- 1Department of Renal Medicine, Royal Prince Alfred Hospital, Sydney, NSW, Australia
- 2Transplantation Services, Royal Prince Alfred Hospital, Sydney, NSW, Australia
- 3Institute of Academic Surgery, Royal Prince Alfred Hospital, Sydney, NSW, Australia
- 4Sydney Medical School, University of Sydney, Sydney, NSW, Australia
- 5Charles Perkins Centre, University of Sydney, Sydney, NSW, Australia
Introduction: Wound complications can cause considerable morbidity in kidney transplantation. Closed-incision negative pressure wound therapy (ciNPWT) systems have been efficacious in reducing wound complications across surgical specialties. The aims of this study were to evaluate the use of ciNPWT, Prevena™, in kidney transplant recipients and to determine any association with wound complications.
Material and methods: A single-center, prospective observational cohort study was performed in 2018. A total of 30 consecutive kidney transplant recipients deemed at high risk for wound complications received ciNPWT, and the results were compared to those of a historical cohort of subjects who received conventional dressings. Analysis for recipients with obesity and propensity score matching were performed.
Results: In total, 127 subjects were included in the analysis. Of these, 30 received a ciNPWT dressing and were compared with 97 subjects from a non-study historical control group who had conventional dressing. The overall wound complication rate was 21.3% (27/127). There was no reduction in the rate of wound complications with ciNPWT when compared with conventional dressing [23.3% (7/30) and 20.6% (20/97), respectively, p = 0.75]. In the obese subset (BMI ≥30 kg/m2), there was no significant reduction in wound complications [31.1% (5/16) and 36.8% (7/19), respectively, p = 0.73]. Propensity score matching yielded 26 matched pairs with equivalent rates of wound complications (23.1%, 6/26).
Conclusion: This is the first reported cohort study evaluating the use of ciNPWT in kidney transplantation. While ciNPWT is safe and well tolerated, it is not associated with a statistically significant reduction in wound complications when compared to conventional dressing. The findings from this study will be used to inform future studies associated with ciNPWT in kidney transplantation.
1 Introduction
Wound complications, including surgical site infections (SSIs) and wound dehiscence, affect between 3% and 27% of kidney transplant recipients (1–4). Risk factors include obesity, diabetes mellitus, age, and immunosuppression (1, 2, 4). SSIs are associated with delayed graft function (DGF) and inferior graft survival, while deep/organ-space infections can lead to graft loss (2, 5, 6). In our unit, the wound complication rate was historically 7.7% (7). However, management can be complex and, in our experience, may include multistage debridement, abdominal wall reconstruction, delayed wound closure with open negative pressure wound therapy (NPWT), and prolonged wound healing (8). This leads to extended hospitalization, increased costs, and negative patient experiences (9–13).
Therefore, a proactive approach to preventing wound complications in kidney transplant recipients is needed to minimize morbidity and associated costs (2, 9, 11, 14). Preventive strategies include closed-incision negative pressure wound therapy (ciNPWT), “Prevena™” Incision Management system (KCI USA, Inc., San Antonio, TX, USA) (15). The WHO recommends using ciNPWT to prevent SSI in high-risk wounds (16). ciNPWT has been successfully utilized in other surgical disciplines including obstetrics (17, 18) and general surgery (19–21). However, there is a lack of evidence for the use of ciNPWT in transplant recipients, with only a single case report on the use of the Prevena™ dressing on a kidney transplant recipient being published (22). It is hypothesized that ciNPWT is associated with a reduction in wound complications in kidney transplantation.
2 Methods
2.1 Study design
This is a prospective observational cohort study. A total of 30 consecutive “high-risk” kidney transplant recipients were selected by the transplant surgeons to receive a ciNPWT dressing (intervention) over a 3-month period. The outcomes were compared to those of a non-study historical control group of subjects who received conventional hydrocolloid surgical dressing over a 12-month period. The study was conducted in a single-center tertiary referral transplant unit (Royal Prince Alfred Hospital Sydney, Australia) in 2018. A detailed methodology is provided in the thesis format of this study, and a study design diagram and flowchart are also presented in Supplementary File 1 (23).
All subjects who received ciNPWT provided informed consent for participation in the study. This included information about the nature of the Prevena™ ciNPWT dressing (i.e., it differed from standard treatment), duration of treatment and follow-up, and complications associated with the device. Furthermore, a waiver of consent was obtained for the historical control group. Institutional ethics [Sydney Local Health District Human Research Ethics Committee (HREC/18/CRGH/127)] approval was obtained for this study, and the Prevena™ ciNPWT device had approval to be used in the hospital. This study conforms to the Declaration of Helsinki.
A total of 30 consecutive “high-risk” kidney transplant recipients were selected to receive a ciNPWT dressing if they satisfied the inclusion and exclusion criteria. The inclusion criteria for receiving a ciNPWT dressing were as follows: had a single kidney transplant with a closed incision and showed any of the following: body mass index (BMI) equal to or over 25 kg/m2, diabetes, previous history of abdominal surgery via the same incision (i.e., re-transplant), or any additional immunosuppression (defined as deviation from standard unit low- or intermediate-risk induction immunosuppression) (24). Patients were excluded from receiving a Prevena™ dressing if there was a contraindication to its use, including allergy to the dressing, inadequate wound hemostasis, or skin cellulitis prior to the application of ciNPWT. A 3-month period was chosen as a practical time frame to allow for consecutive recruitment and sufficient time for follow-up over 12 months, while the number of subjects (n = 30) was based on the number of Prevena™ dressings made available and donated in kind for the study by the company KCI-ACELITY, which was within the acceptable sample size limit for a pilot study
2.1.1 Study treatment
Patients receiving a kidney transplant underwent general anesthesia. Prophylactic antibiotics [intravenous (IV) cephazolin] were given preoperatively within 2 h of the surgical incision. Patients were shaved with clippers as required. A three-way 18-Fr indwelling catheter was inserted and attached to the irrigation fluid bag. Aqueous or alcoholic betadine was used for skin preparation prior to draping. Standard protocol immunosuppression included induction with pulse methylprednisolone on days 0 and 1, followed by prednisolone 30 mg, and tapering to 10 mg by month 3. In addition, basiliximab and a tacrolimus with mycophenolate were given (25).
