- 1China International Neuroscience Institute (China-INI), Beijing, China
- 2Department of Neurosurgery, Xuanwu Hospital, Capital Medical University, Beijing, China
- 3Chinese Academy of Medical Sciences, Peking Union Medical College, Beijing, China
- 4Department of Evidence-Based Medicine, Xuanwu Hospital, Capital Medical University, Beijing, China
- 5Beijing Escope Technology Inc, Beijing, China
- 6Department of Interventional Radiology, Xuanwu Hospital, Capital Medical University, Beijing, China
Introduction: Intracranial atherosclerotic disease (ICAD) is one of the most important etiologies of ischemic stroke, especially in Asia. Although medical treatment was recommended as the first-line therapy for ICAD, the recurrent stroke rate was still high in severe stenosis of ICAD despite aggressive medical treatment. Traditionally, the degree of luminal stenosis is used as the principal index for stroke risk stratification in patients with ICAD, while recent evidence suggested that symptomatic atherosclerotic plaques were characterized by plaque features and hemodynamics. This prospective, longitudinal, and nested case-control study aims to identify multimodal imaging predictors of high-risk patients with ICAD refractory to medical treatment and explore a refined risk stratification model based on the above multimodal imaging predictors.
Methods: This prospective, longitudinal, and nested case-control study includes 400 symptomatic patients with ICAD with 50–99% of stenosis treated with aggressive medical therapy. All patients who meet the eligibility criteria are assessed by multimodal imaging examination from three aspects, including lumen stenosis, plaque characteristics, and hemodynamic features. The enrolled patients receive aggressive medical management, including antiplatelet therapy and cardiovascular risk control. The primary outcome is ischemic stroke or death attributable to the lesion of the target vessel within 1 year. The secondary endpoints are (1) any stroke or death; (2) all-cause mortality; (3) any stroke out of the territory of the responsible lesion; (4) functional outcome with the modified Rankin Scale (mRS).
Ethics and Dissemination: This study has been approved by the ethics committee of our center ([2021]083) and has been prospectively registered (Registration No: ChiCTR2100048832). Study findings will be disseminated through peer-reviewed publications and presentations at scientific meetings.
Introduction
Intracranial atherosclerotic disease (ICAD) is one of the most important etiologies of ischemic stroke, especially in Asia. Approximately 33–50% of cases of stroke and more than 50% of transient ischemic attacks (TIA) are attributed to ICAD, which is the most common cause of stroke burden in China (1–4). Contrary to Caucasian patients, patients in Asia with ICAD have a high occurrence rate of severe stenosis and multiple risk factors, such as hyperlipidemia, diabetes mellitus, a family history of stroke, smoking, heavy drinking, and overweight (5–7).
The primary treatments for ICAD are medical treatments and endovascular treatments. Endovascular treatments, such as primary angioplasty, balloon-mounted stent, and self-expansion stent, were regarded as a substitution for patients refractory to medical treatment (8). According to previous randomized control trials studies, endovascular treatment for symptomatic ICAD was related to a higher recurrent risk of stroke (19.7–36.2%) than medical treatment (9, 10), while it may provide benefits over medical treatment with tailored patient selection and operator credentialing (11, 12). Medical treatment, comprising antithrombotic therapy, lipid-lowering treatment, and risk factor modification, was recommended as the first-line therapy for ICAD since the Stenting vs. Aggressive Medical Therapy for Intracranial Arterial Stenosis (SAMMPRIS) trial suggested that medical treatment was superior to the endovascular treatment. However, the recurrent stroke rate ranged from 12.2 to 23% per year in patients with severe stenosis of ICAD even under aggressive medical treatment (8–10, 13, 14).
A great deal of effort has been invested in early identification of high-risk patients with ICAD despite aggressive medical treatment for alternative therapy, such as endovascular therapy, but with limited effect. Traditionally, the degree of luminal stenosis referred to the Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) criteria is used as the principal and is somehow the only index for stroke risk stratification in patients with ICAD, which is simple but may not be sufficiently distinguishable (15). Recent evidence suggested that symptomatic atherosclerotic plaques were characterized not only by a severe degree of luminal stenosis but also by the features such as plaque instability and hemodynamic compromise (16).
