- Laboratory of Neuropsychology, Nencki Institute of Experimental Biology of Polish Academy of Sciences, Warsaw, Poland
Many studies revealed a link between temporal information processing (TIP) in a millisecond range and speech perception. Previous studies indicated a dysfunction in TIP accompanied by deficient phonemic hearing in children with specific language impairment (SLI). In this study we concentrate in SLI on phonetic identification, using the voice-onset-time (VOT) phenomenon in which TIP is built-in. VOT is crucial for speech perception, as stop consonants (like /t/ vs. /d/) may be distinguished by an acoustic difference in time between the onsets of the consonant (stop release burst) and the following vibration of vocal folds (voicing). In healthy subjects two categories (voiced and unvoiced) are determined using VOT task. The present study aimed at verifying whether children with SLI indicate a similar pattern of phonetic identification as their healthy peers and whether the intervention based on TIP results in improved performance on the VOT task. Children aged from 5 to 8 years (n = 47) were assigned into two groups: normal children without any language disability (NC, n = 20), and children with SLI (n = 27). In the latter group participants were randomly classified into two treatment subgroups, i.e., experimental temporal training (EG, n = 14) and control non-temporal training (CG, n = 13). The analyzed indicators of phonetic identification were: (1) the boundary location (α) determined as the VOT value corresponding to 50% voicing/unvoicing distinctions; (2) ranges of voiced/unvoiced categories; (3) the slope of identification curve (β) reflecting the identification correctness; (4) percent of voiced distinctions within the applied VOT spectrum. The results indicated similar α values and similar ranges of voiced/unvoiced categories between SLI and NC. However, β in SLI was significantly higher than that in NC. After the intervention, the significant improvement of β was observed only in EG. They achieved the level of performance comparable to that observed in NC. The training-related improvement in CG was non-significant. Furthermore, only in EG the β values in post-test correlated with measures of TIP as well as with phonemic hearing obtained in our previous studies. These findings provide another evidence that TIP is omnipresent in language communication and reflected not only in phonemic hearing but also in phonetic identification.
Introduction
Characteristics and Associated Features of Specific Language Impairment
Specific Language Impairment (SLI, diagnosed as F.80.1 and F.80.2 according to ICD 10; Pużyński and Wciórka, 2000) is a form of developmental language impairment in which children demonstrate difficulties in understanding and/or producing speech. However, their general cognitive functioning and non-verbal intelligence remain within the normal range. The language impairment cannot be explained by hearing problems, neurological and speech mechanism abnormalities or environmental factors. The prevalence of SLI is estimated to be approximately 7% among 5-year-olds (Tomblin et al., 1997).
Specific language impairment generates pervasive social problems possibly in relations to future lower academic achievements. Hence, there is a necessity to identify the causal factors of SLI and to create the efficient speech therapy which may provide language disordered children with the same opportunities as their typically developing peers.
Although developmental language disorders have been investigated for almost 150 years, the neural basis of SLI still remains unclear. One theoretical approach assumes difficulties associated with deficient perception of auditory input. In the early seventies Tallal and colleagues revealed that SLI children are less efficient in discriminating between verbal (Tallal and Piercy, 1974; Tallal, 1975) and non-verbal (Tallal and Piercy, 1973; Tallal, 1975) sounds presented in rapid succession. Since that time, the researchers considered difficulties in temporal information processing (TIP) of rapidly changing acoustic events as one of the core problems in SLI.
This indication is in line with a long discussion about the relationship between TIP and language in norm and pathology. Several subject populations, including children with language-learning-impairment (Benasich and Tallal, 2002; Fitch and Tallal, 2003), children or adults with dyslexia (Tallal et al., 1995; Rey et al., 2002) and patients with aphasia following left hemisphere brain lesions (Swisher and Hirsh, 1972; von Steinbüchel et al., 1999; Wittmann et al., 2004; Fink et al., 2006) displayed deficits in the perception of temporal order of two stimuli presented in rapid succession. They indicated elevated temporal order threshold (TOT), i.e., they needed longer time interval between two sounds to report correctly their temporal relation ‘before-after.’
In our previous study (Szelag et al., 2015) the coexistence of deficient TIP and disordered phonemic hearing was confirmed inter alia in children with SLI. We indicated that they displayed higher TOT (of about 184 ms) than normal peers (about 96 ms) accompanied by deficient phonemic hearing. In the present study we verify in children with SLI the co-occurrence of these deficits with disordered phonetic identification using the voice-onset-time (VOT) which may be considered as a sensitive measure combining TIP with phonetic aspects of speech perception.
TIP in Voiced/Voiceless Categorization
Speech perception requires neural encoding of both spectral acoustic and temporal cues. Different speech sounds (consonants and vowels) vary in their spectrotemporal characteristics. Whereas vowels present a relatively steady-state pattern of formants, stop consonants are temporally much more transient and acoustically variable (Obleser et al., 2007). Subjects with deficient TIP have accompanying difficulties in stop consonants reception which are critical in time, whereas such deficits rarely affect the perception of vowels. Moreover, stop consonant – vowel syllables (e.g., /TO/ and /DO/) are distinguished by acoustic differences in time between the onset of the consonant (stop release burst) and the onset of the following vowel (voicing). Such voiced/unvoiced categorical perception is the most frequently studied phonetic feature. It is measured with the VOT phenomenon defined as the time interval between the release from stop closure (onset of the consonant) and the onset of vibration of the vocal folds (onset of the following vowel, Lisker and Abramson, 1964; Molfese, 1980). It is worth mentioning that different languages are characterized by various temporal cues for VOT, thus, the relationship between the burst and laryngeal pulsing. The positive VOT reflects a situation when the laryngeal pulsing is preceded by a burst. In contrast, in case of negative VOT the burst is preceded by the laryngeal pulsing (Szelag and Szymaszek, 2014).
Some languages like American English or German use only the positive VOT, reflected in values from 01 to 20 ms for voiced categorizations, like /BA/, /DA/, /GA/ and longer intervals of around 30–80 ms for voiceless categorizations, like /PA/, /TA/, /KA/ (King et al., 2008). In other languages, e.g., Slavic or French the negative VOT is also observed. The voiced contrast detection varies along a continuum of VOT values. Among Slavic-speaking (including Polish) environments these values are located from –90 to 20 ms. Thus, the differentiation between voiced/unvoiced contrast bases on both negative and positive VOT.
Independently of these cross-linguistic differences in voiced/unvoiced categorical perception some language universalia emerge. They comprise a similar time gap of some tens of milliseconds with either positive or negative values critical for such differentiation in a given language. The efficient TIP in the millisecond time range seems to be crucial for the categorical voiced/unvoiced contrast perception, independently of the natural language. VOT phenomenon as an important aspect of phonetic identification in speech perception has been a topic of many studies including both normal and clinical populations (Giraud et al., 2007; King et al., 2008; Doellinger et al., 2011; Chobert et al., 2012).
Timing-Related Approaches to Remediation of Language Acquisition
As difficulties in rapid auditory processing were indicated as the crucial deficit at least in some children with SLI and the conventional speech therapy often seems not efficient enough, the interventions based on TIP were developed (Merzenich et al., 1996; Tallal et al., 1996; Wittmann and Fink, 2004; Cohen et al., 2005; Gillam et al., 2008; Given et al., 2008). The widely known remediation software Fast ForWord® was successfully implemented in children with SLI and resulted in improvement of expressive and receptive language skills. In addition, the effectiveness of the Dr. Neuronowski® software, focused on TIP, developed in our Institute (Szelag and Szymaszek, 2016), was verified in children with SLI in our previous study (Szelag et al., 2015). We found that such intervention resulted in lowered TOT values, reflecting improved TIP in comparison to the non-significant change after control non-temporal training. The improvement in TIP was accompanied by amelioration of language skills in both phonemic hearing and global speech comprehension tasks.
Study Aims
In the present study we tested whether children with SLI present the same boundaries for the typical categories of phonetic identification as their healthy peers and whether the application of intervention based on rapid auditory processing may result in improved performance on VOT task.
