- 1Department of Neurology, Mie University Graduate School of Medicine, Tsu, Japan
- 2Department of Dementia Prevention and Therapeutics, Mie University Graduate School of Medicine, Tsu, Japan
- 3Department of Neurology, Clinical Neuroscience Branch, Brain Research Institute, Niigata University, Niigata, Japan
- 4Department of Neurology, Graduate School of Medical Sciences, Kumamoto University, Kumamoto, Japan
- 5Department of Amyloidosis Research, Nagasaki International University, Nagasaki, Japan
- 6Department of Neurology, Juntendo University Urayasu Hospital, Chiba, Japan
- 7Department of Neurology, Graduate School of Medicine, Nippon Medical School, Tokyo, Japan
- 8Department of Neurology, Tokyo Women’s Medical University, Tokyo, Japan
- 9Department of Geriatric Medicine, Tokyo Medical University, Tokyo, Japan
- 10Department of Stroke and Cerebrovascular Medicine, Kyorin University, Tokyo, Japan
- 11Department of Internal Medicine, Nanakuri Memorial Hospital, Fujita Health University, Tsu, Japan
- 12Center for the Promotion of Interdisciplinary Education and Research, Kyoto University, Kyoto, Japan
- 13Translational Research Center for Medical Innovation, Foundation for Biomedical Research and Innovation at Kobe, Kobe, Japan
- 14Center for Cerebral and Cardiovascular Disease Information, National Cerebral and Cardiovascular Center, Osaka, Japan
- 15Department of Preventive Medicine and Epidemiologic Informatics, National Cerebral and Cardiovascular Center, Osaka, Japan
- 16Department of Neurology, National Cerebral and Cardiovascular Center, Suita, Japan
- 17Department of Neurology, Graduate School of Medical Science, Kyoto Prefectural University of Medicine, Kyoto, Japan
Objectives: Clinical characteristics of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) include migraine, recurrent stroke, white matter lesions, and vascular dementia. CADASIL is one of the most common hereditary cerebral small vessel diseases. Clinical presentation of CADASIL varies and a racial gap may exist between the Asian and Caucasian populations. This is the first nationwide epidemiological survey which aimed to elucidate the clinical features of CADASIL in Japan. Moreover, the registration database of CADASIL was constructed.
Methods: Subjects included CADASIL patients who visited the hospitals (totally 1,448 hospitals) certified by the Japanese Society of Neurology and/or Japan Stroke Society in 2016. This study consisted of a two-step survey; patients with CADASIL were identified genetically by the first questionnaire, and their clinical features were assessed by the second questionnaire. Selected 6 hospitals registered the data of all CADASIL patients using a Research Electronic Data Capture (REDCap) system for the second questionnaire.
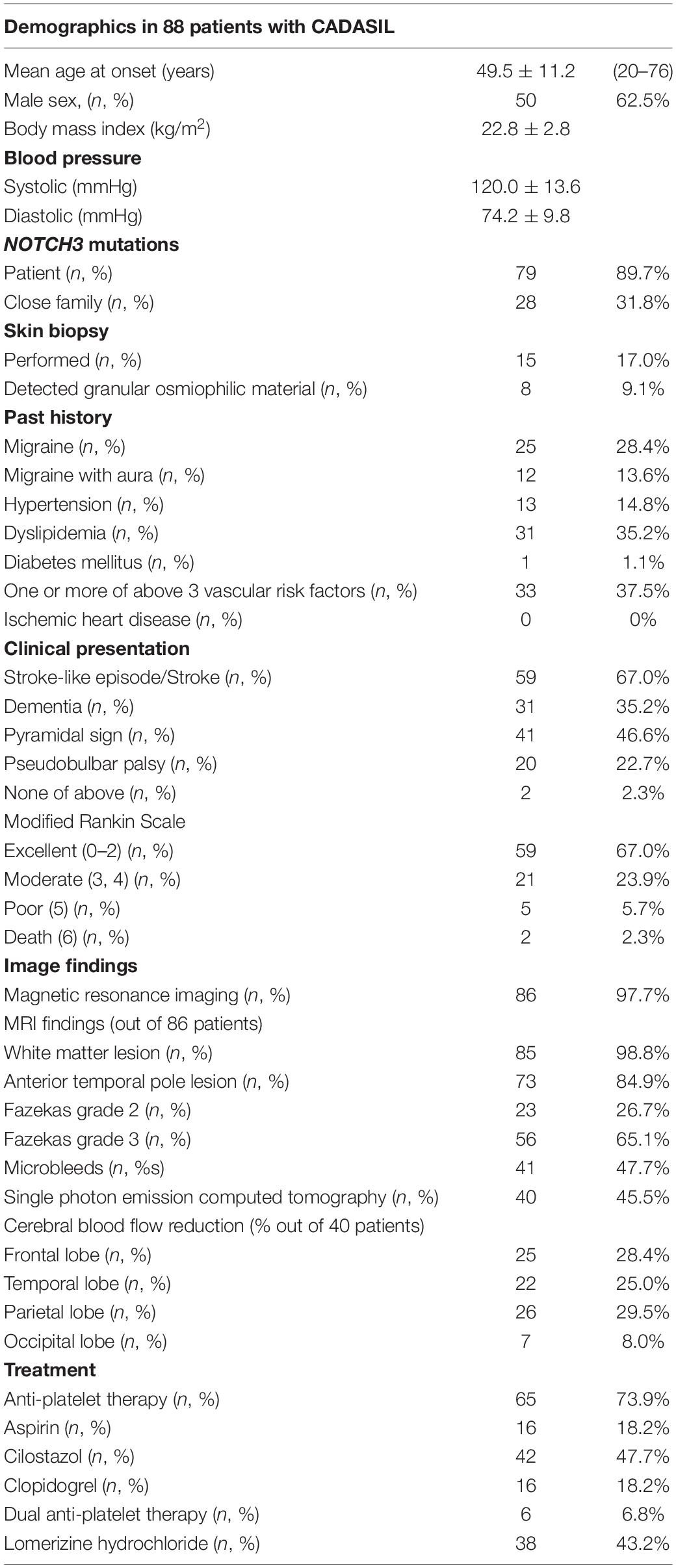
Results: Based on the criteria, 88 patients (50 male and 38 female) with CADASIL were enrolled. The mean age of symptom onset was 49.5 years. Sixteen (18.2%) patients had an elderly onset (>60 years). Thirteen patients (13.6%) had history of migraine with aura and 33 patients (37.5%) had vascular risk factor(s). From among the 86 patients who were examined using magnetic resonance imaging, abnormal deep white matter lesions were detected in 85 patients (98.8%), WMLs extending to anterior temporal pole in 73 patients (84.9%), and cerebral microbleeds in 41 patients (47.7%). Anti-platelet therapy was received by 65 patients (73.9%). Thirty-eight patients (43.2%) underwent treatment with lomerizine hydrochloride. Thirty-four different mutations of NOTCH3 were found in exons 2, 3, 4, 5, 6, 8, 11, 14, and 19. Most of the mutations existed in exon 4 (n = 44, 60.3%). The prevalence rate of CADASIL was 1.20 to 3.58 per 100,000 adults in Japan.
