- 1Department of Neurosurgery, The First Affiliated Hospital of Nanchang University, Nanchang University, Nanchang, China
- 2Department of Thoracic Surgery, Jingshan Union Hospital of Huazhong University of Science and Technology, Wuhan, China
- 3Department of Endocrinology, Morbid Obesity and Preventive Medicine, Faculty of Medicine, Oslo University Hospital, Oslo, Norway
- 4Department of Neurosurgery, Central Theater General Hospital of Chinese PLA, Wuhan, China
- 5Department of Neurosurgery, Beijing Chaoyang Hospital, Capital Medical University, Beijing, China
Background: Disturbed serum calcium levels are related to the risk of stroke. However, previous studies exploring the correlation between serum calcium and the clinical outcome of ischemic stroke (IS) have shown inconsistent results.
Object: The study aimed to investigate the relationship between admission serum calcium and 30-day mortality in patients with IS.
Methods: A total of 876 IS patients from a Norwegian retrospective cohort were included for secondary analysis. The exposure variable and the primary outcome were albumin-corrected serum calcium (ACSC) at baseline and all-cause mortality within 30 days after the first admission, respectively. Multivariable logistic regression analysis was used to estimate the risk of 30-day mortality according to ACSC levels. Moreover, the potential presence of a non-linear relationship was evaluated using two-piecewise linear regression with a smoothing function and threshold level analysis. The stability of the results was evaluated by unadjusted and adjusted models.
Results: The result of multiple regression analysis showed that ACSC at baseline was positively associated with the incidence of 30-day mortality after adjusting for the potential confounders (age, gender, serum glucose, hypertension, atrial fibrillation/atrial flutter, renal insufficiency, heart failure, chronic obstructive pulmonary disease, pneumonia, paralysis, and aphasia) (OR = 2.43, 95% CI 1.43–4.12). When ACSC was translated into a categorical variable, the ORs and 95% CIs in the second to the fourth quartile vs. the first quartile were 1.23 (0.56, 2.69), 1.16 (0.51, 2.65), and 2.13 (1.04, 4.38), respectively (P for trend = 0.03). Moreover, the results of two-piecewise linear regression and curve-fitting revealed a linear relationship between ACSC and 30-day mortality.
Conclusion: ACSC is positively associated with 30-day mortality in IS patients, and the relationship between them is linear.
Introduction
Stroke can cause a low quality of life for patients and their families, as well as a great burden and loss for society due to high rates of disability and mortality (1). Ischemic stroke (IS), which is the main subtype of stroke, accounts for about 60–80% (1) of all stroke cases according to the latest evidence. Given this, early risk stratification after acute IS may contribute to improving clinical decision-making.
Calcium is the most abundant mineral in the human body (2), widely taking part in various crucial physiological processes including signal transduction, maintenance of the stability of the cell membrane, coagulation process, movement of the smooth muscle or skeletal muscle, and endocrine function (3, 4). Serum calcium level in a normal physiological situation is strictly controlled to remain within a narrow range (5). Moreover, dyscalcemia has been demonstrated to be related to the risk of cardiovascular and cerebrovascular diseases (6–8).
To date, a limited amount of studies have addressed the association between serum calcium levels and IS outcomes, with conflicting results (9–13). In these studies, both low (10) and high levels (12) of serum calcium have been reported to correlate with poor outcomes of IS. One study reported a U shape association between serum calcium levels and in-hospital all-cause mortality (11). Furthermore, Asian and North American patients were the main subjects in previous studies, which failed to consider European populations. However, not only do the incidence rate and morbidity of stroke vary in different populations (14), in addition to the calcium metabolism (15).
Serum calcium is susceptible to serum albumin levels (16) and albumin-corrected serum calcium (ACSC) calculated according to the classic formula (17) is increasingly used in place of serum calcium in many clinical studies (10–13). Therefore, this study was designed to assess the correlation between ACSC and 30-day mortality in IS patients based on a Norwegian retrospective cohort.
Methods
Data Source
Initial data were downloaded from the public database “DRYAD” (www.datadryad.org). In this database, Tazmini et al. (18) authorized the use of their data in the DRYAD database. Thus, this secondary research based on the raw data for a different research hypothesis was permitted. In addition, the original corresponding author, Kiarash Tazmini, was listed as a co-author with their consent for the contribution of their team in data collection and making their data publicly available.
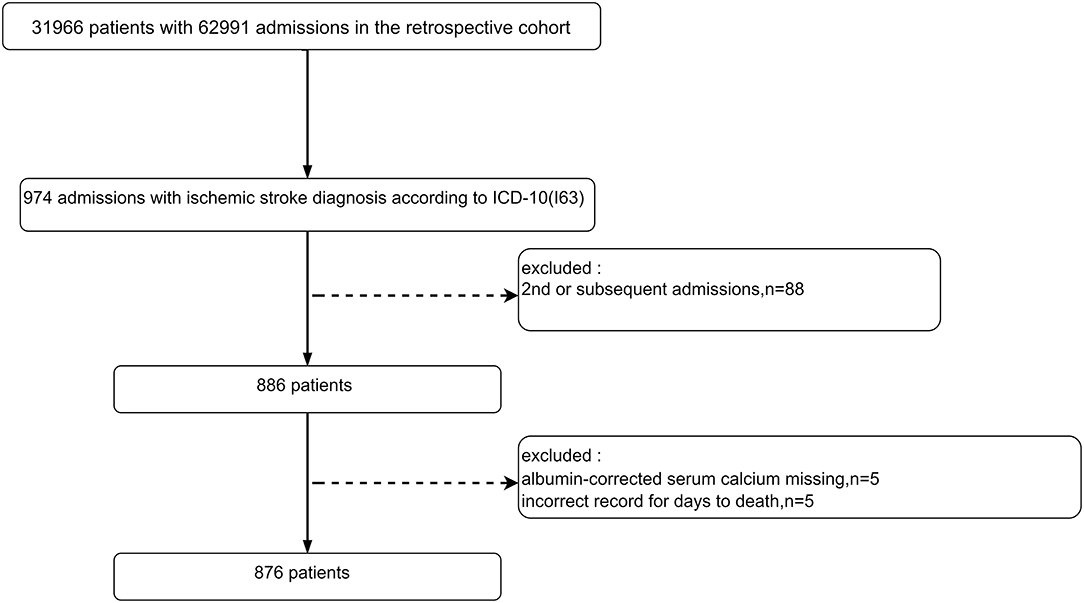
The original research was a single-center retrospective cohort study that included 31,966 unique patients (62,991 registered admission information) who visited the emergency department of the Diakonhjemmet Hospital in Oslo (Norway) from 2010 to 2015. In this study, a total of 974 visits (admission information) with a principal diagnosis of IS (ICD-10, I63) were selected from the raw cohort according to the International Classification of Diseases 10th revision (ICD-10). The raw data included information on multiple hospitalizations for the same patient, but only the first one for each patient was considered in this study. A total of 886 unique IS patients were identified according to their first admission information after excluding the second or subsequent admissions. Subsequently, 10 patients were excluded for missing ACSC information (n = 5) or wrong death information (n = 5). Ultimately, 876 IS patients were included (Figure 1).
