- College of Basic Medical Science, Zhejiang Chinese Medical University, Hangzhou, China
Objectives: The association between gout and dementia, Alzheimer's disease (AD), or vascular dementia (VD) is not fully understood. The aim of this meta-analysis was to evaluate the risk of all-cause dementia, AD, and VD in gout patients with or without medication.
Methods: Data sources were PubMed, Embase, the Cochrane Library, and reference lists of included studies. This meta-analysis included cohort studies assessing whether the risk of all-cause dementia, AD, and VD was associated with gout. The risk of bias was assessed using the Newcastle-Ottawa Quality Assessment Scale (NOS). The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system was used to access the overall certainty of evidence. Risk ratios (RR) with 95% confidence intervals (CI) were pooled using a random-effects model, and publication bias was assessed with funnel plots and Egger's test.
Results: A total of six cohort studies involving 2,349,605 individuals were included in this meta-analysis, which were published between 2015 and 2022. The pooling analysis shows that the risk of all-cause dementia was decreased in gout patients [RR = 0.67, 95% CI (0.51, 0.89), I2 = 99%, P = 0.005, very low quality], especially in gout patients with medication [RR = 0.50, 95% CI (0.31, 0.79), I2 = 93%, P = 0.003, low quality]. The risk of AD [RR = 0.70, 95% CI (0.63, 0.79), I2 = 57.2%, P = 0.000, very low quality] and VD [RR = 0.68, 95% CI (0.49, 0.95), I2 = 91.2%, P = 0.025, very low quality] was also decreased in gout patients. Despite the large heterogeneity, the sensitivity analysis indicated that the results were robust, and there was little evidence of publication bias.
Conclusion: The risk of all-cause dementia, AD, and VD is decreased in gout patients, but the quality of evidence is generally low. More studies are still needed to validate and explore the mechanisms of this association.
Systematic review registration: https://www.crd.york.ac.uk/prospero/#recordDetails, identifier: CRD42022353312.
1. Introduction
Dementia is one of the most common diseases with high morbidity, mortality, and reduced quality of life, especially in the elderly population (Frederiksen et al., 2020). Notably, the number of dementia patients or related cognitive impairment is expected to increase to 115 million by 2050 as the world enters an aging era (Bai et al., 2022). A study that estimated the prevalence of young-onset dementia using a large study population and multiple national health datasets showed a trend toward younger onset of dementia (Ryan et al., 2022). Currently, approximately two-thirds of dementia patients live in low- and middle-income countries, and the average total national expenditure on dementia is accounting for 0.45% of the national gross domestic product (GDP) in these countries. The cost of dementia care increases with the severity of dementia and the number of comorbidities, with total national dementia costs estimated to range from $1.04 million per year in Vanuatu to $150 billion per year in China (Mattap et al., 2022). Therefore, it is important to actively and effectively prevent the onset and progression of dementia and its related diseases such as Alzheimer's disease (AD) and vascular dementia (VD).
Gout is a debilitating disease with long-term complications, including joint damage and urate deposit stones (Neilson et al., 2022). It is estimated that there are ~41.2 million prevalent gout cases globally, with 7.4 million gout cases annually and nearly 1.3 million disabled gout patients in 2017 (Safiri et al., 2020). Gout is the most common inflammatory arthritis in adults. Recently, the dysregulation of the immune system has received much attention in the pathogenesis of neurodegenerative diseases and is even considered as important as the classical hypothesis of pathological protein aggregation. A prospective cohort study of 375,894 individuals showed that immune-mediated disorders were significantly associated with an increased risk of dementia (Zhang et al., 2022). In addition, several observational studies have reported that gout and hyperuricemia might increase the risk of dementia, although the conclusions of different studies are inconsistent (Hong et al., 2015; Lu et al., 2016; Singh and Cleveland, 2018; Chuang et al., 2021; Mikhailichenko et al., 2021; Min et al., 2021; Dehlin et al., 2022; Kim et al., 2022). One recent meta-analysis on a similar topic has been published. This meta-analysis by Pan et al. included four cohorts and found that gout and hyperuricemia did not increase the risk of dementia but might decrease the risk of AD (Pan et al., 2021). However, this pooled analysis included patients with high uric acid who were not clearly diagnosed with gout (Latourte et al., 2018), and there may have been patient selection bias. Furthermore, the inclusion of gout patients with or without medication intervention during the follow-up period was not properly differentiated. Recently, several large samples and well-designed cohort studies involving new evidence about the risk of all-cause dementia have been published (Chuang et al., 2021; Min et al., 2021; Kim et al., 2022). Because of the importance of the issue, the limitations of the previous review, and the availability of new evidence, we conducted a systematic review and meta-analysis to evaluate the association between gout alone and the risk of all-cause dementia.
2. Methods
This meta-analysis is reported according to the updated guidance and exemplars for reporting systematic reviews (PRISMA 2020 statement; Page et al., 2021), and our protocol was registered on PROSPERO (CRD42022353312).
2.1. Data sources
PubMed, EMBASE, and Cochrane Library were searched without any restrictions from inception to 16 August 2022. The subject terms (Emtree in Embase, MeSH in PubMed) and corresponding keywords were used. Search terms included those related to dementia, Alzheimer's disease, and gout and its variants. The reference lists of retrieved studies and previous meta-analyses were also checked to identify other studies that might be eligible for inclusion. The full search strategy for these databases is provided in Supplementary Tables 1–3.
2.2. Study selection
The retrieved initial records were imported into NoteExpress reference management software, and duplicate records were removed. In total, two authors (XL Li and XM Hu) independently reviewed titles and abstracts to exclude irrelevant records and then classified the remaining records according to inclusion, exclusion, or uncertainty. For records that were uncertain, the full text was read to ensure eligibility for inclusion. Any disputes were resolved through group discussion.
2.3. Eligibility criteria
Studies were considered eligible if they met the following criteria: (a) patients: dementia, Alzheimer's disease (AD), or vascular dementia (VD); (b) exposure: a definitive diagnosis of gout, not just hyperuricemia, which is only a biochemical precursor to gout; (c) comparator: healthy people or non-gout sufferers; (d) outcomes: a presentation of quantitative point estimates [hazard ratios (HRs), relative risks (RRs), or odds ratios (ORs)] and a variance of the estimates of the association between gout and the risk of dementia, AD, or VD and also a description of adjustment for potential confounders (Shang et al., 2021; Cao et al., 2022); and (e) type of study: cohort study, either prospective or retrospective.
