- 1Division of Neurology, University of Alberta, Edmonton, AB, Canada
- 2Department of Biomedical Engineering, University of Alberta, Edmonton, AB, Canada
- 3Department of Psychiatry, University of Alberta, Edmonton, AB, Canada
The limbic system is presumed to have a central role in cognitive performance, in particular memory. The purpose of this study was to investigate the relationship between limbic white matter microstructure and neuropsychological function in temporal-lobe epilepsy (TLE) patients using diffusion tensor imaging (DTI). Twenty-one adult TLE patients, including 7 non-lesional (nlTLE) and 14 with unilateral mesial temporal sclerosis (uTLE), were studied with both DTI and hippocampal T2 relaxometry. Correlations were performed between fractional anisotropy (FA) of the bilateral fornix and cingulum, hippocampal T2, neuropsychological tests. Positive correlations were observed in the whole group for the left fornix and processing speed index. In contrast, memory tests did not show significant correlations with DTI findings. Subgroup analysis demonstrated an association between the left fornix and processing speed in nlTLE but not uTLE. No correlations were observed between hippocampal T2 and test scores in either the TLE group as a whole or after subgroup analysis. Our findings suggest that integrity of the left fornix specifically is an important anatomical correlate of cognitive function in TLE patients, in particular patients with nlTLE.
Introduction
Temporal-lobe epilepsy (TLE) is the most common focal epilepsy syndrome and is often associated with cognitive comorbidity in particular for patients with mesial temporal sclerosis (MTS) (Hermann et al., 2009). Previous magnetic resonance imaging (MRI) volumetric studies have demonstrated that cognitive deficits in TLE are most strongly predicted by white matter volume (Hermann et al., 2002, 2003). Diffusion tensor imaging (DTI) permits the measurement of specific white matter tissue characteristics and thus has potential advantages over a non-specific technique like volumetric MRI (Basser et al., 1994). In vivo DTI of the fornix in TLE patients has shown histological correlates between fractional anisotropy (FA) and axonal membranes (Concha et al., 2010) thereby validating the technique as a non-invasive indicator of white matter micro-structural characteristics in human brain. Diffusion parameters of the uncinate fasciculus, inferior fronto-occipital fasciculus, arcuate fasciculus, and cingulum have been demonstrated to correlate with verbal memory, naming performance, and fluency in TLE patients (Flugel et al., 2006; Diehl et al., 2008; McDonald et al., 2008; Riley et al., 2010). In the present study, we focused on the fornix and cingulum, two prominent structures in the limbic white matter network, which have been demonstrated to be abnormal in TLE, albeit with differences between non-lesional TLE (nlTLE) and TLE with unilateral MTS (uTLE) (uTLE subjects have been demonstrated to have reduced FA of the fornix and cingulum while nlTLE have not) (Concha et al., 2005a,b, 2009). The purpose of this study was to assess correlations between white matter structure using DTI and cognitive function in a group of adult TLE patients.
Materials and Methods
Standard Protocol Approvals, Registrations, and Patient Consents