The original research was approved by the Norwegian Regional Committee for Medical and Health Research Ethics South East as a quality study, which did not require ethical approval (19). Informed consent was also not necessary because all the data were anonymously processed (19). Thus, separate ethical approval was not required for this secondary analysis. Finally, this study complied with the Helsinki Declaration.
All the laboratory indicators were taken from the first laboratory results after admission. Serum calcium (mmol/L), serum-albumin (g/L), serum-sodium (mmol/L), serum-potassium (mmol/L), serum-glucose (mmol/L), serum-phosphate (mmol/L), and serum-magnesium (mmol/L) were recorded in the original data.
The ACSC levels in the previous study were calculated according to a standard formula and the epidemiological data of the northern European population (20). And the calculation formula is as follows: ACSC = measured serum-calcium level + 0.020 × (41.3–serum-albumin) where 41.3 g/L is the albumin median (19). The unit of ACSC and serum calcium was converted to mg/dl.
Co-morbidities
Secondary diagnostic information was used to identify co-morbidities including diabetes (ICD-10: E10-E14), hypertension (ICD-10: I10), hyperlipemia (ICD-8: E78), atrial fibrillation/atrial flutter (ICD-10: I48), heart failure (ICD-10: I50), renal insufficiency (ICD-10: N18), chronic obstructive pulmonary disease (ICD-10: J42–44), coronary heart disease (ICD-10: I25), chronic obstructive pulmonary disease (ICD-10: J42–44), cancer (ICD-10: C0-C9, Z51.0-3), malnutrition (ICD-10: E40-E46), and pneumonia (ICD-10: J98, J69, J11-18). Moreover, common complications associated with strokes such as paralysis (ICD-10: G80-G83), epilepsy (ICD-10: G40), cognitive disorder (ICD-10: F06), and aphasia (ICD-10: F80, R47) were also considered. All the above-mentioned co-morbidities were processed into categorical variables to facilitate the statistical analysis.
Outcome
The primary outcome was all-cause mortality within 30 days after the first admission.
Missing Data
The missing data of covariates of all 876 patients, in the final analysis, are shown in Table 1. Serum-phosphate and serum-magnesium were missing a large portion of information and were thus converted into categorical variables to address the lower statistical power and potential bias caused by excluding missing data. Dummy variables were used to identify the missing values of the covariate (21).
Multiple imputations based on five replications and a chained equation approach in the R MI procedure were used to deal with missing data (22). Moreover, a comparison between primary data and interpolation data was performed for the sensitivity analysis. The result is shown in Supplementary Figure 2 and Supplementary Table 1.
Statistical Analysis
Statistical analysis was performed using EmpowerStats (www.empowerstats.com, X&Y Solutions, Inc., Boston, MA) and the statistical software package R (http://www.R-project.org, The R Foundation).
Mean ± standard or median (interquartile range) were used to describe continuous variables and categorical variables were expressed as percentages. One-way ANOVA test for continuous variables with normal distribution, Kruskal–Wallis H-test for continuous variables with skewed distribution, and chi-square tests (or Fisher's exact test) for categorical variables were used to analyze differences between or among groups.
Multiple logistic regression analysis was used to assess the specific relationships between the exposure (ACSC) and outcome (30-day mortality); odds ratio (OR) and 95% confidence interval (CI) were used to evaluate the risk.
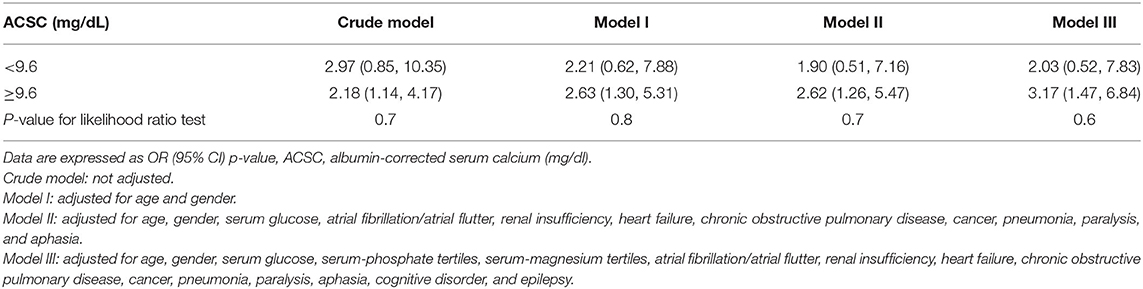
Four models were built to control for the effect of confounding factors: (1) crude model, i.e., unadjusted. (2) Model I, which was adjusted for age and gender. (3) Model II, which was adjusted for age, gender, serum glucose, atrial fibrillation/atrial flutter, renal insufficiency, heart failure, chronic obstructive pulmonary disease, cancer, pneumonia, paralysis, and aphasia. Covariates were included as potential confounders in the final models if they changed the estimates of admission albumin-corrected serum calcium on 30-day mortality by more than 10% or were significantly associated with 30-day mortality (23); gender as a basic variable was also included in the fully adjusted model. (4) Model III, which was additionally adjusted for serum-phosphate tertiles, serum-magnesium tertiles, cognitive disorder, and epilepsy based on Model II (In Model III, the potential influence of missing variables and clinical significance of the other two neurological complications were considered).