Plaque features including specific plaque components, such as large lipid core, thin fiber cap, incomplete fiber cap, intra-plaque hemorrhage, and inflammatory cell infiltration around the lesions, have been collectively characterized as “plaque instability,” which has been demonstrated to be associated with risk of distal embolization or cerebral blood flow deficits (17–19). High-resolution MRI (HR-MRI), which is an emerging effective non-invasive technique, has been used to assess the above features in practice. For instance, intra-plaque hemorrhage and plaque enhancement were detected on HR-MRI in more than 1/2 and 2/3 of patients with ischemic stroke caused by ICAD, respectively (20–22). Hemodynamic compromise, namely hypoperfusion, is another important dimension of ICAD assessment and would impair the washout of debris during distal intracranial circulation, leading to artery-to-artery embolism infarction and increasing the risk of ischemic events (23). Studies have reported that the hazard ratio of ischemic stroke in ICAD patients with hemodynamic compromise was higher with a range of 2–3.5 compared with ICAD patients without hemodynamic compromise after adjusting age and other cardiovascular risk factors (24–27). Cerebral hypoperfusion could be evaluated by several imaging techniques, including CT perfusion, perfusion-weighted imaging, and arterial spin labeling (ASL). In contrast to CT perfusion and perfusion-weighted imaging, ASL is a non-invasive magnetic resonance perfusion imaging method used to visualize and quantify cerebral bleed flow without exogenous contrast agents. Computational fluid dynamics (CFD) simulation is an effective supplement to ASL for hemodynamic evaluation, which could be applied for the interpretation of local flow properties such as translesional pressure drop, wall shear stress, and filtration rate of low-density lipoprotein (28–30). In this study, we have described the methods and procedures used in this prospective, longitudinal, and nested case-control study.
Methods and Analysis
Study Design
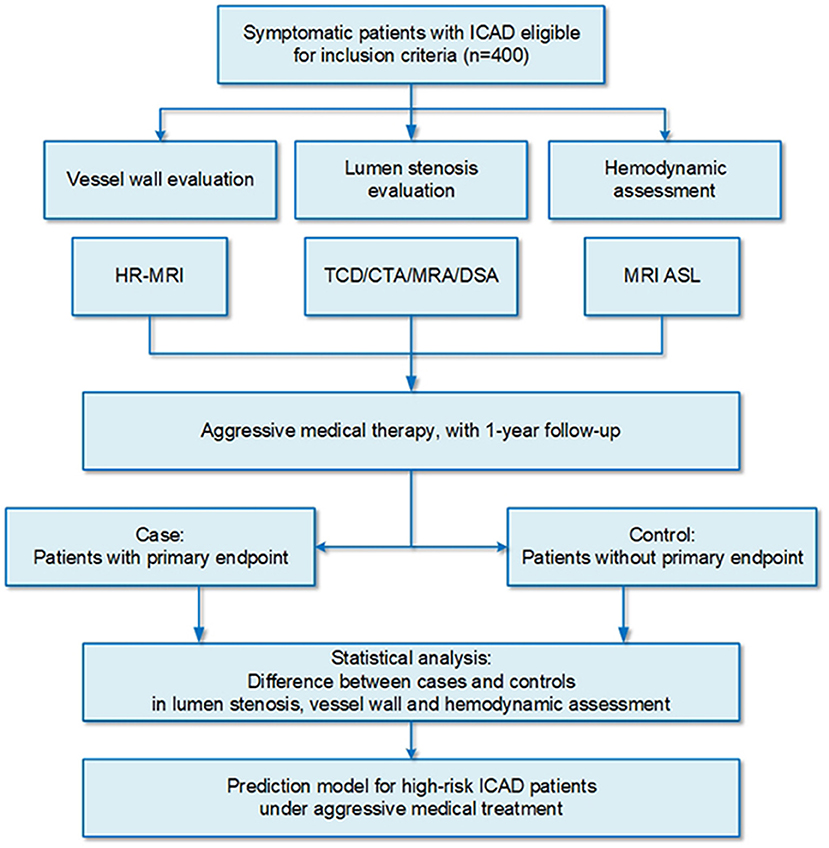
This is a prospective, longitudinal, and nested case-control study of patients with ICAD who are treated with aggressive medical therapy in a tertiary hospital in northern China (Trial Registration No: ChiCTR2100048832) (Figure 1). This study aims to (i) identify multimodal imaging predictors of refractory to medical treatment of high-risk patients with ICAD and (ii) explore and validate a refined risk stratification model for patients with ICAD based on the above multimodal imaging predictors.
