- 1HCI Lab, Yonsei University, Seoul, Republic of Korea
- 2HAII Corporation, Seoul, Republic of Korea
- 3Department of Neurology, Seoul Hospital, Ewha Womans University College of Medicine, Seoul, Republic of Korea
Background: Dysarthria is a motor speech disorder caused by various neurological diseases, particularly stroke. Individuals with post-stroke dysarthria experience impaired speech intelligibility, communication difficulties, and a reduced quality of life. However, studies on the treatment of post-stroke dysarthria are lacking. Digital speech therapy applications have the advantages of being personalized and easily accessible. However, evidence for their efficacy is not rigorous. Moreover, no studies have investigated both the acute to subacute, and chronic phases of stroke. This study aims to investigate the efficacy and feasibility of digital speech therapy applications in addressing these gaps in dysarthria treatment.
Methods and design: This study is a multicenter, prospective, randomized, evaluator-blinded non-inferiority trial. We aim to recruit 76 participants with post-stroke dysarthria. Eligible participants will be stratified based on the onset period of stroke into acute to subacute, and chronic phases. Participants will be randomized in a 1:1 to receive either a personalized digital speech therapy application or conventional therapy with a workbook for 60 min daily, 5 days a week, for 4 weeks. The primary outcome is the improvement in speech intelligibility. This will be measured by how accurately independent listeners can transcribe passages read by the participants. Secondary outcomes, which include speech function, will be evaluated remotely by speech-language pathologists. This includes the maximum phonation time, oral diadochokinetic rate, and percentage of consonants correct. Participants’ psychological well-being will also be assessed using self-report questionnaires, such as depressive symptoms (Patient Health Questionnaire-9) and quality of life (Quality of Life in the Dysarthric Speaker scale). The trial will also assess the feasibility, participant adherence, and usability of the application. Rigorous data collection and monitoring will be implemented to ensure patient safety.
Conclusion: This trial aims to investigate the efficacy and feasibility of digital speech therapy applications for treating post-stroke dysarthria. The results could establish foundational evidence for future clinical trials with larger sample sizes.
Clinical trial registration: Clinicaltrials.gov, identifier: NCT05865106.
1 Introduction
Stroke is a leading cause of mortality and long-term disability (1). Stroke can elicit a range of neurological deficits or handicaps, including speech and language problems. Dysarthria is a neurologic motor speech disorder caused by disruptions in the cranial nerve and muscular control of the speech mechanism. It is estimated that approximately 22–60% of stroke patients experience dysarthria within the first week following a stroke (2–4). Approximately 35% of patients continue to exhibit dysarthria even after 6 months (5). Moreover, post-stroke dysarthria significantly impairs speech intelligibility and articulation movements (2). This impairment leads to substantial communication difficulties, emotional distress, social isolation, and poor quality of life (6, 7).
Interventions for dysarthria involve a range of strategies, such as neurorehabilitation, behavioral exercises, and social support (2). Behavioral speech exercises strengthen the breathing and oral muscles to improve speech control. Strategies such as slowing down speech or controlling pitch are also used to enhance speech intelligibility. The treatment plan should be personalized based on the patient’s prior communication ability, recovery stage, and needs (8).
Despite the critical need for effective treatment of dysarthria, traditional therapies are often limited by their monotonous repeated nature. Consequently, these challenges can result in reduced treatment adherence (9). Moreover, the significant time and effort required by physicians and speech-language pathologists (SLPs), often restricts patient access to necessary therapeutic resources (10). Studies have indicated that only approximately one-third of patients receive adequate speech therapy, with substantial variations in therapy frequency (11). Given these limitations, personalized dysarthria treatment becomes necessary because of the diverse patterns of speech impairment in post-stroke dysarthria caused by different stroke lesions (12). These observations highlight the urgent need for alternative approaches, such as personalized digital speech therapy.
Compared with traditional in-clinic methods, these digital applications offer substantial benefits. These include the ability for patients to engage in various speech exercises from home. They improve treatment precision, enhance economic efficiency, ensure patient safety, maintain continuity of care, and support self-management (13, 14). This approach reduces the need for clinic visits and related expenses (15, 16). Furthermore, digital therapy is especially beneficial during the COVID-19 pandemic or endemic as it allows patients to continue their therapy without interruption (17).
Additionally, there is a noticeable lack of high-quality, comprehensive research on the treatment of post-stroke dysarthria. Many existing randomized controlled trials on post-stroke dysarthria have been on a small scale, limiting their ability to provide conclusive evidence regarding the effectiveness of various treatments (18). This significant research gap highlights the importance of our study, which aims to investigate both the efficacy and feasibility of a personalized digital speech therapy application for participants with post-stroke dysarthria.
This study aims to investigate the efficacy and feasibility of a digital speech therapy application for patients with post-stroke dysarthria. Ultimately, our trial seeks to establish our digital speech therapy application as a comprehensive solution. Our application strives to enhance the accessibility and practicality of post-stroke dysarthria. It provides personalized treatments tailored to each patient’s unique needs, thereby overcoming the limitations of traditional rehabilitation methods (12). We hypothesize that our digital speech therapy application would be non-inferior to conventional speech therapies in enhancing patient speech intelligibility and function for post-stroke dysarthria. Additionally, we anticipate positive impacts of our digital speech therapy application on psychological well-being, such as reduced depression and improved quality of life in patients with post-stroke dysarthria. Furthermore, this trial will assess the feasibility of this innovative approach in a clinical setting. It will focus on critical aspects such as recruitment and retention rates, participant adherence, safety, and gathering user feedback.
2 Methods and analysis
2.1 Trial design
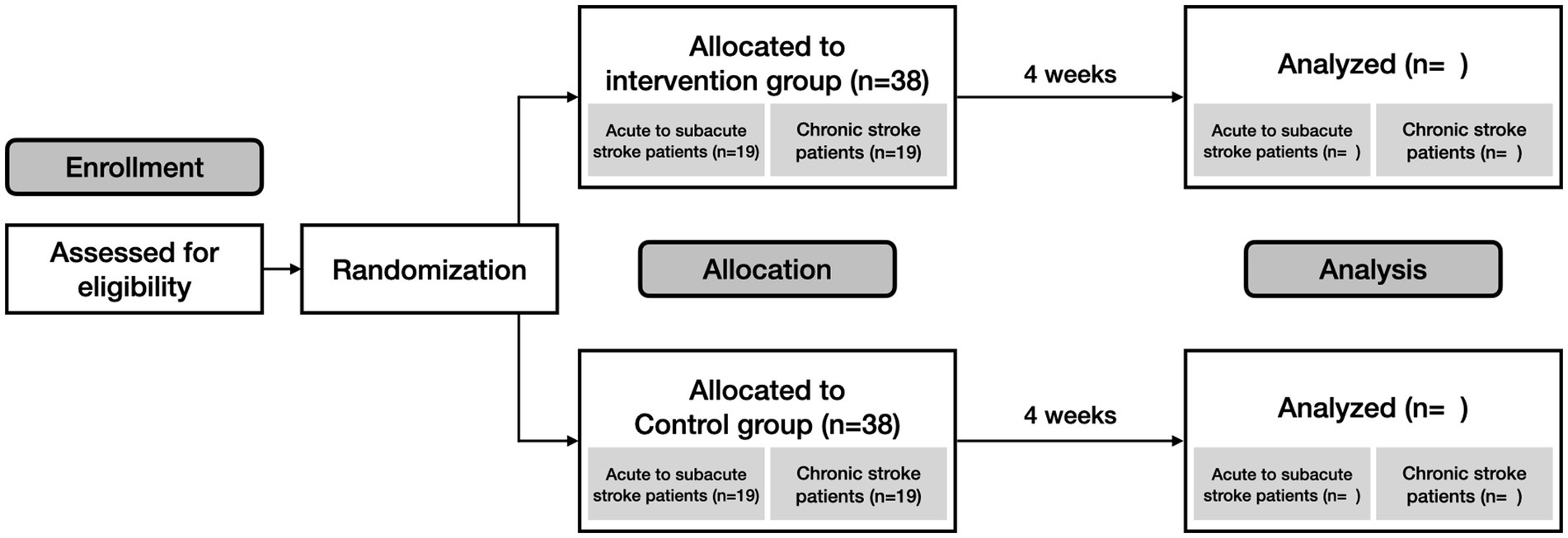
This study is a multicenter, prospective, randomized, evaluator-blinded, non-inferiority trial designed to evaluate the efficacy and feasibility of a digital speech therapy application for post-stroke dysarthria (Figure 1). Participants will be recruited from three stroke centers in South Korea: Ewha Womans University Seoul Hospital, Mokdong Hospital, and National Rehabilitation Center. The trial will be conducted in accordance with the Declaration of Helsinki (19) and received ethics approval from the Institutional Review Board (Approval number: EUMC 2023–02-002, and NRC-2023-01-007). It is registered on clinicaltrials.gov as NCT05865106.