A two-piecewise linear regression model and curve fitting were used to examine the potential linear relationship and threshold effect.
Sensitivity Analysis
ACSC quartiles were also used to test the stability of multiple regression results, and the linear tests were performed by assigning medians to each ACSC quartile as a continuous variable in the models (24).
An E-value was used to explore the potential of unmeasured confounding between ACSC and 30-day mortality. The E-value was defined as the required magnitude for an unmeasured confounder to overturn the observed association between ACSC and 30-day mortality (25).
Results
Baseline Characteristics of Participants
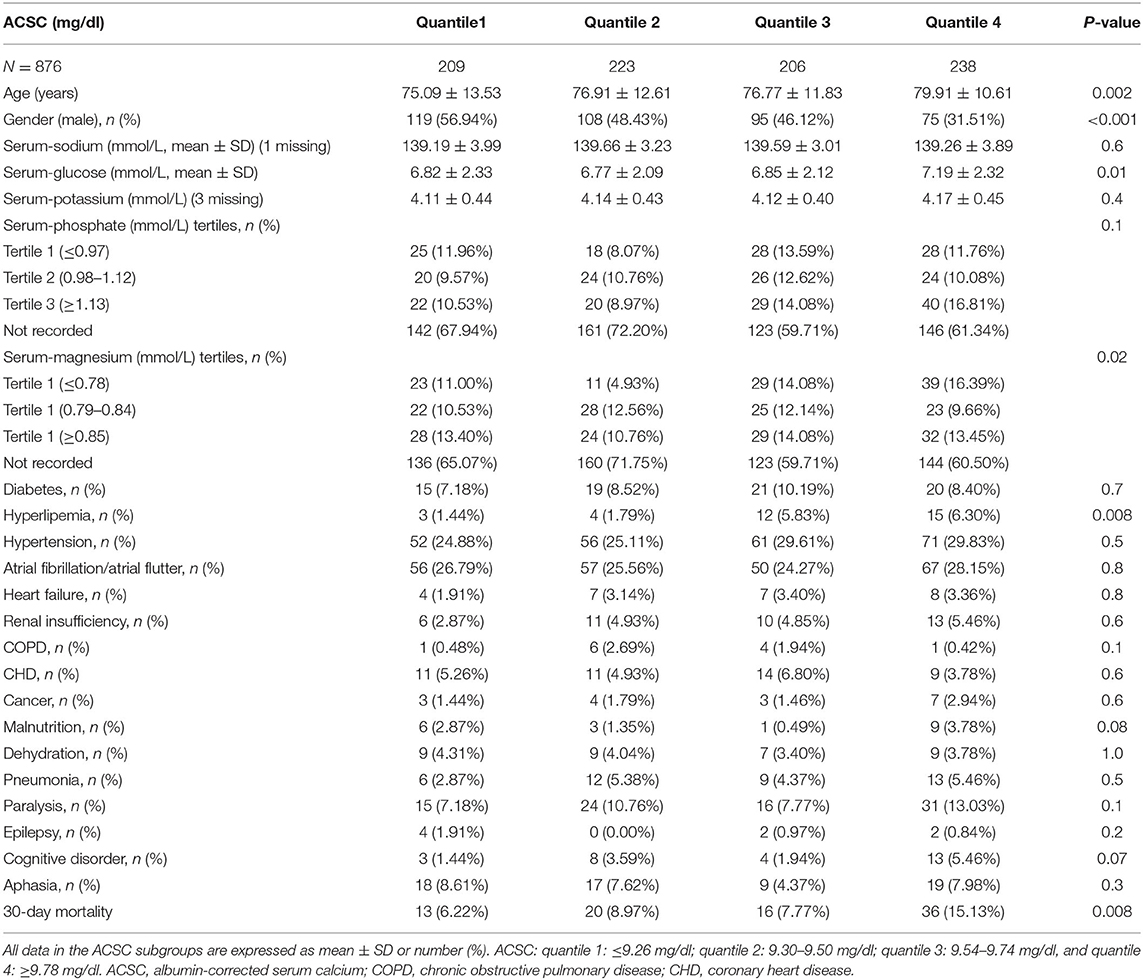
The average age of participants was 77.26 ± 12.26 (34–100) years and 54.68% were female. The baseline characteristics and co-morbidities of participants are listed in Table 1 by ACSC quartiles. Age, gender, serum-glucose, serum-magnesium (tertiles), hyperlipemia, and 30-day mortality of the ACSC quartile groups were statistically different (all p < 0.05).
