- 1Departments of Medicine and Geriatrics, University of Montreal, Montreal, QC, Canada
- 2Research Centre of the Geriatric University Institute of Montreal, Montreal, QC, Canada
- 3Department of Medicine, Division of Geriatric Medicine, Sir Mortimer B. Davis Jewish General Hospital and Lady Davis Institute for Medical Research, McGill University, Montreal, QC, Canada
- 4Research Center of the Centre Hospitalier de l’Université de Montréal, Montreal, QC, Canada
- 5Leenaards Memory Center, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland
Background: Motoric cognitive risk syndrome (MCR) is a pre-dementia stage. The existence of stable and transient MCR, their related clinical characteristics and their association with incident dementia is a matter of debate.
Objective: This study aims to examine the clinical characteristics and the time course associated with new onset, transient and stable MCR, and their association with incidence of probable dementia in community-dwelling older adults living in the province of Quebec (Canada).
Design: Quebec elderly population-based observational cohort study with 3 years of follow-up.
Setting: Community dwellers.
Subjects: A subset of participants (n = 1,113) from the “Quebec Longitudinal Study on Nutrition and Successful Aging” (NuAge) cohort.
Methods: Participants with MCR were identified at baseline and after 1 year of follow-up. Socio-demographic characteristics, 30-item Geriatric depression scale (GDS) score, cardiovascular risk factors and diseases were recorded at baseline. Incidence of probable dementia was measured at annual follow-up visits over a 3-year period.
Results: Over the period of the first year of follow-up, the prevalence of MCR was 8.5% with 4.3% having new onset MCR, 2.8% transient MCR and 1.4% stable MCR. A higher 30-item GDS score was reported with new onset and transient MCR, and the highest prevalence of cerebrovascular diseases was shown with stable MCR compared to non-MCR participants (p < 0.05). MCR was associated with overall incidence of probable dementia, regardless of its status (Hazard Ratio ≥ 1.86, p ≤ 0.034).
Conclusion: Greater prevalence of depressive symptoms and cerebrovascular diseases were reported, respectively, with new onset and transient MCR, and stable MCR. The association of MCR with incidence of probable dementia remains significant, regardless of MCR subtypes.
Highlights
– Depressive symptoms were more prevalent with new onset and transient Motoric Cognitive Risk syndrome (MCR) compared to non-MCR participants.
– The highest prevalence of cerebrovascular diseases was shown with stable MCR.
– MCR is associated with incidence of probable dementia, regardless of its subtypes (i.e., new onset, transient and stable).
Introduction
Motoric cognitive risk syndrome (MCR) is a clinical syndrome characterized by slow gait speed and cognitive complaint (Verghese et al., 2013). MCR is an at-risk state for dementia and is proposed to be a transitional state between normal cognition to dementia, such as mild cognitive impairment (MCI; Sekhon et al., 2017). MCR diagnosis, compared to MCI, does not require extensive cognitive testing and neuroimaging, and thus is easy to perform in clinical practice. This enhances its applicability for risk screening of dementia, particularly in the elderly population (Verghese et al., 2013; Sekhon et al., 2017). The usefulness of an MCR diagnosis is two-fold: first, it identifies individuals at risk of dementia and second, it facilitates preventive actions addressing modifiable risk factors among those identified as being at-risk (Verghese, 2021; Mullin et al., 2022). Recent, systematic reviews showed that up to 40% of late-onset dementia may be prevented or at least delayed by addressing modifiable risk factors (Livingston et al., 2020; Curran et al., 2021). MCR has, thus, became a clinical target for more accurate characterization of dementia risk.
The incidence of MCR is high and ranges between 5% and 10% (Verghese et al., 2014a,b; Mullin et al., 2022). Thus, not all individuals with MCR will develop dementia, suggesting that the MCR state may be with time stable, transient or progress to dementia. Since its first description in 2013, no study has examined MCR status may be transient. The possible of transient MCR underscores two main research questions: first, are there clinical characteristics which may explain stable and transient status of MCR, and second, does it influence its predictive value for dementia?
MCR has been associated with cardiovascular risk factors and diseases, as well as brain abnormalities, including microvascular ischemic lesions (Sekhon et al., 2019; Beauchet et al., 2020). In addition, the highest rate of conversion to dementia has been reported for vascular dementia (Verghese et al., 2013). Both characteristics suggest that stable MCR may be associated with cardiovascular risk factors and diseases leading to irreversible brain damage. MCR has also been associated with depressive symptoms (Beauchet et al., 2021; Xu et al., 2022). First, individuals with MCR frequently reported more depressive symptoms compared to their non-MCR counterparts (Beauchet et al., 2021). Second, individuals with depressive symptoms are at risk of MCR (Meiner et al., 2020). As depressive symptoms are reversible, we hypothesized that the reversibility of MCR may be related to these symptoms. The present study thus aims to examine the clinical characteristics and the time course associated with new onset, transient and stable MCR, and the association of these 3 types of MCR with incidence of probable dementia in community-dwelling older adults living in the province of Quebec (Canada).