The exclusion criteria were as follows: (a) conference abstracts or letters to editors; (b) duplicate publication; (c) patients diagnosed with increased uric acid or hyperuricemia; and (d) incomplete data or no interested outcome.
2.4. Data extraction
We designed a data extraction form in Excel (Microsoft Corporation, USA). In total, two authors (XL Li and YJ Tang) independently extracted information from eligible cohorts. The following data were obtained from each study: first author, time of publication, country, number of events, number of exposures, confounders, and so on. The extracted data were cross-checked, and disagreements were resolved through discussion.
2.5. Study quality
The Newcastle-Ottawa Quality Assessment Scale (NOS; Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp) was used to evaluate the quality of the included studies from three aspects: selection, comparison, and outcomes. Scores for cohort and case–control studies ranged from 0 to 9 stars, with higher stars indicating higher study quality. NOS stars ≥7, 4–6, and 0–3 were of high, moderate, and low quality, respectively.
2.6. Evidence certainty
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system (Balshem et al., 2011) was used to access the overall certainty of evidence. By the GRADE system, the certainty of evidence derived from cohort studies receives an initial grade of low quality. The quality of evidence from cohort studies can be improved at larger effect sizes (RR ≥ 2 or ≤ 0.5), dose–response gradients, or attenuation by plausible confounding after excluding various factors that could lead to downgrading (Guyatt et al., 2011). Finally, the evidence of outcomes can be graded as high, moderate, low, or very low.
2.7. Data synthesis
The Stata software (version 14) was used to conduct the data analysis. We assessed heterogeneity using the chi-square test and I2 value, and P < 0.1 or I2 > 50% indicated that heterogeneity was great, and thus, the random-effects model was adopted (Higgins et al., 2003). Otherwise, a fixed-effect model was employed according to a previous high-quality meta-analysis (Lei et al., 2022; Qu et al., 2022). The sensitivity analysis was performed to verify the robustness of the overall results and to explore sources of heterogeneity. The subgroup analysis was performed according to whether patients with gout received medication intervention. Finally, funnel plots, Begg's test, and Egger's regression test were used to detect publication bias (Egger et al., 1997).
3. Results
3.1. Study selection
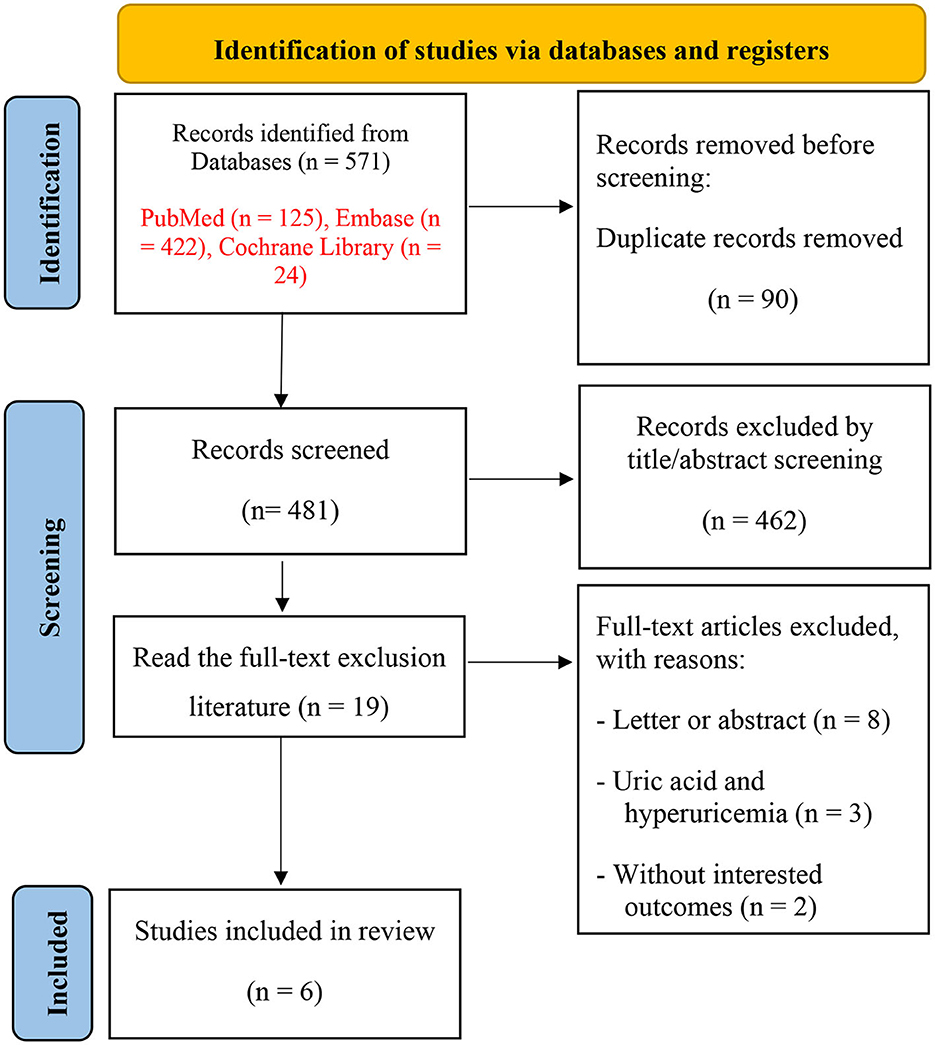
Our search yielded 571 related records, and 90 were excluded due to duplication. Of these, 462 were eliminated by screening titles and abstracts for being irrelevant to our topic. The remaining 19 studies were scrutinized for further assessment. Finally, a total of six cohort studies (Hong et al., 2015; Lu et al., 2016; Singh and Cleveland, 2018; Chuang et al., 2021; Min et al., 2021; Kim et al., 2022) were included in the meta-analysis. The study selection process is shown in Figure 1.
