- 1Laboratory for Movement Biomechanics, Department of Health Sciences and Technology, ETH Zürich, Zürich, Switzerland
- 2Department of Biomedical Engineering, University of Basel, Basel, Switzerland
- 3Centre for Clinical Motion Analysis, University Children's Hospital Basel, Basel, Switzerland
- 4Department of Orthopaedics, University Children's Hospital Basel, Basel, Switzerland
- 5Singapore-ETH Centre, Future Health Technologies Program, CREATE Campus, Singapore, Singapore
Introduction: In toe walking children, impaired maturation of neuromotor control often leads to persistent use of immature motor programs. Understanding the underlying etiology of toe walking in children with cerebral palsy (CP) and idiopathic toe walking (ITW) is crucial for advancing rehabilitation strategies. This study examined gait adaptations and H-reflex responses to varied weight-bearing conditions to determine whether children with ITW and CP exhibit distinct neuromotor control strategies compared to typically developing (TD) peers.
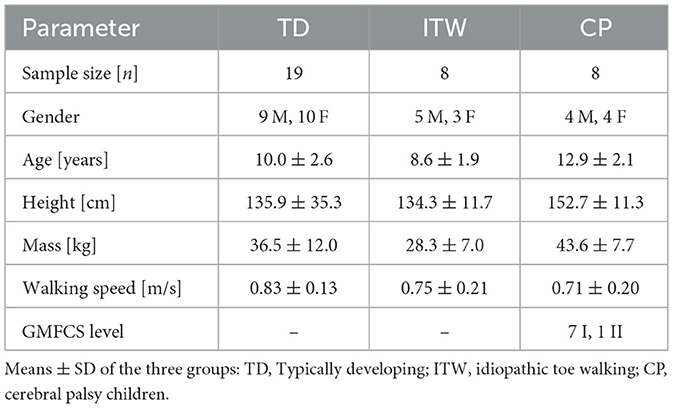
Methods: Eight children with CP (mean age 12.9 ± 2.1 years), eight with ITW (8.6 ± 1.9 years), and 19 TD children (10.0 ± 2.6 years) walked on a treadmill under three conditions: normal bodyweight, 30% bodyweight unloading, and 30% additional bodyweight. Linear mixed-effects models assessed spatiotemporal gait parameters, margin of stability, gait variability, and H-reflex responses.
Results: Bodyweight unloading increased single-limb support time, while reducing double-limb support time and antero-posterior margin of stability across groups (p < 0.01). ITW children exhibited increased gait variability (p < 0.01) under bodyweight unloading, while CP children showed no change. H-reflex amplitudes decreased under bodyweight unloading in TD children, while CP children exhibited hyperreflexia (p < 0.05).
Discussion: The findings of this exploratory study suggest that toe walking is associated with distinct adaptive strategies in ITW and CP children to compensate for environmental challenges. In ITW, increased variability under bodyweight unloading may reflect exploratory motor control, whereas CP children relied on stiffening strategies, marked by reduced variability and hyperreflexia, indicating limited adaptability and less efficient gait patterns. These results imply that similar biomechanical constraints evoke divergent neuromotor adaptations in ITW and CP children.
1 Introduction
Walking is the most fundamental form of human locomotion, essential for autonomy, wellbeing, and active participation in daily life. During early gait development, walking is initially unstable and highly variable. Novice walkers make initial floor contact with a flatfoot pattern (Alvarez et al., 2008) before gradually developing a heel strike within the first year of independent walking, enhancing gait stability and efficiency (Hallemans et al., 2006; Zeininger et al., 2018). This developmental shift relies on complex neuromotor adaptations, including the maturation and proper control of muscle reflexes, which play a critical role in supporting posture and coordinating movement during walking (Geyer and Herr, 2010; Ivanenko and Gurfinkel, 2018). However, some children do not develop a heel strike pattern, resulting in persistent toe walking, which may indicate an underlying neurodevelopmental pathology. The most common neurodevelopmental disorder in early childhood is cerebral palsy (CP) (Cans et al., 2004), with an estimated prevalence of 1.6 per 1000 live births (McIntyre et al., 2022). Here, lesions in the central nervous system could arise from damage to the fetal or infant brain (Rosenbaum et al., 2007), leading to reduced supra-spinal involvement in gait adaptation and regulation (Cappellini et al., 2020). Impaired supra-spinal and spinal control mechanisms lead to irregular locomotion, frequently resulting in persistent toe walking patterns, which affect balance and increase the risk of falling (Schlough et al., 2020).
When CP and other medical causes of toe walking are excluded, the pattern is classified as idiopathic toe walking (ITW). ITW affects up to 5% of children (Engstrom and Tedroff, 2012) and is often associated with developmental delays (Shulman et al., 1997), yet its underlying etiology remains unclear. In both CP and ITW, toe walking has been linked to impaired proprioception, motor control difficulties, balance deficits, reduced ankle range of motion, shortened calf muscles, increased energy expenditure, and a heightened risk of falls (Ruzbarsky et al., 2016; De Oliveira et al., 2021; Boyer and Patterson, 2018). In children with CP, spasticity characterized by increased muscle tone and hyperreflexia is a common contributor to abnormal gait patterns. Although hyperreflexia is difficult to quantify through standard motion analysis, it remains a key target in clinical management. Current treatments, such as physiotherapy, orthoses, Botox injections, and surgery, all primarily address the symptoms rather than the underlying causes (Bauer et al., 2022; Novak et al., 2020). However, toe walking and balance issues often persist despite intervention (van Kuijk et al., 2014), leading to frustration for patients and families when treatments fail to provide lasting improvements (Bartoletta et al., 2021; Gillani et al., 2021). A deeper understanding of the neuromotor etiology underlying toe walking is essential before targeted therapies that enhance stability, mobility, and quality of life for affected children can be developed.
Dynamic stability during walking can be assessed using the inverted pendulum model, which assumes balance is maintained when the center of mass (CoM) remains within the base of support (BoS) (Bruijn and van Dieen, 2018; Winter, 1995). The margin of stability (MoS), defined as the distance between the extrapolated CoM (XCoM) and the center of pressure (CoP), is a commonly used measure to assess dynamic stability during walking (Hof et al., 2005). When CoP data are unavailable, foot markers can approximate BoS boundaries (Hof et al., 2005; Watson et al., 2021; Curtze et al., 2024). This metric provides valuable insights into walking strategies and fall risk. However, interpretation of MoS can be complex and paradoxical. A common interpretation is that individuals with reduced stability adopt more cautious gait patterns. Children with ITW often display lower MoS, suggesting a reliance on a falling forward strategy to maintain momentum (Gwerder et al., 2025a), which may serve as a compensatory mechanism for greater energy absorption at initial contact (Gwerder et al., 2025a). Conversely, the opposite relationship can also occur. Stable individuals may afford seemingly unstable gait patterns, while those with poor stability adopt more cautious and stable gait patterns (Sangeux et al., 2024). In typically developing (TD) children, for example, MoS decreases with age, reflecting improved control of the CoM (Ivanenko et al., 2007; Hallemans et al., 2018). Children with CP, in contrast, exhibit impaired pendular energy transmission during gait (van den Hecke et al., 2007), often resulting in increased MoS as a strategy to enhance stability within the limits of reduced motor coordination (Rethwilm et al., 2021; Tracy et al., 2019). These findings underscore the complexity of interpreting MoS solely in terms of stability and further emphasize that gait is a flexible, adaptive process shaped by both biomechanical constraints and neuromotor control strategies.
In early stages of motor development, proprioceptive and visual feedback mechanisms used for gait adaptation are incomplete, indicating immature neuronal feedback systems (Berger et al., 1985). At this stage, motor programs are still undergoing refinement, characterized by higher gait variability, excessive muscle co-activation and strong reflex responses (Berger et al., 1985). With experience and practice, both supra-spinal and spinal control improve, reducing gait variability and enhancing walking efficiency, but also providing high level control to avoid environmental challenges (Ivanenko et al., 2007). In addition to voluntary motor control, reflex responses play a crucial role in gait adaptation. One approach to probe spinal control and neural excitability is the electrically triggered short latency Hoffmann-reflex (H-reflex), which provides insights into spinal circuit function and neuromuscular adaptations (Ivanenko and Gurfinkel, 2018; Theodosiadou et al., 2023). Its amplitude is known to vary with postural demands, the phase of the gait cycle, and different weight-bearing conditions (Capaday and Stein, 1986; Cecen et al., 2018; Angulo-Kinzler et al., 1998; Kim et al., 2024). In TD children, the maturation process is reflected in the progressive suppression of the H-reflex during walking, suggesting increasing corticospinal tract (CST) involvement and greater central inhibition (Hodapp et al., 2007a). In contrast, children with CP exhibit reduced somatosensory integration and impaired maturation of feedforward processes (Berger et al., 1985; Lorentzen et al., 2019), resulting in a continued reliance on immature motor programs with excessive co-contraction of agonist and antagonist muscles (Lorentzen et al., 2019). Although the H-reflex is rhythmically modulated during gait, its overall suppression is impaired in CP children, resembling spinal control patterns observed in toddlers before CST maturation (Hodapp et al., 2007a). Similarly, children with ITW may exhibit immature supra-spinal control, leading to altered neuromotor control strategies and increased but uncoordinated muscular contractures (Veerkamp et al., 2023). Importantly, atypical H-reflex activity has also been reported in ITW children in prone position (Rossi et al., 2018). Most studies investigating reflex control in toe walking children have been conducted under static conditions (Rossi et al., 2018; Leonard et al., 1990; Marzbani et al., 2016), offering only limited insights into how these mechanisms operate during walking and thus contribute to dynamic balance control. However, dynamic tasks such as walking introduce additional stability demands not captured in static assessments and therefore warrant investigation.
