- Department of Neuroscience, Physiology and Pharmacology, University College London, London, UK
The axon initial segment (AIS), a site of action potential initiation, undergoes activity-dependent homeostatic repositioning to fine-tune neuronal activity. However, little is known about the behavior of GABAA receptors (GABAARs) at synapses made onto the axon and especially the AIS. Here, we study the clustering and lateral diffusion of GABAARs in the AIS under baseline conditions, and find that GABAAR lateral mobility is lower in the AIS than dendrites. We find differences in axonal clustering and lateral mobility between GABAARs containing the α1 or α2 subunits, which are known to localize differentially to the AIS. Interestingly, we find that chronic activity driving AIS repositioning does not alter GABAergic synapse location along the axon, but decreases GABAAR cluster size at the AIS. Moreover, in response to chronic depolarization, GABAAR diffusion is strikingly increased in the AIS, and not in dendrites, and this is coupled with a decrease in synaptic residency time of GABAARs at the AIS. We also demonstrate that activation of L-type voltage-gated calcium channels is important for regulating GABAAR lateral mobility at the AIS during chronic depolarization. Modulation of GABAAR diffusion dynamics at the AIS in response to prolonged activity may be a novel mechanism for regulating GABAergic control of information processing.
Introduction
The axon initial segment (AIS), a neuronal subdomain enriched with ion channels, scaffolding components and cytoskeletal elements, serves as a key site for action potential initiation, and separates neuronal input and output domains (Rasband, 2010). Several proteins, including Na+ channels, the scaffolds ankyrin-G (ankG) and β IV-spectrin, and the cellular adhesion molecule neurofascin 186, form a protein-dense segment of approximately 20 μm in length, located near to the cell soma (Rasband, 2010). The AIS can also translocate away from the cell soma in response to altered neuronal activity patterns (elevated extracellular K+, Grubb and Burrone, 2010), with all AIS proteins tested (including ankG, NaV channels and NF 186) found to undergo a distal shift of approximately 10 μm along the axon. This structural plasticity, which depends on activation of voltage-gated calcium channels, results homeostatically in increased thresholds for action potential firing (Grubb and Burrone, 2010; O'Leary et al., 2010).
The AIS also receives GABAergic input from axo-axonic interneurons, which contact AIS-localized postsynapses containing clusters of GABAARs, while other neurotransmitter receptors are primarily absent from the AIS (Kole and Stuart, 2012). GABAARs are the major mediators of fast synaptic inhibition in the brain, though evidence suggests that axo-axonic inputs can also be depolarizing or excitatory (Szabadics et al., 2006; Khirug et al., 2008; Kole and Stuart, 2012), thus possibly providing a dual function. It is clear that synapses made onto the AIS can control cell excitability, firing frequency and input–output relationship (Klausberger and Somogyi, 2008; Kole and Stuart, 2012). GABAARs containing α1, α2, or α3 subunits are found enriched at synapses while α4, α5, and α6 are found primarily extrasynaptically (Luscher et al., 2011). Of the synaptic α subunits, α2 subunits (and α3 in some cell types) are enriched at the AIS, while few GABAARs at the AIS contain the α1 subunit (Nusser et al., 1996; Brünig et al., 2001; Panzanelli et al., 2011). While GABAAR membrane dynamics have been well studied in dendrites, including their lateral diffusion into and out of synapses (Thomas et al., 2005; Bannai et al., 2009; Muir et al., 2010), virtually nothing is known about the clustering and lateral mobility of GABAARs at the AIS. Moreover, whether GABAergic AIS synapses shift away from the soma in response to chronic depolarization, or whether the diffusion dynamics of GABAARs at the AIS can be modified is unknown.
Here, we investigate the subunit-specific differences between α1- and α2- containing GABAARs in terms of their clustering and lateral mobility at the AIS. We find that α2 clusters are more numerous in the axon than α1 clusters, and that GABAAR lateral mobility at the AIS is lower for α2- vs. α1-containing GABAARs. While the AIS moves away from the cell body in response to chronic depolarization, GABAergic pre- and postsynaptic elements remain fixed in position along the axon. In contrast, GABAAR lateral mobility in the AIS and proximal axon is specifically increased in response to chronic depolarization, coupled with decreased residency time at AIS synapses and reduced GABAAR cluster size in the AIS. Increased AIS-GABAAR lateral mobility is caused by activation of L-type VGCCs, which also drives AIS translocation (Grubb and Burrone, 2010). Our results provide a novel mechanism for modulation of GABAergic synapses under conditions of prolonged activity, which could have important implications for control of neuronal activity and information processing.
Materials and Methods
Cell Culture and Drug Treatments
We used standard culture of primary dissociated hippocampal neurons from E18 embryonic rats as described previously (Banker and Goslin, 1991). For chronic depolarization, the extracellular potassium concentration was elevated from 5 to 15 mM by adding KCl from a 1M stock solution. Nifedipine was from Tocris. KCl (15 mM) and nifedipine (5 μM) treatments were made at 12 DIV for 48 h, and all experiments were performed at 14 DIV. Transfection of ankG-GFP (a kind gift from V. Bennett, HHMI) was made by calcium phosphate precipitation at 10 DIV as previously described (Twelvetrees et al., 2010). Transfection of mGFP was made by lipofectamine 2000 (Invitrogen) at 11 DIV, with 72 h expression before staining.
Live-Cell Imaging
Imaging media used for quantum dot tracking experiments (Muir et al., 2010) contained 125 mM NaCl, 5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 10 mM D-glucose, 10 mM HEPES and was adjusted to pH 7.4 with NaOH before use. Cells were imaged under perfusion (4 ml/min) and heating (35–37°C). Fluorescence was captured using an Olympus microscope (BX51WI) with a 60x Olympus objective coupled to an EM-CCD camera (Ixon, Andor). Excitation was provided by a mercury spiked xenon arc lamp (Cairn). Appropriate filters were chosen for QDs, alexa dyes, and FM 4–64.
Live labeling of the AIS (Schafer et al., 2009) was performed by mixing 1 μl of anti- pan-neurofascin (pan-NF, neuromab) with 0.35 μl of anti-mouse alexa 488 (Invitrogen). This mixture was incubated on ice for 15 min to allow coupling. Then, 100 μl block solution (imaging media containing 10% horse serum) was added and the solution kept at room temperature (RT) for 2–3 min. For parallel QD labeling of GABAARs, rabbit anti- α1 or α2 (1:100, Synaptic Systems, both recognizing extracellular epitopes) was added to the pan-NF/alexa solution. Coverslips were incubated for 8 min at RT by inverting onto this solution spotted on film. Quantum dots (anti-rabbit 605 nm QD, 0.5 nM, Invitrogen) were attached with a subsequent 2 min labeling step in block solution, as above. Coverslips were washed 6–8 times in imaging media after each step. QD movies were of 200 frames, acquired at 8.5 Hz (movie length = 23.5 s). To minimize the amount of GABAAR internalization within the recording period, movies were recorded within 15 min of QD labeling. Labeling of active presynaptic terminals with FM 4–64 (Invitrogen) was performed by 1 min incubations in 1 ml imaging media, first with 1 μm FM 4–64 + 60 mM KCl, followed by 0.2 μm FM 4–64. Coverslips were then washed extensively before imaging.
