- 1Department of Internal Medicine, Faculty of Medicine, Alexandria University, Alexandria, Egypt
- 2Department of Clinical and Chemical Pathology, Faculty of Medicine, Alexandria University, Alexandria, Egypt
Introduction: Lupus is a diverse autoimmune disease with autoantibody formation. Lupus nephritis carries a grave prognosis. Complement involvement, namely, C1q deficiency, is linked to activity and renal involvement and could help in their assessment. LN therapies include plasma exchange, immune adsorption, and probably hemodiafiltration with online endogenous reinfusion (HFR), together with traditional immunosuppressive therapies.
Aim: The aim of this study was to evaluate the role of HFR in improving signs and symptoms of systemic lupus erythematosus (SLE) activity and laboratory parameters in cases not responding to traditional immunosuppressive therapy.
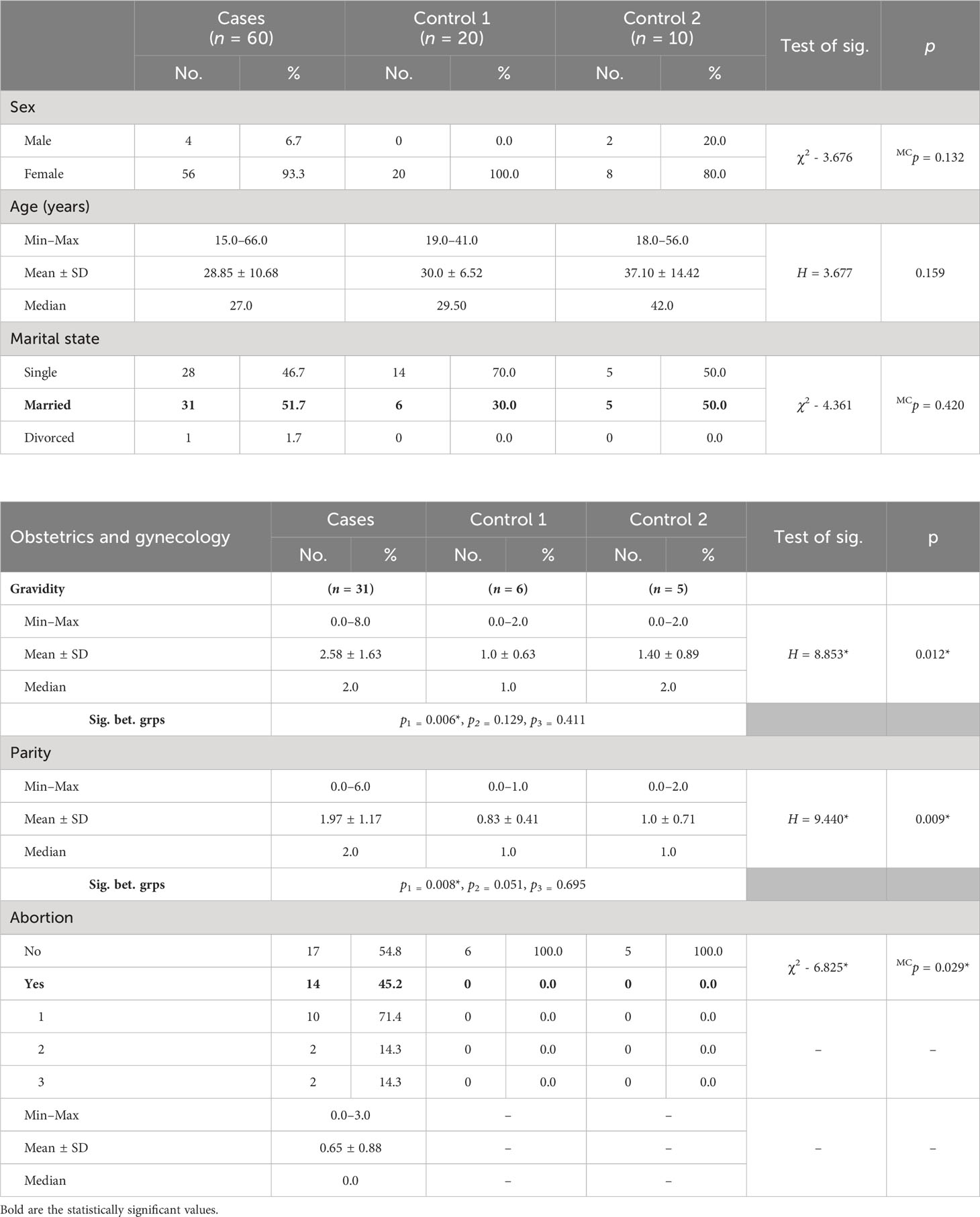
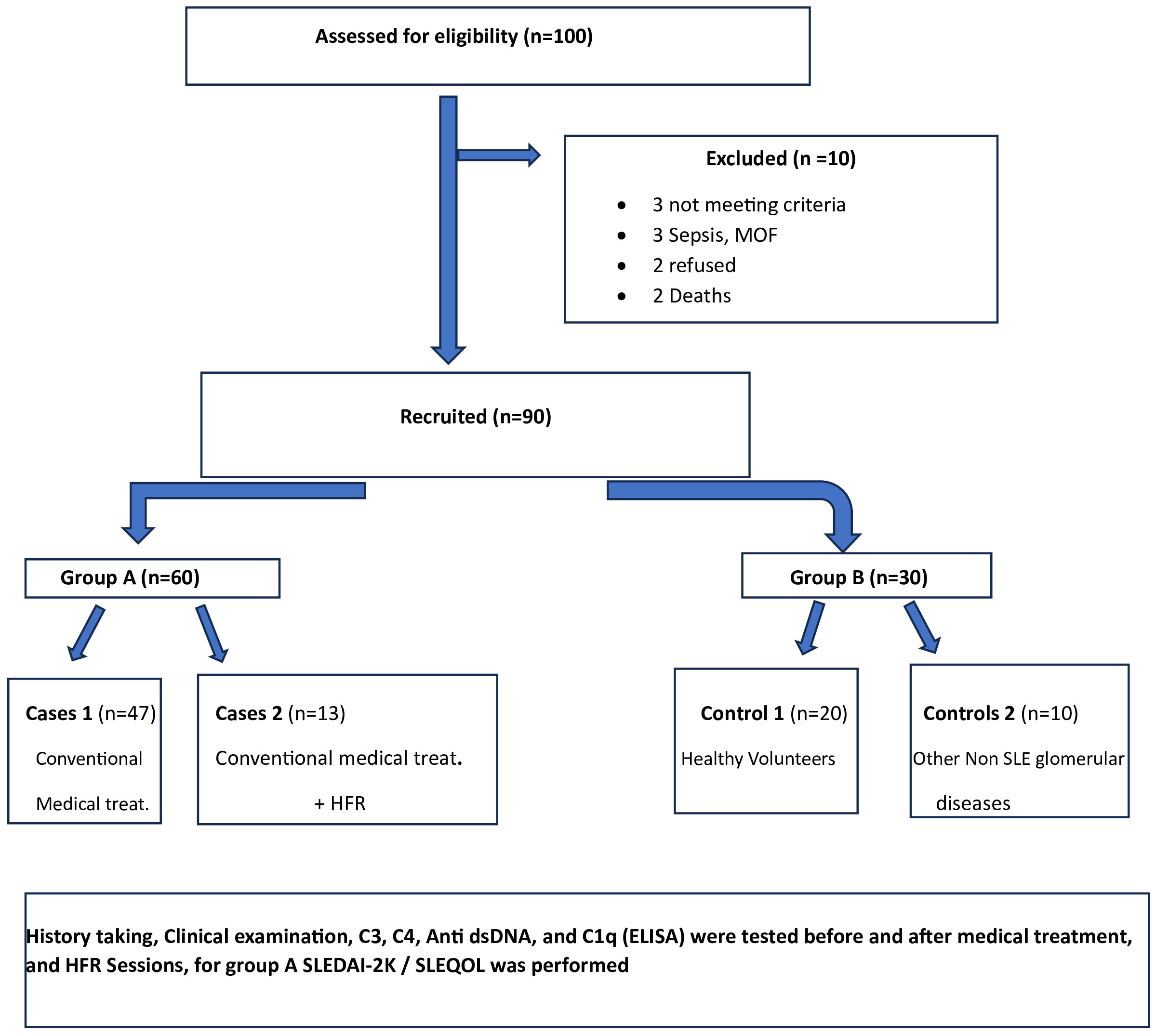
Settings and design: A controlled clinical study was conducted on 60 patients with lupus from Group A that was subdivided into two groups: cases 1 (47 patients), those who received traditional medical treatment, and cases 2 (13 patients), those who underwent HFR in addition to medical treatment. Group B consisted of two subgroups: control 1, composed of 20 healthy age- and sex-matched volunteers, and control 2, consisting of 10 cases with different glomerular diseases other than lupus.