Materials and methods
Design
The database of the “Quebec Longitudinal Study on Nutrition and Successful Aging” (NuAge) was used for the present study (Gaudreau et al., 2007). NuAge is a population-based cohort study carried out in the Province of Quebec (Canada) in generally healthy community-dwelling older adults who were evaluated annually over a 3-year period. The data collection of NuAge has been previously reported (Gaudreau et al., 2007). In summary, 1,793 older adults aged between 67 and 84 without cognitive impairment (i.e., Modified Mini-Mental State (3MS) score > 79/100) and without major physical disability (i.e., able to walk 300 m and climb 10 stairs without rest), living independently in the community and willing to commit to up to a 5-year follow-up were recruited between November 2003 and June 2005 (Teng and Chui, 1987; Gaudreau et al., 2007). Among them, 1,753 (97.8%) agreed to the integration of their data and biosamples into the NuAge Database and Biobank for future studies and 1,526 (85.1%) were followed over a 3-year period. Participants with missing values for MCR and dementia status at the end of follow-up were excluded and, thus, 1,113 (62.1%) participants were selected for the present study.
Assessment
Age, sex, number of reported drugs taken daily were recorded at baseline. Weight (kg) and height (cm) were also measured at baseline. Overweight or obesity was defined as body mass index (BMI) ≥25 kg/m2 and underweight was defined as <18.5 kg/m2. Currently smoking (yes versus no) was also noted. The Physical Activity Scale for the Elderly (PASE) was used to determine a low physical activity level defined by being below the lowest tertile (i.e., <69.1 for females and <87.7 for males; Washburn et al., 1999). Hypertension was self-reported using the Older Americans Resources and Services Multidimensional Functional Assessment (OARS) questionnaire and defined as the use of anti-hypertensive medications and/or a blood pressure measure >140/90 mmHg (Fillenbaum, 1988). Diabetes, regardless of its type, was recorded if it was self-reported in the OARS questionnaire, or if antidiabetic medications were reported. Data on the presence of cardiovascular diseases associating heart, peripheral and cerebrovascular diseases was collected through the OARS questionnaire. Depressive symptomatology was assessed using the 30-item GDS (Yesavage et al., 1982). Subjective cognitive complaint was recorded and defined as a “yes” response to the question “Do you feel you have more problems with memory than most?” from the 30-item GDS and/or as impairment in memory recorded using the memory item of the Functional Autonomy Measurement System (SMAF; Hebert et al., 1988). Finally, gait speed was assessed using a standardized procedure. Participants were asked twice to walk at their usual pace over a 4-m distance. Time was measured in seconds between the second and the fourth meter with a stopwatch and the best time of the two trials was used. Gait speed was calculated in meters per second on the last 3 m. The follow-up period was 3 years. Each year, the full standardized assessment performed at baseline was repeated.
Definition of motoric cognitive risk syndrome
MCR was determined using information collected at baseline and after the first annual follow-up. MCR was defined as a combination of subjective cognitive complaint and slow walking speed in the absence of dementia and gait disability (Verghese et al., 2013). Slow walking speed was defined as a walking speed at least one standard deviation (SD) below the age-appropriate mean values established in the present cohort. Participants were divided into two sex groups and four age groups, as described by Verghese et al. (2013). The slow gait speed cut-offs were: for male <1.09 m/s for age group 67–72, <1.00 m/s for age group 73–77, <0.97 m/s for age group 78–84 and <0.93 m/s for age group ≥85; and for female <1.04 m/s for age group 67–72, <0.97 m/s for age group 73–77, <0.91 m/s for age group 78–84 and <0.81 m/s for age group ≥85. Based on the time course of MCR between baseline assessment and year 1 of follow-up, 3 subtypes of MCR were defined: new onset MCR (no MCR at baseline but MCR after 1 year), transient MCR (MCR at baseline but no MCR after 1 year) and stable MCR (MCR at baseline and after 1 year). Figure 1 shows the distribution of MCR subgroups in selected NuAge participants.
