- 1 Geriatric Research, Education and Clinical Center, Wm. S. Middleton Memorial Veterans Hospital, Madison, WI, USA
- 2 Wisconsin Alzheimer’s Disease Research Center, University of Wisconsin School of Medicine and Public Health, Madison, WI, USA
- 3 Neuroscience Training Program, University of Wisconsin-Madison, Madison, WI, USA
- 4 Department of Mathematics and Computer Science, Ripon College, Ripon, WI, USA
Dyslipidemia is common in adults and contributes to high rates of cardiovascular disease and may be linked to subsequent neurodegenerative and neurovascular diseases. This study examined whether lower brain volumes and cognition associated with dyslipidemia could be observed in cognitively healthy adults, and whether apolipoprotein E (APOE) genotype or family history of Alzheimer’s disease (FHAD) alters this effect. T1-weighted magnetic resonance imaging was used to examine regional brain gray matter (GM) and white matter (WM) in 183 individuals (58.4 ± 8.0 years) using voxel-based morphometry. A non-parametric multiple linear regression model was used to assess the effect of high-density lipoprotein (HDL) and non-HDL cholesterol, APOE, and FHAD on regional GM and WM volume. A post hoc analysis was used to assess whether any significant correlations found within the volumetric analysis had an effect on cognition. HDL was positively correlated with GM volume in the bilateral temporal poles, middle temporal gyri, temporo-occipital gyri, and left superior temporal gyrus and parahippocampal region. This effect was independent of APOE and FHAD. A significant association between HDL and the Brief Visuospatial Memory Test was found. Additionally, GM volume within the right middle temporal gyrus, the region most affected by HDL, was significantly associated with the Controlled Oral Word Association Test and the Center for Epidemiological Studies Depression Scale. These findings suggest that adults with decreased levels of HDL cholesterol may be experiencing cognitive changes and GM reductions in regions associated with neurodegenerative disease and therefore, may be at greater risk for future cognitive decline.
Introduction
Low levels of high-density lipoprotein (HDL) cholesterol, a form of dyslipidemia, is common within the United States population, afflicting greater than 25% of Americans (Ghandehari et al., 2008) and is associated with cardiovascular and neurodegenerative disease. The cardiovascular benefits of HDL cholesterol are well established; however, its role in neurological and cognitive health, along with the role of other lipids, is still not well known and is a topic of clinical interest.
Numerous epidemiological studies have shown a protective association between HDL cholesterol and cognitive impairment and dementia (Bonarek et al., 2000; Merched et al., 2000; van Exel et al., 2002; Singh-Manoux et al., 2008). However, imaging studies attempting to show a relationship between HDL levels and brain pathology have been inconclusive (Wolf et al., 2004; den Heijer et al., 2005). Beyond the risk of stroke, cardiovascular disease, which is closely linked to dyslipidemia, has been shown to be a major risk factor for neuropathology and dementia (Meyer et al., 1999; Luchsinger and Mayeux, 2004; Rosendorff et al., 2007). Further, HDL cholesterol has shown anti-inflammatory (Cockerill et al., 2001; Trompet et al., 2008) and antioxidant (Paterno et al., 2004) systemic effects, which have been implicated as protective against dementia. Oxidative stress, including lipid peroxidation, has been shown to be the mediator of the pathologic effects of numerous risk factors of Alzheimer’s disease (AD; Guglielmotto et al., 2010). Lastly, lifestyle interventions proven to increase HDL cholesterol levels (Ashen and Blumenthal, 2005) including “healthy” diet, regular exercise, weight control, and smoking cessation have also been shown to provide neuroprotective effects (Panza et al., 2004; Ward et al., 2005; Kramer et al., 2006; Swan and Lessov-Schlaggar, 2007). Together these findings suggest that individuals with low HDL cholesterol levels may be more susceptible to neuropathological changes and future development of neurodegenerative and neurovascular diseases.
In addition to the positive effects of HDL and brain health, there appear to be associations between low HDL cholesterol and the neuropathologic mechanisms inherent to neurodegenerative disease. HDL contains apolipoprotein E (APOE) and facilitates reverse cholesterol transport, which implies the transport of other types of cholesterol from various tissues, including the brain, to the liver. APOE has a major physiological role in the regulation of lipid and lipoprotein homeostasis and additionally, the APOE-ε4 isoform is a well-known risk factor for AD (Raber et al., 2004). The ε2 and ε4 isoforms have been shown to influence HDL cholesterol levels in opposite directions (Mahley, 1988) and multiple findings indicate that the association between APOE-ε4 and decreased levels of HDL increase the susceptibility to AD (Hoshino et al., 2002; Raygani et al., 2006). Further, low HDL and the APOE-ε4 genotype are both associated with increased incidence of atherosclerosis, a significant contributor to cerebral hypoperfusion (de la Torre, 2004), and stroke (Schneider et al., 2005; Saidi et al., 2007). Cerebrovascular and Alzheimer’s pathological changes frequently coincide in cases of dementia and may act synergistically in producing cognitive decline (Xuereb et al., 2000). Beta-amyloid (Aβ), a pathological hallmark of AD, binds to HDL, maintaining its solubility in cerebrospinal fluid (CSF) and plasma. This HDL-Aβ interaction prevents the deposition of Aβ into the brain and can serve as a marker for neurodegenerative disease (Koudinov et al., 2001). APOE-ε4 has been shown to adversely affect this HDL-Aβ interaction and has been implicated as a risk factor for cerebral amyloid angiopathy, another prominent pathologic hallmark of AD (Mulder and Terwel, 1998).
Systemic HDL cholesterol is highly correlated with HDL found in CSF (Fagan et al., 2000). HDL is the only lipoprotein type found within CSF and has the critical role of maintaining intracellular cholesterol homeostasis between brain cells (Pitas et al., 1987). This transportation of lipids has an effect on membrane biosynthesis during neuronal regeneration, synaptic remodeling, and synaptic vesicle biogenesis, and has been implicated as a potential player in the development of neurodegenerative disease (Koudinov and Koudinova, 2001). An interesting association exists between cholesterol and various tauopathies, classically observed in specific neurodegenerative diseases, including the production of neurofibrillary tangles (NFT) in AD and Niemann–Pick type C disease and the formation of glial fibrillary acidic protein and lipoxidative damage in some frontotemporal dementias (FTD; Distl et al., 2001; Fan et al., 2001; Ohm et al., 2003). Since HDL is the lipoprotein responsible for the efflux of cholesterol within cells of the brain, it may be that deficient levels or dysfunction of HDL cholesterol may contribute to certain tauopathies or dysgenesis of synaptic processes, such that individuals with dyslipidemia may be more susceptible to neurodegenerative disease.
