- 1First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, China
- 2National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion, Tianjin, China
- 3Tianjin University of Traditional Chinese Medicine, Tianjin, China
The vascular mild cognitive impairment (VaMCI) is generally accepted as the premonition stage of vascular dementia (VaD). However, most studies are focused mainly on VaD as a diagnosis in patients, thus neglecting the VaMCI stage. VaMCI stage, though, is easily diagnosed by vascular injuries and represents a high-risk period for the future decline of patients’ cognitive functions. The existing studies in China and abroad have found that magnetic resonance imaging technology can provide imaging markers related to the occurrence and development of VaMCI, which is an important tool for detecting the changes in microstructure and function of VaMCI patients. Nevertheless, most of the existing studies evaluate the information of a single modal image. Due to the different imaging principles, the data provided by a single modal image are limited. In contrast, multi-modal magnetic resonance imaging research can provide multiple comprehensive data such as tissue anatomy and function. Here, a narrative review of published articles on multimodality neuroimaging in VaMCI diagnosis was conducted,and the utilization of certain neuroimaging bio-markers in clinical applications was narrated. These markers include evaluation of vascular dysfunction before tissue damages and quantification of the extent of network connectivity disruption. We further provide recommendations for early detection, progress, prompt treatment response of VaMCI, as well as optimization of the personalized treatment plan.
1. Introduction
Due to the process of ageing, the incidence rates of cerebral vascular diseases and neurodegenerative diseases like Alzheimer’s disease (AD) and dementia have drastically increased (GBD, 2016). By 2050, the total number of dementia patients is expected to reach 1.52 million people. Data show that 25% of them will be from the Chinese population (GBD, 2016). As the second most common dementia after AD, VaD has a great impact on life quality of patients and brings a heavy burden to the family and society (Plassman et al., 2007). Owing to its high prevalence and potential reversibility, VaD has attracted great attention (O'brien, 2006; Plassman et al., 2007). As the precursor stage of VaD (Wentzel et al., 2001), VaMCI’ s early prediction and intervention play an important role in delaying the transition to VaD (Ravaglia et al., 2006; Jak et al., 2009; Jongsiriyanyong and Limpawattana, 2018). Therefore, early diagnosis and risk factor reduction are clinical strategies to delay the disease progression. At present, there is no reliable method for early diagnosis and recognition of VaMCI. In recent years,the American Academy of Neurology and European Academy of Neurology recommends the use of neuroimaging for the evaluation of dementia patients, due to its ability to identify the pathological cause of dementia syndrome and unearth reliable imaging markers for the early diagnosis (Knopman et al., 2001; Filippi et al., 2012).
Here, we review different multimodal neuroimaging methods such as rs-fMRI, DTI, ASL perfusion imaging, as well as their synergy for the diagnosis of VaMCI patients. The correlation of different neuroimaging features with the cognitive function of these patients is further summarized to provide recommendations for the successful evaluation of dementia progression and prevention by advanced and quantitative neuroimaging technologies.
2. Certain vascular risk factors underlie the pathological mechanisms of VaMCI
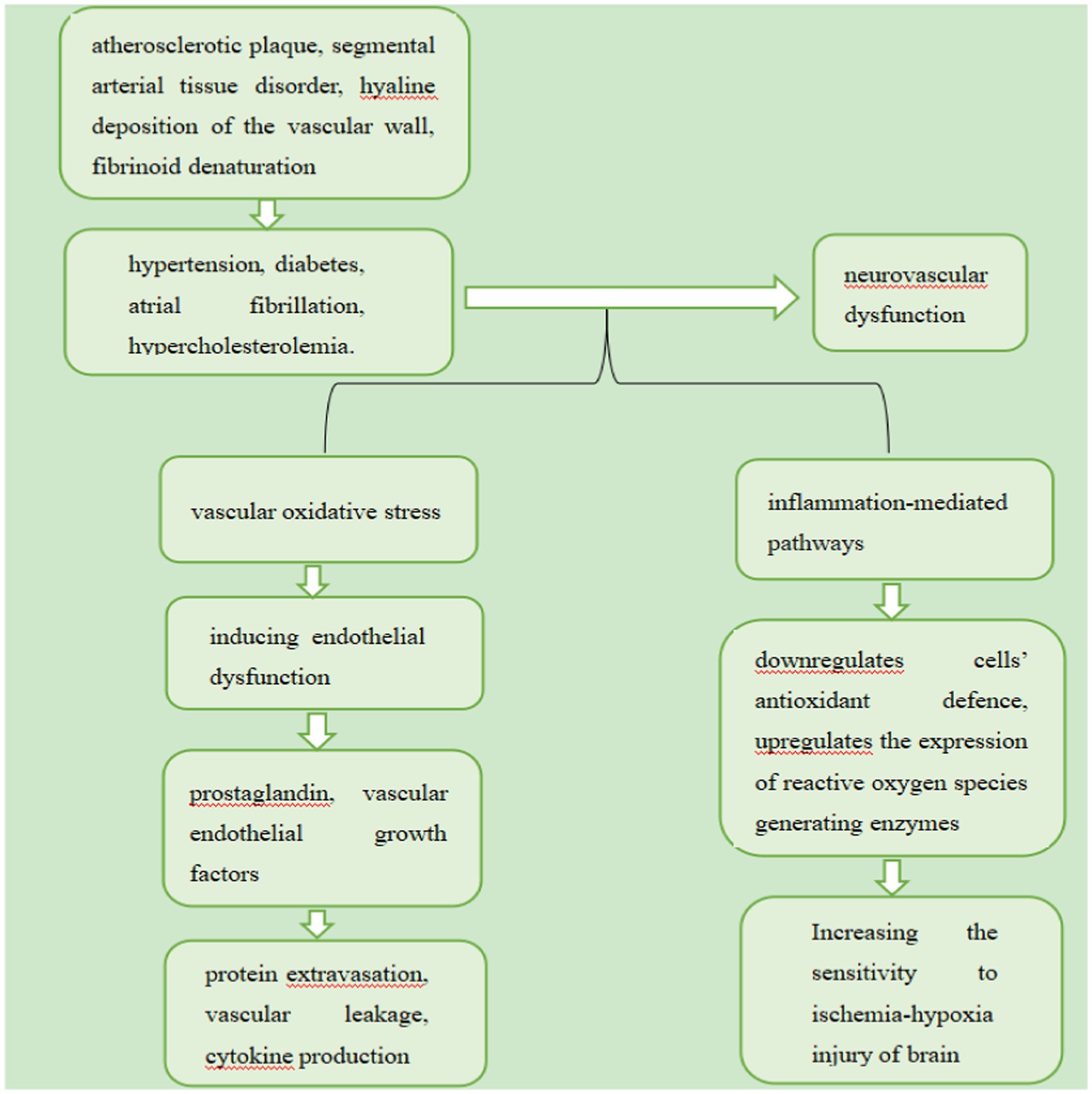
VaMCI is mainly induced by vascular risk factors, which include hypertension, diabetes, atrial fibrillation and hypercholesterolemia (Gorelick et al., 2011). They may induce neurovascular dysfunction through vascular oxidative stress and inflammation-mediated pathways. Oxidative stress promotes the release of prostaglandin and vascular endothelial growth factors by inducing endothelial dysfunction, which in turn promotes protein extravasation, vascular leakage, and cytokine production (Gorelick et al., 2011). On the other hand, inflammation downregulates cells’ antioxidant defence and upregulates the expression of reactive oxygen species generating enzymes (Gorelick et al., 2011; El-Sahar et al., 2021). This vicious cycle holds the potential to destroy the microenvironment of the brain, thus increasing its sensitivity to ischemia-hypoxia injury (Iadecola et al., 2009), Additionally, Vascular risk factors are related to various vascular pathologies, including atherosclerotic plaque, segmental arterial tissue disorder, hyaline deposition of the vascular wall and fibrinoid denaturation (Thal et al., 2012; Caplan, 2015). These vascular diseases reduce the cerebral blood flow (CBF) of perforating arteries, which supply subcortical nuclei, cortical projection fibres, and commissural fibres (Dey et al., 2016). As a result, connections between the cerebral cortex and subcortical regions, as well as between intracortical regions, are disrupted, which can lead to cognitive impairment (Dey et al., 2016). Certain vascular risk factors underlie the pathological mechanisms of VaMCI showed in the Figure 1.
3. Basic MRI features of VaMCI
Magnetic resonance imaging (MRI) is the key neuroimaging modality and has high sensitivity and specificity for detecting pathological changes, including small vessel disease (Mijajlović et al., 2017). A study indicated that the presence of moderate or severe white matter hyperintensities (WMH) on MRI is a hallmark of VaMCI, and extensive white matter damage in the temporal lobe, cingulate gyrus, bilateral lateral ventricles and other areas in VaMCI patients (Yu et al., 2017). Moreover, it was found that the number of lacunar infarcts in VaMCI was 3 times that in normal people, and white matter lesions, frontal Angle and third ventricle widening were also significantly more than in normal people (Meyer et al., 2005; Vermeer et al., 2007). Another studies revealed that VaMCI may display a single critical site infarction was sufficient to cause VaD (Snowdon et al., 1997), more than 2 multiple lacunar infarcts outside the brain stem (Snowdon et al., 1997; Chen et al., 2009), and Intracranial hemorrhage at critical sites, or ≥2 intracranial hemorrhage (Sachdev et al., 2014). Valenti and Li et al. reported that nearly one-third of VaMCI patients had at least one CMB and more than one-third had CMBs in multiple regions (Valenti et al., 2016; Li et al., 2021). They also found that more percentages of severe WMH, cerebral microbleeds (CMBs), enlarged perivascular spaces (EPVS) and cerebral atrophy compared with healthy controls.
4. Resting-state functional magnetic resonance imaging
4.1. Definition
rs-fMRI is a non-invasive neuroimaging technique that measures brain local functional connections at rest and is based on brain low-frequency (<0.1 Hz) MRI signal fluctuations with blood oxygen level dependence (BOLD; Biswal et al., 1995). Moreover, patients are scanned in quiet. It is generally accepted that rs-fMRI can effectively investigate brain networks. In other words, BOLD fMRI is applied to analyze the synchronization between individual cortical areas. Then, functional connections are delineated to illustrate the correlation between isolated regions and spontaneous neuron activities under resting-state (Biswal et al., 1995; Greicius et al., 2003; Fox and Raichle, 2007). Data show that these BOLD signals are not direct indicators of neuron activities. Instead, they reflect local fluctuation of deoxyhemoglobin concentration determined by blood flow, blood volume and oxygen metabolism (Raichle and Mintun, 2006). With advances in fMRI, the research on the pathways underlying the brain connections has expanded from the structural to the functional level of investigation. It has been revealed that the abnormal brain FC detected by the fMRI predated conventional structural changes (e.g., encephalotrophy) and clinical symptoms (Sheline and Raichle, 2013). Indeed, rs-fMRI provides a promising way to explore the changes of spontaneous neural activities related to various brain diseases (Greicius, 2008; Fox and Greicius, 2010). Changes in low-frequency BOLD signal fluctuations were observed in patients with AD, epilepsy and Parkinson’s disease (PD; He et al., 2007; Wu et al., 2009; Luo et al., 2011). Sun et al. reported abnormality of functional connections located between the posterior cingulate cortex (PCC) and frontal as well as temporal regions in patients with VaMCI (Sun et al., 2011). A study found that the functional activities of medial prefrontal cortex, bilateral cingulate gyrus/precuneus and left inferior parietal lobule in patients with AD or mild cognitive impairment (MCI) have changed (Zhang et al., 2012). Similarly, it has been reported that the decreased functional activity of five clusters including the right inferior temporal gyrus, the left medial prefrontal gyrus, the left anterior cingulate gyrus (ACG), the right wedge and the right middle occipital gyrus is related to the severity of AD (Wang et al., 2017).
