- Cognition and Brain Sciences Unit, Medical Research Council, Cambridge, UK
What determines the laterality of activation in motor cortex for words whose meaning is related to bodily actions? It has been suggested that the neuronal representation of the meaning of action-words is shaped by individual experience. However, core language functions are left-lateralized in the majority of both right- and left-handers. It is still an open question to what degree connections between left-hemispheric core language areas and right-hemispheric motor areas can play a role in semantics. We investigated laterality of brain activation using fMRI in right- and left-handed participants in response to visually presented hand-related action-words, namely uni- and bi-manual actions (such as “throw” and “clap”). These stimulus groups were matched with respect to general (hand-) action-relatedness, but differed with respect to whether they are usually performed with the dominant hand or both hands. We may expect generally more left-hemispheric motor cortex activation for hand-related words in both handedness groups, with possibly more bilateral activation for left-handers compared to right-handers. In our study, both participant groups activated motor cortex bilaterally for bi-manual words. Interestingly, both groups also showed a left-lateralized activation pattern to uni-manual words. We argue that this reflects the effect of left-hemispheric language dominance on the formation of semantic brain circuits on the basis of Hebbian correlation learning.
Introduction
The question of how our experience with the world shapes the representation of concepts and meaning in the human mind and brain, or how semantic knowledge is “embodied,” is a matter of continuous intense debate (Harnad, 1990; Barsalou, 1999; Wilson, 2002). In neuroscience, this debate has led to the related question of whether, and how, perceptual and motor areas of the brain activate during, and contribute to, semantic and conceptual processing. Some authors have made the strong claim that these perceptuo-motor areas are essential elements of semantic representations (Barsalou, 1999; Pulvermüller, 1999; Kiefer and Spitzer, 2001; Glenberg and Kaschak, 2002; Kiefer et al., 2008; Kiefer and Pulvermuller, 2011). It has been suggested that semantic representations are stored in widely distributed brain networks, whose topographies reflect the sensory and motor experiences associated with concepts during language acquisition (Hebb et al., 1971; Braitenberg and Pulvermüller, 1992). Although a number of studies have shown by now that perceptuo-motor systems are activated during the retrieval of semantic information, the influence of individual experience and the type of sensorimotor experience on these distributed networks is not yet fully understood.
Several studies have already shown that parts of the motor system are involved in conceptual processing for objects and words (Decety and Grezes, 1999; Fadiga and Craighero, 2004; Pulvermüller, 2005; Martin, 2007; Hauk et al., 2008b; Cattaneo and Rizzolatti, 2009). Furthermore, there is growing evidence that experience with actions, for example in basketball players, ice hockey players, or dancers, shapes the way we understand actions performed by others (Calvo-Merino et al., 2006; Cross et al., 2006; Kiefer et al., 2007; Weisberg et al., 2007; Aglioti et al., 2008; Rocca et al., 2008; Willems and Hagoort, 2009; Hoenig et al., 2011) and language comprehension as well (Beilock et al., 2008; Lyons et al., 2010). The effect of experience on word semantics is less clear. Compelling evidence in favor of embodied semantic representations stems from experiments on action-words referring to actions of different effectors (“pick,” “kick,” “lick”; Pulvermüller et al., 2001; Hauk et al., 2004; Pulvermuller et al., 2009). Although motor activation during language comprehension has been described as inconsistent (Postle et al., 2008), a recent review showed surprising consistencies of semantic-somatotopic activations reported from different labs for different languages (Kemmerer and Gonzalez-Castillo, 2010). The model of “somatotopy of action-words” led to precise functional–anatomical hypotheses that were tested with most available neuroimaging techniques (Pulvermüller, 2005).
The lateralization of word-evoked activity in cortical motor areas, and the factors that determine it, are still not clear. Consistent results were obtained for activity in the left language-dominant hemisphere in a range of fMRI studies looking at words and sentences (Tettamanti et al., 2005; Aziz-Zadeh et al., 2006; Ruschemeyer et al., 2007; Kemmerer et al., 2008; Boulenger et al., 2009; Raposo et al., 2009). Also, a transcranial magnetic stimulation (TMS) study on action-word processing reported left-hemispheric facilitation of responses to hand-words after hand–motor cortex stimulation in a lexical decision task, but only after left- compared to right-hemispheric stimulation (Pulvermüller et al., 2005a). Tomasino et al. (2008) found a similar effect in a mental imagery task, but not in silent reading. Activation to action-words evoked in the right non-dominant hemisphere has been less consistent across studies. Hauk et al. (2004) found bilateral activation for all three action-word types. Tettamanti et al. (2005) reported activation for action-related auditory sentences only in the left hemisphere, except for an area in right middle temporal cortex activated by hand-related sentences. Aziz-Zadeh et al. (2006) found a somatotopic pattern for literal visually presented action-phrases only in the left hemisphere. Similarly, Boulenger et al. (2009) reported somatotopy for action-sentences only for the left hemisphere, but for both literal and idiomatic sentences. In the study of Raposo et al. (2009), hand-words in isolation and in literal action-sentences activated cortical areas in both hemispheres, while leg-words activated the left hemisphere only in isolation, but bilaterally in literal sentence context. Kemmerer et al. (2008) investigated several sub-categories of action-verbs. They reported left-lateralized activation in cortical motor areas for “speaking verbs” and “change-of-state” verbs, the categories “running verbs,” “hitting verbs,” and “cutting verbs” produced bilateral activation. Ruschemeyer et al. (2007) found activation for hand-related words along (post/pre)central gyrus not only in the left hemisphere, but also in right postcentral gyrus. Electrophysiological studies show similar inconsistencies. The ERP studies of Pulvermüller et al. (2001) and Hauk and Pulvermüller (2004) suggested bilateral activation of motor areas. However, using TMS Pulvermüller et al. (2005a) found effects on hand- and leg-word processing only in the left hemisphere, and leg- and face-word-specific activation was localized to the left hemisphere using magnetoencephalography (Pulvermüller et al., 2005b).
Two factors are of particular interest with respect to the lateralization of motor cortex activation in action-word comprehension: (1) General language dominance, i.e., the fact than in most left- and right-handers core language functions are located in the left cerebral hemisphere (e.g., Knecht et al., 2000); and (2) individual experience with the corresponding actions, i.e., the way we perform them ourselves or observe them in our environment. A promising way to study these factors is to test types of actions that are performed differently between different participant groups, such as hand-action-words in right- and left-handers. In a recent study, Willems et al. (2009) presented words relating to actions commonly performed with the dominant hand to left- and right-handers, while measuring the BOLD response in a lexical decision task. Based on their results, these authors suggest that hand-related action-words activated premotor cortex (BA6) more strongly in the left hemisphere for right-handers, and more strongly in the right hemisphere for left-handers. This provides evidence for embodiment of semantic processing, and supports the view that embodied semantic representations of action-words depend on the individual experiences with the corresponding actions. However, peaks of activation were scattered across large parts of area BA6, in particular in the right hemisphere. It is therefore not clear whether it reflected activity specific to hand-actions in hand-motor cortex. A more fundamental question in this context is whether semantic networks between core areas in the left hemisphere and right motor cortex in isolation, i.e., not in combination with its left-hemispheric counterpart, are plausible and possible.
