- 1Department of Neurosurgery, The First Hospital of Jilin University, Changchun, China
- 2Department of Anesthesiology, The First Hospital of Jilin University, Changchun, China
- 3Department of Neurosurgery, Tongji Shanxi Hospital, Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Third Hospital of Shanxi Medical University, Taiyuan, China
Background: Cerebrovascular disease, among the most prevalent neurological disorders, poses a substantial threat to human health with its elevated mortality and disability rates, placing considerable strain on healthcare systems. Although several studies in recent years have suggested a potential association between digestive system diseases and cerebrovascular diseases, the findings remain inconsistent.
Methods: Genome-wide association study (GWAS) summary data for 12 digestive diseases and cerebrovascular diseases were used to conduct Mendelian randomization (MR) analysis. In this investigation, we endeavored to elucidate the causal relationship between digestive system diseases and cerebrovascular diseases. Employing a comprehensive approach, including two-sample MR (TSMR), multivariate MR (MVMR), and two-step MR analysis, we leveraged summary statistics data obtained from published GWAS. The primary analysis method employed was inverse variance weighted (IVW), with MR-Egger and weighted median (WM) as secondary methods. Sensitivity analysis included heterogeneity testing, horizontal multivariate testing, MR-PRESSO, and a “leave-one-out” method. Additionally, the F-statistic was utilized to assess the strength of instrumental variables, ensuring robust results.
Results: In the TSMR analysis, this study found a significant causal relationship between genetically predicted gastroesophageal reflux disease (GERD) and any stroke (AS), any ischemic stroke (AIS), large-artery atherosclerotic stroke (LAS), intracranial aneurysm (IA), and subarachnoid hemorrhage (SAH). In MVMR analysis, this study found that even after adjusting for systolic blood pressure (SBP), body mass index (BMI) and type 2 diabetes (T2D), the causal relationship remains exist. In the two-step MR mediation analysis, it was found that BMI, SBP and T2D play mediating role in the causal relationship between GERD and cerebrovascular diseases.
Conclusion: This study indicates a clear positive causal relationship between GERD and cerebrovascular diseases, and this causal association remains significant even after adjusting for BMI, SBP and T2D. The mediation MR analysis suggests that BMI, SBP and T2D may mediate the causal relationship between GERD and the risk of cerebrovascular diseases.
Introduction
Digestive and cerebrovascular diseases are two major disease categories affecting global health, and they involve, respectively, the body’s digestive and circulatory systems. The state of health of these two systems is closely linked, and a number of studies are gradually revealing the complex relationships that may exist between them.
Digestive disorders, such as inflammatory bowel disease (IBD), liver disease, and GERD, encompass a variety of conditions that impact food digestion, absorption, and excretion. This group of diseases is increasingly prevalent worldwide, garnering significant attention within the medical community. In contrast, cerebrovascular diseases, including strokes and cerebral aneurysms, result in disruptions or abnormalities in blood supply to the brain. While traditionally associated with elderly individuals, cerebrovascular diseases are now affecting younger and middle-aged populations more frequently, imposing a substantial burden on the healthcare system. These conditions are not only a leading cause of disability and mortality (1) but also contribute significantly to the overall social health system challenges (2).
Several studies have examined the association between various digestive disorders and the risk of stroke. In a prospective study involving Taiwanese individuals, the incidence of stroke, including ischemic and hemorrhagic strokes, was notably higher in those with gallstone disease compared to those without (3). A separate prospective study investigating the relationship between peptic ulcer and stroke found that peptic ulcer increased the risk of large atherosclerotic stroke, even after adjusting for factors such as age, gender, hypertension, and diabetes (4). Furthermore, research has indicated that patients diagnosed with Crohn’s disease (CD) may have a heightened risk of stroke based on several studies (5–7). A combined analysis of patients with CD and ulcerative colitis (UC) revealed that 1.3 to 6.4% of adults with inflammatory bowel disease (IBD) and 3.3% of children with IBD experienced cerebrovascular complications (8). Similarly, Ludvigsson’s et al. (9) study identified a positive correlation between celiac disease and cerebral hemorrhage and ischemic stroke. However, conflicting findings emerged from another study, which reported no significant disparity in stroke risk between adults with celiac disease and the general population (10). Despite the collective body of literature investigating the link between digestive disorders and cerebrovascular disease, the findings are often influenced by multiple confounding variables, thus complicating the ability to establish consistent outcomes.
In traditional observational epidemiologic studies, causal inference is often hindered by confounding and the potential for reverse causation (11). However, the method of Mendelian randomization (MR) offers an alternative approach to uncovering causal relationships between exposures and outcomes. Just as in the case of Mendel’s laws of inheritance, genes are passed on randomly from parents to offspring, leading to offspring genotypes that are typically uncorrelated with confounding variables in the population. Furthermore, genotypes are established at the moment of conception and remain constant, thus circumventing the issue of reverse causation. Previous observational studies have identified a correlation between certain digestive diseases and a heightened likelihood of developing cerebrovascular disease. Our study is to explore the causal relationship between digestive system diseases and cerebrovascular diseases through two-sample MR, multivariate MR, and mediated MR. By utilizing MR, this research methodology bypasses the impact of confounding variables often present in observational studies, leading to more reliable outcomes. Consequently, these findings offer valuable insights for clinical strategies aimed at mitigating the risk of cerebrovascular disease subsequent to digestive disorders.
Methods
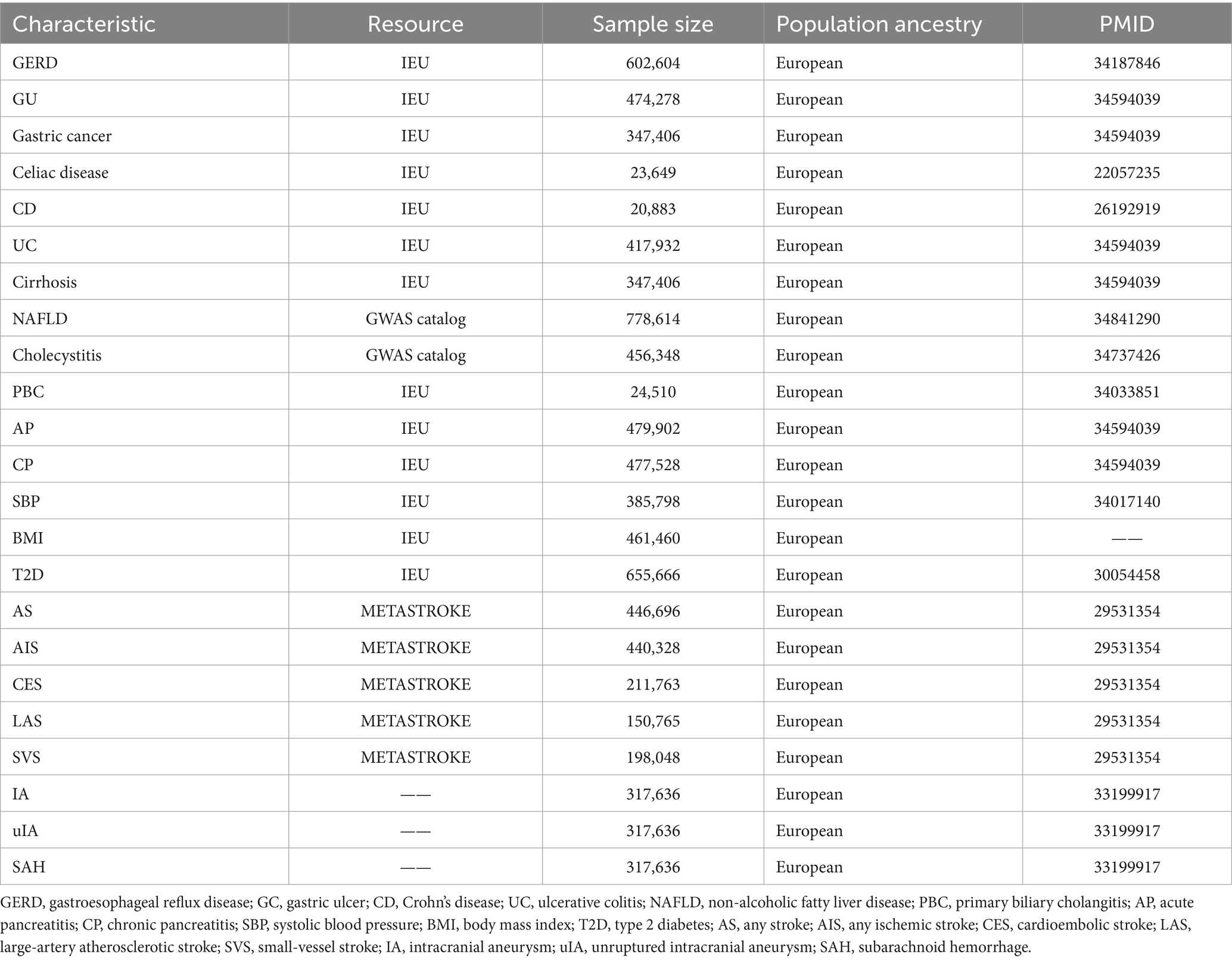
Detailed information of all GWAS data was listed in Table 1.
Digestive system diseases data source
GWAS data for GERD, gastric ulcer (GU), gastric cancer, celiac disease, UC, CD, acute pancreatitis (AP), chronic pancreatitis (CP), cirrhosis of the liver, and primary biliary cholangitis (PBC) were obtained from the Integrated Epidemiology Unit open Genome-Wide Association Study (IEU open GWAS) database (website: https://gwas.mrcieu.ac.uk/) in the present study; for non-alcoholic fatty liver disease (NAFLD), from the GWAS catalog (website: https://www.ebi.ac.uk/gwas/home); and for cholecystitis, from the UK Biobank (website: https://pan.ukbb.broadinstitute.org/). The sample size ranged from 20,883-778,614. All studies are multi-centre, large-sample, repeatedly validated gene-disease association studies conducted at the genome-wide level.
Stroke data sources
The GWAS summary data for stroke were sourced from the MEGASTROKE Consortium (12). This study comprised 29 investigations, encompassing a total of 67,162 cases and 454,450 controls. The MEGASTROKE Consortium defined stroke as a rapidly developing focal (or global) disturbance of cerebral function lasting more than 24 h or leading to death, with no apparent cause other than vascular origin. The MEGASTROKE Consortium classified strokes into AS (40,585 cases and 406,111 controls), AIS (34,217 cases and 406,111 controls), LAS (4,373 cases and 146,392 controls), CES (7,193 cases and 204,570 controls), and SVS (5,386 cases and 192,662 controls) according to the Trial of Org 10,172 in acute stroke treatment classification. To reduce population stratification bias, we restricted the stroke population to individuals of European ancestry.
