- Department of Acupuncture, Longhua Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, China
Background: Alzheimer's disease (AD) is a neurodegenerative disease characterized by progressive memory deficits, cognitive decline, and spatial disorientation. Non-pharmacological interventions to treat AD have been reported in many meta-analyses (MAs), but robust conclusions have not been made because of variations in the scope, quality, and findings of these reviews.
Objective: This work aimed to review existing MAs to provide an overview of existing evidence on the effects of five non-pharmacological interventions in AD patients on three outcomes: Mini-Mental State Examination (MMSE), activities of daily living (ADL), and Alzheimer's Disease Assessment Scale-cognitive section (ADAS-cog).
Methods: The databases PubMed, Cochrane Library, Embase, and Web of Science were searched to collect MAs of non-pharmacological interventions for AD. Two reviewers independently conducted literature screening, data extraction, and quality assessment. We assessed the quality of MAs with the Measurement Tool to Assess Systematic Reviews (AMSTAR) 2 and assessed the evidence quality for significant outcomes using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system.
Results: We found 10 eligible MAs, which included between three (133 patients) and 15 randomized trials (1,217 patients), and five non-pharmacological interventions, namely, acupuncture therapy (40%), exercise intervention (30%), music therapy (10%), cognitive intervention (10%), and repetitive transcranial magnetic stimulation (rTMS) (10%). All the included MAs were critically low to low quality by AMSTAR 2. Acupuncture therapy and exercise intervention showed the preliminary potential to improve ADL and MMSE. rTMS and acupuncture therapy show benefits in decreasing ADAS-cog, and there were some evidence of improved MMSE with cognitive intervention. All these outcomes scored very low quality to moderate quality of evidence on the GRADE system.
Conclusions: Non-pharmacological therapy shows promise for the treatment of AD, but there is still a lack of high-quality evidence. In the future, the quality of the original research needs to be improved, and strictly designed MAs should be carried out following methodological requirements.
Introduction
Dementia is a common public health problem globally, affecting approximately 47 million people worldwide, and is expected to increase to 131.5 million by 2050 (Alzheimer's Disease International, 2015; Arvanitakis et al., 2019). Alzheimer's disease (AD) is a neurodegenerative disease characterized by progressive memory deficits, cognitive decline, and spatial disorientation. Among all types of dementia, AD accounts for 60–80%. An estimated 5.8 million Americans aged 65 and older are living with AD in 2020 (Alzheimer's Association, 2020). The incidence of AD in China was reported as 6.25 cases per 1,000 person-years, and the median standardized mortality ratio was 1.94:1 (Chan et al., 2013). With the progressive death of neurons and damage to brain tissue, AD becomes more severe over time. The progression of AD will lead to problems with memory and, eventually, physical disability (Alzheimer's Association, 2020). AD patients also often have other concomitant diseases that may exacerbate progression and symptoms (Haapasalo et al., 2015). AD is officially listed as the sixth leading cause of death (Xu et al., 2020). With the aging of populations, the high cost of AD imposes an increasingly heavy burden worldwide on patients, family members, healthcare systems, and society (Maresova et al., 2018; Pedroza et al., 2019).
Currently, the main pharmacotherapeutic options for AD are cholinesterase inhibitors (donepezil, rivastigmine, and galantamine) and memantine (O'Brien et al., 2017). However, none of these can slow down or stop the damage and destruction of neurons or the progression of the disease (Alzheimer's Association, 2020). Therefore, more potential and effective treatments need to be assessed. In recent years, increasing attention has been focused on complementary and alternative therapies for AD; among them, non-pharmacological therapies play an important complementary role (Olazaran et al., 2010; Zucchella et al., 2018).
Meta-analyses (MAs) have been conducted to summarize the efficacy of different types of non-pharmacologic therapies for AD (García-Casares et al., 2017; Cammisuli et al., 2018; Sá et al., 2019). However, strong conclusions have not been reached owing to the wide variation in scope, quality, and outcomes. Although study (Kishita et al., 2020) has shown that non-pharmacological interventions improve depression, anxiety, and quality of life in people with dementia, they did not identify the different types of dementia. Therefore, as an overview, we evaluated the quality of MAs of non-pharmacological therapies and summarized the evidence on their effects on the Mini-Mental State Examination (MMSE), activities of daily living (ADL), and Alzheimer's Disease Assessment Scale-cognitive section (ADAS-cog) outcomes in patients with AD.
Methods
Protocol and Registration
We undertook an overview of MAs in line with Preferred Reporting Items for Overview of systematic reviews (Bougioukas et al., 2018), together with the prospective protocol registered in INPLASY (registration no. INPLASY202070014). Ethics approval was not required.
Literature Search
A comprehensive search of four databases comprising PubMed, Cochrane Library, Embase, and Web of Science was undertaken up to 15 May 2020 for MAs evaluating non-pharmacological interventions currently used in AD patients. References from related articles were also searched. There were no language restrictions. The details of the search strategy are given in Supplementary Material 1.
Inclusion Criteria
The inclusion criteria are: (1) population: participants diagnosed as AD; (2) intervention: specific non-pharmacological intervention including, but not limited to, acupuncture therapy, music therapy, exercise interventions, etc., was performed alone or in combination with other treatments; (3) control: medication or placebo or another type of treatment corresponding to the intervention was applied as a comparison; (4) outcome: outcome measures were quantitatively reported as MMSE, ADL, or ADAS-cog; and (5) study: MA of randomized clinical trials (RCTs).
The participants were diagnosed as AD by common criteria such as the National Institute of Neurological and Communicative Disorders and Stroke Alzheimer's Disease and Related Disorders Association (McKhann et al., 1984; Dubois et al., 2007), or it was clearly stated that the subjects of the study were AD patients.
Exclusion Criteria
The exclusion criteria included: (1) AD patients combined with other diseases and mixed samples (AD participants and other types of dementia, mild cognitive impairment, or other related neurocognitive disorders), (2) immunotherapies (vaccine, monoclonal antibodies) and nutritional components (nutraceuticals) administered, (3) systematic reviews (SRs) without MAs of outcomes, (4) SRs with network MAs or Bayesian MAs, and (5) protocols, meeting abstracts, and MAs without full text.
Study Selection
All retrieved studies were imported into Endnote X9 software, and duplicate results were deleted. Based on the selection criteria, two independent researchers (Liaoyao Wang, Yijun Zhan) selected the relevant studies after screening titles and abstracts. Inter-assessor discrepancies would be resolved by discussion or arbitration of a third reviewer (Jian Pei).
The full text of all potential articles was obtained for a detailed evaluation according to the inclusion/exclusion criteria, and the final relevant studies were shortlisted. Any disagreement was resolved by a discussion with a third reviewer (Jian Pei) or by final group consensus.
Data Extraction
A standard form was used for data extraction from all MAs, containing the following: year of publication, first author, database, number of included trials and participants, type of interventions, outcomes, quality assessment tools, main findings, etc. Data were extracted by two independent reviewers (Yiwen Cai, Yijun Zhan). Any discrepancies were resolved by consensus after all the authors re-reviewed the study.
