- Department of Environmental Health Sciences, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, USA
The carotid body (CB) is a primary chemosensory organ for arterial hypoxia. Inhibition of K channels in chemosensory glomus cells (GCs) are considered to be responsible for hypoxic chemoreception and/or chemotransduction of the CB. Hypoxic sensitivity of large-conductance calcium-activated K (BK) channels has been established in the rat CB. Our previous work has shown the BK channel β2 subunits are more expressed in the CB of the DBA/2J mouse than that of the A/J mouse. Because the DBA/2J mouse is more sensitive to hypoxia than the A/J mouse, our general hypothesis is that BK channels play a role in the sensitivity of the mouse CB to mild hypoxia. We performed vigorous analysis of the gene expression of α, β2, and β4 subunits of BK channels in the CB. We found that α and β2 subunits were expressed more in the CB of the DBA/2J mice than that of the A/J mice. No differences were found in the β4 subunit expression. These differences were not seen in the neighboring tissues, the superior cervical ganglion and the carotid artery, suggesting that the differences are CB specific. Further, the sensitivity of BK channels in GCs to mild hypoxia was examined in patch clamp experiments using undissociated CBs. Iberiotoxin significantly inhibited K current of GCs in the DBA/2J mice, but not in the A/J mice. When reducing PO2 to ∼70 mmHg, K current reversibly decreased in GCs of the DBA/2J, but not of the A/J mice. In the presence of iberiotoxin, mild hypoxia did not inhibit K current in either strains. Thus, the data suggest that BK channels in GCs of the DBA/2J mice are sensitive to mild hypoxia. Differential expression of BK channel β subunits in the CBs may, at least in part, explain the different hypoxic sensitivity in these mouse strains.
Introduction
The carotid body (CB) is a primary chemosensory organ for arterial hypoxia. Its excitation by hypoxia induces a variety of responses in the cardiovascular, respiratory, renal, and endocrine systems to compensate decreased oxygen levels. The mechanisms of hypoxic chemoreception and chemotransduction in the CB are still not totally understood, but a general consensus is that the glomus cell (GC, or Type I cell) is a major chemosensory element (Gonzalez et al., 1994; Shirahata and Sham, 1999; Kumar and Prabhakar, 2007). A widely accepted model assumes that hypoxic inhibition of oxygen-sensitive K channels induces depolarization of GCs, followed by an activation of voltage-dependent Ca2+ channels, an influx of Ca2+, and a release of neurotransmitters from GCs. Neurotransmitters bind to their receptors in the afferent nerve endings, which generates increased action potentials (Gonzalez et al., 1994; Peers, 1997; Shirahata and Sham, 1999; Lopez-Lopez and Perez-Garcia, 2007). The efforts for defining oxygen-sensitive K channels in the CB reveal several molecular identities. For example, in the rabbit GC, Kv4 family, in particular Kv4.3 α subunits, appears to be the molecular correlate of the oxygen-sensitive K channel (Sanchez et al., 2002). On the other hand, large-conductance calcium-activated K channels (BK channels; Peers, 1997) and background TASK-like K channels (Williams and Buckler, 2004) were identified to be oxygen sensitive in the rat GC. In the cat GC, oxygen-sensitive K current is mediated via a delayed rectifier, but neither BK channels nor fast-inactivating K channels (Kv4 family) appear to be responsible (Chou and Shirahata, 1996). Further, Kv3 family in GCs of C57BL/6J mice is inhibited by hypoxia (Perez-Garcia et al., 2004). These differences have been attributed to species differences (Shirahata and Sham, 1999; Lopez-Lopez and Perez-Garcia, 2007; Peers and Wyatt, 2007). We can also consider these species differences as variable protein expression in the CB due to background genetic differences. Another factor that needs to be contemplated is oxygen tension. Some studies have indicated that the inhibition of K channels is PO2 dependent (Lopez-Lopez et al., 1989; Montoro et al., 1996; Shirahata and Sham, 1999), although many studies have applied only severe hypoxia. Thus, it is possible that each oxygen-sensitive K channel contributes to GCs’ function at specific levels of oxygen tension.
Recent studies in inbred strains of mice have suggested that the CB in the DBA/2J strain is more sensitive to moderate hypoxia than that in the A/J strain (Tankersley et al., 1994; Rubin et al., 2003; Campen et al., 2004). Investigating the differences in the CB between these inbred strains of mice could offer deeper understanding of the CB function and its relation to genetics. Our previous study found that gene expression of BK channel β2 subunits are more expressed in the CB of the DBA/2J mice compared to the A/J mice (Balbir et al., 2007). Further, K current in the CB of DBA/2J mice was inhibited by mild hypoxia (Yamaguchi et al., 2004). Thus, our general hypothesis is that BK channels play a role in the sensitivity of the mouse CB to mild hypoxia. In this study, we performed more vigorous analysis of gene expression of α, β2, and β4 subunits of BK channels. Further, we have examined if the differences in BK channel expression between the DBA/2J and the A/J mice correlate with the response of GCs to mild hypoxia.
Materials and Methods
Tissue Preparation
All animal experiments were conducted in accordance with the NIH guidelines (Guide for the Care and Use of Laboratory Animals, National Academy Press Washington, DC, 1996), and the protocols were approved by the Animal Care and Use Committee of the Johns Hopkins University. DBA/2J and A/J mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). Some mice were bred in the facility of the Johns Hopkins Bloomberg School of Public Health where the temperature (∼22°C) and the light cycle (12 h light and 12 h dark) were controlled. Water and mouse chow (Harlan TEKLAD, Madison) were provided ad libitum before all experiments. Preparation of the CB was performed as described before (Yamaguchi et al., 2004) with some modifications. In short, male mice (4–6 weeks old) were deeply anesthetized by peritoneal injection of 1.5 g/kg urethane. The carotid bifurcations were exposed. After the heart was removed to avoid bleeding in the neck, the CBs were quickly harvested together with the carotid bifurcation and immersed in ice-cold phosphate-buffered saline (for RT-PCR studies) or modified Krebs solution (for patch clamp experiments). Fat and connective tissues were removed using a stereoscopic microscope.