Materials and Methods
Participants
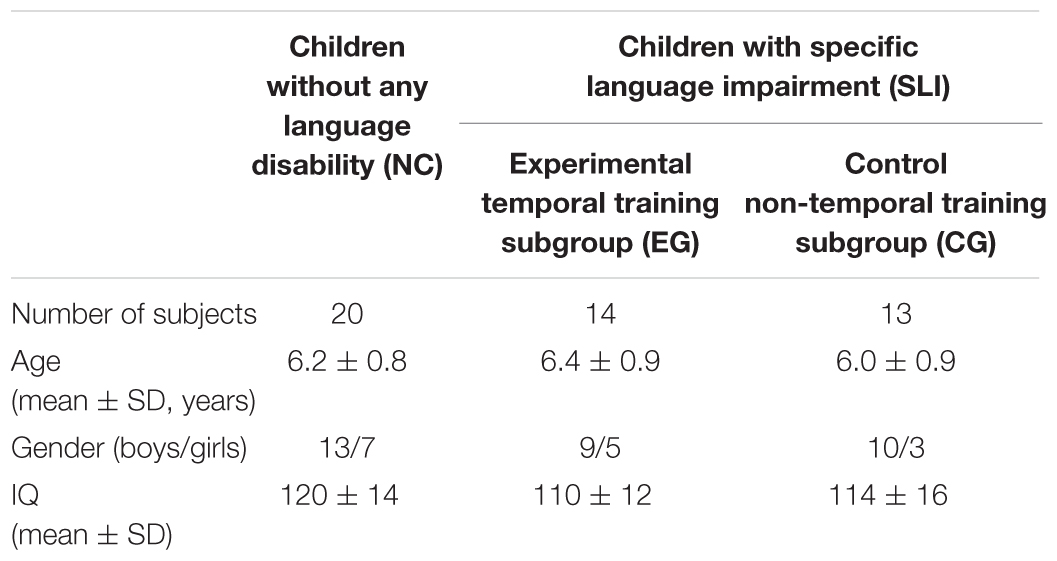
Forty-seven right-handed (Edinburgh Inventory) children aged between 5 and 8 years participated in the study. They were classified into two groups: (1) normal children without any language disability (NC, n = 20) and (2) children affected by SLI (n = 27). In the latter group children were randomly assigned using the RITA® software (Pahlke et al., 2004) into two intervention subgroups, i.e., experimental temporal training subgroup (EG, n = 14) and control non-temporal training subgroup (CG, n = 13).
All children were monolingual Polish native speakers. NC were recruited at kindergartens in the area of Warsaw, whereas children with SLI at either the Early Intervention Centre or the Children’s Memorial Health Institute in Warsaw. All participants showed normal hearing level (ANSI, 2004) which was verified with screening audiometry for 500, 1000, 2000, and 4000 Hz frequencies (audiometer AS 208). These frequencies covered the frequency spectrum of auditory stimuli presented in this study. All children had normal level of non-verbal intelligence (IQ at least 85 or higher, measured by the Raven’s Colored Progressive Matrices; Szustrowa and Jaworowska, 2003).
In case of children with SLI the developmental language impairment was defined as reduced language competency, evidenced by the Test for Assessment of Global Language Skills (TAGLS; Tarkowski, 2001). It constitutes the screening assessment tool for language development in Polish children. All participants with SLI obtained the overall standard language score on at least two standard language subtests below or equal 4th sten. The exclusion criteria were neurological and psychiatric disorders, attention deficits or socio-emotional disorders (as determined by the parental report) and the participation in any other therapy program during our data collection which might have influenced the obtained results.
All three groups (NC, EG, and CG) were balanced according to age, gender, non-verbal IQ based on either one-way analysis of variance (ANOVAs) (for age and IQ) or Pearson’s chi-squared test (for gender). Controlling such variables was important for the efficacy of the applied interventions. It was a blinded randomized controlled study. The detailed subject characteristics are given in Table 1.
Ethics Statement
The study protocol was approved by the Bioethical Commission at the Warsaw Medical University (Permission No. KB/162/2010). Prior to testing a written informed consent was obtained from the parents of each child participating in the study; children provided a verbal approval.
Stimuli and Experimental Procedures
The study comprised both assessment and intervention procedures. The assessment procedures included three tasks: (1) phonetic identification tested with VOT Task (Szelag and Szymaszek, 2014), (2) phonemic hearing with Phoneme Discrimination Test (PDT, Szelag et al., 2015), and (3) TIP with auditory TOT (Fink et al., 2006). These assessment procedures in SLI group were performed twice, i.e., before (pre-test) and, next, after (post-test) the applied intervention. In case of NC the phonetic identification data were collected at the beginning of the study, this group reminded without any intervention.
Assessment Procedures
Measurement of phonetic identification
Voice-onset-time task is a sensitive tool for evaluation of phonological deficits on a basis of phonetic identification during speech perception. The task has built-in the millisecond TIP component which is crucial for a differentiation between voiced and unvoiced category at the initial bilabial consonant. It was achieved by parametrizing a single acoustic temporal dimension of VOT across the synthetized spectrum of presented stimuli. The stimulus continuum was selected on a basis of our previous studies (Szelag and Szymaszek, 2014).
A series of voiced/unvoiced stimuli were created on a basis of the Polish word /TOMEK/ (with unvoiced initial consonant, naturally spoken with a female voice, in English: Tom). In all created stimuli the segment /OMEK/ was spectrally identical. The voiced/unvoiced contrast was achieved by the manipulation (Adobe Audition 3.0) in a single acoustic dimension of VOT within the initial consonant in semi-synthesized word TOMEK. The created stimuli differed in VOT values that separated the onset of the stop burst and subsequent voicing, thus, the relationship between the burst and laryngeal pulsing. It created a continuum of VOT values comprising 13 stimuli: –90, –80, –70, –60, –50, –40, –30, –20, –10, 0, +5, +10, and +20 ms. For VOT from –90 to –10 ms the burst was preceded by the laryngeal pulsing (negative VOT), while from +5 to +20 ms the laryngeal pulsing was preceded by a burst (the positive VOT; for explanations of positive vs. negative VOTs see section “Introduction”). Accordingly, in Slavic languages (including Polish) the word of –90 ms VOT is identified as DOMEK, whereas, that of 20 ms as TOMEK with transition zone (chance level identification) for VOTs of –30 and –20 ms. Illustrative waveforms of two endpoint stimuli of applied VOT continuum are shown in Figure 1.
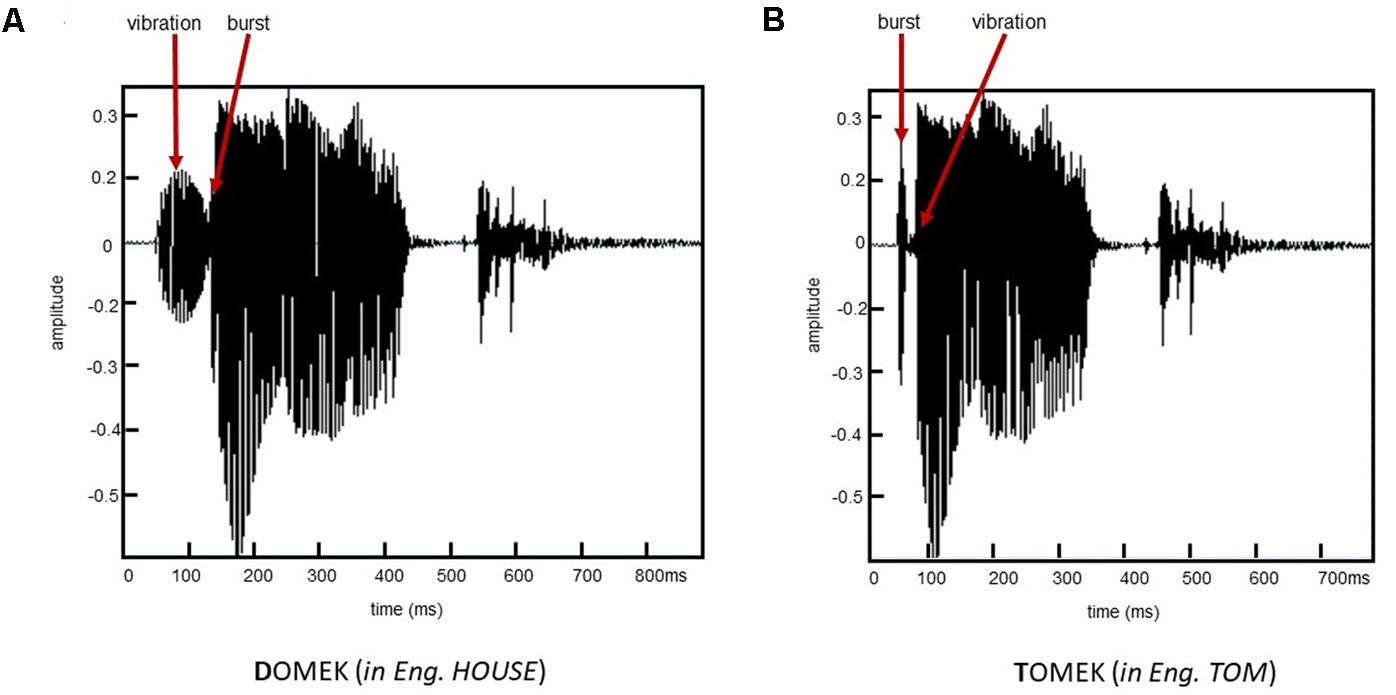
FIGURE 1. Waveforms of two words from the endpoints of applied VOT spectrum: (A) VOT of –90 ms identified as DOMEK and (B) Voice-onset-time (VOT) of 20 ms identified as TOMEK.
