- 1Department of Neurology, Taizhou Second People’s Hospital, Taizhou, China
- 2Department of Neurology, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 3Department of Neurology, Huashan Hospital, Fudan University, Shanghai, China
- 4Department of Neurology, Nanjing Drum Tower Hospital, Nanjing, China
- 5Department of Neurology, Tianjin Medical University General Hospital, Tianjin, China
Objective: There is a lack of longitudinal studies that directly compare the quality of life (QoL) and investigate the impact of clinical factors on QoL across different excessive daytime sleepiness (EDS) statuses in Parkinson’s disease (PD); therefore, we aimed to compare QoL and reveal the potential heterogeneous predictors of QoL between patients with PD with and without EDS.
Methods: We collected clinical data among 306 patients with PD over 2 years. EDS was assessed by the Epworth Sleepiness Scale and QoL was measured with the 39-item Parkinson’s Disease Questionnaire.
Results: We found that at both baseline and follow-up, patients with PD with EDS had poorer QoL and suffered more non-motor symptoms including depression and clinical probable rapid eye movement sleep behavior disorder (cpRBD). The generalized linear mixed model analysis indicated that the major predictors of QoL in PD with EDS were the akinetic-rigid type, disease duration, and total levodopa equivalent dose, while in PD without EDS, the primary determinants of QoL were Hoehn and Yahr, Mini-Mental State Examination (MMSE), and cpRBD.
Conclusion: Patients with PD with EDS presented with poorer QoL. Besides, the baseline predictors of future QoL differed between patients with PD with and without EDS. These findings remind clinicians to target specific clinical factors when attempting to improve QoL among patients with PD.
Introduction
Parkinson’s disease (PD) is usually characterized by motor manifestations, but non-motor symptoms (NMS) are also highly prevalent in PD and even more disabling than motor impairment. Excessive daytime sleepiness (EDS), the main manifestation of daytime sleep disturbance, has been reported to affect about one-third of patients with PD (Feng et al., 2021). EDS not only negatively affects social function and quality of life in patients with PD, but also increases the burden on caregivers.
Quality of life (QoL) is an important index to consider in managing PD because it concerns both the functional and emotional status of patients. The NMS, especially sleep and mood disorders, have been recently shown to exert a great effect on QoL in patients with PD (Gokcal et al., 2017; Palmeri et al., 2019). Previous studies demonstrated that patients with PD with EDS tended to have worse QoL than those without EDS (Xiang et al., 2019; Yoo et al., 2019). A recent study among patients with advanced PD with long-term disease duration showed that EDS was independently related to worse QoL after adjusting relevant factors (Sun et al., 2018). However, the impact of EDS on QoL has not been clarified in longitudinal studies. Besides, the influence of clinical factors on QoL across different EDS statuses in PD has not been addressed. Identifying the potential predictors of QoL might be helpful for clinicians to apply more precise pharmacological and non-pharmacological interventions. Therefore, the aim of our study was to compare QoL and identify the predictors of QoL between patients with PD with and without EDS.
Materials and Methods
Study Design and Participants
All subjects were consecutively enrolled at Huashan Hospital, Fudan University from April 2009 to December 2017. The study was approved by the Human Studies Institutional Review Board, Huashan Hospital, Fudan University. All patients provided written informed consent in accordance with the Declaration of Helsinki.
The clinical diagnosis of PD was made by two senior neurologists specializing in movement disorders based on the United kingdom PD Society Brain Bank criteria (Hughes et al., 1992). In total, 306 patients with PD without histories of stroke, epilepsy, encephalitis, traumatic brain injury, malignancies, cardiac events, or severe psychiatric illness were enrolled. The patients with PD with a family history in their first-degree relatives or have onset age younger than 50 years old were suggested to take gene tests, and those with mutation frequency were excluded. Participants were required to take a comprehensive and extensive assessment annually for 2 years at the Movement Disorder Center of Huashan Hospital.
Clinical and Neuropsychological Assessments
Demographic and clinical data were collected. The patients with PD were divided into PD with EDS group and PD without EDS group based on the Epworth Sleepiness Scale (ESS) scores at baseline (Chen et al., 2002). The ESS scores of ≥10 indicated a diagnosis of EDS. The dosages of anti-parkinsonian drugs were converted into a total daily levodopa equivalent dose (LED) for standardization (Thobois, 2006). Motor function was evaluated using the Hoehn and Yahr (H and Y) stage and the original version of Unified Parkinson’s Disease Rating Scale (UPDRS) Part III (UPDRS-III) scores (Fahn, 1987). Patients were classified as tremor-dominant type, akinetic-rigid type, and mixed type based on the UPDRS items according to the previous study (Schiess et al., 2000). The overall NMS was determined by the non-motor symptoms questionnaire (NMSQ; Chaudhuri et al., 2006). The severity of depressive symptoms was assessed by the Geriatric Depression Rating Scale (GDS), and a score of ≥10 was regarded as depression (Ertan et al., 2005). The rapid eye movement (REM) Sleep Behavior Disorder Screening Questionnaire (RBDSQ) was used as a screening tool of clinical probable RBD (cpRBD), and a score of ≥6 was determined as cpRBD (Wang et al., 2015). Olfactory function was determined using Sniffin’s sticks Screening 12 Test (SS-12) (Pinkhardt et al., 2019). The overall cognitive function of all patients was assessed using the Mini-Mental State Examination (MMSE). A comprehensive battery of neuropsychological tests was administered by two specialized physicians to examine the following cognitive domains: executive function (Stroop Color-Word Test, SCWT; Trial Making Test-B, TMT-B), attention (Symbol Digital Modalities Test, SDMT; and Trial Making Test-A, TMT-A), visuospatial function (Clock Drawing Task, CDT), memory (Auditory Verbal Learning Test, AVLT), and language (Animal Fluency Test, AFT; Boston Naming Test, BNT) (Song et al., 2020).