RT-PCR Analysis of BK Channel Subunits
The CBs were isolated in ice-cold phosphate-buffered saline, collected in ice-cold TRIzol solution (Invitrogen, CA, USA), and frozen at −80°C until use. Total RNA isolation and DNase treatment were similar to what has been described previously (Balbir et al., 2007). RNA was reverse-transcribed to obtain first strand cDNA using SuperScript III™ (Invitrogen). We performed real-time PCR targeting BK channel subunits (α, β2, β4) together with β-actin (Act) as a reference gene. TaqMan7 gene expression assays (Applied Biosystems) were used for PCR amplification of cDNA following the manufacture’s instruction. The primers were purchased from Applied Biosystems: Mm00516078_m1 for BKα, Mm00511481_m1 for BKβ2, Mm00465684_m1 for BKβ4, and 4352341E for Act. ABI PRIZM7 7000HT Sequence Detection System (Applied Biosystems) was used. The protocol for PCR amplification was: 2 min at 50°C, 10 min at 95°C, 50 cycles of 95°C for 15 s followed by 60°C for 1 min. Individual cDNA samples were run in triplicates for each gene. After completion of real-time amplification, the amplification efficiency, crossing point, and corrected crossing point (cCP) of each run were determined using the methods of data analysis for real-time (DART) PCR (Peirson et al., 2003) and four-parameter logistic model (FPLM; Tichopad et al., 2003). CAmpER 1.2 software (Center for Biotechnology, Bielefeld University1) provided a simple and computerized tool for these analytical methods. Subsequently, average and SD of all readings in efficiency and cCP were calculated. The readings outside of mean ± 3SD were eliminated from further analysis of the data. Also, if one reading in the triplicates was three cCP away from other two readings, it was eliminated from further analysis. A mean of the triplicates was calculated to represent a gene transcript of a particular sample. Statistical analysis was performed with Student’s t-test using Prizm 4.0 (GraphPad Software Inc.) and with the Relative Expression Software Tool (REST 2009; Qiagen). REST is based on the efficiency-calibrated model and employs randomization tests to determine differences in target gene expression between experiments. Differences were considered significant if p < 0.05.
Expression of BK Channel Subunits in the Superior Cervical Ganglion and the Carotid Artery
We further examined if gene expression of BK channel subunits differ in the superior cervical ganglion (SCG) and the carotid artery between the two strains of mice. RNA extraction and RT were performed as described above, and a conventional multiplex PCR was performed following the manufacture’s instruction (Qiagen). Primers were designed based on the published nucleotide sequences of mice BK channel subunits (GenBank2). The primers’ sequences were: BKα subunit (forward: CTCATCATCCCGGTGACCAT; reverse: GGATTCTATTGGGTTTGACGAG); BKβ2 subunit (forward: TTTATATGGACCAGTGGCCG; reverse: TGTTGATCCGTTGGATCCTCT); BKβ4 subunit (forward: GGTGTCTTACGAGTACACGGAA; reverse: TTGCGTTTCTTCATGGCTTC); Act (forward: CGTGAAAAGATGACCCAGATCA; reverse: ATTTCCCTCTCAGCTGTGGTG). The final concentrations of primers were 1 μM for β-actin and 5 μM for other primers. The conditions for PCR amplification were as follows. The template was denatured at 95°C for 15 min followed by 25 cycles of denaturation at 94°C for 30 s, annealing of primers at 60°C for 1.5 min, extension at 72°C for 1.5 min. Final cycle was 72°C for 10 min. The PCR products were purified with QIAquick PCR Purification Kit (Qiagen), and the second multiplex PCR was performed. The conditions for PCR amplification were the same as the first PCR except for applying 20 cycles. The final PCR products were separated by 2% agarose gel electrophoresis. The image of gel electrophoresis was captured and analyzed by Kodak Electrophoresis Documentation and Analysis System 290 (Eastman Kodak Co.). The density of the each band was measured and the expression of each BK channel subunit was normalized relative to the expression of β-actin in the same sample.
BK Channel Activity
Patch clamp experiments were performed using undissociated CBs as described before (Yamaguchi et al., 2004). The CB was localized by gently pulling the SCG, and then, the SCG was removed. Subsequently, the tissue was warmed in normoxic Krebs solution (in mM: NaCl, 118; KCl, 4.7; CaCl2, 1.8; KH2PO4, 1.2; MgSO4A7H2O, 1.2; NaHCO3, 22; glucose, 11.1; pH, 7.4 with 5% CO2/Air) at 37°C for 60 min, and then placed in a recording chamber which was attached to a stage of an upright microscope (Axioskop 2, Zeiss). After the tissue was superfused with Krebs solution containing 0.0375% collagenase (Type IX, Sigma) for 40 min, the CB was visualized using a water immersion lens (ACHROPLAN, 40×, Zeiss) combined with an infrared differential interference video camera (DAGE MTI Inc., Michigan City, IN, USA; Figures 3A,B). GCs were easily identified from their morphology as seen in Figure 3B. All experiments were performed at 37.0°C while the tissue was continuously superfused with Krebs solution (1.22 mL/min) using a peristaltic pump (Minipuls 3, Gilson). A conventional tight seal whole cell recording was applied. The patch electrodes (Corning 8161, Warner Instrument Co.) were pulled using a puller (Sutter Instrument Co.), and their resistance was between 4 and 6 MΩ. The composition of the internal solution was: (in mM) K gluconate, 90; KCl, 33; NaCl, 10; CaCl2, 1; EGTA, 10; MgATP, 5; HEPES, 10; pH 7.2 with KOH. Voltage-dependent whole cell current was evoked by voltage clamp pulses from −80 mV of holding potential to +60 with 10 mV increments for 100 ms. Single pulse protocol (from −80 mV of holding potential to +20 mV) was also used. A specific BK channel blocker, iberiotoxin (Alomone Labs), was included in Krebs solution. Hypoxia was applied by switching normoxic Krebs to hypoxic Krebs solution saturated with 5% CO2/95% N2. PO2 in the chamber was measured with a small oxygen electrode (MI-730 Oxygen Electrode, OM-4 Oxygen Meter; Microelectrodes, Inc.) in separate experiments in which the patch clamp experiments were simulated. The current was amplified and filtered at 2 kHz with a low pass Bessel filter using an Axopatch 200B patch clamp amplifier (Axon Instruments). The signals were digitized by a Digidata 1320A (Axon Instruments). A window based personal computer and pCLAMP 8.1 (Axon Instrument) were used for acquisition and processing of data.