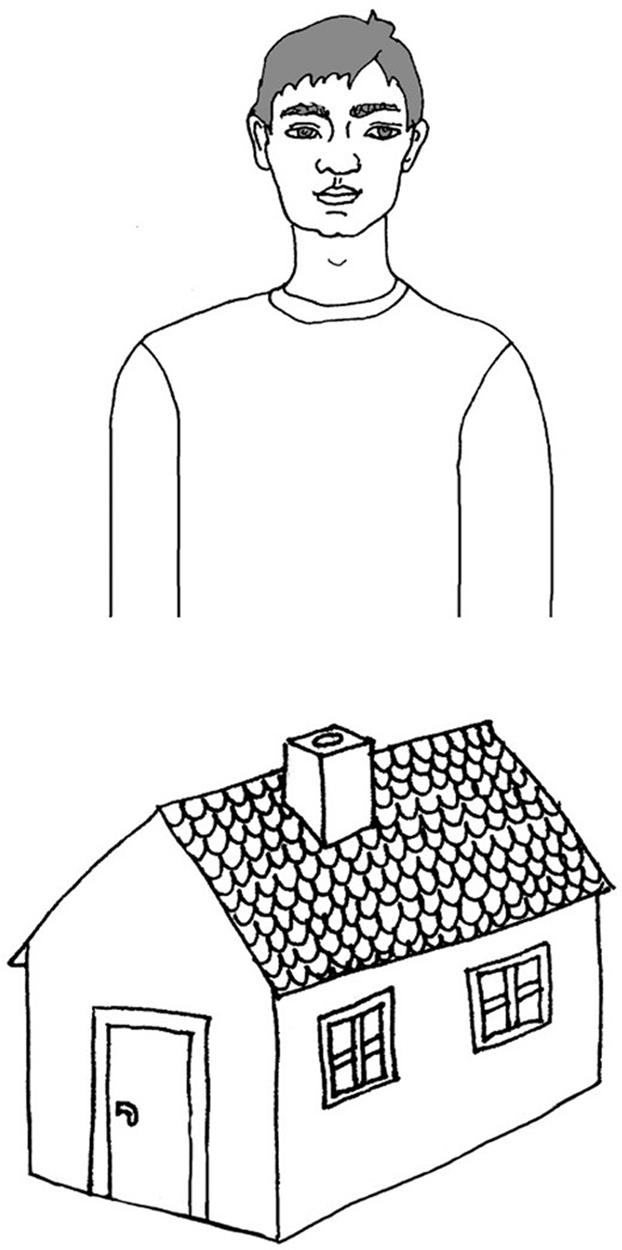
Children were presented with these stimuli binaurally through headphones at a comfortable listening level. The measurement based on identification of presented words as either /TOMEK/ or /DOMEK/. Children were asked to listen to the presented words and to associate each stimulus they heard with one of two pictures. These pictures were presented on one response card (format A4, Figure 2). The upper picture displayed a boy named Tom (in Polish: /TOMEK/) and the lower picture a house (in Polish: /DOMEK/).
In each child the experiment was preceded with an introductory practice session. First, the children were introduced to the above two pictures, hearing examples of /DOMEK/ and /TOMEK/ with the VOT corresponding to continuum endpoints (-90 and 20 ms). Next, 16 presentations (8 repetitions of 2 words from the VOT continuum endpoints, i.e., –90 ms and 20 ms) were randomly presented. After each presentation children were requested to point to the proper picture and a feedback on the correctness achieved was given. The introductory practice session ended when four responses in a row were correct. Then, the experiment started without any feedback on the correctness achieved.
The measurement comprised 78 presentations (6 series, each consisted of the 13 basic stimuli presented in random order).
Outcome measures
We analyzed the percent of voiced distinctions within the whole VOT spectrum.
In further analyses the psychometric (sigmoid) function2 was adjusted to all responses given by each child (Treutwein and Strasburger, 1999; Strasburger, 2001). This function based on the percent of voiced /DOMEK/ identifications for each of the 13 applied VOT values. The sigmoid function was the Z-shape curve limited in the range from 0 to 1 values. The 0 value for a defined VOT stimulus corresponded to a lack of any voiced identification in child’s responses, whereas the value 1 meant 100% of such detections. The sigmoid function (f(x)) was defined by the formula:
where α reflects the categorical boundary location (x coordinate of ‘half way up”) corresponding to 50% voiced/unvoiced discriminations and β indicates the slope of the identification curve. In such generalized logistic model differences in categorical precision are reflected by a shallower slope of the function, i.e., the higher β the more flat curve (less categorical perception) corresponding to the worse performance. The additional abbreviations in the above formula are:
• x – particular VOT values (from –90 to +20 ms) reflecting the spectrum
applied here;
• exp – exponential function, i.e., a logarithmic function based on a natural logarithm e = 2.78 (the Euler’s number);
In our data analysis both α and β values were used as indicators of phonetic identification (see section “Results”).
Measurements of phonemic hearing and TIP
As stated before, the efficiency of phonemic hearing was evaluated with the PDT, whereas that of TIP with auditory TOT task. The measurement procedures of PDT and TOT were described in detail in our earlier report (Szelag et al., 2015). In the present study we refer to some data collected previously and published in Szelag et al. (2015; see Tables 2, 3, p. 9). From the subject pool published in this previous report (n = 32) we selected the data of 27 children with SLI (considering EG and CG subgroups) who were tested also with the VOT task in the present study. These previous data were used here to test in EG and CG the correlations between phonemic hearing (or TIP) and β value used as an indicator of phonetic identification in VOT task performance. Below we summarize briefly the measurement procedures of phonemic hearing and TIP.
Phoneme Discrimination Test comprises 64 paired-words in which 75% pairs were different, e.g., górnik – kurnik (in English: miner – hen house) and 25% the same, e.g., mama –mama (in English: mother – mother). The task was to judge whether two words within the presented pair were the same or different. Responses were given by pointing to one of the two response cards, corresponding to these two situations. In case of different paired-words they did not match in one phoneme. The differed paired-words contrasted for place of articulation, plosive, fricative, voicing, or nasality.
In TIP the measurement based on auditory TOT defined as the minimum time gap between two auditory stimuli presented in rapid succession that is necessary for a participant to report correctly their temporal order, i.e., the relation before-after at 75% correctness. The stimuli were paired 1 ms clicks presented monaurally (i.e., to each ear separately) with various inter-stimulus-intervals (ISIs). The task was to report the order of two clicks, thus: left–right or right–left. ISIs varied adaptively from 1 to 600 ms, according to the adaptive maximum-likelihood-based algorithm (Treutwein, 1997) until the TOT was located with a probability of 95% inside a ±5 ms interval around the currently estimated threshold (Treutwein, 1995).
Intervention Procedures
As mentioned above, in children with SLI two types of interventions were applied, i.e., experimental temporal intervention (in EG subgroup) and control non-temporal intervention (in CG). Detailed description of both intervention programs was provided in Szelag et al. (2015).
Experimental temporal intervention
Experimental temporal intervention procedure used the multimedia intervention program Dr. Neuronowski.® It was designed in our Institute on the basis of our previous prototyping interventions addressed TIP (Szelag et al., 2014; Szymaszek et al., 2017). This software consists of nine various modules containing 46 basic computer games. The crucial aspect of this software is that the majority of games involved TIP in the millisecond time range, sequencing abilities or duration judgment. Moreover, the temporal-based games were extended by tasks exercising other cognitive functions, i.e., language comprehension, attention, working memory and executive functions. The software was auto adaptive, i.e., task difficulty changed adaptively based on correctness of the actual child’s performance. The tasks difficulty was modified according to numerous parameters, i.e., number, length and presentation rate of verbal and non-verbal stimuli, rate of modified speech, various ISIs in stimuli presented sequentially, application of various distractors, time limitations for responses.
Non-temporal control intervention
Non-temporal control intervention was based on freely available computer games (e.g., Memory or Tetris), as well as on educational speech-therapy exercises. The combination of such software trained phonemic hearing, articulation and vocabulary, as well as attention, working memory and executive functions. Contrary to the experimental temporal intervention, none of these tasks engaged any rapid auditory processing in the millisecond time range.
Study Protocol
The assessment procedures and the intervention programs (Figure 3) were conducted with each child individually. The intervention (experimental and control) consisted of 24 sessions of 1-h each, performed 3 times per week.