Assessment of Methodological Quality
We used A Measurement Tool to Assess Systematic Reviews 2 (AMSTAR 2) (Shea et al., 2017) to assess the methodological quality of the included MAs. AMSTAR 2 is a comprehensive critical appraisal tool for SRs of randomized and non-randomized studies that have simpler response categories than the original AMSTAR and, more importantly, is not intended to generate an overall score but instead focuses on weaknesses in critical domains (Shea et al., 2017). A psychometric study found that AMSTAR 2 is a valid and moderately reliable appraisal tool (Lorenz et al., 2019). The process of assessment was independently performed by two of the authors, and any discrepancies were resolved by final consensus among all the authors. AMSTAR 2 has 16 items in total, each of which is evaluated as “yes,” “partial yes,” or “no.” Seven items (2, 4, 7, 9, 11, 13, and 15) were considered as critical domains that critically affected the validity of a review and its conclusions. Overall confidence in the results of the review was rated as “high” (none or one non-critical weakness), “moderate” (more than one non-critical weakness but no critical flaws), “low” (one critical flaw ± non-critical weaknesses), and “critically low” (more than one critical flaw ± non-critical weaknesses) (Shea et al., 2017). The details of the 16 domains of AMSTAR 2 are shown in Supplementary Material 2.
Assessment of Evidence Quality
The Grading of Recommendation, Assessment, Development, and Evaluation (GRADE) (Guyatt et al., 2008) system was used to assess the evidence quality for each outcome on four degrees (high, moderate, low, and very low quality) by two authors independently. There are now more than 110 organizations from 19 countries that have endorsed or use GRADE to judge the quality of evidence bearing on clinical questions and to develop corresponding clinical practice guidelines (Gordon and Guyatt, 2020). We used the GRADE assessment of evidence quality, with the following criteria. For each significant outcome, we initially awarded high quality because these were based on RCTs. We then downgraded the confidence according to five aspects: risk of bias, inconsistency, indirectness, imprecision, and publication bias. A final consensus among all the authors resolved any discrepancies among two of the authors.
Results
Study Selection Process
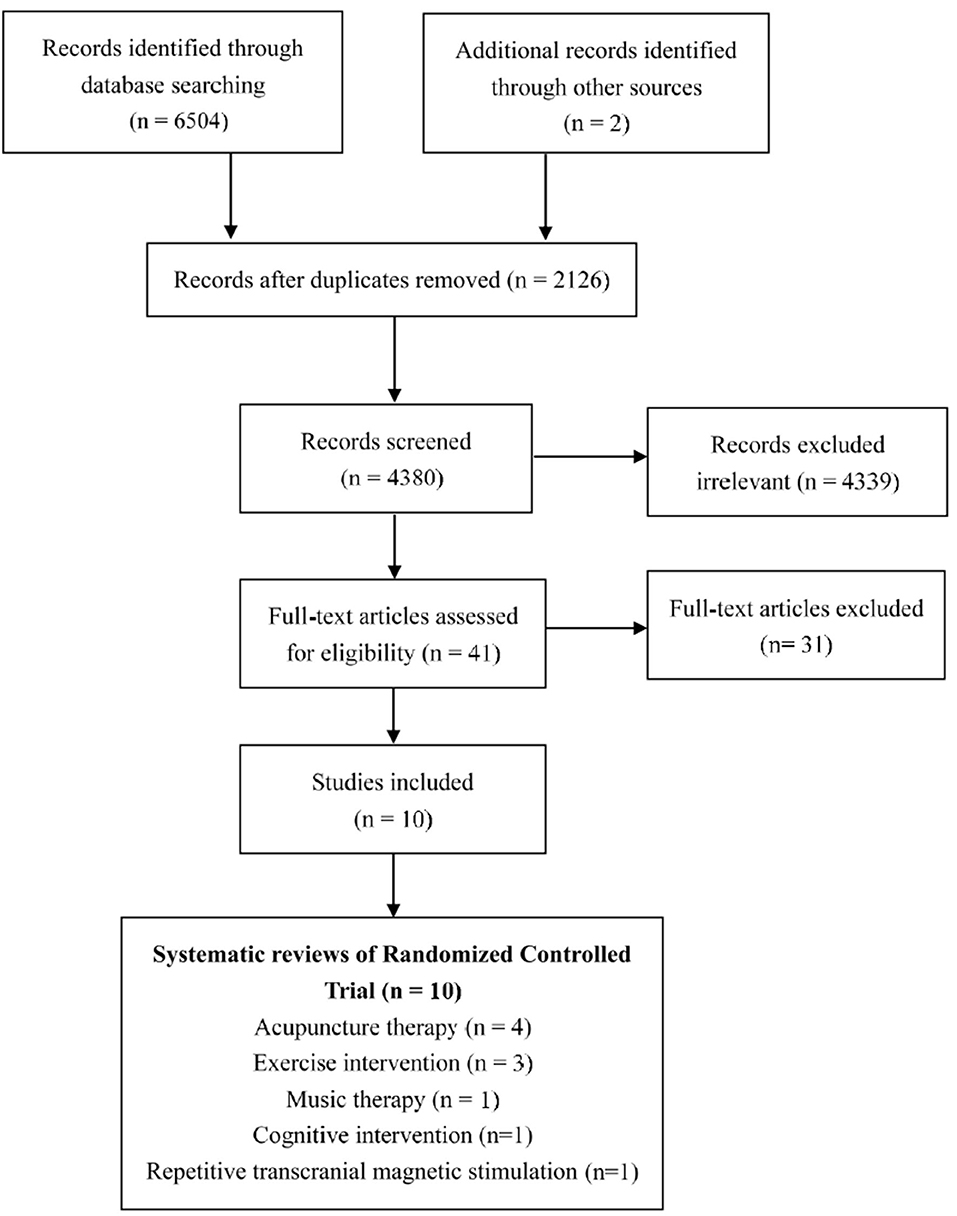
The searches identified 6,504 published studies, and after screening the titles and removing duplicates, 41 potentially eligible studies were selected for a closer scrutiny by retrieving the full text. Finally, we included 10 MAs (Lee et al., 2009; Alves et al., 2013; Rao et al., 2014; Zhou et al., 2015, 2017; Dong et al., 2018; Du et al., 2018; Huang et al., 2019; Jia et al., 2019; Wang et al., 2020). The study selection process is shown in Figure 1.