All chemicals were obtained from Sigma Aldrich Inc., unless otherwise indicated. For data analysis, current size was measured between 93 and 97 ms from the start of a step pulse application, and the data were expressed as mean ± SEM (pA or % of control). Prism 4.0 was used to determine statistical differences, and Student’s t-test or ANOVA was applied. Values were considered significant if p < 0.05.
Results
Expression of BK Channel Subunits
A total of 16 samples from DBA/2J mice and 15 samples from A/J mice were used for this set of experiments. Not all genes were examined from a single cDNA sample, but the expression of Act was always analyzed as a control gene together with genes of interest. Table 1 shows the cCP values of each experimental set (BK subunits with Act). Small discrepancies were observed in the final number of samples between the DART and FPLM analyses. Because DART and FPLM use different criteria for determining amplification efficiency and CP, some samples were excluded by one method, but not necessarily by another method. Thus, the numbers of tests included for the statistics differ between the DART method and the FPLM method.
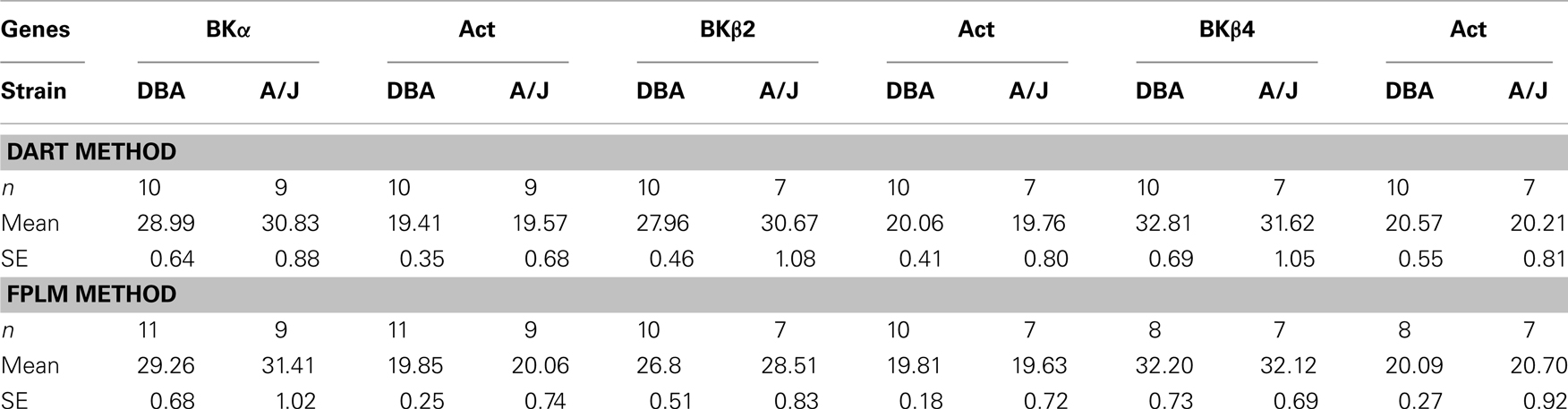
Comparisons between the two strains were made using the differences of cCP (ΔcCP) between the gene of interest and Act (Figures 1A,B). REST analysis was also performed for the comparison between the two strains using Act as a reference gene (Figure 1C). The results of both methods (ΔcCP and REST) clearly showed that BKα subunits and BKβ2 subunits were expressed significantly less in the CB of the A/J mice compared to that of the DBA/2J mice. No differences were observed in BKβ4 subunit expression between the strains.