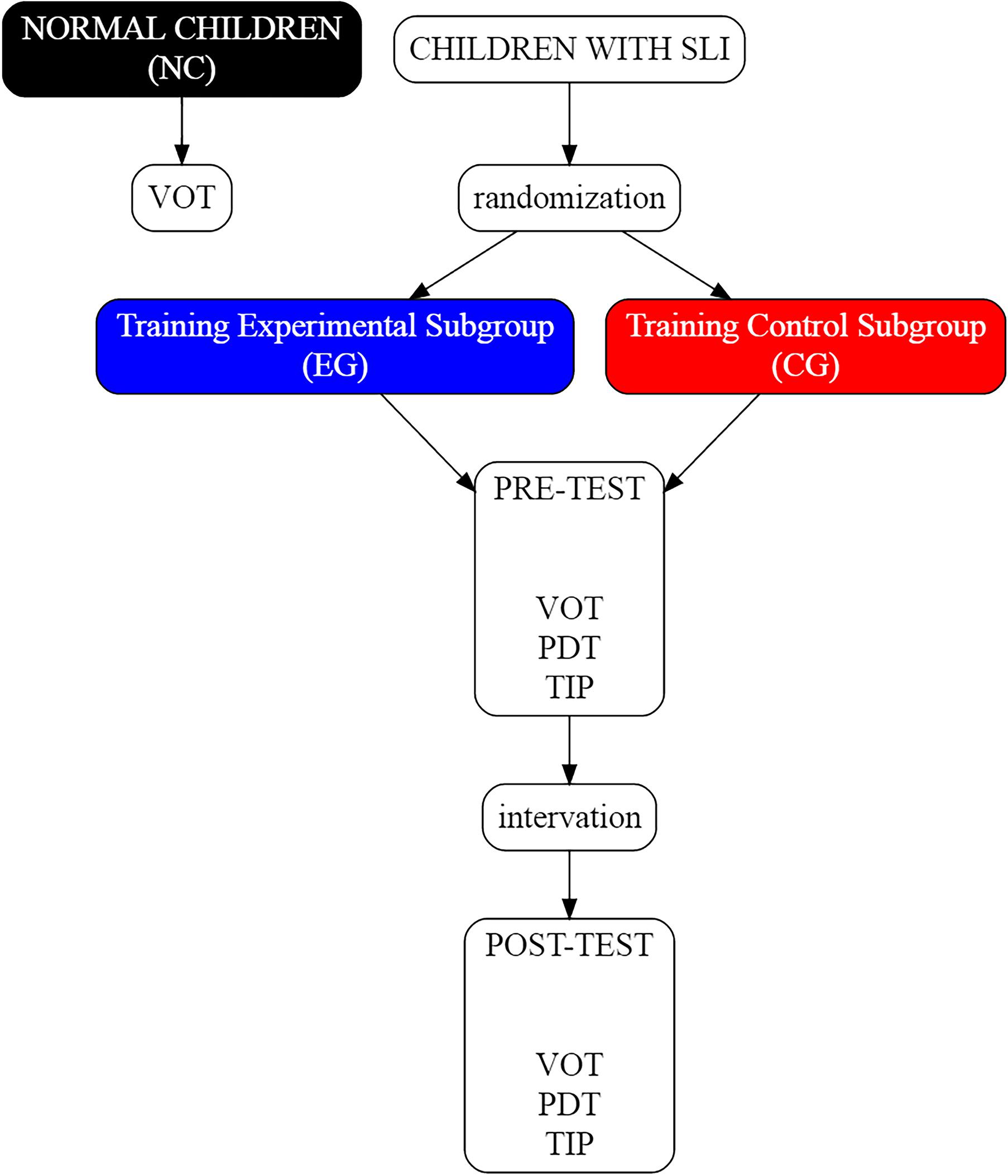
FIGURE 3. Schema of the study. In NC the VOT task was performed once, whereas in EG and CG subgroups all three assessment tasks: VOT, Phoneme Discrimination Test (PDT), and temporal information processing (TIP) were conducted twice, i.e., before and after the intervention.
Statistical Analyses
Statistical analyses comprised four Steps. They included: (1) comparison of phonetic identification between NC and SLI children, (2) training-related differences separately in EG and CG, (3) post-test performance in EG and CG in comparison to NC, and (4) relationships in EG and CG between the phonetic identification indexed with the function slope (β) and results of phonemic hearing and TIP in pre- and in post-test assessment, separately. In Steps 1–3 (Figure 4) we analyzed both α and β3 values using the U Mann–Whitney test (Step 1), Wilcoxon Signed-Rank test (Step 2) and Kruskal–Wallis one-way ANOVA followed by the U Mann–Whitney tests (Step 3).
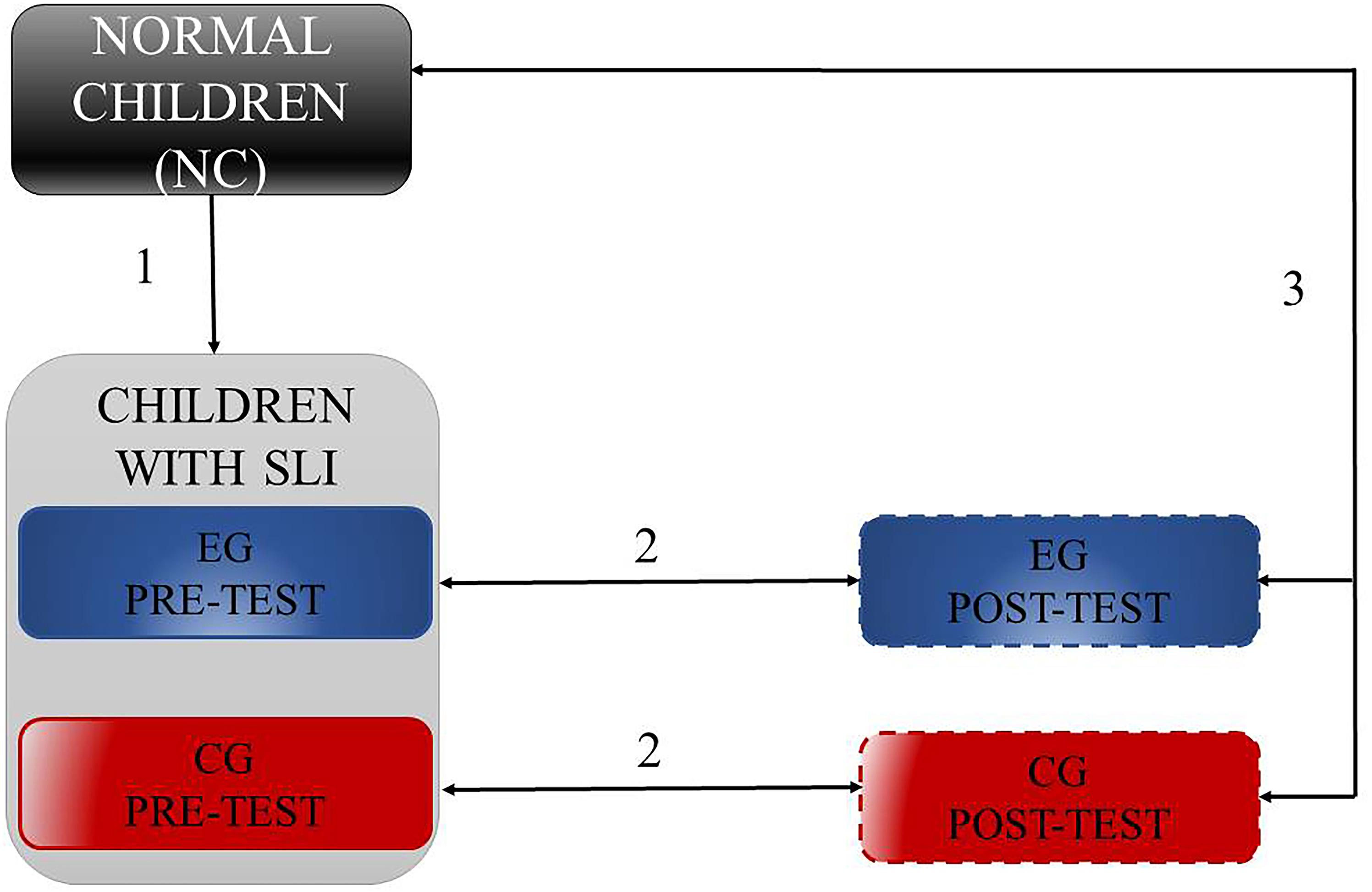
FIGURE 4. Schema of performed comparisions in Steps 1–3, where (1) reflects camparisons of phonetic identification between NC and SLI, (2) training-related differences in EG and CG, and (3) post-test performance in EG and CG in comparison to NC.
Additionally, in Step 1 these comparisons were extended by 2-way ANOVA with the percent of voiced responses for the whole spectrum of VOT values in NC and SLI. This ANOVA aimed at testing the differences in phonetic identification for particular VOT stimuli within the voiced, unvoiced and transition categories4. It included ‘Group’ (NC vs. SLI) as between-subject factor and ‘VOT value’ (13 values: –90, –80, –70, –60, –50, –40, –30, –20, –10, 0, +5, +10, and +20 ms) as a within-subject factor5. In SLI group only pre-test (summed EG and CG) data were considered.
In Step, 4 using Spearman correlations, we tested the relationships between phonetic identification indexed with β values and other cognitive skills reflected by phonemic hearing and TIP in EG and CG subgroups in pre- and in post-test assessment, separately. Referring to Szelag et al. (2015), TOT was the indicator of TIP efficiency and PDT of phonemic hearing.
Results
Phonetic Identification in NC and SLI
Between-group differences for the boundary location (assessed with α, Figure 5) were non-significant (U = 244, p = 0.59). The obtained results indicated the similar boundaries for voiced/unvoiced distinctions in NC (α = –24.5) and SLI (α = –22.2).