The standard approach to the retroperitoneum is via an oblique incision in the iliac fossa in this unit. End-to-side vascular anastomoses were performed for the renal artery and vein, and a modified Lich–Gregoir technique was used for the ureterocystostomy (26). Surgical drains were placed in the retroperitoneum at the discretion of the surgeon and were removed when the drain volume was <50 ml in 24 h. Fascial closure was performed in a continuous or interrupted fashion with 0-loop PDS® or 0 nylon and the skin closed continuously with 4/0 Monocryl® (poliglecaprone) (both from Ethicon, Johnson & Johnson Medical NV, Machelen, Belgium). Postoperative care consisted of a combined multidisciplinary medical, surgical, and nursing team approach on a specialized transplant ward according to unit protocol (25).
2.1.2 Wound dressing
The conventional dressing is a hydrocolloid “Comfeel® Plus” (Coloplast Pty Ltd., Victoria, Australia) dressing applied for 7 days. All patients were followed up over a 3-month period in the Outpatient Department by independent transplant staff. The wound outcomes were recorded in the clinical notes and maintained in a surgical outcomes REDCap database as routine practice.
The intervention group had a Prevena™ ciNPWT applied to the surgical site at the conclusion of the kidney transplant under sterile conditions in the operating theater (OT). The Prevena™ dressing is a polyurethane foam dressing lined with 0.019% silver ionic dressing and is placed over the surgical wound after skin closure. The dressing attaches to a battery-powered suction, connected to a 45-ml canister, and provides continuous negative pressure at −125 mmHg (Figure 1A). After 7 days of therapy, the dressings were removed and the wounds assessed (Figure 1B) and redressed for 7 days with a Comfeel® dressing. Follow-up wound assessments were performed according to a standardized wound assessment form on days 14 and 30 and up to 3 months at the outpatient clinic by an independent, blinded assessor. Data were maintained in the REDCap database as part of the study.
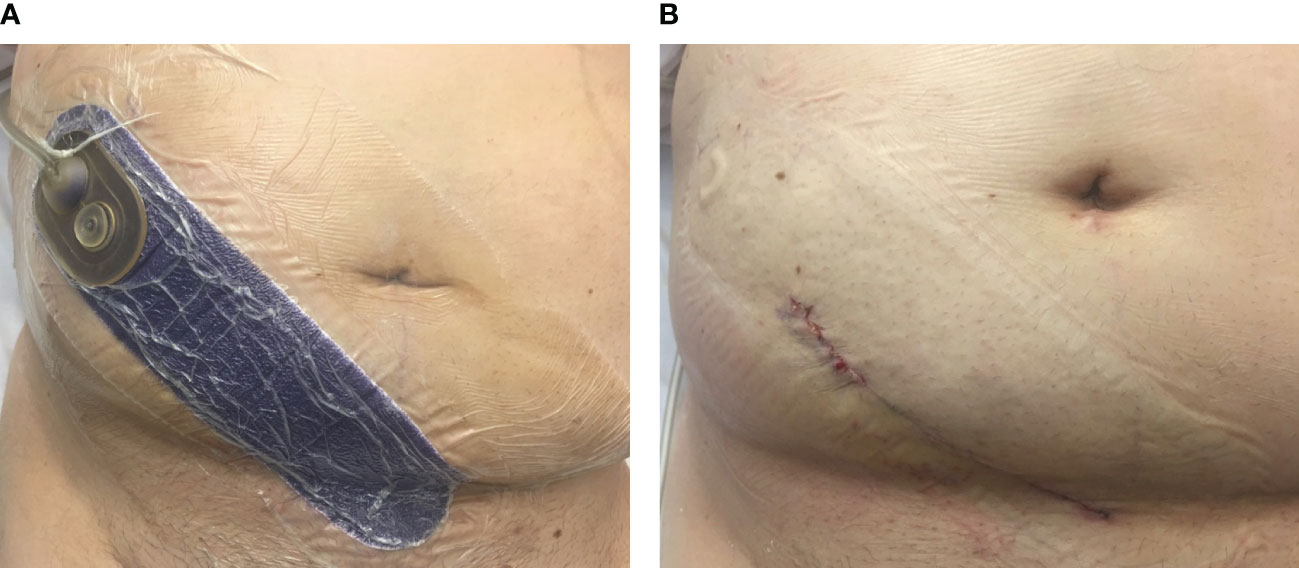
Figure 1 Clinical image of the closed-incision negative pressure wound therapy (ciNPWT) in situ. (A) “Prevena™” dressing applied to the kidney transplant incision in the right iliac fossa and connected to an integrated pump (not shown). (B) Wound appearance of the surgical incision after removal of ciNPWT (image obtained with patient consent).
2.1.3 Outcomes
The primary composite outcome was the presence of wound complications within 3 months of transplantation. Secondary outcomes included SSI, wound dehiscence, return to the OT for wound-related reasons, the presence of a clinically significant hematoma or perigraft fluid collection (PFC) requiring intervention, use of open NPWT dressing for wound management, length of stay, and time to complete wound healing. The baseline characteristics of the recipient and donor and the surgical factors and complications were collected and reported.
2.1.4 Definitions
Wounds were considered healed when the skin edge remained intact and approximated at the surgical incision site with no fluid drainage and no infection (27). In this study, a “wound complication” was defined as any SSI, wound dehiscence, perigraft lymphocele, or hematoma.
SSIs were defined according to the Australian Commission on Safety and Quality in Health Care guidelines (Supplementary File 2) (28), and wounds were assessed according to the “ASEPSIS” criteria developed by Wilson et al. (29) (Supplementary File 3).
