- 1Department of Anesthesiology, The Third Central Clinical College of Tianjin Medical University, Tianjin, China
- 2Tianjin Key Laboratory of Extracorporeal Life Support for Critical Diseases, Artificial Cell Engineering Technology Research Center, Tianjin Institute of Hepatobiliary Disease, Tianjin, China
- 3Department of Anesthesiology, The Third Central Hospital of Tianjin, Tianjin, China
- 4College of Medicine, State Key Laboratory of Medicinal Chemical Biology, Key Laboratory of Bioactive Materials for Ministry of Education, Nankai University, Tianjin, China
- 5Department of Anesthesiology, The Third Central Clinical College of Tianjin Medical University, Tianjin, China
Backgrounds: Aging-related impairment of cerebral blood flow regulation leads to the disruption of neuronal micro-environmental homeostasis. Anesthetics should be carefully selected for aging patients since they have less cognition capacity. Effects and mechanisms of propofol or isoflurane have been widely investigated. However, how different combinations of propofol and isoflurane affect neurons and the mechanism still needs to be demonstrated.
Methods: We cultured rat hippocampal neurons and established a hypoxic injury model to imitate the micro-environment of aging brains. Three different combinations of propofol and isoflurane were applied to find out an optimum group via Cell Counting Kit-8 (CCK8) assay, lactic acid dehydrogenase (LDH) assay, real-time qPCR, and immunofluorescence of key proteins. Then BiP was silenced by small interfering RNA (siRNA) to explore the mechanism of how isoflurane and propofol affect neurons. Endoplasmic reticulum (ER) stress was measured by Western blot and immunofluorescence. To detect GABAAR α1 subunit proteostasis and its function, real-time qPCR, immunoprecipitation, and Western blot were carried out.
Results: Hypoxic neurons showed no different changes on cell viability, LDH leakage, and ER stress after treatment with 1% isoflurane and 1.2 μg/ml of propofol. Hypoxic neurons showed a sharp increase of LDH leakage and ER stress and a decrease of cell viability after treatment with 1.4% isoflurane and 0.6 μg/ml of propofol or 0.5% isoflurane and 1.8 μg/ml of propofol. After knockdown of BiP, the application of 1% isoflurane and 1.2 μg/ml of propofol led to the decrease of GABAAR α1 subunit protein content and viability of cell, as well as aggravation of ER stress.
Conclusion: A combination of 1% isoflurane and 1.2 μg/ml of propofol causes the least damage than do other dosages of both two drugs, and endogenous BiP plays an important role in this process.
Introduction
Globally, 50% of all the elderly people are evaluated to undergo at least one surgical procedure. Evidence proves that about 25% of all the elderly having major surgery will have an identifiable fall in cognitive function, and 50% of these patients will suffer permanent damage (Dodds et al., 2017). Perioperative neurocognitive disorder (PND) is the overarching term for cognitive impairment in the preoperative or postoperative period and is associated with increased mortality (Evered et al., 2018). It is characterized as a decline in cognitive functions including memory, attention, information processing, and cognitive flexibility (Hovens et al., 2012). Aging has been reported as one of the major risk factors (Shoair et al., 2015). Aging elicits multifaceted functional impairment in cerebral microcirculation, leading to cerebral hypoperfusion, deprivation of brain oxygen and nutrition supply, oxidative injury, and neurovascular uncoupling (Daulatzai, 2017; Toth et al., 2017). Elder people have less reserve of neurological function and are less able to resist surgery- and anesthesia-induced cognitive impairment than younger people. As a result, when treating aging patients, special caution is needed to prevent surgery- and anesthesia-induced cognitive impairment with regard to the choice and depth of anesthesia, dosage, and duration of perioperative anesthetics, and surgical strategy.
Propofol (2,6-diisopropylphenol) is a widely used intravenous anesthetic agent (Zhong et al., 2018). In addition to its sedative effects, propofol has a protective effect against cerebral ischemia–reperfusion injury in animal models, which can reduce infarction size and improve neurologic scores (Li et al., 2014; Shi et al., 2014). More specifically, propofol at a subanesthetic dose has a neuroprotective effect on cerebral ischemia–reperfusion rats, but not at higher doses (Wang et al., 2009). Isoflurane is an isomeride of enflurane, which is commonly used in inhalation anesthesia, but it can also induce neurogenetic damage and neurocognitive disorder and even accelerate the process of Alzheimer’s disease (Perucho et al., 2010; Zuo et al., 2018). Neurotoxicity of isoflurane is positively correlated with dose and duration (Wei et al., 2008; Wang H. et al., 2014). Previous studies showed that isoflurane minimum alveolar concentration (MAC) value was 1.45 ± 0.17%; 1.9% isoflurane, equivalent to 1.3 MAC, was sufficient to induce general anesthesia in rats (Boruta et al., 2012), while a minimal infusion rate at 40 mg·kg−1·h−1 was required using propofol alone to induce general anesthesia in rats (Logginidou et al., 2003). Our previous study confirmed that a single use of anesthetic dose propofol (40 mg·kg−1·h−1) or isoflurane (1.9%) aggravated cognitive impairment of aging rats with cerebral hypoperfusion, while a combination of sub-anesthesia dose propofol and isoflurane (1% isoflurane plus 20 mg·kg−1·h−1 propofol) did not (Bu et al., 2020). However, the effects of different combinations of propofol and isoflurane on hippocampal neurons remain to be explored.
The endoplasmic reticulum (ER) is a vast membranous network and a unique environment for protein folding, secretion, lipid biosynthesis, and calcium homeostasis (Kim et al., 2008), which are all required for maintaining normal cell function. ER stress is a subcellular pathological process of impairment in ER homeostasis. A number of insults have been shown to induce protein misfolding in the ER and cause ER stress, such as ischemia, nutrient deprivation, alterations in calcium concentrations, and oxidative stress (Martindale et al., 2006; Minamino et al., 2010; Wang et al., 2011). ER stress has been associated with isoflurane-induced cognitive impairment and neurodegenerative conditions such as Alzheimer’s disease (Cai et al., 2014; Ge et al., 2015; Xiang et al., 2017). A previous study also revealed that ER stress is involved in the neuroprotection of propofol (Wang L. et al., 2014). It remains unknown whether ER stress is involved in the effect of combined use of isoflurane and propofol on neurons. Furthermore, ER-localized molecular chaperone, BiP, one of the heat shock protein-70 family, has protective effects by attenuating ER stress (Feaver et al., 2008; Fu et al., 2008). BiP can be induced by oxidative stress (Dickhout et al., 2005). Therefore, we also investigated the involvement of BiP in the combined effects of isoflurane and propofol.
Synaptic inhibition in the brain mainly relies on GABA signaling. The GABAA receptors (GABAARs) are the major inhibitory receptors in the central nervous system and can mediate fast postsynaptic inhibitory effects. The α1 subunit-typed GABAAR is the most abundant composition subtype (Liu and Wong-Riley, 2004), and the α1 subunit is the key to GABAAR activity (Williams and Akabas, 2002; Kelley et al., 2008). GABAARs are assembled from their component subunits in the ER (Jacob et al., 2008); thus, protein homeostasis of GABAAR α1 subunit was used as an evaluation index for functional damage of neurons.
Therefore, the purpose of this study was to compare the effects of three different combination methods of propofol and isoflurane on hypoxic hippocampus neurons and to screen out the combination method with the least damage, so as to provide a safer general anesthesia strategy for patients with fragile cognitive function in clinical work; and the potential mechanism is discussed to provide a reference for subsequent basic scientific research.
Materials and Methods
Hippocampal Neuron Culture and Treatment
Primary hippocampal neuronal cultures from 16- to 18-day-old Sprague–Dawley rat embryos were prepared as described previously (Kaech and Banker, 2006), with modifications. Briefly, the hippocampus was removed and dissociated into a single-cell suspension. Neurons were plated at an average density of 5 × 105 cells/ml in supplemented Neurobasal medium on poly-D-lysine-coated glass coverslips. Neurons were maintained at 37°C in a humidified atmosphere of 95% air/5% CO2 and were used 7 days later. The confluency of cells was 80–90%. The purity of the cultured primary hippocampal neurons was determined by immunocytochemistry with an antibody against microtubule-associated protein-2 (MAP-2). The percentage of cultured neurons was above 95 ± 2.6% (Supplementary Figure 1). For each group, the operations were replicated six times.
