- 1Clinical Medicine Division, Korea Institute of Oriental Medicine, Daejeon, South Korea
- 2Research and Development Institute, CY Pharma Co., Seoul, South Korea
- 3Chung-Yeon Central Institute, Gwangju, South Korea
- 4Chung-Yeon Korean Medicine Hospital, Gwangju, South Korea
- 5Department of Oriental Neuropsychiatry, Dong-eui University College of Korean Medicine, Busan, South Korea
Background: This systematic review aimed to evaluate the effectiveness (functional outcomes and clinical symptoms) and safety (incidence of adverse events) of herbal medicine (HM) as monotherapy or adjunctive therapy to conventional treatment (CT) for traumatic brain injury (TBI).
Methods: We comprehensively searched 14 databases from their inception until July 2019. Randomized controlled trials (RCTs) using HM as monotherapy or adjunctive therapy to treat TBI patients were included. The primary outcome was functional outcomes, consciousness state, morbidity, and mortality. Meta-analysis was performed to calculate a risk ratio (RR) or mean difference (MD) with 95% confidence intervals (CIs), when appropriate data were available. Methodological quality of RCTs and the strength of evidence were also assessed.
Results: Thirty-seven RCTs with 3,374 participants were included. According to meta-analysis, HM as a monotherapy (RR 1.29, 95% CI: 1.21–1.37) or an adjunctive therapy to CT (RR 1.21, 95% CI: 1.16–1.27) showed significantly better total effective rate based on clinical symptoms, compared to CT alone. Subgroup analysis showed that HM had significantly improved post-concussion syndrome, dizziness, headache, epilepsy, and mild TBI, but not traumatic brain edema, compared to CT. Moreover, HM combined with CT had significantly improved post-concussion syndrome, mental disorder, headache, epilepsy, and mild TBI-like symptoms, but not cognitive dysfunction and posttraumatic hydrocephalus, compared to CT alone. When HM was combined with CT, functional outcomes such as activities of daily living and neurological function were significantly better than in patients treated using CT alone. In terms of the incidence of adverse events, HM did not differ from either CT (RR 0.88, 95% CI: 0.33–2.30) or placebo (RR 2.29, 95% CI: 0.83–6.32). However, HM combined with CT showed better safety profile than CT alone (RR 0.64, 95% CI: 0.44–0.93). Most studies had a high risk of performance bias, and the quality of evidence was mostly rated “very low” to “moderate,” mostly because the included studies had a high risk of bias and imprecise quantitative synthesis results.
Conclusion: The current evidence suggests that there is insufficient evidence for recommending HM for TBI in clinical practice. Therefore, further larger, high-quality, rigorous RCTs should be conducted.
Introduction
External force to the head can cause varying degrees of organic and/or functional abnormalities in the brain, ranging from mild to fatal. Traumatic brain injury (TBI) can be defined as “an alteration in brain function, or other evidence of brain pathology, caused by an external force” (1). TBI is a major threat to public health worldwide. In particular, this condition is an important cause of death and hospitalization (2). According to data from the Centers for Disease Control and Prevention (CDC) (3), the most common external causes of TBI are falls (common in childhood and in the elderly) and road traffic accidents (common in young adults). These results were confirmed in epidemiological studies carried out in Europe (2, 4). A recent systematic review of 82 population-based studies reporting the worldwide prevalence of TBI concluded that approximately 300 cases per 100,000 people occur per year, especially in Asia, with about 380 cases per 100,000, which is higher than the worldwide average (5).
Depending on the area and severity of the initial trauma, the severity of TBI can vary and is classified as mild, moderate, or severe using tools like the Glasgow Coma Scale (GCS) (6), which is based on the patient's state of consciousness (6). Many patients with TBI, even mild TBI, experience post-concussion syndrome (PCS), which involves a complex of symptoms including headache, dizziness, cognitive impairment, and neuropsychiatric symptoms (7). Moreover, TBI can cause persistent, sometimes life-long consequences, even in moderate or mild cases, and it can be associated with long-term negative outcomes that markedly reduce quality of life (QoL) of survivors, such as excess mortality, vegetative state, physical disability, cognitive impairment, depression, anxiety, psychosis, and seizures (8). In addition, TBI may be related to neurodegenerative diseases such as dementia (9), but not Parkinson's disease (10).
According to the CDC report (3), nearly half of patients with moderate-to-severe TBI undergoing inpatient rehabilitation experience pathological changes in their cognitive function between 1 and 5 years after injury (11). Therefore, to prevent long-term negative consequences and improve QoL, TBI requires long-term management as well as acute, post-injury treatment.
Complementary and integrative medicine (CIM) approaches, including acupuncture and herbal medicine (HM), are often used to supplement the limitations of conventional medicine (12, 13), improve effectiveness, and sometimes reduce side effects, even in the management of TBI (14, 15). In particular, HM has been used to manage brain trauma such as hemorrhage-related hydrocephalus (16), as well as long-term neurological diseases such as stroke (17), cerebral palsy (18), Parkinson's disease (19), vascular dementia (20), and Alzheimer's disease (21). In the field of brain trauma, common HMs such as Goreisan have been shown to prevent chronic subdural hematoma recurrence (22, 23), and the mechanism may involve the regulation of aquaporin, a water channel (24–26). Similarly, some HMs such as Yokukansan (27) and Xuefu Zhuyu decoction (28) have beneficial effects on TBI-related behavioral changes or cognitive impairment. In the management of TBI, HMs may have beneficial effects through complex mechanisms; they may reduce tumor necrosis factor-α or nitric oxide expression, improve blood-brain-barrier permeability, and reduce brain water content (29). However, no studies have yet synthesized all the clinical evidence for the effectiveness and safety of HM as an adjunctive or alternative therapy for various outcomes of TBI, including functional outcomes (mobility and global disability), mortality, quality of life, global clinical improvement, and adverse events. The present systematic review aimed to evaluate the effectiveness and safety of HM on these outcomes in TBI compared to placebo, no treatment, and conventional treatment (CT), to inform clinicians, policy makers, and patients in how to manage this disease.
Methods
Study Registration
The protocol of this systematic review has been published and registered in PROSPERO (registration number, CRD42018116559) (30), and the study was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (31) and the Cochrane Handbook for Systematic Reviews of Interventions (32).
Data Sources and Search Strategy
As previously described, the following 14 databases were searched comprehensively: five English-language databases (Medline via PubMed, EMBASE via Elsevier, the Cochrane Central Register of Controlled Trials [CENTRAL], the Allied and Complementary Medicine Database [AMED] via EBSCO, and the Cumulative Index to Nursing and Allied Health Literature [CINAHL] via EBSCO), five Korean-language databases (Oriental Medicine Advanced Searching Integrated System [OASIS], Korean studies Information Service System [KISS], Research Information Service System [RISS], Korean Medical Database [KMbase], and Korea Citation Index [KCI]), three Chinese-language databases (China National Knowledge Infrastructure [CNKI], Wanfang Data, and VIP), and one Japanese database (CiNii). The initial search date was December 2, 2018 and we conducted an updated search on July 27, 2019 to retrieve more up-to-date and comprehensive evidence. Additionally, we searched the reference lists of the relevant articles and performed a manual search on Google Scholar to identify further eligible studies. We also included “gray literature,” such as degree theses and conference proceedings, as well as the literature published in journals. There was no restriction on language, publication date, or publication status. The search strategies for all databases are available in Supplemental Digital Content 1.
Inclusion Criteria
Types of Studies
We included randomized controlled trials (RCTs) and excluded quasi-RCTs that used an inappropriate randomization method such as alternate allocation or allocation by birth date. Studies were excluded if they used the term “randomization” (随机) but failed to detail the randomization methods used. We included both parallel and crossover studies. Other study designs, such as in vivo, in vitro, case reports, and retrospective studies were excluded.
Types of Participants
We included studies involving patients diagnosed with TBI through medical or radiological examination, regardless of target symptoms, disease severity, sex, age, or race. We included all studies involving TBI patients, even if the diagnostic method of TBI was not clearly stated. We excluded studies that included participants with drug allergies or other serious medical conditions, such as cancer, liver disease, or kidney disease.
Types of Interventions
We included studies that used HM as a treatment intervention, regardless of which formulation of HM was used (e.g., decoction, tablets, capsules, pills, powders, and extracts); however, we only included studies in which HM was administered orally. We excluded studies that failed to detail the composition of the HM used, except when patent medicines were used whose composition could be found by searching the Internet. Studies comparing different types of HM were excluded. As control interventions, we included placebo, no treatment, and CT including surgery, medication, rehabilitation treatment, and psychotherapy for acute management and rehabilitation, which are baseline treatments for TBI. In the present study, acute management was defined as any treatment administered to stabilize the patients immediately after the injury (within 1 month). Rehabilitation was defined as any treatment of long-term impairments that aimed to restore to their previous level of health and was administered more than 1 month after injury (33). We included studies that combined HM with other therapies if the other therapies were used equally in both the treatment and control groups.
Types of Outcome Measures
The primary outcome measure was functional outcome, measured using the following validated scales: Barthel index (BI) (34), functional independence measurement (35), Fugl–Meyer assessment (36), and Glasgow Outcome Scale (GOS) (37). We also analyzed consciousness state measured using validated scales such as the GCS (38), with morbidity and mortality as primary outcome measures.
The secondary outcome measures were QoL, measured using validated assessment tools such as the 36-Item Short Form Health Survey (SF-36) (39), and adverse events (AEs), measured using the Treatment Emergent Symptom Scale (TESS) (40) or the incidence. We also analyzed the total effective rate (TER) as a secondary outcome; this is a non-validated outcome measure that is processed secondarily using certain evaluation criteria, such as improvement in clinical symptoms based on clinician ratings. In TER assessment, participants are generally classified as “cured” (痊愈), “markedly improved” (顯效), “improved” (有效), or “non-responsive” (無效) after treatment. The TER is calculated using the following formula: TER = N1 + N2 + N3/N, where N1, N2, N3, are the number of patients who are cured, markedly improved, and improved, respectively, while N is the total sample size. This outcome was considered a secondary outcome in this review as it lacks a unified standard and can be potentially heterogeneous.
Study Selection
As previously reported, two researchers (B. Lee and C-Y Kwon) independently selected the studies according to the above inclusion criteria. After removing duplicates, we screened the titles and abstracts of the retrieved studies for relevance; we then evaluated the full texts of the selected studies for final inclusion. Any disagreement was resolved through discussion with the other authors.