In our study, QoL was measured with the 39-item Parkinson’s disease Questionnaire (PDQ-39) (Tsang et al., 2002). The PDQ-39 (or PDQ-39 subdomain) summary index (SI) was standardized from the original PDQ-39 (or PDQ-39 subdomain) scores by dividing the score by the maximum possible points and then, multiplying by 100. The PDQ-39 SI (or PDQ-39 subdomain SI) ranges from 0 to 100, with higher scores representing poorer QoL.
Statistical Analysis
Analyses were conducted using SPSS Statistics 19. Proportions were compared using the chi-square test, and in cases where sample sizes were too small, significance tests were performed using Fisher’s exact test. The Kolmogorov-Smirnov test was used to determine the normality of continuous variable distributions. Because the two groups were not balanced in terms of sex, comparisons of continuous variables between two groups were analyzed by a multiple linear regression model for normally distributed variables, and by a generalized linear model for non-normally distributed variables.
To identify which baseline variables were associated with longitudinal variation in PDQ-39 scores, the generalized linear mixed models (GLMMs) were developed. The GLMMs allowed us to take into account the repeated measurements of PDQ-39 in the same subject, which were not independent but correlated. The baseline and follow-up of PDQ-39 were all included as the dependent variable. The associations between baseline variables (sex, age, education, BMI, disease duration, H and Y stage, UPDRS-III, akinetic-rigid type, ESS, RBDSQ, GDS, SS-12, MMSE, and LED) and PDQ-39 SI were first tested using univariate analysis, and variables with p < 0.2 were then included for further multivariate analyses. As a result, 12 variables (i.e., sex, age, education, disease duration, H and Y stage, UPDRS-III, akinetic-rigid type, RBDSQ, GDS, SS-12, MMSE, and LED) were examined to identify the main predictors of QoL in patients with PD without EDS, while 9 variables (i.e., sex, education, disease duration, H and Y stage, UPDRS-III, akinetic-rigid type, RBDSQ, GDS, and LED) were included to determine the major associated factors of QoL in patients with PD with EDS. Results were considered significant at p < 0.05.
Results
Baseline Demographic and Clinical Characteristics of Patients With Parkinson’s Disease With and Without Excessive Daytime Sleepiness
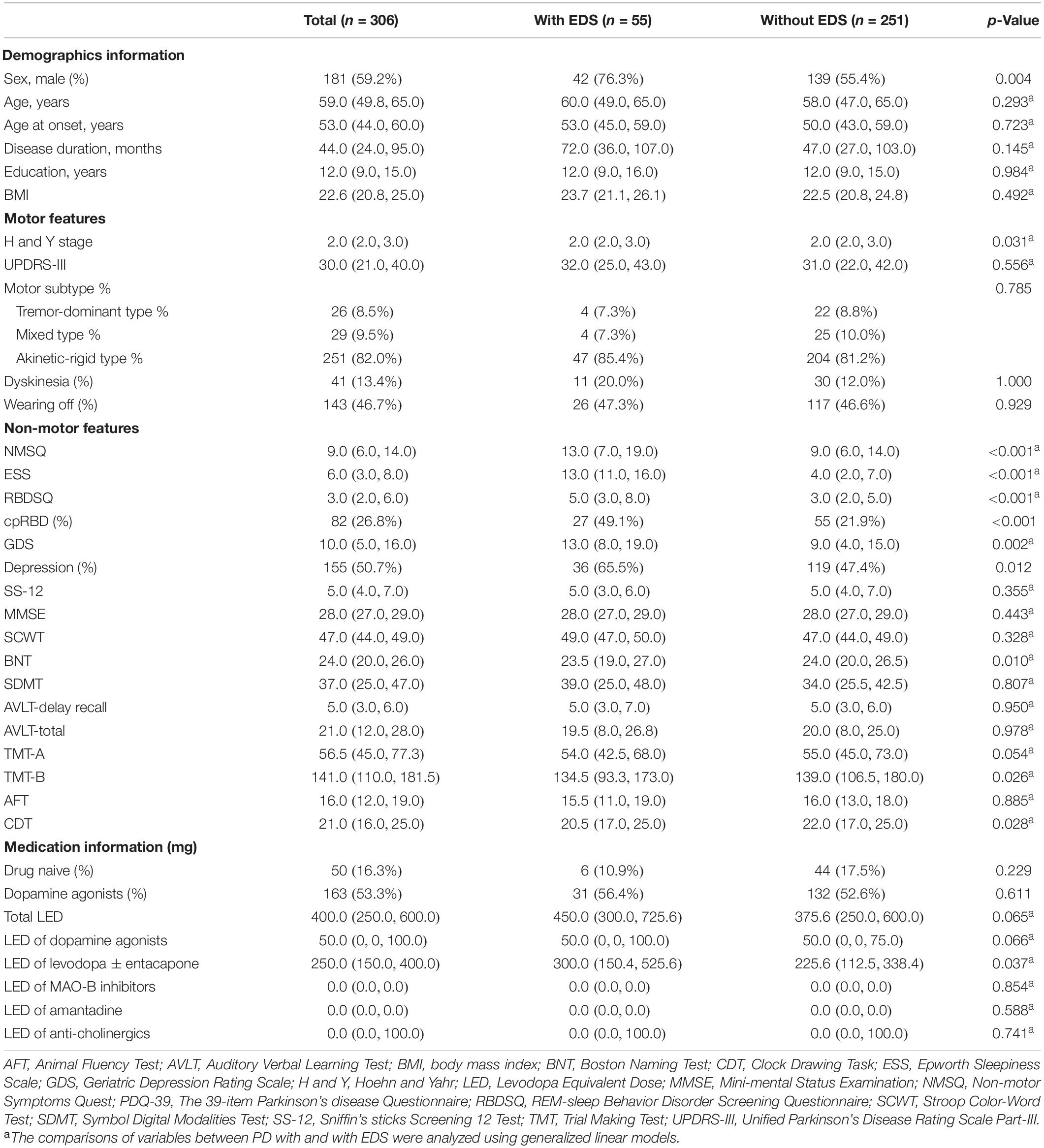
The baseline demographic data and clinical features of patients with PD with (n = 55) and without EDS (n = 251) were shown in Table 1. The PD with EDS group presented with a greater proportion of male patients than the PD without EDS group (76.3% vs. 55.4%, p = 0.004), similar to previous studies (Tholfsen et al., 2015; Zhu et al., 2016). Two groups were consistent in terms of age, age at onset, disease duration, BMI, and education level.
