- 1Department of Nephrology, The Affiliated Traditional Chinese Medicine Hospital, Southwest Medical University, Luzhou, China
- 2Institute of Integrated Chinese and Western Medicine, Southwest Medical University, Luzhou, Sichuan, China
Rhabdomyolysis (RM) induced by electric blankets is exceedingly rare, with only three cases identified in our literature review. Both RM and Guillain–Barré syndrome (GBS) present with similar clinical manifestations of myalgia and muscle weakness, posing a potential challenge for accurate diagnosis in clinical settings. This report presents the case of a 22-year-old man who developed RM subsequent to the use of an electric blanket. Despite undergoing plasma exchange and renal replacement therapy, the patient continued to exhibit poor muscle strength in both lower limbs. Subsequent comprehensive evaluation revealed the presence of concurrent GBS. Following a 5-day course of intravenous gamma globulin treatment, the patient experienced rapid recovery of muscle strength and was discharged. Additionally, we reviewed seven cases from the literature of coexistent RM and GBS. This indicated that investigation of the timing of onset of muscle strength decline in RM patients could help to identify potential concurrent neurological or muscular disorders. In cases in which concurrent GBS and RM cannot be definitively ascertained during early hospitalization, prioritizing plasma exchange treatment may lead to improved patient outcomes.
1 Introduction
The causes of rhabdomyolysis (RM) usually include trauma, drugs, high temperature, strenuous exercise, toxins, and virus infections (1), but acute RM caused by overheating of electric blankets is rarely reported. Guillain–Barré syndrome (GBS) is an immune-mediated polyneurogenic neuropathy with an estimated 100,000 new cases worldwide each year (2). The typical symptom of GBS is rapidly progressive bilateral limb weakness, and some patients initially show muscle or nerve root pain (3), similar to the classic