- 1Université Paris Cité, Université Paris Saclay, ENS Paris Saclay, CNRS, SSA, INSERM, Centre Borelli, Paris, France
- 2Service de Chirurgie Orthopédique, Traumatologie et Réparatrice des Membres, Service de Santé des Armées, HIA Percy, Clamart, France
- 3Service de Neurologie, Service de Santé des Armées, HIA Percy, Clamart, France
- 4SYSNAV, Vernon, France
- 5Université Paris Saclay, Université Paris Cité, ENS Paris Saclay, CNRS, SSA, INSERM, Centre Borelli, Gif-sur-Yvette, France
- 6Universitꃩ Sorbonne Paris Nord, L2TI, UR 3043, Villetaneuse, France
- 7ENGIE Lab CRIGEN, Stains, France
- 8Ecole du Val-de-Grãâce, Service de Santé des Armées, Paris, France
Introduction: The aim of this study was to realize a systematic review of the different ways, both clinical and instrumental, used to evaluate the effects of the surgical correction of an equinovarus foot (EVF) deformity in post-stroke patients.
Methods: A systematic search of full-length articles published from 1965 to June 2021 was performed in PubMed, Embase, CINAHL, Cochrane, and CIRRIE. The identified studies were analyzed to determine and to evaluate the outcomes, the clinical criteria, and the ways used to analyze the impact of surgery on gait pattern, instrumental, or not.
Results: A total of 33 studies were included. The lack of methodological quality of the studies and their heterogeneity did not allow for a valid meta-analysis. In all, 17 of the 33 studies involved exclusively stroke patients. Ten of the 33 studies (30%) evaluated only neurotomies, one study (3%) evaluated only tendon lengthening procedures, 19 studies (58%) evaluated tendon transfer procedures, and only two studies (6%) evaluated the combination of tendon and neurological procedures. Instrumental gait analysis was performed in only 11 studies (33%), and only six studies (18%) combined it with clinical and functional analyses. Clinical results show that surgical procedures are safe and effective. A wide variety of different scales have been used, most of which have already been validated in other indications.
Discussion: Neuro-orthopedic surgery for post-stroke EVF is becoming better defined. However, the method of outcome assessment is not yet well established. The complexity in the evaluation of the gait of patients with EVF, and therefore the analysis of the effectiveness of the surgical management performed, requires the integration of a patient-centered functional dimension, and a reliable and reproducible quantified gait analysis, which is routinely usable clinically if possible.
Introduction
The number of hemiplegic stroke survivors is constantly increasing (1). They classically develop spastic equinovarus foot (EVF), posing a challenge for rehabilitation (2, 3). The position in plantar flexion and inversion results from an imbalance in hindfoot forces due to muscular hypertonia associated with loss of effective motor control. The development of EVF is associated with muscle over-activity of the calf muscles, triceps surae, tibialis posterior, flexor hallucis longus, flexor digitorum longus (FDL), and brevis muscles, combined with paresis or weakness of the antagonist muscles, the tibialis anterior (TA), peroneus longus, and brevis. Over time, flexible deformities typically evolve into fixed deformities as a result of muscle shortening consequent to prolonged contracture (4). This raises serious problems with footwear, upright stance, transfer, and gait. For severe deformities, non-operative treatment is usually unsatisfactory; neuro-orthopedic surgery is recognized as effective in improving foot position in spastic EVF (5, 6), usually achieving improvement in functional scores [most notably the Goal Attainment Scale (GAS)] (7–11). Triceps spasticity, ankle range of motion, and gait velocity are improved. In addition, the need for walking aids is reduced (2, 6, 12–15). There are many surgical techniques, which act on the tendons, in order to lengthen them, to transfer them or both. The most frequently performed intervention is split anterior tibialis tendon transfer (SPLATT) (16). Most of the time we lengthen the triceps surae through the Achilles tendon lengthening (TL) or through the gastrocnemius and soleus aponeurectomy to treat equinus position (17). Other tendon transfers are the extensor hallucis longus transfer (18), anterior transfer of the FDL (19), split posterior tibial transfer (20), and peroneus brevis transfer (21). Another type of surgical procedure aims to act directly on the nerves to reduce the spasticity of certain muscles. These are called selective neurotomies (SN). In EVF, most of the time, they concern the branches of the tibial nerve and, by reducing its caliber, make it possible to reduce spasticity (22–24). More rarely, bony procedures (BPs) are necessary. The details of these different techniques and the current management strategy have been described in a more generic literature review on EVF of all etiologies (16), as well as following the DELPHI method (25). The choice of the techniques used and their possible combination is based on the clinical examination of the patient through a multidisciplinary approach (15, 16). If non-operative techniques are insufficient, neuro-orthopedic surgery can correct the equinus, reduce spasticity, and provide plantigrade support. However, it is essential to accurately analyze the gait disorder in a dynamic manner to propose a program adapted to the patient without risking aggravating his condition. The evaluation of the outcome of the surgery must also be based on a quantified and objective method of analysis and not only on a subjective clinical impression. Today, there is no standard, validated method for assessing gait improvement after post-stroke EVF surgery. Postoperative outcomes are heterogeneously assessed by quality of life scales, functional methods, such as the GAS, and by data from clinical examination, including splinting or barefoot walking. Change in gait pattern is difficult to analyze objectively and quantitatively, especially in longitudinal follow-up of patients. Quantified gait analysis techniques are effective but difficult to implement in routine clinical practice (26, 27). EVF is often evaluated by mixing different etiologies (TBI and cerebral palsy), and it seems important to us to evaluate a homogeneous patient population, here post-stroke EVF (28). The aim of this study was to realize a systematic review of the different ways, especially instrumental, used to evaluate the effects of surgical correction of post-stroke EVF deformities, including SN, tendon lengthening (TL), tendon transfer (possibly associated with TL), or BP, in post-stroke patients.
Methods
In this review, we defined a stroke as “an acute neurological dysfunction of vascular origin with sudden (within seconds) or at least rapid (within hours) occurrence of symptoms and signs corresponding to the involvement of focal areas of the brain” (29). Details of the protocol for this systematic review were registered on PROSPERO and can be accessed at https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022300497.
Search strategy and selection criteria
The literature search and analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (30) and Meta-analysis of Observational Studies in Epidemiology (MOOSE) (31). We searched the MEDLINE (via PubMed), Cochrane Central, and Embase electronic databases to identify articles published before 1 June 2021 that measured the efficacy of neuro-orthopedic surgery on the gait of hemiplegic post-stroke hemiplegic patients. In addition, the gray literature was searched in Google Scholar, Opengrey.eu, Greylit.org, WorldCat, World Health Organization Clinical Trials Search Portal, ClinicalTrials.gov, and the European Union Clinical Trials Register. All reference lists and bibliographies of included studies were also reviewed for relevant articles. The following MeSH headings and keywords were used: “equinus, equinovarus, foot deformity, foot deformities, hemiplegia, hemiparesis, stroke, cerebrovascular disorders, orthopedics, neuro-orthopedic, neurorehabilitation, surgery, and gait analysis”.
Selection criteria
As case series are probably the most frequent type of surgical report in the literature (32), it was decided not to restrict the selection to a specific study design. As a consequence, studies were included if they used either within-group pre-post treatment comparisons or between-group comparisons in a (at best randomized) controlled design. In addition, studies were required to meet the following inclusion criteria:
– investigating stroke in adults (irrespective of the phase of recovery);
– investigating the efficacy of surgical correction of EVF deformity (lengthening, release and/or transferring of muscles and/or tendons, and neurotomy);
– being written as a full-length article in the English, German, French, or Dutch language and being published in a peer-reviewed journal. If two or more articles were published by the same group, and if (within these articles) the etiology of the EVF deformity was comparable, only the study with the highest number of patients was included.
