- 1College of Teacher Education, Guangdong University of Education, Guangzhou, China
- 2Shenzhen Bao’an Traditional Chinese Medicine Hospital, Guangzhou University of Chinese Medicine, Shenzhen, China
- 3The Second Clinical College, Guangzhou University of Chinese Medicine, Guangzhou, Guangdong, China
- 4Key Laboratory of Brain, Cognition and Education Sciences (South China Normal University), Ministry of Education, Guangzhou, Guangdong, China
- 5School of Psychology, Center for Studies of Psychological Application, and Guangdong Key Laboratory of Mental Health and Cognitive Science, South China Normal University, Guangzhou, China
Acupuncture, a cornerstone of Chinese integrative medicine, demonstrates clinical efficacy for pain- and inflammation-related conditions through extensive clinical practices. Using multimodal neuroimaging techniques (fMRI and PET), numerous studies have investigated acupuncture’s impact on brain structure and function. However, a systematic integration of these findings is still lacking. To address this issue, we conducted a meta-analysis using the activation likelihood estimation (ALE) approach to identify brain regions that consistently modulated by verum acupuncture in both healthy populations and patients. Critically, establishing neural signatures in healthy populations provides a foundational baseline for normative brain responses, which is essential to detect pathological deviations in patient cohorts. Subsequent behavioral domain analyses elucidated the functional profiles of these significant clusters. To characterize whole brain functional connectivity architecture that associated with identified regions, we employed meta-analytic connectivity modeling (MACM) and seed-based resting-state functional connectivity (rsFC) analyses. Our analyses consistently implicated the bilateral inferior parietal lobule (extending into postcentral gyrus) and right thalamus, key regions within the somatosensory pain processing network, as core neural substrates that modulated by acupuncture in healthy populations. Functional connectivity analyses further demonstrated that these brain clusters engaged a distributed pain-processing network, including bilateral supplementary motor area (SMA), superior temporal gyrus (STG), and insular cortex. Taken together, these findings provide a mechanistic neurobiological basis for acupuncture-induced pain processing and identify potential therapeutic targets within the pain-associated neural circuitry.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD42024562566, identifier CRD42024562566.
1 Introduction
Acupuncture, a complementary therapy that rooted in traditional Chinese medicine, demonstrates significant clinical potentials in modulating physiological functions and alleviating symptoms across diverse disorders (Kaptchuk, 2002). This core procedure involves inserting needles into specific anatomical points (acupoints), producing measurable therapeutic effects, particularly analgesia (Lin and Chen, 2008). The therapeutic foundation of acupuncture rests on stimulating specific acupoints along meridian pathways to regulate corresponding organ functions and systemic homeostasis. Clinically, the sensory sensation known as “Deqi,” that usually characterized by descriptors such as soreness (suan), numbness (ma), distension (zhang), and pain (tong), is often considered indicative of effective needle manipulation and physiological engagement (Yang et al., 2013). To enhance therapeutic efficacy, traditional manual needle manipulation frequently incorporate some adjunctive techniques like electroacupuncture or laser stimulation (Jun et al., 2015). Despite its established clinical utility, the precise neural mechanisms underpinning acupuncture’s modulation of sensory processing and broader therapeutic effects remain incompletely understood, particularly in pain management.
Given acupuncture’s capacity to modulate brain activity, it serves as a valuable tool for probing neural mechanisms using non-invasive functional imaging techniques (Cao et al., 2020; Dhond et al., 2007). Notably, functional magnetic resonance imaging (fMRI), particularly resting-state (rs-fMRI) which captures intrinsic brain dynamics without task demands, is capable to characterize acupuncture-induced neural responses in both healthy populations and patients (Cai et al., 2018; Feng et al., 2011; Hui et al., 2009). Compared to sham acupuncture, verum acupuncture in healthy populations typically elicited activation in several pain-processing regions (e.g., insular, thalamus, anterior cingulate cortex, postcentral gyrus, and supplementary motor cortex) and hypoactivation in limbic and default mode network (Chae et al., 2013). In clinical practice, patients with comorbid psychiatric and neurological disorders frequently suffer from refractory pain syndromes, including depression-associated pain, migraines and post-stroke pain. Previous neuroimaging studies (Lin et al., 2025; Qian et al., 2025; Wu et al., 2017) found that verum acupuncture-induced neural modulations were primarily centered in sensory regions and insular, while heterogeneous activity in other brain regions might reflect pathological signatures with specific diseases. Consistent with fMRI findings, positron emission tomography (PET) demonstrated congruent regional perfusion and metabolic changes within these brain areas (Huang et al., 2007; Lai et al., 2009; Yang et al., 2014). Therefore, these findings indicate a core set of brain regions, which is critical for processing acupuncture’s somatic effects and mediating its broader physiological regulation. For instance, the insular cortex acts as an integrative hub linking sensory inputs with autonomic networks for homeostatic control. The thalamus and postcentral gyrus are fundamental for transforming peripheral inputs into consciously perceived perception (Rao et al., 2024). However, bridging acupuncture’s clinical efficacy with the inconsistently neural substrates remains a critical barrier to its mechanistic understanding. Substantial heterogeneity across studies in populations (healthy vs. patient), experimental designs (task vs. rest, verum vs. sham), as well as methodological approaches, has obstructed the identification of reproducible neural signatures supporting acupuncture’s core mechanisms.
To establish robust neural signatures of acupuncture, we conducted the activation likelihood estimation (ALE) meta-analysis approach for quantifying spatial convergence across neuroimaging studies (Eickhoff et al., 2009, 2012). ALE statistically models reported peak coordinates (foci) from individual neuroimaging studies as spatial probability distributions. Significant spatial convergence of foci across studies is identified by comparing the modeled results against an empirically derived null distribution of random spatial association. This method has demonstrated robust reliability in synthesizing spatially heterogeneous neuroimaging findings across cognitive, affective, and clinical domains. Although prior meta-analyses have examined acupuncture’s neural correlates of acupuncture (Huang et al., 2012; Park et al., 2017), the evolving literature necessitates an updated synthesis by incorporating contemporary findings. In this current study, we hypothesize that specific brain circuits, particularly within the central pain-processing network, exhibit sufficient reproducibility across independent datasets and may serve as candidate biomarkers underlying acupuncture’s therapeutic mechanism. To address this gap and test our hypothesis, we employ ALE meta-analysis to systematically identify consistent brain regions modulated by verum acupuncture across published fMRI and PET studies in both healthy individuals and patient populations.
2 Materials and methods
2.1 Literature search and study criteria
Neuroimaging studies utilizing acupuncture interventions were identified through systematic literature searches in PubMed, Web of Science, and Scopus. The search strategy employed combinations of the following key terms: “functional magnetic resonance imaging,” “acupuncture,” and “positron emission tomography.” After removing duplicates, 170 neuroimaging studies were retained for further analysis. Inclusion criteria were defined as follows:
(1) The paper was written in English and strictly reviewed before publication.