2.2 Data collection and analysis
All data were collected and managed using a secure REDCap database (30). Comparisons were made between those who received conventional dressing and those who received ciNPWT dressing in the unmatched cohort. Analysis of an obese subset (BMI ≥30 kg/m2) and propensity score matching (PSM) were performed (see Supplementary File 1 for the study design diagram and flowchart). Adverse events and deviations from the protocol were recorded. Missing data were noted and the data was analyzed accordingly. Continuous variables were presented as median with interquartile range, and the Mann–Whitney U test was used to compare non-normally distributed variables. Categorical variables were presented as number and percentage of the total and were compared using the χ2 statistic. Fisher’s exact test was used where the cell counts were less than 5. Power and sample size estimations were also performed prior to the study with an online tool (clincalc.com) using existing data on high-risk wound complications of the lower abdomen (17), where the rate of surgical complications in the control group was 16% and that in the intervention (ciNPWT) group was 5%. At a significance level of 0.05 and at 80% power, an estimated 236 subjects would be required to test the efficacy of ciNPWT. The results of this study were used to calculate the number needed to treat (NNT) with ciNPWT in order to prevent a wound complication and to calculate the post-hoc power of the study (clincalc.com). Binary logistic regression was used to determine the predictors associated with wound complications. Collinearity and outliers were assessed. Statistical significance was set at p < 0.05, and all statistical analyses were performed using the Statistical Package for the Social Sciences software, version 25.0 (SPSS Inc., Chicago, IL, USA).
PSM was performed in order to reduce selection bias and balance the variables between the study groups (31). PSM analysis was performed, where the 97 subjects in the conventional dressing group were matched to 30 subjects in the ciNPWT group based on similar propensity scores. A logistic regression model was used to estimate the propensity scores, which predicted the probability of being assigned to either the conventional dressing or the ciNPWT group (32). The covariates used for calculating the propensity scores were chosen based on the potential to predict outcome and treatment assignment (33), which included age, obesity, diabetes, history of smoking, and the use of additional immunosuppression. The nearest neighbor 1:1 propensity matching without replacement was used. A caliper width of 0.2 of the standard deviation of the logit of the propensity score was used (34). Adequacy of matching was performed, indicating that matching improved the overall balance. Diagnostic plots were generated and confirmed adequate matching (see Supplementary File 4) (35).
3 Results
In the unmatched cohort, 30 kidney transplant recipients received the ciNPWT dressing; in the same calendar year, 97 kidney transplant recipients received conventional dressing. There were 127 subjects in total, all of whom had follow-up and were included in the analysis. Table 1 summarizes the demographic data for the recipients and donors and the surgical factors in the unmatched cohort, obese subset, and PSM cohort.
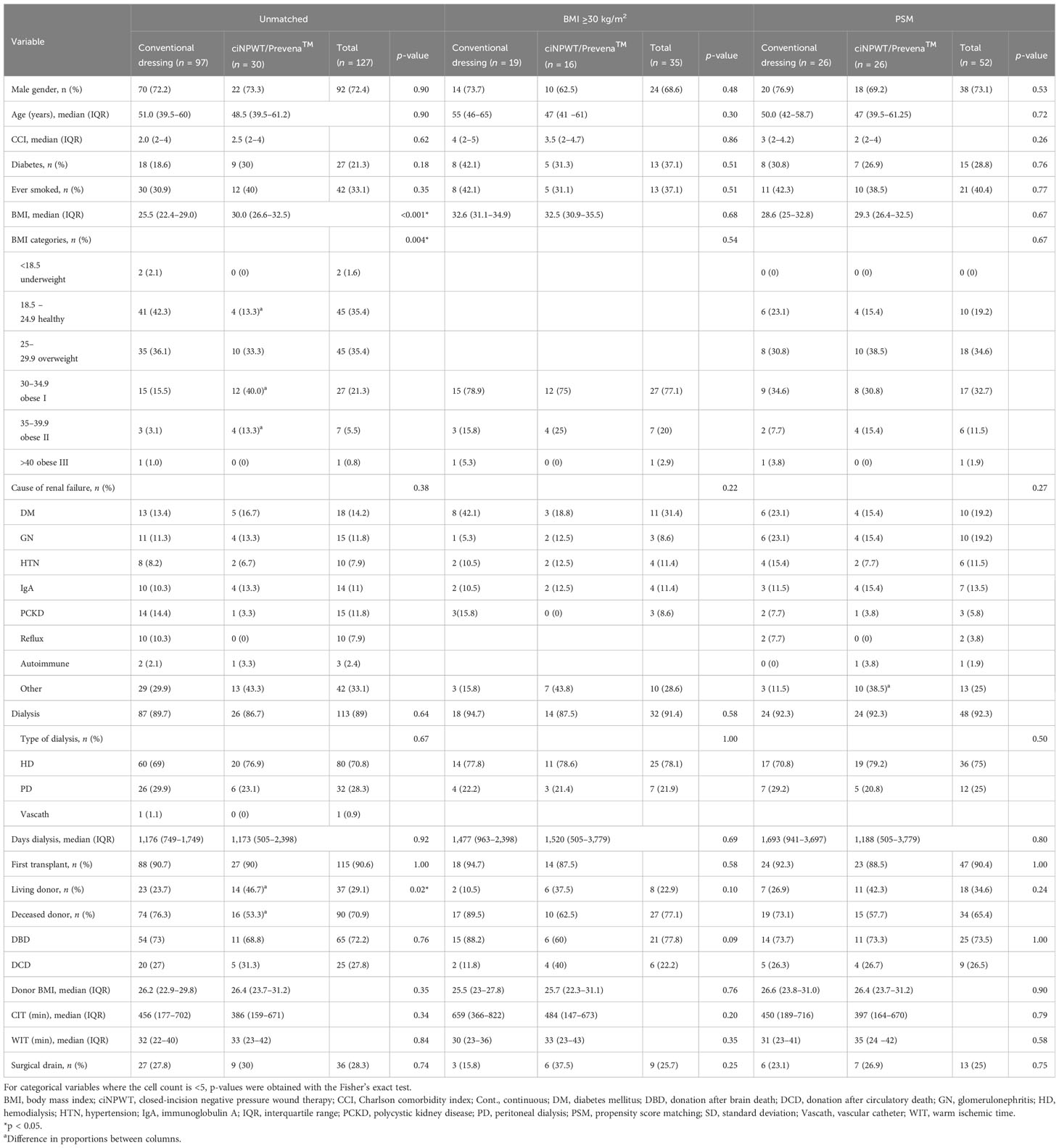
Table 1 Demographic data and donor and surgical data of kidney transplant recipients who had conventional dressing or ciNPWT with unmatched, obese, and propensity score matching analyses.
