- 1Department of Biomedical Sciences, Madda Walabu University Goba Referral Hospital, Bale-Robe, Ethiopia
- 2Department of Human Physiology, School of Medicine, College of Medicine and Health Science, University of Gondar, Gondar, Ethiopia
- 3Department of Epidemiology and Biostatistics, Institute of Public Health, College of Medicine and Health Science, University of Gondar, Gondar, Ethiopia
- 4Department of Reproductive Health, Institute of Public Health, College of Medicine and Health Science, University of Gondar, Gondar, Ethiopia
- 5Department of Anatomy, School of Medicine, College of Medicine and Health Science, University of Gondar, Gondar, Ethiopia
Introduction: Functional connectivity (FC) is the correlation between brain regions’ activities, studied through neuroimaging techniques like fMRI. It helps researchers understand brain function, organization, and dysfunction. Hyperthyroidism, characterized by high serum levels of free thyroxin and suppressed thyroid stimulating hormone, can lead to mood disturbance, cognitive impairment, and psychiatric symptoms. Excessive thyroid hormone exposure can enhance neuronal death and decrease brain volume, affecting memory, attention, emotion, vision, and motor planning.
Methods: We conducted thorough searches across Google Scholar, PubMed, Hinari, and Science Direct to locate pertinent articles containing original data investigating FC measures in individuals diagnosed with hyperthyroidism.
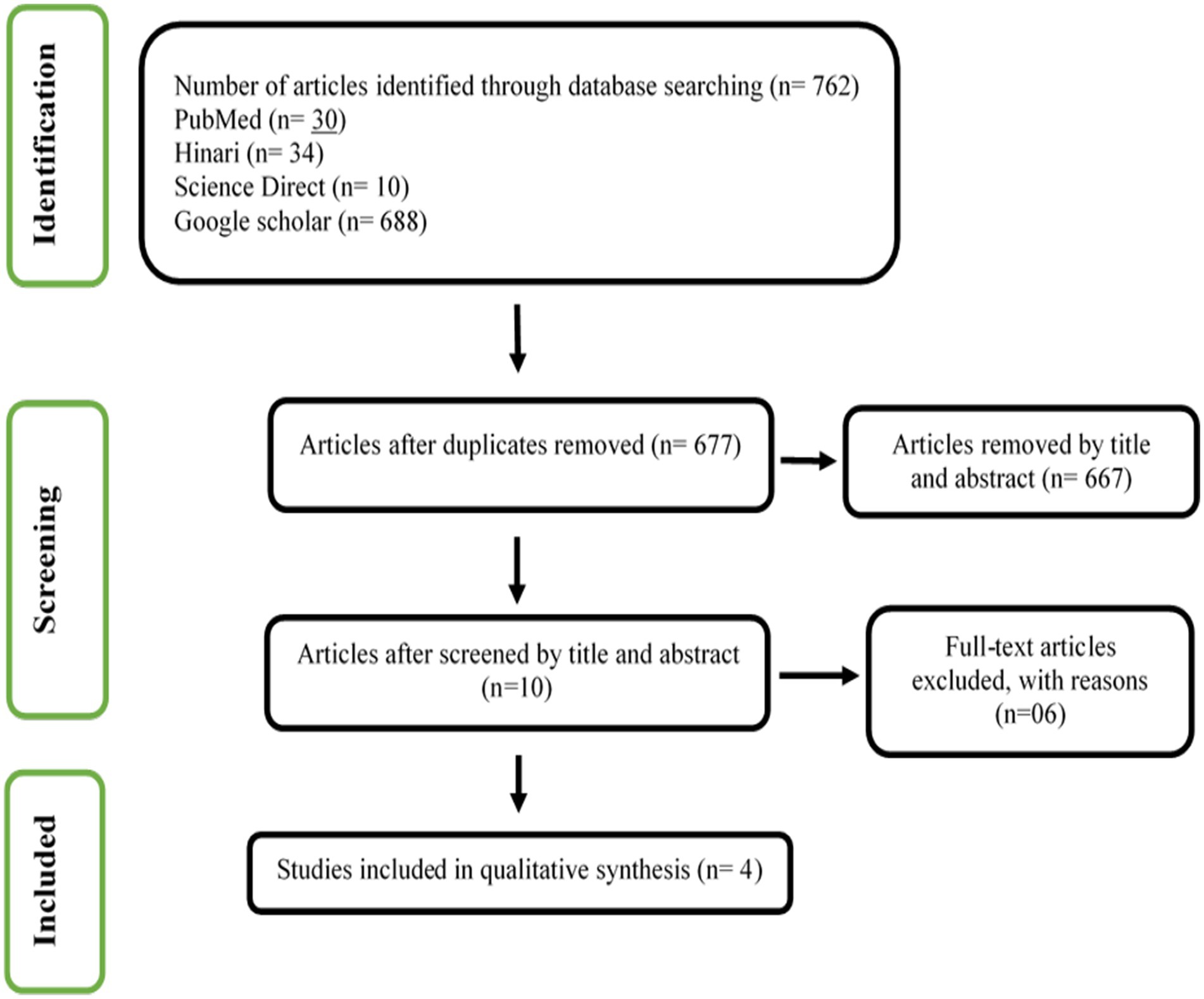
Results: The systematic review identified 762 articles, excluding duplicates and non-matching titles and abstracts. Four full-text articles were included in this review. In conclusion, a strong bilateral hippocampal connection in hyperthyroid individuals suggests a possible neurobiological influence on brain networks that may affect cognitive and emotional processing.
Systematic Review Registration: PROSPERO, CRD42024516216.