Fixed-Cell Imaging
Co-staining for ankG and GABAergic synapse components (α1 and α2-GABAARs, gephyrin, VGAT, all primary antibodies from Synaptic Systems, except ankG, neuroMab and γ2, a kind gift from J. M. Fritschy) was performed using standard immunofluorescence techniques. All primary antibodies were used at 1:100 with secondary staining at 1:500 with alexa 488/594 or cy5. Surface staining of GABAARs (α1 or α2, both extracellular epitope) was made with an initial step in block solution lacking detergent. For analysis of AIS and cluster position, approximately 30 neurons were analysed per condition from images of the whole cell including 100 μm of axon (zoom = 0.7). For analysis of GABAAR cluster size, images at 4 × zoom (25 μm length) were taken of the AIS and two regions of proximal dendrite chosen at random for each cell, and approximately 15 neurons per condition were imaged. All settings were kept constant across experiments. Confocal imaging was performed with Zeiss Pascal and Zeiss 700 microscopes equipped with 63× plan Apochromat oil objectives (NA 1.4).
Image Analysis
AIS position was measured from ankG staining using an automated detection routine similar to that used in (Grubb and Burrone, 2010). Briefly, the axon was traced in ImageJ (NIH) and straightened using the “Straighten” plugin. A running average of ankG intensity along the axon was made (window, W = 20 pixels approximately 5 μm). This image was then scaled such that its pixel intensities range from 0 to 1. Starting from the soma edge (x = 0), AIS start and end positions are defined as where the scaled ankG intensity first exceeds 0.33 and then drops below 0.33, respectively. To account for the size of the smoothing window, W/2 (approximately 2.5 μm) was added to output values of AIS start and end position. Good agreement was found between AIS start and length measurements as determined by this routine compared to analysis by manual inspection.
For analysis of the position of GABAergic synapse components along axons, the straightened image of the axon (as above) was used. Manual logging of each cluster position along the axon (up to 100 μm from the cell soma) was performed in ImageJ. Clusters were defined to be on the axon if their position overlapped with ankG staining and were classified as being before or within the AIS by manual inspection of cluster and AIS position (from ankG staining). GABAAR cluster size was analysed using the “Analyse Particles” function in ImageJ. AIS and dendrite images were first intensity-thresholded (constant across experiments). AIS clusters were classified as those overlapping with AIS/ankG staining.
Analysis of QD-GABAAR trajectories was performed as previously described (Muir et al., 2010) using custom detection and tracking software written in Mathematica (Wolfram Research). Instantaneous diffusion coefficients were calculated from the squared displacement across sequential trajectory segments of five frames, using the 2D diffusion relation, <x2> = 4Dt. Diffusion coefficients were then pooled within like groups/conditions. To analyse QD-GABAAR dynamics in different neuronal regions (i.e., AIS, proximal axon or sample dendrites), neuronal regions were first identified according to neurofascin staining and morphology and then isolated in ImageJ using the selection brush tool. For analysis of GABAAR diffusion in synapses, QD-GABAARs were defined as synaptic if within 0.75 μm of the center of FM 4–64 puncta. GABAAR residency time for each cell was given by the mean duration of QD-GABAAR trajectory segments during which the particle was diffusing within a synapse (defined as above).
To determine AIS and dendrite diameter, confocal images of 25 μm regions (zoom factor 4) were taken. Processes were straightened using the ImageJ Straighten plugin and then thresholded at the same value. To obtain the diameter of the process, the thresholded area was divided by the image length (25 μm).
Statistical Analysis
All experiments were performed on neurons from at least 3 individual preparations. Unless otherwise stated, p-values given are from two-tailed Student's t-tests (equal variance) and values are given as mean ± s.e.m. Error bars represent s.e.m. For multiple comparisons (i.e., Figures 5A–C), One-Way ANOVA followed by Bonferroni correction was used. GABAergic synapse position along axons and GABAAR diffusion coefficients were not normally-distributed. Differences between conditions in these quantities were tested using the non-parametric Mann-Whitney U-test (implemented in R).
Results
GABAAR Clustering and Lateral Mobility at the Axon Initial Segment
Subunit composition of GABAARs is key to determining their subcellular localization. Previous studies have shown that GABAARs containing the α2 subunit are preferentially targeted to the AIS compared to those containing the α1 subunit (Nusser et al., 1996) but whether this is due to subunit-specific differences in receptor diffusion dynamics remains unknown. We investigated the surface clustering and diffusion dynamics of GABAARs at the AIS containing either the α1 or α2 subunits using both immunofluorescence and single particle tracking. We surface stained with antibodies to either α1 or α2 subunits and co-stained for ankG to mark the AIS. Both α1 and α2 subunits were found clustered in dendrites as previously described (Nusser et al., 1996; Brünig et al., 2001). GABAARs containing the α2 subunit were also routinely found in clusters along axons (Figure 1A), while α1 was seen to be more diffuse, but exhibited a clustered distribution in approximately 20% of neurons, (which are likely interneurons, Brünig et al., 2001). In these cells, limited α1 clustering along axons could be seen (Figure 1A). In agreement with the literature (Nusser et al., 1996), we found that α2 clusters were far more numerous in the AIS than α1 clusters (α2: 3.6 ± 0.4, α1: 1.6 ± 0.3, p = 0.002, Figure 1B; quantification was from neurons exhibiting clustered GABAAR distribution only), confirming that the α2 subunit is enriched at the AIS compared to α1.
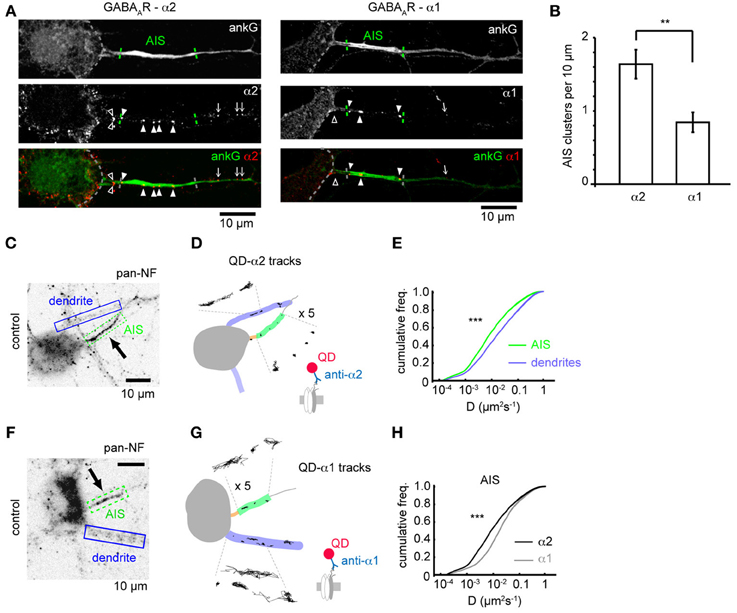
Figure 1. GABAAR clustering and lateral mobility at the axon initial segment. (A) Example neurons stained for ankG and the GABAAR α2 (left) and α1 subunits (right). Outline of soma, AIS position and gephyrin cluster positions along the axon are indicated (open arrowhead, cluster before AIS; closed arrowhead, cluster within AIS; arrow, cluster beyond AIS). (B) Clusters (normalized per 10μ M of AIS length) of the α2 subunit are more numerous in the AIS than the clusters of the α1 subunit, (p = 0.01, n = 5 experiments). (C) Neurofascin (pan-NF) staining delineates the AIS (green). Dendrites (blue) are also identified. (D) Subdomains in (C) overlaid with QD-α2 tracks (also shown zoomed in × 5 for clarity). (E) Cumulative frequency plots of instantaneous α2-GABAAR diffusion coefficient in AIS (green, n = 40,681) and dendrites (blue, n = 316,774); 97 cells, 13 experiments, (p < 2 × 10−16). (F,G) As in (C,D) for QD-α1 labeling. (H) α1- and α2-GABAAR diffusion coefficients in the AIS (gray, α1, n = 10,999, 45 cells; black, α2, n = 40,681, 97 cells, 13 experiments). α2-GABAARs are less mobile in the AIS than α1-GABAARs (median D; α 2 = 0.008 μm2s−1, α 1 = 0.014 μm2s−1, p < 2 × 10−16).