Approval of the project was obtained from the University of Alberta Health Research Ethics Board and informed consent was obtained from all participants.
Subjects
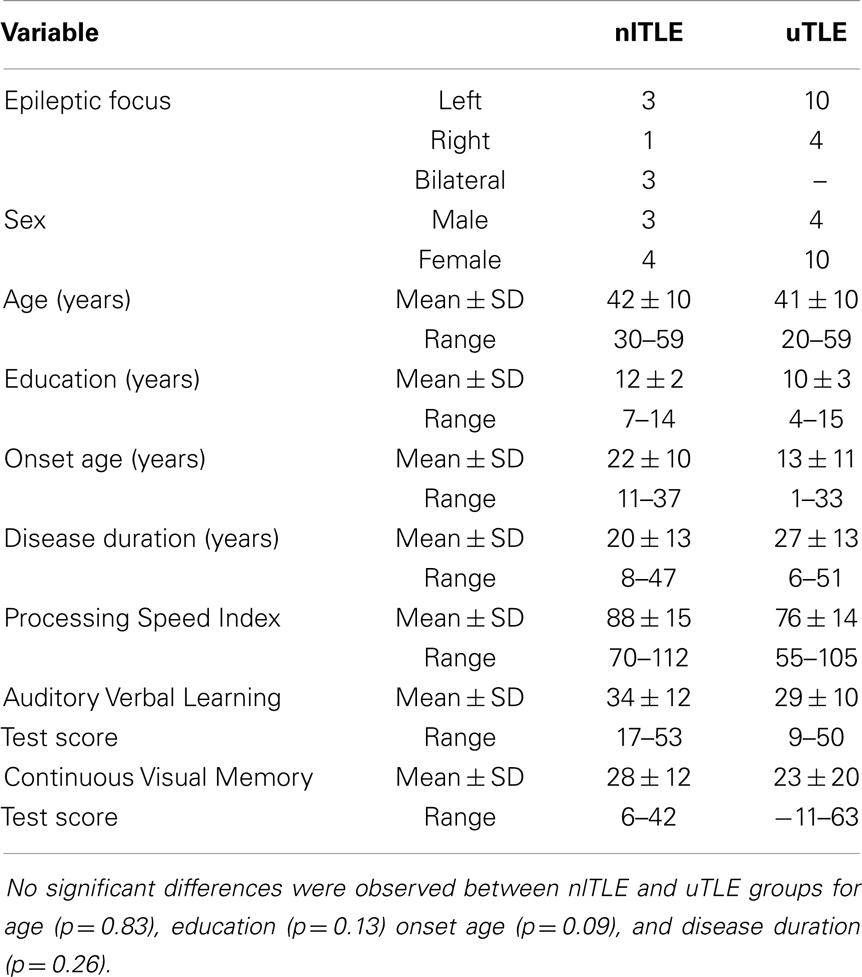
Twenty-one left hemisphere language dominant TLE patients were studied, including 7 nlTLE patients (3 left, 1 right, 3 bilateral) and 14 with uTLE (10 left, 4 right) (Table 1). No significant differences were observed between nlTLE and uTLE group means of age (nlTLE: 42, uTLE: 41, p = 0.86), onset age (nlTLE: 22, uTLE: 13, p = 0.10), or disease duration (nlTLE: 20, uTLE: 27, p = 0.21). The median number of prescribed anti-epileptic drugs per patient was two in both groups. Of note, a similar number of patients in each group were prescribed topiramate (nlTLE: 3, uTLE: 4), which has been shown to negatively impact cognitive ability, particularly verbal memory, and fluency (Thompson et al., 2000). TLE diagnosis was based on ictal semiology of complex partial seizures, confined temporal-lobe interictal, and ictal electroencephalogram findings. Absence of structural lesions outside of the temporal-lobe was confirmed on clinical MRI. MTS was defined based on hippocampal T2 greater than two standard deviations of controls. Patients were considered non-lesional if hippocampal T2 was within two standard deviations of control values. Left hemisphere language dominance was confirmed by either dichotic listening or intracarotid sodium amytal tests. The patient group used in this analysis is a subset of the study group previously reported (Concha et al., 2009) exclusions were made for: insufficient neuropsychological test data, neuropsychological testing greater than 36 months from the time of research MRI, or atypical language dominance. The median length of time between research MRI and neuropsychological evaluation was 1 month (range 0–35 months), 14 patients having less than 6 months between MRI and neuropsychological evaluation. Additional patient information has been previously reported (Concha et al., 2009).
Neuropsychological Evaluation
The neuropsychological tests used were: Processing Speed Index (Digit Symbol and Symbol Search subtests of the WAIS-III) (n = 19), that has been reported to be sensitive to white matter abnormalities (Axelrod et al., 2001; Drew et al., 2009), the Rey Auditory Verbal Learning Test delayed recall score (AVLT) (n = 21) as a measure of verbal memory, and the Continuous Visual Memory Test total raw score (CVMT) as a measure of figural memory (n = 21). Standardized scores adjusted for both age and education were used.
Image Acquisition
Cerebrospinal fluid-suppressed diffusion tensor images were acquired in 9:30 min using a 1.5 T Siemens Sonata MRI scanner (Siemens Medical Systems, Erlangen, Germany). The sequence consisted of 26 contiguous 2 mm thick axial slices positioned to provide coverage of the limbic tracts with an in-plane resolution of 2 mm × 2 mm (interpolated to 1 mm × 1 mm × 2 mm). Diffusion-sensitized images were acquired in six directions, with a b value of 1000 s/mm2. Full details of the DTI protocol have been previously provided (Concha et al., 2005a,b). T2 images for the quantification of hippocampal T2 were obtained using a modified CPMG sequence with 32 echoes (TR = 4.43 s; TE1 = 9.1 ms, echo spacing = 9.1 ms), producing 10 coronal 3 mm thick slices with a 3 mm interslice gap in 8:13 min (voxel size 1.2 mm × 1.2 mm× 3 mm interpolated to 0.6 mm × 0.6 mm × 3 mm).