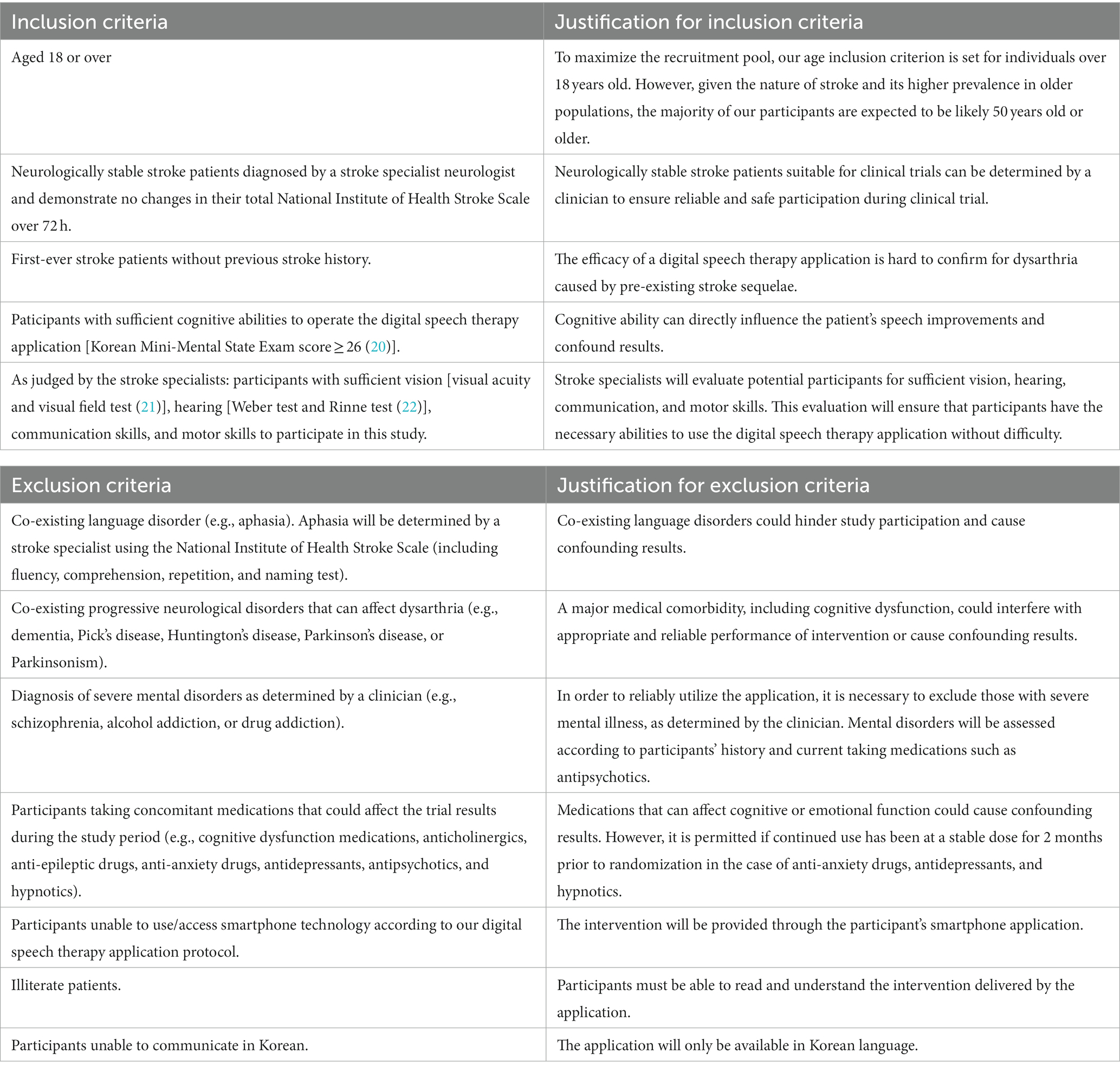
Participants will be recruited based on eligibility criteria (Table 1). Prior to enrollment, participants will receive a detailed explanation of the trial. Following this, written informed consent will be obtained from all participants. Subsequently, the participants’ demographic information and medical histories will be collected. Next, the participants will be randomly assigned to either the intervention or the control group. Randomization will be stratified based on the acute to subacute, and chronic phases of stroke.
2.2 Inclusion procedure
The principal investigator will screen and exclude participants with abnormalities in oral structures based on speech assessments. A comprehensive list of eligibility criteria is presented in Table 1. Stroke specialists will identify participants with dysarthria using the National Institute of Health Stroke Scale (NIHSS) criteria (23). The NIHSS is utilized for its ease of administration and standardized approach, which minimizes interpersonal variation in assessments. Also, the sub-section of “10. speech” is suitable for initially identifying dysarthria severity in stroke patients. We aim to recruit participants with both mild-to-moderate and severe dysarthria. Mild-to-moderate dysarthria is characterized by slurred speech that remains understandable, and severe dysarthria involves mostly unintelligible speech or the inability to speak without evidence of aphasia. The eligible participants will be referred to a research coordinator to verify their suitability for the trial. To educate the participants, the principal investigator will provide detailed information about the study, such as intervention options, associated risks, and expected benefits. All recruitment and screening processes will be thoroughly documented.
Participants will be clearly informed that they have the right to withdraw from the study at any point without any consequences to their future medical care. Withdrawal criteria will include voluntary withdrawal by the participant, a usage rate falling below 40%, a need for immediate medical care that precludes continued participation, and any adverse events that are directly related to the digital therapy application. Additionally, the research team reserves the right to withdraw participants if there is significant noncompliance with the study protocol or if their health status changes to the extent that continued use of the application is deemed unsuitable or unsafe.
2.3 Randomization
Participants will be randomly allocated in a 1:1 ratio to either the intervention group or control group. An independent third-party entity will oversee the randomization process to ensure impartiality.
Additionally, the trial will stratify participants based on the onset period of their stroke: acute to subacute phase (within 1 month of onset) and chronic phase (from 1 month to 6 months of onset) (24). After confirming participants’ eligibility, we will assign each participant a sequential number for group allocation. For each stratum, we will actively allocate participants in a 1:1 ratio to either the intervention or the control group. A secure computerized system will generate an unpredictable and concealed allocation sequence. A blinded researcher will manage this sequence in opaque envelopes, which will be opened after the participant’s enrollment and baseline measurements are complete.
To ensure group balance and methodological rigor within each stratum, we will use permuted blocks of sizes two and four (25). The block size and allocation sequence details will remain confidential and inaccessible to the team members involved in recruitment, treatment, and assessment until the conclusion of the study.