(2) Multimodal acupuncture approaches were included, such as traditional needle acupuncture, electroacupuncture, and laser acupuncture.
(3) Acupoints must be conducted in human subjects aged at least 18 years instead of animal models or computational stimulations.
(4) During statistical analysis, whole brain voxel-wise comparison is necessary to extract the specific coordinates for each experiment. Thus, studies using region-of-interest (ROI)-based analysis or functional connectivity were not taken into consideration.
(5) Study contrasts contained two types: pretreatment vs. posttreatment and sham control vs. real acupuncture.
(6) Significant findings of neuroimaging research were reported as 3D coordinates in Talairach or Montreal Neurological Institute (MNI) space.
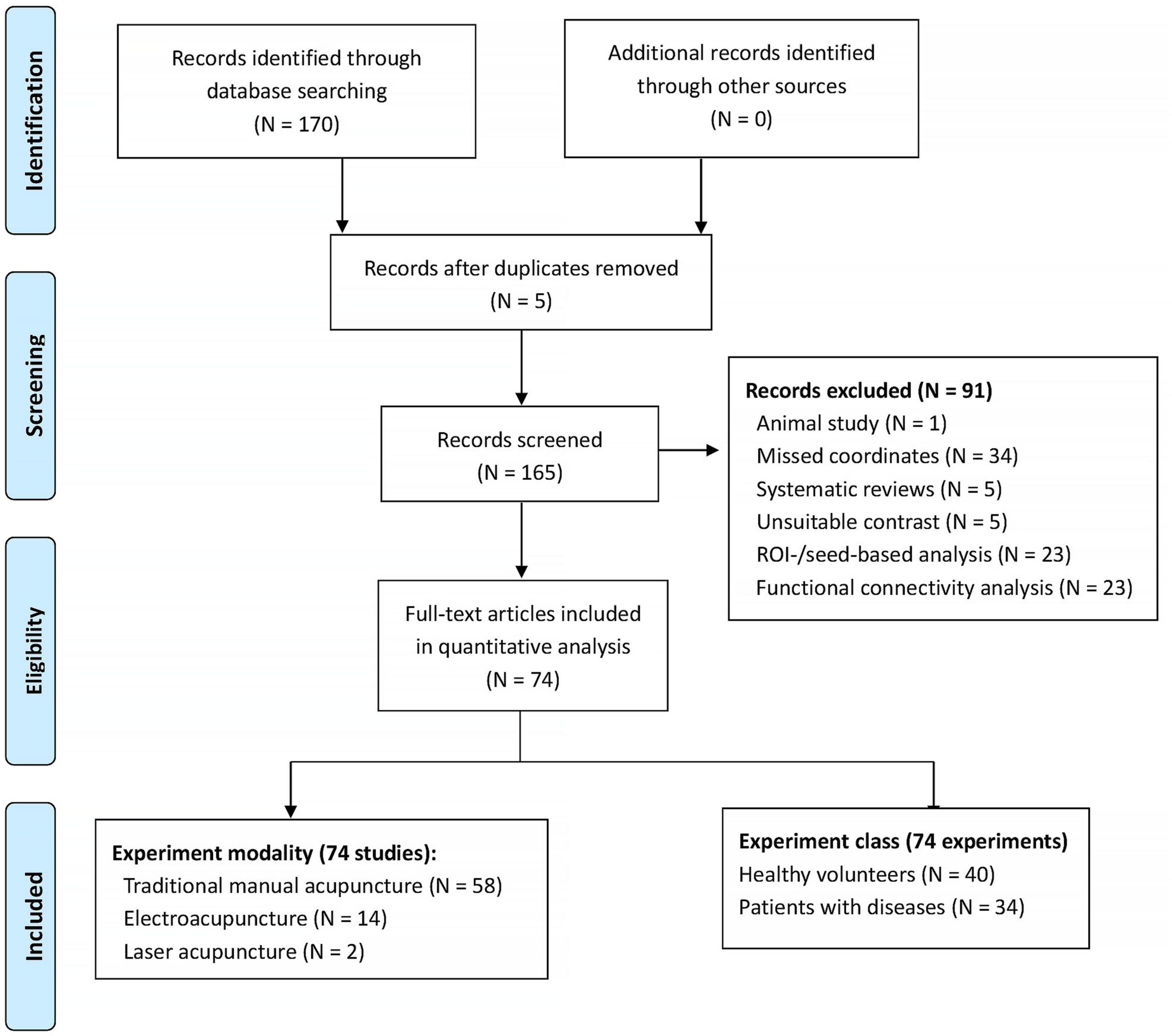
Given potential divergences in acupuncture’s neuroimaging effects between healthy populations and clinical cohorts, we conducted separate literature searches for each group. The study encompassed diverse clinical conditions, such as ischemic stroke, migraine, depression, and chronic pain syndromes. For each experiment, the statistical threshold was set at p < 0.05 level. Multiple comparisons correction was not mandatory and depended on the study. After excluding those studies without any coordination information, we extracted the coordinates for each experiment for further analysis. For the same neuroimaging datasets with different statistical approaches, we combined their coordinates to avoid potential statistical bias. The literature review was finalized by the end of June 2024. This review study has been registered at PROSPERO (CRD42024562566). Figure 1 shows the basic flowcharts of our literature research. For the detailed characteristics of the included literatures, please refer to Supplementary Table 1 in the supplemental materials.
2.2 ALE analysis
In this study, we applied ALE meta-analysis approach to examine spatial convergences over all of the foci (that is, the coordinates) across neuroimaging studies, which was computed using GingerALE3.0.2. This approach calculates spatial convergence of activation foci against a null hypothesis of random spatial distribution, thereby identifying statistically significant clusters within distinct brain areas. Firstly, the ALE model estimation generated a modeled activation (MA) map that was built by the Gaussian distributions. The full-width half-maximum (FWHM) was determined by the sample size of corresponding experiment. Subsequently, we calculated the union of the MA maps across all inclusion experiments for each voxel and analyzed the significances using a cluster-thresholded approach. Consistent with a priori ALE study (Muller et al., 2018), we determined a significance threshold was set at a cluster-level inference of p < 0.05 and a cluster-forming threshold of p < 0.001. To address potential confounds from experimental paradigms, we conducted independent ALE meta-analyses for two subgroups: sham acupuncture vs. real acupuncture, and pre-acupuncture vs. post-acupuncture conditions. In addition, we methodically excluded 4 non-fMRI studies in healthy populations and 3 non-fMRI studies in patient cohorts for resolving the modality-specific differences between PET and fMRI findings.
2.3 Behavioral domain (BD) analysis
Following ALE analysis, the operation functions of each significant brain cluster was identified using behavioral domain analysis. This behavioral system is classified into six primary domains: cognition, action, perception, emotion, interoception, or pharmacology. We utilized the Brain Annotation Toolbox (Liu et al., 2019) to identify those functional profiles of significant clusters based on Neurosynth activation maps (Yarkoni et al., 2011). The mode of permutation analysis was settled as “clusters” and number of permutation times was 1000. The brain functions significantly associated with the given ROIs at p < 0.05 level.