Image Processing
Bilateral fornix and cingulum were investigated using deterministic tractography. By transferring DTI images to a personal computer running DTIstudio 2.5 (Johns Hopkins University, Baltimore, MD, USA), tracts were depicted using fiber assignment by continuous tracking algorithm. Placement of tract-selection regions of interest (ROI) for each fiber bundle was based on methods outlined previously (Mori et al., 1999; Wakana et al., 2004). The FA threshold was set at 0.3 for all tracts. The FA threshold of 0.3 is commonly used in the deterministic tractography literature and is the threshold that we have used in our previous tractography studies of the fornix and cingulum (Mori et al., 1999; Concha et al., 2005a,b, 2009). If more than one streamline for a tract passed through a voxel, repeated coordinates were discarded and the voxel was only included once in the analysis. Mean diffusion parameters for each white matter tract were calculated in each patient. For this study, the crus of the fornix and the temporal portion of the cingulum were analyzed between the axial levels of the mammillary bodies and the fusion of the crura of the fornices. The T2 signal decay was fitted to a mono-exponential curve by voxel across the multi-echo coronal images. T2 values for each hippocampus were calculated by averaging within ROIs manually drawn on two consecutive slices (Concha et al., 2005a,b).
Statistical Analysis
Statistical tests
Analyses were performed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA). Spearman correlations were used to evaluate the relationship between FA of each tract and neuropsychological test scores. With a total of 12 tests performed for the primary analysis, Bonferroni correction sets a significance level of p < 0.0042.
Primary analysis
The primary analysis was made studying the entire group (nlTLE, uTLE, right, left, and bilateral TLE combined) based on the hypothesis that correlations would be observed between white matter measures and neuropsychological tests and that the previously reported differences in white matter DTI measures and neuropsychological tests seen in nlTLE and uTLE and right and left TLE would provide an adequate range of values to demonstrate significant correlations.
Secondary analysis
Where significant correlations were observed in the preliminary analysis, subsequent analysis was performed looking at the nlTLE and uTLE groups and right and left TLE groups separately. Correlations between hippocampal T2 and neuropsychological test scores were also assessed based on the hypothesis that changes in either white matter diffusion parameters or cognitive test scores could be secondary to mesial temporal pathology.
Results
Patient Neuropsychological Test Scores
After examining whole group summary statistics of neuropsychological test scores [Processing Speed: 80 ± 15 (55–112); AVLT: 32 ± 12 (9–57); CVMT: 23 ± 17 (−11–63)], differences between nlTLE and uTLE subgroups were also investigated (Table 1). When compared to the average processing speed standard score of 100 (SD 15), the nlTLE group mean of 88 is low average but still within normal limits, whereas the uTLE group score of 76 is mildly deficient. Considering AVLT, for which the mean standard score is 50 (SD 10), both groups are within normal limits for AVLT but nlTLE patients scored higher (54) than uTLE (42). Both groups were impaired (<first percentile) on the CVMT (nlTLE: 24, uTLE: 23) (Table 1).
Primary Analysis (Entire TLE Group)
Higher FA values for the left fornix were associated with higher Processing Speed indices [r(p)′ = 0.62, p = 0.004] (Table 2). No other correlations were observed between Processing Speed, AVLT, CVMT, and FA of the fornix and cingulum (Table 2).
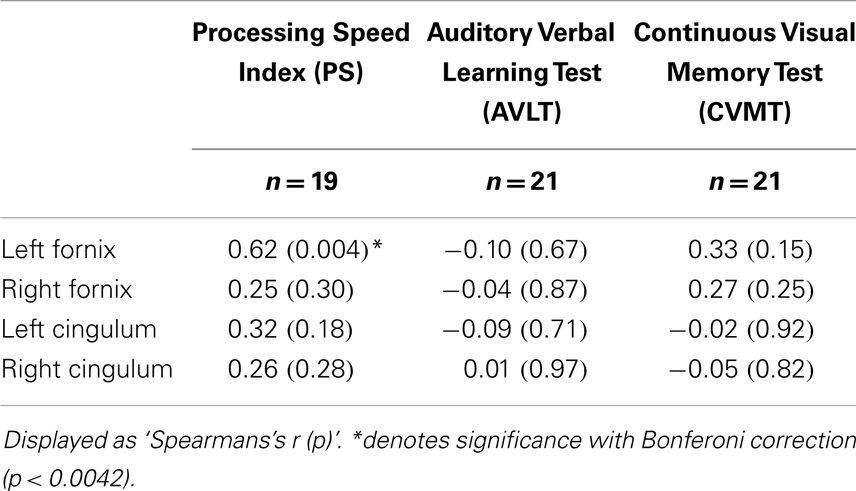
Table 2. Spearman rho correlations of neuropsychological test scores and fractional anisotropy (FA) of the fornix and cingulum in TLE patients.