Conclusion: This questionnaire-based study revealed clinical features and treatment status in Japanese CADASIL patient, although it may not be an exhaustive search. We have constructed the REDCap database for these CADASIL patients.
Introduction
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is an autosomal dominant disease and a common type of ischemic cerebral small artery disease and subcortical vascular dementia (Roman et al., 2002). First identified in 1996, mutations in the NOTCH3 gene cause CADASIL and lead to small vessel arteriopathy in the central nervous system (Joutel et al., 1996). Patients with CADASIL commonly exhibit migraines with aura around at the age of 30 years, subcortical ischemic events at 50 years, and cognitive impairment between 50 and 60 years (Chabriat et al., 2009). Brain magnetic resonance imaging (MRI) displays extensive white matter hyperintensities including those in the anterior temporal lobe and external capsule. Although CADASIL is genetic small vessel disease, several reports have revealed the presence of vascular risk factors, such as hypertension, hyperlipidemia, and diabetes mellitus (Chabriat et al., 2009).
Although, Mizuta et al. (2017) found no significant difference in the clinical features of CADASIL between Japan and other countries, a nationwide survey in Japan was not conducted until 2019. The prevalence of migraines in Japanese CADASIL patients has been reported to be approximately 26.9–44.3% (Santa et al., 2003; Ueda et al., 2015; Mizuta et al., 2017; Koizumi et al., 2019), whereas a much lower prevalence of migraine has been reported in other Asian countries (Kim et al., 2006; Wang et al., 2011; Kang and Kim, 2015). While the clinical features of Asian CADASIL patients could be different from Caucasian patients (Kim et al., 2006; Wang et al., 2011; Choi et al., 2013; Kang and Kim, 2015), Japanese CADASIL patients might have other clinical characteristics.
Herein, this study aimed to elucidate the epidemiological and clinical features of Japanese CADASIL patients based on a Japanese nationwide survey. Moreover, we constructed a database for patients with CADASIL for future clinical studies.
Materials and Methods
Study Design and Subjects (Figure 1)
We conducted a two-step postal survey in 1,448 hospitals in all 47 Japanese prefectures. This study was carried out in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Mie University Graduate School of Medicine (permit number 2918).
The Two-Step Postal Survey
First-Step Postal Survey
Target subjects were CADASIL patients who visited the hospitals between April 1st, 2011 and June 30th, 2016. The hospitals surveyed were certified by the Japanese Society of Neurology and/or Japan Stroke Society in 2016 (in total 1,448 hospitals) and covered all 47 prefectures in Japan. In the first survey, a simple questionnaire asked whether there were any patients with CADASIL, and if so, how many.
Second-Step Survey
We obtained a response to the first questionnaires from 687 hospitals (47.4%). Of those, 78 hospitals had one or more patients with CADASIL and agreed to the next step. We sent a second questionnaire to the 78 hospitals to obtain further details. Detailed clinical information including age, sex, age at the onset (excluding migraine), height, weight, blood pressure (BP), birthplace, clinical presentations (stroke-like episodes, dementia, pyramidal sign, pseudobulbar palsy), migraine (with or without aura), ischemic stroke, hemorrhagic stroke, past medical history (hypertension, hyperlipidemia, diabetes mellitus, renal dysfunction, ischemic heart disease, and atrial fibrillation), type of the mutation of NOTCH3 gene, family history, treatment, modified Rankin Scale (Japanese version) (Shinohara et al., 2006), findings of magnetic resonance imaging (MRI) including Fazekas scale (Fazekas et al., 1987), findings of single photon emission computed tomography (SPECT), and skin biopsy were requested. We received a response to the second questionnaire from 38 hospitals (48.7%) and 61 patients with cases defined as CADASIL were enrolled.
REDCap Survey
We sent out a simple second-step questionnaire to six core hospitals, including Mie University Hospital, Niigata University Hospital, Kumamoto University Hospital, National Cerebral and Cardiovascular Center, Kyoto Prefectural University of Medicine Hospital, and Juntendo University Urayasu Hospital with questions regarding age, sex, age at onset, birthplace, and type of NOTCH3 mutation. After the two-step postal survey, data for core hospitals were also collected using a Research Electronic Data Capture (REDCap) system (Harris et al., 2009). The REDCap database was built to elucidate the natural history, clinical features, and clinical studies for patients with CADASIL in Japan. After obtaining the consent and approval of the Local Institutional Review Board (IRB) Committee to participate, the physician sent a registration form to the secretariat at National Cerebral and Cardiovascular Center of Japan. Detailed data same as the second postal survey was obtained from REDCap. A written consent form was obtained from each patient. REDCap survey enrolled a total 29 patients with CADASIL and all data remained anonymous without any patients’ identifying clinical information.
Diagnosis of CADASIL
The diagnosis of CADASIL was based on the genetic criteria that either the patient or a third-degree relative had NOTCH3 mutations. Seventy-nine patients were identified to have NOTCH3 mutations, and the other nine patients had the gene mutations present in their relatives.
Statistical Analysis
Three types of prevalence rates of CADASIL: the most conservative estimate, the moderate estimate, and the most aggressive estimate, were estimated from the questionnaire sampling process and the Japanese population in 2017, i.e., 106,367,000 adults (over 18 years old) data from the Ministry of Internal Affairs and Communications.
The most conservative estimate was based on the assumption that there were no patients in the not answered site in the first survey, and that the number of patients of the not answered site in the second survey was at least one. The moderate estimate was based on the assumption that there were no patients in the not answered site in the first survey, and that the percentage of patients in the not answered site in the secondary survey was same that in the answered site. The most aggressive estimate was based on the assumption that the percentage of patients in the not answered site in the first survey was same that in the answered site, and that the percentage of patients in the not answered site in the secondary survey was same that in the answered site.