Methodological quality assessment and data extraction
The Oxford CEBM levels of evidence were used to grade the selected studies (33). The methodological index for non-randomized studies (MINORS) was applied to further assess the quality of each study (34). Few validated instruments are available to assess the methodological quality of observational or non-randomized studies. MINORS is a validated list designed to assess the methodological quality of non-randomized (surgical) studies (either comparative or non-comparative) comprising 12 items, the last four items of which apply only to comparative studies. Items are scored as 0 (not reported), 1 (reported but inadequate), or 2 (reported and adequate). The maximum score is 16 for non-comparative studies and 24 for comparative studies. Because no (randomized) controlled trials were identified, no pooling of data was possible, neither in a meta-analysis nor in a best-evidence synthesis. We collected all the modes of evaluation of walking that were used, including the instrumental analysis of gait, the clinical analysis, the scores and scales used, and the subjective feelings of the patients. The GAS (35–37) was also reported if it was used.
Results
Study selection
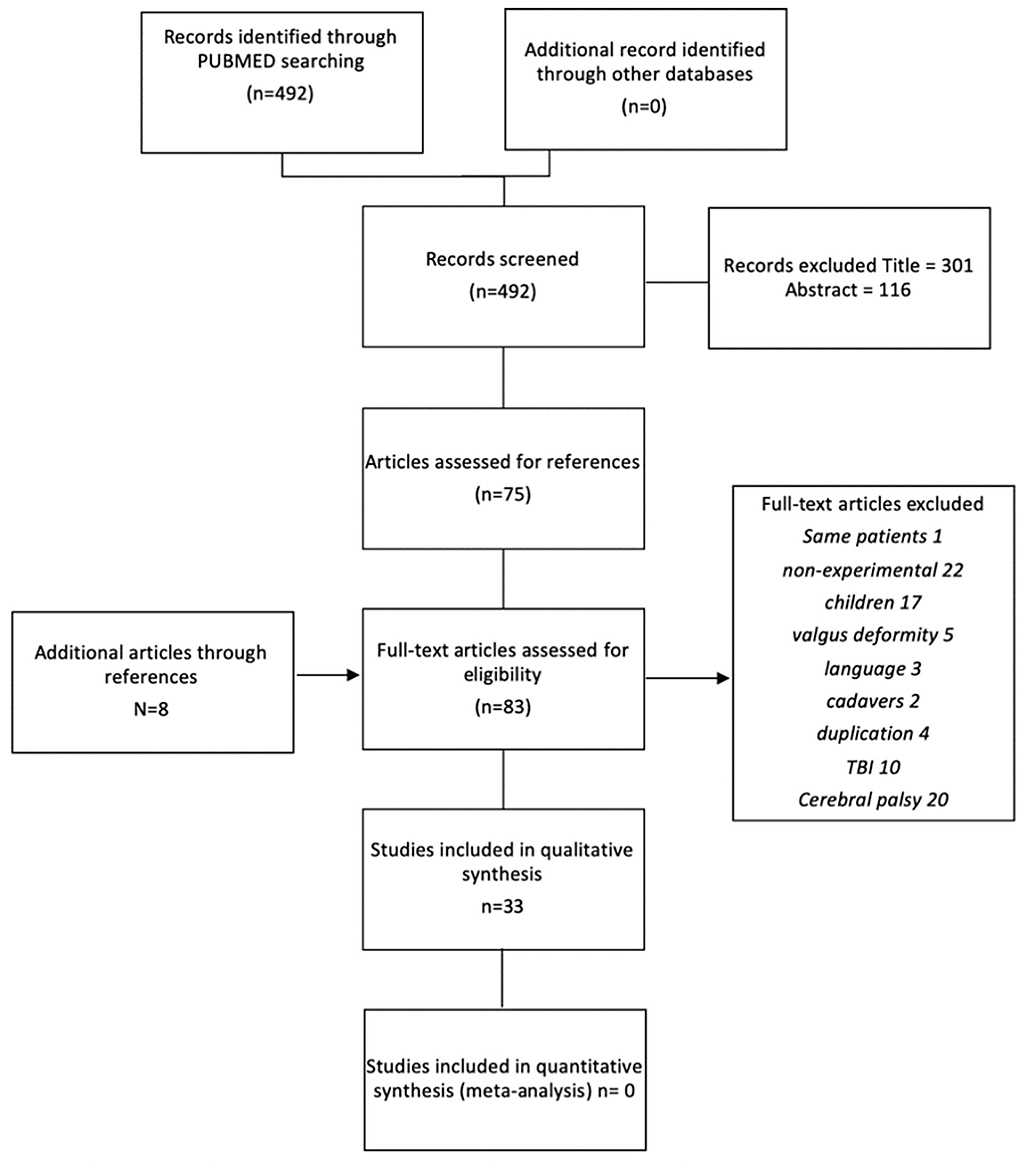
Figure 1 shows the study selection process as a flowchart. The initial systematic search strategy in PubMed identified 492 relevant citations (available on request). The search in the other databases did not yield additional articles. On the basis of the title, 301 studies were excluded for the following reasons: non-surgical studies; botulinum toxin evaluation; pediatric populations; other pathologies, such as cerebral palsy or Traumatic Brain Injury (TBI); other neurological foot deformities; or non-neurological deformity. Another 116 studies were excluded based on their abstracts. Recurrent reasons for exclusion were the use of interventions that did not fit within our definition, such as reduction by external fixator or successive cast, and the use of patient populations with an etiology other than stroke. The full texts of the remaining 75 studies were examined. Screening the references of these studies revealed eight additional articles. From these 83 initially selected studies, 50 studies were excluded in the second instance.
Methodological quality
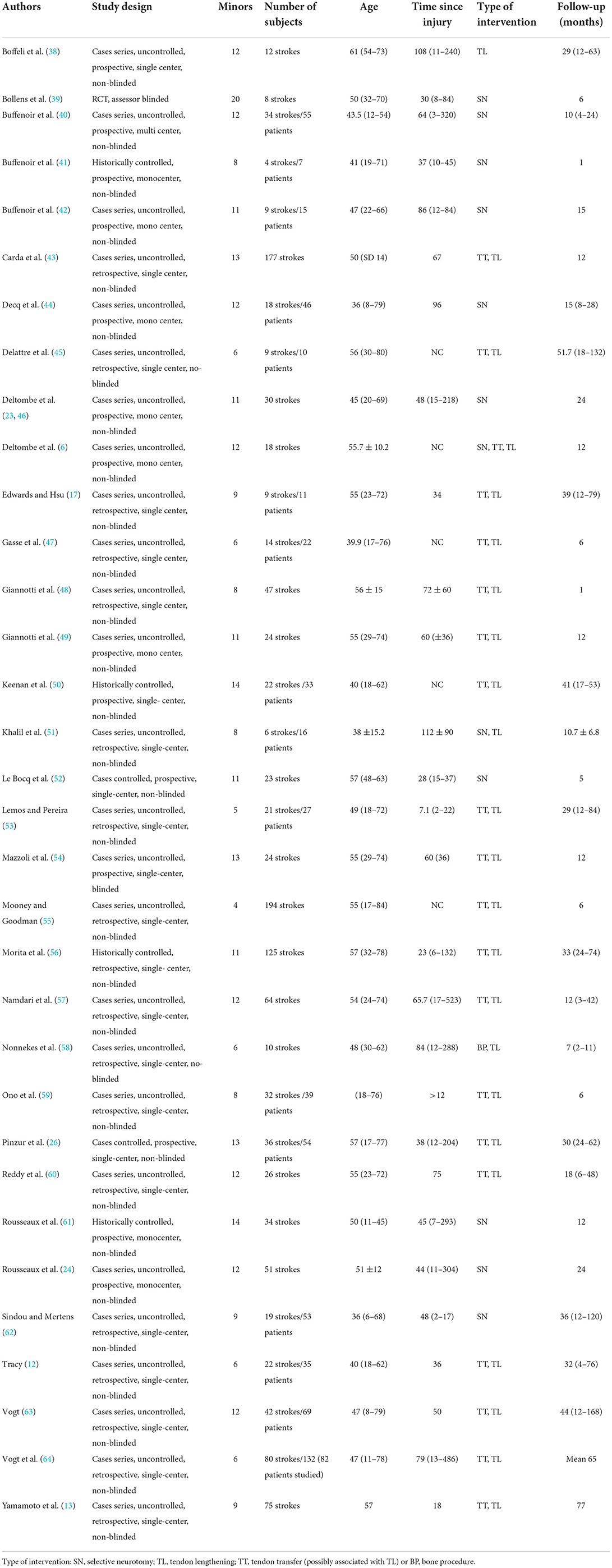
The characteristics of the different articles included in the study are summarized in Table 1. The methodological quality of the studies and their heterogeneity did not allow for a valid meta-analysis. The median score was 11/16, which is low. Only 17 of 33 studies involved exclusively stroke patients.