2.4 Meta-analytic connectivity modeling (MACM) approach
We performed meta-analytic connectivity modeling (MACM) approach to characterize whole-brain co-activation profiles for each significant cluster derived from ALE analysis (Laird et al., 2009). Using this technique, a well-known meta-analysis of whole brain functional connectivity patterns with the amygdala was conducted (Robinson et al., 2010). Using the peak coordinates of each significant brain cluster, we created spherical regions of interest (ROIs) with an 8-mm diameter. Then, we searched all experiments that discovered increased activation (that is, Context-Normal Mapping, Activation-Activations only, and Diagnosis-Normals) for each ROI. Given the outcomes of our behavioral domain analysis, we restricted the task-related condition to Somesthesis-Pain in Perception. The whole brain coordinates in matching-search criteria literature were downloaded from the BrainMap database (Laird et al., 2005). By using GingerALE3.0.2, significant convergences of functionally connected regions were identified at a cluster-level inference of p < 0.05 and cluster-forming threshold of p < 0.001 (Eickhoff et al., 2017).
2.5 Seed-based functional connectivity (FC) analysis in healthy populations
To reproduce the whole-brain FC patterns in real resting-state fMRI datasets, we used Zang’s public datasets from the 1000 Functional Connectomes Project1 to perform the seed-based FC analysis. We totally recruited 185 young volunteers (70 males, age: 21.19 ± 1.84 years old). Using a 3T MRI scanner, both a resting-state fMRI dataset (matrix size = 64 × 64 mm2, voxel size = 3.125 × 3.125 × 3.6 mm3, TR = 2 s, number of slices = 33, 225 volumes) and 3D T1-weighted structural images were archived. We preprocessed the rs-fMRI images by using DPABI_v6.0 (Yan et al., 2016) and SPM12. The main procedures included: first 10 volumes were discarded, Slice timing, Realignment and motor correction (exclusion criteria: head transitions < 1.5 mm, rotations < 1.5°, or mean Framewise Displacement < 0.27 mm), Covariances regression (Friston 24-motion parameters, signals from cerebrospinal fluid, and signal from white matter), Spatial normalization in MNI standard space by DARTEL method, Smooth with 8 mm3 FWHM, Linear detrend, and Bandpass filtering (0.01∼0.08 Hz). Then, we selected the ALE-derived cluster as the seed and calculated functional connectivity with other voxels over the whole brain. After normalizing all the FC maps, one-sample t-tests were applied to examine significant brain clusters (significant level at p < 0.01 with Bonferroni correction).
3 Results
3.1 ALE analysis
Finally, we separately identified 39 studies (38 experiments, 723 subjects, and 1032 foci) in the healthy populations and 28 studies (32 experiments, 918 subjects, and 434 foci) in the patients. We only found that the bilateral inferior parietal lobule/postcentral gyrus (IPL/PostCG, BA40, peak: 62, −18, 18 and −62, −20, 18) and right thalamus (THAL, peak: 8, −12, 6) were significantly convergent in the healthy participants (Figures 2A, B). For the healthy populations, there were 16 experiments comparing sham and real acupuncture conditions and 22 experiments utilizing pre-post or block design methodologies. However, we did not observe any significances in the