- 1Department of Neurology and Institute of Neurology, Ruijin Hospital Affiliated to Shanghai Jiaotong University School of Medicine, Shanghai, China
- 2Ruijin Hospital North Affiliated to Shanghai Jiaotong University School of Medicine, Shanghai, China
- 3CAS Center for Excellence in Brain Science and Intelligence Technology, Shanghai, China
Background: Genetic factors have a well-known influence on Parkinson's disease (PD) susceptibility; however, no previous studies have investigated the influence of SNCA mutations on the natural history of PD using a prospective follow-up study. The aim of this study was to assess the risk factors of variation of SNCA on the prognosis symptoms of PD patients.
Methods: Fifty PD patients were recruited with 38 v-PSG confirmed PD+RBD patients, and the median follow-up period was 30 months. All patients underwent a comprehensive clinical evaluation at baseline and follow-up, and six SNPs of SNCA (rs356165, rs3857053, rs1045722, rs894278, rs356186, and rs356219) were analyzed. Cox proportional hazards regression models and Kaplan–Meier plot analysis were used to assess the associations between the SNCA variation and the primary and secondary progression outcomes.
Results: Based on the clinical assessment, we found that hyposmia was substantially easier to aggravate. Regression analysis showed that patients with the T allele of rs1045722 and the G allele of rs356219 presented a 34 and 20% decreased risk of progression to the H-Y stage, respectively (p = 0.022; p = 0.005). While for rs894278, G allele patients showed a 47% decreased risk of olfactory dysfunction (p = 0.029). Further subgroup analysis showed that PD+RBD patients with rs356219/G exhibited a 30% and 20% decreased risk of progression on the H-Y stage and MoCA score (p = 0.038; p = 0.045).
Conclusions: Our results indicated that genetic variation in SNCA may contribute to variability natural progression of PD and could possibly be used as a prognostic marker.
Introduction
Parkinson's disease (PD) is a multifactorial neurodegenerative disease associated with a combination of motor and non-motor features, while motor disability and cognitive decline may have poor influence on the quality of life in patients with PD (Zhang et al., 2005; Barnett, 2016; Chahine et al., 2016; Sveinbjornsdottir, 2016). The rates of clinical symptom progression and severity in PD are highly heterogeneous. Currently, the prognostic factors include gender, age at onset, disease subtype, and early cognitive status (Konno et al., 2016), while it remains largely unknown what factors indeed influence the long-term clinical progression and outcomes, especially in different subtypes of PD.
Previous studies have shown that genetic mutations play important roles in the pathogenesis of the sporadic form of PD, including SNCA, Parkin, PINK1, DJ-1, and LRRK2, in which SNCA is one of the most prominent hallmarks (Houlden and Singleton, 2012; Han et al., 2015; Tatura et al., 2016). For a better understanding of the progression of disease, a comprehensive analysis between clinical evaluations and genotypes in PD is necessary.
Rapid eye movement behavior disorder (RBD) is one of the dominant non-motor symptoms, which could appear before or after the motor symptoms. Multiple studies have confirmed that RBD is a risk factor for motor impairment and cognitive dysfunction (Postuma et al., 2012; Chahine et al., 2016; Li et al., 2017). Besides, PD patients encoded by the gene SNCA were several supportive evidence in RBD (Paul et al., 2018). In this study, we would investigate whether SNCA variants are associated with the development of the natural clinical evolution and relevant to RBD subtype. Thus, identifying prognostic markers that would influence disease progression would dramatically accelerate research into PD etiology and strengthen precision management of PD.
Methods
Participants
All study participants were recruited from the Department of Neurology, Ruijin Hospital affiliated with Shanghai JiaoTong University School of Medicine. At baseline, 50 PD participants were recruited to (1) be diagnosed according to the UK Brain Bank or current clinical diagnostic criteria by at least two movement disorders specialists (Hughes et al., 1992); (2) be 50 to 80 years old; (3) not have dementia or psychiatric disease; and (4) not have severe primary diseases, such as cardiac, liver, renal, and hematopoietic system diseases. Patients were divided into RBD (+) and RBD (–) groups according to the results of video polysomnography (v-PSG), based on the diagnostic criteria of the International Classification of Sleep Disorders (ISCD)-II criteria. In addition, all patients were designed to a follow-up after an average of 30 months. This study was approved by the ethics committee of Ruijin Hospital, Shanghai JiaoTong University School of Medicine. All participants or their guardians provided written informed consent.