Introduction
Functional connectivity (FC) refers to the statistical correlation between the activities of different brain regions, typically observed through neuroimaging techniques such as functional magnetic resonance imaging (fMRI) (Müller, 2013; Cao et al., 2022; Ursino et al., 2022). Studies of it aim to understand how different brain regions communicate and coordinate their activities during various cognitive processes or in different states (Cao et al., 2022). Analyses of it have become increasingly important in neuroscience, offering valuable information about brain function, organization, and dysfunction. Researchers use these analyses to explore normal brain function, investigate neurological and psychiatric disorders, and assess the effects of interventions or treatments on brain connectivity patterns (Wojtalik et al., 2018).
Hyperthyroidism is defined as a high serum level of free thyroxin (FT4) and/or triiodothyronin (T3) and a suppressed thyroid stimulating hormone (TSH) level (Samuels, 2014; Ross et al., 2016). Thyroid hormone (TH) is essential for normal brain development and may also promote recovery and neuronal regeneration after brain injury (Liu and Brent, 2018; Talhada et al., 2019). Thyroid hormones are essential for appropriate growth, reproduction, and regulation of energy metabolism, neuronal development, and cognitive and behavioral development (Stasiolek, 2015; Taylor et al., 2018; Mathew et al., 2020). The mechanisms include the regulation of neuronal plasticity processes, stimulation of angiogenesis and neurogenesis, as we as modulating the dynamics of cytoskeletal elements, and intracellular transport processes (Talhada et al., 2019).
It is clear that without optimal thyroid function, mood disturbance, cognitive impairment, and other psychiatric symptoms can emerge (Lekurwale et al., 2023). In animal studies, changes in the release pattern of acetylcholine and monoamines have been found in the hippocampus and frontal cortex of experimentally induced hyperthyroid rats, along with associated functional changes (Eslami-Amirabadi and Sajjadi, 2021). Particularly in severe cases, thyroid dysfunction can result in a variety of emotional and cognitive disorders, such as executive function deficiencies, depression, anxiety, and irritability (Samuels, 2014; Stasiolek, 2015).
Related to the morphological changes of hyperthyroid individuals in the brain, exposure to excess thyroid hormones has been shown to enhance neuronal death and decrease brain volume (Folkestad et al., 2020), which leads to more severe atrophy of the amygdala (Wu et al., 2016; Eslami-Amirabadi and Sajjadi, 2021) and hippocampus (Wu et al., 2016; Eslami-Amirabadi and Sajjadi, 2021; Quinlan et al., 2022). Hyperthyroid patients exhibited reduced grey matter volume in regions associated with memory, attention, emotion, vision, and motor planning (Zhang et al., 2014).
The exploration of functional connectivity between brain regions is deemed essential to elucidate the neuropsychiatric symptoms associated with hyperthyroidism and the impact of elevated thyroid hormone levels on the adult brain (Cao et al., 2022; Lekurwale et al., 2023). Thyroid hormones play a crucial role in functional connectivity under physiological conditions (Schroeder and Privalsky, 2014). In the brain, T4 is converted to active T3 by type 2 deiodinase produced by glial cells, highlighting the importance of these hormones in brain development and function (Fingeret, 2024). Studies revealed functional connectivity changes in hyperthyroid patients, an increase in functional connectivity in the rostral temporal lobes, which is integrated with the cognitive control network (Göbel et al., 2020), lower amplitude of low-frequency fluctuations (ALFF) was found in the patients in the right posterior cingulate cortex, and increased functional connectivity in the bilateral anterior and posterior insula, and importantly, in the left anterior lobe of the cerebellum (Göbel et al., 2020). Research has shown that thyroid hormone functions may play a crucial role in modulating functional connectivity in early-course schizophrenia, impacting cognition and functional outcomes (George et al., 2023), resting-state brain network functional connectivity, and shedding light on the intricate relationship between thyroid function and brain network dynamics (Li et al., 2022).
Despite the significance of certain brain regions in emotional and cognitive regulation, there is a notable gap in research pertaining to the interactions between and within these regions in hyperthyroid patients. This review highlights hyperthyroidism’s potential impact on connectivity between brain regions and improves our understanding of the functional connectivity of targeted regions.
Method
Registration and protocol
This study protocol is registered with the International Prospective Register of Systematic Reviews website (PROSPERO; registration number CRD42024516216).
Eligibility criteria
Hyperthyroid patients: all patients who have elevated serum FT3 or FT4 levels, and decreased TSH levels (Ross et al., 2016; Toyib et al., 2019).
Pre/post studies: one experimental session was performed before and one after the end of administration of medications or procedures to assess the impact of medications like anti-thyroid drugs, radioiodine therapy, beta blockers, and thyroidectomy on patients with hyperthyroidism (Doubleday and Sippel, 2020).
We applied the PICO method as a selection criteria for articles:
Population: hyperthyroid patients.
Interventions: thyroid hormone thyroxin replacement therapies, for example, levothyroxine.
Study type: randomized controlled trials, case–control studies, and quasi-experimental studies.
Cases: hyperthyroid patients.
Control: healthy controls.
Outcomes: primary outcome– brain functional connectivity.
Outcome assessment time: There was no limit to the outcome assessment time.
Publication year and language: English-language literature, with publication year not limited. List of countries: all countries in the world.
Search strategy and selection criteria
Four databases– PubMed, Hinari, Science Direct, and Google Scholar– were used to identify studies about brain functional connectivity from the inception date to November 21, 2023. Using title, abstract, and keywords, we searched out the primary studies using the keywords selected: brain, connectivity, network, hyperthyroidism, and their synonyms using AND, OR, and NOT filters as described in Supplementary file 2. This systematic review was prepared according to the instructions of the PRISMA guideline.
Data extraction
We developed a form to extract the suitable data, including the following details: (1) characteristics of the papers (authors, publication year, and country); (2) characteristics of the participants (sample size, age range, and drug use); (3) study design and measurement method; (4) method of analysis; and (5) results. Two authors (ET and LM) independently extracted the data, and disagreements were resolved by discussing with the third author (MG).