Surgical intervention
In view of the multitude of possible procedures, we preferred to classify the type of procedures performed into the following four categories: selective neurotomy (SN), tendon lengthening (TL), tendon transfer (possibly associated with TL), or bone procedure (BP). The studies and their associated categories are reported in Table 1. Ten studies (30%) evaluated only SNs, one study (3%) evaluated only TL procedures, 19 studies (58%) evaluated tendon transfer procedures, and only two studies (6%) evaluated the combination of tendon and neurological procedures, of which one evaluated only TL and one both TT and TL. Only one study (3%) evaluated exclusively BP procedures.
Clinical assessment
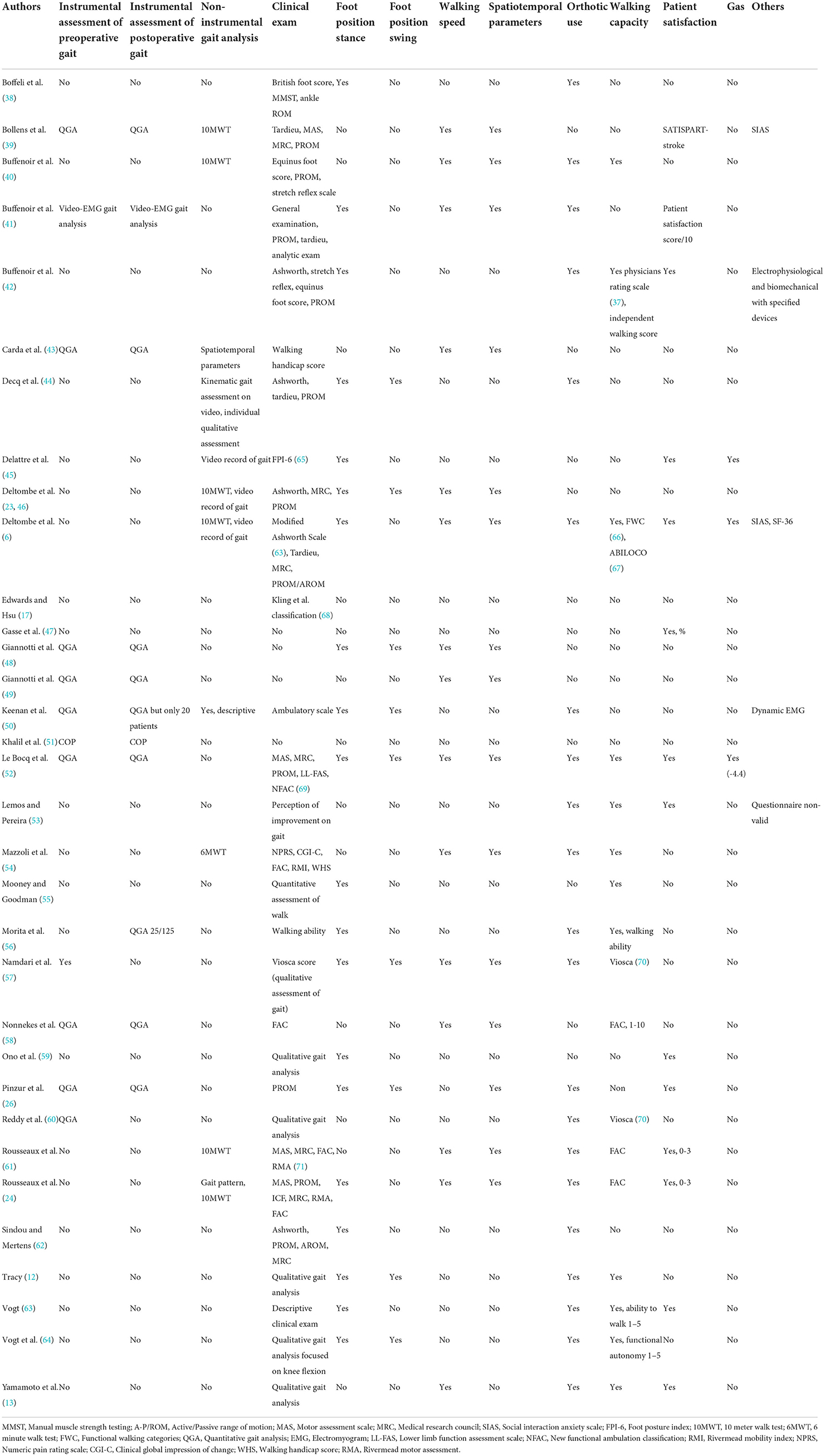
The criteria used in the clinical evaluation of patients are reported in Table 2. There are a wide variety of scales, most of which have been validated in other indications, such as cerebral palsy (e.g., the Physicians Rating Scale) (65). Some seem relevant but are rarely used, such as the FPI-6 (72). Only three studies performed a GAS, one of which did not meet the Turner-Stokes criteria (11). The measurement of passive range of motion (ROM) was the most recurring criterion found, although its relevance for gait improvement was not assessed. On the contrary, the position of the foot during the oscillation phase, which is an essential element, was considered in only eight studies. Only 14 studies assessed patient satisfaction, mostly with simple numerical scales. Patient reported outcome measures were widely used, and only SF-36 (6) or SATISPART Stroke (39). GAS was used only one time (6), and a kind of unvalidated GAS one time (52).
Gait assessment
Instrumental gait analysis refers to all modes of gait analysis using objective and quantified parameters. The types of instrumental analyses that were performed in the included studies are presented in Table 2. Quantitative gait analysis (QGA) is the gold standard for the study of human gait using reflectors attached to the body. It consists of video (kinematic and kinetic recording using digital cameras), an optoelectronic system, and a force platform. BP refers to a simple baropodometric and COP displacement study, and some studies performed only an elementary analysis including speed and number of steps. BP refers to a simple baropodometric and COP displacement study, and some studies performed only an elementary analysis including speed and number of steps.
Eleven studies conducted a non-instrumental walking analysis. Six studies used the 10-Meter Walk Test (10MWT), and one study used the 6-Minute Walk Test (6MWT). Note that the 6MWT did not show any correlation with the functional scores achieved in this study (54). In one study, only the walking velocity was evaluated over 10 m (56). Four studies used sensorless video recording, allowing for secondary measurement of analytical joint mobility by several observers (6, 23, 44, 45). Instrumental gait analysis was performed in only 10 studies (26, 39, 41, 43, 48–52, 58) (Table 3), and only six studies associated it with clinical and functional analyses (Table 2). It was only performed pre-operatively in one study and postoperatively in one study. In two studies, it was not performed on all the patients who were operated on. Regarding spatiotemporal parameters, the most frequently used were elementary parameters that did not require any specific indicators, such as speed or step length.