Results
Study Patients
A total of 90 patients with CADASIL were registered from 44 hospitals (38 hospitals by questionnaires and 6 hospitals using the REDCap system). Of those, we excluded 2 patients as they had no family history nor they filled the genetic diagnostic criteria. Data from 88 CADASIL patients were collected and analyzed (Figure 1).
Age at the Onset and Geographic Data
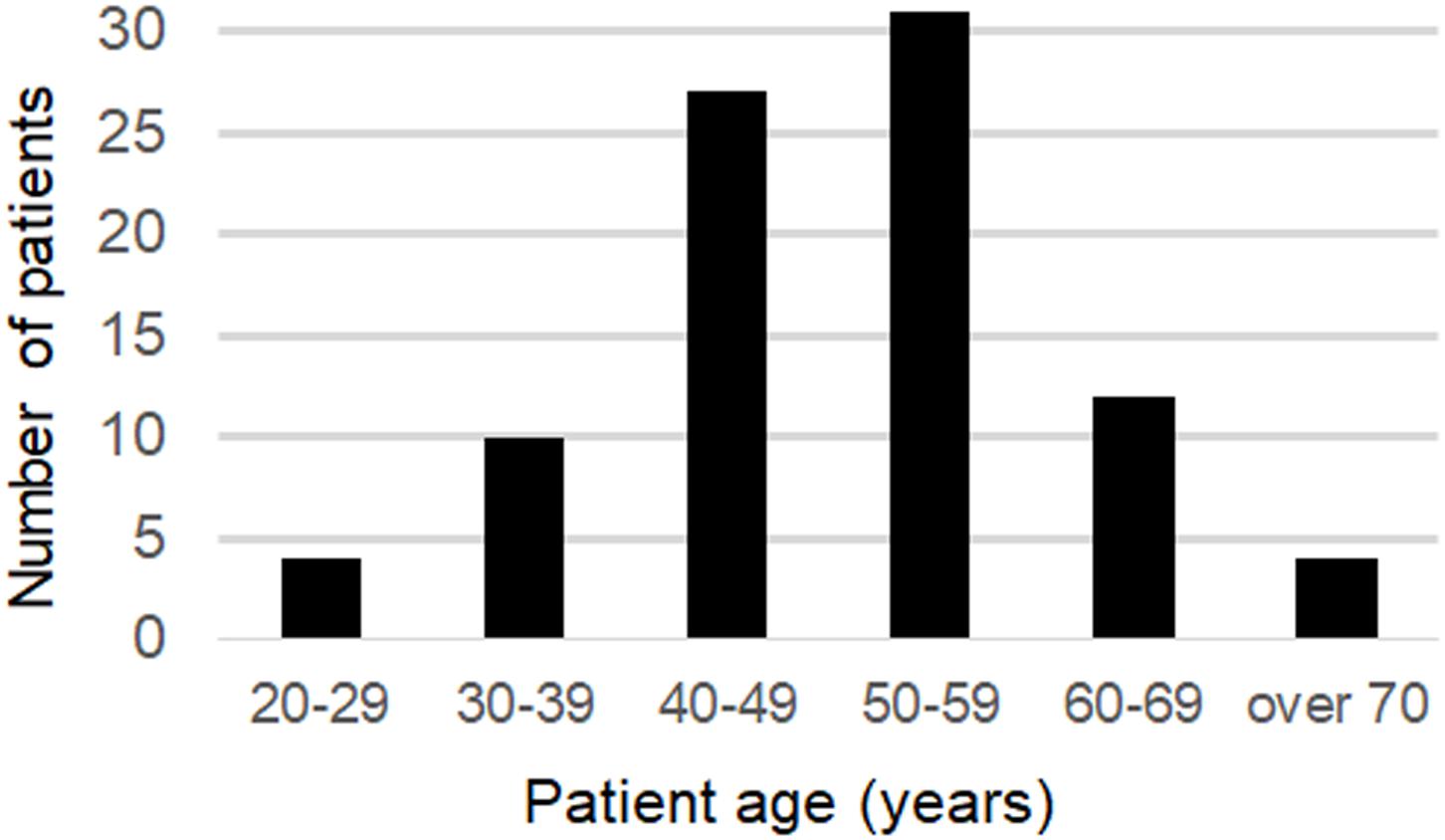
The mean age at onset was 49.5 years old (20–76) for all patients and for the 58 patients (65.9%), the age of onset was between 40 and 59 years old. Sixteen of the patients had an onset over 60 years of age (18.2%), and 4 patients had an onset over 70 years of age (Figure 2).