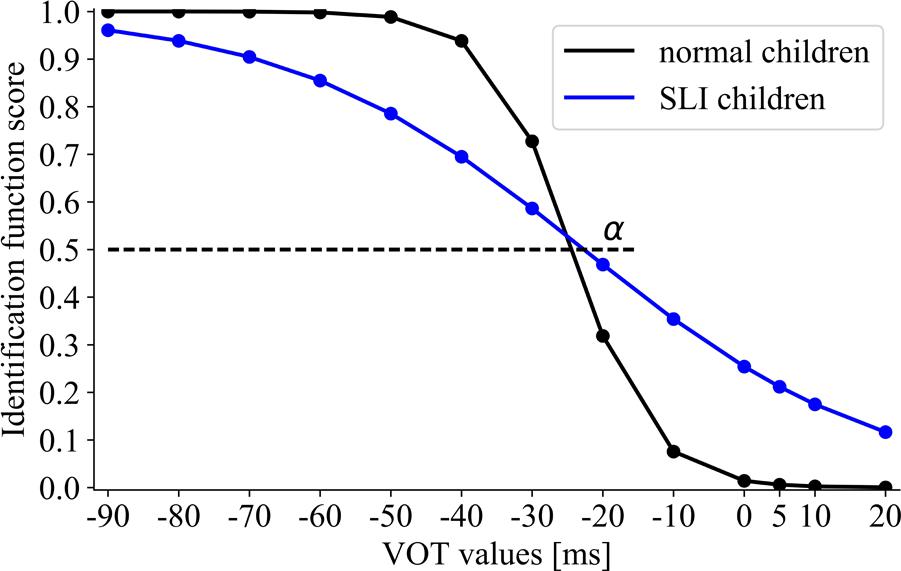
FIGURE 5. The sigmoid function for NC and specific language impairment (SLI) groups. The boundary location (α) corresponds to the VOT value at which 50% of voiced/unvoiced identifications were detected.
Significant between-group differences for the slope of identification function (assessed with β, Figure 5) were found (U = 96, p = 0.0002). The NC group presented the steeper slope (β = 3.62) than the SLI group (β = 26.3), corresponding to better performance in the former group.
Analysis of variance with the voiced identification scores for the VOT spectrum revealed a significant effect of ‘VOT value’ [F(12/540) = 271.593, p < 0.001, η2= 0.858] modified by the interaction ‘VOT value’ × ‘Group’ [F(12/540) = 9.313, p < 0.001, η2= 0.171]. In both groups the results were patterned by two phonetic categories (Figure 6). The voiced category comprised the VOT values ranged from –90 to –40 ms, whereas, the unvoiced one from –10 to 20 ms, independently of the group. The ranges of these two categories were established on a basis of significant jump in the identification score between –40 and –30 ms (p < 0.001 in both groups) indicating the voiced category and between –20 and –10 ms (p < 0.001 in both groups) for the unvoiced category. Despite these similarities in both groups, the better performance within each category was observed in NC than in SLI (‘VOT value’ × ‘Group’ interaction).
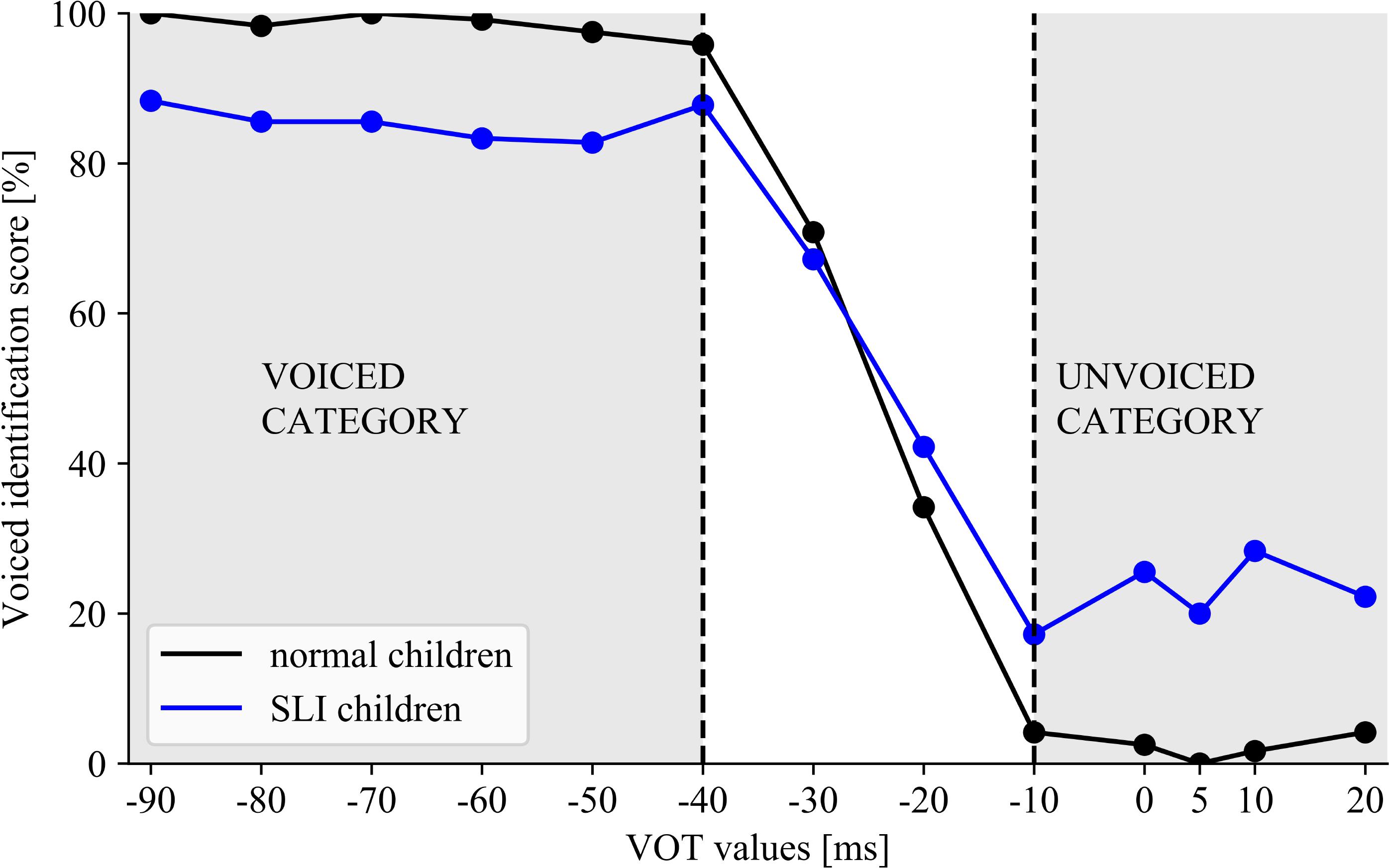
FIGURE 6. The voiced identification score for the presented VOT spectrum in NC and SLI indicating the similar ranges of voiced/unvoiced categories in both groups with poorer performance in SLI than in NC.
The Effect of Applied Experimental vs. Control Intervention
The effect of training on the boundary location (Figure 7) was non-significant in EG (Z = 1.10; p = 0.27; αpre-test = –21.80 and αpost-test = –19.93) and in CG (Z = 0.73; p = 0.47; αpre-test = –22.63 and αpost-test = –24.34). Thus, the categorical boundary location remained relatively stable following each type of intervention.
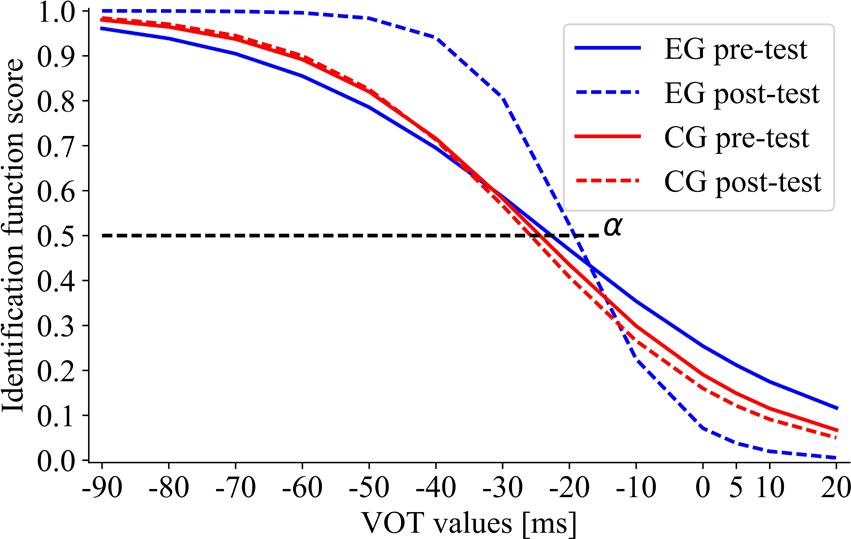
FIGURE 7. The sigmoid function for EG and CG subgroups in pre- and post-test performance. The boundary location (α) corresponds to the VOT value at which 50% of voiced/unvoiced identifications were detected.
The training-related changes in the slope of identification function indicated significantly lower β in EG in post- than in pre-test (Z = 2.73; p = 0.007; βpre-test = 29.59, βpost-test = 6.72), corresponding to improved performance (Figure 7). In contrast, β in CG did not differ significantly between post- and pre-test (Z = 0.52; p = 0.60; βpre-test = 22.77, βpost-test = 19.80), indicating the similar level of performance in pre- and post-test.