Associationist theories of word meaning assume that semantic networks for action-words are formed during language development by means of Hebbian principles (Hebb et al., 1971; Pulvermüller, 2005): Connections between core language areas and the corresponding cortical motor areas are strengthened when the child hears the action-word and either performs the action herself or observes somebody else doing it. With respect to action execution and observation, there is ample evidence that both activate similar cortical motor areas (see Rizzolatti and Craighero, 2004; Cattaneo and Rizzolatti, 2009, for reviews), which is often interpreted as support for the theory that we map observed actions onto our own motor repertoire. Lateralization of this activation is commonly assumed to be contralateral with respect to the effector, although lateralization has been reported to be smaller for complex movements, in particular in left-handers (Solodkin et al., 2001). A similar pattern of results was obtained for action observation in right- and left-handers (Rocca et al., 2008). Both left- and right-handers activate bilateral motor areas during bi-manual action execution (Solodkin et al., 2008). To our knowledge, bilateral motor cortex activation for left- and right-handers during observation of bi-manual movements has not been directly demonstrated yet. However, in the light of the evidence for uni-manual action observation, and in the context of the mirror neuron framework, this is a reasonable assumption.
How does this translate to predictions for brain activation patterns during action-word comprehension? If – as claimed by the above-mentioned associationist theories – the meaning of action-words is represented by strengthened connections between core language areas and motor cortex, then we should expect stronger connections in the language-dominant left hemisphere. For uni-manual action-words in right-handers, this leads to a straightforward prediction: Reading of these words should lead to left-lateralized activation in motor cortex. For bi-manual words, we predict that both right- and left-handers should produce more bilateral activation than for uni-manual words. Language dominance may still result in stronger activation in the left hemisphere, but the fact that left and right motor cortices are co-activated during bi-manual movements may result in an activation of the whole cell assembly, even if only left motor cortex is directly connected to core language areas. The most interesting case are uni-manual action-words for left-handers: Do their neuronal representations comprise cross-hemispheric connections between language and motor areas? Correlated activation in left-hemispheric perisylvian language areas and right-hemispheric hand-motor cortex might be more difficult to map onto each other, because there are no direct cortico-cortical links between non-homotopic areas of the hemispheres. We therefore argue that it is likely that even for left-handers, we will observe left-lateralized activation to uni-manual action-words in motor cortex. This would support theories that assume correlation learning as the basis for the formation of semantic brain circuits.
Materials and Methods
Participants
Data from 21 healthy native English speakers entered the analysis, comprising two groups of 10 right-handers (RHds) and 11 left-handers (LHds), respectively. They had normal or corrected-to-normal vision and no history of neurological or psychiatric disorder. The mean age of all participants was 25.8 (RHds: 24; LHds: 27.4) years (SD 4.8; RHds: 3.8; LHds: 5.2). Most of the participants were pre-selected from the CBU Volunteer Panel. Handedness was then determined by a 10-item version of the Edinburgh handedness inventory (RHds: 87; LHds: −82; Oldfield, 1971). Furthermore, handedness was confirmed in a rating experiment on the 55 uni-manual words that actually occurred in the experiment (see below for details on stimuli). Participants were asked “Which hand would you use to perform these actions?” and could give ratings for each word on a scale from 1 (“always left”) to 7 (“always right”; 4 for “both”). In this context, “both” meant that the corresponding action had to be performed using both hands at the same time. Right-handers had a mean rating of 5.7 (SD 0.4), and left-handers of 2.1 (SD 0.5). Participants were paid for their participation. Ethical approval was obtained from the Cambridge Local Research Ethics Committee.
Materials
The word stimuli consisted of 110 hand-action-related English words, which could be sub-divided into “uni-manual” (UM; actions most commonly performed with the dominant hand only, e.g., “throw,” n = 55), and “bi-manual” (BM; actions most commonly performed with both hands, e.g., “clap,” n = 55), see Table A1 in Appendix. The experiment also contained 55 leg-related words (actions that are most commonly performed with the legs, e.g., “walk”), 100 non-action-related words, and 100 pronounceable pseudowords, which will not be the focus of this study. 165 strings of hash marks (e.g., “####”) were used as a low-level baseline for the contrasts of interest. They were matched for length to UM, BM, and leg-words. This resulted in a total of 530 stimuli.
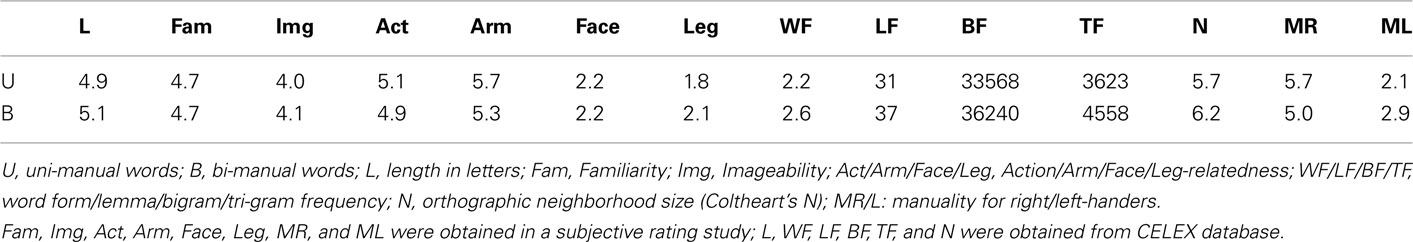
UM and BM words were matched on several relevant psycholinguistic variables by means of two-tailed two-sample t-tests, obtained from the CELEX psycholinguistic data base [number of letters, word form/lemma, and bi-/tri-gram frequencies, orthographic neighborhood size (“Coltheart’s N”)]. In a separate rating study using a different group of right-handed participants, we evaluated and matched our stimuli along the dimensions familiarity, imageability, and action-relatedness [e.g., “How familiar is this word to you, e.g., do you use or hear it frequently? (7 = very familiar; 1 = not familiar at all)” or “Does this word remind you of an action you could perform yourself? (1: does not remind me of an action I can perform myself at all; 7: reminds me very much of an action I can perform myself)”]. Two-tailed two-sample t-tests did not reveal any significant differences between UM and BM words for these variables. The length of UM and BM words ranged from three to nine letters (average: 5.0 letters). “Manuality” was assessed for UM and BM words in separate rating studies before (with different right-handed participants) and after (with the same participants) the fMRI experiment. Participants were asked to answer the question “Which hand would you use to perform these actions?” on a 7-point scale (1: always left; 4: both; 7: always right). In this context, “both” referred to both hands simultaneously. The first rating study established that UM words were significantly more associated with the dominant hand than BM words (5.4 vs. 4.6, p = 0.001). The post-experiment rating revealed that this was the case for both right- (5.7 vs. 5.0) and left-handers (2.1 vs. 2.9) separately, and that UM words differed between right- and left-handers (5.7 vs. 2.1; all p < 0.001). Interestingly, also BM words differed in their manuality ratings between right- and left-handers (5.0 vs. 2.9, p < 0.001), indicating that BM words had a similar bias toward the dominant hand for both participant groups. This could be due to the fact that some bi-manual actions involve a “dominant” and a “non-dominant” hand. For example, opening a jar requires one rather passive hand holding the jar, and one actively unscrewing the lid. This may be reflected in biased ratings toward the dominant hand. The mean values for the psycholinguistic variables used in the matching process are presented in Table 1.
