 10.1016/j.jcf.2020.08.005
Bezzerri, V., Lucca, F., Volpi, S., and Cipolli, M. (2020). Does cystic fibrosis constitute an advantage in COVID-19 infection? Ital. J. Pediatr. 46, 1–3. doi: 10.1186/s13052-020-00909-1
Bhatnagar, R., Tecklenborg, S., Segurado, R., Watt, P., McAuley, N., Fitzpatrick, P., et al. (2023). Impact of COVID-19 pandemic on health care system, work, and mental well-being of people with cystic fibrosis. Irish J. Med. Sci. 23, 1–8. doi: 10.1007/s11845-023-03391-w
Biondo, C., Midiri, A., Gerace, E., Zummo, S., and Mancuso, G. (2022). SARS-CoV-2 infection in patients with cystic fibrosis: what we know so far. Life 12:2087. doi: 10.3390/life12122087
Bitossi, C., Frasca, F., Viscido, A., Oliveto, G., Scordio, M., Belloni, L., et al. (2021). SARS-CoV-2 entry genes expression in relation with interferon response in cystic fibrosis patients. Microorganisms 9:93. doi: 10.3390/microorganisms9010093
Blevings, P. J., Moore, J. E., and Millar, B. C. (2022). Cystic fibrosis: mutations, modulators and microbiology. J. Prev. Diagnost. Treat. Strat. Med. 1:30. doi: 10.4103/jpdtsm.jpdtsm_10_22
Borch, L., Holm, M., Knudsen, M., Ellermann-Eriksen, S., and Hagstroem, S. (2022). Long COVID symptoms and duration in SARS-CoV-2 positive children-a nationwide cohort study. Eur. J. Pediatr. 181, 1597–1607. doi: 10.1007/s00431-021-04345-z
Bradley, B. T., Maioli, H., Johnston, R., Chaudhry, I., Fink, S. L., Xu, H., et al. (2020). Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet 396, 320–332. doi: 10.1016/S0140-6736(20)31305-2
Burgel, P. R., and Goss, C. (2021). COVID-19 outcomes in people with cystic fibrosis. Curr. Opin. Pulmon. Med. 27, 538–543. doi: 10.1097/MCP.0000000000000823
Carr, S. B., McClenaghan, E., Elbert, A., Faro, A., Cosgriff, R., Abdrakhmanov, O., et al. (2022). Factors associated with clinical progression to severe COVID-19 in people with cystic fibrosis: a global observational study. J. Cyst. Fibr. 21, e221–e231. doi: 10.1016/j.jcf.2022.06.006
Chams, N., Chams, S., Badran, R., Shams, A., Araji, A., Raad, M., et al. (2020). COVID-19: a multidisciplinary review. Front. Publ. Health 8:383. doi: 10.3389/fpubh.2020.00383
Chen, N., Zhou, M., Dong, X., Qu, J., Gong, F., Han, Y., et al. (2020). Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395, 507–513. 10.1016/S0140-6736(Zhaoet al., 2022)30211-7
Colombo, C., Alicandro, G., Daccó, V., Gagliano, V., Morlacchi, L. C., Casciaro, R., et al. (2021). SARS-CoV-2 infection in cystic fibrosis: a multicentre prospective study with a control group, Italy, February-July 2020. PLoS ONE 16:e0251527. doi: 10.1371/journal.pone.0251527
Colombo, C., Burgel, P. R., Gartner, S., van Koningsbruggen-Rietschel, S., Naehrlich, L., Sermet-Gaudelus, I., et al. (2020). Impact of COVID-19 on people with cystic fibrosis. Lancet Respirat. Med. 8, e35–e36. doi: 10.1016/S2213-2600(20)30177-6