Dyslipidemia is a known risk factor for neuropathology, however, the association between lipids and voxel-wise brain volume markers in late middle-age have not been studied. In this report, we examine regional associations between HDL and non-HDL cholesterol levels, APOE genotype, and family history of AD (FHAD) on brain gray matter (GM) and white matter (WM) volume in cognitively healthy late middle-aged and older adults using voxel-based morphometry (VBM). We hypothesized that subjects with more significant vascular risk factors would have detectable differences in brain volume even during middle-age. This result would suggest that interventions to reduce dyslipidemia may also reduce the risk of neurodegenerative disease.
Materials and Methods
One hundred eighty-three participants (62 men, 121 women) with a mean age of 58.4 years (SD = 8.0) underwent magnetic resonance imaging (MRI) and cognitive testing as part of a study analyzing cognition, brain structure, and brain function in individuals with risk factors for AD. Ninety-eight (54%) of these participants were recruited from the Wisconsin Registry for Alzheimer’s Prevention (WRAP; Sager et al., 2005). The WRAP is a longitudinal cohort evaluated for pre-clinical markers of AD. Forty-eight of the WRAP participants had a parent with either autopsy-confirmed or probable AD as defined by NINCDS-ADRDA criteria (McKhann et al., 1984) and the remaining fifty had no known first-degree FHAD (with parents surviving until at least age 70 without dementia). The remaining 85 participants were recruited from the community and were selected to match the demographics of the subjects from the WRAP.
Participants were administered a comprehensive battery of neuropsychological tests and structural MRI scans to examine changes in cognition and brain structure associated with various vascular risk factors. All participants completed a detailed health history questionnaire and had blood laboratory tests collected. Data collected were APOE genotype, non-fasting lipid panel (including LDL, HDL, and total cholesterol), systolic and diastolic blood pressure (BP), and height and weight (for BMI calculation). APOE genotype was determined using enzyme-linked immunosorbent assay (ELISA) and non-fasting lipid panel was measured using enzymatic procedures with standard reagents. BP was measured with the participant seated and at rest using an automated BP machine. Body height and weight were collected to the nearest 0.5-inch and 1 pound respectively. The battery of neuropsychological tests (Spreen and Strauss, 1998) included the following: portions of the Wechsler Adult Intelligence Scale-Third Edition (WAIS-III); the Rey Auditory Verbal Learning Test (RAVLT); Trail Making Test A and B; the Mini-Mental State Examination (MMSE); the Wide Range Achievement Test (WRAT-3); the Brief Visuospatial Memory Test-Revised (BVMT-R); the Judgment of Line Orientation (JLO); the Controlled Oral Word Association Test (COWAT); the Boston Naming Test (BNT); the Center for Epidemiological Studies Depression (CESD) Inventory.
Exclusion criteria included: self-reported history of current major Axis I psychiatric disease or history of major medical conditions (i.e., traumatic brain injury, cancer, diabetes, or diagnosed neurological condition); evidence of dementia based on neuropsychological test performance; contraindications for MRIs; ingestion of anti-depressants, mood stabilizers, anti-psychotics, and/or anxiety medications.
All subjects gave written informed consent under a protocol approved by the University of Wisconsin Human Subjects Institutional Review Board. This study was performed in a manner that was in accordance with the Declaration of Helsinki.
Brain Imaging
Magnetic resonance imaging was performed using a General Electric 3.0 Tesla SIGNA (Waukesha, WI, USA) MRI system. A 3D inversion recovery prepared fast gradient echo pulse sequence provided high-resolution T1-weighted structural images. The whole brain was imaged in the axial plane with the following parameters: inversion time = 600 ms, fast gradient echo read-out with TR/TE/flip = 9 ms/1.8 ms/20°; acquisition matrix = 256 × 192 × 124 (interpolated to 256 × 256 × 124); field of view = 240 mm; slice thickness = 1.2 mm (124 slices); ± 16 kHz receiver bandwidth. A neuroradiologist examined all images for evidence of any major neurovascular disease or structural abnormality that would affect brain function or structural anatomy (i.e., neoplasms, traumatic brain injury, or major infarctions) that would exclude the subject from the analysis.
Global Volume Measurements
Global brain volumes were calculated from T1-weighted images using the cross-sectional method of Structural Image Evaluation, using Normalization, of Atrophy (SIENAX) within the FSL 3.3 software suite (Smith et al., 2002). The SIENAX parameters were set for a three-class segmentation of tissue type and at a 0.3 threshold for segmenting the brain from extra-axial soft tissue. SIENAX analysis yielded whole-brain volumetric data, in units of cubic millimeters, for three different tissue types: CSF, GM, and WM.
Voxel-Based Morphometry
T1-weighted MR scans were processed with statistical parametric mapping software (SPM5) using the following supervised protocol: (a) T1-weighted images were segmented using VBM5/SPM5 to obtain segmentation parameters; (b) T1-weighted images were segmented into GM and WM [using the parameters from step (a)] and rigidly aligned using the rigid-body component of the transformations produced in step (a); (c) rigidly aligned segments were coregistered using a fast diffeomorphic image registration algorithm (DARTEL; Ashburner, 2007), which included estimating the flow fields, warping the images, and modulating the images; (d) the modulated images from DARTEL were normalized to Montreal Neurological Institute (MNI) space using an affine transformation estimated from the DARTEL GM segments and the a priori GM probability maps in SPM5; and (e) the images were smoothed with an 8 mm FWHM Gaussian filter.
Statistical output for VBM analyses was constrained in a tissue specific way using WM and GM masks. The GM mask was defined as the intersection between: (1) voxels with GM probability exceeding 40% or a combined gray plus WM probability of 80% and GM probability being greater than WM probability; and (2) voxels identified as brain using Wake Forest University Pick Atlas regions for all lobes, the cerebellum, midbrain, and brainstem (Maldjian et al., 2003). The WM mask was defined as the intersection between: (1) voxels with WM probability exceeding 40% or a combined WM plus GM probability of 80% and WM probability being greater than GM probability; and (2) voxels identified as brain as above. The resulting percentage thresholds were applied to the a priori SPM5 templates using ImCalc to create the masks.
Statistical Analysis
A non-parametric Wilcoxon linear model was used to analyze the effect of non-fasting lipids on GM and WM separately (Trivedi et al., 2008). The models included the statistical predictors HDL cholesterol and non-HDL cholesterol, as well as the two-way interactions between these predictors and FHAD status and APOE status. APOE status was separated into three genotypical subgroups: ε2) one or both alleles containing the ε2 genotype; ε3) both alleles containing the ε3 genotype; ε4) one or both alleles containing the ε4 genotype. One subject possessed a ε2/ε4 genotype and was excluded from the model. APOE genotype was defined as three dichotomous variables (presence/absence of a genotypical subgroup) in the model. FHAD was entered into the model as a dichotomous variable; history/no history of first degree relative with AD. Age, education, gender, systolic and diastolic BP, and BMI were also included as covariates because of their known or potential effects on brain structure. Additionally, total GM and WM volume were entered into their respective models to ameliorate total GM or WM volume as possible confounders of regional volume differences.
