- 1Department of Internal Medicine, Division of Nephrology, University Medical Center Groningen, University of Groningen, Groningen, Netherlands
- 2Department of Surgery, University Medical Center Groningen, University of Groningen, Groningen, Netherlands
- 3Department of Urology, University Medical Center Groningen, University of Groningen, Groningen, Netherlands
Introduction: Urologic complications (UCs) and urinary tract infections (UTIs) are common after kidney transplantation. Intraoperative stent placement at the vesicoureteric anastomosis reduces UC risk, but increases UTI risk.
Methods: In 2014 our stenting protocol changed from external ureteric stent (ES) to internal double J stent (DJ). We retrospectively studied the occurrence of UCs and UTIs in relation to ES or DJ in 697 kidney recipients.
Methods: An ES was used in 403 patients (57.8%), in 294 (42.2%) a DJ. ES was removed 7-12 days and DJ 3-4 weeks post-operative. Induction immunosuppression was the same in both groups. Primary outcomes at 6 months follow-up were UC (urinary leakage/ureter stenosis) and UTI; they were related to stenting procedure and clinical and transplant characteristics. The incidence of UCs was similar for ES (8.4%) and DJ (6.8%), p=0.389. ES use was a significant risk factor for UTI (OR 1.69 (1.15-2.50), p=0.008). Post-transplant hospitalization was significantly shorter in the DJ group. Despite more acute rejection episodes with ES (ES/DJ: 16.4%/6.1%, p<0.001), no clinical relevant differences in graft outcomes existed.
Discussion: A DJ is, compared to ES, associated with a lower incidence of UTIs and comparable occurrence of UCs and is therefore the preferred technique for stenting the vesicoureteric anastomosis.
Introduction
For patients with end stage renal disease, kidney transplantation is the preferred treatment option (1). Important risk factors for graft loss and morbidity after kidney transplantation are urological complications (UCs) (2). The most frequent UCs are urinary leakage and ureter stenosis, which are mostly located at the vesicoureteric anastomosis and occur in the first month after kidney transplantation (3). The introduction of the extravesical Lich-Gregoire surgical technique and later on the full thickness surgical technique for the vesicoureteric anastomosis resulted in less UCs (4). In addition, intraoperative placement of a stent at the vesicoureteric anastomosis further contributed to a reduction in UCs (5, 6). Paradoxically, the presence of a stent may introduce other complications, such as urinary tract infections (UTIs) (5, 6), which is the most common infectious complication after kidney transplantion (7, 8). UTIs after kidney transplantation can, like UCs, negatively affect graft- and patient outcomes (9). Currently, there is no consensus about the optimal stenting protocol for preventing UCs and UTIs after kidney transplantation.
Used techniques for stenting the vesicoureteric anastomosis are: an external ureteric stent (ES), which is a flexible catheter that drains externally, percutaneously, from the donor kidney pyelum through the suprapubic bladder region, and the completely internal double J stent (DJ). The use of an ES has several advantages, such as removal without cystoscopy, selective monitoring of the urine output from the kidney graft, and generally a shorter period of a foreign body in situ. However, besides an extra external connection of the urinary tract, hospitalization is generally longer (1-2 weeks post-operative) with ES due to the in-hospital removal of the stent (usually day 7-12 post-transplant) and the indwelling catheter. There is no consensus and very little literature concerning the optimal stent duration for ES usage (10). In contrast, using a DJ, the indwelling catheter usually can be removed in the first postoperative week, and the patient may be discharged. The DJ stent is removed weeks after transplantation via cystoscopy, usually in the outpatient setting. There is a large variation in DJ stent duration, usually weeks longer than with ES (11). Overall, as compared to ES, using a DJ contributed to a significant reduction in length of hospital stay and healthcare related costs (12–15). However with the longer duration of the stent, the risk of vesicoureteric reflux and cystoscopic removal may result in more UTIs (6, 11).
Only a few studies comparing the use of a DJ versus (vs.) an ES in kidney transplantation have been performed, using a variety of definitions and presenting varying results regarding incidence of UC and UTI (16–19). In September 2014 our kidney transplantation center changed the intra-operative vesicoureteric anastomosis stenting protocol from using an ES to using a DJ, aiming to reduce post-transplant hospital stay and costs without affecting short- and long term transplant outcomes. The current analysis aimed to clarify whether the change from an ES to a DJ stenting protocol, has indeed resulted in comparable results with respect to UCs, UTIs, patient- and graft outcomes. Furthermore, we aimed to identify possible risk factors for UCs and UTIs in the first months after kidney transplantation.
Materials and methods
Patients
We performed a retrospective analysis of all consecutive adult kidney transplant recipients between September 2012 and December 2016 at the University Medical Center Groningen, the Netherlands. Data were obtained using a local registry of all kidney transplant recipients providing the baseline characteristics and kidney transplant outcomes. The data obtained from the patient medical records, contained the type of ureteric stents, indwelling catheter, UCs and UTIs. At admission for kidney transplantation, patients had given consent for using their data for future research and our local Medical Ethical Board waived the need for further consent. All data were anonymized and filed for analyses.
Transplant procedure
All kidney transplantations were performed by a team of experienced transplant surgeons. The kidney allograft was placed in the right or left iliac fossa via an extra-peritoneal approach. The vesicoureteric anastomosis was performed using a modified extravesical Lich-Gregoir technique or a full thickness technique. From September 2012 until September 2014 all kidney transplant recipients received an ES peri-operative. An 8-French, 125 cm enteral feeding tube used as splint, was placed in the graft pyelum and drained externally suprapubic through the bladder wall, hereby stenting the vesicoureteric anastomosis. The ES was removed by gently pulling between 7-12 days post-operative (depending on tightness of the ES and transplant function), without antibiotic prophylaxis. The peri-operatively placed indwelling catheter was usually removed 1-2 days after ES removal and patients were regularly checked for adequate bladder evacuation afterwards. Unto successful indwelling catheter removal, patients stayed in the hospital.
From September 2014 onwards the stenting protocol changed with a transition period of 3 months. In the new protocol a 7-French, 16 cm, internal double J stent was placed peri-operatively, draining from the graft pyelum to the bladder. Based on patient characteristics, like medical history and presence of diuresis, and surgical conditions a ES could still be used. The indwelling catheter was removed on day 5 post-operative and patients were regularly checked for adequate bladder evacuation afterwards. Patients stayed in hospital until successful indwelling catheter removal. The DJ stent was removed 3-4 weeks post-operative at the outpatient clinic via cystoscopy. Around cystoscopic DJ stent removal, patients received antibiotic prophylaxis with ciprofloxacin 500mg twice daily, starting 12 hours before unto 24 hours after removal.
The standard immunosuppressive regimen consisted of prednisolone, mycophenolate mofetil and tacrolimus or ciclosporin. All patients received induction immunosuppressive therapy with the IL-2 receptor blocker Basiliximab and perioperative antibiotic prophylaxis with a single dose of cefazoline 2000mg. During the first 6 months after transplantation patients received prophylaxis against Pneumocystis jiroveci with co-trimoxazole 400/80 mg once daily.
Outcomes
Primary outcomes were the occurrence of UCs and/or UTIs during the first six months after kidney transplantation. UCs were defined as urinary leakage or ureter stenosis requiring an intervention (surgical, percutaneous and/or stent placement). Bladder retention, defined as failure of adequate bladder evacuation requiring indwelling catheter placement or intermittent self-catheterization, was not defined as UC because it is not considered a complication related to stenting of the vesicoureteric anastomosis. A UTI was defined as the clinical suspicion of UTI with leukocyturia and/or a positive urinary culture (≥105 CFU/ml) and requiring antibiotic treatment, excluding asymptomatic bacteriuria (ASB). A non-invasive UTI was defined as a UTI without signs or symptoms of tissue invasion and/or bacteremia.