Univariate Analysis Related to 30-Day Mortality
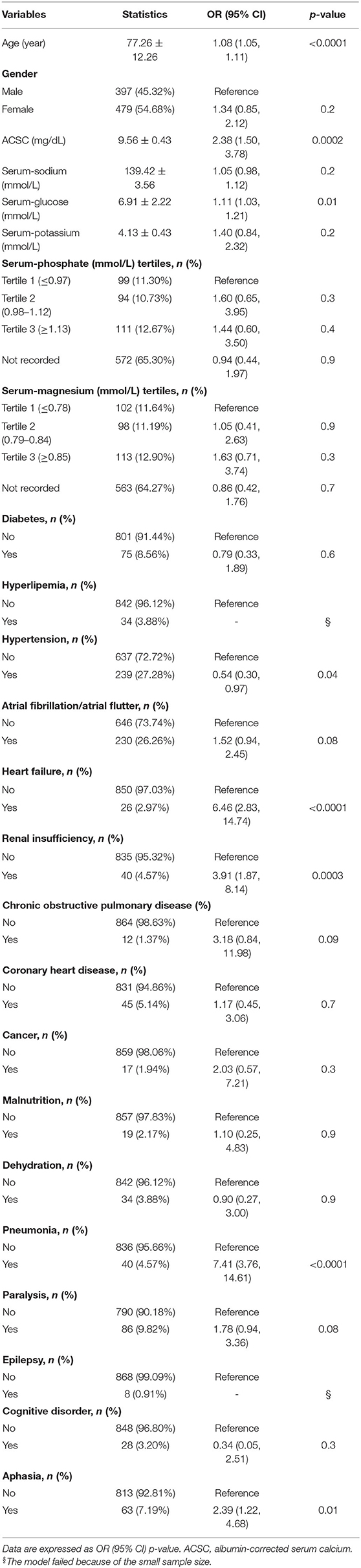
The outcome of 30-day mortality was chosen as a dependent variable, and univariate analysis was used to investigate which independent variable was related to 30-day mortality. The results indicated that age (OR = 1.08, 95% CI 1.05–1.11), ACSC (OR = 2.38, 95% CI 1.50–3.78), serum-glucose (OR = 1.11, 95% CI 1.03–1.21), hypertension (yes vs. no: OR = 0.54, 95% CI 0.30–0.97), heart failure (yes vs. no: OR = 6.46, 95% CI 2.83–14.74), renal insufficiency (yes vs. no: OR = 3.91, 95% CI 1.87–8.14), pneumonia (yes vs. no: OR = 7.41, 95% CI 3.76–14.61) and aphasia (yes vs. no: OR = 2.39, 95% CI 1.22–4.68) were all associated with 30-day mortality (Table 2).
Multivariate Logistic Regression Analysis of ACSC and 30-Day Mortality
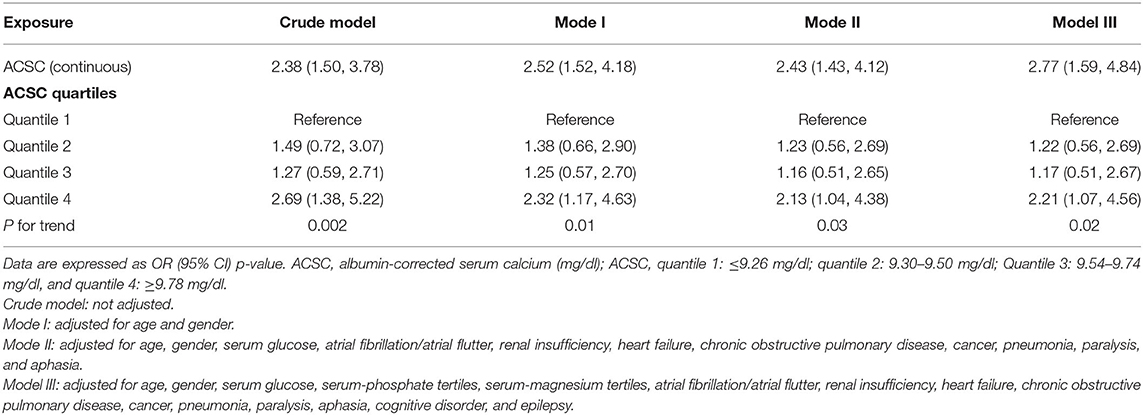
ACSC was chosen as the independent variable and 30-day mortality as the dependent variable in the multiple regression equation. Other variables were used as covariates to adjust the model to prove the stability of the results, and four models were built. No covariates were adjusted in the crude model and the result showed that ACSC was independently associated with 30-day mortality (OR = 2.38, 95% CI 1.50–3.78). The result in the Model I also revealed that ACSC was independently related to 30-day mortality (OR = 2.52, 95% CI 1.52–4.18) after adjusting for age and gender. Moreover, the result was similar in the Model II adjusted for covariates such as age, gender, serum glucose, hypertension, atrial fibrillation/atrial flutter, renal insufficiency, heart failure, chronic obstructive pulmonary disease, pneumonia, paralysis, and aphasia (OR = 2.43, 95% CI 1.43–4.12). In Model III which additionally adjusted for serum-phosphate tertiles, serum-magnesium tertiles, cognitive disorder, and epilepsy based on Model II, the result was also robust (OR = 2.77, 95% CI 1.59–4.84) (Table 3).
Sensitivity Analysis
The results of the linear trend tests of the four models all showed that higher ACSC quartile groups were significantly related to an increased risk of 30-day mortality. And the p-values for the trends in the crude model, Model I, Model II, and Model III were 0.002, 0.01, 0.03, and 0.02, respectively (Table 3).
An E-value was calculated to assess the sensitivity to unmeasured confounding. The primary findings were stable unless an unmeasured confounder existed and was highly positively related to ACSC (OR ≥ 4.29) and 30 day-mortality (OR ≥ 4.29).
Curve Fitting and Two-Piecewise Linear Regression Model of ACSC and 30-Day Mortality
Curve fitting and two-piecewise linear regression analysis were used to investigate a potential non-linear association between ACSC and 30-day mortality. The result of curve fitting adjusted for age, gender, serum glucose, hypertension, atrial fibrillation/atrial flutter, renal insufficiency, heart failure, chronic obstructive pulmonary disease, pneumonia, cognitive disorder, epilepsy, paralysis, and aphasia showed a curve that continued to rise (Figure 2), and the results for the other three models were the same (Supplementary Figure 1). Furthermore, despite two different effective sizes based on the demarcation point (9.6 mg/dl) of the curve fitting was observed in the two-piecewise linear regression analysis adjusted according to Model II, the p-value for the likelihood ratio test was 0.7 (Table 4).