Individual effects of interest were assessed for statistical significance via the permutation method. For each test conducted, the effect under consideration was randomly permuted leaving the remaining effects in the model 1000 times. The maximal statistic across the entire brain was then saved to generate an empirical distribution of the effect of interest under the null hypothesis thus providing strong control over the Type I error rate. Observed statistics at each voxel were then compared to the empirical distribution. The empirical threshold (p < 0.05) was used to assess the GM areas affected by the vascular risk factors considered.
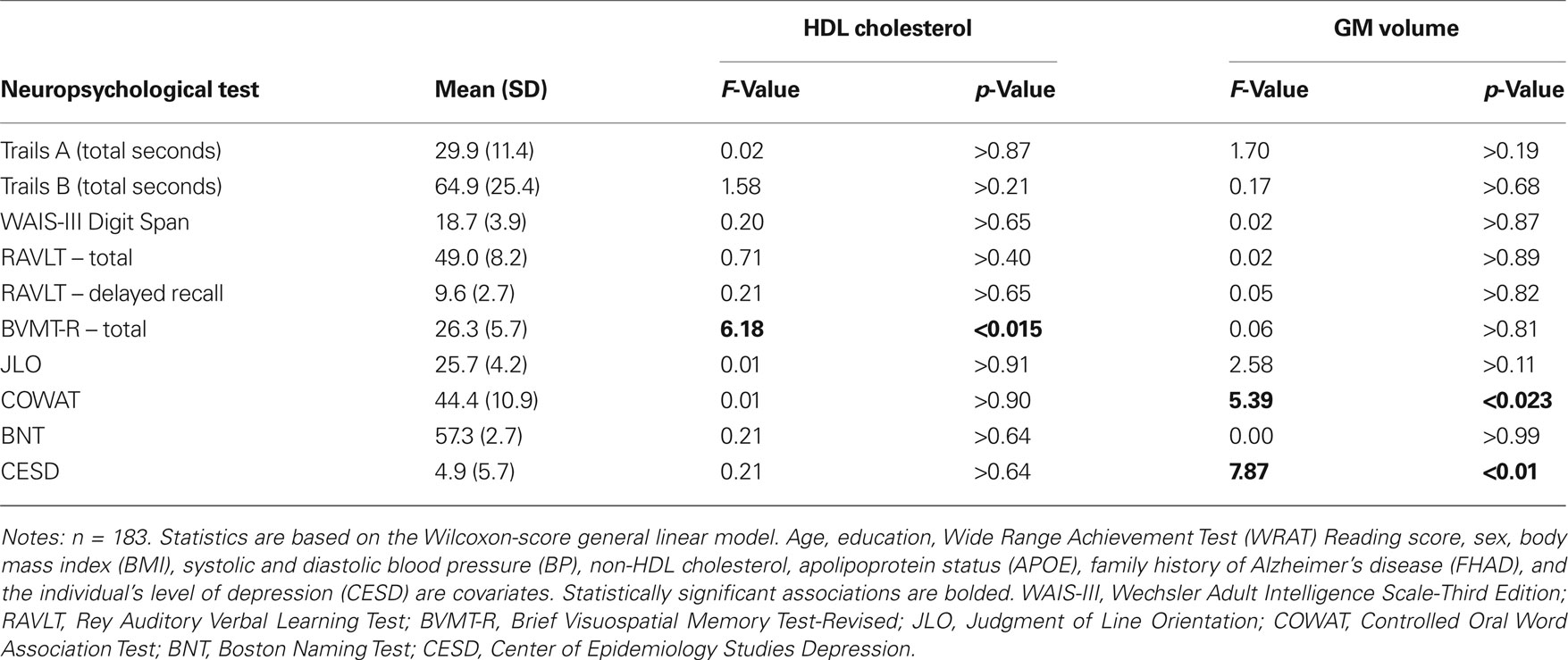
The relationship between HDL cholesterol, GM volume, and several measures of cognition were assessed using a non-parametric Wilcoxon-score general linear model (Hettmansperger and McKean, 1998) for each neuropsychological variable. The relationship between raw scores on Trail Making Test A and B, the Digit Span portion of the WAIS-III, the RAVLT total and delayed recall, the BVMT-R total, COWAT, BNT, JLO, and CESD and HDL cholesterol and GM volume were examined while controlling for the effects of other vascular risk factors (BMI, systolic and diastolic BP, and non-HDL cholesterol), age, depression (using CESD scores), gender, and intelligence (education and WRAT reading performance).
The GM volume values used for the cognition analysis were extracted from a voxel that maximally represented the correlation between HDL and GM volume. This region was determined by the following: (1) significant Z-scores were submitted to a peak (local extremum) search algorithm that identified multiple loci; (2) loci separated by less than 10 mm were consolidated by a center of mass calculation (Burton et al., 2004); (3) the maximum Z-score found through the center of mass calculation defines the voxel of maximal representation.
Results
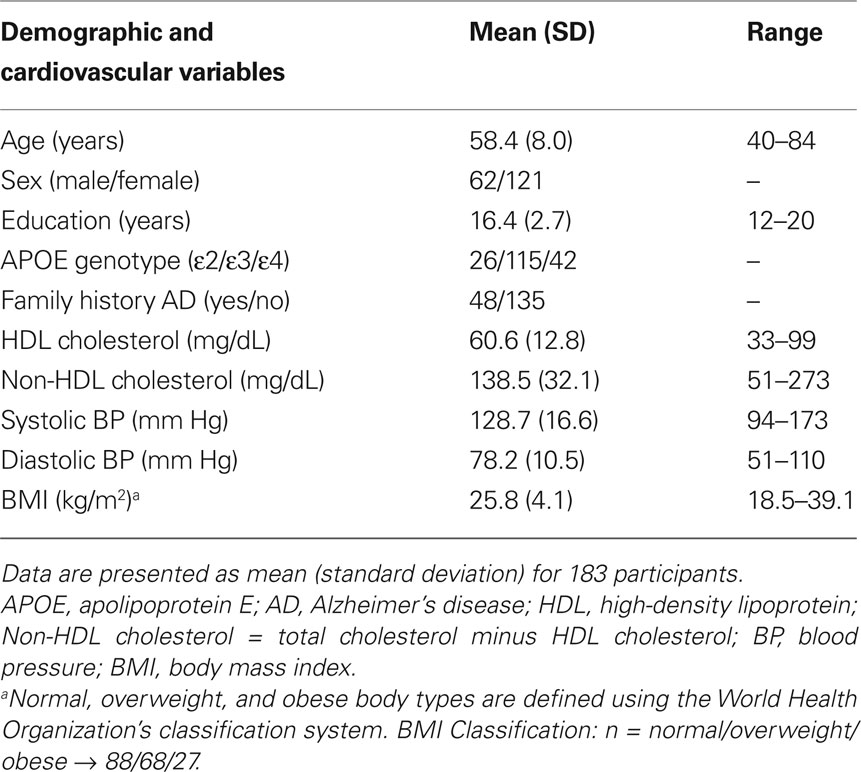