Secondary Analysis
nlTLE and uTLE groups
After splitting the TLE patients into subgroups (nlTLE n = 7, uTLE n = 14), other trends became evident. The correlation between FA of the left fornix and Processing Speed was explained by a strong correlation in the nlTLE group [r(p)′ = 0.90, p = 0.02], whereas the uTLE group showed no correlation [r(p)′ = 0.40, p = 0.18] (Figure 1).
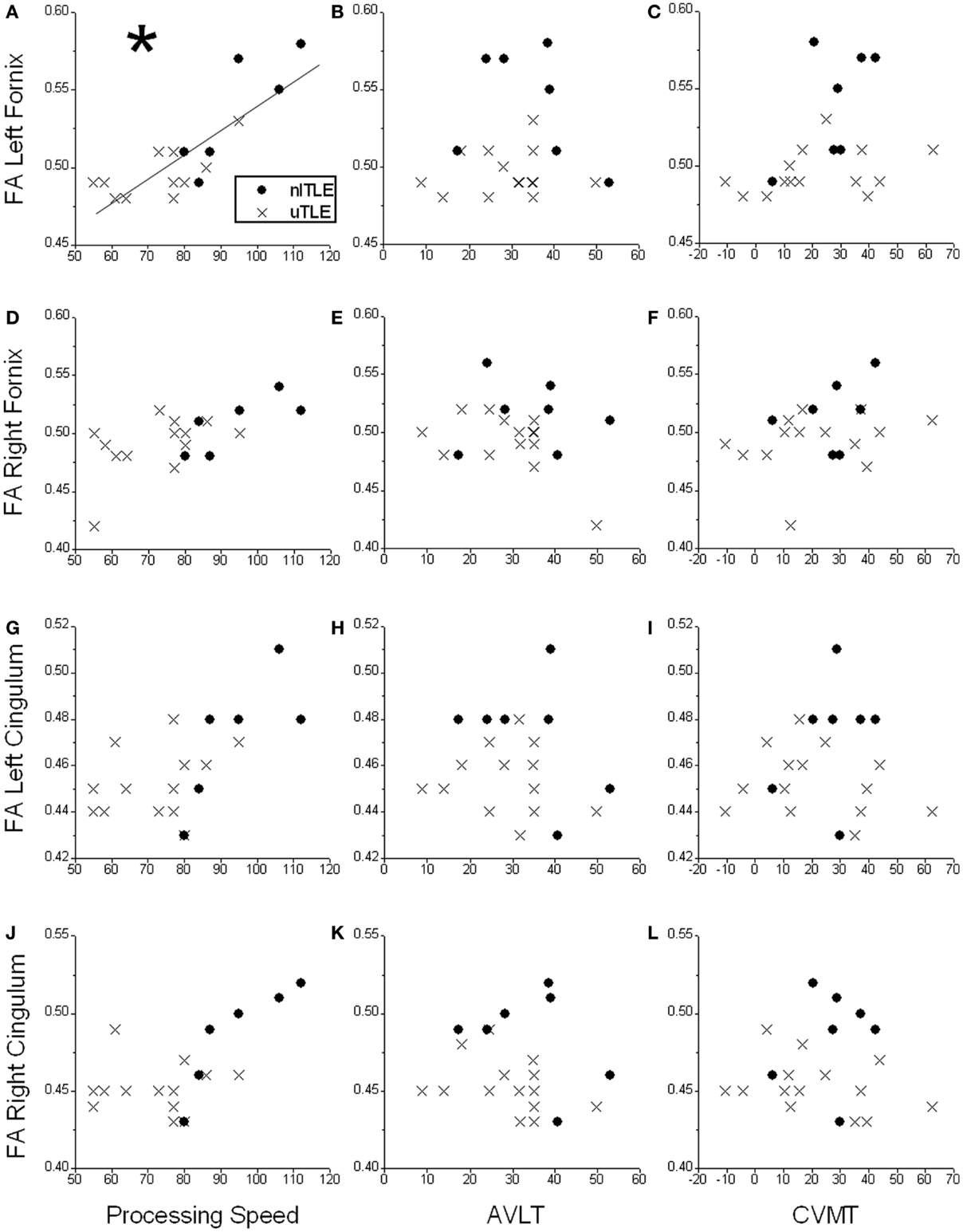
Figure 1. Scatter plots displaying correlations of neuropsychological test scores [Processing Speed Index, Auditory Verbal Learning Test (AVLT), and Continuous Visual Memory Test (CVMT)] and fractional anisotropy (FA) of the left fornix (A–C), right fornix (D–F), left cingulum (G–I), and right cingulum (J–L) in patients with temporal-lobe epilepsy without mesial temporal sclerosis (nlTLE) and with mesial temporal sclerosis (uTLE). *A significant positive correlation is seen between Processing Speed and FA of the left fornix which is primarily driven by the nlTLE subjects. No other significant correlations were observed for the other comparisons.