3.2. Study characteristics
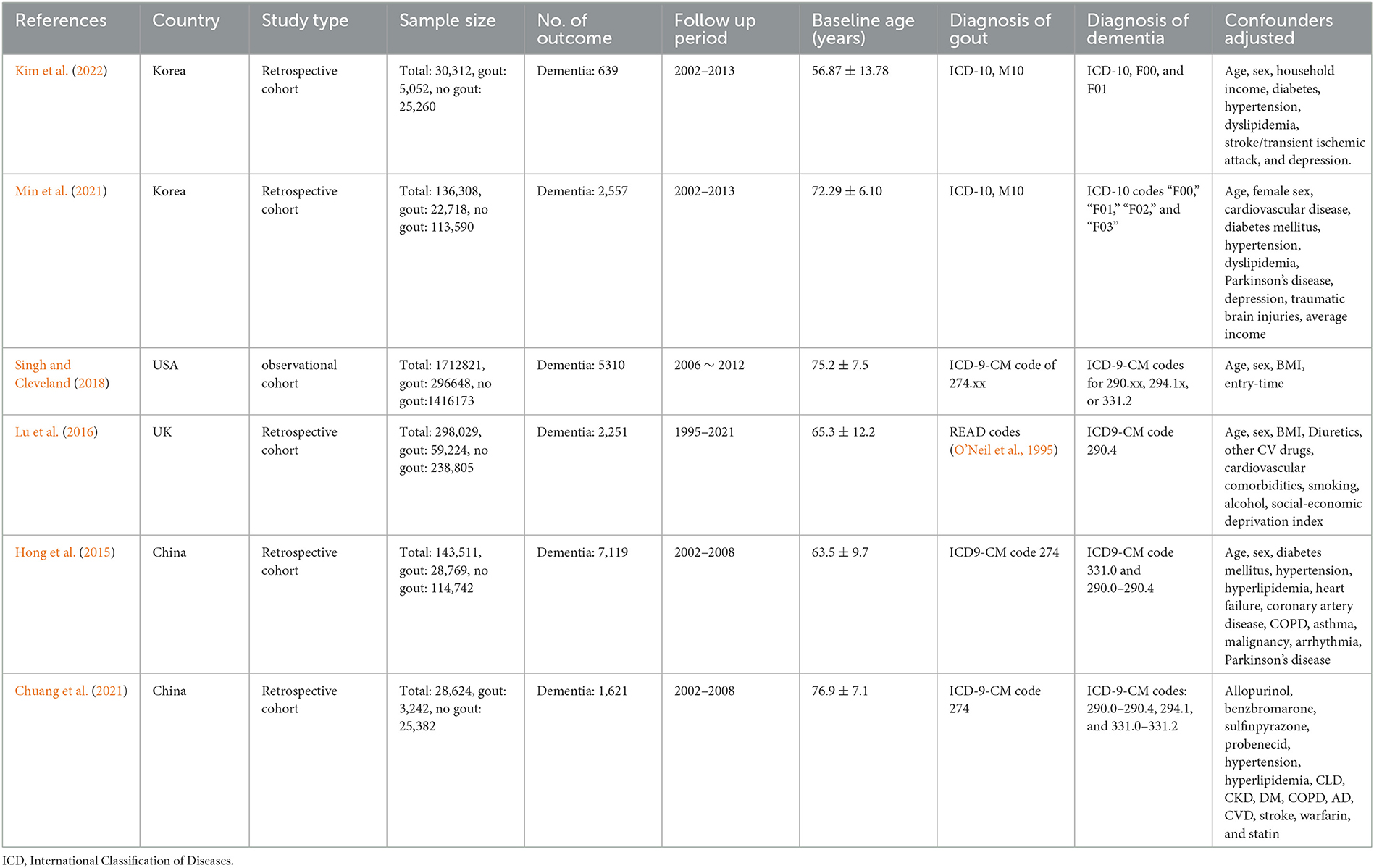
A total of six cohort studies (Hong et al., 2015; Lu et al., 2016; Singh and Cleveland, 2018; Chuang et al., 2021; Min et al., 2021; Kim et al., 2022) involving 2,349,605 individuals (415,653 with gout and 19,497 with dementia events) were included. The studies were published between 2015 and 2022, with the sample size ranging from 28,624 to 1,712,821, and the follow-up years were from 2.3 to 11 years. In total, two studies were conducted in Korea (Min et al., 2021; Kim et al., 2022), two in China (Hong et al., 2015; Chuang et al., 2021), and another two were performed in the United States (Singh and Cleveland, 2018) and the United Kingdom (Lu et al., 2016), respectively. In the original study included, the diagnosis of dementia and gout was mostly classified and diagnosed according to the standard of “International Classification of Diseases (ICD).” The adjusted confounders are slightly different in included studies, and age, sex, and body mass index (BMI) were the most common adjusted confounders. The characteristics of six cohort studies are summarized in Table 1.
3.3. Quality assessment
All six cohorts (Hong et al., 2015; Lu et al., 2016; Singh and Cleveland, 2018; Chuang et al., 2021; Min et al., 2021; Kim et al., 2022) have a score of ≥7, which indicates that the studies included in this meta-analysis are of high quality. Details of the risk of bias are summarized in Table 2.
3.4. Gout and risk of dementia
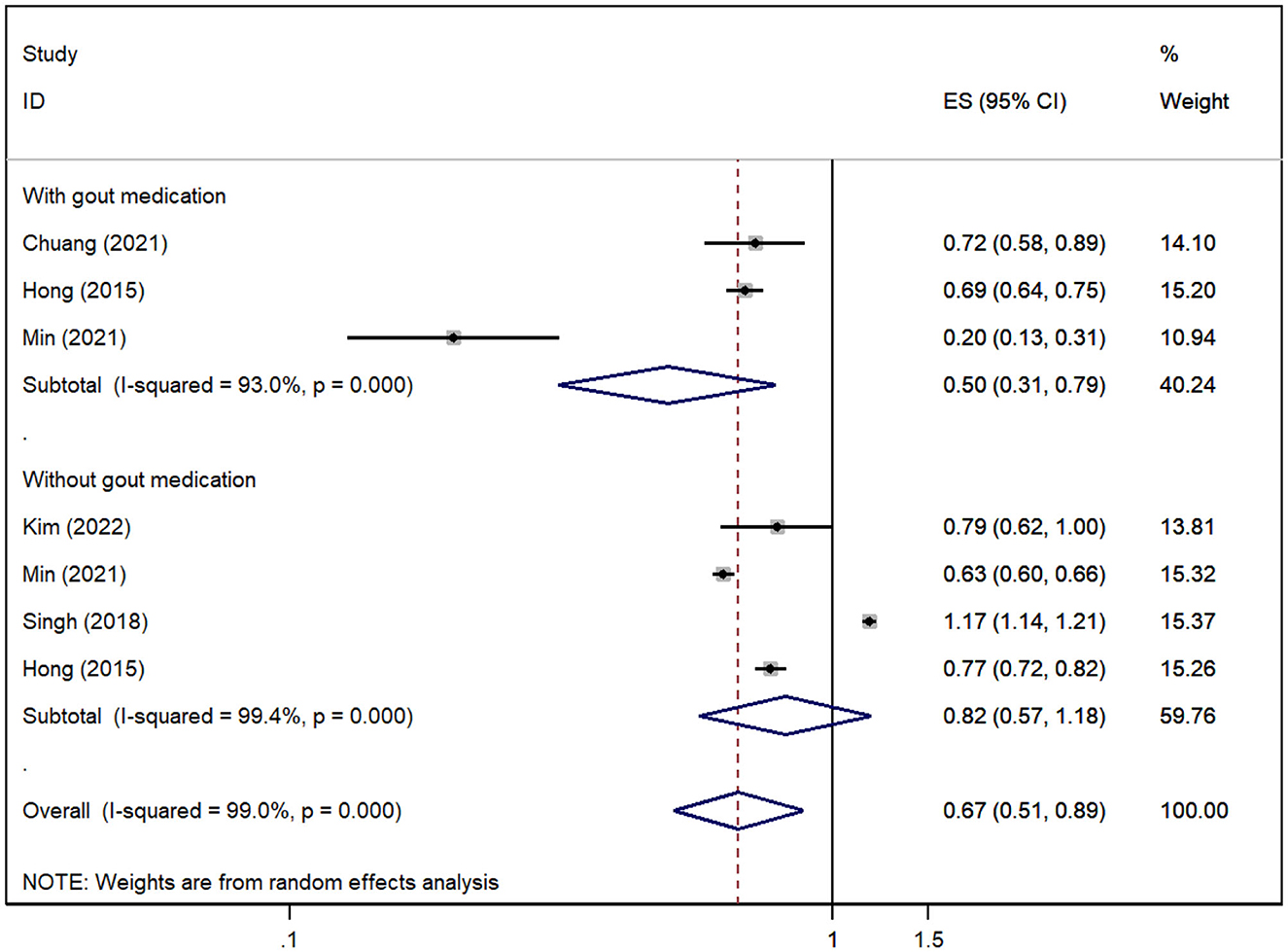