Patient Selection
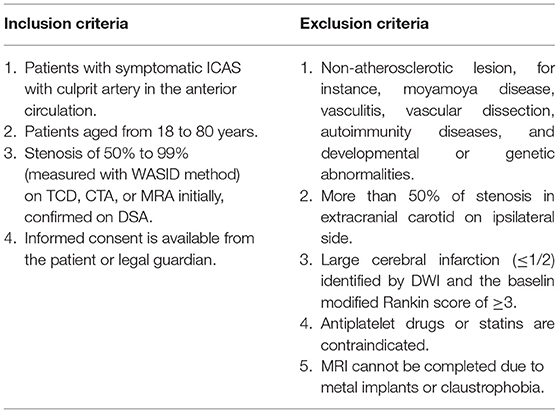
Patients diagnosed with symptomatic ICAD (≥ 50% stenosis) in the anterior circulation in our center will be enrolled in this study from December 2021 to November 2024. Participants will be required to sign informed consent prior to data collection and documentation. All patients will receive the cerebral artery imaging examination. Patients with more than 50% of stenosis in the extracranial carotid on the ipsilateral side will be excluded through transcranial Doppler ultrasound. Detailed inclusion and exclusion criteria are described in Table 1.
Study Procedures
Baseline Evaluations and Data Collection
All eligible patients are comprehensively evaluated from three aspects by non-invasive methods: lumen stenosis, plaque characteristics, and hemodynamics. Magnetic resonance angiography (MRA) is preferred to assess lumen stenosis at supplemented target lesion, while other examinations, such as transcranial Doppler ultrasound, CT angiography, or digital subtraction angiography, could be used as an alternative. The measurement of stenosis degree will be defined by the WASID criteria (15). The location and length of the lesion are measured. As for the assessment of plaque characteristics, HR-MRI (an imaging technique for wall visualization) is planned to perform with 3 Tesla and 3D sequences to assess the arterial remodeling index, plaque burden, and signal intensity. Hemodynamics is quantitatively evaluated by the MRI technology of artery spin labeling (ASL). Hypoperfusion will be defined as regional cerebral blood flow <90% of normal flow referring contralateral territory (31). Arterial transit artifact on ASL is defined as a focus or curvilinear hyperintensity located bordering the region of perfusion defects, which is a highly sensitive indicator of cerebral ischemia (32).
In addition, CFD simulation is applied to analyze the three-dimensional properties of the blood flow, including translesional pressure drop, wall shear stress, and filtration rate of low-density lipoprotein for a comprehensive evaluation of hemodynamic features. The above multimodal imaging examinations are necessary to carried out at the time of recruitment. All imaging parameters are described in Online Supplementary Appendix I.