Despite evidence that children with ITW and CP rely on altered motor control strategies, the interplay between reflex modulation, dynamic stability, and gait variability, particularly under challenging environmental demands, remains poorly understood. Although they both suffer from toe walking, the underlying reasons might not be identical, and the H-reflex may serve as a tool to interrogate distinct neuromotor control strategies. Moreover, walking under different weight-bearing conditions provides a unique window into how the neuromotor system adapts to environmental challenges. By examining spatiotemporal gait parameters, dynamic stability, and H-reflex modulation during treadmill walking under different weight-bearing conditions, this study aims to clarify whether TD, ITW, and CP children employ distinct movement strategies and reflex responses to adapt to environmental challenges. These insights may contribute to the design of more effective, individualized rehabilitation strategies for children who exhibit toe walking patterns.
2 Materials and methods
2.1 Participants
This exploratory study was conducted in accordance with the Declaration of Helsinki and received approval from the Zurich cantonal ethics committee (BASEC-No 2019-00678). All participants were provided with detailed verbal and written explanations of the measurement process prior to participation in the study. For children, written informed consent was obtained from legal guardians, while those aged 14 years and older additionally provided their own consent before participation.
Nineteen TD children, eight with ITW, and eight with mild CP (spastic hemiparesis, GMFCS levels I and II, aged 6–18 years, participated in this study (Table 1). Exclusion criteria were (a) any acute or chronic neurological or musculoskeletal conditions other than ITW or CP, (b) injuries or surgeries affecting the lower extremities within the past year, (c) Botox treatment within the previous 6 months, (d) acute pain, (e) vertigo or balance problems, (f) unable to walk on a treadmill for 4 min, or (g) use of medication that could affect balance.
2.2 Experimental setup and procedure
2.2.1 Overview
Prior to measurement, the optimal location for eliciting an H-reflex response was marked (see details under 3.2.2. H-reflex). After electrode placement and the attachment of 62 whole-body reflective markers (Supplementary material, 1. Marker Set), individualized stimulus intensity was established using H-reflex recruitment curves (RC, see details under 3.2.2. H-reflex) while participants walked barefoot on a treadmill (h/p/cosmos quasar, h/p/cosmos sports & medical GmbH, Germany). Kinematic data were recorded at 200 Hz using a 10-camera optical motion-capture system (VICON Motion Systems Ltd, Oxford, UK). At the start of the experiment, each participant familiarized themself with treadmill walking while wearing a safety harness and selected a comfortable walking speed, which was maintained across all conditions.
Three weight-bearing conditions were tested in a randomized order, with each lasting 4 min: 1) baseline condition with normal bodyweight (BW100), 2) 30% bodyweight unloading (BW070) achieved using a bodyweight support system (Ergolet Walking Sling, Winncare Nordic ApS, DK), and c) 30% bodyweight loading (BW130) applied using a weight vest (EZ Vest Pro, Kensui LLC, US) (Figure 1).
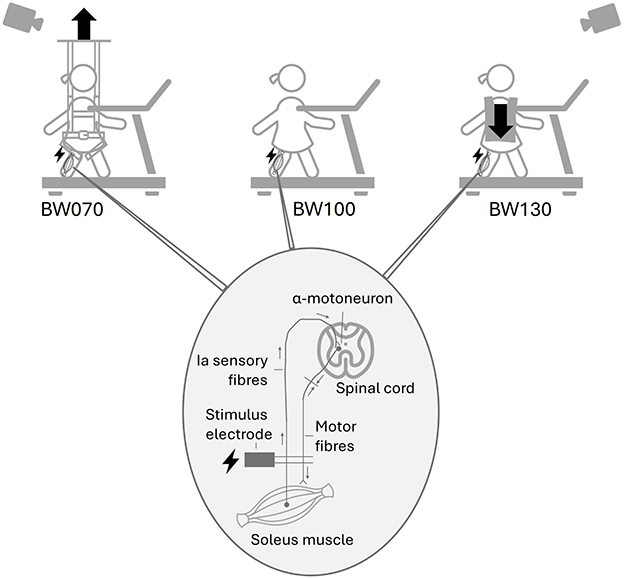
Figure 1. Study setup: Children walked barefoot on a treadmill under three different weight-bearing conditions: normal bodyweight (BW100), 30% bodyweight unloading (BW070), achieved using a bodyweight support system, and 30% additional bodyweight (BW130), applied using a weight vest. Below, the H-reflex mechanism is illustrated, showing the stimulus electrode triggering the nerve in the back of the knee, the Ia sensory fibers connecting with the α-motoneuron in the spinal cord, projecting to the soleus muscle fibers, adapted from Gwerder et al. (2025b).
2.2.2 H-reflex
The H-reflex was electrically elicited in the non-dominant leg for TD children, while for ITW and CP children, stimulation was applied to the more affected leg. The dominant leg was chosen by asking participants: “If you were kicking a ball, which leg would strike the ball?” (van Melick et al., 2017). A wireless electromyography (EMG) electrode (PicoEMG, Cometa, Italy, 3,000 Hz sampling frequency) was positioned on the soleus muscle. The tibial nerve was then identified in the popliteal fossa in a prone position using a movable electrode, with a rectangular pulse (1ms duration) delivered by a constant current stimulator (DS7AH, Digitimer, UK) (Kim et al., 2024; Gwerder et al., 2025b). Once an H-reflex was successfully elicited (visible H-wave in soleus EMG signal), a self-adhesive stimulation cathode (Ag/AgCl Kendall™ H124SG, CardinalHealth, Switzerland) was fixed at the location identified by the movable electrode.
To standardize the H-reflex measurement, optimal stimulation intensity was firstly determined by quantifying the subject-specific H-reflex recruitment curve (RC) during walking (Kim et al., 2024; Gwerder et al., 2025b). Here, a series of stimuli were delivered between heel strike and mid-stance (identified as the gait phase most sensitive to H-reflex modulation Chalmers and Knutzen, 2002; Krauss and Misiaszek, 2007 using MATLAB (R2018a, The MathWorks, Inc., Natick, United States; RRID:SCR_001622). The stimulus intensity was increased from 10 to 15 mA in increments of approximately 1 mA until the M-wave peak-to-peak amplitude reached a plateau (Mmax) (Palmieri et al., 2004). Due to discomfort or pain, it was not possible to reach Mmax for every participant during the stimulation procedure, whereby the stimulation was stopped at the point of discomfort. Gaussian and sigmoid functions were fitted to the data to extract peak-to-peak amplitudes of H-reflex and the associated M-wave where possible. Optimal stimulus intensity was then standardized to the intensity corresponding to 80% of each participant's individual maximum H-reflex amplitude. This intensity was chosen because it reliably produces a sufficiently large and reproducible reflex response that also remains sensitive to changes, while minimizing the risk of antidromic collision with the M-wave since this intensity can be identified on the ascending phase of the H-reflex curve (Kim et al., 2024; Palmieri et al., 2004; Crone et al., 1990; Bouguetoch et al., 2020).
Finally, patients were tested over a period of 4 min of treadmill walking for each weight-bearing condition. Throughout the measurement period, electrical stimulation to elicit an H-reflex was applied approximately every 9–16 s to avoid familiarization (Chen and Zhou, 2011), and was delivered between heel strike and mid-stance similar to the procedures used to determine the optimal stimulation intensity. Marker kinematic data and EMG signals were measured to determine gait events, dynamic stability parameters, and peak-to-peak reflex responses.
2.3 Data analysis
To ensure analysis of steady-state walking, the first and last 30 s of each 4-min walking trial were excluded to avoid transients. Gait events were detected based on kinematic data from foot markers, using a validated algorithm shown to have high accuracy in identifying gait patterns in toe walking children (Ghoussayni et al., 2004; Visscher et al., 2021). The spatiotemporal gait parameters evaluated from each stride included: stride time, single- and double-limb support time, step width, and stride length. The mean and standard deviation (SD) were calculated, with SD serving as a measure of gait variability. Spatiotemporal parameters were analyzed for the stimulated leg only.
2.3.1 Dynamic stability
To assess dynamic stability, the margin of stability (MoS) was calculated in both the antero-posterior (MoSAP) and medio-lateral (MoSML) directions (Hof et al., 2005). To support an accurate CoM estimation in toe walking children, an OpenSim model (OpenSim v4.3, RRID:SCR_002683) was additionally constructed that included all segment kinematics. Here, the model was scaled to represent each participant's anthropometry using marker trajectories captured during a standing trial, while a linear scaling method was used to estimate each segment's mass based on the overall bodyweight. Inverse and body kinematics tools were used to compute the CoM and extrapolated CoM (XCoM) throughout the measured trials:
where CoMv was the velocity of the CoM adjusted for treadmill walking, g the gravitational constant and l the length from the CoM to the midpoint between the two malleoli markers.
The MoS was determined at heel strike, as this moment dictates foot placement for the stance phase (Hof, 2008) and is important to maintain balance (Wallard et al., 2014). The boundaries of the base of support (BoS) were defined using the first metatarsal marker for antero-posterior and the fifth metatarsal marker for the medio-lateral limits:
Note that this approach results in an overestimation of the MoS compared to methods that use the CoP (Hof and Curtze, 2016).
2.3.2 H-reflex
For each participant and condition, average peak-to-peak amplitudes of the H-reflex (30–45 ms after stimulus) were calculated, based on average 14 stimuli per condition (see Figure 2 for example). Some stimulations fell outside early stance phase, mainly due to marker occlusion issues, and were hence excluded (Simonsen and Dyhre-Poulsen, 1999). Since it was not possible to identify an Mmax value for each child from the RCs, but to still ensure consistency and comparability, the inclusion of H-reflex responses was dependent upon stable muscle activity during the M-wave time window. Specifically, the EMG amplitude within this window was required to fall within ± 20% of the condition-specific mean (Kim et al., 2024; Gwerder et al., 2025b). Additionally, H-reflex amplitudes were normalized (H-reflexnorm) to average baseline EMG (bEMG) activity, assessed 50 ms before each stimulation, but after heel strike (Palmieri et al., 2004).