Secondary outcomes were graft function after 6 months [defined as creatinine clearance (ml/min) and proteinuria (g/24h)], and delayed graft function (defined as need for dialysis in the first week after transplantation, with the exception of dialysis to treat immediate post-operative hyperkalemia without further need for kidney replacement therapy during the first week).
Statistical analysis
Categorical data are presented as number and percentages, continuous data as median and interquartile range (IQR) or mean and standard deviation (depending on data distribution). To compare continuous variables, unpaired t-test or Mann-Whitney U test were used when appropriate. The Chi-square test, Fisher’s exact test or Fisher-Freeman-Halton test were used to compare proportions. Time to occurrence of UTI and UC were analyzed with Kaplan-Meier curves and Log Rank test was used to calculate the Hazard ratio (HR) and the 95% confidence interval (CI) to compare ES and DJ. Univariate analyses were performed to compare groups with and without UTI and UC. Multivariate binary backward and forward LR logistic regression was performed with the use of ES or DJ and additional variables with a p-value ≤0.1 by univariate analysis. Thereafter the binary logistic regression was repeated with method enter with the remaining significant variables and the use of ES or DJ to identify the possible influence of the used type of stent on the occurrence of UTI and UC. Multiple imputation (5 times) was performed for missing values and binary logistic regression was repeated with the imputed data to verify that the missing values did not influence the outcome. All reported p-values are two tailed and a p-value <0.05 was considered statistically significant. Analyses were conducted with SPSS version 23 (IBM corp., Armonk, USA) and GraphPad Prism version 9 (GraphPad Software, La Jolla, USA).
Results
Patient characteristics
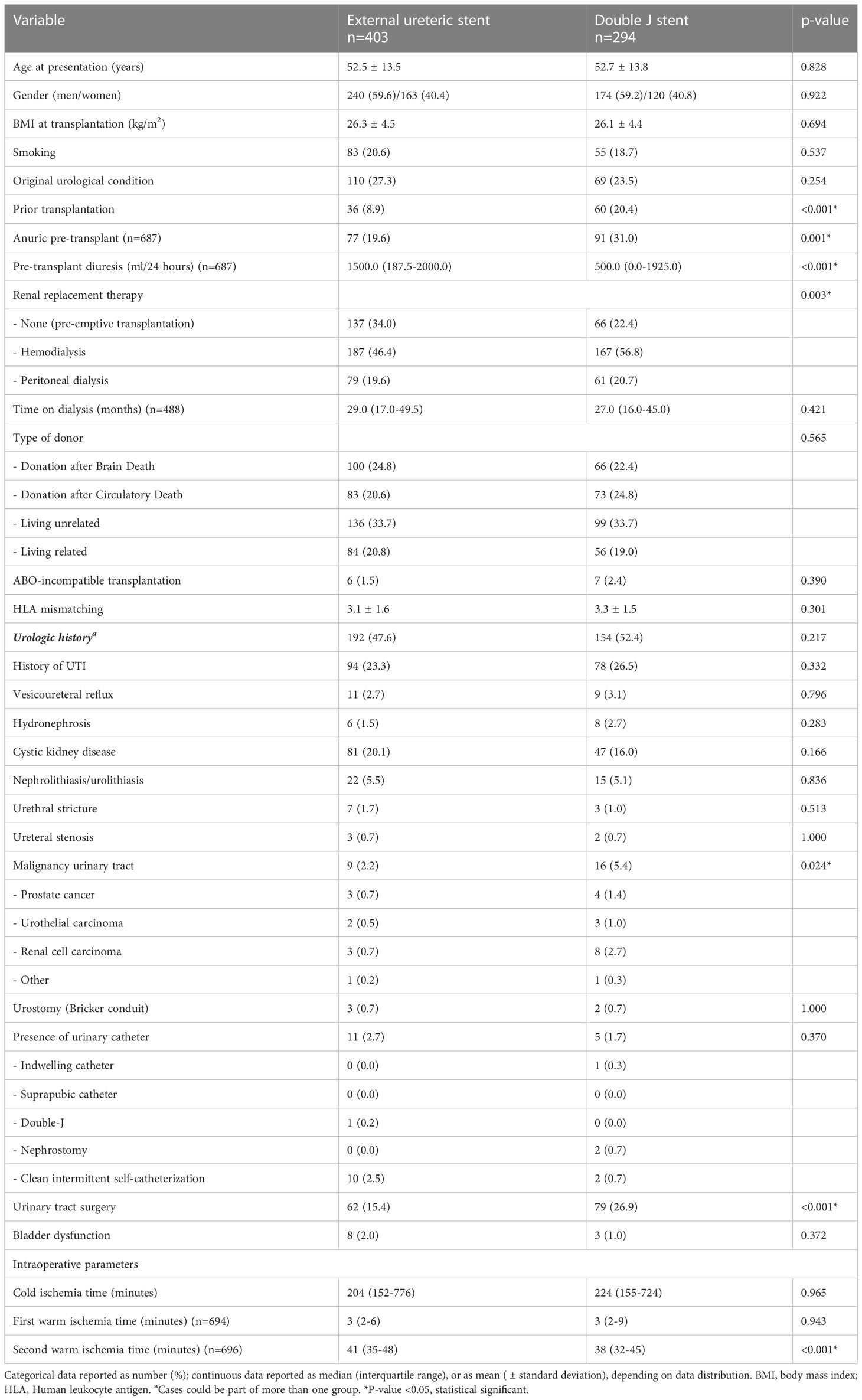
During the inclusion period, 711 consecutive kidney transplants were performed of which 14 were excluded due to graft loss ≤1 week after transplantation. Of the remaining 697 kidney transplants, 13 were lost to follow-up for UC as outcome (deceased n=7, transplantectomy n=4, transplant failure without transplantectomy n=2) and 10 were lost to follow-up for UTI as outcome (deceased n=4, transplantectomy n=4, transplant failure without transplantectomy n=2). In 403 (57.8%) patients an ES was placed during the transplant procedure and in 294 (42.2%) patients a DJ. After December 2014, in 107 cases a ES was used due to patient and/or surgical characteristics. The mean recipient age at transplantation was 53 years for both groups and 40% were women. Patient characteristics of the ES and DJ group were mostly similar (Table 1). Pre-transplant diuresis volume was higher in the ES group (ES 1500 (IQR 187.5-2000) ml/24 hours, DJ 500 (IQR 0-1925) ml/24 hours; p<0.001) as was the number of pre-emptive transplantation (ES 33.3% vs. DJ 22.4%). There was no difference in donor type or presence of pre-transplant urologic history (ES 47.6% vs. DJ 52.4%; p=0.217).