Results
Identification of eligible studies
Figure 1 shows the result of our screening process. We identified 762 articles with our searching strategy. Duplicate articles (n = 85) were excluded. The articles that according to title and abstract did not match the selection criteria (n = 667) were also excluded. Finally, four articles out of 10 available full-text articles were included in this systematic review. The details of the excluded six articles are presented in Supplementary file S1.
Characteristics of included studies
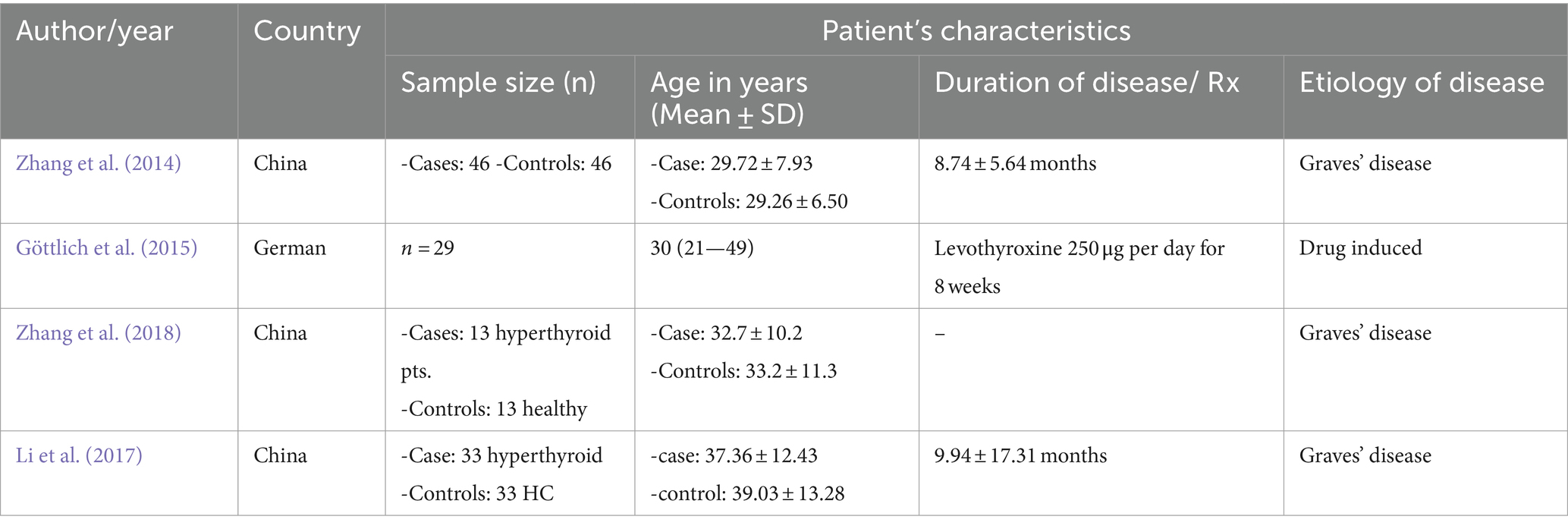
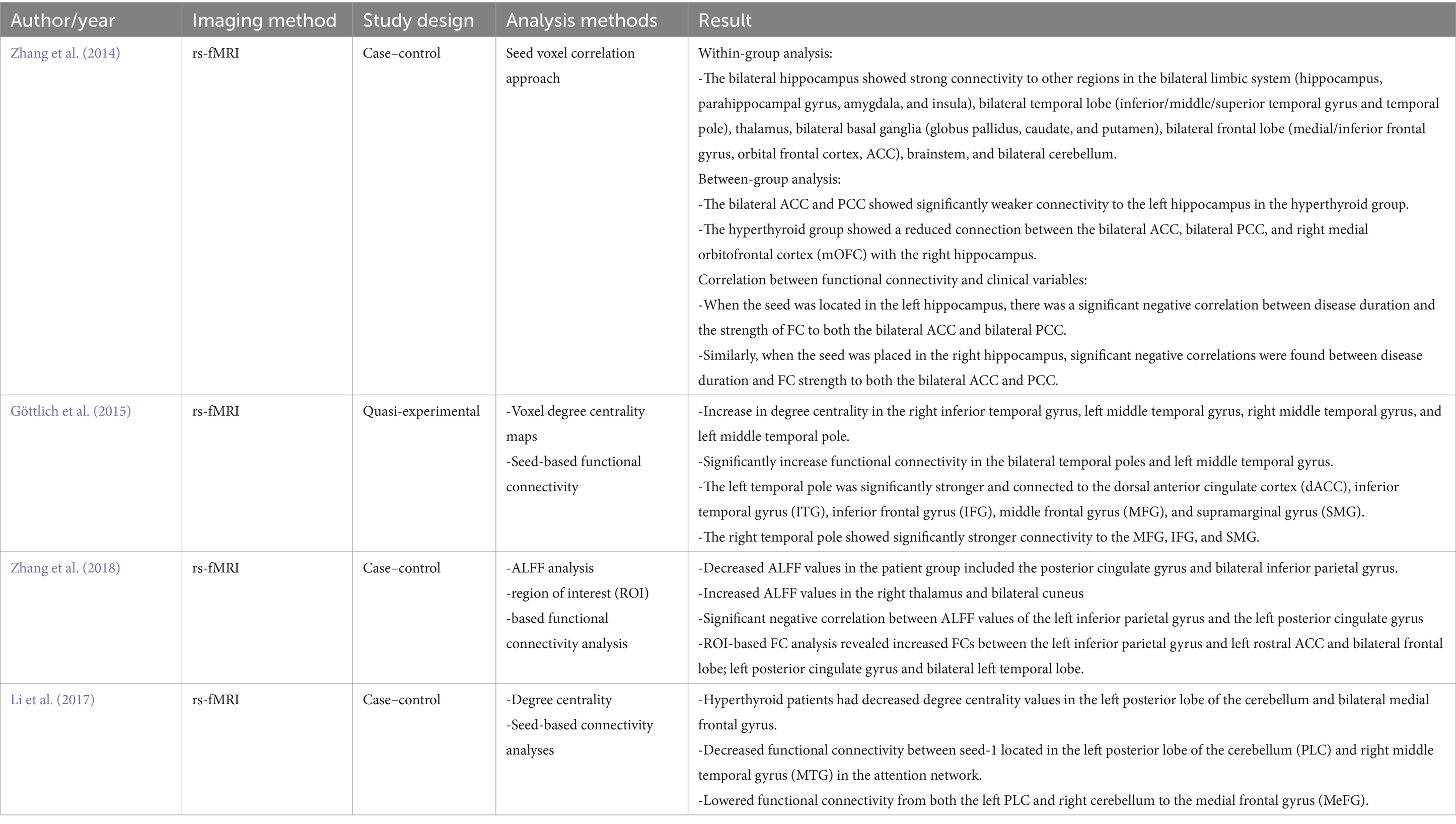
The included studies were either case–control or quasi-experimental studies. The etiology of the disease in the three studies was Graves’ disease, and one drug-induced pre-and post-study. They were all small studies, with the largest sample size of 47. General characteristics of the studies, like the first author’s name, year of publication, country, sample size (case/control or pre-post), age range of participants, and drug use for the study, are shown in Table 1, and the imaging method, study design, analysis method, and results are presented in Table 2.