Characteristics of the Included Studies
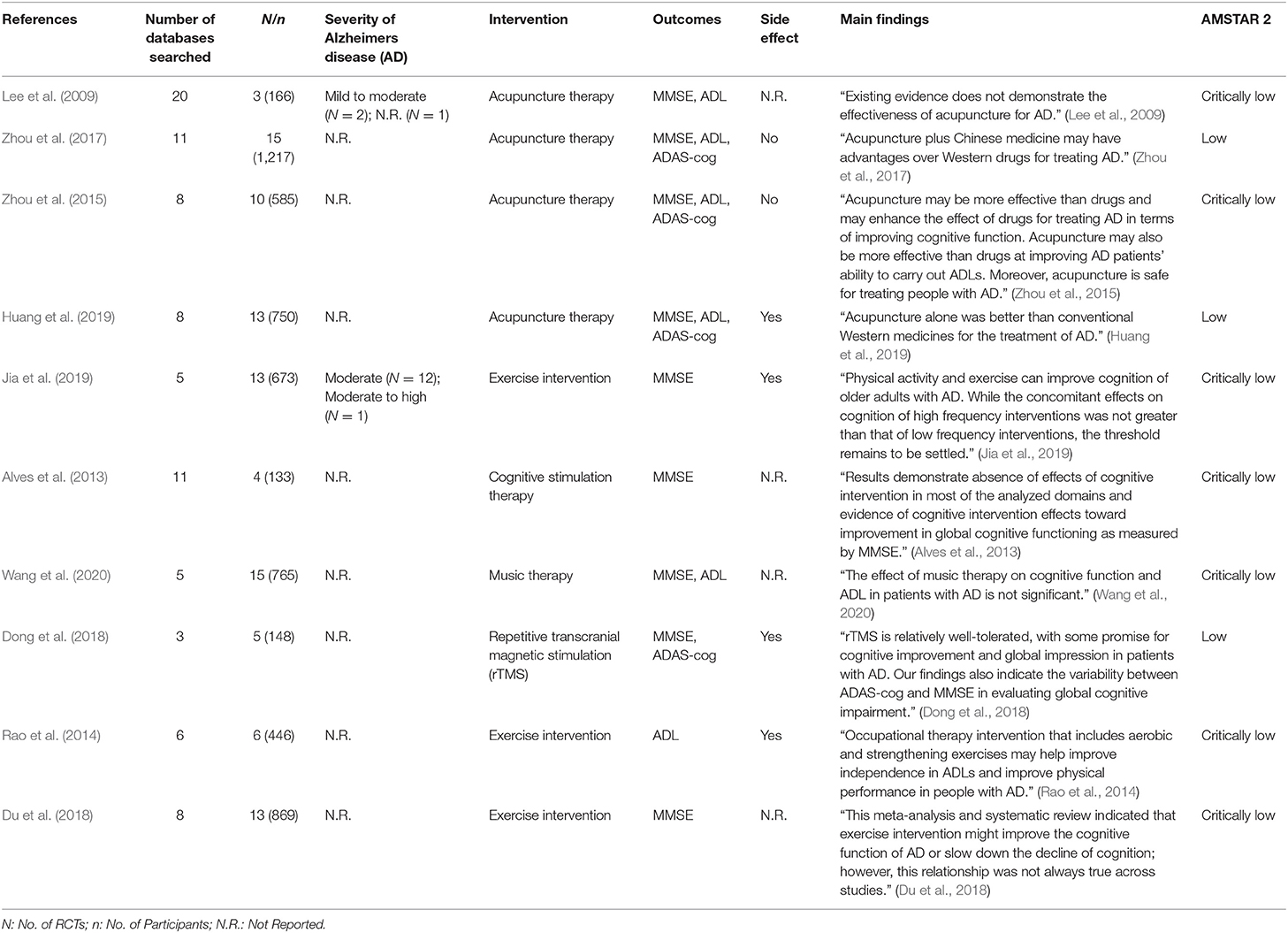
The 10 selected MAs reported 3–15 RCTs with 133–1,217 AD patients. Four MAs (Lee et al., 2009; Zhou et al., 2015, 2017; Huang et al., 2019) assessed the effects of acupuncture therapy, three (Rao et al., 2014; Du et al., 2018; Jia et al., 2019) assessed exercise (aerobic exercise/physical activity/cycle ergometer exercise/brisk walking, etc.), one (Alves et al., 2013) assessed cognitive stimulation therapy (memory-training program/attention-stimulating activities/cognitive training), one (Wang et al., 2020) assessed music therapy, and one (Dong et al., 2018) evaluated repetitive transcranial magnetic stimulation (rTMS). The details of the included MAs are shown in Table 1, and the specific type, content, intensity, and duration of each of the five interventions evaluated in these MAs are shown in Supplementary Material 3.
Methodological Quality of the Included MAs
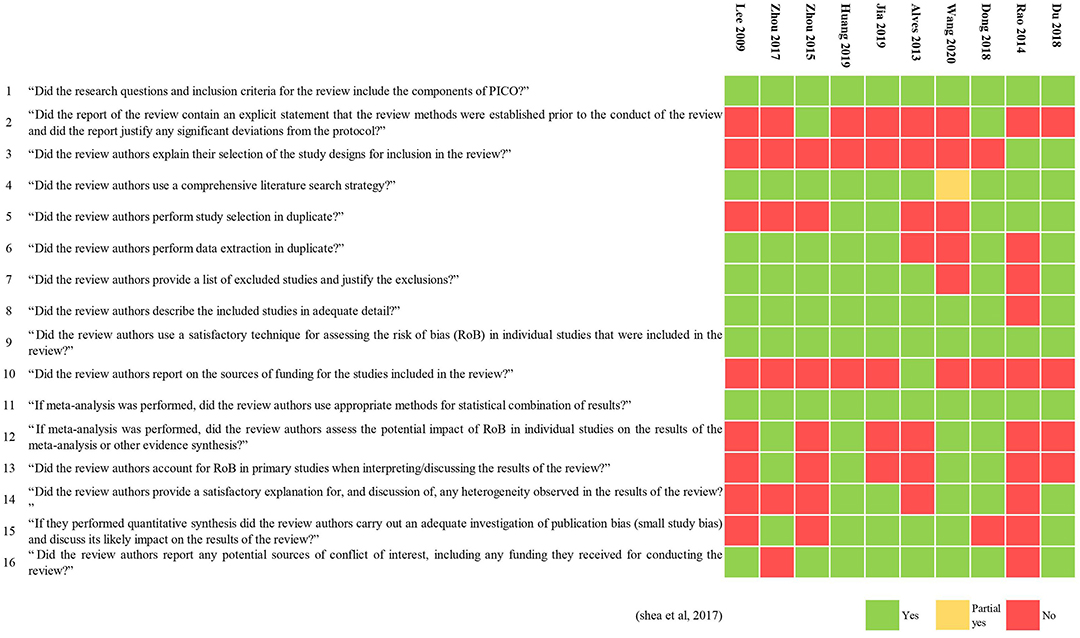
The methodological quality of three MAs (Zhou et al., 2017; Dong et al., 2018; Huang et al., 2019) was low, and that of all the others was critically low. All included MAs specified their inclusion and exclusion criteria, including PICO (population, interventions, comparators, and outcomes), undertook a comprehensive literature search, used a useful technique for assessing the risk of bias, and used appropriate methods for the statistical combination of results. Only two reviews (Zhou et al., 2015; Dong et al., 2018) had registered and had a protocol before performing the review. Six MAs (Alves et al., 2013; Zhou et al., 2017; Du et al., 2018; Huang et al., 2019; Jia et al., 2019; Wang et al., 2020) discussed publication bias. The results of the evaluation by AMSTAR 2 are shown in Table 1 and Figure 2.
Evidence Quality of Outcomes
This overview focused on three outcomes: MMSE, ADL, and ADAS-cog. The evidence synthesis for each outcome by the GRADE system is summarized below and in Table 2. There were 10 (41.67%) moderate-quality evidences, 11 (45.83%) low-quality evidences, and three (12.50%) very low-quality evidence.