2.4 Blinding
Due to the nature of speech therapy, the complete blinding of participants to their assigned interventions is challenging. We will implement evaluator blinding to mitigate this issue and ensure unbiased outcome assessments (26). Evaluators will be thoroughly trained to understand the study protocol and recognize potential biases. Their role is to analyze the trial results objectively without influence from other factors. Evaluators will not interact face-to-face with participants. Instead, they will remotely listen to patient recordings via a web system and evaluate the outcomes. This method ensures evaluators focus exclusively on outcome assessment. They will remain unaware of the participants’ group assignments and will not be involved in other stages of the research.
2.5 Intervention methods
2.5.1 Intervention group
Participants in the intervention group will use a digital speech therapy application designed explicitly for post-stroke dysarthria. Participants will use the application independently, without requiring continuous assistance from caregivers or medical professionals. In addition, occasional guidance or support from family members will be permitted. As part of our regular monitoring process, researchers will follow up on the extent of family assistance to ensure it remains appropriate and consistent for the therapy protocol. However, the primary aim is for participants to manage the therapy autonomously. This approach promotes independence and self-reliance during treatment. Participants will engage in speech therapy for 60 min each day, 5 days a week, for 4 weeks. The flexibility of the application should enable participants to complete their therapy in either a single session or multiple sessions throughout the day. Before therapy begins, researchers will introduce the application to participants and guide them through its usage. Additionally, we will provide a manual booklet to ensure that participants can use the application confidently and independently.
As the majority of stroke patients are older adults, the application is designed to be user-friendly (27). It features elder-friendly functionalities and provides progress updates through both text and voice descriptions to ensure accessibility. Design elements such as button size and spacing are tailored to accommodate the potential fine motor and vision challenges faced by older users (28).
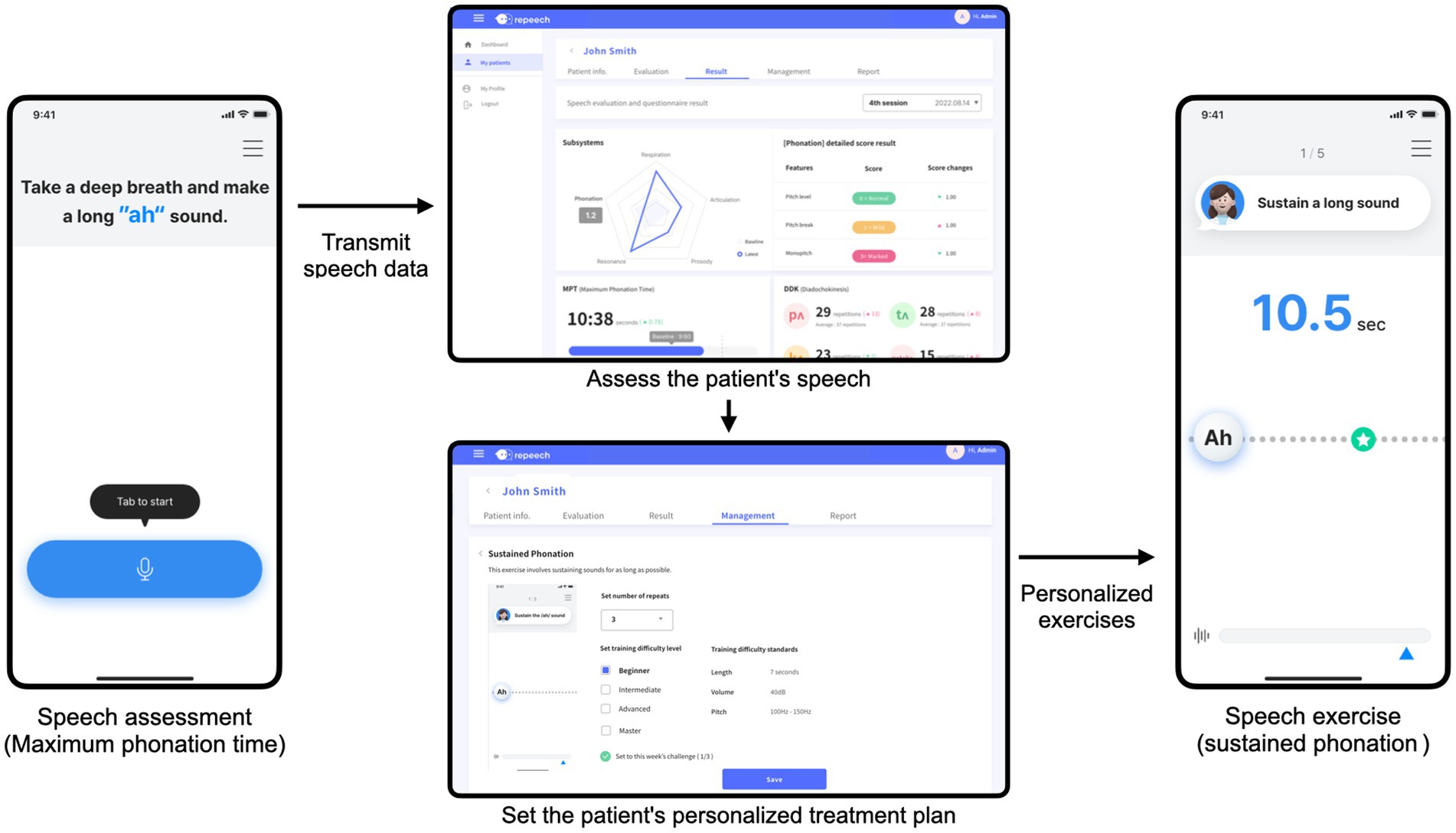
Given the variability in speech impairment patterns due to different stroke lesions in post-stroke dysarthria (29), this application provides a personalized approach to speech therapy. When participants log into the application, it prompts them to perform four speech assessment tasks to evaluate their current speech conditions. The application uses a smartphone microphone to measure the ambient noise levels before each task to ensure optimal recording conditions. The assessment proceeds only if the noise level is <50 dB. Four assessment tasks were adapted from traditional dysarthria assessments. Each task comes with detailed instructions and sample demonstrations to help participants perform assessments efficiently.
• Sustained Vowel: Participants are instructed to sustain the vowels/a/, /i/, and/u/, using their regular speaking tones. Participants are to perform the task twice, and the longer attempt between the two is recorded for analysis (30).
• Diadochokinetic (DDK) Rate: Participants are required to rapidly repeat syllables /pǝ/, /tǝ/, and /kǝ/, along with the sequence/pǝtǝkǝ/ each for at least 5 s. Subsequently, their performance is recorded for analysis (31).
• Word Reading: Participants are asked to read a set of 30 words from the Urimal Test of Articulation and Phonology 2 (UTAP2). The UTAP2 is specifically designed to assess articulation accuracy in Koreans (32).
• Passage Reading: Participants are instructed to read the passage in a comfortable voice at a natural pace. This assessment evaluates the patient’s articulation and overall speech intelligibility. The Gaeul Passage is the standard for examining motor speech disorders in South Korea (33).
The recorded speech assessment data will be sent to a web system. The SLPs will review the recorded assessments remotely. This process enables them to identify specific patterns of dysarthric speech impairment using a web system, as shown in Figure 2. Based on these evaluations, a personalized treatment plan specifies the types and levels of exercise required. As an illustration, participants with respiratory impairment may be assigned the “sustained phonation” exercise. SLPs can also set target goals, including phonation duration, volume, and repetition. Conversely, participants struggling with articulation may perform reading exercises. Considering the common articulation error patterns observed in participants with dysarthria, we arranged the minimal pair sets. If a patient has difficulty stopping sounds, the SLP can set minimal pairs such as/p/ and /f/, or/b/and/v/. This plan is then uploaded to the patient’s application. Subsequently, the patient performs the prescribed speech exercises. The therapeutic goals are set at the beginning of each week. Based on the patient’s performance, the difficulty level of the exercises is adjusted during weekly reviews. If a patient completes the therapy tasks for 1 week, the subsequent week’s exercises are prescribed at a higher difficulty level. This adjustment ensures the continuous progression and adaptation of the patient’s improved speech skills.