Data Extraction
Using a standardized data collection form in Excel 2007 (Microsoft, Redmond, WA, USA), two researchers (B. Lee and C-Y Kwon) independently extracted and double-checked the data from the included studies. Discrepancies were resolved through discussion with the other authors.
Using a predefined data collection form, we extracted information regarding the first author's name, publication year, country, institutional review board (IRB), informed consent, sample size, and number of dropouts, diagnostic criteria, participant details, intervention, comparisons, duration of intervention and follow-up, outcome measures, outcomes, and AEs. We also extracted details of the HM used, including the name, source, dosage form, and dosage of each medical substance, as well as the principles, rationale, and interpretation of the intervention in terms of the Consolidated Standards of Reporting Trials Extension for Chinese Herbal Medicine Formulas 2017 (41). If the data were insufficient or ambiguous, we contacted the corresponding authors of the included studies via e-mail to request additional information.
Quality Assessment
As previously reported, two researchers (B. Lee and C-Y Kwon) independently evaluated the risk of bias of the included studies and the quality of evidence of the main findings. We resolved discrepancies through discussion with other researchers.
We assessed the methodological quality of the included studies using the Cochrane Collaboration's risk of bias tool (42). The following items were evaluated as either “low risk,” “unclear,” or “high risk”: (1) random sequence generation, (2) allocation concealment, (3) blinding of participants and personnel, (4) blinding of outcome assessment, (5) completeness of outcome data, (5) selective reporting, and (6) other biases. In particular, we assessed other bias categories with an emphasis on baseline imbalance between the treatment and control groups in terms of participant characteristics such as mean age, sex, or disease severity, because baseline imbalance in factors that are strongly related to outcome measures can cause bias when estimating the intervention effect.
The quality of evidence for each main finding was assessed using the Grading of Recommendations Assessment, Development, and Evaluation approach (43), which uses the online program GRADEpro (https://gradepro.org/). The following items were evaluated as either “very low,” “low,” “moderate,” or “high”: risk of bias, inconsistency, indirectness, and imprecision of the results, and probability of publication bias.
Data Synthesis and Analysis
As previously described, we conducted descriptive analyses of the participants' details, interventions, and outcomes for all included studies. Using Review Manager version 5.3 software (Cochrane, London, UK), a meta-analysis was performed across studies that used the same types of intervention, comparison, and outcome measure. We pooled the dichotomous data using the risk ratio (RR) with 95% confidence intervals (CIs) and the continuous data using the mean difference (MD) with 95% CIs. We assessed clinical heterogeneity by comparing the distribution of important participant factors, such as age, sex, disease severity, and specific types of TBI, and we compared intervention factors such as co-interventions and control interventions among the included studies. Furthermore, statistical heterogeneity between the studies was assessed using both the chi-squared test and the I2 statistic; I2 ≥ 50% indicated substantial heterogeneity, while those ≥75% indicated high heterogeneity. In the meta-analyses, a random-effects model was used when the heterogeneity was significant (I2 ≥ 50%), while a fixed-effects model was used when the heterogeneity was not significant or when the number of studies included in the meta-analysis was <5, where estimates of inter-study variance have poor accuracy (44, 45). If the necessary data were available, we performed subgroup analyses to explain the heterogeneity or to assess whether the treatment effects varied between subgroups categorized according to the following criteria: (1) objective of interventions, such as acute management or rehabilitation, assessed in terms of time frame following injury; (2) severity of TBI, and (3) target symptoms, such as headache, dizziness, cognitive disorder, or mental disorder. To ascertain the robustness of the meta-analysis result, we conducted a sensitivity analyses by excluding (1) studies with a high risk of bias and (2) outliers that were numerically distant from the rest of the data.
Reporting Bias
We assessed reporting biases, such as publication bias, using funnel plots if more than 10 studies were included in the meta-analysis.
Results
Study Description
We identified 27,258 studies through database searching and one study from the references of the relevant studies. After removing duplicated studies, we considered 626 studies relevant after screening of the titles and abstracts. Among these, we finally included 37 studies with 3,374 participants (46–82) in the qualitative synthesis, and 33 studies with 3,000 participants (46–48, 50, 51, 53–59, 61–74, 76–82) in meta-analysis after screening of the full-text articles (Figure 1).
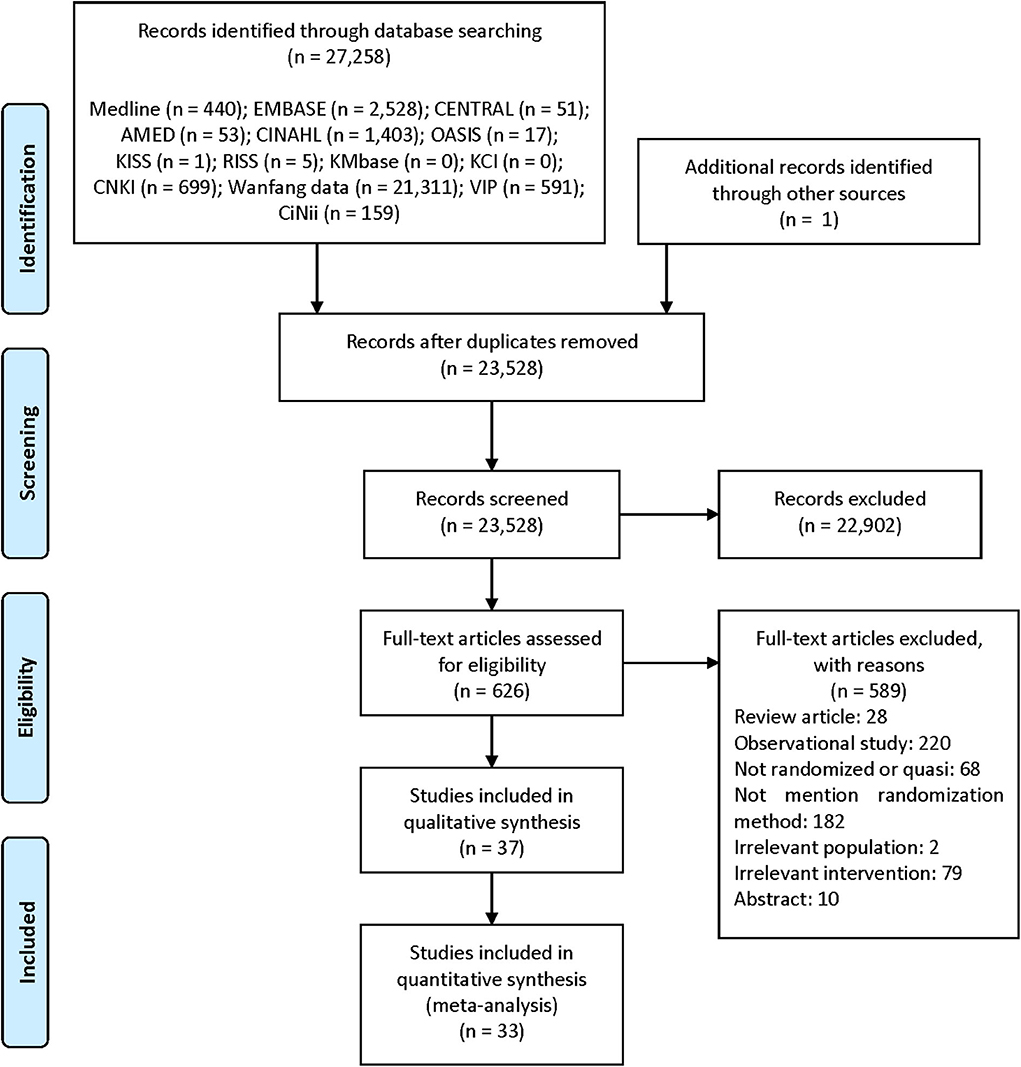
Figure 1. PRISMA flow diagram of the literature screening and selection processes Moher et al. (83). AMED, Allied and Complementary Medicine Database; CENTRAL, Cochrane Central Register of Controlled Trials; CINAHL, Cumulative Index to Nursing and Allied Health Literature; CNKI, China National Knowledge Infrastructure; KCI, Korea Citation Index; KISS, Koreanstudies Information Service System; KMbase, Korean Medical Database; OASIS, Oriental Medicine Advanced Searching Integrated System; RISS, Research Information Service System.
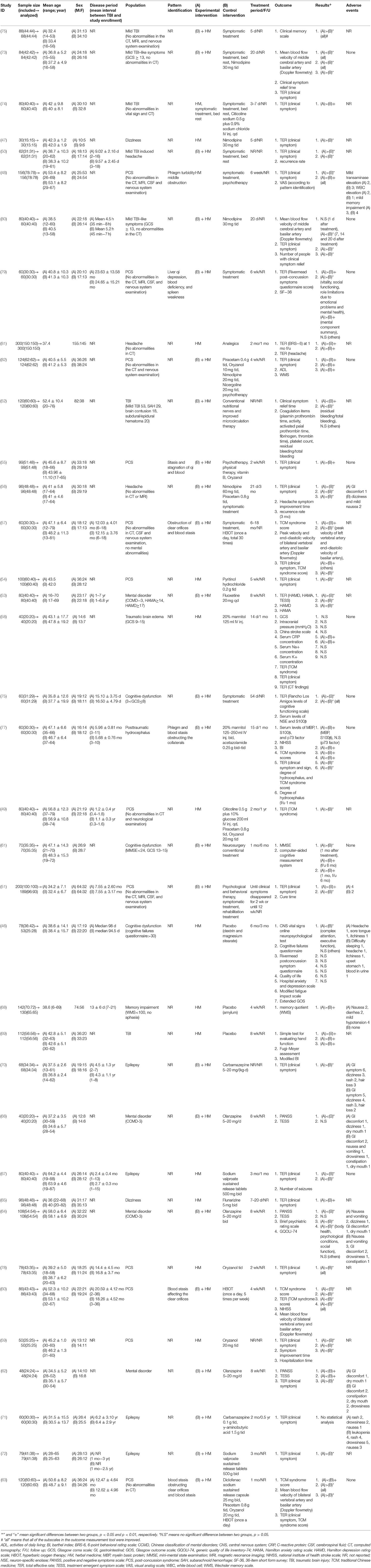
We have summarized the general characteristics of the included studies in Table 1. One study was conducted in New Zealand (46) and all others were conducted in China. The median sample size of the included studies was 80 participants (range: 30–300 participants), meanwhile, the median treatment period was 5 weeks (range: 3 days to 18 months). Eighteen studies (46, 49–51, 53, 57, 60, 63, 67, 68, 70–72, 76–80) reported the disease period of the participants; three of these (50, 68, 80) conducted treatment for acute management (from onset of injury to 1 month post-injury), while 11 (49, 51, 53, 57, 60, 63, 67, 70, 72, 77, 79) reported rehabilitation-focused treatment (>1 month post-injury). With regards to the specific symptoms treated, the included studies recruited patients with PCS (12 studies) (48, 49, 51, 54, 55, 57, 59, 60, 63, 78, 79, 82), mental disorder (four studies) (53, 62, 64, 66), cognitive dysfunction (four studies) (46, 61, 68, 76), epilepsy (four studies) (67, 70–72), mild TBI (four studies) (73–75, 80), headache (three studies) (50, 56, 81), dizziness (two studies) (47, 65), brain edema (one study) (58), and hydrocephalus (77).