Discussion
Reviewing the available evidence, we find significant changes in brain functional connectivity among hyperthyroid patients. These alterations imply that hyperthyroidism may impact brain networks neurobiologically. Studying connectivity patterns in healthy individuals and those with hyperthyroidism can help us understand disruptions in thyroid dysfunction networks, clarify cognitive and emotional symptoms in thyroid disorders, and guide future therapeutic interventions targeting neural circuits. In hyperthyroid patients, alterations in functional connectivity have been observed, particularly in regions associated with emotion regulation, memory, and cognitive processing (Chen et al., 2021). Changes in FC observed in hyperthyroid patients can be attributed to several mechanisms and could explain the manifestations of different disorders.
Recent advancements in neuroimaging techniques have shed light on the intricate neural alterations accompanying this disorder (Minnerop et al., 2018; Yen et al., 2023). Among these, changes in FC within the brain have emerged as a critical area of investigation. The observed connectivity between hyperthyroid patients and healthy controls suggests shared neural circuitry, potentially crucial for detecting the hippocampal memory system’s operation in humans (Liu et al., 2017; Ma et al., 2022).
One of the central findings of the reviewed papers is the disruption in connectivity patterns involving the hippocampus and cingulate cortex. Zhang et al’s. (2014) study found that hyperthyroid individuals show weakened connectivity between the bilateral ACC and PCC and the hippocampi. This suggests that hyperthyroidism affects the limbic system, which is crucial for memory consolidation and emotional regulation. The alterations may indicate cognitive or mental disorders associated with the hippocampus and other brain areas (Li et al., 2017; Yao et al., 2022). Hyper-connectivity patterns may affect the functional connectivity of the default mode network, potentially impacting episodic memory and self-representation (Zanão et al., 2017; Staffaroni et al., 2018; Ursino et al., 2022). The direct effects of thyroid hormones on these brain regions contribute to their functional integrity and connectivity (Biswas and Dey, 2014). Thyroid hormones have receptors in the cingulate cortices and hippocampi. T3 and T4 influence neurotransmitter systems such as glutamate (Ritchie and Yeap, 2015; Zhu et al., 2022), and gamma-aminobutyric acid (GABA) (Yi et al., 2014; Prisciandaro et al., 2021), which are crucial for synaptic transmission and neuronal plasticity in the cingulate cortices (Prisciandaro et al., 2021). Alterations in thyroid hormone levels can disrupt the balance of excitatory and inhibitory neurotransmission, leading to changes in neural connectivity and function within the ACC and PCC and impairing hippocampal function, leading to deficits in memory consolidation, emotional processing, and spatial navigation (Bavarsad et al., 2019).
Moreover, the correlation between FC strength and clinical variables provides valuable insights into the progression of the disease (Zhang et al., 2014). A significant negative correlation was found between disease duration and FC strength between the hippocampi and cingulate cortices (Zhang et al., 2014; Milton et al., 2022). This suggests that as the disease progresses, there is a decline in the integrity of neural circuits linking these regions (Zhi et al., 2018; Johansson et al., 2023), due to adaptive changes or neuronal damage in hyperthyroid patients. In addition, chronic hyperthyroidism could lead to structural (Zhang et al., 2014; Zhe et al., 2021; Duda et al., 2023; Xiong et al., 2023), and functional changes in the hippocampi and cingulate cortices, affecting their connectivity patterns. This is clinically important in identifying neuroimaging markers that can be used to track the progression of hyperthyroidism and assess the effectiveness of treatment interventions (Clerc, 2020).