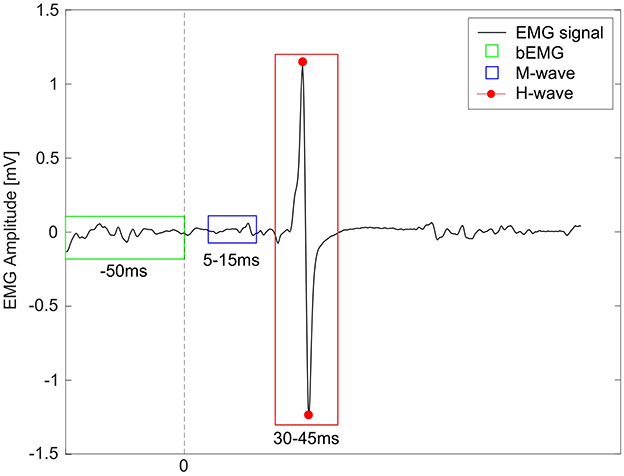
Figure 2. Example of a soleus EMG signal (black) from a child with idiopathic toe walking. The dashed line at 0 represents the timing of the electrical stimulus. The green box represents the window for the baseline EMG (bEMG), the blue box for the M-wave, and the red box shows the peak-to-peak amplitude of the H-reflex (highlighted with red points).
2.4 Statistical analysis
Linear mixed-effects models were used to analyse gait parameters, gait variability, and H-reflex adaptations across different weight-bearing conditions and groups. Dependent variables included mean and variability measures of stride time, single- and double-limb support time, stride length, step width, MoSAP, and MoSML, as well as H-reflex amplitude, H-reflexnorm, and bEMG. Group (TD, ITW, and CP) and bodyweight condition (BW070, BW100, BW130) were treated as fixed effects, along with their interaction, while participants were included as random effects in each model. BW100 served as the baseline for all comparisons. Statistical significance was set at p ≤ 0.05 and adjusted for multiple comparisons where appropriate using the false discovery rate method (Benjamini and Hochberg, 1995). All analyses were conducted in RStudio (v2024.04.2, R Core Team, Austria, RRID:SCR_000432).
3 Results
3.1 Gait adaptations
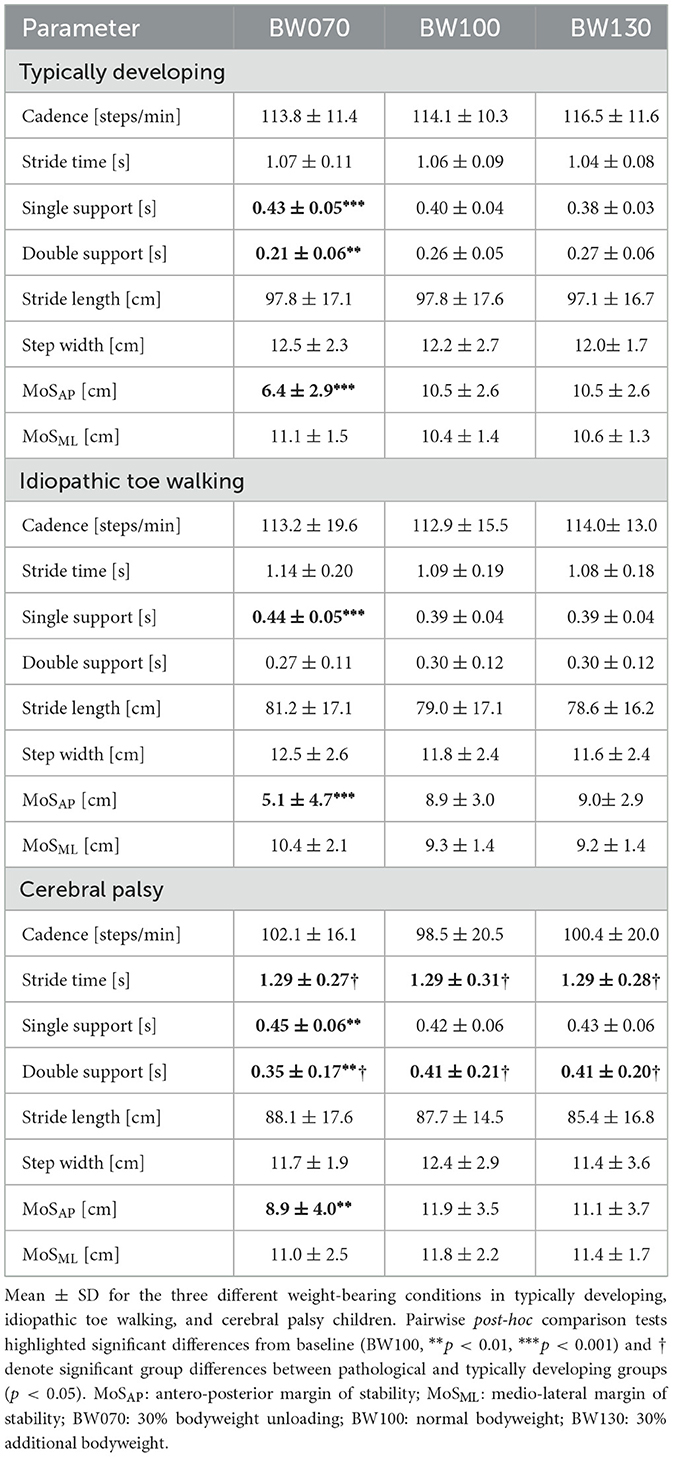
An average of 145 strides per participant and condition was analyzed. Under the BW070 condition, single-limb support time significantly increased (p < 0.001, Table 2 and Supplementary material, 2. Statistical Analysis Table S1) by 8% in TD, 13% in ITW, and 7% in CP children compared to BW100. On the other hand, double-limb support time decreased by 19% in TD, 10% in ITW, and 15% in CP children respectively. CP children exhibited significantly increased double-limb support time compared to TD children (p < 0.001, Table 2 and Supplementary material, 2. Statistical Analysis Table S1). MoSAP was significantly decreased in BW070 by 39% in TD, 43% in ITW, and 26% in CP children compared to BW100 (p < 0.01, Figure 3).
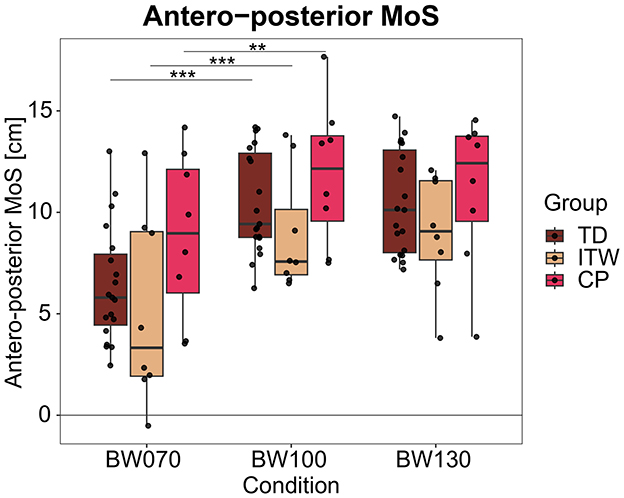
Figure 3. Antero-posterior margin of stability (MoS) for the three different weight-bearing conditions normal bodyweight (BW100), 30% bodyweight unloading (BW070), and 30% additional bodyweight (BW130) for typically developing (TD, dark red), idiopathic toe walking (ITW, orange), and cerebral palsy (CP, pink) children. ** (p < 0.01), *** (p < 0.001) show significant differences from baseline (BW100).
For BW130, no significant differences were found in any groups compared to BW100.
3.2 Gait variability
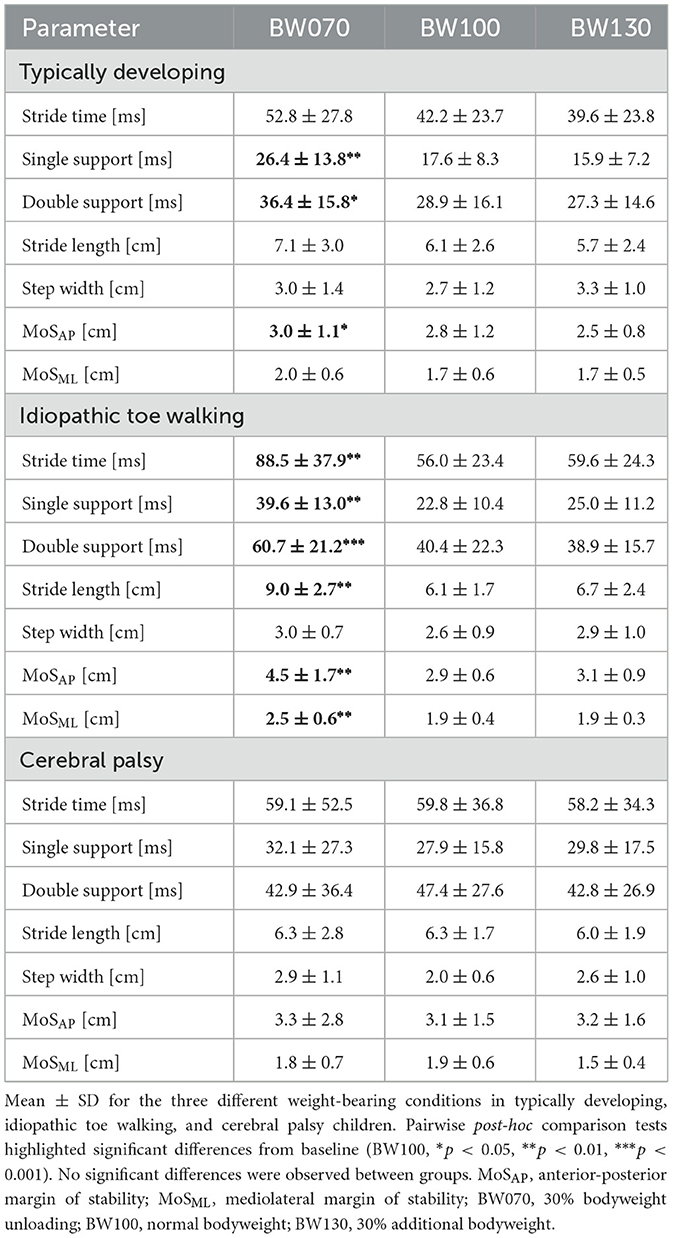
In the BW070 condition, variability in single- and double-limb support times increased significantly in TD children by 50% and 26%, respectively, and in ITW children by 74% and 50% compared to BW100. MoSAP variability also rose significantly, by 7% in TD and 55% in ITW children in the bodyweight unloading condition (p < 0.05, Table 3 and Supplementary material, 2. Statistical Analysis Table S2).
