- 1Department of Psychiatry, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand
- 2Department of Orthopedics, Hip Fracture Research Unit, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand
- 3Department of Medicine, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand
- 4Immunosciences Lab Inc., Los Angeles, CA, United States
- 5Cyrex Labs LLC, Phoenix, AZ, United States
- 6Sichuan Provincial Center for Mental Health, Sichuan Provincial People’s Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
- 7Key Laboratory of Psychosomatic Medicine, Chinese Academy of Medical Sciences, Chengdu, China
- 8Department of Psychiatry, Medical University of Plovdiv, Plovdiv, Bulgaria
- 9Research Institute, Medical University of Plovdiv, Plovdiv, Bulgaria
- 10Kyung Hee University, Seoul, Republic of Korea
- 11Cognitive Impairment and Dementia Research Unit, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand
Introduction: Delirium is accompanied by immune response system activation, which may, in theory, cause a breakdown of the gut barrier and blood–brain barrier (BBB). Some results suggest that the BBB is compromised in delirium, but there is no data regarding the gut barrier. This study investigates whether delirium is associated with impaired BBB and gut barriers in elderly adults undergoing hip fracture surgery.
Methods: We recruited 59 older adults and measured peak Delirium Rating Scale (DRS) scores 2–3 days after surgery, and assessed plasma IgG/IgA levels (using ELISA techniques) for zonulin, occludin, claudin-6, β-catenin, actin (indicating damage to the gut paracellular pathway), claudin-5 and S100B (reflecting BBB damage), bacterial cytolethal distending toxin (CDT), LPS-binding protein (LBP), lipopolysaccharides (LPS), Porphyromonas gingivalis, and Helicobacter pylori.
Results: Results from univariate analyses showed that delirium is linked to increased IgA responses to all the self-epitopes and antigens listed above, except for LPS. Part of the variance (between 45–48.3%) in the peak DRS score measured 2–3 days post-surgery was explained by independent effects of IgA directed to LPS and LBP (or bacterial CDT), baseline DRS scores, and previous mild stroke. Increased IgA reactivity to the paracellular pathway and BBB proteins and bacterial antigens is significantly associated with the activation of M1 macrophage, T helper-1, and 17 cytokine profiles.
Conclusion: Heightened bacterial translocation, disruption of the tight and adherens junctions of the gut and BBB barriers, elevated CDT and LPS load in the bloodstream, and aberrations in cell–cell interactions may be risk factors for delirium.
Background
The estimated incidence of postoperative delirium in older adult patients with hip fractures is between 8.2%–24.0% (1–3). The major clinical characteristics of this acute and fluctuating neuropsychiatric syndrome are disturbances of attention, awareness, and other cognitive components (4). In hip fracture patients, delirium is associated with several adverse outcomes, such as an increased hospital stay, medical complications, poorer functional recovery, and an increased mortality rate (5–7). Older age, delirium history, premorbid dementia, multiple comorbidities, and functional dependency are common risk factors for postoperative delirium (2, 3, 8).
Hip fracture and surgery in older adults may initiate a complex neuro-pathophysiological process which leads to cognitive impairments and often delirium (9). The hypothesized pathophysiology of delirium comprises peripheral activation of the immune-inflammatory response system (IRS), neuroinflammation, oxidative stress, neurotransmitter dysregulation, and neural circuit disconnection (10, 11). Delirium in older adults following hip fracture surgery is associated with increased white blood cell numbers and neutrophil/lymphocyte ratio indicating aseptic inflammation (11). A recent meta-analysis shows that the delirium diagnosis is associated with a higher neutrophil/lymphocyte ratio in various critical care settings (12). Furthermore, we showed that delirium is characterized by IRS activation and a relative deficiency in the compensatory immunoregulatory system (CIRS), which prevents hyperinflammation (13). The increased IRS/CIRS ratio in delirium is indicated by increased macrophage M1; T helper (Th)1, and Th17 profiles, and a relative deficiency in Th2 and T regulatory (Treg) profiles (13).
There is now evidence that interactions between activated IRS pathways (e.g., M1 macrophage), breakdown of the BBB, and increased gut permeability play a role in neuropsychiatric disorders (14–17). Although previous studies have associated post-surgery delirium with BBB breakdown (18), there is no direct evidence that the breakdown of the paracellular pathway is associated with delirium. Nevertheless, there is some data on associations between gut microbiota diversities and postoperative delirium (19, 20). Few studies supported the hypothesis of an association between insomnia and abnormalities in the gut-brain axis (21, 22).
In the context of schizophrenia, notable correlations were identified between cognitive deficits or positive symptoms and biomarkers of IRS activation, including dysfunction of paracellular adherens junctions (e.g., IgA to E-cadherin and β-catenin) and tight junctions (e.g., IgA to occludin and zonulin), bacterial translocation (e.g., elevated IgA/IgM to Gram-negative bacteria), as well as disruption of the blood–brain barrier (e.g., increased IgA to occludin and β-catenin) (23, 24). A recent review and meta-analysis show that schizophrenia, major depression, bipolar disorder, and chronic fatigue syndrome are accompanied by indicants of leaky gut, with increased serum lipopolysaccharides (LPS) or antibodies directed to LPS of Gram-negative gut-commensal bacteria, LPS-binding protein (LBP), and zonulin (25, 26). In addition, increased IgG or IgA responses directed against Helicobacter pylori or Porphyromonas gingivalis may be associated with neurocognitive deficits in Alzheimer’s disease (27–29).
The hepatic secreted LBP may bind LPS in the systemic circulation, thereby forming an LPS-LBP complex, which consequently activates inflammatory cascades via the Toll-Like Receptor (TLR) 4 complex (30, 31). Some Gram-negative bacteria, including Helicobacter species, produce a bacterial cytolethal distending toxin (CDT), which is involved in IRS activation, host cell DNA intoxication, and apoptosis (32). Increased expression of LPS, the LPS-LBP complex, and CDT in the serum may indicate the breakdown of the gut barrier, with consequent increased translocation of bacterial antigens through the gut epithelium barrier into the lamina propria layer and the adjacent lymphatic and vascular systems (33–36). This bacterial translocation may occur via (a) the transcellular pathway and increased reactivity to actin may indicate this process (37, 38); and (b) the paracellular pathway, which comprises tight junctions (TJs) and adherens junctions (AJs) as major components (39, 40). Increased IgA/IgM/IgG responses to TJs (including zonulin, occludin, claudin-5/6) and AJs (including β-catenin) are biomarkers indicating the breakdown of the paracellular pathway (26).
Invasion of bacterial antigens through a dysfunctional intestinal barrier may trigger inflammatory cells inside the lamina propria layer to produce chemokines and pro-inflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin (IL)-1, and IL-6, which may further expand the inflammatory signal to the systemic level (41). Conversely, systemic inflammation may damage the intestinal barrier and cause increased gut permeability due to, for example, increased levels of IL-1β, IL-6, and TNF-α (15, 26, 42). Reciprocal interactions between increased gut permeability and systemic IRS activation were demonstrated in disease models such as inflammatory bowel disease (43), cancer (44, 45), schizophrenia (46), depression (47, 48), and Alzheimer’s dementia (49, 50), but data in delirium is lacking.
Interestingly, the intestinal epithelial barrier and the BBB share some TJ and AJ-associated proteins such as occludin, claudin-5/6, β-catenin, and actin (24, 51). Some epitopes are more specific to the BBB, such as claudin-5 and S100 calcium-binding protein B (S100B) (52–54). Therefore, increased IgA/IgM/IgG responses to these epitopes may indicate increased damage to the gut (leaky gut) and BBB (leaky brain) barriers (24, 55). Despite recent evidence demonstrating increased IgA/IgM levels to gut and BBB breakdown epitopes in schizophrenia (24, 56, 57), depression (42, 58), autism (59), and Alzheimer’s disease (50, 60), no such data were reported in delirium.
Hence, the current study aims to examine (a) whether delirium severity in post-surgery patients with hip fracture is predicted by disturbances of the gut epithelial and the BBB as measured by using IgA/IgG responses to antigens and self-epitopes including LPS, LBP, CDT, zonulin, occludin, claudin-5, claudin-6, S100B, β-catenin, actin, P. gingivalis and H. pylori; and (b) whether these IgA/IgG responses are associated with the IRS response in post-surgery older adults.
Methods
Participants
A cohort of 59 elderly individuals with hip fractures who were admitted to the Hip Fracture Pathway Inpatient Care at King Chulalongkorn Memorial Hospital in Bangkok, Thailand, was enrolled in our study from June 2019 to February 2020. Patients who were 65 years of age or older and presented with a low-energy impact hip fracture, subsequently underwent surgery for the fracture, and were transferred to the surgery intensive care unit (SICU) or orthopedic wards, were included in the study. Individuals who met the following criteria were excluded from the study: major psychiatric illness (including schizophrenia, substance use disorders, bipolar disorder, psycho-organic disorders), a lifetime history of neuro-inflammatory and neurodegenerative diseases (including Alzheimer’s and Parkinson’s disease, multiple sclerosis), (auto) immune disorders (including psoriasis, systemic lupus erythematosus, inflammatory bowel disease, rheumatoid arthritis), coma, intracranial hemorrhage, pathologic fractures, and hip fractures from a high impact accidents. Individuals who have experienced remission of depression, modest neurocognitive disorders, or stroke without developing post-stroke disabilities within a year of the acute event may qualify for inclusion in the study.
Clinical assessments
In addition to bedside interviews, sociodemographic and clinical information was gathered via electronic medical records. The cognitive status and delirium levels were evaluated at baseline, 24 h prior to the surgery. The cognitive status, delirium score, and diagnosis were evaluated daily for 3 days after the procedure. At the patient’s bedside, the Delirium Rating Scale, Revised-98-Thai Version (DRS-R-98-T) was utilized to assess the degree and manifestation of delirium until 3 days after the surgery (29, 30). This evaluation occurred on the evening of day zero, the day before the surgery, as well as in the morning and evening. The DRS-R-98-T demonstrates satisfactory inter-rater reliability and sensitivity and specificity in detecting delirium (29, 30). The severity of sleep–wake cycle disturbance was evaluated using the first item of the DRS-R-98-T (peak values of day 1 and 2), which ranged from normal (0 point) to severe (3 point) sleep disruption (61). As a result, scores two and three were merged into a single score (2), producing an ordinal variable with values of zero, one, and two. Data was gathered regarding the administration of benzodiazepines, opiates, anticholinergic medications, and psychiatric drugs, in addition to pertinent perioperative and postoperative clinical information including blood loss, operative duration, and the need for restraint because of agitation. Acute coronary events, arrhythmias, severe hypertension, and atrial fibrillation were among the cardiovascular complications obtained after surgery. The BMI was calculated by dividing the square of the subject’s height by their body weight (in kilograms).
The study protocol (registration number 528/61) was assessed and authorized by the institutional review board of the Faculty of Medicine, Chulalongkorn University. It adhered to the principles and procedures outlined in the International Guideline for the Protection of Human Subjects, the Belmont Report, the CIOMS Guideline, and the International Conference on Harmonization in Good Clinical Practice (ICH-GCP). Consent forms for the study were duly signed by every patient or their first-degree relatives.
Determination of antibodies by ELISA
Aside from the clinical evaluation, venous blood samples were collected at 7 a.m. on day 0. Blood samples were stored at −80°C until thawed and then forwarded to the lab for IgA/IgG and cytokine/chemokine testing. IgA-and IgG antibodies to the epitopes were measured using ELISA methods. LPS from Escherichia coli, Salmonella, Shigella, Klebsiella, and Pseudomonas, LBP, and actin were purchased from Sigma-Aldrich (St. Louis, MO, United States), and occludin, zonulin, claudin-5, claudin-6, S100B, β-catenin, CDT, P. gingivalis, and S100B were synthesized by Bio-Synthesis® (Lewisville, TX, United States). H. pylori was purchased from the American Type Culture Collection® (Manassas, VA, United States). Jackson-Immuno Research® offered affinity pure goat anti-human IgA α-chain-specific and anti-human IgG, FC-specific (West Grove, PA United States). The IgG and IgA assays were performed as discussed previously (62). In brief: all antigens were prepared at a concentration of 1 mg/mL in 0.01 M phosphate buffer saline (PBS) pH 7.4. The optimal amount of each antigen was found to be one microgram in 100 microliters of 0.01 M carbonate buffer at pH 9.6, which was added to different wells of Costar ELISA plates. Plates were incubated at 25°C for 4 h, followed by overnight incubation at 4°C. In the next step, the unbound antigens were removed, and plates were washed 3 times with PBS containing 0.05% Tween 20, and 200 microliters of 2% bovine serum albumin (BSA) was added to block the non-coated regions of ELISA plate wells. Plates were kept at 4°C overnight, and after the BSA were washed, dried, and kept at 4°C until used. Calibrators, controls, and patients’ sera dilution at 1:50 for IgA and 1:100 for IgG in 0.01 M PBS pH 7.4 with 2% BSA and 0.05% Tween 20 were added to separate wells and incubated at room temperature for 1 h. Several wells contained all the reagents, but no serum was used to measure the background or blank ODs. After repeated washing and removing unattached serum proteins, alkaline phosphatase labeled anti-human IgA at 1:400 or anti-human IgG at 1:800 was added to separate sets of microwell plates and incubated for another hour. After repeating the washing procedure, adding 1 mg/mL of substrate para-nitrophenylphosphate, and incubating at room temperature for 30 min, a yellow color formed proportionately to the antibody concentration in the samples. The reaction was then halted with 60 microliters of 2 N NaOH, which created the endpoint color, which was measured at 405 nm with an ELISA reader. The antibody index was computed as follows: antibody index = (OD of sample − OD blank)/(OD of calibrator − OD of blank).