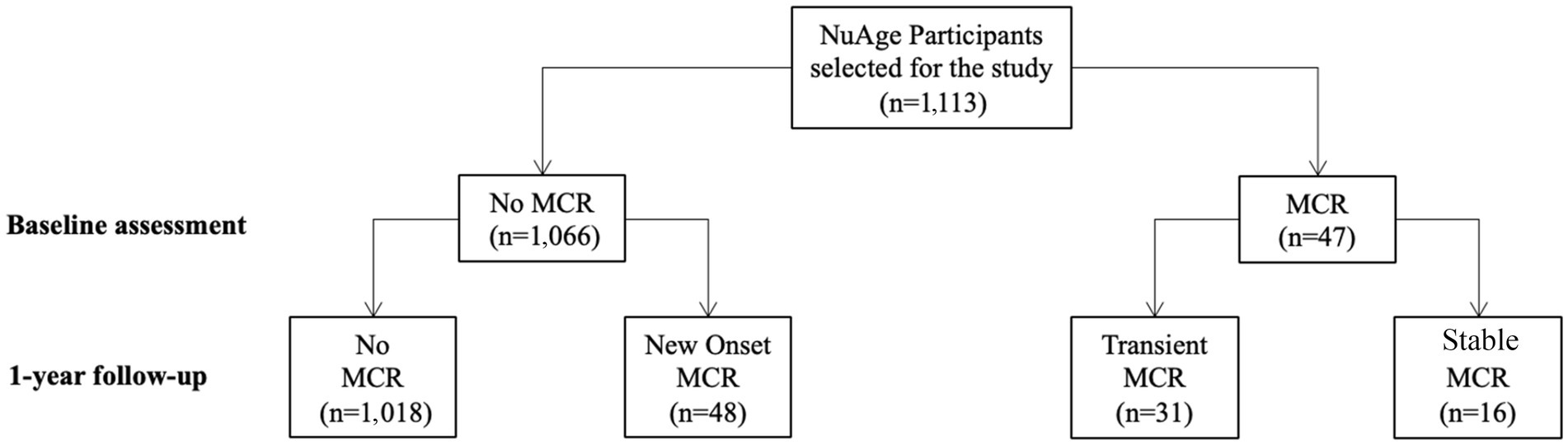
Figure 1. Flow diagram of NuAge participants according to the presence or absence of Motoric cognitive risk syndrome at baseline and 1-year follow-up.
Definition of probable dementia
Cognitive performance was evaluated at baseline and at each annual subsequent visit using the 3MS score (Teng and Chui, 1987). Incidence of probable dementia was considered present if the 3MS score was ≤79/100, which is a sensitive threshold value to screen dementia, and the simplified Instrumental Activities of Daily Leaving (IADL) score was ≤3/4 (Lawton and Brody, 1969; Teng and Chui, 1987).
Standard protocol approval and patient consents
The Research Ethics Boards (REB) of the University Institute of Geriatrics of Sherbrooke and the “Institut universitaire de gériatrie de Montréal” approved the NuAge protocol. Written informed consent for research was obtained for all NuAge participants. The REB of the “Centre intégré universitaire de santé et de services sociaux de l’Estrie – Centre hospitalier universitaire de Sherbrooke”(CIUSSS de l’Estrie-CHUS) approved the NuAge Database and Biobank. The present study was approved by the REB of the Jewish General Hospital (Montreal, Quebec, Canada). The NuAge data set used in this study was obtained from the NuAge Database team on May 07, 2019.
Statistics
The participants’ baseline characteristics were described using means, standard deviation (SD), and percentages. Participants were assigned to one of the following four groups according to their MCR status: No MCR, new onset MCR, transient MCR and stable MCR. Comparisons between groups were performed using Kruskal-Wallis, Mann–Whitney, Chi-square or Fisher’s exact tests. Cox regressions were also performed to examine the association of MCR subtypes, used as independent variables (separated model for each variable), with incidence of probable dementia. Unadjusted and adjusted models were examined by age, sex, polypharmacy, number of school years, the 30-item GDS and cardiovascular risk factors and diseases. Values of p ≤ 0.001 were considered statistically significant for group comparisons (because of multiple comparisons) and those <0.05 were considered significant for Cox regression models. All statistics were performed using SPSS (version 26.0; SPSS, Inc., Chicago, IL).
Results
Over the period of the first year of follow-up, the prevalence of MCR was 8.5%, of which 4.3% had new onset MCR, 2.8% transient MCR and 1.4% stable MCR. The prevalence of cerebrovascular diseases differed significantly per group (p ≤ 0.001; Table 1), with the highest prevalence demonstrated in the stable MCR group (p ≤ 0.001; Table 2). The 30-item GDS and 3MS scores were different across MCR subgroups (p ≤ 0.001); new onset MCR and transient MCR groups had higher 30-item GDS scores than the non-MCR group (p ≤ 0.001). The transient MCR group had a lower 3MS score than its non-MCR counterpart (p ≤ 0.001). Both walking speed at baseline and year 1 differed between MCR subgroups, the lowest value reported in the stable MCR group (p ≤ 0.001). Similarly, subjective cognitive complaint differed among MCR subgroups, the highest prevalence being reported in stable MCR (p ≤ 0.001). Overall incidence of probable dementia also differed among MCR subgroups (p ≤ 0.001). It was higher in the transient MCR group compared to the non-MCR group, whereas only trend was reported for the other subgroups (p = 0.035 for new onset MCR and p = 0.003 for stable MCR).