Our digital speech therapy application integrates a comprehensive behavioral speech therapy exercise based on the established literature (2, 34). An introductory video at the beginning of the exercise explains the participant’s objectives and processes. The application provides real-time visual and auditory feedback during exercise to enhance participants’ immediate responsiveness. Moreover, the application utilizes a sophisticated signal processing algorithm to analyze voice data captured via the smartphone’s microphone. This algorithm assesses various speech acoustic variables, such as sound volume, pitch, reading speed, and pronunciation accuracy. Each exercise comes with predefined thresholds against which participant performance is measured. This enables the application to offer immediate and personalized feedback based on the individual’s progress and performance against these benchmarks. Additionally, the participants can listen to their recordings and receive additional feedback. This comprehensive feedback mechanism promotes patient engagement and constant improvement in speech therapy (35). Detailed procedures for the speech exercises are provided in Supplementary material.
Maintaining adherence and continuing to participate in the study is a crucial goal. Researchers will systematically offer direct feedback to participants regarding their performance and adherence. This approach encourages continuous patient engagement throughout the therapeutic process. To maintain patient adherence, the researchers will monitor the daily progress of each patient. Researchers will contact participants whenever a decrease in the usage of the application is observed. They will reach out through text messages or phone calls to understand the reasons behind the reduction in their usage. The researchers will then provide encouragement and support to reengage participants in the therapy process. Additionally, the application features daily and monthly calendars for visual progress tracking. It also records the duration of daily activities, fostering motivation and consistency among the participants. Moreover, the participants are encouraged to contact the research team for assistance with application usage challenges, including user errors and interface navigation. Researchers will promptly respond to concerns communicated via phone, email, or text to offer immediate support in resolving issues.
2.5.2 Control group
Participants allocated to the control group will undergo conventional therapy for post-stroke dysarthria over 4 weeks. In the absence of a standard protocol for post-stroke dysarthria treatment in South Korea, we have developed a conventional speech therapy workbook. This workbook integrates various clinically validated behavioral therapy techniques (2, 34). Its content is aligned with the intervention group’s digital speech therapy application to ensure consistency in treatment approaches. Participants will engage in the workbook for 60 min daily, 5 days a week, for 4 weeks to match the intervention group’s treatment dose and frequency. The workbook ensures treatment consistency across the multiple centers involved in this clinical trial. Before starting therapy, the researchers will guide the participants using the conventional therapy workbook.
In line with the intervention group, occasional guidance or support from family members will be allowed. This approach ensures that both groups have access to similar levels of support. By doing so, we promote equality in the treatment conditions across the groups. During regular follow-ups, researchers will monitor the extent and nature of any assistance family members provide. This monitoring will help ensure that the level of support remains balanced and appropriate for both groups.
Researchers will conduct daily follow ups to ensure adherence to conventional therapy workbook. During these interactions, researchers will discuss the participants’ progress, address challenges, and provide support and encouragement to maintain consistent participation in therapy. This approach ensures that the control group receives the same level of attention and support as the intervention group, thereby maintaining trial integrity. After the clinical trial, participants in the control group will also have access to the digital speech therapy application.
2.6 Outcome measurements
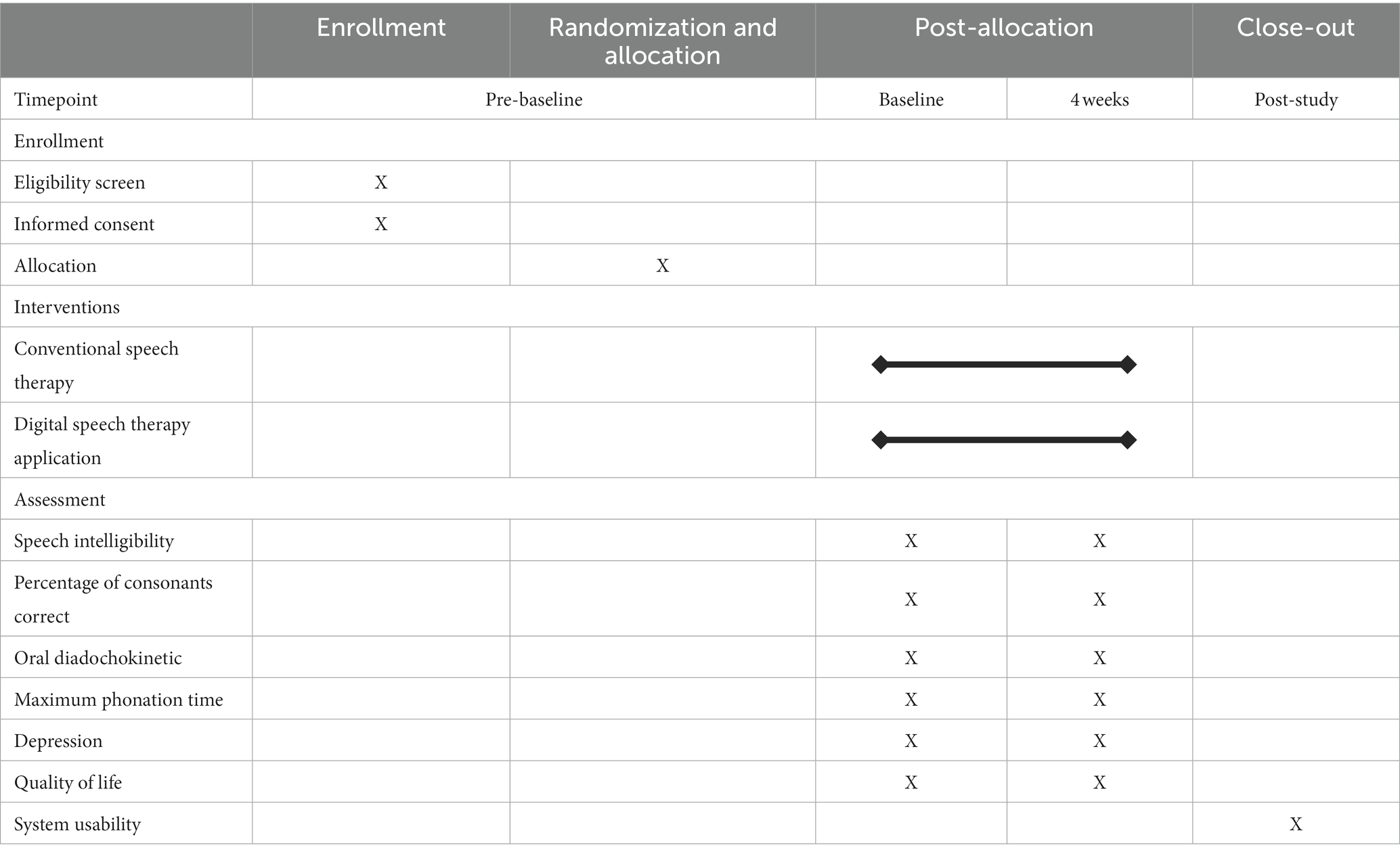
The study will span 4 weeks, and the details are provided in Table 2. Three assessment stages will be conducted during the process.