Antibodies
The primary antibodies include anti-MAP-2 (1:1,000, Abcam, catalog no. ab32454) anti-GABAAR a1 (1:1,000, Abcam, catalog no. ab94585), anti-BiP (1:1,000, Abcam, catalog no. ab21685), anti-CHOP (1:500 for immunofluorescence, 1:1,000 for Western blotting, Cell Signaling Technology, catalog no. 2895), anti-β-actin (1:1,000, Abcam, catalog no. ab8226), anti-pan-cadherin (1:1,000, Abcam, catalog no. ab16505), anti-β-tubulin (1:1,000, Abcam, catalog no. ab18207), and anti-ubiquitin (1:1,000, Abcam, catalog no. ab134953). The secondary antibodies include Goat Anti-Mouse IgG H&L [horseradish peroxidase (HRP)] (1:2,000, Abcam, catalog no. ab205718), Goat Anti-Mouse IgG H&L (HRP; 1:2,000, Abcam, catalog no. ab250719), Goat Anti-Rabbit IgG H&L (Alexa Fluor® 488; 1:1,000 for immunofluorescence, ab150077), and Goat Anti-Mouse IgG H&L (Alexa Fluor® 594; 1:1,000 for immunofluorescence, ab150120).
Grouping 1
To investigate the effects of different dosage for isoflurane and propofol on the cell viability and cytotoxicity, hippocampal neurons were cultured in five groups (n = 6/group): (1) control group (C); (2) hypoxia-injured group (H); (3) hypoxia-injured cells treated with 1% isoflurane and 1.2 μg/ml propofol group (IP1); (4) hypoxia-injured cells treated with 1.4% isoflurane and 0.6 μg/ml propofol group (IP2); and (5) hypoxia-injured cells treated with 0.5% isoflurane and 1.8 μg/ml propofol group (IP3).
Grouping 2
To explore the role of BiP on the hippocampal neurons treated by the combination of 1% isoflurane and 1.2 μg/ml of propofol, small interfering RNA (siRNA) was performed at 3 days in vitro (DIV) for 72 h. The transfected hippocampal neurons were cultured in six groups (n = 6 for each group): (1) control-transfected cells (Con-siRNA + C) group; (2) hypoxia-treated control-transfected cells (Con-siRNA + H) group; (3) hypoxia-treated control-transfected cells treated with 1% isoflurane and 1.2 μg/ml of propofol (Con-siRNA + IP1) group; (4) control BiP-transfected cells (BiP-siRNA + C) group; (5) hypoxia-treated BiP-transfected cells (BiP-siRNA + H) group; and (6) hypoxia-treated BiP-transfected cells treated with 1% isoflurane and 1.2 μg/ml of propofol (BiP-siRNA + IP1) group.
Anoxic Treatment and Anesthesia
To mimic the ischemia–hypoxia condition, the neurons in the Con-siRNA + H, Con-siRNA + IP1, BiP-siRNA + H, and BiP-siRNA + IP1 groups were subjected to hypoxia (Hofmeijer et al., 2014). Briefly, at 7 DIV, primary hippocampal neurons were placed in an incubator at 37°C with 5% CO2, 3% O2, and 92% N2 for 3 h. After hypoxia treatment, 1.2 μg/ml of propofol was added to the culture medium in the Con-siRNA + IP1 and BiP-siRNA + IP1 groups. Then, the culture plates were immediately placed in an airtight and thermostatic chamber with internal electric fans and inlet and outlet valves (Benzonana et al., 2013). Isoflurane was delivered to the chamber at a rate of 2 l/min using a vaporizer with 5% CO2, 21% O2, and 74% N2. An anesthetic analyzer (Datex-Ohmeda, UK) was used to monitor the effluent isoflurane concentration. The concentration of isoflurane was maintained at 1% for 3 h. The cells were then exposed to the fresh neuronal culture medium and normoxic conditions (95% air, 5% CO2) for 24 h. In other groups, the hippocampal neurons were placed in the incubator under the condition of 5% CO2, 21% O2, and 74% N2 balanced.
RNA Interference
SiRNA against rat BiP (targeting sense: 5′-GAGGCGUAUUUGGGAAAGATT-3′; antisense: 5′-UCUUUCCCAAAUACGCCUCTT-3′) and a control siRNA (targeting sense: 5′-UUCUCCGAACGUGUCACGUTT-3′; antisense: 5′-ACGUGA CACGUUCGGAGAATT-3′) were purchased from GeneChem Company (Shanghai, China). The siRNAs were transfected into neurons using lentivirus at 3 DIV (Wong and Lazinski, 2002). After 8 h, the medium was replaced, and the cells were incubated at 37°C for 72 h. Western blot was used to confirm the BiP silencing.
Cell Viability Assay
Cell viability was assessed by using the Cell Counting Kit-8 (CCK8; Dojindo Laboratories, Kumamoto, Japan) according to the manufacturer’s instructions. Briefly, cells were cultured in 96-well plates; 24 h after the anesthetic treatment, 10 μl of CCK8 solution was added to each well, followed by 1 h of incubation at 37°C. The absorbance values were measured at 450 nm by using a microplate reader (Elx 800, Bio-TEK, Winooski, VT, USA).
Lactic Acid Dehydrogenase Release
Twenty-four hours after the anesthetic treatment, lactic acid dehydrogenase (LDH) release was detected by using the LDH Assay kit (Abcam, UK). The cell culture plates were centrifuged at 600 g for 10 min, and supernatants (10 μl/well) were extracted into another 96-well-plate. Then, a 100 μl LDH reaction mix was added to each well and incubated at room temperature for 30 min. The absorbance values were measured at 490 nm on the microplate reader (BioTek, Winooski, VT, USA).
Quantitative Real-Time Polymerase Chain Reaction
The expression of mRNA was detected by qRT-PCR. Total RNA was extracted from cells according to the protocols of the manufacturers and purified with RNA 4 Aqueous kit (Ambion Inc., Austin, TX, USA). Total RNA concentration was measured by spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). cDNA was synthesized using a PrimeScript RT reagent kit (Takara, Japan). Then, cDNA was used as a template for qPCR with Premix Ex TaqII (Takara, Japan) on Applied Biosystems 7500 RT-PCR System (Applied Biosystems, Foster City, CA, USA). The mRNA levels were normalized to GAPDH. Relative quantification was achieved by the comparative 2ΔΔ method (Schmittgen and Livak, 2008). The nucleotide sequences of the PCR primers (Sangon Biotech, Shanghai, China) are as follows:
• XBP1s mRNA: (forward 5′-GATGAATGCCCTGGTTACTG-3′;
• reverse 5′-AGATGTTCTGGG GAGGTGAC-3′)
• ATF6 mRNA: (forward 5′-AAGTGAAGAACCATTACTTTATATC-3′;
• reverse 5′-TTTCTGCTGGCTATTTGT-3′)
• ATF4 mRNA: (forward 5′-CATTCCTCGATTCCAGCAAAGCAC-3′;
• reverse5′-TTCTCCAACATCCAATCTGTCCCG-3′)
• GABAAR α1 mRNA: (forward 5′-TGTCTTTGGAGTGACGACC-3′;
• reverse 5′-ATCCCACGCATACCCTCTCT-3′)
• GRPDH mRNA: (forward 5′-AACAGCAACTCCCACTCTTC-3′;
• reverse 5′-CCTCTCTTGCTCAGTGTCCT-3′)
Immunofluorescence
Primary antibodies used were anti-MAP-2 and anti-CHOP. Staining was visualized with a Zeiss LSM 510 Meta confocal system (10×, 20×, and 40× objectives), and a 405-nm diode laser, a 488-nm Ar laser, and a 594-nm HeNe laser were used for excitation of fluorophores.
Co-immunoprecipitation
Cell proteins were extracted by Pierce™ Classic Magnetic IP/Co-IP Kit (Thermo Fisher Scientific, Waltham, MA, USA, 88804) according to the manuals. Protein co-immunoprecipitation (Co-IP) was performed by using Pierce™ Classic Magnetic IP/Co-IP Kit as well. Protein samples were run on 8% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) gel and probed with appropriate antibodies by Western blotting.
Western Blot
To obtain total cellular protein, neurons were lysed at 4°C in radioimmunoprecipitation assay (RIPA) buffer (Solarbio, R0010) mixed with phenylmethylsulfonyl fluoride (PMSF; Solarbio, P0100). Membrane protein fractions were obtained with a Mem-PER Plus Membrane Protein Extraction Kit (Thermo Fisher Scientific, Waltham, MA, USA). Protein lysates were resolved by SDS-PAGE gel and transferred to Immun-Blot polyvinylidene difluoride (PVDF) membranes (Solarbio). Membranes were incubated in blocking buffer (5% milk, 0.1% Tween20 in Tris-buffered saline) for 1 h and probed overnight with primary antibody at 4°C. Blots were rinsed thrice (0.1% Tween20 in Tris-buffered saline, 5 min each), followed by incubation with peroxidase-conjugated secondary antibody for 2 h at room temperature. Bands were visualized by exposing blots to X-ray film after incubation with freshly made chemiluminescent reagent (EMD Millipore, Billerica, MA, USA) and were quantified using Image Pro Plus.
