- 1Eaton-Peabody Laboratories, Massachusetts Eye and Ear, Boston, MA, United States
- 2Program in Speech and Hearing Bioscience and Technology, Division of Medical Sciences, Harvard Medical School, Boston, MA, United States
- 3Department of Otolaryngology—Head and Neck Surgery, Harvard Medical School, Boston, MA, United States
Introduction: Among all the sounds occurring at any given time, people are often interested in listening to just one. Some competing sounds are merely background noise, whereas others distract attention from target sounds and are less easily suppressed. During active listening, the central auditory pathway unmixes target and distractor sounds based on temporal differences across three orders of magnitude – from millisecond differences in acoustic temporal fine structure to slower perceptual grouping factors that stretch out to multiple seconds. We developed an approach to directly measure central auditory encoding of multiplexed target and distractor sound features in human listeners to determine which timescales are most impacted by the presence of distracting sounds.
Methods: Target sounds contained nested features along four timescales, including temporal fine structure (~500 Hz), temporal envelope (~25–80 Hz), envelope changes (~7 Hz), and slower changes reflecting whether target stimuli were randomly arranged or formed a repeating pattern (~0.5 Hz). Targets were presented with competing sounds that provided variable distraction levels: either a highly distracting melody or a less distracting noise. Neural synchronization to each timescale was simultaneously measured for target and distractor sounds from electroencephalogram (EEG) recordings during a listening task.
Results: Sustained shifts from random to regular sequence arrangements were reliably perceived, yet did not evoke a pattern recognition potential, nor neural synchronization changes at any timescale. Synchronization to relatively slow changes in envelope transitions of the target sound deteriorated with the addition of more distracting sounds while synchronization to more rapid fluctuations in the fine structure or envelope were unaffected by varying distraction level. Categorizing trials by task performance revealed a conjunction of enhanced entrainment to slower temporal features in the distractor sound and reduced synchronization to the target sound on error trials.
Discussion: By designing a stimulus paradigm that leveraged the temporal processing capabilities of the auditory nervous system, we were able to simultaneously quantify multiple target and distractor sound features reproduced in the EEG. This paradigm identified synchronization processes which may prove valuable for research on clinical populations who report difficulty suppressing awareness of distracting sounds.
1 Introduction
Listening requires people to suppress all the sounds they are hearing except for the sound source in their attentional spotlight. Often characterized as the “cocktail party problem” in the context of speech-on-speech masking, the simultaneous presentation of sound from multiple sources challenges the abilities of both the auditory system to separate out the sources and the central mechanisms of attention to selectively attend to a target sound while suppressing task-irrelevant sounds (Cooke et al., 2008). Difficulties in this domain may contribute to difficulties understanding speech in noisy environments, a common complaint of individuals claiming a hearing difficulty without hearing threshold shifts (Tremblay et al., 2015; Parthasarathy et al., 2020; Cancel et al., 2023).
To successfully attend to one sound in an auditory scene, the incoming auditory signal must be separated to form distinct auditory objects. Temporal cues that group together across different timescales are key features utilized by the auditory nervous system to separate different auditory objects (Sollini et al., 2022). Integration across different timescales has been demonstrated in the cortex (Lerner et al., 2011; Norman-Haignere et al., 2022) but encoding of some of these features can occur much earlier in the auditory pathway (Kuwada et al., 2002). More distracting background environments may degrade the encoding of intended target temporal features (Choi et al., 2014) such that measurements of temporal encoding across timescales in distracting environments would provide insight into auditory distraction.
The high temporal fidelity of the auditory nervous system allows measurements of synchronization to stimulus features (Galambos et al., 1981). Measurements of this synchronization, commonly called frequency following responses (FFRs), have been done in response to stimuli ranging from simple tones to repeated syllables. These include synchronization to the temporal fine structure of stimuli (see Krizman and Kraus, 2019 for a review), and synchronization to the amplitude envelope of stimuli, often more specifically termed envelope following responses (EFRs; see Shinn-Cunningham et al., 2017 for review). Most frequently, studies of these following responses measure and report on responses to a single feature of the stimuli, but natural stimuli often contain multiplexed temporal features (Rosen, 1992). Even paradigms which record multiple parallel EFRs typically utilize envelope rates within a narrow frequency range (Perez-Abalo et al., 2001; Linares et al., 2010; Encina-Llamas et al., 2021). FFRs and EFRs in different frequency ranges have been associated with different generators in the auditory pathway and thus reflect different levels of processing, with high frequency FFRs generated primarily in the brainstem and low-frequency EFRs around 40 Hz generated in the cortex (Kuwada et al., 2002; Picton et al., 2003; Chandrasekaran and Kraus, 2010; Parthasarathy and Bartlett, 2012; Coffey et al., 2019). Studies of following responses also typically present the stimuli in quiet or acoustically simple background noise (Zhu et al., 2013; Bharadwaj et al., 2015), thus providing no insight into how temporal synchronization may be challenged by difficult listening environments.
While speech is the canonical stimulus for listening in a noisy environment, the variability in speech stimuli, especially naturally produced speech, reduces the ability to measure synchronization to the various features with specificity. Following responses require hundreds of trials to average to reliably measure responses (Bharadwaj and Shinn-Cunningham, 2014). Isolated syllables can be presented repeatedly to measure an FFR (Kraus et al., 2016), but the fundamental frequency of the temporal fine structure in natural speech varies across a range of hundreds of hertz (Stevens, 1971) precluding averaging.
In contrast, simple stimuli can be designed with well-controlled properties that can be repeated across presentations. This allows for synchronization to be measured even to stimulus features beyond temporal fine structure and amplitude envelopes, such as frequency modulation (Parthasarathy et al., 2020). Behaviorally, simple stimuli have often been presented under passive listening conditions (Zhu et al., 2013; Bharadwaj and Shinn-Cunningham, 2014; Barascud et al., 2016; Herrmann and Johnsrude, 2018; Herrmann et al., 2019; Calcus et al., 2022) or in detection-based tasks (Durlach et al., 2003b) but more complex perceptual judgments can be utilized as well (Southwell et al., 2017). Competitors can also be designed to minimize energetic masking in the auditory periphery (Durlach et al., 2003a,b)—an important contributor to difficulty hearing in noisy environments but which is confounded with cognitive effects such as distraction (Brungart et al., 2001; Kidd et al., 2016).
Using novel stimuli, we report here a paradigm to simultaneously measure synchronization across multiplexed timescales in various levels of distraction. This paradigm allows us to probe the neurophysiological effects of auditory distraction on target encoding and measure distraction-sensitive features which may prove useful as objective markers of susceptibility to auditory distraction.
2 Materials and methods
2.1 Participants
Participants were recruited from the general population via word of mouth, flyers, and advertisements on the Mass General Brigham participant recruitment website. All procedures were approved by the Mass General Brigham Institutional Review Board and took place at Mass Eye and Ear between September 2022 and February 2025. After providing informed consent, 127 study participants were screened for normal cognitive function (telephone Montreal Cognitive Assessment ≥18), English fluency (self-reported), age (18–60 years), mental health status (Beck’s Depression Inventory total score < 31), middle ear status (unremarkable otoscopy), and hearing status. Hearing status was assessed through pure tone audiometry by a licensed audiologist. Inclusion required normal audibility (≤ 25 dB HL) across the low- and mid-frequencies (0.5 up to 2 kHz) corresponding to our test stimulus and no more than mild to moderate hearing loss across the higher frequency range (3–8 kHz) with no indication of tinnitus or sound sensitivity. In total, 65 study participants passed the screening criteria and participated in the study. Of these, 6 participants failed to learn the task and were excluded from analyses, leaving a final sample of 59 participants that provided data for either the target-alone vs. melodic distractor experiment (n = 38, 7 male) or the melodic vs. melody-matched noise distractor experiment (n = 21, 8 male). Participants completed both remote, tablet-based testing and an in-person session with EEG recording.
2.2 Stimuli
Target, melodic distractor, and melody-matched noise distractor stimuli were generated with pre-compensation for transducer response properties, allowing for equal-level output across frequencies. Examples of the stimuli used are available as Supplementary material.
2.2.1 Random or repeating target stimulus
For the laboratory-based EEG task, target stimuli consisted of concatenated sinusoidally amplitude modulated (SAM) tones (516.8 Hz carrier frequency). Five SAM rates were used to produce all random or patterned sequences: 27, 41, 54, 68, and 82 Hz. The duration of the individual SAM tones was set to 143 ms (corresponding to 6.8 Hz) to ensure that each SAM token had completed an integer number of AM and carrier frequency periods at the point of concatenation. A sequence of each SAM tokens could then either be repeated to produce a pattern segment, or a new sequence could be pseudo-randomly selected for each cycle to form random segments (restricting selection to avoid repeating the last SAM tone of the previous cycle as the first SAM tone of the next cycle). The full set of permutations of the five SAM tokens was used in different pattern stimuli (120 pattern stimuli) and a corresponding number of random segments was generated. Target stimuli for the tablet-based testing were the same, with slight numerical variations to durations and AM rates to account for differences in sampling rates across different hardware.
2.2.2 Melodic distractor
Melodic distractor stimuli were generated by the Magenta RL Tuner (Jaques et al., 2017). The Magenta RL Tuner is a neural network melody generator with reward functions explicitly based on Western musical theory in addition to note sequence predictions learned from its training data. These melodies are thus more compelling than random tone sequences while avoiding effects of familiarity with known melodies. Magenta-created melodies that repeated a phrase multiple times were rejected to avoid confusion of repetition in the melody with repetition in the target stimuli.
The output of the Magenta RL Tuner is a series of note and rest durations and corresponding note heights. This output was transposed into pure tones in the frequency range below the target stimulus, maintaining a 1/3 octave protected band below the sideband of the 82 Hz SAM tone to minimize energetic masking of the target stimulus in the frequency domain (Kidd et al., 2002). This melody was then duplicated 3 octaves higher, producing the identical melody in a frequency range above the target stimulus, again maintaining a 1/3 octave band from the 82 Hz SAM sideband. Melodies that could not be transposed into these frequency regions were rejected. As per the target stimuli, notes in the melody were also amplitude modulated but at a lower rate; note durations and SAM rate were adjusted to the hardware specific to remote or lab-based testing in order to produce an integer number of SAM periods per note.
2.2.3 Melody-matched noise distractor
A noise stimulus was synthesized from the pure tone frequencies that comprised the notes of a corresponding melody. Instead of distinct note presentations of each frequency individually, all frequencies in the matched noise lasted the entire duration of the stimulus. The amplitude of each frequency component was scaled to the proportion of beats in the melody containing the corresponding note. Additionally, the starting phrase of each frequency component in the noise stimulus was randomized. The imposed envelope of the melody to be matched (rest periods with zero amplitude and SAM during note periods) was then applied to the noise stimulus. The matched noise stimuli thus have the same magnitude spectra as the melodies while having different spectrograms.