triad of RM (muscle weakness, muscle pain, and myoglobinuria) (4). Diagnosis of RM can be established through clinical manifestations and laboratory tests; however, GBS can be easily overlooked when it coexists with RM. Here, we report a rare case of acute RM caused by overheating of an electric blanket in a patient who also had GBS. Informed written consent was obtained from the patient for the publication of this case report and its associated images and tables.
2 Case presentation
We report a 22-year-old male patient who was previously healthy. One week previously, he turned the temperature of the electric blanket to the highest setting and maintained it for 24 h, then he developed nausea, vomiting, brown urine, general weakness, and muscle pain. No specific treatment was given. None of his family members had experienced similar symptoms. On the day of admission, the patient’s symptoms worsened, and he also developed cough, dyspnea, edema, palpitations, and chest tightness. Physical examination showed: temperature 36.2°C; heart rate 93 beats/min; respiration 24 beats/min; blood pressure 163/100 mmHg; poor mental status; respiratory sounds of both lungs were slightly lower, wet rhonchi could be heard in the left lower lung; lower limb edema; muscle strength of both upper limbs 3/5; and muscle strength of both lower limbs 2/5. The remaining physical examination was normal.
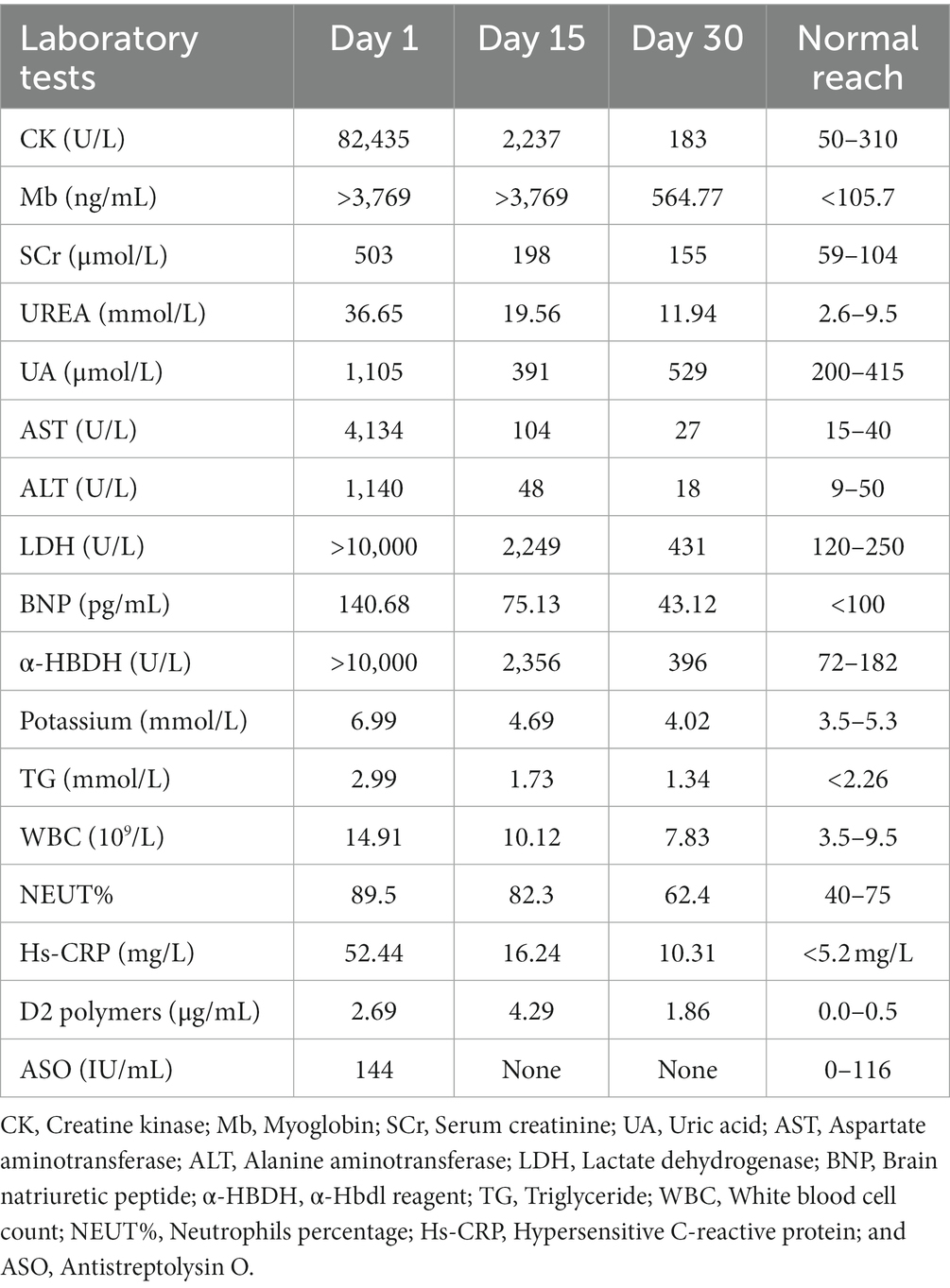
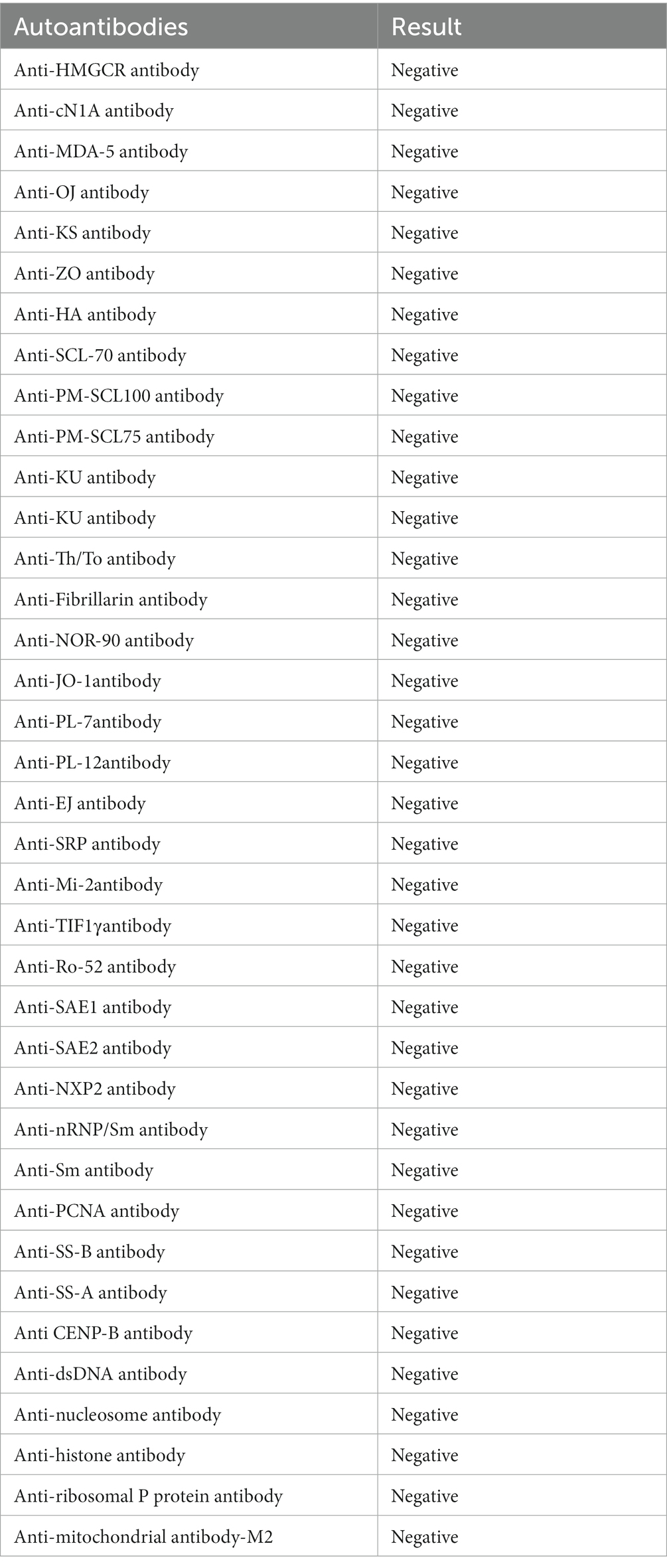
Upon admission, his blood analysis showed that white blood cell count was 14.91 × 109/L (normal range: 3.5 × 109–9.5 × 109/L), hypersensitive C-reactive protein level was 52.44 mg/L (normal range: <5.2 mg/L), and neutrophil percentage was 89.5% (normal range: 40–75%). These results showed that the patient had a systemic inflammatory reaction. To clarify the cause of inflammation, we tested him for endotoxin, (1–3)-β-D-glucan, BB virus DNA, cytomegalovirus DNA, SARS-CoV-2 DNA, sputum and urine bacterial culture, and routine