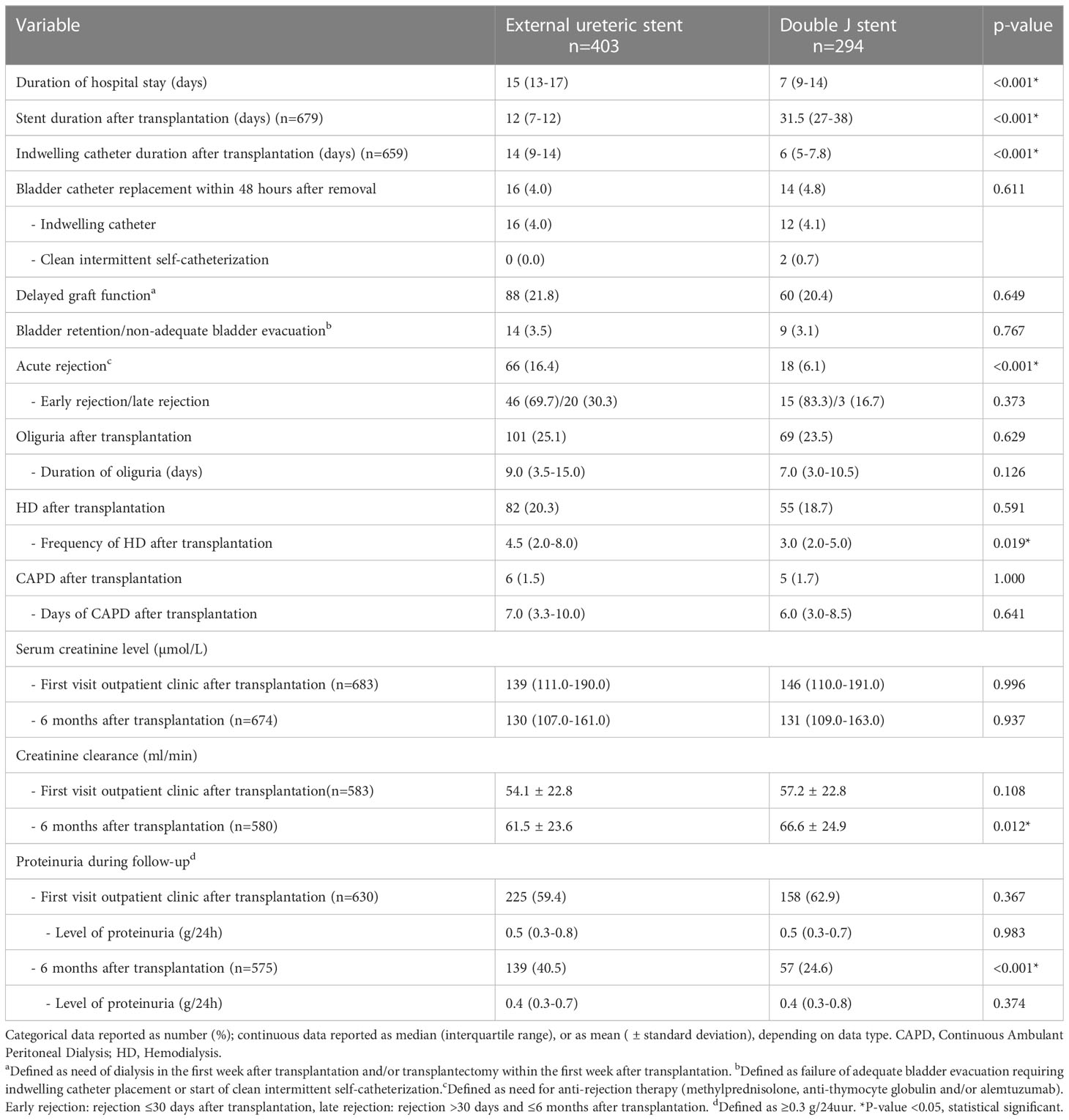
A reduction in hospital stay after kidney transplantation was seen after the change in protocol, median of 7 (IQR 9-14) days after DJ placement and a median of 15 (IQR 13-17) days after ES placement (p<0.001). As expected, duration of stent placement was longer in the DJ group compared to the ES group (31.5 days vs. 12 days; p<0.001). With respect to clinical outcome (6 months follow-up), no clear differences were found in the occurrence of bladder retention (ES 3.5% vs. DJ 3.1%; p=0.767) and serum creatinine (Table 2). Calculated creatinine clearance showed a minor but clinical not relevant difference between ES and DJ (61.5 ml/min vs. 66.6 ml/min). Acute rejection was seen more frequently in the ES group (16.4%) compared to the DJ group (6.1%).
Urological complications
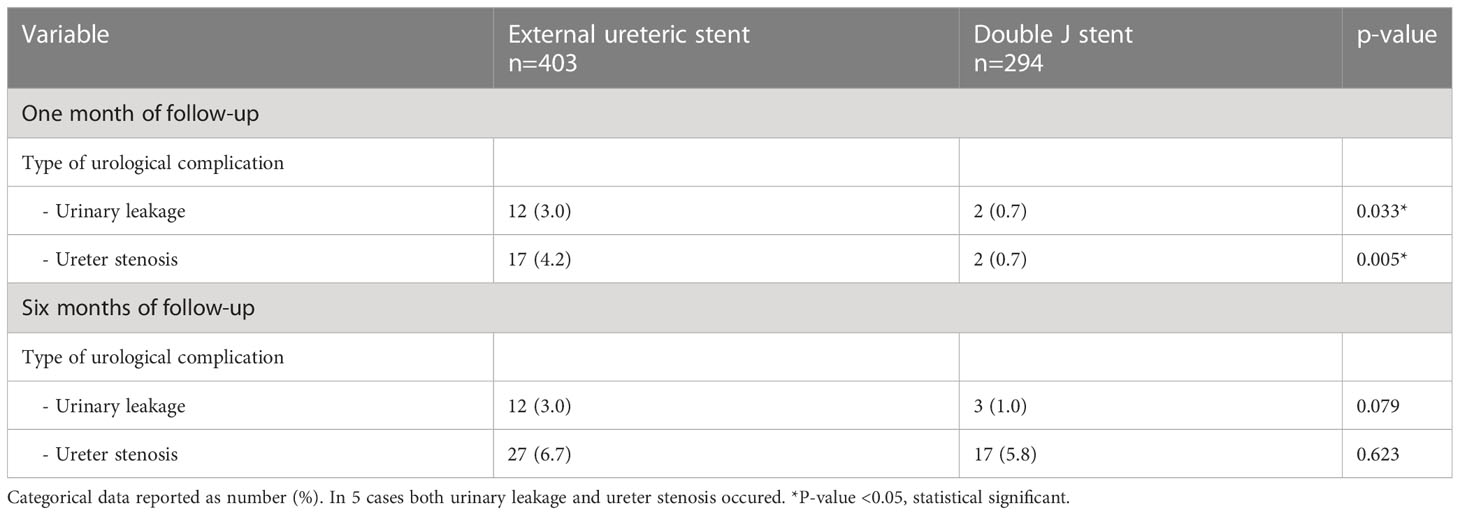
The occurrence of an UC requiring intervention within 6 months after transplantation was similar between the ES group and DJ group; 34 (8.4%) vs. 20 (6.8%) patients, p=0.389, HR 1.27 (95% CI 0.74-2.18) (Figure 1). Also, no differences existed between the incidence of urinary leakage and ureter stenosis (Table 3). In both the ES and DJ group, the majority of the UCs occurred in the first month after transplantation, but there still was a significant difference in UC occurrence in favor of DJ (ES 24 (6.0%) vs. DJ 4 (1.4%) patients, p=0.002, HR 4.48 (95% CI 2.12-9.48) (Supplemental Figure 1). After one month of follow-up there was a clear lower incidence of urinary leakage and ureter stenosis in the DJ group. In addition, almost all urinary leakages occurred in the first month after transplantation (Table 3).