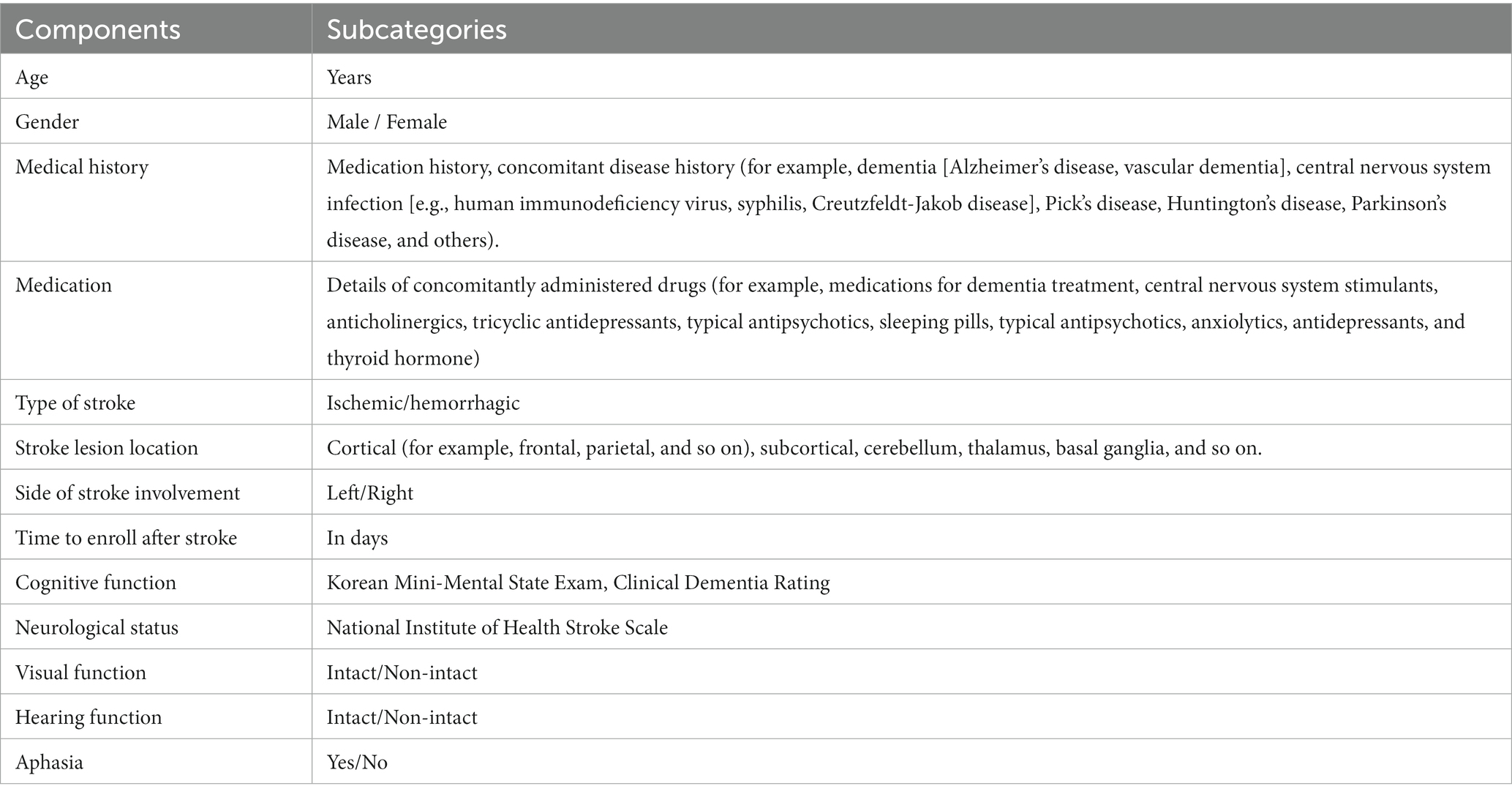
Demographic and clinical characteristics will be recorded after enrollment in the study. This initial recording includes demographic data (age and sex), medical history, stroke information (type and location of stroke and post-stroke duration), level of impairment measured using the NIHSS (23), cognitive status determined using the Korean Mini-Mental State Examination (K-MMSE) score (20), and Clinical Dementia Rating (CDR) tests (36). The demographic and clinical characteristics of the participants are shown in Table 3.
Subsequently, the participants will undergo a comprehensive assessment for post-stroke dysarthria. Psychological well-being questionnaires will also be administered to assess depression and quality of life. Participants will engage in the post-evaluation phase 4 weeks after the baseline assessment. We will repeat the speech assessments and psychological questionnaires from the baseline evaluations. Additional evaluations of usability and adherence rates will be conducted.
2.6.1 Outcomes
The primary outcome is the change in speech intelligibility following the intervention. Speech intelligibility is crucial for effective communication and reflects how well a listener understands a patient’s speech (37). Speech intelligibility will be calculated using a passage-reading assessment. Participants will be instructed to read a passage aloud comfortably and naturally using the application for assessment. The application will record their speech and automatically upload the recordings to a secure server. Next, three independent SLPs will listen to the recordings via a web system. After hearing the passage once, they will transcribe it as they understand it. We will compare these transcriptions with the original passages to calculate the percentage of correctly transcribed words. Each participant’s final score will be the average of the scores of all three evaluators (38).
For secondary outcomes, the study will assess the participants’ speech function and psychological well-being through voice recordings and surveys via the application. The SLPs will listen to the recorded voices of the participants and rate each speech function measure. The maximum phonation time (MPT) assesses the ability to sustain vowel sound (39). Oral diadochokinetic (DDK) rate evaluates the speed, regularity, and accuracy of articulatory movements, with performance quantified by the number of syllables repeated rapidly and accurately (31). The percentage of consonants correct (PCC) is determined by comparing transcriptions from word-reading tasks with the original text to objectively assess the accuracy of consonant sounds (32, 40).
Self-report questionnaires will be used to assess psychological well-being. The Patient Health Questionnaire-9 (PHQ-9) evaluates the severity of depressive symptoms (41). The Quality of Life in the Dysarthric Speaker (QOL-Dys) scale quantifies how dysarthria affects various aspects of life. It specifically assesses the impact on daily activities (42, 43). These measures provided vital insights into the effects of interventions on communication and psychological health.
2.6.2 Feasibility
A comprehensive evaluation will be conducted to assess the feasibility of this trial. First, the trial will estimate the number of potential participants, the duration of recruitment, and the success rate of screening to secure a representative sample size. Additionally, retention rates will be monitored throughout the trial. These measurements will ensure a rigorous sample size for future studies. Second, the participants’ adherence to digital therapy applications will be measured. This will include tracking the frequency and completeness of the application usage and participant engagement with the treatment protocol using a robust data collection and management system. Third, participant feedback will be collected to assess the acceptability and effectiveness of the digital therapy. We will use the System Usability Scale (SUS) questionnaire to measure the usability of the digital speech therapy application in terms of efficiency and user satisfaction (44). Additionally, interviews will be conducted with the application user to collect qualitative insights such as participants’ experiences, challenges, and suggestions for improvement. This comprehensive feedback will aid in a better understanding of the application’s usability and effectiveness and contribute to future development and enhancements. Finally, we will monitor patient safety and potential adverse events and uphold ethical standards. Digital speech therapy applications include the real-time monitoring of patient log data to swiftly identify and address usage errors or technical issues. Rigorous testing of the application’s performance will also focus on stability and user interface to assess its impact on therapy effectiveness and user experience. This approach ensures the safety and reliability of the digital tools throughout the study.
2.7 Analysis
The primary objective of this trial is to investigate the non-inferiority of the intervention group compared to the control group in terms of changes in speech intelligibility from baseline to post-treatment. The hypothesis is as follows:
Where μt is the mean change in speech intelligibility score in the intervention group using the digital speech therapy application, and μc is the mean change in the control group receiving conventional speech therapy. It is assumed that there is no difference between the two groups, and this study seeks to establish the non-inferiority of the groups. The non-inferiority margin δ is set at 19 points, based on the assumption that a difference of 1 to 19 points on the speech intelligibility scale is clinically non-inferior (45).