Based on the results we computed five IgA/IgG z-unit-based composite scores as (a) z transformation of IgA/IgG to LPS (z LPS) + z CDT + z G. gingivalis + z H. pylori (labeled IgA/IgG Bacterial, reflecting increased bacterial load); (b) z occludin + z zonulin + z claudin-6 (labeled IgA TJs, reflecting damage to the endothelial TJs); (c) z claudin-5 + z S100B (labeled IgA/IgG BBB, reflecting damage to the BBB especially when also IgA TJs are present), (d) z catenin + z actin (labeled IgA/IgG CATACT); and (e) z LPS + z LBP (labeled IgA/IgG LPS + LBP, reflecting increased LPS load in the plasma).
Cytokine and chemokine assays
The methodology for analyzing cytokines and chemokines has been previously documented (13, 62). To summarize, the study employed the Bio-Plex ProTM Human Chemokine Assays manufactured by Bio-Rad Laboratories, Inc. in the United States of America. The Bio-Plex® 200 System (Carlsbad, California), was utilized to analyze the samples. 11.0% was the intra-assay CV for all analytes. In the data analysis, we utilized fluorescence intensities while subtracting the blank analyte values, as these intensities are a more reliable substitute for concentrations, particularly when examining numerous plates. The levels of IL-2, IL-10, IL-12, and IL-13 were omitted from the analyses focusing on a particular cytokine, because too many values were below the detection limit. Nonetheless, the values of those cytokines were included when creating immunological profiles because quantifiable levels of specific cytokines may contribute to the IRS/CIRS composites. The IRS and CIRS immune profiles, as well as the IRS/CIRS ratio (11, 62), were the primary immunological outcome determinants in this investigation. IRS was conceptualized as z-unit-based composite score based on z M1 (z IL-1β + z IL-6 + zTNF-α + z CXCL8 + z CCL3 + z IL-2 + z IL-12 + z interferon-γ + z IL-17). CIRS was conceptualized as z IL-4 + z IL-9 + z IL-13 + z IL-10 + z IL-1RA. The IRS/CIRS ratio was conceptualized as z IRS − z CIRS. Since IL-9 and IL-13 may have dual roles, we have recomputed the IRS/CIRS ratio without those two cytokines (labeled as IRS/CIRS2). There was a strong correlation between both IRS/CIRS and IRS/CIRS2 (r = 0.904, p < 0.001).
Statistics
To ascertain relationships between sets of categorical data, the X2-test was utilized. Conversely, analysis of variance (ANOVA) was employed to investigate differences in scale variables between groups. The primary outcome analysis was the quantitative DRS-R-98 scale score, which was predicted by the explanatory variables, which are biomarkers measured 1 to 2 days prior. Pearson’s product moment correlation coefficients were computed to ascertain the relationships between the DRS scores and the IRS, CIRS, and IRS/CIRS data, as well as the IgA/IgG reactivity to self-epitopes and antigens. To examine the relationship between the dichotomized peak DRS scores on days 2–3 and the IgA/IgG responses on day 0 (which were entered as input variables), binary logistic regression and generalized estimating equation (GEE) analysis with repeated measurements were applied. We calculated the odds ratio (OR) with 95% confidence intervals and parameter estimates (B with SE values) for the logistic regressions; Nagelkerke values were utilized as pseudo-R2 effect sizes. While allowing for confounding variables, GEE was utilized to examine the relationships between the repeated DRS score measurements (days 2 and 3) and the IgA/IgG reactivities and DRS score on day 0. The study employed multiple regression analysis to investigate the relationship between predictors (such as IgA and IgG responses) and outcome variables (such as DRS scores), while controlling for confounding variables (such as age and sex). The effect size was determined using R2, and multivariate normality, collinearity, and multicollinearity were consistently assessed using Cook’s distance and leverage, tolerance and VIF, and the White and modified Breusch-Pagan tests for homoscedasticity, respectively. Furthermore, an automatic step-up method was implemented, incorporating values of 0.05 p-to-enter and 0.06 p-to-remove. All bootstrapped regression analyses were conducted using 5,000 samples; in cases where the results did not concur, the bootstrapped results are displayed. We accounted for the following variables in all regression analyses: gender, age, mild cognitive impairment, prior stroke, BMI, surgical duration, time to surgery, estimated blood loss during surgery, and use of deliriogenic medications. The statistical analysis was conducted using IBM SPSS for Windows version 28 (version 2022). A significance level of 0.05 was applied, and two-tailed tests were utilized. For numerous associations and comparisons, a False Discovery Rate (FDR) p correction was implemented. The a priori estimated sample size for a multiple regression analysis using G*Power analysis with an effect size of 0.30, an alpha of 0.05, a power of 0.80, and five predictors is around 49. Partial Least Squares (PLS) analysis using SmartPLS (SmartPLS) (23) was employed to examine whether the effects of age on the increases in the DRS score from baseline to 2–3 days later were mediated by IgA responses to different antigens.
Results
Sociodemographic and clinical data
The sociodemographic, clinical, and immune data of the study population are demonstrated in Table 1. To differentiate patients with increased DRS scores on days 2 and 3 from those with lower values, we computed the peak DRS values on day 2 and 3 and dichotomized the values using a visual binning method. Consequently, we examined two study groups, namely post-surgery patients with and without increased peak DRS values 2–3 days after surgery, using a cut-off value of ≥4. There were no significant differences between the two study groups in sex, education years, BMI, blood loss volume, duration from fall to hospital admission, hospitalization to surgery, or total duration of hospitalization. As expected (because higher age is a risk factor for delirium), patients in the high peak DRS group were significantly older than those in the low peak DRS group. The high-peak DRS group showed significantly higher IRS and IRS/CIRS scores than the low-peak DRS group, while there were no significant differences in CIRS scores between the groups.

Table 1. Socio-demographic, clinical and immune data in older adults divided into those with lower and higher peak Delirium Rating Scale, (DRS) scores on days 2 and 3 after surgery (peak DRS).
Prediction of DRS score by biomarkers
Table 2 shows the results of binary logistic regression analyses with the high peak DRS group (reference group: low peak DRS group) as the dependent variable and the IgA bacterial, TJs, BBB, CATACT, and LPS + LBP composite scores. The high peak DRS group was significantly associated with baseline IgA scores across all five composite scores. Table 2 shows the results of GEE analyses with the DRS scores on days 2 and days 3 as dependent variables (repeated measures) and the IgA values to antigens on days 0 and 1 as predictors (repeated measures). As such, the IgA values at days 0 and 1 predicted the DRS values on days 2 and 3, respectively. We found that the 5 IgA values directed to the bacterial, TJs, BBB, CATACT, and LPS + LBP composite scores significantly predicted the DRS scores on days 2 and 3. The same table shows the results of the effects of the separate IgA values on the DRS values. We found that all IgA values directed to all epitopes, except LPS, were significantly associated with the DRS values some days later. Supplementary Figure 1 shows a clustered bar graph showing the IgA values for the 5 composites and all separate IgA values in post-surgery patients with low vs. high peak DRS scores. Supplementary Figure 2 shows that there were no significant differences in any of the IgG antibody values directed to the different epitopes.
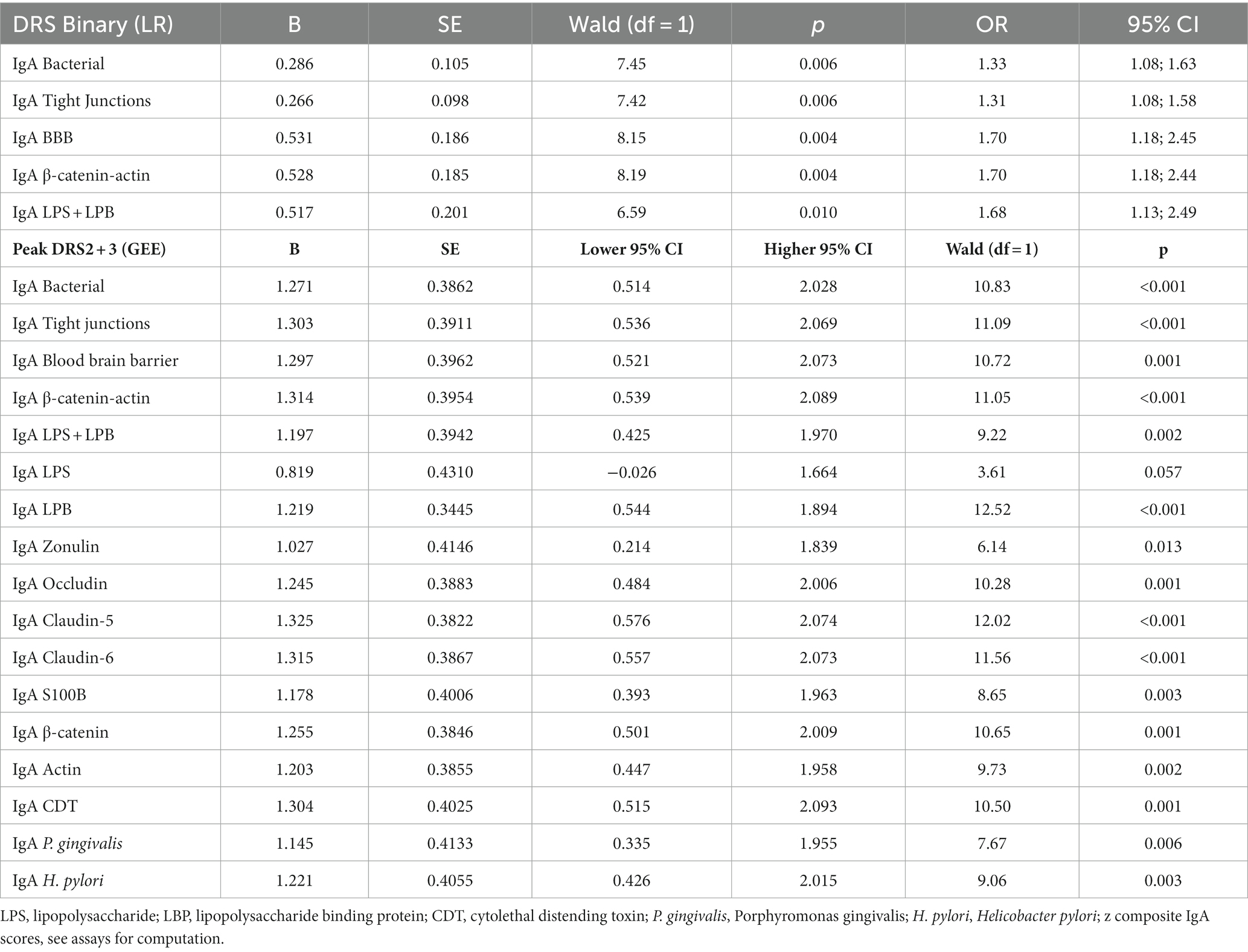
Table 2. Results of binary logistic regression analysis (LR) and generalized estimating equations (GEE) with the Delirium Rating Scale (DRS), either as binary or continuous score, as dependent variables, and IgA responses to self-antigens as explanatory variables.
Intercorrelation matrix between IgA responses, DRS, and IRS/CIRS data
Table 3 shows the intercorrelation matrix between the five IgA composite scores and the day 1, day 2, and peak DRS scores, as well as the IRS, CIRS, and IRS/CIRS values. The results indicate that there is no significant correlation between the five baseline IgA values and the DRS score on day 1, whereas there were highly significant associations with the peak DRS values. To examine the associations between the baseline IgA composite scores and the actual changes in the DRS scores from day 0 to days 2 and 3, we computed the residualized peak DRS values after regression on the DRS Day 0 values. These actual changes in DRS values were significantly associated with all five IgA composite scores. There were also significant correlations between the baseline IgA levels and the bacterial, TJs, BBB, CATACT, and LPS + LBP composite scores and the baseline IRS and IRS/CIRS scores, but less with the CIRS values. In addition, the IRS/CIRS2 ratio was significantly correlated with the bacterial (r = 0.414, p = 0.002), TJs (r = 0.438, p = 0.001), BBB (r = 0.356, p = 0.007), CATACT (r = 0.394, p = 0.003), and LPS + LBP (r = 0.390, p = 0.003) composite scores. All correlations remained significant after the FDR p correction.
