- Department of Psychiatry and Behavioral Medicine, Nova Southeastern University, Fort Lauderdale, FL, United States
Objective: HIV infection is associated with impaired cognition, and as individuals grow older, they may also experience age-related changes in mental abilities. Previous studies have shown that computer-based cognitive training (CCT) and transcranial direct current stimulation (tDCS) may be useful in improving cognition in older persons. This study evaluated the acceptability of CCT and tDCS to older adults with HIV-associated neurocognitive disorder, and assessed their impact on reaction time, attention, and psychomotor speed.
Methods: In a single-blind randomized study, 46 individuals with HIV-associated mild neurocognitive disorder completed neuropsychological assessments and six 20-min training sessions to which they had been randomly assigned to one of the following conditions: (1) CCT with active tDCS; (2) CCT with sham tDCS, or (3) watching educational videos with sham tDCS. Immediately after training and again 1 month later, participants completed follow-up assessments. Outcomes were evaluated via repeated measures mixed effects models.
Results: Participant ratings of the intervention were positive. Effects on reaction time were not significant, but measures of attention and psychomotor speed suggested positive effects of the intervention.
Conclusion: Both CCT and tDCS were highly acceptable to older persons with HIV infection. CCT and tDCS may improve cognitive in affected individuals.
Clinical Trial Registration: [www.ClinicalTrials.gov], identifier [NCT03440840].
Introduction
While there has been significant progress in the treatment of HIV infection using multiple antiretroviral medications, HIV-association neurocognitive disorders (HANDs) continue to be seen in affected individuals, even when their viral loads are non-detectable (Heaton et al., 2010). HANDs are clinically significant because of their impact on patients’ everyday functioning (Thames et al., 2011a,b, 2013), medication adherence (Hinkin et al., 2002, 2004; Thames et al., 2013), and quality of life (Tozzi et al., 2003; Degroote et al., 2013, 2014; Moore et al., 2014). In older persons, HANDs may have an additive or even synergistic effect in older persons, combining the influences of chronic HIV infection and cognitive aging (Valcour et al., 2004; Wendelken and Valcour, 2012).
Few treatments are available for HAND. Stimulants can improve cognition in HAND but may be abused by vulnerable individuals and have undesirable adverse effects (Hinkin et al., 2001). Other medication treatments have been studied, but none has demonstrated clear efficacy (Antinori et al., 2007; Schifitto et al., 2007, 2009; Sacktor et al., 2011; Nakasujja et al., 2013; Decloedt et al., 2016). Other researchers have suggested that computer-delivered cognitive training (CCT) may be useful in HAND (Vance et al., 2012, 2019; Cody and Vance, 2016), but specialized CCT software is not always readily available or affordable. Further, many programs created for CCT are not inherently interesting, reducing users’ motivations for continued use after completing a study for which they were compensated. Another approach may be to use computer gaming software that is already available as a CCT intervention (Zelinski and Reyes, 2009; Bonnechère et al., 2020). CCT software developers have sought to increase the inherent interest in their programs through gamification (Green and Seitz, 2015) to enhance their inherent interest, but many computer games are on the market now and often available at little or no cost. In addition, existing games depend on sustained use by players for their commercial success. Games such as these are interesting to players and include elements that engage them. First-person shooters (in which players use weapons to shoot at fictional enemies) can affect sustained attention and reaction time (Green and Seitz, 2015), however, some players may object to this type of game’s violent content (Wu and Spence, 2013; Green and Seitz, 2015).
Another established genre are games that provide players the simulated experience of car racing. These games require attention and psychomotor speed while using content that may be less objectionable. Use of one car racing game, created for a research study, was associated with better mental functioning in older persons (Anguera et al., 2013). Other researchers have commented on the possible usefulness of commercial computer games in addressing mental functioning in persons 50 years of age and older (Basak et al., 2008; Zelinski and Reyes, 2009; Belchior et al., 2012, 2016; Bavelier and Green, 2016). Car racing games can engage and hold players’ interest, potentially allowing them to continue cognitive training over extended periods. Researchers have shown that an off-the-shelf game that demanded mental speed resulted in longer use by older individuals when compared to a typical CCT program (Belchior et al., 2012, 2016). Game play has been related to long-term mental training results, with effects evident in other cognitive domains besides those specifically trained (Cardoso-Leite and Bavelier, 2014). Gaming has been shown, for example, to have a positive impact on the ability to regulate and direct mental processes (Anguera et al., 2013). Games may thus be effective for training and can engage users in a sustained fashion.
Players’ adherence to regular game play, however, may also be limited even if games are more engaging that other forms of cognitive training. Recent research on ways to encourage users of self-help software has shown that specific game characteristics, such as daily activities to be completed, can help users establish regular play in brief sessions (e.g., 15 min) (Alexandrovsky et al., 2019, 2021). In addition, the effect of type of game play on player motivation may vary across the lifespan (Birk et al., 2017).
Transcranial direct current stimulation (tDCS) in combination with CCT has been shown to improve cognitive functioning (Coffman et al., 2012; Martin et al., 2013, 2014; Trumbo et al., 2016; Lawrence et al., 2017, 2018; Leshikar et al., 2017). tDCS is implemented by applying moistened sponge electrodes to a person’s scalp and passing through a very small direct current (1–2 mA). tDCS research has shown that it can have a positive impact on various mental abilities, including verbal problem solving (Cerruti and Schlaug, 2009), working memory (Fregni et al., 2005; Zaehle et al., 2011; Heimrath et al., 2012), and learning (Clark et al., 2012; Floel et al., 2012).
How tDCS affects mental function is not definitively established, however, it has been shown to stimulate brain-derived neurotrophic growth factor (BDNF) in the motor cortex (Fritsch et al., 2010). This may be especially relevant in treating persons with HAND as BDNF is affected in HIV infection (Bachis et al., 2012; Avdoshina et al., 2013), and implicated in cognitive decline in older persons (Buchman et al., 2016). Increases in BDNF might be expected to exert a positive effect on mental functioning in persons with HAND. It may be noted, however, that multiple mechanisms for the effect of tDCS on cognition have been suggested (Yamada and Sumiyoshi, 2021).
We previously completed a pilot study of game-based CCT comparing its combination with active and sham tDCS in persons 50 years and older with HAND (Ownby and Acevedo, 2016). Results suggested that the intervention was acceptable to participants and that it may have had positive effects on their attention and working memory. In the follow-up study reported here, we further explore the acceptability and efficacy of a game-based CCT intervention combined with tDCS in older persons with HIV infection. We hypothesized that CCT with tDCS would be acceptable to persons 50 years of age and older with HAND. We also hypothesized that CCT would be associated with improved reaction time, psychomotor speed, and attention and that the combination of active tDCS with CCT would be superior to CCT alone.
Materials and Methods
Participants
Participants were individuals 50 years of age and older with HIV infection. Diagnosis of HAND was established through review of recent laboratory results, clinical evaluation, and neuropsychological testing. All participants stated they subjectively experienced cognitive difficulties and, after assessment, were found to have impairment of mental functioning in two or more cognitive domains while not having dementia, thus meeting Frascati criteria for mild neurocognitive disorder (Antinori et al., 2007). Potential participants were excluded if they had characteristics that might have increased risk to them from tDCS, such as seizures or bipolar disorder (Galvez et al., 2011; Brunoni et al., 2013). Use of many psychotropic medications was also an exclusion criterion, as the pharmacologic activity of many of these drugs can affect tDCS (Medeiros et al., 2012; McLaren et al., 2018). Medications that were exclusions included those affecting serotonin, such as many antidepressants, dopamine, such as stimulants and antipsychotics, and gamma-amino butyric acid, such as benzodiazepines. Left-handed participants were excluded as our intent was to stimulate the dominant dorsolateral prefrontal cortex.
Procedures
Recruitment and Determination of Eligibility
Participants were first recruited from individuals who had been in a previous study. We also recruited from local service providers for persons with HIV. A number of participants referred friends or acquaintances. We distributed flyers in several areas of Broward County, Florida, known to have a high prevalence of HIV infection, as well as advertising in a local newspaper and creating a Facebook page.
Interested individuals were contacted for a telephone interview to establish that they had complaints of cognitive difficulties, using questions published by the European AIDS Clinical Society (European AIDS Clinical Society, 2015). In this interview, we inquired about use of medications that might lead to exclusion, and whether the person was willing to be in a study of CCT and tDCS. All were being treated for HIV infection and had been on their current medication regimen for at least the past month. Individuals who, from this telephone interview, appeared likely to be eligible were asked to come to our offices for individual assessment.
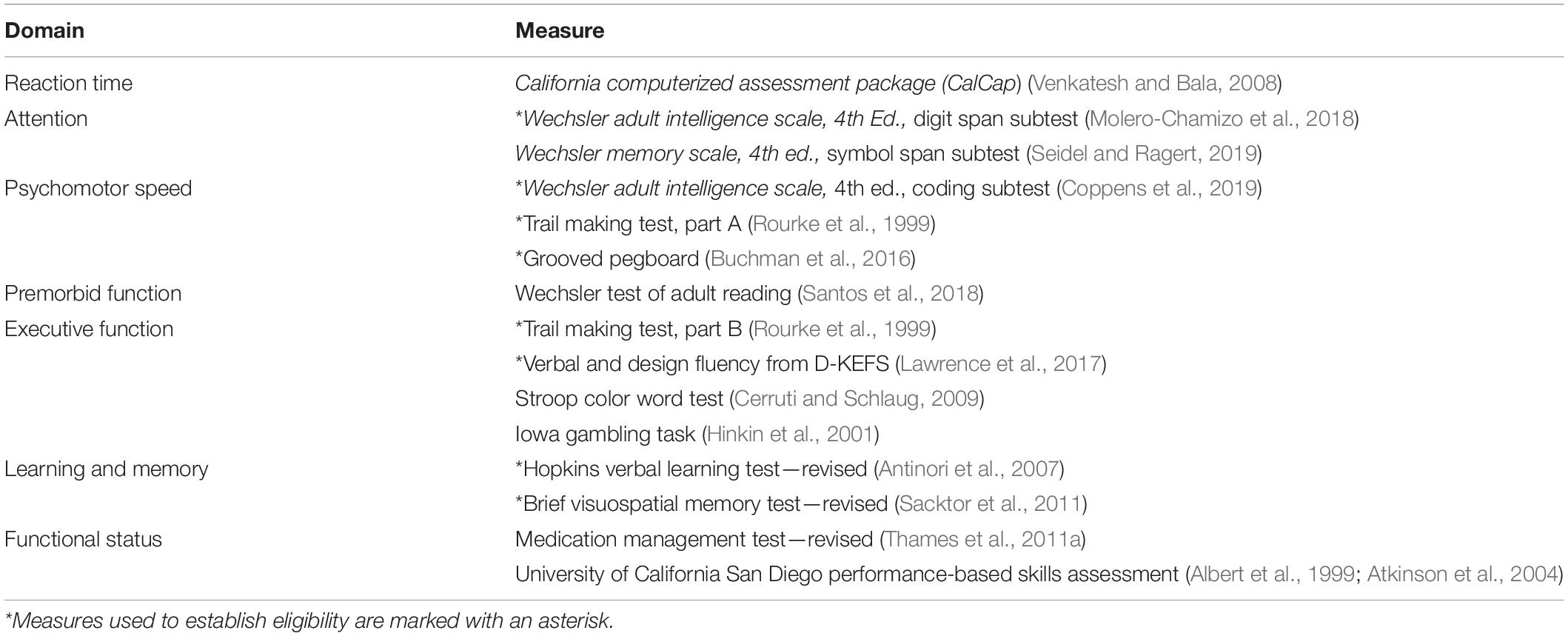
At this assessment, potential participants completed a series of cognitive assessments (marked with an asterisk in Table 1). The battery was selected to allow evaluation of areas often affected in HANDs (Woods et al., 2009). Attention and working memory were evaluated with the Digit Span subtests of the Wechsler Adult Intelligence Scale, 4th edition, or WAIS-IV (Wechsler, 2008). Psychomotor speed was evaluated with the Coding subtest of the WAIS-IV and Grooved Pegboard Test (Lafayette Instrument Company, 2002). Executive function was measured with the Trail Making Test, Part B (Lezak, 2004). Verbal fluency was assessed with the Verbal Fluency test of the Delis-Kaplan Executive Function System (Delis et al., 2001). Verbal learning and memory were assessed with the Hopkins Verbal Learning Test—Revised or HVLT-R (Brandt and Benedickt, 2001), and visual learning and memory with the Brief Visuospatial Memory Test, or BVMT-R (Benedict et al., 1996).