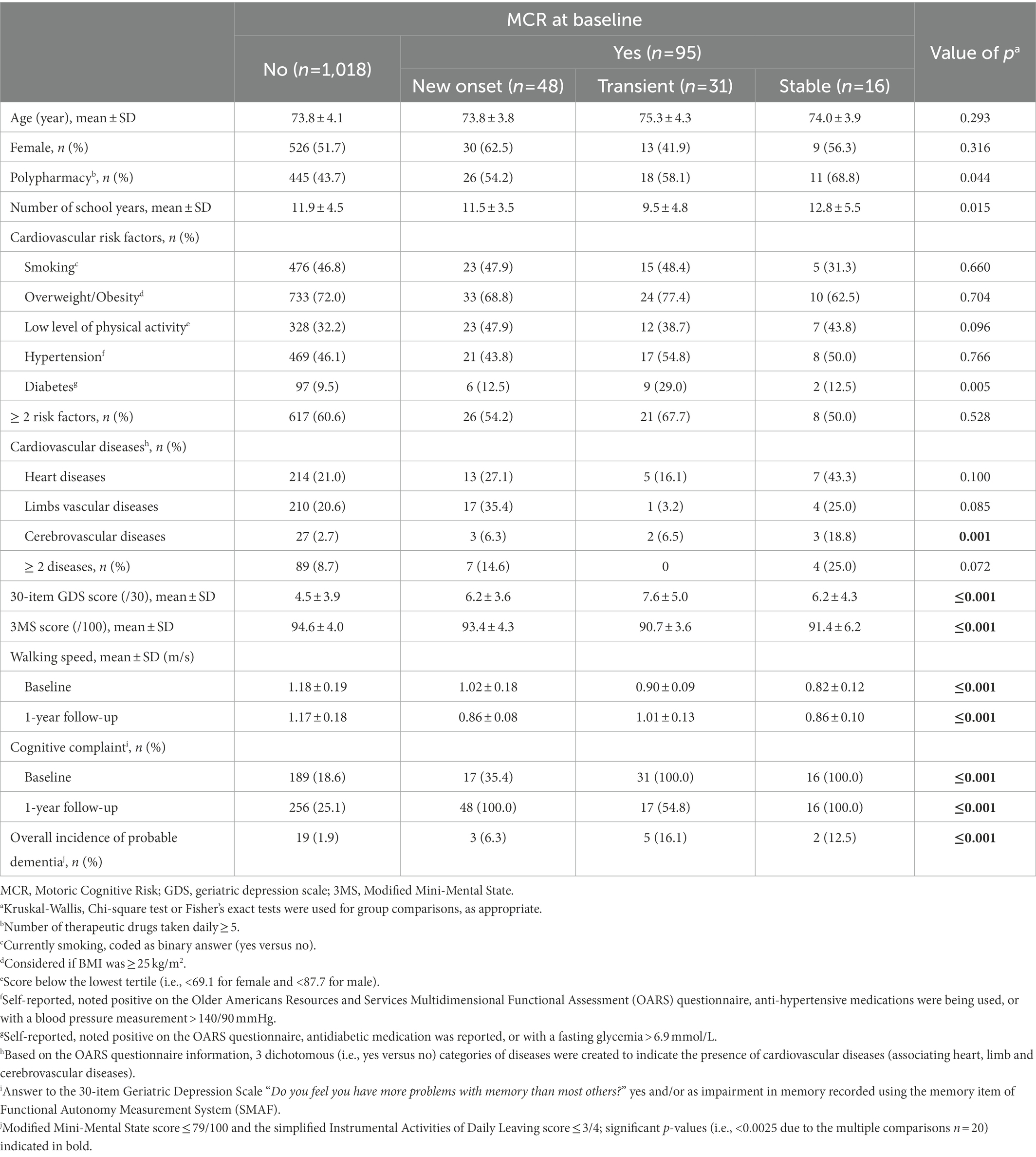
Table 1. Characteristics of participants according to their motoric cognitive risk syndrome status (n = 1,113).
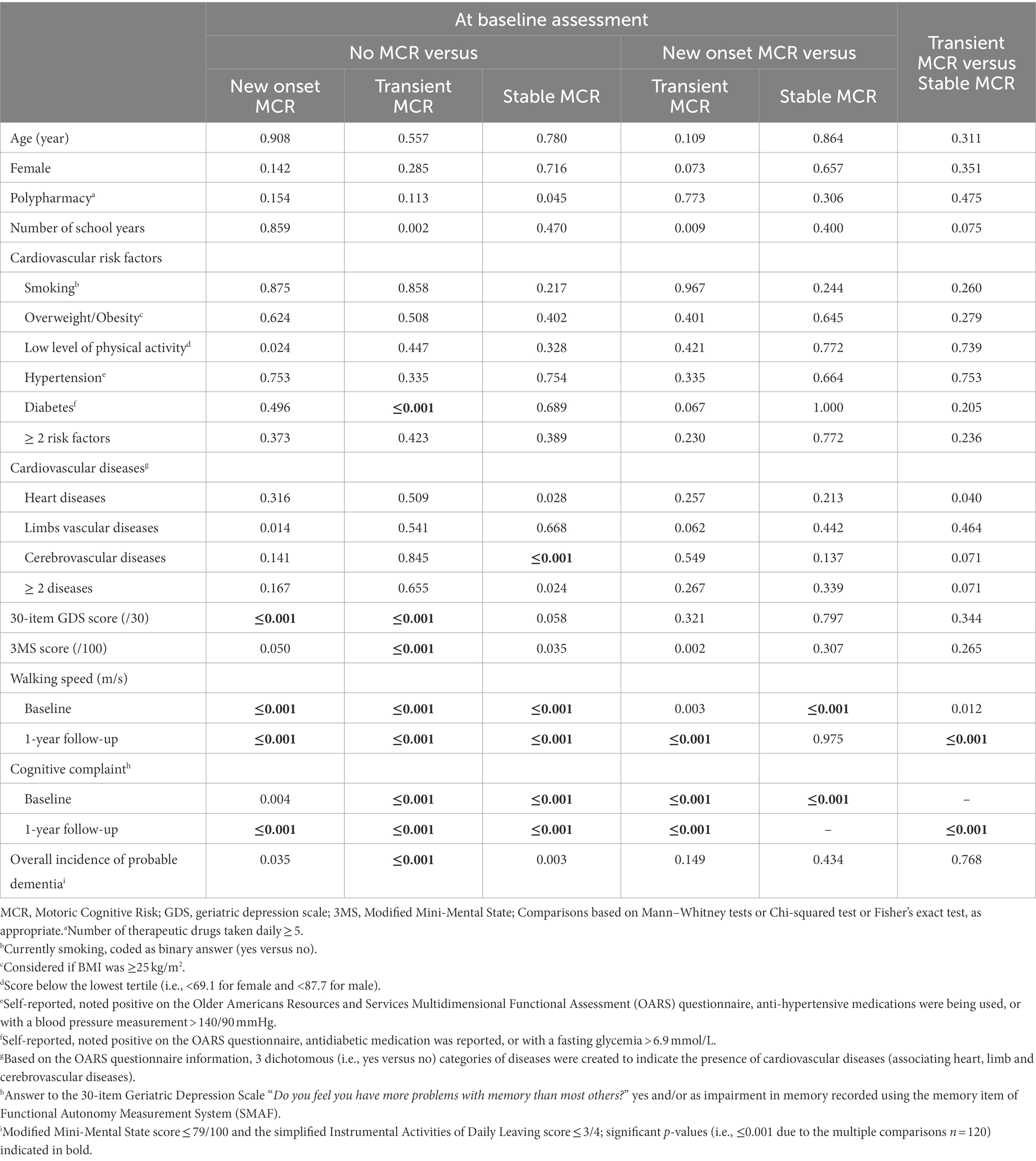
Table 2. Value of p comparisons between groups of participants defined according to their motoric cognitive risk syndrome status at baseline (n = 1,113).
Unadjusted Cox regressions revealed that transient and stable MCR (Hazard ratio (HR) ≥ 1.88 with p ≤ 0.011; Table 3) were