Children with ITW exhibited a substantial increase in variability in the BW070 condition, with at least a 32% rise across all parameters except step width. In contrast, children with CP showed no significant changes in any variability parameters under bodyweight unloading compared to baseline.
For bodyweight loading conditions (BW130), no significant differences in variability were observed compared to baseline for any group. Additionally, no significant differences between groups were observed for gait variability parameters.
3.3 H-reflex
Across all groups and conditions, a total of 1,338 reflexes were elicited (757 in TD, 307 in ITW, and 281 in CP). A total of 876 (65%) were included in the analysis and the included stimuli were applied on average at 26% of the gait cycle, or 0.29s ± 0.11s following foot contact.
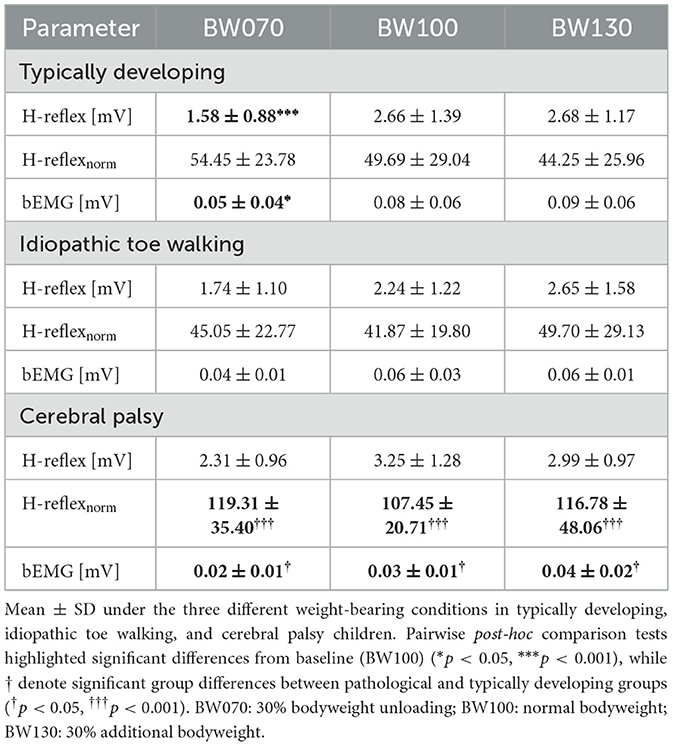
Post-hoc comparisons in H-reflex revealed significant decreases for BW070 compared to BW100 in TD children (41%, p < 0.001) with similar trends in ITW (22%), and CP (29%, Table 4 and Supplementary material, 2. Statistical Analysis Table S3, Figure 4A). Furthermore, CP children exhibited significant reduced bEMG values compared to TD children (p < 0.05). Finally, there was a significant group difference with CP children showing 116% higher H-reflexnorm at BW100 compared to TD children (p < 0.001, Figure 4B).
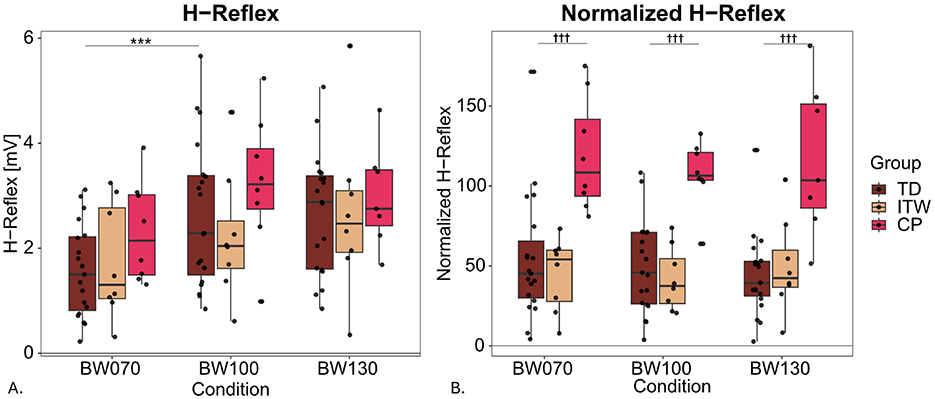
Figure 4. Hofmann reflex (H-reflex) peak-to-peak amplitudes for non-normalized (A) and normalized data (B) for the three different weight bearing conditions normal bodyweight (BW100), 30% bodyweight unloading (BW070), and 30% additional bodyweight (BW130) for typically developing (TD, dark red), idiopathic toe walking (ITW, orange), and cerebral palsy (CP, pink) children. *** show significant differences from baseline (BW100, p < 0.001), while ††† denote significant group differences between pathological and typically developing (TD) groups (p < 0.001).
4 Discussion
A deeper understanding of the interplay between dynamic stability, reflex control, and walking strategies in children with ITW and CP is essential for advancing rehabilitation strategies and improving balance, functional mobility, and quality of life. This study aimed to clarify whether TD, ITW, and CP children employ distinct movement strategies and reflex responses to adapt to environmental challenges. Our findings reveal that bodyweight unloading resulted in longer single-limb support time, shorter double-limb support time, and decreased MoSAP in all three cohorts. These changes suggest well-functioning adaptations in temporal gait regulation under challenging environments, and a shift in movement strategy to accommodate the reduced forward momentum during bodyweight unloading. Notably, ITW children exhibited significantly greater variability during bodyweight unloading compared to baseline (BW100), suggesting increased motor exploration to identify an optimal strategy for adapting to gait challenges. Since no baseline differences were observed compared to TD peers, ITW children appeared stable during normal walking but struggled to adjust to the altered unloading condition, leading to increased variability and instability. In contrast, CP children exhibited no significant differences in variability compared to baseline (BW100) for either additional loading or unloading, suggesting either a reliance on a stiffening strategy that persisted even under challenging conditions, or a limited ability to explore alternative motor strategies. One plausible explanation for this lack of adaptation is that children with CP already exhibit impairments and highly individualized movement strategies under normal conditions, which may remain unchanged in the short-term even when facing the additional challenge of bodyweight unloading.
TD children showed a significant reduction in H-reflex amplitude during bodyweight unloading, indicating intact and responsive H-reflex modulation. Children with CP generally exhibited significantly higher H-reflexnorm amplitudes compared to TD children. These findings indicate hyperreflexia, or a lack of supra-spinal inhibition in the CP group, possibly suggesting differently developed sensorimotor systems. Interestingly, in ITW children there was no difference in H-reflexnorm compared to their TD peers. This result suggests that spinal reflex excitability might be preserved in ITW children. It further indicates that, although their gait pattern is atypical, these children may have developed a stable and well-regulated motor strategy that supports toe walking as a consistent, learned locomotion pattern rather than one primarily driven by abnormal reflex activity. Larger cohort and longitudinal studies from the onset of walking may help to clarify whether this pattern is adaptive or indicative of persistent neurodevelopmental differences.
4.1 Mean gait adaptations
This study found that bodyweight unloading resulted in longer single-limb support and shorter double-limb support time compared to baseline across all three groups, indicating an adapted temporal regulation in response to a more challenging environment (Herssens et al., 2021). It is unlikely that unloading in this way has ever been experienced by the participants, resulting in a first-time reaction and immediate change in temporal gait parameters. Similar trends have previously been reported in healthy adults under bodyweight unloading conditions (Kim et al., 2024; Gwerder et al., 2025b; Apte et al., 2018). According to the inverted pendulum model, a reduced double-limb support time limits the duration available for CoM redirection to the next step, restricting opportunities for postural adjustments (Adamczyk and Kuo, 2009). This may contribute to a less efficient, more variable, and potentially unstable gait pattern. Notably, this timing adaptation was consistent across all three groups and did not appear to be significantly influenced by toe walking behavior. Additionally, MoSAP significantly decreased under the bodyweight unloading condition for all groups. A lower MoSAP likely reflects reduced forward momentum, indicating either weaker forward propulsion and diminished push-off forces or a reservation to move into an unfamiliar posture. However, since the harness mitigated any fall possibility but also somewhat restricted forward leaning, it is more likely that an impaired push-off force hindered effective forward projection of the CoM, such that maintenance of stability in the antero-posterior direction remained challenging (Kulkarni et al., 2023).