The sample size required for the non-inferiority trial was determined using a statistical formula (46). This involved setting the significance level (alpha) to 0.025 for a one-sided test and targeting a power of 80% (β = 0.2) (47) with a standard deviation of 24.9 (48). This calculation revealed that 28 participants are required for each group. A total of 56 participants are required for the trial. To account for a possible dropout rate of 30%, we aim to recruit a total of 76 participants, with 38 participants in each group.
Analyses will be performed using SPSS software (version 27; IBM Corp., Armonk, New York). Descriptive statistics will summarize the demographic data and outcome measures. For interval estimates, 95% confidence intervals will be provided. An intention-to-treat (ITT) analysis will include all randomized participants. Additionally, we will conduct a per-protocol (PP) analysis which includes only participants who fully adhered to the intended protocol.
First, the Shapiro–Wilk test will assess of the normality of the distributions. Based on these results, the appropriate parametric (independent two-sample t-test) or non-parametric test (Mann–Whitney U test) will be chosen for between-group comparisons. Then, mixed repeated-measures analysis of variance (ANOVA) will evaluate the changes over time within subjects and between groups, with fixed effects for time, group, and their interactions. Mauchly’s test will check sphericity by applying the Greenhouse–Geisser, if necessary. For significant findings, Tukey’s HSD tests will conduct post-hoc analyses with Bonferroni correction to control for the overall type I error rate at 0.05. The benchmark for statistical significance is set at p < 0.05.
We will address missing data using multiple imputation techniques and the Last Observation Carried Forward (LOCF) method. This approach aims to minimize bias and ensure the robustness of efficacy outcomes (49). Subgroup and sensitivity analyses will be conducted to explore the effects of participant characteristics and institutional factors on the primary efficacy results. In addition to the primary analysis, we will conduct subgroup analyses to examine the efficacy of our digital speech therapy application in various settings. Subgroup analyses include comparing its use during hospitalization with post-discharge home use. These analyses aim to provide a deeper understanding of how the setting influences the effectiveness of the therapy.
Furthermore, additional subgroup analyses will focus on the effects of the interventions across different stroke phases. Since participants are stratified based on the onset period of their stroke into acute to subacute, and chronic phases, these subgroup analyses will explore how the efficacy of the intervention varies between these two distinct stroke phases. We will perform regression analyses within each group stratified by the stroke onset period. If the statistical analysis reveals significant mean differences between various groups, post-hoc tests will be conducted to identify the specific groups among which these differences exist. This approach will provide valuable insights into whether the timing of a post-stroke intervention influences the efficacy of digital speech therapy. No interim analyses are planned to maintain the integrity of the non-inferiority margin or control for type I error rate.
3 Discussion
This protocol aims to evaluate a digital speech therapy application for post-stroke dysarthria in a randomized clinical trial. This will establish the efficacy and feasibility of the intervention and contribute vital data to inform future trials. The scarcity of rigorous research on post-stroke dysarthria treatment means that these findings will be significant for researchers and clinicians in this field (18, 50).
Previous research has demonstrated the efficacy of behavioral speech treatment in patients with chronic post-stroke dysarthria. For instance, previous study showed a significant improvement in patients with post-stroke dysarthria after 16 sessions over 4 weeks (51). Similarly, another study reported positive outcomes from 60-min sessions administered four times a week for 1 month in patients with at least 6 months of post-stroke dysarthria (52). However, these studies may not fully address error patterns and stroke lesion characteristics across stroke recovery phases (53–55). They recognized the importance of early intervention, which has been shown to significantly enhance outcomes (34, 56, 57). Our protocol aims to fill this research gap by stratifying patients into acute to subacute, and chronic phases. This approach offers a comprehensive view of the effect of treatment on the stroke recovery spectrum.
Our study proposes digital speech therapy designed to address the limitations of traditional in-clinic or in-hospital treatments for post-stroke dysarthria. Considering the diverse error patterns in participants with post-stroke dysarthria, our system emphasizes the delivery of personalized therapies through speech assessment. We aim to enhance treatment efficacy and improve patient adherence using a patient’s smartphone application.
Maintaining long-term commitment to post-stroke treatment can be challenging (58, 59). Patients may exercise less frequently than suggested (60), struggle to achieve daily treatment goals (61), or discontinue therapy entirely (59). Digital solutions offered for comfort in patients’ homes are expected to encourage more intensive speech treatment (6) and better adherence. Digital speech therapy applications enable easier access to speech therapy, increase engagement, and may reduce costs (62, 63). However, this study has several limitations. First, the limited sample size might limit the applicability of our results. Second, our digital speech therapy application is designed exclusively for Koreans and targeted at native Korean speakers. This language limitation could limit the generalizability to non-Korean populations. Third, the lack of direct supervision by clinicians or SLPs in digital speech therapy interventions could affect patient motivation and result in higher dropout rates (59, 64). Finally, a significant challenge arises from the high proportion of older participants, which is typical in stroke patient demographics (65). Despite considering the design of our application for the elderly population, these participants may experience lower usability and adherence to digital treatment because of their unfamiliarity with digital technology.
Despite these limitations, this study has several strengths. First, it aims to provide new evidence for the use of digital speech therapy in the treatment of post-stroke dysarthria. This could increase the adoption of digital treatment in the field. Second, the study is designed to investigate whether a digital speech therapy application is effective and feasible for stroke participants, regardless of their severity and recovery stage.
In conclusion, this trial explores the efficacy and feasibility of digital speech therapy applications in treating post-stroke dysarthria. Our study aims to provide significant insights that will aid future research by refining effect size estimates and enhancing power analysis. By addressing the complexities of post-stroke speech therapy through a digital application, we expect to enhance treatment strategies. Through our trial, we expect to be able to provide evidence of digital therapeutics for the treatment of post-stroke dysarthria.
Ethics statement
The studies involving humans were approved by the EWHA Womans University Seoul Hospital Institutional Review Board (EUSMC-2021-12-011) and the National Rehabilitation Center Institutional Review Board (NRC-2023-01-007). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individuals for the publication of any potentially identifiable images or data included in this article.
Author contributions
YK: Conceptualization, Methodology, Software, Writing – original draft, Writing – review & editing. MK: Writing – original draft, Writing – review & editing. JK: Funding acquisition, Writing – review & editing. T-JS: Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Technology development Program (S3301230) funded by the Ministry of SMEs and Startups (MSS, Korea). This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HI22C073600, RS-2023-00262087 to TS).
Conflict of interest
The authors declare the following financial interests/personal relationships, which may be considered as potential competing interests: JK is a founder of the HAII Corp, makers of digital speech therapy application. YK, and MK are partly employed by HAII Corp.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1305297/full#supplementary-material
References
1. Feigin, VL, Brainin, M, Norrving, B, Martins, S, Sacco, RL, Hacke, W, et al. World stroke organization (WSO): global stroke fact sheet 2022. Int J Stroke. (2022) 17:18–29. doi: 10.1177/17474930211065917
2. Duffy, JR . Motor speech disorders: substrates, differential diagnosis, and management. 4th Edn.) ed. St. Louis, Missouri: Elsevier (2020).