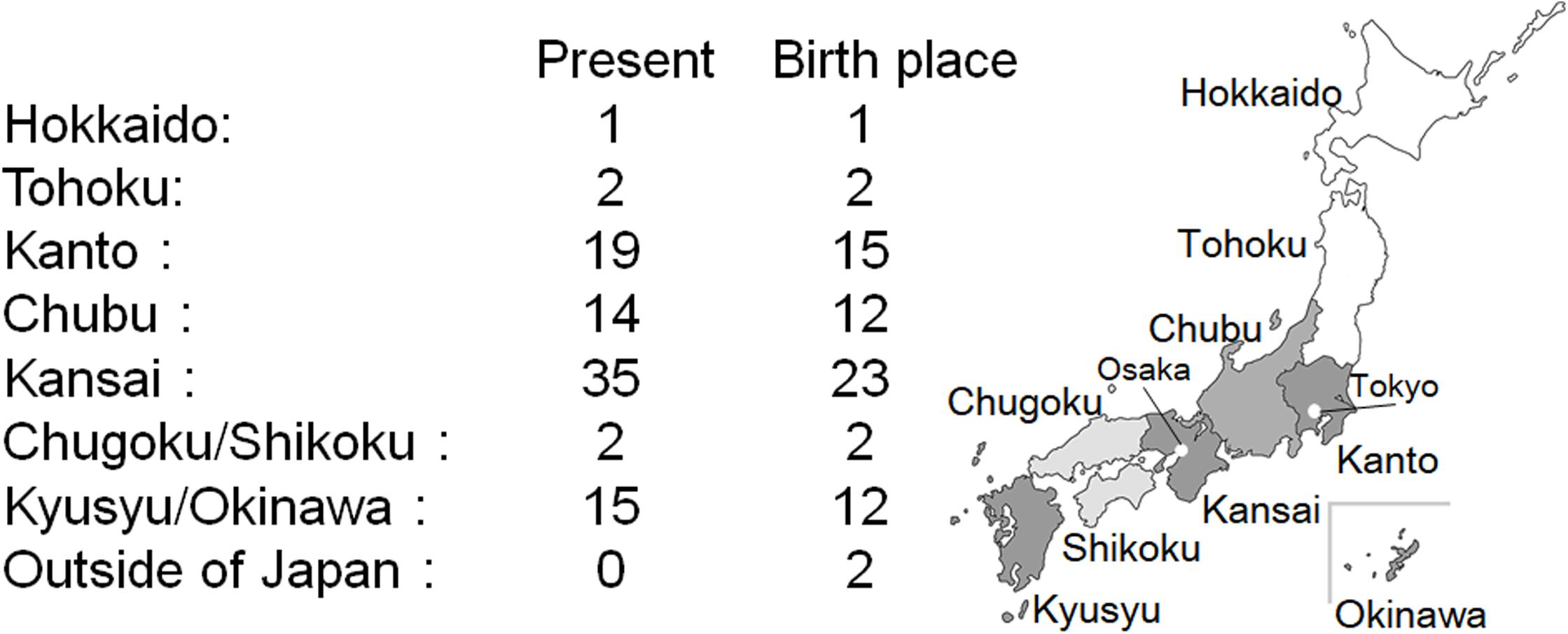
The current residence region and place of birth are shown in Figure 3. Kansai region has the highest number of CADASIL patients, followed by the Kanto and Kyusyu/Okinawa region. Birthplace tends to be the same as the current residence. Only a few patients with CADASIL were found in Hokkaido, Tohoku, and Chugoku/Shikoku regions. Two patients were born in out of Japan, and their birthplace was Taiwan.
Demographic and Clinical Data in Japanese CADASIL Patients (Table 1)
Body mass index (BMI) was calculated using the patients’ height and weight, and the mean BMI was 22.8 kg/m2. Mean systolic BP was 120.0 mmHg, and mean diastolic BP was 74.2 mmHg. NOTCH3 mutations were detected in 79 patients (89.7%) and 28 patients (31.8%) had a close family member with NOTCH3 mutations. Nineteen patients were identified to have NOTCH3 mutations in both the patients and their relatives. Skin biopsy was performed in 15 patients (17.0%), and granular osmiophilic material (GOM) was detected in 8 patients (9.1%). The detection rate of GOM was 53.3%.
From past history, migraine was noted in 25 patients (28.4%) and migraine with aura in 12 patients (13.6%). Vascular risk factors including hypertension, dyslipidemia, and diabetes mellitus were detected in 13 (14.8%), 31 (35.2%), and 1 (1.1%) patients, respectively. There were 33 patients (37.5%) who possessed one or more of these vascular risk factors. None of the patients had any ischemic heart disease.
Stroke and the stroke-like episode was the most common symptom in 59 patients (67.0%) and dementia was reported in 31 patients (35.2%). Pyramidal sign and pseudobulbar palsy were detected in 41 (46.6%) and 20 (22.7%) patients, respectively. Two patients (2.3%) did not show these before mentioned symptoms. Modified Rankin scale outcome at the time of this investigation was excellent [0–2] in 59 (67.0%), moderate [3, 4] in 21 (23.9%), poor [5] in 5 (5.7%), and death [6] in 2 patients (2.3%).
Regarding the neuroimaging findings, brain MRI was performed in 86 patients (97.7%), from among whom, white matter lesions were detected in 85 patients (98.8%) and anterior temporal lobe lesions were detected in 73 patients (84.9%). Fazekas score 2 was reported in 23 patients (26.7%) and score 3 for 56 patients (65.1%). Cerebral microbleeds were detected in 41 patients (47.7%). Although, SPECT was performed in 53 patients (60.2%), findings suggestive of CADASIL were reported in only 40 patients (45.4%). Decreased in cerebral blood flow was detected 25 patients in frontal lobe (62.5%, out of 40 patients), in 22 patients in temporal lobe (55.0%), in 26 patients in parietal lobe (65.0%), and in seven patients in occipital lobe (17.5%).
Sixty-five patients (73.9%) underwent an antiplatelet therapy, 16 (18.2%) had aspirin, 42 (47.7%) had cilostazol, and 16 patients had (18.2%) clopidogrel. Dual antiplatelet therapy was given to 6 patients (6.8%). Thirty-eight patients were treated with lomerizine hydrochloride (43.2%).