patients with a variety of diseases. For the sham and real acupuncture comparison, our analysis revealed significantly convergent activity in the bilateral IPL/PostCG (peak: 64, −16, 16 and −60, −18, 18) and right thalamus (peak: 8, −12, 6). In the pre-post subgroup, significantly convergent activities were localized to the right IPL/PostCG (peak: 64, −18, 18). These finding robustly align with our core findings (IPL/PostCG and thalamus) that derived from sham-controlled experimental design in the healthy populations. Moreover, our results only revealed significant convergences of neural activity in the bilateral IPL/PostCG (peak: 62, −22, 22 and −62, −20, 22) and right thalamus (peak: 8, −14, 4) for the fMRI datasets of healthy subjects.
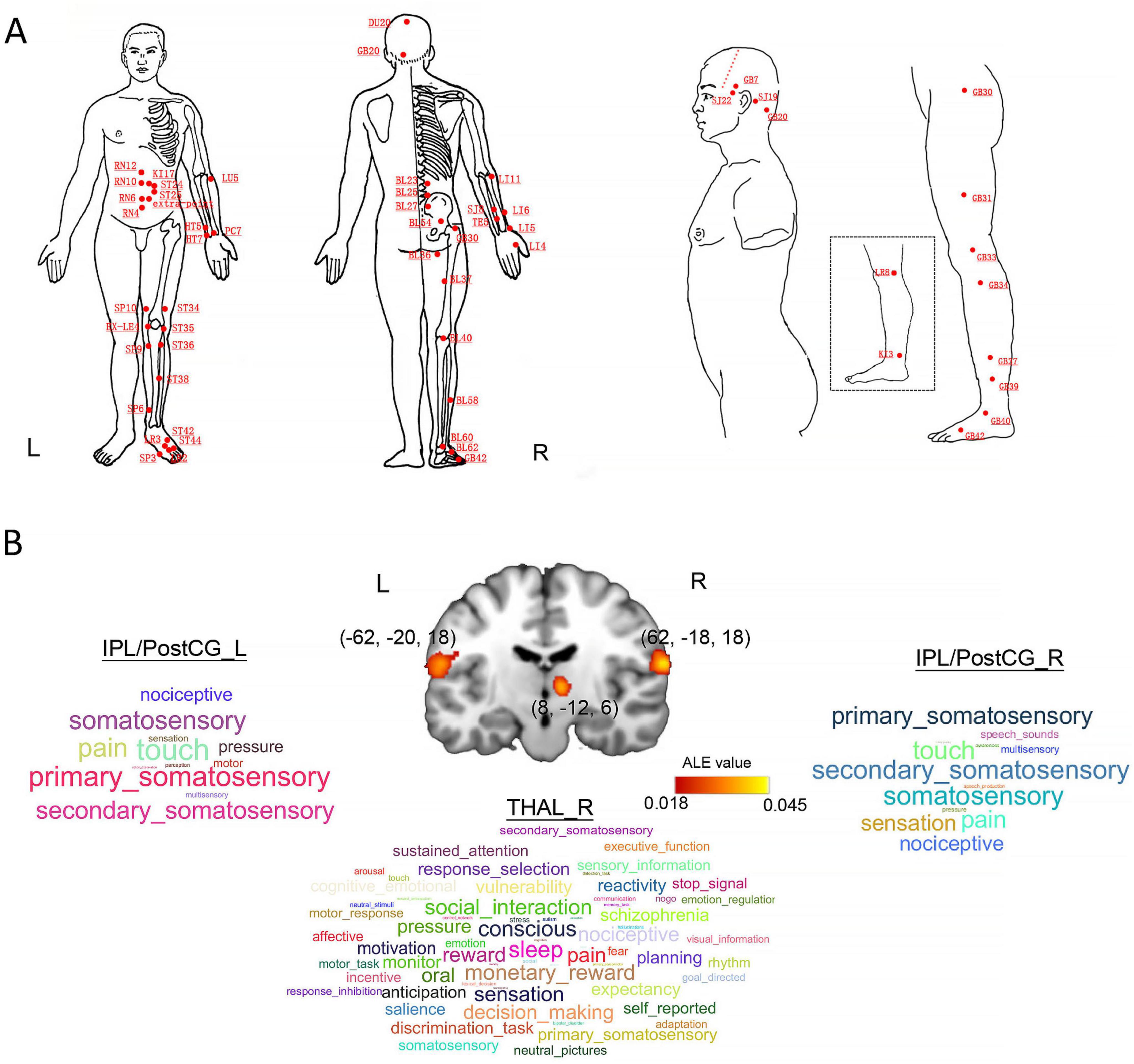
Figure 2. Locations of acupoints, statistical results for the ALE meta-analysis study, and behavioral domain (BD) analysis. (A) All of the relevant acupoints in those selected literatures. (B) Visualized demonstration of our ALE meta-analysis and corresponding behavioral domains for each brain cluster. CV4, Guanyuan; CV6, Qihai; CV10, Xiawan; CV12, Zhongwan; KI17, Shangqu; ST24, Huaroumen; ST25, Tianshu; HT5, Tongli; HT7, Shenmen; LU5, Qi-ze; PC7, Daling; SP10, Xuehai; EX-LE4, Neixiyan; SP3, Taibai; SP6, Sanyinjiao; SP9, Yinlingquan; ST34, Liangqiu; ST35, Dubi; ST36, Zusanli; ST38, Tiaokou; ST42, Chongyang; ST44, Neiting; LR2, Xingjian; SP3, Taibai; BL23, Shenshu; BL25, Dachangshu; BL27, Xiaochangshu; BL36, Chengfu; BL54, Zhibian; BL37, Yinmen; BL40, Weizhong; BL58, Feiyang; BL60, Kunlun; BL62, Shenmai; GB42, Diwuhui; LR8, Ququan; KI3, Taixi; GB31, Fengshi; GB33, Xiyangguan; GB34, Yanglingquan; GB37, Guangming; GB39, Xuanzhong; GB40, Qiuxu; GB42, Diwuhui; TE22, Erheliao; GB7, Qubin; TB19, Sanyangluo; GB20, Fengchi. IPL/PostCG, inferior parietal lobule/postcentral gyrus; THAL, thalamus.
3.2 Behavioral domain results
We identified that the right IPL/PostCG and left IPL/PostCG were significantly associated with 12 and 13 functions with a significant level at p < 0.05, such as pain, sensation, perception, and primary somatosensory (Figure 2B). Similarly, Figure 2B also presents that the right thalamus is significantly correlated with 66 functions, including pain, consciousness, nociceptive, and sensation.
3.3 MACM results
After searching the eligible neuroimaging literatures, our study acknowledged 108 experiments with 1590 participants for the right IPL/PostCG, 189 experiments with 2757 participants for the left IPL/PostCG, as well as 391 experiments with 6031 participants for the right thalamus. Using the MACM approach, we detected some common regions that were significantly coactivated, including the bilateral supplemental motor areas, bilateral superior temporal gyrus, bilateral putamen, and bilateral insular (Figure 3).
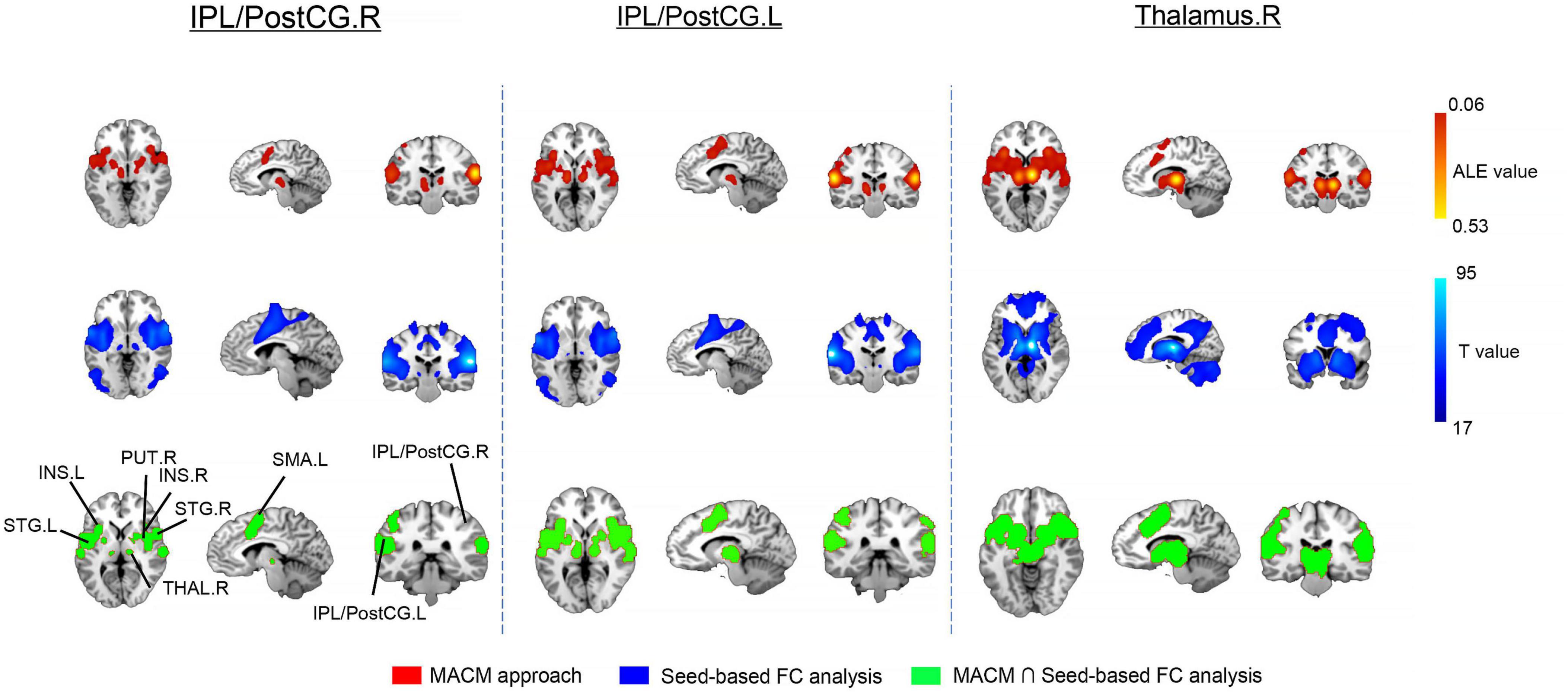
Figure 3. Results of meta-analytic connectivity modeling (MACM) and whole brain seed-based functional connectivity (FC) analysis in healthy participants. For the latter analysis, we only retained 80% top for the significant brain clusters. IPL/PostcG, inferior parietal lobule/postcentral gyrus; INS, insular; SMA, supplemental motor areas; THAL, thalamus; STG, superior temporal gyrus; PUT, putamen; R, right; L, left.