Methods and materials: Serum C1q was determined before and after the HFR as well as induction by medical treatment. Disease activity was assessed using SLEDAI-2K with a responder index of 50; quality of life was assessed using SLEQOL v2, and HFR was performed for the non-responder group.
Results: C1q was lower in cases. It can efficiently differentiate between SLE patients and healthy controls with a sensitivity of 81.67% and a specificity of 90%. It can also efficiently differentiate between SLE patients and the control 2 group (non-lupus patients with renal glomerular disease) with a sensitivity of 83.33% and a specificity of 100%. C1q was more consumed in proliferative lupus, and correlated with anti-ds DNA, C3, and C4.
Conclusions: C1q efficiently discriminates lupus patients and correlates with proliferative forms. HFR might ameliorate lupus activity and restore C1q.
Introduction
Lupus can affect virtually every organ with a relapsing and remitting course. Women are affected more frequently than men (1). Presumed etiopathogenesis includes genetic, epigenetic, ethnic, immunoregulatory, hormonal, and environmental factors (2–5). Urine abnormalities with or without deranged laboratory parameters are common, and may eventually develop in up to 75% of patients with systemic lupus erythematosus (SLE) (3).
Complement involvement, namely, C1q deficiency, predisposes to SLE owing to a reduced ability to clear apoptotic cells. Genetic deficiencies of many classical pathways play a role in SLE (6, 7) Quantifying disease activity is very important to monitor and control. Many disease activity indices aid in monitoring and quantifying; among those indices is the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI), which is based on the presence of 24 descriptors in nine organ systems; it has been used in both research and clinical settings. It is considered a predictive variable and outcome measure in prognostic studies of lupus (8, 9). SLEDAI-2K is a modification of SLEDAI to allow any ongoing disease activity in the descriptors: skin rash, alopecia, mucosal ulcers, and proteinuria. Therefore, SLEDAI-2K included any inflammatory rash, alopecia, or mucosal ulcers and new, recurrent, or persistent proteinuria >0.5 g/24 h (10).
Looking for new therapies is always a dynamic process to help serve that group of patients, and among those therapies are clearance-based approaches. Hemodiafiltration with endogenous reinfusion (HFR) is a dialytic method that combines the processes of diffusion, convection, and adsorption. It can adsorb proinflammatory cytokines and could improve LN prognosis through the counterbalance of the immune-modulatory response (11). There are a plethora of previous studies that confirmed its role in reducing inflammatory cytokines and even light chains. In this study, we tried to explore its role as one of the treatment armamentariums for SLE patients through its amelioration of ongoing inflammation and, subsequently, autoantibody generation given the relatively easy settings to perform it using a regular hemodialysis machine.
Materials and methods
This study is a controlled simple manual randomized unblinded explanatory clinical study that was conducted in Alexandria University Hospitals after the approval of the ethical committee. Eligibility criteria included SLE patients in activity with lupus nephritis. The outcome was the improvement in signs and symptoms together with laboratory parameters of SLE patients in activity especially for patients who are resistant or intolerant to conventional therapy.
It included two groups (Table 1), Group A consisted of 60 SLE patients in activity, subdivided into 47 patients (cases 1) receiving the conventional immunosuppressive therapy (corticosteroids, mycophenolate mofetil, and cyclophosphamide) and 13 patients (cases 2) who underwent HFR in addition to medical treatment. Group B was subdivided into control 1, composed of 20 age- and sex-matched healthy volunteers who have no history of any disease, and control 2, composed of 10 cases with different glomerular diseases other than SLE; all patients were subjected to history taking and clinical examination plus routine laboratory investigations in addition to immune laboratory investigations and determination of C3, C4, anti-ds DNA, and C1q (12–17), which was done using enzyme-linked immunosorbent assay (ELISA) before and after treatment and HFR sessions. Disease activity and quality of life were assessed using both SLEDAI-2K (18) and SLEQOL (19), respectively.
For Group A, 16 patients underwent renal biopsy (26.7%): 1 patient had inadequate (6.3%), 2 patients had class II (12.5%), 2 patients had class III (12.5%), 8 patients had class IV (50%), 2 patients had class IV+V (12.5%), and 1 patient had class VI (6.3%) biopsy findings.
The human C1q ELISA is an assay performed for the quantitative detection of human C1q. An anti-human C1q coating antibody is adsorbed onto microwells. Human C1q present in the sample or standard binds to antibodies adsorbed to the microwells; following incubation, unbound biological components are removed during a wash step and a biotin-conjugated anti-human C1q is captured by the first antibody. After incubation, the unbound biotin-conjugated anti-human C1q is removed during a wash step, and streptavidin-HRP is added and binds to the biotin-conjugated anti-human C1q antibody. Then, after yet another incubation, the unbound streptavidin-HRP is removed during a wash step, and substrate solution reactive with HRP is added to the wells; a colored product is formed in proportion to the amount of human C1q present in the sample or standard. The reaction was terminated by the addition of acid and absorbance was measured at 450 nm. A standard curve was prepared from seven human C1q standard dilutions and the human C1q sample concentration was determined.
Samples were collected in plain tubes, centrifugation was performed to obtain cell-free plasma, and the samples were aliquoted and stored frozen at −20°C until analyzed.