NOTCH3 Mutations
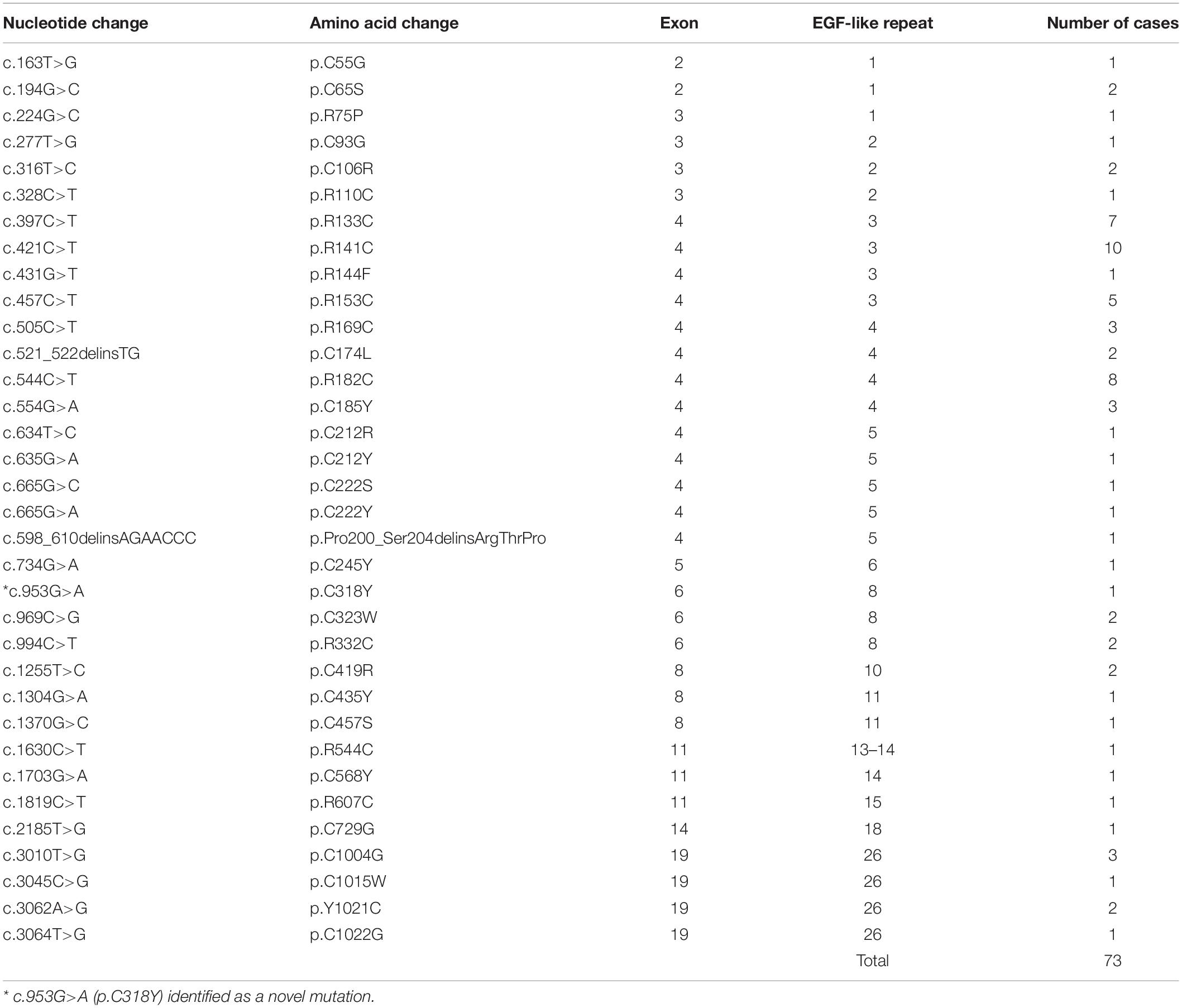
Of the total 88 CADASIL patients, we confirmed NOTCH3 gene mutations in 73 patients (83.0%) (Table 2). There was no description on the type of gene mutation in 15 patients. Thirty-four different mutations of NOTCH3 were found in exons 2, 3, 4, 5, 6, 8, 11, 14, and 19. Most of the mutations existed in exon 4 (n = 44, 60.3%), and the most common mutations were p.R141C, p.R182C, p.R133C, and p.R153C. A novel mutation (p.C318Y) was found in one case.
Prevalence Rate of CADASIL in Japan
In terms of the most conservative estimate, 128 CADASIL patients were estimated and the prevalence rate was 1.20 per 100,000 adults. In terms of the moderate estimate, 180.6 CADASIL patients were estimated and the prevalence rate was 1.70 per 100,000 adults. In terms of the most aggressive estimate, 380.7 CADASIL patients were estimated and the prevalence rate was 3.58 per 100,000 adults. The prevalence rate of CADASIL in Japan was estimated to between 1.20 to 3.58 per 100,000 adults.
Discussion
This is the first nationwide study for Japanese CADASIL patients. We show that the prevalence rate of migraines in Japanese CADASIL patients to be lower than European populations. The presence of vascular risk factors with hypertension and/or diabetes mellitus had a lower tendency than other European and Asian populations. The prevalence rate of CADASIL was 1.20 to 3.58 per 100,000 adults, and Kansai, Kanto, and Kyusyu/Okinawa regions had the largest distribution of CADASIL patients in Japan.
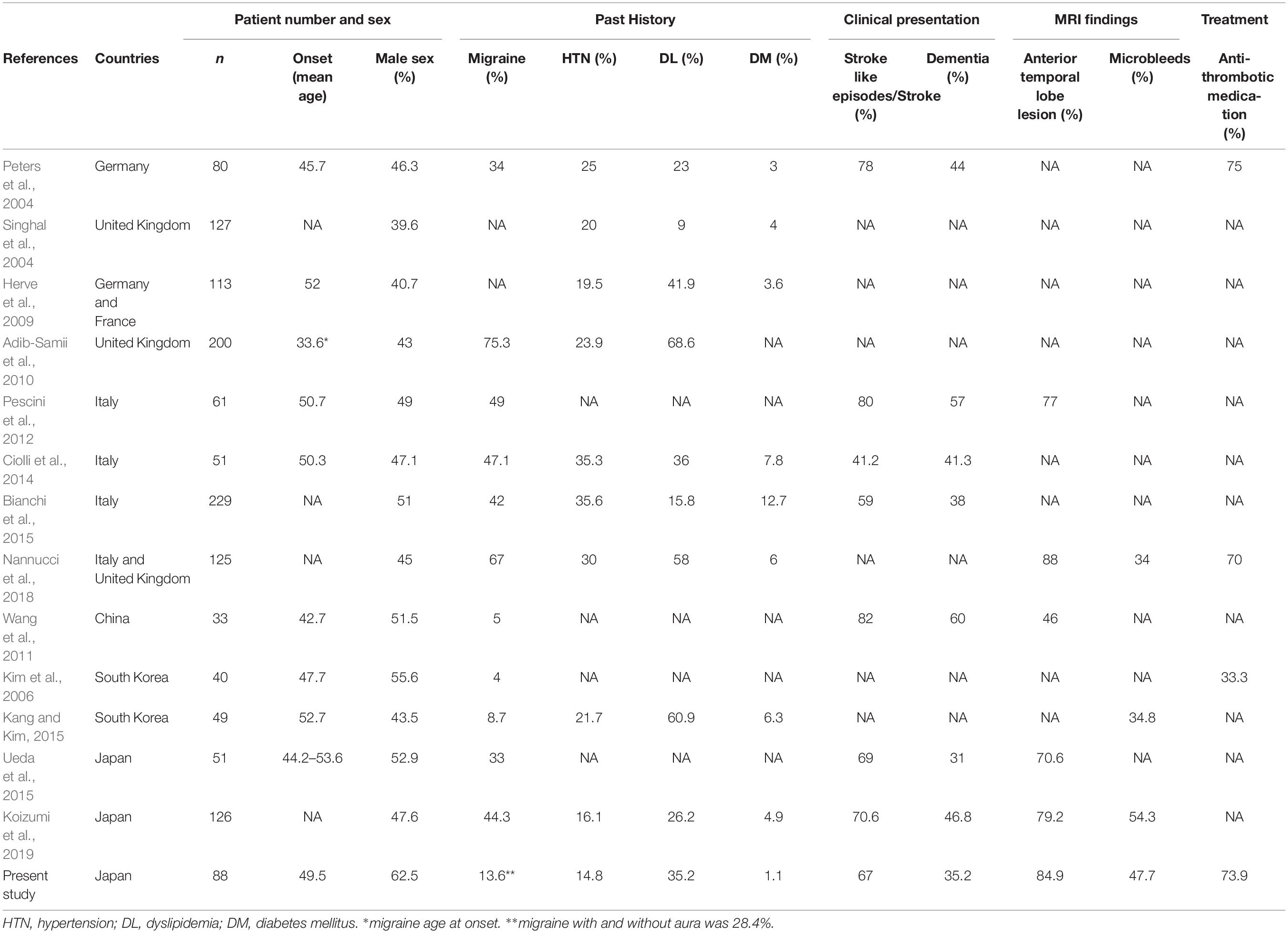
We also described a tendency of a low prevalence rate of migraine with aura in Japanese patients (13.6%) compared to the European counterparts. Table 3 shows the difference in CADASIL patients between Japan and other countries (Peters et al., 2004; Singhal et al., 2004; Kim et al., 2006; Herve et al., 2009; Adib-Samii et al., 2010; Wang et al., 2011; Pescini et al., 2012; Ciolli et al., 2014; Bianchi et al., 2015; Kang and Kim, 2015; Ueda et al., 2015; Nannucci et al., 2018; Koizumi et al., 2019). This table reveals a trend: a higher prevalence rate of migraine in European countries (34–75.3%) (Peters et al., 2004; Adib-Samii et al., 2010; Pescini et al., 2012; Ciolli et al., 2014; Bianchi et al., 2015; Nannucci et al., 2018), but a much lower prevalence in other Asian countries such as China and South Korea (4–8.7%) (Kim et al., 2006; Wang et al., 2011; Kang and Kim, 2015). Similarly, the nationwide survey showed that prevalence rate of migraine in the general population was reported to be lower in Japan than other European countries (Rasmussen et al., 1991; Gobel et al., 1994; Sakai and Igarashi, 1997); Japan appears midway in the prevalence rate scale of migraines between European and other Asian countries. In terms of past histories, the presence of vascular risk factors with hypertension and/or diabetes mellitus had a lower tendency than other European and Asian populations (Peters et al., 2004; Singhal et al., 2004; Herve et al., 2009; Ciolli et al., 2014; Bianchi et al., 2015; Kang and Kim, 2015; Nannucci et al., 2018). The prevalence rate