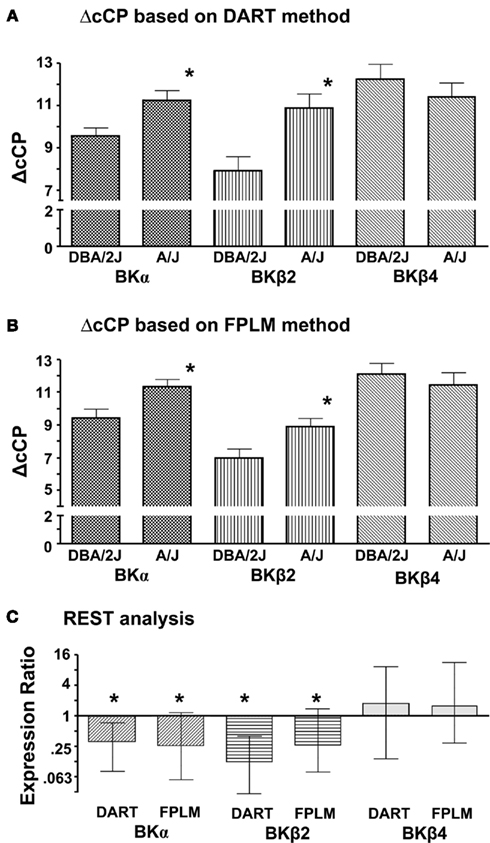
Figure 1. Differences in the expression of BK channel subunits between the CB of the DBA/2J mice and the CB of the A/J mice. Individual cDNA samples were run in triplicates for each gene. A mean of the triplicates was calculated to represent a CP of a gene of a particular sample. Act was used as a reference gene. (A) Corrected CPs were obtained with the DART method. ΔcCP for each gene of a particular sample was calculated as (cCPgene − cCPAct). Data are presented as mean ± SEM. *, Significantly different from the samples of the DBA/2J mice (unpaired t-test, p < 0.05). Larger ΔcCP means the gene expression is lower. (B) Corrected CPs were obtained with the FPLM method. ΔcCP for each gene of a particular sample was calculated as (cCPgene − cCPAct). Data are presented as mean ± SEM. *, Significantly different from the samples of the DBA/2J mice (unpaired t-test, p < 0.05). (C) Corrected CPs of the A/J mice were compared with those of the DBA/2J mice using the REST analysis. The expression ratio lower than 1 means that the expression of the gene in the CB of the A/J mice is lower compared to that of the DBA/2J mice. Data are presented as mean ± SEM. *, Significantly different from the samples of the DBA/2J mice (p < 0.05).
Expression of BK Channel Subunits in the SCG and the Carotid Artery
We examined five SCGs and four carotid arteries in each strain. To show the difference of the gene expression pattern, CBs were also examined. Clear bands of expected sizes for the BKα, BKβ2, and BKβ4 subunits together with Act were observed in the CB samples (Figure 2A), showing the validity of the methods. The density of the each band was normalized relative to the band of Act in the same sample (Figure 2B). The decreased expression in BKα and BKβ2 subunits in the CB of the A/J mice compared to the DBA/2J mice was observed (data not shown). BKβ4 subunits were the dominant β subunits in the SCG and the band of BKβ2 subunits was weakly seen. No significant differences in the expression of BK channel subunits were observed between the strains. In the carotid artery, the BKα subunit was expressed without significant differences between the strains. As expected (Gribkoff et al., 2001; Orio et al., 2002), no expression of BKβ2 and BKβ4 subunits were observed in the artery.
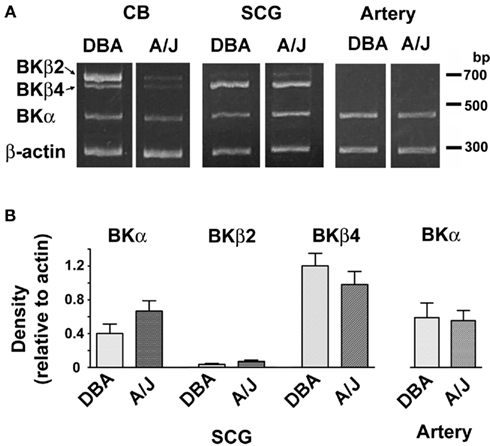
Figure 2. (A) Agarose gel electrophoresis of RT-PCR products for BK channel subunits in the CB, the SCG, and the carotid artery (Artery). Multiplex PCR was performed for β-actin, BKα, BKβ2, and BKβ4. (B) Semi-quantitative analysis of gene expression for BK subunits was shown. The density of the each band was measured and normalized relative to the expression of β-actin in the same sample. Note that no strain differences were observed in the SCG (n = 5) and the artery (n = 4).
BK Channel Activity
After collagenase perfusion, GCs can be readily visualized as shown in Figure 3B and giga ohm seals were routinely obtained. The whole cell configuration was established, Cm was measured by applying a short 10 mV pulse from holding potential −80 mV, and current–voltage relationships for K current was obtained as described above. Mean membrane capacitance of GCs in the DBA/2J mice was 5.9 ± 1.7 pF (n = 12) and that in the A/J mice was 5.9 ± 1.3 pF (n = 11). These values were not significantly different. Depolarization induced outward currents with activation threshold around −40 to −30 mV in both strains. Variations in current size and kinetics were seen in both strains (Figures 3C,D). When inactivation was seen, it was slight and slow during the 100-ms depolarizing pulse in both strains. In some cells no inactivation was observed. Examples of the current records and mean I–V relationships were shown in Figures 3C,D. No statistical differences in the I–V relationships were found between the strains. To examine the BK channel component, a single step protocol was applied with or without iberiotoxin (200 nM; Alomone Laboratories), a selective blocker for BK channels. After 5 min superfusion with iberiotoxin-containing Krebs, K current in GCs of the DBA/2J mice was significantly attenuated (63 ± 5% of control; n = 18) and the effect was reversible (104 ± 6% of control; Figure 3E). On the other hand, K current in GCs of the A/J mice was not significantly affected by iberiotoxin 200 nM (98 ± 5% of control; Figure 3E).
Figure 3. For patch clamp experiments, undissociated CBs were used. The setup (A) and the image of the CB (B) are shown. Examples of K current records and mean I–V relationships in the DBA/2J (C) and the A/J mouse (D) are presented. Whole current traces were evoked by voltage steps from −80 to +60 mV with 10 mV increments for 100 ms. To construct a current–voltage curve, the mean current measured between 93 and 97 ms from the start of each voltage step. Mean currents were plotted versus the test pulse. Bars represent SEM. No statistical differences were seen between the strains. (E) The effect of iberiotoxin (a BK channel blocker; 200 nM) on outward current in GCs. The outward current was evoked by a test pulse from −80 to +20 mV for 100 ms. Iberiotoxin significantly and reversibly decreased outward current in GCs of the DBA/2J mice. However, K current in the A/J mice was not significantly affected by iberiotoxin. *, Significantly different from control and recovery. The amplitudes of K current (control and recovery) were significantly larger in GCs of the DBA/2J mice. Cont, control; Ibx, iberiotoxin; Rec, recovery.