Cognitive impairment for the purpose of establishing the diagnosis of mild neurocognitive disorder was defined as a score in at least two ability areas that was below population norms by at least one standard deviation. Participants were treated for HIV infection that included ongoing laboratory measures of treatment effects (HIV-1 viral load and CD4 cell counts). Individuals in the study brought recent laboratory results, allowing us to verify their HIV status and know their current treatment and immune status. All medications were also brought to this visit to allow verification of current medication use. Persons who met entry criteria then completed the additional assessments as described in the next section.
Acceptability
We used several strategies to evaluate the feasibility and acceptability of the CCT with tDCS intervention to participants. We used a questionnaire based on the Technology Acceptance Model, or TAM (Venkatesh et al., 2003; Venkatesh and Bala, 2008), the dimensions of which have received substantial support for use with digital health technologies (Binyamin and Zafar, 2021). The model specifies that users’ perceptions of an application’s ease of use and usefulness are related to their future intention to use the application. We hypothesized that if the intervention were viewed favorably by participants, their average rating on the Usefulness and Ease of Use scales of this questionnaire would be significantly different from the midpoint of the scale in a positive direction. Participants rated subscale items on a seven-point Likert-type scale anchored at one end with “strongly disagree” and at the other “strongly agree.”
Another scale was developed based on a model balancing risks and benefits of a treatment was used to develop a questionnaire assessing users’ perceptions of the balance between an intervention’s risks and benefits (Atkinson et al., 2004, 2005). Participants were asked, for example, if they experienced benefits from the intervention and adverse effects from it. They were then asked to provide an overall judgment as to whether the benefits of the intervention outweighed its adverse effects. Spontaneous comments about the intervention made by participants during training sessions were recorded verbatim.
Cognitive Measures
In order to evaluate possible cognitive effects of the intervention more comprehensively, participants additional assessments after determination of their eligibility. Use of these measures allowed tests of the study’s hypothesis that participants receiving the active interventions would display better performance than control participants in reaction time, attention, and psychomotor speed.
In order to evaluate intervention effects on participants’ reaction time, they completed the California Computerized Assessment Package (Miller, 2013). To further evaluate the effects of the intervention on executive functions, participants also completed the Stroop Color Word Test (Golden, 1978), the Iowa Gambling Task (Bechara et al., 2005), and the Design Fluency subtest of the Delis-Kaplan Executive Function System (Delis et al., 2001). Finally, to assess whether the intervention had an impact on everyday functional performance, participants completed the Medication Management Test—Revised, a measure of the person’s ability to understand and carry out medication-related tasks (Albert et al., 1999) and the University of San Diego Scales of Observed Performance (Patterson et al., 2001), assessing their ability to perform everyday tasks such as making a medical appointment and paying a bill. Finally, in order to provide an estimate of participants’ premorbid level of functioning, they completed the Wechsler Test of Adult Reading (Wechsler, 2001).
Other Self-Report Measures
The assessment battery also included the Patient’s Assessment of Own Functioning or PAOF (Chelune et al., 1986). This measure asks the individual to self-report their experience of mental problems in several domains such as language, perception, and memory. It has been used in other studies of HAND (Rourke et al., 1999). We also used the Center for Epidemiological Studies Depression scale or CESD (Radloff, 1977) to assess participants’ symptoms of depression. All self-report assessments were completed using computer software that read questions aloud and enabled participants to record their responses by tapping on the computer screen.
Compensation
After these initial assessments were done, individuals in the study were asked to return to begin the intervention. Participants received compensation for their involvement, US $80 for the baseline and follow-up sessions, and $40 for each intervention visit.
Computer-Based Cognitive Training
At the first training visit (after completion of baseline assessments), participants were assigned to intervention group using a computer-created randomization scheme. The scheme was generated via random numbers in a predetermined block (n = 3) randomization scheme. Participants were enrolled by the study coordinator (who was blind to treatment assignment) and assigned by the unblinded principal investigator who also conducted all training sessions.
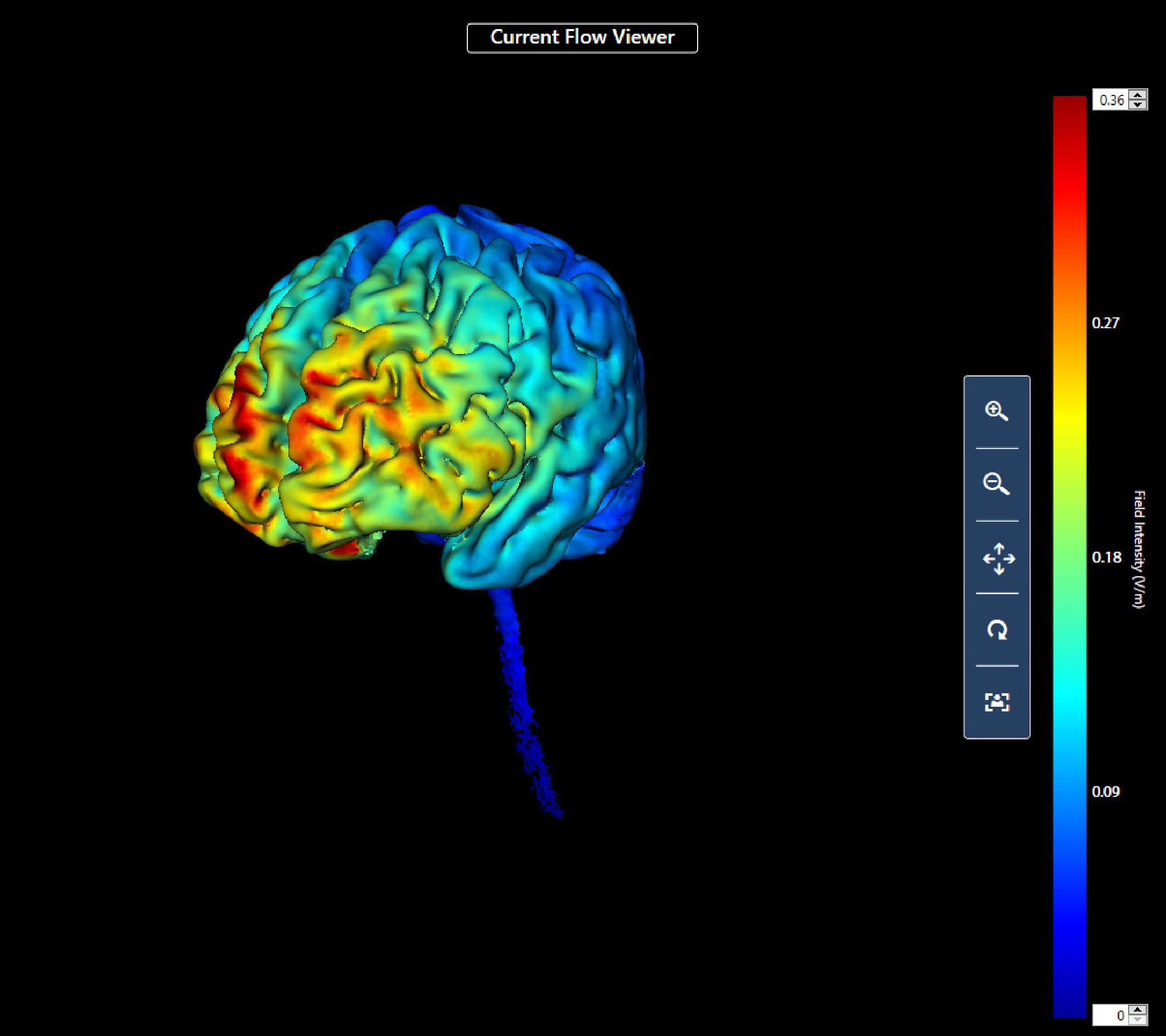
First, procedures regarding the administration of tDCS and the use of the game controller (an Xbox game controller with USB interface to a Windows computer). The participant sat in front of and to the right of the researcher; the participant could not see the direct current device for tDCS or the researcher taking notes during training. For all participants, the anode electrode was located over the left dorsolateral prefrontal cortex (10–20 system F3) and the cathode over the right supraorbital area (FP2) (Acharya et al., 2016). This montage was used because of the likely effect of stimulation at this site on the left dorsolateral prefrontal cortex. This area of the brain was chosen for stimulation as it is known to be involved in complex executive functions such as control of cognitive processes and attention (Leh et al., 2010; Brosnan and Wiegand, 2017), and previous research has shown that its stimulation may affect relevant executive cognitive functions (Elmer et al., 2009; Javadi and Walsh, 2012; Trémolière et al., 2018; Angius et al., 2019). We also completed a computer simulation analysis of the likely current density that would result from the chosen montage (Figure 1) using commercially available software (HD Explore; Soterix Medical, Woodbridge NJ, United States).
Soterix EASYPads, doubled sponges with dimensions of 5 cm × 5 cm (Soterix Medical: New York) were used as electrodes. Approximately 7 cc of sterile saline was used to moisten them. They were positioned by the researcher and then fixed in place with a head band. Current for the tDCS intervention was supplied with an iontophoresis device (ActivaDose II; Gilroy, CA: Activatek). Flat rubberized carbon electrodes were inserted into the moistened sponges. Impedances were assessed before each session and kept below 20K ohms prior to stimulation.
We told people in the study that they might experience minor discomfort at the beginning of the session and that the experience might persist or fade away during the intervention (Brunoni et al., 2013). We then asked the persons in the study to pay attention to the computer while the game was set up and the tDCS intervention was begun. Individuals in the active tDCS group received a current of 1.5 mA, ramping up over 30 s and continuing for 20 min. Individuals in the sham tDCS group and the control group received the ramping up current for 30 s which was then ramped down over 30 s.
The cognitive training intervention in this study was a commercially available car racing game GT Racing 2 (Gameloft SE: Paris, France). This game was chosen because it was easy for most persons and was positively reviewed by a large number of users. We inferred from these characteristics that the participants in the study would find the content tolerable and might even enjoy playing the game. The game includes a several different race courses and types of races to enhance player interest. Game play required that each participant complete each course before moving on to the next. Everyone in the study randomized to CCT was able to complete at least the first four courses during six training sessions.
We encouraged participants to complete each gaming session at their desired pace, although the game imposed some restrictions on their progress. Players had to finish each race in one of the top three places, or navigate a course within a predetermined time, before moving on to the next course. We required as well that participants do each course a minimum of five times. Persons in the study completed six training sessions over a 2-week period. After each intervention session, participants were asked to provide ratings of their thinking, their mood, and how much discomfort they had experienced during the intervention.
Assessments and intervention sessions were completed within 3 weeks from the baseline evaluation. After participants finished the sixth intervention session, they returned to complete cognitive and self-report assessment. Cognitive evaluations were completed by staff who did not know the participant’s intervention group assignment. About 30 days after completing the intervention sessions and follow-up evaluations, persons in the study were asked to return to again complete assessments. All data were collected in the General Clinical Research Center at the Center for Collaborative Research on the campus of Nova Southeastern University beginning in January 2018 and ending in November 2019. The study was concluded at the end of the period of funding support.