associated with incidence of probable dementia. Adjusted Cox regressions showed that all MCR subtypes were associated with incidence of probable dementia (HR ≥ 1.86 with p ≤ 0.034).
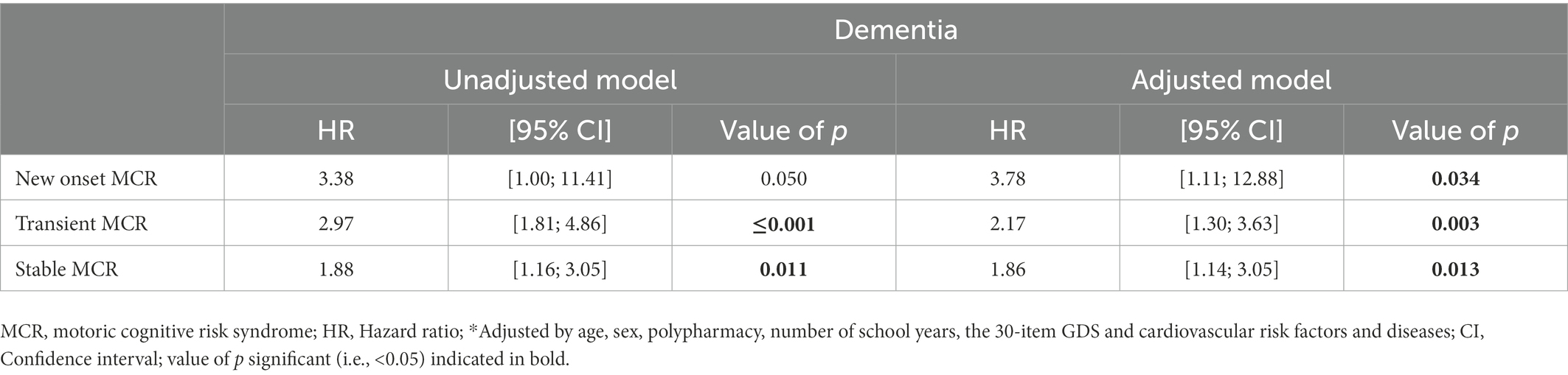
Table 3. Cox regressions showing the association of Motoric Cognitive Risk syndrome status (i.e., new onset, transient and stable used as independent, separated model) and overall incidence of probable dementia (used as dependent variable; n = 1,113).