Intracranial aneurysm data sources
The summary statistics for individuals of European ancestry with intracranial aneurysms were obtained from a genome-wide association study (13). This study comprised 23 cohorts, totaling 79,429 individuals. It included three combined datasets: GWAS summary data for all intracranial aneurysms (ruptured, unruptured, and unknown rupture status) (n = 7,495); for unruptured intracranial aneurysms (n = 2,070), and for aneurysmal subarachnoid hemorrhage (aSAH) (n = 5,140).
Metabolic factors data sources
The GWAS data for systolic blood pressure (SBP), body mass index (BMI) and type 2 diabetes (T2D) are sourced from the IEU GWAS database. The sample size for systolic blood pressure is 385,798, for BMI, it is 461,460, and for T2D, it is 655,666.
Study design
As shown in Figure 1, firstly, this study used two-sample Mendelian randomization (TSMR) analysis to explore the causal relationship between digestive system diseases and cerebrovascular diseases. The Bonferroni correction method was applied for multiple testing corrections, with a significance threshold set at p < 5.21 × 10−4 considered a significant causal relationship, and 5.21 × 10−4 < p < 0.05 considered a suggestive causal relationship. Secondly, a significant exposure factors identified from the TSMR results and metabolic factors (SBP, BMI and T2D) were subjected to a MVMR analysis with cerebrovascular diseases. Thirdly, a two-step MR analysis was employed to explore whether metabolic factors play a mediating role between genetically predicted digestive system diseases and cerebrovascular diseases. Separate two-sample MR analyses were conducted to investigate the causal relationships between digestive system diseases and metabolic factors, as well as between metabolic factors and cerebrovascular diseases. To determine the mediating role of metabolic factors in the causal relationship between digestive system diseases and cerebrovascular diseases, the causal effect between genetically predicted digestive system diseases and cerebrovascular diseases, referred to as the total effect, was defined as β1. The causal effect of digestive system diseases on metabolic factors was defined as β2, and the causal effect of metabolic factors on cerebrovascular diseases was defined as β3. Here, β2 × β3 represents the mediation effect, and β2 × β3/β1 represents the percentage of the mediating effect.
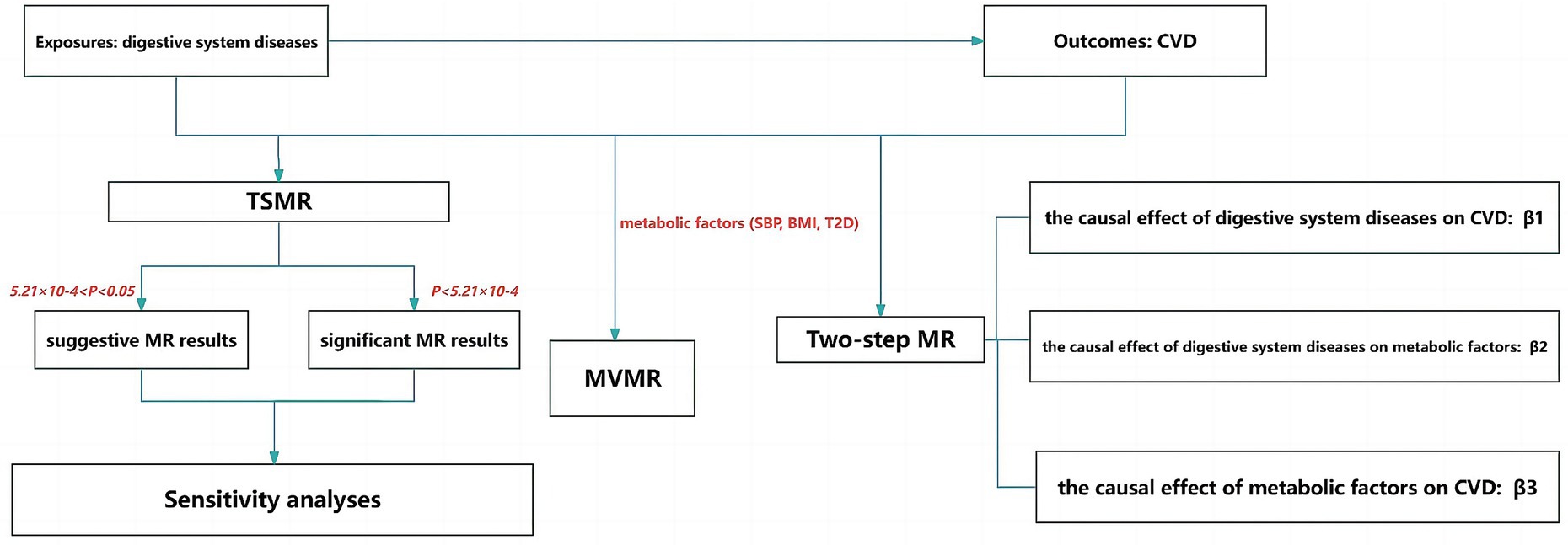
Figure 1. Flow chart of the study. TSMR, two-sample MR; MVMR, multivariate MR; CVD, cerebrovascular diseases.
Instrumental variable selection
In order for genetic variation to qualify as a valid IV for causal inference in MR studies, three core assumptions must be satisfied. First, genetic variation must be strongly correlated with exposure. Secondly, genetic variation should not be associated with confounders of the exposure and outcome relationship. Thirdly, genetic variation should only be associated with outcomes through the exposure being studied. Therefore, we select single nucleotide polymorphisms (SNPs) associated with exposure factors at the genome-wide significance level (p < 5 × 10−8). And Linkage disequilibrium (LD) was removed with R2 < 0.001 and clumping window size >10,000 kb. SNPs with minor allele frequency (MAF) ≤0.01 and with palindrome were excluded. The F-statistic (beta2/se2) was used to assess the power of every SNP. SNPs with F-statistic <10 were excluded. Finally, we excluded SNPs that were associated with confounders or outcomes according to the PhenoScanner database.1
Statistical analysis
We perform MR analysis using the TwoSampleMR package and MR-PRESSO package in R software (version 4.2.2).
In this study, many methods were employed to assess the causal relationship between digestive system diseases and cerebrovascular diseases, including the Wald ratio method, inverse variance weighted (IVW), MR-Egger method, and weighted median method (WM).
The Wald ratio method is a primary approach that uses a single SNP as IV to assess the causal relationship between exposure and outcome. Additionally, when more than one IV is available, this method can be used to calculate the causal effect for each IV. The IVW method is the main method in our study. When genetic variations are effective IVs, each variant provides a consistent estimate of the causal effect. Therefore, IVW method usually provides a consistent estimate of the causal effect of the risk factor on the outcome only when all genetic variations are effective IVs (14). If one or more variants are ineffective, the estimate may be biased (14). MR-Egger method and WM are used as secondary methods. The MR-Egger method not only provides consistent estimates of causal effects under InSIDE (INstrument Strength Independent of Direct Effect) assumption but also assesses the presence of horizontal pleiotropy. Under the InSIDE assumption, the intercept of the MR-Egger analysis can be interpreted as the average pleiotropic effect of the included genetic variations. If the average pleiotropic effect is zero, IVW method gives consistent estimates of the causal effect. Conversely, if the intercept of the MR-Egger analysis is non-zero, indicating a non-zero average pleiotropic effect, or if the InSIDE assumption is violated, it suggests bias in the IVW estimates. Additionally, testing the intercept of the MR-Egger analysis can evaluate the level of horizontal pleiotropy in the instrumental variables (15). Similarly, the weighted median method often yields robust estimates when up to 50% of SNPs are ineffective IVs (16).
Sensitivity analysis
To ensure the impartial results, a series of sensitivity analyses were performed. Cochran’s IVW Q statistic was used to quantify the heterogeneity of the IVs. And p-value >0.05 of Cochran’s IVW Q indicated no heterogeneity. Additionally, the MR-Egger regression intercept method was employed to estimate horizontal pleiotropy of SNPs, with a p-value >0.05 suggesting no horizontal pleiotropy (17). In the presence of horizontal pleiotropy, MR-Pleiotropy Residual Sum and Outlier (MR-PRESSO) were used to further detect it. If the MR-PRESSO global test indicated the presence of horizontal pleiotropy, SNPs with outliers demonstrating horizontal pleiotropy were excluded, and the IVW analysis was reperformed to decrease the heterogeneity of causal effects (18). Finally, a leave-one-out analysis was conducted as a sensitivity analysis. The leave-one-out method assesses the independent impact of each SNP on the outcome (19). This approach aimed to obtain more robust results.
Results
In exploring the genetically predicted causal association of digestive diseases as exposure factors on the risk of cerebrovascular disease, a series of IVs’ selecting processes were performed and a total of 376 SNPs were extracted from the GWAS summary data of 12 digestive system disorders, ranging from 1 to 121 SNPs. In exploring the genetically predicted causal association of metabolic factors on the risk of cerebrovascular disease, a total of 382 SNPs were extracted from the GWAS summary data of SBP, BMI and T2D, respectively. All F-statistic >10. The detailed information of IVs extracted for all exposure factors is shown in Supplementary Table S1.