Seven studies recruited participants based on pattern identification (an approach of some East-Asian traditional medicines, including traditional Chinese medicine, which enables individual treatment by categorizing the signs and symptoms of patients into a series of syndrome concepts): five based on “blood stasis” (55, 57, 60, 63, 77), two on “phlegm” (48, 77), and one on “liver qi depression, blood deficiency, and spleen weakness” (79). Eleven studies compared HM with CT (47–49, 54, 58, 59, 65, 67, 74, 78, 81), three compared HM with a placebo (46, 68, 69), and 23 compared HM plus CT with CT alone (50–53, 55–57, 61, 70, 73, 75–77, 79, 80, 82). The CTs included symptomatic treatment, routine rehabilitation care, psychotherapy, and Western medication. Nine studies (46, 49, 56, 58, 61, 67, 71, 77, 81) conducted follow-up after treatment, with the range of follow-up periods being 1 month to 1 year. Various outcome measures were used depending on the target population, with the most frequently used outcome being TER, assessed in 29 studies (46–51, 53–60, 62, 63, 65, 67, 70, 72–74, 76–82). Ten studies (46, 50, 52, 56, 57, 59, 64, 69, 76, 77) reported IRB approval, and 20 (46, 48, 50–52, 56–61, 63, 64, 66, 69, 76–79, 82) reported that they had received consent from the participants.
The included studies used a variety of HMs, with the most common being Xuefuzhuyu decoction (six studies) (50, 60, 62, 66, 67, 72), followed by the patented drug Yangxue Qingnao granules (four studies) (68, 73, 80, 82). In total, 89 different herbs were used in the included studies, with the most frequently used being Cnidii Rhizoma (27 studies), followed by Angelicae Gigantis Radix (25 studies), Persicae Semen (19 studies), Carthami Flos (17 studies), Bupleuri Radix (16 studies), Paeoniae Radix Rubra (16 studies), and Acori Graminei Rhizoma (15 studies) (Supplemental Digital Content 2).
Risk of Bias
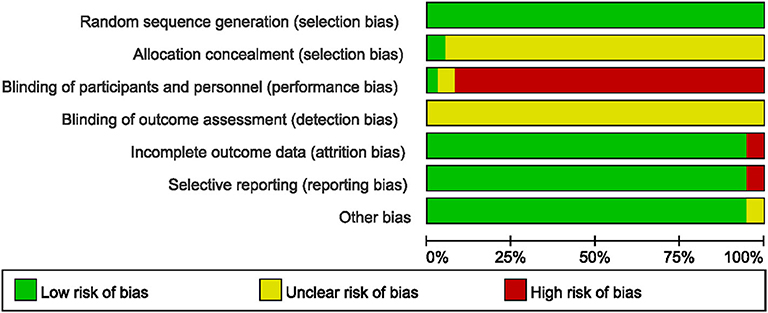
All the included studies reported appropriate random sequence generation methods; however, only two used a sealed opaque envelope (79) or independent allocation manager (46) to conceal allocation. Only one study (46) appropriately blinded both the participants and personnel, and two studies (68, 69) used placebo drugs as a control intervention but did not report appropriate blinding of personnel. None of the included studies reported blinding of the outcome assessor. Two studies (51, 68) that performed per-protocol analysis were assessed as having a high risk of attrition bias, while two (50, 51) that reported only TER, a secondary processed outcome without the raw data, were assessed as having a high risk of reporting bias. Thirty-five studies (46–51, 53–60, 62–82) reported no significant baseline difference in demographic data between the two groups, and were rated as having low risk of bias in the other potential sources of bias domains (Figures 2, 3).
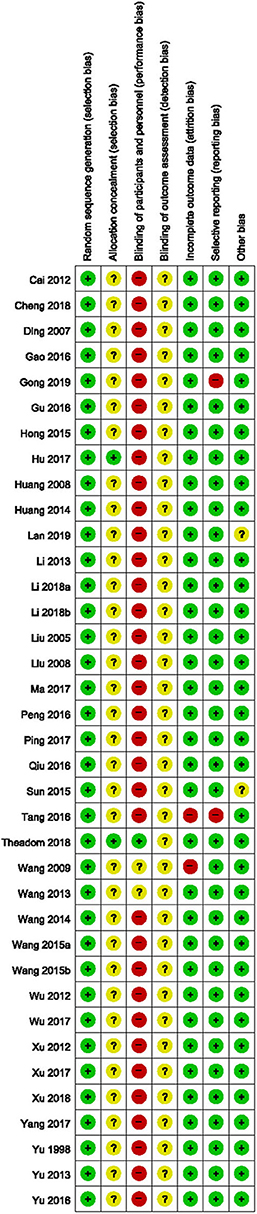
Figure 2. Risk of bias summary for all included studies. Low, unclear, and high risk, respectively, are represented with the following symbols: “+”, “?”, and “–”.
HM vs. CT
Effectiveness
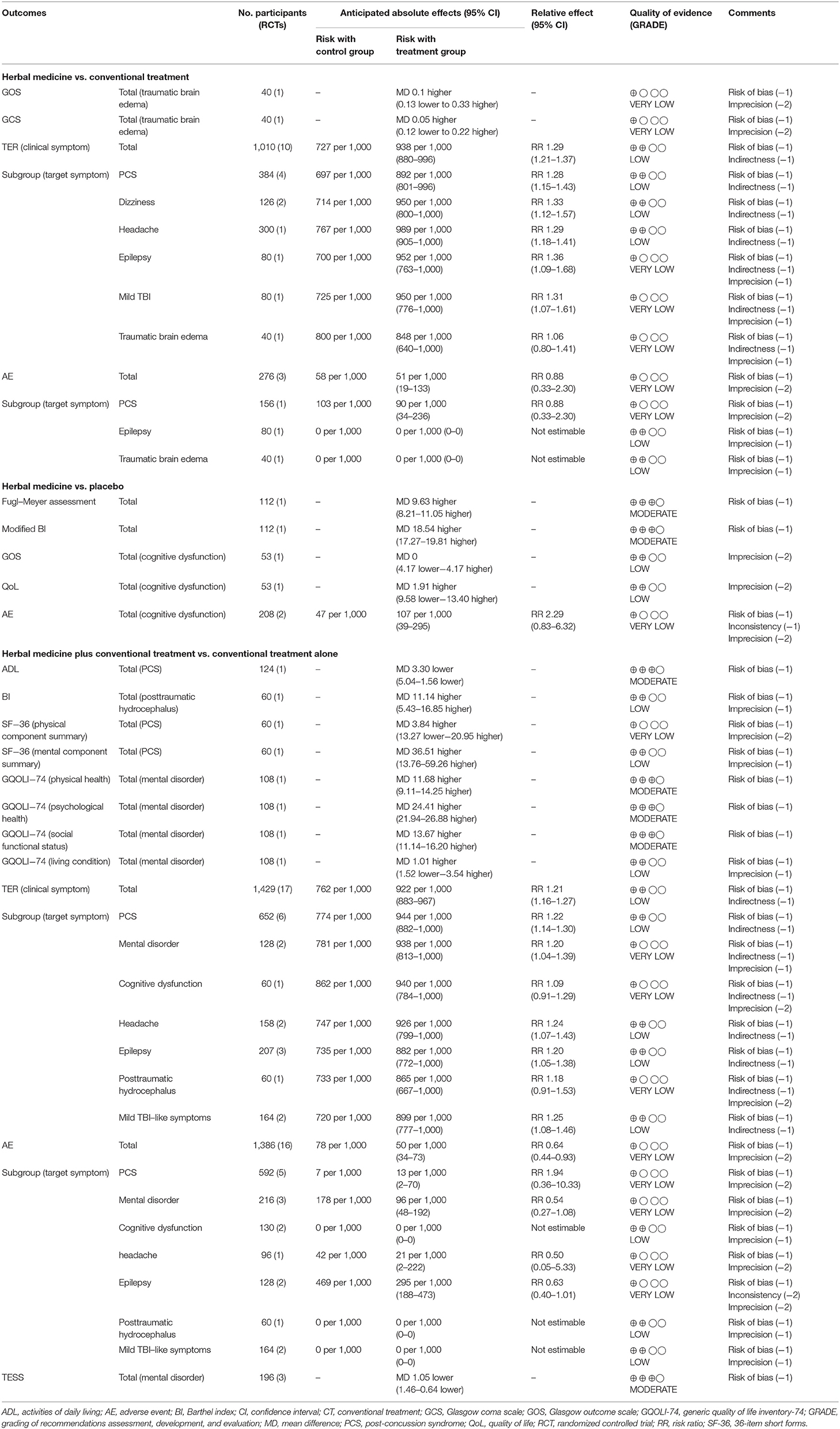
Eleven studies (47–49, 54, 58, 59, 65, 67, 74, 78, 81) were included in the comparison of effectiveness: five (48, 49, 54, 59, 78) were conducted on patients with PCS, (47, 65) two on patients with dizziness, one each on patients with headache (81), epilepsy (67), mild TBI (74), and traumatic brain edema (58). Although there were no differences in the functional outcomes and states of consciousness between two groups, HM group showed significantly better outcomes in TER based on clinical symptoms, symptom improvement time, and duration of hospitalization.
In one study involving traumatic brain edema (58), the groups did not differ in terms of functional outcome, as measured using the GOS, after 1 month of post-intervention follow-up (MD: 0.10, 95% CI: −0.13 to 0.33), nor did they differ in terms of consciousness state, measured using the GCS after 14 days of intervention (MD: 0.05, 95% CI: −0.12–0.22). In addition, the two groups did not differ in terms of intracranial pressure or neurological function, measured using the China stroke scale after treatment. However, in 10 studies, the TER based on clinical symptoms was significantly improved in the HM group (RR: 1.29, 95% CI: 1.21–1.37, I2 = 0%). In a subgroup analysis based on the target symptoms of TBI, the HM group showed significantly better outcomes in patients with PCS, dizziness, headache, epilepsy, and mild TBI of all causes except traumatic brain edema (Table 2; Figure 4) (Supplemental Digital Content 3).