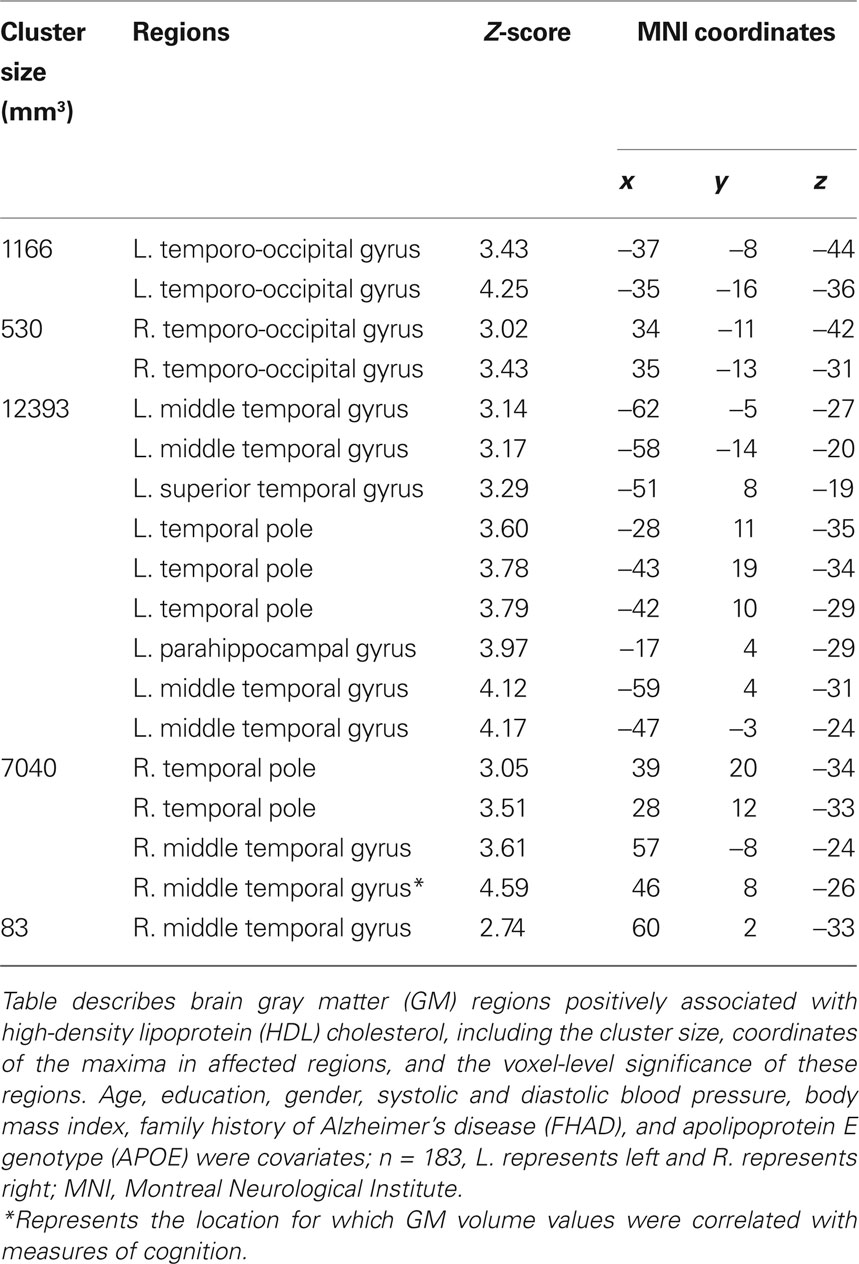
Participant demographics and vascular risk factors are shown in Table 1. We found that HDL cholesterol was positively correlated with GM volume in the bilateral temporal poles, middle temporal gyri, temporo-occipital gyri, and left superior temporal gyrus and parahippocampal region (Figure 1). Table 2 shows the maximum Z-scores before center of mass consolidation, cluster size, and voxel MNI coordinates of these significant areas. This effect was independent of APOE and FHAD, age, education, gender, systolic and diastolic BP, BMI, and total GM volume. HDL cholesterol had no effect on WM volume and non-HDL cholesterol had no effect on either GM or WM volume in this sample. Figure 2 displays GM volume as a function of HDL cholesterol corrected for by calculated residuals from APOE, FHAD, non-HDL cholesterol, age, education, gender, systolic and diastolic BP, BMI, and total GM volume at the voxel of maximal representation.
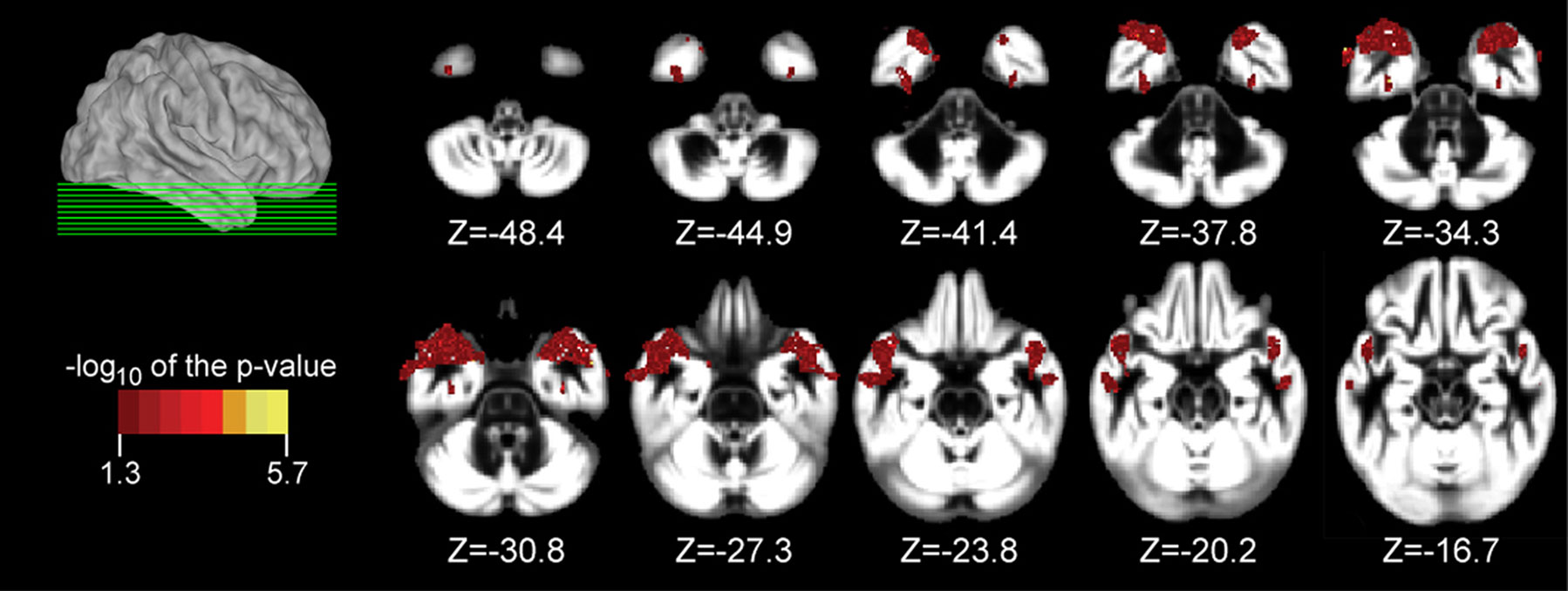
Figure 1. Axial slices from (Z = −48. 4 to −16.7 mm in 4.47 mm increments) overlaid with regions showing a significant association between gray matter (GM) volume and high-density lipoprotein (HDL) cholesterol at a p < 0.05 (corrected with a permutation test) using a non-parametric Wilcoxon linear model. Age, education, gender, systolic and diastolic blood pressure, body mass index, non-HDL cholesterol, family history of Alzheimer’s disease, and apolipoprotein E genotype were entered into the model as covariates of no interest. The location of axial slices are displayed on a lateral view of the cortical surface of the right hemisphere from CARET (Van Essen, 2005). Significant regions included the temporal pole, middle temporal gyrus, and temporo-occipital gyrus bilaterally as well as the left superior temporal gyrus and parahippocampal region. These brain images are viewed as left equal to left and right equal to right. Data are presented for 183 participants.