Results of TSMR
MR results of digestive system diseases on stroke
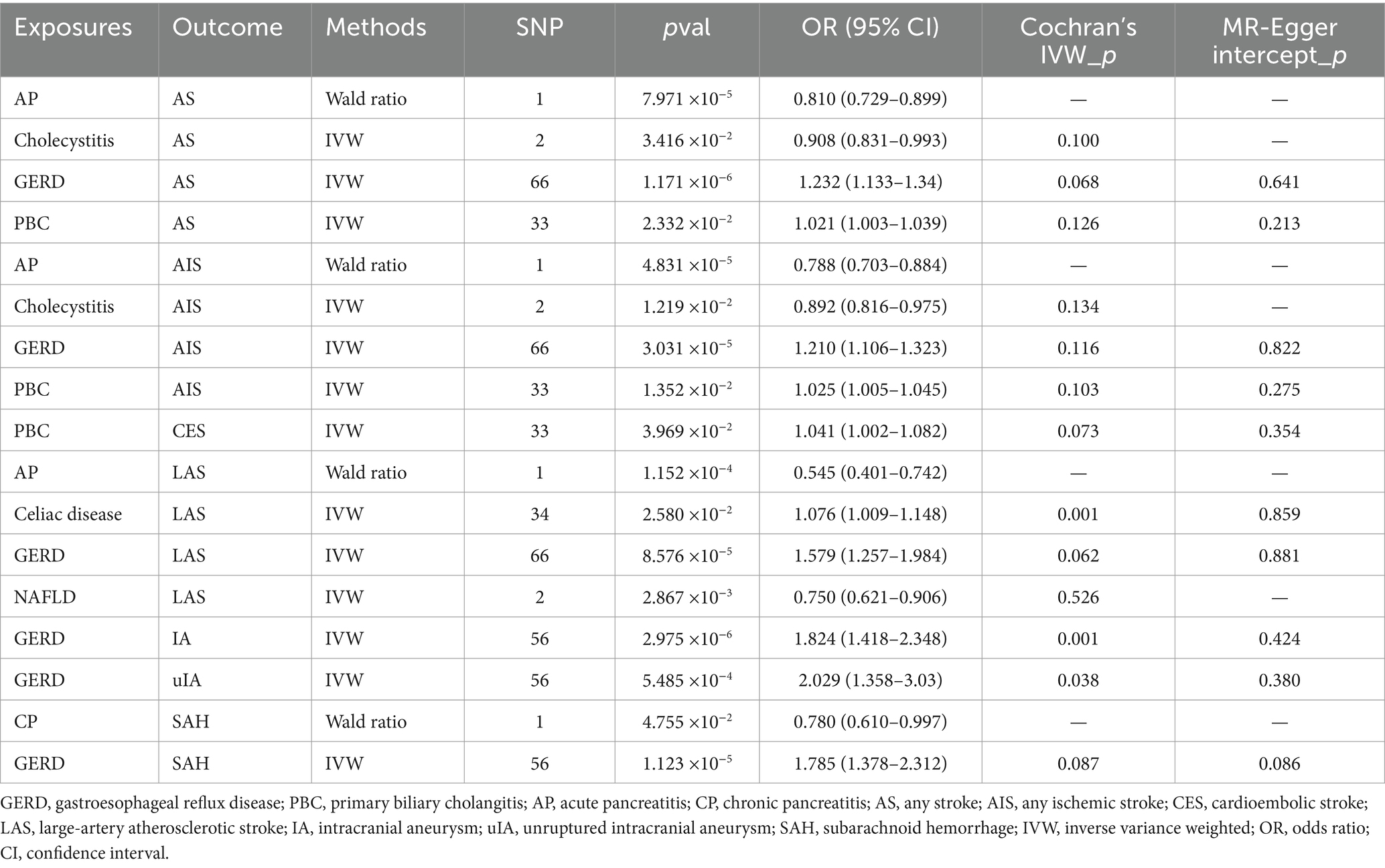
As shown in Table 2; Supplementary Tables S2, S3; Figure 2, when genetically predicted digestive system diseases were considered as the exposure factor, we observed significant positive causal relationships between gastroesophageal reflux disease (GERD) and AS (OR: 1.232, 95% CI: 1.133–1.340, p = 1.171 × 10−6), AIS (OR: 1.210, 95% CI: 1.106–1.323, p = 3.031 × 10−5), and LAS (OR: 1.579, 95% CI: 1.257–1.984, p = 8.576 × 10−5), with no evidence of heterogeneity or horizontal pleiotropy. Additionally, genetically predicted primary biliary cholangitis showed suggestive causal relationships with AS (OR: 1.021, 95% CI: 1.003–1.039, p = 0.023), AIS (OR: 1.025, 95% CI: 1.005–1.045, p = 0.014), and CES (OR: 1.041, 95% CI: 1.002–1.082, p = 0.040), with no observed heterogeneity or horizontal pleiotropy. And, cholecystitis demonstrated suggestive causal relationships with AS (OR: 0.908, 95% CI: 0.831–0.993, p = 0.034) and AIS (OR: 0.892, 95% CI: 0.816–0.975, p = 0.012), without heterogeneity, but the test for horizontal pleiotropy could not be performed with only including 2 SNPs. In the IVW method, a suggestive positive causal relationship between celiac disease and LAS (OR: 1.076, 95% CI: 1.009–1.148, p = 0.026) was observed, without horizontal pleiotropy but with heterogeneity so the weighted median method results were considered as evaluation method. However, this suggestive causal relationship disappeared in the weighted median method, indicating no causal relationship between genetic prediction of celiac disease and LAS. Genetically predicted NAFLD showed a suggestive negative causal relationship with LAS (OR: 0.750, 95% CI: 0.621–0.906, p = 0.003), with no observed heterogeneity. However, due to only including 2 available SNPs, the test of horizontal pleiotropy was not performed. Genetically predicted acute pancreatitis exhibited significant negative causal relationships with AS (OR: 0.810, 95% CI: 0.729–0.899, p = 7.971 × 10−5), AIS (OR: 0.788, 95% CI: 0.703–0.884, p = 4.831 × 10−5), and LAS (OR: 0.545, 95% CI: 0.401–0.742, p = 1.152 × 10−4). However, considering only one IV, the MR-Egger intercept and Cochran’s Q tests for its pleiotropy and heterogeneity were not feasible, and visualization of the results was not possible. Therefore, no definitive causal relationship between acute pancreatitis and AS, AIS, and LAS can be concluded.
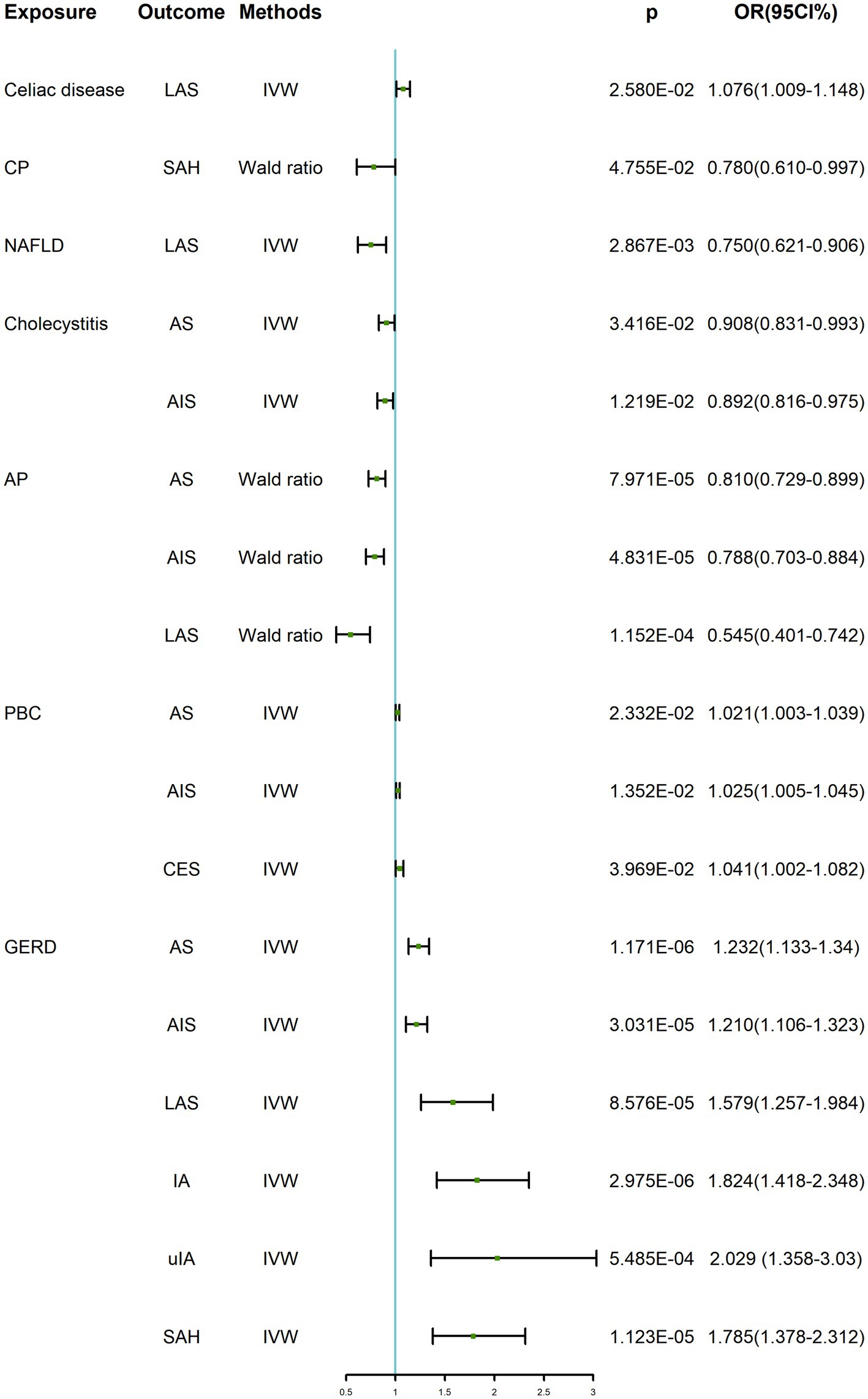
Figure 2. TSMR positive results of digestive system diseases on cerebrovascular diseases. GERD, gastroesophageal reflux disease; PBC, primary biliary cholangitis; AP, acute pancreatitis; CP, chronic pancreatitis; AS, any stroke; AIS, any ischemic stroke; CES, cardioembolic stroke; LAS, large-artery atherosclerotic stroke; IA, intracranial aneurysm; uIA, unruptured intracranial aneurysm; SAH, subarachnoid hemorrhage; IVW, inverse variance weighted; OR, odds ratio; CI, confidence interval.
MR results of digestive system diseases on intracranial aneurysms
In the IVW, we found that genetically predicted GERD presented significant positive causality with IA (OR: 1.824, 95% CI: 1.418–2.348, p = 2.975 × 10−6) and SAH (OR: 1.785, 95% CI: 1.378–2.312, p = 1.123 × 10−5), and suggestive positive causality with uIA (OR: 2.029, 95% CI: 1.358–3.030, p = 5.485 × 10−4). However, heterogeneity was found for both IA and uIA analyses, so the weighted median was suggestive; this causality remained under the weighted median approach, so the results were considered robust. In all analyses, there was no horizontal pleiotropy. In addition, there was a suggestive causal relationship between genetically predicted chronic pancreatitis and SAH (OR: 0.780, 95% CI: 0.610–0.997, p = 0.048), which was not considered to be established. Similarly, there was only one SNP, heterogeneity and horizontal pleiotropy could not be detected, and visualization of the results was not possible. All results were shown in Table 2; Supplementary Tables S4, S5; Figure 2.