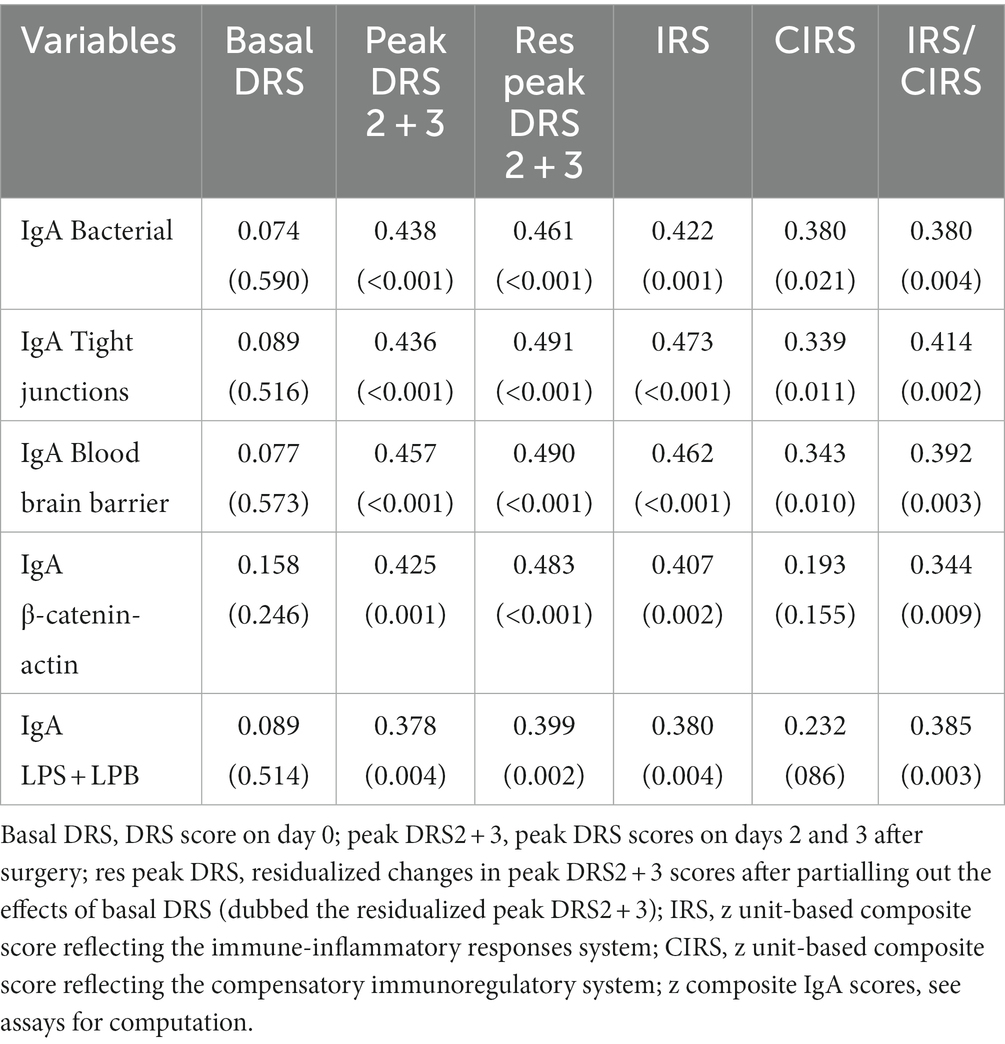
Table 3. Intercorrelation matrix between the Delirium Rating Scale (DRS) scores, immune indices, and IgA responses to self-antigens.
While all IgA responses were correlated with the peak DRS and residualized peak DRS scores, not one of the five IgG composites was correlated with the clinical scores, even without FDR p correction. On the other hand, the IRS/CIRS ratio was significantly correlated with IgG CATACT (r = 0.299, p = 0.025), LBP + LPS (r = 0.361, p = 0.006), TJs (r = 0.342, p = 0.010) and BBB (t = 3.11, p = 0.020) composite scores. These correlations remained significant after the FDR p correction. Insomnia was not significantly correlated with IgA or IgG levels to bacterial, TJ, BBB, CATACT, and LPS + LBP composites, even without FDR p correction.
Results of multiple regression analysis
Table 4 shows the results of multiple regression analyses with the DRS scores as dependent variables and the IgA composites as well as the DRS values on days 0 and 1, and demographic data and known risk factors for delirium (age, sex, previous stroke) as explanatory variables. Regression #1 shows that 48.3% of the variance in the peak DRS scores could be explained by the regression on IgA to CDT, DRS days 0, and previous stroke (all three positively associated). Figure 1 shows the partial regression of peak DRS values on IgA to CDT. Regression #2 examines the same variables, except that DRS days 0 and 1 were excluded from the analysis. This regression shows that 47.1% of the variance in the peak DRS score was explained by the regression on IgA claudin-6, a history of stroke, age, and SSRI treatment before admission; 30.1% of the variance in the DRS score on day 0 could be explained by age and SSRI. Regression #4 found that 45.0% of the variance in the peak DRS scores is associated with DRS Day 1, IgA CATACT, and a history of previous stroke (all three are positively associated). Figure 2 shows the partial regression of peak DRS scores on IgA to the CATACT composite. Deleting IgA to CDT and CATACT showed that the IgA BBB composite was the third most significant explanatory variable that, together with age and previous stroke, explained 39.6% of the variance in the peak DRS values.
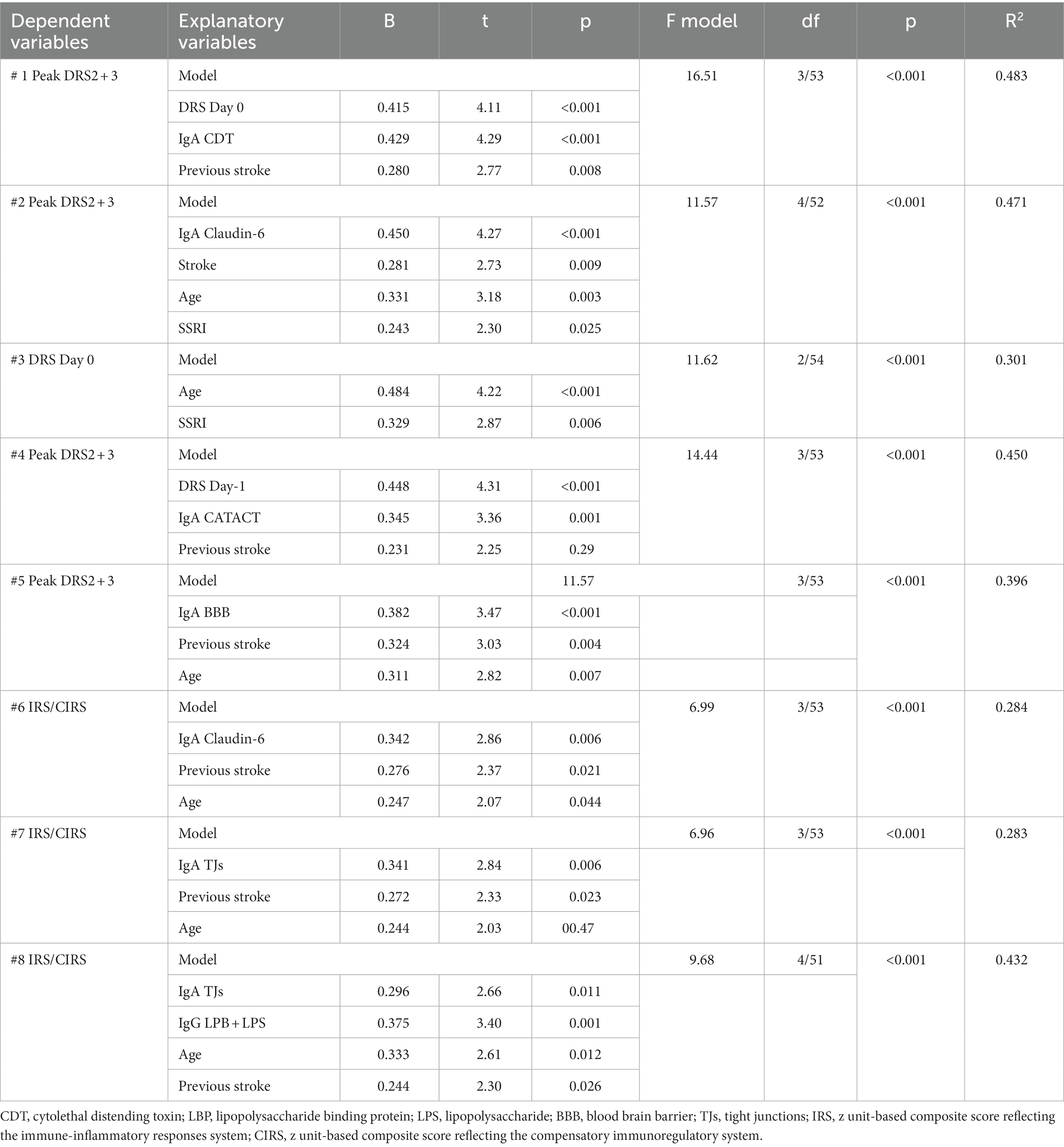
Table 4. Results of multiple regression analyses with the peak Delirium Rating Scale, Revised-98-Thai version (DRS) scores on days 2 and 3 post-surgery, DRS score on day 1, and immune indices as dependent variables.
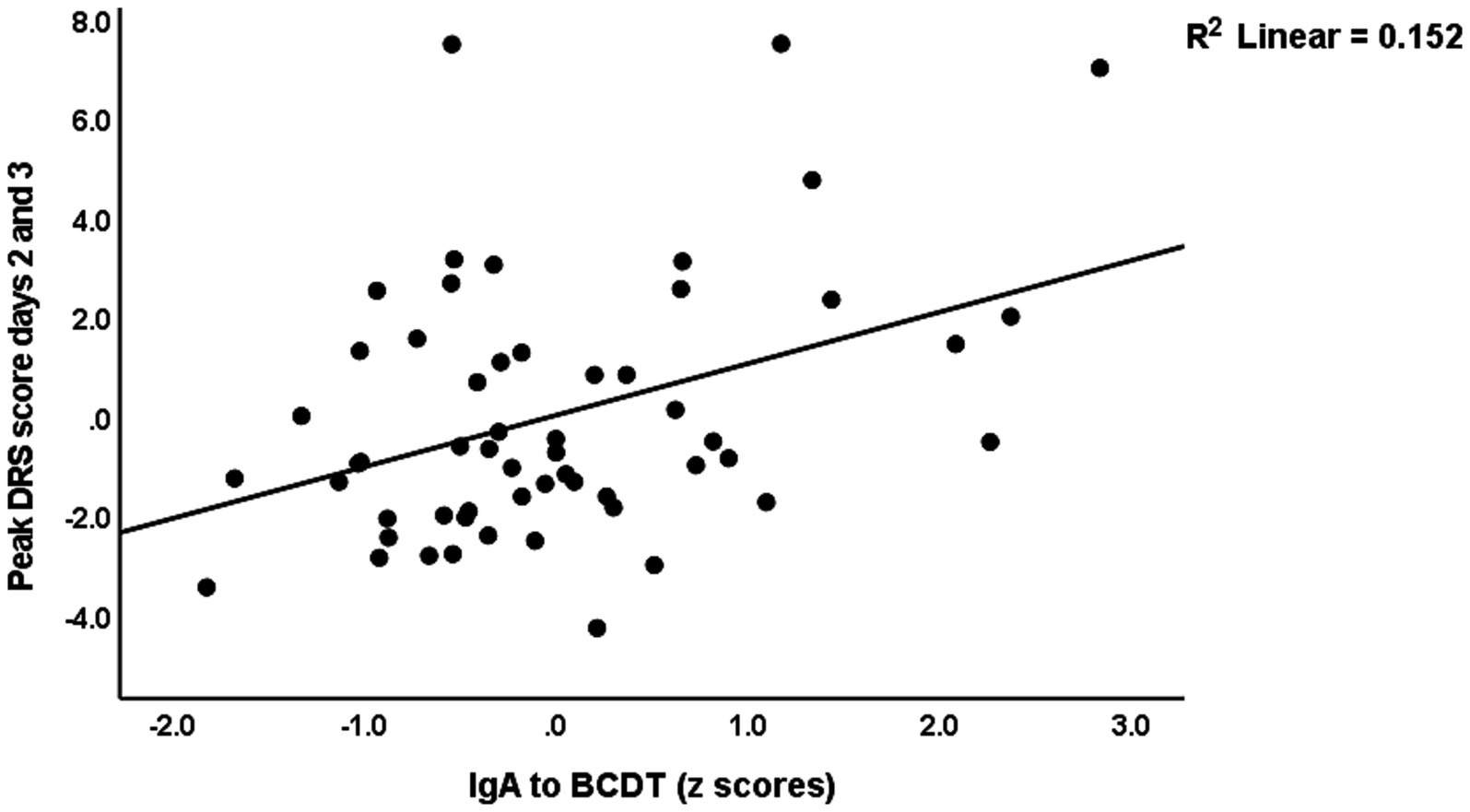
Figure 1. Partial regression of peak Delirium Rating Scale (DRS) scores on IgA to bacterial cytolethal distending toxin (BCDT).