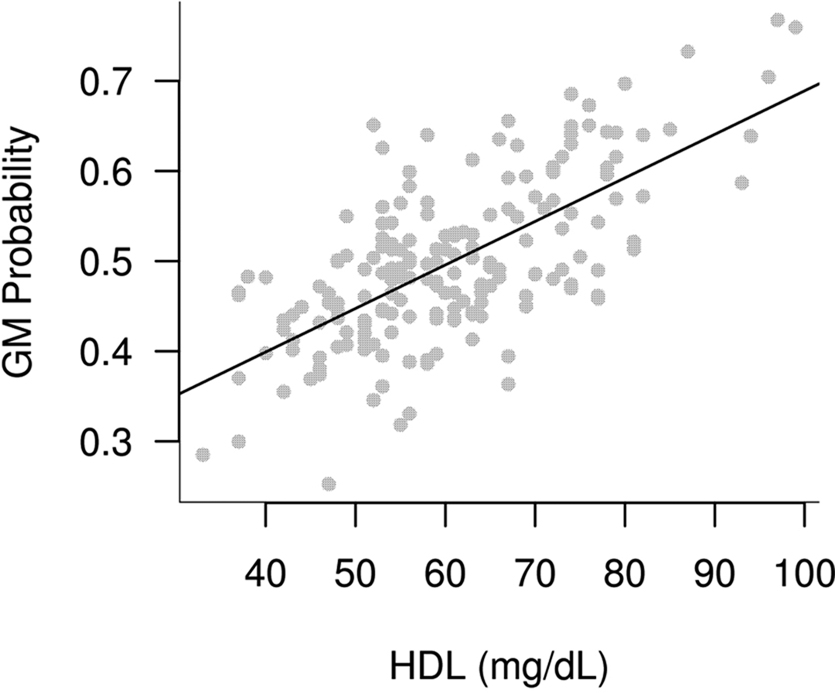
Figure 2. Plot of brain gray matter (GM) volume versus high-density lipoprotein cholesterol at the region which maximally represented the correlation between HDL and GM volume. This plot is adjusted for covariates in the model and shows the positive association between HDL and brain GM volume.
There was a significant association between HDL cholesterol and BVMT and GM volume and COWAT and CESD (depression) for this cross-sectional study group. Table 3 summarizes the mean, standard deviation, F-value, and p-value for each neuropsychological measure with respect to HDL cholesterol and GM volume.
Discussion
The present findings suggest that low levels of HDL cholesterol are associated with lower cognitive function and lower bilateral anterior temporal and temporo-occipital GM volume. These findings were in regions associated with neurodegenerative disease.
Currently, in the United States alone, more than 5 million people suffer from AD. By the year 2050 this number is expected to triple (Alzheimer’s Association, 2008; Tschanz et al., 2004). Statins have been linked to reduction of AD in epidemiologic studies, but clinical trials have not shown a disease modifying effect (van Marum, 2008). Statins are traditionally a treatment for dyslipidemia, but produce modest to no specific change in HDL cholesterol levels (Hausenloy and Yellon, 2008). The effect of traditional HDL-increasing medications such as niacin (Kamanna et al., 2009) and fibrates, on neuropathological markers of AD should be explored further. One recent study in mice found that nicotinamide, a niacin metabolite, lowered tau related neuropathology (Green et al., 2008).
Low HDL cholesterol is associated with risk for cognitive decline and dementia (Bonarek et al., 2000; Merched et al., 2000; van Exel et al., 2002; Singh-Manoux et al., 2008). However, at the time of writing, only two studies have examined regional brain volume as a function of HDL cholesterol. Wolf et al. found a positive relationship between HDL cholesterol and hippocampal and total brain volume in 86 older adults (age 75–85) across a range of cognitive impairment from normal to demented. In non-demented older adults, lower HDL was associated with smaller hippocampal volume(Wolf et al., 2004). A larger study attempted to replicate this finding but did not find any change in hippocampal volume with respect to HDL, regardless of cognitive status (den Heijer et al., 2005). Consistent with the larger study, we did not find an association between hippocampal volume and HDL levels; our findings were mostly localized to the anterior temporal lobes bilaterally. However, our study population was significantly younger [mean age 58 years versus 78 (Wolf et al., 2004) and 72 (den Heijer et al., 2005) years] but similar to Wolf et al., contained a disproportionate fraction (66%) of women. Neither of the aforementioned studies explored regional volumetric associations in areas outside of the hippocampus.
Our study demonstrated a significant association between HDL cholesterol level and visuospatial memory, as measured by the BVMT-R. Previous studies have shown low HDL cholesterol levels to be a risk factor for cognitive deficits and decline (van Exel et al., 2002; Singh-Manoux et al., 2008). The temporal pole, the most anterior region of the temporal lobe, has many limbic connections and plays a role in facial recognition and interpretation of other visual stimuli (Olson et al., 2007). Additionally, a study examining the cognitive effects of bilateral lesions in the lateral temporal neocortex sparing medial temporal structures, similar to our regions of interest, found deficits in both verbal and visuospatial memory when compared to controls (Cheung and Chan, 2003).Therefore, it may be that deficits in visuospatial function may be an early sign of deleterious anterior or lateral temporal lobe changes and that deficient HDL cholesterol levels may play a role in this subtle cognitive change.
The ε4 isoform of APOE and positive FHAD are both well-known risk factors for the development of dementia; however, our study found no effect of APOE or FHAD on brain GM or WM and no interactive effect with HDL cholesterol. The subjects in this study were on average younger than the typical age of onset in AD and others have observed that APOE effects on the brain may be age-dependent and occur later in life (Cherbuin et al., 2007). In our study only 10% of subjects were older than 65 years and of these subjects only three individuals were ε4 carriers.