HFR (for non-responder cases) is a two-chamber single-use filter that employs separated convection, diffusion, and adsorption. In the convective phase (Synclear 02) of the first stage, pure ultrafiltrate (plasmatic water) passes through a sorbent cartridge containing hydrophobic styrene resin (Suprasorb®; Bellco Srl, Mirandola, Italy) consisting of numerous pores and channels that add to its extensive surface area, where adsorption takes place, and then finally diffusion ensues.
Statistical analysis
Data were fed to the computer and analyzed using IBM SPSS software package version 20.0. (Armonk, NY: IBM Corp) (20, 21) Qualitative data were described using numbers and percent. The Kolmogorov–Smirnov test was used to verify the normality of distribution. Quantitative data were described using range (minimum and maximum), mean, standard deviation, and median. The significance of the obtained results was judged at the 5% level.
The following tests were used in the study:
1. Chi-square test
This was used to compare categorical variables between different groups.
2. Fisher’s exact or Monte Carlo correction
This was used as correction for chi-square when more than 20% of the cells have an expected count of less than 5.
3. Paired t-test
This was used to compare normally distributed quantitative variables between two periods.
4. Pearson coefficient
This was used to correlate between two normally distributed quantitative variables.
5. Mann–Whitney test
This was used to compare abnormally distributed quantitative variables between two studied groups.
6. Kruskal–Wallis test
This was used to compare abnormally distributed quantitative variables between more than two studied groups, and post hoc (Dunn’s multiple comparisons test) was used for pairwise comparisons.
7. Wilcoxon signed ranks test
This was used to compare abnormally distributed quantitative variables between two periods.
8. Spearman coefficient
This was used to correlate between two distributed abnormally quantitative variables.
9. Receiver operating characteristic curve (ROC)
It was generated by plotting sensitivity (TP) on the Y axis versus 1 − specificity (FP) on the X axis at different cutoff values. The area under the ROC curve denotes the diagnostic performance of the test. An area of more than 50% gives acceptable performance and an area of approximately 100% gives the best performance for the test. The ROC curve also allows comparison of performance between two tests.