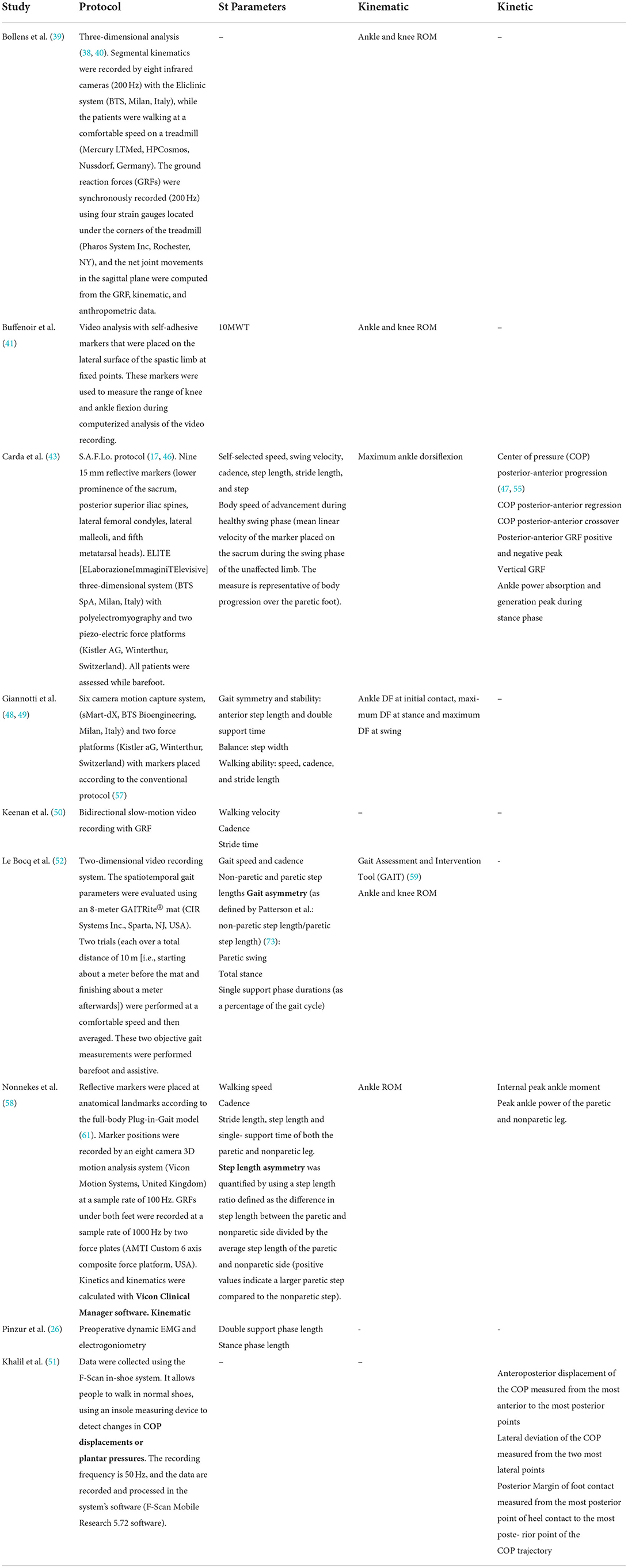
Table 3. Characteristics and methodology of instrumental gait analysis performed to assess postoperative hemiplegic gait.
Three studies evaluated symmetry by various means. Le Bocq et al. (52) used non-paretic step length divided by paretic step length as defined by Patterson et al. (73); for Nonnekes et al. (26), step length asymmetry was quantified by using a step length ratio defined as the difference in step length between the paretic and non-paretic sides divided by the average step length of the paretic and non-paretic sides (positive values indicate a larger paretic step compared to the non-paretic step). For Giannotti et al. (48, 49), gait stability and symmetry were represented by anterior step length and double support time but without any analysis. The authors did not recover the raw data (51, 58), and data analysis was performed by engineers or software, so the method used to obtain the results was not explicit and therefore not reproducible. Kinematics analysis evaluated ankle ROM and sometimes knee ROM. Ankle dynamic ROM was the most frequently used criterion. The only criteria used in the analysis were closed-chain joint kinematics and spatiotemporal parameters. No scores or other assessment methods were used. To the best of our knowledge, inertial measurement systems, largely used in other context of walk assessment in other conditions (74), have never been studied in this type of study.
Outcomes
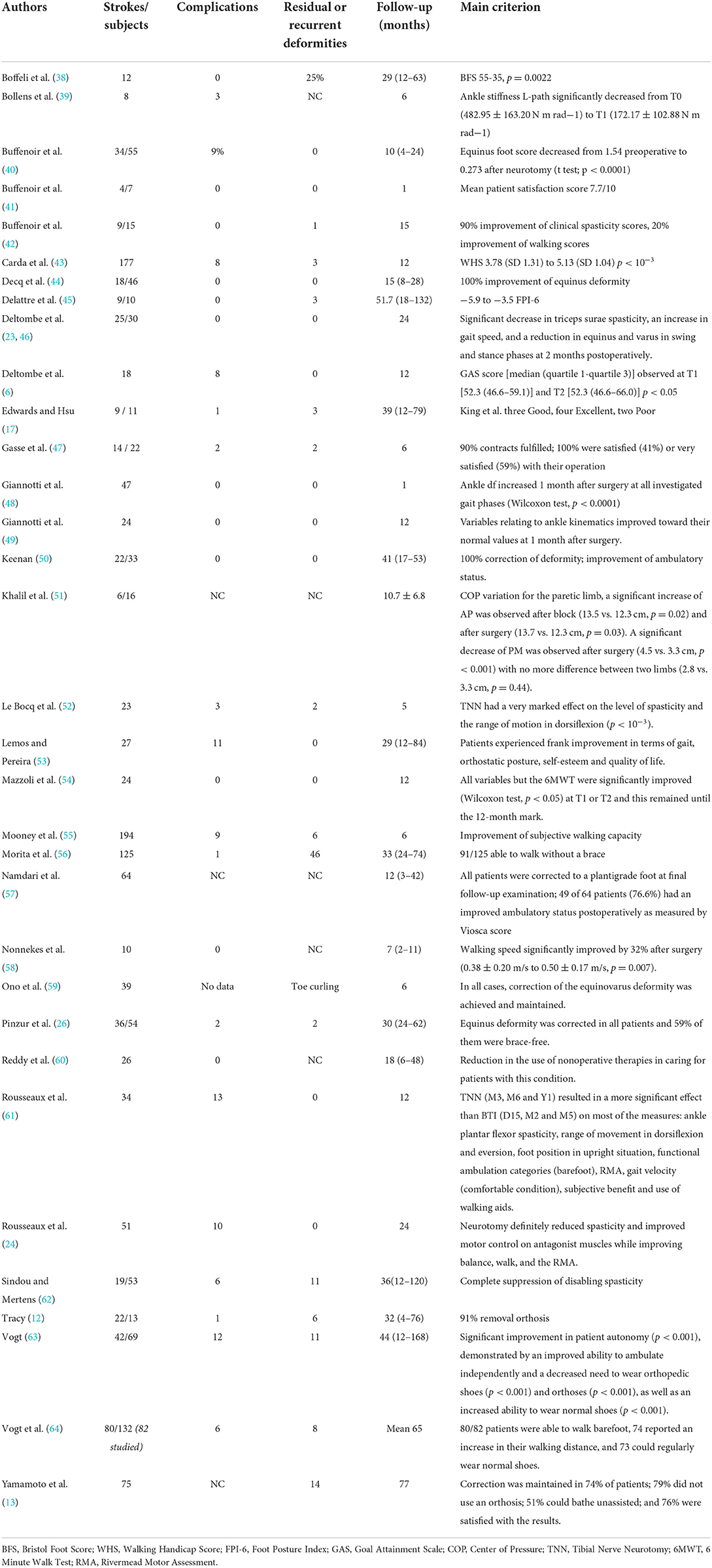
Functional results according to the main criterion used by the studies, as well as complications, and follow-up in months are summarized in Table 4. None of the studies had unfavorable results, and the complication rate was quite low. However, the wide variety of procedures and the differences in the collection of complications or residual deformities did not yield global conclusions. The response to the primary endpoint of the studies is reported in the right-hand column. There was no homogeneity in the evaluation criteria.