Mesiotemporal brain regions are classically affected in prodromal AD (Bell-McGinty et al., 2005; Whitwell et al., 2007). Given the added risk for development of AD within our study population, changes were hypothesized to be found within the mesiotemporal region. With the exception of a small cluster within the parahippocampal gyrus, the affected regions in our study involve mostly the anterolateral portions of the temporal lobes, a pattern more closely representing areas affected in the semantic form of FTD (Rosen et al., 2002). Although mesiotemporal regions are considered the hallmark for prodromal AD, atrophy within the temporal pole and to a lesser extent the middle temporal gyrus significantly predict pre-clinical AD (Desikan et al., 2008). The temporal pole and middle temporal gyrus have also been described as regions susceptible to NFT formation in both normal aging and pre-clinical dementia (Braak and Braak, 1995; Delacourte et al., 1999). Lastly, small bilateral clusters within the temporo-occipital gyrus were associated with HDL levels in our study. The temporo-occipital cortex, in particular the fusiform gyrus, is affected in AD (Halliday et al., 2003) and in posterior variants of AD (Tang-Wai and Mapstone, 2006).
There were some limitations of this study. First, non-fasting, instead of the more accurate fasting cholesterol values were collected for this study. However, it should be noted that studies have shown insignificant clinical differences between fasting and non-fasting levels of HDL and non-HDL cholesterol (Craig et al., 2000). Non-fasting measurements are used in the clinical setting and are supported by the United States Preventive Services Task Force (2002). Next, medications for the management of dyslipidemia were not included in this analysis secondary to the wide range of medication types, dosages, and periods of use. Next, the design of this study was cross-sectional, which only allowed for inferences at a single time point. Temporal lobe volume may be influenced by (unmeasured) genetic or lifestyle factors such as diet and exercise that also influence HDL. A longitudinal study is needed to clarify if the brain GM changes associated with HDL cholesterol are representative of progressive brain changes and concomitant cognitive decline. Next, our study employed a lesser known non-parametric statistical method in substitute of more common parametric methodologies. Non-parametric methods allow for the inclusion of potential statistical outliers but can lessen their influence on the end result when compared to parametric methods and thus, help to lower the risk of type 1 error. Next, the sample in this study was two-thirds women. Although the effect of gender was controlled for in our statistical model, the relatedness of the changes inherent to menopause to our finding is apparent. Studies have associated the abrupt, earlier loss of gonadal function and subsequent elevation of luteinizing hormone (LH) in menopausal women to neurodegenerative changes. LH was shown both in vitro and in vivo to promote Aβ deposition (Meethal et al., 2005). Further, post-menopausal women experience a significant reduction in their levels and function of HDL cholesterol, which puts them at increased risk for cardiovascular disease (Collins, 2008). Future studies should be conducted to analyze the potential interactive effect of gender and age on HDL cholesterol in relation to neurodegenerative disease. Finally, this study population was self-selected from a group of educated volunteers; these findings may not be representative of the general population.
In summary, these findings suggest that adults with lower levels of HDL also have lower GM volumes in temporal regions with related deficits in cognitive function. Whether the effects we observed place individuals at greater risk for future cognitive decline and dementia is still unknown and a topic of continued research in our laboratory.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was supported by NIH R01 AG021155, NIH P50 AG033514 (Wisconsin Alzheimer’s Disease Research Center) and a Merit Review grant from the Department of Veterans Affairs. The resources and use of facilities at the Wm. S. Middleton Memorial Veterans Hospital, Madison, WI is also gratefully acknowledged. We thank Maritza Dowling, PhD, Jodi Barnet, MS, and Andrew Alexander, PhD for their contributions.
References
Alzheimer’s Association. (2008). Alzheimer’s disease facts and figures. Alzheimers Dement. 4, 110–133.
Ashen, M. D., and Blumenthal, R. S. (2005). Clinical practice. Low HDL cholesterol levels. N. Engl. J. Med. 353, 1252–1260.
Bell-McGinty, S., Lopez, O. L., Meltzer, C. C., Scanlon, J. M., Whyte, E. M., Dekosky, S. T., and Becker, J. T. (2005). Differential cortical atrophy in subgroups of mild cognitive impairment. Arch. Neurol. 62, 1393–1397.
Bonarek, M., Barberger-Gateau, P., Letenneur, L., Deschamps, V., Iron, A., Dubroca, B., and Dartigues, J. F. (2000). Relationships between cholesterol, apolipoprotein E polymorphism and dementia: a cross-sectional analysis from the PAQUID study. Neuroepidemiology 19, 141–148.
Braak, H., and Braak, E. (1995). Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol. Aging 16, 271–278; discussion 278–284.
Burton, H., Sinclair, R. J., and McLaren, D. G. (2004). Cortical activity to vibrotactile stimulation: an fMRI study in blind and sighted individuals. Hum. Brain Mapp. 23, 210–228.
Cherbuin, N., Leach, L. S., Christensen, H., and Anstey, K. J. (2007). Neuroimaging and APOE genotype: a systematic qualitative review. Dement. Geriatr. Cogn. Disord. 24, 348–362.
Cheung, M. C., and Chan, A. S. (2003). Memory impairment in humans after bilateral damage to lateral temporal neocortex. Neuroreport 14, 371–374.
Cockerill, G. W., Huehns, T. Y., Weerasinghe, A., Stocker, C., Lerch, P. G., Miller, N. E., and Haskard, D. O. (2001). Elevation of plasma high-density lipoprotein concentration reduces interleukin-1-induced expression of E-selectin in an in vivo model of acute inflammation. Circulation 103, 108–112.
Collins, P. (2008). HDL-C in post-menopausal women: an important therapeutic target. Int. J. Cardiol. 124, 275–282.
Craig, S. R., Amin, R. V., Russell, D. W., and Paradise, N. F. (2000). Blood cholesterol screening influence of fasting state on cholesterol results and management decisions. J. Gen. Intern. Med. 15, 395–399.
Delacourte, A., David, J. P., Sergeant, N., Buee, L., Wattez, A., Vermersch, P., Ghozali, F., Fallet-Bianco, C., Pasquier, F., Lebert, F., Petit, H., and Di Menza, C. (1999). The biochemical pathway of neurofibrillary degeneration in aging and Alzheimer’s disease. Neurology 52, 1158–1165.
de la Torre, J. C. (2004). Alzheimer’s disease is a vasocognopathy: a new term to describe its nature. Neurol. Res. 26, 517–524.
den Heijer, T., Hofman, A., Koudstaal, P. J., and Breteler, M. M. (2005). Serum lipids and hippocampal volume: the link to Alzheimer’s disease? Ann. Neurol. 57, 779–780; author reply 7780.