We then used single-particle tracking with quantum dots to investigate the lateral mobility of GABAARs, combined with neurofascin live-labeling (Schafer et al., 2009), to mark the AIS, which reliably labels the AIS as seen by comparison with ankG-GFP expression (Supplementary Figure 1). As expected, QD-α2-GABAAR labeling was seen in the AIS, axon and somatodendritic region (Figures 1C,D). Interestingly, α2-GABAAR lateral mobility was much lower in the AIS than in dendrites (AIS: median D = 0.008 μm2s−1, dendrites: median D = 0.016, p < 2 × 10−16, Figure 1E), as has previously been observed for lipid diffusion (Nakada et al., 2003). Recent studies suggest that the diameter of a tubular membrane can affect diffusion measurements (Renner et al., 2011). To assess whether differences in AIS and dendrite diameter could explain the difference in GABAAR diffusion between these two compartments, we used transfection of membrane GFP (mGFP) and ankG immunostaining to quantify AIS and dendrite diameter (Supplementary Figure 2). We found that typical AIS and dendrite diameters were both approximately 1 μm, and were not significantly different (p = 0.5), confirming that different tubular diameter could not account for observed differences in AIS and dendritic GABAAR diffusion.
We also analysed the diffusion dynamics of GABAARs containing the α1 subunit (Figures 1F,G). Interestingly, while α1-GABAARs were less mobile at the AIS than dendrites (AIS: median D = 0.014 μm2s−1, dendrites: median D = 0.022 μm2s−1), they were much more mobile than α2-GABAARs, particularly at the AIS (median D; α 1 = 0.014 μm2s−1, α 2 = 0.008 μm2s−1, p < 2 × 10−16, Mann-Whitney U-test, Figure 1H), but also in dendrites (median D; α 1 = 0.022 μm2s−1, α 2 = 0.016 μm2s−1). Using the ratio of median D-values (dendrite/AIS) as a measure of lateral mobility restriction in the AIS suggests that α2-GABAARs are more stable in the AIS membrane than their α1-containing counterparts (median Ddend/DAIS: α 1 = 1.6, α 2 = 2.0), which likely underpins the enriched expression of α2 subunit-containing GABAARs observed in this region.
The AIS Shifts Distally on Chronic Depolarization, but Gabaergic Synapse Positions Are not Affected
Recent studies have shown that the AIS can undergo activity-dependent structural plasticity (Kole and Stuart, 2012), and that the AIS can shift distally along the axon in response to chronic depolarization (Grubb and Burrone, 2010). However, whether GABAergic synapses made onto axons (at axo-axonic synapses) also move distally, or adapt to changes in activity, remains unknown. We studied these synapses both under control conditions and after chronic depolarization (15 mM KCl, 48 h, Grubb and Burrone, 2010) by using immunostaining for key GABAergic synapse components (α2, gephyrin, VGAT) and co-staining with ankG (Figures 2A,A'). As previously demonstrated (Grubb and Burrone, 2010), chronic depolarization caused a distal shift in AIS start position (control AIS start position: 8.0 ± 0.8 μm from soma, KCl: 12.3 ± 0.9 μm, p = 0.002, Figure 2B) while AIS length was unaffected (p > 0.05, Figure 2B'). In contrast to AIS translocation, we found no difference in the number (control: 8.4 ± 0.6; KCl: 8.7 ± 0.6, p > 0.05, Figure 2C), or position of α2-GABAAR clusters along axons between control and KCl-treated neurons (Figures 2D,E, p > 0.05). In agreement with this, a significant decrease in the ratio of axonal α2-GABAAR clusters in AIS/before AIS was seen (control: 2.0 ± 0.2; KCl: 1.2 ± 0.1, p = 0.004, Figure 2F), further suggesting that α2-GABAAR cluster positions along the axon remain fixed compared to homeostatic AIS repositioning.
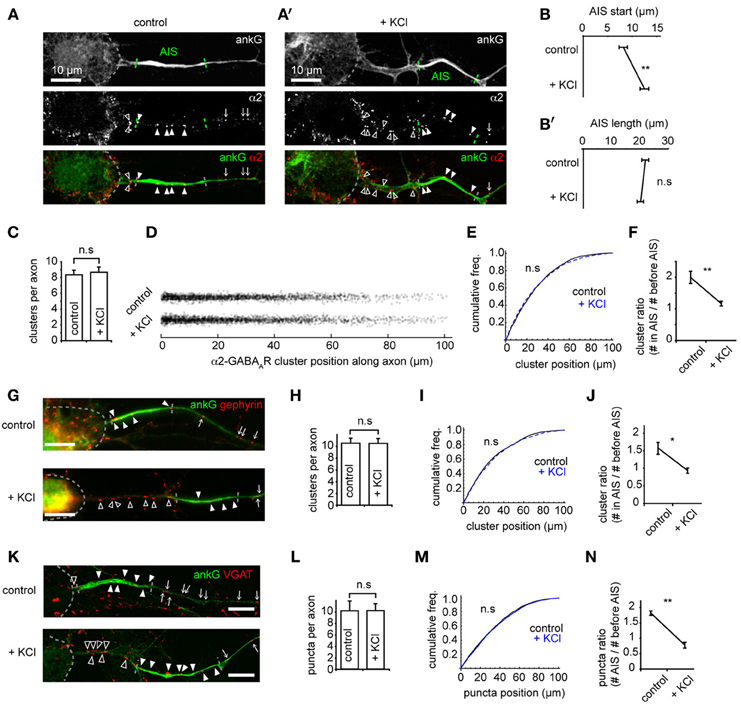
Figure 2. The AIS shifts distally on chronic depolarization, but GABAergic synapse positions are not affected. (A) As Figure 1A. Neuron stained for ankG and GABAAR-α2 subunit. Soma, AIS and GABAAR cluster positions along the axon are indicated (open arrowhead: cluster before AIS; closed arrowhead: cluster within AIS; arrow: cluster beyond AIS). AIS endpoints are determined from ankG intensity. (A') as (A), for a KCl-treated neuron. (B) AIS start position is greater after KCl treatment (p = 0.007, n = 10 experiments); (B') AIS length is not affected (p > 0.05). (C) Number of GABAAR clusters per axon is unchanged (p > 0.05). (D) Positions of all GABAAR clusters from control and KCl pools. (E) GABAAR cluster position along axons (control, black, n = 1944 clusters; KCl, blue, dashed, n = 2130 clusters, p > 0.05, Mann-Whitney U-test). (F) Cluster ratio (in AIS / before AIS) is lower in KCl-treated neurons, p = 0.004, n = 5 experiments. (G) Example neurons stained for ankG and gephyrin (labeled as in A). Top: control, bottom: KCl. Scale bar = 10 μm. (H) Total number of gephyrin clusters per axon is not significantly different (p > 0.05, n = 5). (I) Cumulative frequency plot of gephyrin cluster position along axons (control, black, n = 1585 clusters; KCl, blue, dashed, n = 1686 clusters). Distributions not significantly different (p > 0.05). (J) Gephyrin cluster ratio (in AIS/before AIS) is reduced in KCl-treated neurons, (control: 1.9 ± 0.1; KCl: 0.8 ± 0.1, p = 0.008, n = 5 preps). Under control conditions, axons contained on average 2.5 ± 0.4 gephyrin clusters before their AIS and 3.8 ± 0.5 clusters within their AIS; in KCl treated neurons, axons contained 3.6 ± 0.6 clusters before their AIS and 3.2 ± 0.5 within their AIS. (K) Example neuron stained for ankG and VGAT (labeled as in A). Top: control, bottom, KCl. Scale bar = 10 μm. (L) Total number of VGAT puncta per axon is not significantly different (p > 0.05, n = 5). (M) Cumulative frequency plot of VGAT puncta position along axon (control, black, n = 2219 clusters; KCl, blue, dashed, n = 1962 clusters). Distributions not significantly different (p > 0.05), suggesting that the tight coupling between pre- and post-inhibitory synapses is not affected by chronic depolarization. (N) Cluster ratio (in AIS/before AIS) is lower in KCl treated neurons, (control: 1.9 ± 0.1; KCl: 0.8 ± 0.1, p = 0.003, n = 5 experiments). Under control conditions, axons contained on average 1.7 ± 0.3 VGAT puncta before their AIS and 3.1 ± 0.5 puncta within their AIS; in KCl treated neurons, axons contained 3.3 ± 0.2 puncta before their AIS and 2.7 ± 0.5 within their AIS.