Post-Test Performance in EG and CG in Comparison to NC
Between-group comparisons for the boundary location (Figure 8) were non-significant (H = 3.5, p = 0.18; αEG = –19.93, αCG = –24.34, αNC = –24.54).
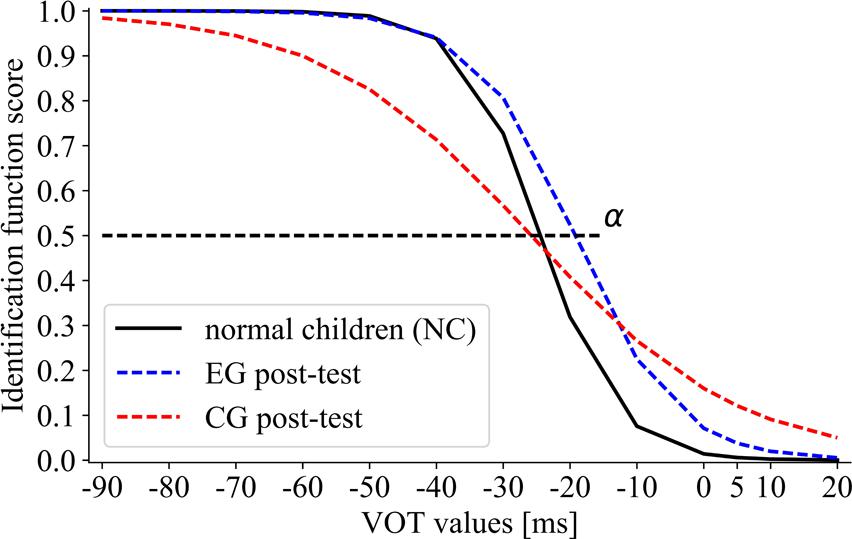
FIGURE 8. The sigmoid function for post-test performance of EG and CG subgroups in comparison to NC. The boundary location (α) corresponds to the VOT value at which 50% of voiced/unvoiced identifications were detected.
Significant between-group differences in the slope of identification function were observed (H = 7.61, p = 0.03) between CG and NC (U = 62, p = 0.02; βCG = 19.80, βNC = 3.62). On the contrary, differences between EG and NC were non-significant (U = 139, p = 0.99; βEG = 6.72, βNC = 3.62). The obtained results indicated that EG in post-test reached the level of NC performance, whereas the post-test performance in CG remained still significantly below that of NC.
Relationships Between Phonetic Identification and Other Cognitive Skills
In pre-test the β values did not correlate significantly with TOT (r = 0.23, p = 0.43 for EG and r = –0.09, p = 0.78 for CG) and PDT (r = 0.48, p = 0.09 for EG and r = 0.22, p = 0.47 for CG) in any subgroup. In contrast, in post-test only in EG significant correlations were found between β and TOT (r = 0.58, p = 0.03), as well as between β and PDT scores (r = 0.56, p = 0.04). For CG correlations between β and TOT (r = 0.25, p = 0.42), as well as between β and PDT scores (r = 0.15, p = 0.62) were non-significant.
Discussion
Considering the aims of our study, the discussion of obtained results is focussed, firstly, on the differences in phonetic identification between children with SLI and healthy peers, followed by the training-related changes in such identification. Finally, we concentrate on relationships between the level of phonetic identification assessed with the VOT test and results of TIP and phonemic hearing obtained in our previous study (Szelag et al., 2015).
Voicing Contrast Detection in Children With SLI on the Background of Healthy Controls
Although some existing literature studies concern the phonetic identification in children with SLI, the boundaries of categorical perception of voiced/unvoiced detection in such children have been rarely studied. Therefore, the important result of the present study was the indication of non-significant differences between the boundary location (α) in children with SLI and NC (Figure 5). Moreover, in both these groups the similar ranges of categories for voiced/unvoiced detection were distinguished. The voiced category was identified at VOT values from –90 to –40 ms, while the unvoiced category from –10 to 20 ms (ANOVA, Figure 6) which is congruent with the previous reports in Polish subjects (Rojczyk, 2010). Besides these similarities, deficient voiced contrast detection reported in this study was reflected in significantly lower correctness within these categories in children with SLI than in NC (Figures 5, 6). It was evidenced in statistical analyses (ANOVA) as well as in a shallower slope of identification function (higher β) values in children with SLI which corresponds to worse performance.
As mentioned above, speech perception difficulties have been frequently reported in SLI in literature studies. For example, Ziegler et al. (2005, 2011) investigated the perception of speech in noise considering various features, such as: voicing, manner and place of articulation. Although the perception of all these features was impaired in children with SLI, as compared to age-matched healthy controls, the voicing was impaired to a greater extent. It was interpreted as the strongest deficit compared to other features of speech perception. The deficient voiced categorical perception may reflect the deteriorated millisecond TIP which is incorporated in such voiced/unvoiced categorization (Benasich and Tallal, 2002; Fitch and Tallal, 2003; Szelag et al., 2015).
As indicated before, particular languages are characterized by specific boundaries for voiced detection (see section “Introduction”). Nevertheless, literature studies provided evidence that infants who experienced any linguistic environment are sensitive to some universal boundaries of phonetic identification which were located at VOT values between –30 and 30 ms (Lasky et al., 1975; Streeter, 1976). It was concluded that infants have a specialized biological predisposition to discriminate an universal set of phonetic contrasts (Eimas, 1991). The process of language acquisition during child development involves reorganization of this universal sensitivity under the influence of specific environmental conditions. Based on this view, infants at around 6 months of life transfer from a language-general to a language-specific mode of speech perception with phonetic boundaries typical for the experienced language. It was evidenced for Spanish (Lasky et al., 1975) as well as for French (Hoonhorst et al., 2009). One may expect that the skilled functioning in the range of some tens of milliseconds is crucial for language development from early years of life. In the VOT task, similarly as in the TIP task, it is necessary to perceive effectively two sequential events (e.g., burst–vibration or two sounds) separated in time by some tens of milliseconds. Such a statement is supported by the literature evidence on shared neural network for rapid auditory processing and speech processing (e.g., Zaehle et al., 2004).
A number of studies has revealed deficient categorical contrast detection in children with SLI. For example, Sussman (1993) compared the performance of VOT in syllables, using the discrimination and identification methods. During discrimination measurement the performance in children with SLI was comparable to that reported in healthy controls. In contrast, in identification they were significantly less accurate. The impaired phonetic identification in VOT continuum in children with SLI was further confirmed by Gerrits and de Bree (2009). Our results are congruent with these literature reports (Figures 5, 6). Although several studies indicated lower accuracy of phonetic identification in children with SLI, our important finding was the observation that categorical boundaries for voiced/unvoiced identification were still preserved and remained comparable to those observed in healthy controls. At this point what should be noted is the similar boundary location reflected in α (Figure 5) as well as typical voiced/unvoiced category ranges (Figure 6) in 5–8-year-old normally developing children and in SLI ones. The indication of the typical temporal framework for phonetic identification in SLI seems to be promising in the context of speech neurorehabilitation.
Training-Related Changes in Voiced Contrast Detection
Despite existing literature controversies on the contribution of deficient TIP and declined temporal precision to deficient speech perception (Fink et al., 2006; Vander Werff and Burns, 2011; Parbery-Clark et al., 2012), our results confirmed that training in TIP improved the phonetic identification measured by the VOT task. As stressed before, although the boundaries of categorical voiced distinctions in children with SLI were the same as in healthy peers, the correctness of performance in SLI was significantly lower (Figures 5, 6). Only in EG after temporal intervention we observed significant improvement reflected in lowered β values (βpre-test = 29.59 vs. βpost-test = 6.72). On the contrary, in CG after control intervention no significant differences were revealed (Figure 7).
Furthermore, it should be stressed that only following temporal intervention, the children with SLI reached the level of performance comparable to that observed in NC (non-significant differences between EG and NC). In contrast, following non-temporal intervention (in CG), the level of performance still remained below that of NC (significant differences between CG and NC, Figure 8).
Previous studies indicated beneficial effects of various interventions based on rapid auditory processing which resulted in amelioration of speech reception evidenced, e.g., by phonemic hearing in children with SLI (e.g., Tallal et al., 1996; Szelag et al., 2015). However, in the present study we confirmed that increased temporal precision in auditory processing resulted in more effective phonetic identification which seems to be more complex and nuanced than simple correct/incorrect phoneme differentiation measured with phonemic hearing tests.
In our previous studies, the effectiveness of intervention based on TIP was investigated in aphasic patients using the prototype version of Dr. Neuronowski® software (Szelag et al., 2014; Szymaszek et al., 2017). Patients were trained in sequencing two sounds presented in a rapid succession. It resulted in significant improvement of both TIP and speech reception (evidenced in phonemic hearing, global speech comprehension and VOT tests).