Human Subjects Approval and Trial Registration
Study procedures were approved by the Institutional Review Board of Nova Southeastern University (protocol number 2017-410). This study was registered on ClinicalTrials.gov (NCT03440840).
Data Analyses
Planned sample size was determined prior to beginning the study using the mixed effects model simulation routine in PASS 16 (Hintze, 2018). The power analysis showed that a final sample size of 90 (30 per treatment group) would have a power of 0.88 to detect interactions of group membership with time (number of evaluations) with a small effect size (Cohen, 1988; Brydges, 2019).
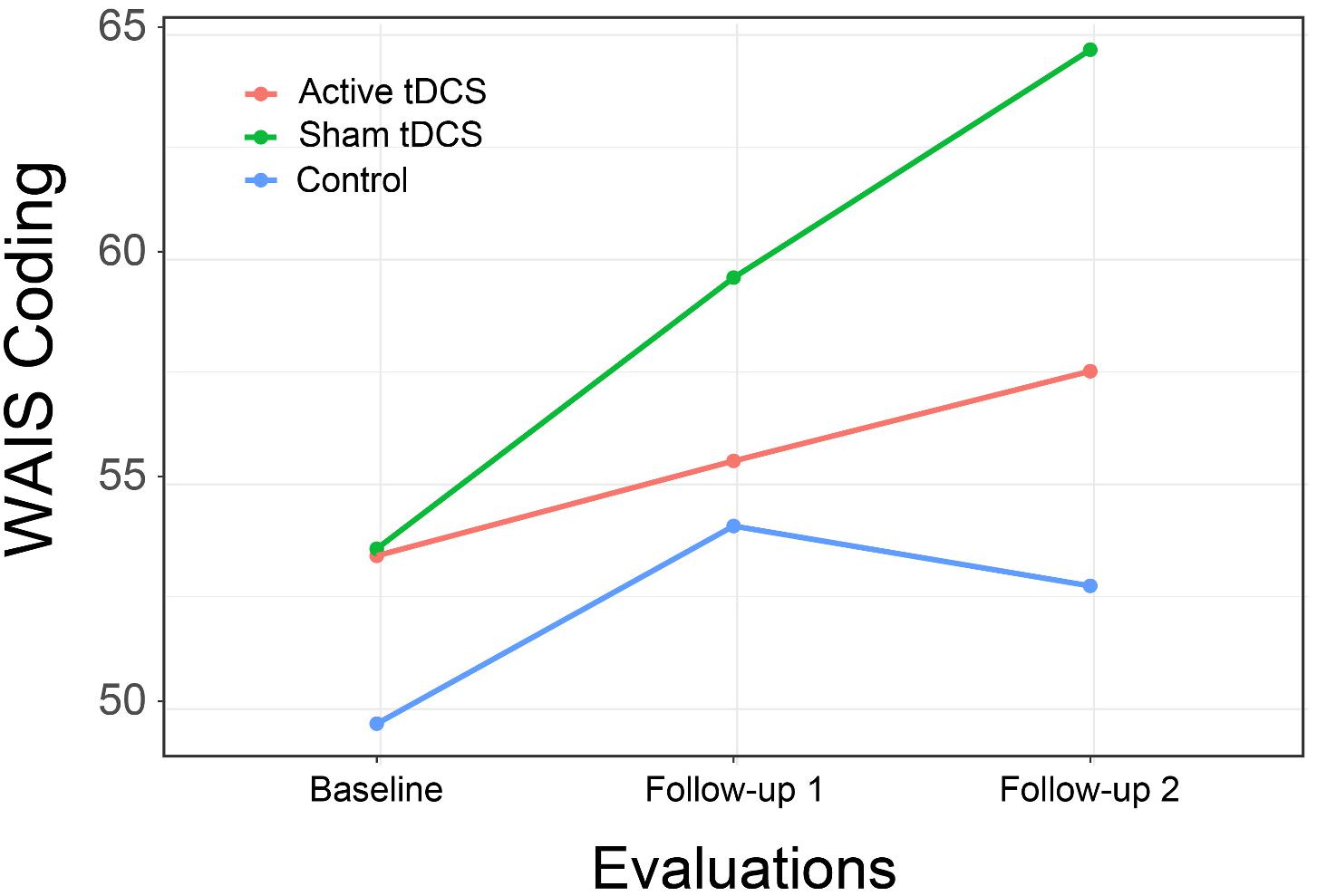
Data analyses were completed in several steps. Preliminary analyses of data and descriptive statistics were obtained using SPSS version 26 (Armonk NY: IBM). Chi-square and one-way ANOVA tests evaluating the relations of participant ratings of the acceptability and feasibility of the intervention to group assignment were also completed in SPSS. Analyses of treatment effects were completed using R version 4.0.2 (R Core Team, 2020) package lme4 (Bates et al., 2015) for mixed effects models. Significance of model effects (interaction of treatment group assignment with time) was assessed using the likelihood ratio test (Kuznetsova et al., 2017). We evaluated outcomes both through tests of statistical significance as well as approximations of effect size from χ2-values from likelihood ratio tests and t-values obtained in tests of between-group differences obtained using emmeans (Lenth, 2020). Effect sizes were converted to the more familiar d statistic using the package esc (Ludecke, 2019). As a post hoc assessment not included in the original study protocol the probability of finding the observed number of treatment effects in the hypothesized direction was evaluated using the exact binomial test in the package stats.
Results
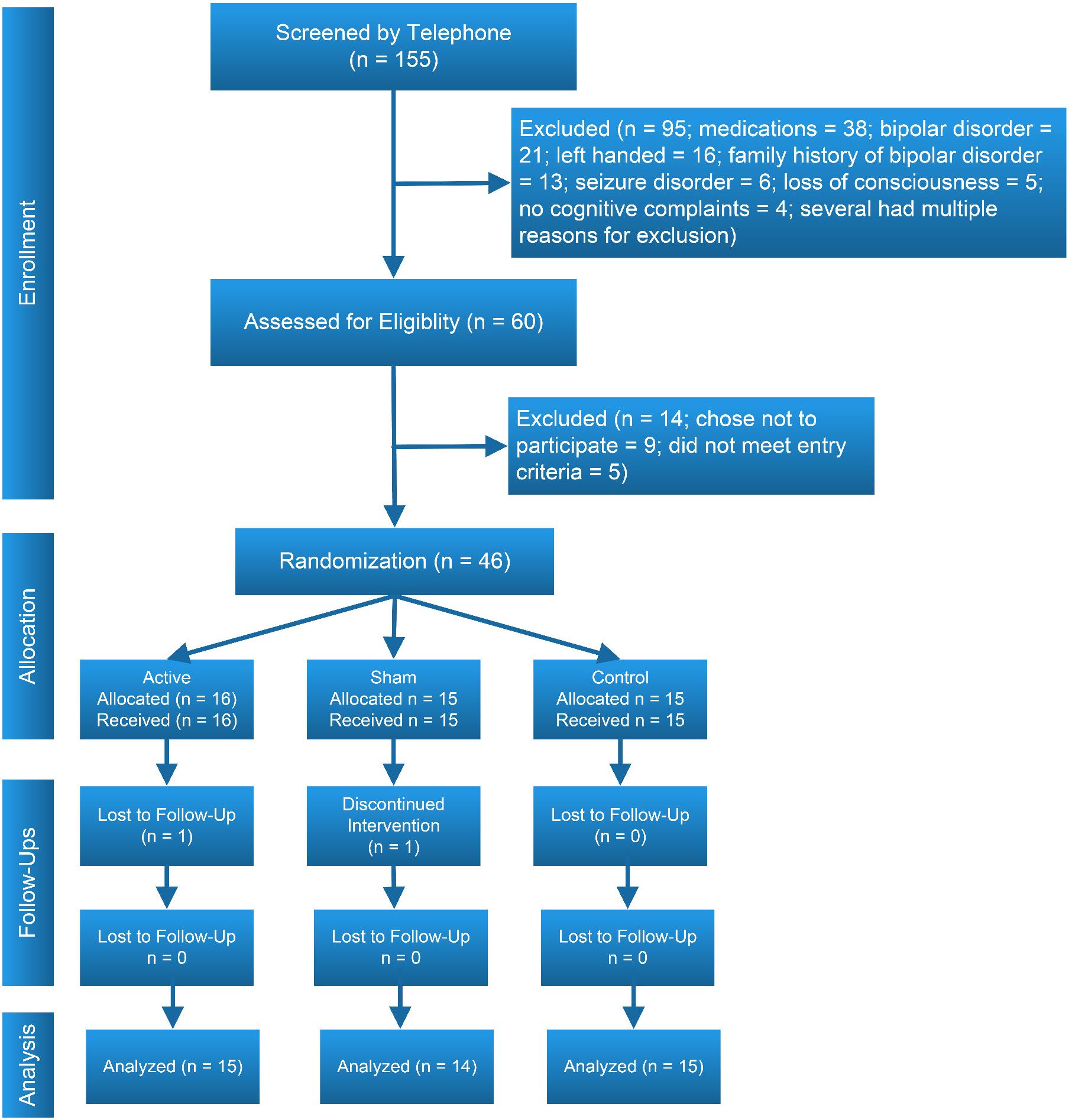
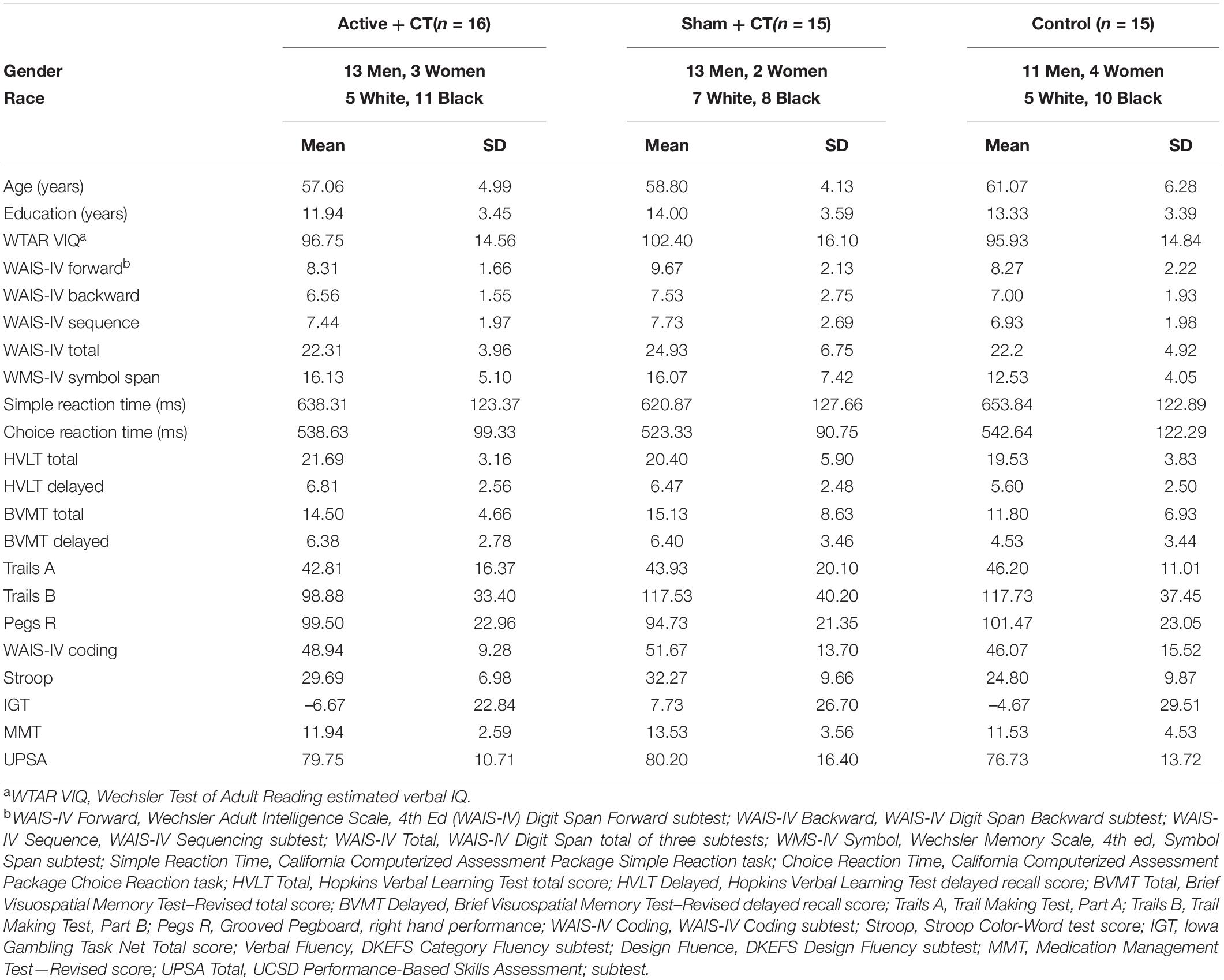
The study’s CONSORT diagram (Moher, 1998) is presented in Figure 2. Demographic, educational and baseline scores for cognitive and functional measures for participants by treatment group are presented in Table 2. We screened 155 potential participants, and included 46 in the study. Reasons for excluding potential participants are listed in Figure 2. The most frequent reasons for exclusion were use of psychotropic medications, and a personal or family history of bipolar disorder. We thus were not able to include the full number of participants in the study as originally envisioned (planned N = 30 per group).