4.2. Common clinical indicators of rs-fMRI
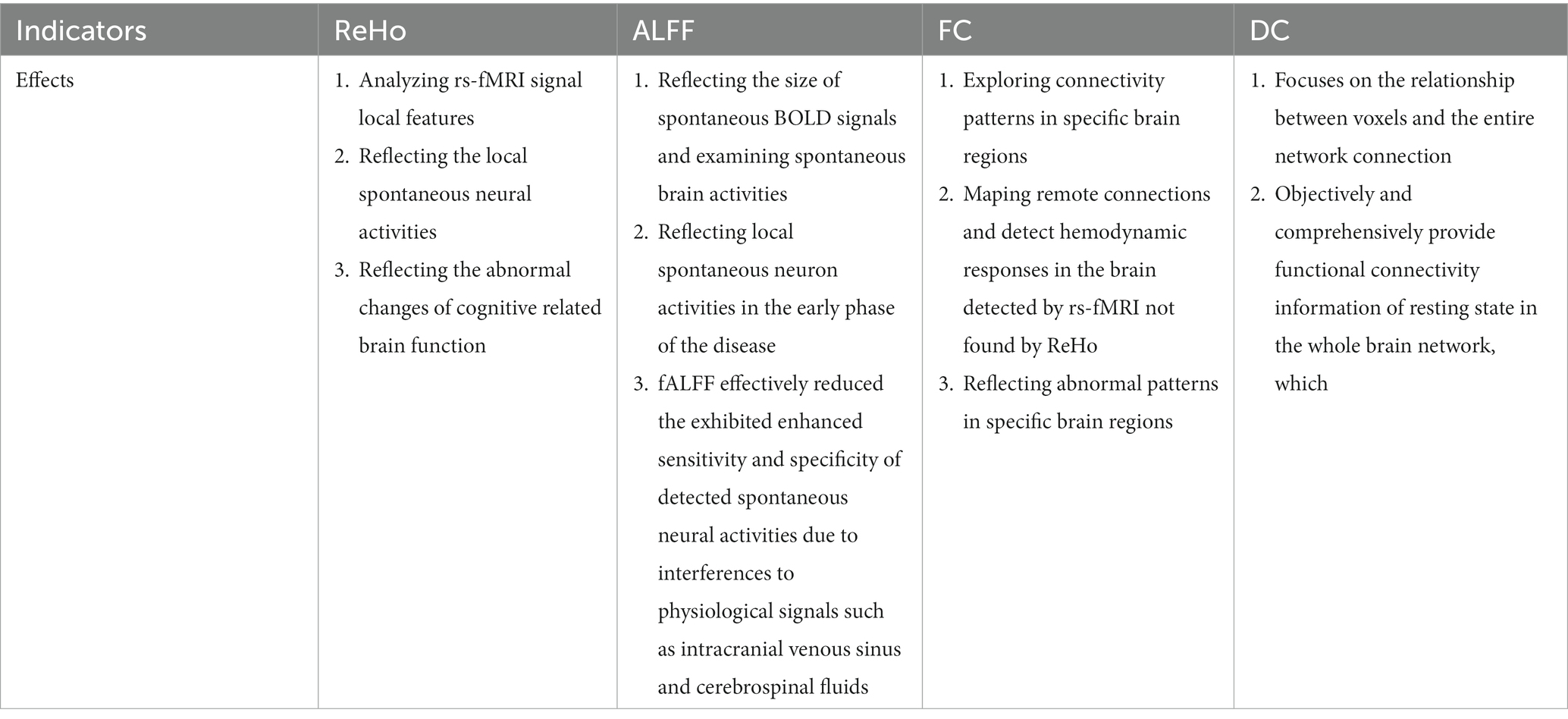
The ommon clinical indicators of rs-fMRI showed in Table 1.
4.2.1. Regional homogeneity
ReHo refers to the correlation between a voxel time series and its locally adjacent voxel time series, which effectively quantifies the synchronization of the BOLD time series between a voxel and its locally adjacent voxel (Zang et al., 2004; Peng et al., 2016). It is an important method for analyzing rs-fMRI signal local features. As it reflects the local spontaneous neural activities it has been widely used to explore indigenous brain activities (Zang et al., 2004; Peng et al., 2016). Therefore, factors determining ReHo value include spatial adjacency and functional homogeneity of time series. These factors provide valuable spatiotemporal information from a neurobiological perspective (Jiang and Zuo, 2016). It has been further demonstrated that ReHo can be used as an imaging biomarker to monitor and/or identify AD pathology (Zhang et al., 2012). Additionally, ReHo has been demonstrated to be significantly associated with a patient’s cognitive performance (Zhang et al., 2012; Liu Y. et al., 2014). Data showed that alterations in intracranial atherosclerosis decrease CBF delivery and efficiency, resulting in the inconsistency of amplitude and/or phase of the BOLD signal of the single neural cluster. Therefore, it is generally accepted that a low ReHo value reflects impaired cerebral perfusion (Tu et al., 2020). Meanwhile, it was demonstrated that ReHo was successfully used in clinical research of various diseases, including attention deficit hyperactivity disorder (ADHD), AD and MCI (Zhang et al., 2012; Wang et al., 2013). Zuo et al. found that ReHo was significantly reduced in the left cerebellum and right lentiform nucleus of VaMCI patients (Zuo et al., 2019). Meanwhile, it was hypothesized that the low ReHo value might be related to diminished neuron activities as the mean ReHo value of the left ACG was negatively correlated with the trail making test (TMT; Tu et al., 2020). Additionally, ReHo reduction of VaMCI patients was closely related to MoCA scores, demonstrating that fMRI-based measurement might indicate brain dysfunction (Zuo et al., 2019). In patients with VaMCI, Diciotti et al. reported that a remarkably negative association between ReHo and MoCA scores, with higher ReHo in the left posterior cerebellum of patients with outstanding integral cognitive impairment, with higher ReHo in the middle cingulate cortex bilaterally of patients with worse executive functions. The findings revealed that ReHo is significantly correlated with measurements of the cognitive disorders (Diciotti et al., 2017). Orsolini et al. also revealed that patients with cognitive decline of cerebrovascular disease showed significantly lower ReHo in the right insula, the left superior frontal gyrus, and the bilateral anterior cingulated cortex, which belonging to networks involved in inhibition and attention (Orsolini et al., 2021). In short, the application of ReHo can reflect the abnormal changes of cognitive related brain function.
4.2.2. Amplitude of low-frequency fluctuation
ALFF is a rs-fmri derived way that primarily measures the total power of the BOLD time course over a specific frequency range (0.01 ~ 0.08 Hz; Zang et al., 2007). It reflects the size of spontaneous BOLD signals and designs to examine spontaneous brain activities (Nugent et al., 2015). As a non-invasive technique of rs-fMRI, ALFF is more advanced than conventional MRI for the diagnosis of advanced pathological changes in cerebral vascular diseases and can reflect local spontaneous neuron activities in the early phase of the disease (Zang et al., 2007). Furthermore, the changes in local spontaneous neuron activities of ALFF can be detected in animals and humans (Logothetis et al., 2001; Moosmann et al., 2003; Pelled and Goelman, 2004; Yang et al., 2007). Recent studies indicated that cognitive impairment patients had abnormal ALFF within the PCC. For VaMCI patients, the alterations of ALFF in brain regions were predominantly found in the default mode network (DMN). Compared with the healthy control group, the ALFF was reduced in the bilateral medial prefrontal cortex (anterior DMN), precuneus (posterior DMN) and posterior parietal cortex (Yi et al., 2012; Wang et al., 2019; Zhuang et al., 2021). Moreover, the decrease in ALFF was positively correlated with the impairment of cognitive functions as assessed by Montreal Cognitive Assessment (MOCA), suggesting that the spontaneous neuron activities were associated with cognitive decline (Yang, 2021). Additionally, reduced ALFF in the precuneus was significantly correlated with the cognitive disability of AD and MCI patients measured by the minimum mental state examination (MMSE; Oakes et al., 2007; Wen et al., 2013).
Previous studies have revealed that ALFF is extensively affected by other physiological noises. Zou et al. proposed a method based on the fractional amplitude of low-frequency fluctuation (fALFF), which was defined as the ratio of ALFF and the given low-frequency band sum (Zou et al., 2008). Compared with ALFF, the fALFF effectively reduced the exhibited enhanced sensitivity and specificity of detected spontaneous neural activities due to interferences to physiological signals such as intracranial venous sinus and cerebrospinal fluids (Zou et al., 2008). For instance, fALFF has been widely applied in the diagnosis of AD (Yang et al., 2018), MCI (Pan et al., 2017), and the amnestic mild cognitive impairment (Zhou et al., 2020). Additionally, a study found that the fALFF values of right frontal lobe, left hippocampus and right cingulate gyrus were significantly increased in patients with cognitive impairment after acute cerebellar infarction, and the fALFF value of posterior cerebellum decreased significantly (Fan et al., 2019).
4.2.3. Functional connections
Seed-based FC analysis is a correlation analysis method for exploring connectivity patterns in specific brain regions (Sala-Llonch et al., 2015). Anatomically separated brain regions are found to fluctuate synchronously and exhibit strong FC, thus forming a complex functional network (Biswal et al., 1995). FCs between different brain regions correspond well to their nerve fiber connections, indicating a strong anatomical basis for FCs (Greicius et al., 2009; Honey et al., 2009). In addition, FC has also been proved to be closely related to regional CBF and metabolism, such as regions with strong connections show more obvious CBF (Liang et al., 2013), higher oxygen consumption (Wu et al., 2009) and glucose metabolism (Tomasi et al., 2013). Therefore, it can be used to map remote connections and detect hemodynamic responses in the brain detected by rs-fMRI not found by ReHo (Peng et al., 2016). Multiple literatures have shown that FC is also associated with dynamic changes in local neuronal ensemble activity, which reflects the neural flexibility or the dynamic range that affects the adaptability and efficiency of the nervous system (Garrett et al., 2013; Nomi et al., 2017). Regional neural variability and brain network dysfunction (Dennis and Thompson, 2014; Colasanti et al., 2016) in patients with stroke (Kielar et al., 2016), multiple sclerosis (AS; Petracca et al., 2017), AD (Scarapicchia et al., 2018) and other neurological disorders (Zoller et al., 2017). Some researches suggesting that FC is highly relevant to the cognitive performance of a specific field, including DMN, executive control network and dorsal attention network, all of which are closely related to attention and execution (Barkhof et al., 2014; Shaw et al., 2015). In the FC analysis, the selections for regions of interest (ROIs) are not consistent. Existing studies show that the main focus is mainly on the PCC connections and their critical role in brain cognitive function and memory (Ding et al., 2015). Ding et al. reported that FC of PCC and the left thalamus were significantly reduced in patients with VaMCI (Ding et al., 2015). In VaMCI patients, a significant reduction of FC was found in the right inferior frontal gyrus, the right middle frontal gyrus, bilateral precentral gyrus, and the right postcentral/superior parietal lobule (Zuo et al., 2019). Additionally, MOCA scores were positively correlated with the decrease of FC in the anterior cingulate cortex and posterior parietal cortex, suggesting that the local FC was related to cognitive impairment (Wang et al., 2021). In brief, FC can reflect abnormal patterns in specific brain regions.