Beyond hippocampal-cingulate alterations, hyperthyroidism is also associated with changes in FC involving regions crucial for cognitive processing and emotional regulation (Göttlich et al., 2015). Increased degree centrality was observed in temporal regions, including the right inferior temporal gyrus, left middle temporal gyrus, right middle temporal gyrus, and left middle temporal pole. Additionally, there was a significant increase in FC within the bilateral temporal poles and left middle temporal gyrus (Göttlich et al., 2015; Zhang et al., 2018), indicating heightened connectivity within temporal regions. Notably, the left temporal pole exhibited stronger connections with various regions, including the dACC, ITG, and frontal gyrus, underscoring the widespread impact of hyperthyroidism on functional brain networks. Degree centrality refers to the number of connections a node (brain region) has with other nodes in the network (Yoo et al., 2017; Jia et al., 2019). The heightened degree centrality indicates increased functional connectivity and communication within these temporal regions. This indicates increased synchronization and information exchange within these regions (Csató, 2017; Lorenzini et al., 2022). The study suggests that hyperthyroid patients’ cognitive deficits may be linked to disrupted functional coordination within the default mode network (DMN), emphasizing the significance of interhemispheric connectivity (Zhi et al., 2018; Berron et al., 2020; Wang et al., 2023).
Conversely, decreased ALFF was noted in regions such as the posterior cingulate gyrus and bilateral inferior parietal gyrus (Zhang et al., 2018), suggesting reduced neural activity. In association with this, Milton et al.’s ROI-based functional connectivity analysis reveals changes in connectivity patterns in the inferior parietal gyrus and posterior cingulate gyrus, indicating complex regional dynamics (Uddin et al., 2009). Additionally, disruptions in FC were observed in cerebellar-frontal circuits, with decreased connectivity between the left PLC and MTG within the attention network (Li et al., 2017). Besides its motor coordination ability, the cerebellum increasingly recognized for its role in cognitive functions, including attention (Li et al., 2017; Liu et al., 2017; Yao et al., 2022). Dysfunction within the cerebellum and frontal regions impairs the coordination and modulation of attention networks (Arif et al., 2020). Damage to the tract and disruptions in neuronal synchronization between the cerebellum and frontal cortex may contribute to decreased functional connectivity (Wang et al., 2023). Cognitive ability is affected by reduced connectivity between cortical regions, particularly the prefrontal cortex, and sub-cortical regions in schizophrenia (Sheffield and Barch, 2016), bipolar disease (Ursino et al., 2022), depression (Liu et al., 2020), traumatic brain injury (Morelli et al., 2021; Nakuci et al., 2021), stroke (Wang et al., 2023), and functional seizure (Foroughi et al., 2020).
Taken together, these findings highlight the complex nature of the brain changes linked to hyperthyroidism. The dysregulation of thyroid hormones affects multiple pathways and mechanisms within the brain, leading to diverse neurological manifestations. This complexity underscores the need for a comprehensive understanding and management of the neurological aspects of hyperthyroidism. The findings open the door for additional research into the functional implications of these connectivity changes and how they might impact the mental and emotional health of hyperthyroid patients, in addition to expanding our understanding of the brain mechanisms underlying thyroid dysfunction (Ritchie and Yeap, 2015; Eslami-Amirabadi and Sajjadi, 2021). Combining these many viewpoints allows for a more thorough understanding of the complex relationship between thyroid function and brain connections.
Limitations
This systematic review had some limitations.
• The exploration of functional connectivity in neuroscience has encountered limitations, with a paucity of comprehensive studies on the intricate networks that govern brain function. To advance our understanding of the dynamic relationships between distinct brain regions, there is a pressing need for more extensive studies on brain functional connectivity in patients with hyperthyroidism.
• A significant limitation frequently encountered in research is the small sample size. Small sample sizes can magnify individual differences and chance variations, making it challenging to draw robust conclusions or to establish the true effect of an intervention or phenomenon. The studies included in this review had a small sample size, with a minimum of 13 and a maximum of 46.
• All studies used fMRI as the imaging technique. It has limitations compared to other connectivity techniques, including lower temporal resolution (vs. EEG/MEG), sensitivity to motion artifacts, and reliance on blood flow measurement. Techniques like EEG and MEG offer better temporal resolution.
Conclusion and recommendation
In conclusion, research on brain functional connectivity among patients with hyperthyroidism suggests a potential neurobiological impact of hyperthyroidism on intricate brain networks. This study found strong bilateral hippocampal connectivity across various brain regions, suggesting a fundamental neural network. Alterations in connectivity patterns suggest a potential hub role in hyperthyroid states, affecting cognitive and emotional processing. These findings highlight the complex nature of brain changes linked to hyperthyroidism and suggest the need for further investigations into the functional effects of these connectivity alterations on mental and emotional well-being.