10. Sensitivity
It shows the capacity of the test to correctly identify diseased individuals in a population (“true positives”). The greater the sensitivity, the smaller the number of unidentified cases (“false negatives”).
11. Specificity
It shows the capacity of the test to correctly exclude individuals who are free of the disease (“true negatives”). The greater the specificity, the fewer “false positives” will be included.
12. Positive predictive value (PPV)
It shows the probability of the disease being present, among those with positive diagnostic test results.
13. Negative predictive value (NPV)
It shows the probability that the disease was absent, among those whose diagnostic test results were negative.
Sample size
A sample of 60 patients with SLE (22) is required to estimate an average change in signs and symptoms after HFR dialysis of 68% (23), with an effect size for new treatment regimen of 3.4 (23) with 95% confidence level and a study power of 80%. The sample size was calculated using PASS Software.
Results
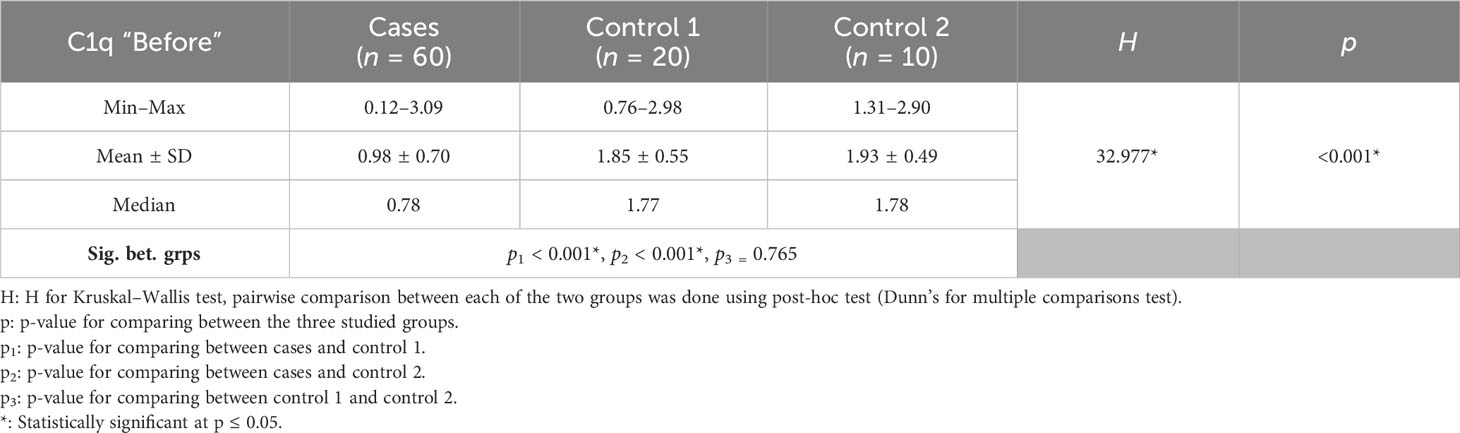
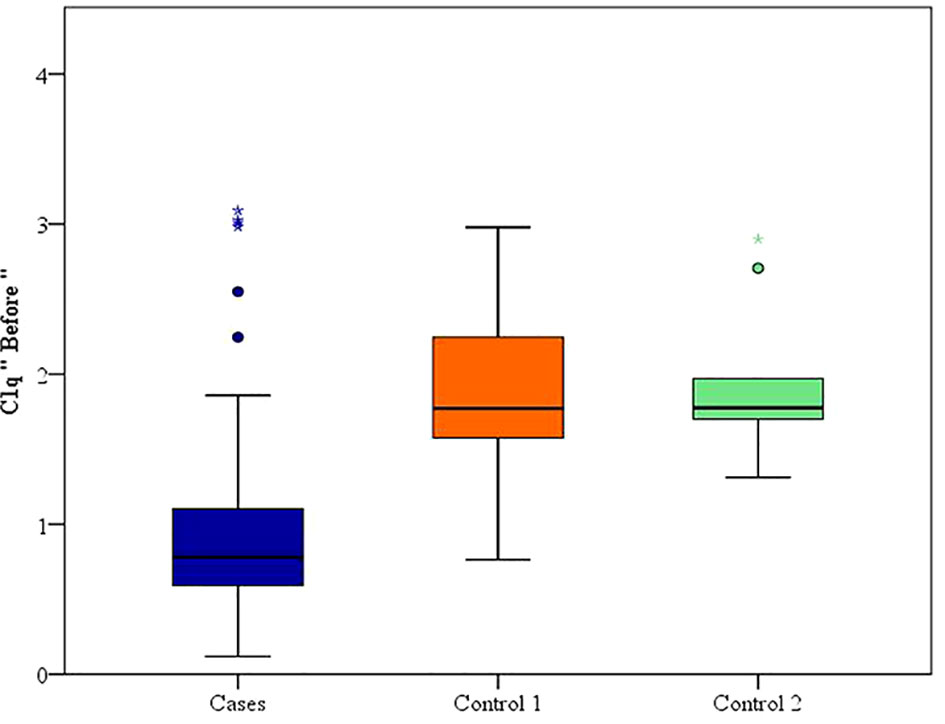
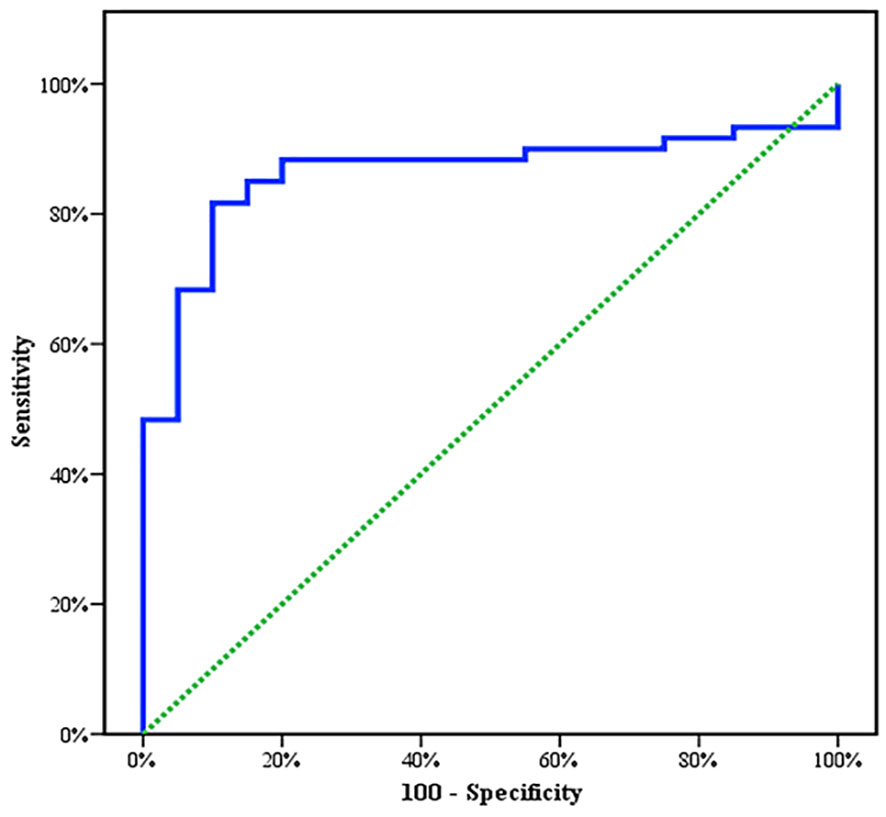
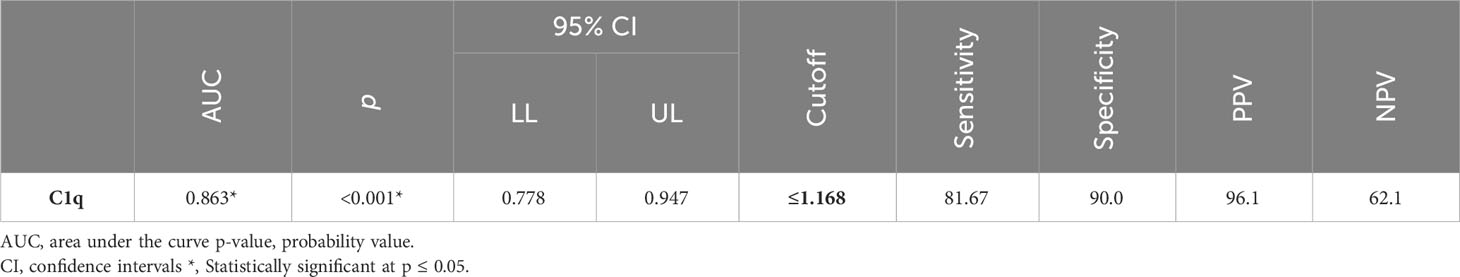
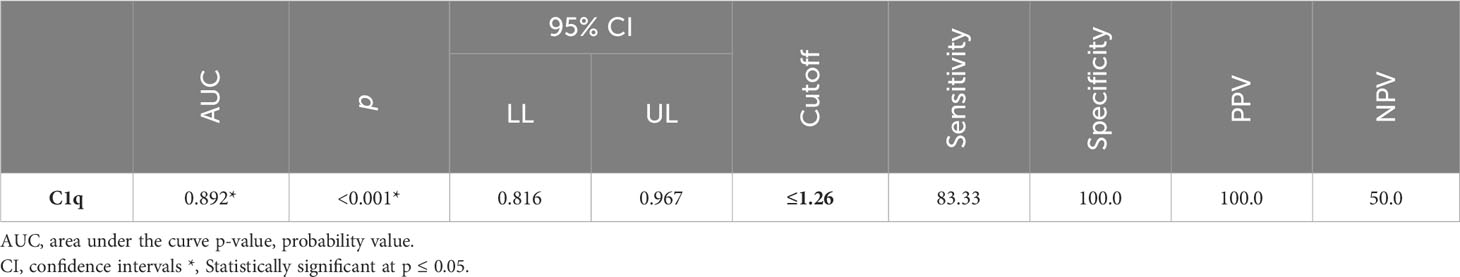
Between January 2018 and January 2020, among 100 patients in Alexandria University Hospitals enrolled in the study, 10 were excluded (due to refusal, sepsis, not meeting the criteria, and death). The recruited 90 patients were divided into Group A including 60 patients (subdivided into cases 1 and 2) and Group B including 30 patients (subdivided into controls 1 and 2) as depicted in the cohort diagram. (Table 2) C1q can efficiently differentiate between SLE patients and both controls 1 and 2 (Figure 1). The ROC curve for C1q in Figure 2 shows that it can efficiently differentiate between SLE patients and healthy controls with a sensitivity of 81.67% and a specificity of 90% (Table 3); in addition, it shows that it can efficiently differentiate between SLE patients and control 2 (non-lupus patients with other glomerular diseases) with a sensitivity of 83.33% and a specificity of 100% (Table 4).