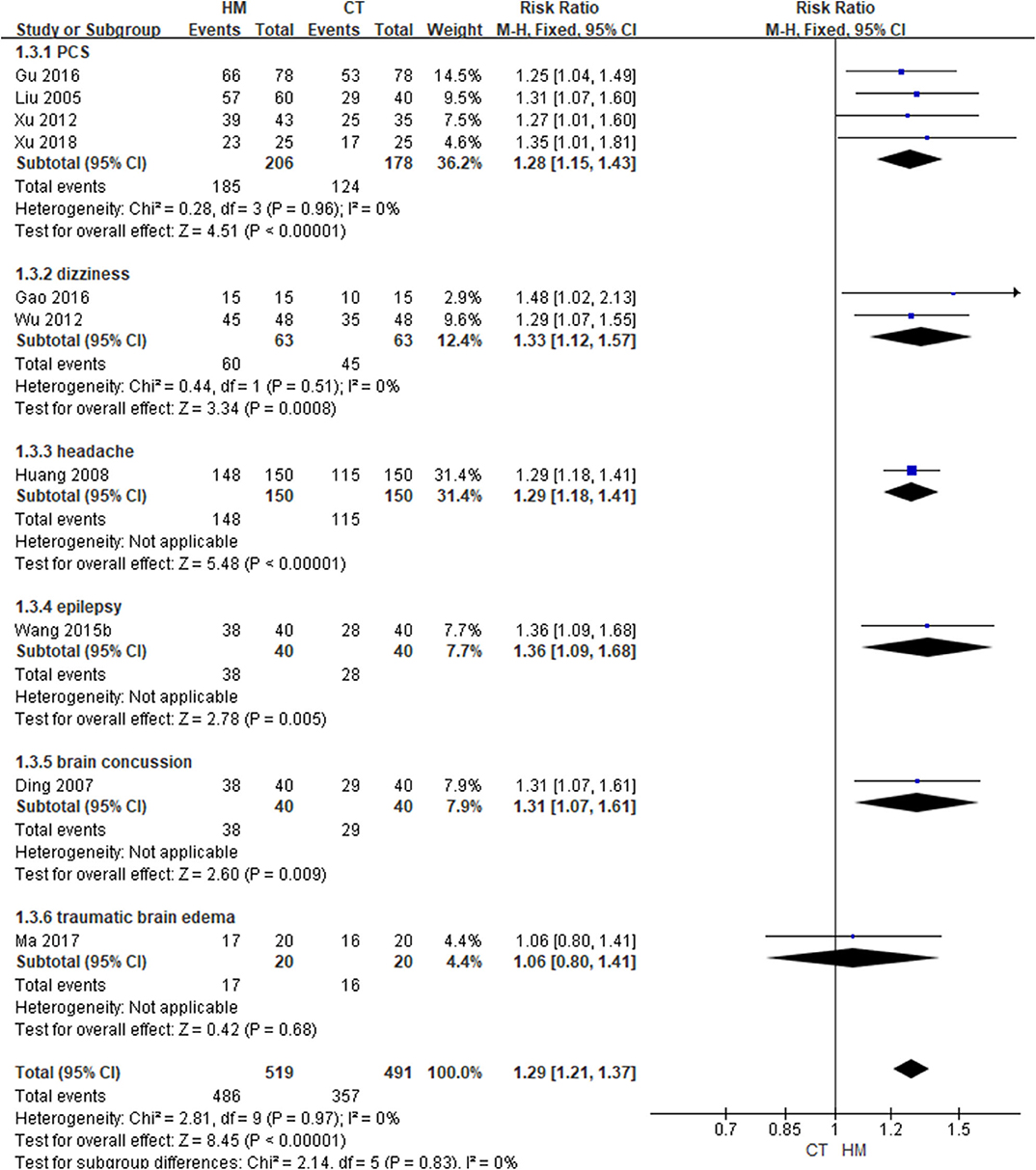
Figure 4. Total Effective rate based on clinical symptoms (Comparison of herbal medicine vs. conventional treatment).
In a study by Xu et al. (59), when HM was administered to patients with PCS, the symptom improvement time and hospitalization time were significantly shorter than in the CT group (P < 0.05, all). Wang and Tian (67) reported that, when HM was administered to patients of epilepsy, the number of seizures was significantly lower than in the CT group (P < 0.05).
Safety
Three studies reported AEs during the intervention, and a meta-analysis of these showed no difference in the incidence of AEs between the two groups (RR: 0.88, 95% CI: 0.33–2.30; Table 2) (Supplemental Digital Content 3).
HM vs. Placebo
Efficacy
Three studies (46, 68, 69) compared HM with a placebo. Two of these (46, 68) were conducted on patients with cognitive dysfunction, while the other one (69) did not include participants with specific symptoms. Collectively, the functional outcomes showed inconsistent results between studies, and there was no significant difference in QoL between two groups. However, memory impairment was improved more in the HM group.
In a study by Wang (69), the HM group showed improved functional outcomes, as assessed using the Fugl–Meyer assessment (MD: 9.63, 95% CI: 8.21–11.05) and modified BI (MD: 18.54, 95% CI: 17.27–19.81), after 8 weeks of treatment. Additionally, hand function in the HM group was significantly better than in the placebo group (P < 0.01). After patients with cognitive dysfunction were treated ifor 6 months (46), physical disability was measured using the GOS and QoL measured by the QoL after brain injury scale showed no significant differences between the two groups (GOS: MD, 0.00; 95% CI: −4.17 to 4.17; QoL after brain injury scale: MD, 1.91; 95% CI: −9.58 to 13.40; Table 2) (Supplemental Digital Content 3). In addition, after intervention, there were no significant differences between the groups in terms of neurobehavioral sequelae, mood, or fatigue. However, complex attention and executive functioning in the HM group were significantly better than in the placebo group (P < 0.05). In a study by Wang et al. (68) involving patients with memory impairment, the HM group showed significantly better memory quotient, measured using the Wechsler Memory Scale, than the placebo group after 4 weeks of treatment (P < 0.01). The results of sensitivity analysis by excluding low quality studies (that had 4 or less low risk of bias on the seven domains of the risk of bias tool) were consistent in GOS and QoL (Supplemental Digital Content 4).
Safety
Two studies (46, 68) recruiting patients with cognitive dysfunction reported AEs during the treatment period. There was no difference in the incidence of AEs between the two groups (RR: 2.29, 95% CI: 0.83–6.32, and I2 = 79%; Table 2; Figure 4) (Supplemental Digital Content 3), nor was there any difference between the two groups in a sensitivity analysis that excluded studies with a high risk of bias (Supplemental Digital Content 4).
HM Plus CT vs. CT Alone
Effectiveness
Twenty-three studies (50–53, 55–57, 61, 70, 73, 75–77, 79, 80, 82) compared effectiveness between HM plus CT and CT alone. Seven of these (51, 55, 57, 60, 63, 79, 82) were conducted on patients with PCS, four (53, 62, 64, 66) on patients with mental disorder, three on patients with epilepsy (70–72), three on patients with mild TBI (73, 75, 80), two on patients with cognitive dysfunction (61, 76), two on patients with headache (50, 56), and one each on patients with hydrocephalus (77) and TBI (52). In summary, the function and TER of various symptoms were significantly improved when HM was added to CT. However, there were inconsistent results in QoL between studies.
Huang and Li (82) conducted 4 weeks of treatment in patients with PCS; they found that activities of daily living were significantly better in the HM plus CT group than in the CT alone group (MD: −3.30, 95% CI: −5.04 to −1.56). Ping (77) conducted 15 days of treatment in patients with post-traumatic hydrocephalus; their results showed that functional outcomes, as measured using BI, were significantly better in the HM group (MD: 11.14, 95% CI: 5.43–16.85) (Table 2) (Supplemental Digital Content 3). When HM was added to the CT, there was a significant difference in neurological function after treatment compared to that with CT alone, as measured using the National Institute of Health Stroke Scale (NIHSS) (P < 0.01), and degree of hydrocephalus differed significantly between the groups after 1 month of post-intervention follow-up (P < 0.05) (77).
Two studies (64, 79) reported the QoL of patients after treatment. One (79) showed that patients with PCS treated using HM had significantly better mental component summary score, as measured using the SF-36 scale, than the CT alone group after 6 weeks of treatment (MD: 36.51, 95% CI: 13.76–59.26). However, there was no difference in physical component summary score (MD: 3.84, 95% CI: −13.27–20.95). Another study (64) treated patients with mental disorder for 8 weeks. The HM group showed significantly better scores in the areas of physical health, psychological health, and social functional status domain, measured using the generic QoL inventory 74. However, there was no difference between the groups in terms of living condition (physical health: MD, 11.68, 95% CI, 9.11–14.25; psychological health: MD, 24.41, 95% CI, 21.94–26.88; social functional status: MD, 13.67, 95% CI, 11.14–16.20; living condition: MD, 1.01, 95% CI,−1.52–3.54). The HM group showed significantly better TER, based on clinical symptoms (17 studies; RR: 1.21, 95% CI: 1.16–1.27, I2 = 0%) (Figure 5). In a subgroup analysis according to target symptoms of TBI, there were significant differences in PCS, mental disorder, headache, epilepsy, and mild TBI-like symptoms, but not in cognitive dysfunction or post-traumatic hydrocephalus (Table 2) (Supplemental Digital Content 3). However, a sensitivity analysis that excluded studies with a high risk of bias showed no difference in TER based on clinical symptoms between the two groups (Supplemental Digital Content 4).