Referring to some theories on SLI, these children presented impaired working memory and selective attention which affected the phonological processes (Bishop et al., 1999; McArthur and Bishop, 2001; Vandewalle et al., 2012). Although, two intervention programs applied here (temporal vs. control) contained exercises focused on cognitive functions, like working memory, attention or executive functions, only the temporal intervention (addressed TIP) caused the improvement in speech perception, i.e., phonetic identification (studied here), as well as phonemic hearing and global speech comprehension (Szelag et al., 2015). Thus, one may emphasize that efficient temporal framework is fundamental for broader aspects of speech perception, but the training in working memory and attention incorporated in both applied interventions was not sufficient enough to improve speech perception skills in children with SLI.
For better understanding training-related benefits reported here in the context of our previous reports (Szelag et al., 2015), we conducted correlation analyses between phonetic identification indexed with β and phonemic hearing (indexed with the percent of errors) or TIP (TOT in millisecond time range).
Correlations Between Phonetic Identification, Phonemic Hearing and TIP
Literature evidence indicated that some aspects of speech reception, i.e., both phonetic identification and phonemic hearing are rooted in millisecond temporal frame (Pöppel, 1997, 2009). In that context, we investigated whether the TOT values and the effectiveness of phonemic hearing tested in our previous study (Szelag et al., 2015) correlated with the β values obtained here, considered as the indicator of voiced contrast identification efficiency.
Only in EG in post-test both these measures (TOT and phonemic hearing) correlated significantly with the β values. Thus, the lower β (better contrast detection) was accompanied by better phonemic hearing and lower TOT (better TIP performance). It may suggest the existence of a neural mechanism underlying speech perception rooted in TIP which was improved during temporal intervention. The application of such exercises may result in a transfer of improvement from the trained non-verbal timing processing into the untrained verbal processing, i.e., some aspects of speech perception in which the temporal component is built-in. Hence, such transfer of improvement was documented in amelioration of both phonemic hearing and phonetic identification. One may hypothesize that following the temporal training the improved temporal acuity resulted in more coherent processing of both speech and non-speech stimuli. Such correlations in pre-test in both groups were non-significant probably due to more variable and less precise subjects’ responses. In CG the applied intervention did not influence TIP, thus, the preserved declined millisecond time frame resulted in non-significant relation ‘timing-speech perception.’
Final Remarks
The obtained results revealed that children with SLI, despite lower correctness in phonetic identification, present the same as their healthy peers boundary location for categorical voicing contrast detection. Temporal intervention in children with SLI resulted in significant improvement of phonetic identification as compared to non-temporal control intervention. In CG intervention based on cognitive functions such as: working memory, attention, executive functions extended by typical speech therapy exercises (non-temporal control intervention) did not benefit speech perception assessed by phonetic identification.
Author Contributions
AS: data acquisition, conducting therapy sessions, analysis and interpretation of data, and manuscript writing. AD: subject recruitment, data acquisition, conducting therapy sessions, analysis and interpretation of data, and contribution to manuscript writing. PU: analysis of psychometric function. ES: conceptualization and study design, analysis and interpretation of data, and manuscript writing. All the authors: final approval.
Funding
The research was supported by the INNOTECH- K1/IN1/30/159041/NCBR/12 grant from the National Centre for Research and Development, Poland.
Conflict of Interest Statement
ES and AS are the creators of the software package Dr. Neuronowski®, realized as a part of a project at the Nencki Institute with funding from the National Centre for Research and Development in Poland. The rights to the software lie with the Nencki Institute that has an agreement with Harpo Ltd., the company commercializing this software. ES and AS are not the owners of this technology nor do they have a direct financial arrangement with Harpo Ltd. The authors state that it does not affect the scientific validity of the results.
The remaining authors state that the research was realized in the absence of any commercial or financial relationships that could generate any potential conflict of interest.
Acknowledgments
The authors would like to thank Anna Bombinska for her technical assistance during the data collection.
Footnotes
- ^VOT value of 0 ms reflects an overlapping of burst and vibration onsets.
- ^In our preliminary data analyses, the sigmoid function seemed to be best fitted to data obtained in this study.
- ^α and β exponents did not have a normal distribution.
- ^Polish-speaking children perceived voiced category at VOT: –90, –80, –70, –60, –50, –40 ms, unvoiced at: –10, 0, +5, +10, +20 ms with the transition zone (a chance level performance) located for –30 and –20 ms. These categories were established on the basis of literature (Rojczyk, 2010) and our previous studies (Szelag and Szymaszek, 2014; Oron et al., 2015) as well as were confirmed in the present study (ANOVA, see below).
- ^The data presented normal distribution.
References
Benasich, A. A., and Tallal, P. (2002). Infant discrimination of rapid auditory cues predicts later language impairment. Behav. Brain Res. 136, 31–49. doi: 10.1016/S0166-4328(02)00098-0
Bishop, D. V. M., Carlyon, R. P., Deeks, J. M., and Bishop, S. J. (1999). Auditory temporal processing impairment: neither necessary nor sufficient for causing language impairment in children. J. Speech Lang. Hear. Res. 42, 1295–1310. doi: 10.1044/jslhr.4206.1295
Chobert, J., François, C., Habib, M., and Besson, M. (2012). Deficit in the preattentive processing of syllabic duration and VOT in children with dyslexia. Neuropsychologia 50, 2044–2055. doi: 10.1016/j.neuropsychologia.2012.05.004
Cohen, W., Hodson, A., O’Hare, A., Boyle, J., Durrani, T., McCartney, E., et al. (2005). Effects of computer-based intervention through acoustically modified speech (Fast ForWord) in severe mixed receptive - expressive language impairment outcomes from a randomized controlled trial. J. Speech Lang. Hear. Res. 48, 715–729. doi: 10.1044/1092-4388(2005/049)
Doellinger, M., Burger, M., Hoppe, U., Bosco, E., and Eysholdt, U. (2011). Effects of consonant-vowel transitions in speech stimuli on cortical auditory evoked potentials in adults. Open Neurol. J. 5, 37–45. doi: 10.2174/1874205X01105010037
Eimas, P. D. (1991). “Comment: some effects of language acquisition on speech perception,” in Modularity and the Motor Theory of Speech, eds I. G. Mattingly and M. Studdert-Kennedy (Hillsdale, MI: Erlbaum), 111–116.
Fink, M., Churan, J., and Wittmann, M. (2006). Temporal processing and context dependency of phoneme discrimination in patients with aphasia. Brain Lang. 98, 1–11. doi: 10.1016/j.bandl.2005.12.00
Fitch, R. H., and Tallal, P. (2003). Neural mechanisms of language-based learning impairments: insights from human populations and animal models. Behav. Cogn. Neurosci. Rev. 2, 155–178. doi: 10.1177/1534582303258736
Gerrits, E., and de Bree, E. (2009). Early language development of children at familial risk of dyslexia: speech perception and production. J. Commun. Disord. 42, 180–194. doi: 10.1016/j.jcomdis.2008.10.004
Gillam, R. B., Loeb, D. F., Hoffman, L. M., Bohman, T., Champlin, C. A., Thibodeau, L., et al. (2008). The efficacy of Fast ForWord language intervention in school-age children with language impairment: a randomized controlled trial. J. Speech Lang. Hear. Res. 51, 97–119. doi: 10.1044/1092-4388(2008/007)
Giraud, A. L., Kleinschmidt, A., Poeppel, D., Lund, T. E., Franckowiak, R. S., and Laufs, H. (2007). Endogenous cortical rhythms determine cerebral specialization for speech perception and production. Neuron 56, 1127–1134. doi: 10.1016/j.neuron.2007.09.038
Given, B. K., Wasserman, J. D., Chari, S. A., Beattie, K., and Eden, G. F. (2008). A randomized, controlled study of computer-based intervention in middle school struggling readers. Brain Lang. 106, 83–97. doi: 10.1016/j.bandl.2007.12.001
Hoonhorst, I., Colin, C., Markessis, E., Radeau, M., Deltenre, P., and Serniclaes, W. (2009). French native speakers in the making: from language-general to language-specific voicing boundaries. J. Exp. Child Psychol. 104, 353–366. doi: 10.1016/j.jecp.2009.07.005
King, K. A., Campbell, J., Sharma, A., Martin, K., Dorman, M., and Langran, J. (2008). The representation of voice onset time in the cortical auditory evoked potentials of young children. Clin. Neurophysiol. 119, 2855–2861. doi: 10.1016/j.clinph.2008.09.015
Lasky, R. E., Syrdal-Lasky, A., and Klein, R. E. (1975). VOT discrimination by four to six and a half month old infants from Spanish environment. J. Exp. Child Psychol. 20, 215–225. doi: 10.1016/0022-0965(75)90099-5
Lisker, L., and Abramson, A. S. (1964). A cross-language study on voicing in initial stops. Acoustical measurements. Word 20, 384–422. doi: 10.1080/00437956.1964.11659830
McArthur, G. M., and Bishop, D. V. M. (2001). Auditory perceptual processing in people with reading and oral language impairments: current issues and recommendations. Dyslexia 7, 150–170. doi: 10.1002/dys.200
Merzenich, M. M., Jenkins, W. M., Johnston, P., Schreiner, C., Miller, S. L., and Tallal, P. (1996). Temporal processing deficits of language-learning impaired children ameliorated by training. Science 271, 81–84. doi: 10.1126/science.271.5245.77
Molfese, D. L. (1980). Hemispheric specialization for temporal information: implications for the perception of voicing cues during speech perception. Brain Lang. 11, 285–299. doi: 10.1016/0093-934X(80)90129-7
Obleser, J., Zimmermann, J., Van Meter, J., and Rauschecker, J. P. (2007). Multiple stages of auditory speech perception reflected in event-related fMRI. Cereb. Cortex 17, 2251–2257. doi: 10.1093/cercor/bhl133
Oron, A., Szymaszek, A., and Szelag, E. (2015). Temporal information processing as a basis for auditory comprehension: clinical evidence from aphasic patients. Int. J. Lang. Commun. Disord. 50, 604–615. doi: 10.1111/1460-6984.12160
Pahlke, F., König, I. R., and Ziegler, A. (2004). Randomization in treatment arms (RITA): ein randomisierungs-programm für klinische studien. Inform. Biomet. Epidemiol. Med. Biol. 35, 1–22.