Colombo, C., Cipolli, M., Daccò, V., Medino, P., Alghisi, F., Ambroni, M., et al. (2022). Clinical course and risk factors for severe COVID-19 among Italian patients with cystic fibrosis: a study within the Italian Cystic Fibrosis Society. Infection 50, 671–679. doi: 10.1007/s15010-021-01737-z
Coomes, E. A., and Haghbayan, H. (2020). Interleukin-6 in COVID-19: a systematic review and meta-analysis. Rev. Med. Virol. 30, 1–9. doi: 10.1002/rmv.2141
Corvol, H., de Miranda, S., Lemonnier, L., Kemgang, A., Reynaud Gaubert, M., Chiron, R., et al. (2020). First wave of COVID-19 in French patients with cystic fibrosis. J. Clin. Med. 9:3624. doi: 10.3390/jcm9113624
Cosgriff, R., Ahern, S., Bell, S. C., Brownlee, K., Burgel, P. R., Byrnes, C., et al. (2020). A multinational report to characterise SARS-CoV-2 infection in people with cystic fibrosis. J. Cyst. Fibr. 19, 355–358. doi: 10.1016/j.jcf.2020.04.012
Demirci, M., Tokman, H., Uysal, H., Demiryas, S., Karakullukcu, A., Saribas, S., et al. (2019). Reduced Akkermansia muciniphila and Faecalibacterium prausnitzii levels in the gut microbiota of children with allergic asthma. Allergologia et Immunopathologia 47, 365–371. doi: 10.1016/j.aller.2018.12.009
Diamond, M. S., and Kanneganti, T. D. (2022). Innate immunity: the first line of defense against SARS-CoV-2. Nat. Immunol. 23, 165–176. doi: 10.1038/s41590-021-01091-0
Dittrich, A. S., Kühbandner, I., Gehrig, S., Rickert-Zacharias, V., Twigg, M., Wege, S., et al. (2018). Elastase activity on sputum neutrophils correlates with severity of lung disease in cystic fibrosis. Eur. Respirat. J. 51:2017. doi: 10.1183/13993003.01910-2017
Earhart, A., Holliday, Z., Hofmann, H., and Schrum, A. (2020). Consideration of dornase alfa for the treatment of severe COVID-19 acute respiratory distress syndrome. N. Microbes N. Infect. 35:100689. doi: 10.1016/j.nmni.2020.100689
Echeverria-Esnal, D., Martin-Ontiyuelo, C., Navarrete-Rouco, M. E., De-Antonio, M., Ferrandez, O., Horcajada, J. P., et al. (2020). Could azithromycin play a role in the treatment of COVID-19? A review. Authorea 2020:16292378. doi: 10.22541/au.159170711.16292378
Fainardi, V., Longo, F., Chetta, A., Esposito, S., and Pisi, G. (2020). SARS-CoV-2 infection in patients with cystic fibrosis. An overwiew. Acta Bio Medica 91:e2020035. doi: 10.23750/abm.v91i3.10391
Flume, P., Rowe, S., Miller, S., Sorscher, E., Elkins, M., Robinson, M., et al. (2019). “Pulmonary complications of cystic fibrosis,” in Seminars in Respiratory and Critical Care Medicine. New York, NY: Thieme Medical Publishers.
Flume, P. A., Saiman, L., and Marshall, B. (2022). The impact of COVID-19 in cystic fibrosis. Archivos de Bronconeumologia 58:466. doi: 10.1016/j.arbres.2021.12.003
Gaudio, D. (2020). SARS-CoV-2 and COVID-19: between pathophysiology complexity and therapeutic uncertainty. Physiol. Rev. 2020, 1−10.