Right and left TLE
Analysis of right TLE (n = 5) and left TLE (n = 13) demonstrated a positive correlation between FA of the left fornix and processing speed in left TLE [r(p)′ = 0.60, p = 0.04].
Correlations between hippocampal T2 relaxometry and neuropsychology
Correlations were not observed between any of the neuropsychological test scores and left or right hippocampal T2 in the total patient group. As well, no correlation was seen between left hippocampal T2 and processing speed in the nlTLE group.
Discussion
Quantitative MRI studies have shown white matter volume correlates with verbal and figural memory (Hermann et al., 2002, 2003). Recent DTI studies have demonstrated reduced FA in cerebral white matter in multiple brain regions and specific white matter tracts (Concha et al., 2005a,b, 2009; Gross et al., 2006; Focke et al., 2008; Schoene-Bake et al., 2009) with several reports demonstrating correlations between memory and DTI changes in TLE (Diehl et al., 2008; McDonald et al., 2008; Riley et al., 2010). While memory deficits are considered central to the clinical phenotype of TLE, broader cognitive measures such as intelligence, executive function and motor speed have also been demonstrated to be reduced (Hermann et al., 1997; Oyegbile et al., 2004).
The goal of this study was to investigate the relationship between limbic white matter microstructure and cognition in TLE. The primary observation of this study was that correlations were only observed between FA of the left fornix and processing speed for the TLE group as a whole with no correlations observed for the verbal (AVLT) and non-verbal (CVMT) memory tests nor for FA of the other tracts (right fornix and bilateral cingulum) and any of the cognitive measures.
It was observed that the Processing Speed Index, a WAIS-III measure that is most sensitive to brain disorders affecting white matter (Hawkins, 1998) such as traumatic brain injury (Axelrod et al., 2001) and multiple sclerosis (Drew et al., 2009), only correlated with left fornix FA whereas it is usually considered a measure of more widespread white matter function. Wernicke–Korsakoff syndrome, largely based in mammillary body pathology (Zubaran et al., 1997), has also been linked to deficits in one of the two subtests making up the Processing Speed Index, the Digit Symbol-Coding test, in addition to the expected deficit in memory test scores (Jacobson et al., 1990; Oscar-Berman et al., 2004). While there are no other specific references to the relationship between the left fornix specifically and Processing Speed, the link between Processing Speed and limbic structures both for Wernicke–Korsakoff as well as this study suggests an important role of the limbic system specifically in what has been considered a measure of more widespread white matter functioning. While the absence of correlations with other white matter structures suggests a unique role for the left fornix, given the limited sample size, false negative results cannot be ruled out, therefore, further work is required to better understand the relationship between Processing Speed and specific white matter tracts.
While caution must be taken in drawing strong conclusions from the secondary analysis, in particular given the small sample size of some of the subgroups, several interesting findings are observed in particular when looking at the nlTLE and uTLE subgroups.
We observed that the association between left fornix FA and Processing Speed is unique to the nlTLE group despite the greater deficit in intelligence scores previously shown to be evident in uTLE patients (McMillan et al., 1987). Of note, we have previously reported correlations between FA of the fornix and disease duration in nlTLE (Concha et al., 2009). Together these observations, albeit preliminary, are consistent with fornix degeneration and progressive cognitive dysfunction being secondary to ongoing seizures in nlTLE. Of note, the absence of correlations between hippocampal T2 and cognition suggests that the observed correlations between the left fornix and Processing Speed are not a secondary effect (i.e., our findings are not consistent with the nlTLE subjects developing hippocampal degeneration which then leads to downstream degeneration of the fornix and subsequent to this reduced FA).
The dissociation of findings between subgroups suggests the potential for seizure-related white matter and cognitive deterioration being unique to the nlTLE group, supporting the distinctiveness of the nlTLE and uTLE disease states. This idea has also been suggested and supported in recent DTI research (Concha et al., 2009; Kim et al., 2010; Shon et al., 2010). The fact that no correlations were observed in uTLE patients despite greater neuropsychological impairment suggests factors other than fornix damage are responsible for cognitive deficits in this group.