Regarding motor symptoms, the H and Y stage was statistically higher in patients with PD with EDS than in those without EDS, but the comparison of motor severity by UPDRS-III showed no significant difference. In the matter of motor subtypes and motor complications, the frequency of akinetic-rigid type, dyskinesia, and wearing off was similar between the two groups.
As to non-motor features, patients with PD with EDS scored remarkably higher on ESS, NMSQ, RBDSQ, and GDS. Correspondingly, the proportion of cpRBD and depression were increased in patients with PD with EDS, when compared with patients with PD without EDS. Although no significant difference was observed in MMSE, patients with PD with EDS had poorer performance on BNT, TMT-B, and CDT than patients with PD without EDS. With respect to medication use, the proportion of drug-naive and the presence of dopamine agonists use were similar between the two groups. Furthermore, the LEDs of different anti-parkinsonism drugs were compared. The patients with PD with EDS tended to have more compound levodopa (p = 0.037) and a marginally increased use of dopamine agonists (p = 0.066).
Clinical Characteristics of Patients With Parkinson’s Disease With and Without Excessive Daytime Sleepiness at 2 Years
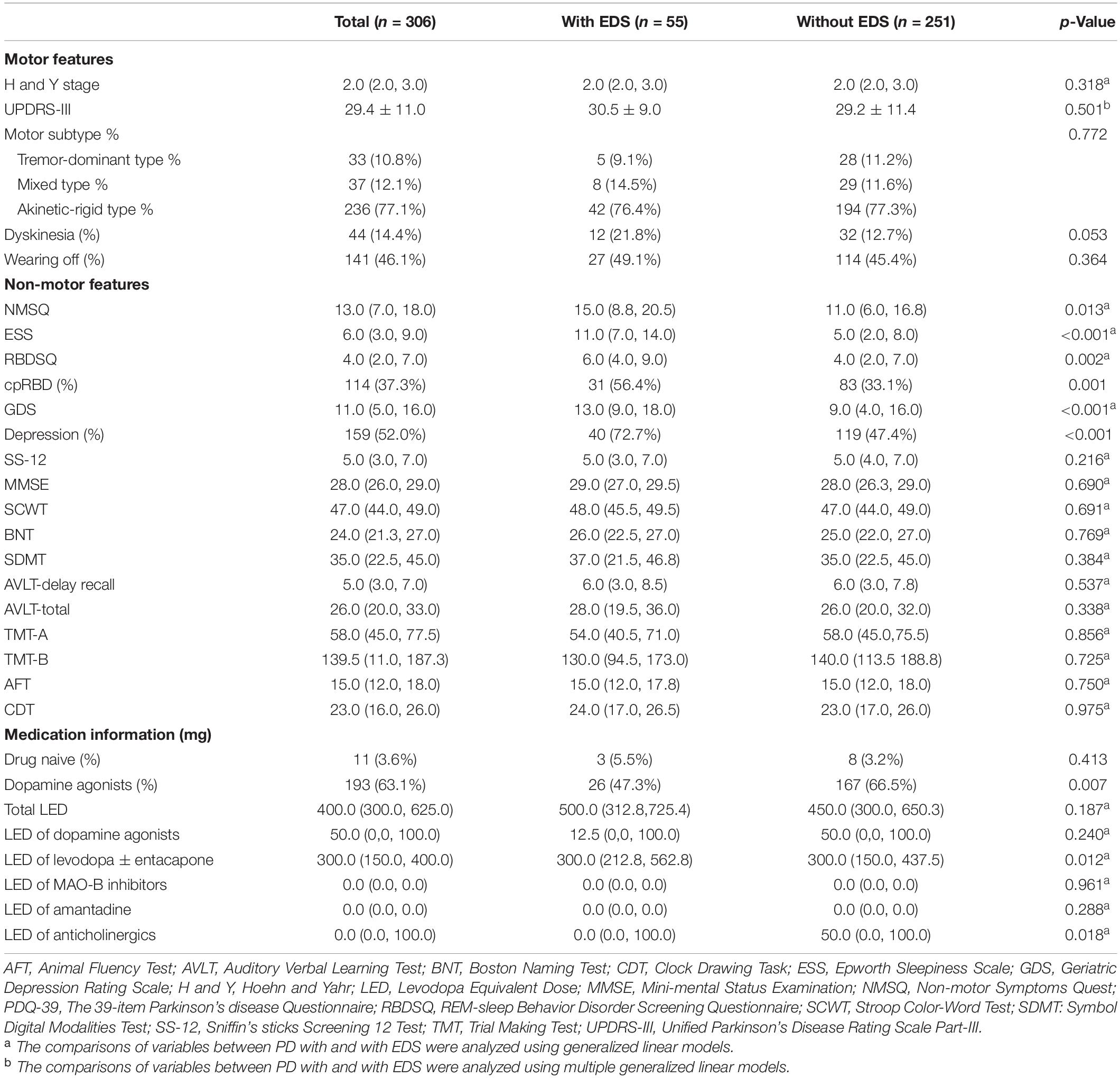
We compared the follow-up clinical information between the two groups (Table 2). The patients with PD with EDS had higher scores on ESS, NMSQ, RBDSQ, and GDS, while cognitive function and motor features were consistent between the two groups. The presence of dopamine agonists use was less in patients with PD with EDS than patients with PD without EDS, perhaps as a result of medication adjustment strategy (p = 0.007). Besides, patients with PD with EDS were prone to take higher doses of compound levodopa (p = 0.012) and lower doses of anticholinergics (p = 0.018).