Treatment Management
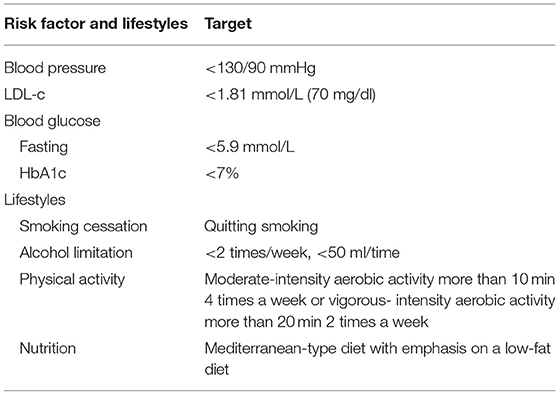
All participants are treated with optimal medical therapy according to the guidelines for ICAD from the American Heart Association/American Stroke Association, which indicates medical management, including antithrombotic therapy and vascular risk factor management, as the first-line therapy (8, 13). Antithrombotic therapies include anticoagulant and antiplatelet agents, which are recommended for all patients with ICAD without contraindications. Anticoagulant agents are recommended for cardiogenic embolism. Antiplatelet agents are more commonly used in patients with ICAD compared with anticoagulant agents. A 3-month dual antiplatelet therapy (e.g., aspirin 100 mg per day and clopidogrel 75 mg per day) is recommended for patients with early-arriving minor stroke and high-risk TIA, while it is not suitable for long-term treatment. Then, single antiplatelet therapy, including aspirin or clopidogrel, is applied throughout their lifetime for the prevention of secondary stroke in patients with ICAD. In addition, all patients with ICAD are required to follow the vascular risk factor management, which comprises the management of low-density lipoprotein (LDL-C), blood pressure, blood glucose level [fasting glucose level and hemoglobin A1c (HbA1c)], and lifestyle changes, such as Mediterranean diet and physical activity (see Table 2). Mediterranean diet is a low-fat diet emphasizing monounsaturated fat, plant-based food, and fish consumption with salt limitation of fewer than 6 grams per day (8). All medical management will be directed and monitored through the internet or telephone by investigators.
Endpoint Evaluation
The primary endpoint of this study is be the ischemic stroke or death attributable to the lesion of the target vessel within 1 year after enrollment. The secondary endpoints are (1) any stroke or death; (2) all-cause mortality; (3) any stroke or death out of the territory of the responsible lesion, including hemorrhagic stroke, non-local ischemic stroke, and non-responsible vascular death; (4) functional outcome with the modified Rankin Scale (mRS).
Ischemic stroke is defined as the sudden onset of a new focal neurological deficit, such as hemiplegia, hemianopia, aphasia, dysphagia, sensory disorders, or confusion, lasting over 24 h, with new cerebral infarctions detected by MRI or CT. The classification of ischemic stroke for the responsible lesion is referred to as infarction location, as infarction in or out of the territory of the symptomatic intracranial artery. Hemorrhagic stroke is defined as the sudden onset of neurological deficits, with parenchymal, subarachnoid, or intraventricular hemorrhage detected by CT or MRI (33).
Follow-Up Assessment
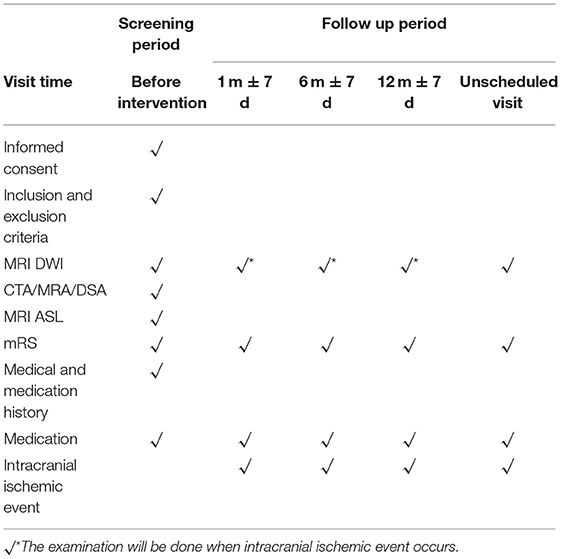
All participants are followed up at 1 month, 6 months, and 1 year after enrollment on the internet or telephone (Table 3). We have established a follow-up system to direct and supervise the participants for better communication, feedback, and compliance. Unscheduled visits for any reason, as well as delayed visits within 7 days of the visit window, are recorded. If a participant reports ischemic symptoms, such as the sudden onset of disorder of motility or sensation, headache, dizziness, imbalance, epilepsy, and/or disorders of consciousness, we will arrange examinations of MRI or CT immediately for the patient and evaluate whether the therapy strategy should be altered. All cerebral arteries including extracranial and intracranial arteries are evaluated per patient at follow-up. During the follow-up period, the drug compliance evaluation scale are also be assessed.