Procedure
The main experiment was run in two blocks of approximately 11 min duration. Stimuli were presented in an event-related design, with stimulus duration of 150 ms and a stimulus onset asynchrony of 2.5 s. Participants were instructed to silently but attentively read the stimuli. They were not informed about the purpose of the experiment until after the fMRI session. Stimuli were presented in a randomized order by means of DMDX software and viewed via a back-projection screen located in front of the scanner and a mirror placed on the head coil. Stimuli fell within a visual angle of less than 4°.
The language session was followed by a motor localizer session of about 8 min duration. Participants were presented visual cues (each for 20 s) indicating a particular body part (left finger, right finger, left foot, right foot, tongue), and had to move the corresponding body part (index finger in the case of fingers) for as long as the cue was on the screen. They were instructed not to move any other part of the body, in particular not the head. The cue “rest” indicated that participants should not move at all. Each cue type was presented four times in randomized order.
Imaging Methods
Participants were scanned in a 3-T Siemens (Munich, Germany) Tim Trio magnetic resonance system using a head coil. Echo-planar imaging (EPI) sequence parameters were TR = 2 s, TE = 30 ms, and flip angle = 78°. The functional images consisted of 32 slices covering the whole-brain (slice thickness 3 mm, interslice distance 0.75 mm, in-plane resolution 3 mm × 3 mm). Imaging data were processed using SPM5 software (Wellcome Department of Imaging Neuroscience, London, UK1).
Images were corrected for slice timing and then realigned to the first image using sinc interpolation. Any non-brain parts were removed from the T1-weighted structural images by using a surface-model approach (“skull-stripping”; Smith, 2002). The EPI images were coregistered to these skull-stripped structural T1 images by using a mutual information coregistration procedure (Maes et al., 1997). The structural MRI was normalized to the 152-subject T1 template of the Montreal Neurological Institute (MNI). The resulting transformation parameters were applied to the coregistered EPI images. During the spatial normalization process, images were resampled with a spatial resolution of 2 mm × 2 mm × 2 mm. Finally, all normalized images were spatially smoothed with a 10-mm full-width half-maximum Gaussian kernel. This sequence of pre-processing steps was automated using software tools developed at the Cognition and Brain Sciences Unit2.
Single-participant statistical contrasts were computed by using the general linear model based on the canonical hemodynamic response function (Friston et al., 1998). The hash mark condition served as a low-level baseline condition for the contrasts of interest. Low-frequency noise was removed with a high-pass filter (time constant 128 s). Each stimulus type described above was modeled as a separate event type, i.e., as separate columns of the design matrix. Group data were analyzed with a random-effects analysis. For visual display, Figures report results at p = 0.001, uncorrected. This lenient threshold was chosen for display because we both wanted to present data at the whole-brain level and we had specific hypotheses about the pattern of activation in motor cortex. Stereotaxic coordinates for voxels with maximal z values within activation clusters are reported in the MNI standard space (which resembles very closely the standardized space of Talairach and Tournoux, 1988; see Brett et al., 2002).
A similar sequence of processing steps was applied to the motor localizer data, except that a high-pass filter had a time constant of 200 s, the duration of stimuli (20 s) was included in the creation of the design matrix, and activation peaks were localized at a family wise-error-corrected (FWE) significance threshold of 0.05.
ROI Analysis
Regions of interest were defined and analyzed using the Marsbar utility in SPM5 (Brett et al., 2002). Parameter estimates were extracted for peak voxels within areas of interest, as described below. The data were smoothed using a Gaussian kernel of 10 mm FWHM during pre-processing (see above), such that activation values at individual voxels represent a weighted average of voxels within the neighborhood. We therefore did not consider it necessary to apply ROIs in the shape of spheres or cubes.
ROI coordinates were computed based on activation peaks for uni-manual (UM) and bi-manual (BM) words in the left and right hemisphere, respectively. Because this revealed several slightly different peak coordinates in the left hemisphere, the mean activation was used for further analysis. In the left hemisphere, bi-manual words produced pericentral activation at an FDR-corrected threshold ([−50 −6 50] and [−54 −8 46]). Uni-manual words activated an area close-by ([−54 −8 46] and sub-cluster [−48 −4 52]), which was significant at an uncorrected level of p < 0.001 in the whole-brain analysis, but at a family wise-error-corrected level of p < 0.05 for a small volume correction within a sphere of 6 mm radius around the first peak for bi-manual words. As a mean coordinate for the pericentral area of the left hemisphere, this yielded [−52 −6 48] (note rounding to nearest even number due to spatial resampling during normalization). In right post/pre-central areas, only bi-manual words produced marginally significant activation at FDR-corrected values around the coordinate [60 −4 38], which was used for analysis, while uni-manual words did not produce any reliable right-hemispheric activation.
Parameter estimates were subjected to an ANOVA including the three factors Laterality, Manuality, and Handedness (the latter as a between-subjects factor). Specific comparisons were performed using one-tailed paired or two-sample t-tests where appropriate. The directed hypothesis that the difference between uni-manual and bi-manual words should be smaller in the left compared to the right hemisphere was tested by a one-tailed t-test for the subtraction [Right(uni-manual–bi-manual) − Left(uni-manual–bi-manual)].
Results
Whole-Brain Analyses
The main intention behind this study was to test specific hypotheses about ROIs in motor cortex. The whole-brain analysis only served the purpose to define those ROIs. The hypotheses were then tested using independent contrasts. Results for all hand-related words against the baseline condition (hash marks), collapsed across right- and left-handers, are presented in Figure 1 and Table 2. Peak activations occurred mainly in the left hemisphere, such as in left inferior temporal and fusiform, middle temporal, and inferior frontal cortex. Activation was also present in left anterior postcentral sulcus, close to the border to pre-central sulcus. This peak was approximately 19 mm lateral and anterior to the peak voxel in the right finger localizer task (Table 5), which is consistent with similar results reported in previous studies: While we observed a peak at [−50 −8 50], Ruschemeyer et al. (2007) reported [−44 −15 59] and Tomasino et al. (2007) [−50 −24 56] for their hand-related action-word categories. The only area significantly activated in the right hemisphere was middle temporal gyrus.