The two groups were similar across most baseline characteristics in the unmatched cohort (Table 1); however, the median recipient BMI was 30 kg/m2 (range, 26.6–32.5 kg/m2) in the ciNPWT group, which was higher compared to the 25.5 kg/m2 (range, 22.4–29.0 kg/m2) in the conventional dressing group (p < 0.001). In the ciNPWT group, there were more living donors compared with the conventional dressing group [46.7% (14/30) and 23.7% (23/97), respectively, p = 0.02].
The outcomes and complications for all kidney transplant recipients and the subset analyses are shown in Table 2. The primary study outcome of wound complications occurred in 21.3% (27/127) of all subjects overall. There was no significant difference in the wound complication rates between the ciNPWT (23.3%, 7/30) and conventional dressing (20.6%, 20/97) groups (p = 0.75). For secondary outcomes, the overall rate of SSI was 7.1% (9/127). The SSI rate was 13.3% (4/30) in the ciNPWT group and was 5.2% (5/97) in the conventional dressing group, which was not significantly different (p = 0.21). The overall wound dehiscence rate was 14.2% (18/127), of which 20% (6/30) occurred in the ciNPWT group and 12.4% (12/97) in the conventional dressing group, although the difference was not significant (p = 0.29) (Table 2). Deep fascial dehiscence occurred in three (3.1%) subjects in the conventional dressing group only, two of whom suffered concomitant deep SSI. The rates of return to OT for wound complications, intervention for PFC, hematoma, use of open NPWT, time to wound healing, and median length of stay were not different between the groups (Table 2). The median ASPESIS score was 15.5 (IQR = 4.2–30) in the ciNPWT group, which was higher compared to the ASEPSIS score of 0 (IQR = 0–5.5) in the conventional dressing group (p = 0.02).
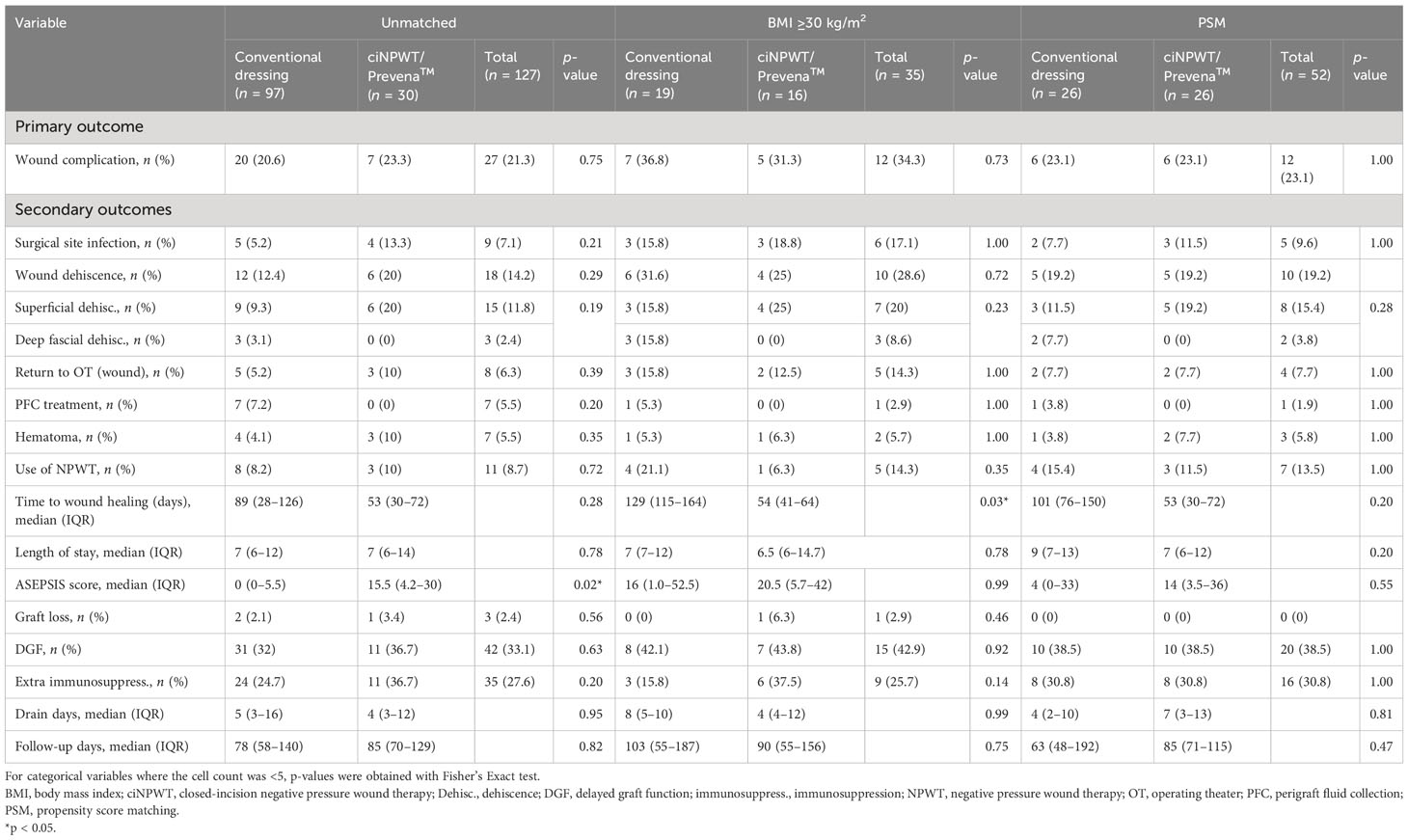
Table 2 Outcomes and complications in kidney transplant recipients who had conventional dressing or ciNPWT with unmatched, obese, and propensity score matching analyses.
There was one death, as well as three recipients with graft loss over the 3-month postoperative period (Table 2). There was no difference in DGF or the rates of additional immunosuppression between the dressing types. Median follow-up times were similar, 85 days (IQR = 70–129 days) and 78 days (IQR = 58–140 days) for those in the ciNPWT and conventional dressing groups, respectively (p = 0.82) (Table 2).