2.3 Psychophysical assessments of distraction
2.3.1 Temporal pattern classification: speed vs. accuracy task
Participants completed a speeded discrimination task in which they were asked to respond whether the target stimulus on each trial formed a pattern or random arrangement. Target stimuli lasted 4 cycles, approximately 2.9 s. A game mechanic was employed, to assess speed vs. accuracy tradeoffs. A score counter hidden from view counted down from 1,000 from the start of audio playback. When participants responded, they either gained (if correct) or lost (if incorrect) the points left on the counter and the score from that trial was displayed on screen. If participants failed to respond within 1 s after stimulus presentation concluded, participants lost 350 points. Blocks of trials with the target alone and the target paired with melodic distractor stimuli at different levels (18–0 dB SNR at 6 dB steps) were randomly interleaved for a total of 120 random trials and 120 pattern trials representing the full set of repeated cycles (i.e., 5!). For the melodic distractor vs. noise distractor experiment, the blocks consisted of the target alone, the target paired with melodic distractor stimuli at 12 dB SNR, and the target paired with the matched noise distractor stimuli at 12 dB SNR. Testing was self-directed and performed remotely via with a tablet computer (Microsoft Surface Pro 2, Pro 7, Redmond, WA) and calibrated closed-back circumaural headphones (Sennheiser HD280, Wedemark, Germany).
Participants underwent several stages of task familiarization before psychophysical data collection and were required to complete 50 practice trials at each phase or score ≥ 70% correct on any 10-trial block, to advance to the next familiarization stage. In practice stage 1, participants were familiarized with categorizing pattern vs. random sequences of the SAM target sound. In stage 2, participants performed the temporal pattern classification task in the presence of the distractor at 18 dB SNR and then 12 dB SNR. In practice stage 3, participants were familiarized with the speeded reaction time test format described above.
2.3.2 Temporal pattern classification: laboratory-based accuracy task
After completing at-home testing, participants came for a lab-based EEG recording session. The lab-based task used the same stimuli as the speed vs. reaction task but was designed to minimize motion artifacts during stimulus presentation. The laboratory-based task target stimulus was always 12 cycles (~9 s) in duration, with the first four cycles in a random arrangement and the subsequent 8 cycles were either in a random or patterned arrangement. Polarity of the stimulus alternated between cycles. Participant responses were recorded via a touchscreen tablet (Microsoft Surface Pro 4, Redmond, WA), where the virtual response buttons were provided once stimulus presentation for a given trial was complete. Auditory stimuli were delivered bilaterally through insert ear headphones (EarTone 3A, Oaktree Products, Chesterfield, MO).
Participants in the target-alone vs. melodic distractor experiment completed one block with the target stimuli alone, and one block with the target and the distractor melodies at 12 dB SNR. For the melodic vs. melody-matched noise distractor experiment, the block with target alone was replaced by a block with the target and matched noise distractor as 12 dB SNR. Each block consisted of 240 trials: 120 random-pattern trials and 120 random-random trials.
2.4 EEG processing and analysis
We recorded 64-channel EEG (BioSemi ActiveTwo system, Wilmington, NC), along with electrodes on the left and right mastoid, lateral to the lateral canthus of each eye, and underneath each eye. The BioSemi system utilizes an active common mode sensing electrode and passive driven electrode in lieu of a typical ground and reference electrode setup; these are placed in the cap at PO1 and PO2, respectively. The raw EEG data were imported into the EEGLAB data structure for analysis in MATLAB (MathWorks, Natick, MA). The data were filtered between 1 and 3,000 Hz using zero-phase Butterworth filters and re-referenced to the average of the left and right mastoids. Particularly noisy channels were identified using channel statistics as implemented in the FASTER pipeline (Nolan et al., 2010) and excluded from the rest of the analysis.
Epochs were individualized for each level of synchronization. For FFR analysis, cycle-length epochs time-locked to the start of each cycle were extracted. For EFR analysis, token-length epochs were taken time-locked to the start of the corresponding SAM tokens. Epochs for envelope change following response (ECFR) analysis were two-token lengths centered on the start of every other token (to avoid overlapping epochs). Regardless of window length, the phase-locking value (PLV) was calculated for each channel across epochs (subtracting the negative polarity from the positive polarity PLV for FFR analysis), and the root mean square was taken across all channels included in the analysis (Zhu et al., 2013). The average value of the PLV in frequency bins unrelated to the stimuli provided a measurement of the noise floor.
The noise floor of the PLV is numerically bounded by the number of trials (Zhu et al., 2013). The comparisons of ECFR by magnitude of change in AM rate and for various following rates by trial response accuracy consisted of comparisons between conditions with different numbers of trials. To keep noise floors consistent, conditions with greater numbers of trials were randomly subsampled across 600 bootstrap iterations to the number of trials in the condition with the fewest trials.
We measured the sustained pattern recognition response following the procedure outlined by Southwell et al. (2017). Using the FieldTrip Toolbox, we filtered the data between 0.1 and 110 Hz (fifth order Butterworth filters), downsampled the data to 256 Hz, re-referenced to the average of all EEG channels, and subtracted the 1 s prestimulus baseline from the trial epoch. We then rejected trials where the average power exceeded the across-trial mean average power by two standard deviations, low-pass filtered the remaining data at 30 Hz (fifth order Butterworth filter), and applied denoising source separation for trial related activity, keeping the first five components. The sustained pattern response was the root mean square across channels of the resulting average across trials. We quantified whether the sustained response was sensitive to the pattern by subtracting the mean of the sustained response from the two target cycles before the transition point from the mean of the sustained response from the third and fourth target cycles after transition (the second and third repeats of the pattern). The same time points were used to evaluate the sustained response in the random condition. We also estimated a noise floor for this difference by bootstrapping the difference observed with a random timepoint selected as the nominal transition point.
2.5 Auditory periphery modelling
To model responses in the auditory periphery, we utilized the model published by Verhulst et al. (2018), version 1.2. Structures represented in the model include the middle ear, basilar membrane, inner hair cells, auditory nerve fibers (at various thresholds and spontaneous rates), the cochlear nucleus, and inferior colliculus. Model parameters were set to their defaults, and inputs to the model were an example target stimulus, the target stimulus with a melodic distractor at 12 dB signal-to-noise ratio (SNR), and the target stimulus with the corresponding matched noise distractor at 12 dB SNR.
2.6 Statistical analysis
Statistical comparisons were made via t-tests, paired t-tests, and repeated measures analysis of variance (rmANOVA). Tests were done in MATLAB using the functions ttest (one-sample and paired t-tests), ttest2 (two-sample t-tests), and ranova (rmANOVA), with the function multcomp used for pairwise comparisons following rmANOVA. We control the family-wise error rate using the Bonferroni-Holm method for tests making comparisons between the same conditions. To meet the assumptions of parametric statistical testing, accuracy scores in the behavioral temporal pattern classification task were converted to rationalized arcsine units (Studebaker, 1985). For ease of comprehension, the untransformed percent correct values are reported in the text.
3 Results
3.1 Target context and synchronization
Target stimuli were designed to produce synchronization to acoustic features at nested timescales, similar to ethologically relevant stimuli while producing distinct neural signatures that can be measured in the frequency range with EEG (Figures 1A,B). Target stimuli acoustic features included a constant carrier frequency; SAM at one of five different rates; and changes between SAM rates at regular time intervals (Figure 1C). Represented in the EEG, these features produce an FFR, EFRs, and a waveform that follows the changes between SAM rates. SAM tones were also arranged into either patterned or random segments expected to produce a sustained pattern recognition potential that would be greater when the target context was patterned than when it was random (Barascud et al., 2016; Southwell et al., 2017; Herrmann and Johnsrude, 2018; Herrmann et al., 2019). While we were able to observe sustained potentials for both types of stimuli (Figure 1D), the sustained potential after transition to a patterned context did not differ from the sustained potential before the transition, and no difference was observed between random-pattern and random-random stimuli (Figure 1E; paired sample t-test p = 0.93).
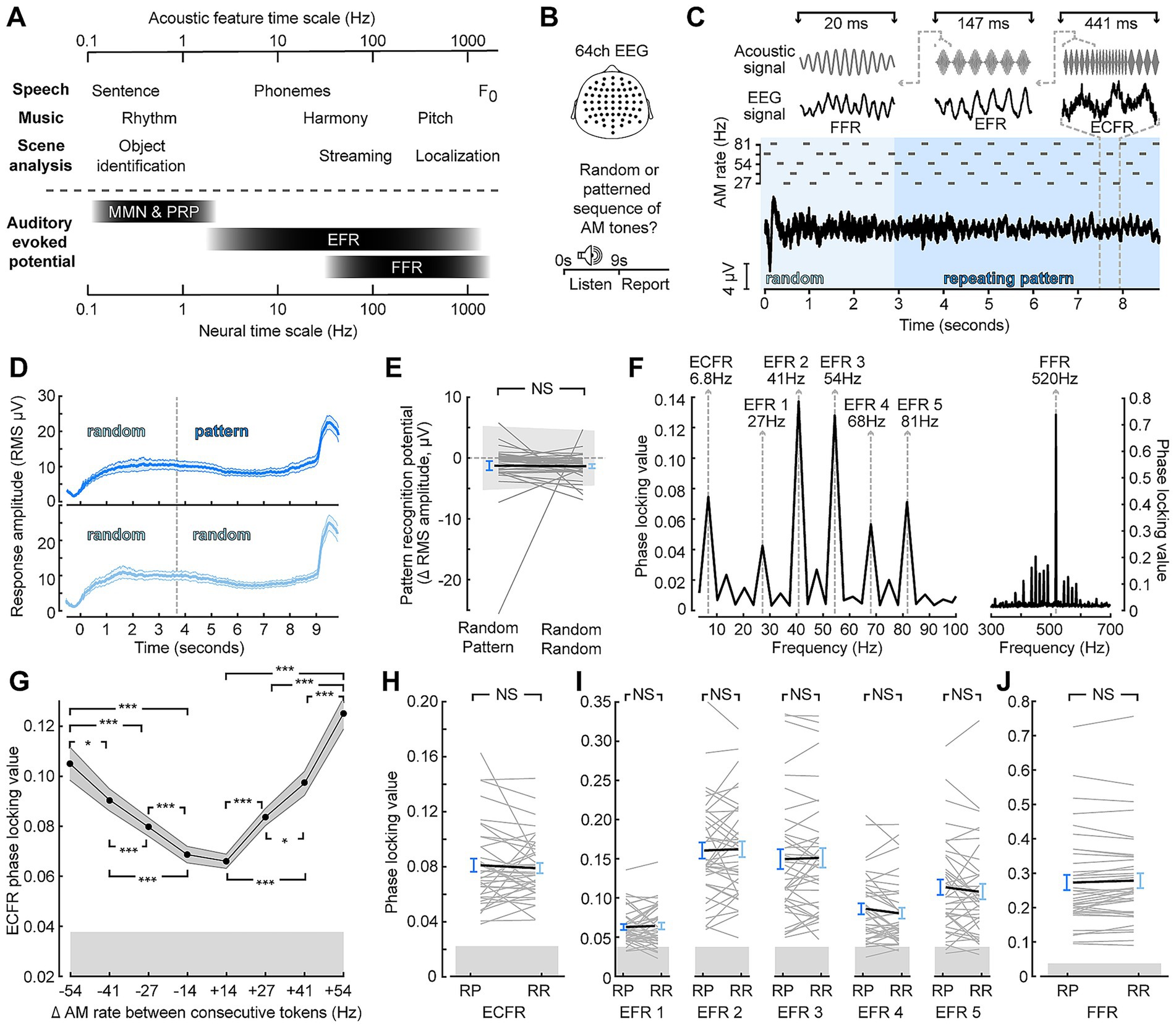
Figure 1. Synchronization across timescales is unaffected by target context. (A) Comparison of temporal features of ecologically relevant sounds (top) and EEG signals (bottom) at concomitant timescales. MMN—mismatched negativity; PRP—pattern recognition potential (B) 64-channel EEG was collected as participants listened to a stimulus and made a perceptual report whether the stimulus was random throughout or switched to a repeating pattern. (C) Illustration of the stimulus, which consisted of a sequence of SAM tones with a carrier frequency of 517 Hz, and corresponding event-related potentials. Insets at top illustrate stimulus features (left to right: carrier frequency, amplitude envelope, and changes between AM rates) and corresponding averaged EEG waveforms (left to right: FFR, EFR, ECFR). (D) Sustained responses to random-pattern (top, dark blue) and random-random (bottom, light blue) stimuli taking the RMS across channels (E) Quantification of pattern recognition potential by subtracting the two cycles before the effective transition point of random-pattern stimuli from the two cycles following the transition point for individual subjects (thin, gray) and group mean (thick, black) to random-pattern (RP) and random-random (RR) stimuli (F) Phase locking value spectrum of an example subject with spectral peaks corresponding to the identified feature of the target sound. (G) Envelope change following response (ECFR) phase locking value (PLV) magnitude scales with the difference in SAM rate between two adjacent tokens. (H–J) Envelope change following responses (H), envelope following responses (I), and frequency following responses (J) are insensitive to random-pattern (RP) versus random-random (RR) context. Error bars represent the standard error of the mean. NS, not significant (p > 0.05); *p < 0.05; ***p < 0.001.