4.2.4. Degree centrality
DC is a graph-based brain network measurement method, which calculates the temporal correlation between a single voxel and other intracerebral voxels within a mask at the voxel level (Zuo et al., 2012). In other words, Consider each voxel as a node and calculate the number of functional connections between each node and other nodes (Buckner et al., 2009). The larger the DC of a node, the more important the node is in the whole brain network, and the stronger its information communication abilities (Zhu et al., 2019). DC focuses on the relationship between voxels and the entire network connection, also be used to detect abnormal changes in functional connectivity in brain (Buckner et al., 2009; Gao et al., 2016). Unlike ALFF which reflects local brain activities, voxel-level DC can objectively and comprehensively provide functional connectivity information of resting state in the whole brain network, which is different from traditional functional neural research methods such as regional homogeneity (Zuo et al., 2012; Adriana et al., 2013). It explains the relationship betweesn the local brain activities and the whole brain network. Moreover, compared with other methods such as ALFF and ReHo (Huang et al., 2015; Shao et al., 2015), DC does not involve defining ROIs and assessing connectivity across the human brain at voxel level (Zuo and Xing, 2014; Shao et al., 2015), which can provide valuable information for the changes of nodes in human brain connections caused by diseases (Adriana et al., 2013). It has high repeatability (Zuo and Xing, 2014). At present, DC has been widely used to explore the neurobiological mechanism and pathophysiological mechanism of brain network changes in various diseases (Lou et al., 2015; Shen et al., 2015). Therefore, DC method has attracted a lot of attention and has been used to explore the neural mechanisms of several diseases, such as Alzheimer’s disease (Guo et al., 2016), alcohol dependence (Luo et al., 2017), attention deficit hyperactivity disorder (Wang et al., 2017), and Parkinson’s disease (Guo et al., 2020). Abnormal DC has been observed in MCI, AD and PD patients (Grau-Olivares et al., 2010; Li et al., 2017). Studies have found that increased DC values in temporal gyrus and hippocampus may be associated with impaired memory function (Feng et al., 2021). Yang et al. demonstrated that DC reduction was significantly correlated with the Hamilton Anxiety Scale (HAMA) in VaMCI patients, suggesting that VaMCI patients may be more likely to develop symptoms of anxiety (Yang, 2021). Existing literature reported that many studies combined DC and FC to explore changes in functional patterns in patients with neurological diseases (Cui et al., 2016; Guo et al., 2020).
5. Diffusion tensor imaging
The hyperintensity in White Matter (WM) is associated with impaired executive and overall cognitive function of the brain (Dao et al., 2018). Specifically, the white matter tract is essential for the maintenance of the normal brain cortex and cortico-subcortical connections. Therefore, the integrity of WM plays a key role in the synchronous activities and neural activation of the brain functional network. DTI can be used as a sensitive method to explore the neural mechanisms of different cognitive impairments (Liu et al., 2019). It is an MRI technique that directly measures the integrity of the brain white matter (WM; Wang et al., 2013). Compared with conventional techniques, it is more sensitive in detecting cognitive impairments and has a higher correlation with the patient’s cognitive function, especially in the early stage of neurological diseases (Xu et al., 2010; Wang et al., 2013). It explores the integrity of WM in patients with dementia or cognitive impairments, and even detects minor changes in the complex brain structural networks, creating the great potential to discover early stages of the disease and to optimize personalized treatment regimens (Sabri et al., 1999; Buckner et al., 2009; Xu et al., 2010; Wang et al., 2013). Recent studies revealed that the assessment for correlation between the WM damage and the cognitive function by DTI was superior to those by T2 weighted or FLAIR sequences (O’Sullivan et al., 2004a,b). In other words, the dispersion of lesions and normal white matter on DTI was increased, and the average diffusion rate of normal white matter was related to the performance of functional tests. These correlations remain significant after controlling age, gender, brain volume and T1/T2 lesion volume (O’Sullivan et al., 2004a,b). No significant correlation between neuropsychological scores of T2 lesions. Additionally, DTI appeared as the most sensitive technique to assess structural WM microstructural damage in patients with cerebrovascular diseases (Banerjee et al., 2016). It was further demonstrated that DTI reflected the processes of the white matter tracts (e.g., cortical tract and spinal tract) and the overall extent and shape of the water proton diffusion by measuring the water proton diffusivity inside the brain tissue (Nir et al., 2013). It also finally clarifies the microstructural integrity of WM. It was demonstrated that fractional anisotropy (FA) appeared as a widely used DTI measurement method in clinical studies to describe diffusion anisotropy. It was further demonstrated that DTI was very sensitive to changes in the integrity of WM and neuron connections, with higher values indicating stronger axonal integrity and lower values suggesting incomplete or loss of neuron connections (Nir et al., 2013).
Another common method of DTI measurement is the mean diffusivity (MD), which is used to characterize the pattern of water diffusion within a tissue, reflecting the average amplitude of diffusion in various directions. Research found that MD was seen to correlate negatively and FA to correlate positively with global and selective cognitive performance in patients with VaMCI (Xu et al., 2010). Moreover, FA and MD correlated with patients’ memory and attention executive scores (Xu et al., 2010). Additionally, FA and MD within the cingulate bundle correlated with verbal memory scores in non-demented elderly with cerebral small vessel disease, while MD in the frontal lobe was associated with psychomotor speed performance (Tuladhar et al., 2015). Mascalchi et al. reported that patients of VaMCI with increased MD substantially corresponded to area of decreased FA in WM exhibiting. The result reveals that a substantially symmetric damage of the long WM tracts in terms of increased MD and decreased FA emerged (Mascalchi et al., 2014). Another study found that MoCA scores were positively correlated with FA as well as MD (negative correlation) of almost the global cerebral hemispheres to the patients with VaMCI, in an almost symmetrical fashion. The study indicates that the cognitive deficits are consistently sustained by the microstructural damage of the normal-appearing WM revealed by DTI (Mascalchi et al., 2019). In summary, loss of microstructural integrity in WM is usually reflected in decreased FA and/or increased MD (Beaulieu, 2002; Sen and Basser, 2005).
6. Arterial spin labelling
ASL perfusion imaging is a promising non-invasive tool for assessing CBF, which can be used to discover certain vascular features of early cognitive impairment (Yoshiura et al., 2009; Iturria-Medina et al., 2016). It is generally accepted that imaging of CBF patterns not only provides direct information of cerebral tissue perfusion but has also been used as a marker for the functional integrity of brain tissue (Roman and Pascual, 2012). It has been widely used to clinically evaluate patients with cognitive impairments (Roman and Pascual, 2012). Data show that CBF changes may be present even in asymptomatic dementia risk individuals (Thambisetty et al., 2010; Lunau et al., 2012; Okonkwo et al., 2014). This indicates that CBF measurements hold the ability to detect subclinical brain pathologies. Additionally, the reduction in total CBF was not only confirmed to be present in dementia patients but was also associated with structural signs of brain ageing and cognitive decline in non-dementia individuals (Bisschops et al., 2004; Ruitenberg et al., 2005; Appelman et al., 2008; Vernooij et al., 2008). In summary, cerebral hypoperfusion may appear at an early stage of cognitive function impairment. Meanwhile, other authors’ results showed that alterations in CBF also reflected the possible effects of vascular risk factors (Pase et al., 2012; Henriksen et al., 2014) especially those that led to encephalopathy (Meltzer et al., 2000; Zonneveld et al., 2015).
Numerous evidence indicated that cerebral hypoperfusion caused by vascular diseases led to neuron and astrocyte death, impaired brain volume and neuron function, thereby serving as a biomarker for cognitive decline (Jack et al., 2010; Wierenga et al., 2014). Henriksen et al. revealed that typical CBF changes preceded the appearance of subjective cognitive difficulties (Henriksen et al., 2017). Global CBF was shown to be associated with encephalatrophy, ischemic lesions and cognitive decline (Ruitenberg et al., 2005; Appelman et al., 2008; Vernooij et al., 2008). Moreover, it was shown that the ischemic brain injury and cognitive changes suggested a decrease of global CBF (Brundel et al., 2012; van der Veen et al., 2015; Zonneveld et al., 2015). It was demonstrated that the cerebral flow correlated with cognition in patients with VaD, in whom the CBF was significantly reduced mainly in the frontal, parietal, and temporal cortices (Schuff et al., 2009; Gao et al., 2013).
7. Synergy of multimodality neuroimaging
7.1. Synergy of BOLD and ASL
As is one of the most important factors contributing to VaMCI (Pasi et al., 2015), the cerebral small vessel disease (CSVD) may cause endothelial cell damage, abnormal perfusion, and disruption of the brain structure as well as damage in brain functional connections (Wallin et al., 2018; Thrippleton et al., 2019), which in turn results in dysregulation of the neurovascular unit (NVU) composed of neurons, astrocytes, and blood vessels (Muoio et al., 2014). The NVU plays a crucial role in maintaining the homeostasis and the normal function of the brain microenvironment (Pasley and Freeman, 2008; Helman and Murphy, 2016). Under physiological conditions, the microvascular flow matches well with neurons and astrocytes in the NVU, termed neurovascular coupling (Girouard, 2006). The occurrence of CSVD may disturb its coupling and lead to disorders in cerebral blood supply and neuron activities, which are regarded as the main possible cause of cognitive impairment (Caruso et al., 2019; Moretti and Caruso, 2020). Currently, most studies focused mainly on ASL perfusion imaging to find CBF perfusion or on a single imaging technique that embodied neuronal activities, which did not comprehensively reflect dysregulated neurovascular coupling. Many authors suggest that CBF fusion and neuron activities be regarded as a functional complex (Liu et al., 2021). Other authors’ results showed that FC in brain regions had a similar pattern to CBF, which generated synergy with cerebral perfusion and neuronal activities in different voxels (Liang et al., 2013; Zhu et al., 2013; Jann et al., 2015). The significant correlation between BOLD and ASL perfusion imaging, respectively, represented by the two is a measurable indicator. The abnormal CBF distribution was found to be consistent with FC changes in VaD patients (Schuff et al., 2009; Gao et al., 2013). Liu et al. combined ReHo with CBF via the synergy of BOLD and ASL perfusion imaging and used the overall ReHo-CBF correlation coefficient and ReHo/CBF ratio to measure the intrinsic links between neuron activities and vascular responses (Liu et al., 2021). They found that the overall ReHo-CBF correlation was significantly lower, and the ReHo/CBF ratio was significantly abnormal in patients with cognitive impairment compared with healthy individuals. It showed an indication of more severe neurovascular coupling impairment in the patients with cognitive impairment. Meanwhile, the study suggested that a coupling exists between cerebral perfusion and functional activities in patients with mild cognitive impairment. It indicated abnormal neurovascular coupling in the early stages, and the development of the disease might be related to disease severity and cognitive impairment (Liu et al., 2021).