Statistical Analysis
The data were analyzed with SPSS 24 (SPSS Science Inc.; Chicago, IL, USA). Data are presented as mean ± standard deviation (SD). All data were analyzed using one-way ANOVA with Tukey post hoc comparisons. P < 0.05 was the criterion for statistical significance.
Results
1% Isoflurane and 1.2 μg/ml of Propofol Caused the Least Damage to the Hypoxic Neurons
First, hippocampal neurons were exposed to hypoxic condition to mimic aging-related cerebral hypoperfusion. Cell viability and cytotoxicity were evaluated to explore the effects of the different drug combinations. Compared with that of the normal neurons (group C: 100.00 ± 12.42), the cell viability of hypoxia-injured neurons decreased significantly (group H: 75.23 ± 10.04, P < 0.05). Compared with the cell viability of group H, no significant change of cell viability was found in group IP1 (hypoxia-injured cells treated with 1% isoflurane and 1.2 μg/ml propofol group; group H vs. IP1: 75.23 ± 10.04 vs. 73.48 ± 12.73, P > 0.05). Cell viability decreased distinctly in group IP2 (hypoxia-injured cells treated with 1.4% isoflurane and 0.6 μg/ml propofol group; 39.02 ± 6.75, P < 0.05) and IP3 (hypoxia-injured cells treated with 0.5% isoflurane and 1.8 μg/ml propofol group; 41.50 ± 5.21, P < 0.05; Figure 1A) compared with group H. Meanwhile, anoxia resulted in ascension of cytotoxicity in hippocampal neurons (C vs. H: 15.85 ± 2.54 vs. 21.35 ± 2.93, P < 0.05). There was no significant difference in cytotoxicity between group H and IP1 (H vs. IP1: 21.35 ± 2.93 vs. 21.79 ± 3.19, P > 0.05). However, in comparison with group H, cytotoxicity increased sharply in group IP2 (39.02 ± 6.75, P < 0.05) and IP3 (32.66 ± 4.85, P < 0.05; Figure 1B).
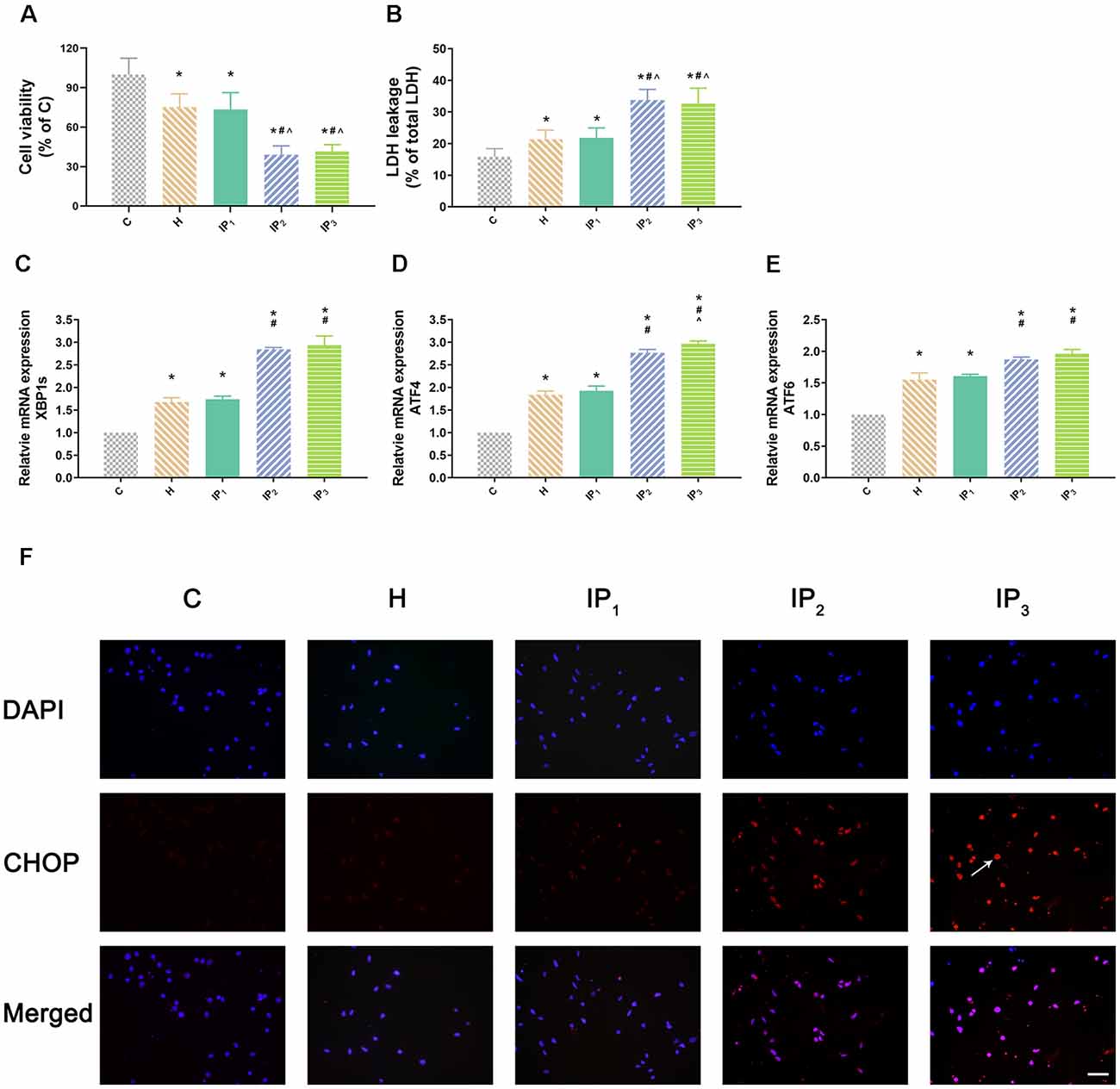
Figure 1. One percent isoflurane and 1.2 μg/ml of propofol caused the least damage to the hypoxic neurons. (A) Statistical graph of the neurons viability determined by the Cell Counting Kit-8 (CCK-8) assay. Data are expressed as the mean ± standard deviation (SD; n = 6/group). Note that hypoxia resulted in a significant decrease in cells viability. IP2 and IP3 aggravated this injury but IP1 did not. (B) Statistical graph of the neurons cytotoxicity determined by the lactic acid dehydrogenase (LDH) assay. Data are expressed as the mean ± SD (n = 6/group). Note that hypoxia resulted in a significant increase in cells cytotoxicity. IP2 and IP3 aggravated this injury but IP1 did not. (C–E) mRNA level analysis of XBP1s, ATF4, and ATF6 in neurons. Data are expressed as the mean ± SD (n = 6/group). Note that hypoxia resulted in a significant increase in transcription of XBP1s, ATF4, and ATF6. IP2 and IP3 aggravated this injury but IP1 did not. (F) Immunofluorescent microscopy for CHOP. Nuclear was stained by DAPI; CHOP antibody was marked by rhodamine Red-X. Note that IP2 and IP3 caused an increase in expression of CHOP but IP1 did not. *P < 0.05 compared with group C; #P < 0.05 compared with group H; ∧P < 0.05 compared with group IP1. Scale bars = 200 μm.