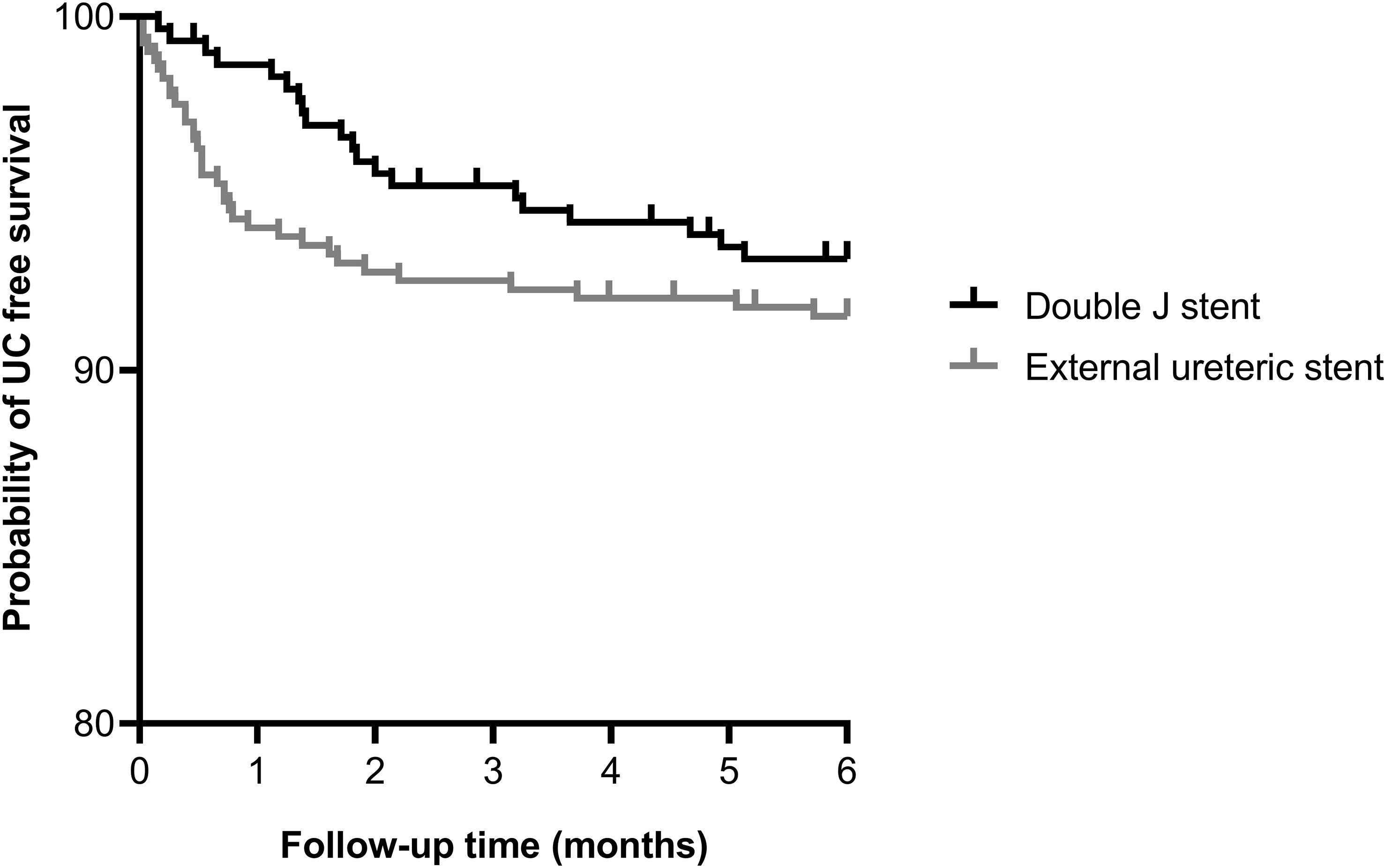
Figure 1 Kaplan-Meier curve showing the probability of urological complication free survival within 6 months after kidney transplantation for external ureteric stent (n=403, UC n=34) and double J stent (n=294, UC n=20); HR 1.27 (95% CI 0.74-2.18), p=0.389. Thirteen cases were censored before an urological complication occurred.
In univariate analysis, multiple risk factors for developing an UC within 6 months after kidney transplantation were identified (Table 4). These factors mainly relate to kidney donor quality and posttransplant urinary flow. In both groups no relation existed between duration (days) of stent placement and the occurrence of UC. In 5 kidney transplant procedures both an acute rejection and a UC occurred, in 4 (80%) the acute rejection occurred prior to the UC. In 1 (20%) case the acute rejection was detected after the UC and if this case was defined as no acute rejection the difference between those with and without UC in acute rejection remains unchanged. Both backward and forward logistic regression analysis, with the occurrence of UC as dependent outcome, failed to show the independent contribution of ≥1 variable, whether ES/DJ use was factored in or not. The use of imputed data did not change these results.
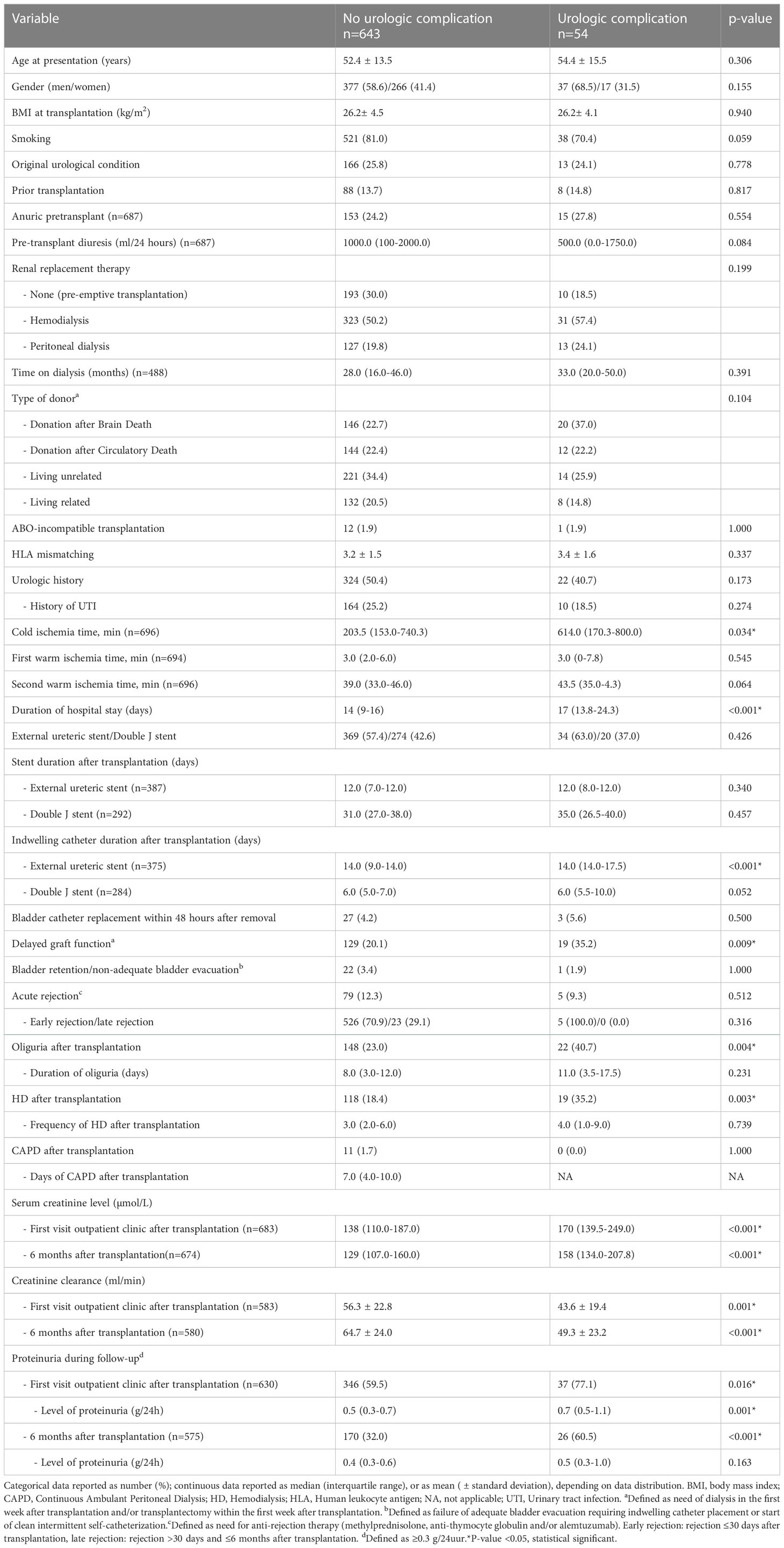
Table 4 Analysis of risk factors for an urologic complication within 6 months after kidney transplantation.