Discussion
Our findings show that MCR may be transient and that specific characteristics are related to this reversibility. Depressive symptoms were more prevalent in participants with new onset and transient MCR, whereas cerebrovascular diseases were more prevalent in participants with stable MCR. In addition, the MCR subtypes did not influence the association with incidence of probable dementia, which was significant in all subtypes.
The reversibility from MCR to a normal condition has not been previously examined. We reported that there are individuals with transient MCR after only 1 year of follow-up. MCR is a pre-dementia stage like MCI. MCI reversibility has previously been reported in community-based cohorts (Malek-Ahmadi, 2016; Gao et al., 2018). Both MCR and MCI are transitional stages between normal cognition and dementia, and their definition is based on clinical characteristics which may change over the time. The impact of interindividual variability on clinical characteristics may be important when dichotomous outcomes are used (Haidich, 2010). Both improvement in medical conditions and the practice effect, which refers to improvement in test performance because of prior exposure to testing, are individual-based factors that may lead to the reversibility of MCR, especially if the disease related to MCR is transitional like depression compared to irreversible conditions, such as cerebrovascular brain lesions (Haidich, 2010; Tractenberg and Pietrzak, 2011).
We reported in the present study that both reversibility of MCR to a normal condition and participants with new onset MCR had a higher 30-item GDS score compared to their counterparts. The relationship of MCR and depressive symptoms has previously been reported (Sekhon et al., 2019; Beauchet et al., 2021; Xu et al., 2022). For instance, we observed that there was a higher prevalence of depressive symptomatology in adults with MCR compared to those without MCR in the Canadian population (Sekhon et al., 2019). Furthermore, it has been demonstrated that depressive symptoms are associated with incident MCR (Xu et al., 2022). Our finding underscores a new component of the relationship between depressive symptoms and MCR, which is the reversibility of MCR. A possible explanation of this characteristic may be related to the reversibility of depressive symptoms. Both individuals with MCR and depressive symptoms report subjective cognitive complaint (Sekhon et al., 2019; Beauchet et al., 2021; Xu et al., 2022). In addition, individuals with depressive symptoms may have a slow walking speed compared to their healthy counterparts (Sekhon et al., 2019; Xu et al., 2022). Thus, we suggest that the cause of MCR in individuals with transient and new onset MCR may be attributed to the depressive symptoms, and that the fluctuation of depressive symptomatology with time may explain the fluctuation of MCR diagnosis.
In contrast to transient and new onset MCR, individuals with stable MCR had a higher prevalence of cerebrovascular diseases when compared to non-MCR individuals. First, this finding is consistent with a previous meta-analysis which showed that MCR was significantly associated with cardiovascular diseases and risk factors (Beauchet et al., 2018). Second, the stability of diagnosis of MCR with time suggests that MCR may be related to irreversible brain lesions. Unfortunately, it is not possible to confirm this hypothesis with the NuAge study because we do not have a brain imaging. MCR has been associated with both cortical atrophy and microvascular ischemic brain abnormalities (Beauchet et al., 2018; Blumen et al., 2021). In addition, it has been shown that MCR is a greater predictor of vascular dementia compared to Alzheimer’s disease (Verghese et al., 2013; Sekhon et al., 2017; Verghese, 2021; Mullin et al., 2022). For instance, the risk of developing dementia reported in the first publication involved a hazard ratio [HR] of 3.3 (95% Confidence interval (CI): 1.55–6.90), which increased to 12.8 (95% CI: 4.98–32.97) for vascular dementia (Verghese et al., 2013). When combined, these previous results and our present findings suggest that the mechanism of stable MCR may be related to cerebrovascular lesions.
Finally, we found that regardless of its subtype (i.e., new onset, transient or stable), MCR is significantly associated with incidence of probable dementia. This result confirms the strong relationship between MCR and dementia reported in a recent meta-analysis (Mullin et al., 2022). The novelty of our results lies in the fact that the reversibility of MCR did not influence the occurrence of probable dementia. We suggested that the reversibility of MCR is related to depressive symptoms. A systematic review and meta-analysis of community-based cohort studies has shown that late-life depressive symptomatology is associated with an increased risk for incident dementia, regardless of its type (i.e., Alzheimer’s disease (AD) and non-AD; Diniz et al., 2013).
The present study has some limitations. First, the NuAge population was composed of relatively healthy older adults, which could affect the generalization of our results. Second, although we were able to control for many characteristics likely to modify the association, residual confounding might still be present. As confounding factors can impact both the magnitude and direction of the association, it is difficult to speculate on the impact of residual confounding factors on the associations found in the study. Third, we selected 62.1% of participants from the initial set of NuAge cohort due to selection criteria and accessible data for this study, that may be considered as a selection bias. Indeed, the sample selection of this study may not accurately reflect the older population. Fourth, the diagnosis of dementia may be underestimated, explaining its low incidence. Indeed, this diagnosis is usually based on an interdisciplinary meeting and more exhaustive information. In our case, we used the dementia threshold of the 3MS combined with an abnormal simplified IADLs score. Fifth, there is no brain imaging or other biomarker variables that can be used as a reference indicator for dementia risk or neurodegeneration/pathology status (e.g., lesions or vascular problems).