1% Isoflurane and 1.2 μg/ml of Propofol Induced the Least Endoplasmic Reticulum Stress
Second, we detected the expression of the element of the ER stress canonical biomarkers: XBP1, ATF4, and ATF6. Results showed that hypoxia increased the mRNA expression of XBP1, ATF4, and ATF6. Compared with that in the control group, mRNA expression of XBP1 (1.68 ± 0.09, P < 0.05), ATF4 (1.83 ± 0.09, P < 0.05), and ATF6 (1.55 ± 0.11, P < 0.05) increased in group H. Compared with that in group H, mRNA expression of XBP1 did not change significantly in group IP1 (1.74 ± 0.07, P > 0.05) but increased distinctly in groups IP2 (2.84 ± 0.04, P < 0.05) and IP3 (2.94 ± 0.20, P < 0.05; Figure 1C). The expression of ATF4 mRNA did not change significantly in group IP1 (1.92 ± 0.10, P > 0.05) but increased distinctly in groups IP2 (2.76 ± 0.08, P < 0.05) and IP3 (2.97 ± 0.06, P < 0.05; Figure 1D). Likewise, the expression of ATF6 mRNA did not change significantly in group IP1 (1.61 ± 0.03, P > 0.05) but increased distinctly in groups IP2 (1.87 ± 0.04, P < 0.05) and IP3 (1.96 ± 0.07, P < 0.05; Figure 1E). CHOP, a pro-apoptotic transcription factor, plays a critical role in ER stress-induced apoptosis (Biwer and Isakson, 2017). Results of immunofluorescence showed that expression of CHOP rose visibly in groups IP2 and IP3 (Figure 1F). The expression of BiP protein increased distinctly in group H (134.94 ± 6.09, P < 0.05) compared with group C (100.00 ± 4.79). Compared with that in group H, the expression of BiP protein increased distinctly in groups IP1 (215.39 ± 24.17, P < 0.05), IP2 (182.78 ± 12.66, P < 0.05), and IP3 (176.36 ± 16.78, P < 0.05; Figures 2A,B). BiP is an indicator that can relieve ER stress. Compared with that in group H, the expression of BiP in group IP2 and IP3 also increased, but it was far less than that in group IP1. In combination with other indicators of ER stress, we concluded that the combination of 1% isoflurane and 1.2 μg/ml of propofol induced ER stress was the least among the three compound drug groups.
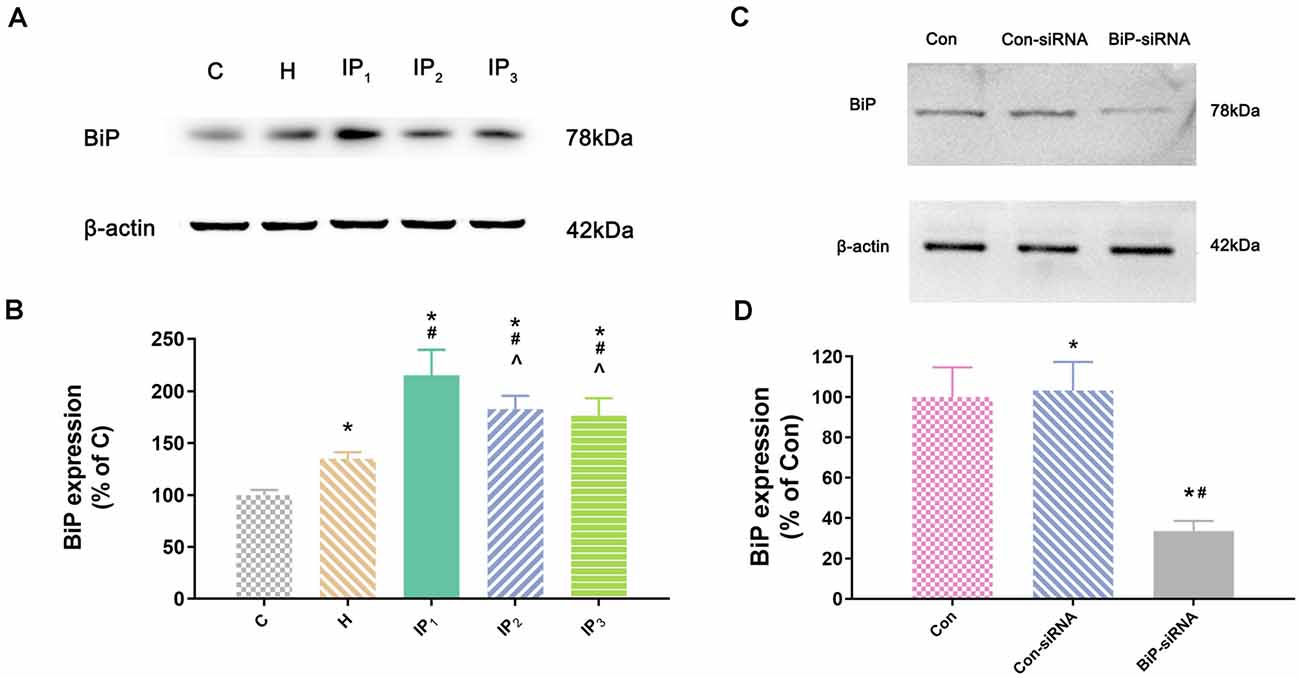
Figure 2. The expression of BiP in hippocampal neurons. (A) The expression of BiP in hippocampal neurons was determined by Western blotting. (B) Statistical graph of the expression of BiP. Note that treatment with isoflurane and propofol caused distinctly increase in BiP expression of hypoxic neurons. Data are expressed as the mean ± SD (n = 6/group). (C) Verifying siRNA targeting BiP on BiP protein expression. BiP-siRNA significantly down-regulates BiP protein expression. (D) Statistical graph of the expression of BiP. Data are expressed as the mean ± SD (n = 6/group). *P < 0.05 compared with group Con; #P < 0.05 compared with group Con-siRNA; ∧P < 0.05 compared with group IP1.
Damage to the Hypoxic Neurons Caused by Anesthetics Was Alleviated by Raising Endogenous Binding Immunoglobulin Protein
To identify the mechanisms by which 1% isoflurane and 1.2 μg/ml of propofol caused the least damage to the hypoxic neurons, we determined the effect of endogenous BiP by using of BiP-siRNA in rat hippocampal neurons. Western blot showed that in comparison with control group or lentiviral vector-negative control treated neurons, the expression of BiP protein was significantly dampened in the BiP-siRNA transfected cells (Con-siRNA vs. BiP-siRNA: 103.16 ± 14.20 vs. 33.53 ± 5.06, P < 0.05; Figures 2C,D).
No significant change of the viability of neurons was found between Con-siRNA + H and Con-siRNA + IP1 groups (Con-siRNA + H vs. Con-siRNA + IP1: 76.80 ± 11.21 vs. 71.60 ± 14.96, P > 0.05; Figure 3A). However, the viability of neurons in the BiP-siRNA + IP1 group was significantly reduced in comparison with that of the BiP-siRNA + H group (BiP-siRNA + IP1 vs. BiP-siRNA + H: 30.35 ± 5.38 vs. 47.81 ± 7.17, P < 0.05; Figure 3A). BiP siRNA interference caused obvious damage of IP1 on hypoxic neurons, which suggested that 1% isoflurane and 1.2 μg/ml of propofol affected neurons by raising endogenous BiP.
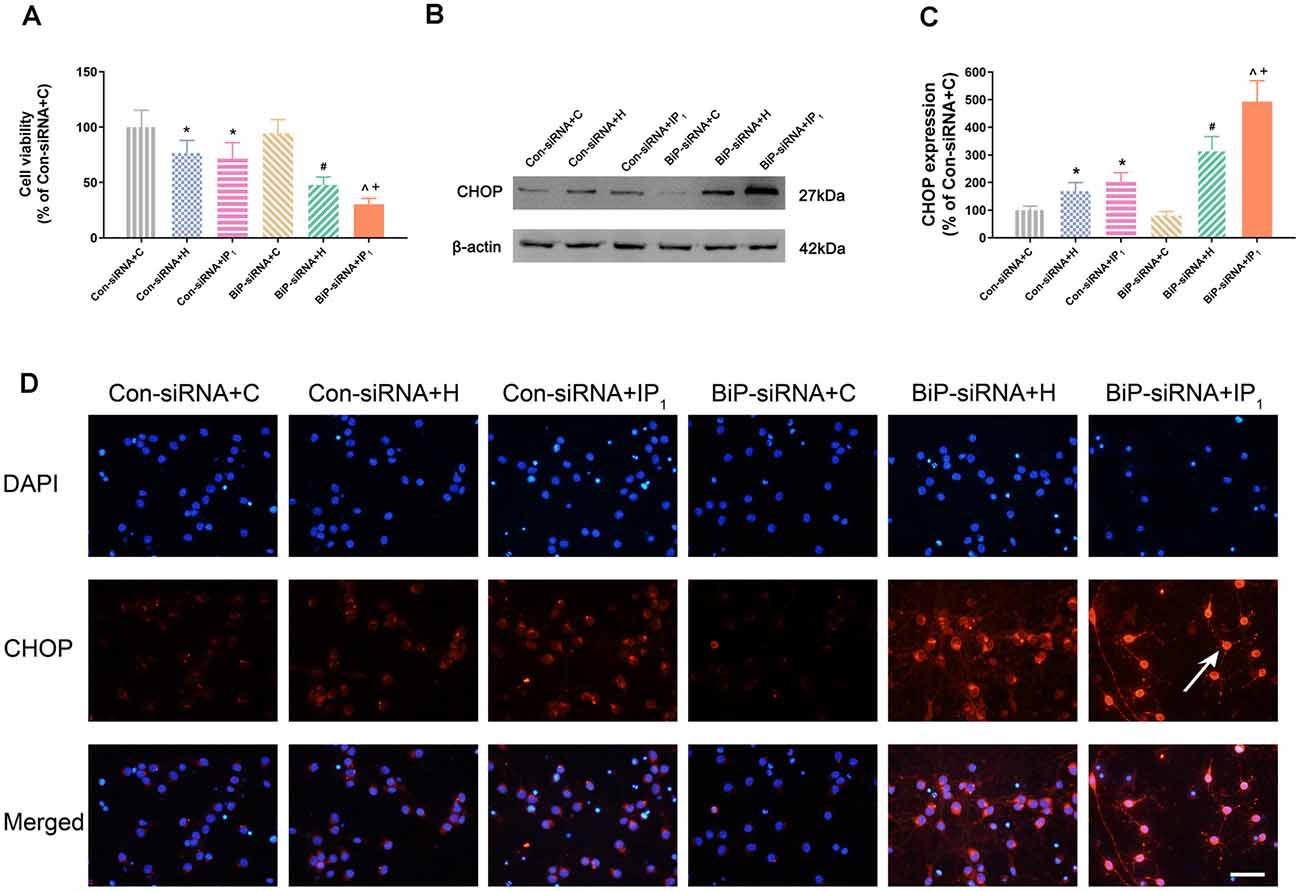
Figure 3. Knockdown of BiP by siRNA aggravated the neuronal injury and endoplasmic reticulum (ER) stress caused by anesthetics. (A) Statistical graph of the neurons viability determined by the Cell Counting Kit-8 (CCK8) assay. Data are expressed as the mean ± SD (n = 6/group). (B,C) The expression of CHOP in hippocampal neurons was determined by Western blotting. Data are expressed as the mean ± SD (n = 6/group). One percent isoflurane and 1.2 μg/ml of propofol significantly up-regulated CHOP protein expression in neurons after treatment with BiP siRNA. *P < 0.05 compared with group Con-siRNA + C; #P < 0.05 compared with group Con-siRNA + H; ∧P < 0.05 compared with group Con-siRNA + IP1; +P < 0.05 compared with group BiP-siRNA + H. (D) Immunofluorescent microscopy for CHOP. Nuclear was stained by DAPI; CHOP antibody was marked by rhodamine Red-X. Scale bars = 50 μm.