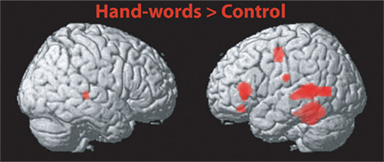
Figure 1. Results of the whole-brain analysis for the whole group of subjects (right- and left-handers) and for the contrast Hand-Words (uni-manual and bi-manual) vs. Hash Marks. Data are presented at a threshold p < 0.001 uncorrected, cluster size > 20 voxels. MNI coordinates for peak activations are presented in Table 1.
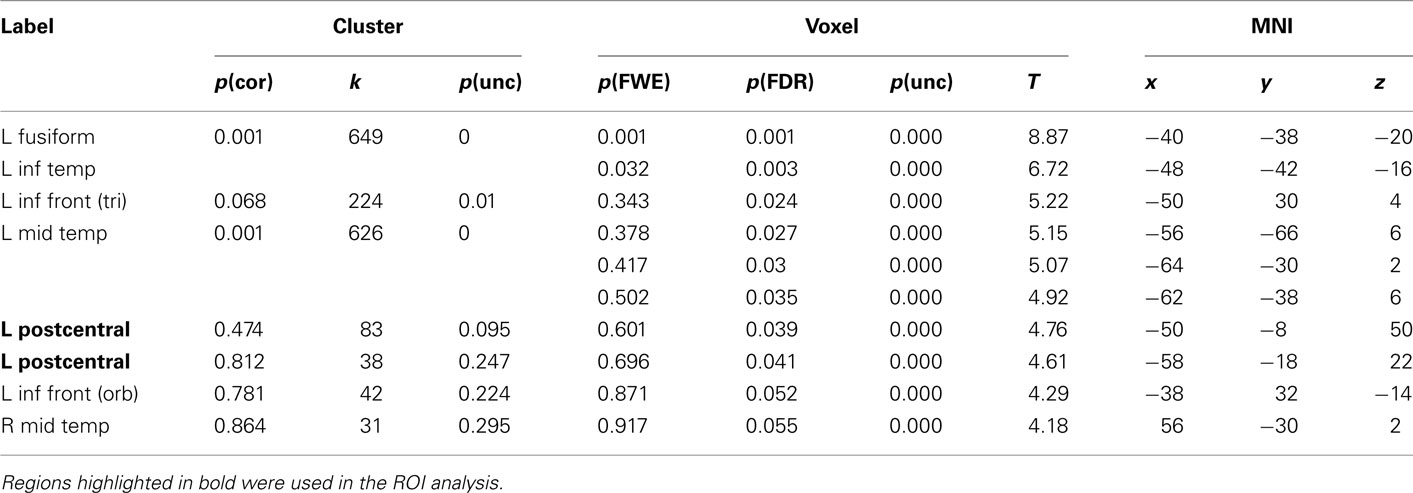
Table 2. Montreal Neurological Institute coordinates and SPM5 group statistics for voxels that were most strongly activated by Hand-Words vs. Hash Marks.
The results for uni-manual and bi-manual words are displayed separately in Figure 2 and Tables 3 and 4, again for all participants combined. Both word groups activated areas in perisylvian and inferior temporal cortex comparable to those presented in Figure 1, namely left fusiform and inferior frontal cortex, as well as middle temporal cortices bilaterally. Importantly, while both word groups activated left pericentral brain areas, only bi-manual words produced additional right-lateralized activation. For uni-manual words, the smallest distance between an activation peak for words and the peak voxel for right finger movements was 23 mm. For bi-manual words, this minimal distance was 20 mm in the left and 29 mm in the right hemisphere. In all cases, word-related activation was localized lateral and anterior to the localizer activation. It should also be noted that activations for the localizer movements was widespread even at conservative statistical thresholds (cluster sizes of ∼2200 at 0.05 FWE corrected, see Table 5).
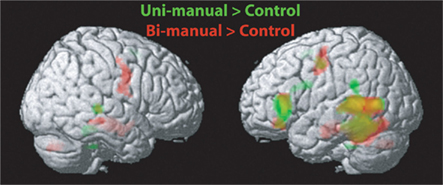
Figure 2. Results of the whole-brain analysis for the whole group of subjects (right- and left-handers) and for the contrasts Uni-Manual Words (green) and Bi-Manual (red) Words vs. Hash Marks, respectively. Data are presented at a threshold p < 0.001 uncorrected. MNI coordinates for peak activations are presented in Tables 2 and 3.
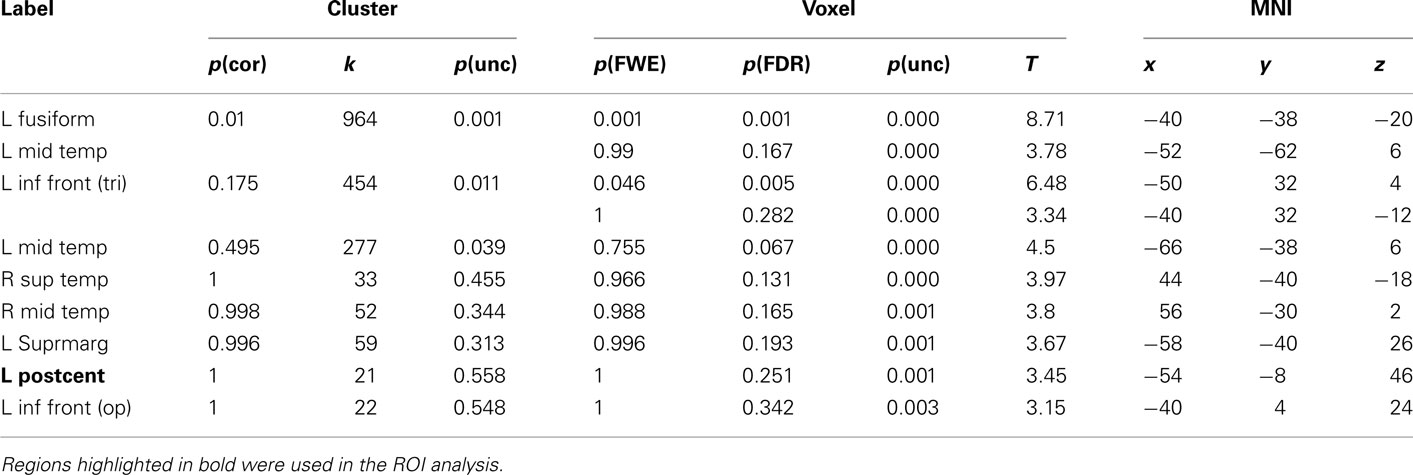
Table 3. Montreal Neurological Institute coordinates and SPM5 group statistics for voxels that were most strongly activated by Uni-manual Words vs. Hash Marks.