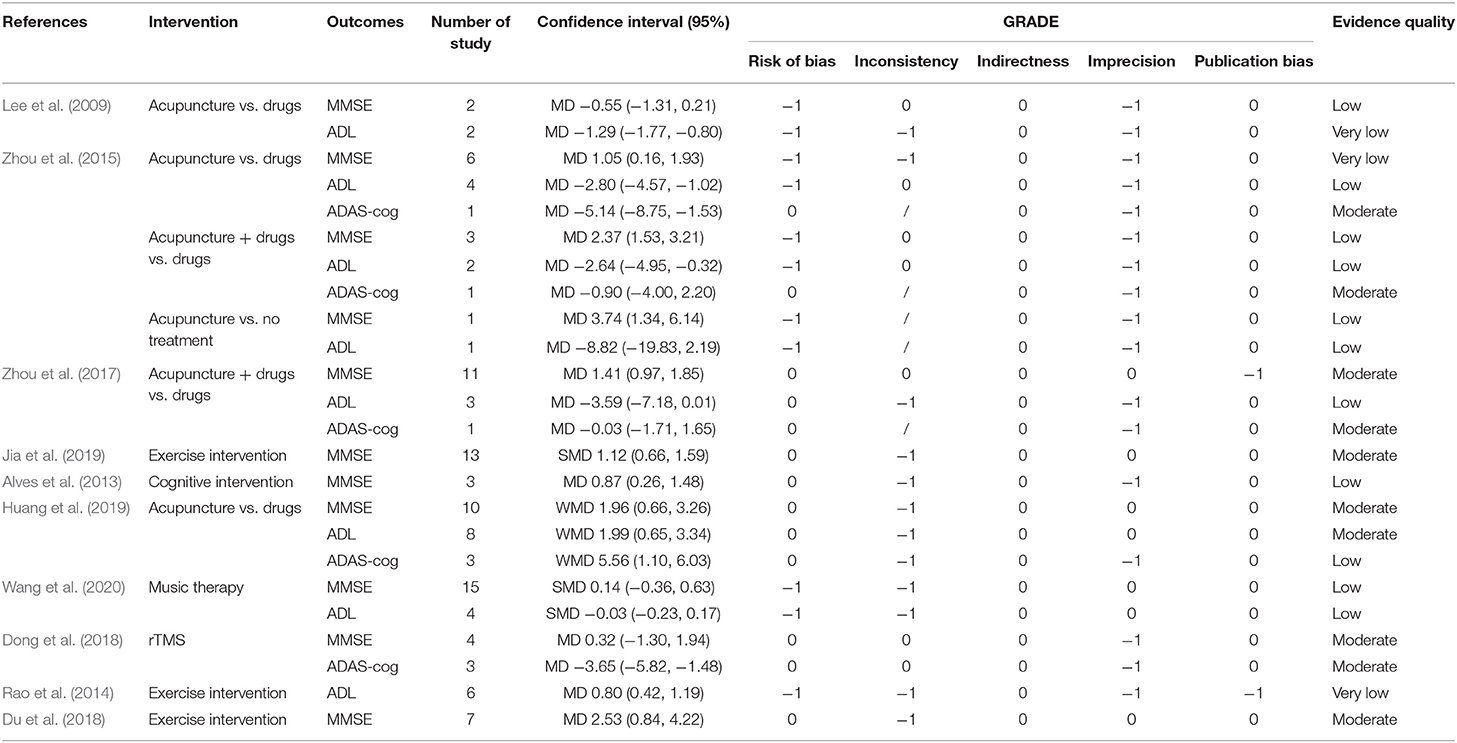
Table 2. Quality of evidence by Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system.
MMSE
Nine MAs (Lee et al., 2009; Alves et al., 2013; Zhou et al., 2015, 2017; Dong et al., 2018; Du et al., 2018; Huang et al., 2019; Jia et al., 2019; Wang et al., 2020) reported the MMSE. Three of the interventions (acupuncture therapy, exercise, and cognitive intervention) improved MMSE significantly. Acupuncture vs. drugs (MD = 1.05, 95% CI: 0.16, 1.93; very low quality; Zhou et al., 2015; WMD = 1.96, 95% CI: 0.66, 3.26; moderate-quality; Huang et al., 2019) and acupuncture vs. no treatment (MD = 3.74, 95% CI: 1.34, 6.14; low quality; Zhou et al., 2015) improved the MMSE. A combination of acupuncture and drugs vs. drugs alone (MD = 2.37, 95% CI: 1.53, 3.21; low quality; Zhou et al., 2015; MD = 1.41, 95% CI: 0.97, 1.85; moderate-quality; Zhou et al., 2017) improved the MMSE. Exercise intervention (SMD = 1.12, 95% CI: 0.66, 1.59; moderate-quality; Jia et al., 2019; MD = 2.53, 95% CI: 0.84, 4.22; moderate-quality; Du et al., 2018) and cognitive intervention (MD = 0.87, 95% CI: 0.26, 1.48; low quality) (Alves et al., 2013) significantly improved the MMSE.
ADL
Six MAs (Lee et al., 2009; Rao et al., 2014; Zhou et al., 2015, 2017; Huang et al., 2019; Wang et al., 2020) reported on ADL. Acupuncture and exercise intervention demonstrated a significant effect on ADL. Acupuncture vs. drugs (MD = −1.29, 95% CI: −1.77, −0.80; very low quality; Lee et al., 2009; MD=-2.80, 95% CI: −4.57, −1.02; low quality; Zhou et al., 2015; WMD = 1.99, 95% CI: 0.65, 3.34; moderate-quality) (Huang et al., 2019) improved the ADL. A combination of acupuncture and drugs vs. drugs alone (MD=-2.64, 95% CI: −4.95, −0.32; low quality) (Zhou et al., 2015) improved ADL. Exercise intervention (MD = 0.80, 95% CI: 0.42, 1.19; very low quality) (Rao et al., 2014) improved ADL.
ADAs-Cog
Only four MAs (Zhou et al., 2015, 2017; Dong et al., 2018; Huang et al., 2019) reported the effects of two interventions (acupuncture and rTMS) on ADAS-cog. Two reported superior effects of acupuncture than drugs (MD = −5.14, 95% CI: −8.75, −1.53; moderate-quality; Zhou et al., 2015; WMD = 5.56, 95% CI: 1.10, 6.03; low quality; Huang et al., 2019) with regards to ADAS-cog. tTMS compared with sham rTMS (MD = −3.56, 95% CI: −5.82, −1.48; moderate-quality) (Dong et al., 2018) decreased the ADAS-cog.
Discussion
Main Findings
In this overview, the different non-pharmacological interventions showed potential in reducing or improving the relevant outcomes, reflecting the clinical efficacy of these interventions. Exercise intervention showed the preliminary potential to improve ADL and MMSE. The cognitive intervention improved MMSE. rTMS may decrease ADAS-cog, and there was some evidence of improved MMSE, ADAS-cog, and ADL with acupuncture therapy. Efficacy was lacking for music therapy on the included outcomes for AD patients.
We used AMSTAR 2 and the GRADE system to assess the methodological quality and evidence quality of 10 MAs of non-pharmacological therapy for AD. The results for methodological quality by AMSTAR 2 showed that only two studies (Zhou et al., 2015; Dong et al., 2018) provided a protocol and had been registered. The lack of registration may result in a great adjustment of the research process as expected. Five of the MAs (Rao et al., 2014; Dong et al., 2018; Du et al., 2018; Huang et al., 2019; Jia et al., 2019) were screened and selected by two researchers, which was beneficial to accurate literature selection. The sources of funding are not reported in any of the included MAs, which may reduce the credibility of the research results due to potential conflicts of interest. Therefore, MAs of non-pharmacological therapy for AD should be registered and have a reported protocol before the study is carried out, and we should pay attention to any observed heterogeneity of the results of the review. The evidence quality of all the included outcomes was degraded. This overview of 10 studies and 24 outcomes showed that the quality of evidence was low and very low (58.3%); moderate evidence accounted for only 41.7%, and there was no high-quality evidence. Despite the potential efficacy of non-pharmacological interventions, the strength of evidence for all outcomes is still unsatisfactory.