Demographics and Clinical Assessment
Disease stage was determined using the modified Hoehn-Yahr (H-Y) staging (Hoehn and Yahr, 2001), while the motor subscale of Unified Parkinson's Disease Rating Scale (UPDRS) was used to evaluate motor symptoms (Movement Disorder Society Task Force on Rating Scales for Parkinson's Disease., 2003). Moreover, a series of comprehensive questionnaires in addition to the evaluation of motor symptoms were also administered for every patient, including the Non-Motor Symptom Questionnaire (NMSQ), REM Sleep Behavior Disorder Screening Questionnaire (RBDSQ), Scale for Outcomes in PD-Autonomic (SCOPA-AUT), olfaction test (SS-16), Mini-Mental State Examination (MMSE), and Montreal Cognitive Assessment (MoCA). The NMSQ was used to evaluate non-motor symptoms, while the SCOPA-AUT was employed to assess autonomic dysfunction (Verbaan et al., 2007). An olfaction test (SS-16) was also performed to assess the olfactory function through a 16-item odor identification (Hummel et al., 1997; Krismer et al., 2017). The MMSE and MoCA were performed to evaluate the cognitive function resulting from PD. In addition, an overnight v-PSG examination was performed for each patient to confirm the diagnosis of RBD, meeting the ISCD-II.
Genotyping
Previous studies have shown that SNCA rs356186 and rs356219 were associated with PD (Han et al., 2015), while rs356165, rs3857053, rs1045722, and rs894278 were associated with RBD (Li et al., 2017; Toffoli et al., 2017). Thus, we measured these six SNPs in the recruited patients (primers seen in Supplemental Table 1). Genomic DNA was extracted from lymphocytes of whole blood using the QIAamp DNA extraction kit (Qiagen, Valencia, CA, USA). The primers for sequences of SNP were designed by using Primer bank online design software and checked by the Shanghai BioSune Applied Biotechnology Company. Genotypes of the six loci were obtained directly by DNA sequencing run on a 3730xl DNA analyzer (Applied Biosystems, Foster City, CA, USA). The results were further analyzed by using the Chromas software (Version 2.6.5).
Statistical Analysis
Continuous data were presented as the mean ± standard deviation (SD), while discontinuous data were presented as median values (quartile). Paired samples t-test was conducted for the comparison of the differences in continuous data, and the Mann–Whitney U-test was performed to compare the difference in ranked data (H-Y stage) between the patients at baseline and the last visit. To assess associations between the SNCA variation and progression outcomes, Cox proportional hazards regression models were used to estimate hazard ratios (HRs) with 95% confidence intervals (95% CI) adjusted for age and sex and Kaplan–Meier plot and log rank test to analyze disease risk. Categorical variables were compared with the χ2 or Fisher exact test to assess the difference between the RBD (+) and RBD (−) group at baseline. All tests were two-sided, and the threshold of significance was p < 0.05. All statistical analyses were performed using SPSS statistical software (Version 22.0).
Results
Demographic and Clinical Data
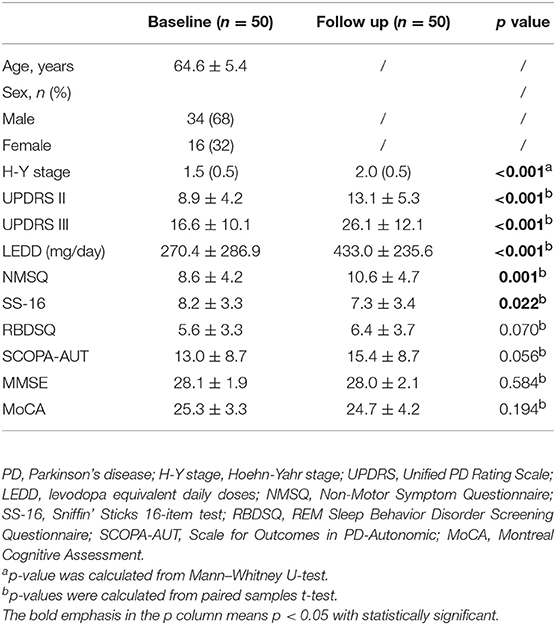
Fifty PD patients were enrolled in this study at baseline, and the cohort was followed up for a mean of 30 months for symptom progression. Demographics and clinical features of the baseline and follow-up data are listed in Table 1. The average age of the recruited PD patients at baseline was 64.6 years old, and 34 (68%) patients were male. The follow-up data showed a significantly higher H-Y stage, UPDRS scores of parts II and III, LEDD, and NMSQ than at the baseline (p ≤ 0.001, Table 1). Moreover, the aggravation of olfactory dysfunction was also found in the follow-up data (p = 0.022, Table 1). However, regarding the RBDSQ, SCOPA-AUT, MMSE, and MoCA scores, no significant difference was observed after the 30-month period.