Gephyrin, a key scaffold protein of GABAARs at synapses, is also clustered at AIS synapses (Panzanelli et al., 2011). Immunostaining for gephyrin and ankG showed that gephyrin formed numerous clusters along the AIS, and also further along axons (Figure 2G). As for GABAAR clusters, we found that the number (Figure 2H) and position (Figure 2I) of gephyrin scaffolds was unaffected by chronic depolarization, leading to a significant decrease in the ratio of gephyrin clusters in AIS/before AIS (Figure 2J). We then used staining for the vesicular GABA transporter VGAT to investigate whether the positioning of presynaptic terminals along the axon was similarly unaffected by chronic depolarization. Similarly, we found no change in the position of GABAergic presynaptic terminals (Figures 2K–N). To confirm that clusters of GABAergic synaptic components found along axons represented bona fide GABAergic synapses, we performed co-labeling for GABAARs (γ2 subunit) and VGAT (Supplementary Figure 3). We found that a high proportion (85%) of GABAAR clusters along the axon were closely opposed to VGAT clusters, and that this value was similar to that for GABAAR clusters along dendrites, confirming that GABAergic synapses form along axons. Taken together, these results suggest that the entire GABAergic synapse remains fixed in position during chronic depolarization, and that the tight pre-post coupling of GABAergic synapses (Dobie and Craig, 2011) along the axon is not significantly disrupted during AIS structural plasticity.
Changes in GABAAR Cluster Size and Lateral Mobility at the AIS in Response to Chronic Depolarization
Using confocal microscopy we then examined GABAAR cluster size in the AIS and dendrites on chronic depolarization. Under control conditions, GABAAR clusters were larger in the AIS than dendrites (mean size: AIS, 0.145 ± 0.007 μm2; dendrites, 0.087 ± 0.013 μm2, p = 0.0002, Figures 3A,A'). On chronic depolarization, a small but significant decrease in cluster size was seen in the AIS (control: 0.145 ± 0.007, KCl, 0.124 ± 0.004 μm2, p = 0.03, Figure 3B). However, dendritic cluster size was slightly but not significantly increased (control, 0.087 ± 0.013, KCl, 0.095 ± 0.006 μm2, p = 0.2, Figure 3C). Thus, while the position of pre and postsynaptic elements of the GABAergic synapse are uncoupled from activity-dependent AIS translocation, chronic activity drives an AIS-specific reduction in the postsynaptic size of GABAergic synapses.
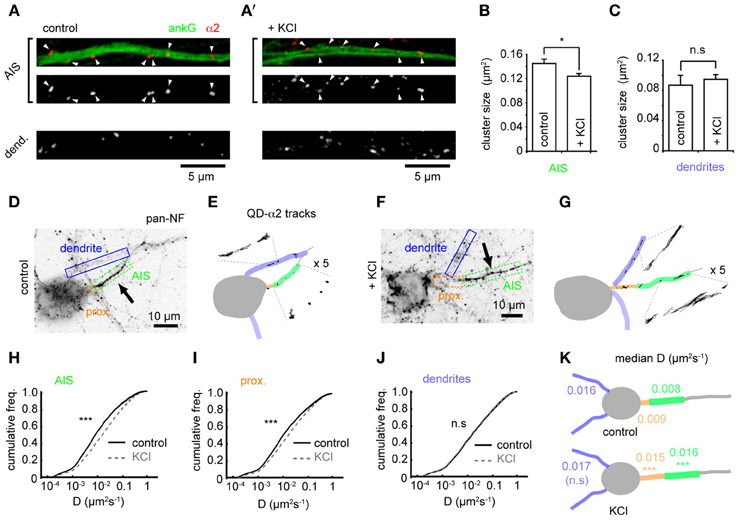
Figure 3. Changes in GABAAR cluster size and lateral mobility at the AIS in response to chronic depolarization. (A) α2 clustering in AIS and dendrites from control neuron. Top panel: AIS, ankG (green), α2 (red). Arrowheads: clusters in AIS. (A') As (A), for KCl-treated neuron. (B) α2-GABAAR cluster size in the AIS is decreased by KCl treatment (p = 0.03, n = 5 experiments). (C) α2-GABAAR cluster size in dendrites is unaffected (p > 0.05). (D) As Figure 1F. Neurofascin live labeling delineates the AIS (boxed in green). Proximal axon (orange) and dendrites (blue) can also be identified from background staining. (E) Map of subcompartments shown in (A), overlaid with QD- α2 tracks. (F,G) As in (C,D) for KCl-treated neuron. (H–J) Instantaneous α2-GABAAR diffusion coefficient in control (black) and KCl conditions (gray, dashed) in AIS (H), proximal axon (I) and dendrites (J). Control, 97 cells; KCl, 111 cells; 10 experiments. In the AIS, median D increased 2-fold, ncontrol = 40,681, nKCl = 49,235, p < 2 × 10−16; in the proximal axon, median D increased 1.7-fold, ncontrol = 11,314, nKCl = 19,979, p < 2 × 10−16; in dendrites, lateral mobility was unaffected, ncontrol = 316,962, nKCl = 463,969, p > 0.05. (K) Summary of median instantaneous α2-GABAAR diffusion coefficient in control and KCl conditions.