Urinary tract infections
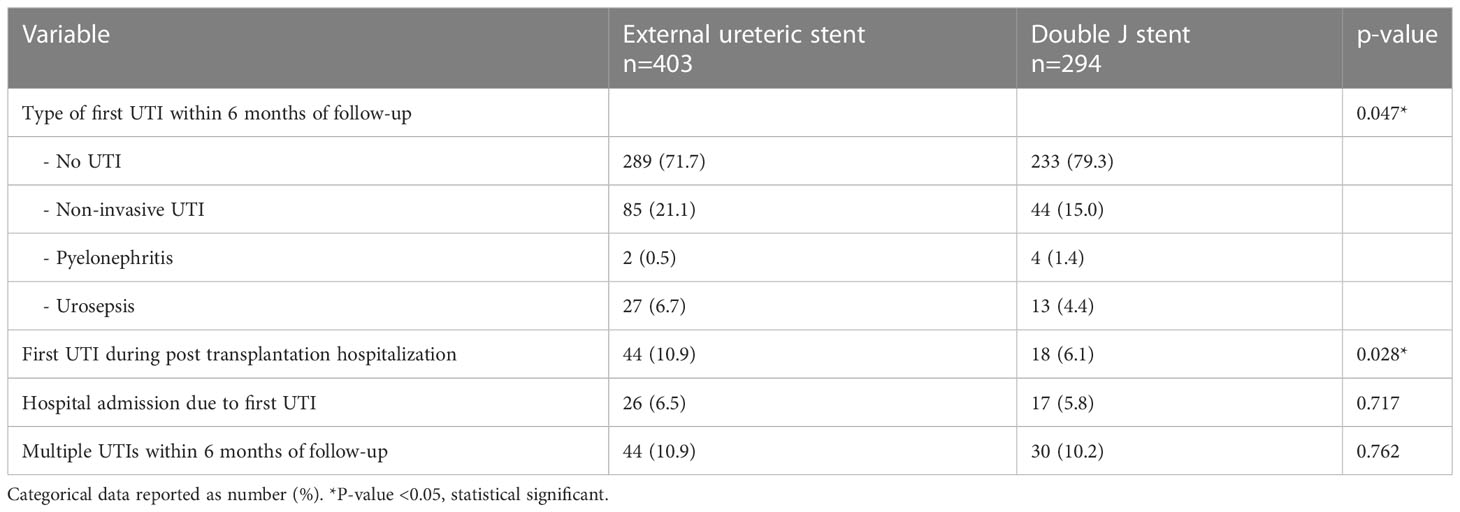
In the ES group more patients experienced at least one UTI within the first 6 months after kidney transplantation compared to the DJ group: 114 (28.3%) vs. 61 (20.7%) patients, p=0.023, HR 1.43 (95% CI 1.06-1.93) (Figure 2). Both non-invasive UTIs and invasive UTIs were more common in the ES group. The occurrence of multiple UTIs within 6 months of follow-up did not differ between ES and DJ (Table 5). No differences in urine diagnostic test results of the first UTI episode between ES and DJ existed; in both groups UTI was urine culture proven in around 86% (data not shown). The number of UTIs per month (expressed as total amount of UTI’s per person divided by the duration of the follow-up period) did not differ between ES and DJ (0.08 UTI/month vs. 0.07 UTI/month). In the UTI group, in 13.8% the first UTI occurred before the ES was removed, while in the DJ group in 60.0% the first UTI occurred before the DJ was removed. A UTI during post-transplantation hospitalization was more common in the ES group, probably influenced by longer hospitalization and indwelling catheter placement as per protocol (ES 10.9% vs. DJ 6.1%, p=0.028). At one month of follow-up, the first UTI occurred in 85 cases with a comparable incidence between ES and DJ; 56 (13.9%) vs. 29 (9.9%), p=0.109, HR 1.44 (95% CI 0.94-2.21) (Supplemental Figure 2). Between one and six months of follow-up, the first UTI occurred in 90 cases (ES n=58 and DJ n=32).
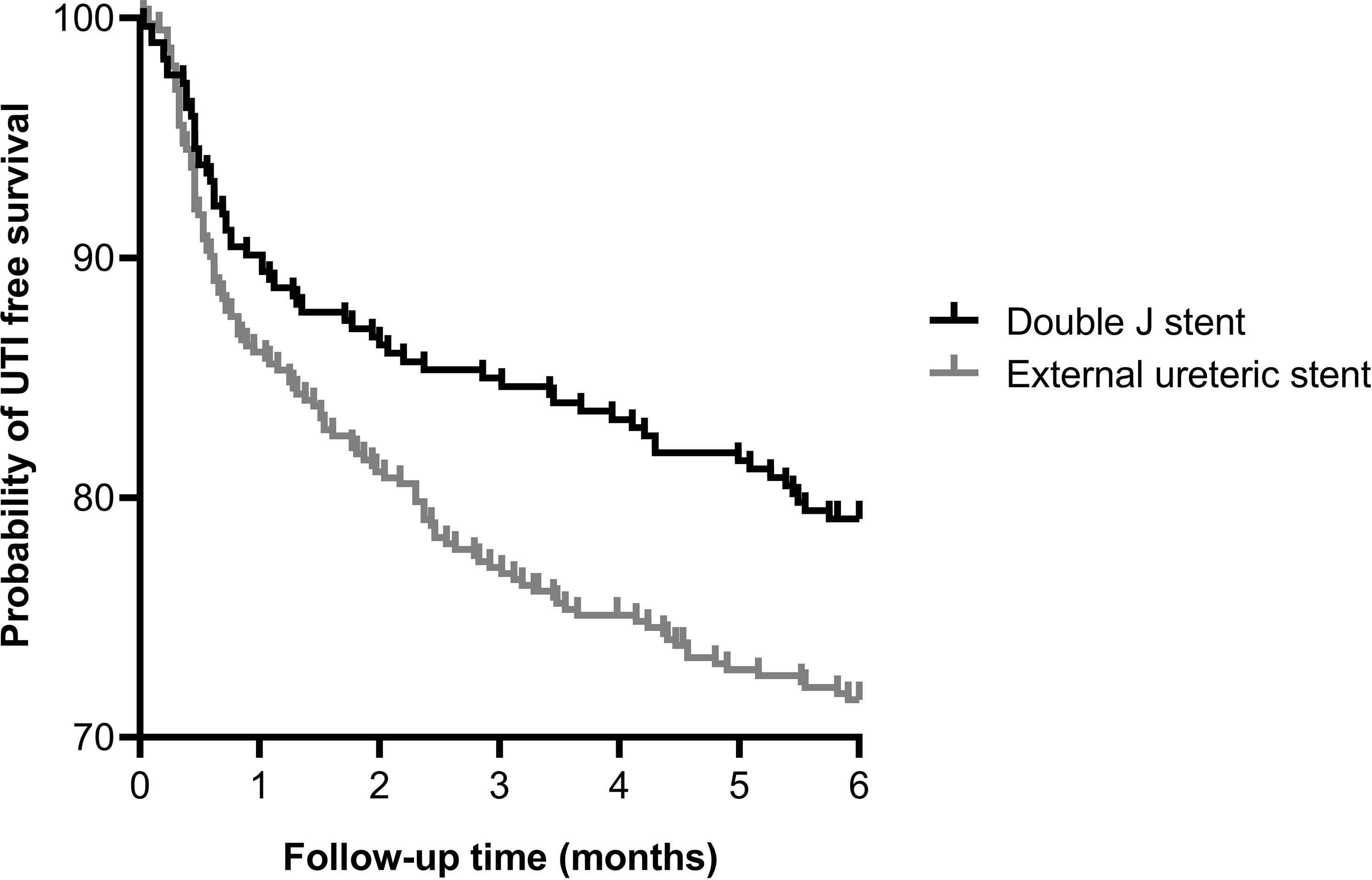
Figure 2 Kaplan-Meier curve showing the probability of UTI free survival within 6 months after kidney transplantation for external ureteric stent (n=403, UTI n=114) and double J stent (n=294, UTI n=61); HR 1.43 (95% CI 1.06–1.93), p=0.023. Ten cases were censored before a urinary tract infection occurred.