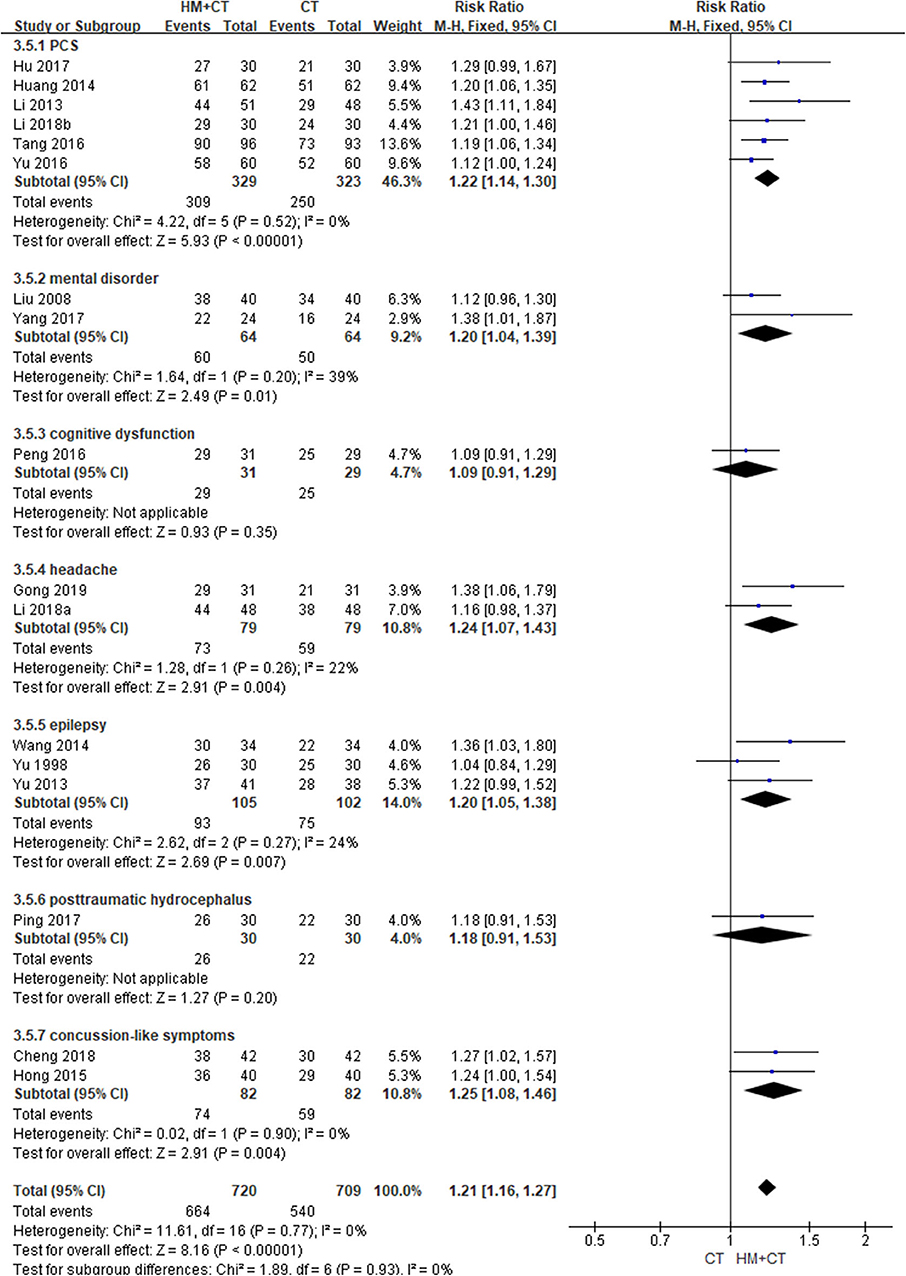
Figure 5. Total effective rate based on clinical symptoms (Comparison of herbal medicine combined with conventional treatment vs. conventional treatment alone).
When HM plus CT was administered to treat patients with PCS, neurological function, as measured using the NIHSS, was better than when CT alone was used (P < 0.05) (60), and cure time was significant shorter in the combination group (P < 0.05) (51). In patients with mental disorder after TBI, symptoms of depression (53), anxiety (53), and schizophrenia (62, 64, 66) were significantly better in the combination group than in the CT alone group (P < 0.05 in all cases). Furthermore, when HM plus CT was administered, cognitive function, as measured using the mini-mental state examination, was significantly improved (P < 0.05) (61), and the recurrence rate of headache was significantly lower than in the CT group (P < 0.05 in all cases) (50, 56). Two studies showed that clinical symptom relief time was significantly shorter in the combination group (P < 0.05 in all cases) (52, 73).
Safety
Sixteen studies (51, 55, 56, 61–64, 66, 70, 71, 73, 76, 77, 79, 80, 82) reported the incidence of AEs during the treatment period. The meta-analysis showed that the incidence of AEs was significantly lower in the HM plus CT group than in the CT alone group (RR: 0.64, 95% CI: 0.44–0.93, and I2 = 34%). Three studies (62, 64, 66) reported TESS scores after treatment in patients with mental disorder. The results showed that TESS scores were significantly lower in the combination group than in the CT group (MD: −1.05, 95% CI: −1.46 to −0.64, and I2 = 85%; Table 2; Figure 6) (Supplemental Digital Content 3).
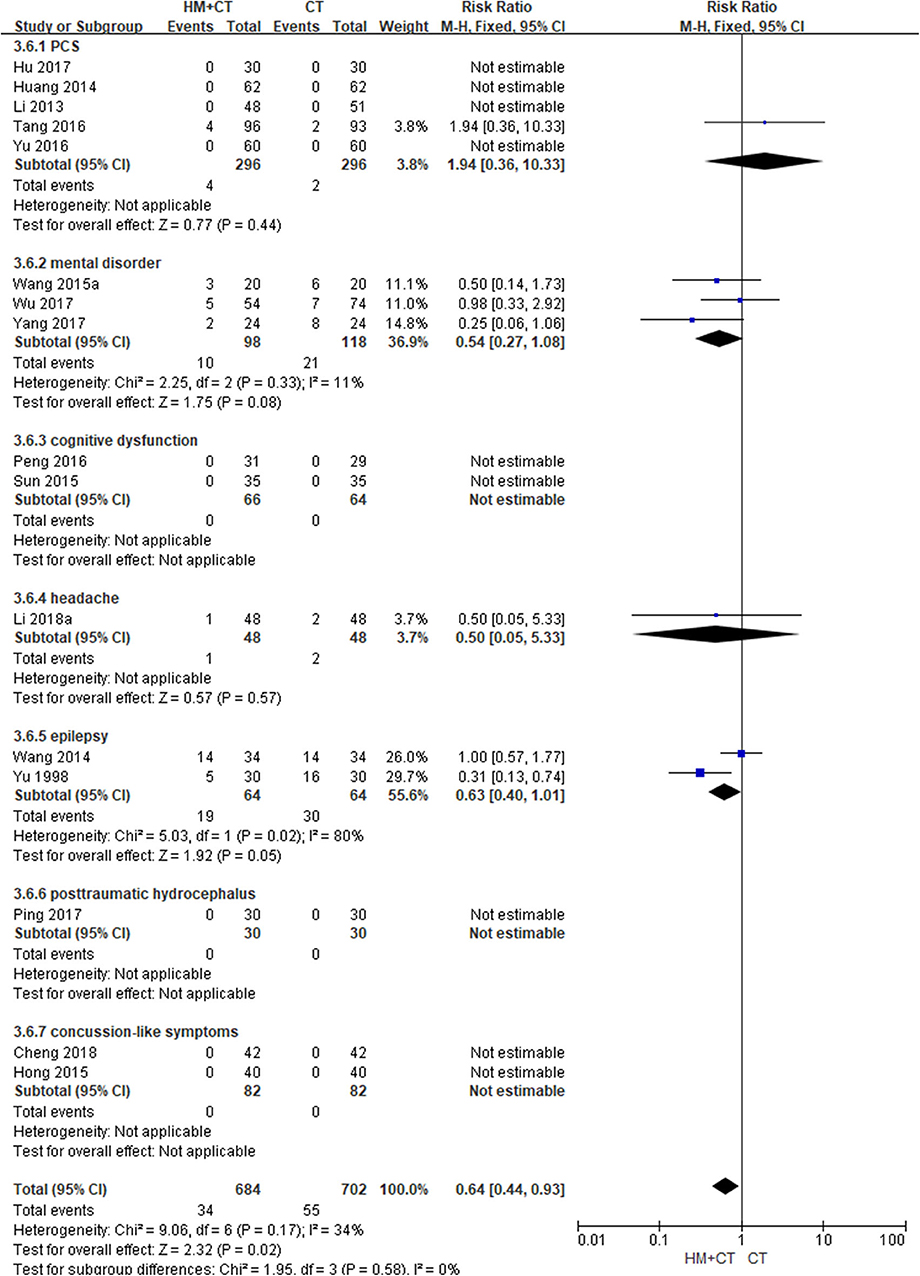
Figure 6. Adverse event (Comparison of herbal medicine combined with conventional treatment vs. conventional treatment alone).
Quality of Evidence
In the studies that compared HM with CT, the quality of evidence was graded as “very low” or “low” (Table 2). Additionally, the quality of evidence was graded as “very low” to “moderate” in studies that compared HM with a placebo, as well as in those that compared HM plus CT with CT alone (Table 2). The main reason for these low grades was the high risk of bias of the included RCTs. Furthermore, most findings had low precision because they did not fulfill the optimal sample size and had wide CIs. Indirect outcome measures also lowered the quality of evidence, especially in studies that measured TER as an outcome.
Publication Bias
No evidence of publication bias emerged from the funnel plots of TER based on clinical symptoms in studies that compared the effectiveness of HM with that of CT, or in studies that compared the effectiveness of HM plus CT with that of CT alone. Furthermore, the funnel plot comparing AE incidence between the HM plus CT group and the CT alone group was also symmetrical (Figure 7).