Ghazy, R. M., Almaghraby, A., Shaaban, R., Kamal, A., Beshir, H., Moursi, A., et al. (2020). A systematic review and meta-analysis on chloroquine and hydroxychloroquine as monotherapy or combined with azithromycin in COVID-19 treatment. Sci. Rep. 10:22139. doi: 10.1038/s41598-020-77748-x
Gilbert, J. A., Blaser, M. J., Caporaso, J. G., Jansson, J. K., Lynch, S. V., Knight, R., et al. (2018). Current understanding of the human microbiome. Nat. Med. 24, 392–400. doi: 10.1038/nm.4517
Gu, S., Chen, Y., Wu, Z., Chen, Y., Gao, H., Lv, L., et al. (2020). Alterations of the gut microbiota in patients with coronavirus disease 2019 or H1N1 influenza. Clin. Infect. Dis. 71, 2669–2678. doi: 10.1093/cid/ciaa709
Hadi, Y. B., Lakhani, D. A., Naqvi, S. F., Fatima, N. U., and Sarwari, A. R. (2021). Outcomes of SARS-CoV-2 infection in patients with cystic fibrosis: a multicenter retrospective research network study. Respirat. Med. 188:106606. doi: 10.1016/j.rmed.2021.106606
Hall, V. G., Solera, J. T., Al-Alahmadi, G., Marinelli, T., Cardinal, H., Poirier, C., et al. (2022). Severity of COVID-19 among solid organ transplant recipients in Canada, 2020–2021: a prospective, multicentre cohort study. CMAJ 194, E1155–E1163. doi: 10.1503/cmaj.220620
Hisert, K. B., Heltshe, S. L., Pope, C., Jorth, P., Wu, X., Edwards, R. M., et al. (2017). Restoring cystic fibrosis transmembrane conductance regulator function reduces airway bacteria and inflammation in people with cystic fibrosis and chronic lung infections. Am. J. Respirat. Crit. Care Med. 195, 1617–1628. doi: 10.1164/rccm.201609-1954OC
Hoffmann, M., Kleine-Weber, H., Schroeder, S., Krüger, N., Herrler, T., Erichsen, S., et al. (2020). SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181, 271–280.e8. doi: 10.1016/j.cell.2020.02.052
Hong, N., Yu, W., Xia, J., Shen, Y., Yap, M., Han, W., et al. (2020). Evaluation of ocular symptoms and tropism of SARS-CoV-2 in patients confirmed with COVID-19. Acta Ophthalmol. 98, e649–e655. doi: 10.1111/aos.14445
Jafarpour, R., Pashangzadeh, S., and Dowran, R. (2021). Host factors: implications in immunopathogenesis of COVID-19. Pathol. Res. Pract. 228:153647. doi: 10.1016/j.prp.2021.153647
Jardel, S., Touzet, S., Poupon-Bourdy, S., Nove-Josserand, R., Durieu, I., Reynaud, Q., et al. (2018). Cystic fibrosis related diabetes before lung transplantation impacts survival but not long-term renal function. Revue d'Épidémiologie et de Santé Publique 66, S324–S325. doi: 10.1016/j.respe.2018.05.235
Jin, Y. H., Cai, L., Cheng, Z. S., Cheng, H., Deng, T., Fan, Y. P., et al. (2020). A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Milit. Med. Res. 7, 1–23. doi: 10.1186/s40779-020-0233-6
Jung, A., Orenti, A., Dunlevy, F., Aleksejeva, E., Bakkeheim, E., Bobrovnichy, V., et al. (2021). Factors for severe outcomes following SARS-CoV-2 infection in people with cystic fibrosis in Europe. ERJ Open Res. 7:411. doi: 10.1183/23120541.00411-2021
Junkins, R. D., McCormick, C., and Lin, T. J. (2014). The emerging potential of autophagy-based therapies in the treatment of cystic fibrosis lung infections. Autophagy 10, 538–547. doi: 10.4161/auto.27750