There has been much investigation into the extent and severity of cognitive decline in chronic TLE. Verbal memory peaks earlier and declines faster in TLE patients, especially in left TLE (Helmstaedter and Elger, 2009). A recent prospective study has demonstrated abnormal 4 year trajectory for memory as well as executive function and motor speed in TLE which was associated with baseline MRI abnormalities, lower baseline intelligence, older age, and longer duration of epilepsy (Hermann et al., 2006). As well, general intelligence has been demonstrated to be significantly reduced in earlier onset TLE, including patients without MTS (Kaaden and Helmstaedter, 2009). The cross-sectional nature of our data makes it difficult to draw conclusions regarding whether structural (fornix) or functional (processing speed) changes are progressive. Longitudinal studies are required to evaluate the relationship between functional and structural changes seen in TLE and determine whether nlTLE and uTLE may follow different trajectories.
With respect to division of our TLE sample, an appealing avenue of analysis would be to study group differences between right and left epileptic foci, as has been previously reported (Diehl et al., 2008; McDonald et al., 2008). The primary objective of this study was to use a population of patients with TLE to look for correlations between limbic white matter structure and cognitive function. The study design intentionally included uTLE, nlTLE as well as TLE patients with both right and left epileptic foci as this design was expected to provide a range of both structural and functional abnormalities [i.e., based on the literature uTLE patients were expected to have reduced limbic white matter FA (Concha et al., 2009) and lower cognitive test scores compared to nlTLE (McMillan et al., 1987) and right and left TLE patients were expected to expand the range of scores in particular tests of verbal and figural memory] (Powell et al., 2007). While it remains interesting to look at right and left differences, unfortunately, due to limitations of sample size in particular for the right TLE group (n = 5), it is difficult to compare right and left TLE in this study. While the left TLE group did show a significant correlation between FA of the left fornix and processing speed, the absence of positive findings in the right TLE group is most likely explained by the low sample size.
Conclusion
In conclusion, our findings suggest that integrity of the left fornix specifically is an important anatomical correlate of cognitive function, in particular processing speed, in TLE patients. Furthermore, the differences in correlations of limbic white matter FA versus cognitive test scores in the subgroup analysis suggest that uTLE and nlTLE are distinctly different clinical and anatomical entities.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Operating support provided by the Canadian Institutes of Health Research (DWG, Christian Beaulieu) as well as salary support by Alberta Innovates – Health Solutions (Christian Beaulieu).
Abbreviations
AVLT, Rey auditory verbal learning test recall score; CPMG, Carr Purcell Meibom Gill sequence; CVMT, continuous visual memory test total score; DTI, diffusion tensor imaging; FA, fractional anisotropy; MRI, magnetic resonance imaging; MTS, mesial temporal sclerosis; nlTLE, non-lesional temporal-lobe epilepsy; r(p)′, Spearman rho correlation.; ROI, region of interest; SD, standard deviation; TLE, temporal-lobe epilepsy; uTLE, temporal-lobe epilepsy with unilateral mesial temporal sclerosis; WAIS-III, Wechsler adult intelligence scale third edition.
References
Axelrod, B. N., Fichtenberg, N. L., Liethen, P. C., Czarnota, M. A., and Stucky, K. (2001). Performance characteristics of postacute traumatic brain injury patients on the WAIS-III and WMS-III. Clin. Neuropsychol. 15, 516–520. doi: 10.1076/clin.15.4.516.1884
Basser, P. J., Mattiello, J., and LeBihan, D. (1994). MR diffusion tensor spectroscopy and imaging. Biophys. J. 66, 259–267. doi:10.1016/S0006-3495(94)80775-1
Concha, L., Beaulieu, C., Collins, D. L., and Gross, D. W. (2009). White-matter diffusion abnormalities in temporal-lobe epilepsy with and without mesial temporal sclerosis. J. Neurol. Neurosurg. Psychiatr. 80, 312–319. doi:10.1136/jnnp.2007.139287
Concha, L., Beaulieu, C., and Gross, D. W. (2005a). Bilateral limbic diffusion abnormalities in unilateral temporal lobe epilepsy. Ann. Neurol. 57, 188–196. doi:10.1002/ana.20334
Concha, L., Gross, D. W., and Beaulieu, C. (2005b). Diffusion tensor tractography of the limbic system. AJNR Am. J. Neuroradiol. 26, 2267–2274.