Estimated Primary Clinical Outcomes in PD Patients With SNCA Variation
Changes >2.5 to 5.2 points on the UPDRS-III motor score represent clinically meaningful differences (Shulman et al., 2010). Thus, we defined a rapid motor progression, based on the mean 2.6 years of follow-up, as a 10-point increase of the UPDRS-III score (mean of 4 points per year) as a previous study performed (Paul et al., 2018). For the other motor indexes, including the UPDRS-II, H-Y stage, and LEDD, and non-motor symptoms, no significantly changed point has been defined. As a 10-point increase of the UPDRS-III score was exactly the average variation between the baseline and follow-up data, we thus specified the mean deviation from the baseline to first follow-up examinations as the cutoff value for further analyses of clinical outcomes, as shown in Table 2.
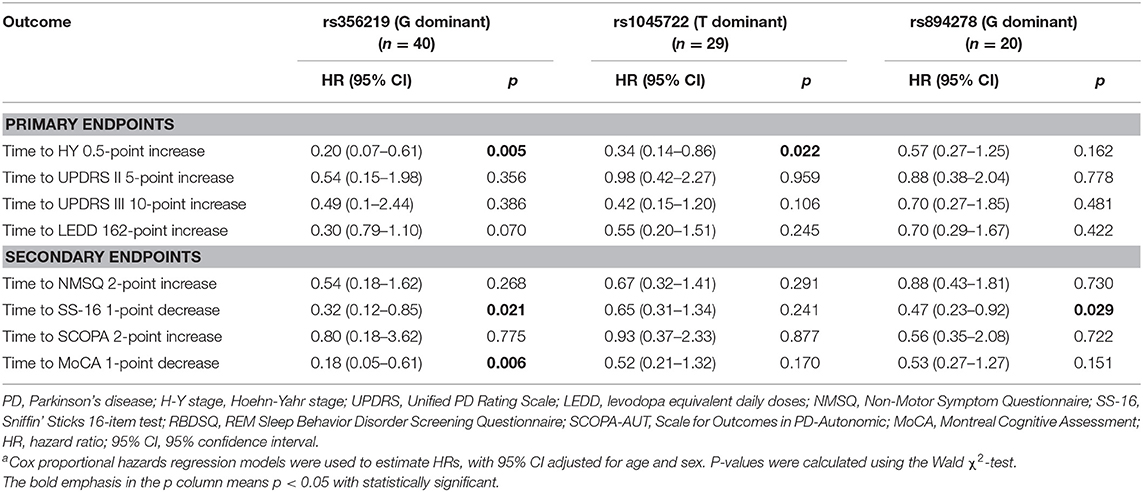
The progression of motor symptoms was set as the primary endpoints, including the H-Y stage, UPDRS II, UPDRS III, and LEED. For the H-Y stages, we defined a change of 0.5 points as disease progression. The results showed that the patients with the G allele of rs356219 and the T allele of rs1045722 of SNCA presented a 20% and 34% decreased risk of progression on the H-Y stage, respectively (p = 0.005, 95% CI: 0.07–0.61; p = 0.022, 95% CI: 0.14–0.86, Table 2). The Kaplan–Meier analysis also indicated that, compared to the patients with the WT genotype, the patients with G dominant models of rs356219 exhibited a longer progression-free-survival (PFS) time to reach a 0.5-point increase of the H-Y stage (42 vs. 24 months, p = 0.002, Figure 1A). For the UPDRS, we defined a 5-point change for the UPDRS II and a 10-point change for the UPDRS III as the progression index. Moreover, for the LEDD, we defined the subtraction of the mean 162 point as the cutoff value. However, no statistical significance was identified among rs356219, rs1045722, and rs894278 for the UPDRS or LEDD.