Trends suggest that ITW children exhibited lower MoSAP compared to TD children across all conditions, while CP children showed an increase. Similar findings have been reported previously (Gwerder et al., 2025a), where a reduced MoSAP in ITW children potentially indicates reliance on an immature, falling forward movement strategy to maintain forward momentum, which includes upper body anchoring to simplify balance control due to a reduced number of degrees of freedom (Gwerder et al., 2025a; Assaiante et al., 2005). Conversely, CP children exhibited the opposite trend, with increased MoSAP compared to TD children, also consistent with previous findings (Sangeux et al., 2024). CP children possibly exhibit impaired pendular energy transmission during gait (van den Hecke et al., 2007). To optimize, they elevate their CoM to improve pendular efficiency and facilitate limb clearance (Zollinger et al., 2016), resulting in increased MoS as a strategy to enhance stability within the limits of reduced motor coordination (Rethwilm et al., 2021; Tracy et al., 2019). This suggests that CP children adopt a more conservative gait strategy, maintaining higher safety margins to compensate for reduced strength, impaired sensorimotor integration, and immature foot trajectory control (Tracy et al., 2019; Zarkou et al., 2020). Due to deficits in intersegmental coordination and muscle activity, they seem to rely on alternative movement strategies with higher safety margins to minimize fall risk as far as possible (Cappellini et al., 2016).
No significant differences were observed under additional bodyweight loading conditions, possibly because children are accustomed to carrying additional weight, such as heavy backpacks. As a result, the added load may not have been sufficiently novel or challenging to elicit changes in gait parameters.
4.2 Gait variability
During bodyweight unloading, temporal parameters of gait variability significantly increased in TD children, likely reflecting the greater challenge posed by reduced weight-bearing compared to increased loading. This condition potentially demands a more active gait control strategy (Collins and Kuo, 2013). In novel or demanding environments, the sensorimotor control system often increases variability as an adaptive mechanism, allowing for movement exploration and fine-tuning to meet new stability demands (Abram et al., 2022). Interestingly, no differences were observed in spatial parameters of gait variability in TD children. Why temporal, rather than spatial, aspects of gait variability are specifically adapted under different challenging conditions remains unclear. One possible explanation is that temporal parameters of task performance are more vulnerable to disruption than spatial parameters (Bakir et al., 2013).
TD children exhibited similar responses to healthy adults (Kim et al., 2024; Gwerder et al., 2025b), suggesting effective adaptations to challenging conditions and well-functioning compensatory strategies. Children with ITW showed no differences at baseline, indicating that their gait is well-adapted to normal walking conditions. However, during bodyweight unloading, variability increased across all parameters except step width. This aligns with Fanchiang et al. (2016), who reported greater gait variability in ITW children compared to healthy controls when walking on different surfaces. These findings suggest that although ITW children may have developed a stable walking strategy on level ground, they might prioritize medio-lateral over antero-posterior stability, potentially explaining the preserved step width variability, while exploring alternative movement strategies in other directions when confronted with challenging conditions. For future studies interested in long-term adaptations, it would be valuable to investigate longer exposure durations, as there is some preliminary evidence, that motor control can improve with time in children with ITW (Clark et al., 2010).
In contrast, CP children in our study generally exhibited reduced variability, consistent with a limited repertoire of movements and behavioral strategies (Delcour et al., 2018; Hadders-Algra, 2010). Here, they are thought to rely on a stiffening response with increased muscle co-contractions, producing a more rigid gait pattern when faced with challenges (Lorentzen et al., 2019). However, to fully assess this, future studies should analyse gait variability in combination with muscle activity across several muscles and throughout the entire gait cycle. In the present study, only bEMG immediately preceding the stimulation was examined, which may not accurately reflect overall muscle activation patterns. Additionally, between-subject variability, reflected in the high standard deviations, was notably elevated in CP children. This suggests that while some children exhibited very high others showed very low gait variability, reflecting the heterogeneity in strategies to regulate task performance among various forms of CP. Although the inclusion criteria aimed to create a relatively homogeneous group (spastic hemiparesis and GMFCS levels I and II), individual differences in motor impairments and functional abilities inevitably contributed to the observed variability. Participants were classified in similar GMFCS levels, but heterogeneity in lesion location, muscle tone, prior interventions, and individual differences in strength and motor control likely also influence their movement strategy. These individualized strategies under normal conditions may remain unchanged in the short-term even when facing the additional challenge of bodyweight unloading. Our protocol only provided brief exposure to bodyweight unloading. Our protocol only provided brief exposure to bodyweight unloading, which is likely insufficient to produce neuromotor adaptation in children with mild CP (Cherng et al., 2007). Finally, the absence of variability modification might also indicate the prioritization of stability over flexibility and adaptability, potentially resulting in higher energy expenditure and reflecting the nervous system's limited ability to finely regulate motor output (Delcour et al., 2018; Hak et al., 2013).
4.3 H-reflex
Our study observed a significant decrease in H-reflex amplitude under bodyweight unloading in TD children with similar trends in ITW and CP children. This response suggests a healthy regulation of postural control, similar to that observed in young adults (Kim et al., 2024; Gwerder et al., 2025b), and has also been reported during challenging tasks in CP children (Conner et al., 2022). The unfamiliar and challenging nature of bodyweight unloading likely requires greater supra-spinal involvement to maintain stability, shifting motor control away from automatic, reflex-driven adjustments toward more active regulation (Dragunas and Gordon, 2016; Skiadopoulos et al., 2020; Sibley et al., 2007). Additionally, altered sensory feedback (e.g. haptic, proprioceptive) under reduced loading conditions may provide limited support for normal postural control mechanisms, necessitating further active neural adaptation to the situation (Marchant et al., 2020). Given that both ITW and CP children often present with sensory processing difficulties (Williams et al., 2010; Pavao et al., 2015), it is a reasonable assumption that they would be even more affected by these changes. In contrast, bodyweight loading did not significantly alter H-reflex amplitudes in any group. However, children with CP exhibited significantly increased H-reflexnorm amplitudes compared to TD children, potentially indicating reduced central inhibition and hyperreflexia (Leonard et al., 1990; Hodapp et al., 2007b). While younger children with CP have shown H-reflex responses comparable to their TD peers in previous research (Cappellini et al., 2016), the age-related increase in reflex suppression typically observed in TD children under normal conditions appears diminished in those with CP, likely reflecting delayed CST maturation (Leonard et al., 1990; Cappellini et al., 2016). Combined with the trend toward reduced gait variability, this suggests that children with CP may have limited capacity and repertoire to explore alternative motor programs, leading to compensatory but less efficient gait strategies and reduced stability. Furthermore, overreliance on reflexive mechanisms is thought to compensate for muscle weakness and poor motor control, assisting lower limb muscles in stabilizing the CoM (Brunner and Götz-Neumann, 2023). This aligns with our findings where CP children exhibited trends toward lower bEMG activity directly before the stimulus, a well-documented issue in CP (Thompson et al., 2011). Thus, increased H-reflexnorm activity in CP children may represent an adopted strategy to counteract instability under different weight-bearing conditions.
In contrast to the hyperreflexia observed in children with CP, those with ITW displayed H-reflex responses similarly to their TD peers, although without the expected modulation. Maybe the upper body anchoring to simplify balance control due to a reduced number of degrees of freedom also limits H-reflex modulation in ITW children (Gwerder et al., 2025a; Assaiante et al., 2005). Existing literature presents conflicting findings. Rossi et al. (2018) observed varying H-reflex responses in ITW children in prone postures, with some showing increased, decreased, or even absent H-reflex activity compared to TD children. Children with ITW often exhibit sensory processing difficulties (Rossi et al., 2018; Williams et al., 2010), suggesting potential issues in the peripheral nervous system, which might explain the heterogeneity of H-reflex responses observed in the study from Rossi et al. (2018). These findings contrast with forward dynamic simulations that suggest increased plantarflexor reflex gains in ITW children and an altered neural control strategy similar to that of CP children (Veerkamp et al., 2023). These discrepancies may indicate that the central nervous system adjusts reflex gains dynamically to match functional demands. It would be important to ensure that such observations do not stem from methodological differences, and therefore studies including greater sample sizes are required in the future. Furthermore, while the inclusion of sensory mechanisms marks a significant step forward, the potential influence of cognitive and psychological factors that may also contribute to the observed differences, should not be overlooked.
4.4 Clinical perspectives
The findings of this study suggest that children with ITW and CP adopted distinct movement strategies in response to varying weight bearing conditions. CP children showed reduced gait variability and hyperreflexia, suggesting immature development of their sensorimotor systems. In contrast, children with ITW exhibited increased variability modulation and H-reflex responses comparable to those of TD peers, possibly indicating that these children take an atypical developmental path during early walking acquisition and altered sensorimotor adaptation. These patterns suggest an altered maturation of neuromotor control and spinal processing centers, highlighting the difficulty of compensating for underlying biomechanical constraints. Therefore, intervention strategies should aim to support the development and refinement of those motor strategies. Evidence suggests that reflex modulation can be learned, even in individuals with neurological disorders (Thompson and Wolpaw, 2021; Chen et al., 2005). Understanding the neuromotor etiology of toe walking lays the groundwork for developing more effective, individualized therapeutic approaches. Unlike Botulinum toxin A or other spasticity-reducing interventions, newer approaches aim to address the underlying causes more directly and with fewer side effects. Potential interventions could include spinal reflex conditioning (Thompson and Wolpaw, 2021), vibration therapy (Fanchiang et al., 2015), real-time feedback walking training (Cappellini et al., 2020; Brunner and Götz-Neumann, 2023), or enhancing sensory inputs through techniques such as stochastic resonance stimulation (Zarkou et al., 2018), plausibly addressing multiple systems simultaneously. However, further research is needed to refine these strategies and explore their long-term benefits.