Figure 2. Partial regression of peak Delirium Rating Scale (DRS) scores on IgA to the β-catenin-actin (CATACT) complex.
The same table shows the results of multiple regression analyses with the IRS/CIRS values as the dependent variables and IgA/IgG responses to different epitopes as the explanatory variables. Entering the IgA responses to the separate epitopes (regression #6) showed that 28.4% of the variance in the IRS/CIRS ratio is explained by IgA claudin-6, previous stroke, and age (all positively associated). The regression of IRC/CIRS2 on the same variables showed that claudin-6 was a significant predictor (β = 0.404, t = 3.21, p = 0.002). Entering the five IgA composite scores (regression #7) shows that 28.3% of the variance in the IRS/CIRS ratio is explained by IgA to TJs, previous strokes, and age as explanatory factors. Entering IRS/CIRS2 as a dependent variable showed that TJ was the most significant composite (β = 0.431, t = 3.51, p = 0.001), whereas age and stroke were not significant. Finally, in regression #7, we have also added the IgG responses and found that 43.2% of the variance in the IRS/CIRS ratio (regression #8) was explained by IgG to LBP + LPS day 0 and the same variables as in regression #7. Figure 3 shows the partial regression of the IRS/CIRS ratio on IgG to LBP + LPS. Entering IgG to CATACT (β =0.266, t = 2.33, p = 0.024), and TJs (β =0.270, t = 2.31, p = 0.025) instead of LBP + LPS in regression #8 showed that these IgG responses were also significant.
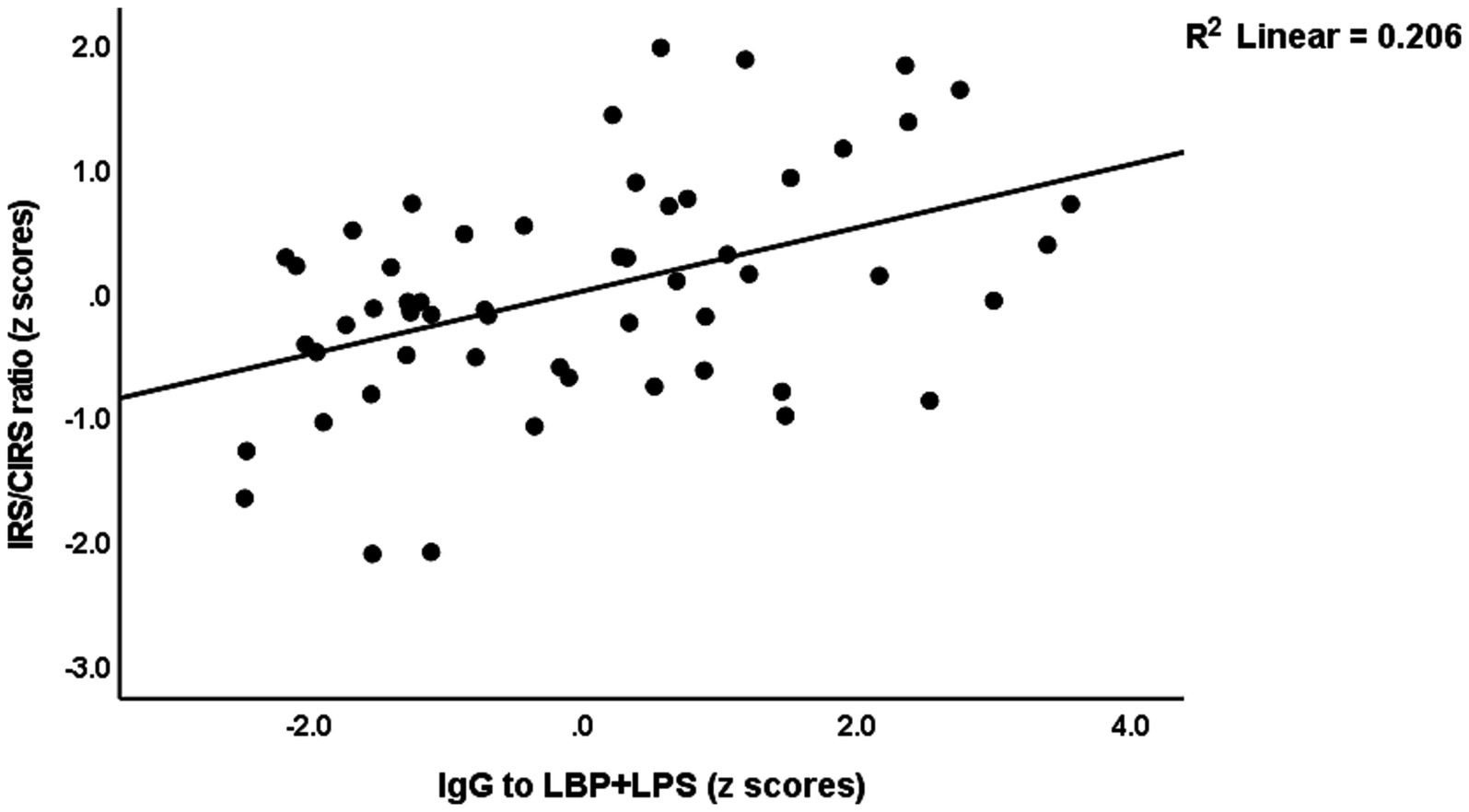
Figure 3. Partial regression of the immune response system (IRS)/compensatory immunoregulatory system (CIRS) on IgA to the lipopolysaccharide (LPS)—LPS-binding protein (LBP + LPS) complex.
Figure 4 shows the results of a mediation PLS analysis with the changes in the DRS score from baseline to a few days later as the dependent variable and the IgA responses (entered as a factor extracted from the 5 IgA indices; labeled IgA responses) as the mediator between age and the changes in the DRS score. The IgA responses factor shows adequate validity (Cronbach’s alpha = 0.92; explained variance = 0.81, all loadings > 0.66) and the model quality fit data are adequate (SRMR = 0.036). There was a significant specific indirect effect of age on the DRS score that was completely mediated by the IgA responses (t = 1.98, p = 0.048). There was a significant direct effect of age on the IgA responses, and a significant total effect of age on the DRS score (t = 2.65, p = 0.008).
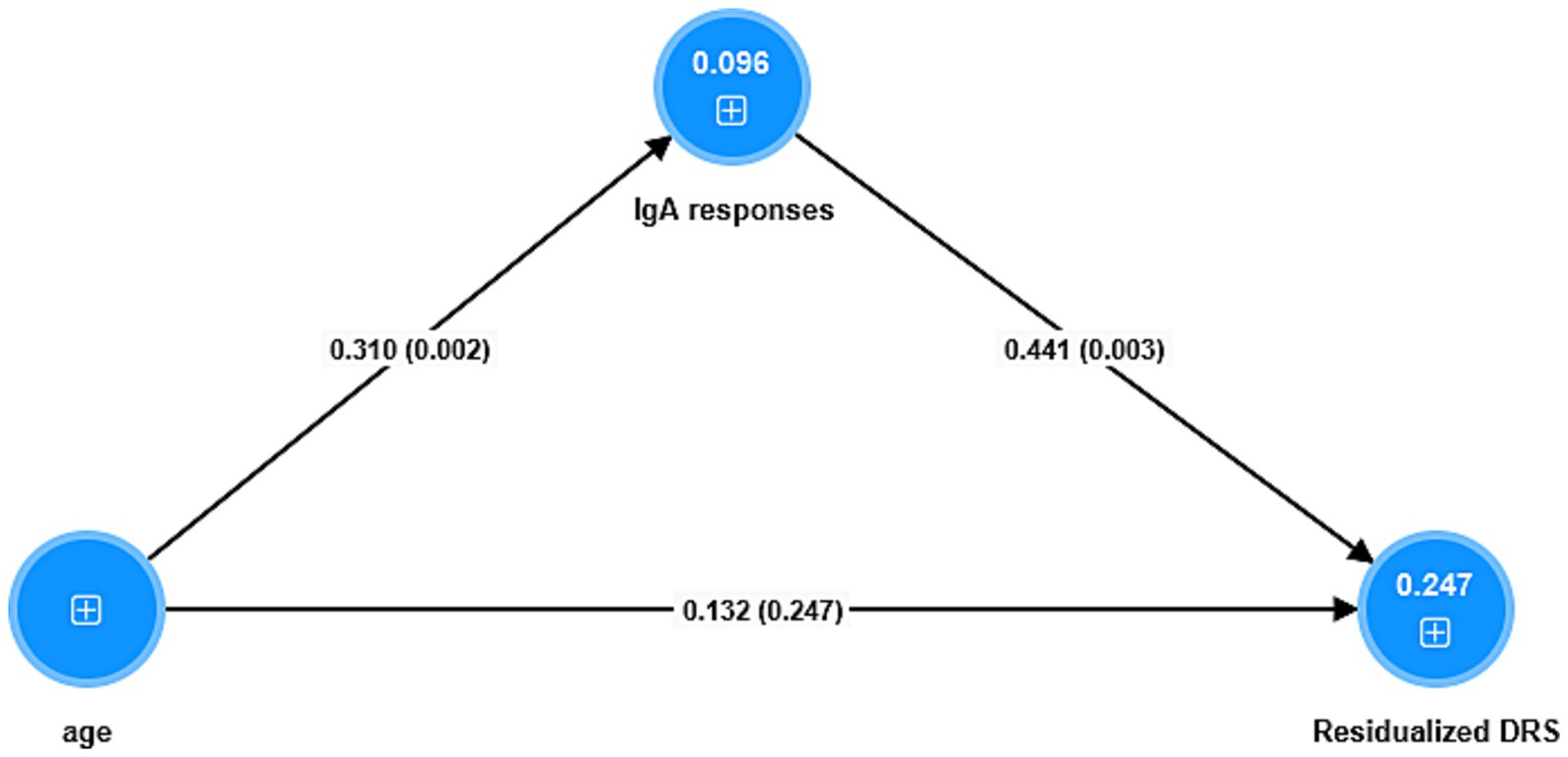
Figure 4. Results of partial least squares analysis. The outcome is the residualized (res) endpoint Delirium Rating Scale (DRS) score value (baseline values covaried out). The predictors are a factor extracted from 5 IgA responses (labeled IgA responses) to antigens, and age whereby the latter is allowed to predict the IgA responses. Shown are the pathway coefficients (with p-values). The figures in the blue circles denote the explained variances. The IgA responses combine blood brain barrier, lipopolysaccharides, lipoprotein binding protein, bacterial load in the bloodstream, catenin and actin, and tight junctions proteins.
Discussion
IgA biomarkers of delirium severity
The first major finding of this study is that delirium and DRS scores are predicted by IgA responses to bacterial, LPS-LBP, TJ, BBB breakdown, and CATACT in older adults with hip fractures. To our knowledge, this is the first study demonstrating an association between delirium severity and IgA responses to LPS, LPB, CDT, H. pylori, zonulin, occludin, claudin-5, claudin-6, β-catenin, and actin. These findings extend those of previous publications showing an association between delirium and P. gingivalis (63), and S100B (64, 65).
Older adults who were exposed to hip fracture surgery, trauma with connective tissue injury, perioperative state, ICU stay, dehydration and hypoxemia, infection, and metabolic impairment are more vulnerable to developing delirium syndrome, especially when they have neurocognitive impairments (6). These precipitating factors may aggravate various upstream processes of circadian dysregulation (66), HPA axis dysregulation (67), oxidative stress (68), and neuro-inflammation (69), which consequently and interactively may cause neurotransmitter dysregulation (70) and neuronal network disconnection (71), which are clinically expressed as delirium (9, 10, 72). Therefore, the immune findings from this study indicate that, apart from the above risk factors, bacterial translocation (LPS-LPB) (73, 74), gut-blood–brain barrier dysfunctions [zonulin (75), occludin and claudin (76, 77)] are involved, as indicated by animal models and human studies.
Interestingly, the current immune findings in delirium partly overlap with previous findings in schizophrenia. Thus, in schizophrenia, several studies reported significant associations with increased bacterial translocation (78–80), breakdown of transcellular and paracellular tight/adherens junction barriers (23, 81, 82), as well as the BBB (24, 83). Moreover, our study demonstrated a significant association between increased levels of IgA in P. gingivalis, H. pylori, and CDT (and their z composite score) and delirium severity. P. gingivalis is a Gram-negative bacterium found in the oral mucosa and is reported to be involved with periodontitis and low-grade systemic inflammation-related diseases such as atherosclerosis, diabetes, cancer, depression, and schizophrenia (84, 85). Similarly, H. pylori infection directly contributes to localized inflammation and is indirectly associated with cardiovascular disease, metabolic syndrome, autoimmune diseases, and Parkinson’s disease (86, 87). Both bacteria were evidently associated with the development of Alzheimer’s disease and other dementia syndromes (88–90).