To investigate if the alteration in α2-GABAAR AIS cluster size after chronic activity is due to altered GABAAR diffusion dynamics in the AIS, we compared GABAAR diffusion dynamics between control and chronically depolarized conditions (Figures 3D–G). Chronic depolarization led to a striking increase in GABAAR lateral mobility in the AIS (control-AIS: median D = 0.008 μm2s−1, KCl-AIS: 0.016 μm2s−1, p < 2 × 10−16, Figures 3H,K), and GABAAR diffusion rates also increased in the proximal axon (between soma and AIS start, control-PA: median D = 0.009 μm2s−1, KCl-PA: 0.015 μm2s−1, p < 2 × 10−16, Figures 3I,K). In contrast, GABAAR lateral mobility in dendrites was unaffected (control: median D = 0.016 μm2s−1, KCl: 0.017 μm2s−1, p > 0.05, Figures 3J,K). These data suggest that chronic depolarization has a subdomain-specific effect on α2-GABAAR diffusion dynamics. Diffusion dynamics of α1-containing GABAARs in the AIS increased only slightly on chronic depolarization, exhibiting a much smaller change than that seen for α2-GABAARs in this region (control: median D = 0.014 μm2s−1, KCl: median D = 0.017 μm2s−1, p < 2 × 10−16, Supplementary Figure 4), and α1-GABAAR lateral mobility was unaffected in dendrites (control: median D = 0.022 μm2s−1, KCl: median D = 0.022 μm2s−1, p > 0.05). Taken together, these results suggest that the subdomain-specific modulation of GABAAR lateral diffusion in response to chronic depolarization primarily affects α2-containing GABAARs.
Chronic Depolarization Affects Synaptic and Extrasynaptic GABAARs, and Reduces GABAAR Residency Time at AIS Synapses
To investigate whether increased AIS-α2-GABAAR diffusion dynamics altered receptor behavior at synapses, we labeled presynaptic inputs with FM 4–64. Active presynaptic terminals (FM-positive puncta) were routinely found along neurofascin labeled AISs (Figures 4A,B). Chronic activity increased α2-GABAAR diffusion both inside and outside synapses made onto the AIS, with similar increases in each domain (median Dsyn increased 1.8-fold from 0.009 to 0.016 μm2s−1, Figure 4C; median Dext increased 1.8-fold from 0.010 to 0.018 μm2s−1, Figure 4D, both p < 2 × 10−16). We also analysed the mean time spent by GABAARs at synapses. We found that synaptic α2-GABAAR residency time at the AIS was significantly decreased (control: 3.1 ± 0.6 s, KCl: 0.9 ± 0.2 s, p = 0.001, Figure 4E), suggesting reduced occupancy of synaptic sites, consistent with the decrease in GABAAR cluster size observed above. In contrast, chronic depolarization did not affect GABAAR lateral mobility in dendrites, either at synapses or outside synapses (median Dsyn, control = 0.013 μm2s−1, KCl = 0.014 μm2s−1; median Dext, control = 0.018 μm2s−1, KCl = 0.018 μm2s−1; both p > 0.05, Mann-Whitney U-test, Figures 4F,G). Moreover, mean synaptic residency times for GABAARs in dendrites were similar between control and KCl conditions (control: 1.6 ± 0.2 s, KCl: 1.5 ± 0.2 s, p > 0.05, Figure 4H). Taken together, these data further suggest that chronic activity has a region-specific effect on GABAAR diffusion dynamics, with increased diffusion and decreased stability of α2-GABAARs at AIS synapses upon chronic depolarization.
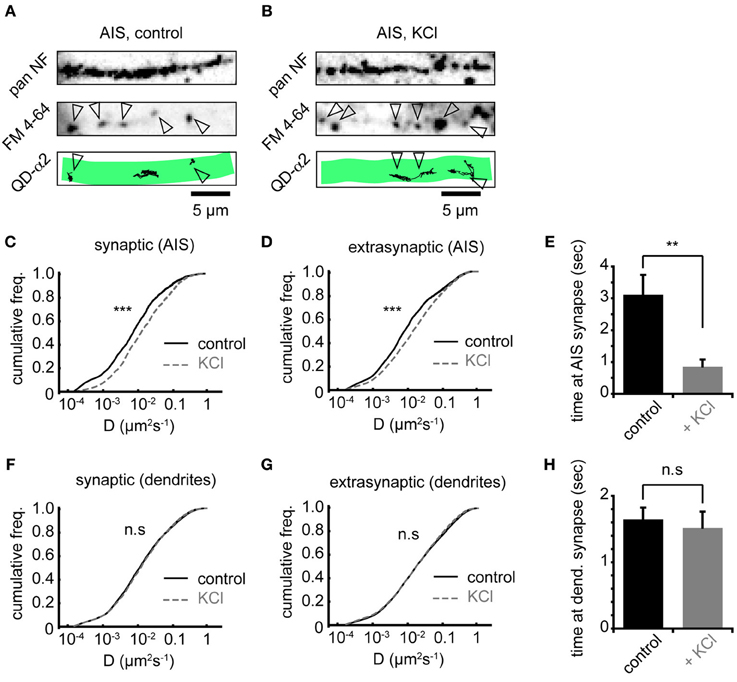
Figure 4. Chronic depolarization affects synaptic and extrasynaptic GABAARs, and reduces GABAAR residency time at AIS synapses. (A) Control neuron AIS labeled by pan NF (top), FM 4–64 loading (middle, arrowheads = synapses) and with QD-α2 tracks shown (bottom). (B) As in (A), but for KCl-treated neuron. (C) Chronic depolarization increases GABAAR lateral mobility in the AIS at synapses (1.85-fold increase, ncontrol = 1922, nKCl = 1620, p < 2 × 10−16). (D) GABAAR lateral mobility in the AIS also increases outside synapses (1.89-fold increase, ncontrol = 4915, nKCl = 8077, p < 2 × 10−16). (E) Mean time spent by GABAARs at AIS synapses decreases significantly on chronic depolarization (control: n = 32 cells; KCl, n = 26 cells, p = 0.001). (F,G) GABAAR lateral mobility in dendrites is unaffected (p > 0.05) by chronic depolarization, both in synapses, ncontrol = 17581, nKCl = 13145 (F) and outside synapses, ncontrol = 75514, nKCl = 73135 (G). (H) Mean time spent by GABAARs at synapses in dendrites is unaffected by chronic depolarization (control: n = 32 cells; KCl, n = 26 cells, p > 0.05).
Distal Shift in AIS Position and Increased GABAAR Lateral Mobility Depend on l-Type VGCCs
To further understand the mechanisms underlying changes in AIS-GABAAR diffusion dynamics, we investigated the role of L-type voltage-gated Ca2+ channels (VGCCs), whose activity was previously reported to drive activity-dependent AIS translocation (Grubb and Burrone, 2010). We tested whether the L-type calcium channel blocker nifedipine (5 μM) could prevent both the distal AIS shift and the increase in GABAAR lateral mobility at the AIS. Immunostaining for ankG confirmed that blockade of L-type VGCCs could indeed prevent AIS translocation (Figures 5A–C). In agreement with the literature, we found that the shift in AIS start position on chronic depolarization was prevented by nifedipine treatment (AIS start position, control: 8.2 ± 1.3 μm; KCl: 14.0 ± 1.2 μm; KCl + nifed: 9.1 ± 0.6 μm, p < 0.05 (control vs. KCl), p < 0.05 (KCl vs. KCl + nifed), p > 0.05 (control vs. KCl + nifed) (Figure 5B). Moreover, no change in AIS length was found under either condition (control: 22.8 ± 1.8 μm; KCl: 28.9 ± 2.0 μm; KCl + nifed: 25.8 ± 1.5 μm, p > 0.05 for all comparisons, Figure 5C). We then analysed α2-GABAAR diffusion dynamics under these conditions (Figures 5D,D', E,E',F,F'). The robust increase in α2-GABAAR diffusion in the AIS upon chronic depolarization (median D, control: 0.009 μm2s−1; KCl: 0.023 μm2s−1, p < 2 × 10−16, Mann-Whitney U-test) was greatly reduced upon nifedipine treatment (median D, KCl + nif: 0.014 μm2s−1, an 1.64-fold reduction from KCl alone, p < 2 × 10−16, Mann-Whitney U-test, Figure 5G). α2-GABAAR lateral mobility in dendrites was similar across control, KCl and KCl + nifedipine conditions (median D control, 0.019; KCl, 0.020; KCl + nif, 0.020 μm2s−1, Figure 5H). Thus, Ca2+ influx through L-type VGCCs controls both an activity-dependent shift in AIS location and increased AIS-GABAAR lateral mobility.