4.5 Limitations
The study setup was complex, and several limitations should be acknowledged. The biggest limitation was the limited participation pool for ITW and CP children, potentially due to the restrictive eligibility criteria in order to include homogenous groups and to obtain relevant data. This study provides first evidence on how ITW and CP children adapt to different weight-bearing conditions and their underlying peripheral control mechanisms. However, larger studies are needed to confirm these findings. Additionally, due to the absence of ground reaction force data, the boundaries of the BoS were approximated using foot marker positions, which may have led to an overestimation of the MoS and did not account for medio-lateral ankle strategies. Furthermore, measurements were conducted on a treadmill at fixed speeds, which may not fully replicate the biomechanics of natural overground walking (Hollman et al., 2016). Walking speed usually changes under different weight-bearing conditions (Apte et al., 2018). However, instead of adjusting step width or walking speed, our participants may have used other strategies, such as foot placement or CoM regulation to adjust their gait patterns. Moreover, while the bodyweight support system effectively reduced loading, it also imposed movement constraints, particularly in the medio-lateral direction. This limitation may have restricted natural hip abduction and step width adjustments. Finally, the relatively short exposure time of 4 min per condition was intentionally chosen to minimize participant burden and avoid fatigue, particularly in children. While this duration allowed us to observe immediate responses to altered bodyweight, it may not have been sufficient to induce measurable gait or reflex adaptations beyond the initial learning phase. However, given our sample size and measurement setup, it is difficult to draw firm conclusions about such time-dependent adaptations. Future studies interested in long-term adaptations may therefore consider extending the duration or frequency of walking trials to better capture the evolution of motor adjustments under altered weight-bearing conditions. While this complex experimental setup may not have allowed complete movement freedom, it did allow for the capture of extended walking protocols.
5 Conclusion
A deeper understanding of the neuromotor etiology of toe walking under challenging conditions is crucial for developing targeted interventions. In this exploratory study, ITW children showed increased gait variability during bodyweight unloading, suggesting exploratory motor control under gait challenges. In contrast, CP children relied on a stiffening strategy, with unchanged variability and hyperreflexia, indicating limited capacity to explore alternative motor strategies, seemingly resulting in less efficient, compensatory gait patterns and reduced stability. These findings suggest that toe walking in both ITW and CP reflect distinctly different neuromotor adaptations to similar underlying biomechanical constraints, which should be confirmed in larger cohorts. A multifaceted approach targeting various sensorimotor and neuromotor control systems may hold the greatest promise for understanding gait stability, toward improving the overall quality of life in toe walking children.
Data availability statement
Due to ethical and privacy constraints, the data underlying this study cannot be shared publicly. It might be possible for the corresponding author to grant access to the datasets upon request and pending approval in accordance with ethical guidelines.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Canton Zürich (BASEC-No 2019-00678). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
MG: Conceptualization, Data curation, Formal analysis, Investigation, Software, Visualization, Writing – original draft, Writing – review & editing. RV: Conceptualization, Funding acquisition, Software, Writing – review & editing. AS: Data curation, Formal analysis, Investigation, Writing – review & editing. SH: Formal analysis, Software, Writing – review & editing. YK: Software, Writing – review & editing. RZ: Conceptualization, Writing – review & editing. RB: Conceptualization, Funding acquisition, Supervision, Writing – review & editing. WT: Conceptualization, Supervision, Writing – review & editing. EV: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing. NS: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Swiss National Science Foundation (32003B_200903/1). MG's position was partially funded by InnoSuisse (49953.1 IP-LS and 120.497 IP-LS) and Ausbildungsstiftung Kanton Schwyz.
Acknowledgments
The authors would like to thank Angela Frautschi, Ursina Camenzind, Carla Hetreau, and Anna Schaub for their assistance with data collection and all the children and their families for their participation. Additionally, we would like to acknowledge the Ostschweizer Kinderspital for their support with participant recruitment.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnhum.2025.1701454/full#supplementary-material
References
Abram, S. J., Poggensee, K. L., Sanchez, N., Simha, S. N., Finley, J. M., Collins, S. H., et al. (2022). General variability leads to specific adaptation toward optimal movement policies. Curr. Biol. 32, 2222–2232. doi: 10.1016/j.cub.2022.04.015
Adamczyk, P. G., and Kuo, A. D. (2009). Redirection of center-of-mass velocity during the step-to-step transition of human walking. J. Exp. Biol. 212, 2668–2678. doi: 10.1242/jeb.027581
Alvarez, C., De Vera, M., Chhina, H., and Black, A. (2008). Normative data for the dynamic pedobarographic profiles of children. Gait Posture 28, 309–315. doi: 10.1016/j.gaitpost.2008.01.017
Angulo-Kinzler, R. M., Mynark, R. G., and Koceja, D. M. (1998). Soleus H-reflex gain in elderly and young adults: modulation due to body position. J. Gerontol. 53A, M120–M125. doi: 10.1093/gerona/53A.2.M120
Apte, S., Plooij, M., and Vallery, H. (2018). Influence of body weight unloading on human gait characteristics: a systematic review. J. Neuroeng. Rehabil. 15:53. doi: 10.1186/s12984-018-0380-0
Assaiante, C., Mallau, S., Viel, S., Jover, M., and Schmitz, C. (2005). Development of postural control in healthy children: a functional approach. Neural Plast. 12, 109–118. doi: 10.1155/NP.2005.109
Bakir, M. S., Gruschke, F., Taylor, W. R., Haberl, E. J., Sharankou, I., Perka, C., et al. (2013). Temporal but not spatial variability during gait is reduced after selective dorsal rhizotomy in children with cerebral palsy. PLoS ONE. 8:e0069500. doi: 10.1371/journal.pone.0069500
Bartoletta, J., Tsao, E., and Bouchard, M. A. (2021). Retrospective analysis of nonoperative treatment techniques for idiopathic toe walking in children: outcomes and predictors of success. Phys. Med. Rehabil. 13, 1127–1135. doi: 10.1002/pmrj.12520
Bauer, J. P., Sienko, S., and Davids, J. R. (2022). Idiopathic toe walking: an update on natural history, diagnosis, and treatment. J. Am. Acad. Orthop. Surg. 30, 1419–1430. doi: 10.5435/JAAOS-D-22-00419
Benjamini, Y., and Hochberg, Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. 57, 289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x
Berger, W., Quintern, J., and Dietz, V. (1985). Stance and gait perturbations in children: developmental aspects of compensatory mechanisms. Electroencephalogr. Clin. Neurophysiol. 61, 385–395. doi: 10.1016/0013-4694(85)91030-2
Bouguetoch, A., Grospretre, S., and Martin, A. (2020). Optimal stimulation parameters for spinal and corticospinal excitabilities during contraction, motor imagery and rest: a pilot study. PLoS ONE. 15:e0235074. doi: 10.1371/journal.pone.0235074
Boyer, E. R., and Patterson, A. (2018). Gait pathology subtypes are not associated with self-reported fall frequency in children with cerebral palsy. Gait Posture 63, 189–194. doi: 10.1016/j.gaitpost.2018.05.004
Bruijn, S. M., and van Dieen, J. H. (2018). Control of human gait stability through foot placement. J. R. Soc. Interface 15, 1–11. doi: 10.1098/rsif.2017.0816
Brunner, R., and Götz-Neumann, K. A. (2023). Critical view on the importance of treating spasticity and new options to improve function in patients wit cerebral palsy GMFCS I-III. Med. Res. Arch. 11, 1–12. doi: 10.18103/mra.v11i4.3812
Cans, C., Surman, G., McManus, V., Coghlan, D., Hensey, O., Johnson, A., et al. (2004). Cerebral palsy registries. Semin. Pediatr. Neurol. 11, 18–23. doi: 10.1016/j.spen.2004.01.004
Capaday, C., and Stein, R. B. (1986). Amplitude modulation of the soleus h-reflex in the human during walking and standing. J. Neurosci. 6, 1308–1313. doi: 10.1523/JNEUROSCI.06-05-01308.1986
Cappellini, G., Ivanenko, Y. P., Martino, G., MacLellan, M. J., Sacco, A., Morelli, D., et al. (2016). Immature spinal locomotor output in children with cerebral palsy. Front. Physiol. 7:48. doi: 10.3389/fphys.2016.00478
Cappellini, G., Sylos-Labini, F., Dewolf, A. H., Solopova, I. A., Morelli, D., Lacquaniti, F., et al. (2020). Maturation of the locomotor circuitry in children with cerebral palsy. Front. Bioeng. Biotechnol. 8:998. doi: 10.3389/fbioe.2020.00998
Cecen, S., Niazi, I. K., Nedergaard, R. W., Cade, A., Allen, K., Holt, K., et al. (2018). Posture modulates the sensitivity of the H-reflex. Exp. Brain Res. 236, 829–885. doi: 10.1007/s00221-018-5182-x
Chalmers, G. R., and Knutzen, K. M. (2002). Soleus H-reflex gain in healthy elderly and young adults when lying, standing, and balancing. J. Gerontol. 57, B321–B329. doi: 10.1093/gerona/57.8.B321