In summary, data show that the ReHo-CBF correlation coefficient measured the spatial distribution consistency between cerebral blood supply and neuron activities at the whole brain voxel level, while ReHo/CBF ratio represented the strength of connections between the neuron supplied by a CBF unit and the surrounding brain areas (Liu et al., 2021).
7.2. Interactions between the structure and function of cerebrum
The human brain has been modelled as a large-scale integrated complex network in functional and structural domains (Zhang et al., 2011). It is increasingly recognized that the anatomical constraints are imposed on FC by structural connections in the network (Honey et al., 2009). In turn, FC exerts influence on the structural connections through the brain plasticity (Hagmann et al., 2010; Guerra-Carrillo et al., 2014). The demonstration of the association between structure and function holds huge potential to expand our understanding of how the association between brain structure and function affects human cognition and behavior (Wang et al., 2015). This close and complex association is of increasing interest to researchers and will contribute to a better understanding of the intrinsic integration of neural resources and will advance our knowledge about the neuropathological basis of brain - related diseases. Here, we present an conclusion of the existing literature on the imaging approaches for examination of brain pathologies in patients to explore the relationship between the functional abnormalities in the brain and its structural damage which further targeted elucidation of the related central mechanisms underlying neurological pathologies.
7.2.1. Synergy of BOLD-fMRI and DTI
Both DTI and BOLD-fMRI as discussed previously are highly sensitive modalities for measuring the structural and functional brain abnormalities in cognitively impaired patients. The close association between the brain structure and function indicates the integration of these two modalities as a valuable tool for exploring the pathogenesis of brain pathologies (Ye and Bai, 2018). DTI and BOLD imaging of spontaneous neuron activities showed the relationship between the brain structure connections (Koch et al., 2002; De Luca et al., 2006). Thus proving them as tools for identifying subtle WM changes and intrinsic connections between different cortical areas (Hagmann et al., 2008; van Eimeren et al., 2010). DTI and fMRI have been increasingly shown to detect early structural and functional brain alterations related to VaMCI, especially in the last 2 years (Ye and Bai, 2018). A close link between them has been confirmed by a series of studies (van Eimeren et al., 2010; Khalsa et al., 2014). The researches also revealed that brain regions showing prominent FC are also structurally connected in DTI anatomy, regions presenting stronger FC are also more significantly connected in structure (Hagmann et al., 2008; Khalsa et al., 2014). Moreover, it has been documented that the structures with abnormal DTI were generally consistent across the brain regions associated with abnormal FC changes (Ye and Bai, 2018). Therefore, Combining DTI and fMRI findings may be highly valuable for the study of early and specific brain alterations in VaMCI.
7.2.2. Synergy of BOL-fMRI and VBM
Voxel-based morphometry (VBM) obtains anatomical information of a patient’s brain by high-resolution 3D T1 weighted imaging and utilizes the voxel-based cortical morphometric analysis method to quantitatively calculate the cortical grey matter (GM) density or volume of each 3D voxel in the brain structure images. Then the differences of anatomical structure were obtained, and the changes of gray matter in brain were evaluated. As a common measure in VBM measurements, the brain grey matter volume (GMV) predicts the presence of cognitive impairment and the rate of cognitive decline (Mungas et al., 2002). In addition to cerebral alterations directly related to cerebrovascular damage, the global or local GM atrophy may also be responsible for cognitive impairment in patients with cerebral vascular diseases (Laakso et al., 1996; Fein et al., 2000; Mungas et al., 2001). A VBM study showed extensive volume atrophy in patients with VaMCI, especially in the frontal cortex and subcortical regions (Thong et al., 2014). Specifically, in the study of Seo, the thinning of frontal cortex in patients VaMCI is closely related to executive dysfunction (Seo et al., 2010). Fein et al. also found that the severity of cognitive impairment is significantly correlated with GM atrophy, and the GM atrophy of hippocampus and frontal lobe are predictors of brain cognitive impairment (Fein et al., 2000; Mungas et al., 2001, 2002; Mok et al., 2005). Moreover, the GM atrophy was shown to reflect the loss of neurons or other types of neuropathological outcomes (Markus, 2007). Consequently, GM atrophy has been a cause of cognitive decline (e.g., memory loss, attention/executive dysfunction, language impairment, visuospatial function, and depression of VaMCI patients; Lei et al., 2016; Li et al., 2017; Lyu et al., 2019).
Because the fact that regions of interest were not predefined, VBM provided unbiased whole-brain comparisons between the studied patients’ groups, making it an ideal method for exploratory cross-sectional studies (Stebbins et al., 2016). Hence, the established synergy between BOL-fMRI and VBM provided comprehensive visualization of characteristic changes in brain function and structure. As an early stage of VD, VaMCI is regarded as the most important subtype of vascular disease caused by CSVD (Peng, 2019). Indeed, CSVD was typically accompanied by changes in the brain function and structure (Ter Telgte et al., 2018) and GMV atrophy was observed in the frontal, temporal versus parietal cortical areas, pons, thalamus, caudate nucleus, and hippocampus of VaMCI patients (Seo et al., 2010, 2012; Thong et al., 2014). These morphological changes interacted with functional activities (Koch et al., 2002; Liu C. et al., 2014; Tu et al., 2020). For patients with ischemic vascular diseases, GM atrophy strongly correlated with dementia severity and it was an independent predictor for the cognitive decline of patients with cerebral vascular diseases (Fein et al., 2000; Mungas et al., 2002). Yang et al. found that GMV of the right precentral gyrus and right inferior temporal gyrus of VaMCI patients decreased (Yang, 2021) and this was related to ALFF reduction, indicating that CSVD patients might be exposed to impairments of the brain structure and function (Zhao et al., 2015; Lyu et al., 2019).
8. Technical problems or difficulties
Although many neuroimaging studies have reported the brain structure and functional characteristics related to cognitive function in patients with VaMCI, most of the previous studies evaluated the single-modality image information. Due to the different imaging principles, the data provided by single-modality images are limited. In contrast, multi-modality magnetic resonance imaging can provide multiple comprehensive data such as tissue anatomy function and find imaging markers related to the occurrence and development of VaMCI, but there are still some technical problems to be solved: (1) The b value selected in each study is different, which needs to be optimized combined with signal-to-noise ratio, image quality and scanning time. (2) Different research institutions use different scanning instruments, imaging parameters and reconstruction algorithms, which can improve the comparability between different studies by establishing standardized image acquisition and post-processing processes. (3) The method of manually drawing the region of interest is greatly affected by subjective factors, and semi-automatic or automatic segmentation techniques can be used to improve the repeatability of imaging parameters. (4) By establishing standardized data analysis methods and evaluation methods, the comparison and feasibility of different studies are improved. Different data analysis methods and evaluation methods may affect the analysis of structure and function.
9. Conclusion
This article conducts a narrative review in the clinical applications of multimodal MRI diagnostic approaches for VaMCI. The close association between the brain structure and function indicates that the integration of rs-fMRI, DTI and ASL patterns may be valuable for the detailed neuroimaging exploration of the pathogenesis of VaMCI. These findings provide a valuable basis for a better understanding of VaMCI pathophysiological mechanisms, its unique features for recognition and diagnosis, as well as provide suggestions for possible targets in the development and evaluation of new cognitive and pharmacological interventions of VaCI pathologies. In future, we can conquer the limits of single-modality analysis and advance the diagnostic and prognostic potential on single patient examination.
Author contributions
QL contributed to the conceptualization of the study, literature search. XZ and QL performed the quality appraisal and manuscript development. All authors contributed to the article and approved the submitted version.
Funding
This work has received funding by National Natural Science Foundation of China (No. 82174492) and Tianjin Science and Technology Plan Project (No. 20YFZCSY00810).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adriana, D. M., Xi-Nian, Z., Clare, K., Rebecca, G., Maarten, M., Ariel, S., et al. (2013). Shared and distinct intrinsic functional network centrality in autism and attention-deficit/hyperactivity disorder. Biol. Psychiatry 74, 623–632. doi: 10.1016/j.biopsych.2013.02.011
Appelman, A. P., Van Der GY, V. K. L., Tiehuis, A. M., Witkamp, T. D., Mali, W. P., et al. (2008). Total cerebral blood flow, white matter lesions and brain atrophy: the SMART-MR study. J. Cereb. Blood Flow Metab. 28, 633–639. doi: 10.1038/sj.jcbfm.9600563
Banerjee, G., Wilson, D. J., Jäger, H. R., and Werring, D. J. (2016). Novel imaging techniques in cerebral small vessel diseases and vascular cognitive impairment. Biochim. Biophys. Acta 1862, 926–938. doi: 10.1016/j.bbadis.2015.12.010
Barkhof, F., Haller, S., and Rombouts, S. (2014). Resting-state functional Mr imaging: a new window to the brain. Radiology 272, 29–49. doi: 10.1148/radiol.14132388
Beaulieu, C. (2002). The basis of anisotropic water diffusion in the nervous system – a technical review. NMR Biomed. 15, 435–455. doi: 10.1002/nbm.782
Bisschops, R. H., Van Der GY, M. W. P., and Van Der, G. J. (2004). High total cerebral blood flow is associated with a decrease of white matter lesions. J. Neurol. 251, 1481–1485. doi: 10.1007/s00415-004-0569-y
Biswal, B., Yetkin, F., Haughton, V., and Hyde, J. S. (1995). Functional connectivity in the motor cortex of resting human brain using Echo-planar Mri. Magn. Reson. Med. 34, 41–537.