of dyslipidemia was in the range of previous reports (Peters et al., 2004; Herve et al., 2009; Adib-Samii et al., 2010; Ciolli et al., 2014; Kang and Kim, 2015; Nannucci et al., 2018; Koizumi et al., 2019). The prevalence rate of hypertension and diabetes mellitus in Japan (14.8 and 1.1%) seems to be much lower than the other countries (20–35.6% and 3–12.7%) (Peters et al., 2004; Singhal et al., 2004; Herve et al., 2009; Adib-Samii et al., 2010; Ciolli et al., 2014; Bianchi et al., 2015; Kang and Kim, 2015; Nannucci et al., 2018) similar to the previous report (Mizuta et al., 2017). The mean age of onset in Japanese CADASIL patients is within the range of other countries (42.7–52 years old) (Peters et al., 2004; Kim et al., 2006; Herve et al., 2009; Wang et al., 2011; Pescini et al., 2012; Ciolli et al., 2014; Kang and Kim, 2015).
The prevalence rate of CADASIL was slightly lower than in other countries. Razvi et al. (2005) showed that the prevalence of CADASIL was 1.98 per 100,000 adults in Scotland, and Bianchi et al. (2015) described the prevalence at 4.1 per 100,000 adults in Italy. Uchino et al. (2002) reported that CADASIL was found in one of 2,030 stroke patients. It had been estimated that the incidence of new stroke was 220,000 per year in Japan (Takashima et al., 2017). Although, the number of patients with CADASIL expected to increase by approximately 108 per year from previous reports, we could identify only 88 patients in this study. There is a high possibility that the recognition of CADASIL varies depending on the facility, and there might be many undetected cases. Moreover, our study indicates that there is a regional difference in prevalence in Japanese CADASIL patients. Kansai, Kanto, and Kyusyu/Okinawa region have the three largest areas of the distribution of CADASIL patients. Note that we sent the questionnaire to all regions of Japan, and moreover, got replies from them all. Kanto region has the largest population, followed by Kansai, Chubu, and the Kyusyu/Okinawa region according to the 2015 data of Statistic Bureau, Ministry of Internal Affairs and Communications. Our study showed that Kansai region was the largest area in terms of distribution of CADASIL, indicating a predominance in the western part of Japan. In accordance with this observation, Ueda et al. (2015) reported a similar distribution of CADASIL patients in Japan and reported fewer numbers of CADASIL patients in the Hokkaido and Tohoku region. These results might depend on recognizing CADASIL itself in some hospitals in Japan.
The majority of NOTCH3 gene mutations existed in exon 4 in Japan. This result is similar to the previous Japanese reports (Ueda et al., 2015; Mizuta et al., 2017; Koizumi et al., 2019). Previous studies showed a high frequency of NOTCH3 gene mutation in exon 4 in Asian and European populations except Italy (Markus et al., 2002; Peters et al., 2004; Adib-Samii et al., 2010; Wang et al., 2011; Bianchi et al., 2015). Our study showed a lower prevalence of NOTCH3 mutations in exon 3, especially the R75P, than previous reports in Japan (Ueda et al., 2015; Mizuta et al., 2017; Koizumi et al., 2019).