3.4 Seed-based whole brain FC analysis
For all ROIs, our statistical maps were thresholded at p < 0.01 with Bonferroni correction and further reported widely distributed brain clusters. Noticeably, the bilateral supplemental motor areas, bilateral putamen, and bilateral insular that functionally connected with the bilateral IPL/PostCG, and right thalamus in the healthy participants as well (Figure 3).
4 Discussion
Our ALE meta-analysis establishes robust neural substrates underlying verum acupuncture, revealing consistently convergent patterns across neuroimaging studies. Crucially, significant clusters within the bilateral IPL/PostCG and right thalamus emerged as core neural signatures modulated by acupuncture in healthy populations. Behavioral domain analysis revealed that these brain regions are functionally specialized for somatosensory processing with distinct roles in pain perception, nociception, and multisensory integration. Importantly, MACM revealed significant functional coupling between these key regions (the bilateral IPL/PostCG and right thalamus) and critical hubs of the central pain-processing network. This interconnected circuitry primarily encompassed the bilateral SMA, STG, insular, and putamen. Moreover, this functional network architecture was validated independently by whole brain seed-based FC analysis using rs-fMRI dataset from 185 healthy young adults. To sum up, these convergent multimodal findings highlight the bilateral IPL/PostCG and right thalamus as pivotal neural substrates. These regions orchestrate a distributed but functionally integrated network underlying acupuncture’s effects, providing a mechanistic framework for investigating acupuncture-induced pain processing and advancing its clinical translation. While informative for elucidating the neurophysiological foundations of acupuncture, these findings may not directly translate to clinical analgesic mechanisms due to the lack of significant convergence in patient-derived neuroimaging datasets. Future research contrasting neural responses between healthy controls and clinical cohorts will be essential to identify therapeutic-specific neural substrates.
Following verum acupuncture, our meta-analysis robustly identified the bilateral IPL/PostCG and right thalamus as core targets in healthy individuals. This consistent neural activity establishes their role in mediating acupuncture’s modulatory effects on somatic sensation, particularly pain perception. Of note, neuroimaging studies (Jin et al., 2018; Li L. L. et al., 2018; Nierhaus et al., 2015) targeting major limb acupoints (e.g., Sanzuli, Sanyinjiao, and Tongli) reliably engage these brain areas. Behavioral domain analysis further clarifies that the bilateral IPL/PostCG sustains somesthetic awareness and directs spatial attention to bodily stimuli, potentially induced by self-reported deqi sensation during needle manipulation. The IPL’s specialization in mediating spatially-directed bodily attention is well-established (Jung et al., 2015; Vandenberghe et al., 2001) and aligns with our findings. Crucially, this neural activity pattern was failed to reproduce in our patient populations, revealing disease-specific modulation of acupuncture’s neural targets. Although several fMRI studies have reported IPL/PostCG engagement in patients (Cho et al., 2013; Li Z. et al., 2018; Tan et al., 2017), the absence of significant spatial convergence in our meta-analysis suggests fundamentally distinct pain regulatory mechanisms in clinical populations. We hypothesize that this divergence may arises from dysfunctional attentional allocation toward somatic signals or reorganized functional integration within core pain-processing circuits in patients. As the key hub in sensory pathways, the thalamus gates conscious pain perception and dynamically facilitates sensory transmission to cortical regions (Chen et al., 2014). In patients, acupuncture modulated thalamocortical functional connectivity associated with motor-related brain areas (Li Z. et al., 2018; Xie et al., 2014), suggesting adaptation of neurocircuitry for therapeutic analgesia. In summary, our findings suggest that verum acupuncture in healthy individuals consistently modulates neural activity within the bilateral IPL/PostCG and right thalamus, which are core hubs for somatic sensory processing and allocations of bodily attention.
Our MACM and seed-based FC analyses converged to reveal a distributed brain network with key hubs in the bilateral IPL/PostCG and right thalamus, exhibiting extensive functional connectivity to the insular, SMA, and STG. This circuit subserves integrated sensory processing, pain modulation, as well as higher-order cognitive evaluation of bodily state awareness (Duerden and Albanese, 2013; Legrain et al., 2011; Price, 2000; Tian et al., 2025). In addition, this functional network architecture demonstrates significant reproducibility across neuroimaging studies employing diverse acupuncture protocols (Jung et al., 2015; Theysohn et al., 2014). Within this circuitry, the insular serves as a pivotal integration hub, synthesizing sensory inputs with cognitive and affective processes to form an interoceptive representation of bodily states (Kurth et al., 2010). As demonstrated in our study and existing literatures, the insula serves as a core node within the pain matrix in healthy individuals, which facilitates pain integration and interoceptive processing (Kong et al., 2006; Simone et al., 2025). In patients with chronic low back pain, investigator observed that low-frequency oscillations in the insula were associated with immediate pain alleviation following ankle acupuncture (Xiang et al., 2019). By synthesizing these findings with prior evidence (Lai et al., 2025; Wen et al., 2021; Zyloney et al., 2010), we propose that acupuncture stimulation may alter the brain responses to pain stimuli by interfering with the functional connectivity between insular and other brain region. Further research remains necessary to validate this hypothesis. Supplementary motor area, a key region for motor planning and pain modulation, was activated by stimulating specific acupuncture points (Han et al., 2019). Therefore, our findings delineate a core large-scale whole brain functional architecture that consistently modulated by verum acupuncture with diverse protocols in the healthy populations. This network functionally integrates sensory-discriminative components (IPL/PostCG and thalamus), affective-cognitive regions (insular and STG), and motor-sensory gating (SMA), providing a neurobiological framework for acupuncture’s simultaneous modulation of pain perception and somatic awareness.
Given the spatial convergence of brain regions, our findings further identify attentional modulation as a plausible central mechanism underlying the acupuncture’s therapeutic effects. This interpretation is grounded in the well-established Tripartite Attention Network Framework, encompassing alerting, orienting, and executive control systems (Posner and Dehaene, 1994). Specifically, the alerting network facilitates rapid detection of salient stimuli by transiently suppressing the executive control network, thereby prioritizing sensory and cognitive processing of relevant events (Callejas et al., 2004; Posner and Dehaene, 1994). That is, it monitors threats to bodily integrity and coordinates defensive responses to noxious stimuli (Apkarian et al., 2005; Reddan and Wager, 2018; Legrain et al., 2011). As core acupuncture targets, we propose that right IPL acts as a key neural substrate for sustaining attentional focus on salient somatic states. Therefore, we hypothesize that acupuncture-induced activity in this key region is to mediate the dynamic coordination between the alerting and executive control attentional systems. This mechanism of inter-network attentional recalibration provides a unified neurocognitive model for acupuncture’s clinical efficacy, particularly its capacity to normalize threat-response patterns to bodily signals through dynamic network reconfiguration (Corbetta and Shulman, 2002; Sarter et al., 2001). Nevertheless, further empirical investigation is required to characterize this mechanism across heterogeneous populations.