The results of this study also demonstrated a significant association between delirium and IgA responses to the LPS + LPB complex and CDT. LPS (endotoxin) and its systemic response protein secreted from the liver (LBP), jointly form the LPS-LPB complex (91). Therefore, these IgA responses are indicants of increased bacterial load in peripheral blood (25). Moreover, the LPS-LBP complex in peripheral blood may further activate the innate immune system and pro-inflammatory cytokines via activating the TLR-4 pathway (34, 35). Elevated CDT may dysregulate the immune-inflammatory system by breaking the intra-cellular and intra-nucleus cascades, leading to an apoptotic process in epithelial barrier cells and acquired immune lymphocytes, and activating pro-inflammatory cytokine-secreting macrophages (92, 93).
Our study also shows significant correlations between IgA directed to claudin-5, occludin, zonulin, and tight junctions, and increased DRS-R-T scores. Tight junctions are located at the apical intercellular area of the intestinal epithelial cell lines, and function as a barrier between the intraluminal and systemic parts of the gastrointestinal tract (94). Claudins are transmembrane proteins located at the inner and outer rims of the tight junctions and coupled with occludins, which maintain the structural integrity of the tight junctions, and function as gatekeepers of the paracellular route (95, 96). Zonulin, on the other hand, induces the breakdown of the tight junctions, and increased zonulin levels indicate a leaky gut (97).
Adherens junctions (AJs) are located underneath the tight junction in the paracellular pathway and comprise the transmembrane E-cadherin protein, cytoplasmic alpha-and beta-catenin binding proteins, and cytoskeletal actin components (98). AJs support inter-epithelial barrier functions, maintain cell and tissue architecture, cytoskeletal regulation, and cell signaling and gene transcription (99, 100). All in all, these IgA findings imply a leaky gut, namely the breakdown of TJs and AJs and increased translocation of common Gram-negative or Gram-positive microbiota or their detrimental antigens into the systemic circulation. These mechanisms are frequently associated with TLR-4 complex activation and increased inflammatory signaling, which may result in systemic diseases (31, 101).
It should be stressed that the delirious patients in our study showed significantly increased IgA reactivity to S100B, and claudin-5, which are specific products of BBB breakdown. As such, these findings, together with increased IgA reactivity to TJ and AJ antigens, not only indicate gut barrier impairments but also represent damage to the BBB. Significant evidence of the disruption of the gastrointestinal (gut) and blood–brain barriers (BBB) has been observed in individuals with neurocognitive impairments, autism, and schizophrenia (24, 42, 59, 102). Notably, certain conditions that increase the likelihood of delirium are also linked to both increased intestinal permeability and the translocation of LPS or bacteria from the gut into the bloodstream causing tissue damage (103), bone fracture (104, 105), aging (106), stroke (107), sepsis (108, 109), liver failure (110, 111), uremia (112, 113), alcohol (114, 115), malnutrition (116, 117), and psychological stressors (118, 119). Furthermore, these risk variables were also linked to BBB disintegration (120–127), which is considered a prevalent and significant contributing factor to delirium (128).
IgA and IgG reactivity, IRS/CIRS, and cell–cell interactions in delirium
The second major finding of this study is that IRS activation in the postoperative period is strongly associated with IgA responses to paracellular and BBB composites and bacterial antigens, and in addition to IgG levels directed to TJs, and LBP + LPS and β-catenin/actin. We previously reported that the onset and severity of delirium are significantly correlated with IRS activation, including increased M1 (with IL-6, IL-8, and TNF-α), Th1, Th17, and T cell growth profiles (13). Consequently, our results indicate that leaky barriers and bacterial antigens increase the risk of delirium in part by activating the IRS. It is interesting to note that IgA/IgG responses observed in our study were strongly associated with IRS activation and did not impact the CIRS.
Moreover, natural polyreactive IgA antibodies (PABs) such as those determined here may induce immune-inflammatory responses and contribute to inflammatory disorders and autoimmunity (28, 129). Furthermore, PAB administration to the brain may cause damage to neuronal circuits (65), and low-affinity PABs may serve as precursors for high-affinity pathogenic Abs (66). Therefore, our data imply that increased IgA reactivity may further enhance IRS activation (130–133). Moreover, increased IgA directed to β-catenin may also implicate cadherin signaling, cell–cell interactions, and thus intracellular signal transduction, cell proliferation, cell migration, and apoptosis (134).
Limitations
A limitation of this study could be the smaller sample size of older adults with hip fractures. Nevertheless, the a priori estimated sample size was at least 49 when considering an effect size of 0.30, an alpha of 0.05, and a power of 0.80. Furthermore, the actual power for the regression analyses performed in this study (see Table 4) with the DRS scores as dependent variables, alpha = 0.05, n = 59, and 3 predictors (see Table 1) was 0.999, indicating a well-powered study. Future studies should be carried out to replicate these findings in other countries and cultures. An open question is whether these biomarkers are traits (risk factors of post-injury delirium) or state biomarkers of delirium. Therefore, future research should measure these biomarkers before trauma and surgery and again after surgery. It would also be interesting to assess the enterotype of gut dysbiosis in delirium to examine whether gut dysbiosis contributes to delirium via the increased leaky gut.
Another concern could be that BBB breakdown composite markers measured in the blood may be insufficient to conclude that increased BBB permeability is a risk factor for delirium. Nevertheless, many different findings in patients with delirium indicate that BBB breakdown is involved: (a) damage to the most important proteins of the paracellular pathway (as indicated by our findings) plays a role in BBB disruption (135). Furthermore, alterations in the tight junction protein complexes (as detected in our study) are known to result in increased paracellular permeability leading to increased BBB permeability (136). (b) peripheral inflammation with increased levels of some pro-inflammatory cytokines as detected in delirium (13) is known to lead to BBB disruption (137, 138). (c) Previously, we have shown increased autoimmunity against neuronal self-epitopes indicating glial fibrillary acidic protein, neurofilament protein, glial fibrillary acidic protein, myelin basic protein, myelin oligodendrocyte glycoprotein, metabotropic glutamate receptors mGluRs 1 and 5, N-Methyl-D-Aspartate receptor (NMDAR) GLU1 (NR1) and GLU2 (NR2) (62). In addition, these indicants of damage to neural tissue epitopes are associated with signs of peripheral immune activation and severity of delirium (62). Future research should measure other specific biomarkers of BBB breakdown in delirious patients, such as increased leakage of gadolinium as assessed using magnetic resonance imaging, increased CSF plasminogen and fibrinogen, and increased peripheral blood neuron-specific enolase (NSE) (139, 140).
Lastly, the difference in age between patients with high and low peak DRS scores and the knowledge that IgA levels may increase with age (141) may be perceived by some as a limitation of this study. Nevertheless, our study is not a case–control study, but a cohort study that examines the predictive effects of baseline risk factors (IgA levels and age, and other) on the onset of delirium 2–3 days later. As such, the primary outcome of this cohort study is the regression of the changes in the DRS scores on the basal risk factors. Since age is a risk factor of delirium (13), it is natural that in our cohort study, those with higher DRS scores have a higher age. To identify the risk factors for increased DRS scores, including IgA responses to self-epitopes, and bacterial antigens, age, and comorbidities, we conducted a prospective cohort study with patients exposed to the same injury. Therefore, the primary analyses of this study are the multiple regression analyses that characterize the risk factors for delirium severity and not the comparison between patients with low and high DRS scores. These are only displayed to illustrate the mean values of the measured variables. It should be noted that matching the delirium and control groups by age would result in selection bias due to the partial restriction imposed by group selection. This methodology would result in gains or losses in multiple regression analyses and, by implication, imprecision. Any such selection or matching based on age has the potential to introduce substantial bias into the regression results (13). By employing mediation analysis, we investigated the relationships between age, IgA responses, and changes in the DRS score. Our findings indicate that IgA responses play a substantial mediating role in the effects of age on changes in the DRS score. In other words, our prospective cohort study found that higher IgA responses can be considered as biomarkers of post-surgery delirium in old adults. It is important to replicate and validate our cohort study in different nations and cultures. Additionally, case–control studies should investigate whether these potential predictive biomarkers may be used as diagnostic biomarkers.
Conclusion
Aberrations in the tight and adherens junctions of the paracellular pathway of the gut and BBB barriers, increased bacterial translocation and LPS and DCT load in the systemic circulation, and cell–cell interactions are identified as risk factors of delirium in older adults after hip fracture surgery. IgA/IgG reactivity to the antigens measured here may contribute to IRS activation, which is another pathophysiological factor leading to delirium and which could mediate at least in part the effects of IgA/IgG responses on delirium. Consequently, leaky gut, translocation of bacterial antigens, IRS activation, and BBB disruption are new drug targets to treat and potentially prevent delirium. The data from animal and human studies demonstrate the use of antioxidants, including zinc and glutamine, norfloxacin, infliximab, tofacitinib, CKD-506, and larazotide acetate to restore the leaky gut barrier and prevent or attenuate bacterial translocation (142–146). Minocycline, raparixin, atorvastatin, melatonin, and mesenchymal stromal cell therapy may promote BBB restoration in various neurological conditions (147, 148).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand (registration number 528/61). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
PT: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. YT: Data curation, Investigation, Methodology, Project administration, Resources, Validation, Writing – review & editing. ST: Conceptualization, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing. SS: Data curation, Formal Analysis, Investigation, Methodology, Resources, Software, Validation, Writing – review & editing. AV: Data curation, Investigation, Methodology, Resources, Software, Writing – review & editing. MM: Conceptualization, Data curation, Formal Analysis, Methodology, Resources, Software, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by a Ratchadapisek Sompoch Endowment Fund of the Faculty of Medicine, Chulalongkorn University (RA62/014).
Acknowledgments
The authors gratefully acknowledge the help of all psychiatry, orthopedic, and anesthesiology nursing staff and residents involved in the execution of this study.
Conflict of interest
AV was employed by Immunosciences Lab Inc. and Cyrex Labs LLC.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1294689/full#supplementary-material
References
1. Yang, Y, Zhao, X, Gao, L, Wang, Y, and Wang, J. Incidence and associated factors of delirium after orthopedic surgery in elderly patients: a systematic review and meta-analysis. Aging Clin Exp Res. (2020) 33:1493–506. doi: 10.1007/s40520-020-01674-1
2. Wu, J, Yin, Y, Jin, M, and Li, B. The risk factors for postoperative delirium in adult patients after hip fracture surgery: a systematic review and meta-analysis. Int J Geriatr Psychiatry. (2021) 36:3–14. doi: 10.1002/gps.5408
3. Yang, Y, Zhao, X, Dong, T, Yang, Z, Zhang, Q, and Zhang, Y. Risk factors for postoperative delirium following hip fracture repair in elderly patients: a systematic review and meta-analysis. Aging Clin Exp Res. (2017) 29:115–26. doi: 10.1007/s40520-016-0541-6
4. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM–5). Washington DC, USA: American Psychiatric Publishing. (2013).