Brundel, M., van den Berg, E., Reijmer, Y. D., de Bresser, J., Kappelle, L. J., and Biessels, G. J. (2012). Cerebral haemodynamics, cognition and brain volumes in patients with type 2 diabetes. J. Diabetes Complicat. 26, 205–209. doi: 10.1016/j.jdiacomp.2012.03.021
Buckner, R. L., Sepulcre, J., Talukdar, T., Krienen, F. M., Liu, H., Hedden, T., et al. (2009). Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. J. Neurosci. 29, 1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009
Caplan, L. (2015). Lacunar infarction and small vessel disease: pathology and pathophysiology. J. Stroke 17, 2–6. doi: 10.5853/jos.2015.17.1.2
Caruso, P., Signori, R., and Moretti, R. (2019). Small vessel disease to subcortical dementia: a dynamic model, which interfaces aging, cholinergic dysregulation and the neurovascular unit. Vasc. Health Risk Manag. 15, 259–281. doi: 10.2147/VHRM.S190470
Chen, X., Wen, W., Anstey, K. J., et al. (2009). Prevalence, incidence, and risk factors of lacunar infarcts in a community sample. Neurology 73, 266–272. doi: 10.1212/WNL.0b013e3181aa52ea
Colasanti, A., Guo, Q., Giannetti, P., et al. (2016). Hippocampal Neuroinflammation, functional connectivity, and depressive symptoms in multiple sclerosis. Biol. Psychiatry 80, 62–72. doi: 10.1016/j.biopsych.2015.11.022
Cui, Y., Li, S. F., Gu, H., Hu, Y. Z., Liang, X., Lu, C. Q., et al. (2016). Disrupted brain connectivity patterns in patients with type 2 diabetes. AJNR Am. J. Neuroradiol. 37, 2115–2122. doi: 10.3174/ajnr.A4858
Dao, E., Hsiung, G. R., and Liu-Ambrose, T. (2018). The role of exercise in mitigating subcortical ischemic vascular cognitive impairment. J. Neurochem. 144, 582–594. doi: 10.1111/jnc.14153
De Luca, M., Beckmann, C. F., De Stefano, N., Matthews, P. M., and Smith, S. M. (2006). fMRI resting state networks define distinct modes of long-distance interactions in the human brain. NeuroImage 29, 1359–1367. doi: 10.1016/j.neuroimage.2005.08.035
Dennis, E., and Thompson, P. (2014). Functional brain connectivity using Fmri in aging and Alzheimer's disease. Neuropsychol. Rev. 24, 49–62. doi: 10.1007/s11065-014-9249-6
Dey, A., Stamenova, V., Turner, G., Black, S. E., and Levine, B. (2016). Pathoconnectomics of cognitive impairment in small vessel disease: a systematic review. Alzheimers Dement. 12, 45–831. doi: 10.1016/j.jalz.2016.01.007
Diciotti, S., Orsolini, S., Salvadori, E., Giorgio, A., Toschi, N., Ciulli, S., et al. (2017). Resting state fMRI regional homogeneity correlates with cognition measures in subcortical vascular cognitive impairment. J. Neurol. Sci. 373, 1–6. doi: 10.1016/j.jns.2016.12.003
Ding, W., Cao, W., Wang, Y., Sun, Y., Chen, X., Zhou, Y., et al. (2015). Altered functional connectivity in patients with subcortical vascular cognitive impairment--a resting-state functional magnetic resonance imaging study. PLoS One 10:e0138180. doi: 10.1371/journal.pone.0138180
El-Sahar, A. E., Shiha, N. A., El Sayed, N. S., and Ahmed, L. A. (2021). Alogliptin attenuates lipopolysaccharide-induced Neuroinflammation in mice through modulation of TLR4/MYD88/NF-κB and mi RNA-155/SOCS-1 signaling pathways. Int. J. Neuropsychopharmacol. 24, 158–169. doi: 10.1093/ijnp/pyaa078
Fan, L., Hu, J., Ma, W., Wang, D., Yao, Q., and Shi, J. (2019). Altered baseline activity and connectivity associated with cognitive impairment following acute cerebellar infarction: a resting-state fMRI study. Neurosci. Lett. 23, 199–203. doi: 10.1016/j.neulet.2018.11.007
Fein, G., Di Sclafani, V., Tanabe, J., Cardenas, V., Weiner, M. W., Jagust, W. J., et al. (2000). Hippocampal and cortical atrophy predict dementia in subcortical ischemic vascular disease. Neurology 55, 1626–1635. doi: 10.1212/WNL.55.11.1626
Feng, Y., Li, Y., Tan, X., Liang, Y., Ma, X., Chen, Y., et al. (2021). Altered gray matter volume, functional connectivity, and degree centrality in early-onset type 2 diabetes mellitus. Front. Neurol. 12:697349. doi: 10.3389/fneur.2021.697349
Filippi, M., Agosta, F., Barkhof, F., Dubois, B., Fox, N. C., Frisoni, G. B., et al. (2012). EFNS task force: the use of neuroimaging in the diagnosis of dementia. Eur. J. Neurol. 19, 1487–1501. doi: 10.1111/j.1468-1331.2012.03859.x
Fox, M. D., and Greicius, M. (2010). Clinical applications of resting state functional connectivity. Front. Syst. Neurosci. 4:19. doi: 10.3389/fnsys.2010.00019
Fox, M., and Raichle, M. (2007). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 8, 700–711. doi: 10.1038/nrn2201
Gao, C. H., Liu, W. H., Liu, Y. L., Ruan, X. H., Chen, X., Liu, L. L., et al. (2016). Decreased subcortical and increased cortical degree centrality in a nonclinical college student sample with subclinical depressive symptoms: a resting-state fMRI study. Front. Hum. Neurosci. 10:617. doi: 10.3389/fnhum.2016.00617
Gao, Y. Z., Zhang, J. J., Liu, H., et al. (2013). Regional cerebral blood flow and cerebrovascular reactivity in Alzheimer's disease and vascular dementia assessed by arterial spinlabeling magnetic resonance imaging. Curr. Neurovasc. Res. 10, 49–53. doi: 10.2174/156720213804806016
Garrett, D., Samanez-larkin, G., Macdonald, S., et al. (2013). Moment-to-moment brain signal variability: a next frontier in human brain mapping? Neurosci. Biobehav. Rev. 37, 610–624. doi: 10.1016/j.neubiorev.2013.02.015
GBD (2016). Global, regional, and national burden of Alzheimer's disease and other dementias, 1990–2016: a systematic analysis for the global burden of disease study.
Girouard, H. (2006). Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J. Appl. Physiol. 100, 328–335. doi: 10.1152/japplphysiol.00966.2005
Gorelick, P., Scuteri, A., Black, S., DeCarli, C., Greenberg, S. M., Iadecola, C., et al. (2011). Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42, 2672–2713. doi: 10.1161/STR.0b013e3182299496
Grau-Olivares, M., Arboix, A., Junque, C., Arenaza-Urquijo, E. M., Rovira, M., and Bartres-Faz, D. (2010). Progressive gray matter atrophy in lacunar patients with vascular mild cognitive impairment. Cerebrovasc. Dis. 30, 157–166. doi: 10.1159/000316059
Greicius, M. (2008). Resting-state functional connectivity in neuropsychiatric disorders. Curr. Opin. Neurol. 21, 424–430. doi: 10.1097/WCO.0b013e328306f2c5
Greicius, M., Krasnow, B., Reiss, A., and Menon, V. (2003). Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. U. S. A. 100, 8–253. doi: 10.1073/pnas.0135058100
Greicius, M., Supekar, K., Menon, V., and Dougherty, R. F. (2009). Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb. Cortex 19, 72–78. doi: 10.1093/cercor/bhn059
Guerra-Carrillo, B., Mackey, A. P., and Bunge, S. A. (2014). Resting-state fMRI: a window into human brain plasticity. Neuroscientist 20, 522–533. doi: 10.1177/1073858414524442
Guo, Z., Liu, X., Hou, H., Wei, F., Liu, J., and Chen, X. (2016). Abnormal degree centrality in Alzheimer’s disease patients with depression: a resting-state functional magnetic resonance imaging study. Exp. Gerontol. 79, 61–66. doi: 10.1016/j.exger.2016.03.017
Guo, M., Ren, Y., Yu, H., Yang, H., Cao, C., Li, Y., et al. (2020). Alterations in degree centrality and functional connectivity in Parkinson's disease patients with freezing of gait: a resting-state functional magnetic resonance imaging study. Front. Neurosci. 14:582079. doi: 10.3389/fnins.2020.582079
Hagmann, P., Cammoun, L., Gigandet, X., Meuli, R., Honey, C. J., Wedeen, V. J., et al. (2008). Mapping the structural core of human cerebral cortex. PLoS Biol. 6:e159. doi: 10.1371/journal.pbio.0060159
Hagmann, P., Sporns, O., Madan, N., Cammoun, L., Pienaar, R., Wedeen, V. J., et al. (2010). White matter maturation reshapes structural connectivity in the late developing human brain. Proc. Natl. Acad. Sci. U. S. A. 107, 19067–19072. doi: 10.1073/pnas.1009073107
He, Y., Wang, L., Zang, Y., Tian, L., Zhang, X., Li, K., et al. (2007). Regional coherence changes in the early stages of Alzheimer’s disease: a combined structural and resting-state functional MRI study. NeuroImage 35, 488–500. doi: 10.1016/j.neuroimage.2006.11.042
Helman, A. M., and Murphy, M. P. (2016). Vascular cognitive impairment: modeling a critical neurologic disease in vitro and in vivo. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 1862, 975–982. doi: 10.1016/j.bbadis.2015.12.009
Henriksen, O. M., Hansen, N. L., Osler, M., Mortensen, E. L., Hallam, D. M., Pedersen, E. T., et al. (2017). Sub-clinical cognitive decline and resting cerebral blood flow in middle aged men. PLoS One 12:e0169912. doi: 10.1371/journal.pone.0169912
Henriksen, O. M., Jensen, L. T., Krabbe, K., Guldberg, P., Teerlink, T., and Rostrup, E. (2014). Resting brain perfusion and selected vascular risk factors in healthy elderly subjects. PLoS One 9:e97363. doi: 10.1371/journal.pone.0097363
Honey, C., Sporns, O., Cammoun, L., Gigandet, X., Thiran, J. P., Meuli, R., et al. (2009). Predicting human resting-state functional connectivity from structural connectivity. Proc. Natl. Acad. Sci. U. S. A. 106, 40–2035. doi: 10.1073/pnas.0811168106
Huang, X., Zhong, Y. L., Zeng, X. J., Zhou, F., Liu, X. H., Hu, P. H., et al. (2015). Disturbed spontaneous brain activity pattern in patients with primary angle-closure glaucoma using amplitude of low-frequency fluctuation: a fMRI study. Neuropsychiatr. Dis. Treat. 11, 1877–1883. doi: 10.2147/NDT.S87596
Iadecola, C., Park, L., and Capone, C. (2009). Threats to the mind: aging, amyloid, and hypertension. Stroke 40, S40–S44. doi: 10.1161/STROKEAHA.108.533638
Iturria-Medina, Y., Sotero, R. C., Toussaint, P. J., Mateos-Pérez, J. M., and Evans, A. C. (2016). Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat. Commun. 7:11934. doi: 10.1038/ncomms11934
Jack, C. R. Jr., Knopman, D. S., Jagust, W. J., Shaw, L. M., Aisen, P. S., Weiner, M. W., et al. (2010). Hypo-thetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 9, 119–128. doi: 10.1016/S1474-4422(09)70299-6