Endoplasmic Reticulum Stress-Related Apoptosis Caused by Anesthetics Was Alleviated by Raising Endogenous Binding Immunoglobulin Protein
There was no difference in the expression of CHOP between the Con-siRNA + H and Con-siRNA + IP1 groups (Con-siRNA + H vs. Con-siRNA + IP1: 168.97 ± 30.67 vs. 202.73 ± 33.55, P > 0.05; Figures 3B–D). However, compared with that in the BiP-siRNA + H group, the expression of CHOP raised significantly in the BiP-siRNA + IP1 group (BiP-siRNA + IP1 vs. BiP-siRNA + H: 492.91 ± 75.60 vs. 318.79 ± 47.82, P < 0.05; Figures 3B–D).
Disturbance of γ-Aminobutyric Acid A Type Receptor α1 Subunit Proteostasis Caused by Anesthetics Was Alleviated by Raising Endogenous Binding Immunoglobulin Protein
We tested the mRNA expression of GABAAR α1 in the hippocampal neurons by RT-qPCR assay. We found that there was no difference in the mRNA expression of GABAAR α1 between the Con-siRNA + H and Con-siRNA + IP1 groups (Con-siRNA + H vs. Con-siRNA + IP1: 78.29 ± 12.34 vs. 75.84 ± 13.51, P > 0.05; Figure 4A). However, compared with that in the BiP-siRNA + H group, the mRNA expression of GABAAR α1 was significantly down-regulated in the BiP-siRNA + IP1 group (BiP-siRNA + H vs. BiP-siRNA + IP1: 48.65 ± 8.49 vs. 32.27 ± 5.40, P < 0.05; Figure 4A). It suggests that transcription was inhibited when neurons were treated with 1% isoflurane and 1.2 μg/ml of propofol after BiP was knocked down.
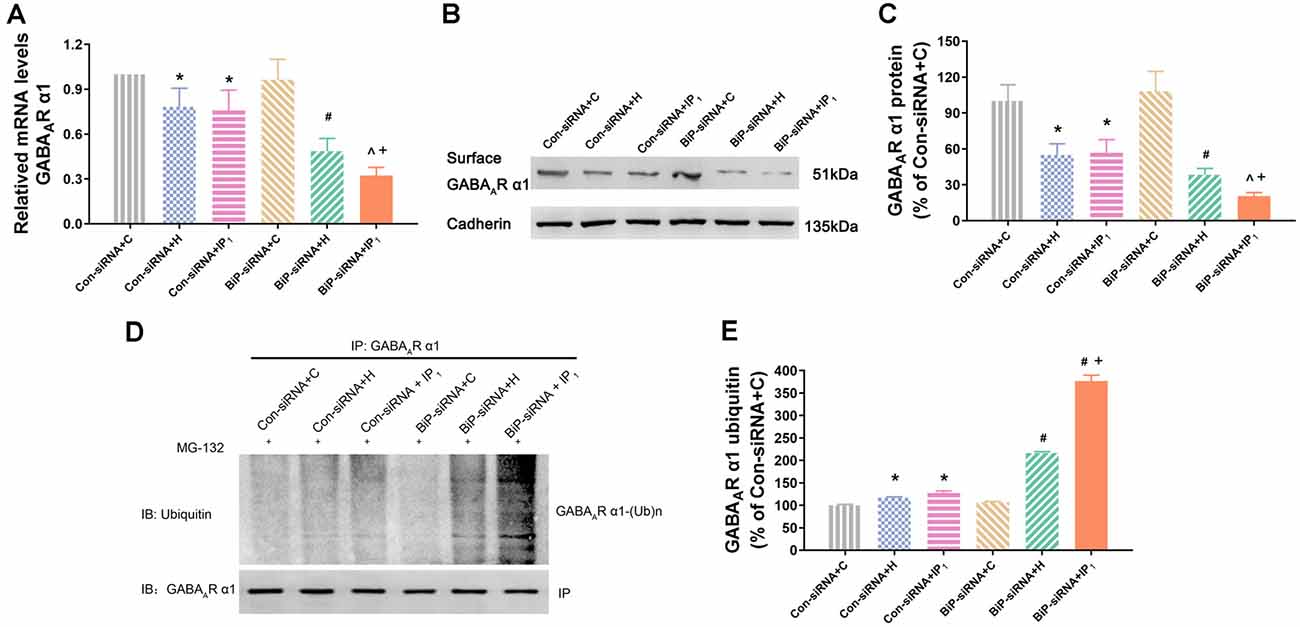
Figure 4. Knockdown of BiP by siRNA aggravated the disturbance of GABAAR α1 subunit protein homeostasis caused by anesthetics. (A) mRNA level analysis of GABAAR α1 subunit in neurons. Note that 1% isoflurane and 1.2 μg/ml of propofol resulted in a significant decrease of GABAAR α1 subunit transcription in neurons after treatment with BiP siRNA. (B,C) The expression of GABAAR α1 subunit in hippocampal neurons was determined by Western blotting. Note that 1% isoflurane and 1.2 μg/ml of propofol resulted in a significant decrease of GABAAR α1 subunit expression in neurons after treatment with BiP siRNA. (D,E) Ubiquitin GABAAR α1 of expression level increased in hippocampal neurons was determined by immunoprecipitation and Western blotting. Note that 1% isoflurane and 1.2 μg/ml of propofol resulted in a significant increase of GABAAR α1 subunit degradation in neurons after treatment with BiP siRNA. Data are expressed as the mean ± SD (n = 6/group). *P < 0.05 compared with group Con-siRNA + C; #P < 0.05 compared with group Con-siRNA + H; ∧P < 0.05 compared with group Con-siRNA + IP1; +P < 0.05 compared with group BiP-siRNA + H.
Similarly, western blot analysis showed that no significant change of the expression of GABAAR α1 protein was found between Con-siRNA + H and Con-siRNA + IP1 groups (Con-siRNA + H vs. Con-siRNA + IP1: 54.93 ± 9.37 vs. 56.82 ± 10.91, P > 0.05; Figures 4B,C). However, compared with that in the BiP-siRNA + H group, the expression of GABAAR α1 protein reduced significantly in the BiP-siRNA + IP1 group (BiP-siRNA + H vs. BiP-siRNA + IP1: 38.31 ± 5.29 vs. 20.55 ± 2.93, P < 0.05; Figures 4B,C).