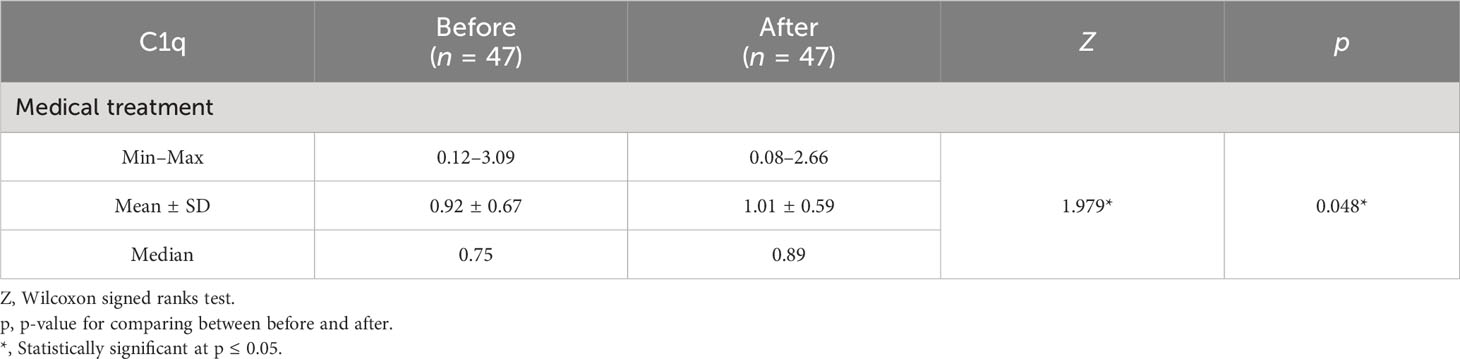
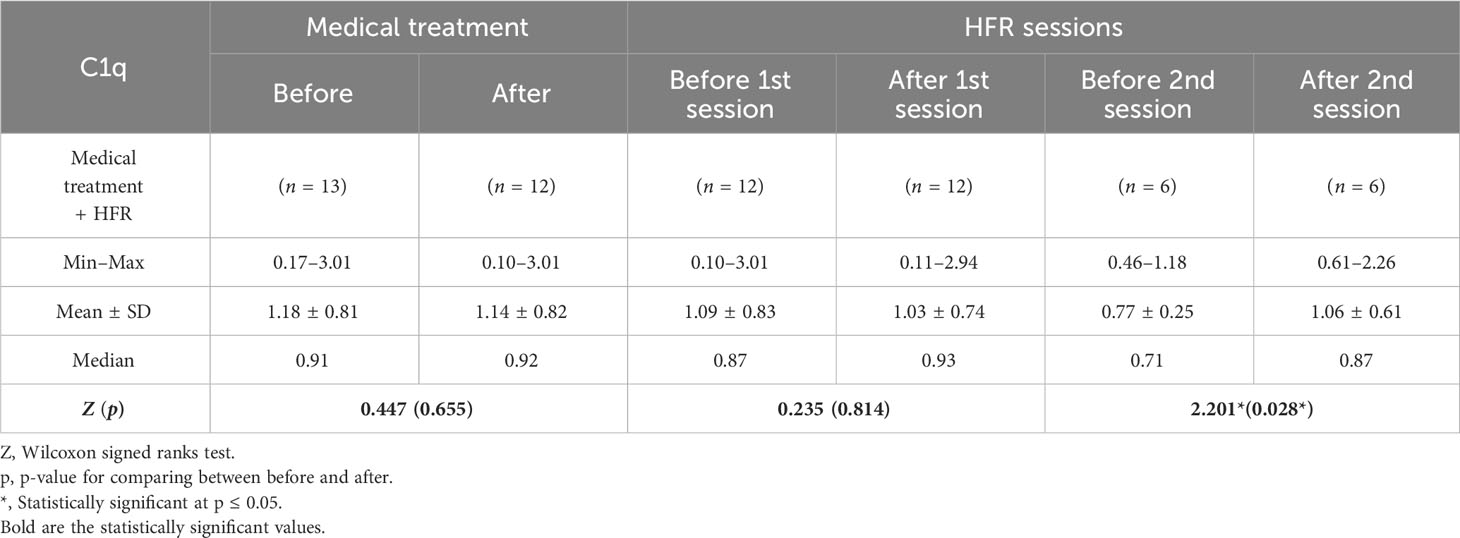
A difference with statistical significance was noted before and after medical treatment in the cases 1 group where C1q levels increased (Table 5). In the cases 2 subgroup, the second session of HFR showed a statistically significant difference in C1q levels before and after the session (Table 6) (Figure 3). Regarding SLEDAI-2K reduction, a statistically significant difference for both cases 1 and 2 before and after treatment was observed. A statistically significant difference was noted in the casse 1 subgroup regarding platelet count drop before and after medical treatment. Anti-ds DNA levels dropped significantly after medical treatment in cases 1. C3 levels increased significantly in cases 1 after medical treatment in comparison to before medical treatment.
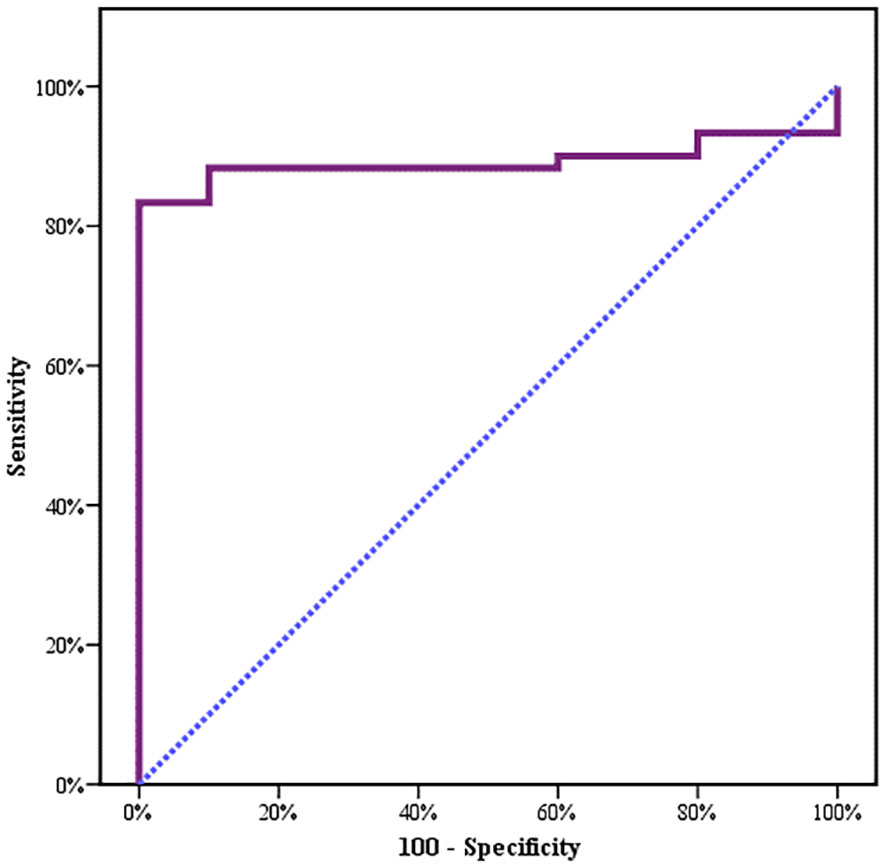
Figure 3 Second session of HFR showed a statistically significant difference in C1q levels before and after the session.
A total of 16 patients had renal biopsy (26.7%): 1 had inadequate (6.3%), 2 had class II (12.5%), 2 patients had class III (12.5%), 8 patients had class IV (50%), 2 patients had class IV+V (12.5%), and 1 patient had class VI (6.3%) biopsy findings.