Kaore, S. N., and Kaore, N. M. (2021). Arginine and citrulline as nutraceuticals: efficacy and safety in diseases. Nutraceuticals 55, 925–944. doi: 10.1016/B978-0-12-821038-3.00055-0
Karakaş Çelik, S., Çakmak Genç, G., Pişkin, N., Açikgöz, B., Altinsoy, B., Kurucu Işsiz, B., et al. (2021). Polymorphisms of ACE (I/D) and ACE2 receptor gene (Rs2106809, Rs2285666) are not related to the clinical course of COVID-19: a case study. J. Med. Virol. 93, 5947–5952. doi: 10.1002/jmv.27160
Khezri, M. R., Zolbanin, N. M., Ghasemnejad-Berenji, M., and Jafari, R. (2021). Azithromycin: immunomodulatory and antiviral properties for SARS-CoV-2 infection. Eur. J. Pharmacol. 905:174191. doi: 10.1016/j.ejphar.2021.174191
Kournoutou, G. G., and Dinos, G. (2022). Azithromycin through the Lens of the COVID-19 treatment. Antibiotics 11:1063. doi: 10.3390/antibiotics11081063
Lam, J. C., Somayaji, R., Surette, M. G., Rabin, H. R., and Parkins, M. D. (2015). Reduction in Pseudomonas aeruginosa sputum density during a cystic fibrosis pulmonary exacerbation does not predict clinical response. BMC Infect. Dis. 15, 1–12. doi: 10.1186/s12879-015-0856-5
Lambrecht, B., Prins, B., and Hoogsteden, H. (2001). Lung dendritic cells and host immunity to infection. Eur. Respirat. J. 18, 692–704. doi: 10.1183/09031936.01.18040692
Leylabadlo, H. E., Ghotaslou, R., Feizabadi, M. M., Farajnia, S., Moaddab, S. Y., Ganbarov, K., et al. (2020). The critical role of Faecalibacterium prausnitzii in human health: an overview. Microb. Pathog. 149:104344. doi: 10.1016/j.micpath.2020.104344
Lim, J. T., Ly, N. P., Willen, S. M., Iwanaga, K., Gibb, E. R., Chan, M., et al. (2022). Food insecurity and mental health during the COVID-19 pandemic in cystic fibrosis households. Pediatr. Pulmonol. 57, 1238–1244. doi: 10.1002/ppul.25850
Lotti, V., Merigo, F., Lagni, A., Di Clemente, A., Ligozzi, M., Bernardi, P., et al. (2022). CFTR modulation reduces SARS-CoV-2 infection in human bronchial epithelial cells. Cells 11, 1347. doi: 10.3390/cells11081347
Mainbourg, S., Philit, F., Touzet, S., Nove-Josserand, R., Durupt, S., Sénéchal, A., et al. (2019). Cystic fibrosis-related diabetes before lung transplantation is associated with lower survival but does not affect long-term renal function. Pediatr. Pulmonol. 54, 977–983. doi: 10.1002/ppul.24307
Majka, G., Mazurek, H., Strus, M., Ciszek-Lenda, M., Szatanek, R., Pac, A., et al. (2021). Chronic bacterial pulmonary infections in advanced cystic fibrosis differently affect the level of sputum neutrophil elastase, IL-8 and IL-6. Clin. Exp. Immunol. 205, 391–405. doi: 10.1111/cei.13624
Marques, L. S., Boschiero, M. N., Sansone, N. M. S., Brienze, L. R., and Marson, F. A. L. (2023). Epidemiological profile of hospitalized patients with cystic fibrosis in Brazil due to severe acute respiratory infection during the COVID-19 pandemic and a systematic review of worldwide COVID-19 in those with cystic fibrosis. Healthcare 2023:31936. doi: 10.3390/healthcare11131936