Parbery-Clark, A., Anderson, S., Hittner, E., and Kraus, N. (2012). Musical experience offsets age-related delays in neural timing. Neurobiol. Aging 33, 1483.e1–1483.e4. doi: 10.1016/j.neurobiolaging.2011.12.015
Pöppel, E. (1997). A hierarchical model of temporal perception. Trends Cogn. Sci. 1, 56–61. doi: 10.1016/S1364-6613(97)01008-5
Pöppel, E. (2009). Pre-semantically defined temporal windows for cognitive processing. Philos. Trans. R. Soc. Ser. B Biol. Sci. 364, 1887–1896. doi: 10.1098/rstb.2009.0015
Pużyński, S., and Wciórka, J. (2000). Klasyfikacja zaburzeń psychicznych i zaburzeń zachowania w ICD-10. Opisy kliniczne i wskazówki diagnostyczne. Krakow-Warszawa: Uniwesyteckie Wydawnictwo Medyczne “Versalius”, Instytut Psychiatrii i Neurologii.
Rey, V., de Martino, S., Espesser, R., and Habib, M. (2002). Temporal processing and phonological impairment in dyslexia: effect of phoneme lengthening on order judgement of two consonants. Brain Lang. 80, 576–591. doi: 10.1006/brln.2001.2618
Rojczyk, A. (2010). Temporal and Spectral Parameters in Perception of the Voicing Contrast in English and Polish. Katowice: Wydawnictwo Uniwersytetu Śla̧skiego.
Strasburger, H. (2001). Converting between measures of slope of the psychometric function. Percept. Psychophys. 63, 1348–1355. doi: 10.3758/BF03194547
Streeter, L. A. (1976). Language perception of 2-month-old infants shows effects of both innate mechanisms and experience. Nature 259, 39–41. doi: 10.1038/259039a0
Sussman, J. E. (1993). Perception of formant transition cues to place of articulation in children with language impairments. J. Speech Hear. Res. 36, 1286–1299. doi: 10.1044/jshr.3606.1286
Swisher, L., and Hirsh, I. J. (1972). Brain damage and the ordering of two temporally successive stimuli. Neuropsychologia 10, 137–152. doi: 10.1016/0028-3932(72)90053-X
Szelag, E., Dacewicz, A., Szymaszek, A., Wolak, T., Senderski, A., Domitrz, I., et al. (2015). The application of timing in therapy of children and adults with language disorders. Front. Psychol. 12:1714. doi: 10.3389/fpsyg.2015.01714
Szelag, E., Lewandowska, M., Wolak, T., Seniow, J., Poniatowska, R., Pöppel, E., et al. (2014). Training in rapid auditory processing ameliorates auditory comprehension in aphasic patients: a randomized controlled pilot study. J. Neurol. Sci. 338, 77–86. doi: 10.1016/j.jns.2013.12.020
Szelag, E., and Szymaszek, A. (2014). Test do Badania Rozumienia mowy u dzieci i dorosłych: Nowe spojrzenie na zegar mózgowy. Sopot: GWP.
Szelag, E., and Szymaszek, A. (2016). Dr Neuronowski - Pomysł na Bystry umysł i płynna̧ mowę. Usprawnianie zegara neuronalnego wspomaga moc naszego umysłu. Poznań: Harpo.
Szustrowa, T., and Jaworowska, A. (2003). Manual for Raven’s Progressive Matrices. Coloured Progressive Matrices. Warszawa: Polskie Towarzystwo Psychologiczne.
Szymaszek, A., Wolak, T., and Szelag, E. (2017). The treatment based on temporal information processing reduces speech comprehension deficits in aphasic subjects. Front. Aging Neurosci. 11:98. doi: 10.3389/fnagi.2017.00098
Tallal, P. (1975). A different view of “auditory processing factors in language disorders”. J. Speech Hear. Disord. 40, 413–415. doi: 10.1044/jshd.4003.413
Tallal, P., Miller, S., and Fitch, R. H. (1995). Neurobiological basis of speech: a case for the preeminence of temporal processing. Irish J. Psychol. 16, 194–219. doi: 10.1080/03033910.1995.10558057
Tallal, P., Miller, S. L., Bedi, G., Byma, G., Wang, X., Nagarajan, S. S., et al. (1996). Language comprehension in language-learning impaired children improved with acoustically modified speech. Science 271, 81–84. doi: 10.1126/science.271.5245.81
Tallal, P., and Piercy, M. (1973). Deficits of non-verbal auditory perception in children with developmental aphasia. Nature 241, 468–469. doi: 10.1038/241468a0
Tallal, P., and Piercy, M. (1974). Developmental aphasia: rate of auditory processing and selective impairment of consonant perception. Neuropsychologia 12, 83–93. doi: 10.1016/0028-3932(74)90030-X
Tomblin, J. B., Records, N. L., Buckwalter, P., Zhang, X., Smith, E., and O’Brien, M. (1997). Prevalence of specific language impairment in kindergarten children. J. Speech Lang. Hear. Res. 40, 1245–1260. doi: 10.1044/jslhr.4006.1245
Treutwein, B. (1995). Adaptive psychophysical procedures. Vis. Res. 35, 2503–2522. doi: 10.1016/0042-6989(95)00016-X
Treutwein, B., and Strasburger, H. (1999). Fitting the psychometric function. Percept. Psychophys. 61, 87–106. doi: 10.3758/BF03211951
Vander Werff, K. R., and Burns, K. S. (2011). Brain stem responses to speech in younger and older adults. Ear Hear. 32, 168–180. doi: 10.1097/AUD.0b013e3181f534b5
Vandewalle, E., Boets, B., Ghesquière, P., and Zink, I. (2012). Auditory processing and speech perception in children with specific language impairment: relations with oral language and literacy skills. Res. Dev. Disabl. 33, 635–644. doi: 10.1016/j.ridd.2011.11.005
von Steinbüchel, N., Wittmann, M., Strasburger, H., and Szelag, E. (1999). Auditory temporal order judgment is impaired in patients with cortical lesions in posterior regions of the left hemisphere. Neurosci. Lett. 264, 168–171. doi: 10.1016/S0304-3940(99)00204-9
Wittmann, M., Burtscher, A., Fries, W., and von Steinbüchel, N. (2004). Effects of brain-lesion size and location on temporal-order judgment. Neuroreport 15, 2401–2405. doi: 10.1097/00001756-200410250-00020
Wittmann, M., and Fink, M. (2004). Time and language-critical remarks on diagnosis and training methods of temporal-order judgment. Acta Neurobiol. Exp. 64, 341–348.
Zaehle, T., Wüstenberg, T., Meyer, M., and Jäncke, L. (2004). Evidence for rapid auditory perception as the foundation of speech processing: a sparse temporal sampling fMRI study. Eur. J. Neurosci. 20, 2447–2456. doi: 10.1111/j.1460-9568.2004.03687.x
Ziegler, J. C., Pech-Georgel, C., George, F., Alario, F. X., and Lorenzi, C. (2005). Deficits in speech perception predict language learning impairment. Proc. Natl. Acad. Sci. U.S.A. 102, 14110–14115. doi: 10.1073/pnas.0504446102
Keywords: temporal information processing (TIP), specific language impairment (SLI), voice-onset-time, phonetic identification, temporal intervention, voicing contrast detection
Citation: Szymaszek A, Dacewicz A, Urban P and Szelag E (2018) Training in Temporal Information Processing Ameliorates Phonetic Identification. Front. Hum. Neurosci. 12:213. doi: 10.3389/fnhum.2018.00213
Received: 20 October 2017; Accepted: 08 May 2018;
Published: 06 June 2018.
Edited by:
Deana Davalos, Colorado State University, United StatesReviewed by:
Marianne Latinus, INSERM U930 Imagerie et Cerveau, FranceYoshitaka Nakajima, Kyushu University, Japan
Copyright © 2018 Szymaszek, Dacewicz, Urban and Szelag. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aneta Szymaszek, YS5zenltYXN6ZWtAbmVuY2tpLmdvdi5wbA==
 Aneta Szymaszek
Aneta Szymaszek Anna Dacewicz
Anna Dacewicz Paulina Urban
Paulina Urban Elzbieta Szelag
Elzbieta Szelag