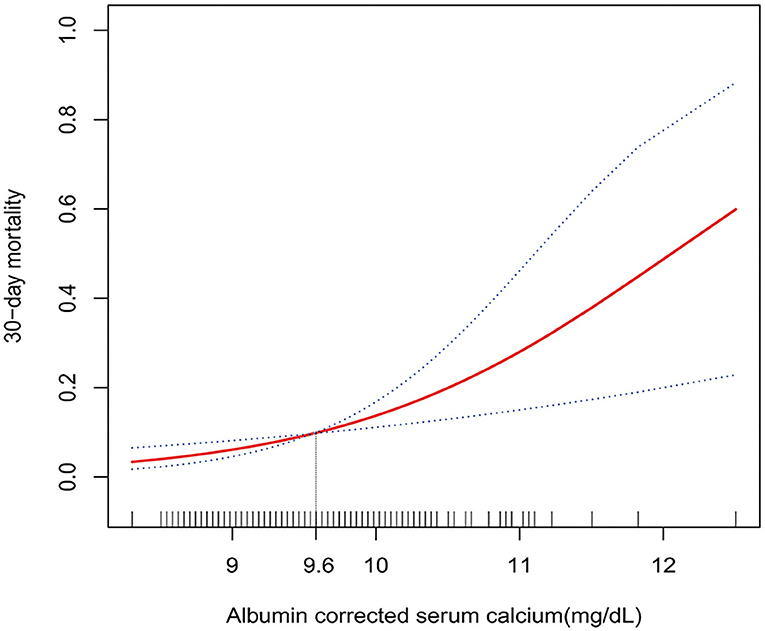
Figure 2. Multivariate adjusted smooth curve-fitting for association between ACSC and 30-day mortality. Same confounder factors as in model III: age, gender, serum glucose, serum-phosphate tertiles, serum-magnesium tertiles, atrial fibrillation/atrial flutter, renal insufficiency, heart failure, chronic obstructive pulmonary disease, cancer, pneumonia, paralysis, aphasia, cognitive disorder, and epilepsy, were adjusted. The red line represents the best-fit line, and the blue lines are 95% CIs. The potential demarcation point was 9.6 mg/dl according to the smoothing spline plots.
The other three models showed similar results and the p-values for the likelihood ratio test were all >0.05 (Table 4). Thus, no threshold effect was observed according to the demarcation point, indicating that the relationship between ACSC and 30-day mortality was linear.
Discussion
This study revealed an appreciable positive association between ACSC and 30-day mortality in IS patients. The relevant results were also significant after adjustment of the three different models with potential confounding factors. ACSC was then translated into a categorical variable (quartile) to analyze the sensitivity of the results, and the results were stable (OR values gradually increased significantly, from the second to the fourth quartile, and the values of p for trend were <0.05 in the four models). Furthermore, the results of curve fitting and the two-piecewise linear regression model revealed a stable linear relationship.
Despite the pivotal role of calcium ions in neuronal damage after ischemic events having been demonstrated as early as 1998 (26), few studies have focused on the correlation between baseline serum calcium and the clinical outcome in patients with IS. In a retrospective cohort study involving 173 IS patients, Buck et al. (9) explored between admission serum calcium and infarct volumes on diffusion-weighted imaging and their results revealed that high serum calcium levels were related to small cerebral infarct area and good outcomes. The study of Buck et al. (9) adjusted for serum glucose, blood pressure, co-morbidities, and stroke subtype in the final model, but serum albumin which might affect the serum calcium level was not considered. However, another study based on the Virtual International Stroke Trials Archive demonstrated that elevated 72–96-h serum calcium levels are related to 3-month functional outcome, but earlier (<4.5 h) serum calcium is not associated with the functional outcome (10). The result revealed that serum calcium levels can reflect a secondary epiphenomenon of stroke. However, the variation of intra-individual calcium levels, in reality, is ~2% (27), and the transportation of extracellular into neuronal cells would not significantly alter serum calcium levels. In 2010, an Israeli clinical study including 784 patients revealed that too high and too low serum calcium levels were both correlated with long-term mortality in female stroke patients (11). Moreover, a Korean cohort study that included 1915 IS patients showed that elevated admission ACSC levels are related to a short-time functional outcome and long-term mortality in IS patients (12). Admission serum calcium and ACSC were both included in the analysis, and the results of ACSC showed a significantly increased risk of all-cause death with the ACSC level elevating, but there were no positive results for serum calcium. Given the physiologic characteristic that more than 50% of calcium ions are combined with albumin and the difficulty for the measurement of ionized calcium in clinical practice, ACSC might be a better parameter than serum calcium to assess the effect of calcium. Most recently, a China national stroke cohort study demonstrated a high risk of long-term mortality of IS patients in the top quartile group of ACSC levels, with no statistical difference between patients of different genders (13).
Heterogeneity between the various study populations may have led to the inconsistency in the findings. Epidemiologic evidence showed that the incidence and mortality of stroke differed in various populations (14), in addition to calcium metabolism in different ethnicity (15). It was noteworthy that the relationship between serum calcium level and risk of stroke in Swedish and Korean populations also showed opposite results (7, 8). This study added evidence of the correlation between ACSC and clinical outcomes in IS patients from a different populations. Compared with other countries at a similar economical level, Nordic countries were with cold climate (28), short sunshine time (29), and plant-based diet pattern (30) which may impact the vitamin-D intake and serum calcium level. However, due to the nature of a retrospective study, the ethnicity, diet, climate, and environment of the selected sample group could not be included in the analysis. Prospective, large sample size studies that included these population characteristics are required in the future to accurately explore the risk range of serum calcium for Norwegian IS patients. In addition, due to the raw data of this study are from a large emergency cohort, ACSC may have a broad application prospect for IS patients admitted to emergency departments especially in primary or smaller Emergency Departments as a brief blood biomarker that could be quickly obtained at admission. Moreover, the causality of admission serum calcium level and short-term mortality could not be determined in this study. If future studies can demonstrate that admission elevated serum calcium cause increased mortality, clinical treatment including intravenous rehydration, enhancing kidney clearance of calcium (loop diuretics, calcitonin, and haemodialysis), calcium channel blocker, and limiting calcium and Vitamin D supplementation, might be beneficial to improve ischemic stroke patient outcomes.