Role of Non-pharmacological Interventions
Exercise Intervention
According to a prospective cohort study, exercise is one of the healthy lifestyle factors that contribute to a lower risk of AD (Dhana et al., 2019). There is now strong evidence linking regular physical activity or exercise to higher cognitive function, decreased cognitive decline, and reduced risk of AD (Brown et al., 2013). Exercise may also play a role in maintaining/improving brain health by increasing the peripheral concentrations of blood brain-derived neurotrophic factor (BDNF) (Marinus et al., 2019). Research suggests that the mechanisms of physical exercise include enhancing Aβ clearance rates or reducing deposition and promoting hippocampal synaptic plasticity. It has been found that physical activity counteracts AD-associated declines in mitochondrial and immune system function (Intlekofer and Cotman, 2013). Physical exercise affects many mechanisms at the cellular and molecular levels. It improves the production of neurotrophic factors, neurotransmitters, and hormones and promotes neuronal survival and neuroplasticity (Mahalakshmi et al., 2020). However, evidence that short-term, single-component exercise interventions promote cognitive function and prevent cognitive decline or dementia in older adults is largely insufficient (Brasure et al., 2018). Our overview found that exercise intervention effectively improved MMSE (very low quality of evidence) and ADL (moderate quality of evidence). Exercise is an available intervention for AD that is worthy of further research and promotion.
Cognitive Intervention
Cognitive intervention is typically classified as cognitive stimulation, cognitive training, and cognitive rehabilitation. Enhanced brain plasticity may be a component of the mechanism underpinning the cognitive improvements associated with cognitive interventions (Jeong et al., 2016). However, the benefits on ADAS-Cog are generally not clinically significant, and difficulties with blinding of patients and the use of adequate placebo controls make comparison with the results of dementia drug treatments problematic (Huntley et al., 2015). Our overview found that cognitive intervention was effective for the MMSE, similar to the previous study above (Huntley et al., 2015).
Music Therapy
The results of study suggested that music therapy is effective in enhancing cognitive function and mental well-being, which can be recommended as an alternative approach to managing AD-associated symptoms (Lyu et al., 2018). Three independent, yet interconnected, mechanisms can be hypothesized to underlie the beneficial effect that music plays in enhancing memory in persons with AD. These include activation of dopaminergic pathways, sympathetic arousal, and default neuronal connectivity (Peck et al., 2016). However, although the evidence for short-term improvement in mood and reduction in behavioral disturbance is consistent, there are no high-quality longitudinal studies that demonstrated the long-term benefits of music therapy (McDermott et al., 2013). Our results concur with those from the cited studies.
rTMS
TMS is a non-invasive and painless technique that generates an electric current-inducing modulation in cortical excitability, which can stimulate and regulate the cortical function of the brain (Rabey and Dobronevsky, 2016). There is strong evidence that rTMS exerts significant neuroprotective and pro-cognitive effects in AD through the expression of hippocampal BDNF (Yulug et al., 2018). The study shows that rTMS can significantly improve cognitive ability in patients with mild to moderate AD. Stimulation of multiple sites and long-term treatment improved AD-associated cognitive performance, but heterogeneity among the studies was inevitable and may have influenced our results (Lin et al., 2019). Our overview found that rTMS was effective on ADAS-cog but not on MMSE.
Acupuncture Therapy
Acupuncture therapy is a unique non-pharmacologic therapy in Chinese medicine that may protect neurons from degeneration and promote axonal regeneration in neurodegenerative diseases such as AD (Li et al., 2014). Related research has demonstrated that acupuncture improves spatial learning and memory ability by ameliorating the dendritic structure (Kan et al., 2018). It is also suggested that acupuncture improves the cognitive function of AD by regulating glucose metabolism, enhancing neurotransmission, and reducing oxidative stress, Aβ protein deposition, and neuronal apoptosis. However, it is still challenging to clarify which specific signaling pathway contributes to the acupuncture effect (Cao et al., 2016). Recently, there has been increasing evidence that acupuncture may be an effective and safe way to treat AD. However, the results among SRs and MAs show varied and heterogeneous effects, making it difficult for clinicians and policymakers to conclude overall treatment efficacy (Lee et al., 2009; Zhou et al., 2015, 2017; Huang et al., 2019). Our overview found that acupuncture was effective for the MMSE, ADL, and ADAS-cog. We suggest that the role of acupuncture should be considered in the development of AD clinical practice guidelines.
Suggestions for Future Research
The objective of any AD treatment is to achieve clinically meaningful symptom reduction in patients. Despite the promise of emerging therapies, existing pharmacological treatments dominate the AD treatment landscape (Ivashchenko et al., 2018), despite the lack of evidence on their effectiveness and inevitable side effects. For example, some antipsychotics are associated with an increased risk of stroke and death in individuals with dementia (Maust et al., 2015; Ralph and Espinet, 2018). In a subgroup of persons with AD, antipsychotics were associated with increased fracture (Watt et al., 2020). Given this, non-pharmacological therapy presents a potentially significant supplementary treatment for AD. The effectiveness of non-pharmacological therapy, including psychosocial interventions, for improving cognition or slowing down the progression of cognitive impairment in AD patients has been confirmed (Duan et al., 2018). When dealing with AD patients, it is suggested that clinicians should not forget non-pharmacological interventions.
“Determining the effectiveness of non-pharmacologic therapies can be difficult because of the large number of unique therapies tested, the diversity of therapeutic aims (from the improved overall quality of life to improvements in specific symptoms), the diverse stages of dementia represented (from mild to moderate to severe), the diverse types of dementia that may be present among participants in a particular study given the pervasiveness of mixed dementia, and the lack of standard methods for carrying out any individual therapy. With these multiple factors to consider, it is challenging to group together and compare non-pharmacologic therapies” (Alzheimer's Association, 2020). Therefore, future research needs to focus on the type and the form of non-pharmacological interventions that are most effective for the different severities of AD. Preliminary data suggest benefits on three outcomes related to cognitive function and quality of life. Furthermore, methodologically rigorous and adequately powered primary studies are necessary for each non-pharmacological intervention, reporting on consistently defined core outcomes in patients with AD.
Strengths and Weaknesses of the Review
A comprehensive evaluation of the efficacy of multiple non-pharmacologic interventions in patients with AD is currently lacking, so we summarized and compared the determining efficacy of five non-pharmacologic therapies evaluated in 10 MAs. To our knowledge, this overview of MAs is the first to compare multiple non-pharmacologic strategies to facilitate decision making systematically. To reduce the risk of bias, we only included MAs of RCTs and excluded narrative reviews and reviews with non-RCTs and observational cohort studies. We assessed the quality of the reviews against the 16 domains of AMSTAR 2, which, compared with AMSTAR, has a wider range of applications and more scientific evaluation methods (Shea et al., 2007, 2017). We assessed the significant outcomes by the GRADE system to determine the strength of evidence. Most of the evidence of the included primary trials were acknowledged as of poor quality. We also identified the deficiencies of current studies and make recommendations for further research on non-pharmacological interventions for AD.