To determine whether combination of 1% isoflurane and 1.2 μg/ml of propofol influenced the proteasome degradation of the GABAAR α1 subunit, hippocampal neurons were immunoprecipitated using anti-α1 antibody and blotted for ubiquitin. We found no significant change of the intensity of ubiquitinated GABAAR α1 subunit between the Con-siRNA + H and Con-siRNA + IP1 groups (Con-siRNA + H vs. Con-siRNA + IP1: 116.71 ± 1.74 vs. 127.12 ± 4.77, P > 0.05; Figures 4D,E). However, compared with that in the BiP-siRNA + H groups, the intensity of ubiquitinated GABAAR α1 subunit increased distinctly in the BiP-siRNA + IP1 group (BiP-siRNA + H vs. BiP-siRNA + IP1: 216.36 ± 3.08 vs. 376.62 ± 13.13, P < 0.05; Figures 4D,E). The results revealed that 1% isoflurane and 1.2 μg/ml of propofol attenuated proteasome degradation of the GABAAR α1 subunit by increasing endogenous BiP.
Discussion
Our data demonstrate that 1% isoflurane and 1.2 μg/ml of propofol have a neuroprotective effect, which is related to the up-regulation of an ER resident chaperone, BiP. BiP expression is thought to be a key cellular component of this effect, since the protective effect of 1% isoflurane and 1.2 μg/ml of propofol was abolished by knocking down endogenous BiP through siRNA.
Aging is a key factor that contributes to cerebral hypoperfusion (Toth et al., 2017), which is emerging as a major contributor to cognitive decline and degenerative processes leading to dementia (Alsop et al., 2010; Chao et al., 2010). Animal study has proved that chronic cerebral hypoperfusion caused by severe bilateral carotid stenosis led to mild cognitive impairment and slightly structural changes in the brains of aged rats (Wang et al., 2020). It has been generally accepted that the metabolic demand by local neuronal-glial activity for oxygen and glucose is tightly coupled to cerebral blood flow delivery (Roy and Sherrington, 1890). Thus, in this study, we cultured rat hippocampal neurons under hypoxia condition to imitate the microenvironment change of aging brains. After hypoxia, cell viability decreased and LDH leakage increased significantly, which could confirm that hypoxia induced neuron damage.
Previous studies showed that 1.9% isoflurane, equivalent to 1.3 MAC, was sufficient to induce general anesthesia in rats (Boruta et al., 2012), while a minimal infusion rate at 40 mg·kg−1·h−1 was required using propofol alone to induce general anesthesia in rats (Logginidou et al., 2003). The infusion rate of 20 mg·kg−1·h−1 led to an estimated mean propofol plasma concentration of 1.2 μg/ml. Therefore, in our study, doses were carefully selected combining isoflurane and propofol (1% and 1.2 μg/ml, 1.4% and 0.6 μg/ml, or 0.5% and 1.8 μg/ml) to imitate general anesthesia in vivo.
A large number of previous studies have demonstrated the dose-dependent effects of propofol and isoflurane. In vitro study showed that a high dose of isoflurane (treatment at a dose of 2% for 6 h) induced apoptosis by causing ER stress but a lower dose isoflurane (treatment at a dose of 1% for 1, 3, and 6 h) did not (Wang et al., 2014). In vivo study suggested that isoflurane (treatment at a dose of 1.3% for 4 h) caused cognitive impairment in aged rats. Inhibition of ER stress overactivation contributed to the relief of isoflurane-induced histopathologic changes (Ge et al., 2015). Moreover, Coghlan et al. (2018) confirmed that the effect of isoflurane was dose dependent, showing no statistical difference from control in aggregated, mislocalized protein at 0.5 MAC, an intermediate response at 0.75 MAC, and the most significant response at 1.0 MAC. Our previous study showed that propofol at doses of 10 or 20 mg·kg−1·h−1 infused at the onset of reperfusion for 30 min could provide neuroprotection to transient middle cerebral artery occlusion rats but 30 mg·kg−1·h−1 could not (Wang et al., 2009). Thal et al. (2014) showed that infusion of propofol (36 or 72 mg·kg−1·h−1) resulted in aggravation of neurologic dysfunction, increased 28-day mortality rate, and impaired posttraumatic neurogenesis. In vitro study demonstrated that the neuroprotective effect of propofol increased in a dose-dependent manner within 10 μM and decreased in a dose-dependent manner beyond 10 μM. The increase of endogenous BiP was the key to propofol’s neuroprotection (Wang L. et al., 2014). All this evidences could prove that a single high dose of propofol or isoflurane may cause neuron damage and cognitive impairment, which aroused our interest in studying low-dose combination applications. Our previous in vivo study revealed that combination of sub-anesthetic dose isoflurane and propofol (1% isoflurane plus 20 mg·kg−1·h−1 propofol) did not cause cognitive impairment of aged rats with cerebral hypoperfusion as compared with single-use of propofol (20 mg·kg−1·h−1) or isoflurane (1.9%; Bu et al., 2020). In the present study, we compared the effects of three different dosages of isoflurane and propofol (1% and 1.2 μg/ml, 1.4% and 0.6 μg/ml, or 0.5% and 1.8 μg/ml) on primary hypoxic hippocampal neurons with the aim of finding the way to minimize the damage caused by anesthetic to vulnerable neurons. In fact, no matter which combination is chosen, anesthetics are unlikely to reverse the damage to neurons caused by hypoxia. All we can do is find a way to administer drugs so that anoxic neurons are not further damaged by anesthetics. Compared with those in the hypoxia group (group H), the neuronal injury indexes of the IP1 (1% isoflurane and 1.2 μg/ml of propofol) treatment group after hypoxia were negative, while there were significant changes in the IP2 (1.4% isoflurane and 0.6 μg/ml of propofol) and IP3 (0.5% isoflurane and 1.8 μg/ml of propofol) group. The protective indicator BiP, which can alleviate cell damage by reducing ER stress, was most significantly increased in the IP1 group. Therefore, we believe that 1% isoflurane and 1.2 μg/ml of propofol are a better anesthetic choice for neurons already injured by hypoxia.
ER stress is the initial response of cells under stress (Zhao et al., 2016). BiP, the key molecular chaperone in the ER, can help to maintain calcium homeostasis (Ouyang et al., 2011); overexpression or induction of BiP possesses anti-apoptosis potential (Xiao-Hong et al., 2006; Li et al., 2008). The CHOP-mediated pathway is involved in ER stress induced neuronal apoptosis (Oida et al., 2008). In our study, an up-regulated level of BiP suggested the involvement of it in the neuroprotection of 1% isoflurane and 1.2 μg/ml of propofol. After knockdown of BiP-siRNA, expression of CHOP and caspase-12 increased and cell viability decreased distinctly when hypoxic neurons were treated with 1% isoflurane and 1.2 μg/ml of propofol. It is suggested that endogenous BiP plays an important role in the neuroprotection of 1% isoflurane and 1.2 μg/ml of propofol.
Neuronal failure of the proteostasis network may cause protein aggregation that leads to neurodegeneration (Ogen-Shtern et al., 2016; Hetz and Saxena, 2017). The α1 subunit of GABAAR, the most prevalent receptor subtype in the brain, is related to cognition (Möhler, 2006; Berry et al., 2008). Neurons expressing α1 GABAAR have been found to mediate sedation (Möhler, 2006). In this study, proteostasis of GABAAR α1 subunit was used to evaluate the role in the neuroprotection of 1% isoflurane and 1.2 μg/ml of propofol. Results showed that 1% isoflurane and 1.2 μg/ml of propofol enhanced GABAAR α1 subunit proteostasis by raising endogenous BiP.
The clinical application value of this study is that it provides a safer anesthetic regimen for elderly patients and patients with reduced cognitive function and neural reserves due to underlying diseases such as chronic cerebral insufficiency, sleep apnea syndrome, and cardiovascular disease. With the improvement of people’s living standards and the progress of medical technology, human society is gradually aging. In clinical practice, anesthesiologists will encounter more patients with fragile brain functions, whose central nervous system is less resistant to damage than normal people. Therefore, we must avoid the cognitive impairment caused by surgery and anesthesia and improve the postoperative quality of life of patients through more careful clinical operation and drug selection.
Taken together, our study confirmed that a combination of 1% isoflurane and 1.2 μg/ml of propofol causes the least damage to hypoxic hippocampal neurons of rats than do other dosages of both two drugs and that endogenous BiP plays an important role in this process.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The animal study was reviewed and approved by Institutional Animal Care and Use Committee of Tianjin Medical University.