In univariate analysis, multiple factors associated with the development of a UTI within 6 months after kidney transplantation were identified (Table 6). In both the ES and DJ group no relation was found between duration (days) of stent placement and the occurrence of a UTI. In 34 kidney transplant procedures both an acute rejection and a UTI occurred. In 27 (79.4% the acute rejection occurred prior to the first UTI and in 7 (20.6%) the acute rejection was detected after the first UTI. If these 7 cases are defined as no acute rejection the difference between those with and without UTI in acute rejection remains significant. Urological complications were more common in the UTI group compared to the no UTI group (17.1% vs. 4.6%, p<0.001). In 30 kidney transplant procedures both a UC and a UTI occurred within 6 months after transplantation. In 21 (70%) cases the UC was discovered prior to the first UTI and in 9 cases the UC was detected simultaneously with or shortly after a UTI. If these 9 cases are defined as no UC the difference between those with and without UTI in UC remains significant. In the final multivariate logistic regression model the type of stenting procedure, ES or DJ, remained significantly associated with the occurrence of an UTI (OR 1.69 (1.15-2.50), p=0.008). Other factors were age, gender, history of UTI, indwelling catheter replacement within 48 hours after removal, and occurrence of urological complications during follow-up (Table 7). The use of imputed data did not change these results.
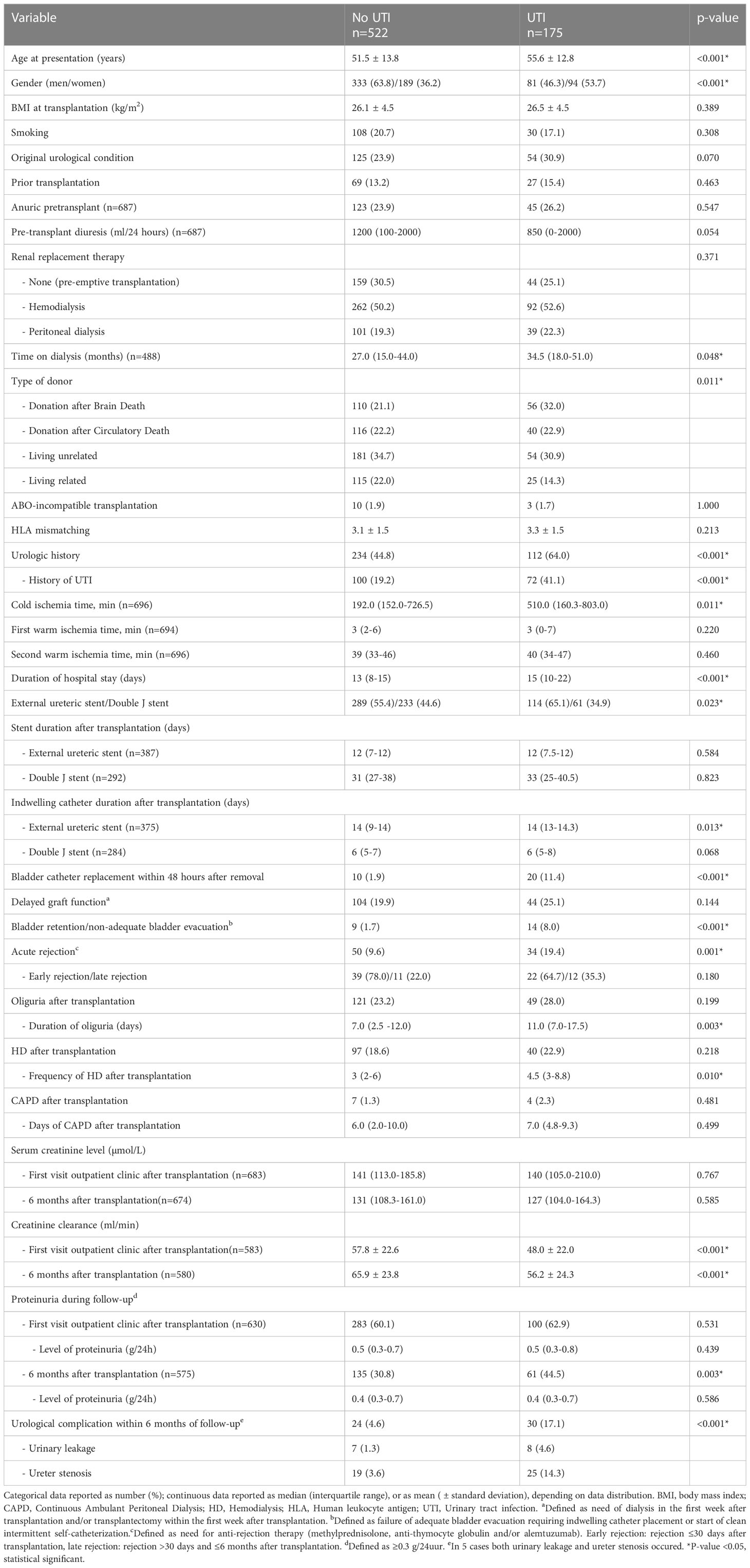
Table 6 Analysis of risk factors for a urinary tract infection within 6 months after kidney transplantation.
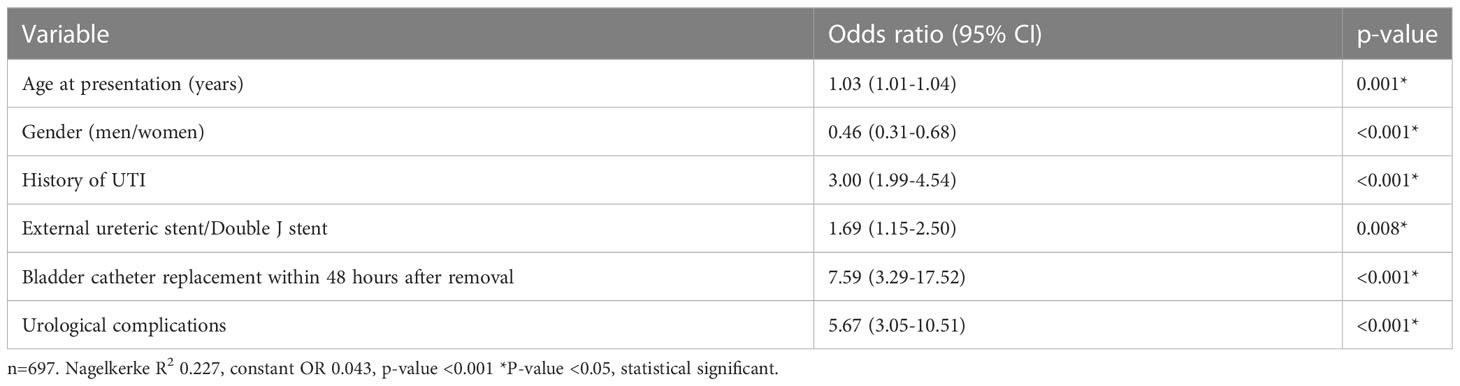
Table 7 Multivariate analysis of factors associated with a urinary tract infection within 6 months after transplantation.
Discussion
Prophylactic stenting of the vesicoureteric anastomosis in kidney transplantation is nowadays standard practice in most kidney transplant centers. There is no consensus on the optimal stenting type and duration (10, 11, 16–19). We compared the outcomes on UC and UTI incidence between a DJ and ES stenting protocol.