Mathew, H. R., Choi, M. Y., Parkins, M. D., and Fritzler, M. J. (2021). Systematic review: cystic fibrosis in the SARS-CoV-2/COVID-19 pandemic. BMC Pulmon. Med. 21, 1–11. doi: 10.1186/s12890-021-01528-0
McClenaghan, E., Cosgriff, R., Brownlee, K., Ahern, S., Burgel, P. R., Byrnes, C. A., et al. (2020). The global impact of SARS-CoV-2 in 181 people with cystic fibrosis. J. Cyst. Fibr. 19, 868–871. doi: 10.1016/j.jcf.2020.10.003
McGonagle, D., Sharif, K., O'Regan, A., and Bridgewood, C. (2020). The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun. Rev. 19:102537. doi: 10.1016/j.autrev.2020.102537
Mehrabani, S. (2020). COVID-19 infection and children: a comprehensive review. Int. J. Prev. Med. 11:20. doi: 10.4103/ijpvm.IJPVM_277_20
Meng, L., Hua, F., and Bian, Z. (2020). Coronavirus disease 2019 (COVID-19): emerging and future challenges for dental and oral medicine. J. Dental Res. 99, 481–487. doi: 10.1177/0022034520914246
Mingione, A., Ottaviano, E., Barcella, M., Merelli, I., Rosso, L., Armeni, T., et al. (2020). Cystic fibrosis defective response to infection involves autophagy and lipid metabolism. Cells 9:1845. doi: 10.3390/cells9081845
Mody, V., Ho, J., Wills, S., Mawri, A., Lawson, L., Ebert, M. C., et al. (2021). Identification of 3-chymotrypsin like protease (3CLPro) inhibitors as potential anti-SARS-CoV-2 agents. Commun. Biol. 4:93. doi: 10.1038/s42003-020-01577-x
Moeller, A., Thanikkel, L., Duijts, L., Gaillard, E. A., Garcia-Marcos, L., Kantar, A., et al. (2020). COVID-19 in children with underlying chronic respiratory diseases: survey results from 174 centres. ERJ Open Res. 6:409. doi: 10.1183/23120541.00409-2020
Mohamed, M. M., El-Shimy, I. A., and Hadi, M. A. (2020). Neutrophil Elastase Inhibitors: A Potential Prophylactic Treatment Option for SARS-CoV-2-Induced Respiratory Complications? (Berlin: Springer), 1–2.
Mondejar-Lopez, P., Quintana-Gallego, E., Giron-Moreno, R. M., Cortell-Aznar, I., de Valbuena-Maiz, M. R., Diab-Caceres, L., et al. (2020). Impact of SARS-CoV-2 infection in patients with cystic fibrosis in Spain: incidence and results of the national CF-COVID-19-Spain survey. Respirat. Med. 170:106062. doi: 10.1016/j.rmed.2020.106062
Naehrlich, L., Orenti, A., Dunlevy, F., Kasmi, I., Harutyunyan, S., Pfleger, A., et al. (2021). Incidence of SARS-CoV-2 in people with cystic fibrosis in Europe between February and June 2020. J. Cyst. Fibr. 20, 566–577. doi: 10.1016/j.jcf.2021.03.017
Nilashi, M., Samad, S., Yusuf, S. Y. M., and Akbari, E. (2020). Can complementary and alternative medicines be beneficial in the treatment of COVID-19 through improving immune system function? J. Infect. Publ. Health 13:893. doi: 10.1016/j.jiph.2020.05.009
Okur, H. K., Yalcin, K., Tastan, C., Demir, S., Yurtsever, B., Karakus, G., et al. (2020). Preliminary report of in vitro and in vivo effectiveness of dornase alfa on SARS-CoV-2 infection. N. Microb. N. Infect. 37:100756. doi: 10.1016/j.nmni.2020.100756
Panou, M.-M. (2018). Role of cellular ion channels in the BK polyomavirus life cycle (PhD thesis). University of Leeds.