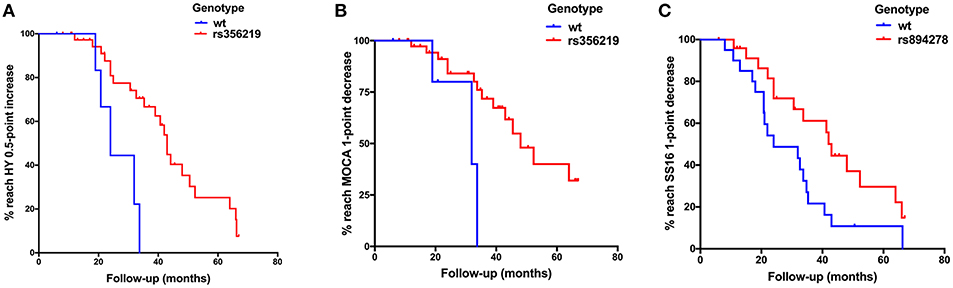
Figure 1. (A) Kaplan–Meier plot of H-Y stage to reach a 0.5-point increase in patients with G dominant models of rs356219 appeared a slower progression than WT genotype (p = 0.002). (B) Kaplan–Meier plot of Montreal Cognitive Assessment (MoCA) to reach a 1-point decrease in patients with G dominant models of rs356219 appeared a slower progression than WT genotype (p = 0.002). (C) Kaplan–Meier plot of SS-16 to reach a 1-point decrease in patients with G dominant models of rs894278 appeared a slower progression than WT genotype (p = 0.024).
Estimated Secondary Clinical Outcomes in PD Patients With SNCA Variation
Non-motor symptom aggravation was defined as the secondary endpoint, including the NMSQ, SCOPA-AUT, SS-16, and MoCA. We defined a two-point increase as non-motor symptom progression, a two-point increase as autonomic nervous disorder aggravation, a one-point decrease as olfactory decline, and a one-point decrease as cognitive decline. We found that the patients carrying the G allele of rs356219 showed a 32% decreased risk of olfactory dysfunction (p = 0.021, 95% CI: 0.12–0.85, Table 2), and the patients with the G allele of rs894278 presented a 47% decreased risk of olfactory dysfunction (p = 0.029, 95% CI: 0.23–0.92, Table 2). The Kaplan–Meier analysis also indicated a longer median PFS time to the SS-16 one-point decrease for the patients with the G allele of rs894278 (42 vs. 24 months, p = 0.024, Figure 1B). Furthermore, we found that the patients carrying the G allele of rs356219 presented an 18% decreased risk of suffering from cognitive decline (p = 0.005, 95% CI: 0.05–0.61, Table 2). The Kaplan–Meier analysis indicated median progression-free survival times of 45.5 months in the group of the G allele of rs356219 and 31.9 months in the remaining population (p = 0.002; Figure 1C).
Subgroup Analysis of Progression of rs356219 of SNCA in PD Patients With RBD
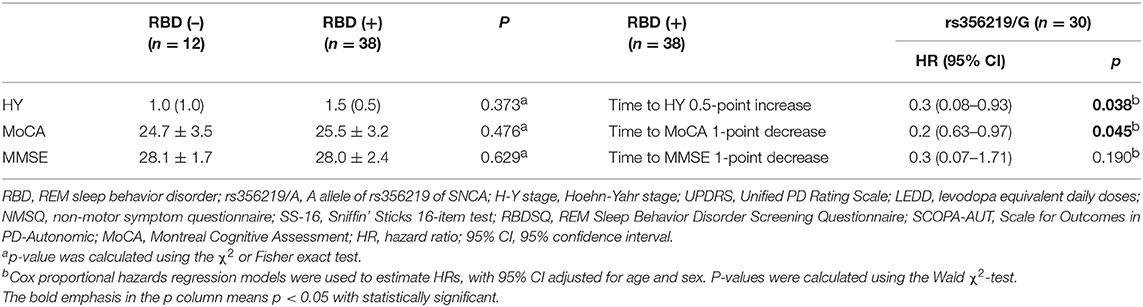
As reported, PD patients with RBD typically presented worse disease progress and cognitive dysfunction (Chahine et al., 2016), and we further analyzed the clinical outcomes in a subgroup of PD patients with RBD or without RBD. However, no significant difference was identified on the H-Y stage, MMSE, or MoCA scores compared to the baseline (Table 3). Given that rs356219 was associated with cognitive function in Table 2, we further investigated the potential predictors of rs356219/G in the RBD subgroup. The results showed that PD+RBD patients with rs356219/G of SNCA presented a 30% and 20% decreased risk of progression on the H-Y stage and MoCA score (p = 0.038, 95% CI: 0.08–0.93; p = 0.045, 95% CI: 0.63–0.97, Table 3).