Our reported UC rates are 8.5% in the ES group vs. 6.8% in the DJ group. Previous research has shown comparable incidences ranging from 0.3-25.0% for ES vs. 0.0-5.4% for DJ (16–19). For UTI incidence, the reported rates in the literature are ranging from 12.5-41.4% for ES vs. 7.6-51.5% for DJ (16–18). Our cohort showed an UTI rate of 28.4% in the ES group vs. 20.9% in the DJ group, both are in the range of previous studies. Compared to the literature, all patients in our analysis were transplanted using the same immunosuppressive regimen, including Basiliximab induction. All patients received co-trimoxazole prophylaxis during the first 6 months after transplantation, whether this contributed to our UTI incidence is questionable. In the only randomized study performed, standard co-trimoxazole prophylaxis had no significant influence on UTI occurrence post-transplantation (20).
The ES group showed significant more UCs in the first month after transplantation compared to the DJ group. After the first month, there is a comparable incidence of UC over the follow-up time. Earlier research also showed that the majority of the UCs occur in the first 4 weeks after transplantation (21). Shorter stent duration in the ES group, 12 days vs. 31.5 days in DJ, could have led to increased early UCs. But, this will not have influenced our results, because each group within our analysis showed no association of stent duration and UC, nor was any other clinical relevant risk factor for UC identified.
Patients in the ES group experienced more UTIs, especially during the first month postoperative, which may be due to the longer presence of the indwelling catheter compared to DJ use and an extra exterior connection of the urinary tract through the percutaneous position of the ES. This is in line with literature, stating that the duration an indwelling catheter is in situ is a risk factor for the development of bacteriuria, especially starting 1-2 weeks after placement (22, 23). Multivariate analysis showed that using an ES and UC are significant and independent risk factors for the occurrence of UTI. Since our data showed more UCs in the ES group, especially in the first month, this could also explain the higher UTI rate in this group. Patients in the DJ group more often had an urological history prior to transplantation, but this was not identified as a risk factor for UTIs. A pre-transplant history of UTIs proved to be a risk factor for post-transplantation UTI. Although there were slightly more patients with a history of UTI in the DJ group, they showed better outcomes on UTIs compared to ES. Likewise older age and female gender were associated with a UTI, but these factors did not influence the difference in UTI between the ES and DJ, since there were no baseline differences between both groups.
In our cohort DJ stent removal was performed after a median of 31.5 days (27–38), which is longer compared to earlier studies (11). The longer duration could potentially have led to more UTIs (24). Although around 60% of the first UTIs in the DJ group occurred before stent removal, our analysis failed to show a relation between stent duration and the occurrence of UTI. In addition, the incidence of UTI was lower in the DJ group as compared to the ES group, despite the fact that the stent was a substantially shorter period in situ in the latter group. Almost all our DJ stent removal procedures were done with antibiotic prophylaxis whereas ES removal is done without, which could have led to more UTIs in the ES group. The prophylaxis only consisted of a short course of antibiotics; therefore we assume there is no or minor influence on UTI incidence.
Although patients with an ES experienced (slightly) more UCs and UTIs, there were no clinical relevant differences in graft outcome 6 months after kidney transplantation. Creatinine clearance was slightly, but significantly, lower in the ES group and in these patients proteinuria was more often present. The higher rates of UCs and UTIs in the ES group could have led to these small, but clinically irrelevant differences (25, 26). Interestingly, there was a significant higher incidence of acute allograft rejection in the ES group, most often in the first 30 days after transplantation, which may also have led to the small differences in graft outcomes at 6 months. We were unable to identify a clear cause for this difference in rejection episodes, as baseline immunological characteristics were comparable between the ES and DJ group. It has been suggested that UCs and UTIs may have a role in triggering immunological phenomena like allograft rejection (27, 28) and vice versa, acute rejection is also a risk factor for early UCs (20). Our analysis showed no association between acute rejection and incidence of UC or UTI. The unexplained increase in acute rejection episodes when using an ES has been reported by others and may also have something to do with more selective monitoring of graft urinary output leading to more biopsies in the early post-operative phase (16, 19, 29).
This study has a few strengths and limitations that need to be addressed. A major strength is the comparable circumstances and baseline characteristics between the two groups. Second, we described two large, representative groups with a longer follow-up compared to previous published cohorts. A limitation of our study is the retrospective design. Although we reviewed all the patients charts and used our local kidney transplantation database, it could be that the UCs and UTIs are not registered correctly. In contrast to other studies, we excluded patients with ASB as having a UTI since there is no need for treatment. This could have led to better results on UTI occurrence, but should not have influenced patient- and graft outcomes. A recent systematic review showed that after kidney transplantation untreated ASB vs. treated ASB have the same outcomes regarding graft function and risk of getting a symptomatic UTI (30). Lastly, because we used data from several years, it could be that modernization of healthcare techniques and improved quality of care has led to better results for the DJ group.
Overall, using a DJ, compared to an ES, for stenting the vesicoureteric anastomosis after kidney transplantation, leads to significantly less urinary tract infections and a shorter hospitalization period post-transplantation which reduces healthcare related costs. Incidence of urological complications was comparable for both groups. There is no indication that a DJ performs worse compared to an ES with regards to graft outcomes. Our data suggests that use of a DJ is the preferred technique for stenting the vesicoureteric anastomosis during kidney transplantation.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
IH participated in data collection, database editing, data analysis and manuscript writing. SM participated in research design, performance of research, data collection, database editing, data analysis, and manuscript writing. AJ, RP, and J-SS participated in research design and manuscript editing. JR participated in data collection and manuscript editing. BD participated in manuscript editing. CS participated in research design, data collection, database editing, data analysis and manuscript editing. IH and SM contributed equally as first author. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneph.2023.1130672/full#supplementary-material
Supplementary Figure 1 | Kaplan-Meier curve showing the probability of urological complication free survival within 1 month after kidney transplantation for external ureteric stent (n=403, UC n=24) and double J stent (n=294, UC n=4); HR 4.48 (95% CI 2.12-9.48), p=0.002. Four cases were censored before an urological complication occurred.
Supplementary Figure 2 | Kaplan-Meier curve showing the probability of UTI free survival within 1 month after kidney transplantation for external ureteric stent (n=403, UTI n=56) and double J stent (n=294, UTI n=29); HR 1.44 (95% CI 0.94-2.21), p=0.109. Three cases were censored before a urinary tract infection occurred.
Abbreviations
ASB, asymptomatic bacteriuria; BMI, body mass index; CAPD, continuous ambulant peritoneal dialysis; CI, confidence interval; DJ, double J stent; ES, external ureteric stent; HD, hemodialysis; HLA, human leukocyte antigen; HR, hazard Ratio; IQR, interquartile range; NA, not applicable; OR, odds ratio; UC, urological complication; UTI, urinary tract infection; vs, versus.
References
1. Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med (1999) 341:1725–30. doi: 10.1056/NEJM199912023412303
2. Thomalla JV, Leapman SB, Filo RS. The use of internalized ureteric stents in renal transplant recipients. Br J Urol (1990) 66:363–68. doi: 10.1111/j.1464-410x.1990.tb14955.x
3. Rigg KM, Proud G, Ross Taylor RM. Urological complications following renal transplantation. Transpl Int (1994) 7:120–26.