On univariable analysis, the variables age, obesity, and male gender were correlated with wound complications, but the dressing type was not (Table 3). On multivariable analysis, increased age was associated with decreased odds of wound complications [odds ratio (OR) = 0.95, 95% confidence interval (CI) = 0.92–0.99, p = 0.017], while obesity was associated with increased odds of wound complications (OR = 4.27, 95% CI = 1.44–12.69, p = 0.009).
3.1 Obese (BMI ≥30 kg/m2) subset
In total, there were 35 subjects with obesity, 16 in the ciNPWT group and 19 in the conventional dressing group. The baseline characteristics are presented in Table 1. The median BMI was 32.5 kg/m2 (IQR = 30.9–35.5 kg/m2) in the ciNPWT group and 32.6 kg/m2 (IQR = 31.1–34.9 kg/m2) in the conventional dressing group (p = 0.68). Wound complications occurred in 34.3% (12/35) of all subjects with obesity (Table 2). There were fewer wound complications in the ciNPWT group (31.3%, 5/16) compared with the conventional dressing group (36.8%, 7/19), although not significant (p = 0.73). Thus, based on these values, the NNT in order to prevent a wound complication in kidney transplant recipients with obesity was 18.
For the obese subset, the overall SSI rate was 17.1% (6/35), while the wound dehiscence rate was 28.6% (10/35), with no significant difference between the conventional and ciNPWT groups (Table 2). Three deep fascial dehiscence occurred in subjects with obesity who had conventional dressings only. Moreover, there was no difference in other secondary outcomes including intervention for PFC, hematoma, use of open NPWT, and return to OT (Table 2). The median time to wound healing in the obese subset was 54 days (IQR = 41–64 days) in the ciNPWT group and 129 days (IQR = 115–164 days) in the conventional dressing group, which was significantly different (p = 0.03). The median ASEPSIS scores were 20.5 (IQR = 5.7–42) and 16 (IQR = 1.0–52.5) in the ciNPWT and conventional dressing groups, respectively, which were not significantly different (p = 0.99) (Table 2).
3.2 Propensity score matching analysis
PSM generated 26 matched pairs in each group, with a total of 52 subjects. The baseline characteristics (Table 1), including BMI, were well matched between the conventional and ciNPWT groups [28.6 kg/m2 (IQR = 25.0–32.8 kg/m2) and 29.3 kg/m2 (IQR = 26.4–32.5 kg/m2), respectively, p = 0.67] (Table 1). The overall wound complications rate was 23.1% (12/52) and was equivalent in the conventional dressing and ciNPWT groups (Table 2). The overall SSI rate was 9.6% (5/52), while the overall wound dehiscence rate was 19.2% (10/52), with no difference between the dressing types for the other outcomes (Table 2).
3.3 Adverse events and deviations from protocol
Minor adverse events included small skin tears from the plastic drape of the ciNPWT in two subjects overall. Deviations from protocol occurred in five subjects. A total of three patients required a return to the OT for graft exploration and early removal of the ciNPWT dressing; of these patients, two developed small superficial wound dehiscence following primary re-closure. The third case developed a device malfunction from the overfilling of the 45-ml canister and was switched to low wall suction. This subject had a small hematoma, which was managed conservatively. The fourth case was non-compliant with wound management and subsequently developed superficial wound dehiscence and SSI, resulting in surgical debridement and open NPWT.
4 Discussion
Wound complications are a common cause of morbidity after kidney transplantation, particularly given the combination of a large lower abdominal incision and risk factors including immunosuppression, overweight/obesity, and diabetes (6, 36). Wound complications can be resource-intensive and complex to manage (11, 13, 37). Strategies to reduce and minimize wound complications are thus required to decrease any associated morbidity in kidney transplant recipients, which was the rationale behind evaluating the closed-incision negative pressure wound management system in this study.
This study reported on the use of a closed-incision negative pressure wound dressing in 30 kidney transplant recipients. The results did not demonstrate an improvement in the primary outcome of wound complications with the use of the ciNPWT dressing compared with conventional dressing in 97 subjects [23.3% (7/30) and 20.6% (20/97), respectively, p = 0.75]. After PSM, the proportion of recipients with obesity was balanced, and the 23.1% (6/26) rate of wound complications was equivalent in both groups, indicating that there was no benefit of the ciNPWT when taking into account all subjects and with balanced covariates. Further analysis in the obese subset (BMI ≥30 kg/m2) showed a reduction in wound complications with the use of the ciNPWT dressing compared to conventional dressing; however, it was not significantly different [31.1% (5/16) and 36.8% (7/19), respectively, p = 0.73], and the NNT to prevent one wound complication in an obese transplant recipient was 18.
The other outcomes including subcategories of SSI and wound dehiscence, return to OT for wound-related reasons, hematoma, use of open NPWT dressing for wound management, PFC treatment, length of stay, readmission to hospital, and time to complete wound healing were not significantly improved with the use of ciNPWT (Table 2).
While most baseline characteristics were similar across the dressing types, in the unmatched analysis, there was a significantly lower median BMI in the conventional dressing group (25.5 kg/m2, IQR = 22.4–29 kg/m2) compared with the ciNPWT group (30 kg/m2, IQR = 26.6–32.5 kg/m2, p < 0.001). This imbalance was likely due to the selection of high-risk patients, including those overweight and obese, which is strongly associated with wound complications (2, 4, 11, 38, 39). Given the potential for a selection bias of high-risk kidney transplant patients receiving ciNPWT, a significantly higher wound complication rate in this group was anticipated; however, there was no difference demonstrated between groups. This may indicate some benefit of the ciNPWT; however, given the small sample size, the results in this study may therefore be subject to a type II error.
The overall wound complication rate in this study was 21.3% (27/127), which was higher than the previously reported 30-day abdominal wall complication rate of 7.7% (64/828) in our unit (7). This may be due to the wider definition of wound complications used in this study. The overall rate of SSI was 7.1% (9/127), and it was 17.1% (6/35) in the obese subset (Table 3). These rates are consistent with those in the literature (2, 40). Open NPWT was utilized in 11 of the 27 (40.7%) patients with wound complications, which was higher than the previously reported 27% (17/64) in our unit (7). This reflects a change in wound management, with more expertise and preference for using NPWT over time.