Discussion
Up to now, few studies have focused on the correlation between clinical evaluation of the progression of PD and genotypes. In our current study, we found higher H-Y stage, UPDRS scores of parts II and III, LEDD, and NMSQ, and worse olfactory function in the 30-month progression of PD. SNCA rs894278/G and rs356219/G were associated with slower olfactory progression. Furthermore, we first found that rs356219/G reduced the risk of disease progression and cognitive impairment, even in the subgroup of PD+RBD. In addition, we first pointed out that SNCA rs1045722/T decreased disease progression.
A previous longitudinal population-based cohort study of 285 PD patients of European ancestry set a faster progression to H-Y stage 3 or a change of one stage as the point to explore motor progression in PD (Paul et al., 2018). This variability may be attributed to factors such as sample size, follow-up duration, and patient demographics. Moreover, it was reported that changes of 2.5 to 5.2 points on the UPDRS-III represent clinically meaningful differences (Shulman et al., 2010). We thus defined a rapid motor progression, based on the mean 2.6 years of follow-up, as a 10-point increase of the UPDRS-III score (mean of 4 points per year) as a previous study performed (Paul et al., 2018). Given that no dominant changed point has been defined for other indexes and a 10-point increase of the UPDRS-III score was exactly the average variation between the baseline and follow-up data, we specified the mean deviation from the baseline to first follow-up examinations as the cutoff value.
Further, we evaluated six SNPs of SNCA to identify its influence on the clinical progression. Based on our analysis, we demonstrated that patients carrying rs894278/G were prone to suffer hyposmia slower. In our previous studies, rs894278/G seems to be a risk allele, since PD patients were more likely to suffer from hyposmia (Chen et al., 2015; Li et al., 2017). However, as previous cross-sectional studies have shown, olfactory dysfunction was not always stable and it did not deteriorate in a linear manner during disease duration (Del Tredici et al., 2002; Braak et al., 2003). Moreover, Michelle E. Fullard's team explained that olfactory dysfunction might just be due to early pathology of the olfactory bulb; thus, it had no further impact on aggravation as the disease progresses deeper and Lewy bodies further deposit (Fullard et al., 2017). Therefore, olfaction score was not a reliable representative of disease progression for individuals. In addition, we found that patients with rs356219/G tend to retain better olfactory function, and it was the first time to investigate the influence of this SNP on hyposmia. In general, further research is required to determine the role of rs894278 and rs356219 in the hyposmia of PD patients.
As for cognitive function, previous studies have reported that ~80% of patients are expected to develop dementia after 10 years from the diagnosis of PD, and 20–30% of patients exhibit impairment that do not meet the criteria for dementia (Hoops et al., 2009; Aarsland and Kurz, 2010). The MMSE was the most widely used instrument used for cognitive assessment, while several independent studies have shown that the MoCA has greater specificity and accuracy than the MMSE (Hoops et al., 2009).
Previous studies have shown the rs356219/G was a significant risk for PD in different populations (Mata et al., 2010; Han et al., 2015; Emelyanov et al., 2018). It has been found that PD patients with rs356219/G had earlier onset age (Brockmann et al., 2013) and worse cognitive function (Campelo et al., 2017), probably affected by the distinction of SNCA expression in brain regions, blood, and plasma (Lesage and Brice, 2012; Brockmann et al., 2013; Burciu et al., 2018), whereas it was firstly shown in our study that rs356219/G reduces the risk of disease progression and cognitive impairment, which was not contradictory with previous studies. Previous studies mainly explored the risk genotypes of PD, while we focused on the disease progression of PD patients. In other words, populations with the G allele of rs356219 presented with higher susceptibility of PD but are more likely to have a relative slower disease progression. Simultaneously, sample limitation, race, or environmental factors are needed to be taken into account.
RBD was a representational prodromal condition of PD, and prospective studies have declared that it would exceed the conversion rate to PD (Gan-Or et al., 2015) and could predict the non–tremor-predominant subtype (Postuma et al., 2015). A previous study has shown that RBD may be associated with at least a subset of PD-associated genes in a 56 RBD cohort (Gan-Or et al., 2015). In our study, however, there was no significant difference on H-Y stage, MMSE, or MoCA scores compared to the baseline in patients with and without RBD. Thus, we further investigated whether there was a difference between the variant of rs356219 loci in the RBD subgroup (n = 38). The results showed that patients with rs356219/G experienced a slower motor progression and cognitive decline, which meant that rs356219/G was a protective factor for PD+RBD patients. In addition, our results firstly indicated that patients with rs1045722/T of SNCA appear to exhibit slower motor progression. This finding was indirectly in line with the self-reliant research that indicated that the A allele of rs1045722 was more frequent in PD patients than in iRBD (Toffoli et al., 2017). More research is required to determine the role of this SNP in the development of PD.