To quantify the synchronization in the EEG to the acoustic features, we utilized frequency-domain analyses of the PLV. Synchronization to target stimulus features was observed for the carrier frequency (FFR) as well as each SAM rate (EFRs; Figure 1F). Additionally, we observed a peak at the rate of changing between different envelopes at 6.8 Hz, which we termed the envelope change following response (ECFR). The ECFR was sensitive to the degree of AM rate difference between consecutive tokens (Figure 1G). Repeated measures ANOVA showed a significant main effect of AM difference (F(7, 259) = 54.94; p < 0.001, Greenhouse–Geisser corrected) and pairwise Tukey HSD comparisons showed statistically significant differences for all pairs between different magnitudes other than −54 to +41 (p = 0.64) and −41 and +27 (p = 0.33).
To examine the effect of patterned or random context on synchronization to target features, we compared PLVs for each acoustic feature between the random-pattern and random-random stimuli (Figures 1H–J). Only epochs from the last 6 stimulus cycles, corresponding to after two cycles of the pattern potion of the random-pattern stimuli, were included in these analyses. Paired t-tests with Bonferroni-Holm correction showed no significant differences in ECFR (p-adj. = 1), EFR at 27 Hz (p-adj. = 1), 41 Hz (p-adj. = 0.75), 54 Hz (p-adj. = 1), 68 Hz (p-adj. = 0.56), and 82 Hz (p-adj. = 1), nor the FFR (p-adj. = 0.85) based on embedded context.
3.2 Distracting effects on behavior
While stimulus context (random vs. patterned) had no measurable effect on EEG following responses across these various timescales, we reasoned that increased attentional demands in the form of information masking or attentional selection could exert a stronger effect (Bharadwaj et al., 2014; Schüller et al., 2023). To address this possibility, we paired the auditory target with distracting melodies, as perception of both melodies and the target stimuli rely on pitch perception cues (Figures 2A,B). In the case of the target stimuli, the pitch cues come from the changing SAM rates, whereas the pitch cues in the melodic distractors come from changes in carrier frequencies. Participants completed a psychophysical task that challenged them to classify a temporal pattern as random or patterned as soon as they felt confident in their response (Figure 2C). Participants were fairly accurate in their judgement (mean: 82% correct), despite the novelty and complexity of the attended sound feature and with only a few cycles of repetition in which to make the judgment (Figure 2D). In fact, the mean response time (2.2 s) was less than the duration of 3 cycles of the stimulus: on average, participants were both making the judgment and effectuating the motor response after fewer than 2 repeats of the pattern cycle.
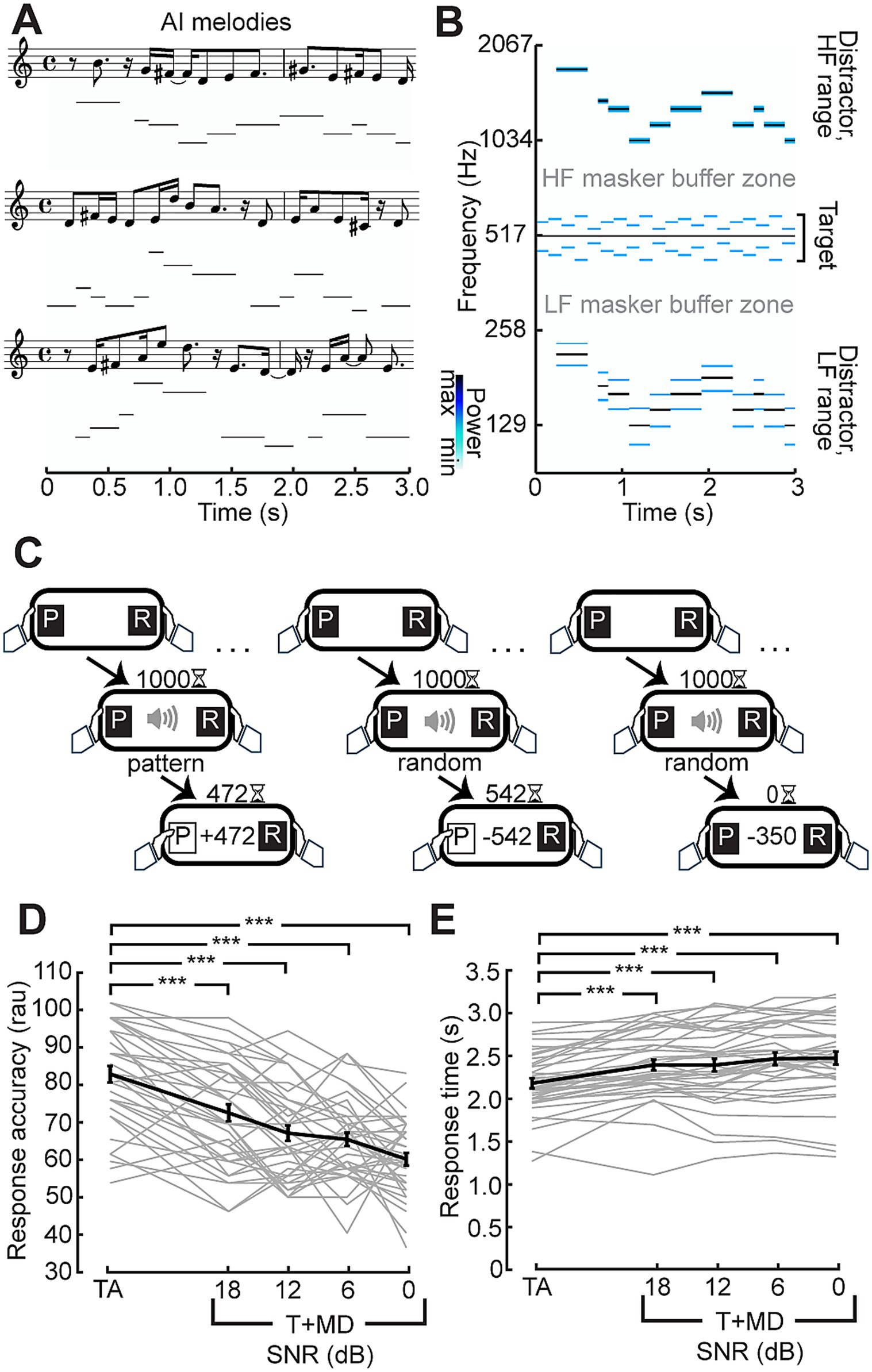
Figure 2. Distraction by AI-generated melodies. (A) Three exemplar melody sequences generated from the Magenta RL Tuner in musical notation and corresponding spectrogram representation. (B) Spectrogram illustrating an exemplar melody transposed above and below the target stimulus, conserving a 1/3 octave buffer zone surrounding the target to minimize energetic masking of the target. Notes in the melody are amplitude modulated at 19.5 Hz producing the lower power sidebands seen in the distractor ranges of the spectrogram. (C) Temporal classification task: Participants were instructed to respond once they were confident whether the target was random or repeating. Correct responses (left) were rewarded with the points remaining on the timer whereas incorrect responses had the points subtracted from their total (middle). Trials where participants did not respond within 1.5 s after stimulus presentation was completed (right) lost participants 350 points. (D,E) Monotonic reduction in accuracy (D) in rationalized arcsine units and increased reaction time (E) with progressively less favorable signal-to-noise ratio (SNR). Individual subjects are shown as thin gray lines, with the group mean shown as a thick black line. Error bars reflect the standard error of the mean. ***p < 0.001.
The melodic distractor successfully impaired performance on the discrimination task. Repeated measures ANOVA showed a statistically significant difference in performance across the conditions both in terms of accuracy (F(4,148) = 44.82; p < 0.001) and response time in seconds (F(4, 148) = 31.73; p < 0.001, Greenhouse–Geisser corrected). Pairwise Tukey HSD comparisons showed responses to the target alone were more accurate (all p < 0.001) and faster (all p < 0.001) than with the melody at each SNR.
Similar effects on behavioral accuracy were observed during the EEG session (Figure 3A). The longer duration of the stimuli used in the EEG session and lack of time pressure on response led to overall higher accuracy (mean: 92 and 80% for target alone and with melodic distractors, respectively). The melodic distractors, however, still significantly impaired response accuracy when compared to the target alone (paired t-test; p < 0.001).