The results of this study were compatible with the results of the previous study on the Chinese cohort (13). This study revealed that higher baseline ACSC levels were associated with 30-day mortality in IS patients. After processing ACSC as a categorical variable (quartiles), our results demonstrated that the top quartile group of ACSC levels most significantly increased the 30-day mortality. This result was also in agreement with the recent study from China (13), whose outcome is 1-year mortality (top quartile group, HR = 1.56, fully adjusted model). Moreover, previous studies had also demonstrated potential correlations between low serum calcium levels and cerebral infarct volume and short-term functional outcome, and U-shape relationship between baseline serum calcium and long-term mortality were shown by Appel et.al. Thus, we used curve fitting and the two-piecewise linear regression analyses to more accurately explore the relationship in this study. The results illustrated a linear relationship without threshold effect between ACSC level and 30-day mortality in IS patients, unlike the non-linear result of a previous study from the study of Appel et al. (11). In addition to differences in study populations, the study by Appel et al. (11) included both ischemic and hemorrhagic stroke patients, and different mediating mechanisms between serum calcium and mortality may account for their results, but this possibility needs to be verified by further studies.
Although extant studies demonstrated that high serum calcium levels were significantly associated with the risk of stroke (7) and clinical outcomes (12, 13), the pathophysiological mechanisms remain undefined. Previous studies revealed the key role of serum calcium in the promotion of vascular calcification, which is a complex process including the promotion of osteogenic/chondrogenic differentiation, vesicle release, cell apoptosis, loss of inhibitors, and extracellular matrix degradation (31, 32), leading to atherosclerosis. Thus, elevated serum calcium levels may accelerate the process of atherosclerosis, cardio-cerebrovascular calcification, and plaque rupture which has been associated with poor clinical outcomes (33–35). In addition, calcium ion is a crucial intracellular messenger, and plays a key role in neuronal damage and cell death (26). Furthermore, mitochondrial damage caused by high calcium concentration may be another mechanism (36). Moreover, recent research has shown that calcium ions could affect the cortical spreading depolarization after ischemic injury by regulating microglia activity (37). The association between elevated extracellular serum calcium levels and microglial calcium overload leading to cortical spreading depolarizations may explain the relationship between serum calcium levels and poor IS outcome. However, the inconsistent results of different studies suggested that there were more complicated mechanisms are needed to explain the effects of different ranges of serum calcium on all-cause mortality for IS patients, and the specific mechanisms need to be further studied.
This study has several advantages. First, the results of univariate analysis, regression coefficient change, and previous literature were used to select the covariates. Second, curve fitting and two-piecewise linear regression were used to explore a potential non-linear relationship, as shown in a previous study. Third, one crude model and four models adjusted with potential variables were used to test the stability of the results. Finally, ACSC was taken as a continuous variable and categorical variable into the multiple regression equation to avoid the contingency of the analysis, and the sensitivity analysis and trend test were used.
However, the following limitations exist. First, owing to the retrospective nature of this study, the non-inclusion of patients with missing ACSC information or wrong death information would lead to selection bias. Thus, the baseline information between the included group and the excluded group was compared, and the results showed no significant differences between the groups (Supplementary Table 2). Second, even though the ACSC level was calculated according to the standard formula, since the relationship between serum albumin and serum calcium could be more complex in the disease state, in addition to some studies having underlined that this formula could overestimate calcium levels (38, 39), further studies are needed to explore the actual relationship among serum calcium, serum albumin, and 30-day mortality. Though first-time laboratory results at admission, which are more likely to reflect the initial state of the patient at the onset, were used, it would be better to examine the dynamic changes in ACSC in future studies to understand the potential mechanism of the associations. Because of the retrospective study design, we could not confirm the time of blood collection, which will influence the ACSC level. Thus, further prospective studies with predesigned identical examination times are required. Third, the presence of unmeasured confounders could not be excluded. Since the secondary analysis originated from a retrospective cohort, variables that were not collected could not be adjusted. E-value was used to explore the potential for unmeasured confounding between ACSC and 30-day mortality and the result showed that an unmeasured confounder was unlikely to explain the entirety of the mortality effect. Pre-stroke medications such as calcium and vitamin D supplements might affect the level of admission to ACSC, and patients with chronic comorbidities were more likely to have a medication history. A stratification analysis was performed on comorbidity and non-comorbidity subgroups, revealing that the results remained stable in both subgroups. This means that the results remain stable even in the non-comorbidity patients who may be less likely to take medication before stroke (Supplementary Table 3). In addition, given that the medication treatment in the hospital would tend to a bias toward the null, it is postulated that the unmeasured confounding of medication treatment in the hospital might underestimate the observed effect. Neurological function (NIHSS score or Norwegian trial Scandinavian Stroke Scale), IS subtypes, and functional status before stroke are important information to evaluate the outcome of stroke patients. Therefore, common complications associated with neurological function including paralysis, epilepsy, cognitive disorder, and aphasia were additionally adjusted, and the results were also robust after adjustment (Model III). Moreover, some studies have revealed a negative association between serum calcium at the baseline and admission neurological function (9, 40). Therefore, adjustment for the neurological function tends to elevate the estimated effect. Finally, the participants in this study were represented by the Norwegian population, and the findings could not necessarily apply to other populations.
Conclusions
Admission albumin-corrected serum calcium in ischemic stroke patients was positively correlated with 30-day mortality, and the relationship between them was almost linear.
Data Availability Statement
The datasets used in this study are publicly available and can be accessed via the database www.Datadryad.org.