4. Kayler L, Kang D, Molmenti E, Howard R. Kidney transplant ureteroneocystotomy techniques and complications: review of the literature. Transplant Proc (2010) 42:1413–20. doi: 10.1016/j.transproceed.2010.04.016
5. Mangus RS, Haag BW. Stented versus nonstented extravesical ureteroneocystostomy in renal transplantation: a metaanalysis. Am J Transplant (2004) 4:1889–96. doi: 10.1111/j.1600-6143.2004.00595.x
6. Wilson CH, Rix DA, Manas DM. Routine intraoperative ureteric stenting for kidney transplant recipients (Review). Cochrane Database Syst Rev (2013) 6:CD004925. doi: 10.1002/14651858.CD004925.pub3
7. Abbot KC, Swanson SJ, Richter ER, Bohen EM, Agodoa LY, Peters TG, et al. Late urinary tract infection after renal transplantation in the united states. Am J Kidney Dis (2004) 44:353–62. doi: 10.1053/j.ajkd.2004.04.040
8. Karuthu S, Blumberg EA. Common infection in kidney transplant recipients. Clin J Am Soc Nephrol (2012) 7:2058–70. doi: 10.2215/CJN.04410512
9. De Souza RM, Olsburgh J. Urinary tract infection in the renal transplant patient. Nat Clin Pract Nephrol (2008) 4:252–64. doi: 10.1038/ncpneph0781
10. Minnee R, Bemelman FJ, Laguna Pes PP, ten Berge IJM, Legemate DA, Idu MM. Effectiveness of a 5-day external stenting protocol on urological complications after renal transplantation. World J Surg (2009) 33:2722–26. doi: 10.1007/s00268-009-0224-y
11. Visser IJ, van der Staaij JPT, Muthusamy A, Willicombe M, Lafranca JA, Dor FJMF. Timing of ureteric stent removal and occurrence of urological complications after kidney transplantation: a systematic review and meta-analysis. J Clin Med (2019) 8:689–704. doi: 10.3390/jcm8050689
12. Bruintjes MHD, Langenhuijsen JF, Kusters A, Hilbrands LB, d’Ancona FCH, Warlé MC. Double J stent is superior to externally draining ureteric stent in enhancing recovery after kidney transplantation – a prospective cohort study. Int J Surg (2019) 71:175–81. doi: 10.1016/j.ijsu.2019.09.031
13. DuBay DA, Lynch R, Cohn J, Ads Y, Punch JD, Pelletier SJ, et al. Is routine ureteral stenting cost-effective in renal transplantation? J Urol (2007) 178:2509–13. doi: 10.1016/j.juro.2007.08.037
14. Tavakoli A, Surange RS, Pearson RC, Parrott NR, Augustine T, Riad HN. Impact of stents on urological complications and health care expenditure in renal transplant recipients: results of a prospective, randomized clinical trial. J Urol (2007) 177:2260–64. doi: 10.1016/j.juro.2007.01.152
15. Mangus RS, Haag BW, Carter CB. Stented lich-gregor. ureteroneocystostomy: case series report and cost-effectiveness analysis. Transplant Proc (2004) 36:2959–61. doi: 10.1016/j.transproceed.2004.10.061
16. Fockens M, Alberts V, Bemelman FJ, Pilar Laguna Pes M, Idu MM. Internal or external stenting of the ureterovesical anastomosis in renal transplantation. Urol Int (2016) 96:152–56. doi: 10.1159/000440702
17. Jakob M, Strupler N, Candinas D, Huynh-Do U, Beldi G. Externalized percutaneous stent versus internal double J stent: short- and long-term complications after kidney transplantation. Transplant Proc (2018) 50:3416–21. doi: 10.1016/j.transproceed.2018.04.042
18. Vogel T, Utech M, Schmidt F, Holscher Keplin W, Diller R, Brockmann J, et al. Double-J versus external ureteral stent in kidney transplantation: a retrospective analysis. Nephro Urol Mon (2015) 4:e27820. doi: 10.5812/numonthly.27820
19. Gomes G, Nunes P, Castelo D, Parada B, Patrao R, Bastos C, et al. Ureteric stent in renal transplantation. Transplant Proc (2013) 45:1099–101. doi: 10.1016/j.transproceed.2013.02.086
20. Singh R, Bemelman FJ, Hodiamond CJ, Idu MM, ten Berg IJM, Geerlings SE. The impact of trimethoprim-sulfamethoxazole as Pneumocystis jiroveci pneumonia prophylaxis on the occurrence of asymptomatic bacteriuria and urinary tract infections among renal allograft recipients: a retrospective before-after study. BMC Infect Dis (2016) 16:90–9. doi: 10.1186/s12879-016-1432-3
21. Minnee RC, Surachno S, Kox C, ten Berge IJM, Aronson DC, Idu MM. Is a selective splinted ureterocystostomy protocol feasible in renal transplantation? an analysis of 475 renal transplantations. Transpl Int (2006) 19:558–62. doi: 10.1111/j.1432-2277.2006.00313.x
22. Hooton TM, Bradley SF, Cardenas DD, Colgan R, Geerlings SE, Rice JC, et al. Diagnosis, prevention and treatment of catheter-associated urinary tract infection in adults: 2009 international clinical practice guidelines from the infectious disease society of America. Clin Infect Dis (2010) 50:625–63. doi: 10.1086/650482
23. Nicolle LE. Urinary catheter associated infections. Infect Dis Clin N Am (2012) 26:13–27. doi: 10.1016/j.idc.2011.09.009
24. Cai JF, Wang W, Hao W, Sun SJ, Su LL, Li X, et al. Meta-analysis of early versus late ureteric stent removal after kidney transplantation. Transplant Proc (2018) 50:3411–15. doi: 10.1016/j.transproceed.2018.08.033
25. Pesce F, Martino M, Fiorentino M, Rollo T, Simone S, Gallo P, et al. Recurrent urinary tract infections in kidney transplant recipients during the first-year influence long-term graft function: a single-center retrospective cohort study. J Nephrol (2019) 32:661–68. doi: 10.1007/s40620-019-00591-5
26. Warzyszynska K, Zawistowski M, Karpeta E, Dziewa N, Kosieradzki M. How early postoperative urinary tract infections affect renal graft function at 1-year follow-up. Transplant Proc (2020) 50:2403–08. doi: 10.1016/j.transproceed.2020.03.033
27. Pelle G, Vimont S, Levy PP, Hertig A, Ouali N, Chassin C, et al. Acute pyelonephritis represents a risk factor impairing long-term kidney graft function. Am J Transplant (2007) 7:899–907. doi: 10.1111/j.1600-6143.2006.01700.x
28. Audard V, Amor D, Devaux D, Pastural M, Baron C, Philippe R, et al. Acute graft pyelonephritis: a potential cause of acute rejection in renal transplant. Transplantation (2005) 80:1128–30. doi: 10.1097/01.tp.0000174343.05590.9f
29. Ooms LSS, Spaans LG, Betjes MGH, IJzermans JNM, Terkivatan T. Minimizing the number of urological complications after kidney transplant: a comparative study of two types of external ureteral stents. Exp Clin Transplant (2017) 2:143–49. doi: 10.6002/ect.2016.0051
Keywords: vesicoureteric anastomosis, external ureteric stent, double J stent, urinary tract infection, urological complication
Citation: Hazenberg IT, Middelkoop SJM, de Joode AAE, Rabbeljee JD, Pol RA, Doornweerd BHJ, Sanders J-SF and Stegeman CA (2023) External ureteric stent versus internal double J stent in kidney transplantation: a retrospective analysis on the incidence of urological complications and urinary tract infections. Front. Nephrol. 3:1130672. doi: 10.3389/fneph.2023.1130672
Received: 23 December 2022; Accepted: 13 April 2023;
Published: 16 May 2023.
Edited by:
Masoud Sadeghi, Islamic Azad University, IranReviewed by:
Miguel Silva-Ramos, Centro Hospitalar Universitário do Porto, PortugalJeffrey J. Gaynor, University of Miami, United States
Copyright © 2023 Hazenberg, Middelkoop, de Joode, Rabbeljee, Pol, Doornweerd, Sanders and Stegeman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ietje T. Hazenberg, aS50LmhhemVuYmVyZ0B1bWNnLm5s
†These authors share first authorship
 Ietje T. Hazenberg
Ietje T. Hazenberg Stephanie J. M. Middelkoop1†
Stephanie J. M. Middelkoop1† Robert A. Pol
Robert A. Pol Jan-Stephan F. Sanders
Jan-Stephan F. Sanders Coen A. Stegeman
Coen A. Stegeman