Data Collection and Management
Data for patients enrolled in the study are collected through electronic case report forms. All de-identified data, including demographics, cerebrovascular events, lesion characteristics, and laboratory examination results, are collected by physicians at study entry and patient follow-up. All participants are expected to complete study visits over the planned 1-year follow-up period. In addition, this study is supervised by an independent Data Safety Monitoring Board (DSMB) that comprises independent experts from neurology, neuroradiology, and neurosurgery. The DSMB oversees the study by training and certificating the study staff, reviewing the general conduct of the trial, and checking the study data for quality and safety. Comprehensive training and regular assessment are carried out for all study staff to standardize data collection and assessment. The DSMB also gives suggestions for continuation, modification, or termination of the study. All adverse events that occurred during the study period are reported to the DSMB.
Sample Size
By referring to the published study on medical management in patients with ICAD under 50 to 99% stenosis, the 1-year recurrence incidence of ischemic stroke is assumed to be 8.98% (5). The hazard ratio of hemodynamics (normal or compromised), which is a major risk factor for stroke, is assumed to be 2.4 (26). In addition, the loss rate of follow-up is assumed to be 10%. Thus, the study needs to recruit around 400 participants.
Statistical Analysis
The results will be analyzed with the intention-to-treat method. All confounding factors that influenced outcomes will be analyzed, such as demographic data, cerebrovascular events, lesion characteristics, and laboratory examination results. Student t-test will be used for continuous variables, while the Mann-Whitney test will be used for ordinal variables. Count data of categorical variables will be analyzed by the Chi-square test or Fisher's exact probability method. P < 0.05 is considered statistically significant.
Subsequent multivariate analysis by logistic regression will be performed to detect independent relevant factors influencing primary endpoints. The regression coefficient of independent variables is defined as the weight of this variable for an evaluation system. If there are difficulties for building an evaluating system by the logistic regression model, we will attempt to establish the decision tree with high sensitivity for the evaluation system by Random Forest regression (34).
Discussion
Intracranial atherosclerotic disease is the primary cause of stroke, especially in Asia, which caused high burdens of mortality and morbidity despite the latest treatment strategies, including medical management, endovascular treatment, and surgery (5). The effect of medical management seems ideal for a majority of patients with ICAD, while the current evaluation system cannot accurately predict those with a high risk of recurrent stroke under aggressive medical management (35). Although recurrent stroke is the multifocal cause in patients with symptomatic ICAD, studies have shown that the culprit lesion of ICAD was the major cause (36). Therefore, treatment for ICAD should be mainly focused on the culprit lesion. For the patients' refractory to medical management, other therapy strategies, such as endovascular treatment or surgery with direct intervention, has been adopted according to the current guideline recommendation (8, 13).
However, early diagnosis of high-risk patients' refractory to medical treatment and early triage to alternative therapy is limited by the current risk stratification system, which is merely based on the degree of luminal stenosis (37). The inclusion of the plaque characteristics and hemodynamics assessment with multimodal imaging method may provide a potential solution to make accurate risk stratification (22). To the best of our knowledge, the current study will be the first research to involve multimodal imaging evaluation from three aspects, including the degree of luminal stenosis, plaque characteristics, and hemodynamics, and plan to establish the prognosis prediction system for patients with ICAD who have medical treatment.