In other set of GCs, hypoxic sensitivity of voltage-dependent outward K current was tested by applying hypoxic Krebs solution. Mild hypoxia (PO2 ∼ 70 mmHg) clearly inhibited outward K current in GCs of the DBA/2J mice, but this level of hypoxia did not affect outward K current in GCs of the A/J mice (Figure 4A). The inhibitory effect was reversible. The effect of O2 tension on K current was further studied using single pulse protocol (Figure 4B). K current was evoked at 1, 3, and 5 min after changing Krebs from normoxia (equilibrated with 21% O2/5% CO2) to hypoxia (equilibrated with 0% O2/5% CO2). PO2 in the chamber was measured in the experiments simulating the patch clamp experiments. PO2 reached 70 mmHg after 5 min of hypoxic superfusion. The degree of inhibition of K current in the DBA/2J mice was dependent on PO2 values in the chamber (Figure 4B, solid line) between the range of 150 and 70 mmHg, while no significant changes were noted in the A/J mice. To evaluate the contribution of BK channels to hypoxic sensitivity, the effect of hypoxia on K current was monitored in the presence of iberiotoxin (200 nM). Mild hypoxia (PO2 ∼ 70 mmHg) did not significantly influence K current in the presence of iberiotoxin in either strain of mice (Figure 4C).
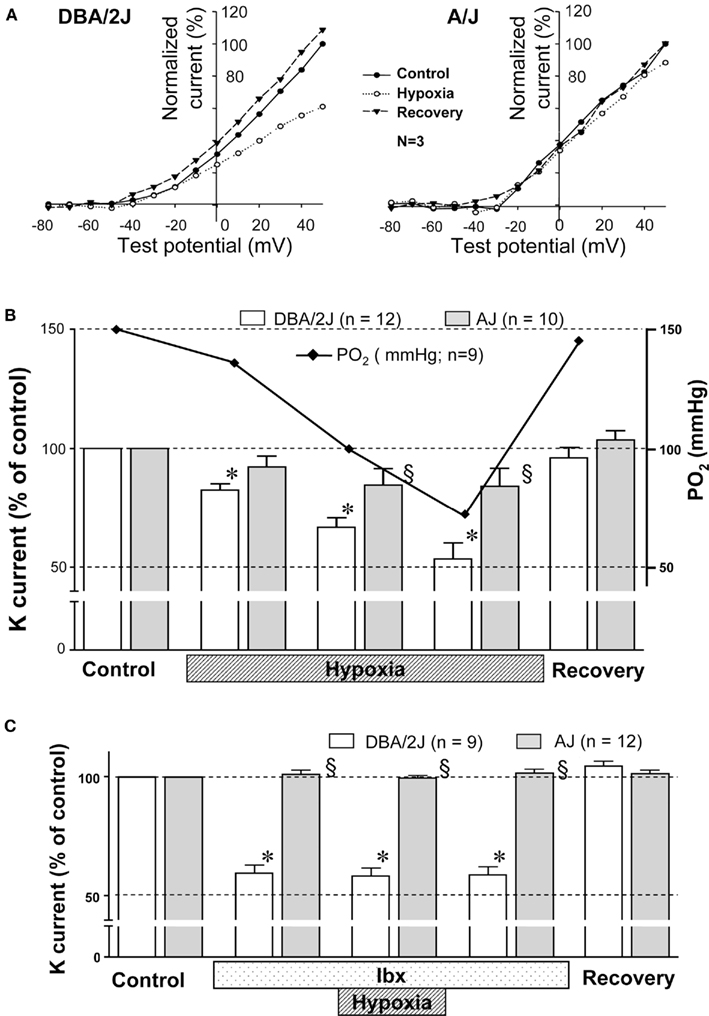
Figure 4. (A) The effect of decreasing O2 on voltage-dependent outward K current in GCs of the DBA/2J (left) and the A/J mice (right). Whole current was evoked by voltage steps from −80 to +50 mV with 10 mV increments for 100 ms. To construct a current–voltage curve, the mean current measured between 93 and 97 ms from the start of each voltage step was plotted versus the test pulse. The current during hypoxia was recorded after 5–10 min of superfusion with hypoxic Krebs solution. Each current was normalized as % of the current measured at +50 mV of test pulse during normoxia. I–V curves were mean values obtained from three GCs in each strain. (B) During control, the CB was superfused with Krebs equilibrated with 5% CO2/air. Subsequently, the superfusate was switched to Krebs equilibrated with 5% CO2/0% O2, and K current was recorded at 1, 3, and 5 min (marked as “Hypoxia”). PO2 in the chamber was measured in the experiments simulating the patch clamp experiments. K current was almost lineally inhibited with decreasing PO2 in GCs of the DBA/2J mice, but not those of the A/J mice. *, Significantly different from control and recovery (p < 0.01). §, Significantly different from the DBA/2J mice. There is no statistical difference between the control and the recovery. (C) The effect of decreasing O2 on voltage-dependent K current of GCs in the DBA/2J and the A/J mice with iberiotoxin (Ibx). Initially the CB was superfused with Krebs equilibrated with 5% CO2/air. Then, Krebs was changed to that containing 200 nM iberiotoxin for 5 min. K current was significantly reduced in GCs of the DBA/2J mice, but not of the A/J mice. Subsequently, the superfusate was switched to Krebs containing iberiotoxin and equilibrated with 5% CO2/0% O2 for 5 min. K current was not further affected in either strain. The effect of iberiotoxin was reversible. *, Significantly different from control and recovery (p < 0.01). §, Significantly different from the DBA/2J mice. There is no statistical difference between the control and the recovery.