Though our findings suggest a consistent neural signature for acupuncture’s effect, several limitations merit rigorous consideration. First of all, substantial methodological variability across neuroimaging studies introduces inherent clinical and neurobiological heterogeneity, including diverse acupoint selections, treatment modalities, patient populations, and control conditions. These heterogeneities impede the clinical translatability of our results and underscore the need for mechanistic stratification in future neuroimaging protocols. Secondly, our meta-analysis synthesized evidences from 32 experiments reporting 434 coordinate foci in the patients. While the ALE methodology is robust to moderate sample sizes, the statistical power still remains constrained for detecting disease-specific effects. Larger-scale prospective neuroimaging studies employing standardized designs are essential to replicate findings and improve effect size estimation. Longitudinal neuroimaging protocols integrating chronic treatment schedules and precise temporal sampling are imperative to elucidate the neuroplastic mechanisms mediating acupuncture’s therapeutic effects. In the end, the inclusion of heterogeneous acupuncture modalities prevents definitive attribution of network modulations to core neurobiological mechanisms owing to the potential technique-specific artifacts. Consequently, we should systematically resolve these variabilities through strict coordinate inclusion criteria and comprehensive subgroup analyses. These limitations delineate essential directions for future research. Future studies integrating mechanism-driven experimental designs, larger samples, temporal profiling, and modality comparisons are needed to transform our neural signatures into clinical translation of acupuncture’s effects.
5 Conclusion
In summary, our findings map consistent neuroanatomical responses to verum acupuncture stimulation in healthy individuals, primarily involving the bilateral IPL/PostCG and right thalamus. These regions are implicated in somatosensory processing and may reflect neural encoding of needling perception rather than disease-modifying effects. Furthermore, we characterized a distributed network that underlies the pain modulation, including bodily signal processing, pain modulation, and allocation to bodily states. Critically, these patterns represent acute neurophysiological responses in pain-free subjects and should be further validated therapeutic efficacy for clinical pain. The absence of convergent fMRI findings in patient cohorts underscores that acupuncture’s putative analgesic mechanisms in pathological states remain unconfirmed by this study.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
CL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. YL: Conceptualization, Writing – review & editing. BN: Supervision, Validation, Writing – review & editing. LW: Writing – review & editing. YZ: Writing – review & editing. JZ: Data curation, Software, Writing – review & editing. DZ: Formal analysis, Project administration, Resources, Supervision, Validation, Writing – review & editing. PZ: Formal analysis, Project administration, Validation, Writing – review & editing. WF: Funding acquisition, Project administration, Resources, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This project is supported by the Joint Funds of the National Natural Science Foundation of China (Grant No. U23A20507); the Innovative Research Team of Acupuncture for Depression and Related Disorders, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine (Guangdong Provincial Hospital of Chinese Medicine), China; Key-Area Research and Development Program of Guangdong Province (2020B1111100007), Shenzhen Bao’an Research Center for Acupuncture and Moxibustion (BAZJ2018239), and Sanming Project of Medicine in Shenzhen (SZSM201806077).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnhum.2025.1494267/full#supplementary-material
Footnotes
References
Apkarian, A. V., Bushnell, M. C., Treede, R. D., and Zubieta, J. K. (2005). Human brain mechanisms of pain perception and regulation in health and disease. Eur. J. Pain 9, 463–484. doi: 10.1016/j.ejpain.2004.11.001
Cai, R. L., Shen, G. M., Wang, H., and Guan, Y. Y. (2018). Brain functional connectivity network studies of acupuncture: A systematic review on resting-state fMRI. J. Integr. Med. 16, 26–33. doi: 10.1016/j.joim.2017.12.002
Callejas, A., Lupiánez, J., and Tudela, P. (2004). The three attentional networks: On their independence and interactions. Brain Cogn. 54, 225–227. doi: 10.1016/j.bandc.2004.02.012
Cao, J., Tu, Y., Wilson, G., Orr, S. P., and Kong, J. (2020). Characterizing the analgesic effects of real and imagined acupuncture using functional and structure MRI. Neuroimage 221:117176. doi: 10.1016/j.neuroimage.2020.117176
Chae, Y., Chang, D. S., Lee, S. H., Jung, W. M., Lee, I. S., Jackson, S., et al. (2013). Inserting needles into the body: A meta-analysis of brain activity associated with acupuncture needle stimulation. J. Pain 14, 215–222. doi: 10.1016/j.jpain.2012.11.011
Chen, S., Wang, S., Rong, P., Liu, J., Zhang, H., and Zhang, J. (2014). Acupuncture for refractory epilepsy: Role of thalamus. Evid. Based Compl. Alternat. Med. 2014:950631. doi: 10.1155/2014/950631
Cho, S.-Y., Kim, M., Sun, J. J., Jahng, G.-H., Kim, H. J., Park, S.-U., et al. (2013). A comparison of brain activity between healthy subjects and stroke patients on fMRI by acupuncture stimulation. Chin. J. Integrat. Med. 19, 269–276. doi: 10.1007/s11655-013-1436-4
Corbetta, M., and Shulman, G. L. (2002). Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 3, 201–215. doi: 10.1038/nrn755
Dhond, R. P., Kettner, N., and Napadow, V. (2007). Neuroimaging acupuncture effects in the human brain. J. Altern. Compl. Med. 13, 603–616. doi: 10.1089/acm.2007.7040
Duerden, E. G., and Albanese, M. C. (2013). Localization of pain-related brain activation: A meta-analysis of neuroimaging data. Hum. Brain Mapp. 34, 109–149. doi: 10.1002/hbm.21416