stool examination; all of which were negative. Only the level of anti-streptolysin O (ASO) increased slightly: 144 IU/mL (normal range: 0–116 IU/mL). Therefore, we thought that his inflammation may have been related to streptococcal infection. Blood laboratory tests (Table 1) showed that he had acute kidney injury (AKI), hyperkalemia, hypercoagulopathy, and hyper-creatine-kinase-emia. The clinical manifestations of myoglobinuria, myalgia, myasthenia, and hyper-creatine-kinase-emia, and a history of exposure to a high-temperature environment, led us to consider that the patient had acute RM caused by heat stroke. His routine 12-lead electrocardiogram showed ventricular rhythm and peaked T waves, which we considered to be associated with hyperkalemia. Arterial blood gas analysis was suggestive of metabolic acidosis. Electromyogram showed myogenic damage to his limbs, with more severe damage in the lower extremities and proximal limbs. The results of his autoantibody profiles (Table 2), thyroid function, thoracic, abdominal and cardiac computed tomography, cardiac color Doppler ultrasonography and left ventricular systolic function measurements, and cranial magnetic resonance imaging were normal.
We used normal saline for fluid resuscitation; sodium bicarbonate for acidosis; calcium gluconate for hyperkalemia; beta-blocker to decrease blood pressure and normalize ventricular rate; meropenem (1 g every 8 h, intravenous guttae) as anti-infective treatment for 7 days; 5,000 IU low molecular weight heparin for anticoagulation; parenteral nutrition support; one plasma exchange (PE); and 11 sessions of bedside continuous renal replacement therapy. After the above treatment, most of the blood laboratory tests returned to normal on day 30 (Table 1). All organ functions were restored, and the 24-h urine output gradually increased and entered the polyuric phase. The patient did not excrete tea-colored urine, and the symptoms of muscle pain, nausea, and vomiting improved (Figure 1).