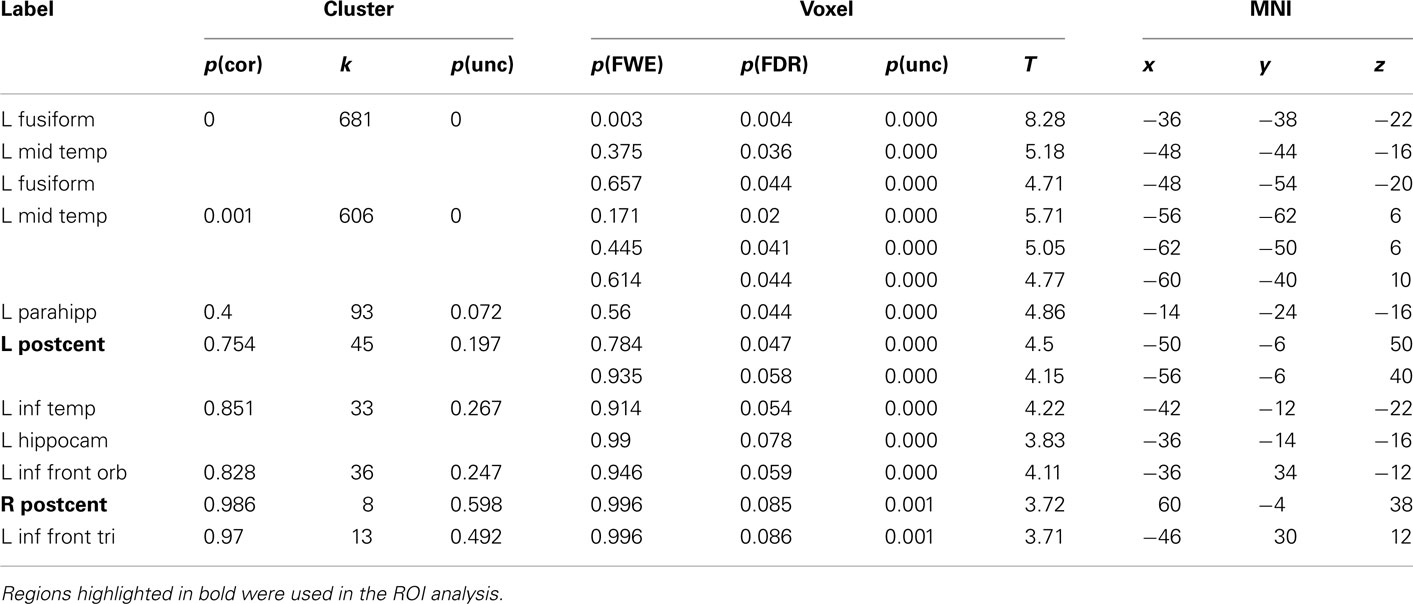
Table 4. Montreal Neurological Institute coordinates and SPM5 group statistics for voxels that were most strongly activated by Bi-manual Words vs. Hash Marks.
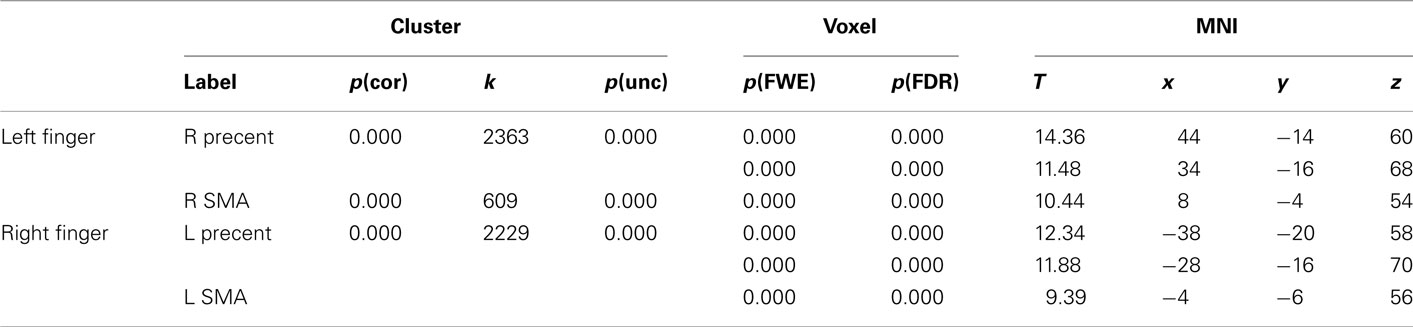
Table 5. Montreal Neurological Institute coordinates and SPM5 statistics for most strongly activated voxels in pre-central gyrus and supplementary motor area to left and right finger movements during the motor localizer scan.
ROI analyses
Figure 3 presents parameter estimates for peak voxels in left and right postcentral gyrus, at mean coordinates of activation peaks obtained for uni-manual and bi-manual words.
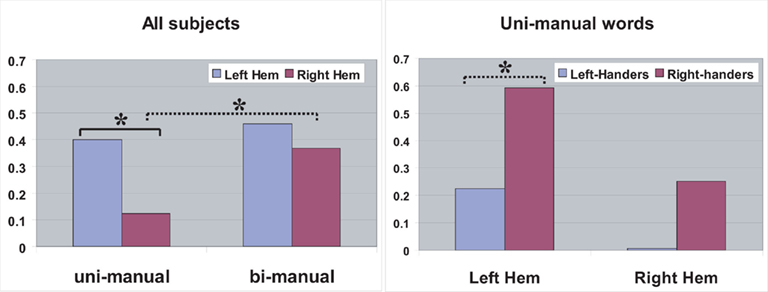
Figure 3. Parameter estimates (arbitrary units) for peak voxels in left (LPC) and right (RPC) pericentral cortex, for all subjects (uni-manual and bi-manual words separately; left) and for left- and right-handers separately (uni-manual words only; right). The difference marked by the solid bracket was significant (p < 0.05), the one marked by the dashed bracket approached significance (p < 0.1).
In a first step, we attempted to replicate previous results by Willems et al. (2010). We therefore contrasted activation to uni-manual words between left and right-handers in LPC and RPC, respectively. For uni-manual words, we predicted larger activation for right-handers compared to left-handers in LPC, and the reverse for RPC. The former prediction was partly supported by a difference in LPC between left- and right-handers [t(19) = 1.59, p = 0.064], while the difference in RPC was non-significant [t(19) = 0.80, p > 0.2]. The difference between left and right-hemispheric peri-central ROIs (left > right) was marginally significant for uni-manual words in right-handers (p = 0.076), but not for left-handers (p = 0.11).