Conclusion
Our study showed that depressive symptoms were more prevalent with new onset and transient MCR, whereas cerebrovascular diseases were more prevalent with stable MCR in Quebec community-dwelling older adults. The MCR subtype status did not influence its association with incidence of probable dementia.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: Access to this research bank can be requested by completing an access request on the NuAge Database and Biobank website (https://nuage.recherche.usherbrooke.ca/en/). Requests to access these datasets should be directed to https://nuage.recherche.usherbrooke.ca/en/.
Ethics statement
The studies involving human participants were reviewed and approved by The Research Ethics Boards (REB) of the University Institute of Geriatrics of Sherbrooke and the “Institut universitaire de gériatrie de Montréal.” The patients/participants provided their written informed consent to participate in this study.
Author contributions
OB and GA: conceived and designed the experiments, analyzed and interpreted the data. PG: performed the experiments. PG and OB: contributed reagents, materials, and analysis tools or data. OB, JM, and GA: writing of the manuscript. PG: revision of manuscript. All authors contributed to the article and approved the submitted version.
Funding
The NuAge Study was funded by the Canadian Institutes of Health Research (CIHR; MOP-62842). The NuAge Database and Biobank are supported by the Fonds de recherche du Québec (FRQ; 2020-VICO-279753), the Quebec Network for Research on Aging, a thematic network funded by the FRQ-Santé, and by the Merck-Frosst Chair funded by La Fondation de l’Université de Sherbrooke. Beauchet and Allali were supported by the National Institute of Health/National Institute on Aging grants PO1 AG03949 and R01AG057548-01A1. The sponsors had no role in the design, execution, analysis and interpretation of data, or writing of the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Beauchet, O., Sekhon, H., Barden, J., Liu-Ambrose, T., Chester, V. L., Szturm, T., et al. (2018). Association of motoric cognitive risk syndrome with cardiovascular disease and risk factors: results from an original study and meta-analysis. J. Alzheimers Dis. 64, 875–887. doi: 10.3233/JAD-180203
Beauchet, O., Sekhon, H., Launay, C. P., Gaudreau, P., Morais, J. A., and Allali, G. (2020). Relationship between motoric cognitive risk syndrome, cardiovascular risk factors and diseases, and incident cognitive impairment: results from the "NuAge" study. Maturitas 138, 51–57. doi: 10.1016/j.maturitas.2020.05.007
Beauchet, O., Sekhon, H., Launay, C. P., Gaudreau, P., Morais, J. A., and Allali, G. (2021). Late-life depressive symptomatology, motoric cognitive risk syndrome, and incident dementia: the "NuAge" study results. Front. Aging Neurosci. 13:740181. doi: 10.3389/fnagi.2021.740181
Blumen, H. M., Schwartz, E., Allali, G., Beauchet, O., Callisaya, M., Doi, T., et al. (2021). Cortical thickness, volume, and surface area in the motoric cognitive risk syndrome. J. Alzheimers Dis. 81, 651–665. doi: 10.3233/JAD-201576
Curran, E., TWH, C., Godbee, K., Abraham, C., Lautenschlager, N. T., and Palmer, V. J. (2021). General population perspectives of dementia risk reduction and the implications for intervention: a systematic review and thematic synthesis of qualitative evidence. PLoS One 16:e0257540. doi: 10.1371/journal.pone.0257540
Diniz, B. S., Butters, M. A., Albert, S. M., Dew, M. A., and Reynolds, C. F. 3rd. (2013). Late-life depression and risk of vascular dementia and Alzheimer's disease: systematic review and meta-analysis of community-based cohort studies. Br. J. Psychiatry 202, 329–335. doi: 10.1192/bjp.bp.112.118307
Fillenbaum, G. G. (1988). Multidimensional Functional Assessment of Older Adults: The Duke Older Americans Resources and Services Procedures, Hillsdale, NJ: Lawrence Erlbaum Associates.