Discussion
In this study we have found that gene expression of BK channel α and β2 subunits in the CB differs between the DBA/2J and the A/J strains of mice. BK channels consist of the four α subunits, which form the pore of the channel, and the auxiliary β subunits (β1–4). Although α subunits alone can form functional channels when expressed in some cell lines, in native tissues they are likely associated with β subunits. Beta subunits significantly influence pharmacology and kinetics of BK channels, and the tissue distribution is distinct. Beta1 mRNA is high in smooth muscle; β2, in chromaffin cells and brain; β3, in testis, pancreas, and spleen; and β4, in brain (Gribkoff et al., 2001; Orio et al., 2002). Because the parenchymal cells of the CB originate from the neural crest, we focused on α, β2, and β4 mRNA levels in the CB of the two strains of mice. Real-time PCR analysis of these subunits showed that mRNA for α and β2 subunits was significantly more expressed in the CB of the DBA/2J than those of the A/J mice (Figure 1).
In our previous comparative gene expression data, BK channel β2 subunits are more expressed in the CB of the DBA/2J than that of the A/J mice at 4 weeks of age (Balbir et al., 2007). The current study utilized more vigorous evaluation with the unbiased estimation of amplification efficiency and cCP using the DART and the FPLM methods. The data agree with our previous observation regarding β subunits expression. The lower expression of BKα subunit gene further suggest that the BK channel numbers may be less in the CB of the A/J mice compared to that of the DBA/2J mice. However, one methodological concern in our gene expression analysis was the possible contamination with other tissues, such as the SCG and blood vessels. This is an inherent problem with the use of an extremely small tissue such as the mouse CB. Thus, we checked if the BK channel subunits were differentially expressed in these tissues between the two strains of mice. No differences in the expression of BKα subunit were observed between the strains in the SCG and the carotid artery. Further, the expression of BKβ2 subunits in the SCG was very weak and indifferent between the strains. The expression of BKβ4 subunits in the SCG was not different between the strains as well. These β subunits were not detected in the carotid artery as shown in previous studies (Gribkoff et al., 2001; Orio et al., 2002). Based on these data, it is reasonable to conclude that the strain differences in the gene expression of BKα and β2 subunits exist in the CB.
The electrophysiology data may reflect the differences in BK channel gene expression between the strains. Iberiotoxin, a selective BK channel antagonist, inhibited K current of GCs in the DBA/2J mice, but not in the A/J mice (Figure 3E). However, RT-PCR analysis (Figure 1; Table 1) suggests that α subunits of BK channels are present, although a smaller amount, in the GCs of the A/J mice. Although speculative, the ineffectiveness of iberiotoxin on K current in GCs of the A/J mice may be, at least in part, explained by the differences in the expression of BKβ subunits. Because BKα and β2 subunits were less expressed in GCs of the A/J mice than those of the DBA/2J mice, relative expression of β4 subunits to β2 subunits as well as relative expression of β4 subunits to α subunits would be much higher in GCs of the A/J mice compared to GCs of the DBA/2J mice. Thus, β4 subunits would contribute more as an associated protein to BK channels in GCs of the A/J mice. It is well known that β subunits have significant influence on the electrophysiological and pharmacological diversity of BK channels. Particularly, β4 subunits have unique influence on BK channel activity. When α subunits are expressed together with β4 subunits in HEK293 or CHO cells, I–V curve of BK current is shifted to the right by 50 mV. Iberiotoxin or charybdotoxin, which inhibits α subunit alone or α+β1 BK channel activity at low nanomolar range, is not effective in α+β4 BK channels until high concentrations (micromolar) are used (Meera et al., 2000; Weiger et al., 2000). Further, β4 subunits decrease BK channel openings at low calcium concentrations (Brenner et al., 2000). If this is the case in the CB, BK channels in GCs of the A/J mice may not have been well activated in our experimental conditions. Their insensitivity to iberiotoxin may be due to a close association of BKα subunits with β4 subunits.
Hypoxic inhibition of voltage-dependent K channels, including BK channels, has been proposed as an early event leading to depolarization of GCs. However, the role of voltage-dependent K channels in GC depolarization in response to hypoxia has been still debated (Buckler, 2007; Lopez-Lopez and Perez-Garcia, 2007; Peers and Wyatt, 2007). Important questions are (1) whether voltage-dependent K channels including BK channels are active at or close to the resting membrane potential of GCs, and (2) whether the inhibition of K channels are fast enough to precede the onset of an increase in chemoreceptor discharge. Wyatt and Peers (1995) have shown that charybdotoxin, a specific BK channel blocker, depolarized rat GCs without other stimuli, indicating that BK channels are active at the resting membrane potential. Consistent with this finding, Pardal et al. (2000) reported that TEA and iberiotoxin increased catecholamine secretion from rat GCs. In a recent review, Peers and Wyatt (2007) further provided detailed discussion which supports a role of BK channels initiating depolarization of GCs during hypoxia. They further argued that hypoxic inhibition of BK channels will maintain depolarization of GCs due to the delay of GC repolarization. In terms of the speed, hypoxic inhibition of voltage-dependent K channels developed faster than tetrodotoxin-induced inhibition of Na channels in the rabbit GCs (Lopez-Lopez and Gonzalez, 1992). Although not studied in detail, the speed of BK channel inhibition was comparable between the hypoxic superfusion and the charybdotoxin superfusion (Wyatt and Peers, 1995). The data indicates that a small drop of O2 tension acts in the order of seconds to inhibit BK channels as charybdotoxin does. We observed K current inhibition at 1 min after switching the superfusate from normoxia to hypoxia (Figure 4B), indicating that BK channel inhibition by lowering O2 develops quickly.