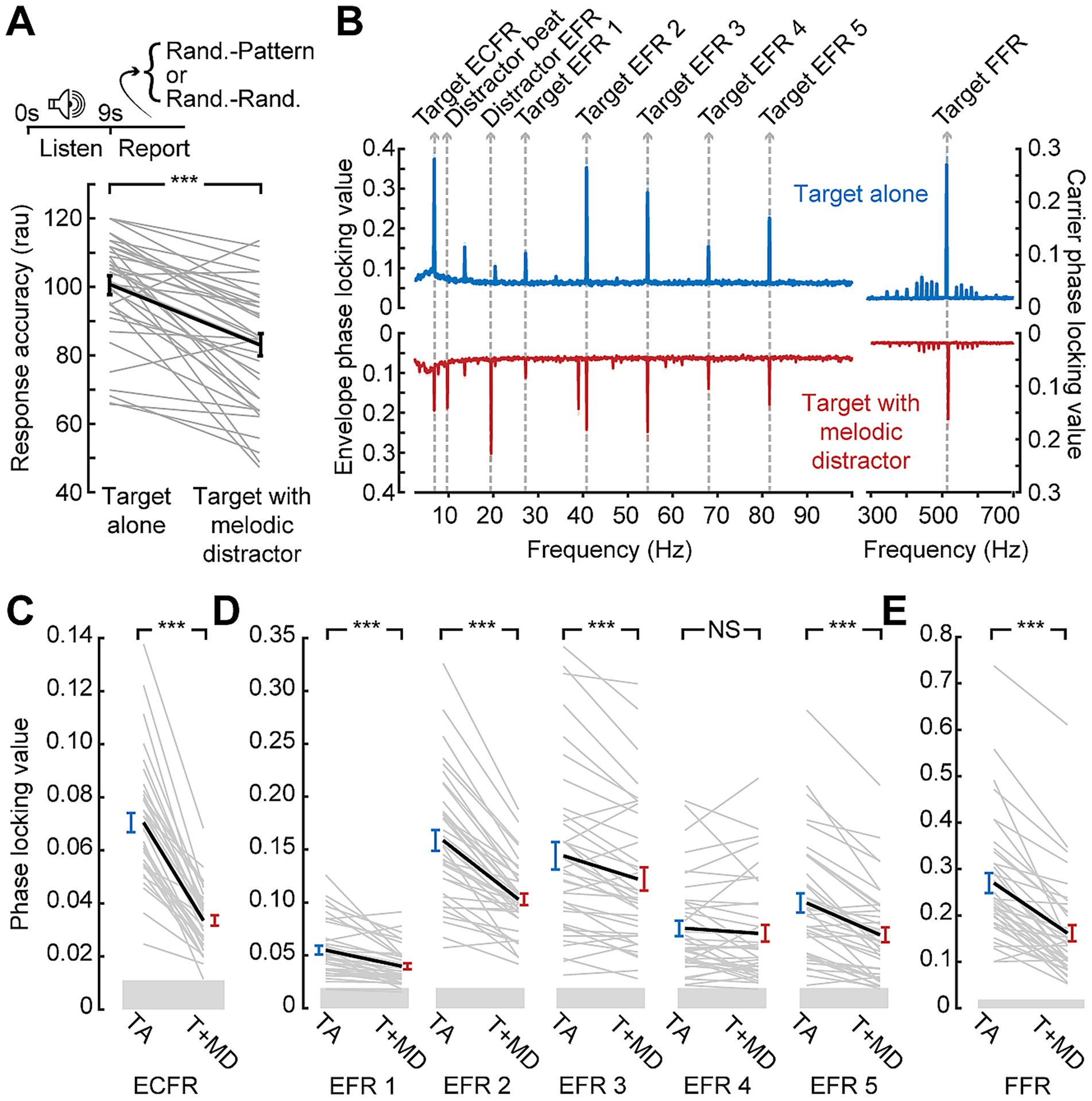
Figure 3. Disrupted synchronization to target sound features in the presence of a distractor. (A) Temporal pattern classification during the EEG session is impaired by the melodic distractor. Note that plotted values are in rationalized arcsine units, while raw accuracy is reported in the text (B) Grand average phase locking value spectra from the block with the target presented alone (blue) or with the melodic distractor (red). Spectral peaks corresponding to target and distractor features identified. (C-E) Reduced ECFR (C), EFR (D), and FFR (E) to the target stimuli when presented with the melodic distractor (T + MD; red) compared to the target alone (TA; blue), except for the EFR at 68 Hz. Data from individual subjects are shown as thin gray lines, with the group mean shown as a thick black line. Error bars reflect the standard error of the mean. NS, not significant (p > 0.05); ***p < 0.001.
3.3 Distracting effects on target synchronization
EEG recordings during temporal pattern classification revealed clearly resolved FFR, EFRs, and ECFR to the target as well as synchronization peaks corresponding to the beat and envelope of the melodic distractor (Figure 3B). However, we noted a clear reduction in synchronization to all three features of the target stimulus when it was presented alongside the melodic distractor (Figures 3C–E). The ECFR PLV was decreased from 0.070 to 0.033 (p-adj. < 0.001); EFR PLVs decreased from 0.055 to 0.040 at 27 Hz (p-adj. < 0.001), from 0.16 to 0.10 at 41 Hz (p-adj. < 0.001), from 0.14 to 0.12 at 54 Hz (p-adj. < 0.001) and from 0.10 to 0.07 at 82 Hz (p-adj. < 0.001); and the FFR decreased from 0.27 to 0.16 (p-adj. < 0.001). The EFR at 68 Hz was not significantly different, with a mean target alone PLV of 0.076 and a mean target with distractor PLV of 0.071 (p-adj. = 0.23).
3.4 Controlling for peripheral effects of melodic distractors
Reduced neural encoding of the target sound in the presence of the melody could be a neurophysiological signature of distraction. Alternatively, it could reflect destructive interference between the neural signals generated by the target and distractor sounds. To better understand the underlying source of the reduced target synchronization, we utilized a biophysical model of the auditory periphery and early central pathway designed to produce modeled outputs up to the level of the auditory brainstem, including EFRs (Figures 4A,B; Verhulst et al., 2018). Population responses from modeled auditory nerve fibers with characteristic frequencies within the target stimulus bandwidth were mostly unaffected by the inclusion of the melodic distractor in the input to the model (Figure 4C). At the level of the simulated EFR, however, synchronization to the target carrier frequency and target envelopes were reduced (Figures 4B,E) similar to our in vivo results (replotted in Figure 4D). The ECFR was not observed in the modeled response.
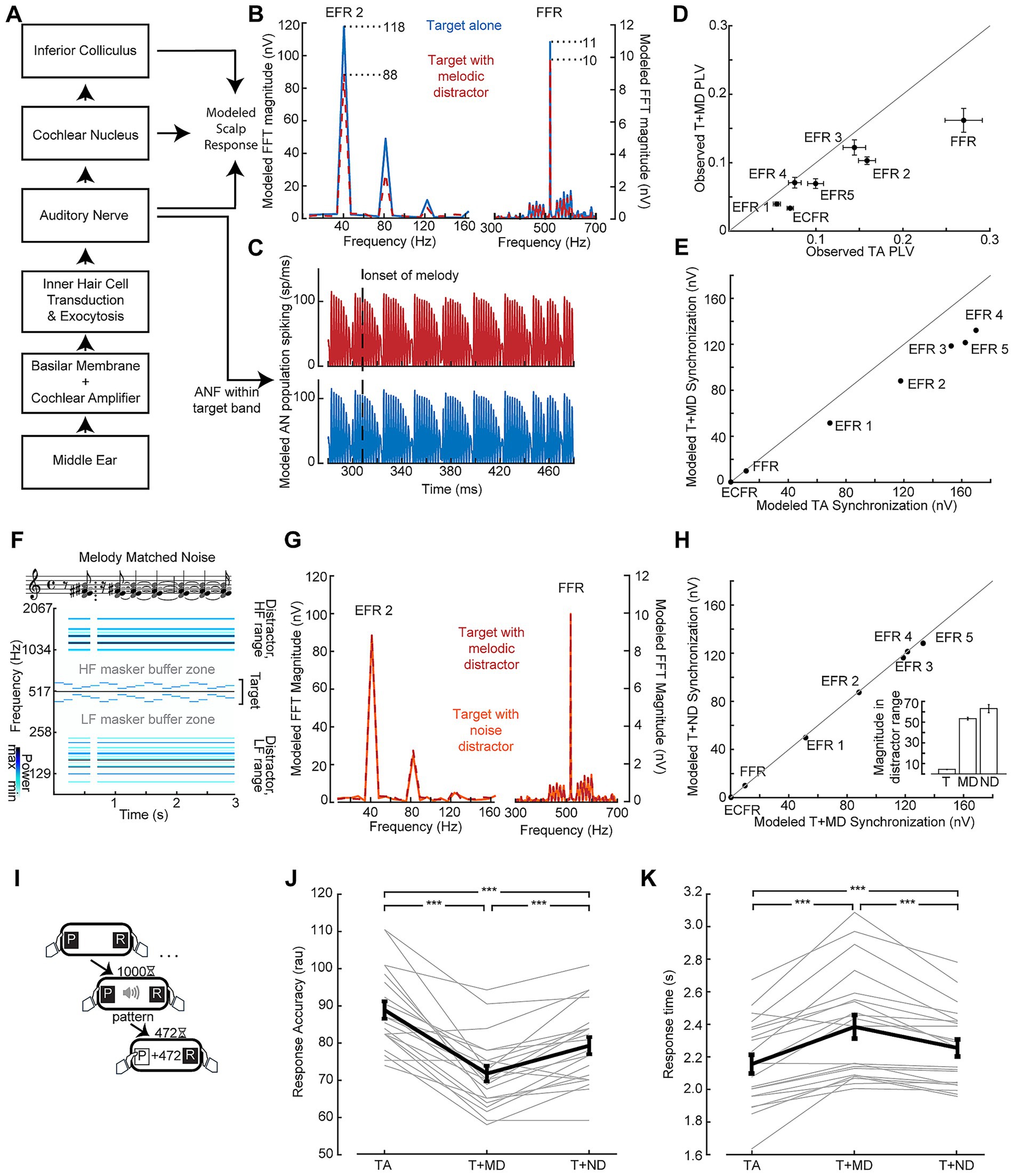
Figure 4. Validating distractor stimuli via model-based simulations of neural synchronization. (A) Simplified representation of the biophysical model from Verhulst et al. (2018) used to evaluate peripheral responses to the stimuli used in this study. (B,C) Simulated spectra of potentials at the scalp (B) and responses in the auditory nerve (C) show weaker envelope and frequency following responses when the target is submitted to the model with the melodic distractor (red) compared to the target alone (blue) despite conserved spiking in the modeled auditory nerve. (D, E) FFR and EFR differences of target-alone vs. target + distractor conditions measured in vivo (D) and simulated by the biophysical model (E). (F) Matched noise stimuli are made by including the frequencies present in the melodies (represented as notes in musical notation) across the entire duration of the stimulus, conserving “rest” periods of silence. The corresponding spectrogram of the matched noise stimulus with a target stimulus is also shown. (G) Simulated scalp potential spectra show similar envelope and frequency following responses when the target is submitted to the model with the melodic distractor (red) and the matched noise distractor (orange). (H) The effect shown in (G) is consistent across envelopes (points lie on the line of unity); inset: total magnitude of the spectrogram outside of the target buffer zone for the target (T), melodic distractor (MD) and matched noise distractor (ND) shows the energy was conserved between melodic and matched noise distractors. (I) The temporal pattern classification task, as per Figure 2. (J,K) Behavioral performance measured by response accuracy (J) and response time (K) is at an intermediate level when the target is paired with the noise distractor (T + ND) compared to the target alone (TA) and target with the melodic distractor (T + MD). Data from individual subjects are shown as thin gray lines, with the group mean shown as a thick black line. Error bars reflect the standard error of the mean. ***p < 0.001.