In the analysis of the obese subset, 3 patients with conventional dressings developed deep fascial dehiscence and 2 had concomitant SSI, requiring reoperative surgery. Furthermore, in this subset, the median time to wound healing was lower in the ciNPWT group when compared with the conventional dressing group [54 days (IQR = 41–64) and 129 days (IQR = 115–164), respectively, p = 0.03] despite similar ASEPSIS scores (Table 2). However, this was no longer significant after PSM, suggesting the presence of confounding factors. The ciNPWT apparently redistributes lateral tensile strength at the superficial and deep suture lines and thus promotes wound healing of the skin and subcutaneous space (41). However, it is unclear how well ciNPWT can mitigate the effects of obesity, such as the traction effect of the abdominal pannus and tension across the transplant incision (11). Furthermore, ciNPWT may mitigate the effect of wound complications in certain conditions, although this remains unclear (41).
The type of dressing used was not associated with wound complications in the univariable analysis (Table 3), nor were surgical drains, diabetes, additional immunosuppression, donor type, or smoking. The multivariable analysis showed that obesity was associated with increased odds of wound complications (OR = 4.27, 95% CI = 1.44–12.69, p = 0.009), which is in keeping with the known risk factors for wound complications.
Evidence for the use of ciNPWT in kidney transplant recipients is lacking. The findings in our study are consistent with reports from other surgical specialties in the literature. A randomized control trial of 2,035 obese, high-risk women undergoing cesarean section demonstrated a 3% reduction in the absolute risk of SSI with ciNPWT compared to standard dressing, which was close to significance on per intention-to-treat analysis (p = 0.06) (42). While a Cochrane review indicated a probable reduction in SSI with ciNPWT compared with standard dressing [8.8% and 13%, respectively; relative risk (RR) = 0.66, 95% CI = 0.55–0.80, I2 = 23%], there was no clear difference in the wound dehiscence rates (5.3% and 6.2%, respectively; RR = 0.88, 95% CI = 0.69–1.13, I2 = 0%) and no effect on seroma or the reoperation rates (43). Some of these results differed from those of our study, which could be due to differences in the definitions of “high risk,” wound type, comorbidity profiles, and the intrinsic differences in healthcare settings.
The reported adverse events from ciNPWT use included skin blistering, which has restricted its use in orthopedic wounds until safety can be established (44). In our study, ciNPWT was well tolerated overall. While two patients suffered small skin tears, this was managed conservatively. Given that there were no significant adverse events, our study does support the safety of this dressing in kidney transplant recipients.
The strengths of this study are as follows: 1) it is the largest reported study evaluating the use of the Prevena™ ciNPWT in kidney transplant recipients; 2) the use of contemporaneous intervention and control groups, which reduced the potential for an era effect on the outcomes; 3) complete follow-up for the majority of patients; and 4) and only a small number of patients violated the study protocol.
The limitations of this study include the study design, given it is a non-randomized and non-blinded cohort, in addition to the small sample size. The sample size of 30 was determined by the number of ciNPWT dressings made available for the study by KCI-Acelity. Although within the required sample size limit for a pilot study, this made it an underpowered study, with post-hoc power calculation of 5.4% for the primary outcome of wound complications. Subset analysis was conducted to account for the imbalance in recipients with obesity in the intervention group. Furthermore, PSM was conducted to address the confounding factors and selection bias. Potential weaknesses include the recruitment and selection bias, a reporting bias or “Hawthorne effect” that describes changes in behavior as a result of being observed (45); however, blinding of the wound assessment for the ciNPWT group aimed to minimize this. Additional limitations include the different data handling and follow-up for the historical control group, although the end outcomes of all surgical outcomes, including wound complications, were routinely recorded for all transplant recipients.
One disadvantage of the Prevena™ ciNPWT dressing is its cost, i.e., AUD $295 per device. Furthermore, Prevena™ is single-use only, and early removal renders the dressing unusable. Future studies should include an economic analysis.
This study indicated no significant difference in the use of the Prevena™ ciNPWT dressing in reducing wound complications in kidney transplant recipients. However, this study provided information on the wound complication rates for kidney transplant recipients in the current era, particularly those who were obese and high-risk at our center. Furthermore, the findings from this study could be useful for informing future studies, including the protocol development for a randomized control trial (Trial ID: NCT03948412). Future research should include comparing different commercially available ciNPWT devices and the identification a subgroup of high-risk renal transplant recipients, such as those with obesity, and tailoring wound care.
5 Conclusion
This is the first reported cohort study evaluating the use of ciNPWT in kidney transplantation. While ciNPWT is safe and was well tolerated, it was not associated with a statistically significant reduction in wound complications when compared to conventional dressing. The findings from this study will be used to inform future studies associated with the use of ciNPWT in kidney transplantation.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Sydney Local Health District Human Research Ethics Committee (HREC/18/CRGH/127). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. AH: Data curation, Writing – original draft, Writing – review & editing. TY: Writing – original draft, Writing – review & editing. CS: Conceptualization, Writing – original draft, Writing – review & editing. DG: Writing – original draft, Writing – review & editing. HP: Writing – original draft, Writing – review & editing. SC: Writing – original draft, Writing – review & editing, Conceptualization. JL: Conceptualization, Formal analysis, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
This manuscript has been presented in thesis format and has fulfilled the requirements for the degree of Master of Philosophy, without conditions, with the Faculty of Medicine and Health, The University of Sydney. KCI and ACELITY, provided the use of the ciNPWT Prevena™ dressings in kind only (no funding or financial support was provided). KCI and ACELITY were not involved in any aspect of this study (this is an investigator led study). We would like to thank the staff working in the transplantation unit at the Royal Prince Alfred Hospital for their assistance with this study. We would like to thank Jim Mathews from the Sydney Informatics Hub, University of Sydney for his statistical support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material templates can be found in the Frontiers Word Templates file.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneph.2024.1352363/full#supplementary-material
Abbreviations
ASEPSIS, additional treatment, serous discharge, erythema, purulent exudate, separation of deep tissues, isolation of bacteria, and stay as inpatient prolonged over 14 days; BMI, body mass index; CCI, Charlson comorbidity index; CI, confidence interval; CIT, cold ischemic time; ciNPWT, closed-incision negative pressure wound therapy; DBD, donation after brain death; DCD, donation after circulatory death; DGF, delayed graft function; DM, diabetes mellitus; GN, glomerulonephritis; HD, hemodialysis; HTN, hypertension; IQR, interquartile range; IV, intravenous; KCI, Kinetics Concepts Incorporated; NNT, number needed to treat; NPWT, negative pressure wound therapy; OR, odds ratio; OT, operating theater; PCKD, polycystic kidney disease; PD, peritoneal dialysis; PDS, polydioxanone; PFC, perigraft fluid collection; PSM, propensity score matching; REDCap, Research Electronic Data Capture; RR, relative risk; SPSS, Statistical Package for the Social Sciences; SSI, surgical site infection; WIT, warm ischemic time.