Gao, Q., Gwee, X., Feng, L., MSZ, N., Feng, L., Collinson, S. L., et al. (2018). Mild cognitive impairment reversion and progression: rates and predictors in community-living older persons in the Singapore longitudinal ageing studies cohort. Dement. Geriatr. Cogn. Dis. Extra. 8, 226–237. doi: 10.1159/000488936
Gaudreau, P., Morais, J. A., Shatenstein, B., Gray-Donald, K., Khalil, A., Dionne, I., et al. (2007). Nutrition as a determinant of successful aging: description of the Quebec longitudinal study Nuage and results from cross-sectional pilot studies. Rejuvenation Res. 10, 377–386. doi: 10.1089/rej.2007.0596
Hebert, R., Carrier, R., and Bilodeau, A. (1988). The Functional Autonomy Measurement System (SMAF): description and validation of an instrument for the measurement of handicaps. Age Ageing 17, 293–302. doi: 10.1093/ageing/17.5.293
Lawton, M. P., and Brody, E. M. (1969). Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 9, 179–186. doi: 10.1093/geront/9.3_Part_1.179
Livingston, G., Huntley, J., Sommerlad, A., Ames, D., Ballard, C., Banerjee, S., et al. (2020). Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396, 413–446. doi: 10.1016/S0140-6736(20)30367-6
Malek-Ahmadi, M. (2016). Reversion from mild cognitive impairment to normal cognition: a meta-analysis. Alzheimer Dis. Assoc. Disord. 30, 324–330. doi: 10.1097/WAD.0000000000000145
Meiner, Z., Ayers, E., and Verghese, J. (2020). Motoric cognitive risk syndrome: a risk factor for cognitive impairment and dementia in different populations. Ann. Geriatr. Med. Res. 24, 3–14. doi: 10.4235/agmr.20.0001
Mullin, D. S., Cockburn, A., Welstead, M., Luciano, M., Russ, T. C., and Muniz-Terrera, G. (2022). Mechanisms of motoric cognitive risk-Hypotheses based on a systematic review and meta-analysis of longitudinal cohort studies of older adults. Alzheimers Dement. 18, 2413–2427. doi: 10.1002/alz.12547
Sekhon, H., Allali, G., and Beauchet, O. (2019). Motoric cognitive risk syndrome and cardiovascular diseases and risk factors in the Canadian population: results from the baseline assessment of the Canadian longitudinal study on aging. Arch. Gerontol. Geriatr. 85:103932. doi: 10.1016/j.archger.2019.103932
Sekhon, H., Allali, G., Launay, C. P., Chabot, J., and Beauchet, O. (2017). The spectrum of pre-dementia stages: cognitive profile of motoric cognitive risk syndrome and relationship with mild cognitive impairment. Eur. J. Neurol. 24, 1047–1054. doi: 10.1111/ene.13331
Teng, E. L., and Chui, H. C. (1987). The modified mini-mental state (3MS) examination. J. Clin. Psychiatry 48, 314–318.
Tractenberg, R. E., and Pietrzak, R. H. (2011). Intra-individual variability in Alzheimer's disease and cognitive aging: definitions, context, and effect sizes. PLoS One 6:e16973. doi: 10.1371/journal.pone.0016973
Verghese, J. (2021). Motoric cognitive risk syndrome: next steps. Eur. J. Neurol. 28, 2467–2468. doi: 10.1111/ene.14949
Verghese, J., Annweiler, C., Ayers, E., Barzilai, N., Beauchet, O., Bennett, D. A., et al. (2014a). Motoric cognitive risk syndrome: multicountry prevalence and dementia risk. Neurology 83, 718–726. doi: 10.1212/WNL.0000000000000717
Verghese, J., Ayers, E., Barzilai, N., Bennett, D. A., Buchman, A. S., Holtzer, R., et al. (2014b). Motoric cognitive risk syndrome: Multicenter incidence study. Neurology 83, 2278–2284. doi: 10.1212/WNL.0000000000001084
Verghese, J., Wang, C., Lipton, R. B., and Holtzer, R. (2013). Motoric cognitive risk syndrome and the risk of dementia. J. Gerontol. Ser. A Biol. Med. Sci. 68, 412–418. doi: 10.1093/gerona/gls191
Washburn, R. A., McAuley, E., Katula, J., Mihalko, S. L., and Boileau, R. A. (1999). The physical activity scale for the elderly (PASE): evidence for validity. J. Clin. Epidemiol. 52, 643–651. doi: 10.1016/S0895-4356(99)00049-9
Xu, W., Bai, A., Liang, Y., and Lin, Z. (2022). Association between depression and motoric cognitive risk syndrome among community-dwelling older adults in China: a 4-year prospective cohort study. Eur. J. Neurol. 29, 1377–1384. doi: 10.1111/ene.15262
Keywords: older adults, cohort study, dementia, clinical screening, epidemiology
Citation: Beauchet O, Matskiv J, Gaudreau P and Allali G (2023) New onset, transient and stable motoric cognitive risk syndrome: Clinical characteristics and association with incidence of probable dementia in the NuAge cohort. Front. Aging Neurosci. 14:1063702. doi: 10.3389/fnagi.2022.1063702
Edited by:
Gro Tangen, Vestfold Hospital Trust, NorwayReviewed by:
Ryota Sakurai, Tokyo Metropolitan Institute of Gerontology, JapanIsabelle Ruth Killane, Technological University Dublin, Ireland
Fang-Yu Cheng, Mackay Medical College, Taiwan
Copyright © 2023 Beauchet, Matskiv, Gaudreau and Allali. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Olivier Beauchet, ✉ b2xpdmllci5iZWF1Y2hldEB1bW9udHJlYWwuY2E=
 Olivier Beauchet
Olivier Beauchet Jacqueline Matskiv2
Jacqueline Matskiv2 Pierrette Gaudreau
Pierrette Gaudreau