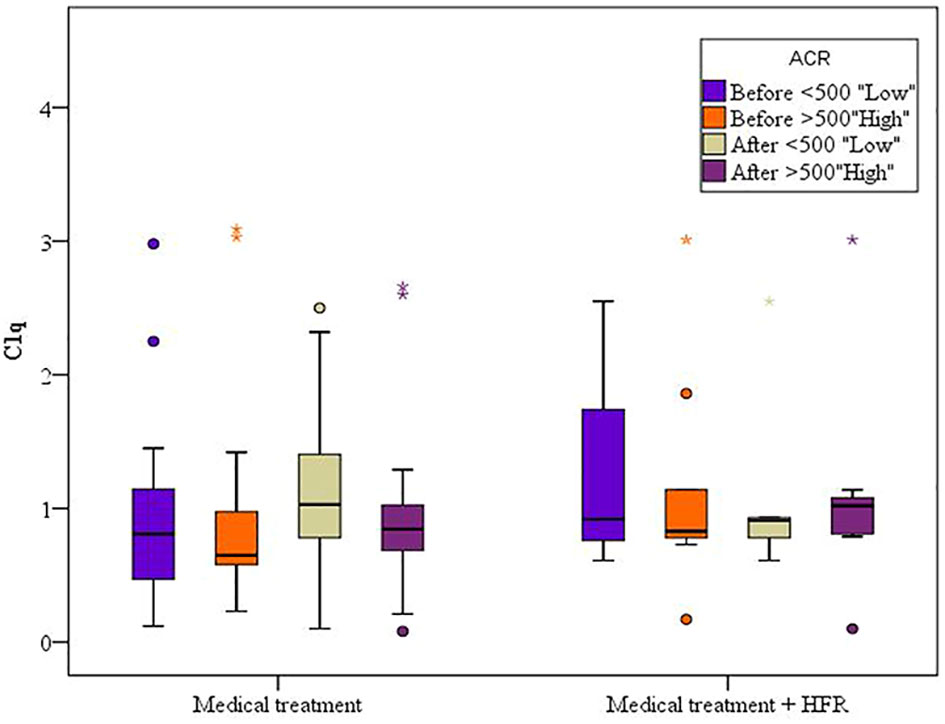
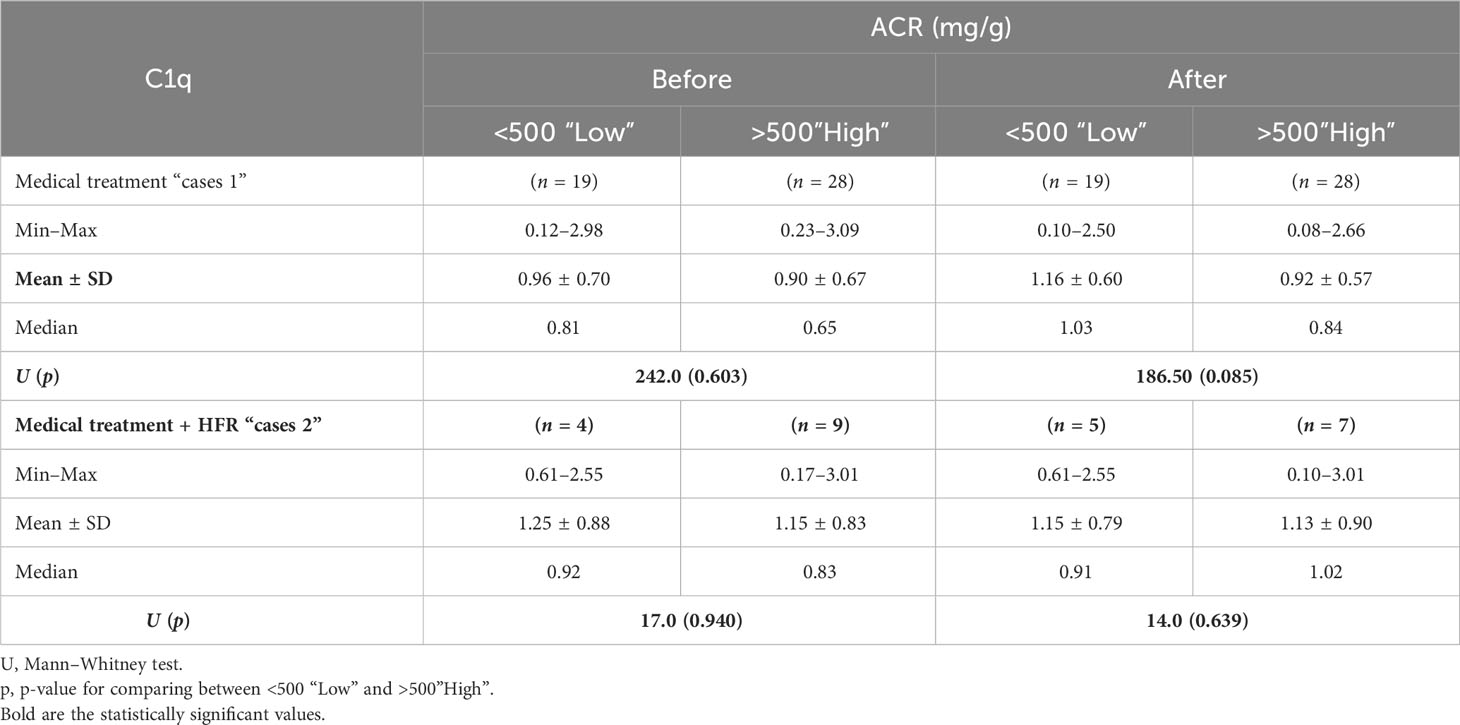
For the cases 1 subgroup, mean levels of C1q increased after treatment when compared to before treatment, and for the group of patients with an albumin/creatinine ratio (ACR) of less than 500 mg/g, mean values were 1.16 ± 0.60 (p = 0.085) and 0.96 ± 0.70 (p = 0.603), respectively, while for the subgroup of patients with an ACR of more than 500 mg/g, mean values were 0.92 ± 0.57 and 0.90 ± 0.67, respectively. For the cases 2 subgroup, mean levels of C1q decreased after treatment when compared to before treatment. In both cases 1 and cases 2 subgroups, C1q values were lower when ACR exceeded 500 mg/g, denoting possible correlation between C1q and lupus nephritis activity. However, the difference was statistically insignificant (Figure 4) (Table 7).
Mean C1q values were low in classes III and IV with mean values of 0.98 ± 0.73 before and 1.02 ± 0.65 after treatment when compared to other classes except class VI, which was lower. This may be linked to C1q deficiency in proliferative lupus, despite it being statistically insignificant (Table 8).
In cases, both C3 and C4 showed a significant positive correlation with C1q. Furthermore, a negative and significant correlation existed between C1q and anti-ds DNA. In the control 2 subgroup, there was a positive correlation between ACR and C1q. For the cases 1 subgroup before medical treatment, there was a statistically significant positive correlation between C1q and both C3 and C4, and a statistically significant negative correlation between C1q and anti-ds DNA. For the cases 2 subgroup after treatment, a significant positive correlation between C1q and SLEQOL was noted. For the cases 1 subgroup after treatment, there was a statistically significant negative correlation between C1q and anti-ds DNA. The study flow chart is depicted in Figure 5.
Discussion
In the present study, we aimed to determine the role of HFR use in a subset of active lupus patients with and without lupus nephritis who are not responding to conventional therapy and/or intolerant to it. Complement system is of paramount role in SLE where C1q as an important complement component and activity marker especially for proliferative LN had highlighted that it could be a possible therapeutic target through its restoration and amelioration of complement activation.
We noticed a statistically significant difference between the case subgroups and both controls 1 and controls 2, regarding C1q; hence, it can significantly discriminate between SLE and both healthy populations as well as non-lupus causes of glomerular disease, and this supports the value of C1q and both the specificity and sensitivity of C1q to lupus patients (24–26).