To better account for the off-frequency effects and interference that contribute to reduced target synchronization in the presence of the melodic distractor, we created a set of matched noise stimuli designed to induce comparable neural interactions with the target as the melodic distractor but less perceptual interference. The matched noise stimuli are comprised of the same frequency components as individual melodic distractor stimuli, but with the energy at each frequency spread out across the duration of the stimulus (Figure 4F). The same SAM applied to the melodic distractor is also applied to the matched noise distractor. When the matched noise stimulus is included in the input to the model, the FFR and EFRs to the target stimulus match the modeled responses with the melodic distractor (Figures 4G,H). Comparing the target + melodic distractor to the target + noise distractor, then, would control for low-level interference effects that are unrelated to distraction.
3.5 Differential distraction effect on target synchronization
As a next step, we set out to test the hypothesis that the matched noise distractor produced an intermediate level of perceptual interference such that temporal classification would be intermediate to target alone and the melodic distractor. We recruited a second cohort of study participants and confirmed that their target-alone performance in the speeded reaction time behavioral assay was in line with the first cohort of study participants (87% vs. 82%; Figure 4I). When the melodic distractor was included, mean accuracy dropped to 72% but improved with the matched noise distractor back to 79%. Repeated measures ANOVA showed a significant main effect of condition (F(2, 40) = 42.13; p < 0.001), with pairwise comparisons showing significant differences between all three conditions (all p < 0.001; Figure 4J). Mean response times were 2.16 s to the target alone, 2.38 s to the target with the melodic distractor, and 2.26 s to the target with the noise distractor. Repeated measures ANOVA showed a significant main effect of condition (F(2, 40) = 31.06; p < 0.001, Greenhouse-Giesser corrected), with pairwise comparisons showing significant differences between all three conditions (all p < 0.001; Figure 4K).
Having demonstrated that target + melody versus target + matched noise distractor supported a more direct comparison of variable distraction loads, we performed another in-lab assessment of combined neural and behavioral recordings. We confirmed that behavioral accuracy during the EEG recordings recapitulated the results from the speeded reaction time temporal classification task (93% vs. 80%; paired t-test p < 0.001; Figure 5A). When the matched noise is paired with the target stimulus, as with the melody, we observe synchronization peaks at the relevant target frequencies (Figure 5B). We also observe peaks corresponding to the envelope of the distractor, but the peak at the “beat” of the melody is not reproduced with the matched noise as it does not contain the note changes present in the melody and the beat may not be perceived.
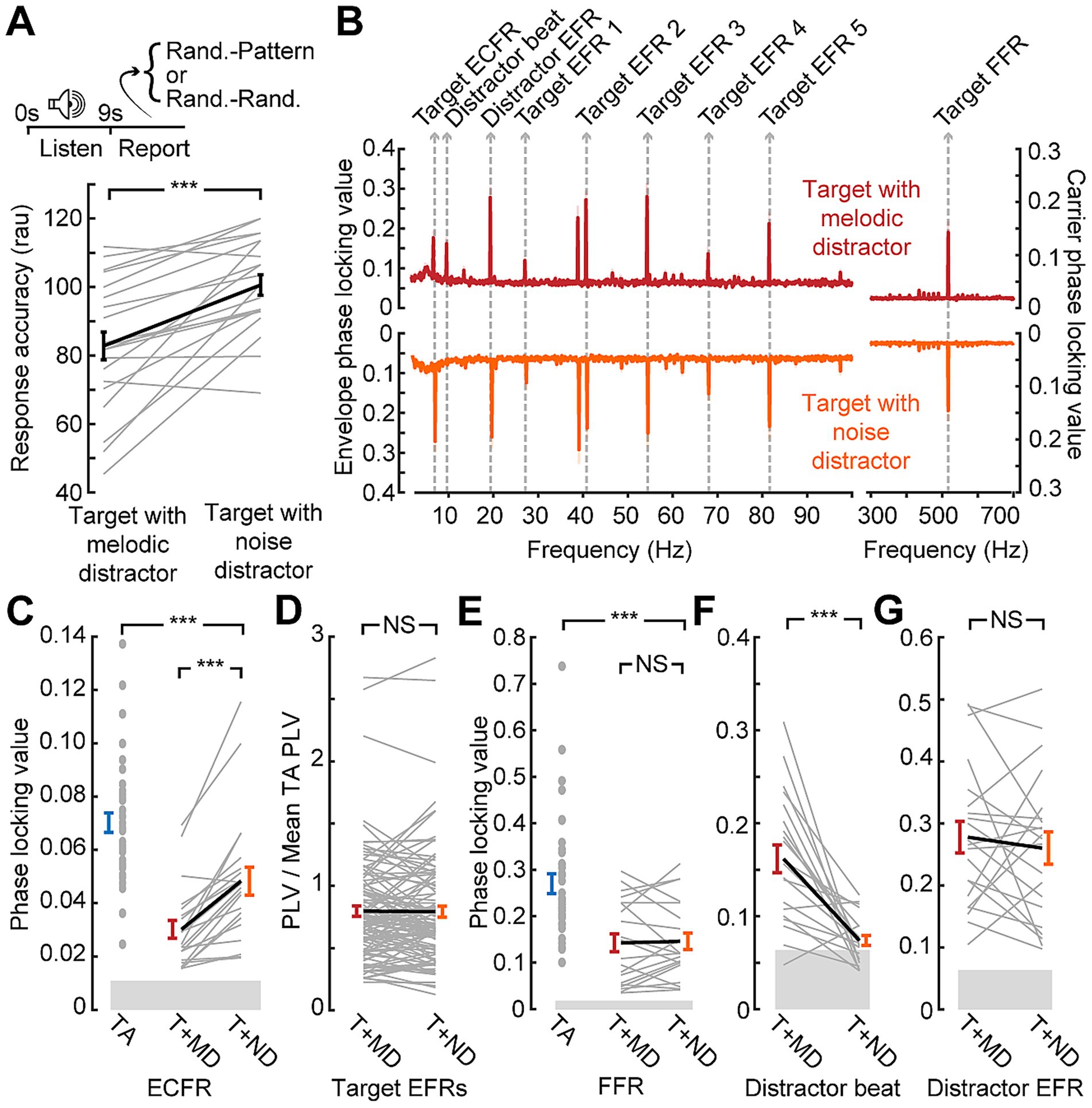
Figure 5. Only the slowest timescale of neural target synchronization, the ECFR, is modulated by distraction level. (A) Data collection paradigm, as per Figures 1, 3, with behavioral performance indicating less impairment by the noise distractor than the melodic distractor. Note that plotted values are in rationalized arcsine units, while raw accuracy is reported in the text (B) Phase locking value spectra of responses to the target with melodic distractor (red) and the target with matched noise distractor (orange). Peaks related to target and distractor stimuli features are labeled. (C) ECFRs to the target with matched noise (T + ND; orange) distractor are greater than those observed for the target with melodic distractor (T + MD; red), but less than those observed for the target alone in the prior cohort (TA; blue). (D, E) EFRs, normalized to the mean target alone PLV and collapsed across AM rate (D), and FFRs (E) do not show significant differences between the melodic distractor and noise distractor conditions. The FFR is reduced relative to the target alone in the prior cohort (TA; blue). (F) The matched noise distractor does not reliably produce synchronization at the melodic beat rate. (G) Synchronization to distractor envelope does not differ by distractor type. Data from individual subjects are shown as thin gray lines, with the group mean shown as a thick black line. Error bars reflect the standard error of the mean. NS, not significant (p > 0.05); ***p < 0.001.
We found that neural synchronization to the target ECFR was significantly greater in the less distraction matched-noise condition than the melody block (mean 0.048 vs. 0.030; p-adj. < 0.001; Figure 5C). Additionally, only two individual subjects went against the group trend. A two-sample t-test also showed that the ECFR in the matched noise block in the second cohort was significantly lower than that observed with the target alone in the prior cohort (p-adj. < 0.001).
While the EFR strength varied across different AM rates, the effects of different conditions appeared to be consistent. We therefore consolidated EFRs across AM rate by normalizing the values to the mean target alone values for each AM rate (Figure 5D). The resulting normalized values showed no difference between EFRs in the melody compared to the matched noise (p-adj. = 0.86). The FFR (Figure 5E) also showed no difference between the melody and the matched noise blocks (p-adj. = 1), but a two-sample t-test comparing the matched noise FFR to the target alone FFR in the prior cohort did show a significant effect (p-adj. < 0.001).
We also examined synchronization to the distractor stimuli at the melody beat rate (Figure 5F) and the distractor SAM rate (Figure 5G). As shown in the spectra, synchronization to the melody beat rate was only clearly present when the melody was presented and approached the noise floor when the matched noise was presented, leading to a significant difference between conditions (p-adj. < 0.001). No differences were observed between melodic and matched distractor EFR (p-adj. = 1). Therefore, of all the target stimulus features that could be reliably measured with both distractors, only the ECFR showed a differential effect of distraction between the more distracting melody condition and the less distracting matched noise condition.
3.6 Synchronization changes based on perceptual report
To strengthen the inference that the differential effect between the melodic and noise distractors on ECFR is due to distraction by the melodies, we examined synchronization on correct versus incorrect response trials (Figure 6A). We pooled participants from both cohorts, and examined the data from the melody block, which was common to both participant cohorts (Figure 6B). Participants with fewer than 15% error trials with the melodic distractor were excluded from this analysis (n = 24). For the remaining participants, following responses were separately calculated for correct and incorrect trials (Figures 6C,D). Because the number of trials, and thus the noise floor of the phase locking value (Zhu et al., 2013), in each trial category varies between participants, we quantified the difference in following rates in terms of an asymmetry index (correct – incorrect)/(correct + incorrect), and only included the asymmetry index for each participant and following rate when at least one category was above the noise floor for that following rate. One-sample t-tests showed that asymmetry indices for the FFR, ECFR, and melody beat response were significantly different than zero (p = 0.03, 0.041, and 0.0018, respectively). We note, however, the small effect size for the FFR. While the result is statistically significant, the mean asymmetry index is −0.026 for FFR, compared to 0.043 for ECFR and −0.11 for the melody beat response. Both target EFRs and the distractor EFR did not differ by response accuracy (p = 0.45, and 0.38, respectively).