Furthermore, we investigated the effects of handedness and manuality of action-words in more detail using a more complex ANOVA design. The ANOVA including the three factors Laterality, Manuality, and Handedness revealed a marginally significant interaction Laterality-by-Manuality [F(1,19) = 3.22, p = 0.089]. Interactions including the between-subject factor Handedness did not reach significance [F(1,19) < 1.2]. We therefore tested hypotheses regarding uni- and bi-manual words on data combined across left- and right-handers. The directed hypothesis that the difference between uni-manual and bi-manual words should be smaller in the left compared to the right hemisphere was confirmed [t(20) = 1.86, p < 0.05]. The difference between left and right-hemispheric ROI activations (left > right) was significant only for uni-manual words [t(20) = 2.41, p < 0.05], but not for bi-manual words [t(20) = 1.38, p = 0.18]. Note that the interaction with Handedness was not significant, and that the analysis for individual groups has lower statistical power than for the overall group. Both ROIs showed activation significantly larger than zero for bi-manual words, as well as for uni-manual words in LPC [all t(20) > 3.3, p < 0.01], but not for uni-manual words in RPC [t(20) = 0.8, p > 0.4].
Discussion
We investigated to what degree lateralization of activation in motor cortex during action-word reading is determined by individual experience and language dominance. We presented right- and left-handed participants with words referring to uni-manual (“throw”) and bi-manual (“clap”) actions in a silent reading paradigm, and analyzed the BOLD signal with respect to laterality in motor/premotor cortex. For bi-manual words, both participant groups activated motor cortex bilaterally. Importantly, both groups also showed the same left-lateralized activation pattern to uni-manual words. We argue that this reflects the effect of left-hemispheric language dominance on the formation of semantic brain circuits on the basis of Hebbian correlation learning.
Motor cortex activation in response to action-words and a significant difference between uni-manual and bi-manual action-words are in line with previous studies on action observation, which have shown that action representations involve the motor system and are shaped by individual experience (Calvo-Merino et al., 2006; Cross et al., 2006; Aglioti et al., 2008; Beilock et al., 2008; Rocca et al., 2008; Willems and Hagoort, 2009; Lyons et al., 2010). We have extended the evidence to word semantics, employing two very specific word categories and participant groups. While in previous studies brain activation distinguished between words referring to different effectors (hand, face, leg; Hauk et al., 2004; Tettamanti et al., 2005; Aziz-Zadeh et al., 2006; Boulenger et al., 2009), the present study extends these findings to more specific sub-groups of action-words for the same effector type, namely uni- and bi-manual action-words. This further confirms that aspects of the meaning of action-words are reflected in activation of the motor system (Pulvermüller, 2005). Previously, it has been argued that cell assemblies representing action-semantics are formed when children simultaneously perform an action and hear the word referring to it (e.g., Pulvermüller, 1999). Recent fMRI data have shown that motor activation to action-words already occurs during early development (James and Maouene, 2009). A more recent study suggests that this motor activation in children is present for words associated with previously observed as well as performed actions, although the latter produced larger activation than the former (James and Swain, 2011). Children were tested shortly after the learning phase, and thus it is as yet unclear how memory consolidation may affect these sensory–motor activation patterns. The investigation of semantic brain networks in the developing brain promises to be an exciting research area in the future.
The activation peaks for uni- and bi-manual action-words around the central sulcus were classified as “postcentral” by our anatomical labeling system (AAL; Tzourio-Mazoyer et al., 2002). However, the activation extended into both post- and pre-central sulcus, and the peaks were about 2 cm anterior–lateral to those obtained in the motor localizer scan for finger movements. This may reflect the fact that most actions are usually accompanied by somatosensory experiences, which are also part of their semantic representations. Similarly, the movements performed by our participants during the localizer scans probably also evoked brain activation in somatosensory areas, which may have shifted activation peaks to more posterior locations. A similar activation pattern has been observed in previous studies (Hauk et al., 2004; Boulenger et al., 2009). Furthermore, different types of action-words (e.g., “hitting” and “cutting” verbs) have been reported to activate different parts of motor cortex (Kemmerer et al., 2008). Small movements of the index finger, as used in our localizer scans, are unlikely to be representative for all kinds of hand/arm movements, and should only be considered as an approximate landmark. A similar argument has been made in a previous study, where face-word activation was anterior to tongue movement activation (Hauk et al., 2004). Future studies should investigate the variability of action-word activation across word types and participant groups in more detail.
It is a general shortcoming of fMRI data that they cannot distinguish between different processing stages, such as early semantic processing and post-access mental imagery, due to their low temporal resolution. A recent fMRI study has demonstrated that action-words in a lexical decision task produce activation patterns in motor areas that are non-overlapping with activation patterns in a mental imagery task (Willems et al., 2010). This demonstrates that motor areas may play different roles in imagery and semantics, and it implies that motor cortex activation in non-imagery tasks cannot be fully explained as reflecting imagery alone. Further fMRI studies have explicitly addressed the issue of mental imagery in fMRI designs, e.g., using priming (Wheatley et al., 2005; Gold et al., 2006) and category-specific word-frequency effects (Hauk et al., 2008a). The most direct way to disentangle early and late semantic and imagery processes is to use fast electrophysiological methods such as EEG and MEG (Hauk and Pulvermüller, 2004; Hauk et al., 2008b; Kiefer et al., 2008; Amsel, 2011). This research has shown that motor system activation to action-words emerges as early as the earliest semantically related brain responses reported so far, thus arguing against a post-understanding, imagery role of such activity (for discussion, see Pulvermuller and Fadiga, 2010).
We could only partly confirm previous results reporting differential activation to uni-manual action-words in left- and right-handers (Willems et al., 2010): In right-handers, activation in left motor regions to uni-manual words was stronger compared to left-handers. This result supports the idea that the way we perform actions ourselves affects the neuronal representation of action-words (Willems et al., 2009). However, the reverse effect in the right hemisphere did not reach significance in the present study. We would like to point out that we here contrasted two types of action-words that were matched with respect to general action-relatedness as well as hand-action-relatedness. We also used a passive reading task, which did not require any motor response or encourage our participants to engage in mental imagery or focus their attention on action-related aspects of our stimuli. We therefore suggest that the small differences we observed between left- and right-handers are due to different lateralization patterns during learning, when subjects use and perceive words during movement execution, action observation, or action imagery (Solodkin et al., 2001; Kloppel et al., 2007; Rocca et al., 2008). Whereas the predominating left-hemispheric activation in perisylvian language areas together with left-hemispheric motor systems activation in right-handers can be mapped by way of strong neuroanatomical links between the language and the motor systems of the left hemisphere, the predominantly right-hemispheric hand-motor activations in left-handers and their co-occurring predominantly left-perisylvian language activations are more difficult to map by way of cortico-cortical connectivity, as neuroanatomical links between these non-homotopic motor regions in the hemispheres do not exist. This laterality difference explains the strong uni-manual action-word response in right-handers and the comparatively weak one seen in left-handers and is also consistent with the hemisphere main effect observed across groups. Therefore, we submit that the difference in lateralization between uni-manual and bi-manual words, across participant groups, may reflect the joint effect of language dominance on the formation of cell assemblies between core language areas and the motor system (Pulvermuller and Fadiga, 2010) and that of correlation of neuronal activity within vs. between cortical hemispheres.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Nadja Tschentscher, Yuanyuan Chen, Clare Cook, David Kemmerer, and three referees for their input at different stages of this work. The authors were supported by the Medical Research Council (UK; MC_US_A060_0050, MC_US_A060_0034), the European Community under the “New and Emerging Science and Technologies” Programme (NEST, Nestcom Project).