As an observational study, several potential limitations must be considered. The collected data may not be as robust as randomized control studies with blinding performance, while the randomized control studies may be needed to further confirm the results of the present study. Data collection and analysis will perform repeated verification and blinding assessment, respectively. Moreover, the study population will be focused on Chinese patients with ICAD; hence, caution must be applied when implementing the findings of this study in other ethnicities.
Ethics and Dissemination
This study has been approved by the ethics committee of our center ([2021]083) and was prospectively registered (Registration No: ChiCTR2100048832). This study will be conducted according to the principles of the Declaration of Helsinki and subsequent amendments (29, 30). Study findings will be disseminated through peer-reviewed publications and presentations at scientific meetings.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Xuanwu Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YM and LJ: conceptualization. TW, JL, and CL: methodology and supervision. BY, RX, LL, KY, CZ, YW, YC, PG, and JC: data curation. TW and JL: project administration. CL: writing – original draft. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the Beijing Science and Technologic Project (Z201100005520019). The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of Interest
CZ was employed by the company Shenzhen Escope Technology Co., Ltd., China.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.803224/full#supplementary-material
References
1. Gorelick PB. Challenges of designing trials for the primary prevention of stroke. Stroke. (2009) 40(3 Suppl):S82–4. doi: 10.1161/STROKEAHA.108.526947
2. Zhang S, Zhou Y, Zhang Y, Gao X, Zhang Q, Wang A, et al. Prevalence and risk factors of asymptomatic intracranial arterial stenosis in a community-based population of Chinese adults. Eur J Neurol. (2013) 20:1479–85. doi: 10.1111/ene.12210
3. Sacco RL, Kargman DE, Gu Q, Zamanillo MC. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The Northern Manhattan Stroke Study. Stroke. (1995) 26:14–20. doi: 10.1161/01.STR.26.1.14
4. Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circ Res. (2017) 120:439–48. doi: 10.1161/CIRCRESAHA.116.308413
5. Wang Y, Zhao X, Liu L, Soo YO, Pu Y, Pan Y, et al. Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: the Chinese Intracranial Atherosclerosis (CICAS) Study. Stroke. (2014) 45:663–9. doi: 10.1161/STROKEAHA.113.003508
6. Yu DD, Pu YH, Pan YS, Zou XY, Soo Y, Leung T, et al. High blood pressure increases the risk of poor outcome at discharge and 12-month follow-up in patients with symptomatic intracranial large artery stenosis and occlusions: subgroup analysis of the CICAS study. CNS Neurosci Ther. (2015) 21:530–35. doi: 10.1111/cns.12400
7. Wu S, Wu B, Liu M, Chen Z, Wang W, Anderson CS, et al. Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol. (2019) 18:394–405. doi: 10.1016/S1474-4422(18)30500-3
8. Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, et al. 2021 Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke. (2021) 52:e364–467. doi: 10.1161/STR.0000000000000375
9. Derdeyn CP, Chimowitz MI, Lynn MJ, Fiorella D, Turan TN, Janis LS, et al. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial. Lancet. (2014) 383:333–41. doi: 10.1016/S0140-6736(13)62038-3
10. Zaidat OO, Fitzsimmons BF, Woodward BK, Wang Z, Killer-Oberpfalzer M, Wakhloo A, et al. Effect of a balloon-expandable intracranial stent vs medical therapy on risk of stroke in patients with symptomatic intracranial stenosis: the VISSIT randomized clinical trial. JAMA. (2015) 313:1240–8. doi: 10.1001/jama.2015.1693
11. Paller AS, Guttman-Yassky E, Irvine AD, Baselga E, de Bruin-Weller M, Jayawardena S, et al. Protocol for a prospective, observational, longitudinal study in paediatric patients with moderate-to-severe atopic dermatitis (PEDISTAD): study objectives, design and methodology. BMJ Open. (2020) 10:e033507. doi: 10.1136/bmjopen-2019-033507
12. Gao P, Wang D, Zhao Z, Cai Y, Li T, Shi H, et al. Multicenter Prospective Trial of Stent Placement in Patients with Symptomatic High-Grade Intracranial Stenosis. AJNR Am J Neuroradiol. (2016) 37:1275–80. doi: 10.3174/ajnr.A4698
13. Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2014) 45:2160–236. doi: 10.1161/STR.0000000000000024
14. Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. (2005) 352:1305–16. doi: 10.1056/NEJMoa043033
15. Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol. (2000) 21:643–6.