Results of multivariate MR analysis
As shown in Table 3 and Figure 3, in the multivariate MR analysis, it was found that when adjusted for BMI, SBP and T2D, GERD was still associated with AS (OR: 1.274, 95% CI: 1.117–1.452, p = 3.06 × 10−4), AIS (OR: 1.250, 95% CI: 1.084–1.441, p = 2.15 × 10−3), LAS (OR: 1.488, 95% CI: 1.077–2.055, p = 1.60 × 10−2), IA (OR: 2.075, 95% CI: 1.472–2.924, p = 3.04 × 10−5), uIA (OR: 2.773, 95% CI: 1.665–4.617, p = 8.84 × 10−5), and SAH (OR: 1.784, 95% CI: 1.231–2.585, p = 3.43 × 10−4) showed a positive causal relationship.
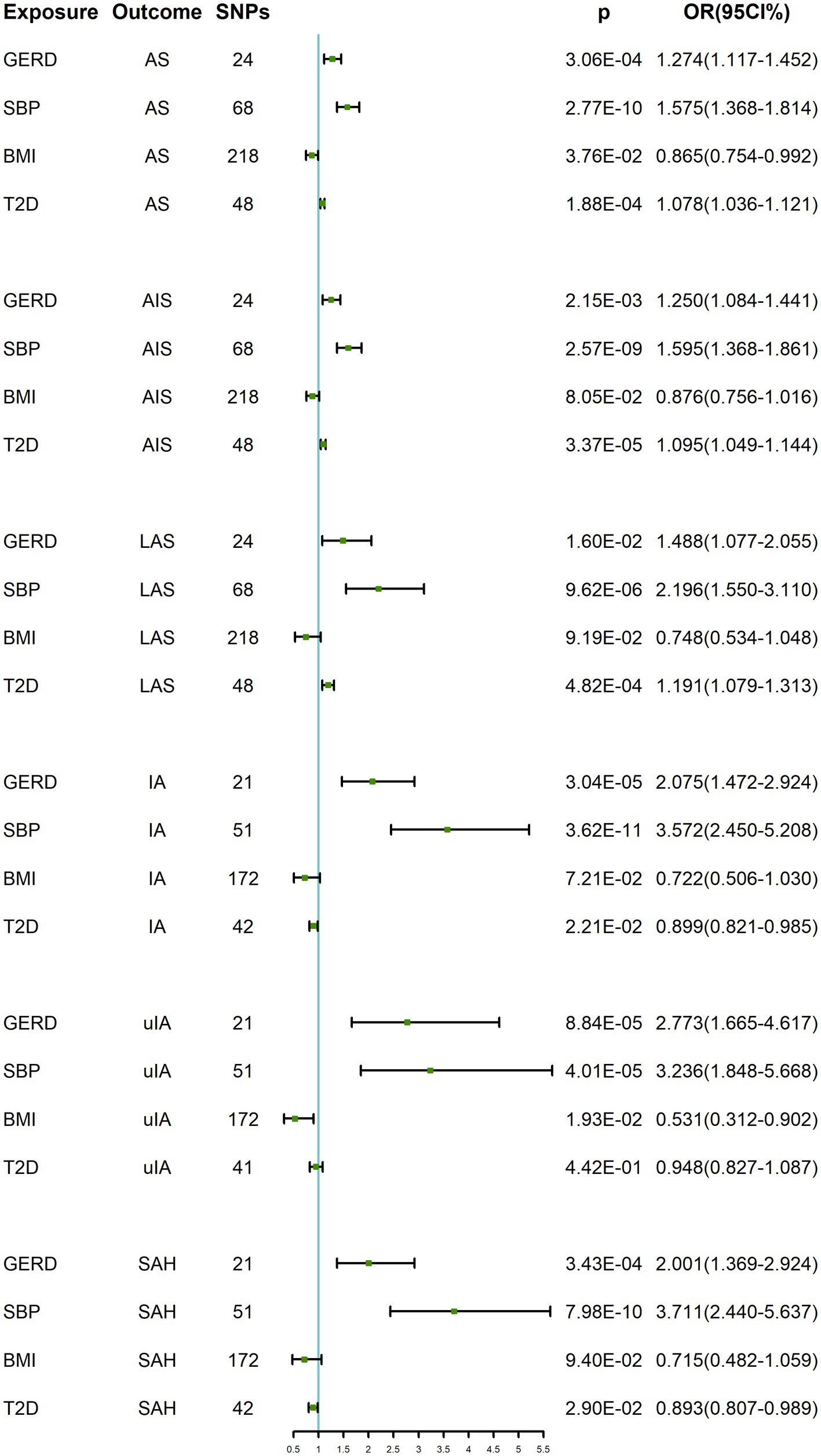
Figure 3. MVMR results of GERD on cerebrovascular diseases after adjusting for BMI, SBP and T2D. GERD, gastroesophageal reflux disease; SBP, systolic blood pressure; BMI, body mass index; T2D, type 2 diabetes; AS, any stroke; AIS, any ischemic stroke; LAS, large-artery atherosclerotic stroke; IA, intracranial aneurysm; uIA, unruptured intracranial aneurysm; SAH, subarachnoid hemorrhage; OR, odds ratio; CI, confidence interval.
Results of two-step MR analysis
MR results of GERD on metabolic factors
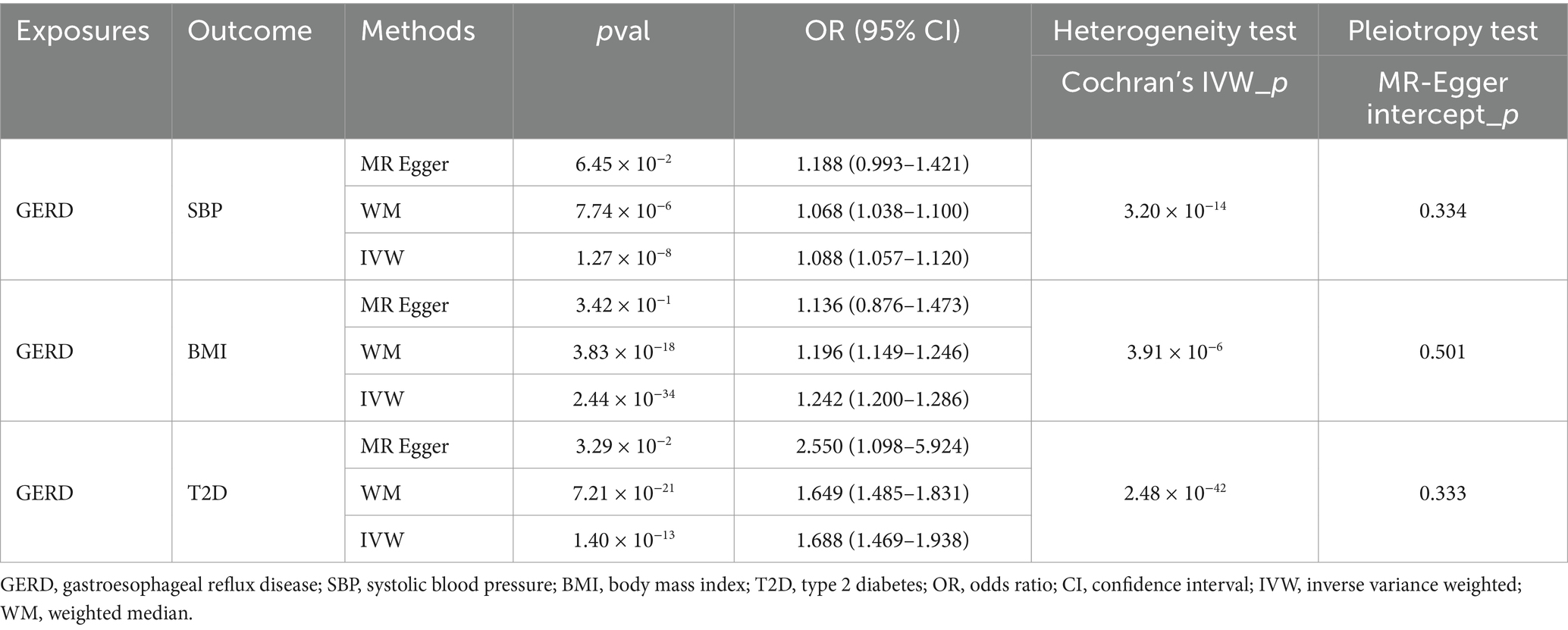
When genetically predicted GERD was used as an exposure factor, a positive causal relationship was found with both SBP (OR: 1.088, 95% CI: 1.057–1.120, p = 1.27 × 10−8), BMI (OR: 1.242, 95% CI: 1.200–1.286, p = 2.44 × 10−34) and T2D (OR: 1.688, 95% CI: 1.469–1.938, p = 1.40 × 10−13), although there was heterogeneity, the results of the weighted median approach remained significant and no horizontal pleiotropy was detected (Table 4), so the results were considered robust.