To date, levodopa remains the mainstay of treatment for PD, and higher doses of levodopa and other drugs may potentially produce better symptomatic control, but more motor complications (Tomlinson et al., 2010). For this reason, it would be meaningful to consider whether genetic effects may also be related to levodopa responsiveness so as to guide neurologists to make individual treatment strategies for PD patients. In our current study, however, no statistical contribution of the genotypes to LEDD progression was found, mainly because our patients were allowed to take PD medications during the entire examination process. Moreover, a longer follow-up period is needed to further verify our results.
In summary, we found that the variations of SNCA could contribute to variability in the natural progression of PD and could possibly be used as a prognostic marker. The limitation of this study is the small sample size; however, it still provides a cue that the genotype may affect the phenotype during PD progression. This study would help us to deeply explore the disease progression of PD and thus to strengthen precision management and contribute to further treatment. The mechanisms of how these variants influenced the pathogenesis and progression of PD are worth exploring in future studies.
Ethics Statement
This study was approved by the ethics committee of Ruijin Hospital, Shanghai JiaoTong University School of Medicine.
Author Contributions
JL and WK conceived and supervised the project and contributed to patients' recruitment. NL, YL, JL, and WK drafted the manuscript. NL and YL performed data management and statistical analyses. NL, YL, MN, LZho, MY, LZhu, and GY contributed to neurological assessment and sample collection of enrolled subjects. All authors read and approved the final version of the manuscript.
Funding
This work was supported by grants from the National Key Research and Development Program (2016YFC1306505), the National Natural Science Foundation of China (81873778), the National Natural Science Foundation of China (81501097), the Shanghai Municipal Education Commission-Basic Key Project (18JC1420300), the Shanghai Clinical Collaboration Construction Project of Chinese and Western Medicine (ZY20182020-FWTX-1104), and the Shanghai Sailing Program (19YF1429800).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all the patients for their generous donations of blood samples. We thank all members of the facilities of Ruijin Hospital for their helpful expertise. We are very grateful to our funders for having provided their support over the past years.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2019.00110/full#supplementary-material
Abbreviations
H-Y stage, Hoehn-Yahr stage; PD, Parkinson's disease; REM, rapid eye movement; RBD, REM behavior disorder; NMS, non-motor symptoms; NMSQ, Non-Motor Symptom Questionnaire; UPDRS, Unified Parkinson's Disease Rating Scale; LEDD, levodopa equivalent daily doses; RBDSQ, RBD Screening Questionnaire; SCOPA-AUT, Scale for Outcomes in Parkinson Disease-Autonomic; SS-16, Sniffin' Sticks 16-item test; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; v-PSG, video polysomnography; ISCD, International Classification of Sleep Disorder; DAT, dopamine transporter.
References
Aarsland, D., and Kurz, M. W. (2010). The epidemiology of dementia associated with Parkinson's disease. Brain Pathol. 20, 633–639. doi: 10.1111/j.1750-3639.2009.00369.x
Braak, H., Del Tredici, K., Rub, U., de Vos, R. A., Jansen Steur, E. N., and Braak, E. (2003). Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol. Aging 24, 197–211. doi: 10.1016/S0197-4580(02)00065-9
Brockmann, K., Schulte, C., Hauser, A. K., Lichtner, P., Huber, H., Maetzler, W., et al. (2013). SNCA: major genetic modifier of age at onset of Parkinson's disease. Mov. Disord. 28, 1217–1221. doi: 10.1002/mds.25469