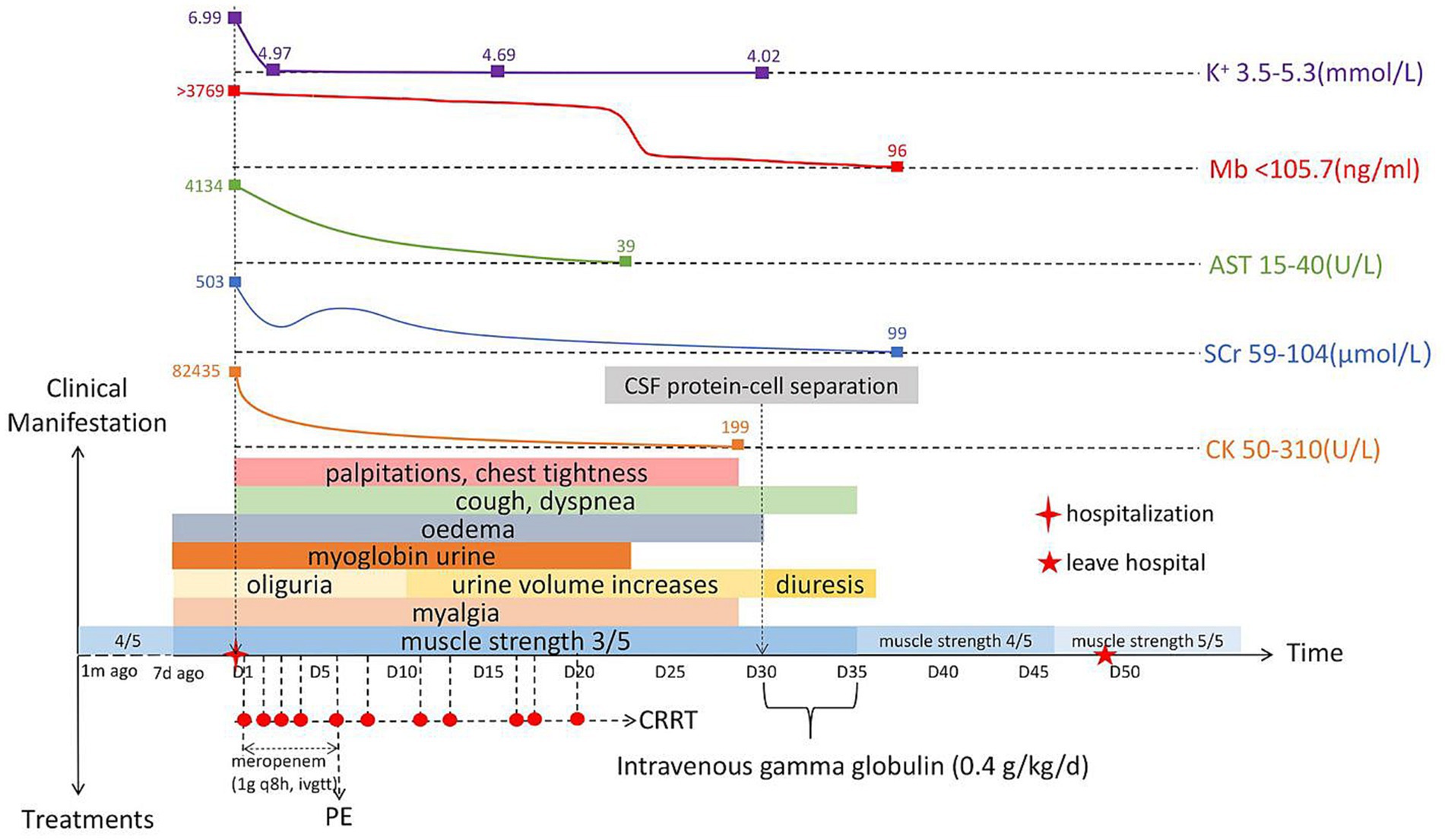
Figure 1. Timeline of the patient’s clinical manifestations, treatments, and laboratory examinations. K+, Blood potassium; Mb, Myoglobin; AST, Aspartate aminotransferase; SCr, Serum creatinine; CK, Creatine kinase; CRRT, Continuous renal replacement therapy; CSF, Cerebrospinal fluid; q8h, Every 8 h; ivgtt, Intravenous guttae; and PE, plasma exchange.
Although the laboratory test results recovered well, the symptoms of weakness in both lower limbs did not improve, and muscle strength was always 3/5. We invited the Department of Neurology to hold a consultation, and they recommended completing the inspection of the 26-item antimyositis antibody profile (Table 2), urinary organic acid, genetic metabolic disease amino acid, and acylcarnitine profiles to judge whether the patient had myopathy. However, all the above-mentioned tests were negative. ASO level was elevated and erythrocyte sedimentation rate increased. Therefore, the possibility of neurological infection could not be ruled out. We completed lumbar puncture on the day 30, cerebrospinal fluid (CSF) analysis showed that white blood cell count was 1 × 106/L (normal range: 0–8 × 106/L), and pathogens were not detected in CSF culture. However, CSF albumin was 824.0 mg/L (normal range: 100–300 mg/L) and total CSF protein 1413.0 mg/L (normal range: 150–450 mg/L). These results showed that the patient had the phenomenon of protein–cell separation in CSF. We inquired again about the patient’s medical history. He disclosed that his lower limbs had been weak 1 month before he was admitted to hospital but it did not affect his walking. He did not think it was important, so he did not tell us this key information before. We estimated that from 1 month to 7 days before admission, his lower limb muscle strength may have been 4/5. Finally, he was diagnosed with GBS by the Department of Neurology. After intravenous gamma globulin (0.4 g/kg/day) was administered for 5 days, the limb muscle strength recovered to 4/5, and to 5/5 at the time of discharge from hospital (Figure 1). He was followed up for 1 year and was recovering well.