Concha, L., Livy, D. J., Beaulieu, C., Wheatley, B. M., and Gross, D. W. (2010). In vivo diffusion tensor imaging and histopathology of the fimbria-fornix in temporal lobe epilepsy. J. Neurosci. 30, 996–1002. doi:10.1523/JNEUROSCI.1619-09.2010
Diehl, B., Busch, R. M., Duncan, J. S., Piao, Z., Tkach, J., and Luders, H. O. (2008). Abnormalities in diffusion tensor imaging of the uncinate fasciculus relate to reduced memory in temporal lobe epilepsy. Epilepsia 49, 1409–1418. doi:10.1111/j.1528-1167.2008.01596.x
Drew, M. A., Starkey, N. J., and Isler, R. B. (2009). Examining the link between information processing speed and executive functioning in multiple sclerosis. Arch. Clin. Neuropsychol. 24, 47–58. doi:10.1093/arclin/acp007
Flugel, D., Cercignani, M., Symms, M. R., O’Toole, A., Thompson, P. J., Koepp, M. J., et al. (2006). Diffusion tensor imaging findings and their correlation with neuropsychological deficits in patients with temporal lobe epilepsy and interictal psychosis. Epilepsia 47, 941–944. doi:10.1111/j.1528-1167.2006.00527.x
Focke, N. K., Thompson, P. J., and Duncan, J. S. (2008). Correlation of cognitive functions with voxel-based morphometry in patients with hippocampal sclerosis. Epilepsy Behav. 12, 472–476. doi:10.1016/j.yebeh.2007.12.011
Gross, D. W., Concha, L., and Beaulieu, C. (2006). Extratemporal white matter abnormalities in mesial temporal lobe epilepsy demonstrated with diffusion tensor imaging. Epilepsia 47, 1360–1363. doi:10.1111/j.1528-1167.2006.00603.x
Hawkins, K. A. (1998). Indicators of brain dysfunction derived from graphic representations of the WAIS-III/WMS-III Technical Manual clinical samples data: a preliminary approach to clinical utility. Clin Neuropsychol 12, 535–551. doi:10.1076/clin.12.4.535.7236
Helmstaedter, C., and Elger, C. E. (2009). Chronic temporal lobe epilepsy: a neurodevelopmental or progressively dementing disease? Brain 132, 2822–2830. doi:10.1093/brain/awp182
Hermann, B., Seidenberg, M., Bell, B., Rutecki, P., Sheth, R., Ruggles, K., et al. (2002). The neurodevelopmental impact of childhood-onset temporal lobe epilepsy on brain structure and function. Epilepsia 43, 1062–1071. doi:10.1046/j.1528-1157.2002.49901.x
Hermann, B., Seidenberg, M., Bell, B., Rutecki, P., Sheth, R. D., Wendt, G., et al. (2003). Extratemporal quantitative MR volumetrics and neuropsychological status in temporal lobe epilepsy. J. Int. Neuropsychol. Soc. 9, 353–362. doi:10.1017/S1355617703930013
Hermann, B. P., Lin, J. J., Jones, J. E., and Seidenberg, M. (2009). The emerging architecture of neuropsychological impairment in epilepsy. Neurol. Clin. 27, 881–907. doi:10.1016/j.ncl.2009.08.001
Hermann, B. P., Seidenberg, M., Dow, C., Jones, J., Rutecki, P., Bhattacharya, A., et al. (2006). Cognitive prognosis in chronic temporal lobe epilepsy. Ann. Neurol. 60, 80–87. doi:10.1002/ana.20872
Hermann, B. P., Seidenberg, M., Schoenfeld, J., and Davies, K. (1997). Neuropsychological characteristics of the syndrome of mesial temporal lobe epilepsy. Arch. Neurol. 54, 369–376. doi:10.1001/archneur.1997.00550160019010
Jacobson, R. R., Acker, C. F., and Lishman, W. A. (1990). Patterns of neuropsychological deficit in alcoholic Korsakoff’s syndrome. Psychol. Med. 20, 321–334. doi:10.1017/S0033291700017633
Kaaden, S., and Helmstaedter, C. (2009). Age at onset of epilepsy as a determinant of intellectual impairment in temporal lobe epilepsy. Epilepsy Behav. 15, 213–217. doi:10.1016/j.yebeh.2009.03.027
Kim, C. H., Koo, B. B., Chung, C. K., Lee, J. M., Kim, J. S., and Lee, S. K. (2010). Thalamic changes in temporal lobe epilepsy with and without hippocampal sclerosis: a diffusion tensor imaging study. Epilepsy Res. 90, 21–27. doi:10.1016/j.eplepsyres.2010.03.002