References
1. Humar A, Ramcharan T, Denny R, Gillingham KJ, Payne WD, Matas AJ. Are wound complications after a kidney transplant more common with modern immunosuppression? Transplantation. (2001) 72:1920–3. doi: 10.1097/00007890-200112270-00009
2. Lynch RJ, Ranney DN, Shijie C, Lee DS, Samala N, Englesbe MJ. Obesity, surgical site infection, and outcome following renal transplantation. Ann surg. (2009) 250:1014–20. doi: 10.1097/SLA.0b013e3181b4ee9a
3. Røine E, Bjørk I, Øyen O. Targeting risk factors for impaired wound healing and wound complications after kidney transplantation. Transplant Proc. (2010) 42:2542–6. doi: 10.1016/j.transproceed.2010.05.162
4. Zrim S, Furlong T, Grace BS, Meade A. Body mass index and postoperative complications in kidney transplant recipients. Nephrology. (2012) 17:582–7. doi: 10.1111/j.1440-1797.2012.01621.x
5. Chen X, Liu L, Nie W, Deng R, Li J, Fu Q, et al. Vacuum sealing drainage therapy for refractory infectious wound on 16 renal transplant recipients. Transplant Proc. (2018) 50:2479–84. doi: 10.1016/j.transproceed.2018.04.014
6. Wszola M, Kwiatkowski A, Ostaszewska A, Gorski L, Kuthan R, Sawicka-Grzelak A, et al. Surgical site infections after kidney transplantation–where do we stand now? Transplantation. (2013) 95:878–82. doi: 10.1097/TP.0b013e318281b953
7. Lau NS, Ahmadi N, Verran D. Abdominal wall complications following renal transplantation in adult recipients - factors associated with interventional management in one unit. BMC surg. (2019) 19:10. doi: 10.1186/s12893-019-0468-x
8. Lam S, Laurence J, Verran D. The surgical approach for obtaining abdominal wall closure in renal transplant recipients with temporary or permanent loss of fascial integrity following emergency reoperative surgery. OBM Transplantation. (2019) 3. doi: 10.21926/obm.transplant.
9. ACSQHC. Australian Commission on Safety and Quality in Health Care. Approaches to Surgical Site Infection Surveillance. Sydney: Australian Commission on Safety and Quality in Health Care (2017).
10. Andersson AE, Bergh I, Karlsson J, Nilsson K. Patients' experiences of acquiring a deep surgical site infection: An interview study. Am J infection control. (2010) 38:711–7. doi: 10.1016/j.ajic.2010.03.017
11. Fockens MM, Alberts VP, Bemelman FJ, van der Pant KA, Idu MM. Wound morbidity after kidney transplant. Prog Transplantation. (2015) 25:45–8. doi: 10.7182/pit2015812
12. Kirkland KB, Briggs JP, Trivette SL, Wilkinson WE, Sexton DJ. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infection Control Hosp Epidemiol. (2015) 20:725–30. doi: 10.1086/501572
13. Willy C, Engelhardt M, Stichling M, Grauhan O. The impact of surgical site occurrences and the role of closed incision negative pressure therapy. Int Wound J. (2016) 13:35–46. doi: 10.1111/iwj.12659
14. ACSQHC. Australian Commission on Safety and Quality in Health Care. National Strategy to Address Health Care Associated Infections. (2003).
15. KCI. Prevena Incision Management System Clinician Guide San Antonio. TX. KCI - Kinetic Concepts Incorporated (2013).
16. Allegranzi B, Zayed B, Bischoff P, Kubilay NZ, de Jonge S, de Vries F, et al. New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Diseases. (2016) 16:e288–303. doi: 10.1016/S1473-3099(16)30402-9
17. Gunatilake RP, Swamy GK, Brancazio LR, Smrtka MP, Thompson JL, Gilner JB, et al. Closed-incision negative-pressure therapy in obese patients undergoing cesarean delivery: A randomized controlled trial. AJP Rep. (2017) 7:e151–e7. doi: 10.1055/s-0037-1603956
18. Hyldig N, Vinter CA, Kruse M, Mogensen O, Bille C, Sorensen JA, et al. Prophylactic incisional negative pressure wound therapy reduces the risk of surgical site infection after caesarean section in obese women: a pragmatic randomised clinical trial. BJOG an Int J obstetrics gynaecol. (2019) 126:628–35. doi: 10.1111/1471-0528.15413
19. Curran T, Alvarez D, Pastrana Del Valle J, Cataldo TE, Poylin V, Nagle D. Prophylactic closed-incision negative-pressure wound therapy is associated with decreased surgical site infection in high-risk colorectal surgery laparotomy wounds. Colorectal Disease. (2019) 21:110–8. doi: 10.1111/codi.14350
20. Li P-Y, Yang D, Liu D, Sun S-J, Zhang L-Y. Reducing surgical site infection with negative-pressure wound therapy after open abdominal surgery: a prospective randomized controlled study. Scandinavian J Surg. (2017) 106:189–95. doi: 10.1177/1457496916668681
21. O’leary PD, Peirce CC, Anglim CB, Burton CM, Concannon CE, Carter CM, et al. Prophylactic negative pressure dressing use in closed laparotomy wounds following abdominal operations: A randomized, controlled, open-label trial: the P.I.C.O. Trial. Ann Surg. (2017) 265:1082–6. doi: 10.1097/SLA.0000000000002098
22. Bozkurt B, Tokac M, Dumlu E, Yalcin A, Kilic M. Our first experience with negative pressure incision management system implemented on the clean surgical incision in the renal transplantation recipient: a case report. Transplant Proc. (2015) 47:1515–7. doi: 10.1016/j.transproceed.2015.04.057
23. Lam S. Evaluating the Closed Incision Negative Pressure Wound Therapy System on Wound Complications in Kidney Transplant Recipients [Masters by Research ]. Sydney: The University of Sydney (2022).