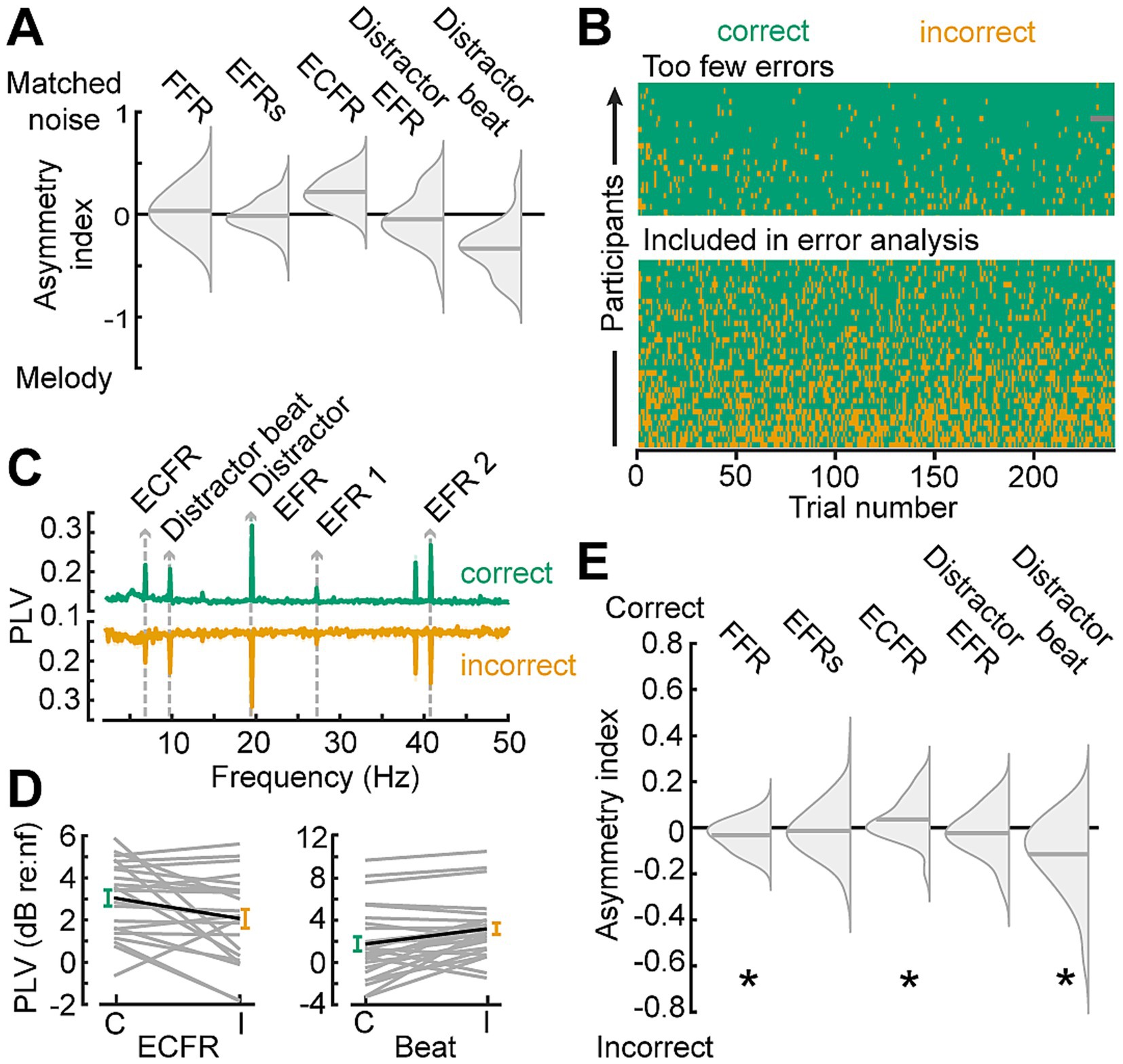
Figure 6. Synchronization to target changes is decreased while synchronization to distractor changes is increased during incorrect trials. (A) Summary of findings in Figure 6, plotted as split violin plots of the asymmetry index between the matched noise and melodic distractor conditions. (B) Raster of trial-by-trial response accuracy for all participants during the melodic distractor block, sorted by overall accuracy. Participants with too few error trials (top section) were excluded from analysis of the error trials. Green represents correct trials, yellow incorrect trials, and gray missing trials due to technical error. (C) Phase locking value spectra of correct (green, top) and incorrect (yellow, bottom) trials, focused on the lower frequency synchronization peaks. (D) The ECFR (left) and melody beat synchronization (right) plotted in dB relative to individual subject noise floors (nf) for correct (green, C) and incorrect (yellow, I) trials. Data from individual subjects are shown as thin gray lines, with the group mean shown as a thick black line. (E) Asymmetry indices for synchronization peaks between correct and incorrect trials show decreased ECFRs and increased synchronization to the melody beat during incorrect trials. FFRs are also slightly elevated during incorrect trials. *p < 0.05.
4 Discussion
Here, we developed a paradigm that allows us to measure neural synchronization across multiple timescales and with multiple stimuli presented, including synchronization to both target and competitor stimuli. This paradigm has yielded an electrophysiological measurement that is sensitive to auditory distraction which may be applied in future studies to populations of interest.
4.1 Synchronization across timescales
We have shown it is possible to measure synchronization across multiple timescales simultaneously with carefully designed stimuli. Past studies of synchronization in the auditory nervous system, even with complex stimuli, have typically focused on a single feature and timescale at a time (Krizman and Kraus, 2019). These studies have found important objective measures that provide insight into various auditory processes and disorders (Kraus et al., 2016; Vercammen et al., 2018; Parthasarathy et al., 2020; Bidelman and Momtaz, 2021) but are limited by only analyzing a single timescale. Features at different timescales may be differentially implicated in various auditory perception tasks (Lerner et al., 2011; Swaminathan et al., 2016; Norman-Haignere et al., 2022) and implicate different auditory structures (Kuwada et al., 2002; Picton et al., 2003; Chandrasekaran and Kraus, 2010; Coffey et al., 2019), so a design that gathers across multiple timescales provides advantages that single features do not, as other recent paradigms demonstrate (Wang et al., 2019; Calcus et al., 2022; Schüller et al., 2023). For instance, Wang et al. (2019) demonstrated that beyond just measuring across different frequency ranges, the combination of stimuli across multiple frequency bands actually improved cochlear place sensitivity for each individual frequency band.
We identified a novel following response, the ECFR. With our target stimulus, the ECFR is elicited by changes between different SAM envelopes which occur at a consistent rate and is sensitive to the magnitude of change (Figure 1G). We also, however, observe a similar following rate to regular changes in a stimulus with the melody beat following rate (Figure 3B). This suggests that the ECFR is part of a larger class of synchronization to changes in ongoing stimuli as has been observed for inter-aural phase difference changes (Undurraga et al., 2016; Parthasarathy et al., 2020) and synchronization to syllable rate in speech stimuli (Assaneo et al., 2019; He et al., 2023). These synchronized responses may also be related to the acoustic change complex (Kim, 2015) traditionally elicited by alternations in ongoing stimuli at slower rates. In fact, at least one other study has reported acoustic change complexes evoked by stimuli with carrier frequency changes at rates similar to the envelope changes used in our study and observed a similar synchronization peak at the rate of change (Calcus et al., 2022).
Synchronization at all timescales was affected by the presence of a competing sound. Previous work with noise stimuli had primarily shown effects at the harmonics of the EFR (Zhu et al., 2013), but our analyses show significant impacts even at the fundamental frequency of both the FFR and the EFR. This occurs despite reducing energetic masking of the target stimulus by keeping frequencies in the distractor separated from the frequencies present in the target. Using a model of the auditory periphery, we show that effects on the FFR and EFRs are partially explained by off-frequency contributors to these following rates. Importantly, the model that we used has no cortical component, despite the generators of the EFRs likely including cortical sources (Kuwada et al., 2002; Picton et al., 2003; Shinn-Cunningham et al., 2017). Future studies that examine following responses when multiple sound sources are presented should take care to account for such low-level interference between stimuli, even in the absence of direct energetic masking.
4.2 No effect of pattern
Previous studies have reported a sustained potential difference related to repeating patterns in complex stimuli using MEG (Barascud et al., 2016) and EEG (Southwell et al., 2017; Herrmann and Johnsrude, 2018; Herrmann et al., 2019). With our target stimuli, however, we did not observe an effect of the pattern or random context. Both the nonsynchronized pattern recognition potential and the synchronized following responses to the low-level features were unchanged by this context. Differences between the target stimuli utilized in our paradigm and previous studies include greater overall predictability in the stimulus, a potentially less-salient feature to differentiate tones, and active listening in our paradigm compared to passive listening in previous studies. The change in the sustained potential during a patterned stimulus has been associated with the predictability of the stimulus (Barascud et al., 2016; Southwell et al., 2017). Previous paradigms involved larger pools of potential combinations to select from; for example, in the work first describing this potential (Barascud et al., 2016), tones were selected from a library of 20 possible tones to build into sequences. While they were able to show effects when the chosen set on a given trial was limited to only 5 of the tones—in line with the five AM tones used in our target stimuli—the trial-to-trial variability of the selected tones contributed to greater uncertainty than in our paradigm. Additionally, the differences between tones in our target stimuli—changes in SAM rate—may be more subtle than changes in frequency (Barascud et al., 2016; Southwell et al., 2017) or coherence of modulation among several tones (Herrmann and Johnsrude, 2018; Herrmann et al., 2019) that have been used previously. Finally, previous studies have employed passive listening to the patterned stimuli, whereas here we asked participants to attend to and report on the pattern or random context of the stimuli.
4.3 Distraction
We characterize the effect of the melodic stimuli on target perception as distraction. More broadly, the effect falls under informational masking (Durlach et al., 2003a). While informational masking is an appropriate description of how the melodic stimuli impair target stimulus perception, we avoided the intentional use of low-level acoustic features classically associated with informational masking. Timing coincidences and manipulations of the same features in both targets and maskers have previously been utilized to produce informational masking, in part through perceptual grouping (Durlach et al., 2003b). As our approach depended on isolating neural entrainment to distinct and independent temporal features of the target and distractor sounds, we could not manipulate distraction through introducing greater temporal co-incidence between sound features. Instead, we sought to manipulate the salience of the distractor rather than manipulating low-level acoustic features to match the target stimuli.
The behavioral results, taken together, are consistent with distraction being a key feature of the impaired target perception. Participants were both less accurate and slower to respond when the target was accompanied by the melodic distractor than when the target was alone (Figures 2D,E, 3A) or when the target was accompanied by the matched noise distractor (Figures 4J,K, 5A). It is also notable that in the EEG session, when participants had a longer time to suppress the distractor and evaluate the target, accuracy was similar between the target alone block for the first cohort (mean 92%) and the matched noise block for the second cohort (mean 91%). The less-distracting matched noise stimuli were behaviorally completely suppressed, given enough time to do so, while the melodies continued to impair target perception.
Additionally, the trade-off between target and distractor synchronization observed in the analysis by response accuracy (Figure 6) is indicative of an attentional shift from the target to the melodic distractor (Kong et al., 2014). When participants make errors—and are thus failing to correctly perceive the target stimulus—we observe increased synchronization to the changes in the distractor and decreased synchronization to changes in the target. If the informational masking effect of the melodies was driven by failures of stream segregation, we would expect synchronization to both target and distractor to be reduced as the features of the combined stream would be ambiguous (Choi et al., 2014). That we instead observe a trade-off indicates that target and melodic stimuli were still segregated, but that the distractor melodies received greater attention on trials when participants made errors.