A total of five cohorts (Hong et al., 2015; Singh and Cleveland, 2018; Chuang et al., 2021; Min et al., 2021; Kim et al., 2022) reported the risk of all-cause dementia in gout patients, and the pooled analysis suggested that the risk of all-cause dementia was decreased in gout patients [RR = 0.67, 95% CI (0.51, 0.89), I2 = 99%, P = 0.005; Figure 2]. Due to significant heterogeneity, we performed sensitivity analysis by removing each study to explore the source of heterogeneity, which suggested that the results were stable after excluding any one study, suggesting that the results of our meta-analysis were robust (Supplementary Figure 1). However, the results for subgroups stratified according to whether they had medication interventions for gout were inconsistent. The risk of all-cause dementia was significantly reduced when gout patients were treated with medication (Hong et al., 2015; Chuang et al., 2021; Min et al., 2021) [RR = 0.50, 95% CI (0.31, 0.79), I2 = 93%, P = 0.003; Figure 2], but no association was found when no medication was administered in gout patients (Hong et al., 2015; Singh and Cleveland, 2018; Min et al., 2021; Kim et al., 2022) [RR = 0.82, 95% CI (0.57, 1.18), I2 = 99.4%, P = 0.285; Figure 2].
3.5. Gout and risk of AD
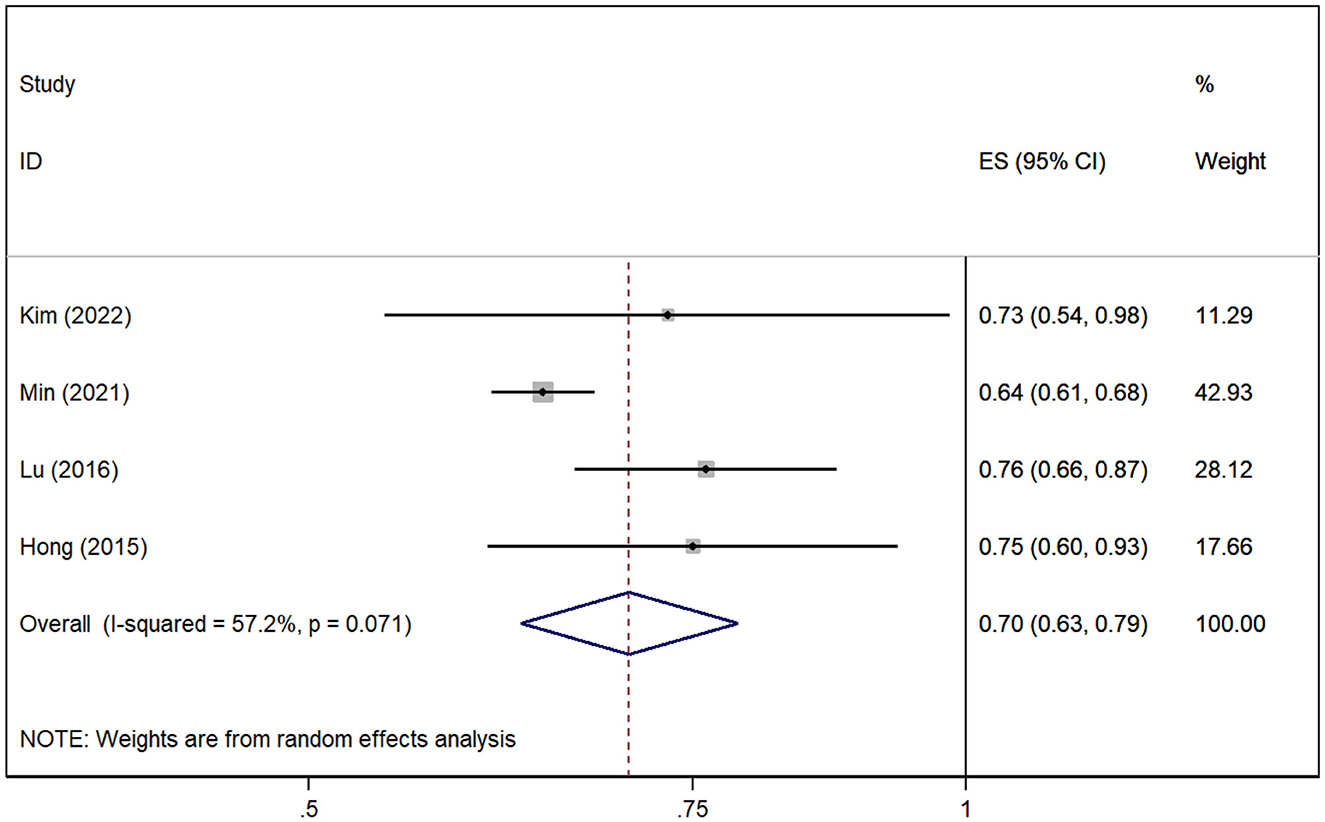
A total of four cohort studies (Hong et al., 2015; Lu et al., 2016; Min et al., 2021; Kim et al., 2022) assessed the association between gout without medication and the risk of AD. The pooled analysis showed that gout patients are associated with decreased risk of AD [RR = 0.70, 95% CI (0.63, 0.79), I2 = 57.2%, P = 0.000; Figure 3]. The sensitivity analysis showed that none of the studies reversed the pooled effect, which means that the results of the risk of AD in gout are robust (Supplementary Figure 2).