Jak, A., Bondi, M., Delano-wood, L., Wierenga, C., Corey-Bloom, J., Salmon, D. P., et al. (2009). Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am. J. Geriatr. Psychiatry 17, 75–368. doi: 10.1097/JGP.0b013e31819431d5
Jann, K., Gee, D. G., Kilroy, E., Schwab, S., Smith, R. X., Cannon, T. D., et al. (2015). Functional connectivity in BOLD and CBF data: similarity and reliability of resting brain networks. NeuroImage 106, 111–122. doi: 10.1016/j.neuroimage.2014.11.028
Jiang, L., and Zuo, X. (2016). Regional homogeneity: a multimodal, multiscale neuroimaging marker of the human connectome. Neuroscientist 22, 486–505. doi: 10.1177/1073858415595004
Jongsiriyanyong, S., and Limpawattana, P. (2018). Mild cognitive impairment in clinical practice: a review article. Am. J. Alzheimers Dis. Other Dement. 33, 500–507. doi: 10.1177/1533317518791401
Khalsa, S., Mayhew, S. D., Chechlacz, M., Bagary, M., and Bagshaw, A. P. (2014). The structural and functional connectivity of the posterior cingulate cortex: comparison between deterministic and probabilistic tractography for the investigation of structure-function relationships. NeuroImage 102, 118–127. doi: 10.1016/j.neuroimage.2013.12.022
Kielar, T., Deschamps, R. K., Chu, R., Jokel, Y. B., Khatamian, J. J., and Chen, J. A. (2016). Meltzer identifying dysfunctional cortex: dissociable effects of stroke and aging on resting state dynamics in MEG and fMRI. Front. Aging Neurosci. 8:40. doi: 10.3389/fnagi.2016.00040
Knopman, D., DeKosky, S. T., Cummings, J., Chui, H., Corey–Bloom, J., Relkin, N., et al. (2001). Practice parameter: diagnosis of dementia (an evidence-based review). Report of the quality standards Subcommittee of the American Academy of Neurology. Neurology 56, 1143–1153. doi: 10.1212/WNL.56.9.1143
Koch, M. A., Norris, D. G., and Hund-Georgiadis, M. (2002). An investigation of functional and anatomical connectivity using magnetic resonance imaging. NeuroImage 16, 241–250. doi: 10.1006/nimg.2001.1052
Laakso, M. P., Partanen, K., Riekkinen, P., Lehtovirta, M., Helkala, E. L., Hallikainen, M., et al. (1996). Hippocampal volumes in Alzheimer’s disease, Parkinson’s disease with and without dementia, and in vascular dementia: an MRI study. Neurology 46, 678–681. doi: 10.1212/WNL.46.3.678
Lei, Y., Su, J., Guo, Q., Yang, H., Gu, Y., and Mao, Y. (2016). Regional gray matter atrophy in vascular mild cognitive impairment. J. Stroke Cerebrovasc. Dis. 25, 95–101. doi: 10.1016/j.jstrokecerebrovasdis.2015.08.041
Li, M., Meng, Y., Wang, M., Yang, S., Wu, H., Zhao, B., et al. (2017). Cerebral gray matter volume reduction in subcortical vascular mild cognitive impairment patients and subcortical vascular dementia patients, and its relation with cognitive deficits. Brain Behav. 7:e00745. doi: 10.1002/brb3.745
Li, X., Shen, M., Jin, Y., Jia, S., Zhou, Z., Han, Z., et al. (2021). The effect of cerebral small vessel disease on the subtypes of mild cognitive impairment. Front. Psychol. 12:685965. doi: 10.3389/fpsyt.2021.685965
Liang, X., Zou, Q., He, Y., and Yang, Y. (2013). Coupling of functional connectivity and regional cerebral blood flow reveals a physiological basis for network hubs of the human brain. Proc. Natl. Acad. Sci. U. S. A. 110, 34–1929. doi: 10.1073/pnas.1214900110
Liu, X., Cheng, R., Chen, L., Gong, J., Luo, T., and Lv, F. (2021). Altered neurovascular coupling in subcortical ischemic vascular disease. Front. Aging Neurosci. 13:598365. doi: 10.3389/fnagi.2021.598365
Liu, X., Cheng, R., Chen, L., Luo, T., Lv, F., Gong, J., et al. (2019). Alterations of white matter integrity in subcortical ischemic vascular disease with and without cognitive impairment: a TBSS study. J. Mol. Neurosci. 67, 595–603. doi: 10.1007/s12031-019-01266-3
Liu, C., Li, C., Yin, X., Yang, J., Zhou, D., Gui, L., et al. (2014). Abnormal intrinsic brain activity patterns in patients with subcortical ischemic vascular dementia. PLoS One 9:e87880. doi: 10.1371/journal.pone.0116352
Liu, Y., Yu, C., Zhang, X., Liu, J., Duan, Y., Alexander-Bloch, A. F., et al. (2014). Impaired long distance functional connectivity and weighted network architecture in Alzheimer's disease. Cereb. Cortex 24, 1422–1435. doi: 10.1093/cercor/bhs410
Logothetis, N., Pauls, J., Augath, M., Trinath, T., and Oeltermann, A. (2001). Neurophysiological investigation of the basis of the Fmri signal. Nature 412, 150–157. doi: 10.1038/35084005
Lou, Y., Huang, P., Li, D., Cen, Z., Wang, B., Gao, J., et al. (2015). Altered brain network centrality in depressed Parkinson’s disease patients. Mov. Disord. 30, 1777–1784. doi: 10.1002/mds.26321
Lunau, L., Mouridsen, K., Rodell, A., Østergaard, L., Nielsen, J. E., Isaacs, A., et al. (2012). Presymptomatic cerebral blood flow changes in CHMP2B mutation carriers of familial frontotemporal dementia (FTD-3), measured with MRI. BMJ Open 2:e000368. doi: 10.1136/bmjopen-2011-000368
Luo, X., Guo, L., Dai, X. J., Wang, Q., Zhu, W., Miao, X., et al. (2017). Abnormal intrinsic functional hubs in alcohol dependence: evidence from a voxelwise degree centrality analysis. Neuropsychiatr. Dis. Treat. 13, 2011–2020. doi: 10.2147/NDT.S142742
Luo, C., Qiu, C., Guo, Z., Fang, J., Li, Q., Lei, X., et al. (2011). Disrupted functional brain connectivity in partial epilepsy: a resting-state Fmri study. PLoS One 7:e28196. doi: 10.1371/journal.pone.0028196
Lyu, H., Wang, J., Xu, J., Zheng, H., Yang, X., Lin, S., et al. (2019). Structural and functional disruptions in subcortical vascular mild cognitive impairment with and without depressive symptoms. Front. Aging Neurosci. 11:241. doi: 10.3389/fnagi.2019.00241
Markus, H. S. (2007). Mild cognitive impairment after lacunar infarction: voxel-based morphometry and neuropsychological assessment. Cerebrovasc. Dis. 23, 323–324. doi: 10.1159/000099129
Mascalchi, M., Ginestroni, A., Toschi, N., Poggesi, A., Cecchi, P., Salvadori, E., et al. (2014). VMCI Tuscany investigators. The burden of microstructural damage modulates cortical activation in elderly subjects with MCI and leuko-araiosis. A DTI and fMRI study. Hum. Brain Mapp. 35, 819–830. doi: 10.1002/hbm.22216
Mascalchi, M., Salvadori, E., Toschi, N., Giannelli, M., Orsolini, S., Ciulli, S., et al. (2019). VMCI-Tuscany study group. DTI-derived indexes of brain WM correlate with cognitive performance in vascular MCI and small-vessel disease. A TBSS study. Brain Imaging Behav. 13, 594–602. doi: 10.1007/s11682-018-9873-5
Meltzer, C. C., Cantwell, M. N., Greer, P. J., Ben-Eliezer, D., Smith, G., Frank, G., et al. (2000). Does cerebral blood flow decline in healthy aging a PET study with partial-volume correction. J. Nucl. Med. 41, 1842–1848.
Meyer, J. S., Quach, M., Thomby, J., Chowdhury, M., and Huang, J. (2005). MRI identifies MCI subtypes: vascular versus neurodegenerative. J. Neuml. Sci. 229, 121–129. doi: 10.1016/j.jns.2004.11.012.
Mijajlović, M. D., Pavlović, A., Brainin, M., Heiss, W. D., Quinn, T. J., Ihle-Hansen, H. B., et al. (2017). Post-stroke dementia - a comprehensive review. BMC Med. 15:11. doi: 10.1186/s12916-017-0779-7
Mok, V., Chang, C., Wong, A., Lam, W. W. M., Richards, P. S., Wong, K. T., et al. (2005). Neuroimaging determinants of cognitive performances in stroke associated with small vessel disease. J. Neuroimaging 15, 129–137. doi: 10.1111/j.1552-6569.2005.tb00297.x
Moosmann, M., Ritter, P., Krastel, I., Brink, A., Thees, S., Blankenburg, F., et al. (2003). Correlates of alpha rhythm in functional magnetic resonance imaging and near infrared spectroscopy. NeuroImage 20, 145–158. doi: 10.1016/S1053-8119(03)00344-6
Moretti, R., and Caruso, P. (2020). Small vessel disease-related dementia: An invalid neurovascular coupling? Int. J. Mol. Sci. 21:1095. doi: 10.3390/ijms21031095
Mungas, D., Jagust, W. J., Reed, B. R., Kramer, J. H., Weiner, M. W., Schuff, N., et al. (2001). MRI predictors of cognition in subcortical ischemic vascular disease and Alzheimer’s disease. Neurology 57, 2229–2235. doi: 10.1212/WNL.57.12.2229
Mungas, D., Reed, B. R., Jagust, W. J., DeCarli, C., Mack, W. J., Kramer, J. H., et al. (2002). Volumetric MRI predicts rate of cognitive decline related to AD and cerebrovascular disease. Neurology 59, 867–873. doi: 10.1212/WNL.59.6.867
Muoio, V., Persson, P. B., and Sendeski, M. M. (2014). The neurovascular unit - concept review. Acta Physiol. 210, 790–798. doi: 10.1111/apha.12250
Nir, T. M., Jahanshad, N., Villalon-Reina, J. E., Toga, A. W., Jack, C. R., Weiner, M. W., et al. (2013). Effectiveness of regional DTI measures in distinguishing Alzheimer's disease, MCI, and normal aging. Neuroimage Clin. 3, 180–195. doi: 10.1016/j.nicl.2013.07.006
Nomi, J., Bolt, T., Ezie, C., Uddin, L. Q., and Heller, A. S. (2017). Moment-to-moment Bold signal variability reflects regional changes in neural flexibility across the lifespan. J. Neurosci. Off. J. Soc. Neurosci. 37, 5539–5548. doi: 10.1523/JNEUROSCI.3408-16.2017
Nugent, A., Martinez, A., D'Alfonso, A., Zarate, C. A., and Theodore, W. H. (2015). The relationship between glucose metabolism, resting-state Fmri Bold signal, and Gabaa-binding potential: a preliminary study in healthy subjects and those with temporal lobe epilepsy. J. Cereb. Blood Flow Metab. 35, 91–583. doi: 10.1038/jcbfm.2014.228
O’Sullivan, M., Morris, R. G., Huckstep, B., Jones, D. K., Williams, S. C. R., and Markus, H. S. (2004a). Diffusion tensor MRI correlates with executive dysfunction in patients with ischaemic leukoaraiosis. J. Neurol. Neurosurg. Psychiatry 75, 441–447. doi: 10.1136/jnnp.2003.014910
O’Sullivan, M., Singhal, S., Charlton, R., and Markus, H. S. (2004b). Diffusion tensor imaging of thalamus correlates with cognition in CADASIL without dementia. Neurology 62, 702–707. doi: 10.1212/01.WNL.0000113760.72706.D2