16. Arenillas JF. Intracranial atherosclerosis: current concepts. Stroke. (2011) 42(1 Suppl):S20–3. doi: 10.1161/STROKEAHA.110.597278
17. Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation. (2003) 108:1664–72. doi: 10.1161/01.CIR.0000087480.94275.97
18. Finn AV, Nakano M, Narula J, Kolodgie FD, Virmani R. Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol. (2010) 30:1282–92. doi: 10.1161/ATVBAHA.108.179739
19. Fuster V, Moreno PR, Fayad ZA, Corti R, Badimon JJ. Atherothrombosis and high-risk plaque: part I: evolving concepts. J Am Coll Cardiol. (2005) 46:937–54. doi: 10.1016/j.jacc.2005.03.074
20. Teng Z, Peng W, Zhan Q, Zhang X, Liu Q, Chen S, et al. An assessment on the incremental value of high-resolution magnetic resonance imaging to identify culprit plaques in atherosclerotic disease of the middle cerebral artery. Eur Radiol. (2016) 26:2206–14. doi: 10.1007/s00330-015-4008-5
21. Liu S, Luo Y, Wang C, Tang R, Sheng Z, Xie W, et al. Combination of Plaque Characteristics, Pial Collaterals, and Hypertension Contributes to Misery Perfusion in Patients With Symptomatic Middle Cerebral Artery Stenosis. J Magn Reson Imaging. (2020) 51:195–204. doi: 10.1002/jmri.26778
22. Liu S, Tang R, Xie W, Chai S, Zhang Q, Luo Y, et al. Plaque characteristics and hemodynamics contribute to neurological impairment in patients with ischemic stroke and transient ischemic attack. Eur Radiol. (2021) 31:2062–72. doi: 10.1007/s00330-020-07327-1
23. Ito Y, Kato N, Matsumura A, Sonobe M. Hemodynamic instability increases new ischemic brain lesions on diffusion-weighted imaging after carotid artery stenting. Neurol Med Chir. (2013) 53:375–80. doi: 10.2176/nmc.53.375
24. Amin-Hanjani S, Pandey DK, Rose-Finnell L, Du X, Richardson D, Thulborn KR, et al. Effect of Hemodynamics on Stroke Risk in Symptomatic Atherosclerotic Vertebrobasilar Occlusive Disease. JAMA Neurol. (2016) 73:178–85. doi: 10.1001/jamaneurol.2015.3772
25. Yamauchi H, Nishii R, Higashi T, Kagawa S, Fukuyama H. Hemodynamic compromise as a cause of internal border-zone infarction and cortical neuronal damage in atherosclerotic middle cerebral artery disease. Stroke. (2009) 40:3730–5. doi: 10.1161/STROKEAHA.109.560011
26. Donahue MJ, Strother MK, Hendrikse J. Novel MRI approaches for assessing cerebral hemodynamics in ischemic cerebrovascular disease. Stroke. (2012) 43:903–15. doi: 10.1161/STROKEAHA.111.635995
27. Wabnitz AM, Derdeyn CP, Fiorella DJ, Lynn MJ, Cotsonis GA, Liebeskind DS, et al. Hemodynamic markers in the anterior circulation as predictors of recurrent stroke in patients with intracranial stenosis. Stroke. (2018). doi: 10.1161/STROKEAHA.118.020840. [Epub ahead of print].