However, there are still some shortcomings. The number of included studies was limited, and some studies of non-pharmacological therapy for AD did not meet the inclusion criteria, such as plasmapheresis (Luengo-Matos et al., 2017), dance (Ruiz-Muelle and López-Rodríguez, 2019), light treatment (Mitolo et al., 2018), spaced retrieval training (Oren et al., 2014), and physical therapy interventions (Zhu et al., 2015). These studies were not evaluated, but their clinical effectiveness cannot be ruled out. We need more scientific and standard studies in the future. Besides that, only three main clinical outcomes (MMSE, ADL, and ADAS-cog) were included. The MMSE is a cognitive test that is the most common and widely used cognitive screening measure as part of the evaluation for AD (Folstein et al., 1975; Tsoi et al., 2015), and it has good validity and reliability (Razani et al., 2009). In AD, the decline in ADL is increasingly recognized as a source of considerable social, health, and economic costs (Alzheimer's Association, 2020), and ADAS-Cog (Rosen et al., 1984) is the most widely used general cognitive measure in clinical trials of AD. Research demonstrates that a three-point decline in ADAS-Cog may be an appropriate minimal clinical relevant change for clinical trials of early AD (Schrag and Schott, 2012). Unfortunately, despite the advantages, other outcomes reflecting AD status were not included, which need to be discussed further.
Conclusion
Although there are gaps in the literature and a lack of high-quality evidence, the findings from this overview suggest that non-pharmacological interventions are important for AD patients. In the future, the quality of the original research needs to be improved, and strictly designed MAs should be carried out following methodological requirements. Higher-quality trials and patient-based MAs are needed to determine the benefits of specific non-pharmacological interventions.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
L-YW and JP designed the study. L-YW and Y-JZ selected the relevant studies. Y-WC and Y-JZ extracted the data. L-YW and Y-WC performed the quality assessment. L-YW wrote the manuscript. All the authors contributed to the writing of this manuscript.
Funding
This project was supported by the grant of the Key Scientific Research Program of Shanghai Municipal Science and Technology Committee in China (16401970300 and 18401970500), the Financing Scheme of Arising Interdisciplinary Subjects of TCM in Shanghai (Shxxjcxk201709), and the TCM genre program of Shanghai Health Bureau [ZY (2018-2020)-CCCX-1006].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2020.594432/full#supplementary-material
Abbreviations
AD, Alzheimer's disease; ADAS-cog, Alzheimer's Disease Assessment Scale-cognitive section; ADL, activities of daily living; AMSTAR, A Measurement Tool to Assess Systematic Reviews; BDNF, brain-derived neurotrophic factor; GRADE, Grading of Recommendations, Assessment, Development and Evaluation; MAs, meta-analyses; MMSE, Mini-Mental State Examination; PICO, population, intervention, comparison, outcome; RCT, randomized clinical trial; rTMS, repetitive transcranial magnetic stimulation; SR, systematic review.
References
Alves, J., Magalhaes, R., Thomas, R. E., Goncalves, O. F., Petrosyan, A., and Sampaio, A. (2013). Is there evidence for cognitive intervention in Alzheimer disease? A systematic review of efficacy, feasibility, and cost-effectiveness. Alzheimer Dis Assoc Disord. 27, 195–203. doi: 10.1097/WAD.0b013e31827bda55
Alzheimer's Association (2020). 2020 Alzheimer's disease facts and figures. Alzheimers Dement 16, 391–460. doi: 10.1002/alz.12068
Alzheimer's Disease International (2015). World Alzheimer Report 2015—the Global Impact of Dementia: An Analysis of Prevalence Incidence, Cost and Trends. Available online at: https://www.alz.co.uk/research/world-report-2015 (accessed August, 2015).
Arvanitakis, Z., Shah, R. C., and Bennett, D. A. (2019). Diagnosis and management of dementia: review. JAMA. 322, 1589–1599. doi: 10.1001/jama.2019.4782
Bougioukas, K. I., Liakos, A., Tsapas, A., Ntzani, E., and Haidich, A. B. (2018). Preferred reporting items for overviews of systematic reviews including harms checklist: a pilot tool to be used for balanced reporting of benefits and harms. J Clin Epidemiol. 93:9–24. doi: 10.1016/j.jclinepi.2017.10.002
Brasure, M., Desai, P., Davila, H., Nelson, V. A., Calvert, C., Jutkowitz, E., et al. (2018). Physical activity interventions in preventing cognitive decline and Alzheimer-type dementia: a systematic review. Ann Intern Med. 168, 30–38. doi: 10.7326/m17-1528
Brown, B. M., Peiffer, J. J., and Martins, R. N. (2013). Multiple effects of physical activity on molecular and cognitive signs of brain aging: can exercise slow neurodegeneration and delay Alzheimer's disease? Mol Psychiatry. 18, 864–874. doi: 10.1038/mp.2012.162
Cammisuli, D. M., Innocenti, A., Fusi, J., Franzoni, F., and Pruneti, C. (2018). Aerobic exercise effects upon cognition in Alzheimer's Disease: a systematic review of randomized controlled trials. Arch Ital Biol. 156, 54–63. doi: 10.12871/00039829201816
Cao, Y., Zhang, L. W., Wang, J., Du, S. Q., Xiao, L. Y., Tu, J. F., et al. (2016). Mechanisms of acupuncture effect on Alzheimer's disease in animal- based researches. Curr Top Med Chem. 16, 574–578. doi: 10.2174/1568026615666150813144942
Chan, K. Y., Wang, W., Wu, J. J., Liu, L., Theodoratou, E., Car, J., et al. (2013). Epidemiology of Alzheimer's disease and other forms of dementia in China, 1990-2010: a systematic review and analysis. Lancet. 381, 2016–2023. doi: 10.1016/s0140-6736(13)60221-4
Dhana, K., Evans, D. A., Rajan, K. B., Bennett, D. A., and Morris, M. C. (2019). Impact of healthy lifestyle factors on the risk of Alzheimer's dementia: findings from two prospective cohort studies. Alzheimer's and Dementia. 15:P207. doi: 10.1016/j.jalz.2019.06.4547
Dong, X., Yan, L., Huang, L., Guan, X., Dong, C., Tao, H., et al. (2018). Repetitive transcranial magnetic stimulation for the treatment of Alzheimer's disease: a systematic review and meta-analysis of randomized controlled trials. PLoS ONE. 13:e0205704. doi: 10.1371/journal.pone.0205704