Footnotes
References
Aglioti, S. M., Cesari, P., Romani, M., and Urgesi, C. (2008). Action anticipation and motor resonance in elite basketball players. Nat. Neurosci. 11, 1109–1116.
Amsel, B. D. (2011). Tracking real-time neural activation of conceptual knowledge using single-trial event-related potentials. Neuropsychologia 49, 970–983.
Aziz-Zadeh, L., Wilson, S. M., Rizzolatti, G., and Iacoboni, M. (2006). Congruent embodied representations for visually presented actions and linguistic phrases describing actions. Curr. Biol. 16, 1818–1823.
Barsalou, L. W. (1999). Perceptual symbol systems. Behav. Brain Sci. 22, 577–609; discussion 610–560.
Beilock, S. L., Lyons, I. M., Mattarelia-Micke, A., Nusbaum, H. C., and Small, S. L. (2008). Sports experience changes the neural processing of action language. Proc. Natl. Acad. Sci. U.S.A. 105, 13269–13273.
Boulenger, V., Hauk, O., and Pulvermuller, F. (2009). Grasping ideas with the motor system: semantic somatotopy in idiom comprehension. Cereb. Cortex 19, 1905–1914.
Braitenberg, V., and Pulvermüller, F. (1992). Model of a neurological theory of speech. Naturwissenschaften 79, 103–117.
Brett, M., Anton, J. L., Valabregue, R., and Poline, J. B. (2002). Region of interest analysis using an SPM toolbox. Paper presented at the 8th International Conferance on Functional Mapping of the Human Brain, Sendai.
Calvo-Merino, B., Grezes, J., Glaser, D. E., Passingham, R. E., and Haggard, P. (2006). Seeing or doing? Influence of visual and motor familiarity in action observation. Curr. Biol. 16, 1905–1910.
Cross, E. S., Hamilton, A. F. D. C., and Grafton, S. T. (2006). Building a motor simulation de novo: observation of dance by dancers. Neuroimage 31, 1257–1267.
Decety, J., and Grezes, J. (1999). Neural mechanisms subserving the perception of human actions. Trends Cogn. Sci. (Regul. Ed.) 3, 172–178.
Fadiga, L., and Craighero, L. (2004). Electrophysiology of action representation. J. Clin. Neurophysiol. 21, 157–169.
Friston, K. J., Fletcher, P., Josephs, O., Holmes, A., Rugg, M. D., and Turner, R. (1998). Event-related fMRI: characterizing differential responses. Neuroimage 7, 30–40.
Glenberg, A. M., and Kaschak, M. P. (2002). Grounding language in action. Psychon. Bull. Rev. 9, 558–565.
Gold, B. T., Balota, D. A., Jones, S. J., Powell, D. K., Smith, C. D., and Andersen, A. H. (2006). Dissociation of automatic and strategic lexical-semantics: functional magnetic resonance imaging evidence for differing roles of multiple frontotemporal regions. J. Neurosci. 26, 6523–6532.
Hauk, O., Davis, M. H., Kherif, F., and Pulvermuller, F. (2008a). Imagery or meaning? Evidence for a semantic origin of category-specific brain activity in metabolic imaging. Eur. J. Neurosci. 27, 1856–1866.
Hauk, O., Shtyrov, Y., and Pulvermuller, F. (2008b). The time course of action and action-word comprehension in the human brain as revealed by neurophysiology. J. Physiol. Paris 102, 50–58.
Hauk, O., Johnsrude, I., and Pulvermüller, F. (2004). Somatotopic representation of action words in human motor and premotor cortex. Neuron 41, 301–307.
Hauk, O., and Pulvermüller, F. (2004). Neurophysiological distinction of action words in the fronto-central cortex. Hum. Brain Mapp. 21, 191–201.
Hebb, D. O., Lambert, W. E., and Tucker, G. R. (1971). Language, thought and experience. Mod. Lang. J. 55, 212–222.
Hoenig, K., Muller, C., Herrnberger, B., Sim, E. J., Spitzer, M., Ehret, G., and Kiefer, M. (2011). Neuroplasticity of semantic representations for musical instruments in professional musicians. Neuroimage 56, 1714–1725.
James, K. H., and Maouene, J. (2009). Auditory verb perception recruits motor systems in the developing brain: an fMRI investigation. Dev. Sci. 12, F26–F34.
James, K. H., and Swain, S. N. (2011). Only self-generated actions create sensori-motor systems in the developing brain. Dev. Sci. 14, 673–678.
Kemmerer, D., Castillo, J. G., Talavage, T., Patterson, S., and Wiley, C. (2008). Neuroanatomical distribution of five semantic components of verbs: evidence from fMRI. Brain Lang. 107, 16–43.
Kemmerer, D., and Gonzalez-Castillo, J. (2010). The two-level theory of verb meaning: an approach to integrating the semantics of action with the mirror neuron system. Brain Lang. 112, 54–76.
Kiefer, M., and Pulvermuller, F. (2011). Conceptual representations in mind and brain: theoretical developments, current evidence and future directions. Cortex. PMID: 21621764. [Epub ahead of print].
Kiefer, M., Sim, E. J., Herrnberger, B., Grothe, J., and Hoenig, K. (2008). The sound of concepts: four markers for a link between auditory and conceptual brain systems. J. Neurosci. 28, 12224–12230.
Kiefer, M., Sim, E. J., Liebich, S., Hauk, O., and Tanaka, J. (2007). Experience-dependent plasticity of conceptual representations in human sensory-motor areas. J. Cogn. Neurosci. 19, 525–542.
Kiefer, M., and Spitzer, M. (2001). The limits of a distributed account of conceptual knowledge. Trends Cogn. Sci. (Regul. Ed.) 5, 469–471.