Discussion
To the best of our knowledge, our review is the only one dealing exclusively with the management of EVF in adult hemiplegic post-stroke patients, focusing on evaluation methods of the impact of the surgery on gait. Indeed, in our opinion, to better specify the management and the consequence of possible surgical procedure in the gait pattern, it is necessary to study cohorts of patients with the same pathology. Some studies mixed patients with amyotrophic lateral sclerosis, multiple sclerosis, Little's disease, TBI, and stroke (42, 44). Moreover, it is the first review to include both the classical clinical analysis and the instrumental analysis of walking, which should become essential in the years to come. It highlights not only the lack of consensus on the clinical criteria used in the evaluation of gait but also the poor access to quantified analysis in routine clinical practice. The main limit of this review lies in the fact that no meta-analysis was possible because of the statistical weaknesses of the included studies. This has already been noticed in previous reviews on the subject (28).
Surgical strategy
There is great heterogeneity of attitudes, divided between neurological and tendinous procedures, due to the fact that they are performed by different surgical teams. For several years, the performance of all these procedures by the same teams has made it possible to refine the indications and to perform combined procedures (6, 15, 16, 25). Few series have analyzed the results of this global management. It is important that future series do not take into account only one procedure or another, but the “neuro-orthopedic” management itself.
Clinical assessment
Numerous scales and measurement methods have been used, but none of them has imposed itself. This clearly shows the absence of a validated generic scale for evaluating walking in hemiplegics. On the contrary, the measurement of joint kinematics in analysis, although easily achievable and reproducible, does not give a good idea of what really happens in the closed chain during walking. Moreover, the type and importance of the global deficit are variable, and the resulting functional discomfort is specific to each patient. For this reason, it is extremely difficult to obtain a functional scale that can be adapted to each situation. Some have simply used a scale of satisfaction and the feelings of the patients with different subjective criteria (53).
The GAS is a method for quantifying progress on personal goals. Turner-Stokes's guide to the GAS is a method for quantifying progress toward personal goals (11). Turner-Stokes's guide and the use of Kiresuk's T-score (75) are the most widely used GAS-based approaches in rehabilitation (37). This personalized analysis allows to directly evaluate the success of the intervention, according to the defined objective. It represents a sensitive and specific analysis of the result. Indeed, in other pathologies, the GAS and the overall clinical impression have shown a significant correlation (7, 9). Moreover, in rehabilitation, the GAS is more sensitive to change than the Barthel Index and the Functional Independence Measure (8–10). In some studies, the GAS was the only scale capable of detecting change after treatment (8, 76). Standard scales sometimes show no change, while the GAS goal is achieved. The main reason for this is that the goals and fixed GAS often do not correspond to any of the items in the standard scales (77). For us, this functional analysis must now be part of the systematic clinical analysis of the outcome of the intervention. The limitation of this method lies in the definition of pre-operative goals, since it must be sufficiently ambitious but still achievable. Regarding the evaluation of spasticity, as explained by Deltombe et al. (22), although the Ashworth scale is commonly used in the literature, it is confounded by contracture, as increased resistance to movement is not only exclusively dependent on stretch reflex activity but also due to increased stiffness as a result of contracture. The Tardieu scale seems more appropriate, especially to evaluate triceps spasticity (78). Reddy et al. (60) used the evaluation of the reduction of non-operative care associated with an EVF deformity, which seems to be a relevant criterion (60).
Gait assessment
Traditional non-instrumental scales have moderate effectiveness. The 6MWT does not appear to be a relevant indicator. As used in the study by Mazzoli et al. (54), it shows no difference pre- and postoperatively, while all functional scores are improved. Its use is, therefore, not relevant in this indication. The 10MWT provides some information, notably on step length and speed. However, this analysis does not detail the intrinsic quality of walking. As we said before, there is no correlation between the analysis of the open and closed chain gaits, and if the analytical analysis is easily done and traceable in the medical record, global analysis of gait is more difficult to assess in an objective way. In some retrospective studies, data concerning the exact position of the foot during the gait cycle were too unequally reproduced in patient files to be properly exploited (64). This pre- and postoperative comparison of the gait analysis seems essential to evaluate the effect of the procedure. For example, the comparison of pre- and postoperative joint kinematics is a reliable and reproducible criterion that can be measured by instrumental analysis (43). For this purpose, instrumental gait analysis methods are of considerable help. The gold standard is QGA, which provides more precise data for assessing surgical outcome, to improve the surgical program in spastic EVF and define more standardized strategies (27). While QGA represents the gold standard, the availability of facilities and immediacy of results makes QGA challenging to use in routine clinical. Indeed, there is no consensus on QGA indices, as such a consensus requires a team of engineers and physicians trained in interpreting such data. There is also a delay between acquisition and final analysis. Finally, the analysis can only be conducted in dedicated premises, often located far from where the patient lives or is being followed. For all these reasons, QGA is difficult for doctors to implement in routine clinical practice and postoperative follow-up. Presently, there are some simple tests for assessing dynamic balance in consultation, basically consisting of observing the patient walking and quantifying gait on an equipped walkway. However, we saw that instrumental gait analyses were scarce and of widely varying quality to evaluate EVF treatment in post-stroke adults. In addition, no validated and reproducible indicators were used. For example, only three studies evaluated pitch symmetry, and the three indicators were different. Most studies used instrumental analysis only to collect simple spatiotemporal data or joint kinematics data, which represents a limited contribution. No team collected the raw signal data for analysis, and they used the parameters provided by the brand's software but not their own algorithm. Moreover, the raw data were not accessible in open source. Moreover, QGA provides precise data on locomotion, but they require large and specific spaces, are very expensive (between €10,000 and €40,000), and hardly suited for everyday medical practice. Recently, a study by Mazzoli et al. (54) showed a good correlation between indices based on ground reaction force and clinical and functional variables. Since the acquisition of ground reaction forces does not require patient preparation, it can be used in clinical routine and especially for postoperative evaluation (79). An alternative is to use combined accelerometric and gyroscopic data on an inertial measurement unit (IMU). IMUs have the advantage of being lightweight, inexpensive, and easy to use in practice. It has been validated for clinical use in gait assessment in patients with osteoarthritis or neurological pathology, such as post-stroke hemiplegia (74, 80–83). Another advantage is the possibility to perform ambulatory measurements over a longer period of time in the patient's environment (84), which is not feasible with QGA. On the contrary, the comparison with the norms of healthy subjects is a criterion that is not often used but seems to be correlated with walking improvement. This cross-sectional step study represents a complementary element in the evaluation of postoperative improvement (26).
Conclusion
Neuro-orthopedic surgery for post-stroke EVF is becoming better defined. However, outcome assessments are not yet well established. The complexity of the evaluation of gait of patients with EVF, and therefore the analysis of the effectiveness of the surgical management performed, requires the integration of a patient-centered functional dimension, as well as a reliable and reproducible quantified gait analysis, and if possible usable in routine clinical practice. Therefore, it seems necessary, in future, to compare the results of a systematic instrumental analysis with the functional results.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
Nl'E: writing and submission of the article. MM, CV, SJ, and BT: writing and correction. AM, NV, LO, and DR: conception and correction. All authors contributed to the article and approved the submitted version.