We explored relations of relevant covariates (age, gender, education, immune status) to mental ability variables with standard measures of association (correlations). As several of these associations were substantial and likely to create confounding relations, they were included as covariates in mixed effects random intercept models. Outcomes assessed were changes in test scores across treatment groups before and after the study intervention.
Acceptability to Participants
As hypothesized, participants rated the intervention significantly more positively than the midpoint of the Usefulness subscale of the TAM scale based on a composite of Likert-type items. The mean rating for all participants was 4.33 (SD = 1.27; scale range 0 “strongly disagree” to 6 “strongly agree), and this rating was significantly greater than the neutral midpoint [t(40) = 6.71, p < 0.001]. We also found that they rated its ease of use positively with a mean rating of 5.04 (SD = 0.95), ratings that were again significantly greater than the scale midpoint [t(43) = 5.04, p < 0.001]. Ratings suggested that overall, they enjoyed the intervention (mean = 4.73; SD = 1.30) and would use it again if given the opportunity (mean = 4.62; SD = 1.25). Participants’ ratings did not vary by treatment group (all ps > 0.35).
On the scale which asked participants to assess the intervention’s risks and benefits, participant ratings again suggested a positive evaluation, with a mean rating of 4.53 (SD = 1.20; scale range 0 “extremely dissatisfied” to 6 “extremely satisfied”) on the item “overall the good outweighs the bad” and a rating of 4.70 (SD = 1.01) on the item “overall satisfaction.” Forty-three of 46 participants (93%) indicated they were satisfied with the intervention.
Participant comments, made spontaneously during or immediately after the training sessions, were consistently positive, for example, “That was fun,” “that was great,” “I really enjoyed that,” and “I have to get me one of these.”
Cognitive and Functional Outcomes
Results of evaluations of study outcomes assessed as the interactions of intervention group across evaluations are available in Table 3.
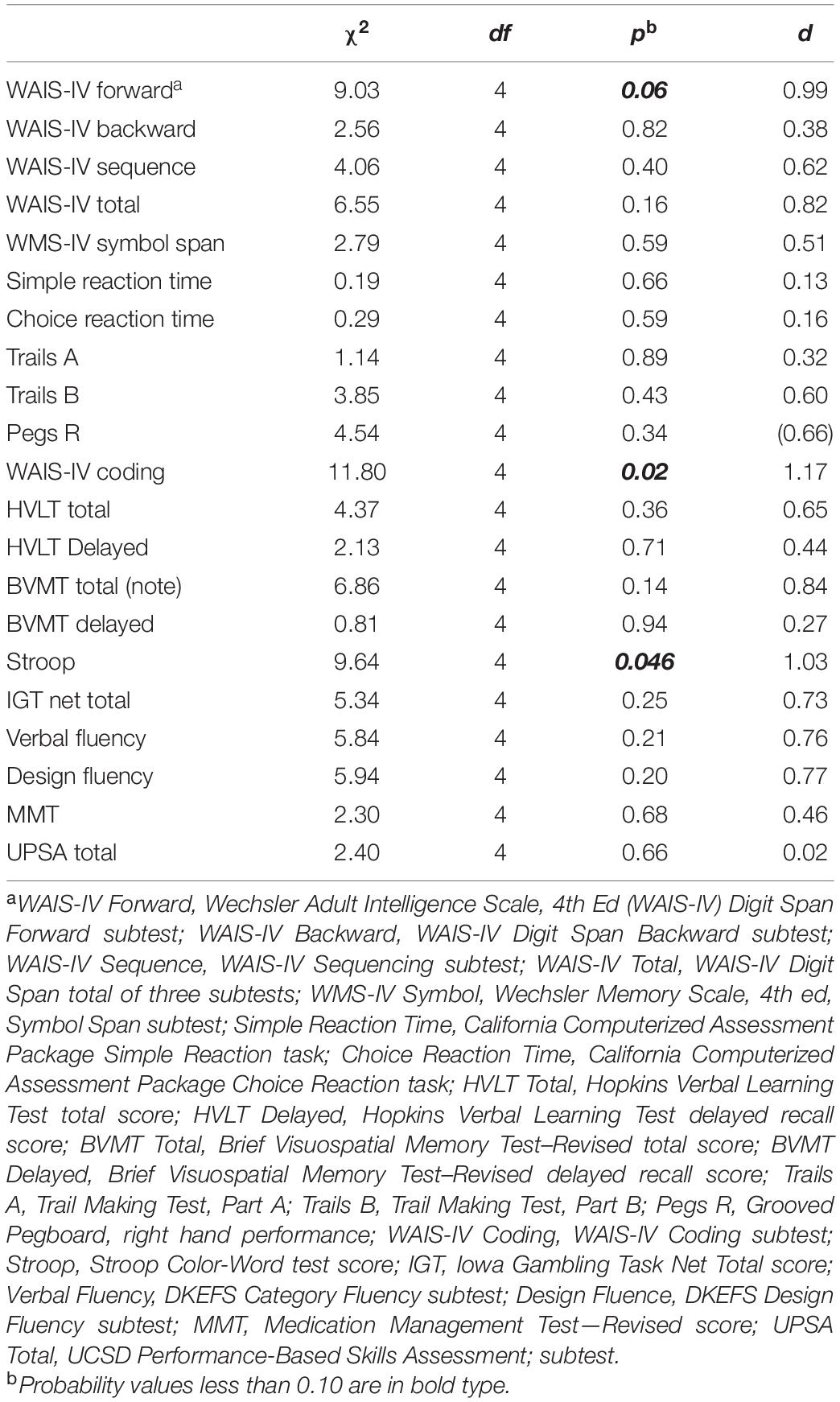
We found mixed evidence across cognitive domains to support the hypothesis that CCT with and without tDCS might result in improvements in cognitive functioning relative to control. For the Digit Span Forward subtest, the interaction of treatment group by time approached statistical significance and represented a large effect size. The difference between active tDCS and the control group (Figure 3) also approached statistical significance [t(61.3) = 2.19, p = 0.08; d = 0.79]. Other subtests hypothesized to assess attention showed similar positive but non-significant interactions.
Results did not support the hypothesis that persons receiving CCT with or without tDCS would show improved reaction time. Although the observed interactions were in the hypothesized direction, they were not significant and represented at best a very small effect size.
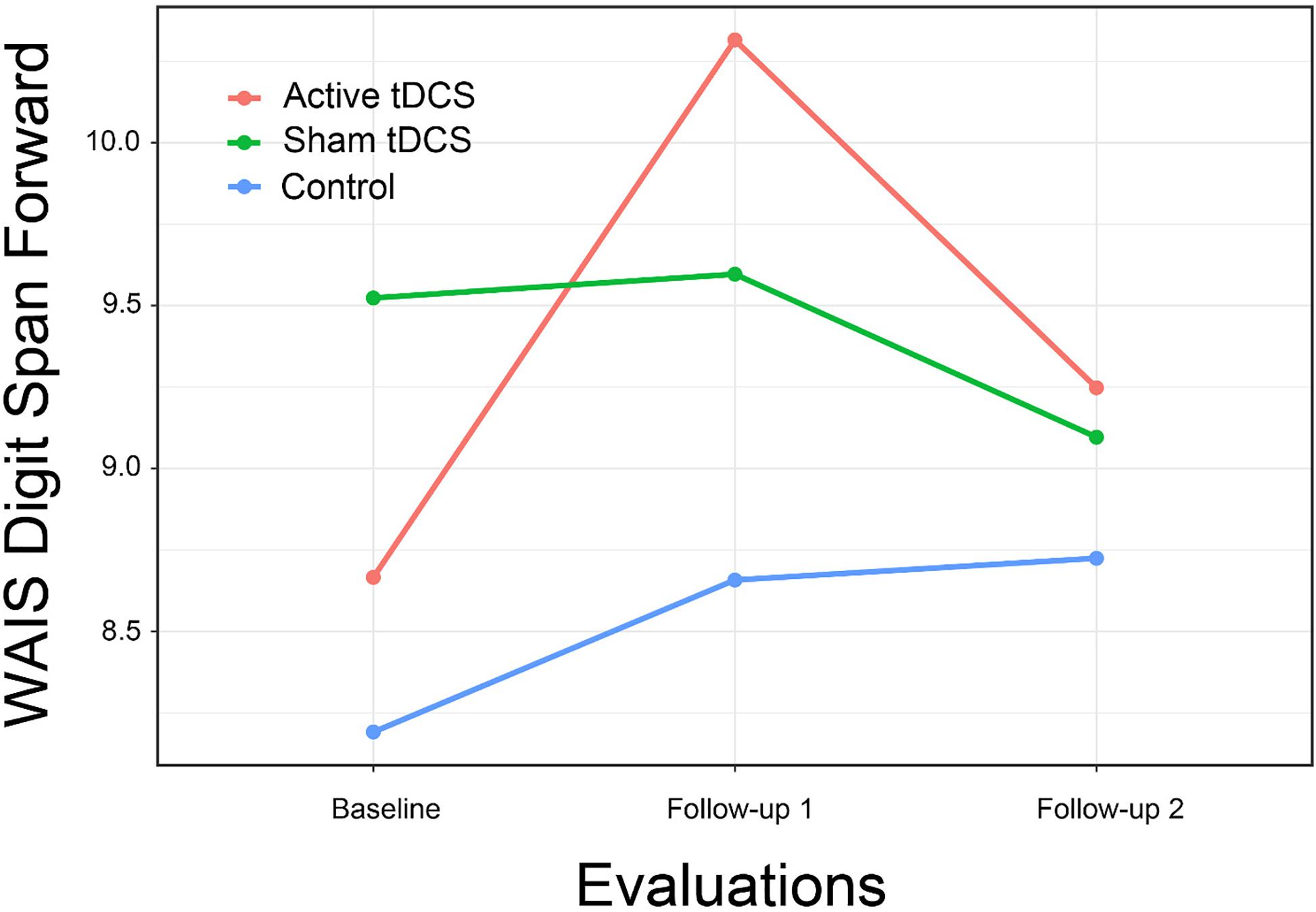
Results again provided limited support for the hypothesis that CCT with tDCS might result in improved psychomotor speed compared to controls (Figure 4). Although the overall interaction of group by time was not significant for Trails B, the comparison of the active treatment group to control approached significance [t(67.2) = 2.37, p = 0.053; d = 0.85], a difference that became significant at second follow-up [t(67.2) = 3.03, p = 0.01; d = 1.09].