Kloppel, S., van Eimeren, T., Glauche, V., Vongerichten, A., Munchau, A., Frackowiak, R. S., Büchel, C., Weiller, C., and Siebner, H. R. (2007). The effect of handedness on cortical motor activation during simple bilateral movements. Neuroimage 34, 274–280.
Knecht, S., Drager, B., Deppe, M., Bobe, L., Lohmann, H., Floel, A., Ringelstein, E. B., and Henningsen, H. (2000). Handedness and hemispheric language dominance in healthy humans. Brain 123(Pt 12), 2512–2518.
Lyons, I. M., Mattarella-Micke, A., Cieslak, M., Nusbaum, H. C., Small, S. L., and Beilock, S. L. (2010). The role of personal experience in the neural processing of action-related language. Brain Lang. 112, 214–222.
Maes, F., Collignon, A., Vandermeulen, D., Marchal, G., and Suetens, P. (1997). Multimodality image registration by maximization of mutual information. IEEE Trans. Med. Imaging 16, 187–198.
Martin, A. (2007). The representation of object concepts in the brain. Annu. Rev. Psychol. 58, 25–45.
Oldfield, R. C. (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9, 97–113.
Postle, N., McMahon, K. L., Ashton, R., Meredith, M., and de Zubicaray, G. I. (2008). Action word meaning representations in cytoarchitectonically defined primary and premotor cortices. Neuroimage 43, 634–644.
Pulvermüller, F. (1999). Words in the brain’s language. Behav. Brain Sci. 22, 253–279; discussion 280–336.
Pulvermüller, F. (2005). Brain mechanisms linking language and action. Nat. Rev. Neurosci. 6, 576–582.
Pulvermuller, F., and Fadiga, L. (2010). Active perception: sensorimotor circuits as a cortical basis for language. Nat. Rev. Neurosci. 11, 351–360.
Pulvermüller, F., Härle, M., and Hummel, F. (2001). Walking or talking? Behavioral and neurophysiological correlates of action verb processing. Brain Lang. 78, 143–168.
Pulvermüller, F., Hauk, O., Nikulin, V. V., and Ilmoniemi, R. J. (2005a). Functional links between motor and language systems. Eur. J. Neurosci. 21, 793–797.
Pulvermüller, F., Shtyrov, Y., and Ilmoniemi, R. (2005b). Brain signatures of meaning access in action word recognition. J. Cogn. Neurosci. 17, 884–892.
Pulvermuller, F., Kherif, F., Hauk, O., Mohr, B., and Nimmo-Smith, I. (2009). Distributed cell assemblies for general lexical and category-specific semantic processing as revealed by fMRI cluster analysis. Hum. Brain Mapp. 30, 3837–3850.
Raposo, A., Moss, H. E., Stamatakis, E. A., and Tyler, L. K. (2009). Modulation of motor and premotor cortices by actions, action words and action sentences. Neuropsychologia 47, 388–396.
Rizzolatti, G., and Craighero, L. (2004). The mirror-neuron system. Annu. Rev. Neurosci. 27, 169–192.
Rocca, M. A., Falini, A., Comi, G., Scotti, G., and Filippi, M. (2008). The mirror-neuron system and handedness: a “right” world? Hum. Brain Mapp. 29, 1243–1254.
Ruschemeyer, S. A., Brass, M., and Friederici, A. D. (2007). Comprehending prehending: neural correlates of processing verbs with motor stems. J. Cogn. Neurosci. 19, 855–865.
Solodkin, A., Hlustik, P., Noll, D. C., and Small, S. L. (2001). Lateralization of motor circuits and handedness during finger movements. Eur. J. Neurol. 8, 425–434.
Solodkin, A., Walsh, R. R., Small, S. L., and Chen, E. E. (2008). Network activation during bimanual movements in humans. Neuroimage 43, 540–553.
Talairach, J., and Tournoux, P. (1988). Co-Planar Stereotaxic Atlas of the Human Brain. New York, NY: Thieme Medical Publishers.
Tettamanti, M., Buccino, G., Saccuman, M. C., Gallese, V., Danna, M., Scifo, P., Fazio, F., Rizzolatti, G., Cappa, S. F., and Perani, D. (2005). Listening to action-related sentences activates fronto-parietal motor circuits. J. Cogn. Neurosci. 17, 273–281.
Tomasino, B., Fink, G. R., Sparing, R., Dafotakis, M., and Weiss, P. H. (2008). Action verbs and the primary motor cortex: a comparative TMS study of silent reading, frequency judgments, and motor imagery. Neuropsychologia 46, 1915–1926.
Tomasino, B., Werner, C. J., Weiss, P. H., and Fink, G. R. (2007). Stimulus properties matter more than perspective: an fMRI study of mental imagery and silent reading of action phrases. Neuroimage 36(Suppl. 2), T128–T141.
Tzourio-Mazoyer, N., Landeau, B., Papathanassiou, D., Crivello, F., Etard, O., Delcroix, N., Mazoyer, B., and Joliot, M. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273–289.
Weisberg, J., van Turennout, M., and Martin, A. (2007). A neural system for learning about object function. Cereb. Cortex 17, 513–521.
Wheatley, T., Weisberg, J., Beauchamp, M. S., and Martin, A. (2005). Automatic priming of semantically related words reduces activity in the fusiform gyrus. J. Cogn. Neurosci. 17, 1871–1885.
Willems, R. M., and Hagoort, P. (2009). Hand preference influences neural correlates of action observation. Brain Res. 1269, 90–104.
Willems, R. M., Toni, I., Hagoort, P., and Casasanto, D. (2009). Body-specific motor imagery of hand actions: neural evidence from right- and left-handers. Front. Hum. Neurosci. 3:39. doi:10.3389/neuro.09.039.2009
Willems, R. M., Toni, I., Hagoort, P., and Casasanto, D. (2010). Neural dissociations between action verb understanding and motor imagery. J. Cogn. Neurosci. 22, 2387–2400.
Appendix
Keywords: embodiment, semantics, word recognition, fMRI
Citation: Hauk O and Pulvermüller F (2011) The lateralization of motor cortex activation to action-words. Front. Hum. Neurosci. 5:149. doi: 10.3389/fnhum.2011.00149
Received: 11 May 2011;
Accepted: 09 November 2011;
Published online: 29 November 2011.
Edited by:
Hans-Jochen Heinze, University of Magdeburg, GermanyReviewed by:
Sara L. Gonzalez Andino, HUG Geneva, SwitzerlandDavid Kemmerer, Purdue University, USA
Michael Schaefer, University Magdeburg, Germany
Copyright: © 2011 Hauk and Pulvermüller. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Olaf Hauk, Cognition and Brain Sciences Unit, Medical Research Council, 15 Chaucer Road, Cambridge CB2 7EF, UK. e-mail:b2xhZi5oYXVrQG1yYy1jYnUuY2FtLmFjLnVr