In the Chinese study done by Pang et al. (27), they revealed that the specificity and prognostic yield for anti-C1q A08 antibodies were superior to either the intact or collagen regions of anti-C1q antibodies in disease relapse.
In our study, a significant difference before and after medical treatment in the case 1 subgroup was noted where C1q levels increased. In the second session of HFR, a statistically significant difference in C1q levels before and after the session was noted, and this may suggest the need for more than just one session of HFR. Thus, C1q is considered a marker of evaluation of activity and response to treatment. These findings may be supported and explained by the improvement in pro-inflammatory state in hemodialysis patients when they transitioned to HFR, as confirmed by reduced IL-6, IL-1β, and TNF-α and increased pre-albumin, in the study done by Borelli et al. (28).
Inflammation and cytokines represent a cornerstone in the etiopathogenesis of SLE; IL-6, retinol-binding protein 4 (RBP4), tumor necrosis factor α (TNF-α), IFN-α, and B-lymphocyte stimulator (BLyS) have all been detected in lupus as in the study done by Solano et al. (23).
Francesco et al. (27) used high-performance liquid chromatography coupled with a quadrupole time-of-flight mass spectrometer for the identification of proteins in the HFR session where ultrafiltrate and the species captured by the resin bed detected neutrophil gelatinase-associated lipocalin, zinc α-2 glycoprotein, and cystatin-C. Moreover, additional uremic toxins such as serotransferrin, β-2-glycoprotein 1, α-1-acid glycoprotein, prostaglandin-H2 D-isomerase, and transthyretin were identified. All of these markers are considered by many like Varghese et al. (29) as biomarkers for glomerular disease.
Moreover, Francesco et al. (23) reported fever and joint pain reduction, and improved skin manifestations and quality of life. After starting SUPRA (three times weekly for 4 h per session) for the patient case they studied, that patient no longer needed any additional plasma exchange treatment. Prednisone and immune suppressors were gradually reduced and eventually discontinued since the improvement of symptoms suggested a trend toward systemic remission. In this study, we had a patient who showed the same marvelous response, especially in the constitutional and musculoskeletal symptoms.
HFR was effective not only in SLE but even beyond, and it has been reported to reduce free light chain in myeloma kidney as revealed by Pasquali et al. (30) where they treated four patients affected by dialysis-dependent acute kidney injury due to biopsy-proven de novo free light chain myeloma cast nephropathy. Each patient received Bortezomib chemotherapy and extracorporeal treatment with the HFR. All patients had a considerable reduction in serum FLC, while serum albumin concentration remained unchanged. Renal function was restored in three out of four patients.
In this study, a statistically significant difference was noted in the cases 1 subgroup regarding platelet count drop before and after medical treatment. In the cases 2 subgroup, there was no statistically significant difference before and after medical treatment despite the fact that platelet count dropped.
Thus, whether HFR exerts a preserving effect on platelet count or function is not known. We had another case with persistent hemoptysis that was not responsive except to plasma exchange, and after the patient underwent HFR, he was free of hemoptysis for 2 weeks. One possible explanation for this is the amelioration of inflammation with subsequent reduction of pulmonary hemorrhage.
In both cases 1 and cases 2 subgroups, C1q values were lower when ACR exceeded 500 mg/g, denoting a possible correlation between C1q and lupus nephritis, despite being statistically insignificant.
Merete Bock et al. In Basel used anti-C1q antibodies as part of routine clinical care, and they aimed to assess the utility of anti-C1q antibodies as a marker of follow-up in lupus. They found that they correlate with activity scores as well as flare and remission and both urine protein creatinine ratio and anti-ds DNA levels, and negatively correlate with C3, C4, and CH50.
In agreement with our study, there was a negative insignificant correlation between ACR, SLEDAI-2K, SLEQOL creatinine, and C1q in the case subgroups. However, C3 and C4 showed a significant positive correlation with C1q. In addition, a negative and significant correlation existed between C1q and anti-ds DNA.
For cases 1 before treatment, there was a considerable negative correlation between C1q and anti-ds DNA, and a considerable positive correlation between C1q and both C3 and C4.
For cases 1 after treatment, there was a statistically significant negative correlation between C1q and both ACR and anti-ds DNA. For cases 2 after treatment, there was a statistically significant positive correlation between C1q and SLEQOL.
Mean C1q values were low in classes III and IV lupus nephritis before and after treatment when compared to other classes except class VI in which it was lower. This could be explained by a link between C1q deficiency and proliferative lupus. The study limitations included a relatively low sample size and the fact that follow-up for longer period would have given more insights into treatment effect. Study strengths are related to the easy, available setting for applying this triple-stage dialysis modality with convection, adsorption, and diffusion combined in one filter using a regular HD machine. It also offers a new treatment modality that needs further validation for SLE patients especially those with LN. To our knowledge, the study is internally and externally valid.
Conclusions
C1q is a sensitive and specific diagnostic marker for lupus patients and may help as a marker of evaluation of activity and response to treatment; in addition, it is correlated with lupus nephritis. It is available, inexpensive, and non-invasive; thus, it may be of benefit for those who refuse or are contraindicated to do a biopsy, as well as to monitor for prediction of flare.
HFR could, through amelioration of inflammation, be a new therapeutic option for lupus patients, especially those with persistent disease activity despite traditional immunosuppressive therapy, and those who are intolerant to other therapies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Faculty of Medicine, Alexandria University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
ME: Writing – review & editing, Conceptualization, Investigation, Methodology, Project administration, Writing – original draft. SN: Writing – review & editing, Supervision. IH: Supervision, Writing – review & editing. IE: Supervision, Writing – review & editing. AM: Writing – review & editing, Supervision.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors express their gratitude and appreciation to Alexandria University Hospitals, namely participating patients, technicians, laboratory workers and administrative staff for their consistent support and guidance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneph.2024.1269852/full#supplementary-material
References
1. Tsokos GC. Systemic lupus erythematosus. N Engl J Med. (2011) 365:2110–21. doi: 10.1056/NEJMra1100359.
2. Jarukitsopa S, Hoganson DD, Crowson CS, Sokumbi O, Davis MD, Michet CJ, et al. Epidemiology of systemic lupus erythematosus and cutaneous lupus erythematosus in a predominantly white population in the United States. Arthritis Care Res (Hoboken). (2015) 67:817–28. doi: 10.1002/acr.22502.
3. Lisnevskaia L, Murphy G, Isenberg D. Systemic lupus erythematosus. Lancet. (2014) 384:1878–88. doi: 10.1016/S0140-6736(14)60128-8.
4. Chakravarty EF, Bush TM, Manzi S, Clarke AE, Ward MM. Prevalence of adult systemic lupus erythematosus in California and Pennsylvania in 2000: estimates obtained using hospitalization data. Arthritis Rheumatol. (2007) 56:2092–4. doi: 10.1002/art.22641.
5. Borchers AT, Keen CL, Shoenfeld Y, Gershwin ME. Surviving the butterfly and the wolf: mortality trends in systemic lupus erythematosus. Autoimmun Rev. (2004) 3:423–53. doi: 10.1016/j.autrev.2004.04.002.