Longitudinal Assessment of Quality of Life in Patients With Parkinson’s Disease With and Without Excessive Daytime Sleepiness
The assessment of QoL by PDQ-39 was displayed in Table 3. Overall, patients with PD with EDS had poorer QoL both at baseline (PDQ-39 SI, 28.2 ± 16.4 vs. 18.4 ± 13.9, p < 0.001) and at follow-up (PDQ-39 SI, 28.6 ± 17.9 vs. 19.8 ± 15.5, p < 0.001). At baseline, compared with the PD without EDS group, the PD with EDS group got higher scores on almost all domains (except stigma and social support), and the three most impaired domains of PDQ-39 were cognition, bodily discomfort, and mobility. At follow-up, PDQ-39 scores were reduced in all domains in patients with PD with EDS, and the three most impaired domains were cognition, bodily discomfort, and stigma.
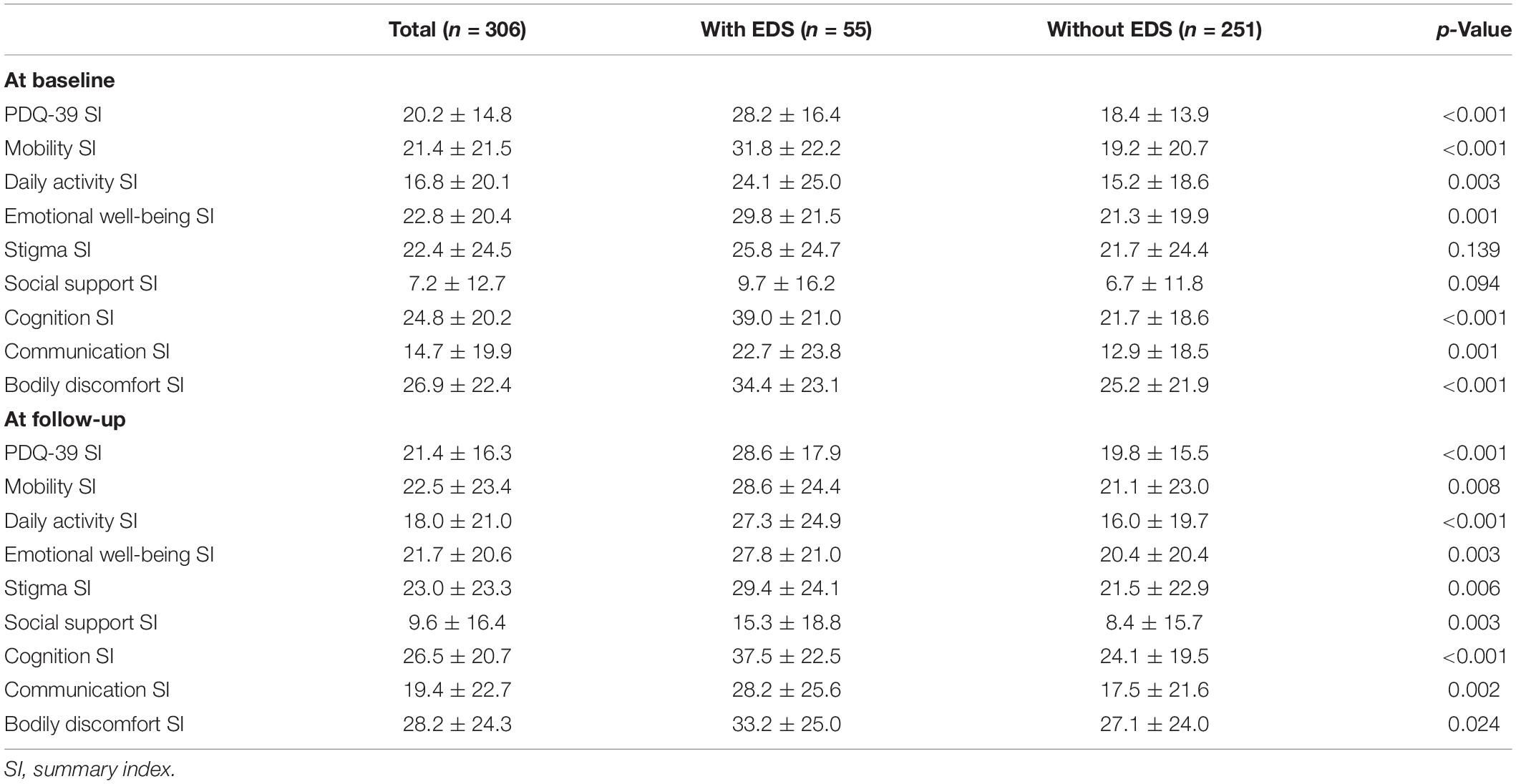
Table 3. Longitudinal assessment of quality of life (QoL) in patients with Parkinson’s diseases (PD) with and without excessive daytime sleepiness (EDS).
Predictors of Quality of Life in Patients With Parkinson’s Disease With and Without Excessive Daytime Sleepiness
The GLMMs analysis showed that greater GDS scores were associated with poorer QoL in all groups. Besides GDS scores, the baseline H and Y stage, MMSE, ESS, and LED remained significant predictors of QoL in the PD group (Table 4). For PD with EDS group, the presence of akinetic-rigid type, disease duration, and total LED at baseline were independently associated with QoL, regardless of relevant factors. As to the PD without EDS group, H and Y stage, MMSE, and RBDSQ were related to reduced QoL.