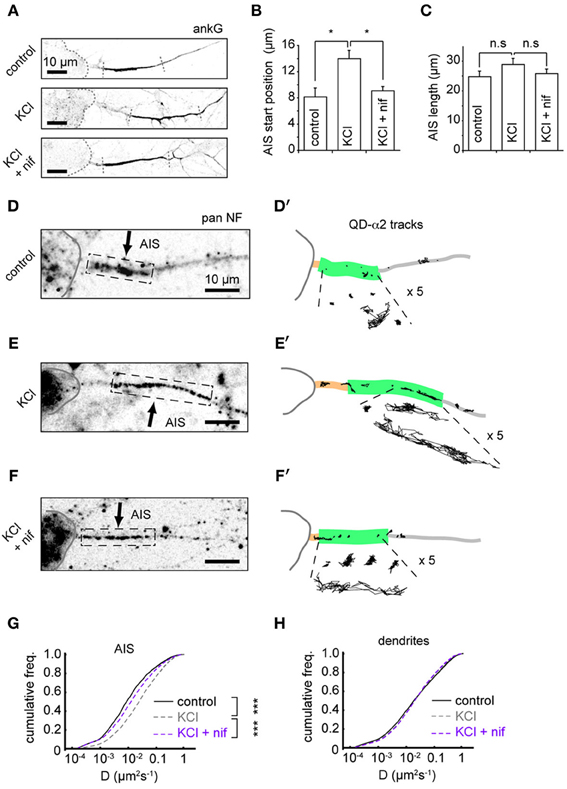
Figure 5. Distal shift in AIS position and increased GABAAR lateral mobility depend on L-type VGCCs. (A) Example ankG staining from control (top), KCl (middle) and KCl + 5 μM nifedipine conditions (bottom). (B) Analysis of AIS start position. One-way ANOVA omnibus test p = 0.01. Pairwise test p-values are Bonferroni-corrected. KCl treatment caused a distal shift in AIS start position (p < 0.05), which was prevented by addition of nifedipine (p < 0.05), n = 5 experiments (control = 150 cells, KCl = 132 cells and KCl+nif = 116 cells). (C) Analysis of AIS length. Omnibus p = 0.28. No change in AIS length was seen in either KCl or KCl + nifedipine (p > 0.05 for both comparisons). (D,D') Example control neuron with AIS location given by pan-NF labeling shown with QD-α2 tracks (those in AIS shown with 5× zoom for clarity). (E,E') As above, for KCl treated neuron. (F,F') For KCl + nifedipine condition. (G) Instantaneous GABAAR diffusion coefficient distributions in the AIS for control (black, n = 9,482, 17 cells), KCl (gray, dashed, n = 15,605, 22 cells) and KCl + nif (purple, dashed, n = 6194, 12 cells). Increase in AIS GABAAR lateral mobility seen on KCl treatment (p < 2 × 10−16, Mann-Whitney U-test) was reduced in presence of nifedipine, (p < 2 × 10−16, Mann-Whitney U-test). (H) As in (D), but for dendrites. Control, n = 76,005; KCl, n = 102,445; KCl + nif, n = 55,821. Dendritic GABAAR mobilities are similar across conditions.
Discussion
In this study, we have investigated the surface behavior of GABAARs at the AIS, both under baseline conditions and in response to changes in neuronal activity that drive AIS structural plasticity (Grubb and Burrone, 2010). We find that surface GABAARs are less mobile at the AIS than in dendrites, but that chronic depolarization drives increased GABAAR lateral mobility and decreased synaptic residency time at the AIS. Intriguingly, both the distal shift in AIS position and increase in GABAAR diffusion dynamics at the AIS depend on L-type VGCC activation, suggesting that these activity-dependent responses are linked.
Virtually nothing is known about the behavior of GABAARs at the AIS. Indeed, to our knowledge, this is the first study to look at the surface trafficking of GABAARs specifically in the AIS. Our investigation into the clustering and lateral mobility of α1- or α2-containing GABAARs revealed interesting differences between receptors containing the two subunits. We find that α2-GABAARs are more numerous in the axon (as shown previously by immunogold electron microscopy, Nusser et al., 1996), and are also found distributed further down the axon, detectable in clusters 100 μm away from the soma. Moreover, α2-GABAARs are less mobile in the surface membrane than α1-containing GABAARs, especially at the AIS. Differences in the membrane dynamics of receptors containing these two subunits may be due to GABAAR targeting mechanisms that are subunit-specific.
We also find that α2-GABAARs at the AIS and proximal axon are far less mobile than those in dendrites (which are approximately twice as mobile as their AIS-localized counterparts). Similarly, GABAAR cluster size in the AIS is almost twice that in dendrites (Figures 3A–C), and GABAAR residency time at synapses in the AIS is longer than for GABAARs in dendrites (Figures 4E,H). These findings suggest that α2-GABAARs are especially stable at AIS synapses. Interestingly, GABAARs also exhibit comparably slower surface dynamics at extrasynaptic sites in the AIS in agreement with the notion that properties of the AIS per se may play a role in regulating GABAAR mobilities in this neuronal subcompartment. Slow surface dynamics at the AIS have been previously reported for lipids (Nakada et al., 2003) and NaV channels (Brachet et al., 2010). This could in part be due to the high density of protein scaffolds and membrane proteins at the AIS (Rasband, 2010). However, it is additionally possible that specific protein interactions between the α2 subunit and ankG or another AIS protein (e.g., neurofascin 186, which can stabilize axo-axonic synapses, Kriebel et al., 2011) may also act as diffusion traps to contribute to the increased stability of GABAARs at the AIS (i.e., low diffusion rate, high residency time and cluster size). The gephyrin scaffold can interact directly with the α1, α2, and α3 subunits (Tretter et al., 2008; Mukherjee et al., 2011; Tretter et al., 2011) and forms clusters at the AIS (Panzanelli et al., 2011; also herein, Figure 2G) suggesting that a complex between GABAARs, gephyrin and AIS proteins may also exist in this region.