Author Contributions
HW helped with conception and design; acquisition, analysis, and interpretation of the data; and critical revision of the article and gave the final approval. ZY helped with critical revision of the article and gave the final approval. XB and TL helped with conception and design; acquisition, analysis, and interpretation of the data; and drafting and critical revision of the article and gave the final approval. DG helped with analysis and interpretation of data acquisition, analysis, and interpretation of the data and gave the final approval. CY and XW helped with conception and design and gave the final approval. JW helped with conception and design; analysis and interpretation of the data; and critical revision of the article and gave the final approval. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the National Natural Science Foundation of China (82071220), Major Support Program of Tianjin Municipal Science and Technology (18YFZCSY00530), and Natural Science Foundation of Tianjin (20JCYBJC31000).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
ATF4, Activating Transcription Factor 4; ATF6, Activating Transcription Factor 6; BiP, binding immunoglobulin protein; CHOP, C/EBP homologous protein; ER, endoplasmic reticulum; GABA, γ-aminobutyric acid; GABAAR, γ-aminobutyric acid A type receptor; UPR, unfolded protein response; XBP1, X-box binding protein 1.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2020.591938/full#supplementary-material.
References
Alsop, D. C., Dai, W., Grossman, M., and Detre, J. A. (2010). Arterial spin labeling blood flow MRI: its role in the early characterization of Alzheimer’s disease. J. Alzheimers Dis. 20, 871–880. doi: 10.3233/JAD-2010-091699
Benzonana, L. L., Perry, N. J., Watts, H. R., Yang, B., Perry, I. A., Coombes, C., et al. (2013). Isoflurane, a commonly used volatile anesthetic, enhances renal cancer growth and malignant potential via the hypoxia-inducible factor cellular signaling pathway in vitro. Anesthesiology 119, 593–605. doi: 10.1097/ALN.0b013e31829e47fd
Berry, R. B., Werner, D. F., Wang, X., Jablonski, M. M., Homanics, G. E., Mittleman, G., et al. (2008). Mice with targeted genetic reduction of GABAA receptor α1 subunits display performance differences in Morris water maze tasks. Neurobiol. Learn. Mem. 90, 580–583. doi: 10.1016/j.nlm.2008.06.004
Biwer, L. A., and Isakson, B. E. (2017). Endoplasmic reticulum-mediated signalling in cellular microdomains. Acta Physiol. 219, 162–175. doi: 10.1111/apha.12675
Boruta, D. T., Sotgiu, G., and Golder, F. J. (2012). Effects of intraperitoneal administration of gabapentin on the minimum alveolar concentration of isoflurane in adult male rats. Lab. Anim. 46, 108–113. doi: 10.1258/la.2011.011127
Bu, X., Li, T., Wang, H., Xia, Z., Guo, D., Wang, J., et al. (2020). Combination of isoflurane and propofol as general anesthesia during orthopedic surgery of perioperative cerebral hypoperfusion rats to avoid cognitive impairment. Front. Med. 7:549081. doi: 10.3389/fmed.2020.549081
Cai, X. H., Li, X. C., Jin, S. W., Liang, D. S., Wen, Z. W., Cao, H. C., et al. (2014). Endoplasmic reticulum stress plays critical role in brain damage after chronic intermittent hypoxia in growing rats. Exp. Neurol. 257, 148–156.
Chao, L. L., Buckley, S. T., Kornak, J., Schuff, N., Madison, C., Yaffe, K., et al. (2010). ASL perfusion MRI predicts cognitive decline and conversion from MCI to dementia. Alzheimer Dis. Assoc. Disord. 24, 19–27. doi: 10.1097/WAD.0b013e3181b4f736
Coghlan, M., Richards, E., Shaik, S., Rossi, P., Vanama, R. B., Ahmadi, S., et al. (2018). Inhalational anesthetics induce neuronal protein aggregation and affect ER trafficking. Sci. Rep. 8:5275. doi: 10.1038/s41598-018-23335-0
Daulatzai, M. A. (2017). Cerebral hypoperfusion and glucose hypometabolism: key pathophysiological modulators promote neurodegeneration, cognitive impairment, and Alzheimer’s disease. J. Neurosci. Res. 95, 943–972. doi: 10.1002/jnr.23777
Dickhout, J. G., Hossain, G. S., Pozza, L. M., Zhou, J., Lhotak, S., and Austin, R. C. (2005). Peroxynitrite causes endoplasmic reticulum stress and apoptosis in human vascular endothelium: implications in atherogenesis. Arterioscler. Thromb. Vasc. Biol. 25, 2623–2629. doi: 10.1161/01.ATV.0000189159.96900.d9
Dodds, C., Kumar, C. M., and Servin, F. D. R. (2017). “Anaesthesia for the elderly patient (Oxford anaesthesia library),” in Cognitive Dysfunction and Sleep Disorders, (Oxford, United Kingdom: Oxford University Press), 137–148.
Evered, L., Silbert, B., Knopman, D. S., Scott, D. A., Dekosky, S. T., Rasmussen, L. S., et al. (2018). Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Br. J. Anaesth. 121, 1005–1012. doi: 10.1016/j.bja.2017.11.087
Feaver, R. E., Hastings, N. E., Pryor, A., and Blackman, B. R. (2008). GRP78 upregulation by atheroprone shear stress via p38-, α2β1-dependent mechanism in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 28, 1534–1541. doi: 10.1161/ATVBAHA.108.167999
Fu, H. Y., Minamino, T., Tsukamoto, O., Sawada, T., Asai, M., Kato, H., et al. (2008). Overexpression of endoplasmic reticulum-resident chaperone attenuates cardiomyocyte death induced by proteasome inhibition. Cardiovasc. Res. 79, 600–610. doi: 10.1093/cvr/cvn128
Ge, H. W., Hu, W. W., Ma, L. L., and Kong, F. J. (2015). Endoplasmic reticulum stress pathway mediates isoflurane-induced neuroapoptosis and cognitive impairments in aged rats. Physiol. Behav. 151, 16–23. doi: 10.1016/j.physbeh.2015.07.008
Hetz, C., and Saxena, S. (2017). ER stress and the unfolded protein response in neurodegeneration. Nat. Rev. Neurol. 13, 477–491. doi: 10.1038/nrneurol.2017.99
Hofmeijer, J., Mulder, A. T., Farinha, A. C., Van Putten, M. J., and Le Feber, J. (2014). Mild hypoxia affects synaptic connectivity in cultured neuronal networks. Brain Res. 1557, 180–189. doi: 10.1016/j.brainres.2014.02.027
Hovens, I. B., Schoemaker, R. G., Van Der Zee, E. A., Heineman, E., Izaks, G. J., and Van Leeuwen, B. L. (2012). Thinking through postoperative cognitive dysfunction: how to bridge the gap between clinical and pre-clinical perspectives. Brain Behav. Immun. 26, 1169–1179. doi: 10.1016/j.bbi.2012.06.004
Jacob, T. C., Moss, S. J., and Jurd, R. (2008). GABAA receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat. Rev. Neurosci. 9, 331–343. doi: 10.1038/nrn2370
Kaech, S., and Banker, G. (2006). Culturing hippocampal neurons. Nat. Protoc. 1, 2406–2415. doi: 10.1038/nprot.2006.356
Kelley, M. H., Taguchi, N., Ardeshiri, A., Kuroiwa, M., Hurn, P. D., Traystman, R. J., et al. (2008). Ischemic insult to cerebellar Purkinje cells causes diminished GABAA receptor function and Allopregnanolone neuroprotection is associated with GABAA receptor stabilization. J. Neurochem. 107, 668–678. doi: 10.1111/j.1471-4159.2008.05617.x
Kim, I., Xu, W., and Reed, J. C. (2008). Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat. Rev. Drug Discov. 7, 1013–1030. doi: 10.1038/nrd2755
Li, J., Ni, M., Lee, B., Barron, E., Hinton, D. R., and Lee, A. S. (2008). The unfolded protein response regulator GRP78/BiP is required for endoplasmic reticulum integrity and stress-induced autophagy in mammalian cells. Cell Death & Differentiation 15, 1460–1471. doi: 10.1111/j.1471-4159.2008.05617.x