3 Discussion
Rhabdomyolysis has been reported to occur in about a third of hospitalized patients with heatstroke (5), and the causes of heatstroke often include poor heat-dissipation mechanisms, strenuous exercise, and high environmental temperatures, and this disease usually occurs in hot weather (6). Overheating of electric blankets is a rare form of heatstroke that occurs in winter, and by reviewing the literature, we found only three patients with RM due to the use of electric blankets in winter; two patients died (7) and one survived (8). These remind us that heatstroke may also occur in cold seasons, which can induce RM.
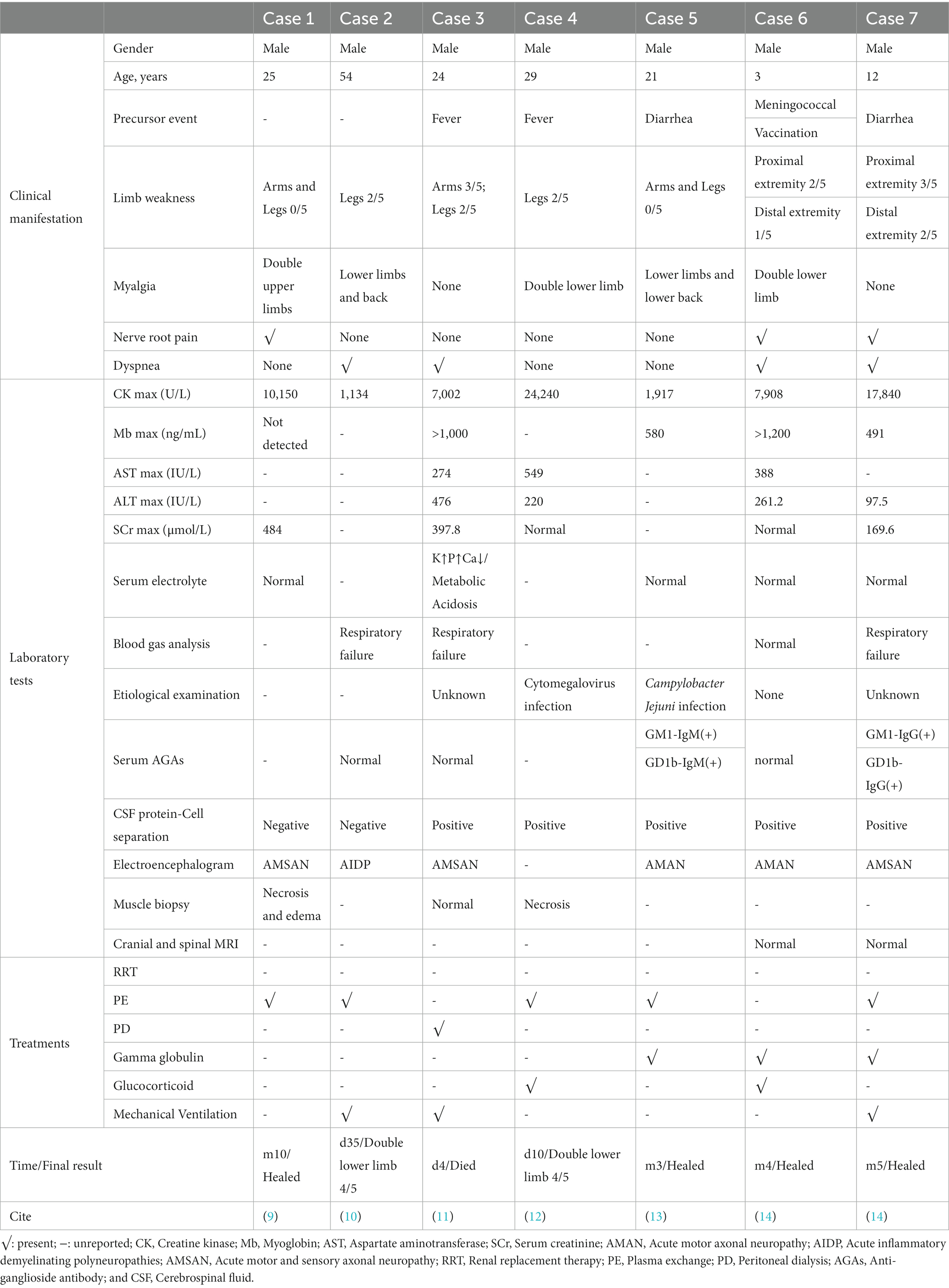
We found seven detailed reports of RM complicated with GBS by reviewing the literature (Table 3), which makes eight cases in total including the present case. All eight patients were male, which means that men are more likely to suffer from RM complicated with GBS. Six patients had precursor events (four infections, one vaccination, and one pyrexia). The proportion of male patients and patients with precursor infections was higher among GBS patients with elevated serum creatine kinase (CK) levels (15), which is in line with our results.