Parada Venegas, D., De la Fuente, M. K., Landskron, G., González, M. J., Quera, R., Dijkstra, G., et al. (2019). Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 277:1486. doi: 10.3389/fimmu.2019.01486
Peckham, D., McDermott, M. F., Savic, S., and Mehta, A. (2020). COVID-19 meets cystic fibrosis: for better or worse? Genes Immun. 21, 260–262. doi: 10.1038/s41435-020-0103-y
Pehote, G., and Vij, N. (2020). Autophagy augmentation to alleviate immune response dysfunction, and resolve respiratory and COVID-19 exacerbations. Cells 9:1952. doi: 10.3390/cells9091952
Pereira, M. R., Mohan, S., Cohen, D. J., Husain, S. A., Dube, G. K., Ratner, L. E., et al. (2020). COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am. J. Transpl. 20, 1800–1808. doi: 10.1111/ajt.15941
Polverino, E., Rosales-Mayor, E., Dale, G. E., Dembowsky, K., and Torres, A. (2017). The role of neutrophil elastase inhibitors in lung diseases. Chest 152, 249–262. doi: 10.1016/j.chest.2017.03.056
Porter, J. C., Inshaw, J., Solis, V. J., Denneny, E., Evans, R., Temkin, M. I., et al. (2023). Nebulised dornase alfa reduces inflammation and improves clinical outcomes in severe COVID-19: a randomised clinical trial. eLife 12:2. doi: 10.7554/eLife.87030.2
Roingeard, P., Eymieux, S., Burlaud-Gaillard, J., Hourioux, C., Patient, R., Blanchard, E., et al. (2022). The double-membrane vesicle (DMV): a virus-induced organelle dedicated to the replication of SARS-CoV-2 and other positive-sense single-stranded RNA viruses. Cell. Mol. Life Sci. 79:425. doi: 10.1007/s00018-022-04469-x
Ruffin, M., Bigot, J., Calmel, C., Mercier, J., Givelet, M., Oliva, J., et al. (2021). Flagellin from Pseudomonas aeruginosa modulates SARS-CoV-2 infectivity in cystic fibrosis airway epithelial cells by increasing TMPRSS2 expression. Front. Immunol. 12:714027. doi: 10.3389/fimmu.2021.714027
Sahebnasagh, A., Saghafi, F., Safdari, M., Khataminia, M., Sadremomtaz, A., Ghaleno, H. R., et al. (2020). Neutrophil Elastase inhibitor (Sivelestat), may be a promising therapeutic option for Management of Acute Lung Injury/acute respiratory distress syndrome or disseminated intravascular coagulation in COVID-19. Authorea 2020:13251. doi: 10.1111/jcpt.13251
Salzberger, B., Buder, F., Lampl, B., Ehrenstein, B., Hitzenbichler, F., Holzmann, T., et al. (2021). Epidemiology of SARS-CoV-2. Infection 49, 233–239. doi: 10.1007/s15010-020-01531-3
Scagnolari, C., Bitossi, C., Frasca, F., Viscido, A., Oliveto, G., Scordio, M., et al. (2020). No detection of SARS-CoV-2 in cystic fibrosis patients at the Regional (Lazio) Reference Center for CF in Italy. J. Cyst. Fibr. 19, 837–838. doi: 10.1016/j.jcf.2020.06.018
Scurati-Manzoni, E., Fossali, E. F., Agostoni, C., Riva, E., Simonetti, G. D., Zanolari-Calderari, M., et al. (2014). Electrolyte abnormalities in cystic fibrosis: systematic review of the literature. Pediatr. Nephrol. 29, 1015–1023. doi: 10.1007/s00467-013-2712-4
Segal, J. P., Mak, J. W., Mullish, B. H., Alexander, J. L., Ng, S. C., Marchesi, J. R., et al. (2020). The gut microbiome: an under-recognised contributor to the COVID-19 pandemic? Therapeut. Adv. Gastroenterol. 13:1756284820974914. doi: 10.1177/1756284820974914
Shirato, K., Kanou, K., Kawase, M., and Matsuyama, S. (2017). Clinical isolates of human coronavirus 229E bypass the endosome for cell entry. J. Virol. 91:16. doi: 10.1128/JVI.01387-16