Du, Z., Li, Y., Li, J., Zhou, C., Li, F., and Yang, X. (2018). Physical activity can improve cognition in patients with Alzheimer's disease: a systematic review and meta-analysis of randomized controlled trials. Clin Interv Aging. 13, 1593–1603. doi: 10.2147/cia.S169565
Duan, Y., Lu, L., Chen, J., Wu, C., Liang, J., Zheng, Y., et al. (2018). Psychosocial interventions for Alzheimer's disease cognitive symptoms: a Bayesian network meta-analysis. BMC Geriatr. 18:175. doi: 10.1186/s12877-018-0864-6
Dubois, B., Feldman, H. H., Jacova, C., DeKosky, S. T., Gateau, P. B., Cummings, J., et al. (2007). Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 6, 734–746. doi: 10.1016/S1474-4422(07)70178-3
Folstein, M. F., Folstein, S. E., and McHugh, P. R. (1975). “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 12, 189–198. doi: 10.1016/0022-3956(75)90026-6
García-Casares, N., Moreno-Leiva, R. M., and García-Arnés, J. A. (2017). Music therapy as a non-pharmacological treatment in alzheimer's disease. A systematic review. Rev. Neurol. 65, 529–538. Available online at: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L623517089
Gordon, M., and Guyatt, G. (2020). Assessment of evidence quality in IBD guidance: the use and misuse of GRADE. Gastroenterology 159, 1209–1215. doi: 10.1053/j.gastro.2020.06.092
Guyatt, G. H., Oxman, A. D., Vist, G. E., Kunz, R., Falck-Ytter, Y., Alonso-Coello, P., et al. (2008). GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336, 924–926. doi: 10.1136/bmj.39489.470347.AD
Haapasalo, A., Pikkarainen, M., and Soininen, H. (2015). Alzheimer's disease: a report from the 7th Kuopio Alzheimer symposium. Neurodegener Dis Manag. 5, 379–382. doi: 10.2217/nmt.15.31
Huang, Q., Luo, D., Chen, L., Liang, F. X., and Chen, R. (2019). Effectiveness of acupuncture for Alzheimer's disease: an updated systematic review and meta-analysis. Curr Med Sci. 39, 500–511. doi: 10.1007/s11596-019-2065-8
Huntley, J. D., Gould, R. L., Liu, K., Smith, M., and Howard, R. J. (2015). Do cognitive interventions improve general cognition in dementia? A meta-analysis and meta-regression. BMJ Open. 5, e005247. doi: 10.1136/bmjopen-2014-005247
Intlekofer, K. A., and Cotman, C. W. (2013). Exercise counteracts declining hippocampal function in aging and Alzheimer's disease. Neurobiol Dis. 57:47–55. doi: 10.1016/j.nbd.2012.06.011
Ivashchenko, A. V., Brooks, L., and Tran, Z. V. (2018). Differential effects of pharmacologic and non-pharmacologic threatments of Alzheimer's disease: a comprehensive summary of evidence and meta-amalysis. Alzheimer's Dementia 14, P676–P677. doi: 10.1016/j.jalz.2018.06.710
Jeong, J. H., Na, H. R., Choi, S. H., Kim, J., Na, D. L., Seo, S. W., et al. (2016). Group- and home-based cognitive intervention for patients with mild cognitive impairment: a randomized controlled trial. Psychother Psychosom. 85, 198–207. doi: 10.1159/000442261
Jia, R. X., Liang, J. H., Xu, Y., and Wang, Y. Q. (2019). Effects of physical activity and exercise on the cognitive function of patients with Alzheimer disease: a meta-analysis. BMC Geriatr. 19:181. doi: 10.1186/s12877-019-1175-2
Kan, B. H., Yu, J. C., Zhao, L., Zhao, J., Li, Z., Suo, Y. R., et al. (2018). Acupuncture improves dendritic structure and spatial learning and memory ability of Alzheimer's disease mice. Neural Regen Res. 13, 1390–1395. doi: 10.4103/1673-5374.235292
Kishita, N., Backhouse, T., and Mioshi, E. (2020). Nonpharmacological interventions to improve depression, anxiety, and quality of life (QoL) in people with dementia: an overview of systematic reviews. J Geriatr Psychiatry Neurol. 33, 28–41. doi: 10.1177/0891988719856690
Lee, M. S., Shin, B. C., and Ernst, E. (2009). Acupuncture for Alzheimer's disease: a systematic review. Int J Clin Pract. 63, 874–879. doi: 10.1111/j.1742-1241.2009.02043.x
Li, X., Guo, F., Zhang, Q., Huo, T., Liu, L., Wei, H., et al. (2014). Electroacupuncture decreases cognitive impairment and promotes neurogenesis in the APP/PS1 transgenic mice. BMC Complement Altern Med. 14:37. doi: 10.1186/1472-6882-14-37
Lin, Y., Jiang, W. J., Shan, P. Y., Lu, M., Wang, T., Li, R. H., et al. (2019). The role of repetitive transcranial magnetic stimulation (rTMS) in the treatment of cognitive impairment in patients with Alzheimer's disease: a systematic review and meta-analysis. J Neurol Sci. 398:184–191. doi: 10.1016/j.jns.2019.01.038
Lorenz, R. C., Matthias, K., Pieper, D., Wegewitz, U., Morche, J., Nocon, M., et al. (2019). A psychometric study found AMSTAR 2 to be a valid and moderately reliable appraisal tool. J Clin Epidemiol. 114, 133–140. doi: 10.1016/j.jclinepi.2019.05.028
Luengo-Matos, S., Polo-Desantos, M., Pablo, J., Orrego, C., and Sanchez-Gomez, L. M. (2017). Assessment of plasmapheresis for Alzheimer's disease systematic review. Int J Technol Assess Health Care. 33:227. doi: 10.1017/S0266462317004044
Lyu, J., Zhang, J., Mu, H., Li, W., Champ, M., Xiong, Q., et al. (2018). The effects of music therapy on cognition, psychiatric symptoms, and activities of daily living in patients with Alzheimer's disease. J Alzheimers Dis. 64, 1347–1358. doi: 10.3233/jad-180183
Mahalakshmi, B., Maurya, N., Lee, S. D., and Bharath Kumar, V. (2020). Possible neuroprotective mechanisms of physical exercise in neurodegeneration. Int J Mol Sci. 21, E5895. doi: 10.3390/ijms21165895
Maresova, P., Dolejs, J., and Kuca, K. (2018). Call for a uniform strategy of collecting Alzheimer's disease costs: a review and meta-analysis. J Alzheimers Dis. 63, 227–238. doi: 10.3233/jad-171028
Marinus, N., Hansen, D., Feys, P., Meesen, R., Timmermans, A., and Spildooren, J. (2019). The impact of different types of exercise training on peripheral blood brain-derived neurotrophic factor concentrations in older adults: a meta-analysis. Sports Med. 49, 1529–1546. doi: 10.1007/s40279-019-01148-z
Maust, D. T., Kim, H. M., Seyfried, L. S., Chiang, C., Kavanagh, J., Schneider, L. S., et al. (2015). Antipsychotics, other psychotropics, and the risk of death in patients with dementia: number needed to harm. JAMA Psychiatry 72, 438–445. doi: 10.1001/jamapsychiatry.2014.3018
McDermott, O., Crellin, N., Ridder, H. M., and Orrell, M. (2013). Music therapy in dementia: a narrative synthesis systematic review. Int J Geriatr Psychiatry 28, 781–794. doi: 10.1002/gps.3895
McKhann, G., Drachman, D., Folstein, M., Katzman, R., Price, D., and Stadlan, E. M. (1984). Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 34, 939–944. doi: 10.1212/wnl.34.7.939