MR results of metabolic factors on cerebrovascular disease
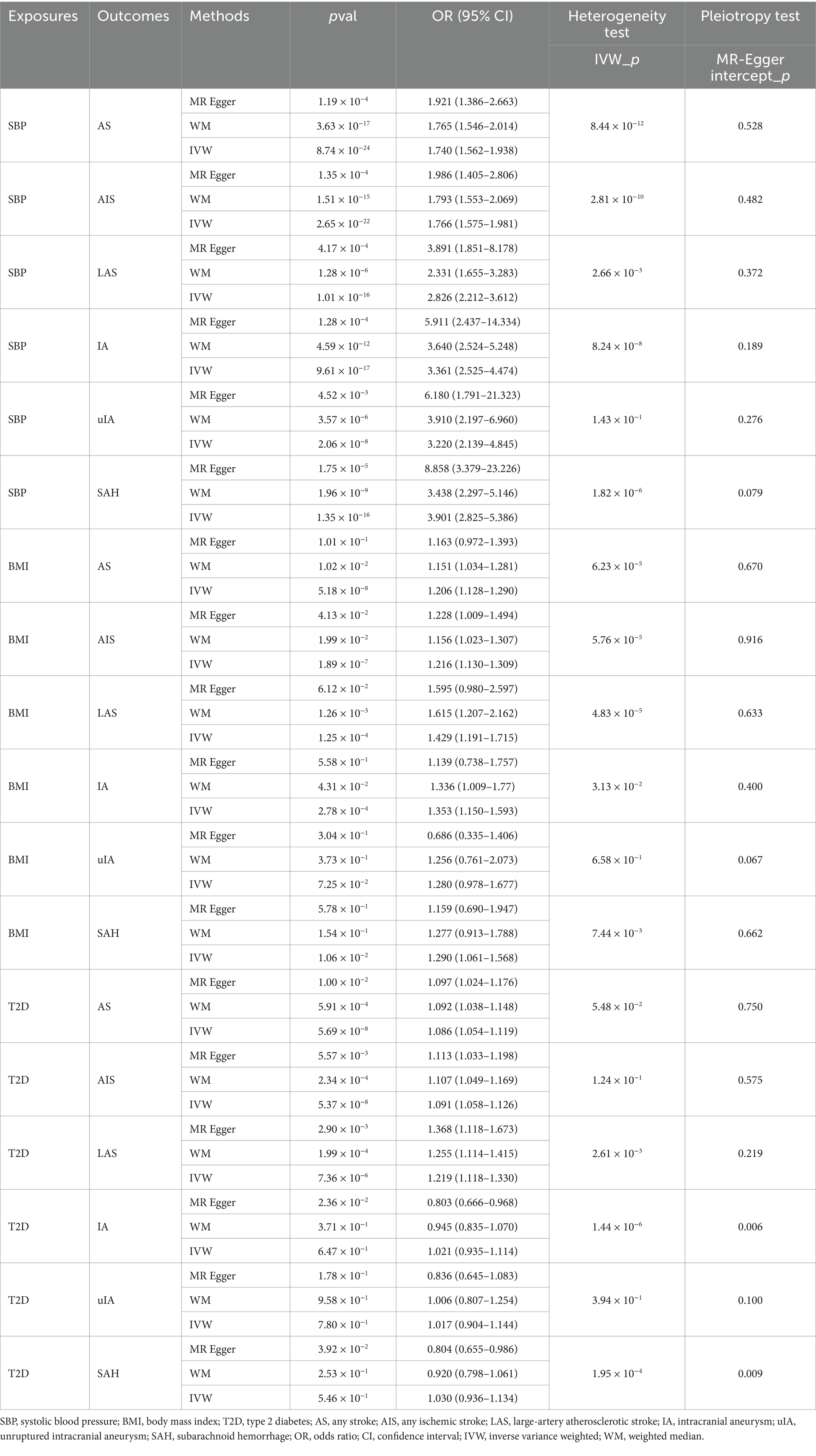
When stroke was considered as the outcome, it was found that genetically predicted SBP had a positive causal relationship with AS (OR: 1.740, 95% CI: 1.562–1.938, p = 8.743 × 10−24), AIS (OR: 1.766, 95% CI: 1.575–1.981, p = 2.649 × 10−22), and LAS (OR: 2.826, 95% CI: 2.212–3.612, p = 1.009 × 10−16). When genetically predicted BMI was considered as the exposure factor, a positive causal relationship was observed with AS (OR: 1.206, 95% CI: 1.128–1.290, p = 5.184 × 10−8), AIS (OR: 1.216, 95% CI: 1.130–1.309, p = 1.887 × 10−7), and LAS (OR: 1.429, 95% CI: 1.191–1.715, p = 1.245 × 10−4). When genetically predicted T2D was considered as the exposure factor, a positive causal relationship was observed with AS (OR: 1.086, 95% CI: 1.054–1.119, p = 5.69 × 10−8), AIS (OR: 1.091, 95% CI: 1.058–1.126, p = 5.37 × 10−8), and LAS (OR: 1.219, 95% CI: 1.118–1.330, p = 7.36 × 10−6). Although there was evidence of heterogeneity in the above analyses, the results of the weighted median method remained significant, and no horizontal pleiotropy was detected (Table 5). Therefore, the results were considered robust.
When intracranial aneurysm was considered as the outcome, it was found that SBP had a positive causal relationship with IA (OR: 3.361, 95% CI: 2.525–4.474, p = 9.605 × 10−17), unruptured IA (uIA) (OR: 3.220, 95% CI: 2.139–4.845, p = 2.055 × 10−8), and subarachnoid hemorrhage (SAH) (OR: 3.901, 95% CI: 2.825–5.386, p = 1.353 × 10−16). In the MR analysis with IA and SAH, there was heterogeneity but no horizontal pleiotropy. The results of the weighted median method still showed a significant causal relationship between them, indicating the robustness of the results. Genetically predicted BMI had a positive causal relationship with IA (OR: 1.353, 95% CI: 1.150–1.593, p = 2.779 × 10−4), with heterogeneity, but the MR results of the weighted median method remained significant, and no horizontal pleiotropy was found. Therefore, the results were considered robust. Although genetically predicted BMI had a significant causal relationship with SAH (OR: 1.290, 95% CI: 1.061–1.568, p = 0.011), there was heterogeneity. When evaluated using the weighted median method, the significance disappeared, and therefore, a causal effect between BMI and SAH could not be concluded. There was no causal relationship between genetically predicted BMI and uIA (OR: 1.280, 95% CI: 0.978–1.677, p = 0.07), and no heterogeneity or horizontal pleiotropy was observed. However, the significant causal relationship were not found in T2D on IA, uIA, and SAH (Table 5).
Results mediation MR analysis
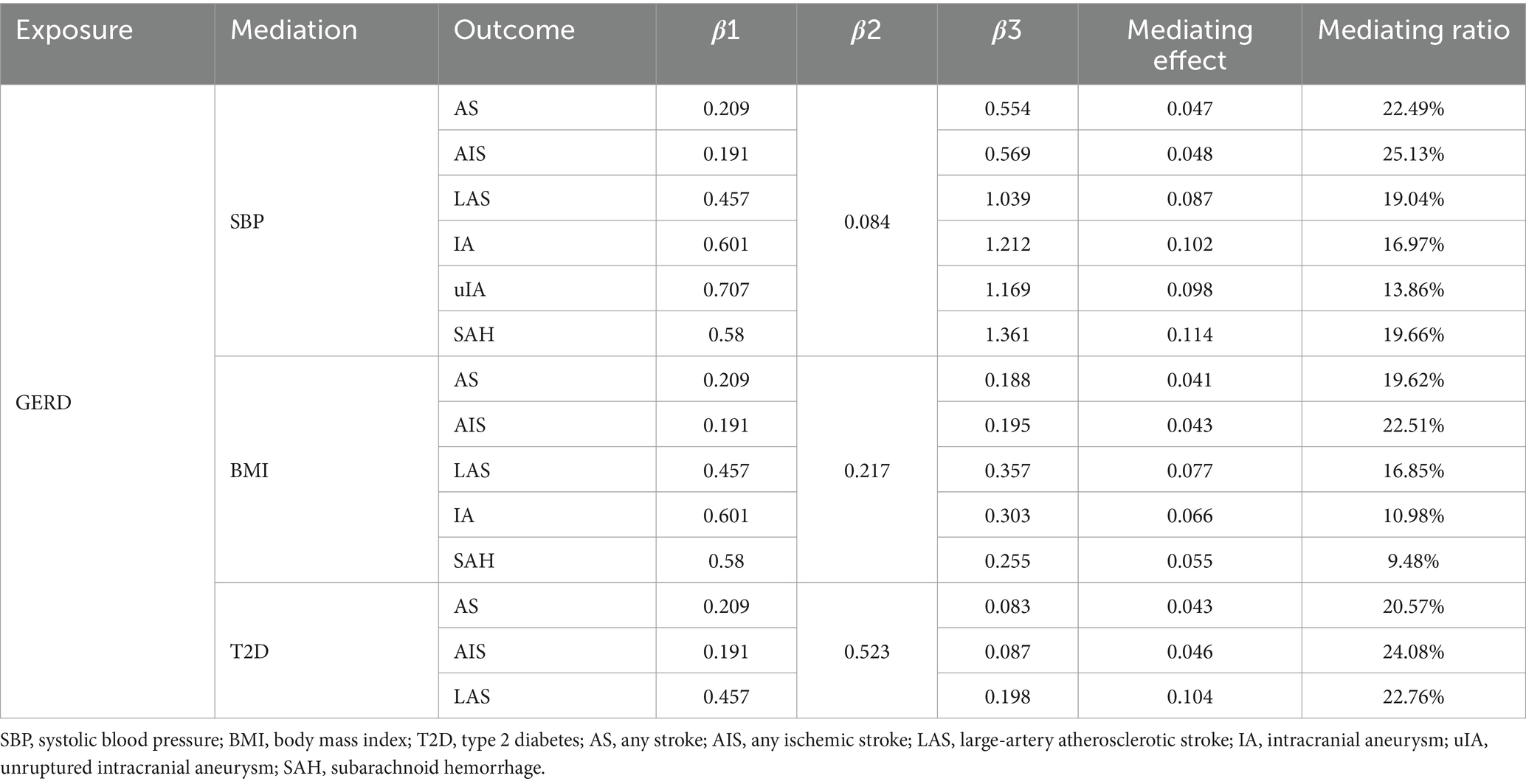
Through two-step MR analysis, it was found that when the outcome was stroke, the causal relationship between GERD and AS, AIS, and LAS was partially mediated by SBP and BMI. Specifically, the mediated effects of GERD, mediated by SBP, on AS, AIS, and LAS were 0.047, 0.048, and 0.087, accounting for 22.49, 25.13, and 19.04% of the total effects, respectively. The mediated effects of GERD, mediated by BMI, on AS, AIS, and LAS were 0.041, 0.043, and 0.077, accounting for 19.62, 22.51, and 16.85% of the total effects, respectively. The mediated effects of GERD, mediated by T2D, on AS, AIS, and LAS were 0.043, 0.046, and 0.104, accounting for 20.57, 24.08, and 22.76% of the total effects, respectively (Table 6).
When the outcome was intracranial aneurysm, SBP and BMI were also identified as factors mediating the causal relationship between GERD and intracranial aneurysm. Specifically, the mediated effects of GERD, mediated by SBP, on IA, unruptured IA (uIA), and subarachnoid hemorrhage (SAH) were 0.102, 0.098, and 0.114, accounting for 16.97, 13.86, and 19.66% of the total effects, respectively. The mediated effects of GERD, mediated by BMI, on IA and SAH were 0.066 and 0.055, accounting for 10.98 and 9.48% of the total effects, respectively (Table 6).
Discussion
This MR study investigated the causal link between digestive system diseases and the development of cerebrovascular diseases. The research findings revealed a significant causal association between gastroesophageal reflux disease (GERD) and the risk of cerebrovascular diseases. This insight underscores the genetic predisposition of GERD as a major risk factor for cerebrovascular conditions. The study’s sensitivity analysis demonstrated consistent and robust causal effects. Moreover, in the multivariable MR analysis, even upon adjusting for variables like BMI, systolic blood pressure (SBP), and type 2 diabetes (T2D), the causal relationship between GERD and cerebrovascular diseases remained evident. Additionally, the two-step MR analysis outcomes indicated that BMI, SBP, and T2D potentially act as partial mediators in the causal pathway linking GERD to cerebrovascular diseases. Therefore, this paragraph will focus solely on discussing the relationship between GERD and cerebrovascular disease.