The distal shift undergone by the AIS in response to chronic depolarization (Grubb and Burrone, 2010; also observed herein) is an intriguing cell biological phenomenon, for which a molecular mechanism remains unclear. It was recently identified that an ankyrin-B based scaffold in the distal axon can define the position of the AIS (Galiano et al., 2012), which could be involved in AIS structural plasticity. Whether creation and insertion of new axon from the soma is required is also currently unknown. In contrast to the movement of the AIS, we find that GABAergic synapses distributed along the axon do not undergo a distal shift, as the positioning of pre- and postsynaptic components tested (GABAARs, gephyrin, VGAT) was found to be unaffected by chronic depolarization. While it is unclear how this may affect the ability of these inputs to regulate the initiation of APs, one possibility is that the resulting increase in the number of synaptic inputs between the soma and the shifted AIS could lead to higher inhibitory shunt acting on conductances reaching the AIS. This would raise the threshold for AP initiation, counterbalancing the chronic activity stimulus and thus acting homeostatically, in concert with the distal shift in AIS position, which causes increased thresholds for action potential initiation (Grubb and Burrone, 2010; O'Leary et al., 2010). Activity-dependent disruption of scaffolding interactions may underlie the observed increase in GABAAR diffusion and decrease in GABAAR cluster size at the AIS. Since the activity-dependent increase in GABAAR mobility at the AIS is also seen extrasynaptically (in gephyrin negative regions) we think it unlikely that alterations in gephyrin-dependent GABAAR stabilization are the primary driver of the increase in GABAAR mobility at the AIS upon chronic depolarization. Rather, a distal shift in AIS position but not GABAergic synapses may uncouple GABAARs from mechanisms that contribute to their stabilization in the axonal membrane. An intriguing possibility is that the AIS-specific mechanisms that stabilize GABAARs in the axon may be weakened in order to allow GABAergic synapses to remain fixed in position and resist the distal shift of the AIS scaffold (including neurofascin 186) in response to chronic depolarization. Increased GABAAR diffusion dynamics in the AIS and proximal axon could be a necessary consequence of such reduced tethering, to allow the preservation of GABAergic synaptic positions along the axon. Moreover, these putative interactions could be disrupted by activation of L-type VGCCs, since inhibition of L-type VGCCs with nifedipine blocks translocation of the AIS, and also partially prevents an increase in AIS-GABAAR diffusion on KCl treatment. Previous studies revealed that acute increases in neuronal activity and spiking (e.g., driven by treatment with 4-AP or glutamate) lead to rapid calcium and calcineurin-dependent GABAAR de-clustering and increased GABAAR diffusion dynamics in dendrites (Bannai et al., 2009; Muir et al., 2010). In contrast we found that chronic treatment with low levels of KCl (15 mM, 48 h), which was shown to cause only a small 10mV depolarization of the resting membrane potential and a suppression of spontaneous spiking (Grubb and Burrone, 2010) only increased GABAAR diffusion at the AIS but not in dendrites, suggesting that mild chronic depolarization (with KCl) cannot drive a sufficient rise in dendritic calcium to activate dendritic calcineurin or alter dendritic GABAAR stability. Interestingly, chronic KCL-dependent AIS repositioning was also recently demonstrated to be calcineurin-dependent suggesting that these conditions may lead to a selective increase in somatic and/or AIS specific calcineurin activity (Evans et al., 2013). This could also account (perhaps in concert with the localization of a specific scaffold such as AnkG to the proximal axon) for a more localized activity-dependent impact on GABAAR diffusion in the proximal axon/AIS (rather than throughout the entire axon). It will be interesting to determine in the future if the activity-dependent increase in GABAAR mobility at the AIS is also dependent on changes in GABAAR phosphorylation state (Muir et al., 2010).
While GABAergic inputs onto the AIS are ideally localized to control action potential initiation (Kole and Stuart, 2012), the nature of these inputs, i.e., whether they are inhibitory or excitatory, is still unresolved. A body of evidence suggests that GABAergic inputs onto the AIS can be depolarizing in the cortex (Szabadics et al., 2006; Khirug et al., 2008; Kole and Stuart, 2012). This is thought to be due to high expression of the Na+/K+/Cl− cotransporter NKCC1 (Khirug et al., 2008) and absence of the K+/Cl− cotransporter KCC2 from the AIS (Hedstrom et al., 2008; Báldi et al., 2010), resulting in a high intracellular [Cl−] and subsequent depolarization on GABAAR activation. Thus, reduced GABAAR cluster size and increased GABAAR diffusion at AIS synapses in response to chronic depolarization could alternatively represent weakening of depolarizing or excitatory GABAergic inputs. In this case, increased GABAAR diffusion dynamics would provide a mechanism to weaken depolarizing inputs in a homeostatic response to chronic elevation of activity.
We conclude that during activity-dependent AIS translocation, occurring in response to chronic depolarization, the positions of GABAergic synapses along the axon are unaffected. However, the AIS shift is coupled with plasticity of GABAAR cluster size and diffusion dynamics at this key neuronal subcompartment. This novel form of plasticity could be important for GABAergic control of information processing in the healthy or diseased brain, for example in epilepsy, where repeated bursts of activity may lead to structural plasticity of the AIS and of axonal GABAAR diffusion dynamics.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: http://www.frontiersin.org/journal/10.3389/fncel.2014.00151/abstract
Supplementary Figure 1. (A) Schematic showing live-labeling of AIS via an antibody to neurofascin. We used an antibody to an extracellular epitope on neurofascin (NF), pre-conjugated to alexa dye. (B) Overlap of pan-NF live labeling with AIS as marked by ankG-GFP, confirming that this approach can reliably label the AIS. Scale bar = 10 μm.
Supplementary Figure 2. (A) mGFP-transfected neuron. Left: ankG staining (AIS); right: mGFP expression, with AIS and sample dendrite labeled. (B) Zoomed regions of AIS and dendrite shown boxed in (A). (C) Process diameter of AIS and dendrites is not significantly different. AIS: 1.0 ± 0.1 μm (n = 14), dendrite: 1.1 ± 0.1 μm (n = 28), p = 0.54).
Supplementary Figure 3. (A) Axon from neuron stained for ankG (top, cyan), γ2-GABAARs (middle, magenta) and VGAT (bottom, yellow), shown merged beneath. Closed arrowheads indicate position of a GABAAR cluster opposed to a VGAT cluster; open arrowheads indicate position of a GABAAR cluster not opposed to a VGAT cluster. (B) Synaptic GABAAR cluster fraction in axons and dendrites is not significantly different. Axon: 0.86 ± 0.03 (n = 30), dendrite: 0.80 ± 0.01 (n = 30), p = 0.08.
Supplementary Figure 4. (A) As Figure 1C. Subcompartments delineated from neurofascin staining overlaid with α1-QD trajectories. (B) As (A), for KCl-treated neuron. (C,D) Instantaneous α1-GABAAR diffusion coefficient in control (black) and KCl conditions (gray, dashed) in AIS (C) and dendrites (D). Control, 45 cells; KCl, 47 cells; 5 experiments. In the AIS, median D increased 1.2-fold, ncontrol = 10,099, nKCl = 18,933, p < 2 × 10−16; in dendrites, lateral mobility was unaffected, ncontrol = 137,377, nKCl = 175,592, p > 0.05.