Eickhoff, S. B., Bzdok, D., Laird, A. R., Kurth, F., and Fox, P. T. (2012). Activation likelihood estimation meta-analysis revisited. Neuroimage 59, 2349–2361. doi: 10.1016/j.neuroimage.2011.09.017
Eickhoff, S. B., Laird, A. R., Fox, P. M., Lancaster, J. L., and Fox, P. T. (2017). Implementation errors in the GingerALE Software: Description and recommendations. Hum. Brain Mapp. 38, 7–11. doi: 10.1002/hbm.23342
Eickhoff, S. B., Laird, A. R., Grefkes, C., Wang, L. E., Zilles, K., and Fox, P. T. (2009). Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: A random-effects approach based on empirical estimates of spatial uncertainty. Hum. Brain Mapp. 30, 2907–2926. doi: 10.1002/hbm.20718
Feng, Y., Bai, L., Ren, Y., Wang, H., Liu, Z., Zhang, W., et al. (2011). Investigation of the large-scale functional brain networks modulated by acupuncture. Magnet. Resonance Imag. 29, 958–965. doi: 10.1016/j.mri.2011.04.009
Han, X., Bai, L., Sun, C., Niu, X., Ning, Y., Chen, Z., et al. (2019). Acupuncture enhances communication between cortices with damaged white matters in poststroke motor impairment. Evidence-Based Compl. Alternat. Med. 2019:4245753. doi: 10.1155/2019/4245753
Huang, W., Pach, D., Napadow, V., Park, K., Long, X., Neumann, J., et al. (2012). Characterizing acupuncture stimuli using brain imaging with FMRI-a systematic review and meta-analysis of the literature. PLoS One 7:e32960. doi: 10.1371/journal.pone.0032960
Huang, Y., Chen, J., Htut, W. M., Lai, X., and Wik, G. (2007). Acupuncture increases cerebral glucose metabolism in human vascular dementia. Int. J. Neurosci. 117, 1029–1037. doi: 10.1080/00207450600936825
Hui, K. K., Marina, O., Claunch, J. D., Nixon, E. E., Fang, J., Liu, J., et al. (2009). Acupuncture mobilizes the brain’s default mode and its anti-correlated network in healthy subjects. Brain Res. 1287, 84–103. doi: 10.1016/j.brainres.2009.06.061
Jin, L., Sun, J., Xu, Z., Yang, X., Liu, P., and Qin, W. (2018). Intersubject synchronisation analysis of brain activity associated with the instant effects of acupuncture: An fMRI study. Acupunct. Med. 36, 14–20. doi: 10.1136/acupmed-2016-011327
Jun, M. H., Kim, Y. M., and Kim, J. U. (2015). Modern acupuncture-like stimulation methods: A literature review. Integr. Med. Res. 4, 195–219. doi: 10.1016/j.imr.2015.09.005
Jung, W. M., Lee, I. S., Wallraven, C., Ryu, Y. H., Park, H. J., and Chae, Y. (2015). Cortical activation patterns of bodily attention triggered by acupuncture stimulation. Sci. Rep. 5:12455. doi: 10.1038/srep12455
Kaptchuk, T. J. (2002). Acupuncture: Theory, efficacy, and practice. Ann. Intern. Med. 136, 374–383. doi: 10.7326/0003-4819-136-5-200203050-00010
Kong, J., White, N. S., Kwong, K. K., Vangel, M. G., Rosman, I. S., Gracely, R. H., et al. (2006). Using fMRI to dissociate sensory encoding from cognitive evaluation of heat pain intensity. Hum. Brain Mapp. 27, 715–721. doi: 10.1002/hbm.20213
Kurth, F., Zilles, K., Fox, P. T., Laird, A. R., and Eickhoff, S. B. (2010). A link between the systems: Functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct. Funct. 214, 519–534. doi: 10.1007/s00429-010-0255-z
Lai, P., Chen, X., Liu, J., Tian, Z., Mao, Y., Jia, C., et al. (2025). Abnormalities of insular functional connectivity in patients with musculoskeletal pain: A meta-analysis of resting-state fMRI studies. Brain Res. Bull. 224:111294. doi: 10.1016/j.brainresbull.2025.111294
Lai, X., Zhang, G., Huang, Y., Tang, C., Yang, J., Wang, S., et al. (2009). A cerebral functional imaging study by positron emission tomography in healthy volunteers receiving true or sham acupuncture needling. Neurosci. Lett. 452, 194–199. doi: 10.1016/j.neulet.2009.01.052
Laird, A. R., Eickhoff, S. B., Kurth, F., Fox, P. M., Uecker, A. M., Turner, J. A., et al. (2009). ALE meta-analysis workflows via the brainmap database: Progress towards a probabilistic functional brain atlas. Front. Neuroinform. 3:23. doi: 10.3389/neuro.11.023.2009
Laird, A. R., Fox, P. M., Price, C. J., Glahn, D. C., Uecker, A. M., Lancaster, J. L., et al. (2005). ALE meta-analysis: Controlling the false discovery rate and performing statistical contrasts. Hum. Brain Mapp. 25, 155–164. doi: 10.1002/hbm.20136
Legrain, V., Iannetti, G. D., Plaghki, L., and Mouraux, A. (2011). The pain matrix reloaded: A salience detection system for the body. Prog. Neurobiol. 93, 111–124. doi: 10.1016/j.pneurobio.2010.10.005
Li, L. L., Liu, X. W., Wu, F., Tong, D. C., Ye, L. P., Tao, H. X., et al. (2018). Electroacupuncture stimulation of language-implicated acupoint tongli (HT 5) in healthy subjects: An fMRI evaluation study. Chin. J. Integr. Med. 24, 822–829. doi: 10.1007/s11655-017-2924-8
Li, Z., Chen, J., Cheng, J., Huang, S., Hu, Y., Wu, Y., et al. (2018). Acupuncture modulates the cerebello-thalamo-cortical circuit and cognitive brain regions in patients of Parkinson’s disease with tremor. Front. Aging Neurosci. 10:206. doi: 10.3389/fnagi.2018.00206
Lin, D., Ren, Q., Ou, Y., Li, L., Peng, D., and Yang, S. (2025). Neuroimaging studies of acupuncture for depressive disorder: A systematic review of published papers from 2014 to 2024. Front. Psychiatry 16:1536660. doi: 10.3389/fpsyt.2025.1536660
Lin, J. G., and Chen, W. L. (2008). Acupuncture analgesia: A review of its mechanisms of actions. Am. J. Chin. Med. 36, 635–645. doi: 10.1142/S0192415X08006107
Liu, Z., Rolls, E. T., Liu, Z., Zhang, K., Yang, M., Du, J., et al. (2019). Brain annotation toolbox: Exploring the functional and genetic associations of neuroimaging results. Bioinformatics 35, 3771–3778. doi: 10.1093/bioinformatics/btz128
Muller, V. I., Cieslik, E. C., Laird, A. R., Fox, P. T., Radua, J., Mataix-Cols, D., et al. (2018). Ten simple rules for neuroimaging meta-analysis. Neurosci. Biobehav. Rev. 84, 151–161. doi: 10.1016/j.neubiorev.2017.11.012
Nierhaus, T., Pach, D., Huang, W., Long, X., Napadow, V., Roll, S., et al. (2015). Differential cerebral response to somatosensory stimulation of an acupuncture point vs. two non-acupuncture points measured with EEG and fMRI. Front. Hum. Neurosci. 9:74. doi: 10.3389/fnhum.2015.00074