Our MR findings are consistent with previous studies on the causal relationship between GERD and cerebrovascular diseases. Sheu et al. (20) explored the risk of stroke in young individuals within a year of GERD diagnosis. They reported that after accounting for other stroke risk factors, young individuals with GERD had a 1.68 times higher likelihood of experiencing a stroke during the one-year follow-up period compared to age- and gender-matched controls. Additionally, Jansson et al. (21) proposed a plausible link between stroke and GERD based on a population-based cross-sectional study conducted in Norway.
Several mechanisms have been proposed to explain the potential links between GERD and the increased risk of cerebrovascular diseases. Firstly, Helicobacter pylori (H. pylori) infection is a crucial mechanism. H. pylori, known as a high-risk factor for digestive diseases (22), can lead to conditions such as peptic ulcer and gastric cancer. The impact of H. pylori on the development of GERD may involve alterations in gastroesophageal barrier function, changes like reflux, or effects on gastric emptying (23). A study involving 156 patients with peptic ulcer and reflux esophagitis demonstrated that the eradication of H. pylori significantly improved GERD symptoms when compared to patients with persistent infection (24). Furthermore, cross-sectional studies have suggested a potential association between H. pylori infection and conditions like atherosclerotic coronary artery disease (25, 26) or ischemic stroke (27, 28). H. pylori can persistently infect and survive in the gastric mucosa, triggering acute and chronic inflammatory responses (22, 29, 30). These inflammatory responses can subsequently initiate and promote atherosclerosis (31). H. pylori can be categorized into two main strains depending on the presence or absence of the cytotoxin-associated gene-A (CagA) gene. Type 1 H. pylori, which possesses the CagA gene, expresses both CagA and vacuolating cytotoxin-A (VacA), whereas type 2 H. pylori lacks its expression (32). VacA has been identified to bind to cell membrane receptors, be internalized by cells, translocate to mitochondria, and trigger inflammation and apoptosis (33). Furthermore, GroEL, a newly recognized virulence factor in H. pylori, acts as a molecular chaperone protein essential for the correct folding of proteins in the bacterium (34). Studies have shown that GroEL also plays a role in the gastric colonization of H. pylori and subsequent initiation of the inflammatory response (35). A MR study examining the causal relationship between H. pylori and stroke revealed that the link between H. pylori and stroke was solely mediated by the pro-inflammatory factor C-reactive protein (CRP) (36). Previous research has consistently demonstrated that elevated CRP levels are correlated with an elevated risk of cardiovascular disease and stroke (37, 38). Several studies have demonstrated that elevated neutrophil counts are linked to increased severity of infarcts and poorer neurological outcomes (39–42). Moreover, H. pylori has the ability to recruit various immune cells, including neutrophils, macrophages, dendritic cells, T and B cells, and IL-8 (43–46). Several mechanisms may contribute to the association between leukocyte counts and increased infarct severity. Atherosclerosis is increasingly viewed as a chronic inflammatory disease (47), and one possibility is that elevated leukocyte counts precede stroke episodes, reflecting the burden of atherosclerotic disease. Leukocytosis is associated with the degree of atherosclerosis (48, 49), is a risk factor for cardiovascular events and stroke (50, 51), and is associated with stroke subtypes (50). In addition, there is evidence that leukocytosis may be associated with plaque instability and induction of acute thrombotic events. Secondly, GERD-induced stimulation of the esophagus and stomach can lead to impaired cardiac conduction signals or autonomic regulation, potentially causing arrhythmias (52). Additionally, GERD-related reflexes, such as the esophagus-heart reflex, could contribute to coronary vasoconstriction and reduced coronary blood flow, potentially leading to cardioembolic stroke (53). Thirdly, In GERD, there is a high prevalence of vagal nerve dysfunction, which is associated with delayed esophageal transit, abnormal peristalsis, and an increased frequency of transient lower esophageal sphincter relaxation (54, 55). Because the immune system directly controls the vagal nerve through the cholinergic anti-inflammatory pathway, dysfunction of the vagal nerve may trigger an exaggerated inflammatory response and the spread of inflammatory mediators into the bloodstream, potentially initiating the common atherosclerotic process and leading to cardiovascular events (56). Finally, cardiovascular autonomic dysfunction mediated by the vagus nerve in GERD patients may lead to an imbalance in the sympathetic and parasympathetic nervous systems in the cerebrovascular system. This alteration could result in a defect in the autoregulation of cerebral blood flow, thereby increasing the risk of cerebrovascular diseases (57, 58). As GERD can seriously increase the risk of atherosclerosis, it can seriously increase the burden on blood vessels, resulting in an increased prevalence of aneurysms.
Several pathological cascades of obesity-induced responses can lead to thrombosis and cardiovascular disease. Obesity is associated with increased inflammatory markers, leading to a state of low-grade chronic inflammation (59). This low-grade inflammation and systemic oxidative stress can be deleterious to endothelial cells, pushing them toward a prothrombotic state. Mechanisms such as platelet reactivity, enhanced coagulation, and impaired fibrinolysis are recognized contributors to this process (60). The most intuitive pathways involve elevated blood pressure and diabetes mellitus, both established causative factors for atherosclerosis in both large and small vessels (61). Furthermore, studies have shown that GERD is associated with various risk factors for vascular disease, including BMI, T2D, SBP, smoking, triglycerides (TG), and insomnia. GERD and obesity often coexist, with weight loss potentially improving GERD symptoms (62). Meta-analysis studies conducted in the US have identified a positive association between increased BMI and GERD incidence (63). Additionally, a recent Mendelian randomization study linked genetically predicted higher BMI, T2D, and smoking to an elevated risk of GERD (64). Turning to diabetes, it is a recognized risk factor for stroke and vascular disease through multiple mechanisms. These include increased production of free oxygen radicals and oxidative stress (65), heightened glycosylation product production (66), increased aldose reductase activity in the polyol pathway (65, 67), and activation of specific protein kinase C (PKC) isoforms (67). Diabetes can also contribute to cerebrovascular disease by inducing autonomic neuropathy, which in turn impacts cerebrovascular sympathetic and parasympathetic innervation (68, 69). Autonomic neuropathy leads to inadequate blood flow autoregulation, rendering cerebral vessels more vulnerable to damage and occlusion (70, 71). Evidence has demonstrated that diabetic autonomic neuropathy causes a consistent rise in sympathetic tone at night, resulting in elevated heart rate and blood pressure levels (72). Additionally, hypertension, a prevalent cardiovascular risk factor, is provoked by prolonged high blood pressure, promoting atherosclerosis and vascular remodeling that leads to thickened arterial walls (73). Hypertension also induces cardiac morphological changes such as left ventricular hypertrophy and left atrial dilatation, which are independent risk factors for stroke (74–76).
In addition, recent research has intensified the focus on the microbial-gut-brain axis, particularly in exploring the interplay between gut microbiota and GERD. Liu et al. (77) observed a lower prevalence of the Actinobacteria phylum among individuals with GERD (78), consistent with the findings of Wang’s et al. (79) MR analysis. Specifically, in a study involving pediatric patients with GERD, higher levels of Aspergillus and Anaplasma phyla were detected, while concentrations of Thickettsia and Actinobacteria phyla were notably reduced (80). Notably, a Japanese study employed a novel method utilizing quantitative 16S rRNA gene PCR to assess total bacterial counts. The results indicated that the relative proportions of taxa such as Aspergillus, Thick-walled Bacteria, Anaplasma, Clostridium, and Actinobacteria exhibited stronger correlations with esophageal diseases than absolute bacterial counts (77). Moreover, a separate MR study revealed a causal link between gut microbes and cerebrovascular disease (19), paving the way for further investigation into the mechanisms of the microbe-gut-brain axis.
In this study, we utilized published GWAS summary data to conduct a thorough MR analysis. This approach enabled us to assess the causal association between digestive system diseases and cerebrovascular diseases more effectively, eliminating confounding factors and reverse causality commonly found in traditional observational studies. Our study benefitted from the substantial sample sizes of all GWAS data and the genetic homogeneity of European populations included, thereby mitigating concerns related to population stratification and genetic variation. Furthermore, we employed sensitivity analysis to enhance the reliability and robustness of our MR findings. Nevertheless, it is important to acknowledge the presence of certain limitations in our study. Firstly, using instruments with an F-statistic >10 only reduces bias to less than a certain level, and the issues with weak instrument bias still occur (81). Secondly, there was significant heterogeneity present in some of the analyses which relate to directional pleiotropy. Confounding by cryptic pleiotropy is a known limitation of MR analyses (82). However, we addressed this possibility by quantifying pleiotropy and by using multiple MR approaches with different modeling assumptions regarding the use of pleiotropic variants in the analyses to further strengthen the validity of our MR models. Finally, there are other limitations, such as the generalizability of the conclusions to other ethnicities and the inability to assess selection bias due to the lack of individual-level data for binary variables.