5. Mosk, CA, Mus, M, Vroemen, JP, van der Ploeg, T, Vos, DI, Elmans, LH, et al. Dementia and delirium, the outcomes in elderly hip fracture patients. Clin Interv Aging. (2017) 12:421–30. doi: 10.2147/CIA.S115945
6. Albanese, AM, Ramazani, N, Greene, N, and Bruse, L. Review of postoperative delirium in geriatric patients after hip fracture treatment. Geriatr Orthopaed Surg Rehabil. (2022) 13:215145932110589. doi: 10.1177/21514593211058947
7. de Miguel, AM, Roca Chacón, O, Martínez-Alonso, M, Serrano Godoy, M, Mas Atance, J, and García, GR. Hip fracture in the elderly patient: prognostic factors for mortality and functional recovery at one year. Rev Esp Geriatr Gerontol. (2018) 53:247–54. doi: 10.1016/j.regg.2018.04.447
8. Smith, TO, Cooper, A, Peryer, G, Griffiths, R, Fox, C, and Cross, J. Factors predicting incidence of post-operative delirium in older people following hip fracture surgery: a systematic review and meta-analysis. Int J Geriatr Psychiatry. (2017) 32:386–96. doi: 10.1002/gps.4655
9. Oldham, MA, Flaherty, JH, and Maldonado, JR. Refining delirium: a Transtheoretical model of delirium disorder with preliminary neurophysiologic subtypes. Am J Geriatr Psychiatry. (2018) 26:913–24. doi: 10.1016/j.jagp.2018.04.002
10. Maldonado, JR. Delirium pathophysiology: an updated hypothesis of the etiology of acute brain failure. Int J Geriatr Psychiatry. (2018) 33:1428–57. doi: 10.1002/gps.4823
11. Thisayakorn, P, Tangwongchai, S, Tantavisut, S, Thipakorn, Y, Sukhanonsawat, S, Wongwarawipat, T, et al. Immune, blood cell, and blood gas biomarkers of delirium in elderly individuals with hip fracture surgery. Dement Geriatr Cogn Disord. (2021) 50:161–9. doi: 10.1159/000517510
12. Sarejloo, S, Shojaei, N, Lucke-Wold, B, Zelmanovich, R, and Khanzadeh, S. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio as prognostic predictors for delirium in critically ill patients: a systematic review and meta-analysis. BMC Anesthesiol. (2023) 23:58. doi: 10.1186/s12871-023-01997-2
13. Thisayakorn, P, Thipakorn, Y, Tantavisut, S, Sirivichayakul, S, and Maes, M. Delirium due to hip fracture is associated with activated immune-inflammatory pathways and a reduction in negative immunoregulatory mechanisms. BMC Psychiatry. (2022) 22:369. doi: 10.1186/s12888-022-04021-y
14. Hietbrink, F, Besselink, MG, Renooij, W, de Smet, MB, Draisma, A, van der Hoeven, H, et al. Systemic inflammation increases intestinal permeability during experimental human endotoxemia. Shock. (2009) 32:374–8. doi: 10.1097/SHK.0b013e3181a2bcd6
15. Chavez, AM, Menconi, MJ, Hodin, RA, and Fink, MP. Cytokine-induced intestinal epithelial hyperpermeability: role of nitric oxide. Crit Care Med. (1999) 27:2246–51. doi: 10.1097/00003246-199910000-00030
16. Yang, R, Han, X, Uchiyama, T, Watkins, SK, Yaguchi, A, Delude, RL, et al. IL-6 is essential for development of gut barrier dysfunction after hemorrhagic shock and resuscitation in mice. Am J Physiol Gastrointest Liver Physiol. (2003) 285:G621–9. doi: 10.1152/ajpgi.00177.2003
17. Clark, E, Hoare, C, Tanianis-Hughes, J, Carlson, GL, and Warhurst, G. Interferon gamma induces translocation of commensal Escherichia coli across gut epithelial cells via a lipid raft-mediated process. Gastroenterology. (2005) 128:1258–67. doi: 10.1053/j.gastro.2005.01.046
18. Taylor, J, Parker, M, Casey, CP, Tanabe, S, Kunkel, D, Rivera, C, et al. Postoperative delirium and changes in the blood-brain barrier, neuroinflammation, and cerebrospinal fluid lactate: a prospective cohort study. Br J Anaesth. (2022) 129:219–30. doi: 10.1016/j.bja.2022.01.005
19. Garcez, FB, Garcia de Alencar, JC, Fernandez, SSM, Avelino-Silva, VI, Sabino, EC, Martins, RCR, et al. Association between gut microbiota and delirium in acutely ill older adults. J Gerontol A Biol Sci Med Sci. (2023) 78:1320–7. doi: 10.1093/gerona/glad074
20. Zhang, Y, Baldyga, K, Dong, Y, Song, W, Villanueva, M, Deng, H, et al. The association between gut microbiota and postoperative delirium in patients. Transl Psychiatry. (2023) 13:156. doi: 10.1038/s41398-023-02450-1
21. Gao, T, Wang, Z, Dong, Y, Cao, J, Lin, R, Wang, X, et al. Role of melatonin in sleep deprivation-induced intestinal barrier dysfunction in mice. J Pineal Res. (2019) 67:e12574. doi: 10.1111/jpi.12574
22. Sgro, M, Kodila, ZN, Brady, RD, Reichelt, AC, Mychaisuk, R, and Yamakawa, GR. Synchronizing our clocks as we age: the influence of the brain-gut-immune axis on the sleep-wake cycle across the lifespan. Sleep. (2022) 45:268. doi: 10.1093/sleep/zsab268
23. Maes, M, Sirivichayakul, S, Kanchanatawan, B, and Vodjani, A. Upregulation of the intestinal Paracellular pathway with breakdown of tight and Adherens junctions in deficit schizophrenia. Mol Neurobiol. (2019) 56:7056–73. doi: 10.1007/s12035-019-1578-2
24. Maes, M, Sirivichayakul, S, Kanchanatawan, B, and Vodjani, A. Breakdown of the Paracellular tight and Adherens junctions in the gut and blood brain barrier and damage to the vascular barrier in patients with deficit schizophrenia. Neurotox Res. (2019) 36:306–22. doi: 10.1007/s12640-019-00054-6
25. Safadi, JM, Quinton, AMG, Lennox, BR, Burnet, PWJ, and Minichino, A. Gut dysbiosis in severe mental illness and chronic fatigue: a novel trans-diagnostic construct? A systematic review and meta-analysis. Mol Psychiatry. (2022) 27:141–53. doi: 10.1038/s41380-021-01032-1
26. Simeonova, D, Ivanovska, M, Murdjeva, M, Carvalho, AF, and Maes, M. Recognizing the leaky gut as a trans-diagnostic target for Neuroimmune disorders using clinical chemistry and molecular immunology assays. Curr Top Med Chem. (2018) 18:1641–55. doi: 10.2174/1568026618666181115100610
27. Rezvani, F, Sayadnasiri, M, and Rezaei, O. The study of memory and executive dysfunction in patients infected with Helicobacter pylori. Neurol Res. (2017) 39:953–8. doi: 10.1080/01616412.2017.1363349
28. Beydoun, MA, Beydoun, HA, Shroff, MR, Kitner-Triolo, MH, and Zonderman, AB. Helicobacter pylori seropositivity and cognitive performance among US adults: evidence from a large national survey. Psychosom Med. (2013) 75:486–96. doi: 10.1097/PSY.0b013e31829108c3
29. Desta, NT. Pathophysiological association between periodontal disease and Alzheimer’s disease: importance of periodontal health in the elderly. J Oral Biosci. (2021) 63:351–9. doi: 10.1016/j.job.2021.08.007
30. Meng, L, Song, Z, Liu, A, Dahmen, U, Yang, X, and Fang, H. Effects of lipopolysaccharide-binding protein (LBP) single nucleotide polymorphism (SNP) in infections, inflammatory diseases, metabolic disorders and cancers. Front Immunol. (2021) 12:681810. doi: 10.3389/fimmu.2021.681810
31. Lucas, K, and Maes, M. Role of the toll like receptor (TLR) radical cycle in chronic inflammation: possible treatments targeting the TLR4 pathway. Mol Neurobiol. (2013) 48:190–204. doi: 10.1007/s12035-013-8425-7
32. Kailoo, S, and Shreya, KY. Cytolethal distending toxin: from genotoxin to a potential biomarker and anti-tumor target. World J Microbiol Biotechnol. (2021) 37:1–18. doi: 10.1007/s11274-021-03117-z
33. Bannerman, DD, and Goldblum, SE. Direct effects of endotoxin on the endothelium: barrier function and injury. Lab Investig. (1999) 79:1181–99.
34. Gabarin, RS, Li, M, Zimmel, PA, Marshall, JC, Li, Y, and Zhang, H. Intracellular and extracellular lipopolysaccharide signaling in Sepsis: avenues for novel therapeutic strategies. J Innate Immun. (2021) 13:323–32. doi: 10.1159/000515740
35. Wang, H, Reddy, ST, and Fogelman, AM. The role of gut-derived oxidized lipids and bacterial lipopolysaccharide in systemic inflammation and atherosclerosis. Curr Opin Lipidol. (2022) 33:277–82. doi: 10.1097/MOL.0000000000000841
36. Camilleri, M. Leaky gut: mechanisms, measurement and clinical implications in humans. Gut. (2019) 68:1516–26. doi: 10.1136/gutjnl-2019-318427
37. Wang, YH. Current progress of research on intestinal bacterial translocation. Microb Pathog. (2021) 152:104652. doi: 10.1016/j.micpath.2020.104652
38. Wiest, R, Lawson, M, and Geuking, M. Pathological bacterial translocation in liver cirrhosis. J Hepatol. (2014) 60:197–209. doi: 10.1016/j.jhep.2013.07.044
39. Monaco, A, Ovryn, B, Axis, J, and Amsler, K. The epithelial cell leak pathway. Int J Mol Sci. (2021) 22:677. doi: 10.3390/ijms22147677
40. Barbara, G, Barbaro, MR, Fuschi, D, Palombo, M, Falangone, F, Cremon, C, et al. Inflammatory and microbiota-related regulation of the intestinal epithelial barrier. Front Nutr. (2021) 8:718356. doi: 10.3389/fnut.2021.718356
41. Yang, W, and Cong, Y. Gut microbiota-derived metabolites in the regulation of host immune responses and immune-related inflammatory diseases. Cell Mol Immunol. (2021) 18:866–77. doi: 10.1038/s41423-021-00661-4
42. Maes, M, Kubera, M, and Leunis, JC. The gut-brain barrier in major depression: intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro Endocrinol Lett. (2008) 29:117–24. Available at: https://www.nel.edu/userfiles/articlesnew/NEL290108A12.pdf.
43. Michielan, A, and D’Incà, R. Intestinal permeability in inflammatory bowel disease: pathogenesis, clinical evaluation, and therapy of leaky gut. Mediat Inflamm. (2015) 2015:628157:1–10. doi: 10.1155/2015/628157
44. Sánchez-Alcoholado, L, Ordóñez, R, Otero, A, Plaza-Andrade, I, Laborda-Illanes, A, Medina, JA, et al. Gut microbiota-mediated inflammation and gut permeability in patients with obesity and colorectal Cancer. Int J Mol Sci. (2020) 21:782. doi: 10.3390/ijms21186782
45. Bindels, LB, Neyrinck, AM, Loumaye, A, Catry, E, Walgrave, H, Cherbuy, C, et al. Increased gut permeability in cancer cachexia: mechanisms and clinical relevance. Oncotarget. (2018) 9:18224–38. doi: 10.18632/oncotarget.24804
46. Yoo, JY, Groer, M, Dutra, SVO, Sarkar, A, and McSkimming, DI. Gut microbiota and immune system interactions. Microorganisms. (2020) 8:1587. doi: 10.3390/microorganisms8101587
47. Du, Y, Gao, XR, Peng, L, and Ge, JF. Crosstalk between the microbiota-gut-brain axis and depression. Heliyon. (2020) 6:e04097. doi: 10.1016/j.heliyon.2020.e04097
48. Bear, T, Dalziel, J, Coad, J, Roy, N, Butts, C, and Gopal, P. The microbiome-gut-brain Axis and resilience to developing anxiety or depression under stress. Microorganisms. (2021) 9:723. doi: 10.3390/microorganisms9040723
49. Jiang, C, Li, G, Huang, P, Liu, Z, and Zhao, B. The gut microbiota and Alzheimer’s disease. J Alzheimers Dis. (2017) 58:1–15. doi: 10.3233/JAD-161141
50. Kesika, P, Suganthy, N, Sivamaruthi, BS, and Chaiyasut, C. Role of gut-brain axis, gut microbial composition, and probiotic intervention in Alzheimer’s disease. Life Sci. (2021) 264:118627. doi: 10.1016/j.lfs.2020.118627
51. Rutsch, A, Kantsjö, JB, and Ronchi, F. The gut-brain Axis: how microbiota and host Inflammasome influence brain physiology and pathology. Front Immunol. (2020) 11:604179. doi: 10.3389/fimmu.2020.604179
52. Haruwaka, K, Ikegami, A, Tachibana, Y, Ohno, N, Konishi, H, Hashimoto, A, et al. Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nat Commun. (2019) 10:5816. doi: 10.1038/s41467-019-13812-z
53. Lindblad, C, Nelson, DW, Zeiler, FA, Ercole, A, Ghatan, PH, von Horn, H, et al. Influence of blood-brain barrier integrity on brain protein biomarker clearance in severe traumatic brain injury: a longitudinal prospective study. J Neurotrauma. (2020) 37:1381–91. doi: 10.1089/neu.2019.6741
54. Wu, H, Brown, EV, Acharya, NK, Appelt, DM, Marks, A, Nagele, RG, et al. Age-dependent increase of blood-brain barrier permeability and neuron-binding autoantibodies in S100B knockout mice. Brain Res. (2016) 1637:154–67. doi: 10.1016/j.brainres.2016.02.026
55. Brescia, P, and Rescigno, M. The gut vascular barrier: a new player in the gut-liver-brain axis. Trends Mol Med. (2021) 27:844–55. doi: 10.1016/j.molmed.2021.06.007
56. Nemani, K, Hosseini Ghomi, R, McCormick, B, and Fan, X. Schizophrenia and the gut-brain axis. Prog Neuro-Psychopharmacol Biol Psychiatry. (2015) 56:155–60. doi: 10.1016/j.pnpbp.2014.08.018
57. Kelly, JR, Minuto, C, Cryan, JF, Clarke, G, and Dinan, TG. The role of the gut microbiome in the development of schizophrenia. Schizophr Res. (2021) 234:4–23. doi: 10.1016/j.schres.2020.02.010
58. Rudzki, L, and Maes, M. From “leaky gut” to impaired glia-neuron communication in depression. Adv Exp Med Biol. (2021) 1305:129–55. doi: 10.1007/978-981-33-6044-0_9
59. Fiorentino, M, Sapone, A, Senger, S, Camhi, SS, Kadzielski, SM, Buie, TM, et al. Blood-brain barrier and intestinal epithelial barrier alterations in autism spectrum disorders. Mol Autism. (2016) 7:49. doi: 10.1186/s13229-016-0110-z
60. Shabbir, U, Arshad, MS, Sameen, A, and Oh, DH. Crosstalk between gut and brain in Alzheimer’s disease: the role of gut microbiota modulation strategies. Nutrients. (2021) 13:690. doi: 10.3390/nu13020690
61. FitzGerald, JM, O’Regan, N, Adamis, D, Timmons, S, Dunne, CP, Trzepacz, PT, et al. Sleep-wake cycle disturbances in elderly acute general medical inpatients: longitudinal relationship to delirium and dementia. Alzheimers Dement. (2017) 7:61–8. doi: 10.1016/j.dadm.2016.12.013
62. Maes, M, Thisayakorn, P, Thipakorn, Y, Tantavisut, S, Sirivichayakul, S, and Vojdani, A. Reactivity to neural tissue epitopes, aquaporin 4 and heat shock protein 60 is associated with activated immune-inflammatory pathways and the onset of delirium following hip fracture surgery. Eur Geriatr Med. (2023) 14:99–112. doi: 10.1007/s41999-022-00729-y
63. Zhang, M, Bai, X, Zhang, Y, and Xie, X. Analysis of risk factors for postoperative delirium in patients with esophageal Cancer. J Clin Med Res. (2021) 2:69–72.