Mitolo, M., Tonon, C., La Morgia, C., Testa, C., Carelli, V., and Lodi, R. (2018). Effects of light treatment on sleep, cognition, mood, and behavior in Alzheimer's disease: a systematic review. Dement Geriatr Cogn Disord. 46, 371–384. doi: 10.1159/000494921
O'Brien, J. T., Holmes, C., Jones, M., Jones, R., Livingston, G., McKeith, I., et al. (2017). Clinical practice with anti-dementia drugs: a revised (third) consensus statement from the British Association for Psychopharmacology. J Psychopharmacol. 31, 147–168. doi: 10.1177/0269881116680924
Olazaran, J., Reisberg, B., Clare, L., Cruz, I., Pena-Casanova, J., Del Ser, T., et al. (2010). Nonpharmacological therapies in Alzheimer's disease: a systematic review of efficacy. Dement Geriatr Cogn Disord. 30, 161–178. doi: 10.1159/000316119
Oren, S., Willerton, C., and Small, J. (2014). Effects of spaced retrieval training on semantic memory in Alzheimer's disease: a systematic review. J Speech Lang Hear Res. 57, 247–270. doi: 10.1044/1092-4388(2013/12-0352)
Peck, K. J., Girard, T. A., Russo, F. A., and Fiocco, A. J. (2016). Music and memory in Alzheimer's disease and the potential underlying mechanisms. J Alzheimers Dis. 51, 949–959. doi: 10.3233/JAD-150998
Pedroza, P., Chakrabarti, S., Chapin, A., Liu, A., Matyasz, T., and Dieleman, J. L. (2019). Costs of Alzheimer's disease and dementia in 188 countries. Alzheimer's Dementia 15:P1635. doi: 10.1016/j.jalz.2019.06.4877
Rabey, J. M., and Dobronevsky, E. (2016). Repetitive transcranial magnetic stimulation (rTMS) combined with cognitive training is a safe and effective modality for the treatment of Alzheimer's disease: clinical experience. J Neural Transm. 123, 1449–1455. doi: 10.1007/s00702-016-1606-6
Ralph, S. J., and Espinet, A. J. (2018). Increased all-cause mortality by antipsychotic drugs: updated review and meta-analysis in dementia and general mental health care. J Alzheimers Dis Rep. 2, 1–26. doi: 10.3233/adr-170042
Rao, A. K., Chou, A., Bursley, B., Smulofsky, J., and Jezequel, J. (2014). Systematic review of the effects of exercise on activities of daily living in people with Alzheimer's disease. Am J Occup Ther. 68, 50–56. doi: 10.5014/ajot.2014.009035
Razani, J., Wong, J. T., Dafaeeboini, N., Edwards-Lee, T., Lu, P., Alessi, C., et al. (2009). Predicting everyday functional abilities of dementia patients with the Mini-Mental State Examination. J Geriatr Psychiatry Neurol. 22, 62–70. doi: 10.1177/0891988708328217
Rosen, W. G., Mohs, R. C., and Davis, K. L. (1984). A new rating scale for Alzheimer's disease. Am J Psychiatry. 141, 1356–1364. doi: 10.1176/ajp.141.11.1356
Ruiz-Muelle, A., and López-Rodríguez, M. M. (2019). Dance for people with Alzheimer's disease: a systematic review. Curr Alzheimer Res. 16, 919–933. doi: 10.2174/1567205016666190725151614
Sá, C. C., da Silva, D. F., Bigongiari, A., and Machado-Lima, A. (2019). Efficacy of cognitive rehabilitation in improving and maintaining daily living activities in patients with alzheimer's disease: a systematic review of literature. Jornal Brasileiro de Psiquiatria. 68, 153–160. doi: 10.1590/0047-2085000000241
Schrag, A., and Schott, J. M. (2012). What is the clinically relevant change on the ADAS-Cog? J Neurol Neurosurg Psychiatry. 83, 171–173. doi: 10.1136/jnnp-2011-300881
Shea, B. J., Grimshaw, J. M., Wells, G. A., Boers, M., Andersson, N., Hamel, C., et al. (2007). Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 7:10. doi: 10.1186/1471-2288-7-10
Shea, B. J., Reeves, B. C., Wells, G., Thuku, M., Hamel, C., Moran, J., et al. (2017). AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 358:j4008. doi: 10.1136/bmj.j4008
Tsoi, K. K., Chan, J. Y., Hirai, H. W., Wong, S. Y., and Kwok, T. C. (2015). Cognitive tests to detect dementia: a systematic review and meta-analysis. JAMA Intern Med. 175, 1450–1458. doi: 10.1001/jamainternmed.2015.2152
Wang, Y., Zhen, T., Liao, Y., Li, L., and Zhang, Y. (2020). A meta-analysis of the effect of music therapy on alzheimer's disease. Int J Clin Exp Med. 13, 317–329. Available online at: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L2003914068
Watt, J. A., Goodarzi, Z., Veroniki, A. A., Nincic, V., Khan, P. A., Ghassemi, M., et al. (2020). Safety of pharmacologic interventions for neuropsychiatric symptoms in dementia: a systematic review and network meta-analysis. BMC Geriatr. 20:212. doi: 10.1186/s12877-020-01607-7
Xu, J., Murphy, S. L., Kockanek, K. D., and Arias, E. (2020). Mortality in the United States, 2018. NCHS Data Brief. 355:1–8
Yulug, B., Hanoglu, L., Khanmammadov, E., Duz, O. A., Polat, B., Hanoglu, T., et al. (2018). Beyond the therapeutic effect of rTMS in Alzheimer's disease: a possible neuroprotective role of hippocampal BDNF?: A minireview. Mini Rev Med Chem. 18, 1479–1485. doi: 10.2174/1389557517666170927162537
Zhou, J., Peng, W. N., Xu, M., Li, W., and Liu, Z. S. (2015). The effectiveness and safety of acupuncture for patients with Alzheimer disease a systematic review and meta-analysis of randomized controlled trials. Medicine 94:e933. doi: 10.1097/md.0000000000000933
Zhou, S., Dong, L., He, Y., and Xiao, H. (2017). Acupuncture plus herbal medicine for Alzheimer's disease: a systematic review and meta-analysis. Am J Chin Med. 45, 1327–1344. doi: 10.1142/s0192415x17500732
Zhu, X. C., Yu, Y., Wang, H. F., Jiang, T., Cao, L., Wang, C., et al. (2015). Physiotherapy intervention in Alzheimer's disease: systematic review and meta-analysis. J Alzheimers Dis. 44, 163–174. doi: 10.3233/jad-141377
Keywords: Alzheheimer's disease, non-pharmacological intervention, overview, meta–analysis, effectiveness
Citation: Wang L-Y, Pei J, Zhan Y-J and Cai Y-W (2020) Overview of Meta-Analyses of Five Non-pharmacological Interventions for Alzheimer's Disease. Front. Aging Neurosci. 12:594432. doi: 10.3389/fnagi.2020.594432
Received: 13 August 2020; Accepted: 15 October 2020;
Published: 25 November 2020.
Edited by:
Gjumrakch Aliev, GALLY International Biomedical Research, United StatesReviewed by:
Guoqiang Xing, Affiliated Hospital of North Sichuan Medical College, ChinaLing Zhao, Chengdu University of Traditional Chinese Medicine, China
Copyright © 2020 Wang, Pei, Zhan and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Pei, bG9uZ2h1YWFjdXBAYWxpeXVuLmNvbQ==
 Liao-Yao Wang
Liao-Yao Wang Jian Pei*
Jian Pei*