Ethics Statement
The original research was approved by the Norwegian Regional Committee for Medical and Health Research Ethics South East as a quality study, which did not need ethical approval. Thus, our secondary analysis based on this original research did not require separate ethical approval. This research was performed according to the Declaration of Helsinki. All data was processed anonymously and therefore written informed consent from the patients/participants was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
Conceptualization and methodology: YL and XM. Data source: KT. Software and writing—original draft preparation: YL. Visualization: XM. Writing—reviewing and editing: YW, MY, and XZ. All authors listed have read and approved the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81960330) (http://www.nsfc.gov.cn) and Jiangxi Provincial Department of Science and Technology (Grant Numbers: 20202BABL206053, 20192BAB205045, and 20161BBI90018).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors specially thank the other authors of the original study. They and KT completed the whole study and shared their data selflessly and kindly (19). They are [the rankings and institutions of these researchers are ranked according to the original reference (19)]: Professor Ståle H. Nymo (Department of Internal Medicine, Diakonhjemmet Hospital, Oslo, Norway), Professor William E. Louch (Institute of Experimental Medical Research, Oslo University Hospital, Ullevål and University of Oslo, Oslo, Norway; Center for Heart Failure Research, University of Oslo, Oslo, Norway), Professor Anette H. Ranhoff (Department of Internal Medicine, Diakonhjemmet Hospital, Oslo, Norway; Department of Clinical Science, University of Bergen, Bergen, Norway), and Professor Erik Øie (Department of Internal Medicine, Diakonhjemmet Hospital, Oslo, Norway; Department of Clinical Science, University of Bergen, Bergen, Norway). The authors are also grateful to Professor Xinglin Chen and Professor Changzhong Chen (Statistical consultant, X&Y Solution, lnc. Boston MA) for their assistance in statistics. Finally, YL would like to especially thank his daughter (Mrs. Yanli Lu) and other family members for their encouragement and kind support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.889518/full#supplementary-material
Abbreviations
IS, ischemic stroke; ACSC, albumin-corrected serum calcium; COPD, chronic obstructive pulmonary disease; CHD, coronary heart disease.
References
1. GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. (2021) 20:795–820. doi: 10.1016/S1474-4422(21)00252-0
3. Kraft MD. Phosphorus and calcium: a review for the adult nutrition support clinician. Nutr Clin Pract. (2015) 30:21–33. doi: 10.1177/0884533614565251
4. Santulli G, Marks AR. Essential roles of intracellular calcium release channels in muscle, brain, metabolism, and aging. Curr Mol Pharmacol. (2015) 8:206–22. doi: 10.2174/1874467208666150507105105
5. Walsh J, Gittoes N, Selby P, Society for Endocrinology Endocrine Emergency Guidance. Emergency management of acute hypercalcaemia in adult patients. Endocr Connect. (2016) 5:G9–11. doi: 10.1530/EC-16-0055
6. Reid IR, Gamble GD, Bolland MJ. Circulating calcium concentrations, vascular disease and mortality: a systematic review. J Intern Med. (2016) 279:524–40. doi: 10.1111/joim.12464
7. Rohrmann S, Garmo H, Malmstrom H, Hammar N, Jungner I, Walldius G, et al. Association between serum calcium concentration and risk of incident and fatal cardiovascular disease in the prospective AMORIS study. Atherosclerosis. (2016) 251:85–93. doi: 10.1016/j.atherosclerosis.2016.06.004
8. Dibaba DT, Xun P, Fly AD, Bidulescu A, Tsinovoi CL, Judd SE, et al. Calcium intake and serum calcium level in relation to the risk of ischemic stroke: findings from the REGARDS study. J Stroke. (2019) 21:312–23. doi: 10.5853/jos.2019.00542
9. Buck BH, Liebeskind DS, Saver JL, Bang OY, Starkman S, Ali LK, et al. Association of higher serum calcium levels with smaller infarct volumes in acute ischemic stroke. Arch Neurol. (2007) 64:1287–91. doi: 10.1001/archneur.64.9.1287
10. Ovbiagele B, Starkman S, Teal P, Lyden P, Kaste M, Davis SM, et al. Serum calcium as prognosticator in ischemic stroke. Stroke. (2008) 39:2231–6. doi: 10.1161/STROKEAHA.107.513499
11. Appel SA, Molshatzki N, Schwammenthal Y, Merzeliak O, Toashi M, Sela BA, et al. Serum calcium levels and long-term mortality in patients with acute stroke. Cerebrovasc Dis. (2011) 31:93–9. doi: 10.1159/000321335
12. Chung JW, Ryu WS, Kim BJ, Yoon BW. Elevated calcium after acute ischemic stroke: association with a poor short-term outcome and long-term mortality. J Stroke. (2015) 17:54–9. doi: 10.5853/jos.2015.17.1.54
13. Zhang JF, Meng X, Jing J, Pan Y, Wang YL, Zhao XQ, et al. Serum calcium and long-term outcome after ischemic stroke: results from the China National stroke registry III. Atherosclerosis. (2021) 325:24–9. doi: 10.1016/j.atherosclerosis.2021.03.030
14. Tsai CF, Thomas B, Sudlow CL. Epidemiology of stroke and its subtypes in Chinese vs. white populations: a systematic review. Neurology. (2013) 81:264–72. doi: 10.1212/WNL.0b013e31829bfde3
15. Andersen S, Noahsen P, Rex KF, Fleischer I, Albertsen N, Jorgensen ME, et al. Serum 25-hydroxyvitamin D, calcium and parathyroid hormone levels in Native and European populations in Greenland. Br J Nutr. (2018) 119:391–7. doi: 10.1017/S0007114517003944
16. Bushinsky DA, Monk RD. Electrolyte quintet: calcium. Lancet. (1998) 352:306–11. doi: 10.1016/S0140-6736(97)12331-5
17. Payne RB, Little AJ, Williams RB, Milner JR. Interpretation of serum calcium in patients with abnormal serum proteins. Br Med J. (1973) 4:643–6. doi: 10.1136/bmj.4.5893.643