References
Báldi, R., Varga, C., and Támas, G. (2010). Differential distribution of KCC2 along the axo-somato-dendritic axis of hippocampal principal cells. Eur. J. Neurosci. 32, 1319–1325. doi: 10.1111/j.1460-9568.2010.07361.x
Bannai, H., Lévi, S., Schweizer, C., Inoue, T., Launey, T., Racine, V., et al. (2009). Activity-dependent tuning of inhibitory neurotransmission based on GABAAR diffusion dynamics. Neuron 62, 670–682. doi: 10.1016/j.neuron.2009.04.023
Brachet, A., Leterrier, C., Irondelle, M., Fache, M.-P., Racine, V., Sibarita, J.-B., et al. (2010). Ankyrin G restricts ion channel diffusion at the axonal initial segment before the establishment of the diffusion barrier. J. Cell Biol. 191, 383–395. doi: 10.1083/jcb.201003042
Brünig, I., Scotti, E., Sidler, C., and Fristchy, J.-M. (2001). Intact sorting, targeting, and clustering of γ-aminobutyric acid A receptor subtypes in hippocampal neurons in vitro. J. Comp. Neurol. 443, 43–55. doi: 10.1002/cne.10102
Dobie, F. A., and Craig, A. M. (2011). Inhibitory synapse dynamics: coordinated presynaptic and postsynaptic mobility and the major contribution of recycled vesicles to new synapse formation. J. Neurosci. 31, 10481–10493. doi: 10.1523/JNEUROSCI.6023-10.2011
Evans, M. D., Sammons, R. P., Lebron, S., Dumitrescu, A. S., Watkins, T. B., Uebele, V. N., et al. (2013). Calcineurin signaling mediates activity-dependent relocation of the axon initial segment. J. Neurosci. 33, 6950–6963. doi: 10.1523/JNEUROSCI.0277-13.2013
Galiano, M. R., Jha, S., Ho, T. S. Y., Zhang, C., Ogawa, Y., Chang, K.-J., et al. (2012). A distal axonal cytoskeleton forms an intra-axonal boundary that controls axon initial segment assembly. Cell 149, 1125–1139. doi: 10.1016/j.cell.2012.03.039
Grubb, M. S., and Burrone, J. (2010). Activity-dependent relocation of the axon initial segment fine-tunes neuronal excitability. Nature 465, 1070–1074. doi: 10.1038/nature09160
Hedstrom, K. L., Ogawa, Y., and Rasband, M. N. (2008). AnkyrinG is required for maintenance of the axon initial segment and neuronal polarity. J. Cell Biol. 183, 635–640. doi: 10.1083/jcb.200806112
Khirug, S., Yamada, J., Afzalov, R., Voipio, J., Khiroug, L., and Kaila, K. (2008). GABAergic depolarization of the axon initial segment in cortical principal neurons is caused by the Na-K-2Cl cotransporter NKCC1. J. Neurosci. 28, 4635–4639. doi: 10.1523/JNEUROSCI.0908-08.2008
Klausberger, T., and Somogyi, P. (2008). Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science 321, 53–57. doi: 10.1126/science.1149381
Kole, M. H. P., and Stuart, G. J. (2012). Signal processing in the axon initial segment. Neuron 73, 235–247. doi: 10.1016/j.neuron.2012.01.007
Kriebel, M., Metzger, J., Trinks, S., Chugh, D., Harvey, R. J., Harvey, K., et al. (2011). The cell adhesion molecule neurofascin stabilizes axo-axonic GABAergic terminals at the axon initial segment. J. Biol. Chem. 286, 24385–24393. doi: 10.1074/jbc.M110.212191
Luscher, B., Fuchs, T., and Kilpatrick, C. L. (2011). GABAA receptor trafficking-mediated plasticity of inhibitory synapses. Neuron 70, 385–409. doi: 10.1016/j.neuron.2011.03.024
Muir, J., Arancibia-Carcamo, I. L., Macaskill, A. F., Smith, K. R., Griffin, L. D., and Kittler, J. T. (2010). NMDA receptors regulate GABAA receptor lateral mobility and clustering at inhibitory synapses through serine 327 on the γ2 subunit. Proc. Natl. Acad. Sci. U.S.A. 107, 16679–16684. doi: 10.1073/pnas.1000589107
Mukherjee, J., Kretschmannova, K., Gouzer, G., Maric, H.-M., Ramsden, S., Tretter, V., et al. (2011). The residence time of GABAARs at inhibitory synapses is determined by direct binding of the receptor α1 subunit to gephyrin. J. Neurosci. 31, 14677–14687. doi: 10.1523/JNEUROSCI.2001-11.2011
Nakada, C., Ritchie, K., Oba, Y., Nakamura, M., Hotta, Y., Iino, R., et al. (2003). Accumulation of anchored proteins forms membrane diffusion barriers during neuronal polarization. Nat. Cell Biol. 5, 626–632. doi: 10.1038/ncb1009
Nusser, Z., Sieghart, W., Benke, D., Fritschy, J. M., and Somogyi, P. (1996). Differential synaptic localization of two major γ-aminobutyric acid type A receptor α subunits on hippocampal pyramidal cells. Proc. Natl. Acad. Sci. U.S.A. 93, 11939–11944. doi: 10.1073/pnas.93.21.11939
O'Leary, T., van Rossum, M. C. W., and Wyllie, D. J. A. (2010). Homeostasis of intrinsic excitability in hippocampal neurones: dynamics and mechanism of the response to chronic depolarization. J. Physiol. (Lond.) 588, 157–170. doi: 10.1113/jphysiol.2009.181024
Panzanelli, P., Gunn, B. G., Schlatter, M. C., Benke, D., Tyagarajan, S. K., Scheiffele, P. et al. (2011). Distinct mechanisms regulate GABAA receptor and gephyrin clustering at perisomatic and axo-axonic synapses on CA1 pyramidal cells. J. Physiol. (Lond.) 589, 4959–4980. doi: 10.1113/jphysiol.2011.216028
Rasband, M. N. (2010). The axon initial segment and the maintenance of neuronal polarity. Nat. Rev. Neurosci. 11, 552–562. doi: 10.1038/nrn2852
Renner, M., Domanov, Y., Sandrin, F., Izeddin, I., Bassereau, P., and Triller, A. (2011). Lateral diffusion on tubular membranes: quantification of measurements bias. PLoS ONE 6:e25731. doi: 10.1371/journal.pone.0025731
Schafer, D. P., Jha, S., Liu, F., Akella, T., McCullough, L. D., and Rasband, M. N. (2009). Disruption of the axon initial segment cytoskeleton is a new mechanism for neuronal injury. J. Neurosci. 29, 13242–13254. doi: 10.1523/JNEUROSCI.3376-09.2009
Szabadics, J., Varga, C., Molnár, G., Oláh, S., Barzó, P., and Tamás, G. (2006). Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science 311, 233–235. doi: 10.1126/science.1121325
Thomas, P., Mortensen, M., Hosie, A. M., and Smart, T. G. (2005). Dynamic mobility of functional GABAA receptors at inhibitory synapses. Nat. Neurosci. 8, 889–897. doi: 10.1038/nn1483
Tretter, V., et al. (2011). Molecular basis of the GABAA receptor α3 subunit interaction with gephyrin. J. Biol. Chem. 31, 37702–37711. doi: 10.1074/jbc.M111.291336
Tretter, V., Jacob, T. C., Mukherjee, J., Fritschy, J.-M., Pangalos, M. N., and Moss, S. J. (2008). The clustering of GABAA receptor subtypes at inhibitory synapses is facilitated via the direct binding of receptor α2 subunits to gephyrin. J. Neurosci. 28, 1356–1365. doi: 10.1523/JNEUROSCI.5050-07.2008
Keywords: gaba receptors, homeostatic plasticity, axon initial segment, calcium, quantum dots, diffusion
Citation: Muir J and Kittler JT (2014) Plasticity of GABAA receptor diffusion dynamics at the axon initial segment. Front. Cell. Neurosci. 8:151. doi: 10.3389/fncel.2014.00151
Received: 29 January 2014; Paper pending published: 24 February 2014;
Accepted: 11 May 2014; Published online: 10 June 2014.
Edited by:
Andrea Barberis, Italian Institute of Technology, ItalyReviewed by:
Enrica Maria Petrini, Italian Institute of Technology, ItalySabine Levi, Institut National de la Santé et de la Recherche Médicale, France
Copyright © 2014 Muir and Kittler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Josef T. Kittler, Department of Neuroscience, Physiology and Pharmacology, University College London, Gower St., London WC1E 6BT, UK e-mail:ai5raXR0bGVyQHVjbC5hYy51aw==
 James Muir
James Muir Josef T. Kittler
Josef T. Kittler