Oakes, T., Fox, A., Johnstone, T., Chung, M. K., Kalin, N., and Davidson, R. J. (2007). Integrating Vbm into the general linear model with Voxelwise anatomical covariates. NeuroImage 34, 500–508. doi: 10.1016/j.neuroimage.2006.10.007
Okonkwo, O. C., Xu, G., Oh, J. M., Dowling, N. M., Carlsson, C. M., Gallagher, C. L., et al. (2014). Cerebral blood flow is diminished in asymptomatic middle-aged adults with maternal history of Alzheimer’s disease. Cereb. Cortex 24, 978–988. doi: 10.1093/cercor/bhs381
Orsolini, S., Marzi, C., Gavazzi, G., Bianchi, A., Salvadori, E., Giannelli, M., et al. (2021). Altered regional brain homogeneity of BOLD signal in CADASIL: a resting state fMRI study. J. Neuroimaging 31, 348–355. doi: 10.1111/jon.12821
Pan, P., Zhu, L., Yu, T., Shi, H., Zhang, B., Qin, R., et al. (2017). Aberrant spontaneous low-frequency brain activity in amnestic mild cognitive impairment: a meta-analysis of resting-state fMRI studies. Ageing Res. Rev. 35, 12–21. doi: 10.1016/j.arr.2016.12.001
Pase, M. P., Grima, N. A., Stough, C. K., Scholey, A., and Pipingas, A. (2012). Cardiovascular disease risk and cerebral blood flow velocity. Stroke 43, 2803–2805. doi: 10.1161/STROKEAHA.112.666727
Pasi, M., Salvadori, E., Poggesi, A., Ciolli, L., del Bene, A., Marini, S., et al. (2015). White matter microstruc-tural damage in small vessel disease is associated with Montreal cognitive assessment but not with mini mental state examination performances: vascular mild cognitive impairment Tuscany study. Stroke 46, 262–264, doi: 10.1161/STROKEAHA.114.007553
Pelled, G., and Goelman, G. (2004). Different physiological Mri noise between cortical layers. Magn. Reson. Med. 52, 6–913. doi: 10.1002/mrm.20229
Peng, C., Chen, Y., Cui, Y., Zhao, D. L., Jiao, Y., Tang, T. Y., et al. (2016). Regional coherence alterations revealed by resting-state Fmri in post-stroke patients with cognitive dysfunction. PLoS One 11:e0159574. doi: 10.1371/journal.pone.0159574
Peng, D., Geriatric Neurology Group, Chinese Society of Geriatrics, Clinical Practice Guideline for Cognitive Impairment of Cerebral Small Vessel Disease Writing Group (2019). Clinical practice guideline for cognitive impairment of cerebral small vessel disease. Aging Med. (Milton) 2, 64–73. doi: 10.1002/agm2.12073
Petracca, C., Saiote, H. A., Bender, F., Arias, C., Farrell, P., Magioncalda, M., et al. (2017). Inglese synchronization and variability imbalance underlie cognitive impairment in primary-progressive multiple sclerosis. Sci. Rep. 7:46411. doi: 10.1038/srep46411
Plassman, B. L., Langa, K. M., Fisher, G. G., Heeringa, S. G., Weir, D. R., Ofstedal, M. B., et al. (2007). Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology 29, 125–132. doi: 10.1159/000109998
Raichle, M. E., and Mintun, M. A. (2006). Brain work and brain imaging. Annu. Rev. Neurosci. 29, 449–476. doi: 10.1146/annurev.neuro.29.051605.112819
Ravaglia, G., Forti, P., Maioli, F., Martelli, M., Servadei, L., Brunetti, N., et al. (2006). Conversion of mild cognitive impairment to dementia: predictive role of mild cognitive impairment subtypes and vascular risk factors. Dement. Geriatr. Cogn. Disord. 21, 8–51. doi: 10.1159/000089515
Roman, G., and Pascual, B. (2012). Contribution of neuroimaging to the diagnosis of Alzheimer’s disease and vascular dementia. Arch. Med. Res. 43, 671–676. doi: 10.1016/j.arcmed.2012.10.018
Ruitenberg, A., Den HT, B. S. L., Van Swieten, J. C., Koudstaal, P. J., Hofman, A., et al. (2005). Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam study. Ann. Neurol. 57, 789–794. doi: 10.1002/ana.20493
Sabri, O., Ringelstein, E. B., Hellwig, D., Schneider, R., Schreckenberger, M., Kaiser, H. J., et al. (1999). Neuropsychological impairment correlates with hypoperfusion and hypometabolism but not with severity of white matter lesions on MRI in patients with cerebral microangiopathy. Stroke 30, 556–566. doi: 10.1161/01.STR.30.3.556
Sachdev, P., Kalaria, R., O’Brien, J., Skoog, I., Alladi, S., Black, S. E., et al. (2014). Diagnostic criteria for vascular cognitive disorders: a VASCOG statement. Alzheimer Dis. Assoc. Disord. 28:206–218, doi: 10.1097/WAD.0000000000000034
Sala-Llonch, R., Bartres-Faz, D., and Junque, C. (2015). Reorganization of brain networks in aging: a review of functional connectivity studies. Front. Psychol. 6:663. doi: 10.3389/fpsyg.2015.00663
Scarapicchia, E. L., Mazerolle, J. D., Fisk, L. J., Ritchie, J. R., and Gawryluk, J. R. (2018). Gawryluk resting state BOLD variability in Alzheimer's disease: a marker of cognitive decline or cerebrovascular status? Front. Aging Neurosci. 10:39. doi: 10.3389/fnagi.2018.00039
Schuff, N., Matsumoto, S., Kmiecik, J., Studholme, C., du, A., Ezekiel, F., et al. (2009). Cerebral blood flow in ischemic vascular dementia and Alzheimer's disease, measured by arterial spin-labeling magnetic resonance imaging. Alzheimers Dement. 5, 454–462. doi: 10.1016/j.jalz.2009.04.1233
Sen, P. N., and Basser, P. J. (2005). A model for diffusion in white matter in the brain. Biophys. J. 89, 2927–2938. doi: 10.1529/biophysj.105.063016
Seo, S. W., Ahn, J., Yoon, U., Im, K., Lee, J. M., Tae, K. S., et al. (2010). Cortical thinning in vascular mild cognitive impairment and vascular dementia of subcortical type. J. Neuroimaging 20, 37–45. doi: 10.1111/j.1552-6569.2008.00293.x
Seo, S. W., Lee, J. M., Im, K., Park, J. S., Kim, S. H., Kim, S. T., et al. (2012). Cortical thinning related to periventricular and deep white matter hyperintensities. Neurobiol. Aging 6:S368. doi: 10.1016/j.neurobiolaging.2010.12.003
Shao, Y., Cai, F. Q., Zhong, Y. L., Huang, X., Zhang, Y., Hu, P. H., et al. (2015). Altered intrinsic regional spontaneous brain activity in patients with optic neuritis: a resting-state functional magnetic resonance imaging study. Neuropsychiatr. Dis. Treat. 11, 3065–3073. doi: 10.2147/NDT.S92968
Shaw, E., Schultz, A., Sperling, R., and Hedden, T. (2015). Functional connectivity in multiple cortical networks is associated with performance across cognitive domains in older adults. Brain Conn. 5, 16–505. doi: 10.1089/brain.2014.0327
Sheline, Y., and Raichle, M. (2013). Resting state functional connectivity in preclinical Alzheimer's disease. Biol. Psychiatry 74, 7–340. doi: 10.1016/j.biopsych.2012.11.028
Shen, Y., Yao, J., Jiang, X., Zhang, L., Xu, L., Feng, R., et al. (2015). Sub-hubs of baseline functional brain networks are related to early improvement following two-week pharmacological therapy for major depressive disorder. Hum. Brain Mapp. 36, 2915–2927. doi: 10.1002/hbm.22817
Snowdon, D. A., Greiner, L. H., Mortimer, J. A., Riley, K. P., Greiner, P. A., and Markesbery, W. A. (1997). Brain infarction and the clinical expression of Alzheimer disease. The nun study. JAMA 277:813–817, doi: 10.1001/jama.1997.03540340047031
Stebbins, G. T., Nyenhuis, D. L., Wang, C., Cox, J. L., Freels, S., Bangen, K., et al. (2016). Gray matter atrophy in patients with ischemic stroke with cognitive impairment. Stroke 39, 785–793. doi: 10.1161/STROKEAHA.107.507392
Sun, Y., Qin, L., Zhou, Y., Xu, Q., Qian, L. J., Tao, J., et al. (2011). Abnormal functional connectivity in patients with vascular cognitive impairment, no dementia: a resting-state functional magnetic resonance imaging study. Behav. Brain Res. 223, 94–388. doi: 10.1016/j.bbr.2011.05.006
Ter Telgte, A., van Leijsen, E. M. C., Wiegertjes, K., Klijn, C. J. M., Tuladhar, A. M., and de Leeuw, F. E. (2018). Cerebral small vessel disease: from a focal to a global perspective. Nat. Rev. Neurol. 14, 387–398. doi: 10.1038/s41582-018-0014-y
Thal, D. R., Grinberg, L. T., and Attems, J. (2012). Vascular dementia: different forms of vessel disorders contribute to the development of dementia in the elderly brain. Exp. Gerontol. 47, 816–824. doi: 10.1016/j.exger.2012.05.023
Thambisetty, M., Beason-Held, L., An, Y., Kraut, M. A., and Resnick, S. M. (2010). APOE epsilon 4 genotype and longitudinal changes in cerebral blood flow in normal aging. Arch. Neurol. 67, 93–98. doi: 10.1001/archneurol.2009.913
Thong, J. Y., Du, J., Ratnarajah, N., Dong, Y., Soon, H. W., Saini, M., et al. (2014). Abnormalities of cortical thickness, subcortical shapes, and white matter integrity in subcortical vascular cognitive impairment. Hum. Brain Mapp. 35, 2320–2332. doi: 10.1002/hbm.22330
Thrippleton, M. J., Backes, W. H., Sourbron, S., Ingrisch, M., van Osch, M. J. P., Dichgans, M., et al. (2019). Quantifying blood-brain barrier leakage in small vessel disease: review and consensus recommendations. Alzheimers Dement 15, 840–858. doi: 10.1016/j.jalz.2019.01.013
Tomasi, D., Wang, G., and Volkow, N. (2013). Energetic cost of brain functional connectivity. Proc. Natl. Acad. Sci. U. S. A. 110, 7–13642. doi: 10.1073/pnas.1303346110
Tu, M. C., Hsu, Y. H., Yang, J. J., Huang, W.-H., Deng, J. F., Lin, S.-Y., et al. (2020). Attention and functional connectivity among patients with early-stage subcortical ischemic vascular disease and Alzheimer's disease. Front. Aging Neurosci. 12:239. doi: 10.3389/fnagi.2020.00239
Tuladhar, A. M., van Norden, A. G., de Laat, K. F., Zwiers, M. P., van Dijk, E. J., Norris, D. G., et al. (2015). White matter integrity in small vessel disease is related to cognition. Neuroimage Clin. 7, 518–524. doi: 10.1016/j.nicl.2015.02.003
Valenti, R., Del Bene, A., Poggesi, A., Ginestroni, A., Salvadori, E., Pracucci, G., et al. (2016). Cerebral microbleeds in patients with mild cognitive impairment and small vessel disease: the vascular mild cognitive impairment (VMCI)-Tuscany study. J. Neurol. Sci. 368, 195–202. doi: 10.1016/j.jns.2016.07.018
van der Veen, P. H., Muller, M., Vincken, K. L., Hendrikse, J., Mali, W. P., van der Graaf, Y., et al. (2015). Longitudinal relationship between cerebral small-vessel disease and cerebral blood flow: the second manifestations of arterial disease-magnetic resonance study. Stroke 46, 1233–1238. doi: 10.1161/STROKEAHA.114.008030