McDonald, C. R., Ahmadi, M. E., Hagler, D. J., Tecoma, E. S., Iragui, V. J., Gharapetian, L., et al. (2008). Diffusion tensor imaging correlates of memory and language impairments in temporal lobe epilepsy. Neurology 71, 1869–1876. doi:10.1212/01.wnl.0000327824.05348.3b
McMillan, T. M., Powell, G. E., Janota, I., and Polkey, C. E. (1987). Relationships between neuropathology and cognitive functioning in temporal lobectomy patients. J. Neurol. Neurosurg. Psychiatr. 50, 167–176. doi:10.1136/jnnp.50.2.167
Mori, S., Crain, B. J., Chacko, V. P., and van Zijl, P. C. (1999). Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann. Neurol. 45, 265–269. doi:10.1002/1531-8249(199902)45:2<265::AID-ANA21>3.0.CO;2-3
Oscar-Berman, M., Kirkley, S. M., Gansler, D. A., and Couture, A. (2004). Comparisons of Korsakoff and non-Korsakoff alcoholics on neuropsychological tests of prefrontal brain functioning. Alcohol. Clin. Exp. Res. 28, 667–675. doi:10.1097/01.ALC.0000122761.09179.B9
Oyegbile, T. O., Dow, C., Jones, J., Bell, B., Rutecki, P., Sheth, R., et al. (2004). The nature and course of neuropsychological morbidity in chronic temporal lobe epilepsy. Neurology 62, 1736–1742. doi:10.1212/01.WNL.0000125186.04867.34
Powell, H. W., Richardson, M. P., Symms, M. R., Boulby, P. A., Thompson, P. J., Duncan, J. S., et al. (2007). Reorganization of verbal and nonverbal memory in temporal lobe epilepsy due to unilateral hippocampal sclerosis. Epilepsia 48, 1512–1525. doi:10.1111/j.1528-1167.2007.01053.x
Riley, J. D., Franklin, D. L., Choi, V., Kim, R. C., Binder, D. K., Cramer, S. C., et al. (2010). Altered white matter integrity in temporal lobe epilepsy: association with cognitive and clinical profiles. Epilepsia 51, 536–545. doi:10.1111/j.1528-1167.2009.02508.x
Schoene-Bake, J. C., Faber, J., Trautner, P., Kaaden, S., Tittgemeyer, M., Elger, C. E., et al. (2009). Widespread affections of large fiber tracts in postoperative temporal lobe epilepsy. Neuroimage 46, 569–576. doi:10.1016/j.neuroimage.2009.03.013
Shon, Y. M., Kim, Y. I., Koo, B. B., Lee, J. M., Kim, H. J., Kim, W. J., et al. (2010). Group-specific regional white matter abnormality revealed in diffusion tensor imaging of medial temporal lobe epilepsy without hippocampal sclerosis. Epilepsia 51, 529–535. doi:10.1111/j.1528-1167.2009.02327.x
Thompson, P. J., Baxendale, S. A., Duncan, J. S., and Sander, J. W. (2000). Effects of topiramate on cognitive function. J. Neurol. Neurosurg. Psychiatr. 69, 636–641. doi:10.1136/jnnp.69.5.636
Wakana, S., Jiang, H., Nagae-Poetscher, L. M., van Zijl, P. C., and Mori, S. (2004). Fiber tract-based atlas of human white matter anatomy. Radiology 230, 77–87. doi:10.1148/radiol.2301021640
Keywords: mesial temporal sclerosis, temporal-lobe epilepsy, neuropsychological assessment, diffusion tensor imaging, processing speed
Citation: Alexander RPD, Concha L, Snyder TJ, Beaulieu C and Gross DW (2014) Correlations between limbic white matter and cognitive function in temporal-lobe epilepsy, preliminary findings. Front. Aging Neurosci. 6:142. doi: 10.3389/fnagi.2014.00142
Received: 11 March 2014; Accepted: 12 June 2014;
Published online: 30 June 2014.
Edited by:
Kenichi Oishi, Johns Hopkins University, USAReviewed by:
JackJ J. Lin, University of California, Irvine, USAKenichi Oishi, Johns Hopkins University, USA
Copyright: © 2014 Alexander, Concha, Snyder, Beaulieu and Gross. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Donald William Gross, Department of Medicine, Division of Neurology, 2E3.19 Walter C Mackenzie Health Sciences Centre, Edmonton, AB T6G 2B7, Canada e-mail:ZG9uYWxkLmdyb3NzQHVhbGJlcnRhLmNh
 Ryan P. D. Alexander
Ryan P. D. Alexander Luis Concha
Luis Concha Thomas J. Snyder
Thomas J. Snyder Christian Beaulieu
Christian Beaulieu Donald William Gross
Donald William Gross