Smith, C. B., Kanner, R. E., Golden, C. A., Klauber, M. R., and Renzetti Jr, A. D. (1980). Effect of viral infections on pulmonary function in patients with chronic obstructive pulmonary diseases. J. Infect. Dis. 141, 271–280. doi: 10.1093/infdis/141.3.271
Sorci, G., Faivre, B., and Morand, S. (2020). Explaining among-country variation in COVID-19 case fatality rate. Sci. Rep. 10:18909. doi: 10.1038/s41598-020-75848-2
Southern, K. W., Clancy, J. P., and Ranganathan, S. (2019). Aerosolized agents for airway clearance in cystic fibrosis. Pediatr. Pulmonol. 54, 858–864. doi: 10.1002/ppul.24306
Stanton, B. A., Hampton, T. H., and Ashare, A. (2020). SARS-CoV-2 (COVID-19) and cystic fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 319, L408–L415. doi: 10.1152/ajplung.00225.2020
Stern, R. C. (1997). The diagnosis of cystic fibrosis. N. Engl. J. Med. 336, 487–491. doi: 10.1056/NEJM199702133360707
Suarez-Reyes, A., and Villegas-Valverde, C. A. (2021). Implications of low-grade inflammation in SARS-CoV-2 immunopathology. Medicc Rev. 23, 42–54. doi: 10.37757/MR2021.V23.N2.4
Tay, M. Z., Poh, C. M., Rénia, L., MacAry, P. A., and Ng, L. F. (2020). The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 20, 363–374. doi: 10.1038/s41577-020-0311-8
Taylor-Cousar, J. L., Shteinberg, M., Cohen-Cymberknoh, M., and Jain, R. (2023). The impact of highly effective cystic fibrosis transmembrane conductance regulator modulators on the health of female subjects with cystic fibrosis. Clin. Therapeut. 45, 278–289. doi: 10.1016/j.clinthera.2023.01.016
Tedbury, P. R., Manfredi, C., Degenhardt, F., Conway, J., Horwath, M. C., McCracken, C., et al. (2023). Mechanisms by which the cystic fibrosis transmembrane conductance regulator may influence SARS-CoV-2 infection and COVID-19 disease severity. FASEB J. 37:e23220. doi: 10.1096/fj.202300077R
Terlizzi, V., Castellani, C., Taccetti, G., and Ferrari, B. (2022a). Dornase alfa in cystic fibrosis: indications, comparative studies and effects on lung clearance index. Ital. J. Pediatr. 48:141. doi: 10.1186/s13052-022-01331-5
Terlizzi, V., Motisi, M. A., Pellegrino, R., Padoan, R., and Chiappini, E. (2022c). Risk factors for severe COVID-19 in people with cystic fibrosis: a systematic review. Front. Pediatr. 10:958658. doi: 10.3389/fped.2022.958658
Terlizzi, V., Parisi, G. F., Ferrari, B., Castellani, C., Manti, S., Leonardi, S., et al. (2022b). Effect of dornase alfa on the lung clearance index in children with cystic fibrosis: a lesson from a case series. Children 9:1625. doi: 10.3390/children9111625
Tharappel, A. M., Samrat, S. K., Li, Z., and Li, H. (2020). Targeting crucial host factors of SARS-CoV-2. ACS Infect. Dis. 6, 2844–2865. doi: 10.1021/acsinfecdis.0c00456
Touret, F., Gilles, M., Barral, K., Nougairède, A., Van Helden, J., Decroly, E., et al. (2020). In vitro screening of a FDA approved chemical library reveals potential inhibitors of SARS-CoV-2 replication. Sci. Rep. 10:13093. doi: 10.1038/s41598-020-70143-6
van Ewijk, B. E., van der Zalm, M. M., Wolfs, T. F., and van der Ent, C. K. (2005). Viral respiratory infections in cystic fibrosis. J. Cyst. Fibr. 4, 31–36. doi: 10.1016/j.jcf.2005.05.011