Although we conducted the first nationwide survey of CADASIL patients in Japan and constructed a registration database of CADASIL, we acknowledge that there are several limitations to our study. First, the number of the patients included in this study were small. Second, the prevalence rate of CADASIL might be lower than expected. However, this limitation may be related to the approach used by our survey study: we sent questionnaires to the departments of neurology of certificated hospitals. Thus, we might have missed patients treated by other departments or clinics. Moreover, there is a possibility that asymptomatic patients or those with only minor symptoms could not be identified. Therefore, the number of individuals with NOTCH3 mutations might be underestimated. An alternative strategy involves asking genetic testing facilities for the number of NOTCH3 mutation-positive individuals, which would ensure the inclusion of all the possible cases of NOTCH3 mutation and thus, guarantee a larger study population. However, genetic testing for CADASIL is performed at several independent institutions, which might make it difficult to include all the positives in a single study. Therefore, we sent the questionnaire survey to hospitals for this study. This study sought the characteristics of CADASIL using a simple, two-step questionnaire, and therefore, detailed information may be lacking. However, through this study, we conducted a large survey involving more than one thousand certificated hospitals and created a novel registration database for future studies. We hope this study enables the increased recognition of CADASIL in Japan.
Conclusion
This is the first nationwide survey of CADASIL in Japan. We have constructed the REDCap database for CADASIL patients in Japan. This database could be expanded and used for future clinical trials.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Mie University Graduate School of Medicine (permit number 2918). Written consent was obtained from the patients who were registered in REDCap survey.
Author Contributions
AS: draft of the manuscript, study concept and design, and acquisition of the data and analysis. KT, AT, TK, IM, MI, and TM: revision of the manuscript, interpretation of the data, and study supervision. TH, HW, MT, and SS: revision of the manuscript and interpretation of the data. HN, OO, AU, YA, TU, KKim, KKit, HH, HF, YM, and AW-H: acquisition of the data and interpretation of the data. HT: revision of the manuscript, study concept and design, and study supervision. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by AMED under Grant Number JP17ek010913h0003.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Dr. Yumi Yamamoto, Dr. Fumiyuki Otsuka, and Dr. Kazuyuki Nagatsuka (National Cerebral and Cardiovascular Center), Dr. Soichiro Shimizu (Tokyo Medical University), Dr. Toshihiko Aso and Dr. Takakuni Maki (Kyoto University), Dr. Takashi Koizumi, Dr. Mao Mukai, and Dr. Jun Matsuura (Kyoto Prefectural University of Medicine), Dr. Masahiro Uemura, (Niigata University), Dr. Makoto Nakajima and Dr. Yihong Ma (Kumamoto University), and Dr. Eiji Nakatani and Dr. Shinsuke Kojima (Foundation for Biomedical Research and Innovation at Kobe), for their kind contribution. We would also like to thank all the patients and families with CADASIL and express our gratitude to all the neurologists, neurosurgeons, and strokologists for answering the questionnaire.
References
Adib-Samii, P., Brice, G., Martin, R. J., and Markus, H. S. (2010). Clinical spectrum of CADASIL and the effect of cardiovascular risk factors on phenotype: study in 200 consecutively recruited individuals. Stroke 41, 630–634. doi: 10.1161/strokeaha.109.568402
Bianchi, S., Zicari, E., Carluccio, A., Di Donato, I., Pescini, F., Nannucci, S., et al. (2015). CADASIL in central Italy: a retrospective clinical and genetic study in 229 patients. J. Neurol. 262, 134–141. doi: 10.1007/s00415-014-7533-2
Chabriat, H., Joutel, A., Dichgans, M., Tournier-Lasserve, E., and Bousser, M. G. (2009). Cadasil. Lancet Neurol. 8, 643–653.
Choi, J. C., Lee, K. H., Song, S. K., Lee, J. S., Kang, S. Y., and Kang, J. H. (2013). Screening for NOTCH3 gene mutations among 151 consecutive Korean patients with acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 22, 608–614. doi: 10.1016/j.jstrokecerebrovasdis.2011.10.013
Ciolli, L., Pescini, F., Salvadori, E., Del Bene, A., Pracucci, G., Poggesi, A., et al. (2014). Influence of vascular risk factors and neuropsychological profile on functional performances in CADASIL: results from the MIcrovascular LEukoencephalopathy Study (MILES). Eur. J. Neurol. 21, 65–71. doi: 10.1111/ene.12241
Fazekas, F., Chawluk, J. B., Alavi, A., Hurtig, H. I., and Zimmerman, R. A. (1987). MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am. J. Roentgenol. 149, 351–356. doi: 10.2214/ajr.149.2.351
Gobel, H., Petersen-Braun, M., and Soyka, D. (1994). The epidemiology of headache in Germany: a nationwide survey of a representative sample on the basis of the headache classification of the International Headache Society. Cephalalgia 14, 97–106. doi: 10.1046/j.1468-2982.1994.1402097.x
Harris, P. A., Taylor, R., Thielke, R., Payne, J., Gonzalez, N., and Conde, J. G. (2009). Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 42, 377–381. doi: 10.1016/j.jbi.2008.08.010
Herve, D., Godin, O., Dufouil, C., Viswanathan, A., Jouvent, E., Pachai, C., et al. (2009). Three-dimensional MRI analysis of individual volume of Lacunes in CADASIL. Stroke 40, 124–128. doi: 10.1161/strokeaha.108.520825
Joutel, A., Corpechot, C., Ducros, A., Vahedi, K., Chabriat, H., Mouton, P., et al. (1996). Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature 383, 707–710. doi: 10.1038/383707a0
Kang, H. G., and Kim, J. S. (2015). Intracranial arterial disease in CADASIL patients. J. Neurol. Sci. 359, 347–350. doi: 10.1016/j.jns.2015.11.029
Kim, Y., Choi, E. J., Choi, C. G., Kim, G., Choi, J. H., Yoo, H. W., et al. (2006). Characteristics of CADASIL in Korea: a novel cysteine-sparing Notch3 mutation. Neurology 66, 1511–1516. doi: 10.1212/01.wnl.0000216259.99811.50
Koizumi, T., Mizuta, I., Watanabe-Hosomi, A., Mukai, M., Hamano, A., Matsuura, J., et al. (2019). The CADASIL scale-J, a modified scale to prioritize access to genetic testing for Japanese CADASIL-suspected patients. J. Stroke Cerebrovasc. Dis. 28, 1431–1439. doi: 10.1016/j.jstrokecerebrovasdis.2019.03.026
Markus, H. S., Martin, R. J., Simpson, M. A., Dong, Y. B., Ali, N., Crosby, A. H., et al. (2002). Diagnostic strategies in CADASIL. Neurology 59, 1134–1138. doi: 10.1212/wnl.59.8.1134
Mizuta, I., Watanabe-Hosomi, A., Koizumi, T., Mukai, M., Hamano, A., Tomii, Y., et al. (2017). New diagnostic criteria for cerebral autosomal dominant arteriopathy with subcortical infarcts and leukocencephalopathy in Japan. J. Neurol. Sci. 381, 62–67. doi: 10.1016/j.jns.2017.08.009
Nannucci, S., Rinnoci, V., Pracucci, G., MacKinnon, A. D., Pescini, F., Adib-Samii, P., et al. (2018). Location, number and factors associated with cerebral microbleeds in an Italian-British cohort of CADASIL patients. PLoS One 13:e0190878. doi: 10.1371/journal.pone.0190878
Pescini, F., Nannucci, S., Bertaccini, B., Salvadori, E., Bianchi, S., Ragno, M., et al. (2012). The cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) scale: a screening tool to select patients for NOTCH3 gene analysis. Stroke 43, 2871–2876.