We suggest exploring how changes in connectivity affect thinking and emotions in hyperthyroidism patients to help develop better mental health treatments. Furthermore, given the recognized challenge of small sample sizes in research, it is advisable for future studies to strive for larger and more representative samples to enhance the reliability and generalizability of the findings. Additionally, researchers should consider diversifying imaging techniques beyond fMRI to overcome its limitations such as lower temporal resolution and susceptibility to motion artifacts.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
ET: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. MG: Conceptualization, Data curation, Methodology, Supervision, Writing – original draft, Writing – review & editing. DeA: Conceptualization, Data curation, Methodology, Writing – original draft, Writing – review & editing. DaA: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. LM: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2024.1383355/full#supplementary-material
References
Arif, Y., Spooner, R. K., Wiesman, A. I., Embury, C. M., Proskovec, A. L., and Wilson, T. W. (2020). Modulation of attention networks serving reorientation in healthy aging. Aging (Albany NY) 12, 12582–12597. doi: 10.18632/aging.103515
Bavarsad, K., Hosseini, M., Hadjzadeh, M. A. R., and Sahebkar, A. (2019). The effects of thyroid hormones on memory impairment and Alzheimer's disease. J. Cell. Physiol. 234, 14633–14640. doi: 10.1002/jcp.28198
Berron, D., van Westen, D., Ossenkoppele, R., Strandberg, O., and Hansson, O. (2020). Medial temporal lobe connectivity and its associations with cognition in early Alzheimer's disease. Brain J. Neurol. 143, 1233–1248. doi: 10.1093/brain/awaa068
Biswas, T., and Dey, S. K. (2014). Association of Thyroid Dysfunction and Mood Disorders and role of imaging: a review. Bangladesh J. 17, 146–152. doi: 10.3329/bjnm.v17i2.28202
Cao, J., Zhao, Y., Shan, X., Wei, H. L., Guo, Y., Chen, L., et al. (2022). Brain functional and effective connectivity based on electroencephalography recordings: a review. Hum. Brain Mapp. 43, 860–879. doi: 10.1002/hbm.25683
Chen, W., Wu, Q., Chen, L., Zhou, J., Chen, H.-H., Xu, X.-Q., et al. (2021). Disrupted spontaneous neural activity in patients with thyroid-associated ophthalmopathy: a resting-state fMRI study using amplitude of low-frequency fluctuation. Front. Hum. Neurosci. 15:676967. doi: 10.3389/fnhum.2021.676967
Clerc, J. (2020). Quantified 123I-thyroid scan based classification of hyperthyroidism. Médecine Nucléaire. 44, 231–249. doi: 10.1016/j.mednuc.2020.07.005
Csató, L. (2017). Measuring centrality by a generalization of degree. CEJOR 25, 771–790. doi: 10.1007/s10100-016-0439-6
Doubleday, A. R., and Sippel, R. S. (2020). Hyperthyroidism. Gland Surg. 9, 124–135. doi: 10.21037/gs.2019.11.01
Duda, M., Faghiri, A., Belger, A., Bustillo, J. R., Ford, J. M., Mathalon, D. H., et al. (2023). Alterations in grey matter structure linked to frequency-specific cortico-subcortical connectivity in schizophrenia via multimodal data fusion. bio Rxiv [Preprint]. doi: 10.1101/2023.07.05.547840
Eslami-Amirabadi, M., and Sajjadi, S. A. (2021). The relation between thyroid dysregulation and impaired cognition/behaviour: an integrative review. J. Neuroendocrinol. 33:e12948. doi: 10.1111/jne.12948
Fingeret, MAEAA . Physiology, thyroid function: Stat pearls. Treasure Island (FL): StatPearls Publishing, (2024).
Folkestad, L., Brandt, F., Lillevang-Johansen, M., Brix, T. H., and Hegedüs, L. (2020). Graves' disease and toxic nodular goiter, aggravated by duration of hyperthyroidism, are associated with alzheimer's and vascular dementia: a registry-based long-term follow-up of two large cohorts. Thyroid 30, 672–680. doi: 10.1089/thy.2019.0672
Foroughi, A. A., Nazeri, M., and Asadi-Pooya, A. A. (2020). Brain connectivity abnormalities in patients with functional (psychogenic nonepileptic) seizures: a systematic review. Seizure 81, 269–275. doi: 10.1016/j.seizure.2020.08.024
George, A. B., Beniwal, R. P., Singh, S., Bhatia, T., Khushu, S., and Deshpande, S. N. (2023). Association between thyroid functions, cognition, and functional connectivity of the brain in early-course schizophrenia: a preliminary study. Ind. Psychiatry J. 32, S76–s82. doi: 10.4103/ipj.ipj_198_23
Göbel, A., Göttlich, M., Reinwald, J., Rogge, B., Uter, J.-C., Heldmann, M., et al. (2020). The influence of thyroid hormones on brain structure and function in humans. Exp. Clin. Endocrinol. Diabetes 128, 432–436. doi: 10.1055/a-1101-9090
Göttlich, M., Heldmann, M., Göbel, A., Dirk, A. L., Brabant, G., and Münte, T. F. (2015). Experimentally induced thyrotoxicosis leads to increased connectivity in temporal lobe structures: a resting state fMRI study. Psychoneuroendocrinology 56, 100–109. doi: 10.1016/j.psyneuen.2015.03.009
Jia, P., Liu, J., Huang, C., Liu, L., and Xu, C. (2019). An improvement method for degree and its extending centralities in directed networks. Physica A 532:121891. doi: 10.1016/j.physa.2019.121891
Johansson, B., Holmberg, M., Skau, S., Malmgren, H., and Nyström, H. F. (2023). The relationship between mental fatigue, depression, and cognition in graves’ disease. European Thyroid J. 12. doi: 10.1530/ETJ-23-0040