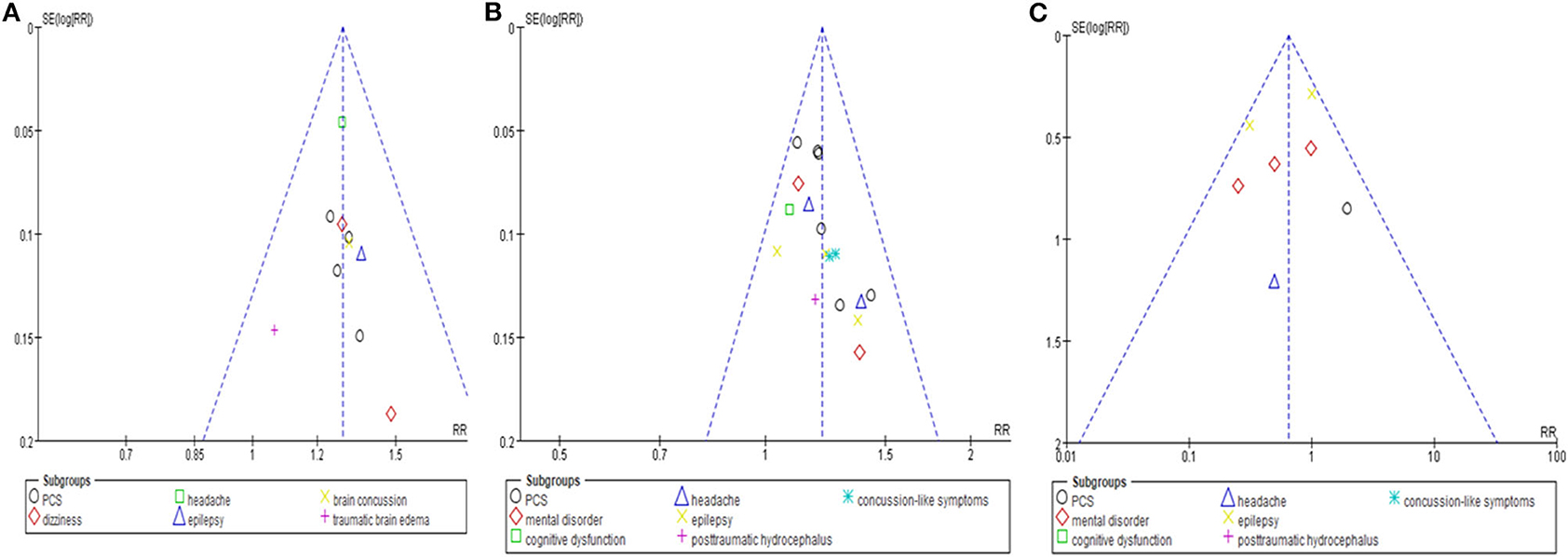
Figure 7. Funnel plots of the meta-analysis. (A) Total effective rate based on clinical symptom. Comparison: herbal medicine vs. conventional treatment. (B) Total effective rate based on clinical symptom. Comparison: herbal medicine combined with conventional treatment vs. conventional treatment. (C) Adverse events. Comparison: herbal medicine combined with conventional treatment vs. conventional treatment.
Discussion
This review aimed to assess the effectiveness and safety of HM as a monotherapy or adjunctive therapy to conventional treatment for TBI. We conducted a comprehensive and systematic search of English, Korean, Chinese, and Japanese-language databases and retrieved a total of 37 RCTs (46–82).
In summary, when comparing HM with CT, there was no conclusive evidence in functional outcome or consciousness state in patients with traumatic brain edema because there was only one study. However, the function measured by Fugl–Meyer assessment, BI, and NIHSS was significantly improved when HM was added to CT in studies that focused on symptomatic treatment or rehabilitation. Results regarding QoL were inconsistent between the two groups after treatment. The present meta-analysis showed that the TER of various symptoms showed significantly better results in the HM group in all comparisons. However, TER is a non-validated outcome measure that is secondarily processed, and thus, assertions regarding HM's effectiveness cannot be made confidently. Regarding the safety of HM, none of the study participants showed obvious abnormalities in electrocardiogram examinations or laboratory tests, such as the blood routine, urine routine, fecal routine, and liver and kidney function tests. There was no difference in the incidence of AEs between the two groups when HM monotherapy was compared with CT or placebo. Conversely, the incidence of AEs and TESS was significantly better in the HM plus CT group than in the CT alone group. However, the risk of bias in the included studies was generally high, whereas the quality of evidence of the main findings was generally low; thus, only limited confidence can be placed in the estimate of the effect, that is, the true effect may be different from the estimate.
Interestingly, pattern identification based on blood stasis was most frequently used in the included studies. In addition, the most commonly used HM was Xuefuzhuyu decoction, and the commonly used single herbs comprising the HM were Cnidii Rhizoma, Angelicae Gigantis Radix, Persicae Semen, Carthami Flos, and Paeoniae Radix Rubra, which improve blood stasis (84, 85). In East-Asian traditional medicine, blood stasis is considered the main pathology in traumatic injury (84). According to this pathological concept, blood stasis-removing therapy is widely used to treat TBI in clinical practice, and some clinical evidence has shown that blood stasis-removing HM is effective in the treatment of TBI (86, 87). Our review does not prove that blood stasis-removing HM is effective in improving TBI, but suggests that this type of herbal medicine is promising in the field of research for TBI treatment in the future.
Many studies have tried to explain the mechanism through which HM functions in TBI, showing that HM decreases neuronal injury by increasing superoxide dismutase and catalase activities, as well as by suppressing the expression of interleukin (IL)-1, IL-6, nuclear factor kappa B, and glial fibrillary acidic protein (88). Another study showed that HM protected a rat model of TBI, possibly via immune-promoting, anti-inflammatory, and neuroprotective effects (89). However, the underlying mechanism of HM in the treatment of TBI is still not fully understood; future studies should address this question to help establish an optimal management strategy for BI.
Our review had the following limitations. Firstly, although we conducted a systematic and comprehensive search in English, Korean, Chinese, and Japanese databases, most studies were conducted and published in China. This may have resulted in reporting biases, such as language and location bias. In addition, many studies assessed TER, which is a secondarily processed outcome measure according to certain criteria, and the meta-analysis showed significant results suggesting better outcomes in the HM group. However, this non-standardized outcome measure may have caused outcome reporting bias, and the results may not have been reliable. Secondly, most of the included studies were not of high quality. In particular, many had a high risk of performance bias. Therefore, our confidence in the effect estimate, as assessed using GRADE methodology, was low. Thirdly, we attempted to perform subgroup analysis in terms of either the objective of intervention (acute management or rehabilitation) or the TBI severity, as described in the study protocol (30). However, few studies clearly specified the objective of intervention or the severity of TBI in a subgroup analysis. Finally, although we performed subgroup analysis according to different target symptoms of TBI to address heterogeneity, we could not resolve clinical heterogeneity because the participants had diverse clinical characteristics and a wide range of interventions were used in the included studies. Relatedly, because the studies showed clinical heterogeneity, we performed only a few quantitative syntheses.
The following recommendations may be considered in future studies. To evaluate the effectiveness of HM in PCS, participants should be enrolled using standardized diagnostic criteria, such as the international statistical classification of diseases and related health problems or the diagnostic and statistical manual of mental disorders. In addition, the multi-compound, multi-target nature of HM may improve a wide range of symptoms after TBI, such as PCS; therefore, the underlying molecular mechanism of HM should be studied. Particularly, priority should be given to HM and/or herb, which are especially known for ameliorating blood stasis, in further HM researches on TBI. To optimize the use of HM during treatment of TBI and to resolve the clinical heterogeneity, future studies should characterize the participants in detail, with particular focus on TBI severity and target symptoms after TBI, such as headache, mental disorder, and cognitive dysfunction, and on the objectives of HM, such as acute management or rehabilitation. In PCS, validated disease specific tools should be adopted to evaluate the effect of HM on various symptoms and deficits; these may include the Rivermead Postconcussion Symptoms Questionnaire, the World Health Organization Disability Assessment Schedule 2.0, and the British Columbia Post-concussion Symptom Inventory-Short Form (90). Finally, only three of the retrieved studies compared HM with a placebo and these showed marked clinical heterogeneity, and thus, we could not draw a definite conclusion about the efficacy of HM. Blinding of participants and personnel using placebo with the same taste, flavor, and formulation should be conducted to avoid performance bias. In future, rigorously conducted, placebo-controlled trials to evaluate the efficacy of HM in TBI should be performed considering the above implications.
Conclusion
The current evidence suggests that there is insufficient evidence for recommending HM for TBI in clinical practice. Although some RCTs reported that HM as an adjuvant therapy to CT may have benefits for some functional outcomes of TBI, the low quality of evidence significantly limited its reliability. Therefore, further rigorous, well-designed, high quality, placebo-controlled RCTs should be conducted to confirm these results.
Data Availability Statement
The data used to support the findings of this study are included in the article.
Author Contributions
This study was conceptualized by JL. BL and C-YK performed the literature search, study selection, data extraction, and quality assessment using the risk of bias tool and GRADE approach. BL analyzed the data and C-YK critically double-checked the data analysis. BL and C-YK drafted the manuscript. All authors interpreted the data and critically reviewed the manuscript. The draft was reviewed and edited by HK, JL, and H-GJ. Resources were provided by JL. This study was supervised by HK and H-GJ. All authors approved the final manuscript.
Conflict of Interest
JL was employed by the company CY Pharma Co.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by both Chung-Yeon Medical Institute (Research Program 2018) and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. NRF-2019R1F1A1059310). The funding source will have no input on the interpretation or publication of the study results.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2020.00772/full#supplementary-material
Abbreviations
AEs, adverse events; AMED, the Allied and Complementary Medicine Database; BI, Barthel index; CDC, the Centers for Disease Control and Prevention; CENTRAL, the Cochrane Central Register of Controlled Trials; CIM, complementary and integrative medicine; CINAHL, the Cumulative Index to Nursing and Allied Health Literature; Cis, confidence intervals; CNKI, China National Knowledge Infrastructure; CT, conventional treatment; GCS, the Glasgow Coma Scale; GOS, Glasgow outcome scale; HM, herbal medicine; IRB, institutional review board; KCI, Korea Citation Index; KISS, Korean studies Information Service System; KMbase, Korean Medical Database; MD, mean difference; NIHSS, the National Institute Of Health Stroke Scale; OASIS, Oriental Medicine Advanced Searching Integrated System; PCS, post-concussion syndrome; PRISMA, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses; QoL, quality of life; RCTs, randomized controlled trials; RISS, Research Information Service System; RR, risk ratio; SF-36, the 36-Item Short Form Health Survey; TBI, traumatic brain injury; TER, total effective rate; TESS, the treatment emergent symptom scale.
References
1. Menon DK, Schwab K, Wright DW, Maas AI. Position statement: definition of traumatic brain injury. Arch Phys Med Rehabil. (2010) 91:1637–40. doi: 10.1016/j.apmr.2010.05.017
2. Majdan M, Plancikova D, Brazinova A, Rusnak M, Nieboer D, Feigin V, et al. Epidemiology of traumatic brain injuries in Europe: a cross-sectional analysis. Lancet Public health. (2016) 1:e76–e83. doi: 10.1016/S2468-2667(16)30017-2
3. Taylor CA, Bell JM, Breiding MJ, Xu L. traumatic brain injury-related emergency department visits, hospitalizations, and deaths - United States 2007 and 2013. MWR Surveill Summ. (2017) 66:1–16. doi: 10.15585/mmwr.ss6609a1
4. Majdan M, Plancikova D, Maas A. Years of life lost due to traumatic brain injury in Europe: A cross-sectional analysis of 16 countries. PLoS Med. (2017) 14:e1002331. doi: 10.1371/journal.pmed.1002331
5. Nguyen R, Fiest KM, McChesney J, Kwon CS, Jette N, Frolkis AD, et al. The international incidence of traumatic brain injury: a systematic review and meta-analysis. Can J Neurol Sci. (2016) 43:774–85. doi: 10.1017/cjn.2016.290
6. Reid P, Erdtmann F, Buono J, Butler D. Systems Engineering to Improve Traumatic Brain Injury Care in the Military Health System: Workshop Summary. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK214924/ (2009).