3.6. Gout and risk of VD
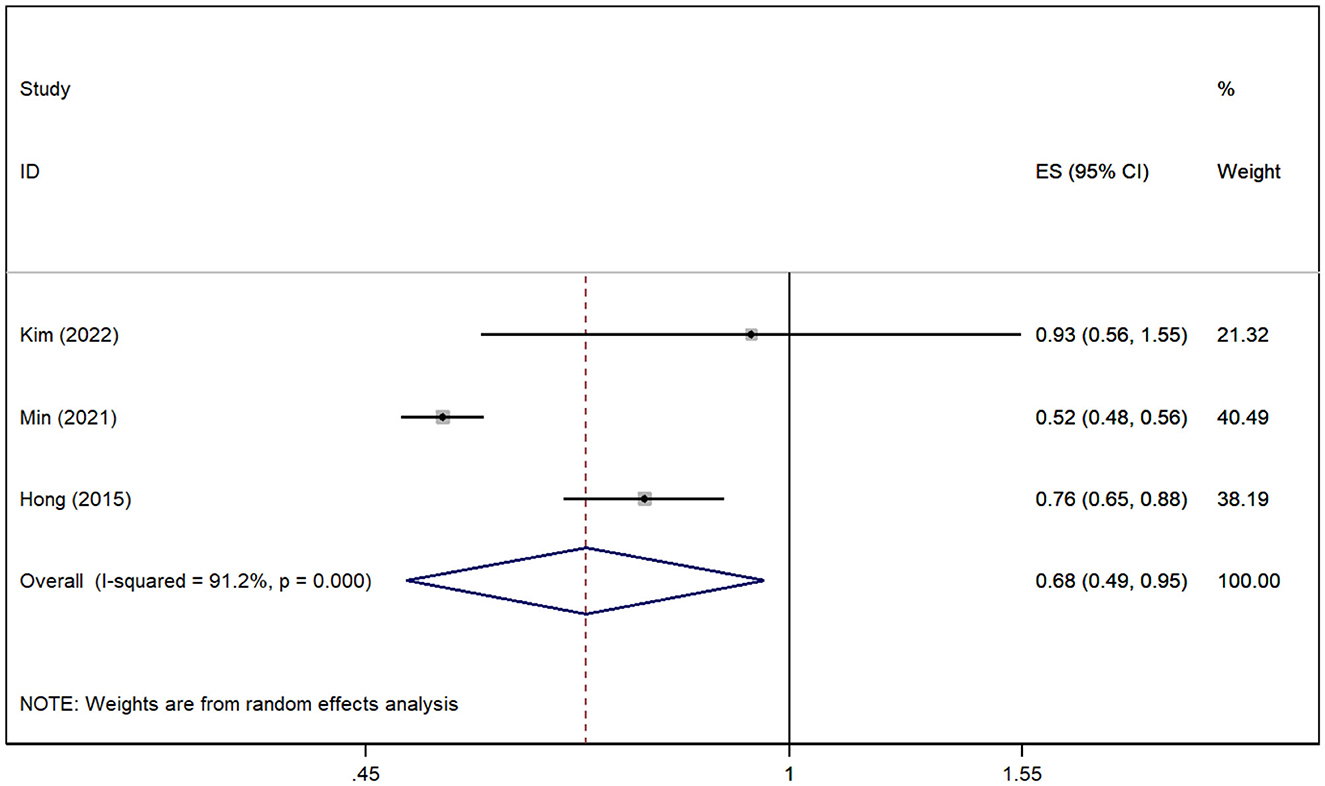
In total, three studies (Hong et al., 2015; Min et al., 2021; Kim et al., 2022) revealed the relationship between gout and the risk of VD. The pooled analysis showed that the risk of VD was reduced in patients with gout [RR = 0.68, 95% CI (0.49, 0.95), I2 = 91.2%, P = 0.025; Figure 4]. Although there was large heterogeneity, the results of sensitivity analysis support the robustness of this result.
3.7. Evidence certainty
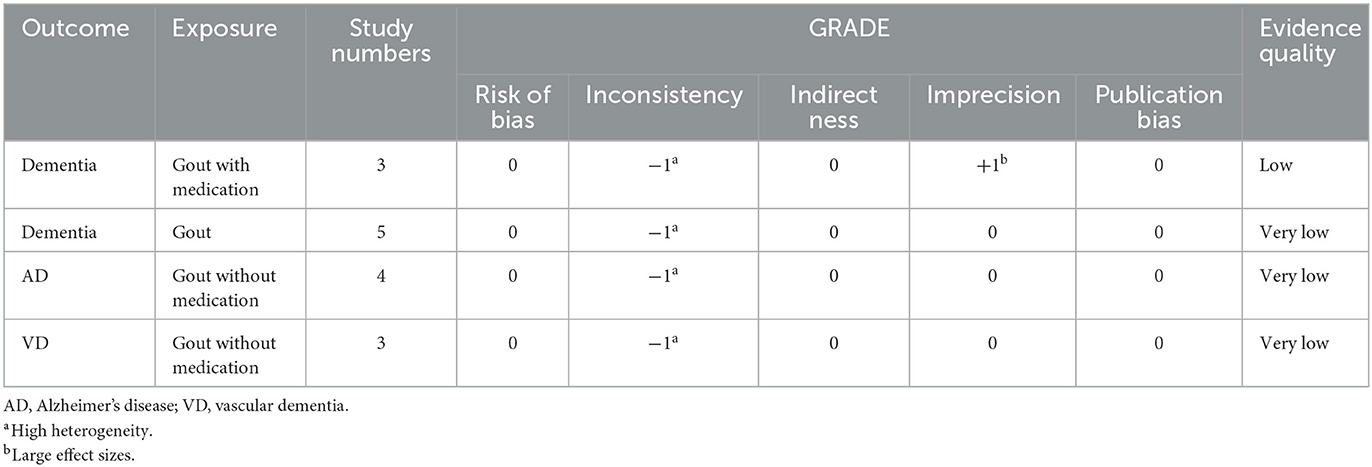
The GRADE level of evidence is very low for the risk of dementia, risk of AD, and risk of VD in gout patients without medication and is low for the risk of dementia in gout patients with medication. GRADE evidence certainty for the outcomes is shown in Table 3.