Lekurwale, V., Acharya, S., Shukla, S., and Kumar, S. (2023). Neuropsychiatric manifestations of thyroid diseases. Cureus 15:e33987. doi: 10.7759/cureus.33987
Li, Y., Qin, Y., Luo, Z., and Zhou, J. (2022). The resting-state brain network functional connectivity changes in patients with acute thyrotoxic myopathy based on independent component analysis. Front. Endocrinol. 13:829411. doi: 10.3389/fendo.2022.829411
Li, L., Zhi, M., Hou, Z., Zhang, Y., Yue, Y., and Yuan, Y. (2017). Abnormal brain functional connectivity leads to impaired mood and cognition in hyperthyroidism: a resting-state functional MRI study. Oncotarget 8, 6283–6294. doi: 10.18632/oncotarget.14060
Liu, Y. Y., and Brent, G. A. (2018). Thyroid hormone and the brain: mechanisms of action in development and role in protection and promotion of recovery after brain injury. Pharmacol. Ther. 186, 176–185. doi: 10.1016/j.pharmthera.2018.01.007
Liu, B., Ran, Q., Liu, D., Zhang, S., and Zhang, D. (2017). Changes in resting-state cerebral activity in patients with hyperthyroidism: a short-term follow-up functional MR imaging study. Sci. Rep. 7:10627. doi: 10.1038/s41598-017-10747-7
Liu, B., Wen, L., Ran, Q., Zhang, S., Hu, J., Gong, M., et al. (2020). Dysregulation within the salience network and default mode network in hyperthyroid patients: a follow-up resting-state functional MRI study. Brain Imaging Behav. 14, 30–41. doi: 10.1007/s11682-018-9961-6
Lorenzini, L., Ingala, S., Collij, L. E., Wottschel, V., Haller, S., Blennow, K., et al. (2022). Functional eigenvector centrality dynamics are related to amyloid deposition in preclinical Alzheimer’s disease. Alzheimers Dement. 18:e064631. doi: 10.1002/alz.064631
Ma, Q., Rolls, E. T., Huang, C. C., Cheng, W., and Feng, J. (2022). Extensive cortical functional connectivity of the human hippocampal memory system. Cortex 147, 83–101. doi: 10.1016/j.cortex.2021.11.014
Mathew, C. J., Jose, M. T., Elshaikh, A. O., Shah, L., Lee, R., and Cancarevic, I. (2020). Is hyperthyroidism a possible etiology of early onset dementia? Cureus 12. doi: 10.7759/cureus.10603
Milton, C. K., O’Neal, C. M., and Conner, A. K. (2022). Functional connectivity of hippocampus in temporal lobe epilepsy depends on hippocampal dominance: a systematic review of the literature. J. Neurol. 269, 221–232. doi: 10.1007/s00415-020-10391-8
Minnerop, M., Gliem, C., and Kornblum, C. (2018). Current progress in CNS imaging of myotonic dystrophy. Front. Neurol. 9:382932. doi: 10.3389/fneur.2018.00646
Morelli, N., Johnson, N. F., Kaiser, K., Andreatta, R. D., Heebner, N. R., and Hoch, M. C. (2021). Resting state functional connectivity responses post-mild traumatic brain injury: a systematic review. Brain Inj. 35, 1326–1337. doi: 10.1080/02699052.2021.1972339
Müller, R.-A. (2013). “Functional connectivity” in Encyclopedia of autism Spectrum disorders. ed. F. R. Volkmar (Springer New York: New York, NY), 1363–1370.
Nakuci, J., McGuire, M., Schweser, F., Poulsen, D., and Muldoon, S. F. (2021). Differential patterns of change in brain connectivity resulting from traumatic brain injury. bio Rxiv. 12, 799–811. doi: 10.1101/2021.10.27.466136
Prisciandaro, J. J., Hoffman, M., Brown, T. R., Voronin, K., Book, S., Bristol, E., et al. (2021). Effects of gabapentin on dorsal anterior cingulate cortex GABA and glutamate levels and their associations with abstinence in alcohol use disorder: a randomized clinical trial. Am. J. Psychiatry 178, 829–837. doi: 10.1176/appi.ajp.2021.20121757
Quinlan, P., Horvath, A., Eckerström, C., Wallin, A., and Svensson, J. (2022). Higher thyroid function is associated with accelerated hippocampal volume loss in Alzheimer’s disease. Psychoneuroendocrinology 139:105710. doi: 10.1016/j.psyneuen.2022.105710
Ritchie, M., and Yeap, B. B. (2015). Thyroid hormone: influences on mood and cognition in adults. Maturitas 81, 266–275. doi: 10.1016/j.maturitas.2015.03.016
Ross, D. S., Burch, H. B., Cooper, D. S., Greenlee, M. C., Laurberg, P., Maia, A. L., et al. (2016). 2016 American Thyroid Association guidelines for diagnosis and Management of Hyperthyroidism and Other Causes of thyrotoxicosis. Thyroid 26, 1343–1421. doi: 10.1089/thy.2016.0229
Samuels, M. H. (2014). Thyroid disease and cognition. Endocrinol. Metab. Clin. 43, 529–543. doi: 10.1016/j.ecl.2014.02.006
Schroeder, A. C., and Privalsky, M. L. (2014). Thyroid hormones, t3 and t4, in the brain. Front. Endocrinol. 5:80680. doi: 10.3389/fendo.2014.00040
Sheffield, J. M., and Barch, D. M. (2016). Cognition and resting-state functional connectivity in schizophrenia. Neurosci. Biobehav. Rev. 61, 108–120. doi: 10.1016/j.neubiorev.2015.12.007
Staffaroni, A. M., Brown, J. A., Casaletto, K. B., Elahi, F. M., Deng, J., Neuhaus, J., et al. (2018). The longitudinal trajectory of default mode network connectivity in healthy older adults varies as a function of age and is associated with changes in episodic memory and processing speed. J. Neurosci. 38, 2809–2817. doi: 10.1523/JNEUROSCI.3067-17.2018
Stasiolek, M. (2015). Neurological symptoms and signs in thyroid disease. Thyroid. Res. 8:A25. doi: 10.1186/1756-6614-8-S1-A25
Talhada, D., Santos, C. R. A., Gonçalves, I., and Ruscher, K. (2019). Thyroid hormones in the brain and their impact in recovery mechanisms after stroke. Front. Neurol. 10:1103. doi: 10.3389/fneur.2019.01103