Chen, S. C., Chen, Y. L., Chen, C. J., Lai, C. H., Chiang, W. H., Chen, W. L., et al. (2005). Effects of surface electrical stimulation on the muscle-tendon junction of spastic gastrocnemius in stroke patients. Disabil. Rehabil. 27, 105–110. doi: 10.1080/09638280400009022
Chen, Y. S., and Zhou, S. (2011). Soleus H-reflex and its relation to static postural control. Gait Postur 33, 169–178. doi: 10.1016/j.gaitpost.2010.12.008
Cherng, R.-J., Liu, C,-. F., Lau, T.-W., and Hong, R.-B. (2007). Effect of treadmill training with body weight support on gait and gross motor function in children with spastic cerebral palsy. Am. J. Phys. Med. Rehabil. 86, 548–55. doi: 10.1097/PHM.0b013e31806dc302
Clark, E., Sweeney, J. K., Yocum, A., and McCoy, S. W. (2010). Effects of motor control intervention for children with idiopathic toe walking: a 5-case series. Pediatr. Phys. Ther. 22, 417–26. doi: 10.1097/PEP.0b013e3181f9d5b8
Collins, S. H., and Kuo, A. D. (2013). Two independent contributions to step variability during over-ground human walking. PLoS ONE. 8:e73597. doi: 10.1371/journal.pone.0073597
Conner, B. C., Spomer, A. M., Bishe, S., Steele, K. M., and Lerner, Z. F. (2022). Soleus H-reflex modulation in cerebral palsy and its relationship with neural control complexity: a pilot study. Exp. Brain Res. 240, 2073–2084. doi: 10.1007/s00221-022-06399-3
Crone, C., Hultborn, H., Mazières, L., Morin, C., Nielsen, J., Pierrot-Deseilligny, E., et al. (1990). Sensitivity of monosynaptic test reflexes to facilitation and inhibition as a function of the test reflex size: a study in man and the cat. Exp. Brain Res. 81, 35–45. doi: 10.1007/BF00230098
Curtze, C., Buurke, T. J. W., and McCrum, C. (2024). Notes on the margin of stability. J. Biomech. 166, 1–8. doi: 10.1016/j.jbiomech.2024.112045
De Oliveira, V., Arrebola, L., De Oliveira, P., and Yi, L. (2021). Investigation of muscle strength, motor coordination and balance in children with idiopathic toe walking: a case-control study. Dev. Neurorehabil. 2021, 1–7. doi: 10.1080/17518423.2021.1899326
Delcour, M., Massicotte, V. S., Russier, M., Bras, H., Peyronnet, J., Canu, M. H., et al. (2018). Early movement restriction leads to enduring disorders in muscle and locomotion. Brain Pathol. 28, 889–901. doi: 10.1111/bpa.12594
Dragunas, A. C., and Gordon, K. E. (2016). Body weight support impacts lateral stability during treadmill walking. J. Biomech. 49, 2662–2668. doi: 10.1016/j.jbiomech.2016.05.026
Engstrom, P., and Tedroff, K. (2012). The prevalence and course of idiopathic toe-walking in 5-year-old children. Pediatrics 130, 279–284. doi: 10.1542/peds.2012-0225
Fanchiang, H. D., Geil, M., Wu, J., Chen, Y. P., and Wang, Y. T. (2015). The effects of vibration on the gait pattern and vibration perception threshold of children with idiopathic toe walking. J. Child Neurol. 30, 1010–1016. doi: 10.1177/0883073814550655
Fanchiang, H. D., Geil, M. D., Wu, J., Ajisafe, T., and Chen, Y. P. (2016). The effects of walking surface on the gait pattern of children with idiopathic toe walking. J. Child Neurol. 31, 858–863. doi: 10.1177/0883073815624760
Geyer, H., and Herr, H. A. (2010). muscle-reflex model that encodes principles of legged mechanics produces human walking dynamics and muscle activities. IEEE Trans. Neural Syst. Rehabil. Eng. 18, 263–273. doi: 10.1109/TNSRE.2010.2047592
Ghoussayni, S., Stevens, C., Durham, S., and Ewins, D. (2004). Assessment and validation of a simple automated method for the detection of gait events and intervals. Gait Posture 20, 266–272. doi: 10.1016/j.gaitpost.2003.10.001
Gillani, S., Rafique, A., Taqi, M., Chatta, M., Masood, F., Ahmad Blouch, T., et al. (2021). Effectiveness of treatment in children with cerebral palsy. Cureus 13, 1–8. doi: 10.7759/cureus.13754
Gwerder, M., Camenzind, U., Wild, S., Kim, Y. K., Taylor, W. R., Singh, N. B., et al. (2025b). Probing gait adaptations: the impact of aging on dynamic stability and reflex control mechanisms under varied weight-bearing conditions. Eur. J. Appl. Physiol. doi: 10.1007/s00421-025-05884-1. [Epub ahead of print].
Gwerder, M., Widmer, M., Scharen, O., Singh, N. B., Sangeux, M., Viehweger, E., et al. (2025a). Assessing dynamic stability in children with idiopathic toe walking during overground walking. Clin Biomech (Bristol). 123:106468. doi: 10.1016/j.clinbiomech.2025.106468
Hadders-Algra, M. (2010). Variation and variability: key words in human motor development. Phys. Ther. 90, 1823–1837. doi: 10.2522/ptj.20100006
Hak, L., Houdijk, H., Beek, P. J., and van Dieen, J. H. (2013). Steps to take to enhance gait stability: the effect of stride frequency, stride length, and walking speed on local dynamic stability and margins of stability. PLoS ONE 8:e0082842. doi: 10.1371/journal.pone.0082842
Hallemans, A., De Clercq, D., Van Dongen, S., and Aerts, P. (2006). Changes in foot-function parameters during the first 5 months after the onset of independent walking: a longitudinal follow-up study. Gait Posture 23, 142–148. doi: 10.1016/j.gaitpost.2005.01.003
Hallemans, A., Verbecque, E., Dumas, R., Cheze, L., Van Hamme, A., Robert, T., et al. (2018). Developmental changes in spatial margin of stability in typically developing children relate to the mechanics of gait. Gait Posture 63, 33–38. doi: 10.1016/j.gaitpost.2018.04.019
Herssens, N., Saeys, W., Vereeck, L., Meijer, K., van de Berg, R., Van Rompaey, V., et al. (2021). An exploratory investigation on spatiotemporal parameters, margins of stability, and their interaction in bilateral vestibulopathy. Sci. Rep. 11:6427. doi: 10.1038/s41598-021-85870-7
Hodapp, M., Klisch, C., Berger, W., Mall, V., and Faist, M. (2007a). Modulation of soleus H-reflexes during gait in healthy children. Exp. Brain Res. 178, 252–260. doi: 10.1007/s00221-006-0730-1
Hodapp, M., Klisch, C., Mall, V., Vry, J., Berger, W., Faist, M., et al. (2007b). Modulation of soleus H-reflexes during gait in children with cerebral palsy. J. Neurophysiol. 98, 3263–3268. doi: 10.1152/jn.00471.2007
Hof, A. L. (2008). The ‘extrapolated center of mass' concept suggests a simple control of balance in walking. Hum. Mov. Sci. 27, 112–125. doi: 10.1016/j.humov.2007.08.003
Hof, A. L., and Curtze, C. A. (2016). stricter condition for standing balance after unexpected perturbations. J. Biomech. 49, 580–585. doi: 10.1016/j.jbiomech.2016.01.021
Hof, A. L., Gazendam, M. G., and Sinke, W. E. (2005). The condition for dynamic stability. J. Biomech. 38, 1–8. doi: 10.1016/j.jbiomech.2004.03.025
Hollman, J. H., Watkins, M. K., Imhoff, A. C., Braun, C. E., Akervik, K. A., Ness, D. K., et al. (2016). A comparison of variability in spatiotemporal gait parameters between treadmill and overground walking conditions. Gait Postur 43, 204–209. doi: 10.1016/j.gaitpost.2015.09.024
Ivanenko, Y. P., Dominici, N., and Lacquantiti, F. (2007). Development of independent walking in toddlers. Exerc. Sport Sci. Rev. 35, 67–73. doi: 10.1249/JES.0b013e31803eafa8
Ivanenko, Y. P., and Gurfinkel, F. S. (2018). Human postural control. Front. Hum. Neurosci. 12:171. doi: 10.3389/fnins.2018.00171
Kim, Y. K., Gwerder, M., Taylor, W. R., Baur, H., and Singh, N. B. (2024). Adaptive gait responses to varying weight-bearing conditions: inferences from gait dynamics and H-reflex magnitude. Exp. Physiol. 109, 754–765. doi: 10.1113/EP091492
Krauss, E. M., and Misiaszek, J. E. (2007). Phase-specific modulation of the soleus H-reflex as a function of threat to stability during walking. Exp. Brain Res. 181, 665–672. doi: 10.1007/s00221-007-0962-8
Kulkarni, A., Cui, C., Rietdyk, S., and Ambike, S. (2023). Humans prioritize walking efficiency or walking stability based on environmental risk. PLoS ONE 18:e0284278. doi: 10.1371/journal.pone.0284278
Leonard, C. T., Moritani, T., Hirschfeld, H., and Forssberg, H. (1990). Deficits in reciprocal inhibition of children with cerebral palsy as revealed by H reflex testing. Dev. Med. Child Neurol. 32, 974–984. doi: 10.1111/j.1469-8749.1990.tb08120.x
Lorentzen, J., Willerslev-Olsen, M., Huche Larsen, H., Farmer, S. F., and Nielsen, J. B. (2019). Maturation of feedforward toe walking motor program is impaired in children with cerebral palsy. Brain 142, 526–541. doi: 10.1093/brain/awz002
Marchant, A., Ball, N., Witchalls, J., Waddington, G., Mulavara, A. P., Bloomberg, J. J., et al. (2020). The effect of acute body unloading on somatosensory performance, motor activation, and visuomotor tasks. Front. Physiol. 11:318. doi: 10.3389/fphys.2020.00318
Marzbani, H., Parvin, S., Amiri, S., Lotfian, M., Kharazi, M. R., Azizi, S., et al. (2016). The correlation between transcranial magnetic stimulation parameters and neuromuscular properties in children with cerebral palsy. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2016, 5473–5476. doi: 10.1109/EMBC.2016.7591965
McIntyre, S., Goldsmith, S., Webb, A., Ehlinger, V., Hollung, S. J., McConnell, K., et al. (2022). Global prevalence of cerebral palsy: a systematic analysis. Dev. Med. Child Neurol. 64, 1494–1506. doi: 10.1111/dmcn.15346
Novak, I., Morgan, C., Fahey, M., Finch-Edmondson, M., Galea, C., Hines, A., et al. (2020). State of the evidence traffic lights 2019: systematic review of interventions for preventing and treating children with cerebral palsy. Curr. Neurol. Neurosci. Rep. 20, 1–21. doi: 10.1007/s11910-020-1022-z
Palmieri, R. M., Ingersoll, C. D., and Hoffman, M. (2004). The Hoffmann reflex: methodologic considerations and applications for use in sports medicine and athletic training research. J. Athl. Train. 39, 286–277.