Burciu, R. G., Seidler, R. D., Shukla, P., Nalls, M. A., Singleton, A. B., Okun, M. S., et al. (2018). Multimodal neuroimaging and behavioral assessment of alpha-synuclein polymorphism rs356219 in older adults. Neurobiol. Aging 66, 32–39. doi: 10.1016/j.neurobiolaging.2018.02.001
Campelo, C. L. C., Cagni, F. C., de Siqueira Figueredo, D., Oliveira, L. G. Jr., Silva-Neto, A. B., Macedo, P. T., et al. (2017). Variants in SNCA gene are associated with Parkinson's disease risk and cognitive symptoms in a Brazilian sample. Front. Aging Neurosci. 9:198. doi: 10.3389/fnagi.2017.00198
Chahine, L. M., Xie, S. X., Simuni, T., Tran, B., Postuma, R., Amara, A., et al. (2016). Longitudinal changes in cognition in early Parkinson's disease patients with REM sleep behavior disorder. Parkinsonism Relat. Disord. 27, 102–106. doi: 10.1016/j.parkreldis.2016.03.006
Chen, W., Kang, W. Y., Chen, S., Wang, Y., Xiao, Q., Wang, G., et al. (2015). Hyposmia correlates with SNCA variant and non-motor symptoms in Chinese patients with Parkinson's disease. Parkinsonism Relat. Disord. 21, 610–614. doi: 10.1016/j.parkreldis.2015.03.021
Del Tredici, K., Rub, U., De Vos, R. A., Bohl, J. R., and Braak, H. (2002). Where does parkinson disease pathology begin in the brain? J. Neuropathol. Exp. Neurol. 61, 413–426. doi: 10.1093/jnen/61.5.413
Emelyanov, A., Kulabukhova, D., Garaeva, L., Senkevich, K., Verbitskaya, E., Nikolaev, M., et al. (2018). SNCA variants and alpha-synuclein level in CD45+ blood cells in Parkinson's disease. J. Neurol. Sci. 395, 135–140. doi: 10.1016/j.jns.2018.10.002
Fullard, M. E., Morley, J. F., and Duda, J. E. (2017). Olfactory dysfunction as an early biomarker in Parkinson's disease. Neurosci. Bull. 33, 515–525. doi: 10.1007/s12264-017-0170-x
Gan-Or, Z., Girard, S. L., Noreau, A., Leblond, C. S., Gagnon, J. F., Arnulf, I., et al. (2015). Parkinson's disease genetic loci in rapid eye movement sleep behavior disorder. J. Mol. Neurosci. 56, 617–622. doi: 10.1007/s12031-015-0569-7
Han, W., Liu, Y., Mi, Y., Zhao, J., Liu, D., and Tian, Q. (2015). Alpha-synuclein (SNCA) polymorphisms and susceptibility to Parkinson's disease: a meta-analysis. Am. J. Med. Genet. B Neuropsychiatr. Genet. 168B, 123–134. doi: 10.1002/ajmg.b.32288
Hoehn, M. M., and Yahr, M. D. (2001). Parkinsonism: onset, progression, and mortality (Reprinted from Neurology, vol 17, 1967). Neurology 57, S11–S26. doi: 10.1212/WNL.17.5.427
Hoops, S., Nazem, S., Siderowf, A. D., Duda, J. E., Xie, S. X., Stern, M. B., et al. (2009). Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology 73, 1738–1745. doi: 10.1212/WNL.0b013e3181c34b47
Houlden, H., and Singleton, A. B. (2012). The genetics and neuropathology of Parkinson's disease. Acta. Neuropathol. 124, 325–338. doi: 10.1007/s00401-012-1013-5
Hughes, A. J., Daniel, S. E., Kilford, L., and Lees, A. J. (1992). Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 55, 181–184. doi: 10.1136/jnnp.55.3.181
Hummel, T., Sekinger, B., Wolf, S. R., Pauli, E., and Kobal, G. (1997). ‘Sniffin' sticks': olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem. Senses 22, 39–52. doi: 10.1093/chemse/22.1.39
Konno, T., Ross, O. A., Puschmann, A., Dickson, D. W., and Wszolek, Z. K. (2016). Autosomal dominant Parkinson's disease caused by SNCA duplications. Parkinsonism Relat. Disord. 22(Suppl. 1), S1–S6. doi: 10.1016/j.parkreldis.2015.09.007
Krismer, F., Pinter, B., Mueller, C., Mahlknecht, P., Nocker, M., Reiter, E., et al. (2017). Sniffing the diagnosis: olfactory testing in neurodegenerative parkinsonism. Parkinsonism Relat. Disord. 35, 36–41. doi: 10.1016/j.parkreldis.2016.11.010
Lesage, S., and Brice, A. (2012). Role of mendelian genes in “sporadic” Parkinson's disease. Parkinsonism Relat. Disord. 18(Suppl. 1), S66–S70. doi: 10.1016/S1353-8020(11)70022-0