Park, J.-Y., Kim, Y.-K., Kim, S.-Y., Lee, H., Choi, C.-J., Chae, Y., et al. (2017). Acupuncture modulates brain neural activity in patients: A systematic review and meta-analysis. Orient. Pharmacy Exp. Med. 17, 111–126. doi: 10.1007/s13596-017-0266-x
Posner, M. I., and Dehaene, S. (1994). Attentional networks. Trends Neurosci. 17, 75–79. doi: 10.1016/0166-2236(94)90078-7
Price, D. D. (2000). Psychological and neural mechanisms of the affective dimension of pain. Science 288, 1769–1772. doi: 10.1126/science.288.5472.1769
Qian, X., Zhang, H., Peng, J., Song, L., Lv, Q., Jia, W., et al. (2025). Acupuncture modulates group neural activity in patients with post stroke sensory impairment: An fMRI study based on inter-subject correlation and inter-subject functional connectivity. Brain Res. Bull. 222:111259. doi: 10.1016/j.brainresbull.2025.111259
Rao, Y., Ge, L., and Wu, J. (2024). A systematic review and coordinate-based meta-analysis of fMRI studies on acupuncture at LR 3 [Systematic Review]. Front. Neurosci. 18:1341567. doi: 10.3389/fnins.2024.1341567
Reddan, M. C., and Wager, T. D. (2018). Modeling pain using fMRI: From regions to biomarkers. Neurosci. Bull. 34, 208–215. doi: 10.1007/s12264-017-0150-1
Robinson, J. L., Laird, A. R., Glahn, D. C., Lovallo, W. R., and Fox, P. T. (2010). Metaanalytic connectivity modeling: Delineating the functional connectivity of the human amygdala. Hum. Brain Mapp. 31, 173–184. doi: 10.1002/hbm.20854
Sarter, M., Givens, B., and Bruno, J. P. (2001). The cognitive neuroscience of sustained attention: Where top-down meets bottom-up. Brain Res. Brain Res. Rev. 35, 146–160. doi: 10.1016/s0165-0173(01)00044-3
Simone, L., Caruana, F., Borra, E., Del Sorbo, S., Jezzini, A., Rozzi, S., et al. (2025). Anatomo-functional organization of insular networks: From sensory integration to behavioral control. Prog. Neurobiol. 247:102748. doi: 10.1016/j.pneurobio.2025.102748
Tan, T. T., Wang, D., Huang, J. K., Zhou, X. M., Yuan, X., Liang, J. P., et al. (2017). Modulatory effects of acupuncture on brain networks in mild cognitive impairment patients. Neural Regen. Res. 12, 250–258. doi: 10.4103/1673-5374.200808
Theysohn, N., Choi, K. E., Gizewski, E. R., Wen, M., Rampp, T., Gasser, T., et al. (2014). Acupuncture-related modulation of pain-associated brain networks during electrical pain stimulation: A functional magnetic resonance imaging study. J. Altern. Compl. Med. 20, 893–900. doi: 10.1089/acm.2014.0105
Tian, X., Russo, S. J., and Li, L. (2025). Behavioral animal models and neural-circuit framework of depressive disorder. Neurosci. Bull. 41, 272–288. doi: 10.1007/s12264-024-01270-7
Vandenberghe, R., Gitelman, D. R., Parrish, T. B., and Mesulam, M. M. (2001). Location- or feature-based targeting of peripheral attention. Neuroimage 14(1 Pt 1), 37–47. doi: 10.1006/nimg.2001.0790
Wen, Q., Ma, P., Dong, X., Sun, R., Lan, L., Yin, T., et al. (2021). Neuroimaging studies of acupuncture on low back pain: A systematic review. Front. Neurosci. 15:730322. doi: 10.3389/fnins.2021.730322
Wu, P., Zeng, F., Yin, C., Xiong, Y., Bai, Y., Wang, D., et al. (2017). Effect of acupuncture plus conventional treatment on brain activity in ischemic stroke patients: A regional homogeneity analysis. J. Tradit. Chin. Med. 37, 650–658. doi: 10.1016/S0254-6272(17)30319-9
Xiang, A., Yu, Y., Jia, X., Ma, H., Liu, H., Zhang, Y., et al. (2019). The low-frequency BOLD signal oscillation response in the insular associated to immediate analgesia of ankle acupuncture in patients with chronic low back pain. J. Pain Res. 12, 841–850. doi: 10.2147/JPR.S189390
Xie, Z., Cui, F., Zou, Y., and Bai, L. (2014). Acupuncture enhances effective connectivity between cerebellum and primary sensorimotor cortex in patients with stable recovery stroke. Evid. Based Compl. Alternat. Med. 2014:603909. doi: 10.1155/2014/603909
Yan, C. G., Wang, X. D., Zuo, X. N., and Zang, Y. F. (2016). DPABI: Data processing & analysis for (resting-state) brain imaging. Neuroinformatics 14, 339–351. doi: 10.1007/s12021-016-9299-4
Yang, M., Yang, J., Zeng, F., Liu, P., Lai, Z., Deng, S., et al. (2014). Electroacupuncture stimulation at sub-specific acupoint and non-acupoint induced distinct brain glucose metabolism change in migraineurs: A PET-CT study. J. Transl. Med. 12:351. doi: 10.1186/s12967-014-0351-6
Yang, X. Y., Shi, G. X., Li, Q. Q., Zhang, Z. H., Xu, Q., and Liu, C. Z. (2013). Characterization of deqi sensation and acupuncture effect. Evid. Based Compl. Alternat. Med. 2013:319734. doi: 10.1155/2013/319734
Yarkoni, T., Poldrack, R. A., Nichols, T. E., Van Essen, D. C., and Wager, T. D. (2011). Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods 8, 665–670. doi: 10.1038/nmeth.1635
Keywords: multimodal neuroimaging, acupuncture, ALE meta-analysis, functional connectivity, pain processing
Citation: Li C, Liu Y, Ning B, Wu L, Zhang Y, Zhou J, Zhang D, Zhou P and Fu W (2025) Identifying robust neural signatures of acupuncture modulation in healthy brains: a multimodal meta-analysis mapping core network. Front. Hum. Neurosci. 19:1494267. doi: 10.3389/fnhum.2025.1494267
Received: 10 September 2024; Accepted: 18 August 2025;
Published: 02 September 2025.
Edited by:
Bum Joon Kim, University of Ulsan, Republic of KoreaReviewed by:
Kevin T. Willeford, Nova Southeastern University, United StatesEva Lendaro, Massachusetts Institute of Technology, United States
Copyright © 2025 Li, Liu, Ning, Wu, Zhang, Zhou, Zhang, Zhou and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Delong Zhang, ZGVsb25nLnpoYW5nQG0uc2NudS5lZHUuY24=; Peng Zhou, enBtbTE5OTlAMTYzLmNvbQ==; Wenbin Fu, ZnV3ZW5iaW5AZ3p1Y20uZWR1LmNu
†Present address: Yamin Liu, School of Acupuncture-Moxibustion and Tuina, Hebei University of Traditional Chinese Medicine, Shijiazhuang, China
‡These authors have contributed equally to this work
 Changhong Li
Changhong Li Yamin Liu
Yamin Liu Baile Ning
Baile Ning Lihua Wu
Lihua Wu Yuan Zhang
Yuan Zhang Junhe Zhou
Junhe Zhou Delong Zhang
Delong Zhang Peng Zhou2*
Peng Zhou2* Wenbin Fu
Wenbin Fu