It is reported that streptococcal infection can be a precursor event of RM (16). Our patient’s ASO level only increased slightly, and no pathogen was cultured in the blood, which is inconsistent with the severity of RM. Therefore, in our case, the cause of RM was more likely to have been heatstroke. Almost 72% of GBS patients have previous infections (17), and streptococcal infection can also be associated with GBS (18). ASO was the only positive test in our patient. However, because no pathogens were cultured from his blood, sputum and CSF, the onset time of GBS was 1 month ago. Therefore, we can only speculate that his GBS may have been related to streptococcal infection.
Both RM and GBS can have clinical manifestations of limb weakness. Eight cases (100%) patients all developed different degrees of limb weakness. All of them had ≤2/5 muscle strength in both lower limbs and were unable to walk. This makes it difficult for us to determine whether they had GBS in the first place, which means that it is easy to miss the diagnosis in the clinic. However, we found that the symptoms of bilateral lower limbs weakness in cases 3, 5, 6, and 7 and our patient all appeared before the CK elevation, indicating that the decrease of muscle strength in these five patients is caused by GBS. This shows that we can consider whether RM patients are complicated with GBS by judging the time sequence of limb weakness and CK elevation.
Seven patients, including ours, showed pain in different regions: four with muscle pain, one with neurogenic pain, and two with combined muscle and neurogenic pain. One of the triad of RM is muscle pain. About 34.5% of GBS patients have pain symptoms, and neuropathic pain and muscle pain are the most common types of pain in GBS patients (19). This means that when RM patients have only muscle pain, it is difficult to determine whether the patient had GBS at first, which may lead to missed diagnosis. Therefore, when RM patients have pain, we need to carefully determine the nature of their pain, especially when the patient has nerve root pain, it is necessary for us to conduct further examination to confirm the presence of GBS.
Rhabdomyolysis is often accompanied by elevated serum aminotransferase levels. However, most of these increases are not from liver cell damage, and are more likely to be derived from skeletal muscle injury (20). Five patients showed varying degrees of elevated serum aminotransferase levels. All of them had severe RM (CK >5,000 U/L) but none had prior liver disease. Therefore, for patients with no previous history of liver disease and decreased muscle strength, if they also have elevated serum aminotransferase levels, we should consider whether they have liver damage or RM.
Five patients, including ours, had dyspnea, and three of them received mechanical ventilation due to respiratory failure. It is reported that about 30% of GBS patients have respiratory failure, and the main mechanism involves respiratory myasthenia, autonomic nerve involvement, and progressive bulbar palsy (21). Few patients with RM have respiratory muscle paralysis that evolves into respiratory failure (22). Therefore, when an RM patient has dyspnea or even respiratory failure caused by respiratory muscle paralysis, we should consider whether they have GBS.
Acute kidney injury is one of the serious complications of RM, with a reported incidence of 13–50%. Its pathological basis is acute tubular necrosis, and its mechanism may be related to renal vasoconstriction, tubular obstruction, and direct cytotoxicity of myoglobin (23). When RM patients are complicated with AKI, a lot of muscle cell contents dissolve into the blood, which makes electrolyte disorder and metabolic acidosis more serious. Four patients, including ours, presented with AKI. However, GBS patients rarely show an increase in serum creatinine. GBS and RM can be caused by infection (16–18), and the clinical manifestations of these two diseases are similar; therefore, it is easy to miss diagnosis. Although AKI is rare in GBS, we think that when it does occur, it is necessary to carry out further muscle enzymatic examination to determine whether the patient is complicated with RM.
Rhabdomyolysis complicated with GBS should be differentiated from diseases such as hereditary myopathies, metabolic myopathies, inflammatory myositis, and myopathies due to sepsis. Muscle biopsy, genetic or serological tests are helpful to make a definite diagnosis. In the literature, three of seven patients underwent muscle biopsy (two cases of muscle necrosis and edema, and one normal case). In our case, to rule out hereditary and metabolic myopathy, we measured the autoantibody, amino acid and acyl carnitine spectrum of hereditary, and metabolic myopathy, but did not find anything unusual. After treatment, CK level returned to normal, so we did not conduct muscle biopsy.
The management of RM is aimed at preventing AKI, and the mechanism of AKI in RM is related to urinary myoglobin. Therefore, the in vitro removal of myoglobin from the blood may be an effective method of prevention and treatment (24), such as with PE and renal replacement therapy (25). Intravenous gamma globulin and PE have been shown to be effective treatments for GBS (26). Therefore, PE may be a necessary and effective treatment for patients with RM complicated with GBS. Unfortunately, PE was not used in the only fatal case among the seven in the literature. Including our patient, six underwent PE, and all these patients recovered and were discharged successfully.