Conclusion
This study indicates a clear positive causal relationship between GERD and cerebrovascular diseases. Moreover, this causal association remains significant even after adjusting for BMI, SBP and T2D. The mediation MR analysis suggests that BMI, SBP and T2D may mediate the causal relationship between GERD and the risk of cerebrovascular diseases.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients/participants or patients/participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
HQ: Conceptualization, Data curation, Formal analysis, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. SS: Supervision, Validation, Writing – review & editing. FY: Supervision, Validation, Writing – review & editing. PH: Data curation, Methodology, Resources, Writing – review & editing. XZ: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We gratefully acknowledge the authors and participants of all GWAS from which we used summary statistics data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1389352/full#supplementary-material
Footnotes
References
1. An, SJ, Kim, TJ, and Yoon, BW. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: an update. J Stroke. (2017) 19:3–10. doi: 10.5853/jos.2016.00864
2. GBD 2016 Causes of Death Collaborators . Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. (2017) 390:1151–210. doi: 10.1016/S0140-6736(17)32152-9
3. Wei, CY, Chung, TC, Chen, CH, Lin, CC, Sung, FC, Chung, WT, et al. Gallstone disease and the risk of stroke: a nationwide population-based study. J Stroke Cerebrovasc Dis. (2014) 23:1813–20. doi: 10.1016/j.jstrokecerebrovasdis.2014.04.024
4. Xu, Z, Wang, H, Lin, Y, Zhai, Q, Sun, W, Wang, Z, et al. The impacts of peptic ulcer on functional outcomes of ischemic stroke. J Stroke Cerebrovasc Dis. (2019) 28:311–6. doi: 10.1016/j.jstrokecerebrovasdis.2018.09.056
5. Xiao, Z, Pei, Z, Yuan, M, Li, X, Chen, S, and Xu, L. Risk of stroke in patients with inflammatory bowel disease: a systematic review and Meta-analysis. J Stroke Cerebrovasc Dis. (2015) 24:2774–80. doi: 10.1016/j.jstrokecerebrovasdis.2015.08.008
6. Keller, JJ, Wang, J, Hwang, YL, Chou, CC, Wang, LH, Hsu, JL, et al. Increased risk of stroke among patients with Crohn’s disease: a population-based matched cohort study. Int J Color Dis. (2015) 30:645–53. doi: 10.1007/s00384-015-2132-y
7. Andersohn, F, Waring, M, and Garbe, E. Risk of ischemic stroke in patients with Crohn's disease: a population-based nested case-control study. Inflamm Bowel Dis. (2010) 16:1387–92. doi: 10.1002/ibd.21187
8. Standridge, S, and de los Reyes, E. Inflammatory bowel disease and cerebrovascular arterial and venous thromboembolic events in 4 pediatric patients: a case series and review of the literature. J Child Neurol. (2008) 23:59–66. doi: 10.1177/0883073807308706
9. Ludvigsson, JF, de Faire, U, Ekbom, A, and Montgomery, SM. Vascular disease in a population-based cohort of individuals hospitalised with coeliac disease. Heart. (2007) 93:1111–5. doi: 10.1136/hrt.2006.097097
10. West, J, Logan, RF, Card, TR, Smith, C, and Hubbard, R. Risk of vascular disease in adults with diagnosed coeliac disease: a population-based study. Aliment Pharmacol Ther. (2004) 20:73–9. doi: 10.1111/j.1365-2036.2004.02008.x
11. Smith, GD, and Ebrahim, S. Data dredging, bias, or confounding. BMJ. (2002) 325:1437–8. doi: 10.1136/bmj.325.7378.1437
12. Malik, R, Chauhan, G, Traylor, M, Sargurupremraj, M, Okada, Y, Mishra, A, et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet. (2018) 50:524–37. doi: 10.1038/s41588-018-0058-3
13. Bakker, MK, van der Spek, RAA, van Rheenen, W, Morel, S, Bourcier, R, Hostettler, IC, et al. Genome-wide association study of intracranial aneurysms identifies 17 risk loci and genetic overlap with clinical risk factors. Nat Genet. (2020) 52:1303–13. doi: 10.1038/s41588-020-00725-7
14. Burgess, S, Foley, CN, and Zuber, V. Inferring causal relationships between risk factors and outcomes from genome-wide association study data. Annu Rev Genomics Hum Genet. (2018) 19:303–27. doi: 10.1146/annurev-genom-083117-021731
15. Burgess, S, and Thompson, SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. (2017) 32:377–89. doi: 10.1007/s10654-017-0255-x
16. Burgess, S, Dudbridge, F, and Thompson, SG. Combining information on multiple instrumental variables in Mendelian randomization: comparison of allele score and summarized data methods. Stat Med. (2016) 35:1880–906. doi: 10.1002/sim.6835
17. Bowden, J, Davey Smith, G, and Burgess, S. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080
18. Verbanck, M, Chen, CY, Neale, B, and Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
19. Qin, H, Yang, F, Hao, P, and Zhang, X. Gut microbiota and cerebrovascular diseases: a Mendelian randomization study. Front Microbiol. (2023) 14:1228815. doi: 10.3389/fmicb.2023.1228815
20. Sheu, JJ, Kang, JH, Lou, HY, and Lin, HC. Reflux esophagitis and the risk of stroke in young adults: a 1-year population-based follow-up study. Stroke. (2010) 41:2033–7. doi: 10.1161/STROKEAHA.110.588558
21. Jansson, C, Nordenstedt, H, Wallander, MA, Johansson, S, Johnsen, R, Hveem, K, et al. Severe symptoms of gastro-oesophageal reflux disease are associated with cardiovascular disease and other gastrointestinal symptoms, but not diabetes: a population-based study. Aliment Pharmacol Ther. (2008) 27:58–65. doi: 10.1111/j.1365-2036.2007.03537.x
22. Kurata, JH, and Nogawa, AN. Meta-analysis of risk factors for peptic ulcer. Nonsteroidal antiinflammatory drugs, Helicobacter pylori, and smoking. J Clin Gastroenterol. (1997) 24:2–17. doi: 10.1097/00004836-199701000-00002
23. Metz, DC, and Kroser, JA. Helicobacter pylori and gastroesophageal reflux disease. Gastroenterol Clin N Am. (1999) 28:971–85. doi: 10.1016/S0889-8553(05)70100-X
24. Ishiki, K, Mizuno, M, Take, S, Nagahara, Y, Yoshida, T, Yamamoto, K, et al. Helicobacter pylori eradication improves pre-existing reflux esophagitis in patients with duodenal ulcer disease. Clin Gastroenterol Hepatol. (2004) 2:474–9. doi: 10.1016/S1542-3565(04)00165-X
25. Sun, L, Zheng, H, Qiu, M, Hao, S, Liu, X, Zhu, X, et al. Helicobacter pylori infection and risk of cardiovascular disease. Helicobacter. (2023) 28:e12967. doi: 10.1111/hel.12967
26. Shmuely, H, Wattad, M, Solodky, A, Yahav, J, Samra, Z, and Zafrir, N. Association of Helicobacter pylori with coronary artery disease and myocardial infarction assessed by myocardial perfusion imaging. Isr Med Assoc J. (2014) 16:341–6.
27. Diomedi, M, Pietroiusti, A, Silvestrini, M, Rizzato, B, Cupini, LM, Ferrante, F, et al. CagA-positive Helicobacter pylori strains may influence the natural history of atherosclerotic stroke. Neurology. (2004) 63:800–4. doi: 10.1212/01.WNL.0000138025.82419.80
28. Pietroiusti, A, Diomedi, M, Silvestrini, M, Cupini, LM, Luzzi, I, Gomez-Miguel, MJ, et al. Cytotoxin-associated gene-A—positive Helicobacter pylori strains are associated with atherosclerotic stroke. Circulation. (2002) 106:580–4. doi: 10.1161/01.CIR.0000023894.10871.2F
29. Tamer, GS, Tengiz, I, Ercan, E, Duman, C, Alioglu, E, and Turk, UO. Helicobacter pylori seropositivity in patients with acute coronary syndromes. Dig Dis Sci. (2009) 54:1253–6. doi: 10.1007/s10620-008-0482-9
30. Buzás, GM . Metabolic consequences of Helicobacter pylori infection and eradication. World J Gastroenterol. (2014) 20:5226–34. doi: 10.3748/wjg.v20.i18.5226
31. Algood, HM, and Cover, TL. Helicobacter pylori persistence: an overview of interactions between H. Pylori and host immune defenses. Clin Microbiol Rev. (2006) 19:597–613. doi: 10.1128/CMR.00006-06
32. Xiang, Z, Censini, S, Bayeli, PF, Telford, JL, Figura, N, Rappuoli, R, et al. Analysis of expression of CagA and VacA virulence factors in 43 strains of Helicobacter pylori reveals that clinical isolates can be divided into two major types and that CagA is not necessary for expression of the vacuolating cytotoxin. Infect Immun. (1995) 63:94–8. doi: 10.1128/iai.63.1.94-98.1995
33. Palframan, SL, Kwok, T, and Gabriel, K. Vacuolating cytotoxin A (VacA), a key toxin for Helicobacter pylori pathogenesis. Front Cell Infect Microbiol. (2012) 2:92. doi: 10.3389/fcimb.2012.00092
34. Pan, KF, Formichella, L, Zhang, L, Zhang, Y, Ma, JL, Li, ZX, et al. Helicobacter pylori antibody responses and evolution of precancerous gastric lesions in a Chinese population. Int J Cancer. (2014) 134:2118–25. doi: 10.1002/ijc.28560
35. Bergonzelli, GE, Granato, D, Pridmore, RD, Marvin-Guy, LF, Donnicola, D, and Corthésy-Theulaz, IE. GroEL of Lactobacillus johnsonii La1 (NCC 533) is cell surface associated: potential role in interactions with the host and the gastric pathogen Helicobacter pylori. Infect Immun. (2006) 74:425–34. doi: 10.1128/IAI.74.1.425-434.2006
36. Guo, X, Tang, P, Zhang, X, and Li, R. Causal associations of circulating Helicobacter pylori antibodies with stroke and the mediating role of inflammation. Inflamm Res. (2023) 72:1193–202. doi: 10.1007/s00011-023-01740-0
37. Georgakis, MK, Malik, R, Björkbacka, H, Pana, TA, Demissie, S, Ayers, C, et al. Circulating monocyte chemoattractant protein-1 and risk of stroke: meta-analysis of population-based studies involving 17180 individuals. Circ Res. (2019) 125:773–82. doi: 10.1161/CIRCRESAHA.119.315380
38. Hong, SI, Kim, JS, Bae, HJ, and Kim, WY. C-reactive protein for stroke detection in the emergency department in patients with dizziness without neurological deficits. Front Neurol. (2021) 12:662510. doi: 10.3389/fneur.2021.662510
39. Buck, BH, Liebeskind, DS, Saver, JL, Bang, OY, Yun, SW, Starkman, S, et al. Early neutrophilia is associated with volume of ischemic tissue in acute stroke. Stroke. (2008) 39:355–60. doi: 10.1161/STROKEAHA.107.490128