4.4 Low-level synchronization insensitive to distraction
The FFR and EFRs were insensitive to differential levels of distraction once off-target effects of competitor stimuli were accounted for by the matched noise stimulus (Figure 5). This result is in line with previous work that has shown FFR and EFR measurements that are insensitive to attentional state (Gutschalk et al., 2008; Varghese et al., 2015) and consistent with primary generators for the FFR and EFRs elicited by our stimuli being in brainstem and early auditory cortex (Kuwada et al., 2002; Picton et al., 2003; Chandrasekaran and Kraus, 2010). While some studies have shown an effect of attention on EFRs at frequencies around 40 Hz, these effects are typically shown with spatially separated streams (Müller et al., 2009; Bharadwaj et al., 2014). Spatial segregation is a helpful auditory cue in natural listening environments, but spatial auditory attention involves different processes than those evoked under non-spatial tasks (Bonacci et al., 2020). The increased EFR observed in other studies may be a result of the recruitment of this spatially-selective system; our stimuli were presented diotically, thus providing no spatialized cues.
4.5 Neural measure sensitive to distraction
We have demonstrated that the ECFR (and indirectly the melody beat following response) is sensitive to the level of auditory distraction. This is consistent with other work showing that low-frequency synchronization to auditory stimuli is sensitive to attentional effects (Elhilali et al., 2009; Xiang et al., 2010; Schüller et al., 2023). It is also possible that low-level differences between the melodic and noise distractors could contribute specifically to the differential effect in the ECFR. While our modeling experiments suggest that the low-level effects on the higher-rate synchronization measures were similar between the two types of distractors, the models do not produce the ECFR. However, such low-level differences would not explain the reduction in ECFR for error trials within the melodic distractor condition. More likely, different combinations of neural generators could explain why the sensitivity to distraction was observed only for the ECFR and not the higher-frequency FFR and EFRs. While we do not have direct evidence of the generator of the ECFR, synchronization at rates similar to the ECFR has been linked to cortical sources which would more likely be susceptible to top-down effects of distraction (Liégeois-Chauvel et al., 2004; Elhilali et al., 2009; Xiang et al., 2010).
Susceptibility to auditory distraction is implicated in a variety of conditions, including difficulty understanding speech in noisy environments (Shinn-Cunningham and Best, 2008; Tierney et al., 2020), tinnitus (McKenna et al., 2014), and neurodevelopmental disorders (O’Connor, 2012; Blomberg et al., 2019; Keehn et al., 2019). While the ECFR cannot serve as a direct measure of these disorders, it may be useful in evaluating this contributing factor. Moreover, there is evidence that auditory distractor suppression may be a skill that can be trained through interactive training software (Whitton et al., 2017; de Larrea-Mancera et al., 2022). Measurement of factors that are receptive to change may prove particularly useful in evaluating therapeutic effects. Future studies may evaluate longitudinal changes in the ECFR as participants receive training in suppressing auditory distractors. Individuals who improve in their ability to suppress distractors may see increases in ECFR when target stimuli are accompanied by highly distracting competitors.
The ECFR, but not the FFR or EFRs, is a neural synchronization measure sensitive to the level of auditory distraction. Being measured alongside the low-level, distraction-insensitive FFR and EFRs provides an internal control for effects at earlier stages of the pathway or generalized disordered temporal processing. Our results point to the potential of paradigms evoking the ECFR and other regular stimulus changes to elicit objective measures of auditory distraction in a variety of contexts.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Mass General Brigham Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
DS: Writing – review & editing, Methodology, Writing – original draft, Software, Visualization, Conceptualization, Formal analysis. JS: Investigation, Writing – review & editing, Data curation, Resources, Methodology. AP: Writing – review & editing, Methodology. KH: Software, Resources, Writing – review & editing. DP: Writing – review & editing, Funding acquisition, Project administration, Conceptualization, Visualization, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. An NIH research grant supported sensory stimulus design and data analysis approaches (P50 DC015857). Internal funds supported project piloting and data collection. DS was supported by a training grant from the National Institute on Deafness and Communication Disorders (T32 DC000038).
Acknowledgments
We thank Gerald Kidd, Hari Bharadwaj, Josh McDermott, and Barbara Shinn-Cunningham for feedback that motivated the development of the matched noise stimulus. Finally, we thank all the participants who agreed to undergo study procedures in order to provide the data described herein.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnhum.2025.1623431/full#supplementary-material
References
Assaneo, M. F., Ripollés, P., Orpella, J., Lin, W. M., de Diego-Balaguer, R., and Poeppel, D. (2019). Spontaneous synchronization to speech reveals neural mechanisms facilitating language learning. Nat. Neurosci. 22, 627–632. doi: 10.1038/s41593-019-0353
Barascud, N., Pearce, M. T., Griffiths, T. D., Friston, K. J., and Chait, M. (2016). Brain responses in humans reveal ideal observer-like sensitivity to complex acoustic patterns. Proc. Natl. Acad. Sci. USA 113, E616–E625. doi: 10.1073/PNAS.1508523113
Bharadwaj, H. M., Lee, A. K. C., and Shinn-Cunningham, B. G. (2014). Measuring auditory selective attention using frequency tagging. Front. Integr. Neurosci. 8:6. doi: 10.3389/FNINT.2014.00006
Bharadwaj, H. M., Masud, S., Mehraei, G., Verhulst, S., and Shinn-Cunningham, B. G. (2015). Individual differences reveal correlates of hidden hearing deficits. J. Neurosci. 35, 2161–2172. doi: 10.1523/JNEUROSCI.3915-14.2015
Bharadwaj, H. M., and Shinn-Cunningham, B. G. (2014). Rapid acquisition of auditory subcortical steady state responses using multichannel recordings. Clin. Neurophysiol. 125, 1878–1888. doi: 10.1016/J.CLINPH.2014.01.011
Bidelman, G. M., and Momtaz, S. (2021). Subcortical rather than cortical sources of the frequency-following response (FFR) relate to speech-in-noise perception in normal-hearing listeners. Neurosci. Lett. 746:135664. doi: 10.1016/j.neulet.2021.135664
Blomberg, R., Danielsson, H., Rudner, M., Söderlund, G. B. W., and Rönnberg, J. (2019). Speech processing difficulties in attention deficit hyperactivity disorder. Front. Psychol. 10:458190. doi: 10.3389/fpsyg.2019.01536
Bonacci, L. M., Bressler, S., and Shinn-Cunningham, B. G. (2020). Nonspatial features reduce the reliance on sustained spatial auditory attention. Ear Hear. 41, 1635–1647. doi: 10.1097/AUD.0000000000000879
Brungart, D. S., Simpson, B. D., Ericson, M. A., and Scott, K. R. (2001). Informational and energetic masking effects in the perception of multiple simultaneous talkers. J. Acoust. Soc. Am. 110, 2527–2538. doi: 10.1121/1.1408946
Calcus, A., Undurraga, J. A., and Vickers, D. (2022). Simultaneous subcortical and cortical electrophysiological recordings of spectro-temporal processing in humans. Front. Neurol. 13:928158. doi: 10.3389/FNEUR.2022.928158
Cancel, V. E., McHaney, J. R., Milne, V., Palmer, C., and Parthasarathy, A. (2023). A data-driven approach to identify a rapid screener for auditory processing disorder testing referrals in adults. Sci. Rep. 13:13636. doi: 10.1038/s41598-023-40645-0
Chandrasekaran, B., and Kraus, N. (2010). The scalp-recorded brainstem response to speech: neural origins and plasticity. Psychophysiology 47, 236–246. doi: 10.1111/j.1469-8986.2009.00928.x
Choi, I., Wang, L., Bharadwaj, H., and Shinn-Cunningham, B. (2014). Individual differences in attentional modulation of cortical responses correlate with selective attention performance. Hear. Res. 314, 10–19. doi: 10.1016/j.heares.2014.04.008
Coffey, E. B. J., Nicol, T., White-Schwoch, T., Chandrasekaran, B., Krizman, J., Skoe, E., et al. (2019). Evolving perspectives on the sources of the frequency-following response. Nat. Commun. 10:5036. doi: 10.1038/s41467-019-13003-w
Cooke, M., Garcia Lecumberri, M. L., and Barker, J. (2008). The foreign language cocktail party problem: energetic and informational masking effects in non-native speech perception. J. Acoust. Soc. Am. 123, 414–427. doi: 10.1121/1.2804952
de Larrea-Mancera, E. S. L., Philipp, M. A., Stavropoulos, T., Carrillo, A. A., Cheung, S., Koerner, T. K., et al. (2022). Training with an auditory perceptual learning game transfers to speech in competition. J. Cogn. Enhanc. 6, 47–66. doi: 10.1007/s41465-021-00224-5
Durlach, N. I., Mason, C. R., Kidd, G., Arbogast, T. L., Colburn, H. S., and Shinn-Cunningham, B. G. (2003a). Note on informational masking (L). J. Acoust. Soc. Am. 113, 2984–2987. doi: 10.1121/1.1570435
Durlach, N. I., Mason, C. R., Shinn-Cunningham, B. G., Arbogast, T. L., Colburn, H. S., and Kidd, G. (2003b). Informational masking: counteracting the effects of stimulus uncertainty by decreasing target-masker similarity. J. Acoust. Soc. Am. 114, 368–379. doi: 10.1121/1.1577562
Elhilali, M., Xiang, J., Shamma, S. A., and Simon, J. Z. (2009). Interaction between attention and bottom-up saliency mediates the representation of foreground and background in an auditory scene. PLoS Biol. 7:e1000129. doi: 10.1371/journal.pbio.1000129
Encina-Llamas, G., Dau, T., and Epp, B. (2021). On the use of envelope following responses to estimate peripheral level compression in the auditory system. Sci. Rep. 11:6962. doi: 10.1038/s41598-021-85850-x
Galambos, R., Makeig, S., and Talmachoff, P. J. (1981). A 40-Hz auditory potential recorded from the human scalp. Proc. Natl. Acad. Sci. USA 78, 2643–2647. doi: 10.1073/PNAS.78.4.2643
Gutschalk, A., Micheyl, C., and Oxenham, A. J. (2008). Neural correlates of auditory perceptual awareness under informational masking. PLoS Biol. 6:e138. doi: 10.1371/journal.pbio.0060138
He, D., Buder, E. H., and Bidelman, G. M. (2023). Effects of syllable rate on Neuro-behavioral synchronization across modalities: brain oscillations and speech productions. Neurobiol. Lang. 4, 344–360. doi: 10.1162/nol_a_00102
Herrmann, B., Buckland, C., and Johnsrude, I. S. (2019). Neural signatures of temporal regularity processing in sounds differ between younger and older adults. Neurobiol. Aging 83, 73–85. doi: 10.1016/j.neurobiolaging.2019.08.028
Herrmann, B., and Johnsrude, I. S. (2018). Neural signatures of the processing of temporal patterns in sound. J. Neurosci. 38, 5466–5477. doi: 10.1523/JNEUROSCI.0346-18.2018
Jaques, N., Gu, S., Bahdanau, D., Hernández-Lobato, J. M., Turner, R. E., and Eck, D. (2017) ‘Sequence tutor: conservative fine-tuning of sequence generation models with KL-control’, in D. Precup and Y. W. Teh (eds) Proceedings of the 34th International Conference on Machine Learning. PMLR (Proceedings of Machine Learning Research), pp. 1645–1654.