4 Conclusion
Rhabdomyolysis caused by overheating of electric blankets reminds us that heatstroke can also occur in cold winters. RM and GBS have some similar symptoms, such as muscle pain and muscle weakness. This means that when RM patients are complicated with GBS, it is easy to cause missed diagnosis at an early stage. By reviewing the literature, we found that we can consider whether RM patients are complicated with GBS by judging the time sequence of limb weakness and CK elevation. In addition, if RM patients have clinical manifestations of respiratory muscle paralysis and nerve root pain, we can make an electroneurogram or CSF puncture to help us judge whether he is complicated with GBS. If GBS patients’ serum aminotransferase and serum creatinine increasing significantly, we can conduct muscle enzyme or muscle biopsy to help us judge whether he is complicated with RM or other neuromuscular diseases. When RM is complicated with GBS, PE should be the first choice of treatment, and it can achieve a better prognosis.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of Affiliated Traditional Chinese Medicine Hospital of Southwest Medical. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
DJ: Writing – original draft. MZ: Data curation, Visualization, Writing – original draft. QH: Supervision, Writing – review & editing. QZ: Supervision, Writing – review & editing. XL: Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Science and Technology Plan Project of Sichuan Province (2022YFS0621), Science and Technology Department Project of Sichuan Province (2022NSFSC1459), and Luzhou Science and Technology Bureau Project (2021LZXNYD-D13).
Acknowledgments
We would like to thank the patient for agreeing to publish the treatment process and results of this case and allow us to comment on it. We would also like to thank the Affiliated Traditional Chinese Medicine Hospital of Southwest Medical University for its support in the treatment and diagnosis of this case.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Torres, PA, Helmstetter, JA, Kaye, AM, and Kaye, AD. Rhabdomyolysis: pathogenesis, diagnosis, and treatment. Ochsner J. (2015) 15:58–69.
2. Sejvar, JJ, Baughman, AL, Wise, M, and Morgan, OW. Population incidence of Guillain-Barré syndrome: a systematic review and meta-analysis. Neuroepidemiology. (2011) 36:123–33. doi: 10.1159/000324710
3. Willison, HJ, Jacobs, BC, and van Doorn, PA. Guillain-Barré syndrome. Lancet. (2016) 388:717–27. doi: 10.1016/S0140-6736(16)00339-1
4. Stahl, K, Rastelli, E, and Schoser, B. A systematic review on the definition of rhabdomyolysis. J Neurol. (2020) 267:877–82. doi: 10.1007/s00415-019-09185-4
5. Thongprayoon, C, Petnak, T, Kanduri, SR, Kovvuru, K, Cheungpasitporn, W, Boonpheng, B, et al. Impact of rhabdomyolysis on outcomes of hospitalizations for heat stroke in the United States. Hosp Pract. (2020) 48:276–81. doi: 10.1080/21548331.2020.1792214
6. Epstein, Y, and Yanovich, R. Heatstroke. N Engl J Med. (2019) 380:2449–59. doi: 10.1056/NEJMra1810762
7. Zhou, Y, Li, L, Liu, L, Jia, D, Zhang, X, Fowler, DR, et al. Heat stroke deaths caused by electric blankets: case report and review of the literature. Am J Forensic Med Pathol. (2006) 27:324–7. doi: 10.1097/01.paf.0000233567.51784.31
8. Gişi, K, Koksal, N, Sayarlıoğlu, M, Koroglu, S, and Çıralık, H. Heat stroke caused by electric blanket: a rare surviving case. Turk J Emerg Med. (2013) 13:86–8. doi: 10.5505/1304.7361.2013.02259
9. Scott, AJ, Duncan, R, Henderson, L, Jamal, GA, and Kennedy, PG. Acute rhabdomyolysis associated with atypical Guillain-Barré syndrome. Postgrad Med J. (1991) 67:73–4. doi: 10.1136/pgmj.67.783.73
10. Hargis, MJ. Atypical asymmetric Guillain-Barré syndrome with acute rhabdomyolysis. J Clin Neuromuscul Dis. (2017) 19:96. doi: 10.1097/CND.0000000000000175