28. Leng X, Lan L, Ip HL, Fan F, Ma SH, Ma K, et al. Translesional pressure gradient and leptomeningeal collateral status in symptomatic middle cerebral artery stenosis. Eur J Neurol. (2018) 25:404–10. doi: 10.1111/ene.13521
29. Liu H, Gong Y, Leng X, Xia L, Wong KS, Ou S, et al. Estimating current and long-term risks of coronary artery in silico by fractional flow reserve, wall shear stress and low-density lipoprotein filtration rate. Biomed Phys Eng Expr. (2018) 4:1–5. doi: 10.1088/2057-1976/aa9a09
30. Lan L, Liu H, Ip V, Soo Y, Abrigo J, Fan F, et al. Regional High Wall Shear Stress Associated With Stenosis Regression in Symptomatic Intracranial Atherosclerotic Disease. Stroke. (2020) 51:3064–73. doi: 10.1161/STROKEAHA.120.030615
31. Li Z, Li N, Qu Y, Gai F, Zhang G, Zhang G. Application of 30T magnetic resonance arterial spin labeling (ASL) technology in mild and moderate intracranial atherosclerotic stenosis. Exp Ther Med. (2016) 12:297–301. doi: 10.3892/etm.2016.3318
32. Nam KW, Kim CK, Ko SB, Yoon BW, Yoo RE, Sohn CH. Regional Arterial Spin Labeling Perfusion Defect Is Associated With Early Ischemic Recurrence in Patients With a Transient Ischemic Attack. Stroke. (2020) 51:186–92. doi: 10.1161/STROKEAHA.119.026556
33. Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2013) 44:2064–89. doi: 10.1161/STR.0b013e318296aeca
34. Segal MR., Machine Learning Benchmarks Random Forest Regression. 679 (2004). Available online at: https://escholarship.org/uc/item/35x3v9t4 (accessed October 1, 2021).
35. Leung TW, Wang L, Soo YO, Ip VH, Chan AY, Au LW, et al. Evolution of intracranial atherosclerotic disease under modern medical therapy. Ann Neurol. (2015) 77:478–86. doi: 10.1002/ana.24340
36. Famakin BM, Chimowitz MI, Lynn MJ, Stern BJ, George MG, Investigators WT. Causes and severity of ischemic stroke in patients with symptomatic intracranial arterial stenosis. Stroke. (2009) 40:1999–2003. doi: 10.1161/STROKEAHA.108.546150
Keywords: intracranial atherosclerosis, medical treatment, vessel wall, hemodynamics, protocol
Citation: Wang T, Luo J, Liu C, Yang B, Xu R, Li L, Yang K, Zhang C, Wang Y, Chen Y, Gao P, Chen J, Jiao L and Ma Y (2022) High-Risk Intracranial Atherosclerotic Stenosis Despite Aggressive Medical Treatment: Protocol for a Prospective Nested Case-Control Study. Front. Neurol. 13:803224. doi: 10.3389/fneur.2022.803224
Received: 27 October 2021; Accepted: 08 March 2022;
Published: 13 April 2022.
Edited by:
Jean-Claude Baron, University of Cambridge, United KingdomReviewed by:
Marialuisa Zedde, IRCCS Local Health Authority of Reggio Emilia, ItalyHaipeng Liu, Coventry University, United Kingdom
Rui Li, Tsinghua University, China
Copyright © 2022 Wang, Luo, Liu, Yang, Xu, Li, Yang, Zhang, Wang, Chen, Gao, Chen, Jiao and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Ma, leavesyan@sina.com; Liqun Jiao, liqunjiao@sina.cn
†These authors have contributed equally to this work
 Tao Wang
Tao Wang Jichang Luo
Jichang Luo Changyi Liu
Changyi Liu Bin Yang1,2
Bin Yang1,2 Ran Xu
Ran Xu Long Li
Long Li Kun Yang
Kun Yang Jian Chen
Jian Chen Liqun Jiao
Liqun Jiao Yan Ma
Yan Ma