3.8. Publication bias
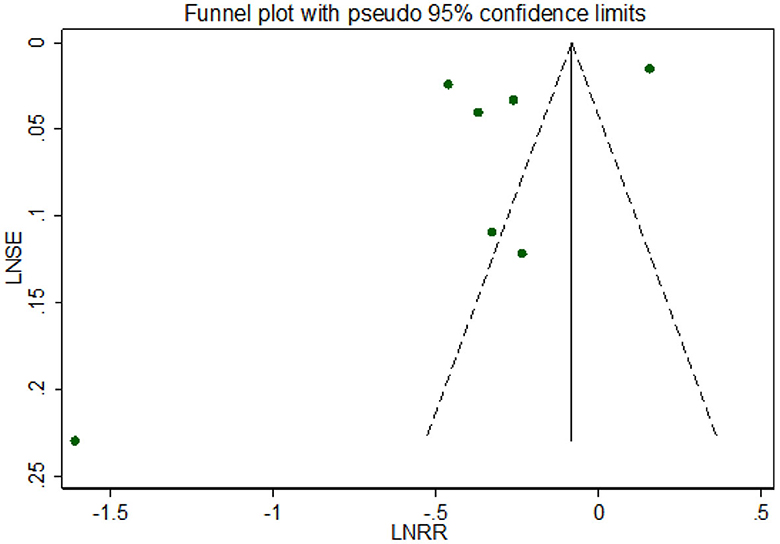
Visual inspection suggested that the funnel plot for all-cause dementia in gout patients was symmetrical but in formal statistical tests, including Egger's test (p = 0.197) and Begg's test (p = 0.881), no publication bias was shown. The funnel plot is shown in Figure 5.
4. Discussion
4.1. Principal findings
Our meta-analysis involving 2,349,605 individuals found a reduced risk of all-cause dementia in patients with gout, particularly in those with a history of medication therapy. Meanwhile, gout seems to be a protective factor for AD and VD although the quality of evidence is low or very low.
4.2. Comparison with previous studies
A total of one meta-analysis by Pan et al. reported that gout and hyperuricemia did not increase the risk of dementia but might decrease the risk of AD (Pan et al., 2021). However, in the four included cohort studies, one study (Latourte et al., 2018) only investigated the risk of dementia and AD in hyperuricemia patients. We all know that hyperuricemia is a biochemical precursor to gout, and nearly two-thirds of patients will not be diagnosed with gout (Clarson et al., 2015). Therefore, this study (Latourte et al., 2018) was excluded from our meta-analysis, thereby reducing patient selection bias and confounding. Moreover, the meta-analysis of Pan et al. (2021) did not distinguish between the special case of adherence to medication in the cohort follow-up, which may introduce confounding in the intervention. Thus, our meta-analysis distinguished between the two types of gout patients and dementia-onset outcomes based on the presence or absence of medication. In comparison, our meta-analysis found that the risk of all-cause dementia was reduced in gout patients, and this phenomenon was more pronounced in gout patients who were treated with medication. This may be due to our inclusion of several large samples of high-quality cohort studies while focusing more on patients with gout and controlling for other confounders. Notably, similar to the results of Pan et al. (2021), we found that the risk of AD was also reduced in gout patients without medication. In addition, we further evaluated the association of gout with the risk of VD, and the risk of VD was also reduced in gout patients, which has not been reported in a previous meta-analysis (Pan et al., 2021).
4.3. Interpretation of findings
To date, whether gout or hyperuricemia contributes to the risk of all-cause dementia has been controversial. There are several main postulated mechanisms of all-cause dementia caused by gout or hyperuricemia, but the more focused ones are chronic inflammation and oxidative stress mechanisms (Campbell et al., 1997; Lin and Beal, 2006; Bhat et al., 2015; Flores-Aguilar et al., 2021; Jurcau, 2021). Chronic inflammation caused by gout or hyperuricemia increases inflammatory cytokine levels in the brain, leading to neurodegenerative lesions. Meanwhile, high inflammatory cytokines damage endothelial cells and activate inflammatory cells, causing oxidative stress and promoting the development of atherosclerosis, a recognized risk factor for dementia (Lin and Beal, 2006; Bhat et al., 2015; Jurcau, 2021). However, based on the result of our meta-analysis, the risk of all-cause dementia, AD, and VD was low in gout patients, especially in patients with medication management. The explanatory mechanism for these findings remains to be explored. An important mechanism may be the pro- and antioxidant effects of uric acid. Uric acid is an important circulating antioxidant and free radical scavenger, which can scavenge peroxynitrite and hydroxyl radicals and reduce oxidative stress, thereby exerting neuroprotective effects (Kanellis and Kang, 2005; Lee et al., 2022). In addition, uric acid may preserve mitochondrial function and inhibit the accumulation of oxygen-free radicals, and mitochondrial dysfunction is considered to be one of the pathogeneses of AD (Yu et al., 1998; Ye et al., 2016). In clinical observations, Euser et al. found that high serum urate levels were associated with a reduced risk of dementia and further improved cognitive function (Euser et al., 2009), and Scheepers et al. found that higher serum uric acid may be protective against dementia including AD and VD (Scheepers et al., 2019). These are consistent with the findings of our meta-analysis. In addition, the gout patients who adhered to drug treatment in this study had a lower risk of dementia, which may be related to some uric acid-lowering drugs to antagonize oxidative stress and improve vascular endothelial cell function (Kryscio et al., 2017). The results of our meta-analysis give great insight to both clinicians and gout patients: first, to insist on proper management of gout, and second, to reduce the psychological burden by recognizing the uncertainty of the dangers of gout in terms of causing dementia or AD.