24. Wan SS, Chadban SJ, Watson N, Wyburn K. Development and outcomes of de novo donor specific antibodies in low, moderate and high immunological risk kidney transplant recipients. Am J Transplantation. (2019) 20:1351–64. doi: 10.1111/ajt.15754
25. Chadban S, Eris J, Wyburn K, Jones C, Patwardhan A, Burton M, et al. Renal Transplant Protocol 2014. 1 ed. Royal Prince Alfred Hospital (2014).
26. Raman A, Lam S, Vasilaras A, Joseph D, Wong J, Sved P, et al. Influence of ureteric anastomosis technique on urological complications after kidney transplantation. Transplant Proc. (2013) 45:1622–4. doi: 10.1016/j.transproceed.2013.01.084
27. Mehrabi A, Fonouni H, Wente M, Sadeghi M, Eisenbach C, Encke J, et al. Wound complications following kidney and liver transplantation. Clin Transplant. (2006) 20:97–110. doi: 10.1111/j.1399-0012.2006.00608.x
28. ACSQHC. Australian Commission on Safety and Quality in Health Care. Surgical Site Infection (SSI) Definition. Canberra: ACT (2012).
29. Wilson A, Sturridge M, Treasure T, Grüneberg R. A scoring method (ASEPSIS) for postoperative wound infections for use in clinical trials of antibiotic prophylaxis. Lancet. (1986) 327:311–2. doi: 10.1016/S0140-6736(86)90838-X
30. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed informatics. (2009) 42:377–81. doi: 10.1016/j.jbi.2008.08.010
31. D'Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. (1998) 17:2265–81. doi: 10.1002/(ISSN)1097-0258
32. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. (2011) 46:399–424. doi: 10.1080/00273171.2011.568786
33. McMurry TL, Hu Y, Blackstone EH, Kozower BD. Propensity scores: methods, considerations, and applications in the Journal of Thoracic and Cardiovascular Surgery. J Thorac Cardiovasc surg. (2015) 150:14–9. doi: 10.1016/j.jtcvs.2015.03.057
34. Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm statistics. (2011) 10:150–61. doi: 10.1002/pst.433
36. Menezes FG, Wey SB, Peres CA, Medina-Pestana JO, Camargo LF. Risk factors for surgical site infection in kidney transplant recipients. Infection Control Hosp Epidemiol. (2008) 29:771–3. doi: 10.1086/589725
37. Cruickshank M, Ferguson J, Bull A. Reducing harm to patients from health care associated infection: the role of surveillance. Chapter 3: Surg site infection–an abridged version Healthcare infection. (2009) 14:109–14. doi: 10.1071/HI09912
38. Lafranca JA, IJzermans JN, Betjes MG, Dor FJ. Body mass index and outcome in renal transplant recipients: a systematic review and meta-analysis. BMC Med. (2015) 13:111. doi: 10.1186/s12916-015-0387-3
39. Johnson DW, Isbel NM, Brown AM, Kay TD, Franzen K, Hawley CM, et al. The effect of obesity on renal transplant outcomes. Transplantation. (2002) 74:675–81. doi: 10.1097/00007890-200209150-00015
40. Adamska Z, Karczewski M, Cichańska L, Więckowska B, Małkiewicz T, Mahadea D, et al. Bacterial infections in renal transplant recipients. Transplant Proc. (2015) 47:1808–12. doi: 10.1016/j.transproceed.2015.03.046
41. Wilkes RP, Kilpad DV, Zhao Y, Kazala R, McNulty A. Closed incision management with negative pressure wound therapy (CIM) biomechanics. Surg innovation. (2012) 19:67–75. doi: 10.1177/1553350611414920
42. Gillespie BM, Webster J, Ellwood D, Thalib L, Whitty JA, Mahomed K, et al. Closed incision negative pressure wound therapy versus standard dressings in obese women undergoing caesarean section: multicentre parallel group randomised controlled trial. BMJ. (2021) 373:n893. doi: 10.1136/bmj.n893
43. Norman G, Goh EL, Dumville JC, Shi C, Liu Z, Chiverton L, et al. Negative pressure wound therapy for surgical wounds healing by primary closure. Cochrane Database Syst Rev. (2020) 5:Cd009261. doi: 10.1002/14651858
44. Webster J, Scuffham P, Stankiewicz M, Chaboyer WP. Negative pressure wound therapy for skin grafts and surgical wounds healing by primary intention. Cochrane Database Syst Rev. (2014) 10:CD009261. doi: 10.1002/14651858.CD009261.pub3
Keywords: wound complication, kidney transplant, closed incision negative pressure wound therapy, closed incision negative pressure, closed incision management
Citation: Lam S, Huynh A, Ying T, Sandroussi C, Gracey D, Pleass HC, Chadban S and Laurence JM (2024) Prospective evaluation of a closed-incision negative pressure wound therapy system in kidney transplantation and its association with wound complications. Front. Nephrol. 4:1352363. doi: 10.3389/fneph.2024.1352363
Received: 08 December 2023; Accepted: 24 January 2024;
Published: 27 February 2024.
Edited by:
Masoud Sadeghi, Islamic Azad University, IranReviewed by:
Luca Neri, Fresenius Medical Care, ItalyPierpaolo Di Cocco, University of Illinois Chicago, United States
Copyright © 2024 Lam, Huynh, Ying, Sandroussi, Gracey, Pleass, Chadban and Laurence. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Susanna Lam, c3VzYW5uYS5zLmxhbUBnbWFpbC5jb20=
 Susanna Lam
Susanna Lam Annie Huynh2
Annie Huynh2 Charbel Sandroussi
Charbel Sandroussi Steve Chadban
Steve Chadban