Desikan, R. S., Fischl, B., Cabral, H. J., Kemper, T. L., Guttmann, C. R., Blacker, D., Hyman, B. T., Albert, M. S., and Killiany, R. J. (2008). MRI measures of temporoparietal regions show differential rates of atrophy during prodromal AD. Neurology 71, 819–825.
Distl, R., Meske, V., and Ohm, T. G. (2001). Tangle-bearing neurons contain more free cholesterol than adjacent tangle-free neurons. Acta Neuropathol. 101, 547–554.
Fagan, A. M., Younkin, L. H., Morris, J. C., Fryer, J. D., Cole, T. G., Younkin, S. G., and Holtzman, D. M. (2000). Differences in the Abeta40/Abeta42 ratio associated with cerebrospinal fluid lipoproteins as a function of apolipoprotein E genotype. Ann. Neurol. 48, 201–210.
Fan, Q. W., Yu, W., Senda, T., Yanagisawa, K., and Michikawa, M. (2001). Cholesterol-dependent modulation of tau phosphorylation in cultured neurons. J. Neurochem. 76, 391–400.
Ghandehari, H., Kamal-Bahl, S., and Wong, N. D. (2008). Prevalence and extent of dyslipidemia and recommended lipid levels in US adults with and without cardiovascular comorbidities: the National Health and Nutrition Examination Survey 2003–2004. Am. Heart J. 156, 112–119.
Green, K. N., Steffan, J. S., Martinez-Coria, H., Sun, X., Schreiber, S. S., Thompson, L. M., and LaFerla, F. M. (2008). Nicotinamide restores cognition in Alzheimer’s disease transgenic mice via a mechanism involving sirtuin inhibition and selective reduction of Thr231-phosphotau. J. Neurosci. 28, 11500–11510.
Guglielmotto, M., Giliberto, L., Tamagno, E., and Tabaton, M. (2010). Oxidative stress mediates the pathogenic effect of different Alzheimer’s disease risk factors. Front. Aging Neurosci. 2, 1–8. doi:10.3389/neuro.24.003.2010.
Halliday, G. M., Double, K. L., Macdonald, V., and Kril, J. J. (2003). Identifying severely atrophic cortical subregions in Alzheimer’s disease. Neurobiol. Aging 24, 797–806.
Hausenloy, D. J., and Yellon, D. M. (2008). Targeting residual cardiovascular risk: raising high-density lipoprotein cholesterol levels. Heart 94, 706–714.
Hettmansperger, T. P., and McKean, J. W. (1998). Robust Nonparametric Statistical Methods. London/New York: Arnold/Wiley.
Hoshino, T., Kamino, K., and Matsumoto, M. (2002). Gene dose effect of the APOE-epsilon4 allele on plasma HDL cholesterol level in patients with Alzheimer’s disease. Neurobiol. Aging 23, 41–45.
Kamanna, V. S., Ganji, S. H., and Kashyap, M. L. (2009). Niacin: an old drug rejuvenated. Curr. Atheroscler. Rep. 11, 45–51.
Koudinov, A. R., Berezov, T. T., and Koudinova, N. V. (2001). The levels of soluble amyloid beta in different high density lipoprotein subfractions distinguish Alzheimer’s and normal aging cerebrospinal fluid: implication for brain cholesterol pathology? Neurosci. Lett. 314, 115–118.
Koudinov, A. R., and Koudinova, N. V. (2001). Essential role for cholesterol in synaptic plasticity and neuronal degeneration. FASEB J. 15, 1858–1860.
Kramer, A. F., Erickson, K. I., and Colcombe, S. J. (2006). Exercise, cognition, and the aging brain. J. Appl. Physiol. 101, 1237–1242.
Luchsinger, J. A., and Mayeux, R. (2004). Cardiovascular risk factors and Alzheimer’s disease. Curr. Atheroscler. Rep. 6, 261–266.
Mahley, R. W. (1988). Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science 240, 622–630.
Maldjian, J. A., Laurienti, P. J., Kraft, R. A., and Burdette, J. H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19, 1233–1239.
McKhann, G., Drachman, D., Folstein, M., Katzman, R., Price, D., and Stadlan, E. M. (1984). Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34, 939–944.
Meethal, S. V., Smith, M. A., Bowen, R. L., and Atwood, C. S. (2005). The gonadotropin connection in Alzheimer’s disease. Endocrine 26, 317–326.
Merched, A., Xia, Y., Visvikis, S., Serot, J. M., and Siest, G. (2000). Decreased high-density lipoprotein cholesterol and serum apolipoprotein AI concentrations are highly correlated with the severity of Alzheimer’s disease. Neurobiol. Aging 21, 27–30.
Meyer, J. S., Rauch, G. M., Crawford, K., Rauch, R. A., Konno, S., Akiyama, H., Terayama, Y., and Haque, A. (1999). Risk factors accelerating cerebral degenerative changes, cognitive decline and dementia. Int. J. Geriatr. Psychiatry 14, 1050–1061.
Mulder, M., and Terwel, D. (1998). Possible link between lipid metabolism and cerebral amyloid angiopathy in Alzheimer’s disease: a role for high-density lipoproteins? Haemostasis 28, 174–194.
Ohm, T. G., Treiber-Held, S., Distl, R., Glockner, F., Schonheit, B., Tamanai, M., and Meske, V. (2003). Cholesterol and tau protein – findings in Alzheimer’s and Niemann Pick C’s disease. Pharmacopsychiatry 36(Suppl. 2), S120–S126.
Olson, I. R., Plotzker, A., and Ezzyat, Y. (2007). The enigmatic temporal pole: a review of findings on social and emotional processing. Brain 130, 1718–1731.
Panza, F., Solfrizzi, V., Colacicco, A. M., D’Introno, A., Capurso, C., Torres, F., Del Parigi, A., Capurso, S., and Capurso, A. (2004). Mediterranean diet and cognitive decline. Public Health Nutr. 7, 959–963.
Paterno, R., Ruocco, A., Postiglione, A., Hubsch, A., Andresen, I., and Lang, M. G. (2004). Reconstituted high-density lipoprotein exhibits neuroprotection in two rat models of stroke. Cerebrovasc. Dis. 17, 204–211.
Pitas, R. E., Boyles, J. K., Lee, S. H., Hui, D., and Weisgraber, K. H. (1987). Lipoproteins and their receptors in the central nervous system. Characterization of the lipoproteins in cerebrospinal fluid and identification of apolipoprotein B,E(LDL) receptors in the brain. J. Biol. Chem. 262, 14352–14360.
Raber, J., Huang, Y., and Ashford, J. W. (2004). ApoE genotype accounts for the vast majority of AD risk and AD pathology. Neurobiol. Aging 25, 641–650.
Raygani, A. V., Rahimi, Z., Kharazi, H., Tavilani, H., and Pourmotabbed, T. (2006). Association between apolipoprotein E polymorphism and serum lipid and apolipoprotein levels with Alzheimer’s disease. Neurosci. Lett. 408, 68–72.