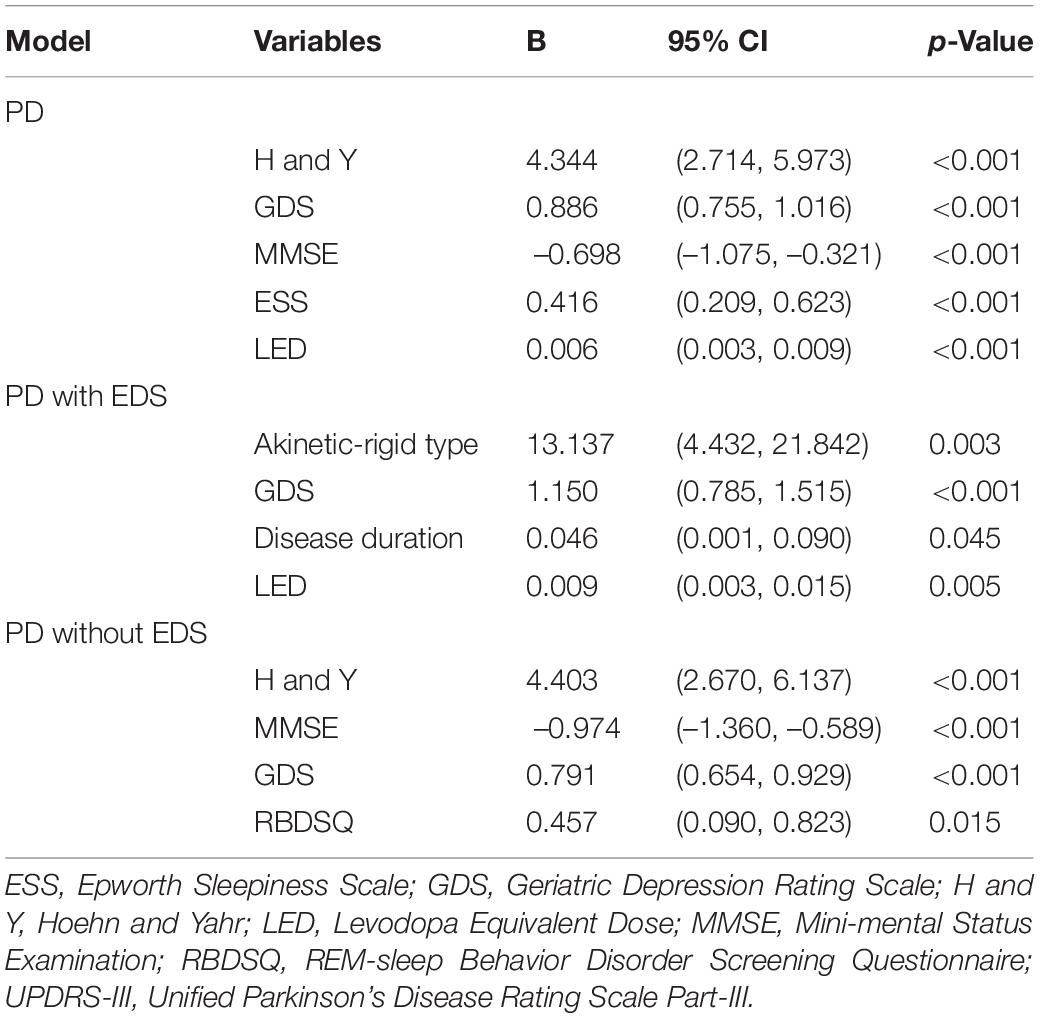
Table 4. Predictors of QoL in PD patients with and without EDS using generalized linear mixed models.
Discussion
This is the first longitudinal study in China exploring the associated factors of QoL in patients with PD with and without EDS. We found that the presence of akinetic-rigid type, as well as disease duration and LED contributed to QoL in patients with PD with EDS. While in patients with PD without EDS, non-motor features including cognitive dysfunction, depression, and cpRBD play more important roles in determining QoL.
We showed that patients with PD with EDS had worse performance in depression and RBD, similar to previous reports (Zhu et al., 2016; Amara et al., 2017; Xiang et al., 2019). All these NMS can be related to neurodegeneration within the brainstem, which is responsible for controlling mood, REM atonia, and sleep-awaken rhythm (Müller and Bohnen, 2013). The expansion of Lewy pathology over time may help explain the association between EDS and other NMS among patients with PD (Abbott et al., 2019). In addition, depression and RBD may profoundly impact the quality of nighttime sleep, which can induce daytime sleepiness as well. As to the relationship between EDS and cognitive deficits, research showed discrepancies (Jester et al., 2019). Studies among de novo patients with PD reported no significant differences in cognitive function between patients with and without EDS (Simuni et al., 2015; Amara et al., 2017). However, in patients with PD with advanced disease stages, those suffering from EDS appeared to have cognitive decline globally and in executive function (Bjornara et al., 2014; Zhu et al., 2016). In our study, patients with PD with EDS displayed worse performance on BNT, TMT-B, and CDT at baseline, but the difference became insignificant at 2-year follow-up. More longitudinal studies are warranted to clarify the association.
One novel finding of our study is that baseline EDS severity was independently related to poor QoL over time in PD after adjusting for relevant clinical factors. The negative impact of EDS on QoL may become prominent as the disease progresses (Gallagher et al., 2010; Yoo et al., 2019). The difficulty to stay awake in activities of daily living may partly account for the association between EDS and worse QoL in patients with PD. Furthermore, the negative impact of EDS on mood, cognition, and other function could also promote a reduction in QoL (Sobreira-Neto et al., 2017). Taken together, it is of great importance for clinicians to recognize EDS early and manage EDS throughout PD.
Our results were in accordance with previous studies suggesting that NMS may play an essential role in determining QoL in PD (Muller et al., 2013; Gokcal et al., 2017). Among all kinds of NMS, depression was shown to be the most consistent predictor of reduced QoL in both PD with and without EDS groups. Since depression is often overlooked by patients and physicians in clinical practice, more attention should be paid to the identification and management of depression to achieve better QoL. In our study, cognitive impairment and cpRBD also produced negative effects on future QoL among patients with PD without EDS. Mild cognitive impairment have been reported to be related to poor QoL in PD by both cross-sectional and longitudinal studies (Lawson et al., 2014, 2016). But the impact of RBD on QoL was inconsistent in different PD research (Suzuki et al., 2013; Kim et al., 2017; Kuhlman et al., 2019) and needs further exploration. These findings suggested that interventions targeting cognitive impairment and RBD might be helpful to improve QoL in patients with PD without EDS. However, we could not exclude the possible ameliorating effect of this treatment in patients with PD with EDS, as this was an observational study.