Conflict of interest
Author MM was employed by company SYSNAV. Author SJ was employed by company ENGIE.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Maaijwee NAMM, Rutten-Jacobs LCA, Schaapsmeerders P, Van Dijk EJ, De Leeuw F-E. Ischaemic stroke in young adults: risk factors and long-term consequences. Nat Rev Neurol. (2014) 10:315–25. doi: 10.1038/nrneurol.2014.72
2. Keenan MA. The management of spastic equinovarus deformity following stroke and head injury. Foot Ankle Clin. (2011) 16:499–514. doi: 10.1016/j.fcl.2011.07.002
3. Lawrence SJ, Botte MJ. Management of the Adult, Spastic, Equinovarus Foot Deformity. Foot Ankle Int. (1994) 15:340–6. doi: 10.1177/107110079401500610
4. Gracies JM. Pathophysiology of spastic paresis. I: Paresis and soft tissue changes Muscle Nerve. (2005) 31:535–51. doi: 10.1002/mus.20284
5. Roper B, Williams A, King J. The surgical treatment of equinovarus deformity in adults with spasticity. Br. Vol. (1978) 4:533–5. doi: 10.1302/0301-620X.60B4.711804
6. Deltombe T, Gilliaux M, Peret F, Leeuwerck M, Wautier D, Hanson P, et al. Effect of the neuro-orthopedic surgery for spastic equinovarus foot after stroke: a prospective longitudinal study based on a goal-centered approach. Eur J Phys Rehabil Med. (2018) 54:853–9. doi: 10.23736/S1973-9087.18.04993-6
7. Rockwood K, Joyce B, Stolee P. Use of goal attainment scaling in measuring clinically important change in cognitive rehabilitation patients. J Clin Epidemiol. (1997) 50:581–8. doi: 10.1016/S0895-4356(97)00014-0
8. Rockwood K, Howlett S, Stadnyk K, Carver D, Powell C, Stolee P. Responsiveness of goal attainment scaling in a randomized controlled trial of comprehensive geriatric assessment. J Clin Epidemiol. (2003) 56:736–43. doi: 10.1016/S0895-4356(03)00132-X
9. Khan F, Pallant JF, Turner-Stokes L. Use of goal attainment scaling in inpatient rehabilitation for persons with multiple sclerosis. Arch Phys Med Rehabil. (2008) 89:652–9. doi: 10.1016/j.apmr.2007.09.049
10. Turner-Stokes L, Williams H, Johnson J. Goal attainment scaling: does it provide added value as a person-centred measure for evaluation of outcome in neurorehabilitation following acquired brain injury? J Rehabil Med. (2009) 41:528–35. doi: 10.2340/16501977-0383
11. Turner-Stokes L. Goal attainment scaling (GAS) in rehabilitation: a practical guide. Clin Rehabil. (2009) 23:362–70. doi: 10.1177/0269215508101742
12. Tracy H. Operative treatment of the plantar-flexed inverted foot in adult hemiplegia. J Bone Jt Surg. (1976) 58:1142–5. doi: 10.2106/00004623-197658080-00019
13. Yamamoto H, Okumura S, Morita S, Obata K, Furuya K. Surgical correction of foot deformities after stroke. Clin Orthop Relat Res. (1992) 282:213–8. doi: 10.1097/00003086-199209000-00027
14. Schuh R, Benca E, Willegger M, Hirtler L, Zandieh S, Holinka J, et al. Comparison of Broström technique, suture anchor repair, and tape augmentation for reconstruction of the anterior talofibular ligament. Knee Surg. Sport Traumatol Arthrosc. (2016) 24:1101–7. doi: 10.1007/s00167-015-3631-7
15. Genêt F, Denormandie P, Keenan MA. Orthopaedic surgery for patients with central nervous system lesions: concepts and techniques. Ann Phys Rehabil Med. (2019) 62:225–33. doi: 10.1016/j.rehab.2018.09.004
16. Allart E, Sturbois-Nachef N, Salga M, Rosselin C, Gatin L, Genet F. Neuro-orthopaedic surgery for equinovarus foot deformity in adults: a narrative review. J Foot Ankle Surg. (2022) 61:648–56. doi: 10.1053/j.jfas.2021.11.012
17. Edwards P, Hsu J, SPLATT. Combined with tendo achilles lengthening for spastic equinovarus in adults: results and predictors of surgical outcome. Foot Ankle. (1993) 14:335–8. doi: 10.1177/107110079301400605
18. Carda S, Molteni F, Bertoni M, Zerbinati P, Invernizzi M, Cisari C. Extensor hallucis longus transfer as an alternative to split transfer of the tibialis anterior tendon to correct equinovarus foot in hemiplegic patients without overactivity of tibialis anterior. J Bone Joint Surg Br. (2010) 92-B:1262–6. doi: 10.1302/0301-620X.92B9.23580
19. Morita S, Yamamoto H, Furuya K. Anterior transfer of the toe flexors for equinovarus deformity due to hemiplegia. J Bone Joint Surg Br. (1994) 76-B:447–9. doi: 10.1302/0301-620X.76B3.8175851
20. Medina PA, Karpman RR, Yeung AT. Split posterior tibial tendon transfer for spastic equinovarus foot deformity. Foot Ankle. (1989) 10:65–7. doi: 10.1177/107110078901000204
21. Lord G, Moati JC. [The treatment of spastic equinovarus in the adult by transplantation of peroneus brevis and lengthening of the tendo-calcaneus (author's transl)]. Rev Chir Orthop Reparatrice Appar Mot. (1979) 65:297–9.
22. Deltombe T, Decq P, Mertens P, Gustin T. Does fascicular neurotomy have long-lasting effects? J Rehabil Med. (2007) 39:421–2. doi: 10.2340/16501977-0073
23. Deltombe T, Gustin T. Selective tibial neurotomy in the treatment of spastic equinovarus foot in hemiplegic patients: a 2-year longitudinal follow-up of 30 cases. Arch Phys Med Rehabil. (2010) 91:1025–30. doi: 10.1016/j.apmr.2010.04.010
24. Rousseaux M, Buisset N, Daveluy W, Kozlowski O, Blond S. Long-term effect of tibial nerve neurotomy in stroke patients with lower limb spasticity. J Neurol Sci. (2009) 278:71–6. doi: 10.1016/j.jns.2008.11.024
25. Salga M, Gatin L, Deltombe T, Gustin T, Carda S, Marque P, et al. International recommendations to manage poststroke equinovarus foot deformity validated by a panel of experts using delphi. Arch Phys Med Rehabil. (2022). doi: 10.1016/j.apmr.2022.07.020. [Epub ahead of print].