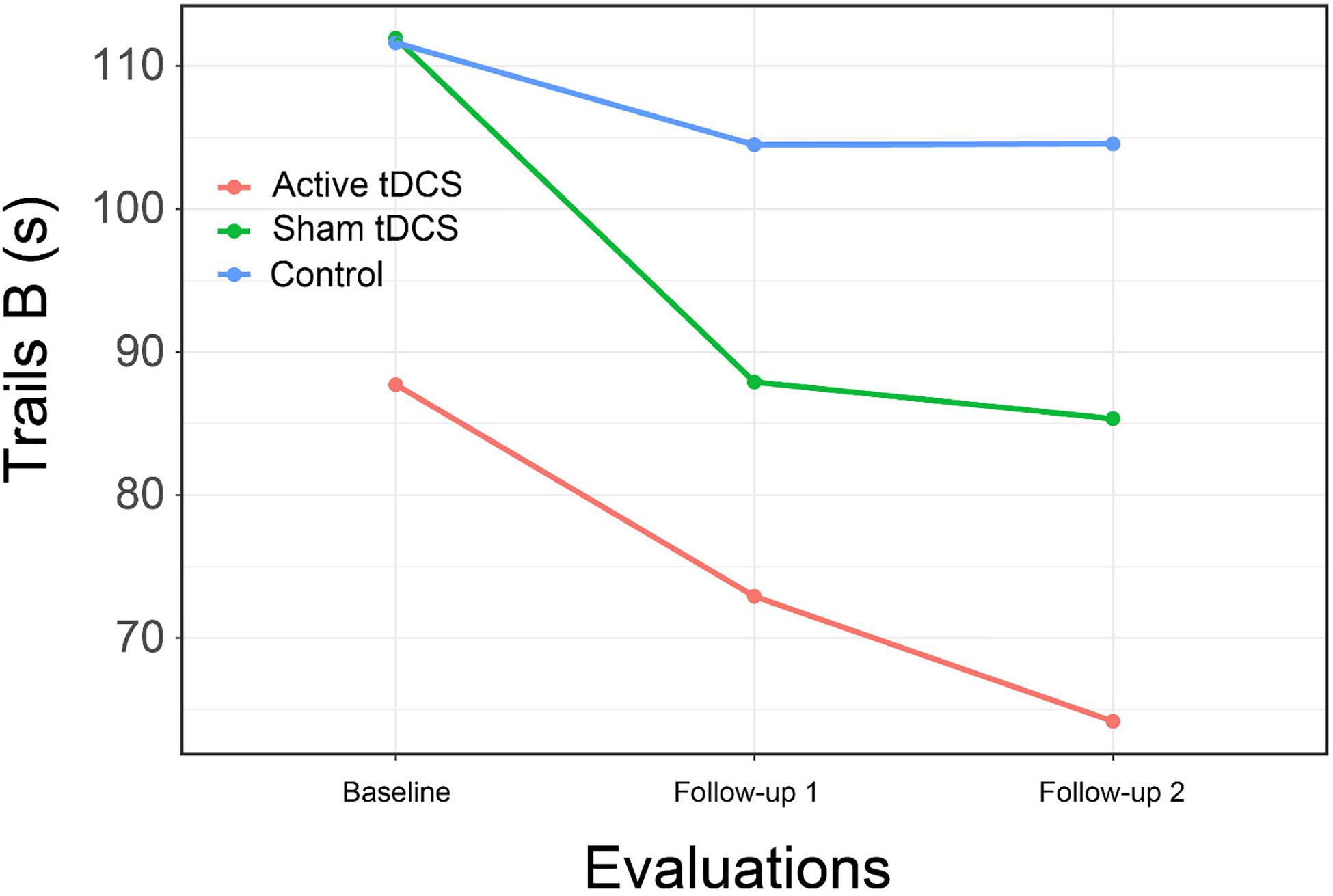
We found support for the hypothesis of improvement in psychomotor speed with a significant interaction of group by time for the WAIS-IV Coding subtest, although examination of the interaction plot suggests the effect was primarily due to the performance of persons in the CCT with sham tDCS (Figure 5). The between group difference from CCT + sham was not significant at immediate follow-up [t(45.6) = 1.02, p = 0.57; d = 0.37] but approached significance at 1-month follow-up [t(45.6) = 2.21, p = 0.08; d = 0.79].
This pattern was again found in results for the HVLT total score, with a significant interaction of group by time resulting from differential improvement in both treatment groups, with significant difference between the CCT + active tDCS group and control at immediate follow-up [t(69.6) = 2.51, p = 0.04; d = 0.90] and a substantial but no longer significant difference at 1-month follow-up [t(69.6) = 2.05, p = 0.11; d = 0.73; Figure 6].
Although we did not pose specific hypotheses for the effects of CCT with or without tDCS on executive function for this study, in exploratory analyses we evaluated the intervention’s effects on this domain with several measures. A significant interaction of group by time was found for the Stroop with a large effect size, but as with several other measures, the finding was related to substantial improvement in the CCT with sham stimulation group. Other interactions were again positive but non-significant, with effect sizes in the moderate range.
As with executive function, we did not propose specific hypotheses for the two functional measures included in the assessments, but explored the intervention’s effect on them. Results suggest a moderate but non-significant effect size for the MMT with a negligible effect size for the UPSA.
Given the large number of outcomes and our use of effect sizes to evaluate treatment effects, in an unplanned post hoc analysis, we evaluated the overall impact of the study intervention based on the number of effect sizes for cognitive variables that were in the hypothesized direction (showing an interaction of group by time favoring one of the active treatment groups). The average effect size was 0.52, with 17 effects equal to or greater than 0.16, a small effect size (Brydges, 2019). The probability of this outcome compared to chance (equal distribution of positive effects across all groups) was significantly different (p = 0.04). It is thus improbable that the observed effects of the intervention were only due to chance. It should be acknowledged, that this analysis included positive effects for either treatment group and did not support the original hypothesis that the effects of CCT with active tDCS would be superior to CCT with sham tDCS.
Self-Report Outcomes
Evaluations of treatment by time interactions of participant self-report on the PAOF subscales [PAOF Memory, χ2 (4) = 1.86, p = 0.76, d = 0.41; Cognition subscale, χ2(4) = 0.37, p = 0.98, d = 0.18] and the CESD [χ2(4) = 3.38, p = 0.50, d = 0.56] did not suggest the existence of between-group differences in response to the intervention. Examination of the interaction plots for the PAOF subscales (not presented) showed that mean scores for all three groups improved to a similar extent over the three evaluations. Participants reported similar levels of depression (CESD) across groups and evaluations, except participants in the active treatment group reported better mood at the 1-month follow-up evaluation. Although the test of between groups differences were not significant, the within-group change from the immediate follow-up to the 1-month follow-up for the CCT with active tDCS group approached significance and represented a large effect size [t(78.2) = 2.17, p = 0.08, d = 0.78].
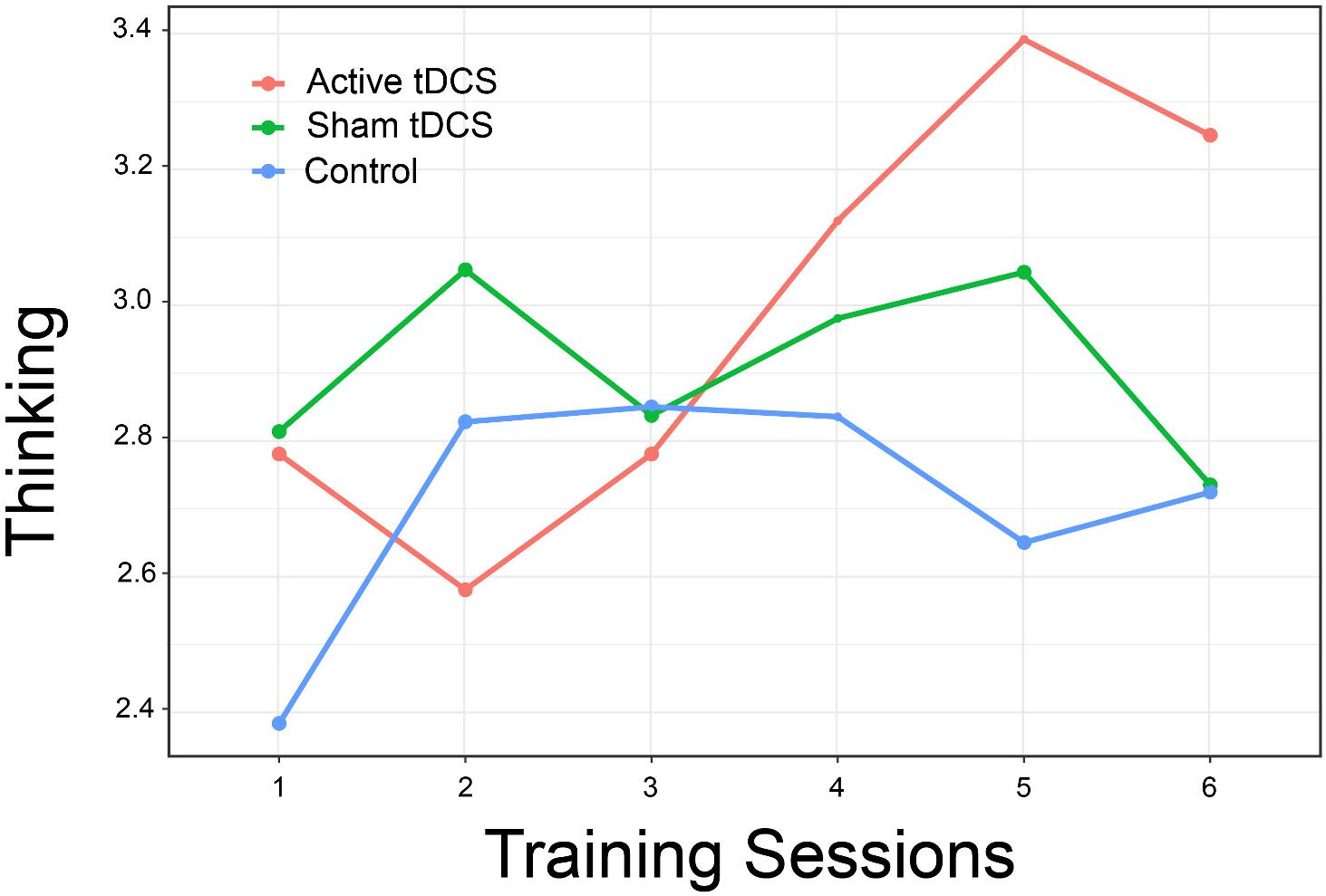
Evaluation of participant ratings of how well they could think and remember resulted in an interaction that again approached statistical significance [χ2(10) = 17.47, p = 0.06, d = 1.59]. Examination of the interaction plot (Figure 7) suggests that persons in the active treatment group reported better ability to think over the final three training sessions, although between-group differences were not statistically significant [t(106.8) = 1.57, p = 0.26, d = 0.56].
Discussion
This study’s goals were to assess the acceptability of CCT using a racing game to persons 50 years of age and older with HAND. The impact of adding tDCS to CCT was also evaluated. As we had a smaller than expected sample, we assessed outcomes not only through tests of statistical significance but also through calculation of effect sizes. Results showed that the majority of participants had positive views of the intervention and believed its benefits outweighed any risks. Ratings of its usefulness and usability were positive.
Our results clearly show that participants found the intervention acceptable. The majority of ratings on factors such as usefulness and ease of use were positive, and nearly all participants indicated that they felt the benefits of the intervention outweighed any drawbacks. Further exploration of ways to make the intervention even more positive for persons with HAND appear warranted.