As to patients with PD with EDS, motor features might exert a more important effect on QoL than NMS. We found that the presence of akinetic-rigid type was significantly associated with a reduction in QoL among patients with PD with EDS. This finding was in line with the predictive role of akinetic-rigid type in progressive disability in patients with PD (Post et al., 2007). The axial symptoms in akinetic-rigid type could become less responsive to pharmacotherapy with disease progression. To retain independence in activities of daily living, more comprehensive measures including physical therapy for fall prevention are highly recommended in this group (Marras et al., 2020).
Previous studies demonstrated that the akinetic-rigid type was more common in early patients with PD with EDS than those without EDS (Amara et al., 2017; Suzuki et al., 2017). Besides, the akinetic-rigid type appeared to have higher ESS scores than the tremor-dominant type, after correcting for relevant factors (Suzuki et al., 2017). The common involvement of non-dopaminergic mechanisms, especially cholinergic dysfunction in akinetic-rigid and EDS pathology, could help explain the association (Müller and Bohnen, 2013). Our study showed that akinetic-rigid type was the predominant motor subtype in PD with EDS group, but we did not observe a significant difference in motor subtypes between the two groups. Perhaps the more advanced stage of our participants may account for the discrepancies.
The LED was another critical determinant of QoL in patients with PD with EDS, which failed to predict QoL among those without EDS. Dopaminergic medications, especially dopamine agonists, were reported to reduce daytime alertness and induce somnolence (Bliwise et al., 2012; Chahine et al., 2017). But other researchers found no difference in ESS scores between different dopaminergic medication and non-dopaminergic medication (Suzuki et al., 2008). Besides, the correlation between dopamine agonist and objective sleepiness evaluated with multiple sleep latency tests was not consistent in different studies (Razmy et al., 2004; Micallef et al., 2009). Thus, studies with both objective and subjective evaluation of daytime sleepiness are needed to verify the effect of different dopamine agonists on EDS. In clinical practice, non-dopaminergic drugs or non-pharmacological interventions should be considered, especially for patients with PD with EDS. This medication strategy may account for the phenomenon that the presence of dopamine agonist use in PD with EDS group was reduced at a 2-year follow-up in our study.
The strengths of our study involve the longitudinal design and comprehensive clinical assessments. In addition, this is the first study to investigate the influence of clinical factors on QoL across positive/negative EDS statuses in PD. We acknowledge some limitations in the current study. One limitation is the short duration of follow-up. There were only slight changes in PDQ-39 scores during the 2-year follow-up and EDS is reported to be non-persistent at an early stage. A long-term follow-up would help reduce the confounding effect of time and initial medication adjustment. Another limitation is the lack of objective methods for evaluating EDS, such as polysomnography (PSG) since subjective assessments of EDS are not always consistent with objective assessment. The PSG may also serve as an effective tool to identify patients with EDS patients caused by sleep-related respiratory disorders. And there was no evaluation of nocturnal sleep quality and nocturnal sleep disturbances in our study. However, some previous studies suggested that EDS in PD was dependent on the disease itself rather than nocturnal disturbances (Suzuki et al., 2008). Lastly, the sex compositions were not balanced between the two groups. However, the higher proportion of male patients in the PD with EDS group was also reported by previous studies, and the effect of sex was taken into account when performing the comparison between groups.
Conclusion
We concluded that the baseline predictors of future QoL differed between patients with PD with and without EDS. The presence of akinetic-rigid type was a critical predictor of reduced QoL in patients with PD with EDS, thus, comprehensive measures were recommended in such patients. As to patients with PD without EDS, more attention should be paid to NMS, including cognitive decline and RBD. The different predictors of QoL and their relationships with EDS need to be confirmed in future trials with larger sample size, long follow-up, and validated evaluation methods.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the Human Studies Institutional Review Board, Huashan Hospital, Fudan University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
LZ: conceptualization, methodology, formal analysis, writing – original draft preparation, and writing – review and editing. YC: conceptualization, methodology, formal analysis, writing – original draft preparation, writing – review and editing and funding acquisition. XL and LW: data curation, validation, formal analysis, and investigation. JW: supervision, project administration, and funding acquisition. YT: writing – review and editing, supervision, project administration, and funding acquisition. XZ: supervision and project administration. All authors involved have read and approved the submitted version.
Funding
This work was supported by the Project from the Ministry of Science and Technology of China (grant number: 2016YFC1306504), the Grants from the National Natural Science Foundation of China (grant number: 81801260) and Fundamental Research Program Funding of Ninth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (JYZZ155).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbott, R. D., Ross, G. W., Duda, J. E., Shin, C., Uyehara-Lock, J. H., Masaki, K. H., et al. (2019). Excessive daytime sleepiness and topographic expansion of Lewy pathology. Neurology 93, e1425–e1432. doi: 10.1212/WNL.0000000000008241
Amara, A. W., Chahine, L. M., Caspell-Garcia, C., Long, J. D., Coffey, C., Hogl, B., et al. (2017). Longitudinal assessment of excessive daytime sleepiness in early Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 88, 653–662. doi: 10.1136/jnnp-2016-315023
Bjornara, K. A., Dietrichs, E., and Toft, M. (2014). Clinical features associated with sleep disturbances in Parkinson’s disease. Clin. Neurol. Neurosurg. 124, 37–43. doi: 10.1016/j.clineuro.2014.06.027
Bliwise, D. L., Trotti, L. M., Wilson, A. G., Greer, S. A., Wood-Siverio, C., Juncos, J. J., et al. (2012). Daytime alertness in Parkinson’s disease: potentially dose-dependent, divergent effects by drug class. Mov. Disord. 27, 1118–1124. doi: 10.1002/mds.25082
Chahine, L. M., Amara, A. W., and Videnovic, A. (2017). A systematic review of the literature on disorders of sleep and wakefulness in Parkinson’s disease from 2005 to 2015. Sleep Med. Rev. 35, 33–50. doi: 10.1016/j.smrv.2016.08.001
Chaudhuri, K. R., Martinez-Martin, P., Schapira, A. H., Stocchi, F., Sethi, K., Odin, P., et al. (2006). International multicenter pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson’s disease: the NMSQuest study. Mov. Disord. 21, 916–923. doi: 10.1002/mds.20844
Chen, N. H., Johns, M. W., Li, H. Y., Chu, C. C., Liang, S. C., Shu, Y. H., et al. (2002). Validation of a Chinese version of the Epworth sleepiness scale. Qual. Life Res. 11, 817–821. doi: 10.1023/a:1020818417949
Ertan, F. S., Ertan, T., Kiziltan, G., and Uygucgil, H. (2005). Reliability and validity of the geriatric depression scale in depression in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry. 76, 1445–1447. doi: 10.1136/jnnp.2004.057984
Fahn, S. (1987). “Unified Parkinson’s disease rating scale,” in Recent Developments in Parkinson’s Disease, Vol. 2, eds S. Fahn, C. D. Marsden, D. B. Calne, and M. Goldstein (Florham Park, NJ: Macmillan Health Care Information), 293–304.