26. Pinzur MS, Sherman R, DiMonte-Levine P. Adult-onset hemiplegia: changes in gait after muscle-balancing procedures to correct the equinus deformity. J Bone Jt Surg [Am]. (1986) 68:1249–57. doi: 10.2106/00004623-198668080-00016
27. Fuller DA, Keenan MAE, Esquenazi A, Whyte J, Mayer NH, Fidler-Sheppard R. The impact of instrumented gait analysis on surgical planning: Treatment of spastic equinovarus deformity of the foot and ankle. Foot Ankle Int. (2002) 23:738–43. doi: 10.1177/107110070202300810
28. Renzenbrink GJ, Buurke JH, Nene A V, Geurts ACH, Kwakkel G, Rietman JS. Improving walking capacity by surgical correction of equinovarus foot deformity in adult patients with stroke or traumatic brain injury: A systematic review. J Rehabil Med. (2012) 44:614–23. doi: 10.2340/16501977-1012
29. Stroke WHO. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO task force on stroke and other cerebrovascular disorders. Stroke. (1989) 20:1407–31. doi: 10.1161/01.STR.20.10.1407
30. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. (2009) 62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005
31. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
32. Ridgway PF, Guller U. Interpreting study designs in surgical research: a practical guide for surgeons and surgical residents. J Am Coll Surg. (2009) 208:635–45. doi: 10.1016/j.jamcollsurg.2009.01.005
33. Howick J, Chalmers I, Glasziou P, Greenhalgh T, Heneghan C, Liberati A, et al. The Oxford Levels of Evidence 2. Oxford Centre for Evidence-Based Medicine (2022). Available online at: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence
34. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. (2003) 73:712–6. doi: 10.1046/j.1445-2197.2003.02748.x
35. Hanlan A, Mills P, Lipson R, Thompson D, Finlayson H. Interdisciplinary spasticity management clinic outcomes using the goal attainment scale: a retrospective chart review. J Rehabil Med. (2017) 49:423–30. doi: 10.2340/16501977-2228
36. Jung Y, Sim J, Park J, Kim J, Kim MY. Usefulness of goal attainment scaling in intensive stroke rehabilitation during the subacute stage. Ann Rehabil Med. (2020) 44:181–94. doi: 10.5535/arm.19087
37. Krasny-Pacini A, Hiebel J, Pauly F, Godon S, Chevignard M, Finlayson H, et al. Goal attainment scaling in rehabilitation : a literature-based update. Ann Phys Rehabil Med. (2013) 56:1–14. doi: 10.1016/j.rehab.2013.02.002
38. Boffeli TJ, Collier RC, Neubauer EF, Malay DS. Surgical outcomes after minimally invasive release of stroke-related equinovarus contracture of the foot and ankle. J Foot Ankle Surg. (2019) 58:1108–17. doi: 10.1053/j.jfas.2019.01.019
39. Bollens B, Gustin T, Stoquart G, Detrembleur C, Lejeune T, Deltombe T, et al. Randomized controlled trial of selective neurotomy versus botulinum toxin for spastic equinovarus foot after stroke. Neurorehabil Neural Repair. (2013) 27:695–703. doi: 10.1177/1545968313491002
40. Buffenoir K, Roujeau T, Lapierre F, Menei P, Menegalli-Boggelli D, Mertens P, et al. Spastic equinus foot: Multicenter study of the long-term results of tibial neurotomy. Neurosurgery. (2004) 55:1130–6. doi: 10.1227/01.NEU.0000140840.59586.CF
41. Buffenoir K, Rigoard P, Lefaucheur J-P, Filipetti P, Decq P. Lidocaine hyperselective motor blocks of the triceps surae nerves: role of the soleus versus gastrocnemius on triceps spasticity and predictive value of the soleus motor block on the result of selective tibial neurotomy. Am J Phys Med Rehabil. (2008) 87:292–304. doi: 10.1097/PHM.0b013e318168bccb
42. Buffenoir K, Decq P, Hamel O, Lambertz D, Perot C. Long-term neuromechanical results of selective tibial neurotomy in patients with spastic equinus foot. Acta Neurochir. (2013) 155:1731–43. doi: 10.1007/s00701-013-1770-5
43. Carda S, Bertoni M, Zerbinati P, Rossini M, Magoni L, Molteni F. Gait changes after tendon functional surgery for equinovarus foot in patients with stroke assessment of temporo-spatial, kinetic, and kinematic parameters in 1 77 PATIENTS. Am J Phys Med Rehabil. (2009) 88:292–301. doi: 10.1097/PHM.0b013e318198b593
44. Decq P, Filipetti P, Cubillos A, Slavov V, Lefaucheur JP, Nguyen JP. Soleus neurotomy for treatment of the spastic equinus foot. Neurosurgery. (2000) 47:1154–61. doi: 10.1097/00006123-200011000-00027
45. Delattre O, Sellenet T, Barnay J-L, Chevillotte T, De Tienda M. Transfer of distal peroneus longus tendon to tibialis anterior by retrograde fixation to treat spastic equinovarus foot in adults: surgical technique and preliminary results. Orthop Traumatol Surg Res. (2021) 14:102935. doi: 10.1016/j.otsr.2021.102935
46. Deltombe T, Bleyenheuft C, Gustin T. Comparison between tibial nerve block with anaesthetics and neurotomy in hemiplegic adults with spastic equinovarus foot. Ann Phys Rehabil Med. (2015) 58:54–9. doi: 10.1016/j.rehab.2014.12.003
47. Gasse N, Luth T, Loisel F, Serre A, Obert L, Parratte B, et al. Fixation of split anterior tibialis tendon transfer by anchorage to the base of the 5th metatarsal bone. Orthop Traumatol Surg Res. (2012) 98:829–33. doi: 10.1016/j.otsr.2012.07.007
48. Giannotti E, Merlo A, Zerbinati P, Longhi M, Prati P, Masiero S, et al. Early rehabilitation treatment combined with equinovarus foot deformity surgical correction in stroke patients: safety and changes in gait parameters. Eur J Phys Rehabil Med. (2016) 52:296–303. Available online at: https://www.minervamedica.it/en/journals/europamedicophysica/article.php?cod=R33Y2016N03A0296
49. Giannotti E, Merlo A, Zerbinati P, Prati P, Masiero S, Mazzoli D. Safety and long-term effects on gait of hemiplegic patients in equinovarus foot deformity surgical correction followed by immediate rehabilitation: a prospective observational study. Eur J Phys Rehabil Med. (2019) 55:169–75. doi: 10.23736/S1973-9087.18.05290-5
50. Keenan MAE, Lee GA, Tuckman AS, Esquenazi A. Improving calf muscle strength in patients with spastic equinovarus deformity by transfer of the long toe flexors to the os calcis. J Head Trauma Rehabil. (1999) 14:163–75. doi: 10.1097/00001199-199904000-00006
51. Khalil N, Chauvière C, Le Chapelain L, Guesdon H, Speyer E, Bouaziz H, et al. Plantar pressure displacement after anesthetic motor block and tibial nerve neurotomy in spastic equinovarus foot. J Rehabil Res Dev. (2016) 53:219–28. doi: 10.1682/JRRD.2014.11.0298
52. Le Bocq C, Rousseaux M, Buisset N, Daveluy W, Blond S, Allart E. Effects of tibial nerve neurotomy on posture and gait in stroke patients: a focus on patient-perceived benefits in daily life. J Neurol Sci. (2016) 366:158–63. doi: 10.1016/j.jns.2016.04.055
53. Lemos R, Pereira A. Subjective outcome of reconstruction of the adult acquired neurological equinovarus foot. Acta Orthop Belg. (2011) 77:652–8. Available online at: http://www.actaorthopaedica.be/assets/1945/14-Lemos_et_al.pdf
54. Mazzoli D, Giannotti E, Rambelli C, Zerbinati P, Galletti M, Mascioli F, et al. Long-term effects on body functions, activity and participation of hemiplegic patients in equino varus foot deformity surgical correction followed by immediate rehabilitation. A prospective observational study. Top Stroke Rehabil. (2019) 26:518–22. doi: 10.1080/10749357.2019.1642651
55. Mooney V, Goodman F. Surgical approaches to lower-extremity disability secondary to strokes. Clin Orthop Relat Res. (1969) 63:142–52. doi: 10.1097/00003086-196903000-00014
56. Morita S, Muneta T, Yamamoto H, Shinomiya K. Tendon transfer for equinovarus deformed foot caused by cerebrovascular disease. Clin Orthop Relat Res. (1998) 350:166–73. doi: 10.1097/00003086-199805000-00023
57. Namdari S, Min JP, Baldwin K, Hosalkar HS, Keenan MA. Effect of age, sex, and timing on correction of spastic equinovarus following cerebrovascular accident. Foot Ankle Int. (2009) 30:923–7. doi: 10.3113/FAI.2009.0923
58. Nonnekes J, Kamps M, Den Boer J, Van Duijnhoven H, Lem F, Louwerens JWK, et al. Tarsal fusion for pes equinovarus deformity improves gait capacity in chronic stroke patients. J Neuroeng Rehabil. (2019) 16:1–6. doi: 10.1186/s12984-019-0572-2
59. Ono K, Hiroshima K, Tada K, Inoue A. Anterior transfer of the toe flexors for equinovarus deformity of the foot. Int Orthop. (1980) 4:225–9. doi: 10.1007/BF00268160
60. Reddy S, Kusuma S, Hosalkar H, Keenan MA. Surgery can reduce the nonoperative care associated with an equinovarus foot deformity. Clin Orthop Relat Res. (2008) 466:1683–7. doi: 10.1007/s11999-008-0250-3
61. Rousseaux M, Buisset N, Daveluy W, Kozlowski O, Blond S. Comparison of botulinum toxin injection and neurotomy in patients with distal lower limb spasticity. Eur J Neurol. (2008) 15:506–11. doi: 10.1111/j.1468-1331.2008.02112.x
62. Sindou M, Mertens P. Selective neurotomy of the tibial nerve for treatment of the spastic foot. Neurosurgery. (1988) 23:738–44. doi: 10.1227/00006123-198812000-00009
63. Vogt JC. Split anterior tibial transfer for spastic equinovarus foot deformity: Retrospective study of 73 operated feet. J Foot Ankle Surg. (1998) 37:2–7. doi: 10.1016/S1067-2516(98)80003-3
64. Vogt JC, Bach G, Cantini B, Perrin S. Split anterior tibial tendon transfer for varus equinus spastic foot deformity: Initial clinical findings correlate with functional results: A series of 132 operated feet. Foot Ankle Surg. (2011) 17:178–81. doi: 10.1016/j.fas.2010.05.009
65. Maathuis KGB, Van Der Schans CP, Van Iperen A, Rietman HS, Geertzen JHB. Gait in children with cerebral palsy: observer reliability of physician rating scale and edinburgh visual gait analysis interval testing scale. J Pediatr Orthop. (2005) 25:268–72. doi: 10.1097/01.bpo.0000151061.92850.74
66. Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke. (1995) 26:982–9.