4.4. Strengths and limitations
The main advantage of our meta-analysis is the application of the GRADE system for grading the quality of evidence, which seems to be relatively rare in previous meta-analyses based on observational studies. Moreover, it is registered in PROSPERO and reported according to the updated PRISMA checklist, promoting transparency of the process and reliability of the results. However, this meta-analysis inevitably has several potential shortcomings. First, although we require a clear diagnosis of gout and a subgroup analysis based on medication taken, there are some clinical heterogeneities in the severity, disease status, and duration of disease in these gout patients which may be inconsistent. Second, there is a certain degree of statistical heterogeneity in the larger I2 of our meta-analysis. Although the sensitivity analysis indicates that the results are relatively robust, it inevitably affects the reliability of the results. These also contributed to the overall low quality of evidence. Finally, although we conducted a comprehensive literature search and excluded conference abstracts (incomplete information could lead to a biased assessment of methodological quality), it was difficult to exclude studies that might have been missed.
5. Conclusion
Our updated meta-analysis found that the risk of all-cause dementia, AD, and VD was reduced in patients with gout, but there was a large heterogeneity, and the mechanism was not clear. More studies are needed to investigate the true relationship between gout and dementia, AD, and VD in future.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
XL: acquisition of data, analysis and interpretation of data, drafting of the article, and approval of the final version to be submitted. XH and YT: acquisition of data, analysis and interpretation of data, and final approval of the version to be submitted. LH and CW: conception and design of the study, revision of the manuscript, and final approval of the version to be submitted. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the National Key Research and Development Project (2022YFC3501204), National Natural Science Foundation of China (81873145), and Natural Science Foundation of Zhejiang Province (LY21H290005).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2023.1051809/full#supplementary-material
References
Bai, W., Chen, P., Cai, H., Zhang, Q., Su, Z., Cheung, T., et al. (2022). Worldwide prevalence of mild cognitive impairment among community dwellers aged 50 years and older: A meta-analysis and systematic review of epidemiology studies. Age Ageing 51, afac173. doi: 10.1093/ageing/afac173
Balshem, H., Helfand, M., Schünemann, H. J., Oxman, A. D., Kunz, R., Brozek, J., et al. (2011). GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 64, 401–406. doi: 10.1016/j.jclinepi.2010.07.015
Bhat, A. H., Dar, K. B., Anees, S., Zargar, M. A., Masood, A., Sofi, M. A., et al. (2015). Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; A mechanistic insight. Biomed. Pharmacother. 74, 101–110. doi: 10.1016/j.biopha.2015.07.025
Campbell, I. L., Stalder, A. K., Chiang, C. S., Bellinger, R., Heyser, C. J., Steffensen, S., et al. (1997). Transgenic models to assess the pathogenic actions of cytokines in the central nervous system. Mol. Psychiatry 2, 125–129. doi: 10.1038/sj.mp.4000225
Cao, G. Y., Chen, Z. S., Yao, S. S., Wang, K., Huang, Z. T., Su, H. X., et al. (2022). The association between vision impairment and cognitive outcomes in older adults: A systematic review and meta-analysis. Aging Ment. Health 27, 350–6. doi: 10.1080/13607863.2022.2077303
Chuang, T. J., Wang, Y. H., Wei, J. C., and Yeh, C. J. (2021). Association between use of anti-gout preparations and dementia: Nested case-control nationwide population-based cohort study. Front. Med. 7, 607808. doi: 10.3389/fmed.2020.607808
Clarson, L. E., Chandratre, P., Hider, S. L., Belcher, J., Heneghan, C., Roddy, E., et al. (2015). Increased cardiovascular mortality associated with gout: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 22, 335–343. doi: 10.1177/2047487313514895
Dehlin, M., Sandström, T. Z., and Jacobsson, L. T. (2022). Incident gout: Risk of death and cause-specific mortality in western Sweden: A prospective, controlled inception cohort study. Front. Med. 9, 802856. doi: 10.3389/fmed.2022.802856
Egger, M., Davey Smith, G., Schneider, M., and Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. Br. Med. J. 315, 629–634. doi: 10.1136/bmj.315.7109.629
Euser, S. M., Hofman, A., Westendorp, R. G., and Breteler, M. M. (2009). Serum uric acid and cognitive function and dementia. Brain 132, 377–382. doi: 10.1093/brain/awn316
Flores-Aguilar, L., Iulita, M. F., Orciani, C., Tanna, N., Yang, J., Bennett, D. A., et al. (2021). Cognitive and brain cytokine profile of non-demented individuals with cerebral amyloid-beta deposition. J. Neuroinflammation 18, 147. doi: 10.1186/s12974-021-02169-0
Frederiksen, K. S., Cooper, C., Frisoni, G. B., Frölich, L., Georges, J., Kramberger, M. G., et al. (2020). A European Academy of Neurology guideline on medical management issues in dementia. Eur. J. Neurol. 27, 1805–1820. doi: 10.1111/ene.14412
Guyatt, G. H., Oxman, A. D., Sultan, S., Glasziou, P., Akl, E. A., Alonso-Coello, P., et al. (2011). GRADE guidelines: 9. Rating up the quality of evidence. J. Clin. Epidemiol. 64, 1311–1316. doi: 10.1016/j.jclinepi.2011.06.004
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. Br. Med. J. 327, 557–560. doi: 10.1136/bmj.327.7414.557
Hong, J. Y., Lan, T. Y., Tang, G. J., Tang, C. H., Chen, T. J., and Lin, H. Y. (2015). Gout and the risk of dementia: A nationwide population-based cohort study. Arthritis Res. Ther. 17, 139. doi: 10.1186/s13075-015-0642-1
Jurcau, A. (2021). Insights into the pathogenesis of neurodegenerative diseases: Focus on mitochondrial dysfunction and oxidative stress. Int. J. Mol. Sci. 22:2111847. doi: 10.3390/ijms222111847
Kanellis, J., and Kang, D. H. (2005). Uric acid as a mediator of endothelial dysfunction, inflammation, and vascular disease. Semin. Nephrol. 25, 39–42. doi: 10.1016/j.semnephrol.2004.09.007