Li, Y., Kang, W., Zhang, L., Zhou, L., Niu, M., and Liu, J. (2017). Hyposmia is associated with RBD for PD patients with variants of SNCA. Front. Aging Neurosci. 9:303. doi: 10.3389/fnagi.2017.00303
Mata, I. F., Shi, M., Agarwal, P., Chung, K. A., Edwards, K. L., Factor, S. A., et al. (2010). SNCA variant associated with Parkinson disease and plasma alpha-synuclein level. Arch. Neurol. 67, 1350–1356. doi: 10.1001/archneurol.2010.279
Movement Disorder Society Task Force on Rating Scales for Parkinson's Disease. (2003). The Unified Parkinson's Disease Rating Scale (UPDRS): status and recommendations. Mov. Disord. 18, 738–750. doi: 10.1002/mds.10473
Paul, K. C., Schulz, J., Bronstein, J. M., Lill, C. M., and Ritz, B. R. (2018). Association of polygenic risk score with cognitive decline and motor progression in Parkinson disease. JAMA Neurol. 75, 360–366. doi: 10.1001/jamaneurol.2017.4206
Postuma, R. B., Adler, C. H., Dugger, B. N., Hentz, J. G., Shill, H. A., Driver-Dunckley, E., et al. (2015). REM sleep behavior disorder and neuropathology in Parkinson's disease. Mov. Disord. 30, 1413–1417. doi: 10.1002/mds.26347
Postuma, R. B., Bertrand, J. A., Montplaisir, J., Desjardins, C., Vendette, M., Rios Romenets, S., et al. (2012). Rapid eye movement sleep behavior disorder and risk of dementia in Parkinson's disease: a prospective study. Mov. Disord. 27, 720–726. doi: 10.1002/mds.24939
Shulman, L. M., Gruber-Baldini, A. L., Anderson, K. E., Fishman, P. S., Reich, S. G., and Weiner, W. J. (2010). The clinically important difference on the unified Parkinson's disease rating scale. Arch. Neurol. 67, 64–70. doi: 10.1001/archneurol.2009.295
Sveinbjornsdottir, S. (2016). The clinical symptoms of Parkinson's disease. J. Neurochem. 139(Suppl. 1), 318–324. doi: 10.1111/jnc.13691
Tatura, R., Kraus, T., Giese, A., Arzberger, T., Buchholz, M., Hoglinger, G., et al. (2016). Parkinson's disease: SNCA-, PARK2-, and LRRK2-targeting microRNAs elevated in cingulate gyrus. Parkinsonism Relat. Disord. 33, 115–121. doi: 10.1016/j.parkreldis.2016.09.028
Toffoli, M., Dreussi, E., Cecchin, E., Valente, M., Sanvilli, N., Montico, M., et al. (2017). SNCA 3′UTR genetic variants in patients with Parkinson's disease and REM sleep behavior disorder. Neurol. Sci. 38, 1233–1240. doi: 10.1007/s10072-017-2945-2
Tomlinson, C. L., Stowe, R., Patel, S., Rick, C., Gray, R., and Clarke, C. E. (2010). Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov. Disord. 25, 2649–2653. doi: 10.1002/mds.23429
Verbaan, D., Marinus, J., Visser, M., van Rooden, S. M., Stiggelbout, A. M., and van Hilten, J. J. (2007). Patient-reported autonomic symptoms in Parkinson disease. Neurology 69, 333–341. doi: 10.1212/01.wnl.0000266593.50534.e8
Keywords: Parkinson's disease, RBD, SNCA, SNP, disease progression
Citation: Luo N, Li Y, Niu M, Zhou L, Yao M, Zhu L, Ye G, Kang W and Liu J (2019) Variants in the SNCA Locus Are Associated With the Progression of Parkinson's Disease. Front. Aging Neurosci. 11:110. doi: 10.3389/fnagi.2019.00110
Received: 27 January 2019; Accepted: 29 April 2019;
Published: 21 May 2019.
Edited by:
Jiawei Zhou, Shanghai Institutes for Biological Sciences (CAS), ChinaReviewed by:
Sofya Pchelina, Petersburg Nuclear Physics Institute (RAS), RussiaBeisha Tang, Central South University, China
Copyright © 2019 Luo, Li, Niu, Zhou, Yao, Zhu, Ye, Kang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ningdi Luo, bHVvbmluZ2RpQDEyNi5jb20=
Wenyan Kang, a3d5bWVkQDE2My5jb20= Jun Liu, amx5MDUyMEBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work
 Ningdi Luo
Ningdi Luo Yuanyuan Li
Yuanyuan Li Mengyue Niu
Mengyue Niu Liche Zhou
Liche Zhou Mengsha Yao
Mengsha Yao Lin Zhu
Lin Zhu Guanyu Ye
Guanyu Ye Wenyan Kang
Wenyan Kang Jun Liu
Jun Liu