Pavao, S. L., Silva, F. P., Savelsbergh, G. J., and Rocha, N. A. (2015). Use of sensory information during postural control in children with cerebral palsy: systematic review. J. Mot. Behav. 47, 291–301. doi: 10.1080/00222895.2014.981498
Rethwilm, R., Bohm, H., Haase, M., Perchthaler, D., Dussa, C. U., Federolf, P., et al. (2021). Dynamic stability in cerebral palsy during walking and running: predictors and regulation strategies. Gait Posture. 84, 329–334. doi: 10.1016/j.gaitpost.2020.12.031
Rosenbaum, P., Paneth, N., Leviton, A., Goldstein, M., and Bax, M. (2007). The definition and classification of cerebral palsy. Dev. Med. Child Neurol. 49, 1–44. doi: 10.1111/j.1469-8749.2007.00201.x
Rossi, E. M., Nevalainen, P., Maenpaa, H., and Lauronen, L. (2018). Soleus H-reflex and its modulation with vibration in idiopathic toe walkers and typically developing children. J. Child Neurol. 33, 351–358. doi: 10.1177/0883073818759104
Ruzbarsky, J. J., Scher, D., and Dodwell, E. (2016). Toe walking: causes, epidemiology, assessment, and treatment. Curr. Opin. Pediatr. 28, 40–46. doi: 10.1097/MOP.0000000000000302
Sangeux, M., Viehweger, E., Romkes, J., and Bracht-Schweizer, K. (2024). On the clinical interpretation of overground gait stability indices in children with cerebral palsy. Sci. Rep. 14, 1–8. doi: 10.1038/s41598-024-76598-1
Schlough, K., Andre, K. S., Owen, M., Adelstein, L., Hartford, M. C., Javier, B., et al. (2020). Differentiating between idiopathic toe walking and cerebral palsy: a systematic review. Pediatr. Phys. Ther. 32, 2–10. doi: 10.1097/PEP.0000000000000659
Shulman, L. H., Sala, D. A., Chu, M. L. Y., McCaul, P. R., and Sandler, B. J. (1997). Developmental Implications of idiopathic toe walking. J Pediatr. 130, 541–546. doi: 10.1016/S0022-3476(97)70236-1
Sibley, K. M., Carpenter, M. G., Perry, J. C., and Frank, J. S. (2007). Effects of postural anxiety on the soleus H-reflex. Hum. Mov. Sci. 26, 103–112. doi: 10.1016/j.humov.2006.09.004
Simonsen, E. B., and Dyhre-Poulsen, P. (1999). Amplitude of the human soleus H reflex during walking and running. J Physiol. 515, 929–939. doi: 10.1111/j.1469-7793.1999.929ab.x
Skiadopoulos, A., Moore, E. E., Sayles, H. R., Schmid, K. K., and Stergiou, N. (2020). Step width variability as a discriminator of age-related gait changes. J. Neuroeng. Rehabil. 17, 1–13. doi: 10.1186/s12984-020-00671-9
Theodosiadou, A., Henry, M., Duchateau, J., and Baudry, S. (2023). Revisiting the use of Hoffmann reflex in motor control research on humans. Eur. J. Appl. Physiol. 123, 695–710. doi: 10.1007/s00421-022-05119-7
Thompson, A. K., and Wolpaw, J. R. (2021). H-reflex conditioning during locomotion in people with spinal cord injury. J Physiol. 599, 2453–2469. doi: 10.1113/JP278173
Thompson, N., Stebbins, J., Seniorou, M., and Newham, D. (2011). Muscle strength and walking ability in diplegic cerebral palsy: implications for assessment and management. Gait Posture 33, 321–325. doi: 10.1016/j.gaitpost.2010.10.091
Tracy, J. B., Petersen, D. A., Pigman, J., Conner, B. C., Wright, H. G., Modlesky, C. M., et al. (2019). Dynamic stability during walking in children with and without cerebral palsy. Gait Posture 72, 182–187. doi: 10.1016/j.gaitpost.2019.06.008
van den Hecke, A., Malghem, C., Renders, A., Detrembleur, C., Palumbo, S., Lejeune, T. M., et al. (2007). Mechanical work, energetic cost, and gait efficiency in children with cerebral palsy. J. Pediatr. Orthop. B 27, 643–647. doi: 10.1097/BPO.0b013e318093f4c3
van Kuijk, A. A., Kosters, R., Vugts, M., and Geurts, A. C. (2014). Treatment for idiopathic toe walking: a systematic review of the literature. J. Rehabil. Med. 46, 945–957. doi: 10.2340/16501977-1881
van Melick, N., Meddeler, B. M., Hoogeboom, T. J., Nijhuis-van der Sanden, M. W. G., and van Cingel, R. E. H. (2017). How to determine leg dominance: the agreement between self-reported and observed performance in healthy adults. PLoS ONE 12:e0189876. doi: 10.1371/journal.pone.0189876
Veerkamp, K., van der Krogt, M. M., Waterval, N. F. J., Geijtenbeek, T., Walsh, H. P. J., Harlaar, J., et al. (2023). Predictive simulations identify potential neuromuscular contributors to idiopathic toe walking. Clin. Biomech. 111:106152. doi: 10.1016/j.clinbiomech.2023.106152
Visscher, R. M. S., Sansgiri, S., Freslier, M., Harlaar, J., Brunner, R., Taylor, W. R., et al. (2021). Towards validation and standardization of automatic gait event identification algorithms for use in paediatric pathological populations. Gait Posture 86, 64–69. doi: 10.1016/j.gaitpost.2021.02.031
Wallard, L., Dietrich, G., Kerlirzin, Y., and Bredin, J. (2014). Balance control in gait children with cerebral palsy. Gait Posture 40, 43–47. doi: 10.1016/j.gaitpost.2014.02.009
Watson, F., Fino, P. C., Thornton, M., Heracleous, C., Loureiro, R., Leong, J. J. H., et al. (2021). Use of the margin of stability to quantify stability in pathologic gait - a qualitative systematic review. BMC Musculoskelet. Disord. 22:597. doi: 10.1186/s12891-021-04466-4
Williams, C. M., Tinley, P., and Curtin, M. (2010). Idiopathic toe walking and sensory processing dysfunction. J. Foot Ankle Res. 3:16. doi: 10.1186/1757-1146-3-16
Winter, D. A. (1995). Human balance and posture control during standing and walking. Gait Posture 3, 193–214. doi: 10.1016/0966-6362(96)82849-9
Zarkou, A., Lee, S. C. K., Prosser, L. A., Hwang, S., and Jeka, J. (2018). Stochastic resonance stimulation improves balance in children with cerebral palsy: a case control study. J. Neuroeng. Rehabil. 15, 1–12. doi: 10.1186/s12984-018-0467-7
Zarkou, A., Lee, S. C. K., Prosser, L. A., and Jeka, J. J. (2020). Foot and ankle somatosensory deficits affect balance, and motor function in children with cerebral palsy. Front. Hum. Neurosci. 14:45. doi: 10.3389/fnhum.2020.00045
Zeininger, A., Schmitt, D., Jensen, J. L., and Shapiro, L. J. (2018). Ontogenetic changes in foot strike pattern and calcaneal loading during walking in young children. Gait Posture 59, 18–22. doi: 10.1016/j.gaitpost.2017.09.027
Keywords: toe walking, dynamic stability, margin of stability, H-reflex, neuromotor control, weight loading and unloading
Citation: Gwerder M, Visscher RMS, Spescha A, Hosseini Nasab SH, Kim YK, Zibold R, Brunner R, Taylor WR, Viehweger E and Singh NB (2025) The effects of weight-bearing manipulations on gait and its underlying neural control mechanisms in toe walking children. Front. Hum. Neurosci. 19:1701454. doi: 10.3389/fnhum.2025.1701454
Received: 08 September 2025; Accepted: 23 October 2025;
Published: 10 November 2025.
Edited by:
Nadia Dominici, VU Amsterdam, NetherlandsReviewed by:
Yi-Ning Wu, University of Massachusetts Lowell, United StatesKrishnakumar Sankar, Rajalakshmi Engineering College, India
Copyright © 2025 Gwerder, Visscher, Spescha, Hosseini Nasab, Kim, Zibold, Brunner, Taylor, Viehweger and Singh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michelle Gwerder, bWljaGVsbGUuZ3dlcmRlckBoZXN0LmV0aHouY2g=
 Michelle Gwerder
Michelle Gwerder Rosa M. S. Visscher
Rosa M. S. Visscher Anusha Spescha1
Anusha Spescha1 Seyyed H. Hosseini Nasab
Seyyed H. Hosseini Nasab Yong K. Kim
Yong K. Kim William R. Taylor
William R. Taylor Elke Viehweger
Elke Viehweger Navrag B. Singh
Navrag B. Singh