64. van Munster, BC, Korevaar, JC, Korse, CM, Bonfrer, JM, Zwinderman, AH, and de Rooij, SE. Serum S100B in elderly patients with and without delirium. Int J Geriatr Psychiatry. (2010) 25:234–9. doi: 10.1002/gps.2326
65. Gao, Y, Duan, J, Ji, H, and Lu, W. Levels of S100 calcium binding protein B (S100B), neuron-specific enolase (NSE), and cyclophilin a (CypA) in the serum of patients with severe craniocerebral injury and multiple injuries combined with delirium transferred from the ICU and their prognostic value. Annals Palliat Med. (2021) 10:3371–8. doi: 10.21037/apm-21-424
66. Fitzgerald, JM, Adamis, D, Trzepacz, PT, O’Regan, N, Timmons, S, Dunne, C, et al. Delirium: a disturbance of circadian integrity? Med Hypotheses. (2013) 81:568–76. doi: 10.1016/j.mehy.2013.06.032
67. Cerejeira, J, Batista, P, Nogueira, V, Vaz-Serra, A, and Mukaetova-Ladinska, EB. The stress response to surgery and postoperative delirium: evidence of hypothalamic-pituitary-adrenal axis hyperresponsiveness and decreased suppression of the GH/IGF-1 Axis. J Geriatr Psychiatry Neurol. (2013) 26:185–94. doi: 10.1177/0891988713495449
68. Pang, Y, Li, Y, Zhang, Y, Wang, H, Lang, J, Han, L, et al. Effects of inflammation and oxidative stress on postoperative delirium in cardiac surgery. Front Cardiovasc Med. (2022) 9:1049600. doi: 10.3389/fcvm.2022.1049600
69. Cunningham, C. Systemic inflammation and delirium: important co-factors in the progression of dementia. Biochem Soc Trans. (2011) 39:945–53. doi: 10.1042/BST0390945
70. Mulkey, MA, Hardin, SR, Olson, DM, and Munro, CL. Pathophysiology review: seven neurotransmitters associated with delirium. Clin Nurse Spec. (2018) 32:195–211. doi: 10.1097/NUR.0000000000000384
71. Haggstrom, L, Welschinger, R, and Caplan, GA. Functional neuroimaging offers insights into delirium pathophysiology: a systematic review. Australas J Ageing. (2017) 36:186–92. doi: 10.1111/ajag.12417
72. Wang, Y, and Shen, X. Postoperative delirium in the elderly: the potential neuropathogenesis. Aging Clin Exp Res. (2018) 30:1287–95. doi: 10.1007/s40520-018-1008-8
73. Hoogland, IC, Houbolt, C, van Westerloo, DJ, van Gool, WA, and van de Beek, D. Systemic inflammation and microglial activation: systematic review of animal experiments. J Neuroinflammation. (2015) 12:114. doi: 10.1186/s12974-015-0332-6
74. Barron, M, Gartlon, J, Dawson, LA, Atkinson, PJ, and Pardon, MC. A state of delirium: deciphering the effect of inflammation on tau pathology in Alzheimer’s disease. Exp Gerontol. (2017) 94:103–7. doi: 10.1016/j.exger.2016.12.006
75. Androsova, G, Krause, R, Winterer, G, and Schneider, R. Biomarkers of postoperative delirium and cognitive dysfunction. Front Aging Neurosci. (2015) 7:112. doi: 10.3389/fnagi.2015.00112
76. Li, K, Wang, J, Chen, L, Guo, M, Zhou, Y, Li, X, et al. Netrin-1 ameliorates postoperative delirium-like behavior in aged mice by suppressing Neuroinflammation and restoring impaired blood-brain barrier permeability. Front Mol Neurosci. (2021) 14:751570. doi: 10.3389/fnmol.2021.751570
77. Zhou, Y, Wang, J, Li, X, Li, K, Chen, L, Zhang, Z, et al. Neuroprotectin D1 protects against postoperative delirium-like behavior in aged mice. Front Aging Neurosci. (2020) 12:582674. doi: 10.3389/fnagi.2020.582674
78. Severance, EG, Gressitt, KL, Stallings, CR, Origoni, AE, Khushalani, S, Leweke, FM, et al. Discordant patterns of bacterial translocation markers and implications for innate immune imbalances in schizophrenia. Schizophr Res. (2013) 148:130–7. doi: 10.1016/j.schres.2013.05.018
79. Wang, C, Zhang, T, He, L, Fu, JY, Deng, HX, Xue, XL, et al. Bacterial translocation associates with aggression in schizophrenia inpatients. Front Syst Neurosci. (2021) 15:704069. doi: 10.3389/fnsys.2021.704069
80. Maes, M, Kanchanatawan, B, Sirivichayakul, S, and Carvalho, AF. In schizophrenia, increased plasma IgM/IgA responses to gut commensal Bacteria are associated with negative symptoms, neurocognitive impairments, and the deficit phenotype. Neurotox Res. (2019) 35:684–98. doi: 10.1007/s12640-018-9987-y
81. Gokulakrishnan, K, Nikhil, J, Vs, S, Holla, B, Thirumoorthy, C, Sandhya, N, et al. Altered intestinal permeability biomarkers in schizophrenia: a possible link with subclinical inflammation. Ann Neurosci. (2022) 29:151–8. doi: 10.1177/09727531221108849
82. Maes, M, Andrés-Rodríguez, L, Vojdani, A, Sirivichayakul, S, Barbosa, DS, and Kanchanatawan, B. In schizophrenia, chronic fatigue syndrome-and fibromyalgia-like symptoms are driven by breakdown of the Paracellular pathway with increased Zonulin and immune activation-associated neurotoxicity. CNS Neurol Disord Drug Targets. (2023) 22:215–25. doi: 10.2174/1871527321666220806100600
83. Pong, S, Karmacharya, R, Sofman, M, Bishop, JR, and Lizano, P. The role of brain microvascular endothelial cell and blood-brain barrier dysfunction in schizophrenia. Complex Psychiatry. (2020) 6:30–46. doi: 10.1159/000511552
84. Martínez, M, Postolache, TT, García-Bueno, B, Leza, JC, Figuero, E, Lowry, CA, et al. The role of the Oral microbiota related to periodontal diseases in anxiety, mood and trauma-and stress-related disorders. Front Psych. (2021) 12:814177. doi: 10.3389/fpsyt.2021.814177
85. Wadhawan, A, Reynolds, MA, Makkar, H, Scott, AJ, Potocki, E, Hoisington, AJ, et al. Periodontal pathogens and neuropsychiatric health. Curr Top Med Chem. (2020) 20:1353–97. doi: 10.2174/1568026620666200110161105
86. Martin-Nuñez, GM, Cornejo-Pareja, I, Clemente-Postigo, M, and Tinahones, FJ. Gut microbiota: the missing link between Helicobacter pylori infection and metabolic disorders? Front Endocrinol. (2021) 12:639856. doi: 10.3389/fendo.2021.639856
87. Budzyński, J, and Kłopocka, M. Brain-gut axis in the pathogenesis of Helicobacter pylori infection. World J Gastroenterol. (2014) 20:5212–25. doi: 10.3748/wjg.v20.i18.5212
88. Liu, NY, Sun, JH, Jiang, XF, and Li, H. Helicobacter pylori infection and risk for developing dementia: an evidence-based meta-analysis of case-control and cohort studies. Aging. (2021) 13:22571–87. doi: 10.18632/aging.203571
89. Borsa, L, Dubois, M, Sacco, G, and Lupi, L. Analysis the link between periodontal diseases and Alzheimer’s disease: a systematic review. Int J Environ Res Public Health. (2021) 18:9312. doi: 10.3390/ijerph18179312
90. Lorenzi, C, Bianchi, N, Pinto, A, Mazzetti, V, and Arcuri, C. The role of periodontal bacteria, Porphyromonas Gingivalis, in Alzheimer’s disease pathogenesis and aggravation: a review. J Biol Regul Homeost Agents. (2021) 35:37–45. doi: 10.23812/21-3supp1-6
91. Tobias, PS, and Ulevitch, RJ. Lipopolysaccharide binding protein and CD14 in LPS dependent macrophage activation. Immunobiology. (1993) 187:227–32. doi: 10.1016/S0171-2985(11)80341-4
92. Smith, JL, and Bayles, DO. The contribution of cytolethal distending toxin to bacterial pathogenesis. Crit Rev Microbiol. (2006) 32:227–48. doi: 10.1080/10408410601023557
93. Scuron, MD, Boesze-Battaglia, K, Dlakić, M, and Shenker, BJ. The Cytolethal distending toxin contributes to microbial virulence and disease pathogenesis by acting as a tri-Perditious toxin. Front Cell Infect Microbiol. (2016) 6:168. doi: 10.3389/fcimb.2016.00168
94. Otani, T, and Furuse, M. Tight junction structure and function revisited. Trends Cell Biol. (2020) 30:805–17. doi: 10.1016/j.tcb.2020.08.004
95. Barmeyer, C, Schulzke, JD, and Fromm, M. Claudin-related intestinal diseases. Semin Cell Dev Biol. (2015) 42:30–8. doi: 10.1016/j.semcdb.2015.05.006
96. Li, W, Yuan, S, Sui, X, Bian, H, Wei, M, Chen, Z, et al. Higher serum occludin after successful reperfusion is associated with early neurological deterioration. CNS Neurosci Ther. (2022) 28:999–1007. doi: 10.1111/cns.13830
97. Fasano, A. All disease begins in the (leaky) gut: role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Res. (2020) 9:1. doi: 10.12688/f1000research.20510.1
98. Meng, W, and Takeichi, M. Adherens junction: molecular architecture and regulation. Cold Spring Harb Perspect Biol. (2009) 1:a002899. doi: 10.1101/cshperspect.a002899
99. Coopman, P, and Djiane, A. Adherens junction and E-cadherin complex regulation by epithelial polarity. Cell Mol Life Sci. (2016) 73:3535–53. doi: 10.1007/s00018-016-2260-8
100. Seo, K, Seo, J, Yeun, J, Choi, H, Kim, YI, and Chang, SY. The role of mucosal barriers in human gut health. Arch Pharm Res. (2021) 44:325–41. doi: 10.1007/s12272-021-01327-5
101. Maes, M, Mihaylova, I, and Leunis, JC. Increased serum IgA and IgM against LPS of enterobacteria in chronic fatigue syndrome (CFS): indication for the involvement of gram-negative enterobacteria in the etiology of CFS and for the presence of an increased gut-intestinal permeability. J Affect Disord. (2007) 99:237–40. doi: 10.1016/j.jad.2006.08.021
102. Liu, F, Duan, M, Fu, H, Zhao, G, Han, Y, Lan, F, et al. Orthopedic surgery causes gut microbiome Dysbiosis and intestinal barrier dysfunction in prodromal Alzheimer disease patients: a prospective observational cohort study. Ann Surg. (2022) 276:270–80. doi: 10.1097/SLA.0000000000005489
103. Deitch, EA, and Bridges, RM. Effect of stress and trauma on bacterial translocation from the gut. J Surg Res. (1987) 42:536–42. doi: 10.1016/0022-4804(87)90029-1
104. Ayan, E, Koksel, O, Polat, A, Tamer, L, Ersöz, G, Demir, M, et al. The role of thoracic trauma in inflammatory responses, apoptosis and bacterial translocation following multiple traumas. Turk J Trauma Emerg Surg. (2013) 19:491–9. doi: 10.5505/tjtes.2013.29660
105. Oztuna, V, Ersoz, G, Ayan, I, Eskandari, MM, Colak, M, and Polat, A. Early internal fracture fixation prevents bacterial translocation. Clin Orthop Relat Res. (2006) 446:253–8. doi: 10.1097/01.blo.0000203468.66055.23
106. Köhler, CA, Maes, M, Slyepchenko, A, Berk, M, Solmi, M, Lanctôt, KL, et al. The gut-brain Axis, including the microbiome, leaky gut and bacterial translocation: mechanisms and pathophysiological role in Alzheimer’s disease. Curr Pharm Des. (2016) 22:6152–66. doi: 10.2174/1381612822666160907093807
107. Crapser, J, Ritzel, R, Verma, R, Venna, VR, Liu, F, Chauhan, A, et al. Ischemic stroke induces gut permeability and enhances bacterial translocation leading to sepsis in aged mice. Aging. (2016) 8:1049–63. doi: 10.18632/aging.100952
108. Haussner, F, Chakraborty, S, Halbgebauer, R, and Huber-Lang, M. Challenge to the intestinal mucosa during Sepsis. Front Immunol. (2019) 10:891. doi: 10.3389/fimmu.2019.00891
109. Deitch, EA. Gut-origin sepsis: evolution of a concept. Surgeon. (2012) 10:350–6. doi: 10.1016/j.surge.2012.03.003
110. Casulleras, M, Zhang, IW, López-Vicario, C, and Leukocytes, CJ. Systemic inflammation and immunopathology in acute-on-chronic liver failure. Cell. (2020) 9:632. doi: 10.3390/cells9122632
111. Sun, J, Zhang, J, Wang, X, Ji, F, Ronco, C, Tian, J, et al. Gut-liver crosstalk in sepsis-induced liver injury. Crit Care. (2020) 24:614. doi: 10.1186/s13054-020-03327-1
112. Rysz, J, Franczyk, B, Ławiński, J, Olszewski, R, Ciałkowska-Rysz, A, and Gluba-Brzózka, A. The impact of CKD on uremic toxins and gut microbiota. Toxins. (2021) 13:252. doi: 10.3390/toxins13040252
113. Chi, M, Ma, K, Wang, J, Ding, Z, Li, Y, Zhu, S, et al. The immunomodulatory effect of the gut microbiota in kidney disease. J Immunol Res. (2021) 2021:1–16. doi: 10.1155/2021/5516035
114. Engen, PA, Green, SJ, Voigt, RM, Forsyth, CB, and Keshavarzian, A. The gastrointestinal microbiome: alcohol effects on the composition of intestinal microbiota. Alcohol Res. (2015) 37:223–36.