3. Mackenzie, C . Dysarthria in stroke: a narrative review of its description and the outcome of intervention. Int J Speech Lang Pathol. (2011) 13:125–36. doi: 10.3109/17549507.2011.524940
4. Darley, FL, Aronson, AE, and Brown, JR. Differential diagnostic patterns of dysarthria. J Speech Hear Res. (1969) 12:246–69. doi: 10.1044/jshr.1202.246
5. O'Mahony, PG, Thomson, RG, Dobson, R, and Rodgers, HJames OF. The prevalence of stroke and associated disability. J Public Health. (1999) 21:166–71. doi: 10.1093/pubmed/21.2.166
6. Dickson, S, Barbour, RS, Brady, M, Clark, AM, and Paton, G. Patients' experiences of disruptions associated with post-stroke dysarthria. Int J Lang Commun Disord. (2008) 43:135–53. doi: 10.1080/13682820701862228
7. Mackenzie, C, Kelly, S, Paton, G, Brady, M, and Muir, M. The living with dysarthria group for post-stroke dysarthria: the participant voice. Int J Lang Commun Disord. (2013) 48:402–20. doi: 10.1111/1460-6984.12017
8. Brady, MC, Clark, AM, Dickson, S, Paton, G, and Barbour, RS. Dysarthria following stroke: the patient's perspective on management and rehabilitation. Clin Rehabil. (2011) 25:935–52. doi: 10.1177/0269215511405079
9. Tamayo-Serrano, P, Garbaya, S, Bouakaz, S, and Blazevic, P. A game-based rehabilitation therapy for post-stroke patients: an approach for improving patient motivation and engagement. IEEE Syst Man Cyber Magaz. (2020) 6:54–62. doi: 10.1109/MSMC.2020.3002519
10. Avan, A, Digaleh, H, Napoli, MD, Stranges, S, Behrouz, R, Shojaeianbabaei, G, et al. Socioeconomic status and stroke incidence, prevalence, mortality, and worldwide burden: an ecological analysis from the global burden of disease study 2017. BMC Med. (2019) 17:191.doi: 10.1186/s12916-019-1397-3
11. Dobkin, BH, and Dorsch, A. New evidence for therapies in stroke rehabilitation. Curr Atheroscler Rep. (2013) 15:1–9. doi: 10.1007/s11883-013-0331-y
12. Macoir, J, Lavoie, M, Routhier, S, and Bier, N. Key factors for the success of self-administered treatments of Poststroke aphasia using technologies. Telemed e-Health. (2019) 25:663–70. doi: 10.1089/tmj.2018.0116
13. Karlsen, C, Ludvigsen, MS, Moe, CE, Haraldstad, K, and Thygesen, E. Experiences of community-dwelling older adults with the use of telecare in home care services: a qualitative systematic review. JBI Evid Synth. (2017) 15:2913–80. doi: 10.11124/JBISRIR-2017-003345
14. Griffin, M, Bentley, J, Shanks, J, and Wood, C. The effectiveness of Lee Silverman voice treatment therapy issued interactively through an iPad device: a non-inferiority study. J Telemed Telecare. (2018) 24:209–15. doi: 10.1177/1357633X17691865
15. Abelson, JS, Kaufman, E, Symer, M, Peters, A, Charlson, M, and Yeo, H. Barriers and benefits to using mobile health technology after operation: a qualitative study. Surgery. (2017) 162:605–11. doi: 10.1016/j.surg.2017.05.007
16. Bowser, DM, Shepard, DS, Nandakumar, A, Okunogbe, A, Morrill, T, Halasa-Rappell, Y, et al. Cost effectiveness of Mobile health for antenatal care and facility births in Nigeria. Ann Glob Health. (2018) 84:592–602. doi: 10.9204/aogh.2364
17. Zhou, X, Snoswell, CL, Harding, LE, Bambling, M, Edirippulige, S, Bai, X, et al. The role of telehealth in reducing the mental health burden from COVID-19. Telemed e-Health. (2020) 26:377–9. doi: 10.1089/tmj.2020.0068
18. Mitchell, C, Bowen, A, Tyson, S, Butterfint, Z, and Conroy, P. Interventions for dysarthria due to stroke and other adult-acquired, non-progressive brain injury. Cochrane Database Syst Rev. (2017) 2017. doi: 10.1002/14651858.CD002088.pub3
19. World Medical A . World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. (2013) 310:2191–4. doi: 10.1001/jama.2013.281053
20. Kim, JM, Shin, IS, Yoon, JS, and Lee, HY. Comparison of diagnostic validities between MMSE-K and K-MMSE for screening of dementia. J Korean Neuropsychiatr Assoc. (2003) 46:124–30. doi: 10.5124/jkma.2003.46.2.124
21. Rowe, F, Brand, D, Jackson, CA, Price, A, Walker, L, Harrison, S, et al. Visual impairment following stroke: do stroke patients require vision assessment? Age Ageing. (2008) 38:188–93. doi: 10.1093/ageing/afn230
22. Isaacson, J, and Vora, NM. Differential diagnosis and treatment of hearing loss. Am Fam Physician. (2003) 68:1125–32.
23. Oh, MS, Yu, K-H, Lee, J-H, Jung, S, Ko, I-S, Shin, J-H, et al. Validity and reliability of a Korean version of the National Institutes of Health stroke scale. J Clin Neurol. (2012) 8:177–83. doi: 10.3988/jcn.2012.8.3.177
24. Bernhardt, J, Hayward, KS, Kwakkel, G, Ward, NS, Wolf, SL, Borschmann, K, et al. Agreed definitions and a shared vision for new standards in stroke recovery research: the stroke recovery and rehabilitation roundtable taskforce. Int J Stroke. (2017) 12:444–50. doi: 10.1177/1747493017711816
25. Broglio, K . Randomization in clinical trials: permuted blocks and stratification. JAMA. (2018) 319:2223–4. doi: 10.1001/jama.2018.6360
26. Schulz, KF, and Grimes, DA. Blinding in randomised trials: hiding who got what. Lancet. (2002) 359:696–700. doi: 10.1016/S0140-6736(02)07816-9
27. Wildenbos, GA, Jaspers, MWM, Schijven, MP, and Dusseljee-Peute, LW. Mobile health for older adult patients: using an aging barriers framework to classify usability problems. Int J Med Inform. (2019) 124:68–77. doi: 10.1016/j.ijmedinf.2019.01.006
28. Barros, AC, Leitão, R, and Ribeiro, J. Design and evaluation of a Mobile user Interface for older adults: navigation, interaction and visual design recommendations. Proced. Comp. Sci. (2014) 27:369–78. doi: 10.1016/j.procs.2014.02.041
29. Palmer, R, and Enderby, P. Methods of speech therapy treatment for stable dysarthria: a review. Adv. Speech Lang. Pathol. (2007) 9:140–53. doi: 10.1080/14417040600970606
30. Kwon, YG, Do, KH, Park, SJ, Chang, MC, and Chun, MH. Effect of repetitive transcranial magnetic stimulation on patients with dysarthria after subacute stroke. Annals of Rehabilitation Medicine. (2015) 39:793–9. doi: 10.5535/arm.2015.39.5.793
31. Ziegler, W . Task-related factors in oral motor control: speech and oral diadochokinesis in dysarthria and apraxia of speech. Brain Lang. (2002) 80:556–75. doi: 10.1006/brln.2001.2614
32. Kim, YT, Park, H, Kang, JK, Kim, JA, Shin, MJ, Kim, S-J, et al. Validity and reliability analyses for the development of Urimal test of articulation and Phonology-2. Commun. Sci. Disord. (2018) 23:959–70. doi: 10.12963/csd.18545
33. Kim, HH , Perceptual, Acoustical, and Physiological Tools in Ataxic Dysarthria Management; A Case Report. Proceedings of the Korean society of phonetic sciences and speech technology conference. (1996) 2:9–22.