Assessment of mental abilities before and after training suggested that the intervention had a positive effect on learning, memory, and motor speed compared to control. Although only a few outcomes were statistically significant or approached it, those which were significant were associated with large effect sizes. Outcomes with moderate or smaller effect sizes may thus have been non-significant due to low power related to the sample size rather than a lack of effect. A post hoc evaluation of the probability of arriving at the observed set of effect sizes by chance suggested that our findings were not due to chance. Further, effect sizes obtained in this study are similar to those reported by other researchers, including in a meta-analysis of studies with older adults (Indahlastari et al., 2021).
Consistent with other research, the combination of cognitive training and anodal tDCS at the left dorsolateral prefrontal cortex was associated with improvement in attention as measured by digit span (Martin et al., 2013; Park et al., 2014; Santos et al., 2018). Contrary to our hypothesis, however, there was no evidence of a substantial impact of the intervention on reaction time. Others have reported that tDCS may have a facilitating effect on reaction time with anodal stimulation over primary motor cortex, but Molero-Chamizo et al. (2018) found that the effect was time dependent. Others have also failed to find an effect of tDCS (Coppens et al., 2019; Seidel and Ragert, 2019) on reaction time. Our hypothesis of an effect on reaction time was based primarily on the nature of the training task (a fast-paced computer game), but neither statistical tests nor inspection of mean plots by groups (not presented) suggested an effect for either of the active treatment groups.
We found more substantial improvements in measures of psychomotor speed, including a significant interaction of group by time for the WAIS-IV Coding subtest. Although the overall interaction for Trails B was not significant, inspection of group mean plots was consistent with relatively greater improvement on this measure for the two active treatment groups, and post hoc between groups tests showed a significant difference between the control and CCT + active tDCS group after treatment while none was found at baseline.
A significant effect was also found for the Stroop Color-Word Test, a measure often interpreted as assessing a person’s ability to inhibit well-learned responses (Scarpina and Tagini, 2017). Others have observed a positive effect of tDCS on the Stroop task (Loftus et al., 2015; Lu et al., 2021; Perrotta et al., 2021), while Frings et al. (2018) found that cathodal stimulation of the left DLPFC disrupted Stroop performance, with the negative effect of cathodal vs. anodal stimulation expected.
We found limited support for the impact of the intervention on functional measures, with a moderate effect size but non-significant results for the Medication Management Test but no evidence of an effect on the UPSA. This is similar results reported by Vance et al. (2021) who found limited effects of cognitive training on various measures of everyday function.
Self-report measures of mood or subjective cognitive functioning did not differ between groups, with one exception. The failure to find group differences may have been related to self-reported improvements across all groups, including the control. The one exception were self-reports of how well the participant perceived their thinking and memory, for which the CCT + active tDCS group gave substantially more positive reports over the last several sessions of the intervention.
The finding of possible treatment effects later during the training period suggests that the intervention’s effects may have continued after training ended, with continuing improvements at 1-month follow-up in psychomotor speed (Trail Making Test, Part B; Figure 3) and verbal learning (Hopkins Verbal Learning Test; Figure 5). We also found an improvement in depression self-report (Center for Epidemiological Studies—Depression) at 1-month follow-up. This possibly delayed effect of tDCS on mood was also reported by Li et al. (2019). Given the evidence for improvement during the second week of training, it is possible that a more intensive and longer intervention might have resulted in greater effects. This possibility should be explored in future studies.
Strengths of the study include the single-blind sham-controlled design, which was effective in our pilot study as it has been in other studies (Woods et al., 2016). We collected all data using a staff member who did not know the participant’s intervention assignment or by way of a computer. These measures reduced the likelihood of bias in outcomes due to experimenter effects. The characteristics of participants (age, gender, education level, cognitive function) made them similar to other persons who might have HAND and be able to benefit from the intervention. Another strength is the clear characterization of study participants with respect to concurrent medication use, although this in turn limited our ability to recruit participants.
This study’s limitations include the smaller than planned sample size. In spite of intensive recruiting efforts in the local community, including newspaper and online advertising, multiple contacts with community organizations and local infectious disease practitioners, and contacts with participants in previous studies we were not able to recruit the planned number of participants for the study. As shown in Figure 1, we were able to contact a number of potential participants that might have been adequate for planned sample size for the study, but exclusion due to validity concerns related to psychotropic medication use and safety concerns related to history of bipolar disorder, a large number of potential participants were not eligible. This limitation in turn may have affected the ability to test the statistical significance of outcomes, although in several cases when we found large effect sizes, we also found statistically significant results. Generalizing our results based only on observed effect sizes is a limitation. While the electrode montage used was chosen to stimulate the left dorsolateral prefrontal cortex via the anode, it should be acknowledged that the right frontal pole was also stimulated via the cathode (Friehs et al., 2021). Finally, it should be acknowledged that while we found a number of positive effects on cognitive measures in the two CCT groups, the original hypothesis that CCT with active tDCS would be superior to CCT with sham tDCS was not supported.
Another limitation is that this study did not employ a double-blind controlled design, raising the possibility of bias caused by the investigators. Further, it should also be noted that the timing of stimulation (concurrent with or prior to the cognitive task) may have affected study outcome. For example, Friehs and Frings (2019) showed that offline (not concurrent) stimulation of the left dorsolateral prefrontal cortex as associated with a greater impact on working memory (assessed as performance on the n-back task). In contrast, Martin et al. (2014) showed that concurrent stimulation was associated with skill acquisition.
As noted above, we did several things to reduce the possibility of effects related to the unblinded experimenter. These included positioning the investigator and the tDCS device out of sight of the participant during stimulation, providing neutral suggestions about what the participants might experience during stimulation, and collecting most self-report data via computer assisted self-interview with the investigators out of the room. All baseline and follow-up assessments were conducted by an assessor blind to the participant’s treatment assignment or by way of a computer without the presence of a researcher. Thus, while we took a number of steps to reduce possible bias in the research design, they did not eliminate it.
HIV-related mental ability problems have implications for the functional status and quality of life for older persons with HAND. Our results, though limited, demonstrate the possibility that CCT with or without tDCS may have a positive impact on cognitive function. We found evidence of a moderate though non-significant effect of the intervention on a test of medication taking, a critically important skill for persons with HIV infection.
Future research should focus on continuing to explore the potential efficacy of CCT and tDCS with this population. A more detailed exploration of factors such as intensity and duration of stimulation and length and frequency of training sessions as well as the optimal timing of follow-up assessments may yield more effective treatment protocols with greater impacts on participants’ functional status.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of Nova Southeastern University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
RO designed the study, obtained external funding, carried out the interventions, and wrote the draft of the manuscript. JK wrote a portion of the manuscript and reviewed the complete manuscript. Both authors contributed to the article and approved the submitted version.
Funding
This work was supported by a grant from the US National Institutes on Aging to RO (grant R21AG056256).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Acharya, J. N., Hani, A., Cheek, J., Thirumala, P., and Tsuchida, T. N. (2016). American Clinical Neurophysiology Society guideline 2: guidelines for standard electrode position nomenclature. J. Clin. Neurophysiol. 33, 308–311. doi: 10.1097/wnp.0000000000000316
Albert, S. M., Weber, C. M., Todak, G., Polanco, C., Clouse, R., McElhiney, M., et al. (1999). An observed performance test of medication management ability in HIV: Relation to neuropsychological status and medication adherence outcomes. AIDS Behav. 3, 121–128.
Alexandrovsky, D., Friehs, M. A., Grittner, J., Putze, S., Birk, M. V., Malaka, R., et al. (2021). Serious snacking: A survival analysis of how snacking mechanics affect attrition in a mobile serious game. CHI 2021 Conference on Human Factors in Computing Systems: Making Waves, Combining Strengths; Virtual Online, Japan. New York, NY: Association for Computing Machinery, 1–18.
Alexandrovsky, D., Friesh, M. A., Birk, M. V., Yates, R. K., and Mandryk, R. L. (2019). Game dynamics that support snacking, not feasting. 6th ACM SIGCHI Annual Symposium on Computer-Human Interaction in Play, CHI PLAY 2019. Barcelona: Association for Computing Machinery, 573–588.
Angius, L., Santarnecchi, E., Pascual-Leone, A., and Marcora, S. M. (2019). Transcranial direct current stimulation over the left dorsolateral prefrontal cortex improves inhibitory control and endurance performance in healthy individuals. Neuroscience 419, 34–45. doi: 10.1016/j.neuroscience.2019.08.052
Anguera, J. A., Boccanfuso, J., Rintoul, J. L., Al-Hashimi, O., Faraji, F., Janowich, J., et al. (2013). Video game training enhances cognitive control in older adults. Nature 501, 97–101. doi: 10.1038/nature12486
Antinori, A., Arendt, G., Becker, J. T., Brew, B. J., Byrd, D. A., Cherner, M., et al. (2007). Updated research nosology for HIV-associated neurocognitive disorders. Neurology 69, 1789–1799.
Atkinson, M. J., Kumar, R., Cappelleri, J. C., and Hass, S. L. (2005). Hierarchical construct validity of the treatment satisfaction questionnaire for medication (TSQM version II) among outpatient pharmacy consumers. Value Health 8(Suppl. 1), S9–S24. doi: 10.1111/j.1524-4733.2005.00066.x
Atkinson, M. J., Sinha, A., Hass, S. L., Colman, S. S., Kumar, R. N., Brod, M., et al. (2004). Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual. Life Outcomes 2:12. doi: 10.1186/1477-7525-2-12
Avdoshina, V., Bachis, A., and Mocchetti, I. (2013). Synaptic dysfunction in human immunodeficiency virus type-1-positive subjects: inflammation or impaired neuronal plasticity? J. Intern. Med. 273, 454–465. doi: 10.1111/joim.12050
Bachis, A., Avdoshina, V., Zecca, L., Parsadanian, M., and Mocchetti, I. (2012). Human immunodeficiency virus type 1 alters brain-derived neurotrophic factor processing in neurons. J. Neurosci. 32, 9477–9484. doi: 10.1523/jneurosci.0865-12.2012
Basak, C., Boot, W. R., Voss, M. W., and Kramer, A. F. (2008). Can training in a real-time strategy video game attenuate cognitive decline in older adults? Psychol. Aging. 23, 765–777. doi: 10.1037/a0013494
Bates, D., Maechler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48.
Bavelier, D., and Green, C. S. (2016). The brain-boosting power of video games. Sci. Am. 315, 26–31. doi: 10.1038/scientificamerican0716-26
Bechara, A., Damasio, H., Tranel, D., and Damasio, A. R. (2005). The Iowa Gambling Task and the somatic marker hypothesis: some questions and answers. Trends Cogn. Sci. 9, 159–162. doi: 10.1016/j.tics.2005.02.002
Belchior, P., Marsiske, M., Leite, W. L., Yam, A., Thomas, K., and Mann, W. (2016). Older adults’ engagement during an intervention involving off-the-shelf videogame. Games Health J. 5, 151–156. doi: 10.1089/g4h.2015.0049
Belchior, P., Marsiske, M., Sisco, S., Yam, A., and Mann, W. (2012). Older adults’ engagement with a video game training program. Act. Adapt. Aging. 36, 269–279. doi: 10.1080/01924788.2012.702307
Benedict, R. B., Schretlen, D., Groninger, L., Dobraski, M., and Sphritz, B. (1996). Revision of the Brief Visuospatial Memory Test: Studies of normal performance, reliability and validity. Psycholog. Assess. 8, 145–153. doi: 10.1080/13854046.2017.1288270
Binyamin, S. S., and Zafar, B. A. (2021). Proposing a mobile apps acceptance model for users in the health area: A systematic literature review and meta-analysis. Health Inform. J. 27:1460458220976737. doi: 10.1177/1460458220976737
Birk, M. V., Friehs, M. A., and Mandryk, R. L. (2017). Age-based preferences and player experience: A crowdsources cross-sectional study. CHI Play ’17: The annual symposium on computer-human interaction in paly. Amsterdam: Association for Computing Machinery, 157–170.