Vernocchi, P., Del Chierico, F., Russo, A., Majo, F., Rossitto, M., Valerio, M., et al. (2018). Gut microbiota signatures in cystic fibrosis: loss of host CFTR function drives the microbiota enterophenotype. PLoS ONE 13:e0208171. doi: 10.1371/journal.pone.0208171
Vitiello, A., Sabbatucci, M., Silenzi, A., Capuano, A., Rossi, F., Zovi, A., et al. (2023). The impact of SARS-CoV-2 infection in patients with cystic fibrosis undergoing CFTR channel modulators treatment: a literature review. Respirat. Res. 24:278. doi: 10.1186/s12931-023-02593-1
Wark, P. A., Tooze, M., Cheese, L., Whitehead, B., Gibson, P. G., Wark, K. F., et al. (2012). Viral infections trigger exacerbations of cystic fibrosis in adults and children. Eur. Respirat. J. 40, 510–512. doi: 10.1183/09031936.00202311
Yang, C., and Montgomery, M. (2021). Dornase alfa for cystic fibrosis. Cochr. Datab. Syst. Rev. 2021:CD001127. doi: 10.1002/14651858.CD001127.pub5
Yapasert, R., Khaw-On, P., and Banjerdpongchai, R. (2021). Coronavirus infection-associated cell death signaling and potential therapeutic targets. Molecules 26:7459. doi: 10.3390/molecules26247459
Ye, Z. W., Yuan, S., Yuen, K. S., Fung, S. Y., Chan, C. P., Jin, D. Y., et al. (2020). Zoonotic origins of human coronaviruses. Int. J. Biol. Sci. 16:1686. doi: 10.7150/ijbs.45472
Yohannes, A. M. (2021). COPD patients in a COVID-19 society: depression and anxiety. Exp. Rev. Respirat. Med. 15, 5–7. doi: 10.1080/17476348.2020.1787835
Yu, C., and Kotsimbos, T. (2023). Respiratory infection and inflammation in cystic fibrosis: a dynamic interplay among the host, microbes, and environment for the ages. Int. J. Mol. Sci. 24:4052. doi: 10.3390/ijms24044052
Yuan, S., Peng, L., Park, J. J., Hu, Y., Devarkar, S. C., Dong, M. B., et al. (2020). Nonstructural protein 1 of SARS-CoV-2 is a potent pathogenicity factor redirecting host protein synthesis machinery toward viral RNA. Mol. Cell 80, 1055–1066.e6. doi: 10.1016/j.molcel.2020.10.034
Zhang, H., Penninger, J. M., Li, Y., Zhong, N., and Slutsky, A. S. (2020). Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intens. Care Med. 46, 586–590. doi: 10.1007/s00134-020-05985-9
Zhao, M. M., Zhu, Y., Zhang, L., Zhong, G., Tai, L., Liu, S., et al. (2022). Novel cleavage sites identified in SARS-CoV-2 spike protein reveal mechanism for cathepsin L-facilitated viral infection and treatment strategies. Cell Disc. 8:53. doi: 10.1038/s41421-022-00419-w
Keywords: cystic fibrosis (CF), SARS-CoV-2, Pseudomonas aeruginosa, immune system, respiratory virus, pathogenesis
Citation: Abolhasani FS, Moein M, Rezaie N, Sheikhimehrabadi P, Shafiei M, Afkhami H and Modaresi M (2024) Occurrence of COVID-19 in cystic fibrosis patients: a review. Front. Microbiol. 15:1356926. doi: 10.3389/fmicb.2024.1356926
Received: 16 December 2023; Accepted: 11 March 2024;
Published: 17 April 2024.
Edited by:
Leiliang Zhang, Shandong First Medical University and Shandong Academy of Medical Sciences, ChinaReviewed by:
Erica Diani, University of Verona, ItalySriram Kumar, Institute of Virology, Münster, Germany
Copyright © 2024 Abolhasani, Moein, Rezaie, Sheikhimehrabadi, Shafiei, Afkhami and Modaresi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Morvarid Shafiei, dr.m.shafiei@pasteur.ac.ir; Hamed Afkhami, hamedafkhami70@gmail.com
†These authors share first authorship
 Fatemeh Sadat Abolhasani1†
Fatemeh Sadat Abolhasani1† Hamed Afkhami
Hamed Afkhami Mohammadreza Modaresi
Mohammadreza Modaresi