Kim, J. H., Yim, D. H., Choi, I. A., Lee, J., Park, H., and Eom, S. Y. (2022). The impact of clinical association between gout and dementia: A nationwide population-based cohort study in Korea. Arthritis Care Res. 2022, acr.24959. doi: 10.1002/acr.24959
Kryscio, R. J., Abner, E. L., Caban-Holt, A., Lovell, M., Goodman, P., Darke, A. K., et al. (2017). Association of antioxidant supplement use and dementia in the prevention of Alzheimer's disease by vitamin E and selenium trial (PREADViSE). J. Am. Med. Assoc. Neurol. 74, 567–573. doi: 10.1001/jamaneurol.2016.5778
Latourte, A., Soumaré, A., Bardin, T., Perez-Ruiz, F., Debette, S., and Richette, P. (2018). Uric acid and incident dementia over 12 years of follow-up: A population-based cohort study. Ann. Rheum. Dis. 77, 328–335. doi: 10.1136/annrheumdis-2016-210767
Lee, J. E., Shin, Y. J., Kim, Y. S., Kim, H. N., Kim, D. Y., Chung, S. J., et al. (2022). Uric acid enhances neurogenesis in a parkinsonian model by remodeling mitochondria. Front. Aging Neurosci. 14, 851711. doi: 10.3389/fnagi.2022.851711
Lei, S., Li, X., Zhao, H., Feng, Z., Chun, L., Xie, Y., et al. (2022). Risk of dementia or cognitive impairment in sepsis survivals: A systematic review and meta-analysis. Front. Aging Neurosci. 14, 839472. doi: 10.3389/fnagi.2022.839472
Lin, M. T., and Beal, M. F. (2006). Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443, 787–795. doi: 10.1038/nature05292
Lu, N., Dubreuil, M., Zhang, Y., Neogi, T., Rai, S. K., Ascherio, A., et al. (2016). Gout and the risk of Alzheimer's disease: A population-based, BMI-matched cohort study. Ann. Rheum. Dis. 75, 547–551. doi: 10.1136/annrheumdis-2014-206917
Mattap, S. M., Mohan, D., McGrattan, A. M., Allotey, P., Stephan, B. C., Reidpath, D. D., et al. (2022). The economic burden of dementia in low- and middle-income countries (LMICs): A systematic review. Br. Med. J. Glob. Health 7, 409. doi: 10.1136/bmjgh-2021-007409
Mikhailichenko, N., Wang, Y. H., Wei, J. C., and Lai, T. J. (2021). Association between anti-inflammatory drug and dementia in patients with gout: A nationwide, population-based nested case-control study. Int. J. Med. Sci. 18, 2042–2050. doi: 10.7150/ijms.55496
Min, K. H., Kang, S. O., Oh, S. J., Han, J. M., and Lee, K. E. (2021). Association between gout and dementia in the elderly: A nationwide population-based cohort study. Am. J. Geriatr. Psychiatry 29, 1177–1185. doi: 10.1016/j.jagp.2021.01.016
Neilson, J., Bonnon, A., Dickson, A., and Roddy, E. (2022). Gout: Diagnosis and management-summary of NICE guidance. Br. Med. J. 378, eo1754. doi: 10.1136/bmj.o1754
O'Neil, M., Payne, C., and Read, J. (1995). Read Codes Version 3: A user led terminology. Methods Inf. Med. 34, 187–192. doi: 10.1055/s-0038-1634585
Page, M. J., Moher, D., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. Br. Med. J. 372, n160. doi: 10.1136/bmj.n160
Pan, S. Y., Cheng, R. J., Xia, Z. J., Zhang, Q. P., and Liu, Y. (2021). Risk of dementia in gout and hyperuricaemia: A meta-analysis of cohort studies. Br. Med. J. Open 11, e041680. doi: 10.1136/bmjopen-2020-041680
Qu, H., Yang, S., Yao, Z., Sun, X., and Chen, H. (2022). Association of headache disorders and the risk of dementia: Meta-analysis of cohort studies. Front. Aging Neurosci. 14, 804341. doi: 10.3389/fnagi.2022.804341
Ryan, B., To, E., Ma'u, E., Chan, A. H. Y., Rivera-Rodriguez, C., Curtis, M. A., et al. (2022). Prevalence of young-onset dementia: Nationwide analysis of routinely collected data. J. Neurol. Neurosurg. Psychiatry 2022, 329126. doi: 10.1136/jnnp-2022-329126
Safiri, S., Kolahi, A. A., Cross, M., Carson-Chahhoud, K., Hoy, D., Almasi-Hashiani, A., et al. (2020). Prevalence, incidence, and years lived with disability due to gout and its attributable risk factors for 195 countries and territories 1990-2017: A systematic analysis of the global burden of disease study 2017. Arthritis Rheumatol. 72, 1916–1927. doi: 10.1002/art.41404
Scheepers, L., Jacobsson, L. T. H., Kern, S., Johansson, L., Dehlin, M., and Skoog, I. (2019). Urate and risk of Alzheimer's disease and vascular dementia: A population-based study. Alzheimers Dement. 15, 754–763. doi: 10.1016/j.jalz.2019.01.014
Shang, X., Zhu, Z., Wang, W., Ha, J., and He, M. (2021). The association between vision impairment and incidence of dementia and cognitive impairment: A systematic review and meta-analysis. Ophthalmology 128, 1135–1149. doi: 10.1016/j.ophtha.2020.12.029
Singh, J. A., and Cleveland, J. D. (2018). Gout and dementia in the elderly: A cohort study of Medicare claims. BMC Geriatr 18, 281. doi: 10.1186/s12877-018-0975-0
Ye, B. S., Lee, W. W., Ham, J. H., Lee, J. J., Lee, P. H., and Sohn, Y. H. (2016). Does serum uric acid act as a modulator of cerebrospinal fluid Alzheimer's disease biomarker related cognitive decline? Eur. J. Neurol. 23, 948–957. doi: 10.1111/ene.12969
Yu, Z. F., Bruce-Keller, A. J., Goodman, Y., and Mattson, M. P. (1998). Uric acid protects neurons against excitotoxic and metabolic insults in cell culture, and against focal ischemic brain injury in vivo. J. Neurosci. Res. 53, 613–625. doi: 10.1002/(SICI)1097-4547(19980901)53:5<613::AID-JNR11>3.0.CO;2-1
Keywords: gout, dementia, Alzheimer's disease, vascular dementia, meta-analysis
Citation: Li X, Huang L, Tang Y, Hu X and Wen C (2023) Gout and risk of dementia, Alzheimer's disease or vascular dementia: a meta-epidemiology study. Front. Aging Neurosci. 15:1051809. doi: 10.3389/fnagi.2023.1051809
Received: 23 September 2022; Accepted: 13 March 2023;
Published: 26 April 2023.
Edited by:
Keren Nitzan, Open University of Israel, IsraelReviewed by:
Francisca Sivera, Hospital General de Elda, SpainXinwei Wu, National Institutes of Health (NIH), United States
Copyright © 2023 Li, Huang, Tang, Hu and Wen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chengping Wen, d2VuZ2NwQDE2My5jb20=
 Xuanlin Li
Xuanlin Li Lin Huang
Lin Huang Yujun Tang
Yujun Tang Xuanming Hu
Xuanming Hu Chengping Wen
Chengping Wen