6. Bongu A, Chang E, Ramsey-Goldman R. Can morbidity and mortality of SLE be improved? Best Pract Res Clin Rheumatol. (2002) 16:313–32. doi: 10.1053/berh.2001.0228.
7. Cooper GS, Dooley MA, Treadwell EL, St Clair EW, Parks CG, Gilkeson GS. Hormonal, environmental, and infectious risk factors for developing systemic lupus erythematosus. Arthritis Rheumatol. (1998) 41:1714–24. doi: 10.1002/(ISSN)1529-0131.
8. Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. (2008) 358:929–39. doi: 10.1056/NEJMra071297.
9. D'Cruz DP, Khamashta MA, Hughes GR. Systemic lupus erythematosus. Lancet. (2007) 369:587–96. doi: 10.1016/S0140-6736(07)60279-7.
10. Lupus Foundation of America. What are the risk factors for developing lupus? . Available online at: http://www.lupus.org/webmodules/webarticlesnet/templates/new_learnunderstanding.aspx?articleid=2237&zoneid=523.
11. Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. (2003) 349:1526–33. doi: 10.1056/NEJMoa021933.
12. Mayer MM. Complement and complement fixation. In: Kabat EA, Mayer MM, editors. Experimental immunochemestriy, 2nd ed. Springfield Illinosis: Charles C. Thomas Publisher (1961). p. 133–239.
13. Kishore U, Reid KB. C1q: structure, function, and receptors. Immunopharmacology. (2000) 49:159–70. doi: 10.1016/S0162-3109(00)80301-X.
14. Gaboriaud C, Juanhuix J, Gruez A, Lacroix M, Darnault C, Pignol D, et al. The crystal structure of the globular head of complement protein C1q provides a basis for its versatile recognition properties. J Biol Chem. (2003) 278:46974–82. doi: 10.1074/jbc.M307764200.
15. Son M, Santiago-Schwarz F, Al-Abed Y, Diamond B. C1q limits dendritic cell differentiation and activation by engaging LAIR-1. Proc Natl Acad Sci U S A. (2012) 109:E3160–3167. doi: 10.1073/pnas.1212753109.
16. Benoit ME, Hernandez MX, Dinh ML, Benavente F, Vasquez O, Tenner AJ. C1q-induced LRP1B and GPR6 proteins expressed early in Alzheimer disease mouse models, are essential for the C1q-mediated protection against amyloid-beta neurotoxicity. J Biol Chem. (2013) 288:654–65. doi: 10.1074/jbc.M112.400168.
17. Kang YH, Urban BC, Sim RB, Kishore U. Human complement Factor H modulates C1q-mediated phagocytosis of apoptotic cells. Immunobiology. (2012) 217:455–64. doi: 10.1016/j.imbio.2011.10.008.
18. Touma Z, Gladman DD, Ibanez D, Urowitz MB. Development and initial validation of the systemic lupus erythematosus disease activity index 2000 responder index 50. J Rheumatol. (2011) 38:275–84. doi: 10.3899/jrheum.100724.
19. Smolen JS, Strand V, Cardiel M, Edworthy S, Furst D, Gladman D, et al. Randomized clinical trials and longitudinal observational studies in systemic lupus erythematosus: consensus on a preliminary core set of outcome domains. J Rheumatol. (1999) 26:504–7.
20. Moser KL, Kelly JA, Lessard CJ, Harley JB. Recent insights into the genetic basis of systemic lupus erythematosus. Genes Immun. (2009) 10:373–9. doi: 10.1038/gene.2009.39.
21. Tsokos GC, Kammer GM. Molecular aberrations in human systemic lupus erythematosus. Mol Med Today. (2000) 6:418–24. doi: 10.1016/S1357-4310(00)01798-6.
22. Daniel W. Biostatistics. In: A foundation for analysis in the health science, 6th edition. John Wiley and sons, Inc, NY (1995).
23. Solano FG, Bellei E, Cuoghi A, Caiazzo M, Bruni F. Radical improvement of signs and symptoms in systemic lupus erythematosus when treated with hemodiafiltration with endogenous reinfusion dialysis. Case Rep Nephrol Dial. (2015) 5:106–12. doi: 10.1159/000381395.
24. Marto N, Bertolaccini ML, Calabuig E, Hughes GR, Khamashta MA. Anti-C1q antibodies in nephritis: correlation between titres and renal disease activity and positive predictive value in systemic lupus erythematosus. Ann Rheum Dis. (2005) 64:444–8. doi: 10.1136/ard.2004.024943.
25. Sinico RA, Rimoldi L, Radice A, Bianchi L, Gallelli B, Moroni G. Anti-C1q autoantibodies in lupus nephritis. Ann N Y Acad Sci. (2009) 1173:47–51. doi: 10.1111/j.1749-6632.2009.04746.x.
26. Mok CC, Ho LY, Leung HW, Wong LG. Performance of anti-C1q, antinucleosome, and anti-dsDNA antibodies for detecting concurrent disease activity of systemic lupus erythematosus. Transl Res. (2010) 156:320–5. doi: 10.1016/j.trsl.2010.07.009.
27. Pang Y, Tan Y, Li Y, Zhang J, Guo Y, Guo Z, et al. Serum A08 C1q antibodies are associated with disease activity and prognosis in Chinese patients with lupus nephritis. Kidney Int. (2016) 90:1357–67. doi: 10.1016/j.kint.2016.08.010.
28. Borrelli S, Minutolo R, De Nicola L, De Simone E, De Simone W, Zito B, et al. Effect of hemodiafiltration with endogenous reinfusion on overt idiopathic chronic inflammation in maintenance hemodialysis patients: a multicenter longitudinal study. Hemodial Int. (2014) 18:758–66. doi: 10.1111/hdi.12178.
29. Varghese SA, Powell TB, Budisavljevic MN, Oates JC, Raymond JR, Almeida JS, et al. Urine biomarkers predict the cause of glomerular disease. J Am Soc Nephrol. (2007) 18:913–22. doi: 10.1681/ASN.2006070767.
Keywords: systemic lupus erythematosus (SLE), C1q, online hemodiafiltration with endogenous reinfusion (HFR), systemic lupus disease activity index 2K (SLEDAI-2K), albumin/creatinine ratio (ACR)
Citation: Elghiriani MA, Naga SS, Hameed IA, Elgohary IE and Mansour AR (2024) The role of online hemodiafiltration with endogenous reinfusion in the treatment of systemic lupus erythematosus activity resistant to conventional therapy. Front. Nephrol. 4:1269852. doi: 10.3389/fneph.2024.1269852
Received: 31 July 2023; Accepted: 05 February 2024;
Published: 22 March 2024.
Edited by:
Peter Kotanko, Renal Research Institute, United StatesReviewed by:
Irma Tchokhonelidze, Tbilisi State Medical University, GeorgiaKhaled Boubes, The Ohio State University, United States
Copyright © 2024 Elghiriani, Naga, Hameed, Elgohary and Mansour. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammed A. Elghiriani, bS5lbGdoZXJpYW55QHlhaG9vLmNvbQ==
 Mohammed A. Elghiriani
Mohammed A. Elghiriani Salah S. Naga1
Salah S. Naga1