34. Godecke, E, Armstrong, E, Rai, T, Ciccone, N, Rose, ML, Middleton, S, et al. A randomized control trial of intensive aphasia therapy after acute stroke: the very early rehabilitation for SpEech (VERSE) study. Int J Stroke. (2021) 16:556–72. doi: 10.1177/1747493020961926
35. Maas, E, Robin, DA, Hula, SNA, Freedman, SE, Wulf, G, Ballard, KJ, et al. Principles of motor learning in treatment of motor speech disorders. Am J Speech Lang Pathol. (2008) 17:277–98. doi: 10.1044/1058-0360(2008/025)
36. Morris, JC . The clinical dementia rating (CDR): current version and scoring rules. Neurology. (1993) 43:2412–4. doi: 10.1212/WNL.43.11.2412-a
37. Yorkston, KM, Dowden, PA, and Beukelman, DR. Intelligibility measurement as a tool in the clinical management of dysarthric speakers. Intellig. Speech Disord. (1992) 1:265–85. doi: 10.1075/sspcl.1.08yor
38. Hirsch, ME, Thompson, A, Kim, Y, and Lansford, KL. The reliability and validity of speech-language pathologists’ estimations of intelligibility in dysarthria. Brain Sci. (2022) 12:1011. doi: 10.3390/brainsci12081011
39. Shin, MJ, Kim, JO, Lee, SB, and Lee, SY. Speech mechanism screening test. Seoul: Hakjisa (2010).
40. Namasivayam, AK, Huynh, A, Granata, F, Law, V, and van Lieshout, P. PROMPT intervention for children with severe speech motor delay: a randomized control trial. Pediatr Res. (2021) 89:613–21. doi: 10.1038/s41390-020-0924-4
41. Spitzer, RL, Kroenke, K, and Williams, JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary care evaluation of mental disorders. Patient health questionnaire. JAMA. (1999) 282:1737–44. doi: 10.1001/jama.282.18.1737
42. Piacentini, V, Zuin, A, Cattaneo, D, and Schindler, A. Reliability and validity of an instrument to measure quality of life in the dysarthric speaker. Folia Phoniatr Logop. (2011) 63:289–95. doi: 10.1159/000322800
43. Long, A, Hesketh, A, and Bowen, AStudy ANR. Communication outcome after stroke: a new measure of the carer's perspective. Clin Rehabil. (2009) 23:846–56. doi: 10.1177/0269215509336055
45. Lousada, M, Jesus, LMT, Hall, A, and Joffe, V. Intelligibility as a clinical outcome measure following intervention with children with phonologically based speech–sound disorders. Int J Lang Commun Disord. (2014) 49:584–601. doi: 10.1111/1460-6984.12095
46. Flight, L, and Julious, SA. Practical guide to sample size calculations: non-inferiority and equivalence trials. Pharm Stat. (2016) 15:80–9. doi: 10.1002/pst.1716
47. Muller, KE, Lavange, LM, Ramey, SL, and Ramey, CT. Power calculations for general linear multivariate models including repeated measures applications. J Am Stat Assoc. (1992) 87:1209–26. doi: 10.1080/01621459.1992.10476281
48. Levy, ES, Moya-Galé, G, Chang, YHM, Freeman, K, Forrest, K, Brin, MF, et al. The effects of intensive speech treatment on intelligibility in Parkinson's disease: a randomised controlled trial. eClinicalMedicine. (2020) 24:100429. doi: 10.1016/j.eclinm.2020.100429
49. Hamer, RM, and Simpson, PM. Last observation carried forward versus mixed models in the analysis of psychiatric clinical trials. Am J Psychiatr. (2009) 166:639–41. doi: 10.1176/appi.ajp.2009.09040458
50. Finch, E, Rumbach, AF, and Park, S. Speech pathology management of non-progressive dysarthria: a systematic review of the literature. Disabil Rehabil. (2020) 42:296–306. doi: 10.1080/09638288.2018.1497714
51. Mahler, LA, and Ramig, LO. Intensive treatment of dysarthria secondary to stroke. Clin Linguist Phon. (2012) 26:681–94. doi: 10.3109/02699206.2012.696173
52. Park, S, Theodoros, D, Finch, E, and Cardell, E. Be clear: a new intensive speech treatment for adults with nonprogressive dysarthria. Am J Speech Lang Pathol. (2016) 25:97–110. doi: 10.1044/2015_AJSLP-14-0113
53. Cramer, SC . Repairing the human brain after stroke: I. Mechanisms Spontaneous Recov Ann Neurol. (2008) 63:272–87. doi: 10.1002/ana.21393
54. Kiran, S, and Thompson, CK. Neuroplasticity of language networks in aphasia: advances, updates, and future challenges. Front Neurol. (2019) 10:295. doi: 10.3389/fneur.2019.00295
55. Robertson, SJ, and Thomson, F. Speech therapy in Parkinson's disease: a study of the efficacy ad long term effects of intensive treatment. Br J Disord Commun. (1984) 19:213–24. doi: 10.3109/13682828409029837
56. Lancaster, GA, Dodd, S, and Williamson, PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract. (2004) 10:307–12. doi: 10.1111/j.2002.384.doc.x
57. Coleman, ER, Moudgal, R, Lang, K, Hyacinth, HI, Awosika, OO, Kissela, BM, et al. Early rehabilitation after stroke: a narrative review. Curr Atheroscler Rep. (2017) 19:1–12. doi: 10.1007/s11883-017-0686-6
58. Behrman, A, Rutledge, J, Hembree, A, and Sheridan, S. Vocal hygiene education, voice production therapy, and the role of patient adherence: a treatment effectiveness study in women with phonotrauma. J Speech Lang Hear Res. (2008) 51:350–66. doi: 10.1044/1092-4388(2008/026)
59. Hapner, E, Portone-Maira, C, and Johns, MM III. A study of voice therapy dropout. J Voice. (2009) 23:337–40. doi: 10.1016/j.jvoice.2007.10.009
60. Leer, EV, and Connor, NP. Predicting and influencing voice therapy adherence using social–cognitive factors and mobile video. Am J Speech Lang Pathol. (2015) 24:164–76. doi: 10.1044/2015_AJSLP-12-0123
61. Leer, EV, and Connor, NP. Patient perceptions of voice therapy adherence. J Voice. (2010) 24:458–69. doi: 10.1016/j.jvoice.2008.12.009
62. Abbadessa, G, Brigo, F, Clerico, M, De Mercanti, S, Trojsi, F, Tedeschi, G, et al. Digital therapeutics in neurology. J Neurol. (2022) 269:1209–24. doi: 10.1007/s00415-021-10608-4
63. Kamoen, O, Maqueda, V, Yperzeele, L, Pottel, H, Cras, P, Vanhooren, G, et al. Stroke coach: a pilot study of a personal digital coaching program for patients after ischemic stroke. Acta Neurol Belg. (2020) 120:91–7. doi: 10.1007/s13760-019-01218-z
64. Ramsberger, G, and Marie, B. Self-administered cued naming therapy: a single-participant investigation of a computer-based therapy program replicated in four cases. Am J Speech Lang Pathol. (2007) 16:343–58. doi: 10.1044/1058-0360(2007/038)
Keywords: dysarthria, stroke, digital, speech therapy, application
Citation: Kim Y, Kim M, Kim J and Song T-J (2024) Efficacy and feasibility of a digital speech therapy for post-stroke dysarthria: protocol for a randomized controlled trial. Front. Neurol. 15:1305297. doi: 10.3389/fneur.2024.1305297
Edited by:
Simona Bonavita, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Junzi Long, Capital Medical University, ChinaGopee Krishnan, Manipal Academy of Higher Education, India
Copyright © 2024 Kim, Kim, Kim and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tae-Jin Song, knstar@ewha.ac.kr
 Yuyoung Kim
Yuyoung Kim Minjung Kim
Minjung Kim Jinwoo Kim1,2
Jinwoo Kim1,2 Tae-Jin Song
Tae-Jin Song