11. Saxena, A, Singh, V, and Verma, N. Guillain-Barre syndrome complicated by acute fatal rhabdomyolysis. Ind J Crit Care Med. (2014) 18:241–3. doi: 10.4103/0972-5229.130577
12. Sakthivadivel, V, Naveenraj, P, Kachhwaha, A, Kumar, D, Anne, PB, Elhence, P, et al. Concurrent acute myositis and Guillain-Barre syndrome in cytomegalovirus infection—a rare case report. BMC Infect Dis. (2020) 20:768. doi: 10.1186/s12879-020-05506-5
13. Satoh, J, Okada, K, Kishi, T, Nagayama, S, and Kuroda, Y. Cramping pain and prolonged elevation of serum creatine kinase levels in a patient with Guillain-Barré syndrome following Campylobacter jejuni enteritis. Eur J Neurol. (2000) 7:107–9. doi: 10.1046/j.1468-1331.2000.00018.x
14. Yang, XY, Han, TL, and Lv, JL. Guillain-Barré syndrome (GBS) complicated by rhabdomyolysis (RML): case reports of 2 children and literature review. Front Pediatr. (2022) 10:1001775. doi: 10.3389/fped.2022.1001775
15. Hosokawa, T, Nakajima, H, Sawai, T, Nakamura, Y, Sano, E, Tsukahara, A, et al. Clinical features of Guillain-Barré syndrome patients with elevated serum creatine kinase levels. BMC Neurol. (2020) 20:214. doi: 10.1186/s12883-020-01796-z
16. Nordal, HH, Kittang, BR, and Bindoff, LA. Rhabdomyolysis after group C streptococcal infection. Infect Dis Rep. (2010) 2:e15. doi: 10.4081/idr.2010.e15
17. Leonhard, SE, van der Eijk, AA, Andersen, H, Antonini, G, Arends, S, Attarian, S, et al. An international perspective on preceding infections in Guillain-Barré syndrome: the IGOS-1000 cohort. Neurology. (2022) 99:e1299–313. doi: 10.1212/WNL.0000000000200885
18. Khatib, HE, Naous, A, Ghanem, S, Dbaibo, G, and Rajab, M. Case report: Guillain-Barre syndrome with pneumococcus—a new association in pediatrics. IDCases. (2017) 11:36–8. doi: 10.1016/j.idcr.2017.11.009
19. Yao, S, Chen, H, Zhang, Q, Shi, Z, Liu, J, Lian, Z, et al. Pain during the acute phase of Guillain-Barré syndrome. Medicine (Baltimore). (2018) 97:e11595. doi: 10.1097/MD.0000000000011595
20. Jo, KM, Heo, NY, Park, SH, Moon, YS, Kim, TO, Park, J, et al. Serum aminotransferase level in rhabdomyolysis according to concurrent liver disease. Kor J Gastroenterol. (2019) 74:205–11. doi: 10.4166/kjg.2019.74.4.205
21. Shang, P, Zhu, M, Baker, M, Feng, J, Zhou, C, and Zhang, HL. Mechanical ventilation in Guillain-Barré syndrome. Expert Rev Clin Immunol. (2020) 16:1053–64. doi: 10.1080/1744666X.2021.1840355
22. Hirohama, D, Shimizu, T, Hashimura, K, Yamaguchi, M, Takamori, M, Awatsu, Y, et al. Reversible respiratory failure due to rhabdomyolysis associated with cytomegalovirus infection. Intern Med. (2008) 47:1743–6. doi: 10.2169/internalmedicine.47.1349
23. Basnayake, K, Cockwell, P, and Hutchison, CA. Rhabdomyolysis and acute kidney injury. N Engl J Med. (2009) 361:1411–3. doi: 10.1056/NEJMc091538
24. Bosch, X, Poch, E, and Grau, JM. Rhabdomyolysis and acute kidney injury. N Engl J Med. (2009) 361:62–72. doi: 10.1056/NEJMra0801327
25. Gupta, A, Thorson, P, Penmatsa, KR, and Gupta, P. Rhabdomyolysis: revisited. Ulster Med J. (2021) 90:61–9.
Keywords: rhabdomyolysis, Guillain–Barré syndrome, heatstroke, electric blanket, acute kidney injury, plasma exchange
Citation: Jiang D, Zhao M, Li X, Hu Q and Zhang Q (2024) Case report and literature review: Acute rhabdomyolysis caused by overheating of electric blanket complicated with Guillain-Barré syndrome. Front. Neurol. 15:1362648. doi: 10.3389/fneur.2024.1362648
Edited by:
Federica Ginanneschi, University of Siena, ItalyReviewed by:
Penghui Wei, Qilu Hospital of Shandong University (Qingdao), ChinaShaobin Yu, Sichuan University, China
Copyright © 2024 Jiang, Zhao, Li, Hu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiongdan Hu, huqiongdan@swmu.edu.cn; Qiong Zhang, lyjean69@163.com
 Dongyang Jiang
Dongyang Jiang Ming Zhao1
Ming Zhao1 Qiongdan Hu
Qiongdan Hu