7. Bazarian JJ, Wong T, Harris M, Leahey N, Mookerjee S, Dombovy M. Epidemiology and predictors of post-concussive syndrome after minor head injury in an emergency population. Brain Inj. (1999) 13:173–89. doi: 10.1080/026990599121692
8. Stocchetti N, Zanier ER. Chronic impact of traumatic brain injury on outcome and quality of life: a narrative review. Crit Care. (2016) 20:148. doi: 10.1186/s13054-016-1318-1
9. Shively S, Scher AI, Perl DP, Diaz-Arrastia R. Dementia resulting from traumatic brain injury: what is the pathology? Arch Neurol. (2012) 69:1245–51. doi: 10.1001/archneurol.2011.3747
10. Kenborg L, Rugbjerg K, Lee PC, Ravnskjær L, Christensen J, Ritz B, et al. Head injury and risk for Parkinson disease: results from a Danish case-control study. Neurology. (2015) 84:1098–103. doi: 10.1212/WNL.0000000000001362
11. Whiteneck GG, Eagye CB, Cuthbert JP, Corrigan JD, Bell JM, Haarbauer-Krupa JK, et al. One and Five Year Outcomes After Moderate-to Severe Traumatic Brain Injury Requiring Inpatient Rehabilitation: Traumatic Brain Injury Report. Available online at: https://www.cdc.gov/traumaticbraininjury/pdf/CDC-NIDILRR-Self-Report-508.pdf (2018).
12. Galgano M, Toshkezi G, Qiu X, Russell T, Chin L, Zhao LR. Traumatic brain injury: current treatment strategies and future endeavors. Cell Transplant. (2017) 26:1118–30. doi: 10.1177/0963689717714102
13. Polich G, Iaccarino MA, Kaptchuk TJ, Morales-Quezada L, Zafonte R. Placebo effects in traumatic brain injury. J Neurotrauma. (2018) 35:1205–12. doi: 10.1089/neu.2017.5506
14. Gau BS, Yang HL, Huang SJ, Lou MF. The use of complementary and alternative medicine for patients with traumatic brain injury in Taiwan. BMC Complement Altern Med. (2012) 12:211. doi: 10.1186/1472-6882-12-211
15. Tan L, Zeng L, Wang N, Deng M, Chen Y, Ma T, et al. Acupuncture to promote recovery of disorder of consciousness after traumatic brain injury: a systematic review and meta-analysis. Evid Based Complement Alternat Med. (2019) 2019:5190515. doi: 10.1155/2019/5190515
16. Park B, Hong SC, Park SW, Yi CS, Ha YK, Choi DJ. Herbal medicine for hemorrhage-related hydrocephalus: a systematic review of randomised controlled trials. Complement Ther Med. (2018) 39:146–53. doi: 10.1016/j.ctim.2018.06.003
17. Han CH, Kim M, Cho SY, Jung WS, Moon SK, Park JM, et al. Adjunctive herbal medicine treatment for patients with acute ischemic stroke: a systematic review and meta-analysis. Complement Ther Clin Pract. (2018) 33:124–37. doi: 10.1016/j.ctcp.2018.09.007
18. Lee B, Kwon CY, Chang GT. Oriental herbal medicine for neurological disorders in children: an overview of systematic reviews. Am J Chin Med. (2018) 46:1701–26. doi: 10.1142/S0192415X18500866
19. Jin XC, Zhang L, Wang Y, Cai HB, Bao XJ, Jin YY, et al. An overview of systematic reviews of Chinese herbal medicine for Parkinson's disease. Front Pharmacol. (2019) 10:155. doi: 10.3389/fphar.2019.00155
20. Chan ES, Bautista DT, Zhu Y, You Y, Long JT, Li W, et al. Traditional Chinese herbal medicine for vascular dementia. Cochrane Database Syst Rev. (2018) 12:CD010284. doi: 10.1002/14651858.CD010284.pub2
21. Zhang Y, Noh K, Song W. Chinese herbal medicines on cognitive function and activity of daily living in senior adults with Alzheimer's disease: a systematic review and meta-analysis. Integr Med Res. (2019) 8:92–100. doi: 10.1016/j.imr.2019.04.006
22. Goto S, Kato K, Yamamoto T, Shimato S, Ohshima T, Nishizawa T. Effectiveness of goreisan in preventing recurrence of chronic subdural hematoma. Asian J Neurosurg. (2018) 13:370–4. doi: 10.4103/ajns.AJNS_174_16
23. Yasunaga H. Effect of Japanese herbal kampo medicine goreisan on reoperation rates after burr-hole surgery for chronic subdural hematoma: analysis of a national inpatient database. Evid Based Complement Alternat Med. (2015) 2015:817616. doi: 10.1155/2015/817616
24. Nakano T, Nishigami C, Irie K, Shigemori Y, Sano K, Yamashita Y, et al. Goreisan prevents brain edema after cerebral ischemic stroke by inhibiting aquaporin 4 upregulation in mice. J Stroke Cerebrovasc Dis. (2018) 27:758–63. doi: 10.1016/j.jstrokecerebrovasdis.2017.10.010
25. Yano Y, Yano H, Takahashi H, Yoshimoto K, Tsuda S, Fujiyama K, et al. Goreisan inhibits upregulation of aquaporin 4 and formation of cerebral edema in the rat model of juvenile hypoxic-ischemic encephalopathy. Evid Based Complement Alternat Med. (2017) 2017:3209219. doi: 10.1155/2017/3209219
26. Nagai K, Isohama Y, Koga T, Ashizuka T, Hisatsune A, Miyata T. Effect of herbal extracts and minerals on aquaporin-mediated water transport across plasma membrane. J Pharmacol Sci. (2005) 97:103.
27. Kan'o T, Han JY, Nakahara K, Konno S, Shibata M, Kitahara T, et al. Yokukansan improves distress of medical staff, and cognitive function and motivation in patients with destructive and aggressive behaviors after traumatic brain injury. Acute Med Surg. (2014) 1:88–93. doi: 10.1002/ams2.24
28. Xing Z, Xia Z, Peng W, Li J, Zhang C, Fu C, et al. Xuefu Zhuyu decoction, a traditional Chinese medicine, provides neuroprotection in a rat model of traumatic brain injury via an anti-inflammatory pathway. Sci Rep. (2016) 6:20040. doi: 10.1038/srep20040
29. Yang B, Wang Z, Sheng C, Wang Y, Zhou J, Xiong XG, et al. Evidence-based review of oral traditional Chinese medicine compound recipe administration for treating weight drop-induced experimental traumatic brain injury. BMC Complement Altern Med. (2016) 16:95. doi: 10.1186/s12906-016-1076-2
30. Lee B, Leem J, Kim H, Jo HG, Yoon SH, Shin A, et al. Herbal medicine for acute management and rehabilitation of traumatic brain injury: a protocol for a systematic review. Medicine (Baltimore). (2019) 98:e14145. doi: 10.1097/MD.0000000000014145
31. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. (2009) 6:e1000100. doi: 10.1371/journal.pmed.1000100
32. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0. The Cochrane Collaboration. Available online at: http://handbook-5-1.cochrane.org/ (2011).
33. Wong V, Cheuk DK, Lee S, Chu V. Acupuncture for acute management and rehabilitation of traumatic brain injury. Cochrane Database Syst Rev. (2013) 28:CD007700. doi: 10.1002/14651858.CD007700.pub3
34. Mahoney FI, Barthel DW. Functional evaluation: the barthel index. Maryland State Med J. (1965) 14:61–5. doi: 10.1037/t02366-000
35. Stineman MG, Maislin G. Validity of functional independence measure scores. Scand J Rehabil Med. (2000) 32:143–4. doi: 10.1080/003655000750045505
36. Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med. (1975) 7:13–31.
37. Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. (1975) 1:480–4. doi: 10.1016/S0140-6736(75)92830-5
38. Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. (1974) 2:81–4. doi: 10.1016/S0140-6736(74)91639-0
39. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. (1992) 30:473–83. doi: 10.1097/00005650-199206000-00002
40. National Institute of Mental Health. TESS (Treatment Emergent Symptom Scale-Write-in). Psychopharmacol Bull. (1985) 21:1069–72.
41. Cheng CW, Wu TX, Shang HC, Li YP, Altman DG, Moher D, et al. CONSORT extension for chinese herbal medicine formulas 2017: recommendations, explanation, and elaboration. Ann Intern Med. (2017) 167:112–21. doi: 10.7326/M16-2977
42. Higgins JPT, Altman DG. Chapter 8: assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions, Version. 5.1.0. The Cochrane Collaboration. Available online at: http://handbook-5-1.cochrane.org/ (2011).
43. Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. (2011) 64:401–6. doi: 10.1016/j.jclinepi.2010.07.015
44. Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. (2010) 1:97–111. doi: 10.1002/jrsm.12
45. Murad MH, Montori VM, Ioannidis JPA, Guyatt G, Rennie D, Meade MO, et al. Chapter 25.1: fixed-effects and random-effects models. In users' guide to the medical literature. In: Guyatt G, Rennie D, Meade MO, Cook DJ, editors. A Manual for Evidence-Based Clinical Practice. 3rd ed. New York, NY: McGraw-Hill (2015).
46. Theadom A, Barker-Collo S, Jones KM, Parmar P, Bhattacharjee R, Feigin VL. MLC901 (NeuroAiD IITM) for cognition after traumatic brain injury: a pilot randomized clinical trial. Eur J Neurol. (2018) 25:1055–62. doi: 10.1111/ene.13653
47. Gao TT, Qian YH, Shen HL. To observe the effect of Baizhu Tianma decoction in the treatment of vertigo after craniocerebral trauma Wendan decoction and Rhizoma Pinelliae. Chin Contin Med Educ. (2016) 8:184–5.
48. Gu HB, Jiang L, Wang SY, Wang P, Chen XT, Li C, et al. Application efficacy of modified Jieyu Tongqiao decoction in the treatment of post-traumatic syndrome based on syndrome differentiation. Chin Med Her. (2016) 13:191–2.
49. Qiu XJ, Ma XW. Therapeutic effect of Buyang Huanwu decoction on 40 cases of craniocerebral trauma sequela. Nei Mongol J Tradit Chin Med. (2016) 35:6–7.