40. Kim, J, Song, TJ, Park, JH, Lee, HS, Nam, CM, Nam, HS, et al. Different prognostic value of white blood cell subtypes in patients with acute cerebral infarction. Atherosclerosis. (2012) 222:464–7. doi: 10.1016/j.atherosclerosis.2012.02.042
41. Mo, X, Li, T, Ji, G, Lu, W, and Hu, Z. Peripheral polymorphonuclear leukocyte activation as a systemic inflammatory response in ischemic stroke. Neurol Sci. (2013) 34:1509–16. doi: 10.1007/s10072-013-1447-0
42. Price, CJ, Menon, DK, Peters, AM, Ballinger, JR, Barber, RW, Balan, KK, et al. Cerebral neutrophil recruitment, histology, and outcome in acute ischemic stroke: an imaging-based study. Stroke. (2004) 35:1659–64. doi: 10.1161/01.STR.0000130592.71028.92
43. Crowe, SE, Alvarez, L, Dytoc, M, Hunt, RH, Muller, M, Sherman, P, et al. Expression of interleukin 8 and CD54 by human gastric epithelium after Helicobacter pylori infection in vitro. Gastroenterology. (1995) 108:65–74. doi: 10.1016/0016-5085(95)90009-8
44. Alvarez-Arellano, L, and Maldonado-Bernal, C. Helicobacter pylori and neurological diseases: married by the laws of inflammation. World J Gastrointest Pathophysiol. (2014) 5:400–4. doi: 10.4291/wjgp.v5.i4.400
45. Xu, Y, Wang, Q, Liu, Y, Cui, R, and Zhao, Y. Is Helicobacter pylori infection a critical risk factor for vascular dementia? Int J Neurosci. (2016) 126:899–903. doi: 10.3109/00207454.2015.1081387
46. Consolazio, A, Borgia, MC, Ferro, D, Iacopini, F, Paoluzi, OA, Crispino, P, et al. Increased thrombin generation and circulating levels of tumour necrosis factor-alpha in patients with chronic Helicobacter pylori-positive gastritis. Aliment Pharmacol Ther. (2004) 20:289–94. doi: 10.1111/j.1365-2036.2004.02074.x
47. Ross, R . Atherosclerosis—an inflammatory disease. N Engl J Med. (1999) 340:115–26. doi: 10.1056/NEJM199901143400207
48. Loimaala, A, Rontu, R, Vuori, I, Mercuri, M, Lehtimäki, T, Nenonen, A, et al. Blood leukocyte count is a risk factor for intima-media thickening and subclinical carotid atherosclerosis in middle-aged men. Atherosclerosis. (2006) 188:363–9. doi: 10.1016/j.atherosclerosis.2005.11.021
49. Elkind, MS, Cheng, J, Boden-Albala, B, Paik, MC, and Sacco, RLNorthern Manhattan Stroke Study. Elevated white blood cell count and carotid plaque thickness: the northern Manhattan stroke study. Stroke. (2001) 32:842–9. doi: 10.1161/01.STR.32.4.842
50. Elkind, MS, Sciacca, RR, Boden-Albala, B, Rundek, T, Paik, MC, and Sacco, RL. Relative elevation in baseline leukocyte count predicts first cerebral infarction. Neurology. (2005) 64:2121–5. doi: 10.1212/01.WNL.0000165989.12122.49
51. Madjid, M, Awan, I, Willerson, JT, and Casscells, SW. Leukocyte count and coronary heart disease: implications for risk assessment. J Am Coll Cardiol. (2004) 44:1945–56. doi: 10.1016/j.jacc.2004.07.056
52. Cuomo, R, De Giorgi, F, Adinolfi, L, Sarnelli, G, Loffredo, F, Efficie, E, et al. Oesophageal acid exposure and altered neurocardiac function in patients with GERD and idiopathic cardiac dysrhythmias. Aliment Pharmacol Ther. (2006) 24:361–70. doi: 10.1111/j.1365-2036.2006.02987.x
53. Chauhan, A, Petch, MC, and Schofield, PM. Effect of oesophageal acid instillation on coronary blood flow. Lancet. (1993) 341:1309–10. doi: 10.1016/0140-6736(93)90817-Z
54. Cunningham, KM, Horowitz, M, Riddell, PS, Maddern, GJ, Myers, JC, Holloway, RH, et al. Relations among autonomic nerve dysfunction, oesophageal motility, and gastric emptying in gastro-oesophageal reflux disease. Gut. (1991) 32:1436–40. doi: 10.1136/gut.32.12.1436
55. Orlando, RC . Pathophysiology of gastroesophageal reflux disease. J Clin Gastroenterol. (2008) 42:584–8. doi: 10.1097/MCG.0b013e31815d0628
57. Töyry, JP, Niskanen, LK, Länsimies, EA, Partanen, KP, and Uusitupa, MI. Autonomic neuropathy predicts the development of stroke in patients with non-insulin-dependent diabetes mellitus. Stroke. (1996) 27:1316–8. doi: 10.1161/01.STR.27.8.1316
58. Ko, SH, Song, KH, Park, SA, Kim, SR, Cha, BY, Son, HY, et al. Cardiovascular autonomic dysfunction predicts acute ischaemic stroke in patients with type 2 diabetes mellitus: a 7-year follow-up study. Diabet Med. (2008) 25:1171–7. doi: 10.1111/j.1464-5491.2008.02567.x
59. Haley, MJ, and Lawrence, CB. Obesity and stroke: can we translate from rodents to patients? J Cereb Blood Flow Metab. (2016) 36:2007–21. doi: 10.1177/0271678X16670411
60. Vilahur, G, Ben-Aicha, S, and Badimon, L. New insights into the role of adipose tissue in thrombosis. Cardiovasc Res. (2017) 113:1046–54. doi: 10.1093/cvr/cvx086
61. Pantoni, L . Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. (2010) 9:689–701. doi: 10.1016/S1474-4422(10)70104-6
62. Anand, G, and Katz, PO. Gastroesophageal reflux disease and obesity. Gastroenterol Clin N Am. (2010) 39:39–46. doi: 10.1016/j.gtc.2009.12.002
63. Corley, DA, and Kubo, A. Body mass index and gastroesophageal reflux disease: a systematic review and meta-analysis. Am J Gastroenterol. (2006) 101:2619–28. doi: 10.1111/j.1572-0241.2006.00849.x
64. Yuan, S, and Larsson, SC. Adiposity, diabetes, lifestyle factors and risk of gastroesophageal reflux disease: a Mendelian randomization study. Eur J Epidemiol. (2022) 37:747–54. doi: 10.1007/s10654-022-00842-z
65. Baynes, JW, and Thorpe, SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. (1999) 48:1–9. doi: 10.2337/diabetes.48.1.1
66. Schmidt, AM, Yan, SD, Wautier, JL, and Stern, D. Activation of receptor for advanced glycation end products: a mechanism for chronic vascular dysfunction in diabetic vasculopathy and atherosclerosis. Circ Res. (1999) 84:489–97. doi: 10.1161/01.RES.84.5.489
67. Idris, I, Gray, S, and Donnelly, R. Protein kinase C activation: isozyme-specific effects on metabolism and cardiovascular complications in diabetes. Diabetologia. (2001) 44:659–73. doi: 10.1007/s001250051675
68. Morita-Tsuzuki, Y, Hardebo, JE, and Bouskela, E. Inhibition of nitric oxide synthase attenuates the cerebral blood flow response to stimulation of postganglionic parasympathetic nerves in the rat. J Cereb Blood Flow Metab. (1993) 13:993–7. doi: 10.1038/jcbfm.1993.124
69. Morita-Tsuzuki, Y, Hardebo, JE, and Bouskela, E. Interaction between cerebrovascular sympathetic, parasympathetic and sensory nerves in blood flow regulation. J Vasc Res. (1993) 30:263–71. doi: 10.1159/000159005
70. Mayhan, WG . Impairment of endothelium-dependent dilatation of cerebral arterioles during diabetes mellitus. Am J Phys. (1989) 256:H621–5. doi: 10.1152/ajpheart.1989.256.3.H621
71. Mayhan, WG, Simmons, LK, and Sharpe, GM. Mechanism of impaired responses of cerebral arterioles during diabetes mellitus. Am J Phys. (1991) 260:H319–26. doi: 10.1152/ajpheart.1991.260.2.H319
72. Karamitsos, DT, Didangelos, TP, Athyros, VG, and Kontopoulos, AG. The natural history of recently diagnosed autonomic neuropathy over a period of 2 years. Diabetes Res Clin Pract. (1998) 42:55–63. doi: 10.1016/S0168-8227(98)00089-8
73. Wiesmann, M, Zerbi, V, Jansen, D, Lütjohann, D, Veltien, A, Heerschap, A, et al. Hypertension, cerebrovascular impairment, and cognitive decline in aged AβPP/PS1 mice. Theranostics. (2017) 7:1277–89. doi: 10.7150/thno.18509
74. Benjamin, EJ, D’Agostino, RB, Belanger, AJ, Wolf, PA, and Levy, D. Left atrial size and the risk of stroke and death. The Framingham Heart Study. Circulation. (1995) 92:835–41. doi: 10.1161/01.CIR.92.4.835
75. Di Tullio, MR, Sacco, RL, Sciacca, RR, and Homma, S. Left atrial size and the risk of ischemic stroke in an ethnically mixed population. Stroke. (1999) 30:2019–24. doi: 10.1161/01.STR.30.10.2019
76. Gardin, JM, McClelland, R, Kitzman, D, Lima, JAC, Bommer, W, Klopfenstein, HS, et al. M-mode echocardiographic predictors of six- to seven-year incidence of coronary heart disease, stroke, congestive heart failure, and mortality in an elderly cohort (the Cardiovascular Health Study). Am J Cardiol. (2001) 87:1051–7. doi: 10.1016/S0002-9149(01)01460-6
77. Liu, N, Ando, T, Ishiguro, K, Maeda, O, Watanabe, O, Funasaka, K, et al. Characterization of bacterial biota in the distal esophagus of Japanese patients with reflux esophagitis and Barrett’s esophagus. BMC Infect Dis. (2013) 13:130. doi: 10.1186/1471-2334-13-130
78. Pei, Z, Yang, L, Peek, RM Jr, Levine, SM, Pride, DT, and Blaser, MJ. Bacterial biota in reflux esophagitis and Barrett’s esophagus. World J Gastroenterol. (2005) 11:7277–83. doi: 10.3748/wjg.v11.i46.7277
79. Wang, K, Wang, S, Chen, Y, Lu, X, Wang, D, Zhang, Y, et al. Causal relationship between gut microbiota and risk of gastroesophageal reflux disease: a genetic correlation and bidirectional Mendelian randomization study. Front Immunol. (2024) 15:1327503. doi: 10.3389/fimmu.2024.1327503
80. Ye, X, Yu, F, Zhou, J, Zhao, C, Wu, J, and Ni, X. Analysis of the gut microbiota in children with gastroesophageal reflux disease using metagenomics and metabolomics. Front Cell Infect Microbiol. (2023) 13:1267192. doi: 10.3389/fcimb.2023.1267192
81. Burgess, S, and Thompson, SGCRP CHD Genetics Collaboration. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. (2011) 40:755–64. doi: 10.1093/ije/dyr036
Keywords: digestive system diseases, stroke, intracranial aneurysm, Mendelian randomization, causal relationship
Citation: Qin H, Suo S, Yang F, Hao P and Zhang X (2024) The role of digestive system diseases in cerebrovascular disease: a comprehensive Mendelian randomization study. Front. Neurol. 15:1389352. doi: 10.3389/fneur.2024.1389352
Edited by:
Chunyu Li, Sichuan University, ChinaReviewed by:
Kimia Jazi, Qom University of Medical Sciences, IranXinglong Yang, The First Affiliated Hospital of Kunming Medical University, China
Copyright © 2024 Qin, Suo, Yang, Hao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xianfeng Zhang, zhangxianf@jlu.edu.cn
 Hao Qin
Hao Qin Shihuan Suo1
Shihuan Suo1 Fan Yang
Fan Yang Xianfeng Zhang
Xianfeng Zhang