18. Tazmini K, Nymo SH, Louch WE, Ranhoff AH, Oie E. Data from: electrolyte imbalances in an unselected population in an emergency department: a retrospective cohort study. Dryad Dataset. (2019). doi: 10.5061/dryad.f3h26j3
19. Tazmini K, Nymo SH, Louch WE, Ranhoff AH, Oie E. Electrolyte imbalances in an unselected population in an emergency department: a retrospective cohort study. PLoS ONE. (2019) 14:e215673. doi: 10.1371/journal.pone.0215673
20. Rustad P, Felding P, Franzson L, Kairisto V, Lahti A, Martensson A, et al. The nordic reference interval project 2000: recommended reference intervals for 25 common biochemical properties. Scand J Clin Lab Invest. (2004) 64:271–84. doi: 10.1080/00365510410006324
21. Vetter C, Devore EE, Wegrzyn LR, Massa J, Speizer FE, Kawachi I, et al. Association between rotating night shift work and risk of coronary heart disease among women. JAMA. (2016) 315:1726–34. doi: 10.1001/jama.2016.4454
22. Bernhardt PW. Model validation and influence diagnostics for regression models with missing covariates. Stat Med. (2018) 37:1325–42. doi: 10.1002/sim.7584
23. Bjerregaard LG, Pedersen DC, Mortensen EL, Sorensen T, Baker JL. Breastfeeding duration in infancy and adult risks of type 2 diabetes in a high-income country. Matern Child Nutr. (2019) 15:e12869. doi: 10.1111/mcn.12869
24. Park SY, Freedman ND, Haiman CA, Le Marchand L, Wilkens LR, Setiawan VW. Association of coffee consumption with total and cause-specific mortality among nonwhite populations. Ann Intern Med. (2017) 167:228–35. doi: 10.7326/M16-2472
25. Haneuse S, VanderWeele TJ, Arterburn D. Using the E-value to assess the potential effect of unmeasured confounding in observational studies. JAMA. (2019) 321:602–3. doi: 10.1001/jama.2018.21554
26. Kristian T, Siesjo BK. Calcium in ischemic cell death. Stroke. (1998) 29:705–18. doi: 10.1161/01.STR.29.3.705
27. Valero-Politi J, Ginard-Salva M, Gonzalez-Alba JM. Annual rhythmic and non-rhythmic biological variation of magnesium and ionized calcium concentrations. Clin Chem Lab Med. (2001) 39:45–9. doi: 10.1515/CCLM.2001.011
28. Nafstad P, Skrondal A, Bjertness E. Mortality and temperature in Oslo, Norway, 1990-1995. Eur J Epidemiol. (2001) 17:621–7. doi: 10.1023/A:1015547012242
29. Moan J, Baturaite Z, Juzeniene A, Porojnicu AC. Vitamin D, sun, sunbeds and health. Public Health Nutr. (2012) 15:711–5. doi: 10.1017/S1368980011002801
30. Kehoe L, Walton J, Flynn A. Nutritional challenges for older adults in Europe: current status and future directions. Proc Nutr Soc. (2019) 78:221–33. doi: 10.1017/S0029665118002744
31. Yang H, Curinga G, Giachelli CM. Elevated extracellular calcium levels induce smooth muscle cell matrix mineralization in vitro. Kidney Int. (2004) 66:2293–9. doi: 10.1111/j.1523-1755.2004.66015.x
32. Reynolds JL, Joannides AJ, Skepper JN, McNair R, Schurgers LJ, Proudfoot D, et al. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. J Am Soc Nephrol. (2004) 15:2857–67. doi: 10.1097/01.ASN.0000141960.01035.28
33. Bugnicourt JM, Leclercq C, Chillon JM, Diouf M, Deramond H, Canaple S, et al. Presence of intracranial artery calcification is associated with mortality and vascular events in patients with ischemic stroke after hospital discharge: a cohort study. Stroke. (2011) 42:3447–53. doi: 10.1161/STROKEAHA.111.618652
34. Pelisek J, Eckstein HH, Zernecke A. Pathophysiological mechanisms of carotid plaque vulnerability: impact on ischemic stroke. Arch Immunol Ther Exp. (2012) 60:431–42. doi: 10.1007/s00005-012-0192-z
35. Magdic J, Cmor N, Kaube M, Hojs FT, Hauer L, Sellner J, et al. Intracranial vertebrobasilar calcification in patients with ischemic stroke is a predictor of recurrent stroke, vascular disease, and death: a case-control study. Int J Environ Res Public Health. (2020) 17:13. doi: 10.3390/ijerph17062013
36. Nicholls DG. A role for the mitochondrion in the protection of cells against calcium overload? Prog Brain Res. (1985) 63:97–106. doi: 10.1016/S0079-6123(08)61978-0
37. Liu L, Kearns KN, Eli I, Sharifi KA, Soldozy S, Carlson EW, et al. Microglial calcium waves during the hyperacute phase of ischemic stroke. Stroke. (2021) 52:274–83. doi: 10.1161/STROKEAHA.120.032766
38. Pfitzenmeyer P, Martin I, D'Athis P, Grumbach Y, Delmestre MC, Blonde-Cynober F, et al. A new formula for correction of total calcium level into ionized serum calcium values in very elderly hospitalized patients. Arch Gerontol Geriatr. (2007) 45:151–7. doi: 10.1016/j.archger.2006.10.006
39. Byrnes MC, Huynh K, Helmer SD, Stevens C, Dort JM, Smith RS, et al. comparison of corrected serum calcium levels to ionized calcium levels among critically ill surgical patients. Am J Surg. (2005) 189:310–4. doi: 10.1016/j.amjsurg.2004.11.017
Keywords: serum calcium, albumin-corrected serum calcium, association, ischemic stroke, 30-day mortality, baseline
Citation: Lu Y, Ma X, Tazmini K, Yang M, Zhou X and Wang Y (2022) Admission Serum Calcium Level and Short-Term Mortality After Acute Ischemic Stroke: A Secondary Analysis Based on a Norwegian Retrospective Cohort. Front. Neurol. 13:889518. doi: 10.3389/fneur.2022.889518
Received: 04 March 2022; Accepted: 09 May 2022;
Published: 15 June 2022.
Edited by:
John Zhang, Loma Linda University, United StatesReviewed by:
Yan Fan, Liaocheng People's Hospital, ChinaTao-Hsin Tung, Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University, China
Copyright © 2022 Lu, Ma, Tazmini, Yang, Zhou and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming Yang, yangmingwh2021@163.com; Xiaobing Zhou, xiaobingzhou1234@163.com; Yang Wang, wangyang6877@163.com
 Yuzhao Lu1
Yuzhao Lu1 Kiarash Tazmini
Kiarash Tazmini Yang Wang
Yang Wang