Li, J., Yu, W., Li, X. T., Qi, S. H., and Li, B. (2014). The effects of propofol on mitochondrial dysfunction following focal cerebral ischemia-reperfusion in rats. Neuropharmacology 77, 358–368. doi: 10.1016/j.neuropharm.2013.08.029
Liu, Q., and Wong-Riley, M. T. (2004). Developmental changes in the expression of GABAA receptor subunits α1, α2 and α3 in the rat pre-Botzinger complex. J. Appl. Physiol. 96, 1825–1831. doi: 10.1152/japplphysiol.01264.2003
Logginidou, H. G., Li, B. H., Li, D. P., Lohmann, J. S., Schuler, H. G., Divittore, N. A., et al. (2003). Propofol suppresses the cortical somatosensory evoked potential in rats. Anesth. Analg. 97, 1784–1788. doi: 10.1213/01.ane.0000090318.16879.a8
Martindale, J. J., Fernandez, R., Thuerauf, D., Whittaker, R., Gude, N., Sussman, M. A., et al. (2006). Endoplasmic reticulum stress gene induction and protection from ischemia/reperfusion injury in the hearts of transgenic mice with a tamoxifen-regulated form of ATF6. Circ. Res. 98, 1186–1193. doi: 10.1161/01.RES.0000220643.65941.8d
Minamino, T., Komuro, I., and Kitakaze, M. (2010). Endoplasmic reticulum stress as a therapeutic target in cardiovascular disease. Circ. Res. 107, 1071–1082. doi: 10.1161/CIRCRESAHA.110.227819
Möhler, H. (2006). GABAA receptor diversity and pharmacology. Cell Tissue Res. 326, 505–516. doi: 10.1007/s00441-006-0284-3
Ogen-Shtern, N., Ben David, T., and Lederkremer, G. Z. (2016). Protein aggregation and ER stress. Brain Res. 1648, 658–666. doi: 10.1016/j.brainres.2016.03.044
Oida, Y., Shimazawa, M., Imaizumi, K., and Hara, H. (2008). Involvement of endoplasmic reticulum stress in the neuronal death induced by transient forebrain ischemia in gerbil. Neuroscience 151, 111–119. doi: 10.1016/j.neuroscience.2007.10.047
Ouyang, Y. B., Xu, L. J., Emery, J. F., Lee, A. S., and Giffard, R. G. (2011). Overexpressing GRP78 influences Ca2+ handling and function of mitochondria in astrocytes after ischemia-like stress. Mitochondrion 11, 279–286. doi: 10.1016/j.mito.2010.10.007
Perucho, J., Rubio, I., Casarejos, M. J., Gomez, A., Rodriguez-Navarro, J. A., Solano, R. M., et al. (2010). Anesthesia with isoflurane increases amyloid pathology in mice models of Alzheimer’s disease. J. Alzheimers Dis. 19, 1245–1257. doi: 10.3233/JAD-2010-1318
Roy, C. S., and Sherrington, C. S. (1890). On the regulation of the blood-supply of the brain. J. Physiol. 11, 85.17–158.17. doi: 10.1113/jphysiol.1890.sp000321
Schmittgen, T. D., and Livak, K. J. (2008). Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 3, 1101–1108. doi: 10.1038/nprot.2008.73
Shi, S. S., Yang, W. Z., Chen, Y., Chen, J. P., and Tu, X. K. (2014). Propofol reduces inflammatory reaction and ischemic brain damage in cerebral ischemia in rats. Neurochem. Res. 39, 793–799. doi: 10.1007/s11064-014-1272-8
Shoair, O. A., Grasso Ii, M. P., Lahaye, L. A., Daniel, R., Biddle, C. J., and Slattum, P. W. (2015). Incidence and risk factors for postoperative cognitive dysfunction in older adults undergoing major noncardiac surgery: a prospective study. J. Anaesthesiol. Clin. Pharmacol. 31, 30–36. doi: 10.4103/0970-9185.150530
Thal, S. C., Timaru-Kast, R., Wilde, F., Merk, P., Johnson, F., Frauenknecht, K., et al. (2014). Propofol impairs neurogenesis and neurologic recovery and increases mortality rate in adult rats after traumatic brain injury. Crit. Care Med. 42, 129–141. doi: 10.1097/CCM.0b013e3182a639fd
Toth, P., Tarantini, S., Csiszar, A., and Ungvari, Z. (2017). Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment and neurovascular uncoupling in aging. Am. J. Physiol. Heart Circ. Physiol. 312, H1–H20. doi: 10.1152/ajpheart.00581.2016
Wang, H., Dong, Y., Zhang, J., Xu, Z., Wang, G., Swain, C. A., et al. (2014). Isoflurane induces endoplasmic reticulum stress and caspase activation through ryanodine receptors. Br. J. Anaesth. 113, 695–707. doi: 10.1093/bja/aeu053
Wang, X., Eno, C. O., Altman, B. J., Zhu, Y., Zhao, G., Olberding, K. E., et al. (2011). ER stress modulates cellular metabolism. Biochem. J. 435, 285–296. doi: 10.1042/BJ20101864
Wang, L., Tang, W., Jiang, T., Lu, P., Li, Y., Sun, A., et al. (2014). Endoplasmic reticulum stress is involved in the neuroprotective effect of propofol. Neurochem. Res. 39, 1741–1752. doi: 10.1007/s11064-014-1369-0
Wang, H. Y., Wang, G. L., Yu, Y. H., and Wang, Y. (2009). The role of phosphoinositide-3-kinase/Akt pathway in propofol-induced postconditioning against focal cerebral ischemia-reperfusion injury in rats. Brain Res. 1297, 177–184. doi: 10.1016/j.brainres.2009.08.054
Wang, J., Yang, C., Wang, H., Li, D., Li, T., Sun, Y., et al. (2020). A new rat model of chronic cerebral hypoperfusion resulting in early-stage vascular cognitive impairment. Front. Aging Neurosci. 12:86. doi: 10.3389/fnagi.2020.00086
Wei, H., Liang, G., Yang, H., Wang, Q., Hawkins, B., Madesh, M., et al. (2008). The common inhalational anesthetic isoflurane induces apoptosis via activation of inositol 1,4,5-trisphosphate receptors. Anesthesiology 108, 251–260. doi: 10.1097/01.anes.0000299435.59242.0e
Williams, D. B., and Akabas, M. H. (2002). Structural evidence that propofol stabilizes different GABAA receptor states at potentiating and activating concentrations. J. Neurosci. 22, 7417–7424. doi: 10.1523/JNEUROSCI.22-17-07417.2002
Wong, S. K., and Lazinski, D. W. (2002). Replicating hepatitis delta virus RNA is edited in the nucleus by the small form of ADAR1. Proc. Natl. Acad. Sci. U S A 99, 15118–15123. doi: 10.1073/pnas.232416799
Xiang, C., Wang, Y., Zhang, H., and Han, F. (2017). The role of endoplasmic reticulum stress in neurodegenerative disease. Apoptosis 22, 1–26.
Xiao-Hong, Y., Li, L., Yan-Xia, P., Hong, L., Wei-Fang, R., Yan, L., et al. (2006). Salusins protect neonatal rat cardiomyocytes from serum deprivation-induced cell death through upregulation of GRP78. J. Cardiovasc. Pharmacol. 48, 41–46. doi: 10.1097/01.fjc.0000242059.89430.ac
Zhao, Y., Han, Y., Bu, D. F., Zhang, J., Li, Q. R., Jin, H. F., et al. (2016). Reduced AKT phosphorylation contributes to endoplasmic reticulum stress-mediated hippocampal neuronal apoptosis in rat recurrent febrile seizure. Life Sci. 153, 153–162. doi: 10.1016/j.lfs.2016.04.008
Zhong, H., Song, R., Pang, Q., Liu, Y., Zhuang, J., Chen, Y., et al. (2018). Propofol inhibits parthanatos via ROS-ER-calcium-mitochondria signal pathway in vivo and vitro. Cell Death Dis. 9:932. doi: 10.1038/s41419-018-0996-9
Keywords: binding immunoglobulin protein, endoplasmic reticulum stress, GABAAR α1 subunit, isoflurane, propofol
Citation: Bu X, Li T, Guo D, Yang C, Wang J, Wang X, Yang Z and Wang H (2020) 1% Isoflurane and 1.2 μg/ml of Propofol: A Combination of Anesthetics That Causes the Least Damage to Hypoxic Neurons. Front. Aging Neurosci. 12:591938. doi: 10.3389/fnagi.2020.591938
Received: 05 August 2020; Accepted: 19 October 2020;
Published: 16 November 2020.
Edited by:
Yiying Zhang, Harvard Medical School, United StatesReviewed by:
Hailong Dong, Fourth Military Medical University, ChinaLize Xiong, Xijing Hospital, The Fourth Military Medical University, China
Copyright © 2020 Bu, Li, Guo, Yang, Wang, Wang, Yang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haiyun Wang, d2h5ODE5QDEyNi5jb20=; Zhuo Yang, emh1b3lhbmdAbmFua2FpLmVkdS5jbg==
† These authors have contributed equally to this work
 Xinyue Bu
Xinyue Bu Tang Li1,2†
Tang Li1,2† Zhuo Yang
Zhuo Yang Haiyun Wang
Haiyun Wang