Our previous study (Balbir et al., 2007) showed that the gene expression of TASK-like K channels were indifferent between the DBA/2J and the A/J strains. Therefore, in our experimental model using these mice, we did not focus on these channels. Our current data clearly indicate that mild hypoxia mainly inhibits BK channels in GCs of the DBA/2J mouse: with application of iberiotoxin, K current was inhibited, and mild hypoxia did not further influence K current. However, our results do not mean that other oxygen-sensitive K channels are not important in hypoxic chemotransduction processes. Several types of oxygen-sensitive K channels in the CB have been identified in several species. These include BK channels and background TASK-like K channels in the rat (Williams and Buckler, 2004), Kv4.1 and Kv4.3 in the rabbit (Sanchez et al., 2002), and Kv3 in the C57Bl/6J mouse (Perez-Garcia et al., 2004). Most of these studies used severe hypoxia such as PO2 ∼ 10 mmHg. The relationship between PO2 and K current inhibition was investigated in some studies: oxygen-sensitive K current decreased lineally in the rabbit (Montoro et al., 1996) and in the cat (Shirahata and Sham, 1999), or maximal inhibition occurred at PO2 of 80 mmHg in the rabbit (Lopez-Lopez et al., 1989). These data suggest a possibility that distinct K channels may be inhibited at different PO2 levels.
It has been long known that hypoxic sensitivity among individuals is variable (Hirshman et al., 1975). Human studies in twins and in individuals over time suggest that hypoxic sensitivity of the CB is genetically controlled (Collins et al., 1978; Kawakami et al., 1982; Nishimura et al., 1991; Thomas et al., 1993). Inbred strains of mice are useful tools to investigate genetic background of physiological phenomena, and several studies have shown differential hypoxic responses between the A/J (lower sensitivity) and the DBA/2J (higher sensitivity) mice (Tankersley et al., 1994; Rubin et al., 2003; Campen et al., 2004). The current study provided some molecular mechanisms of the differential hypoxic sensitivity of the CB in these mice.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors appreciate excellent technical supports given by Ms. Mariko Okumura. This work was supported by AHA 09GRNT2080158, NHLBI HL72293, and HL81345.
Footnotes
References
Balbir, A., Lee, H., Okumura, M., Biswal, S., Fitzgerald, R. S., and Shirahata, M. (2007). A search for genes that may confer divergent morphology and function in the carotid body between two strains of mice. Am. J. Physiol. Lung Cell Mol. Physiol. 292, L704–L715.
Brenner, R., Jegla, T. J., Wickenden, A., Liu, Y., and Aldrich, R. W. (2000). Cloning and functional characterization of novel large conductance calcium-activated potassium channel beta subunits, hKCNMB3 and hKCNMB4. J. Biol. Chem. 275, 6453–6461.
Buckler, K. J. (2007). TASK-like potassium channels and oxygen sensing in the carotid body. Respir. Physiol. Neurobiol. 157, 55–64.
Campen, M. J., Tagaito, Y., Li, J., Balbir, A., Tankersley, C. G., Smith, P., Schwartz, A., and O’Donnell, C. P. (2004). Phenotypic variation in cardiovascular responses to acute hypoxic and hypercapnic exposure in mice. Physiol. Genomics 20, 15–20.
Chou, C. L., and Shirahata, M. (1996). Two types of voltage-gated K channels in carotid body cells of adult cats. Brain Res. 742, 34–42.
Collins, D. D., Scoggin, C. H., Zwillich, C. W., and Weil, J. V. (1978). Hereditary aspects of decreased hypoxic response. J. Clin. Invest. 62, 105–110.
Gonzalez, C., Almaraz, L., Obeso, A., and Rigual, R. (1994). Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol. Rev. 74, 829–898.
Gribkoff, V. K., Starrett, J. E., and Dworetzky, S. I. (2001). Maxi-K potassium channels: form, function, and modulation of a class of endogenous regulators of intracellular calcium. Neuroscientist 7, 166–177.
Hirshman, C. A., McCullough, R. E., and Weil, J. V. (1975). Normal values for hypoxic and hypercapnic ventilaroty drives in man. J. Appl. Physiol. 38, 1095–1098.
Kawakami, Y., Yoshikawa, T., Shida, A., Asanuma, Y., and Murao, M. (1982). Control of breathing in young twins. J. Appl. Physiol. 52, 537–542.
Kumar, P., and Prabhakar, N. (2007). Sensing hypoxia: carotid body mechanisms and reflexes in health and disease. Respir. Physiol. Neurobiol. 157, 1–3.
Lopez-Lopez, J., and Gonzalez, C. (1992). Time course of K+ current inhibition by low oxygen in chemoreceptor cells of adult rabbit carotid body effects of carbon monoxide. FEBS Lett. 299, 251–254.
Lopez-Lopez, J., Gonzalez, C., Urena, J., and Lopez-Barneo, J. (1989). Low pO2 selectively inhibits K channel activity in chemoreceptor cells of the mammalian carotid body. J. Gen. Physiol. 93, 1001–1015.
Lopez-Lopez, J. R., and Perez-Garcia, M. T. (2007). Oxygen sensitive Kv channels in the carotid body. Respir. Physiol. Neurobiol. 157, 65–74.
Meera, P., Wallner, M., and Toro, L. (2000). A neuronal beta subunit (KCNMB4) makes the large conductance, voltage- and Ca2+-activated K+ channel resistant to charybdotoxin and iberiotoxin. Proc. Natl. Acad. Sci. U.S.A. 97, 5562–5567.