Taylor, P. N., Albrecht, D., Scholz, A., Gutierrez-Buey, G., Lazarus, J. H., Dayan, C. M., et al. (2018). Global epidemiology of hyperthyroidism and hypothyroidism. Nat. Rev. Endocrinol. 14, 301–316. doi: 10.1038/nrendo.2018.18
Toyib, S., Kabeta, T., Dendir, G., Bariso, M., and Reta, W. (2019). Prevalence, clinical presentation and patterns of thyroid disorders among anterior neck mass patients visiting Jimma medical center, Southwest Ethiopia. Biomed J Sci Tech Res. 18, 13431–13435. doi: 10.26717/BJSTR.2019.18.003126
Uddin, L. Q., Kelly, A. M., Biswal, B. B., Castellanos, F. X., and Milham, M. P. (2009). Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum. Brain Mapp. 30, 625–637. doi: 10.1002/hbm.20531
Ursino, M., Magosso, E., and Petti, M. (2022). Neural networks and connectivity among brain regions. Brain Sci. 12:346. doi: 10.3390/brainsci12030346
Wang, X., Xia, J., Wang, W., Lu, J., Liu, Q., Fan, J., et al. (2023). Disrupted functional connectivity of the cerebellum with default mode and frontoparietal networks in young adults with major depressive disorder. Psychiatry Res. 324:115192. doi: 10.1016/j.psychres.2023.115192
Wang, Y., Yang, L., and Liu, J. (2023). Causal associations between functional/structural connectivity and stroke: a bidirectional Mendelian randomization study. Biomedicines 11:1575. doi: 10.3390/biomedicines11061575
Wang, M., Zhao, G., Jiang, Y., Lu, T., Wang, Y., Zhu, Y., et al. (2023). Disconnection of network hubs underlying the executive function deficit in patients with ischemic Leukoaraiosis. J. Alzheimers Dis. 94, 1–10. doi: 10.3233/JAD-230048
Wojtalik, J. A., Eack, S. M., Smith, M. J., and Keshavan, M. S. (2018). Using cognitive neuroscience to improve mental health treatment: a comprehensive review. J. Soc. Soc. Work Res. 9, 223–260. doi: 10.1086/697566
Wu, Y., Pei, Y., Wang, F., Xu, D., and Cui, W. (2016). Higher FT4 or TSH below the normal range are associated with increased risk of dementia: a meta-analysis of 11 studies. Sci. Rep. 6:31975. doi: 10.1038/srep31975
Xiong, Y., Ye, C., Sun, R., Chen, Y., Zhong, X., Zhang, J., et al. (2023). Disrupted balance of gray matter volume and directed functional connectivity in mild cognitive impairment and Alzheimer’s disease. Curr. Alzheimer Res. 20, 161–174. doi: 10.2174/1567205020666230602144659
Yao, Y., Lu, C., Chen, J., Sun, J., Zhou, C., Tan, C., et al. (2022). Increased resting-state functional connectivity of the Hippocampus in rats with Sepsis-associated encephalopathy. Front. Neurosci. 16. doi: 10.3389/fnins.2022.894720
Yen, C., Lin, C.-L., and Chiang, M.-C. (2023). Exploring the frontiers of neuroimaging: a review of recent advances in understanding brain functioning and disorders. Life. 13:1472. doi: 10.3390/life13071472
Yi, J., Zheng, J.-y., Zhang, W., Wang, S., Yang, Z.-f., and Dou, K.-f. (2014). Decreased pain threshold and enhanced synaptic transmission in the anterior cingulate cortex of experimental hypothyroidism mice. Mol. Pain 10:1744-8069-10-38. doi: 10.1186/1744-8069-10-38
Yoo, K., Lee, P., Chung, M. K., Sohn, W. S., Chung, S. J., Na, D. L., et al. (2017). Degree-based statistic and center persistency for brain connectivity analysis. Hum. Brain Mapp. 38, 165–181. doi: 10.1002/hbm.23352
Zanão, T. A., Martins Lopes, T., Machado de Campos, B., Nogueira, M. H., Yasuda, C. L., and Cendes, F. (2017). Default mode network in temporal lobe epilepsy: interactions with memory performance. bio Rxiv. :205476. doi: 10.1101/205476
Zhang, W., Liu, X., Zhang, Y., Song, L., Hou, J., Chen, B., et al. (2014). Disrupted functional connectivity of the hippocampus in patients with hyperthyroidism: evidence from resting-state fMRI. Eur. J. Radiol. 83, 1907–1913. doi: 10.1016/j.ejrad.2014.07.003
Zhang, M., Ma, X., Ma, S., and Ling, X. (2018). Resting-state functional connectivity in untreated overt hyperthyroidism (graves’ disease) with mood disorders 2018. Austria, Vienna: European Congress of Radiology.
Zhang, W., Song, L., Yin, X., Zhang, J., Liu, C., Wang, J., et al. (2014). Grey matter abnormalities in untreated hyperthyroidism: a voxel-based morphometry study using the DARTEL approach. Eur. J. Radiol. 83, e43–e48. doi: 10.1016/j.ejrad.2013.09.019
Zhe, X., Zhang, X., and Zhang, D. (2021). Altered gray matter volume and functional connectivity in patients with vestibular migraine. Front. Neurosci. 15:683802. doi: 10.3389/fnins.2021.683802
Zhi, M., Hou, Z., Zhang, Y., Yue, Y., Li, L., and Yuan, Y. (2018). Cognitive deficit-related interhemispheric asynchrony within the medial hub of the default mode network aids in classifying the hyperthyroid patients. Neural Plast. 2018, 1–7. doi: 10.1155/2018/9023604
Keywords: brain, fMRI, functional connectivity, hyperthyroid, resting-state fMRI
Citation: Tesfaye E, Getnet M, Anmut Bitew D, Adugna DG and Maru L (2024) Brain functional connectivity in hyperthyroid patients: systematic review. Front. Neurosci. 18:1383355. doi: 10.3389/fnins.2024.1383355
Edited by:
Nicola Simola, University of Cagliari, ItalyReviewed by:
Annibale Antonioni, University of Ferrara, ItalyHelge Malmgren, University of Gothenburg, Sweden
Copyright © 2024 Tesfaye, Getnet, Anmut Bitew, Adugna and Maru. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ephrem Tesfaye, ephremtesfaye126@gmail.com
 Ephrem Tesfaye
Ephrem Tesfaye Mihret Getnet
Mihret Getnet Desalegn Anmut Bitew
Desalegn Anmut Bitew Dagnew Getnet Adugna
Dagnew Getnet Adugna Lemlemu Maru
Lemlemu Maru