50. Gong XP, Wang T. Analysis of the effect of Xuefu Zhuyu decoction on headache caused by concussion. Chin Health Care Nutr. (2019) 29:310–1.
51. Tang LP. Clinical observation of Huishen Xingnao decoction combined with rehabilitation nursing intervention for post-traumatic brain injury syndrome. J New Chin Med. (2016) 48:192–4.
52. Lan PG, Deng FJ, Mai H. Effect of Huoxue Huayu Chinese medicine of Taohong Siwu decoction on blood coagulation function in traumatic patients. Chin J Mod Drug Appl. (2019) 13:151–3.
53. Liu JG, Wu JL, Wang XM. Clinical study on tongqiao pingyu pill and fluoxetine in the treatment of mental disorder after traumatic brain injury. Chin J Mod Drug Appl. (2008) 2:71–2.
54. Liu XD, Liu YB, Dong N, Liu XL. Clinical observation on 60 cases of post-traumatic brain syndrome treated by integrative Chinese and Western medicine. Xhanxi J TCM. (2005) 21:28–9.
55. Li JM, Shao JB, Tai LW, Wang M, Mu YM. Treatment of 51 cases of post-traumatic brain syndrome with qi and blood stasis syndrome. Shaanxi J Tradit Chin Med. (2013) 34:806–7.
56. Li JF, Yin XY, Song GL. Effect of chuanxiong qingnao granule on headache after brain trauma. Contemp Med Symp. (2018) 16:121–3.
57. Li XX, Wang XJ, Wu JW, Bu JH, Yan H, Huamg XP. Efficacy of Buyang Huanwu decoction combined with hyperbaric oxygen in treating post traumatic brain syndrome. Med Inf. (2018) 31:145–8.
58. Ma ZF. Clinical Effect Observation on Treating Traumatic Brain Edema with Wulingsan and its Effect on the two-Way Adjustment. Gansu College of Traditional Chinese Medicine (2017).
59. Xu J. Clinical effectiveness analysis of modified Wendan decoction in treating post-traumatic brain syndrome. J Clin Med. (2018) 5:152–3.
60. Xu ML, Wu XY, Ma YY, Liu J, Li YF. Effect of Huishen Xingnao decoction combined with hyperbaric oxygen therapy on neurological function recovery and vertebral basilar artery blood flow velocity in patients with blood stasis affecting the clear orifices type post-traumatic brain syndrome. Mod J Integr Tradit Chin Western Med. (2017) 26:3154–6.
61. Sun CZ, Yu Z, Peng YJ, Shao B, Wang YQ. Clinical observation of Huishen Xingnao decoction in treating cognitive dysfunction after traumatic brain injury. Zhejiang J Integr Tradit Chin Western Med. (2015) 25:364–6.
62. Yang SQ, Wang JY. The clinical effect of decoction of traditional Chinese medicine (Xuefe Zhuyu decoction) combined with Olanzapine in the treatment patients with mental disorders caused by brain trauma. Chin Foreign Med Res. (2017) 15:8–9.
63. Yu YL. Effect of Xueshuantong Combined Hyperbaric Chamber on Traumatic Brain Injury Sequelae Stasis Resistance Qing Qiao. Nanjing University of Chinese Medicine (2016).
64. Wu M, Zhao SM, Wu JZ. Clinical observation on treatment of mental disorders caused by brain injury with Xuefu Zhuyu decoction combined with Olanzapine. Zhejiang J Integr Tradit Chin Western Med. (2017) 27:763–5.
65. Wu QQ. Treatment of 48 cases of dizziness after craniocerebral trauma by tongqiao huoxue decoction. Jiangxi J Tradit Chin Med. (2012) 43:52–3.
66. Wang JC, Wang QH, Tian JX. Therapeutic effect of integrated traditional Chinese and Western medicine on patients with mental disorders caused by traumatic brain injury. Chin J Pract Nerv Dis. (2015) 18:50–2.
67. Wang JC, Tian JX. Efficacy Xuefu Zhuyu decoction brain trauma caused by intractable epilepsy observation. Shaanxi J Tradit Chin Med. (2015) 36:653–4.
68. Wang J, Xing L, Xu D. The efficacy of Yangxue Qingnao granule in treatment of brain injury induced memory decreasing. Clin Med J Chin. (2009) 16:37–8.
69. Wang XJ. Effect of Naoxintong capsule combined with rehabilitation nursing on upper limb function of patients with traumatic brain injury. Strait Pharm J. (2013) 25:96–7.
70. Wang XP. Clinical observation of Carbamazepine combined with traditional Chinese medicine in treating 34 patients with epilepsy after brain trauma. Med Forum. (2014) 18:3068–9.
71. Yu DS. Clinical observation on 30 cases of traumatic epilepsy treated by integrative Chinese and Western medicine. Hunan J Tradit Chin Med. (1998) 14:13.
72. Yu LX. Treating 41 cases of epilepsy after traumatic brain injury with modified Xuefu Zhuyu decoction. Zhejiang J Tradit Chin Med. (2013) 48:421.
73. Cheng AL. Analysis on effect of Nimodipine combined with blood-nourishing and brain-clearing granules on cerebral concussion-like incipient symptom after cerebral trauma. Chin J Ration Drug Use. (2018) 15:57–59, 80.
74. Ding N, Hou KQ. Clinical observation on 80 cases of concussion treated by huoxue xingnao decoction. J Commun Med. (2007) 5:40.
75. Cai PH, Shen ZB, Qiu F, Kong LJ, Fei ZM. Effect of “Angong Niuhuang Bolus” on hypomnesis after head concussion. SH J TCM. (2012) 46:62–3.
76. Peng WJ, Wang Y, Xia ZA, Wang WH, Xu JX, Yang B. Effect of Xingnaozhuyu decoction on severe traumatic brain injury patients with cognitive dysfunctions. In: Chin J Chin Mater Med. 2015/Proceedings of Scientific and Technological Paper Writing Training Conference for Practitioners in Grassroots Medical Institutions. Beijing (2016).
77. Ping QN. Effect of Banxia Baizhu Tianma decoction on serum basic protein, S100β and p73 factor in treatment of posttraumatic hydrocephalus. Acta Chin Med. (2017) 32:440–3.
78. Xu LY, Wang JY, Gong L, Cai PH, Qiu F, Kong LJ, et al. Effects of “Danqin Xiaoyu Mixture” on post-traumatic brain syndrome: a report of 43 cases. Acta Univ Tradit Med Sinensis Pharmacol Shanghai. (2012) 26:39–41.
79. Hu SZ. The Clinical Study of Xiaoyao Powder in the Treatment of Post Traumatic Brain Syndrome. Guangzhou University of Chinese Medicine (2017).
80. Hong JJ, Wu HB, Zhao DQ, Zhang JJ. Yangxueqingnao granule combined with nimodipine in the treatment of brain concussion-like symptoms after traumatic brain injury. Pract Clin Med. (2015) 16:51–2, 61.
81. Huang JL, Li YH, Lin ZP. Clinical observation on treatment of headache after traumatic brain injury with di shen xiang ren decoction. Tradit Chin Med J. (2008) 7:33–5.
82. Huang PC, Li XF. Therapeutic effect of Yangxueqingnao granule on post-traumatic brain injury syndrome. Clin Med. (2014) 34:119–21.
83. Moher D, Liberati A, Tetzlaff J, Altman DA, for the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1136/bmj.b2535
84. Park B, You S. Korean studies on blood stasis: an overview. Evid Based Complement Alternat Med. (2015) 2015:316872. doi: 10.1155/2015/316872
85. Xue M, Yang L, Kou N, Miao Y, Wang M, Zhao Q, et al. The effect of xuefuzhuyu oral liquid on aspirin resistance and its association with rs5911, rs5787, and rs3842788 gene polymorphisms. Evid Based Complement Alternat Med. (2015) 2015:507349. doi: 10.1155/2015/507349
86. Kwon CY, Lee B, Leem J, Chung SY, Kim JW. Korean medicine treatments including blood stasis-removing therapy and auriculotherapy for persistent headache after traumatic brain injury: a case report. Explore. (2019) 15:419–24. doi: 10.1016/j.explore.2019.06.001
87. Li HQ, Wei JJ, Xia W, Li JH, Liu AJ, Yin SB, et al. Promoting blood circulation for removing blood stasis therapy for acute intracerebral hemorrhage: a systematic review and meta-analysis. Acta Pharmacol Sin. (2015) 36:659–75. doi: 10.1038/aps.2014.139
88. Keshavarzi Z, Shakeri F, Barreto GE, Bibak B, Sathyapalan T, Sahebkar A. Medicinal plants in traumatic brain injury: neuroprotective mechanisms revisited. Biofactors. (2019) 45:517–35. doi: 10.1002/biof.1516
89. Wang W, Li H, Yu J, Hong M, Zhou J, Zhu L, et al. Protective effects of Chinese herbal medicine Rhizoma Drynariae in rats after traumatic brain injury and identification of active compound. Mol Neurobiol. (2016) 53:4809–20. doi: 10.1007/s12035-015-9385-x
90. WorkSafeBC. Evidence-Based Practice Group Martin CW. Post-Concussion Syndrome (PCS) - Validated Symptom Measurement Tools. Available online at: https://www.worksafebc.com/en/resources/health-care-providers/guides/post-concussion-syndrome-validated-symptom-measurement-tools?lang=en (2018).
Keywords: herbal medicine, traumatic brain injuries, systematic review, East Asian traditional medicine, post-concussion syndrome
Citation: Lee B, Leem J, Kim H, Jo H-G and Kwon C-Y (2020) Herbal Medicine for Traumatic Brain Injury: A Systematic Review and Meta-Analysis of Randomized Controlled Trials and Limitations. Front. Neurol. 11:772. doi: 10.3389/fneur.2020.00772
Received: 29 February 2020; Accepted: 23 June 2020;
Published: 18 September 2020.
Edited by:
Guoqiang Xing, Affiliated Hospital of North Sichuan Medical College, ChinaReviewed by:
Zhang-Jin Zhang, The University of Hong Kong, Hong KongYang Wang, Central South University, China
Noah D. Silverberg, University of British Columbia, Canada
Copyright © 2020 Lee, Leem, Kim, Jo and Kwon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chan-Young Kwon, beanalogue@naver.com
†These authors have contributed equally to this work and share first authorship
 Boram Lee
Boram Lee Jungtae Leem
Jungtae Leem Hyunho Kim3
Hyunho Kim3 Chan-Young Kwon
Chan-Young Kwon