Feng, F., Cai, Y., Hou, Y., Ou, R., Jiang, Z., and Shang, H. (2021). Excessive daytime sleepiness in Parkinson’s disease: a systematic review and meta-analysis. Parkinsonism Relat. Disord. 85, 133–140. doi: 10.1016/j.parkreldis.2021.02.016
Gallagher, D. A., Lees, A. J., and Schrag, A. (2010). What are the most important nonmotor symptoms in patients with Parkinson’s disease and are we missing them? Mov. Disord. 25, 2493–2500. doi: 10.1002/mds.23394
Gokcal, E., Gur, V. E., Selvitop, R., Babacan Yildiz, G., and Asil, T. (2017). Motor and non-motor symptoms in Parkinson’s disease: effects on quality of life. Noro Psikiyatr. Ars. 54, 143–148. doi: 10.5152/npa.2016.12758
Hughes, A. J., Daniel, S. E., Kilford, L., and Lees, A. J. (1992). Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 55, 181–184. doi: 10.1136/jnnp.55.3.181
Jester, D. J., Lee, S., Molinari, V., and Volicer, L. (2019). Cognitive deficits in Parkinson’s disease with excessive daytime sleepiness: a systematic review. Aging Ment. Health 24, 1769–1780. doi: 10.1080/13607863.2019.1660852
Kim, K. T., Motamedi, G. K., and Cho, Y. W. (2017). Quality of life in patients with an idiopathic rapid eye movement sleep behaviour disorder in Korea. J. Sleep Res. 26, 422–427. doi: 10.1111/jsr.12486
Kuhlman, G. D., Flanigan, J. L., Sperling, S. A., and Barrett, M. J. (2019). Predictors of health-related quality of life in Parkinson’s disease. Parkinsonism Relat. Disord. 65, 86–90. doi: 10.1016/j.parkreldis.2019.05.009
Lawson, R. A., Yarnall, A. J., Duncan, G. W., Breen, D. P., Khoo, T. K., Williams-Gray, C. H., et al. (2016). Cognitive decline and quality of life in incident Parkinson’s disease: the role of attention. Parkinsonism Relat. Disord. 27, 47–53. doi: 10.1016/j.parkreldis.2016.04.009
Lawson, R. A., Yarnall, A. J., Duncan, G. W., Khoo, T. K., Breen, D. P., Barker, R. A., et al. (2014). Severity of mild cognitive impairment in early Parkinson’s disease contributes to poorer quality of life. Parkinsonism Relat. Disord. 20, 1071–1075. doi: 10.1016/j.parkreldis.2014.07.004
Marras, C., Chaudhuri, K., Titova, N., and Mestre, T. (2020). Therapy of Parkinson’s disease subtypes. Neurotherapeutics 17, 1366–1377. doi: 10.1007/s13311-020-00894-7
Micallef, J., Rey, M., Eusebio, A., Audebert, C., Rouby, F., Jouve, E., et al. (2009). Antiparkinsonian drug-induced sleepiness: a double-blind placebo-controlled study of L-dopa, bromocriptine and pramipexole in healthy subjects. Br. J. Clin. Pharmacol. 67, 333–340. doi: 10.1111/j.1365-2125.2008.03310.x
Muller, B., Assmus, J., Herlofson, K., Larsen, J. P., and Tysnes, O. B. (2013). Importance of motor vs. non-motor symptoms for health-related quality of life in early Parkinson’s disease. Parkinsonism Relat. Disord. 19, 1027–1032.
Müller, M., and Bohnen, N. (2013). Cholinergic dysfunction in Parkinson’s disease. Curr. Neurol. Neurosci. Rep. 13:377. doi: 10.1007/s11910-013-0377-9
Palmeri, R., Lo Buono, V., Bonanno, L., Sorbera, C., Cimino, V., Bramanti, P., et al. (2019). Potential predictors of quality of life in Parkinson’s disease: sleep and mood disorders. J. Clin. Neurosci. 70, 113–117. doi: 10.1016/j.jocn.2019.08.058
Pinkhardt, E. H., Liu, H., Ma, D., Chen, J., Pachollek, A., Kunz, M. S., et al. (2019). Olfactory screening of Parkinson’s disease patients and healthy subjects in China and Germany: a study of cross-cultural adaptation of the Sniffin’ Sticks 12-identification test. PLoS One 14:e0224331. doi: 10.1371/journal.pone.0224331
Post, B., Merkus, M., de Haan, R., and Speelman, J. (2007). Prognostic factors for the progression of Parkinson’s disease: a systematic review. Mov. Disord. 22, 1839–1851; quiz1988. doi: 10.1002/mds.21537
Razmy, A., Lang, A., and Shapiro, C. (2004). Predictors of impaired daytime sleep and wakefulness in patients with Parkinson disease treated with older (ergot) vs newer (nonergot) dopamine agonists. Arch. Neurol. 61, 97–102. doi: 10.1001/archneur.61.1.97
Schiess, M., Zheng, H., Soukup, V., Bonnen, J., and Nauta, H. (2000). Parkinson’s disease subtypes: clinical classification and ventricular cerebrospinal fluid analysis. Parkinsonism Relat. Disord. 6, 69–76.