67. Caty GD, Arnould C, Stoquart GG, Thonnard J-L, Lejeune TM. ABILOCO: A Rasch-built 13-item questionnaire to assess locomotion ability in stroke patients. Arch Phys Med Rehabil. (2008) 89:284–90. doi: 10.1016/j.apmr.2007.08.155
68. Kling TF, Kaufer H, Hensinger RN. Split posterior tibial-tendon transfers in children with cerebral spastic paralysis and equinovarus deformity. J Bone Jt Surg - Ser A. (1985) 67:186–94.
69. Brun V, Mousbeh Z, Jouet-Pastre B, Benaim C, Kunnert JE, Dhoms G, et al. Clinical assessment of stroke hémiplégic gait: suggestion for a modification of the functional ambulation classification. Ann Réadapt Médecine Phys. (2000) 43:14–20.
70. Viosca E, Martínez JL, Almagro PL, Gracia A, González C. Proposal and validation of a new functional ambulation classification scale for clinical use. Arch Phys Med Rehabil. (2005) 86:1234–8. doi: 10.1016/j.apmr.2004.11.016
71. Lincoln N, Leadbitter D. Assessment of motor function in stroke patients. Physiotherapy. (1979) 65:48–51.
72. Keenan A-M, Redmond AC, Horton M, Conaghan PG, Tennant A. The foot posture index: RASCH analysis of a novel, foot-specific outcome measure. Arch Phys Med Rehabil. (2007) 88:88–93. doi: 10.1016/j.apmr.2006.10.005
73. Patterson KK, Parafianowicz I, Danells CJ, Closson V, Verrier MC, Staines WR, et al. Gait asymmetry in community-ambulating stroke survivors. Arch Phys Med Rehabil. (2008) 89:304–10. doi: 10.1016/j.apmr.2007.08.142
74. Vienne A, Barrois RP, Buffat S, Ricard D, Vidal PP. Inertial sensors to assess gait quality in patients with neurological disorders: a systematic review of technical and analytical challenges. Front Psychol. (2017) 8:1–12. doi: 10.3389/fpsyg.2017.00817
75. Kiresuk TJ, Sherman RE. Goal attainment scaling: A general method for evaluating comprehensive community mental health programs. Community Ment Health J. (1968) 4:443–53. doi: 10.1007/BF01530764
76. Wallen M, O'Flaherty SJ, Waugh M-CA. Functional outcomes of intramuscular botulinum toxin type a and occupational therapy in the upper limbs of children with cerebral palsy: a randomized controlled trial. Arch Phys Med Rehabil. (2007) 88:1–10. doi: 10.1016/j.apmr.2006.10.017
77. Steenbeek D, Gorter JW, Ketelaar M, Galama K, Lindeman E. Responsiveness of goal attainment scaling in comparison to two standardized measures in outcome evaluation of children with cerebral palsy. Clin Rehabil. (2011) 25:1128–39. doi: 10.1177/0269215511407220
78. Patrick E, Ada L. The Tardieu Scale differentiates contracture from spasticity whereas the Ashworth Scale is confounded by it. Clin Rehabil. (2006) 20:173–82. doi: 10.1191/0269215506cr922oa
79. Mazzoli D, Basini G, Prati P, Galletti M, Mascioli F, Rambelli C, et al. Indices of loading and propulsive ability in the gait of patients with chronic stroke with equinus foot deviation: a correlation study. Front Hum Neurosci. (2022) 15:771392. doi: 10.3389/fnhum.2021.771392
80. Barrois R, Gregory T, Oudre L, Moreau T, Truong C, Pulini AA, et al. An automated recording method in clinical consultation to rate the limp in lower limb osteoarthritis. PLoS One. (2016) 11:1–15. doi: 10.1371/journal.pone.0164975
81. Yang S, Zhang J-T, Novak AC, Brouwer B, Li Q. Estimation of spatio-temporal parameters for post-stroke hemiparetic gait using inertial sensors. Gait Posture. (2013) 37:354–8. doi: 10.1016/j.gaitpost.2012.07.032
82. Barrois R, Oudre L, Moreau T, Truong C, Vayatis N, Buffat S, et al. Quantify osteoarthritis gait at the doctor's office: a simple pelvis accelerometer based method independent from footwear and aging. Comput Methods Biomech Biomed Engin. (2015) 18:1880–1. doi: 10.1080/10255842.2015.1072414
83. Mizuike C, Ohgi S, Morita S. Analysis of stroke patient walking dynamics using a tri-axial accelerometer. Gait Posture. (2009) 30:60–4. doi: 10.1016/j.gaitpost.2009.02.017
Keywords: equinovarus foot, hemiplegia, stroke, neuro-orthopedic surgery, gait analysis, IMU
Citation: de l'Escalopier N, Voisard C, Michaud M, Moreau A, Jung S, Tervil B, Vayatis N, Oudre L and Ricard D (2022) Evaluation methods to assess the efficacy of equinovarus foot surgery on the gait of post-stroke hemiplegic patients: A literature review. Front. Neurol. 13:1042667. doi: 10.3389/fneur.2022.1042667
Received: 12 September 2022; Accepted: 10 October 2022;
Published: 09 November 2022.
Edited by:
Maurizio Ferrarin, Fondazione Don Carlo Gnocchi Onlus (IRCCS), ItalyReviewed by:
Stefano Carda, Centre Hospitalier Universitaire Vaudois (CHUV), SwitzerlandPaolo Zerbinati, Ospedale Privato Accreditato Sol et Salus, Italy
Copyright © 2022 de l'Escalopier, Voisard, Michaud, Moreau, Jung, Tervil, Vayatis, Oudre and Ricard. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicolas de l'Escalopier, bmRlbGVzY2Fsb3BpZXJAZ21haWwuY29t
 Nicolas de l'Escalopier
Nicolas de l'Escalopier Cyril Voisard
Cyril Voisard Mona Michaud1,4
Mona Michaud1,4 Albane Moreau
Albane Moreau Laurent Oudre
Laurent Oudre