Keehn, B., Kadlaskar, G., McNally Keehn, R., and Francis, A. L. (2019). Auditory attentional disengagement in children with autism Spectrum disorder. J. Autism Dev. Disord. 49, 3999–4008. doi: 10.1007/s10803-019-04111-z
Kidd, G., Arbogast, T. L., Mason, C. R., and Walsh, M. (2002). Informational masking in listeners with sensorineural hearing loss. JARO 3, 107–119. doi: 10.1007/s101620010095
Kidd, G., Mason, C. R., Swaminathan, J., Roverud, E., Clayton, K. K., and Best, V. (2016). Determining the energetic and informational components of speech-on-speech masking. J. Acoust. Soc. Am. 140, 132–144. doi: 10.1121/1.4954748
Kim, J. (2015). Acoustic change complex: clinical implications. J. Audiol. Otol. 19, 120–124. doi: 10.7874/jao.2015.19.3.120
Kong, Y. Y., Mullangi, A., and Ding, N. (2014). Differential modulation of auditory responses to attended and unattended speech in different listening conditions. Hear. Res. 316, 73–81. doi: 10.1016/j.heares.2014.07.009
Kraus, N., Thompson, E. C., Krizman, J., Cook, K., White-Schwoch, T., and LaBella, C. R. (2016). Auditory biological marker of concussion in children. Sci. Rep. 6, 1–10. doi: 10.1038/srep39009
Krizman, J., and Kraus, N. (2019). Analyzing the FFR: a tutorial for decoding the richness of auditory function. Hear. Res. 382:107779. doi: 10.1016/j.heares.2019.107779
Kuwada, S., Anderson, J. S., Batra, R., Fitzpatrick, D. C., Teissier, N., and D’Angelo, W. R. (2002). Sources of the scalp-recorded amplitude-modulation following response. J. Am. Acad. Audiol. 13, 188–204. doi: 10.1055/S-0040-1715963
Lerner, Y., Honey, C. J., Silbert, L. J., and Hasson, U. (2011). Topographic mapping of a hierarchy of temporal receptive windows using a narrated story. J. Neurosci. 31, 2906–2915. doi: 10.1523/JNEUROSCI.3684-10.2011
Liégeois-Chauvel, C., Lorenzi, C., Trébuchon, A., Régis, J., and Chauvel, P. (2004). Temporal envelope processing in the human left and right auditory cortices. Cereb. Cortex 14, 731–740. doi: 10.1093/cercor/bhh033
Linares, A. E., Filho, O. A. C., and De Souza Martinez, M. A. N. (2010). Auditory steady state response in pediatric audiology. Braz. J. Otorhinolaryngol. 76, 723–728. doi: 10.1590/s1808-86942010000600010
McKenna, L., Handscomb, L., Hoare, D. J., and Hall, D. A. (2014). A scientific cognitive-behavioral model of tinnitus: novel conceptualizations of tinnitus distress. Front. Neurol. 5:196. doi: 10.3389/FNEUR.2014.00196
Müller, N., Schlee, W., Hartmann, T., Lorenz, I., and Weisz, N. (2009). Top-down modulation of the auditory steady-state response in a task-switch paradigm. Front. Hum. Neurosci. 3:2009. doi: 10.3389/NEURO.09.001.2009
Nolan, H., Whelan, R., and Reilly, R. B. (2010). FASTER: fully automated statistical thresholding for EEG artifact rejection. J. Neurosci. Methods 192, 152–162. doi: 10.1016/J.JNEUMETH.2010.07.015
Norman-Haignere, S. V., Long, L. K., Devinsky, O., Doyle, W., Irobunda, I., Merricks, E. M., et al. (2022). Multiscale temporal integration organizes hierarchical computation in human auditory cortex. Nat. Hum. Behav. 6, 455–469. doi: 10.1038/s41562-021-01261-y
O’Connor, K. (2012). Auditory processing in autism spectrum disorder: a review. Neurosci. Biobehav. Rev. 36, 836–854. doi: 10.1016/j.neubiorev.2011.11.008
Parthasarathy, A., and Bartlett, E. (2012). Two-channel recording of auditory-evoked potentials to detect age-related deficits in temporal processing. Hear. Res. 289, 52–62. doi: 10.1016/j.heares.2012.04.014
Parthasarathy, A., Hancock, K. E., Bennett, K., Degruttola, V., and Polley, D. B. (2020). Bottom-up and top-down neural signatures of disordered multi-talker speech perception in adults with normal hearing. eLife 9:e51419. doi: 10.7554/eLife.51419
Perez-Abalo, M. C., Savio, G., Torres, A., Martín, V., Rodríguez, E., and Galán, L. (2001). Steady state responses to multiple amplitude-modulated tones: an optimized method to test frequency-specific thresholds in hearing-impaired children and normal-hearing subjects. Ear Hear. 22, 200–211. doi: 10.1097/00003446-200106000-00004
Picton, T. W., John, M. S., Dimitrijevic, A., and Purcell, D. (2003). Human auditory steady-state responses. Int. J. Audiol. 42, 177–219. doi: 10.3109/14992020309101316
Rosen, S. (1992). Temporal information in speech: acoustic, auditory and linguistic aspects. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 336, 367–373. doi: 10.1098/rstb.1992.0070
Schüller, A., Schilling, A., Krauss, P., Rampp, S., and Reichenbach, T. (2023). Attentional modulation of the cortical contribution to the frequency-following response evoked by continuous speech. J. Neurosci. 43, 7429–7440. doi: 10.1523/JNEUROSCI.1247-23.2023
Shinn-Cunningham, B., Varghese, L., Wang, L., and Bharadwaj, H. (2017). “Individual differences in temporal perception and their implications for everyday listening” in eds. N. Kraus, S. Anderson, T. White-Schwoch, R. R. Fay, and A. N. Popper. The frequency-following response: A window into human communication. Cham: Springer International Publishing, 159–192. doi: 10.1007/978-3-319-47944-6_7
Shinn-Cunningham, B., and Best, V. (2008). Selective attention in normal and impaired hearing. Trends Amplif. 12, 283–299. doi: 10.1177/1084713808325306
Sollini, J., Poole, K. C., Blauth-Muszkowski, D., and Bizley, J. K. (2022). The role of temporal coherence and temporal predictability in the build-up of auditory grouping. Sci. Rep. 12, 14493–14410. doi: 10.1038/s41598-022-18583-0
Southwell, R., Baumann, A., Gal, C., Barascud, N., Friston, K., and Chait, M. (2017). Is predictability salient? A study of attentional capture by auditory patterns. Philos. Trans. R. Soc. B Biol. Sci. 372:20160105. doi: 10.1098/RSTB.2016.0105
Stevens, K. N. (1971). “Sources of inter- and intra-speaker variability in the acoustic properties of speech sound” in Proceedings of the seventh international congress of phonetic sciences. eds. A. Rigault and R. Charbonneau (Boston: De Gruyter), 206–232.
Studebaker, G. A. (1985). A “rationalized” arcsine transform. J. Speech Hear. Res. 28, 455–462. doi: 10.1044/JSHR.2803.455
Swaminathan, J., Mason, C. R., Streeter, T. M., Best, V., Roverud, E., and Kidd, G. (2016). Role of binaural temporal fine structure and envelope cues in cocktail-party listening. J. Neurosci. 36, 8250–8257. doi: 10.1523/JNEUROSCI.4421-15.2016
Tierney, A., Rosen, S., and Dick, F. (2020). Speech-in-speech perception, nonverbal selective attention, and musical training. J. Exp. Psychol. Learn. Mem. Cogn. 46, 968–979. doi: 10.1037/xlm0000767
Tremblay, K. L., Pinto, A., Fischer, M. E., Klein, B. E. K., Klein, R., Levy, S., et al. (2015). Self-reported hearing difficulties among adults with Normal audiograms: the beaver dam offspring study. Ear Hear. 36, e290–e299. doi: 10.1097/AUD.0000000000000195
Undurraga, J. A., Haywood, N. R., Marquardt, T., and McAlpine, D. (2016). Neural representation of interaural time differences in humans—an objective measure that matches behavioural performance. J. Assoc. Res. Otolaryngol. 17, 591–607. doi: 10.1007/s10162-016-0584-6
Varghese, L., Bharadwaj, H. M., and Shinn-Cunningham, B. G. (2015). Evidence against attentional state modulating scalp-recorded auditory brainstem steady-state responses. Brain Res. 1626, 146–164. doi: 10.1016/j.brainres.2015.06.038
Vercammen, C., Goossens, T., Undurraga, J., Wouters, J., and Van Wieringen, A. (2018). Electrophysiological and behavioral evidence of reduced binaural temporal processing in the aging and hearing impaired human auditory system. Trends Hear. 22:733. doi: 10.1177/2331216518785733
Verhulst, S., Altoè, A., and Vasilkov, V. (2018). Computational modeling of the human auditory periphery: auditory-nerve responses, evoked potentials and hearing loss. Hear. Res. 360, 55–75. doi: 10.1016/j.heares.2017.12.018
Wang, L., Bharadwaj, H., and Shinn-Cunningham, B. (2019). Assessing Cochlear-place specific temporal coding using multi-band complex tones to measure envelope-following responses. Neuroscience 407, 67–74. doi: 10.1016/j.neuroscience.2019.02.003
Whitton, J. P., Hancock, K. E., Shannon, J. M., and Polley, D. B. (2017). Audiomotor perceptual training enhances speech intelligibility in background noise. Curr. Biol. 27, 3237–3247.e6. doi: 10.1016/j.cub.2017.09.014
Xiang, J., Simon, J., and Elhilali, M. (2010). Competing streams at the cocktail party: exploring the mechanisms of attention and temporal integration. J. Neurosci. 30, 12084–12093. doi: 10.1523/JNEUROSCI.0827-10.2010
Keywords: distraction, synchronization, frequency following response, envelope following response, informational masking, auditory evoked potential
Citation: Sorensen DO, Sugai JA, Parthasarathy A, Hancock KE and Polley DB (2025) The slowest timescales of neural synchronization reveal the strongest influence of auditory distraction. Front. Hum. Neurosci. 19:1623431. doi: 10.3389/fnhum.2025.1623431
Edited by:
Robert C. Liu, Emory University, United StatesReviewed by:
Heather Read, University of Connecticut, United StatesJens Hjortkjaer, Technical University of Denmark, Denmark
Copyright © 2025 Sorensen, Sugai, Parthasarathy, Hancock and Polley. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David O. Sorensen, ZGF2aWRfc29yZW5zZW5AZy5oYXJ2YXJkLmVkdQ==
†Present address: Aravindakshan Parthasarathy, Department of Communication Science and Disorders, School of Health and Rehabilitation Sciences, University of Pittsburgh, Pittsburgh, PA, United States
 David O. Sorensen
David O. Sorensen Jenna A. Sugai
Jenna A. Sugai Aravindakshan Parthasarathy
Aravindakshan Parthasarathy Kenneth E. Hancock
Kenneth E. Hancock Daniel B. Polley
Daniel B. Polley