Peters, N., Herzog, J., Opherk, C., and Dichgans, M. (2004). A two-year clinical follow-up study in 80 CADASIL subjects: progression patterns and implications for clinical trials. Stroke 35, 1603–1608. doi: 10.1161/01.str.0000131546.71733.f1
Rasmussen, B. K., Jensen, R., and Olesen, J. (1991). A population-based analysis of the diagnostic criteria of the International Headache Society. Cephalalgia 11, 129–134. doi: 10.1046/j.1468-2982.1991.1103129.x
Razvi, S. S., Davidson, R., Bone, I., and Muir, K. W. (2005). The prevalence of cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy (CADASIL) in the west of Scotland. J. Neurol. Neurosurg. Psychiatry 76, 739–741. doi: 10.1136/jnnp.2004.051847
Roman, G. C., Erkinjuntti, T., Wallin, A., Pantoni, L., and Chui, H. C. (2002). Subcortical ischaemic vascular dementia. Lancet Neurol. 1, 426–436. doi: 10.1016/s1474-4422(02)00190-4
Sakai, F., and Igarashi, H. (1997). Prevalence of migraine in Japan: a nationwide survey. Cephalalgia 17, 15–22. doi: 10.1046/j.1468-2982.1997.1701015.x
Santa, Y., Uyama, E., Chui, D. H., Arima, M., Kotorii, S., Takahashi, K., et al. (2003). Genetic, clinical and pathological studies of CADASIL in Japan: a partial contribution of Notch3 mutations and implications of smooth muscle cell degeneration for the pathogenesis. J. Neurol. Sci. 212, 79–84. doi: 10.1016/s0022-510x(03)00109-6
Shinohara, Y., Minematsu, K., Amano, T., and Ohashi, Y. (2006). Modified Rankin scale with expanded guidance scheme and interview questionnaire: interrater agreement and reproducibility of assessment. Cerebrovasc. Dis. 21, 271–278. doi: 10.1159/000091226
Singhal, S., Bevan, S., Barrick, T., Rich, P., and Markus, H. S. (2004). The influence of genetic and cardiovascular risk factors on the CADASIL phenotype. Brain 127(Pt 9), 2031–2038. doi: 10.1093/brain/awh223
Takashima, N., Arima, H., Kita, Y., Fujii, T., Miyamatsu, N., Komori, M., et al. (2017). Incidence, management and short-term outcome of stroke in a general population of 1.4 million Japanese- shiga stroke registry. Circ. J. 81, 1636–1646. doi: 10.1253/circj.cj-17-0177
Uchino, M., Hirano, T., Uyama, E., and Hashimoto, Y. (2002). Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) and CADASIL-like disorders in Japan. Ann. N. Y. Acad. Sci. 977, 273–278.
Ueda, A., Ueda, M., Nagatoshi, A., Hirano, T., Ito, T., Arai, N., et al. (2015). Genotypic and phenotypic spectrum of CADASIL in Japan: the experience at a referral center in Kumamoto University from 1997 to 2014. J. Neurol. 262, 1828–1836. doi: 10.1007/s00415-015-7782-8
Keywords: small vessel disease, CADASIL, NOTCH3, risk factors, migraine, microbleeds
Citation: Shindo A, Tabei K, Taniguchi A, Nozaki H, Onodera O, Ueda A, Ando Y, Urabe T, Kimura K, Kitagawa K, Hanyu H, Hirano T, Wakita H, Fukuyama H, Kagimura T, Miyamoto Y, Takegami M, Saito S, Watanabe-Hosomi A, Mizuta I, Ihara M, Mizuno T and Tomimoto H (2020) A Nationwide Survey and Multicenter Registry-Based Database of Cerebral Autosomal Dominant Arteriopathy With Subcortical Infarcts and Leukoencephalopathy in Japan. Front. Aging Neurosci. 12:216. doi: 10.3389/fnagi.2020.00216
Received: 03 December 2019; Accepted: 19 June 2020;
Published: 14 July 2020.
Edited by:
Ramesh Kandimalla, Texas Tech University Health Sciences Center, United StatesReviewed by:
Yi Li, Cornell University, United StatesHugues Chabriat, Université Paris Diderot, France
Copyright © 2020 Shindo, Tabei, Taniguchi, Nozaki, Onodera, Ueda, Ando, Urabe, Kimura, Kitagawa, Hanyu, Hirano, Wakita, Fukuyama, Kagimura, Miyamoto, Takegami, Saito, Watanabe-Hosomi, Mizuta, Ihara, Mizuno and Tomimoto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Akihiro Shindo, YS1zaGluZG9AY2xpbi5tZWRpYy5taWUtdS5hYy5qcA==
 Akihiro Shindo
Akihiro Shindo Ken-ichi Tabei
Ken-ichi Tabei Akira Taniguchi1
Akira Taniguchi1 Hiroaki Nozaki
Hiroaki Nozaki Osamu Onodera
Osamu Onodera Kazuo Kitagawa
Kazuo Kitagawa Haruo Hanyu
Haruo Hanyu Hideaki Wakita
Hideaki Wakita Hidenao Fukuyama
Hidenao Fukuyama Yoshihiro Miyamoto
Yoshihiro Miyamoto Satoshi Saito
Satoshi Saito Ikuko Mizuta
Ikuko Mizuta Masafumi Ihara
Masafumi Ihara Toshiki Mizuno
Toshiki Mizuno Hidekazu Tomimoto
Hidekazu Tomimoto