Montoro, R. J., Urena, J., Fernandez-Chacon, R., Alvarez de Toledo, G., and Lopez-Barneo, J. (1996). Oxygen sensing by ion channels and chemotransduction in single glomus cells. J. Gen. Physiol. 107, 133–143.
Nishimura, M., Yamamoto, M., Yoshioka, A., Akiyama, Y., Kishi, F., and Kawakami, Y. (1991). Longitudinal analyses of respiratory chemosensitivity in normal subjects. Am. Rev. Respir. Dis. 143, 1278–1281.
Orio, P., Rojas, P., Ferreira, G., and Latorre, R. (2002). New disguises for an old channel: maxiK channel beta-subunits. News Physiol. Sci. 17, 156–161.
Pardal, R., Ludewig, U., Garcia-Hirschfeld, J., and Lopez-Barneo, J. (2000). Secretory responses of intact glomus cells in thin slices of rat carotid body to hypoxia and tetraethylammonium. Proc. Natl. Acad. Sci. U.S.A. 97, 2361–2366.
Peers, C., and Wyatt, C. N. (2007). The role of maxiK channels in carotid body chemotransduction. Respir. Physiol. Neurobiol. 157, 75–82.
Peirson, S. N., Butler, J. N., and Foster, R. G. (2003). Experimental validation of novel and conventional approaches to quantitative real-time PCR data analysis. Nucleic Acids Res. 31, e73.
Perez-Garcia, M. T., Colinas, O., Miguel-Velado, E., Moreno-Dominguez, A., and Lopez-Lopez, J. R. (2004). Characterization of the Kv channels of mouse carotid body chemoreceptor cells and their role in oxygen sensing. J. Physiol. (Lond.) 557, 457–471.
Rubin, A. E., Polotsky, V. Y., Balbir, A., Krishnan, J. A., Schwartz, A. R., Smith, P. L., Fitzgerald, R. S., Tankersley, C. G., Shirahata, M., and O’Donnell, C. P. (2003). Differences in sleep-induced hypoxia between A/J and DBA/2J mouse strains. Am. J. Respir. Crit. Care Med. 168, 1520–1527.
Sanchez, D., Lopez-Lopez, J. R., Perez-Garcia, M. T., Sanz-Alfayate, G., Obeso, A., Ganfornina, M. D., and Gonzalez, C. (2002). Molecular identification of Kvalpha subunits that contribute to the oxygen-sensitive K+ current of chemoreceptor cells of the rabbit carotid body. J. Physiol. (Lond.) 542, 369–382.
Shirahata, M., and Sham, J. S. (1999). Roles of ion channels in carotid body chemotransmission of acute hypoxia. Jpn. J. Physiol. 49, 213–228.
Tankersley, C. G., Fitzgerald, R. S., and Kleeberger, S. R. (1994). Differential control of ventilation among inbred strains of mice. Am. J. Physiol. 267, R1371–R1377.
Thomas, D. A., Swaminathan, S., Beardsmore, C. S., McArdle, E. K., MacFadyen, U. M., Goodenough, P. C., Carpenter, R., and Simpson, H. (1993). Comparison of peripheral chemoreceptor responses in monozygotic and dizygotic twin infants. Am. Rev. Respir. Dis. 148, 1605–1609.
Tichopad, A., Dilger, M., Schwarz, G., and Pfaffl, M. W. (2003). Standardized determination of real-time PCR efficiency from a single reaction set-up. Nucleic Acids Res. 31, e122.
Weiger, T. M., Holmqvist, M. H., Levitan, I. B., Clark, F. T., Sprague, S., Huang, W. J., Ge, P., Wang, C., Lawson, D., Jurman, M. E., Glucksmann, M. A., Silos-Santiago, I., DiStefano, P. S., and Curtis, R. (2000). A novel nervous system beta subunit that downregulates human large conductance calcium-dependent potassium channels. J. Neurosci. 20, 3563–3570.
Williams, B. A., and Buckler, K. J. (2004). Biophysical properties and metabolic regulation of a TASK-like potassium channel in rat carotid body type 1 cells. Am. J. Physiol. Lung Cell Mol. Physiol. 286, L221–L230.
Wyatt, C. N., and Peers, C. (1995). Ca2+-activated K+ channels in isolated type I cells of the neonatal rat carotid body. J. Physiol. (Lond.) 483, 559–565.
Keywords: chemoreceptor, glomus cell, hypoxia, patch clamp, oxygen, RT-PCR
Citation: Otsubo T, Kostuk EW, Balbir A, Fujii K and Shirahata M (2011) Differential expression of large-conductance Ca2+-activated K channels in the carotid body between DBA/2J and A/J strains of mice. Front. Cell. Neurosci. 5:19. doi: 10.3389/fncel.2011.00019
Received: 27 May 2011;
Accepted: 14 September 2011;
Published online: 11 October 2011.
Edited by:
Arianna Maffei, SUNY Stony Brook, USACopyright: © 2011 Otsubo, Kostuk, Balbir, Fujii and Shirahata. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Machiko Shirahata, Department of Environmental Health Sciences, Johns Hopkins Bloomberg School of Public Health, 615 N. Wolfe Street, Baltimore, MD 21205, USA. e-mail:bXNoaXJhaGFAamhzcGguZWR1
†Present address: Toshiki Otsubo, Department of Physiology, Dokkyo University School of Medicine, Mibu, Tochigi, Japan; Alexander Balbir, National Intrepid Center of Excellence for Traumatic Brain Injury, Bethesda, MD, USA; Koichi Fujii, Department of Anesthesiology, Dokkyo University School of Medicine, Mibu, Tochigi, Japan.
 Toshiki Otsubo†
Toshiki Otsubo†