Bonnechère, B., Langley, C., and Sahakian, B. J. (2020). The use of commercial computerised cognitive games in older adults: a meta-analysis. Sci. Rep. 10:15276. doi: 10.1038/s41598-020-72281-3
Brandt, J., and Benedickt, R. H. (2001). Hopkins Verbal Learning Test–Revised: Professional manual. Odessa, FL: Psychological Assessment Resources.
Brosnan, M. B., and Wiegand, I. (2017). The dorsolateral prefrontal cortex, a dynamic cortical area to enhance top-down attentional control. J. Neurosci. 37, 3445–3446. doi: 10.1523/JNEUROSCI.0136-17.2017
Brunoni, A. R., Valiengo, L., Baccaro, A., Zanao, T. A., de Oliveira, J. F., Goulart, A., et al. (2013). The sertraline vs. electrical current therapy for treating depression clinical study: results from a factorial, randomized, controlled trial. JAMA Psychiatry 70, 383–391. doi: 10.1001/2013.jamapsychiatry.32
Brydges, C. R. (2019). Effect size guidelines, sample size calculations, and statistical power in gerontology. Innov. Aging 3:4. doi: 10.1093/geroni/igz036
Buchman, A. S., Yu, L., Boyle, P. A., Schneider, J. A., De Jager, P. L., and Bennett, D. A. (2016). Higher brain BDNF gene expression is associated with slower cognitive decline in older adults. Neurology 86, 735–741. doi: 10.1212/WNL.0000000000002387
Cardoso-Leite, P., and Bavelier, D. (2014). Video game play, attention, and learning: how to shape the development of attention and influence learning? Curr. Opin. Neurol. 27, 185–191. doi: 10.1097/wco.0000000000000077
Cerruti, C., and Schlaug, G. (2009). Anodal transcranial direct current stimulation of the prefrontal cortex enhances complex verbal associative thought. J. Cogn. Neurosci. 21, 1980–1987. doi: 10.1162/jocn.2008.21143
Chelune, G. J., Heaton, R. K., and Lehman, R. A. W. (1986). “Neuropsychological and personality correlates of patients’ complaints of disability,” in Advances in clinical neuropsychology, Vol. 3, eds G. Goldstein and R. E. Tarter (New York, NY: Springer), 95–126. doi: 10.1007/978-1-4613-2211-5_4
Clark, V. P., Coffman, B. A., Mayer, A. R., Weisend, M. P., Lane, T. D., Calhoun, V. D., et al. (2012). TDCS guided using fMRI significantly accelerates learning to identify concealed objects. Neuroimage 59, 117–128. doi: 10.1016/j.neuroimage.2010.11.036
Cody, S. L., and Vance, D. E. (2016). The neurobiology of HIV and its impact on cognitive reserve: A review of cognitive interventions for an aging population. Neurobiol. Dis. 92, 144–156. doi: 10.1016/j.nbd.2016.01.011
Coffman, B. A., Trumbo, M. C., and Clark, V. P. (2012). Enhancement of object detection with transcranial direct current stimulation is associated with increased attention. BMC Neurosci. 13:108.
Cohen, J. (1988). Statistical power analysis for the behavioral sciences, 2nd Edn. New York, NY: Routledge.
Coppens, M. J. M., Staring, W. H. A., Nonnekes, J., Geurts, A. C. H., and Weerdesteyn, V. (2019). Offline effects of transcranial direct current stimulation on reaction times of lower extremity movements in people after stroke: a pilot cross-over study. J. NeuroEng. Rehab. 16:136. doi: 10.1186/s12984-019-0604-y
Decloedt, E. H., Freeman, C., Howells, F., Casson-Crook, M., Lesosky, M., Koutsilieri, E., et al. (2016). Moderate to severe HIV-associated neurocognitive impairment: A randomized placebo-controlled trial of lithium. Medicine 95:e5401. doi: 10.1097/MD.0000000000005401
Degroote, S., Vogelaers, D., and Vandijck, D. M. (2014). What determines health-related quality of life among people living with HIV: an updated review of the literature. Arch Public Health. 72:40.
Degroote, S., Vogelaers, D. P., Vermeir, P., Mariman, A., De, R. A., Van Der Gucht, B., et al. (2013). Socio-economic, behavioural, (neuro)psychological and clinical determinants of HRQoL in people living with HIV in Belgium: a pilot study. J. Int. AIDS Soc. 16:18643. doi: 10.7448/IAS.16.1.18643
Delis, D. C., Kaplan, E., and Kramer, J. H. (2001). Delis-Kaplan Executive Function System (D-KEFS). San Antonio, TX: The Psychological Corporation, 2001.
Elmer, S., Burkard, M., Renz, B., Meyer, M., and Jancke, L. (2009). Direct current induced short-term modulation of the left dorsolateral prefrontal cortex while learning auditory presented nouns. Behav. Brain Funct. 5:29. doi: 10.1186/1744-9081-5-29
European AIDS Clinical Society (2015). Guidelines 8.0. Brussels: European AIDS Clinical Society, 2015.
Floel, A., Suttorp, W., Kohl, O., Kurten, J., Lohmann, H., Breitenstein, C., et al. (2012). Non-invasive brain stimulation improves object-location learning in the elderly. Neurobiol. Aging. 33, 1682–1689. doi: 10.1016/j.neurobiolaging.2011.05.007
Fregni, F., Boggio, P. S., Nitsche, M., Bermpohl, F., Antal, A., Feredoes, E., et al. (2005). Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Exp. Brain Res. 166, 23–30. doi: 10.1007/s00221-005-2334-6
Friehs, M. A., and Frings, C. (2019). Offline beats online: transcranial direct current stimulation timing influences on working memory. Neuroreport 30, 795–799. doi: 10.1097/WNR.0000000000001272
Friehs, M. A., Frings, C., and Hartwigsen, G. (2021). Effects of single-session transcranial direct current stimulation on reactive response inhibition. Neurosci. Biobehav. Rev. 128, 749–765. doi: 10.1016/j.neubiorev.2021.07.013
Frings, C., Brinkmann, T., Friehs, M. A., and van Lipzig, T. (2018). Single session tDCS over the left DLPFC disrupts interference processing. Brain Cogn. 120, 1–7. doi: 10.1016/j.bandc.2017.11.005
Fritsch, B., Reis, J., Martinowich, K., Schambra, H. M., Ji, Y., and Cohen, L. G. (2010). Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron 2010:66. doi: 10.1016/j.neuron.2010.03.035
Galvez, V., Alonzo, A., Martin, D., Mitchell, P. B., Sachdev, P., and Loo, C. K. (2011). Hypomania induction in a patient with bipolar II disorder by transcranial direct current stimulation (tDCS). J. ECT. 27, 256–258. doi: 10.1097/YCT.0b013e3182012b89
Green, C. S., and Seitz, A. R. (2015). The impacts of video games on cognition (and how the government can guide the industry). Policy Insights Behav. Brain Sci. 2, 101–110. doi: 10.1177/2372732215601121
Heaton, R. K., Clifford, D. B., Franklin, D. R., Woods, S. P., Ake, C., Vaida, F., et al. (2010). HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 75, 2087–2096. doi: 10.1212/WNL.0b013e318200d727
Heimrath, K., Sandmann, P., Becke, A., Muller, N. G., and Zaehle, T. (2012). Behavioral and electrophysiological effects of transcranial direct current stimulation of the parietal cortex in a visuo-spatial working memory task. Front. Psychiatry 3:56. doi: 10.3389/fpsyt.2012.00056
Hinkin, C. H., Castellon, S. A., Durvasula, R. S., Hardy, D. J., Lam, M. N., Mason, K. I., et al. (2002). Medication adherence among HIV+ adults: effects of cognitive dysfunction and regimen complexity. Neurology 59, 1944–1950. doi: 10.1212/01.wnl.0000038347.48137.67
Hinkin, C. H., Castellon, S. A., Hardy, D. J., Farinpour, R., Newton, T., and Singer, E. (2001). Methylphenidate improves HIV-1-associated cognitive slowing. J. Neuropsyc. Clin. Neurosci. 13, 248–254. doi: 10.1176/jnp.13.2.248
Hinkin, C. H., Hardy, D. J., Mason, K. I., Castellon, S. A., Durvasula, R. S., Lam, M. N., et al. (2004). Medication adherence in HIV-infected adults: effect of patient age, cognitive status, and substance abuse. AIDS 18(Suppl. 1), S19–S25. doi: 10.1097/00002030-200418001-00004
Indahlastari, A., Hardcastle, C., Albizu, A., Alvarez-Alvarado, S., Boutzoukas, E. M., Evangelista, N. D., et al. (2021). A systematic review and meta-analysis of transcranial direct current stimulation to remediate age-related cognitive decline in healthy older adults. Neuropsychiatr. Dis. Treat. 17, 971–990. doi: 10.2147/NDT.S259499
Javadi, A. H., and Walsh, V. (2012). Transcranial direct current stimulation (tDCS) of the left dorsolateral prefrontal cortex modulates declarative memory. Brain Stimul. 5, 231–241. doi: 10.1016/j.brs.2011.06.007
Kuznetsova, A., Brockhoff, P. B., and Christensen, R. H. B. (2017). lmerTest package: tests in linear mixed effects models. J. Stat. Softw. 82, 1–26.
Lafayette Instrument Company (2002). Grooved Pegboard Test user instructions. Lafayette, IN: Lafayette Instrument Company.
Lawrence, B. J., Gasson, N., Bucks, R. S., Troeung, L., and Loftus, A. M. (2017). Cognitive training and noninvasive brain stimulation for cognition in Parkinson’s disease: A meta-analysis. Neurorehabil Neural. Repair. 31, 597–608. doi: 10.1177/1545968317712468
Lawrence, B. J., Gasson, N., Johnson, A. R., Booth, L., and Loftus, A. M. (2018). Cognitive training and transcranial direct current stimulation for mild cognitive impairment in Parkinson’s Disease: A randomized controlled trial. Parkinson’s Dis. 2018:4318475.
Leh, S. E., Petrides, M., and Strafella, A. P. (2010). The neural circuitry of executive functions in healthy subjects and Parkinson’s disease. Neuropsychopharmacology 35, 70–85. doi: 10.1038/npp.2009.88
Lenth, R. (2020). Estimated marginal means, aka least-squares means. 2020. p. R package version 1.5.0.
Leshikar, E. D., Leach, R. C., McCurdy, M. P., Trumbo, M. C., Sklenar, A. M., Frankenstein, A. N., et al. (2017). Transcranial direct current stimulation of dorsolateral prefrontal cortex during encoding improves recall but not recognition memory. Neuropsychologia 106, 390–397. doi: 10.1016/j.neuropsychologia.2017.10.022
Li, M.-S., Du, X.-D., Chu, H.-C., Liao, Y.-Y., Pan, W., Li, Z., et al. (2019). Delayed effect of bifrontal transcranial direct current stimulation in patients with treatment-resistant depression: a pilot study. BMC Psychiatry 19:180. doi: 10.1186/s12888-019-2119-2
Loftus, A. M., Yalcin, O., Baughman, F. D., Vanman, E. J., and Hagger, M. S. (2015). The impact of transcranial direct current stimulation on inhibitory control in young adults. Brain Behav. 5:e00332.