Rosen, H. J., Gorno-Tempini, M. L., Goldman, W. P., Perry, R. J., Schuff, N., Weiner, M., Feiwell, R., Kramer, J. H., and Miller, B. L. (2002). Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology 58, 198–208.
Rosendorff, C., Beeri, M. S., and Silverman, J. M. (2007). Cardiovascular risk factors for Alzheimer’s disease. Am. J. Geriatr. Cardiol. 16, 143–149.
Sager, M. A., Hermann, B., and La Rue, A. (2005). Middle-aged children of persons with Alzheimer’s disease: APOE genotypes and cognitive function in the Wisconsin Registry for Alzheimer’s Prevention. J. Geriatr. Psychiatry. Neurol. 18, 245–249.
Saidi, S., Slamia, L. B., Ammou, S. B., Mahjoub, T., and Almawi, W. Y. (2007). Association of apolipoprotein E gene polymorphism with ischemic stroke involving large-vessel disease and its relation to serum lipid levels. J. Stroke Cerebrovasc. Dis. 16, 160–166.
Schneider, J. A., Bienias, J. L., Wilson, R. S., Berry-Kravis, E., Evans, D. A., and Bennett, D. A. (2005). The apolipoprotein E epsilon4 allele increases the odds of chronic cerebral infarction detected at autopsy in older persons. Stroke 36, 954–959.
Singh-Manoux, A., Gimeno, D., Kivimaki, M., Brunner, E., and Marmot, M. G. (2008). Low HDL cholesterol is a risk factor for deficit and decline in memory in midlife: the Whitehall II Study. Arterioscler. Thromb. Vasc. Biol. 28, 1556–1562.
Smith, S. M., Zhang, Y., Jenkinson, M., Chen, J., Matthews, P. M., Federico, A., and De Stefano, N. (2002). Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage 17, 479–489.
Spreen, O., and Strauss, E. (1998). A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary, 2nd Edn. New York/Oxford: Oxford University Press.
Swan, G. E., and Lessov-Schlaggar, C. N. (2007). The effects of tobacco smoke and nicotine on cognition and the brain. Neuropsychol. Rev. 17, 259–273.
Tang-Wai, D., and Mapstone, M. (2006). What are we seeing? Is posterior cortical atrophy just Alzheimer disease? Neurology 66, 300–301.
Trivedi, M. A., Schmitz, T. W., Ries, M. L., Hess, T. M., Fitzgerald, M. E., Atwood, C. S., Rowley, H. A., Asthana, S., Sager, M. A., and Johnson, S. C. (2008). fMRI activation during episodic encoding and metacognitive appraisal across the lifespan: Risk factors for Alzheimer’s disease. Neuropsychologia 46, 1667–1678.
Trompet, S., de Craen, A. J., Slagboom, P., Shepherd, J., Blauw, G. J., Murphy, M. B., Bollen, E. L., Buckley, B. M., Ford, I., Gaw, A., Macfarlane, P. W., Packard, C. J., Stott, D. J., Jukema, J. W., and Westendorp, R. G. (2008). Genetic variation in the interleukin-1 beta-converting enzyme associates with cognitive function. The PROSPER study. Brain 131, 1069–1077.
Tschanz, J. T., Corcoran, C., Skoog, I., Khachaturian, A. S., Herrick, J., Hayden, K. M., Welsh-Bohmer, K. A., Calvert, T., Norton, M. C., Zandi, P., and Breitner, J. C. (2004). Dementia: the leading predictor of death in a defined elderly population: the Cache County Study. Neurology 62, 1156–1162.
United States Preventive Services Task Force. (2002). Screening for lipid disorders in adults: recommendations and rationale. Am. Fam. Physician 65, 273–276.
Van Essen, D. C. (2005). A population-average, landmark- and surface-based (PALS) atlas of human cerebral cortex. Neuroimage 28, 635–662.
van Exel, E., de Craen, A. J., Gussekloo, J., Houx, P., Bootsma-van der Wiel, A., Macfarlane, P. W., Blauw, G. J., and Westendorp, R. G. (2002). Association between high-density lipoprotein and cognitive impairment in the oldest old. Ann. Neurol. 51, 716–721.
van Marum, R. J. (2008). Current and future therapy in Alzheimer’s disease. Fundam. Clin. Pharmacol. 22, 265–274.
Ward, M. A., Carlsson, C. M., Trivedi, M. A., Sager, M. A., and Johnson, S. C. (2005). The effect of body mass index on global brain volume in middle-aged adults: a cross sectional study. BMC Neurol. 5, 23.
Whitwell, J. L., Przybelski, S. A., Weigand, S. D., Knopman, D. S., Boeve, B. F., Petersen, R. C., and Jack, C. R. Jr. (2007). 3D maps from multiple MRI illustrate changing atrophy patterns as subjects progress from mild cognitive impairment to Alzheimer’s disease. Brain 130, 1777–1786.
Wolf, H., Hensel, A., Arendt, T., Kivipelto, M., Winblad, B., and Gertz, H. J. (2004). Serum lipids and hippocampal volume: the link to Alzheimer’s disease? Ann. Neurol. 56, 745–748.
Xuereb, J. H., Brayne, C., Dufouil, C., Gertz, H., Wischik, C., Harrington, C., Mukaetova-Ladinska, E., McGee, M. A., O’Sullivan, A., O’Connor, D., Paykel, E. S., and Huppert, F. A. (2000). Neuropathological findings in the very old. Results from the first 101 brains of a population-based longitudinal study of dementing disorders. Ann. N. Y. Acad. Sci. 903, 490–496.
Keywords: HDL, cholesterol, brain, gray, matter, volume, Alzheimer’s, dementia
Citation: Ward MA, Bendlin BB, McLaren DG, Hess TM, Gallagher CL, Kastman EK, Rowley HA, Asthana S, Carlsson CM, Sager MA and Johnson SC (2010) Low HDL cholesterol is associated with lower gray matter volume in cognitively healthy adults. Front. Ag. Neurosci. 2:29. doi: 10.3389/fnagi.2010.00029
Received: 15 March 2010;
Paper pending published: 07 April 2010;
Accepted: 26 June 2010;
Published online: 15 July 2010
Edited by:
George Perry, The University of Texas at San Antonio, USAReviewed by:
Fidel Santamaria, The University of Texas at San Antonio, USAClyde Phelix, The University of Texas at San Antonio, USA
Miguel Pappolla, Medical University of South Carolina, USA
Copyright: © 2010 Ward, Bendlin, McLaren, Hess, Gallagher, Kastman, Rowley, Asthana, Carlsson, Sager and Johnson. This is an open-access article subject to an exclusive license agreement between the authors and the Frontiers Research Foundation, which permits unrestricted use, distribution, and reproduction in any medium, provided the original authors and source are credited.
*Correspondence: Sterling C. Johnson, Geriatric Research, Education and Clinical Center, Wm. S. Middleton Memorial Veterans Hospital, 2500 Overlook Terrace (11G), Madison, WI 53705, USA. e-mail:c2NqQG1lZGljaW5lLndpc2MuZWR1