115. Choudhry, MA, Rana, SN, Kavanaugh, MJ, Kovacs, EJ, Gamelli, RL, and Sayeed, MM. Impaired intestinal immunity and barrier function: a cause for enhanced bacterial translocation in alcohol intoxication and burn injury. Alcohol. (2004) 33:199–208. doi: 10.1016/j.alcohol.2004.05.004
116. Patterson, GT, Osorio, EY, Peniche, A, Dann, SM, Cordova, E, Preidis, GA, et al. Pathologic inflammation in malnutrition is driven by Proinflammatory intestinal microbiota, large intestine barrier dysfunction, and translocation of bacterial lipopolysaccharide. Front Immunol. (2022) 13:846155. doi: 10.3389/fimmu.2022.846155
117. Casafont, F, Sánchez, E, Martín, L, Agüero, J, and Romero, FP. Influence of malnutrition on the prevalence of bacterial translocation and spontaneous bacterial peritonitis in experimental cirrhosis in rats. Hepatology. (1997) 25:1334–7. doi: 10.1002/hep.510250605
118. Brzozowski, B, Mazur-Bialy, A, Pajdo, R, Kwiecien, S, Bilski, J, Zwolinska-Wcislo, M, et al. Mechanisms by which stress affects the experimental and clinical inflammatory bowel disease (IBD): role of brain-gut Axis. Curr Neuropharmacol. (2016) 14:892–900. doi: 10.2174/1570159X14666160404124127
119. Zareie, M, Johnson-Henry, K, Jury, J, Yang, PC, Ngan, BY, McKay, DM, et al. Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut. (2006) 55:1553–60. doi: 10.1136/gut.2005.080739
120. Cash, A, and Theus, MH. Mechanisms of blood-brain barrier dysfunction in traumatic brain injury. Int J Mol Sci. (2020) 21:3344. doi: 10.3390/ijms21093344
121. Knox, EG, Aburto, MR, Clarke, G, Cryan, JF, and O’Driscoll, CM. The blood-brain barrier in aging and neurodegeneration. Mol Psychiatry. (2022) 27:2659–73. doi: 10.1038/s41380-022-01511-z
122. Gao, Q, and Hernandes, MS. Sepsis-associated encephalopathy and blood-brain barrier dysfunction. Inflammation. (2021) 44:2143–50. doi: 10.1007/s10753-021-01501-3
123. Nguyen, JH. Blood-brain barrier in acute liver failure. Neurochem Int. (2012) 60:676–83. doi: 10.1016/j.neuint.2011.10.012
124. Hernandez, L, Ward, LJ, Arefin, S, Ebert, T, Laucyte-Cibulskiene, A, Heimbürger, O, et al. Blood-brain barrier and gut barrier dysfunction in chronic kidney disease with a focus on circulating biomarkers and tight junction proteins. Sci Rep. (2022) 12:4414. doi: 10.1038/s41598-022-08387-7
125. Wei, J, Dai, Y, Wen, W, Li, J, Ye, LL, Xu, S, et al. Blood-brain barrier integrity is the primary target of alcohol abuse. Chem Biol Interact. (2021) 337:109400. doi: 10.1016/j.cbi.2021.109400
126. Beauchesne, E, Desjardins, P, Hazell, AS, and Butterworth, RF. Altered expression of tight junction proteins and matrix metalloproteinases in thiamine-deficient mouse brain. Neurochem Int. (2009) 55:275–81. doi: 10.1016/j.neuint.2009.03.014
127. Welcome, MO, and Mastorakis, NE. Stress-induced blood brain barrier disruption: molecular mechanisms and signaling pathways. Pharmacol Res. (2020) 157:104769. doi: 10.1016/j.phrs.2020.104769
128. Ormseth, CH, LaHue, SC, Oldham, MA, Josephson, SA, Whitaker, E, and Douglas, VC. Predisposing and precipitating factors associated with delirium: a systematic review. JAMA Netw Open. (2023) 6:e2249950. doi: 10.1001/jamanetworkopen.2022.49950
129. Wijarnpreecha, K, Chesdachai, S, Thongprayoon, C, Jaruvongvanich, V, Ungprasert, P, and Cheungpasitporn, W. Association of Helicobacter pylori with the risk of hepatic encephalopathy. Dig Dis Sci. (2017) 62:3614–21. doi: 10.1007/s10620-017-4834-1
130. Aristo Vojdani, EV. Food-associated autoimmunities: When food breaks your immune system: A&G. Beverly Hills, CA: Wilshire, LLC (2019).
131. Dimitrov, JD, Planchais, C, Roumenina, LT, Vassilev, TL, Kaveri, SV, and Lacroix-Desmazes, S. Antibody polyreactivity in health and disease: statu variabilis. J Immunol. (2013) 191:993–9. doi: 10.4049/jimmunol.1300880
132. Zhou, ZH, Tzioufas, AG, and Notkins, AL. Properties and function of polyreactive antibodies and polyreactive antigen-binding B cells. J Autoimmun. (2007) 29:219–28. doi: 10.1016/j.jaut.2007.07.015
133. Zhang, J, Jacobi, AM, Wang, T, Berlin, R, Volpe, BT, and Diamond, B. Polyreactive autoantibodies in systemic lupus erythematosus have pathogenic potential. J Autoimmun. (2009) 33:270–4. doi: 10.1016/j.jaut.2009.03.011
134. Klezovitch, O, and Vasioukhin, V. Cadherin signaling: keeping cells in touch. F1000Res. (2015) 4:550. doi: 10.12688/f1000research.6445.1
135. Erickson, MA, and Banks, WA. Transcellular routes of blood-brain barrier disruption. Exp Biol Med. (2022) 247:788–96. doi: 10.1177/15353702221080745
136. Lochhead, JJ, Yang, J, Ronaldson, PT, and Davis, TP. Structure, function, and regulation of the blood-brain barrier tight junction in central nervous system disorders. Front Physiol. (2020) 11:914. doi: 10.3389/fphys.2020.00914
137. Huang, X, Hussain, B, and Chang, J. Peripheral inflammation and blood-brain barrier disruption: effects and mechanisms. CNS Neuroscience Ther. (2021) 27:36–47. doi: 10.1111/cns.13569
138. Takata, F, Nakagawa, S, Matsumoto, J, and Dohgu, S. Blood-brain barrier dysfunction amplifies the development of Neuroinflammation: understanding of cellular events in brain microvascular endothelial cells for prevention and treatment of BBB dysfunction. Front Cell Neurosci. (2021) 15:661838. doi: 10.3389/fncel.2021.661838
139. Sweeney, MD, Sagare, AP, and Zlokovic, BV. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol. (2018) 14:133–50. doi: 10.1038/nrneurol.2017.188
140. Kadry, H, Noorani, B, and Cucullo, L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barr CNS. (2020) 17:69. doi: 10.1186/s12987-020-00230-3
141. Khan, SR, Chaker, L, Ikram, MA, Peeters, RP, van Hagen, PM, and Dalm, V. Determinants and reference ranges of serum immunoglobulins in middle-aged and elderly individuals: a population-based study. J Clin Immunol. (2021) 41:1902–14. doi: 10.1007/s10875-021-01120-5
142. Camilleri, M, and Vella, A. What to do about the leaky gut. Gut. (2022) 71:424–35. doi: 10.1136/gutjnl-2021-325428
143. Twardowska, A, Makaro, A, Binienda, A, Fichna, J, and Salaga, M. Preventing bacterial translocation in patients with leaky gut syndrome: nutrition and pharmacological treatment options. Int J Mol Sci. (2022) 23:204. doi: 10.3390/ijms23063204
144. Maes, M, and Leunis, JC. Normalization of leaky gut in chronic fatigue syndrome (CFS) is accompanied by a clinical improvement: effects of age, duration of illness and the translocation of LPS from gram-negative bacteria. Neuro Endocrinol Lett. (2008) 29:902–10.
145. Lee, JY, Ma, HW, Kim, JH, Park, IS, Son, M, Ryu, KH, et al. Novel histone deacetylase 6 inhibitor confers anti-inflammatory effects and enhances gut barrier function. Gut Liver. (2022) 17:766–76. doi: 10.5009/gnl220159
146. Hoilat, GJ, Altowairqi, AK, Ayas, MF, Alhaddab, NT, Alnujaidi, RA, Alharbi, HA, et al. Larazotide acetate for treatment of celiac disease: a systematic review and meta-analysis of randomized controlled trials. Clin Res Hepatol Gastroenterol. (2022) 46:101782. doi: 10.1016/j.clinre.2021.101782
147. Qiu, YM, Zhang, CL, Chen, AQ, Wang, HL, Zhou, YF, Li, YN, et al. Immune cells in the BBB disruption after acute ischemic stroke: targets for immune therapy? Front Immunol. (2021) 12:678744. doi: 10.3389/fimmu.2021.678744
Keywords: neuro-immune, leaky gut, bacterial translocation, inflammation, cytokines, biomarkers
Citation: Thisayakorn P, Thipakorn Y, Tantavisut S, Sirivichayakul S, Vojdani A and Maes M (2024) Increased IgA-mediated responses to the gut paracellular pathway and blood–brain barrier proteins predict delirium due to hip fracture in older adults. Front. Neurol. 15:1294689. doi: 10.3389/fneur.2024.1294689
Edited by:
Per Svenningsson, Karolinska Institutet (KI), SwedenReviewed by:
Yang Liu, University Hospital of the Saarland, GermanyClaudia Cantoni, Barrow Neurological Institute (BNI), United States
Copyright © 2024 Thisayakorn, Thipakorn, Tantavisut, Sirivichayakul, Vojdani and Maes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael Maes, dr.michaelmaes@hotmail.com
 Paul Thisayakorn
Paul Thisayakorn Yanin Thipakorn1
Yanin Thipakorn1 Sunee Sirivichayakul
Sunee Sirivichayakul Aristo Vojdani
Aristo Vojdani Michael Maes
Michael Maes