Lu, H., Gong, Y., Huang, P., Zhang, Y., Guo, Z., Zhu, X., et al. (2021). Effect of repeated anodal HD-tDCS on executive functions: Evidence from a pilot and single-blinded fNIRS study. Front. Hum. Neurosci. 14:609. doi: 10.3389/fnhum.2020.583730
Martin, D. M., Liu, R., Alonzo, A., Green, M., and Loo, C. K. (2014). Use of transcranial direct current stimulation (tDCS) to enhance cognitive training: effect of timing of stimulation. Exp. Brain Res. 232, 3345–3351. doi: 10.1007/s00221-014-4022-x
Martin, D. M., Liu, R., Alonzo, A., Green, M., Player, M. J., Sachdev, P., et al. (2013). Can transcranial direct current stimulation enhance outcomes from cognitive training? A randomized controlled trial in healthy participants. Int. J. Neuropsychopharmacol. 16, 1927–1936. doi: 10.1017/s1461145713000539
McLaren, M. E., Nissim, N. R., and Woods, A. J. (2018). The effects of medication use in transcranial direct current stimulation: A brief review. Brain Stimulat. Basic Transl. Clin. Res. Neuromod. 11, 52–58. doi: 10.1016/j.brs.2017.10.006
Medeiros, L. F., de Souza, I. C., Vidor, L. P., de Souza, A., Deitos, A., Volz, M. S., et al. (2012). Neurobiological effects of transcranial direct current stimulation: A review. Front. Psychiatry 3:110.
Miller, E. N. (2013). California Computerized Assessment Package Manual, 2nd ed. Palm Springs, CA: Eric N. Miller, 2013.
Moher, D. (1998). CONSORT: an evolving tool to help improve the quality of reports of randomized controlled trials. Consolidated Standards of Reporting Trials. JAMA 279, 1489–1491. doi: 10.1001/jama.279.18.1489
Molero-Chamizo, A., Alameda Bailén, J. R., Garrido Béjar, T., García López, M., Jaén Rodríguez, I., Gutiérrez Lérida, C., et al. (2018). Poststimulation time interval-dependent effects of motor cortex anodal tDCS on reaction-time task performance. Cogn. Affect. Behav. Neurosci. 18, 167–175. doi: 10.3758/s13415-018-0561-0
Moore, R. C., Fazeli, P. L., Jeste, D. V., Moore, D. J., Grant, I., and Woods, S. P. (2014). Successful cognitive aging and health-related quality of life in younger and older adults infected with HIV. AIDS Behav. 18, 1186–1197. doi: 10.1007/s10461-014-0743-x
Nakasujja, N., Miyahara, S., Evans, S., Lee, A., Musisi, S., Katabira, E., et al. (2013). Randomized trial of minocycline in the treatment of HIV-associated cognitive impairment. Neurology 80, 196–202. doi: 10.1212/wnl.0b013e31827b9121
Ownby, R. L., and Acevedo, A. (2016). A pilot study of cognitive training with and without transcranial direct current stimulation (tDCS) to improve cognition in older persons with HIV-related cognitive impairment. Neuropsychiatr. Dis. Treat. 12, 2745–2754. doi: 10.2147/NDT.S120282
Park, S.-H., Seo, J.-H., Kim, Y.-H., and Ko, M.-H. (2014). Long-term effects of transcranial direct current stimulation combined with computer-assisted cognitive training in healthy older adults. Neuroreport 2014:25. doi: 10.1097/WNR.0000000000000080
Patterson, T. L., Goldman, S., McKibbin, C. L., Hughs, T., and Jeste, D. V. U. C. S. D. (2001). Performance-Based Skills Assessment: development of a new measure of everyday functioning for severely mentally ill adults. Schizophr. Bull. 27, 235–245. doi: 10.1093/oxfordjournals.schbul.a006870
Perrotta, D., Bianco, V., Berchicci, M., Quinzi, F., and Perri, R. L. (2021). Anodal tDCS over the dorsolateral prefrontal cortex reduces Stroop errors. A comparison of different tasks and designs. Behav. Brain Res. 405:113215. doi: 10.1016/j.bbr.2021.113215
R Core Team (2020). R: A language and environment for statistical computing. Vienna: R foundation for statistical computing.
Radloff, L. S. (1977). The CES-D scale: A self-report depression scale for research in the general population. Appl. Psychol. Measur. 1, 385–481. doi: 10.1177/014662167700100306
Rourke, S. B., Halman, M. H., and Bassel, C. (1999). Neurocognitive complaints in HIV-infection and their relationship to depressive symptoms and neuropsychological functioning. J. Clin. Exp. Neuropsychol. 21, 737–756. doi: 10.1076/jcen.21.6.737.863
Sacktor, N., Miyahara, S., Deng, L., Evans, S., Schifitto, G., Cohen, B. A., et al. (2011). Minocycline treatment for HIV-associated cognitive impairment: results from a randomized trial. Neurology 77, 1135–1142. doi: 10.1212/wnl.0b013e31822f0412
Santos, V. S. D. S. D., Zortea, M., Alves, R. L., Naziazeno, C. C. D. S., Saldanha, J. S., Carvalho, S. D. C. R. D., et al. (2018). Cognitive effects of transcranial direct current stimulation combined with working memory training in fibromyalgia: a randomized clinical trial. Sci. Rep. 8:12477. doi: 10.1038/s41598-018-30127-z
Schifitto, G., Yiannoutsos, C. T., Ernst, T., Navia, B. A., Nath, A., Sacktor, N., et al. (2009). Selegiline and oxidative stress in HIV-associated cognitive impairment. Neurology 73, 1975–1981. doi: 10.1212/wnl.0b013e3181c51a48
Schifitto, G., Zhang, J., Evans, S. R., Sacktor, N., Simpson, D., Millar, L. L., et al. (2007). A multicenter trial of selegiline transdermal system for HIV-associated cognitive impairment. Neurology 69, 1314–1321. doi: 10.1212/01.wnl.0000268487.78753.0f
Seidel, O., and Ragert, P. (2019). Effects of transcranial direct current stimulation of primary motor cortex on reaction time and tapping performance: A comparison between athletes and non-athletes. Front. Hum. Neurosci. 13:103. doi: 10.3389/fnhum.2019.00103
Thames, A. D., Arentoft, A., Rivera-Mindt, M., and Hinkin, C. H. (2013). Functional disability in medication management and driving among individuals with HIV: a 1-year follow-up study. J. Clin. Exp. Neuropsychol. 35, 49–58. doi: 10.1080/13803395.2012.747596
Thames, A. D., Becker, B. W., Marcotte, T. D., Hines, L. J., Foley, J. M., Ramezani, A., et al. (2011a). Depression, cognition, and self-appraisal of functional abilities in HIV: An examination of subjective appraisal aersus objective performance. Clin. Neuropsychologist. 25, 224–243. doi: 10.1080/13854046.2010.539577
Thames, A. D., Kim, M. S., Becker, B. W., Foley, J. M., Hines, L. J., Singer, E. J., et al. (2011b). Medication and finance management among HIV-infected adults: The impact of age and cognition. J. Clin. Exp. Neuropsychol. 33, 200–209. doi: 10.1080/13803395.2010.499357
Tozzi, V., Balestra, P., Galgani, S., Murri, R., Bellagamba, R., Narciso, P., et al. (2003). Neurocognitive performance and quality of life in patients with HIV infection. AIDS Res. Hum. Retroviruses. 19, 643–652. doi: 10.1089/088922203322280856
Trémolière, B., Maheux-Caron, V., Lepage, J.-F., and Blanchette, I. (2018). tDCS stimulation of the dlPFC selectively moderates the detrimental impact of emotion on analytical reasoning. Front. Psychol. 9:68. doi: 10.3389/fpsyg.2018.00568
Trumbo, M. C., Matzen, L. E., Coffman, B. A., Hunter, M. A., Jones, A. P., Robinson, C. S. H., et al. (2016). Enhanced working memory performance via transcranial direct current stimulation: The possibility of near and far transfer. Neuropsychologia 93, 85–96. doi: 10.1016/j.neuropsychologia.2016.10.011
Valcour, V., Shikuma, C., Shiramizu, B., Watters, M., Poff, P., Selnes, O., et al. (2004). Higher frequency of dementia in older HIV-1 individuals: the Hawaii Aging with HIV-1 Cohort. Neurology 63, 822–827. doi: 10.1212/01.wnl.0000134665.58343.8d
Vance, D. E., Fazeli, P., Azuero, A., Frank, J. S., Wadley, V. G., Raper, J. L., et al. (2021). Can individualized-targeted computerized cognitive training improve everyday functioning in adults with HIV-associated neurocognitive disorder? Appl. Neuropsychol. 2021, 1–12. doi: 10.1080/23279095.2021.1906678
Vance, D. E., Fazeli, P. L., Cheatwood, J., Nicholson, W. C., Morrison, S. A., and Moneyham, L. D. (2019). Computerized cognitive training for the neurocognitive complications of HIV infection: A systematic review. J. Assoc. Nurses AIDS Care 30, 51–72. doi: 10.1097/JNC.0000000000000030
Vance, D. E., Fazeli, P. L., Ross, L. A., Wadley, V. G., and Ball, K. K. (2012). Speed of processing training with middle-age and older adults with HIV: a pilot study. J. Assoc. Nurses AIDS Care 23, 500–510. doi: 10.1016/j.jana.2012.01.005
Venkatesh, V., and Bala, H. (2008). Technology Acceptance Model 3 and a research agenda on interventions. Dec. Sci. 39, 273–315. doi: 10.1111/j.1540-5915.2008.00192.x
Venkatesh, V., Morris, M. G., Davis, G. B., and Davis, F. D. (2003). User acceptance of information technology: Toward a unified view. MIS Q. 27, 425–478. doi: 10.2307/30036540
Wechsler, D. (2001). The Wechsler Test of Adult Reading. San Antonio: The Psychological Corporation, 2001.
Wechsler, D. (2008). Manual for the Wechsler Adult Intelligence Scale–IV. San Antonio, TX: Pearson Assessment.
Wendelken, L. A., and Valcour, V. (2012). Impact of HIV and aging on neuropsychological function. J. Neurovirol. 18, 256–263. doi: 10.1007/s13365-012-0094-1
Woods, A. J., Antal, A., Bikson, M., Boggio, P. S., Brunoni, A. R., Celnik, P., et al. (2016). A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin. Neurophysiol. 127, 1031–1048. doi: 10.1016/j.clinph.2015.11.012
Woods, S. P., Moore, D. J., Weber, E., and Grant, I. (2009). Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychol. Rev. 19, 152–168. doi: 10.1007/s11065-009-9102-5
Wu, S., and Spence, I. (2013). Playing shooter and driving videogames improves top-down guidance in visual search. Atten. Percept Psychophys. 75, 673–686. doi: 10.3758/s13414-013-0440-2
Yamada, Y., and Sumiyoshi, T. (2021). Neurobiological mechanisms of transcranial direct current stimulation for psychiatric disorders; neurophysiological, chemical, and anatomical considerations. Front. Hum. Neurosci. 15:21. doi: 10.3389/fnhum.2021.631838
Zaehle, T., Sandmann, P., Thorne, J. D., Jancke, L., and Herrmann, C. S. (2011). Transcranial direct current stimulation of the prefrontal cortex modulates working memory performance: combined behavioural and electrophysiological evidence. BMC Neurosci. 12:2. doi: 10.1186/1471-2202-12-2
Keywords: ranscranial direct current stimulation, computer-delivered cognitive training, human immunodeficiency virus, cognition, HIV-associated neurocognitive disorder, mild neurocognitive disorder
Citation: Ownby RL and Kim J (2021) Computer-Delivered Cognitive Training and Transcranial Direct Current Stimulation in Patients With HIV-Associated Neurocognitive Disorder: A Randomized Trial. Front. Aging Neurosci. 13:766311. doi: 10.3389/fnagi.2021.766311
Received: 28 August 2021; Accepted: 20 October 2021;
Published: 15 November 2021.
Edited by:
Wei Wu, Alto Neuroscience, United StatesReviewed by:
David Eugene Vance, University of Alabama at Birmingham, United StatesMaximilian Achim Friehs, University College Dublin, Ireland
Copyright © 2021 Ownby and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Raymond L. Ownby, cm83MUBub3ZhLmVkdQ==
 Raymond L. Ownby
Raymond L. Ownby Jae Kim
Jae Kim