Simuni, T., Caspell-Garcia, C., Coffey, C., Chahine, L. M., Lasch, S., Oertel, W. H., et al. (2015). Correlates of excessive daytime sleepiness in de novo Parkinson’s disease: a case control study. Mov. Disord. 30, 1371–1381. doi: 10.1002/mds.26248
Sobreira-Neto, M. A., Pena-Pereira, M. A., Sobreira, E. S. T., Chagas, M. H. N., Fernandes, R. M. F., Tumas, V., et al. (2017). High frequency of sleep disorders in Parkinson’s disease and its relationship with quality of life. Eur. Neurol. 78, 330–337. doi: 10.1159/000481939
Song, J., Shen, B., Yang, Y. J., Liu, F. T., Zhao, J., Tang, Y. L., et al. (2020). Non-motor symptoms in Parkinson’s disease patients with Parkin mutations: more depression and less executive dysfunction. J. Mol. Neurosci. 70, 246–253. doi: 10.1007/s12031-019-01444-3
Sun, Q., Wang, T., Jiang, T. F., Huang, P., Wang, Y., Xiao, Q., et al. (2018). Clinical profile of Chinese long-term Parkinson’s disease survivors with 10 years of disease duration and beyond. Aging Dis. 9, 8–16. doi: 10.14336/AD.2017.0204
Suzuki, K., Miyamoto, T., Miyamoto, M., Okuma, Y., Hattori, N., Kamei, S., et al. (2008). Excessive daytime sleepiness and sleep episodes in Japanese patients with Parkinson’s disease. J. Neurol. Sci. 271, 47–52. doi: 10.1016/j.jns.2008.03.008
Suzuki, K., Miyamoto, T., Miyamoto, M., Watanabe, Y., Suzuki, S., Tatsumoto, M., et al. (2013). Probable rapid eye movement sleep behavior disorder, nocturnal disturbances and quality of life in patients with Parkinson’s disease: a case-controlled study using the rapid eye movement sleep behavior disorder screening questionnaire. BMC Neurol. 13:18. doi: 10.1186/1471-2377-13-18
Suzuki, K., Okuma, Y., Uchiyama, T., Miyamoto, M., Sakakibara, R., Shimo, Y., et al. (2017). Impact of sleep-related symptoms on clinical motor subtypes and disability in Parkinson’s disease: a multicentre cross-sectional study. J. Neurol. Neurosurg. Psychiatry 88, 953–959. doi: 10.1136/jnnp-2017-316136
Thobois, S. (2006). Proposed dose equivalence for rapid switch between dopamine receptor agonists in Parkinson’s disease: a review of the literature. Clin. Ther. 28, 1–12. doi: 10.1016/j.clinthera.2005.12.003
Tholfsen, L. K., Larsen, J. P., Schulz, J., Tysnes, O. B., and Gjerstad, M. D. (2015). Development of excessive daytime sleepiness in early Parkinson disease. Neurology 85, 162–168. doi: 10.1212/WNL.0000000000001737
Tsang, K. L., Chi, I., Ho, S. L., Lou, V. W., Lee, T. M., and Chu, L. W. (2002). Translation and validation of the standard Chinese version of PDQ-39: a quality-of-life measure for patients with Parkinson’s disease. Mov. Disord. 17, 1036–1040. doi: 10.1002/mds.10249
Wang, Y., Wang, Z. W., Yang, Y. C., Wu, H. J., Zhao, H. Y., and Zhao, Z. X. (2015). Validation of the rapid eye movement sleep behavior disorder screening questionnaire in China. J. Clin. Neurosci. 22, 1420–1424. doi: 10.1016/j.jocn.2015.03.008
Xiang, Y. Q., Xu, Q., Sun, Q. Y., Wang, Z. Q., Tian, Y., Fang, L. J., et al. (2019). Clinical features and correlates of excessive daytime sleepiness in Parkinson’s disease. Front. Neurol. 10:121. doi: 10.3389/fneur.2019.00121
Yoo, S. W., Kim, J. S., Oh, Y. S., Ryu, D. W., and Lee, K. S. (2019). Excessive daytime sleepiness and its impact on quality of life in de novo Parkinson’s disease. Neurol. Sci. 40, 1151–1156. doi: 10.1007/s10072-019-03785-8
Keywords: Parkinson’s disease, excessive daytime sleepiness, quality of life, non-motor symptoms, motor subtype
Citation: Zhang L, Chen Y, Liang X, Wang L, Wang J, Tang Y and Zhu X (2022) Prediction of Quality of Life in Patients With Parkinson’s Disease With and Without Excessive Daytime Sleepiness: A Longitudinal Study. Front. Aging Neurosci. 14:846563. doi: 10.3389/fnagi.2022.846563
Received: 31 December 2021; Accepted: 08 March 2022;
Published: 13 April 2022.
Edited by:
Bogdan O. Popescu, Carol Davila University of Medicine and Pharmacy, RomaniaReviewed by:
Keisuke Suzuki, Dokkyo Medical University, JapanTomoyuki Miyamoto, Dokkyo Medical University Koshigaya Hospital, Japan
Copyright © 2022 Zhang, Chen, Liang, Wang, Wang, Tang and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yilin Tang, dGFuZ3lpbGluQGZ1ZGFuLmVkdS5jbg==; Xiaodong Zhu, enhkMzUxNkB0bXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Lixia Zhang1†
Lixia Zhang1† Yajing Chen
Yajing Chen Xiaoniu Liang
Xiaoniu Liang Lan Wang
Lan Wang Jian Wang
Jian Wang Yilin Tang
Yilin Tang Xiaodong Zhu
Xiaodong Zhu