van Eimeren, L., Grabner, R. H., Koschutnig, K., Reishofer, G., Ebner, F., and Ansari, D. (2010). Structure-function relationships underlying calculation: a combined diffusion tensor imaging and fMRI study. NeuroImage 52, 358–363. doi: 10.1016/j.neuroimage.2010.04.001
Vermeer, S. E., Longstreth, W. T. Jr., and Koudstaal, P. J. (2007). Silent brain infarcts: a systematic review. Lancet Neurol. 6, 611–619. doi: 10.1016/S1474-4422(07)70170-9
Vernooij, M. W., van der Lugt, A., Ikram, M. A., Wielopolski, P. A., Vrooman, H. A., Hofman, A., et al. (2008). Total cerebral blood flow and total brain perfusion in the general population: the Rotterdam scan study. J. Cereb. Blood Flow Metab. 28, 412–419. doi: 10.1038/sj.jcbfm.9600526
Wallin, A., Román, G. C., Esiri, M., Kettunen, P., Svensson, J., Paraskevas, G. P., et al. (2018). Update on vascular cognitive impairment associated with subcortical small-vessel disease. J. Alzheimers Dis. 62, 1417–1441. doi: 10.3233/JAD-170803
Wang, J., Chen, H., Liang, H., Wang, W., Liang, Y., Liang, Y., et al. (2019). Low-frequency fluctuations amplitude signals exhibit abnormalities of intrinsic brain activities and reflect cognitive impairment in leukoaraiosis patients. Med. Sci. Monit. 25, 5219–5228. doi: 10.12659/MSM.915528
Wang, Z., Dai, Z., Gong, G., Zhou, C., and He, Y. (2015). Understanding structural-functional relationships in the human brain: a largescale network perspective. Neuroscientist 21, 290–305. doi: 10.1177/1073858414537560
Wang, X., Jiao, Y., Tang, T., Wang, H., and Lu, Z. (2013). Altered regional homogeneity patterns in adults with attention-deficit hyperactivity disorder. Eur. J. Radiol. 82, 7–1552. doi: 10.1016/j.ejrad.2013.04.009
Wang, B., Niu, Y., Miao, L., Cao, R., Yan, P., Guo, H., et al. (2017). Decreased complexity in Alzheimer’s disease: resting-state fMRI evidence of brain entropy mapping. Front. Aging Neurosci. 9:378. doi: 10.3389/fnagi.2017.00378
Wang, S., Rao, B., Chen, L., Chen, Z., Fang, P., Miao, G., et al. (2021). Using fractional amplitude of low-frequency fluctuations and functional connectivity in patients with post-stroke cognitive impairment for a simulated stimulation program. Front. Aging Neurosci. 13:724267. doi: 10.3389/fnagi.2021.724267
Wang, J. B., Zheng, L. J., Cao, Q. J., Wang, Y. F., Sun, L., Zang, Y. F., et al. (2017). Inconsistency in abnormal brain activity across cohorts of ADHD-200 in children with attention deficit hyperactivity disorder. Front. Neurosci. 11:320. doi: 10.3389/fnins.2017.00320
Wang, J., Zuo, X., Dai, Z., Xia, M., Zhao, Z., Zhao, X., et al. (2013). Disrupted functional brain connectome in individuals at risk for Alzheimer's disease. Biol. Psychiatry 73, 472–481. doi: 10.1016/j.biopsych.2012.03.026
Wen, X., Wu, X., Li, R., Fleisher, A. S., Reiman, E. M., Wen, X., et al. (2013). Alzheimer’s disease related changes in regional spontaneous brain activity levels and inter-region interactions in the default mode network. Brain Res. 1509, 58–65. doi: 10.1016/j.brainres.2013.03.007
Wentzel, C., Rockwood, K., MacKnight, C., Hachinski, V., Hogan, D. B., Feldman, H., et al. (2001). Progression of impairment in patients with vascular cognitive impairment without dementia. Neurology 57, 714–716. doi: 10.1212/WNL.57.4.714
Wierenga, C. E., Hays, C. C., and Zlatar, Z. Z. (2014). Cerebral blood flow measured by arterial spin labeling MRI as a preclinical marker of Alzheimer’s disease. J. Alzheimers Dis. 42, S411–S419. doi: 10.3233/JAD-141467
Wu, C., Gu, H., Lu, H., Stein, E. A., Chen, J. H., and Yang, Y. (2009). Mapping functional connectivity based on synchronized Cmro2 fluctuations during the resting state. NeuroImage 45, 694–701. doi: 10.1016/j.neuroimage.2008.12.066
Wu, T., Long, X., Zang, Y., Wang, L., Hallett, M., Li, K., et al. (2009). Regional homogeneity changes in patients with Parkinson's disease. Hum. Brain Mapp. 30, 10–1502. doi: 10.1002/hbm.20622
Xu, Q., Zhou, Y., Li, Y. S., Cao, W. W., Lin, Y., Pan, Y. M., et al. (2010). Diffusion tensor imaging changes correlate with cognition better than conventional MRI findings in patients with subcortical ischemic vascular disease. Dement. Geriatr. Cogn. Disord. 30, 317–326. doi: 10.1159/000320491
Yang, Q. (2021). Aberrant amplitude of low-frequency fluctuation and degree centrality within the default mode network in patients with vascular mild cognitive impairment. Brain Sci. 11:11. doi: 10.3390/brainsci11111534
Yang, H., Long, X., Yang, Y., Yan, H., Zhu, C. Z., Zhou, X. P., et al. (2007). Amplitude of low frequency fluctuation within visual areas revealed by resting-state functional Mri. NeuroImage 36, 144–152. doi: 10.1016/j.neuroimage.2007.01.054
Yang, L., Yan, Y., Wang, Y., Hu, X., Lu, J., Chan, P., et al. (2018). Gradual disturbances of the amplitude of low-frequency fluctuations (ALFF) and fractional ALFF in Alzheimer Spectrum. Front. Neurosci. 12:975. doi: 10.3389/fnins.2018.00975
Ye, Q., and Bai, F. (2018). Contribution of diffusion, perfusion and functional MRI to the disconnection hypothesis in subcortical vascular cognitive impairment. Stroke Vasc. Neurol. 3, 131–139. doi: 10.1136/svn-2017-000080
Yi, L., Wang, J., Jia, L., Zhao, Z., Lu, J., Li, K., et al. Structural and functional changes in subcortical vascular mild cognitive impairment: a combined voxel-based morphometry and resting-state Fmri study. PLoS One (2012). 7: 0,:e44758, doi: 10.1371/journal.pone.0044758
Yoshiura, T., Hiwatashi, A., Noguchi, T., Yamashita, K., Ohyagi, Y., Monji, A., et al. (2009). Arterial spin labelling at 3-T MR imaging for detection of individuals with Alzheimer’s disease. Eur. Radiol. 19, 2819–2825. doi: 10.1007/s00330-009-1511-6
Yu, Y., Liang, X., Yu, H., Zhao, W., Lu, Y., Huang, Y., et al. (2017). How does white matter microstructure differ between the vascular and amnestic mild cognitive impairment? Oncotarget 8, 42–50. doi: 10.18632/oncotarget.13960
Zang, Y., He, Y., Zhu, C., Cao, Q. J., Sui, M. Q., Liang, M., et al. (2007). Altered baseline brain activity in children with Adhd revealed by resting-state functional Mri. Brain and Development 29, 83–91. doi: 10.1016/j.braindev.2006.07.002
Zang, Y., Jiang, T., Lu, Y., He, Y., and Tian, L. (2004). Regional homogeneity approach to Fmri data analysis. NeuroImage 22, 394–400. doi: 10.1016/j.neuroimage.2003.12.030
Zhang, Z., Liao, W., Chen, H., Mantini, D., Ding, J. R., Xu, Q., et al. (2011). Altered functional-structural coupling of large-scale brain networks in idiopathic generalized epilepsy. Brain 134, 2912–2928. doi: 10.1093/brain/awr223
Zhang, Z., Liu, Y., Jiang, T., et al. (2012). Altered spontaneous activity in Alzheimer's disease and mild cognitive impairment revealed by regional homogeneity. NeuroImage 59, 40–1429.
Zhao, Z. L., Fan, F. M., Lu, J., Li, H. J., Jia, L. F., Han, Y., et al. (2015). Changes of gray matter volume and amplitude of low-frequency oscillations in amnestic MCI: An integrative multi-modal MRI study. Acta Radiol. 56, 614–621. doi: 10.1177/0284185114533329
Zhou, Q. H., Wang, K., Zhang, X. M., Wang, L., and Liu, J. H. (2020). Differential regional brain spontaneous activity in subgroups of mild cognitive impairment. Front. Hum. Neurosci. 14:2. doi: 10.3389/fnhum.2020.00002
Zhu, Y., Qi, S., Zhang, B., He, D., Teng, Y., Hu, J., et al. (2019). Connectome-based biomarkers predict subclinical depression and identify abnormal brain connections with the lateral habenula and thalamus. Front. Psychol. 10:371. doi: 10.3389/fpsyt.2019.00371
Zhu, S., Fang, Z., Hu, S., Wang, Z., and Rao, H. (2013). Resting state brain function analysis using concurrent BOLD in ASL perfusion fMRI. PLoS One 8:8. doi: 10.1371/journal.pone.0065884
Zhuang, Y., Shi, Y., Zhang, J., Kong, D., Guo, L., Bo, G., et al. (2021). Neurologic factors in patients with vascular mild cognitive impairment based on fMRI. World Neurosurg. 149, 461–469. doi: 10.1016/j.wneu.2020.11.120
Zoller, M., Schaer, E., Scariati, M. C., Padula, S., Eliez, D., and De Ville, V. (2017). Disentangling resting-state BOLD variability and PCC functional connectivity in 22q11.2 deletion syndrome. NeuroImage 149, 85–97. doi: 10.1016/j.neuroimage.2017.01.064
Zonneveld, H. I., Loehrer, E. A., Hofman, A., Niessen, W. J., van der Lugt, A., Krestin, G. P., et al. (2015). The bidirectional association between reduced cerebral blood flow and brain atrophy in the general population. J. Cereb. Blood Flow Metab. 35, 1882–1887. doi: 10.1038/jcbfm.2015.157
Zou, Q., Zhu, C., Yang, Y., Zuo, X.-N., Long, X.-Y., Cao, Q.-J., et al. (2008). An improved approach to detection of amplitude of low-frequency fluctuation (alff) for resting-state Fmri: fractional Alff. J. Neurosci. Methods 172, 41–137. doi: 10.1016/j.jneumeth.2008.04.012
Zuo, X. N., Ehmke, R., Mennes, M., Imperati, D., Castellanos, F. X., Sporns, O., et al. (2012). Network centrality in the human functional connectome. Cereb. Cortex 22, 1862–1875. doi: 10.1093/cercor/bhr269
Zuo, X. N., and Xing, X. X. (2014). Test-retest reliabilities of resting-state FMRI measurements in human brain functional connectomics: a systems neuroscience perspective. Neurosci. Biobehav. Rev. 45, 100–118. doi: 10.1016/j.neubiorev.2014.05.009
Keywords: vascular mild cognitive impairment, multimodal neuroimaging, resting-state functional magnetic resonance imaging, diffusion tensor imaging, arterial spin labeled perfusion imaging
Citation: Liu Q and Zhang X (2023) Multimodality neuroimaging in vascular mild cognitive impairment: A narrative review of current evidence. Front. Aging Neurosci. 15:1073039. doi: 10.3389/fnagi.2023.1073039
Edited by:
Shuo Wang, Capital Medical University, ChinaReviewed by:
Mario Mascalchi, University of Florence, ItalyNesrine Salah El Dine El Sayed, Cairo University, Egypt
Copyright © 2023 Liu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuezhu Zhang, eHp6aGFuZ0B0anV0Y20uZWR1LmNu
 Qiuping Liu
Qiuping Liu Xuezhu Zhang1,2*
Xuezhu Zhang1,2*